Abstract
Aims/Introduction
Sodium–glucose cotransporter 2 inhibitors reduce bodyweight (BW) by creating a negative energy balance. Previous reports have suggested that this BW reduction is mainly loss of body fat and that ~20% of the reduction is lean mass. However, the effects of sodium–glucose cotransporter 2 inhibitors on BW and body composition remain unclear. We examined these effects in Japanese patients with type 2 diabetes mellitus treated with insulin.
Materials and Methods
In this open‐label, randomized controlled trial, 49 overweight patients (body mass index ≥23 kg/m2) with inadequate glycemic control (hemoglobin A1c >7.0%) receiving insulin treatment were randomly assigned to receive add‐on ipragliflozin or no additional treatment (control group). Patients were followed for 24 weeks. The goal for all patients was to achieve glycated hemoglobin <7.0% without hypoglycemia. The primary end‐point was a change in BW from baseline to week 24. Body composition was assessed with dual‐energy X‐ray absorptiometry and bioelectrical impedance analysis.
Results
BW change was significantly larger in the ipragliflozin group than in the control group (−2.78 vs −0.22 kg, P < 0.0001). Total fat mass was reduced evenly in the arms, lower limbs and trunk in the ipragliflozin group. Total muscle mass and bone mineral content were maintained, but muscle mass in the arms might have been affected by ipragliflozin treatment.
Conclusions
Ipragliflozin treatment for 24 weeks resulted in reduced BW, mainly from fat mass loss. Muscle mass and bone mineral content were maintained. Further study is necessary to elucidate the long‐term effects of ipragliflozin.
Keywords: Bodyweight, Sodium–glucose cotransporter 2 inhibitor, Treatment drug
Introduction
Bodyweight (BW) gain is a major disadvantage of insulin treatment. Previous reports have suggested that achieving sufficient glycemic control with insulin in inadequately controlled type 2 diabetes patients results in BW gain1, 2.
Sodium–glucose cotransporter 2 (SGLT2) inhibitors improve glycemic control and induce BW reduction by creating a negative energy balance through urinary glucose excretion. Previous studies have reported BW reduction of approximately 3 kg at 24 weeks after initiation of SGLT2 inhibitors3. This BW reduction is thought to result from fluid loss and dehydration in the initial phase, and from fat and muscle mass reduction in the late phase of treatment4. Fat mass reduction is a favorable feature of SGLT2 inhibitors. However, reduction in skeletal muscle mass might have negative effects, especially in elderly or lean patients with type 2 diabetes.
Sarcopenia is a serious condition that affects quality of life and mortality among elderly people5, 6. Diabetes is a risk factor for sarcopenia, probably because of the negative energy balance caused by glucosuria or insulin resistance7. Bone mineral density is another concern related to SGLT2 inhibitors. Previous studies have shown a 1.2‐fold increase in the incidence of bone fracture among patients treated with canagliflozin, although meta‐analysis showed that the difference was not significant8, 9. SGLT2 inhibitors have been preferentially used by obese patients. However, little is currently known about how BW and body composition are affected by SGLT2 inhibitors in the clinical setting.
In the present study, we examined the effect of ipragliflozin on BW and body composition in Japanese patients with type 2 diabetes treated with insulin.
Methods
The present randomized, 24‐week, open‐label, parallel‐group comparative clinical trial enrolled patients treated at Shiga University of Medical Science Hospital, Shiga, Japan, between November 2015 and March 2017. The final date of follow up was 25 October 2017. This trial was registered with the UMIN Clinical Trials Registry (UMIN000018839).
Participants
The participants were outpatients with type 2 diabetes aged #bib10 #bib11 #bib12 #bib13 years with a hemoglobin A1c of 7.0–10.0% (53–85 mmol/mol) and body mass index ≥23 kg/m2. At the time of study enrollment, all patients were treated with insulin therapy alone or with insulin plus oral hypoglycemic agents other than SGLT2 inhibitors. The type of insulin injection (bolus, basal or both) was not specified. All included patients had an estimated glomerular filtration rate >45 mL/min/1.73 m2, received an explanation of the study using an informed consent form and provided written consent for study participation. Exclusion criteria included treatment with SGLT2 inhibitors or loop diuretics; any contraindication to ipragliflozin; severe ketosis; diabetic coma or precoma; history of hospitalization within 6 months for trauma, surgery or infectious disease; and history of cerebral infarction, transient ischemic attack or orthostatic hypotension. Inclusion and exclusion criteria were amended on 15 July 2016 to increase the maximum age from 70 to 75 years. In addition, patients with a history of unstable angina or myocardial infarction were not excluded after 15 July 2016, as long as the patient had been stable for 6 months. The nature and potential risks of the study were explained to all participants, and written informed consent was obtained. The study was carried out in accordance with the principles of the Declaration of Helsinki. The original protocol was approved by the ethics committee of Shiga University of Medical Science on 28 April 2015. The amended protocol was approved on 15 July 2016.
