Abstract
Mesenchymal stem cells (MSCs) are multipotent tissue stem cells that differentiate into a number of mesodermal tissue types, including osteoblasts, adipocytes, chondrocytes and myofibroblasts. MSCs were originally identified in the bone marrow (BM) of humans and other mammals, but recent studies have shown that they are multilineage progenitors in various adult organs and tissues. MSCs that localize at perivascular sites function to rapidly respond to external stimuli and coordinate with the vascular and immune systems to accomplish the wound healing process. Cancer, considered as wounds that never heal, is also accompanied by changes in MSCs that parallels the wound healing response. MSCs are now recognized as key players at distinct steps of tumorigenesis. In this review, we provide an overview of the function of MSCs in wound healing and cancer progression with the goal of providing insight into the development of novel MSC-manipulating strategies for clinical cancer treatment.
Keywords: Mesenchymal stem cells, Regeneration, Cancer, Wound healing, Tumor microenvironment
1. Introduction
Mesenchymal stem cells (MSCs) are multipotent progenitor cells with the potential to differentiate into diverse types of tissue cells, including osteoblasts, adipocytes, chondrocytes and myofibroblasts (Keating, 2012; Singer & Caplan, 2011). This type of tissue stem cell plays an essential role in tissue regeneration and closely interacts with cells of the immune system in the tissue microenvironment during repair from tissue damage (Le Blanc & Davies, 2015; Y. Shi et al., 2012; Uccelli, Moretta, & Pistoia, 2008). Recently, MSCs have also emerged as a new player in the tumor microenvironment, contributing to tumor growth, metastasis and therapeutic resistance (Ridge, Sullivan, & Glynn, 2017; Y. Shi, Du, Lin, & Wang, 2017).
Cancer, regarded as “non-healing wounds” (Dvorak, 1986, 2015), is believed to take advantage of the regenerative functions of host cells to facilitate local cancer growth, resistance to therapy, and metastases to distant organs (Calvo et al., 2015; Krall et al., 2018; Sundaram et al., 2017). In this review, we discuss how MSCs participate in distinct stages of wound healing and tumor “wound” progression, and compare the functions and mechanisms of MSCs in these two pathological processes. Understanding how the tumor microenvironmental cues drive MSCs to regenerate tumor “wounds” will facilitate our deeper understanding of MSC biology in distinct steps of cancer progression, thereby supporting development of new cancer treatments that target MSCs.
2. Tumors are wounds that do not heal: comparison of wound healing to injury with wound healing in tumorigenesis
2.1. Wound healing response to injury
Upon tissue injury, a series of wound healing steps including inflammation, tissue proliferation and remodeling are successively initiated and highly coordinated by the adult tissue cells (Maxson, Lopez, Yoo, Danilkovitch-Miagkova, & Leroux, 2012). Blood clotting, or coagulation, occurs immediately after injury. This is achieved by platelet aggregation and clot formation from fibrinogen-converted fibrin and extracellular matrix (ECM) proteins. Blood clotting serves as the first barrier against blood and water loss as well as invading pathogens. After clot formation, an inflammation stage is initiated by neutrophil infiltration into the injury sites usually within a few hours but up to one day post injury, and is followed by the arrival of monocytes and mast cells one to two days later. The monocytes differentiate into macrophages, which persist long-term at the tissue sites until wound healing is complete. Myeloid cell migration is guided by chemotactic substances such as damage- or pathogen-associated molecular patterns (DAMPs or PAMPs), hydrogen peroxide (H2O2) and chemokines, which are released from the damaged tissues and surrounding stroma in response to injury and invading pathogens (de Oliveira, Rosowski, & Huttenlocher, 2016).
Migration of myeloid cells marks the beginning of the inflammation stage in wound healing from injury. Myeloid cells function to not only phagocytize the dead tissue cells or invading pathogens during the inflammation stage, but also further modulate the next stages of tissue proliferation and remodeling by releasing cytokines, chemokines and other trophic factors (Minutti, Knipper, Allen, & Zaiss, 2017). Along with myeloid cell infiltration, adaptive immune cells such as T lymphocytes are also recruited to the injury sites where they specifically target pathogens and release cytokines to further regulate inflammation, proliferation and tissue remodeling (Havran & Jameson, 2010; Keyes et al., 2016). In the absence of major infection, the inflammatory phase peaks at about two to three days post-injury and gives way to the proliferative phase that lasts about two weeks. Proliferation of three major cell types including epithelial cells (re-epithelialization), mesenchymal cells (fibroplasia) and endothelial cells (revascularization or angiogenesis), occurs concomitantly in the proliferation phase. Through both autocrine and paracrine mechanisms, a plethora of cytokines and trophic factors, such as epidermal growth factor (EGF), transforming growth factor beta (TGFβ), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF), play essential roles in the acceleration of epithelial tissue re-growth, production of collagen and other ECM proteins and formation of new blood vessels (Guo & Dipietro, 2010). Following the proliferative phase, tissue remodeling continues from weeks to years depending on the wound type and size. During tissue remodeling, the disorganized collagen fibers formed during the proliferative phase will rearrange and align along tension lines. Wound contraction occurs in parallel with a reduction in the numbers of macrophages and fibroblasts and a reduction in blood vessels by apoptosis, which together achieve a successful healing process (Hinz et al., 2012).
2.2. Wound healing response to tumorigenesis
Tumors have long been regarded as wounds that fail to complete the normal three stages of wound healing (Dvorak, 1986). During early tumorigenesis, cancer cells are recognized by innate and adaptive immune cell-mediated host surveillance leading to apoptosis and/or necrosis. The dead tumor cells in turn release various DAMPs such as adenosine, high-mobility group box 1 protein (HMGB1), annexins and calreticulin, which serve to initiate the inflammatory response followed by a cascade of events within the wound healing process (Hernandez, Huebener, & Schwabe, 2016). With tumor progression, the surrounding blood vessels become permeable which further triggers platelet and fibrin deposition, and, in turn, starts the program of inflammation, proliferation and ECM remodeling (Kreuger & Phillipson, 2016). As long as tumors are not eradicated by the host immune system or external therapies, the healing steps continue until the tumor burden exceeds the host’s capability to survive. An apparent difference between normal physiological wound healing and tumorigenesis is that the latter possesses one or multiple prolonged, uncompleted phases, and tumors can therefore be considered as an “overhealing wound” (Schafer & Werner, 2008).
Tumor progression is closely associated with the three phases of wound healing. The first phase, inflammation, a hallmark of cancer, is in fact a double–edged sword in tumor development and therapy responses. The innate and adaptive immune cells are phenotypically and functionally plastic depending on their resident microenvironment. Type 1 immune cells are conventionally activated cells, such as “N1” neutrophils, “M1” macrophages and “Th1” or “Tc1” T cells. These cells are mainly considered to be tumoricidal and exert direct cytotoxic effects against tumor cells. In contrast, the alternatively activated type 2 immune cells function to promote distinct steps in cancer progression through release of multiple cytokines and chemokines (Gabrilovich, Ostrand-Rosenberg, & Bronte, 2012; Nowarski, Gagliani, Huber, & Flavell, 2013). During the second phase, the proliferation phase, angiogenesis (revascularization) and desmoplasia (fibroplasia) coordinately support epithelial tumor growth (re-epithelialization) via a collection of growth factors, which are equally essential for wound healing. The third phase, tissue remodeling, completes the wound healing response but fails to be completed in the context of a tumor. However, many of the tissue remodeling activities that occur during wound healing, such as lysyl oxidase (LOX)-induced collagen crosslinking and matrix metalloproteinase (MMP)-mediated collagen rearrangement and alignment, have been shown to be pivotal in tumor cell migration, invasion and metastasis, as well as in resistance to therapy (Cox & Erler, 2011; P. Lu, Weaver, & Werb, 2012).
3. In vitro and in vivo identity of mesenchymal stem cells
MSCs are multipotent stem cells that can differentiate into multiple cell lineages including adipocytes, osteoblasts, chondrocytes, tenocytes and myofibroblasts. In the 1960s and 1970s, this type of adult stem cell was initially identified as a population of bone marrow (BM) cells with colony forming capabilities in vitro and osteogenic cell differentiation potential in vivo (Friedenstein, Chailakhjan, & Lalykina, 1970; Friedenstein, Petrakova, Kurolesova, & Frolova, 1968). In the 1980s and 1990s, these cells were defined as “Mesenchymal Stem Cells” possessing multiple non-hematopoietic mesenchymal lineage cell differentiation capabilities in vitro and in vivo (Caplan, 1991; Prockop, 1997). Afterwards, MSCs were isolated from almost every tissue in the body (umbilical cord, Wharton’s jelly, skin, lung, liver, adipose tissue, muscle, dental pulp, etc.) in addition to the BM (da Silva Meirelles, Chagastelles, & Nardi, 2006). In recent years, MSCs were also isolated from various tumor tissues as “tumor wounds” could stimulate resident MSC proliferation or recruit circulating MSCs (Karnoub et al., 2007; Ren et al., 2012; Y. Shi et al., 2017).
According to criteria by the International Society for Cellular Therapy (ISCT) published in 2006, cultured MSCs should be adherent, fibroblast-like cells with osteogenic, adipogenic and chondrogenic differentiative capacity in vitro. Moreover MSCs must express the surface markers CD105, CD73 and CD90, but not express CD45, CD34, CD14, CD11b, CD79α, CD19 or human leukocyte antigen-DR isotype (HLA-DR) (Dominici et al., 2006). In 2013, ISCT further shared guidelines for the immunological characterization of MSCs, highlighting the functional plasticity of MSCs in the context of different inflammatory milieus (Krampera et al., 2013). In spite of all these efforts in defining MSCs, the boundaries for delineating MSCs, fibroblasts and pericytes still remain largely illusive due to a lack of specific markers for these cell populations (Caplan, 2008; Haniffa, Collin, Buckley, & Dazzi, 2009; Keating, 2012; Nombela-Arrieta, Ritz, & Silberstein, 2011). Nevertheless, MSCs were recently described as “Medicinal Signaling Cells” to reflect their multifaceted functions in wound repair rather than considering their stemness properties (Caplan, 2017). Based upon their robust capacity in regulating every step of wound healing, cultured MSCs have been applied in over 450 clinical trials to treat degenerative diseases and immune disorders (Maxson et al., 2012; Squillaro, Peluso, & Galderisi, 2016).
While the clinical application of ex vivo-expanded MSCs in treating diseases has been established, in vivo exploration of endogenous MSCs has also progressed. Pioneering work by Crisan et al in 2008 demonstrated a perivascular origin of MSCs in multiple human organs. They purified perivascular cells from human skeletal muscle, pancreas, adipose tissue and placenta and showed after long-term culture that these cells maintained the MSC phenotype as well as the trilineage differentiation potential (Crisan et al., 2008). Since then, the endogenous MSCs were believed to reside at perivascular sites in various tissues which may be regarded as a subset of pericytes in vivo (Caplan, 2008, 2017). Genetic lineage tracing assays were also widely applied to identify the differentiation fates of perivascular MSC-like cells in the context of fibrotic diseases and tissue injury models (El Agha et al., 2017). A series of transgenic mice with Cre/loxP technology have recently been created and applied to explore the cellular hierarchies of the perivascular MSCs, such as Nestin-cre (Mendez-Ferrer et al., 2010; Tronche et al., 1999), myxovirus resistant 1 (Mx1)-cre (R. Kuhn, Schwenk, Aguet, & Rajewsky, 1995; Park et al., 2012), Leptin-receptor (Lepr)-cre (Decker et al., 2017; DeFalco et al., 2001; Zhou, Yue, Murphy, Peyer, & Morrison, 2014), and glioma-associated oncogene homolog 1 (Gli1)-cre (Ahn & Joyner, 2004; Kramann et al., 2015; Schneider et al., 2017). Although the differentiation specificity and efficiency of the perivascular MSCs still remain controversial, most reports support the notion that endogenous MSCs are highly plastic upon tissue injury and give rise to myofibroblasts, osteoblasts or adipocytes in bone, lung, liver, kidney, heart, spinal cord, muscle, and skin depending on the injury types or duration. It is also well accepted that cells differentiating from endogenous tissue MSCs play a major role in induction of organ or tissue fibrosis (El Agha et al., 2017). In contrast to the extensive studies that recognize the in vivo identity of MSCs in tissue regeneration, far fewer studies have been carried out to determine the role of MSCs in primary tumor tissues and metastatic sites.