Study Design, Randomization and Allocation
After confirming that a patient satisfied the inclusion criteria and cleared all exclusion criteria, the principle investigator registered the patient in the research registry. Eligible patients were randomized to either the ipragliflozin (Ipra) or control (Control) group at a 1:1 ratio, with patient sex as a stratification factor. A web‐based, password‐protected randomization system based on a computer‐generated random sequence was used. Patient registration and randomization were carried out by a clinical research development office that was independent of the investigators.
Intervention
All participants in the Ipra group received 50 mg of ipragliflozin orally once daily after breakfast for 24 weeks, with no dosage adjustments during the trial. To prevent hypoglycemia at the initiation of ipragliflozin administration, the insulin dosage was decreased by 20%. Sulfonylurea dosage was decreased according to treatment guidelines as follows: for patients taking >2 mg/day of glimepiride, the dose was decreased to ≤2 mg/day; for patients taking >1.25 mg/day of glibenclamide, the dose was decreased to ≤1.25 mg/day; for patients taking >40 mg/day of gliclazide, the dose was decreased to ≤40 mg/day. After 2 weeks of treatment, the insulin dosage was adjusted to achieve the therapeutic goal of hemoglobin A1c <7.0% without hypoglycemia.
Patients in the Control group continued their previous treatments and were allowed to change insulin dosage to achieve therapeutic goals. New antidiabetic agents were not introduced in either group during the 24‐week observation period.
Outcomes
The primary outcome was the change in total BW (TBW) between baseline and 24 weeks in both groups. Secondary outcomes were the changes from baseline to 24 weeks in body fat mass and lean body mass measured with dual‐energy X‐ray absorptiometry (DEXA); changes in navel subcutaneous fat mass, navel visceral fat mass and iliopsoas surface area measured with magnetic resonance imaging (MRI); changes in bone mineral content; and changes in fat mass and lean body mass in the arms, lower limbs and trunk measured with DEXA and bioelectrical impedance analysis (BIA). We also evaluated changes in aspartate transaminase, alanine transaminase, hemoglobin A1c, fasting plasma glucose, serum ketone bodies, triglycerides, and total high‐density lipoprotein, low‐density lipoprotein and remnant‐like particle cholesterol. Safety variables included adverse events, hypoglycemic episodes, standard laboratory analysis findings, physical examination and vital signs. Patients were examined at 0 #bib2 #bib8 #bib16 and 24 weeks during treatment.
Weight and Body Composition
The BW and composition of each participant was recorded with BIA (MC‐780A; Tanita, Tokyo, Japan). Segmental body composition values were calculated by using multiple electrodes with different current frequencies. Fat and muscle mass at the arms, lower limbs and trunk were determined. Visceral and subcutaneous fat area was measured with abdominal BIA (HDS‐2000 DUALSCAN; Omron, Kyoto, Japan). Patients wore a hospital gown during measurements. Measurements were carried out at 0 #bib2 #bib8 #bib16 and 24 weeks after an 8‐ to 16‐h fast. DEXA (GE Healthcare, Madison, WI, USA) was carried out at 0 and 24 weeks to measure whole‐body and segmental body composition.
MRI
Navel subcutaneous and visceral fat area and iliopsoas surface area were measured at 0 and 24 weeks with MRI (Discovery MR750w Expert 3.0T; GE Healthcare Japan, Tokyo, Japan). This test was only carried out for participants who provided additional consent. Participants with extreme BW, claustrophobia, a pacemaker or any metal with the potential to cause harm in a 3T‐MR scanner were excluded from the additional agreement for safety reasons. The aforementioned areas were measured by an independent radiologist who used a three‐dimensional workstation (Aquarius iNtuition; TeraRecon Inc., Foster City, CA, USA). All tests were carried out after fasting on separate dates within 2 weeks of 0 and 24 weeks. Unless an emergency situation occurred, measurements were not revealed to investigators until the database was locked.
Dietary Preference Survey
Patients completed a questionnaire on dietary preferences and hunger at 0 and 24 weeks. Visual analog scores were used to measure: (i) hunger; (ii) desire for oily foods; (iii) desire for sweets; (iv) desire for a Western‐style diet; (v) desire for a Japanese‐style diet; and (vi) desire for meat/protein versus carbohydrate/starchy food.
Statistical Analysis
We calculated our sample size on the basis of results from a study carried out in Europe, which reported that the primary end‐point of difference in BW change between dapagliflozin and placebo groups was 2.08 kg4. The standard deviation of BW change was 2.51 kg in the dapagliflozin group and 2.53 kg in the placebo group; therefore #bib24 patients per group are required to detect differences between the groups (power 80%, two‐sided P = 0.05). We assumed an attrition rate of 5%; thus, 26 patients were required per group. Therefore, the total number of patients was set at 52.
Statistical analyses were carried out with SAS (ver. 9.4; SAS Institute Inc., Cary, NC, USA), according to the principle of intention‐to‐treat. Data are expressed as the mean ± standard deviation for continuous variables and n (%) for categorical variables. The primary outcome, change in TBW from baseline to 24 weeks, was compared between groups with sex‐adjusted analysis of covariance (ancova). Differences in baseline characteristics between the groups were examined with Student's t‐test for continuous variables and with Pearson's χ2‐test for categorical variables. Sensitivity analysis was carried out with ancova to test the effect of insulin dosage. To maintain data independence, data analysis after database lock was carried out by a statistical analyst and an outsourcing company. P < 0.05 was considered statistically significant.
Results
Baseline Clinical Characteristics of Study Participants
A total of 77 eligible patients with type 2 diabetes mellitus were screened at Shiga University of Medical Science Hospital between November 2015 and March 2017; 50 were enrolled. Figure 1 shows the study flowchart and CONSORT diagram. After exclusion of one patient who withdrew consent because of breathing difficulty during MRI examination, 49 patients were randomly assigned to either the Ipra group (n = 25) or the Control group (n = 24). A total of 44 patients completed the study (Ipra group, n = 20; Control group, n = 24). One patient in the Ipra group withdrew consent before starting the intervention because of anxiety about ipragliflozin side‐effects. Two patients in the Ipra group discontinued the intervention, one because of exanthema and one because of liver dysfunction, but continued follow up, including bodyweight and body composition measurements. Two additional patients in the Ipra group withdrew from the intervention, one because of cholecystitis and one because of genital itching. Finally, 48 patients (Ipra, n = 24; Control, n = 24) were included in intention‐to‐treat analysis; however, two patients in the Ipra group were not followed up at 24 weeks and were not included in the primary analysis. Table 1 shows the demographic and baseline characteristics of study participants. None of the baseline characteristics were significantly different between groups. The total daily dose of insulin tended to be slightly higher in the Ipra group at enrollment (Table 1).
Figure 1.