4. MSCs participate in all phases of the wound healing response to injury
Perivascular mesenchymal cells including MSCs play crucial roles through every step of wound healing. Immediately after tissue injury, MSCs express tissue factor (TF) and Factor VIII:c (FVIII) which activate both extrinsic and intrinsic coagulation pathways to facilitate blood clotting (Christy et al., 2017; Sanada et al., 2013). In addition to driving the inflammation stage (described below) MSCs themselves also secrete antimicrobial peptides such as LL-37, hepcidin, β-defensin 2 and lipocalin-2 serving to restrain the invading pathogens (Alcayaga-Miranda et al., 2015; Gupta et al., 2012; Krasnodembskaya et al., 2010; Sung et al., 2016). We next provide an overview of the participation of MSCs in the three stages of wound healing: inflammation, proliferation and tissue remodeling (outlined in Fig. 1).
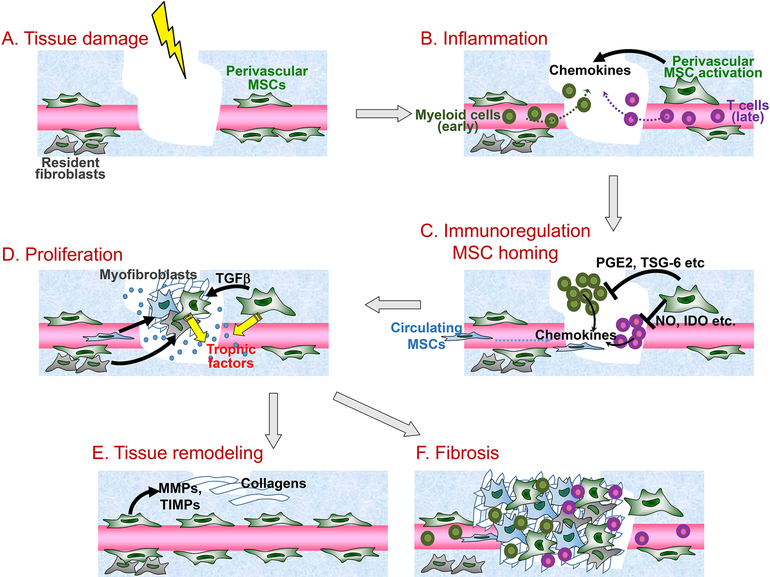
Figure 1. Participation of MSCs in distinct steps of wound healing.
MSCs are involved in all stages of wound healing. (A) After tissue damage, perivascular MSCs are activated. (B) At the inflammation stage, MSCs recruit both innate and adaptive immune cells via secretion of chemokines. (C) Through negative feedback loops, MSCs regulate the immune response (immunoregulation) by suppressing excessive innate and adaptive immune cell activities through release of prostaglandin E2 (PGE2), TSG-6, nitric oxide (NO), indoleamine 2, 3-dioxygenase (IDO) and others. In the meantime, chemokines produced by immune cells and stromal cells further recruit BM- and other tissue-derived MSCs (MSC homing). (D) MSCs then differentiate into myofibroblasts via transforming growth factor beta (TGFβ) signaling and also produce a series of trophic factors to support tissue cell proliferation to accomplish re-epithelialization, fibroplasia and revascularization. (E) At the tissue remodeling stage, MSCs express matrix metalloproteinases (MMPs) as well as tissue inhibitor of metallopeptidases (TIMPs) to re-organize the extracellular matrix (ECM) structures leading to scar formation and wound repair. (F) In the cases of chronic inflammation and infection, the dysregulated MSC differentiation program can result in uncontrolled myofibroblast generation, causing tissue fibrosis.
4.1. Inflammation
In the inflammatory stage, MSCs play dual roles serving as both facilitators and terminators of the inflammation process. At the beginning of inflammation or infection, wound signals such as toll-like receptor (TLR) ligands stimulate bone marrow MSCs to secrete the chemokine CCL-2 which triggers the emigration of monocytes from the BM to the circulation (C. Shi et al., 2011). At the wound sites, paracrine chemokines such as CCL-2, CCL-3, CCL-4, CXCL1, IL-8 and macrophage migration inhibitory factor (MIF) expressed by naïve or TLR ligand-stimulated MSCs serve to recruit monocytes, macrophages and neutrophils (Brandau et al., 2010; L. Chen, Tredget, Wu, & Wu, 2008; Romieu-Mourez et al., 2009). Intravital microscopy, a technique that allows visualizing the biological events in live animals, has enabled us to understand how perivascular mesenchymal cells, including endogenous MSCs, regulate myeloid cell infiltration at the early stage of inflammation in vivo (Proebstl et al., 2012). Upon stimulation by lipopolysaccharide (LPS) or inflammatory cytokines, endogenous perivascular cells (or pericytes) upregulate their expression of the chemokine CXCL1 or MIF, as well as the adhesion molecule intercellular adhesion molecule 1 (ICAM-1). Chemokines serve to recruit neutrophils and monocytes for extravasation whereas adhesion molecules in turn guide these emigrating myeloid cells as they crawl on perivascular cells and move towards the inflammatory foci (Proebstl et al., 2012; Stark et al., 2013).
A myriad of mechanisms have evolved to impede excessive innate immune responses following the peak of inflammation. Dampening of innate immunity is particularly critical in cases of sterile inflammation. Perivascular MSCs indeed play a “gatekeeper” role to limit inflammation-caused tissue damage. Such effects are achieved through a negative feedback mechanism driven by inflammatory mediators such as tumor necrosis factor alpha (TNFα), interleukin-1 (IL-1) and reactive oxygen species (ROS). These factors stimulate MSCs to upregulate cyclooxygenase-2 (COX-2), TNFα-stimulated gene-6 (TSG-6), superoxide dismutase 3 (SOD3) and possibly other effector molecules, to suppress the phagocytic activities of type 1 myeloid cells leading to remission of inflammatory responses (Francois, Romieu-Mourez, Li, & Galipeau, 2012; D. Jiang et al., 2016; R. H. Lee et al., 2009; Nemeth et al., 2009). Notably, the COX-2-prostaglandin E2 (PGE2) pathway and TSG-6 have been extensively studied in MSC-mediated reprograming of M1 macrophages to M2 regulatory macrophages. These regulatory macrophages preferentially release higher levels of cytokines and growth factors to drive the wound healing process into the proliferation stage (Mittal et al., 2016; Nemeth et al., 2009; Ylostalo, Bartosh, Coble, & Prockop, 2012). In addition to these mechanisms, a recent report suggested that engulfment of apoptotic MSCs can also reprogram the host phagocytes to be an immunosuppressive phenotype via production of indoleamine 2, 3-dioxygenase (IDO) which in turn inhibits graft-versus-host disease (GvHD) in mice (Galleu et al., 2017). In addition to modulating monocytes, macrophages and neutrophils, MSCs are also able to repress the functions of other innate immune cell populations including natural killer (NK) cells, dendritic cells and mast cells through PGE2 release and other mechanisms (Aggarwal & Pittenger, 2005; Cui et al., 2016; X. X. Jiang et al., 2005; Spaggiari et al., 2008; Spaggiari, Capobianco, Becchetti, Mingari, & Moretta, 2006; W. R. Su, Zhang, Shi, Nguyen, & Le, 2011).
Compared to the innate immune responses that participate early in the wound healing process, T and B lymphocyte-mediated adaptive immune responses participate at a later time point (Strbo, Yin, & Stojadinovic, 2014). Similar to their dual roles in regulation of innate immune responses, MSCs are capable of augmenting or inhibiting the activities of the adaptive immune cells. When concentrations of environmental stimuli or inflammatory cytokines are low, in vitro cultured MSCs can boost T-cell responses likely through MSC-secreted T-cell chemokines (W. Li et al., 2012; Renner et al., 2009). With the elevation of the T-cell responses, the high levels of T-cell cytokines subsequently turn on the negative feedback mechanisms in MSCs to dampen excessive T-cell reactivity. A key T-cell cytokine in this regulation is IFNγ, which acts independently or with inflammatory mediators including TNFα, IL-1, interleukin 17A (IL-17) and TLR ligands to functionally convert MSCs from a resting state to a highly immunosuppressive stage (X. Han et al., 2014; Opitz et al., 2009; Y. Wang, Chen, Cao, & Shi, 2014). Such an immunosuppression is achieved via cytokine-induced expression of the immunosuppressive molecules inducible nitric oxide synthase (iNOS) and IDO in mouse and human MSCs, respectively (Krampera et al., 2006; Ren et al., 2009; Ren et al., 2008; Sato et al., 2007; J. Su et al., 2014). Other effector molecules and cell populations implicated in MSC-mediated T-cell immunoregulation include PGE2, programmed death ligand 1 (PD-L1), heme oxygenase-1 (HO-1), leukemia inhibitory factor (LIF), IL-6, galectins, Fas ligand, TGF-β and regulatory T cells (reviewed in (Y. Shi et al., 2012; Singer & Caplan, 2011)). Certain chemokines and adhesion molecules that commonly play roles in accelerating immune responses, however, serve to recruit immune cells to form an MSC-immune cell interaction that supports a more robust immunosuppression (Espagnolle, Balguerie, Arnaud, Sensebe, & Varin, 2017; H. K. Lee et al., 2017; Ren et al., 2010; Rubtsov et al., 2017). Such a cytokine-elicited immunoregulatory mechanism was also reported in other endogenous stromal cells such as fibroblastic reticular cells (FRCs) and lymphatic endothelial cells (LECs) suggesting a common negative feedback regulation employed by distinct stromal subsets in vivo (Lukacs-Kornek et al., 2011; Siegert et al., 2011).
4.2. Proliferation
After completion of the inflammation stage, MSCs further functionally contribute to wound healing by participating in the proliferation stage mainly via secretion of trophic factors. In addition to the resident perivascular MSCs, other tissue/organ-derived MSCs are recruited into the wound sites guided by chemokines and adhesion molecules produced during the inflammation stage (Karp & Leng Teo, 2009). The major chemokine-chemokine receptors involved in MSC trafficking include CXCL12-CXCR4, CCL-2-CCR2, CCL27-CCR10, and CCL21-CCR4 (Alexeev, Donahue, Uitto, & Igoucheva, 2013; Belema-Bedada, Uchida, Martire, Kostin, & Braun, 2008; Hu et al., 2013; Kitaori et al., 2009; Sasaki et al., 2008). Coordinated with chemokine-mediated chemotaxis, CD44-hyaluronic acid (HA) and vascular cell adhesion protein 1 (VCAM-1)-α4/β1 integrin interactions, as well as MMP-mediated ECM degradation, facilitate MSCs to transmigrate into the endothelium (Ries et al., 2007; Ruster et al., 2006; Zhu et al., 2006).
Both resident and newly recruited MSCs exert two main effects in the proliferation stage at the wound sites. First, MSCs are directly involved in the tissue repair process through differentiating into multiple mesenchymal lineages. In the 1990s, the exogenously implanted BM-MSCs were shown to have a potent osteogenic potential as detected by the “cube assay” in vivo (Dennis, Haynesworth, Young, & Caplan, 1992; Dennis, Konstantakos, Arm, & Caplan, 1998). Further, by fluorescent protein labeling and in vivo genetic lineage tracing assays, exogenous and endogenous MSCs were demonstrated to possess the capacity to differentiate into other mesenchymal lineages such as adipocytes and myofibroblasts in various injury conditions (Anjos-Afonso, Siapati, & Bonnet, 2004; Kramann et al., 2015; Park et al., 2012; Uezumi, Fukada, Yamamoto, Takeda, & Tsuchida, 2010). Second, MSCs and their lineages serve as a reservoir of trophic agents continuously supplying growth factors to accelerate cell proliferation towards a successful damage repair. The major factors produced by mesenchymal lineages include EGF, PDGF, FGF, VEGF, TGFβ, keratinocyte growth factor (KGF) and hepatocyte growth factor (HGF) (Caplan & Dennis, 2006; Hofer & Tuan, 2016). Most of the wound-related cell types including epithelial cells, endothelial cells, keratinocytes and resident fibroblasts respond to these MSC-derived growth factors for their survival, activation and proliferation to accomplish re-epithelialization, angiogenesis and fibroplasia (Maxson et al., 2012; Zambetti et al., 2016).