Study flowchart and CONSORT diagram. ITT, intention‐to‐treat.
Table 1.
Demographic and baseline characteristics of the full analysis set
| Total | Control group (insulin) | Ipra group (insulin + ipragliflozin 50 mg) | P‐value | |
|---|---|---|---|---|
| No. patients | 48 | 24 | 24 | |
| Age, years (mean ± SD) | 60.6 ± 10.9 | 60.8 ± 12.1 | 60.5 ± 9.8 | 0.577 |
| Sex (male/female) | 27/21 | 14/10 | 13/11 | 0.771 |
| Height, cm (mean ± SD) | 163.0 ± 9.2 | 164.1 ± 9.7 | 162.0 ± 8.7 | 0.427 |
| Bodyweight, kg (mean ± SD) | 74.1 ± 13.9 | 74.6 ± 13.3 | 73.6 ± 14.7 | 0.658 |
| Body mass index, kg/m2 (mean ± SD) | 27.8 ± 4.2 | 27.7 ± 4.5 | 27.9 ± 4.0 | 0.875 |
| Systolic BP, mmHg (mean ± SD) | 143.2 ± 15.4 | 142.5 ± 14.9 | 143.9 ± 16.2 | 0.975 |
| Diastolic BP, mmHg (mean ± SD) | 83.0 ± 10.7 | 83.4 ± 9.6 | 82.5 ± 11.9 | 0.804 |
| Pulse rate, b.p.m. (mean ± SD) | 82.7 ± 11.8 | 80.9 ± 11.1 | 84.5 ± 12.5 | 0.445 |
| HbA1c, % (mean ± SD) | 8.21 ± 0.80 | 8.30 ± 0.65 | 8.12 ± 0.93 | 0.239 |
| eGFR, mL/min/1.73 m2 (mean ± SD) | 86.9 ± 25.7 | 82.9 ± 24.8 | 91.0 ± 26.4 | 0.097 |
| Duration of type 2 diabetes mellitus, years (mean ± SD) | 17.5 ± 9.3 | 19.1 ± 10.7 | 15.9 ± 7.7 | 0.343 |
| Diabetes‐related disease, n (%) | ||||
| Retinopathy | 12 (25.1) | 8 (33.3) | 4 (16.7) | 0.323 |
| Nephropathy | 17 (35.5) | 10 (41.7) | 7 (29.2) | 0.144 |
| Neuropathy | 13 (27.1) | 6 (25.0) | 7 (29.2) | 0.557 |
| Insulin dosage, units/day (mean ± SD) | 32.2 ± 18.7 | 28.6 ± 21.4 | 35.8 ± 15.1 | 0.051 |
| Medications, n (%) | ||||
| Metformin | 27 (57.4) | 15 (65.2) | 12 (50.0) | 0.292 |
| DPP‐4 inhibitor | 15 (31.3) | 9 (37.5) | 6 (25.0) | 0.350 |
| Sulfonylurea | 5 (10.4) | 3 (12.5) | 2 (8.3) | 0.637 |
| Thiazolidinedione | 9 (18.8) | 3 (12.5) | 6 (25.0) | 0.267 |
| α‐GI | 9 (18.8) | 5 (20.8) | 4 (16.7) | 0.712 |
| GLP‐1 | 8 (16.7) | 3 (12.5) | 5 (20.8) | 0.439 |
| Glinide | 7 (14.6) | 3 (12.5) | 4 (16.7) | 0.683 |
| Antihypertensive medication | 29 (60.4) | 13 (54.2) | 16 (66.7) | 0.376 |
| Antihyperlipidemic medication | 32 (66.7) | 18 (75.0) | 14 (58.3) | 0.221 |
| Hypertension, n (%) | 29 (60.4) | 13 (54.2) | 16 (66.7) | 0.376 |
| Dyslipidemia, n (%) | 34 (70.8) | 19 (79.2) | 15 (62.5) | 0.204 |
| Smoking, n (%) | ||||
| Never | 17 (35.4) | 11 (45.8) | 6 (25.0) | 0.315 |
| Former | 23 (47.9) | 10 (41.7) | 13 (54.2) | |
| Current | 8 (16.7) | 3 (12.5) | 5 (20.8) | |
Values are mean ± standard deviation for continuous variables. The P‐values show the difference between the group receiving add‐on ipragliflozin (Ipra group) and the group receiving no additional treatment (Control group) using unpaired t‐tests or the χ2‐test. α‐GI, alpha glucosidase inhibitor; BP, blood pressure; DPP‐4, dipeptidyl peptidase‐4; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1 receptor agonist; HbA1c, hemoglobin A1c; SD, standard deviation.
Primary Outcome
Patients in the Ipra group showed a statistically greater TBW reduction than those in the Control group. The mean change in TBW from baseline to 24 weeks was −2.78 ± 0.40 kg (from 72.34 ± 14.51 to 69.18 ± 14.38 kg) in the Ipra group and −0.22 ± 0.38 kg (from 73.42 ± 13.20 to 73.16 ± 12.69 kg) in the Control group. A difference in TBW loss of −2.56 kg (95% confidence interval −3.67 to −1.45) was observed between the groups (Table 2). The difference in TBW increased and became significant from 2 weeks after Ipra treatment initiation (Figure S1).
Table 2.