4.3. Tissue remodeling
In the tissue remodeling stage, MSCs regulate collagen deposition, degradation and rearrangement through release of MMPs and tissue inhibitors of metalloproteinases (TIMPs). Upon stimulation by TGF-β, IL-1, and TNFα, MSCs overexpress MMP-2, MMP-9, and membrane type-1 MMP (MT1-MMP) which drive ECM degradation and support MSC cell invasion in three-dimensional (3-D) cultures (C. Lu, Li, Hu, Rowe, & Weiss, 2010; Ries et al., 2007). In a rat model of myocardial infarction (MI), implanted MSCs promoted the expression of MMP2 by cardiac fibroblasts and reduced cardiac ventricular fibrosis after MI (Mias et al., 2009). On the other hand, MSCs secrete the MMP inhibitors-TIMPs, which may protect vascular matrix molecules and endothelial cell structures from MMP-induced disruption (Lozito & Tuan, 2011). Recent evidence also suggests that MSC-derived TIMP-1 can function as an anti-angiogenic effector molecule in the inflamed endothelium to reduce the inflammatory response (Zanotti et al., 2016). Lineage tracing of the endogenous MSCs indicate that they can directly give rise to myofibroblasts and contribute to dysregulated ECM remodeling leading to tissue fibrosis. Such roles of MSCs in fibrotic diseases have been extensively reviewed recently (El Agha et al., 2017) and will not be discussed here.
Overall, MSCs are not only beneficial to all steps of wound healing, but also serve as a facilitator for non-healing pathological processes such as fibrosis and cancer when chronic stresses (injury, infection, etc.) are present.
5. MSCs in wound healing response to cancer
Cancer is regarded as an “overhealing wound” and MSCs recently emerged as a new player in the tumor wound microenvironment. Tumor-associated MSCs (TA-MSCs), isolated from malignant tissues, actively participate in tumor-associated inflammation, immunosuppression, tumor growth, angiogenesis and tumor metastasis in various cancer types including breast cancer, ovarian cancer, pancreatic cancer, lymphoma, melanoma and others in both human and mouse tumor models (reviewed in (Y. Shi et al., 2017)). These isolated TA-MSCs also have multipotent differentiation capacities for mesenchymal lineages with an ability to generate fibroblastoid colony-forming units (Karnoub et al., 2007; McLean et al., 2011; Ren et al., 2012). Here we present an overview of the roles of MSCs in cancer progression from the point of view of cancer as a wound (outlined in Fig. 2).
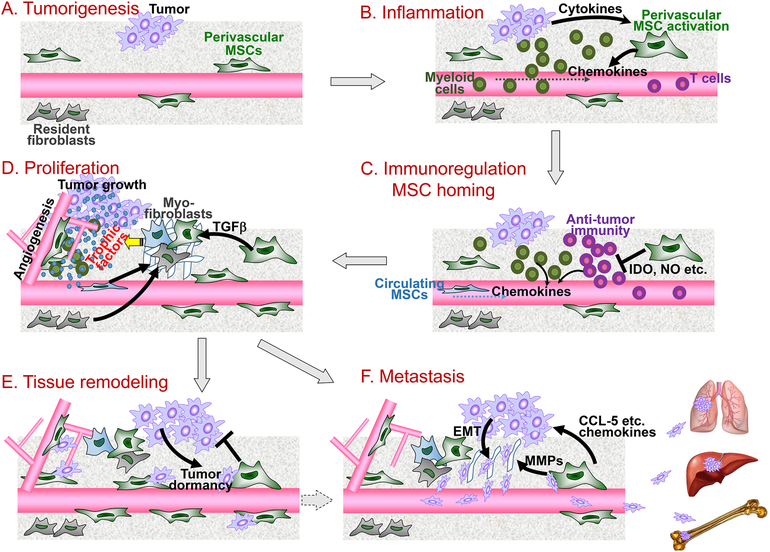
Figure 2. Roles of tumor-associated MSCs (TA-MSCs) in the tumor “wounds”.
TA-MSCs are essential for distinct steps of cancer progression from the view of cancer as “wounds”. (A) During tumorigenesis, perivascular MSCs are activated. (B) Inflammatory cytokines stimulate TA-MSCs to produce myeloid cell chemokines which recruit inflammatory cells to the tumor microenvironment causing an aggravated inflammation. (C) TA-MSCs suppress anti-tumor adaptive immunity through immunosuppressive effector molecules IDO, NO, and others. Guided by the chemotactic cues, circulating MSCs home to the tumor sites. (D) TA-MSCs are further differentiated into myofibroblasts (or CAFs), and both TA-MSCs and myofibroblasts produce trophic factors to support tumor cell proliferation and angiogenesis. (E) At the tissue remodeling stage, TA-MSCs may suppress the outgrowth of tumor cells while impelling them to enter dormancy. Such a dormant cancer stem cell status plays a key role in tumor recurrence and therapeutic resistance. (F) TA-MSCs secrete multiple chemokines and also stimulate the tumor cells to undergo epithelial–mesenchymal transition (EMT) which instigates tumor cell invasion and metastasis to distant organs such as lung, liver and bone.
Dysfunction of MSCs is thought to contribute to tumorigenesis. Indeed, mutation of tyrosine-protein phosphatase non-receptor type 11 (Ptpn11) in MSCs was recently shown to trigger a CCL-3-monocytes-IL-1β axis leading to BM inflammation and myeloproliferative neoplasm in a mouse model (Dong et al., 2016). Similarly, deletion of the Shwachman-Bodian-Diamond syndrome (SBDS) gene in osterix+ mesenchymal progenitor cells caused a p53-S100A8/9-TLR inflammatory response which drove the development of Shwachman-Diamond syndrome (SDS) and myelodysplastic syndrome (MDS) in mice (Zambetti et al., 2016). In contrast to these blood malignancies elicited by abnormal BM-MSCs, it remains largely undefined how tissue MSCs regulate early tumorigenesis events in carcinomas.
If we consider cancer progression in terms of the three stages of wound healing, inflammation, proliferation and tissue remodeling, recent studies using exogenous MSC implantation models showed that MSCs exert either supportive or inhibitory effects on tumor development. While these studies were informative, future efforts should be directed toward a precise understanding of the role of endogenous MSCs in distinct steps of cancer progression. Below we discuss the contribution of MSCs to distinct steps of tumor “wound healing”, although some processes are intermingled.
5.1. Inflammation
5.1.1. TA-MSCs facilitate tumor-associated inflammation
Similar to their effects in wound healing, MSCs play a regulatory role in tumor-associated immune responses. First, TA-MSCs, thought to be derived from healthy MSCs at the tumorigenesis sites, are educated by the tumor inflammatory microenvironment (Ren et al., 2014; Ren et al., 2012), after which they have the capacity to elicit tumor-associated inflammation via secretion of cytokines and chemokines. The tumor and stroma-derived factors such as TNFα and IL-1 stimulate the TA-MSCs to specifically elevate their expression of chemokines, which in turn induce myeloid cell infiltration to exaggerate tumor-associated inflammation (Escobar et al., 2015; Ren et al., 2012; Yu et al., 2017). In mouse and human models of lymphoma, TA-MSCs were shown to express CCL-2, which serves to recruit monocytes and macrophages into the tumor microenvironment to sustain tumor growth (Guilloton et al., 2012; Ren et al., 2012). TA-MSCs also potently recruit neutrophils through overexpression of chemokines CXCL1 and CXCL2, and the neutrophils in turn stimulate primary tumor cell invasion and metastasis to distant organs in mice (Yu et al., 2017). In a human breast cancer xenograft model, the MSC-breast cancer cell interaction provoked recruitment of both macrophages and neutrophils through the colony stimulating factor 1 (CSF1)-CSF1 receptor signaling pathway, resulting in enhanced cancer cell metastases (Chaturvedi, Gilkes, Takano, & Semenza, 2014).
5.1.2. TA-MSCs reprogram innate immune cells
In the tumor microenvironment, TA-MSCs further convert the recruited myeloid cells from a type 1 to a type 2 phenotype by abolishing their phagocytic abilities while activating their “healing” potentials (Biswas & Mantovani, 2010; Coffelt, Wellenstein, & de Visser, 2016). In both in vivo and in vitro models, TA-MSCs isolated from lymphoma or pancreatic carcinoma were shown to polarize macrophages into an M2-like phenotype with increased expression of the alternatively activated macrophage markers (Guilloton et al., 2012; Mathew et al., 2016; Ren et al., 2012). These M2 macrophages function to support tumor growth and depletion of these macrophages substantially reduced TA-MSC-mediated tumor promoting effect in vivo (Mathew et al., 2016; Ren et al., 2012). Similar to the TA-MSC-mediated education of macrophages, TA-MSCs also accelerate a preferential differentiation of leukocytes into immunosuppressive myeloid-derived suppressor cells (MDSCs) (H. W. Chen et al., 2013; Giallongo, Tibullo, et al., 2016; Yen et al., 2013). In vitro, human MSCs induced a differentiation of human peripheral blood leukocytes (PBLs) into MDSCs through secretion of HGF and CXCL3 (H. W. Chen et al., 2013; Yen et al., 2013). In vivo, knockdown of HGF in co-injected MSCs caused a reduction of tumor-infiltrating MDSCs in a human colon cancer xenograft model (Yen et al., 2013). In multiple myeloma and chronic myeloid leukemia, TA-MSCs isolated from the patients’ BM had an elevated ability to induce MDSC expansion compared to healthy donor-derived BM-MSCs (Giallongo, Romano, et al., 2016; Giallongo, Tibullo, et al., 2016). Together, these studies showed that TA-MSCs are a key regulator of the innate immune cell plasticity.
5.1.3. TA-MSCs suppress adaptive immunity
In addition to their capacity to elicit tumor-associated inflammation, TA-MSCs also suppress adaptive immunity in the tumor microenvironment. The role of MSC-mediated immunosuppression in tumor progression was first indicated by the finding that subcutaneous injection of B16 mouse melanoma cells led to tumor growth in allogeneic recipients only when MSCs were co-injected (Djouad et al., 2003). Subsequently, MSC-derived immunosuppressive effector molecules such as NO and IDO were found to ablate anti-tumor T cell and NK cell immunity and facilitate tumor growth (Gazdic et al., 2017; Z. Han et al., 2011; Y. Huang et al., 2014; Ling et al., 2014; Liotta et al., 2015). Besides the production of immunosuppressive factors, TA-MSCs also abrogate adaptive immunity by inducing immunoregulatory cells such as T regulatory cells (Treg) and regulatory CD8+ T cells in vitro and in vivo (Hof-Nahor et al., 2012; Kudo-Saito, Fuwa, Murakami, & Kawakami, 2013; Patel et al., 2010).
Overall, TA-MSCs accelerate tumor-associated inflammation but suppress anti-tumor adaptive immunity. These two mechanisms, though beneficial in a conventional wound healing response, are utilized by the tumor cells to enhance their progression.
5.2. Proliferation
After completion of the inflammation stage of wound healing, there is a transition into the proliferation stage characterized by exogenous tissue precursor cell recruitment and resident tissue cell differentiation and proliferation. TA-MSCs have been shown to actively participate in the proliferative stage in various cancers.
5.2.1. MSC homing to tumors
A preferential homing of MSCs to the tumor, a process equivalent to the homing of MSCs to the wound site in wound healing, is a hallmark of MSC biology in cancer. In both syngeneic and xenogeneic tumor models, systemically delivered luciferase-labeled MSCs or GFP-labeled engrafted BM-MSCs showed a persistent and specific co-localization at the sites of tumor progression (Y. Huang et al., 2014; Kidd et al., 2009). Such tumor-oriented MSC trafficking is coordinated by tumor-secreted paracrine factors and the autocrine BM-MSC-expressed chemoattractants. Many tumor-derived factors such as CCL-2, CCL-25, CXCL16, MIF, IL-6 and antimicrobial peptide LL-37 have been reported to be functional in recruiting MSCs in vitro and in vivo (Coffelt et al., 2009; Dwyer et al., 2007; Jung et al., 2013; Lourenco et al., 2015; Rattigan, Hsu, Mishra, Glod, & Banerjee, 2010; Xu et al., 2012). Complementary to these paracrine mechanisms, BM-MSCs also utilize the CXCL12/CXCR4 autocrine loop to impel their own migration towards tumors as shown in human colorectal cancer and mouse gastric cancer cell models (Menon et al., 2007; Quante et al., 2011).
When arriving at the tumor sites newly recruited MSCs, together with resident MSCs, cooperatively accelerate tumor “wound” healing. The TA-MSCs themselves undergo proliferation and differentiation into myofibroblasts to build a collagen network. Moreover, TA-MSCs support the survival and growth of cancer cells as well as cancer stem cells, and supply angiogenic factors for neovascularization. Again, these natural mechanisms in wound healing are well exploited by malignant cells to support their own progression.
5.2.2. TA-MSC transdifferentiation to myofibroblasts
Myofibroblasts, or tumor-associated fibroblasts (TAFs), are one of the major populations of the tumor stromal cells. They exert diverse effects at distinct steps during cancer progression (Kalluri, 2016). Resident and distant tissue (such as BM)-derived MSCs have been reported as precursors of TAFs. In a syngeneic mouse model of ovarian cancer, the origins of TAFs in the tumor microenvironment have been quantitatively assessed in vivo (Kidd et al., 2012). It was shown that ~40% of tumor stromal cells are BM-derived. Among the TAFs, most fibroblast specific protein (FSP) positive and fibroblast activation protein (FAP) positive TAFs originate from BM-MSCs, whereas α-SMA+ TAFs and perivascular stromal cells (pericytes) are mainly derived from the adipose tissue adjacent to the tumor (Kidd et al., 2012). In various syngeneic and xenograft tumors such as gastric cancer, breast cancer, glioma, pancreatic cancer, ovarian cancer and prostate cancer, exogenously implanted BM-MSCs were shown to differentiate into α-SMA+ vimentin+ myofibroblasts in the tumor microenvironment. This process was largely dependent on the TGFβ/Smad signaling axis (Barcellos-de-Souza et al., 2016; Mishra et al., 2008; Quante et al., 2011; Shangguan et al., 2012; Spaeth et al., 2009; Spaeth et al., 2013). Together, these experiments demonstrate that myofibroblasts are a major MSC-derived cell lineage in the tumor microenvironment.
5.2.3. Trophic effects of TA-MSCs on tumor cells and cancer stem cells
In addition to transitioning into myofibroblasts to sustain cancer progression, TA-MSCs also directly release trophic factors to epithelial and hematologic malignant cells. In particular, TA-MSCs provide survival and pro-proliferative signals to cancer stem cells (CSCs) and tumor-initiating cells (TICs), leading to therapeutic resistance and early relapse (Y. Shi et al., 2017). In the tumor microenvironment, TA-MSCs facilitate tumor initiation and cause an increase in the numbers of aldehyde dehydrogenase (ALDH) positive CSCs in xenograft models of breast cancer, ovarian cancer, colorectal cancer and glioma. These effects were mainly exerted through TA-MSC-derived pro-survival signals such as IL-6, IL-8, CXCL1 CXCL7, and bone morphogenetic protein (BMP) (Hossain et al., 2015; Li, Reinhardt, Herschman, & Weinberg, 2012; S. Liu et al., 2011; McLean et al., 2011; Tsai et al., 2011). In a breast carcinoma xenograft model, cell-cell contact between TA-MSCs and tumor cells led to induction of miR-199a and subsequent repression of FOXP2, a transcriptional regulator inhibiting CSC associated factor.
Thereby, TA-MSCs elicited a propagation of breast CSCs through the miR-199a-FOXP2 axis (Cuiffo et al., 2014). In accordance with these pre-clinical results, extensive bioinformatic and immunohistochemical analyses of human colorectal cancer patient specimens suggested that stromal gene expression is associated with a high frequency of CSCs and disease relapse (Calon et al., 2015). Thus, TA-MSCs directly function to nourish the tumor cells and CSCs in the tumor microenvironment.
5.2.4. TA-MSCs support angiogenesis
In normal wound healing, MSCs release angiogenic factors such as VEGF to foster growth of neovascular vessels for angiogenesis. This mechanism is also executed by TA-MSCs. In syngeneic models of melanoma, lung cancer and colorectal cancer, administration of mouse BM-MSCs promotes tumor growth through increased angiogenesis via the hypoxia-inducible factor 1 (HIF1)-VEGF signaling pathway (Y. Liu et al., 2011; Suzuki et al., 2011). In a human pancreatic cancer xenograft model, GFP-labeled human BM-MSCs, when systemically administered, were found to attach onto the tumor vessel endothelium to induce neovascular sprouting without directly differentiating into endothelial cells in vivo (Beckermann et al., 2008). In addition to MSC-derived VEGF, in a human colorectal cancer xenograft model, TA-MSCs also produce IL-6 which in turn stimulates cancer cells to express endothelin 1 (ET-1) for angiogenesis (W. H. Huang et al., 2013).
Therefore, at the proliferation stage, TA-MSCs exert multifaceted functions to sustain tumor cell survival and proliferation. Several key MSC-regulating signaling pathways such as CXCL12/CXCR4, TGFβ/Smad, IL-6 and VEGF could be potential candidates for developing novel MSC-targeting adjuvant therapeutics.
5.3. TA-MSCs in tissue remodeling
In the last stage of wound healing, host tissues including MSCs evolve self-restricting mechanisms to prevent over-proliferation of different types of tissue cells. Although such capabilities are largely dampened in the tumor microenvironment, MSC-mediated inhibition of epithelial tumor cell outgrowth, driving tumor cells to enter dormancy, as well as suppression of tumor angiogenesis, were indeed observed in many types of cancers. In addition, in response to a hypoxic tumor environment, TA-MSCs modulate the primary tumor ECM, and support tumor cell invasion and metastasis into distant organs. Although such mechanisms are beneficial for the host to accomplish tissue remodeling in wound healing, they instigate cancer progression and metastasis.
5.3.1. TA-MSC-mediated suppression of tumor growth and angiogenesis
It is unclear whether TA-MSC-elicited tumor suppression is a reflection of the self-defending (antibacterial) capacity of MSCs in the early inflammation stage, or a self-restraining mechanism exerted in the tissue remodeling stage of wound healing. Studies of various hematopoietic and non-hematopoietic cancers suggest that TA-MSCs potently inhibit tumor growth. Such inhibition was mediated by MSC-expressed TNF-related apoptosis-inducing ligand (TRAIL), dickkopf-related protein 3 (DKK-3), or cell-cell contact-dependent mechanisms causing tumor cell apoptosis or cell cycle arrest (Khakoo et al., 2006; R. H. Lee, Yoon, Reneau, & Prockop, 2012; Qiao et al., 2008; Ramasamy et al., 2007; Sun et al., 2009).
In human breast carcinoma xenograft models, TA-MSCs also serve to protect tumor cells from stress-induced cell apoptosis and facilitate proliferating tumor cells to enter dormancy and acquire cancer stem cell capabilities in vitro and in vivo. Such a cancer dormancy status is mainly achieved by MSC-released exosomes containing microRNAs such as miR 23b, 127, 197, 222, and 223 which repress the cell cycling regulatory genes, myristoylated alanine-rich C-kinase substrate (MARCKS) and CXCL12, as well as the cell cycle genes, cyclin-dependent kinase 4 (CDK4), cyclin D1 and p21WAF1 (Bliss et al., 2016; Lim et al., 2011; Ono et al., 2014). In an elegant three-dimensional MSC-human breast tumor cell co-culture system, MSCs were observed to be gradually internalized (cannibalized) by cancer cells and such a cannibalism led to the functional alteration of the cancer cells from active to dormant status in vitro and in vivo (Bartosh, Ullah, Zeitouni, Beaver, & Prockop, 2016). In addition to directly restraining the growth of epithelial tumor cells, TA-MSCs were also found to inhibit angiogenesis. In a syngeneic mouse melanoma model, co-administration of MSCs induced endothelial cell apoptosis through MSC-released ROS (Otsu et al., 2009). In a human glioma xenograft model, TA-MSCs suppressed endothelial cell growth by inhibiting the PDGF/PDGFR signaling axis (Ho et al., 2013). Taken together, the role of MSCs in limiting tumor growth could be a reflection of their modulatory effects to terminate the excessive tissue proliferation occurred in a regular wound healing process.
5.3.2. TA-MSCs accelerate tumor cell invasion and metastasis
At the remodeling stage, the final stage in wound healing, the originally disorganized collagen fibers synthesized during the proliferation stage undergo rearrangement, cross-linking and alignment, which are regulated by MMPs, TIMP3, TGFβ and other cytokines (Xue & Jackson, 2015). In the tumor microenvironment, such a remodeling process is more complicated as the driving forces of remodeling are always affected by the earlier inflammation and proliferation processes, resulting in non-healing or over-healing wounds. TA-MSCs have been widely investigated for their potency to accelerate tumor cell invasion and metastasis through remodeling of the ECM (N. Z. Kuhn & Tuan, 2010). Three mechanisms have been proposed in TA-MSC-elicited tumor cell invasion and metastasis, and are outlined below.
First, TA-MSCs secrete chemokines to direct tumor cell invasion. In syngeneic mouse melanoma and human breast carcinoma xenograft models, CCL-2, CCL-5, CCL-9 and CXCL10 are released from TA-MSCs to activate tumor cell migration and invasion which facilitate primary tumor cell metastases to lung, bone and lymph nodes (Chaturvedi et al., 2013; Karnoub et al., 2007; Kudo-Saito et al., 2013; Luo et al., 2014; Swamydas, Ricci, Rego, & Dreau, 2013). As certain chemokines were shown to activate MMPs, the MSC-derived chemokines may also play a role in MMP-mediated ECM remodeling (Swamydas et al., 2013).
Second, TA-MSCs serve as potent drivers for tumor cells to undergo an epithelial-mesenchymal transition (EMT) which in turn stimulates the invasion and metastasis of tumor cells. In a human breast cancer xenograft model, MSCs induced breast tumor cells to undergo EMT via a LOX-Twist signaling pathway. This, in turn, resulted in an elevated capability for tumor cells to metastasize to lung and bone (El-Haibi et al., 2012). Direct contact between MSCs and cancer cells is regarded as a mechanism for upregulation of the EMT-related genes (Martin et al., 2010; Takigawa et al., 2017). In human cancer specimens, the typical EMT markers were found to be expressed at higher levels at the stroma-epithelial invasive edge (Takigawa et al., 2017).
Third, in human breast cancer xenograft and tongue cancer models, human MSCs were shown to modulate collagen deposition through their expression of collagen receptor discoidin domain receptor 2 (DDR2), or to increase collagen I expression in cancer cells. Such ECM modulation further favors tumor cell migration and invasion thus supporting metastases (Gonzalez et al., 2017; Salo et al., 2013).
Therefore, TA-MSCs impede the outgrowth of the primary tumors in the tumor “wound” remodeling stage. On the other hand, through secretion of chemokines and cytokines, TA-MSCs potently drive the primary tumor cells to undergo EMT and metastasize into distant organs.
5.4. TA-MSCs and cancer therapeutic resistance
MSCs were recently identified as a key tumor microenvironmental component that elicits tumor cell resistance to various therapies (Houthuijzen, Daenen, Roodhart, & Voest, 2012). Such a capability is mainly attributed to the pro-survival and cancer stem cell-promoting effects of TA-MSCs, as mentioned above. Following chemotherapy, MSCs are capable of retaining their “stemness” characteristics, proliferative rate and differentiation potential, in part because of their elevated apoptotic threshold (Mueller et al., 2006). Similarly, MSCs were also shown to be radioresistant since they could develop multiple, atypical DNA damage response mechanisms to offset effects of radiation-induced DNA damage (Sugrue, Brown, Lowndes, & Ceredig, 2013).