Change in parameters compared between baseline and week 24
| Control group (insulin) | Ipra group (insulin + ipragliflozin 50 mg) | Difference in changes (95% CI) | P‐value | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Week 24 | Change | Baseline | Week 24 | Change | |||
| Bodyweight (kg) | 73.42 ± 13.20 | 73.16 ± 12.69 | −0.22 ± 0.38 | 72.34 ± 14.51 | 69.18 ± 14.38 | −2.78 ± 0.40 | −2.56 (−3.67, −1.45) | <0.0001 |
| DEXA | ||||||||
| Fat mass (kg) | 22.96 ± 9.37 | 22.94 ± 9.30 | −0.01 ± 0.27 | 23.39 ± 9.16 | 21.32 ± 9.09 | −2.07 ± 0.28 | −2.06 (−2.85, −1.26) | <0.0001 |
| Lean mass (kg) | 43.43 ± 7.43 | 43.20 ± 7.18 | −0.22 ± 0.27 | 41.63 ± 7.72 | 41.02 ± 7.35 | −0.60 ± 0.29 | −0.38 (−1.17, 0.40) | 0.33 |
| BMC (g) | 2080 ± 467 | 2080 ± 480 | 0.6 ± 11.4 | 1999 ± 438 | 1986 ± 445 | −14.0 ± 11.8 | −13.4 (−46.5, 19.6) | 0.42 |
| BIA | ||||||||
| Fat mass (kg) | 22.90 ± 12.83 | 22.63 ± 12.36 | −0.27 ± 0.46 | 22.14 ± 10.61 | 19.93 ± 9.87 | −2.21 ± 0.48 | −1.94 (−3.28, −0.60) | 0.0056 |
| Muscle mass (kg) | 47.78 ± 8.75 | 47.79 ± 8.71 | 0.05 ± 0.33 | 47.14 ± 9.97 | 46.56 ± 9.70 | −0.56 ± 0.34 | −0.61 (−1.56, 0.35) | 0.21 |
| MRI | ||||||||
| Navel subcutaneous fat area (cm2) | 315.0 ± 121.3 | 319.4 ± 143.3 | 6.7 ± 14.1 | 334.9 ± 157.7 | 314.6 ± 165.0 | −18.9 ± 15.7 | −25.6 (−68.3, 17.1) | 0.231 |
| Navel visceral fat area (cm2) | 122.7 ± 46.4 | 131.6 ± 64.2 | 11.3 ± 11.5 | 138.8 ± 101.3 | 125.3 ± 77.4 | −12.0 ± 12.7 | −23.3 (−57.9, 11.3) | 0.180 |
| Iliopsoas muscle surface area (cm2) | 21.2 ± 6.3 | 20.7 ± 8.8 | −0.5 ± 1.2 | 20.6 ± 6.6 | 18.8 ± 8.0 | −1.8 ± 1.3 | −1.3 (−4.9, 2.3) | 0.452 |
| V/S ratio | 0.40 ± 0.11 | 0.42 ± 0.10 | 0.02 ± 0.02 | 0.39 ± 0.14 | 0.40 ± 0.15 | 0.01 ± 0.02 | −0.01 (−0.07, 0.05) | 0.706 |
| HbA1c (%) | 8.30 ± 0.65 | 8.14 ± 0.88 | −0.15 ± 0.17 | 8.12 ± 0.93 | 7.41 ± 0.70 | −0.69 ± 0.18 | −0.53 (−1.03, −0.04) | 0.0035 |
| FPG (mg/dL) | 167.3 ± 51.6 | 150.5 ± 40.6 | −15.8 ± 8.6 | 152.4 ± 44.9 | 121.3 ± 19.6 | −30.5 ± 9.0 | −14.7 (−39.7, 10.3) | 0.243 |
| Total cholesterol (mg/dL) | 173.9 ± 25.1 | 177.3 ± 30.5 | 3.6 ± 4.2 | 186.8 ± 29.6 | 178.4 ± 28.6 | −8.4 ± 4.3 | −12.0 (−24.0, 0) | 0.051 |
| LDL cholesterol (mg/dL) | 96.3 ± 17.4 | 97.0 ± 22.6 | 0.7 ± 17.9 | 103.7 ± 24.8 | 98.5 ± 22.7 | −4.3 ± 19.0 | −5.0 (−16.1, 6.1) | 0.372 |
| HDL cholesterol (mg/dL) | 50.3 ± 8.3 | 49.6 ± 8.2 | −1.0 ± 1.1 | 51.4 ± 12.4 | 53.8 ± 9.6 | 1.4 ± 1.1 | 2.3 (−0.8, 5.5) | 0.143 |
| Triacylglycerol (mg/dL) | 111.9 ± 43.8 | 139.3 ± 82.3 | 32.3 ± 36.1 | 170.3 ± 290.0 | 110.1 ± 75.2 | −57.6 ± 37.5 | −89.9 (−194.6, 14.7) | 0.090 |
| RLP cholesterol (mg/dL) | 4.04 ± 2.28 | 6.02 ± 4.99 | 2.19 ± 1.80 | 7.56 ± 14.03 | 4.73 ± 4.16 | −2.71 ± 1.87 | −4.90 (−10.1, 0.31) | 0.065 |
| AST (units/L) | 26.9 ± 12.5 | 27.3 ± 13.5 | 0.3 ± 2.0 | 26.0 ± 15.9 | 22.4 ± 11.8 | −0.5 ± 2.1 | −0.7 (−6.5, 5.0) | 0.798 |
| ALT (units/L) | 31.8 ± 17.9 | 34.3 ± 20.3 | 2.3 ± 2.3 | 30.9 ± 24.1 | 21.8 ± 10.6 | −3.7 ± 2.4 | −5.9 (−12.6, 0.8) | 0.081 |
| Insulin dosage (units/day) | 28.8 ± 21.6 | 28.6 ± 18.1 | −0.58 ± 1.2 | 28.4 ± 12.3 | 26.2 ± 14.9 | −2.33 ± 1.19 | −1.75 (−5.14, 1.63) | 0.303 |