MSCs themselves are relatively resistant to conventional cancer therapies compared to tumor cells, and more importantly, they can further protect tumor cells from therapy-induced cytotoxicity. In a chronic lymphocytic leukemia model, TA-MSCs were shown to directly interact with leukemia cells to prevent drug-induced cleavage of myeloid cell leukemia 1 (Mcl-1) and poly ADP ribose polymerase (Parp) resulting in drug resistance (Kurtova et al., 2009). In a human ovarian cancer model, a paracrine hedgehog-BMP4 positive feedback loop between ovarian cancer cells and TA-MSCs was defined as the mechanism for drug resistance (Coffman et al., 2016). In response to treatment with the chemotherapeutic drug cisplatin, TA-MSCs also are induced to produce certain fatty acids and the cytokines IL-6 and IL-8 leading to drug resistance in a mouse colon cancer model and a human breast cancer model, respectively (Roodhart et al., 2011; Skolekova et al., 2016). Although there is little experimental evidence showing MSCs directly participate in resistance to the currently promising immunotherapeutics particularly the immune checkpoint blockade, the mesenchymal lineage cells such as myofibroblasts have been revealed to mediate immunotherapy failure via autocrine and paracrine TGFβ signaling and their secretion of CXCL12, MMP9 and ECM proteins in multiple pre-clinical tumor models (Chakravarthy, Khan, Bensler, Bose, & De Carvalho, 2018; Feig et al., 2013; Mariathasan et al., 2018; Zhao et al., 2018). Moreover, when epithelial tumor cells undergo EMT, their “mesenchymal” status favors the development of resistance to immune checkpoint blockade in treatment of breast tumors in mice (Dongre et al., 2017). In human cancer patients, the EMT signature is associated with unique tumor microenvironment and therefore could be a potential biomarker for selecting patients who will benefit from immunotherapeutics (Lou et al., 2016; Mak et al., 2016; L. Wang et al., 2018).
Therefore, TA-MSCs are highly anti-apoptotic and can further help tumor cells to evade different types of cancer therapeutics. A deeper understanding of the robust pro-survival potency of TA-MSCs, as well as the mechanisms underlying MSC-promoted tumor cell dormancy, will benefit development of MSC-targeting approaches to overcome therapeutic resistance and prevent cancer recurrence.
6. Concluding Remarks
Considering tumors as an unresolvable wound, the diverse functions of MSCs in the three stages of regular wound healing and cancer progression are compared (Table 1). MSCs indeed play similar roles in cancer progression, as they do in wound healing, however, such beneficial effects to repair a wound are fully utilized by the tumor cells for further progression and evolution of resistance towards different therapeutics. Increased knowledge of the biology of MSCs in tissue regeneration would highly accelerate a deeper and more comprehensive understanding of the contribution of MSCs to cancer progression. Several questions remain to be answered to achieve the goal of targeting MSCs and mesenchymal lineages as new strategies in clinical cancer research.
Table 1.
MSCs in wound healing responses to injury and cancer
| Wound healing response to injury | Wound healing response to Cancer | ||||
|---|---|---|---|---|---|
| Functions | Mechanisms | Functions | Mechanisms | ||
| Blood clotting Anti-bacterial | MSCs promote blood clotting MSCs are self-defending by elimination of the invading bacteria | TFs; FVIII Secretion of anti-microbial peptides LL-37, hepcidin, (β-defensin 2 and lipocalin-2 etc. | Not known It is unclear whether the tumor-suppressive effects of MSCs are related to such a self-defending capacity by MSCs |
||
| Inflammation | Myeloid cell recruitment | MSCs drive myeloid cell migration from BM to inflamed tissues | Chemokines CCL-2, CCL-3, CCL-4, CXCL1, IL-8, MIF; adhesion molecules ICAM-1, etc. | TA-MSCs recruit monocytes, macrophages and neutrophils to the tumor microenvironment | Chemokines and cytokines CCL-2, CCL-7, CCL-12, CXCL1, CXCL2, CSF1 etc. |
| Myeloid cell and other innate immune cell suppression | MSCs suppress myeloid cell functions upon stimulation by TNFα, IL-1 and ROS | COX-2, PGE2, TSG-6, SOD3, etc. | TA-MSCs polarize the M1 macrophages to an M2 phenotype; TA-MSCs also stimulate MDSC differentiation | CXCL3, HGF etc. | |
| Myeloid cell reprogramming | MSCs convert M1 macrophages to an M2-like type | PGE2, TSG-6, etc. | |||
| T cell recruitment | MSCs augment T-cell infiltration and activities in a low-level inflammatory environment | Chemokines CXCL9, 10, 11; adhesion molecules ICAM-1, VCAM-1 | Not known | ||
| T cell suppression | MSCs exert a robust suppression of T-cells upon stimulation by IFNγ together with other inflammatory cytokines | NO, IDO, PGE2, PD-L1, HO-1, LIF, IL-6, galectin 1, FasL, TGFβ, Treg, etc. | MSCs suppress anti-tumor immunity | NO, IDO, Treg, regulatory CD8+ T cells, etc. | |
| Wound healing response to injury | Wound healing response to Cancer | ||||
| Functions | Mechanisms | Functions | Mechanisms | ||
| Proliferation | MSC homing | MSCs efficiently home to tissue injurysites | Chemokines CXCL12, CCL-2, CCL27 and CCL21; VCAM-1; MMPs; etc. | Exogenously implanted and endogenous MSCs efficiently migrate into the tumor environment | CXCL12, CCL-2, CCL-25, CXCL16, MIF, IL-6, LL-37 etc. |
| MSC differentiation and fibroplasia | MSCs can be differentiated into osteoblasts, adipocytes and myofibroblasts at the injury sites | TGFβ, BMPs, Notch, Wnt, Hedgehogs, etc. | BM-MSCs or adjacent tissue-derived MSCs give rise to distinct types of tumor-associated myofibroblasts | TGFβ etc. | |
| Re-epithelialization | MSCs promote epithelial cell growth | Growth factors EGF, HGF, KGF, PDGF, IGF etc. | TA-MSCs support survival of cancer stem cells and proliferation of tumor cells | IL-6, IL-8, CXCL1, CXCL7, BMPs, miRNAs | |
| Angiogenesis | MSCs enhance angiogenesis | Pro-angiogenic factors VEGF, FGF, PDGF, TGFβ, etc. | TA-MSCs facilitate tumor-associated angiogenesis | VEGF, IL-6, etc. | |
| Tissue remodeling | Inhibition of proliferation | MSCs inhibit tissue cell proliferation | TGFβ, cell-cell contact, MMPs | TA-MSCs suppress epithelial tumor growth but drive them to enter dormancy | TRAIL, DKK-3, cell-cell contact, cannibalization, miR-23b, 127, 197, 222, 223 |
| Inhibition of angiogenesis | MSCs suppress angiogenesis during tissue remodeling | TIMPs, MMPs | TA-MSCs can inhibit angiogenesis | ROS, inhibition of PDGF signaling | |
| Collagen rearrangement | MSCs promote ECM remodeling | MMPs, TIMPs, TGFβ | TA-MSCs modulate collagen organization | DDR2, MMPs | |
| Tissue cell invasion | MSCs stimulate tissue cell invasion | MMPs, collagen rearrangement | TA-MSCs accelerate tumor cell invasion and metastasis | Chemokines CCL-2, CCL-5, CCL-9, CXCL10 etc.; TA-MSCs-induced EMT | |
Abbreviations: TFs, Tissue factors; FVIII, Factor VIII; MIF, Macrophage migration inhibitory factor; ICAM-1, Intercellular adhesion molecule 1; CCL, CC motif chemokine ligand; CXCL, C-X-C motif chemokine ligand; IL, Interleukin; CSF1, Colony stimulating factor 1; COX-2, Cyclooxygenase-2; PGE2, Prostaglandin E2; TSG-6, Tumor necrosis factor (TNF)-stimulated gene-6; SOD3, Superoxide dismutase 3; HGF, Hepatocyte growth factor; VCAM-1, Vascular cell adhesion molecule 1; NO, Nitric oxide; IDO, Indoleamine 2,3-dioxygenase; PD-L1, Programmed death-ligand 1; HO-1, Heme oxygenase 1; LIF, Leukemia inhibitory factor; FasL, Fas ligand; Treg, Regulatory T cell; EGF, Epidermal growth factor; KGF, Keratinocyte growth factor; PDGF, Platelet-derived growth factor; IGF, Insulin-like growth factor; BMP, Bone morphogenetic protein; miRNA, microRNA; VEGF, Vascular endothelial growth factor; FGF, Fibroblast growth factors; PDGF, Platelet-derived growth factor; TGFβ, Transforming growth factor beta; MMP, Matrix metalloproteinase; TRAIL, TNF-related apoptosis-inducing ligand; DKK-3, Dickkopf WNT Signaling Pathway Inhibitor 3; TIMP, Tissue inhibitor of metalloproteinase; ROS, Reactive oxygen species; DDR2, Discoidin domain receptor 2; EMT, Epithelial–mesenchymal transition.
First, the tumor modulating effects mediated by TA-MSCs are largely controversial. The conflicting results could be due to the variance in MSC cell isolation and culture maintenance methods, MSC cell passages, species, cancer models utilized, adoptive cell transplantation time, and doses or duration. From the analysis of the function of MSCs in both wound healing and cancer contexts, it is clear that MSCs are a type of regulatory cell and can function by either enhancing or suppressing distinct steps of the healing process. For example, MSCs can be potently immunosuppressive, but can also enhance inflammation depending on the immune environment in which they reside. Moreover, MSCs are able to both accelerate and inhibit epithelial cell and endothelial cell growth at the proliferation stage and tissue remodeling stage, respectively. In accordance, many feedback loops have been identified in studies of MSCs in both wound healing and cancer (Caplan, 2017; Le Blanc & Davies, 2015; Prockop, 2013; Y. Wang et al., 2014). The functions of MSCs are usually “licensed” upon activation by external stimuli (Krampera, 2011), and MSCs have been proposed to possess an MSC1 or MSC2 status based upon their activation by different TLR ligands (Waterman, Tomchuck, Henkle, & Betancourt, 2010). Such concepts would inspire us to further deliberate the endogenous functions of MSCs in a systematic and dynamic view in the growth, metastasis and drug resistance of various cancers and in other disease processes.
Second, there is currently a lack of well-accepted standards for MSC identification particularly for the identification of endogenous MSCs (da Silva Meirelles, Caplan, & Nardi, 2008; Sacchetti et al., 2016). The distinctions among MSCs, perivascular cells (pericytes) and fibroblasts are still ambiguous (Guimaraes-Camboa et al., 2017; Murphy, Moncivais, & Caplan, 2013). What has been observed or concluded thus far may be from studies of mixed types of mesenchymal cells in vitro and in vivo. Furthermore, the research in the field has mainly relied on exogenous MSC adoptive transfer or ex vivo systems in which conclusions may be less physiologically relevant. Recent efforts have been made to explore endogenous MSCs in pathological contexts, such as fibrotic diseases (El Agha et al., 2017). In the future, well-recognized endogenous markers and transcriptional factors for endogenous MSCs need to be tested and established in both human and experimental animals.
Lastly, it is time to consider harnessing the properties of MSCs for cancer therapy(Marofi, Vahedi, Biglari, Esmaeilzadeh, & Athari, 2017). There are already a few pre-clinical studies in which the homing capacity MSCs to tumors is exploited to utilize MSCs as an excellent in vivo vehicle for delivering tumoricidal agents such as IFNα, IFNβ and TRAIL (Shah, 2012; Stuckey & Shah, 2014). In consideration of the complicated roles of MSCs in cancer progression, especially their function to support tumor dormancy (Bartosh et al., 2016), a more careful and specific tailoring of engineered MSCs need to be conducted for designing future clinical application. Approaches to insert certain suicide genes or genetic modulation to deplete known tumor-promoting genes in engineered MSCs would help develop safer and more efficacious cell-based cancer therapeutics. Furthermore, following the concept of vascular normalization in cancer treatment (Goel et al., 2011), new avenues for conversion of malignancy-facilitating MSCs back to their normal malignancy-inhibitory status, could be a promising direction for future MSC-targeting cancer research. Such an MSC or mesenchymal normalization may also benefit the promising vascular normalization strategies owing to the essential vascular gatekeeper role of endogenous MSCs.
Overall, a deeper exploration of MSC biology from a regenerative perspective will expand our understanding of the multiple functions of MSCs in cancer progression and response to therapy, thereby supporting development of more precise MSC targeted therapy for clinical translation.
Acknowledgdements
This work was supported by grants from the National Institutes of Health (R00-CA188093 to G.R., P30-CA034196 to E.L., and OD018259 to L.S.), a grant from the U.S. Department of Defense (BC170584 to G.R.), and the Young Scholars Program of Shandong University, China (2015wljh46 to P.L.). We appreciate critical reading of the manuscript by Drs. Stephen D. Krasinski, Nadia A. Rosenthal and Edison T. Liu. We apologize to our colleagues whose works are not cited in this manuscript due to space limitations.
Abbreviations
- BM
bone marrow
- BMP
bone morphogenetic protein
- CSC
cancer stem cell
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- HGF
hepatocyte growth factor
- IDO
indoleamine 2,3-dioxygenase
- IL-1
interleukin-1
- iNOS
inducible nitric oxide synthase
- MIF
macrophage migration inhibitory factor
- MMP
matrix metalloproteinase
- MSCs
mesenchymal stem cells
- PDGF
platelet-derived growth factor
- PGE2
prostaglandin E2
- TAF
tumor-associated fibroblasts
- TA-MSCs
tumor-associated MSCs
- TGFβ
transforming growth factor beta
- TIMP
tissue inhibitors of metalloproteinase
- TNFα
tumor necrosis factor alpha
- TSG-6
TNFα-stimulated gene-6
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Aggarwal S, & Pittenger MF (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105(4), 1815–1822. [DOI] [PubMed] [Google Scholar]
- Ahn S, & Joyner AL (2004). Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell, 118(4), 505–516. [DOI] [PubMed] [Google Scholar]
- Alcayaga-Miranda F, Cuenca J, Martin A, Contreras L, Figueroa FE, & Khoury M (2015). Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther, 6, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeev V, Donahue A, Uitto J, & Igoucheva O (2013). Analysis of chemotactic molecules in bone marrow-derived mesenchymal stem cells and the skin: Ccl27-Ccr10 axis as a basis for targeting to cutaneous tissues. Cytotherapy, 15(2), 171–184e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjos-Afonso F, Siapati EK, & Bonnet D (2004). In vivo contribution of murine mesenchymal stem cells into multiple cell-types under minimal damage conditions. J Cell Sci, 117(Pt 23), 5655–5664. [DOI] [PubMed] [Google Scholar]
- Barcellos-de-Souza P, Comito G, Pons-Segura C, Taddei ML, Gori V, Becherucci V, et al. (2016). Mesenchymal Stem Cells are Recruited and Activated into Carcinoma-Associated Fibroblasts by Prostate Cancer Microenvironment-Derived TGF-beta1. Stem Cells, 34(10), 2536–2547. [DOI] [PubMed] [Google Scholar]
- Bartosh TJ, Ullah M, Zeitouni S, Beaver J, & Prockop DJ (2016). Cancer cells enter dormancy after cannibalizing mesenchymal stem/stromal cells (MSCs). Proc Natl Acad Sci U S A, 113(42), E6447–E6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, et al. (2008). VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer, 99(4), 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belema-Bedada F, Uchida S, Martire A, Kostin S, & Braun T (2008). Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell, 2(6), 566–575. [DOI] [PubMed] [Google Scholar]
- Biswas SK, & Mantovani A (2010). Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol, 11(10), 889–896. [DOI] [PubMed] [Google Scholar]
- Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K, et al. (2016). Mesenchymal Stem Cell-Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res, 76(19), 5832–5844. [DOI] [PubMed] [Google Scholar]
- Brandau S, Jakob M, Hemeda H, Bruderek K, Janeschik S, Bootz F, et al. (2010). Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J Leukoc Biol, 88(5), 1005–1015. [DOI] [PubMed] [Google Scholar]
- Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. (2015). Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet, 47(4), 320–329. [DOI] [PubMed] [Google Scholar]
- Calvo F, Ranftl R, Hooper S, Farrugia AJ, Moeendarbary E, Bruckbauer A, et al. (2015). Cdc42EP3/BORG2 and Septin Network Enables Mechano-transduction and the Emergence of Cancer-Associated Fibroblasts. Cell Rep, 13(12), 2699–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI (1991). Mesenchymal stem cells. J Orthop Res, 9(5), 641–650. [DOI] [PubMed] [Google Scholar]
- Caplan AI (2008). All MSCs are pericytes? Cell Stem Cell, 3(3), 229–230. [DOI] [PubMed] [Google Scholar]
- Caplan AI (2017). New MSC: MSCs as pericytes are Sentinels and gatekeepers. J Orthop Res, 35(6), 1151–1159. [DOI] [PubMed] [Google Scholar]
- Caplan AI, & Dennis JE (2006). Mesenchymal stem cells as trophic mediators. J Cell Biochem, 98(5), 1076–1084. [DOI] [PubMed] [Google Scholar]
- Chakravarthy A, Khan L, Bensler NP, Bose P, & De Carvalho DD (2018). TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun, 9(1), 4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Gilkes DM, Takano N, & Semenza GL (2014). Hypoxia-inducible factor-dependent signaling between triple-negative breast cancer cells and mesenchymal stem cells promotes macrophage recruitment. Proc Natl Acad Sci U S A, 111(20), E2120–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Gilkes DM, Wong CC, Kshitiz, Luo W, Zhang H, et al. (2013). Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J Clin Invest, 123(1), 189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HW, Chen HY, Wang LT, Wang FH, Fang LW, Lai HY, et al. (2013). Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J Immunol, 190(10), 5065–5077. [DOI] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Wu PY, & Wu Y (2008). Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One, 3(4), e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy BA, Herzig MC, Montgomery RK, Delavan C, Bynum JA, Reddoch KM, et al. (2017). Pro-coagulant activity of human mesenchymal stem cells. J Trauma Acute Care Surg. [DOI] [PubMed] [Google Scholar]
- Coffelt SB, Marini FC, Watson K, Zwezdaryk KJ, Dembinski JL, LaMarca HL, et al. (2009). The pro-inflammatory peptide LL-37 promotes ovarian tumor progression through recruitment of multipotent mesenchymal stromal cells. Proc Natl Acad Sci U S A, 106(10), 3806–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffelt SB, Wellenstein MD, & de Visser KE (2016). Neutrophils in cancer: neutral no more. Nat Rev Cancer, 16(7), 431–446. [DOI] [PubMed] [Google Scholar]
- Coffman LG, Choi YJ, McLean K, Allen BL, di Magliano MP, & Buckanovich RJ (2016). Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget, 7(6), 6916–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, & Erler JT (2011). Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech, 4(2), 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. (2008). A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell, 3(3), 301–313. [DOI] [PubMed] [Google Scholar]
- Cui R, Rekasi H, Hepner-Schefczyk M, Fessmann K, Petri RM, Bruderek K, et al. (2016). Human mesenchymal stromal/stem cells acquire immunostimulatory capacity upon cross-talk with natural killer cells and might improve the NK cell function of immunocompromised patients. Stem Cell Res Ther, 7(1), 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuiffo BG, Campagne A, Bell GW, Lembo A, Orso F, Lien EC, et al. (2014). MSC-regulated microRNAs converge on the transcription factor FOXP2 and promote breast cancer metastasis. Cell Stem Cell, 15(6), 762–774. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Caplan AI, & Nardi NB (2008). In search of the in vivo identity of mesenchymal stem cells. Stem Cells, 26(9), 2287–2299. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, & Nardi NB (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci, 119(Pt 11), 2204–2213. [DOI] [PubMed] [Google Scholar]
- de Oliveira S, Rosowski EE, & Huttenlocher A (2016). Neutrophil migration in infection and wound repair: going forward in reverse. Nat Rev Immunol, 16(6), 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker M, Martinez-Morentin L, Wang G, Lee Y, Liu Q, Leslie J, et al. (2017). Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat Cell Biol, 19(6), 677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, et al. (2001). Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science, 291(5513), 2608–2613. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Haynesworth SE, Young RG, & Caplan AI (1992). Osteogenesis in marrow-derived mesenchymal cell porous ceramic composites transplanted subcutaneously: effect of fibronectin and laminin on cell retention and rate of osteogenic expression. Cell Transplant, 1(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Dennis JE, Konstantakos EK, Arm D, & Caplan AI (1998). In vivo osteogenesis assay: a rapid method for quantitative analysis. Biomaterials, 19(15), 1323–1328. [DOI] [PubMed] [Google Scholar]
- Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, et al. (2003). Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood, 102(10), 3837–3844. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. [DOI] [PubMed] [Google Scholar]
- Dong L, Yu WM, Zheng H, Loh ML, Bunting ST, Pauly M, et al. (2016). Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature, 539(7628), 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, et al. (2017). Epithelial-to-Mesenchymal Transition Contributes to Immunosuppression in Breast Carcinomas. Cancer Res, 77(15), 3982–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF (1986). Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med, 315(26), 1650–1659. [DOI] [PubMed] [Google Scholar]
- Dvorak HF (2015). Tumors: wounds that do not heal-redux. Cancer Immunol Res, 3(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, et al. (2007). Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res, 13(17), 5020–5027. [DOI] [PubMed] [Google Scholar]
- El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, Scherber CM, et al. (2012). Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci U S A, 109(43), 17460–17465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Agha E, Kramann R, Schneider RK, Li X, Seeger W, Humphreys BD, et al. (2017). Mesenchymal Stem Cells in Fibrotic Disease. Cell Stem Cell, 21(2), 166–177. [DOI] [PubMed] [Google Scholar]
- Escobar P, Bouclier C, Serret J, Bieche I, Brigitte M, Caicedo A, et al. (2015). IL-1beta produced by aggressive breast cancer cells is one of the factors that dictate their interactions with mesenchymal stem cells through chemokine production. Oncotarget, 6(30), 29034–29047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espagnolle N, Balguerie A, Arnaud E, Sensebe L, & Varin A (2017). CD54-Mediated Interaction with Pro-inflammatory Macrophages Increases the Immunosuppressive Function of Human Mesenchymal Stromal Cells. Stem Cell Reports, 8(4), 961–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, et al. (2013). Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A, 110(50), 20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Romieu-Mourez R, Li M, & Galipeau J (2012). Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther, 20(1), 187–195. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Chailakhjan RK, & Lalykina KS (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet, 3(4), 393–403. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Petrakova KV, Kurolesova AI, & Frolova GP (1968). Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation, 6(2), 230–247. [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, & Bronte V (2012). Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol, 12(4), 253–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galleu A, Riffo-Vasquez Y, Trento C, Lomas C, Dolcetti L, Cheung TS, et al. (2017). Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med, 9(416). [DOI] [PubMed] [Google Scholar]
- Gazdic M, Simovic Markovic B, Jovicic N, Misirkic-Marjanovic M, Djonov V, Jakovljevic V, et al. (2017). Mesenchymal Stem Cells Promote Metastasis of Lung Cancer Cells by Downregulating Systemic Antitumor Immune Response. Stem Cells Int, 2017, 6294717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallongo C, Romano A, Parrinello NL, La Cava P, Brundo MV, Bramanti V, et al. (2016). Mesenchymal Stem Cells (MSC) Regulate Activation of Granulocyte-Like Myeloid Derived Suppressor Cells (G-MDSC) in Chronic Myeloid Leukemia Patients. PLoS One, 11(7), e0158392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallongo C, Tibullo D, Parrinello NL, La Cava P, Di Rosa M, Bramanti V, et al. (2016). Granulocyte-like myeloid derived suppressor cells (G-MDSC) are increased in multiple myeloma and are driven by dysfunctional mesenchymal stem cells (MSC). Oncotarget, 7(52), 85764–85775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. (2011). Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev, 91(3), 1071–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez ME, Martin EE, Anwar T, Arellano-Garcia C, Medhora N, Lama A, et al. (2017). Mesenchymal Stem Cell-Induced DDR2 Mediates Stromal-Breast Cancer Interactions and Metastasis Growth. Cell Rep, 18(5), 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloton F, Caron G, Menard C, Pangault C, Ame-Thomas P, Dulong J, et al. (2012). Mesenchymal stromal cells orchestrate follicular lymphoma cell niche through the CCL2-dependent recruitment and polarization of monocytes. Blood, 119(11), 2556–2567. [DOI] [PubMed] [Google Scholar]
- Guimaraes-Camboa N, Cattaneo P, Sun Y, Moore-Morris T, Gu Y, Dalton ND, et al. (2017). Pericytes of Multiple Organs Do Not Behave as Mesenchymal Stem Cells In Vivo. Cell Stem Cell, 20(3), 345–359e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, & Dipietro LA (2010). Factors affecting wound healing. J Dent Res, 89(3), 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, et al. (2012). Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax, 67(6), 533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yang Q, Lin L, Xu C, Zheng C, Chen X, et al. (2014). Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ, 21(11), 1758–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Tian Z, Lv G, Zhang L, Jiang G, Sun K, et al. (2011). Immunosuppressive effect of bone marrow-derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells. J Cell Mol Med, 15(11), 2343–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa MA, Collin MP, Buckley CD, & Dazzi F (2009). Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica, 94(2), 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havran WL, & Jameson JM (2010). Epidermal T cells and wound healing. J Immunol, 184(10), 5423–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez C, Huebener P, & Schwabe RF (2016). Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene, 35(46), 5931–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, et al. (2012). Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol, 180(4), 1340–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM, et al. (2013). Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells, 31(1), 146–155. [DOI] [PubMed] [Google Scholar]
- Hof-Nahor I, Leshansky L, Shivtiel S, Eldor L, Aberdam D, Itskovitz-Eldor J, et al. (2012). Human mesenchymal stem cells shift CD8+ T cells towards a suppressive phenotype by inducing tolerogenic monocytes. J Cell Sci, 125(Pt 19), 4640–4650. [DOI] [PubMed] [Google Scholar]
- Hofer HR, & Tuan RS (2016). Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther, 7(1), 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A, Gumin J, Gao F, Figueroa J, Shinojima N, Takezaki T, et al. (2015). Mesenchymal Stem Cells Isolated From Human Gliomas Increase Proliferation and Maintain Stemness of Glioma Stem Cells Through the IL-6/gp130/STAT3 Pathway. Stem Cells, 33(8), 2400–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthuijzen JM, Daenen LG, Roodhart JM, & Voest EE (2012). The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer, 106(12), 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Yong X, Li C, Lu M, Liu D, Chen L, et al. (2013). CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J Surg Res, 183(1), 427–434. [DOI] [PubMed] [Google Scholar]
- Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, & Hung SC (2013). Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene, 32(37), 4343–4354. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yu P, Li W, Ren G, Roberts AI, Cao W, et al. (2014). p53 regulates mesenchymal stem cell-mediated tumor suppression in a tumor microenvironment through immune modulation. Oncogene, 33(29), 3830–3838. [DOI] [PubMed] [Google Scholar]
- Jiang D, Muschhammer J, Qi Y, Kugler A, de Vries JC, Saffarzadeh M, et al. (2016). Suppression of Neutrophil-Mediated Tissue Damage-A Novel Skill of Mesenchymal Stem Cells. Stem Cells, 34(9), 2393–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. (2005). Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood, 105(10), 4120–4126. [DOI] [PubMed] [Google Scholar]
- Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, et al. (2013). Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun, 4, 1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R (2016). The biology and function of fibroblasts in cancer. Nat Rev Cancer, 16(9), 582–598. [DOI] [PubMed] [Google Scholar]
- Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature, 449(7162), 557–563. [DOI] [PubMed] [Google Scholar]
- Karp JM, & Leng Teo GS (2009). Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell, 4(3), 206–216. [DOI] [PubMed] [Google Scholar]
- Keating A (2012). Mesenchymal stromal cells: new directions. Cell Stem Cell, 10(6), 709–716. [DOI] [PubMed] [Google Scholar]
- Keyes BE, Liu S, Asare A, Naik S, Levorse J, Polak L, et al. (2016). Impaired Epidermal to Dendritic T Cell Signaling Slows Wound Repair in Aged Skin. Cell, 167(5), 1323–1338e1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. (2006). Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med, 203(5), 1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, et al. (2009). Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells, 27(10), 2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, et al. (2012). Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One, 7(2), e30563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, et al. (2009). Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum, 60(3), 813–823. [DOI] [PubMed] [Google Scholar]
- Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, Dougan M, et al. (2018). The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med, 10(436). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, et al. (2015). Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell, 16(1), 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampera M (2011). Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia, 25(9), 1408–1414. [DOI] [PubMed] [Google Scholar]
- Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. (2006). Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells, 24(2), 386–398. [DOI] [PubMed] [Google Scholar]
- Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L, & Therapy, M. S. C. C. o. t. I. S. f. C. (2013). Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy, 15(9), 1054–1061. [DOI] [PubMed] [Google Scholar]
- Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, et al. (2010). Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells, 28(12), 2229–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J, & Phillipson M (2016). Targeting vascular and leukocyte communication in angiogenesis, inflammation and fibrosis. Nat Rev Drug Discov, 15(2), 125–142. [DOI] [PubMed] [Google Scholar]
- Kudo-Saito C, Fuwa T, Murakami K, & Kawakami Y (2013). Targeting FSTL1 prevents tumor bone metastasis and consequent immune dysfunction. Cancer Res, 73(20), 6185–6193. [DOI] [PubMed] [Google Scholar]
- Kuhn NZ, & Tuan RS (2010). Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol, 222(2), 268–277. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, & Rajewsky K (1995). Inducible gene targeting in mice. Science, 269(5229), 1427–1429. [DOI] [PubMed] [Google Scholar]
- Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, et al. (2009). Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood, 114(20), 4441–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Blanc K, & Davies LC (2015). Mesenchymal stromal cells and the innate immune response. Immunol Lett, 168(2), 140–146. [DOI] [PubMed] [Google Scholar]
- Lee HK, Kim HS, Kim JS, Kim YG, Park KH, Lee JH, et al. (2017). CCL2 deficient mesenchymal stem cells fail to establish long-lasting contact with T cells and no longer ameliorate lupus symptoms. Sci Rep, 7, 41258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. (2009). Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell, 5(1), 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Yoon N, Reneau JC, & Prockop DJ (2012). Preactivation of human MSCs with TNF-alpha enhances tumor-suppressive activity. Cell Stem Cell, 11(6), 825–835. [DOI] [PubMed] [Google Scholar]
- Li HJ, Reinhardt F, Herschman HR, & Weinberg RA (2012). Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer Discov, 2(9), 840–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ren G, Huang Y, Su J, Han Y, Li J, et al. (2012). Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ, 19(9), 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, et al. (2011). Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res, 71(5), 1550–1560. [DOI] [PubMed] [Google Scholar]
- Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, et al. (2014). Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res, 74(5), 1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta F, Querci V, Mannelli G, Santarlasci V, Maggi L, Capone M, et al. (2015). Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br J Cancer, 112(4), 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, et al. (2011). Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res, 71(2), 614–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX, Wang CY, et al. (2011). Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem, 286(28), 25007–25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Diao L, Cuentas ER, Denning WL, Chen L, Fan YH, et al. (2016). Epithelial-Mesenchymal Transition Is Associated with a Distinct Tumor Microenvironment Including Elevation of Inflammatory Signals and Multiple Immune Checkpoints in Lung Adenocarcinoma. Clin Cancer Res, 22(14), 3630–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco S, Teixeira VH, Kalber T, Jose RJ, Floto RA, & Janes SM (2015). Macrophage migration inhibitory factor-CXCR4 is the dominant chemotactic axis in human mesenchymal stem cell recruitment to tumors. J Immunol, 194(7), 3463–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozito TP, & Tuan RS (2011). Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J Cell Physiol, 226(2), 385–396. [DOI] [PubMed] [Google Scholar]
- Lu C, Li XY, Hu Y, Rowe RG, & Weiss SJ (2010). MT1-MMP controls human mesenchymal stem cell trafficking and differentiation. Blood, 115(2), 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, & Werb Z (2012). The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol, 196(4), 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, et al. (2011). Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol, 12(11), 1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Ok Lee S, Liang L, Huang CK, Li L, Wen S, et al. (2014). Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene, 33(21), 2768–2778. [DOI] [PubMed] [Google Scholar]
- Mak MP, Tong P, Diao L, Cardnell RJ, Gibbons DL, William WN, et al. (2016). A Patient-Derived, Pan-Cancer EMT Signature Identifies Global Molecular Alterations and Immune Target Enrichment Following Epithelial-to-Mesenchymal Transition. Clin Cancer Res, 22(3), 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. (2018). TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature, 554(7693), 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marofi F, Vahedi G, Biglari A, Esmaeilzadeh A, & Athari SS (2017). Mesenchymal Stromal/Stem Cells: A New Era in the Cell-Based Targeted Gene Therapy of Cancer. Front Immunol, 8, 1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FT, Dwyer RM, Kelly J, Khan S, Murphy JM, Curran C, et al. (2010). Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat, 124(2), 317–326. [DOI] [PubMed] [Google Scholar]
- Mathew E, Brannon AL, Del Vecchio A, Garcia PE, Penny MK, Kane KT, et al. (2016). Mesenchymal Stem Cells Promote Pancreatic Tumor Growth by Inducing Alternative Polarization of Macrophages. Neoplasia, 18(3), 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, & Leroux MA (2012). Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med, 1(2), 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, et al. (2011). Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest, 121(8), 3206–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. (2010). Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature, 466(7308), 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon LG, Picinich S, Koneru R, Gao H, Lin SY, Koneru M, et al. (2007). Differential gene expression associated with migration of mesenchymal stem cells to conditioned medium from tumor cells or bone marrow cells. Stem Cells, 25(2), 520–528. [DOI] [PubMed] [Google Scholar]
- Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, et al. (2009). Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells, 27(11), 2734–2743. [DOI] [PubMed] [Google Scholar]
- Minutti CM, Knipper JA, Allen JE, & Zaiss DM (2017). Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol, 61, 3–11. [DOI] [PubMed] [Google Scholar]
- Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, et al. (2008). Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. [Research Support, Non-U.S. Gov’t]. Cancer research, 68(11), 4331–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal M, Tiruppathi C, Nepal S, Zhao YY, Grzych D, Soni D, et al. (2016). TNFalpha-stimulated gene-6 (TSG6) activates macrophage phenotype transition to prevent inflammatory lung injury. Proc Natl Acad Sci U S A, 113(50), E8151–E8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller LP, Luetzkendorf J, Mueller T, Reichelt K, Simon H, & Schmoll HJ (2006). Presence of mesenchymal stem cells in human bone marrow after exposure to chemotherapy: evidence of resistance to apoptosis induction. Stem Cells, 24(12), 2753–2765. [DOI] [PubMed] [Google Scholar]
- Murphy MB, Moncivais K, & Caplan AI (2013). Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med, 45, e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, et al. (2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med, 15(1), 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nombela-Arrieta C, Ritz J, & Silberstein LE (2011). The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol, 12(2), 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowarski R, Gagliani N, Huber S, & Flavell RA (2013). Innate immune cells in inflammation and cancer. Cancer Immunol Res, 1(2), 77–84. [DOI] [PubMed] [Google Scholar]
- Ono M, Kosaka N, Tominaga N, Yoshioka Y, Takeshita F, Takahashi RU, et al. (2014). Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal, 7(332), ra63. [DOI] [PubMed] [Google Scholar]
- Opitz CA, Litzenburger UM, Lutz C, Lanz TV, Tritschler I, Koppel A, et al. (2009). Toll-like receptor engagement enhances the immunosuppressive properties of human bone marrow-derived mesenchymal stem cells by inducing indoleamine-2,3-dioxygenase-1 via interferon-beta and protein kinase R. Stem Cells, 27(4), 909–919. [DOI] [PubMed] [Google Scholar]
- Otsu K, Das S, Houser SD, Quadri SK, Bhattacharya S, & Bhattacharya J (2009). Concentration-dependent inhibition of angiogenesis by mesenchymal stem cells. Blood, 113(18), 4197–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, et al. (2012). Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell, 10(3), 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, & Rameshwar P (2010). Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol, 184(10), 5885–5894. [DOI] [PubMed] [Google Scholar]
- Prockop DJ (1997). Marrow stromal cells as stem cells for nonhematopoietic tissues. Science, 276(5309), 71–74. [DOI] [PubMed] [Google Scholar]
- Prockop DJ (2013). Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells, 31(10), 2042–2046. [DOI] [PubMed] [Google Scholar]
- Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, et al. (2012). Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med, 209(6), 1219–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, et al. (2008). Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res, 18(4), 500–507. [DOI] [PubMed] [Google Scholar]
- Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. (2011). Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell, 19(2), 257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy R, Lam EW, Soeiro I, Tisato V, Bonnet D, & Dazzi F (2007). Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia, 21(2), 304–310. [DOI] [PubMed] [Google Scholar]
- Rattigan Y, Hsu JM, Mishra PJ, Glod J, & Banerjee D (2010). Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp Cell Res, 316(20), 3417–3424. [DOI] [PubMed] [Google Scholar]
- Ren G, Liu Y, Zhao X, Zhang J, Zheng B, Yuan ZR, et al. (2014). Tumor resident mesenchymal stromal cells endow naive stromal cells with tumor-promoting properties. Oncogene, 33(30), 4016–4020. [DOI] [PubMed] [Google Scholar]
- Ren G, Su J, Zhang L, Zhao X, Ling W, L’Huillie A, et al. (2009). Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells, 27(8), 1954–1962. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell, 2(2), 141–150. [DOI] [PubMed] [Google Scholar]
- Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, et al. (2012). CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell, 11(6), 812–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G, Zhao X, Zhang L, Zhang J, L’Huillier A, Ling W, et al. (2010). Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J Immunol, 184(5), 2321–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner P, Eggenhofer E, Rosenauer A, Popp FC, Steinmann JF, Slowik P, et al. (2009). Mesenchymal stem cells require a sufficient, ongoing immune response to exert their immunosuppressive function. Transplant Proc, 41(6), 2607–2611. [DOI] [PubMed] [Google Scholar]
- Ridge SM, Sullivan FJ, & Glynn SA (2017). Mesenchymal stem cells: key players in cancer progression. Mol Cancer, 16(1), 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries C, Egea V, Karow M, Kolb H, Jochum M, & Neth P (2007). MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood, 109(9), 4055–4063. [DOI] [PubMed] [Google Scholar]
- Romieu-Mourez R, Francois M, Boivin MN, Bouchentouf M, Spaner DE, & Galipeau J (2009). Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol, 182(12), 7963–7973. [DOI] [PubMed] [Google Scholar]
- Roodhart JM, Daenen LG, Stigter EC, Prins HJ, Gerrits J, Houthuijzen JM, et al. (2011). Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell, 20(3), 370–383. [DOI] [PubMed] [Google Scholar]
- Rubtsov Y, Goryunov capital Ka C, Romanov capital A C, Suzdaltseva Y, Sharonov G, & Tkachuk V (2017). Molecular Mechanisms of Immunomodulation Properties of Mesenchymal Stromal Cells: A New Insight into the Role of ICAM-1. Stem Cells Int, 2017, 6516854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruster B, Gottig S, Ludwig RJ, Bistrian R, Muller S, Seifried E, et al. (2006). Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood, 108(12), 3938–3944. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, et al. (2016). No Identical “Mesenchymal Stem Cells” at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Reports, 6(6), 897–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo S, Bitu C, Merkku K, Nyberg P, Bello IO, Vuoristo J, et al. (2013). Human bone marrow mesenchymal stem cells induce collagen production and tongue cancer invasion. PLoS One, 8(10), e77692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada C, Kuo CJ, Colletti EJ, Soland M, Mokhtari S, Knovich MA, et al. (2013). Mesenchymal stem cells contribute to endogenous FVIII:c production. J Cell Physiol, 228(5), 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, & Shimizu H (2008). Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol, 180(4), 2581–2587. [DOI] [PubMed] [Google Scholar]
- Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. (2007). Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood, 109(1), 228–234. [DOI] [PubMed] [Google Scholar]
- Schafer M, & Werner S (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol, 9(8), 628–638. [DOI] [PubMed] [Google Scholar]
- Schneider RK, Mullally A, Dugourd A, Peisker F, Hoogenboezem R, Van Strien PMH, et al. (2017). Gli1+ Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target. Cell Stem Cell, 20(6), 785–800e788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K (2012). Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev, 64(8), 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangguan L, Ti X, Krause U, Hai B, Zhao Y, Yang Z, et al. (2012). Inhibition of TGF-beta/Smad signaling by BAMBI blocks differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and abolishes their protumor effects. Stem Cells, 30(12), 2810–2819. [DOI] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, et al. (2011). Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity, 34(4), 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Du L, Lin L, & Wang Y (2017). Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov, 16(1), 35–52. [DOI] [PubMed] [Google Scholar]
- Shi Y, Su J, Roberts AI, Shou P, Rabson AB, & Ren G (2012). How mesenchymal stem cells interact with tissue immune responses. Trends Immunol, 33(3), 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S, Huang HY, Yang CY, Scarpellino L, Carrie L, Essex S, et al. (2011). Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS One, 6(11), e27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer NG, & Caplan AI (2011). Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol, 6, 457–478. [DOI] [PubMed] [Google Scholar]
- Skolekova S, Matuskova M, Bohac M, Toro L, Durinikova E, Tyciakova S, et al. (2016). Cisplatin-induced mesenchymal stromal cells-mediated mechanism contributing to decreased antitumor effect in breast cancer cells. Cell Commun Signal, 14, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. (2009). Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One, 4(4), e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaeth EL, Labaff AM, Toole BP, Klopp A, Andreeff M, & Marini FC (2013). Mesenchymal CD44 expression contributes to the acquisition of an activated fibroblast phenotype via TWIST activation in the tumor microenvironment. Cancer Res, 73(17), 5347–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, & Moretta L (2008). Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood, 111(3), 1327–1333. [DOI] [PubMed] [Google Scholar]
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, & Moretta L (2006). Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood, 107(4), 1484–1490. [DOI] [PubMed] [Google Scholar]
- Squillaro T, Peluso G, & Galderisi U (2016). Clinical Trials With Mesenchymal Stem Cells:An Update. Cell Transplant, 25(5), 829–848. [DOI] [PubMed] [Google Scholar]
- Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, et al. (2013). Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol, 14(1), 41–51. [DOI] [PubMed] [Google Scholar]
- Strbo N, Yin N, & Stojadinovic O (2014). Innate and Adaptive Immune Responses in Wound Epithelialization. Adv Wound Care (New Rochelle), 3(7), 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuckey DW, & Shah K (2014). Stem cell-based therapies for cancer treatment: separating hope from hype. Nat Rev Cancer, 14(10), 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J, Chen X, Huang Y, Li W, Li J, Cao K, et al. (2014). Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ, 21(3), 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WR, Zhang QZ, Shi SH, Nguyen AL, & Le AD (2011). Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells, 29(11), 1849–1860. [DOI] [PubMed] [Google Scholar]
- Sugrue T, Brown JA, Lowndes NF, & Ceredig R (2013). Multiple facets of the DNA damage response contribute to the radioresistance of mouse mesenchymal stromal cell lines. Stem Cells, 31(1), 137–145. [DOI] [PubMed] [Google Scholar]
- Sun B, Roh KH, Park JR, Lee SR, Park SB, Jung JW, et al. (2009). Therapeutic potential of mesenchymal stromal cells in a mouse breast cancer metastasis model. Cytotherapy, 11(3), 289–298, 281 p following 298. [DOI] [PubMed] [Google Scholar]
- Sundaram GM, Ismail HM, Bashir M, Muhuri M, Vaz C, Nama S, et al. (2017). EGF hijacks miR-198/FSTL1 wound-healing switch and steers a two-pronged pathway toward metastasis. J Exp Med, 214(10), 2889–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DK, Chang YS, Sung SI, Yoo HS, Ahn SY, & Park WS (2016). Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via toll-like receptor 4 signalling. Cell Microbiol, 18(3), 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, et al. (2011). Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Mol Med, 17(7–8), 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamydas M, Ricci K, Rego SL, & Dreau D (2013). Mesenchymal stem cell-derived CCL-9 and CCL-5 promote mammary tumor cell invasion and the activation of matrix metalloproteinases. Cell Adh Migr, 7(3), 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa H, Kitadai Y, Shinagawa K, Yuge R, Higashi Y, Tanaka S, et al. (2017). Mesenchymal Stem Cells Induce Epithelial to Mesenchymal Transition in Colon Cancer Cells through Direct Cell-to-Cell Contact. Neoplasia, 19(5), 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. (1999). Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet, 23(1), 99–103. [DOI] [PubMed] [Google Scholar]
- Tsai KS, Yang SH, Lei YP, Tsai CC, Chen HW, Hsu CY, et al. (2011). Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology, 141(3), 1046–1056. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, & Pistoia V (2008). Mesenchymal stem cells in health and disease. Nat Rev Immunol, 8(9), 726–736. [DOI] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, & Tsuchida K (2010). Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol, 12(2), 143–152. [DOI] [PubMed] [Google Scholar]
- Wang L, Saci A, Szabo PM, Chasalow SD, Castillo-Martin M, Domingo-Domenech J, et al. (2018). EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat Commun, 9(1), 3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen X, Cao W, & Shi Y (2014). Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol, 15(11), 1009–1016. [DOI] [PubMed] [Google Scholar]
- Waterman RS, Tomchuck SL, Henkle SL, & Betancourt AM (2010). A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One, 5(4), e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Menu E, De Becker A, Van Camp B, Vanderkerken K, & Van Riet I (2012). Bone marrow-derived mesenchymal stromal cells are attracted by multiple myeloma cell-produced chemokine CCL25 and favor myeloma cell growth in vitro and in vivo. Stem Cells, 30(2), 266–279. [DOI] [PubMed] [Google Scholar]
- Xue M, & Jackson CJ (2015). Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv Wound Care (New Rochelle), 4(3), 119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen BL, Yen ML, Hsu PJ, Liu KJ, Wang CJ, Bai CH, et al. (2013). Multipotent human mesenchymal stromal cells mediate expansion of myeloid-derived suppressor cells via hepatocyte growth factor/c-met and STAT3. Stem Cell Reports, 1(2), 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylostalo JH, Bartosh TJ, Coble K, & Prockop DJ (2012). Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells, 30(10), 2283–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PF, Huang Y, Han YY, Lin LY, Sun WH, Rabson AB, et al. (2017). TNFalpha-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2+ neutrophils. Oncogene, 36(4), 482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambetti NA, Ping Z, Chen S, Kenswil KJ, Mylona MA, Sanders MA, et al. (2016). Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell Stem Cell, 19(5), 613–627. [DOI] [PubMed] [Google Scholar]
- Zanotti L, Angioni R, Cali B, Soldani C, Ploia C, Moalli F, et al. (2016). Mouse mesenchymal stem cells inhibit high endothelial cell activation and lymphocyte homing to lymph nodes by releasing TIMP-1. Leukemia, 30(5), 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Evans K, Xiao C, DeVito N, Theivanthiran B, Holtzhausen A, et al. (2018). Stromal Fibroblasts Mediate Anti-PD-1 Resistance via MMP-9 and Dictate TGFbeta Inhibitor Sequencing in Melanoma. Cancer Immunol Res, 6(12), 1459–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Yue R, Murphy MM, Peyer JG, & Morrison SJ (2014). Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell, 15(2), 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, et al. (2006). The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells, 24(4), 928–935. [DOI] [PubMed] [Google Scholar]