Values are the mean ± standard deviation for continuous variables of “baseline” and “24 weeks,” and least squares mean ± standard error for continuous variables of “change”. Except for the magnetic resonance imaging (MRI): n = 24 for the group receiving no additional treatment (Control group); n = 22 for the group receiving add‐on ipragliflozin (Ipra group). MRI: n = 20 for the Control group; n = 16 for the Ipra group. Visceral‐to‐subcutaneous fat (V/S) ratio: the P‐values show comparisons of changes between the Ipra group and the Control group using anova adjusted by sex. ALT, alanine transaminase; AST, aspartate transaminase; BIA, bioelectrical impedance analysis; BMC, bone mineral content; CI, confidence interval; DEXA, dual‐energy X‐ray absorptiometry; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; RLP, remnant‐like particle.
Secondary Outcomes
Body composition was evaluated with DEXA. The change in fat mass was significantly different between the groups (−2.07 kg vs −0.01 kg, P < 0.0001). The change in lean mass was somewhat larger in the Ipra group, but this difference was not significant (−0.60 kg vs −0.22 kg, P = 0.3316). Approximately 80% of TBW change resulted from reduced fat mass. Bone mineral content was unchanged in both groups after 6 months of intervention. Body composition was also evaluated with BIA. As with the DEXA measurements, the change in fat mass according to BIA was significantly different between the groups (−2.21 kg in Ipra group vs −0.27 kg in Control group, P < 0.01). Lean body mass measured with DEXA includes muscle, organs and body water, whereas BIA measures skeletal muscle mass. The change in muscle mass measured with BIA was somewhat larger in the Ipra group than in the Control group, but the difference was not significant (−0.56 kg vs 0.05 kg, P = 0.2056). Approximately 80% of TBW change resulted from reduced fat mass. In both analyses, there was no significant difference in muscle or lean mass content from baseline to the end of the trial. Changes in hemoglobin A1c levels were significantly greater in the Ipra group compared with the Control group (−0.69 ± 0.18% vs −0.15 ± 0.17%, P < 0.005). Lipid profiles tended to improve more in the Ipra group than in the Control group, although these improvements were not statistically significant. Other plasma factors were comparable between the groups (Table 2). The difference in changes of insulin dosage showed no significance (Table 2). Adverse events during the trial are shown in Table S1. Hypoglycemia, dehydration, urinary tract infection and exanthema were observed in both the Ipra and Control groups.
Segmental Body Composition
The trunk, arms and lower limbs were separately evaluated with both DEXA and BIA to investigate segmental changes in body composition. Absolute change in fat mass was evident in the trunk (DEXA: −1.31 kg in Ipra group vs 0.22 kg in Control group, P < 0.0001; BIA: −1.35 kg in Ipra group vs −0.16 kg in Control group, P < 0.01; Figure 2a). The percentage change in fat mass was comparable for the trunk, arms and lower limbs (Figure 2b). The absolute change in lean mass in the arms measured with DEXA was significantly greater in the Ipra group than in the Control group (−0.19 kg vs 0.02 kg, P < 0.01), whereas the differences were not significant in the lower limbs and trunk (Figure 2c). To evaluate the areas of visceral fat, subcutaneous fat and iliopsoas muscle, MRI was carried out in selected participants (Table 2). A total of 36 of the 50 patients participated in this analysis. These three areas did not significantly differ between the groups. The visceral‐to‐subcutaneous fat ratio was evaluated as post‐hoc analysis; the change in this ratio did not significantly differ between groups (Table 2). In contrast, abdominal BIA showed significant differences in both visceral and subcutaneous fat changes between groups (Figure S3).
Figure 2.
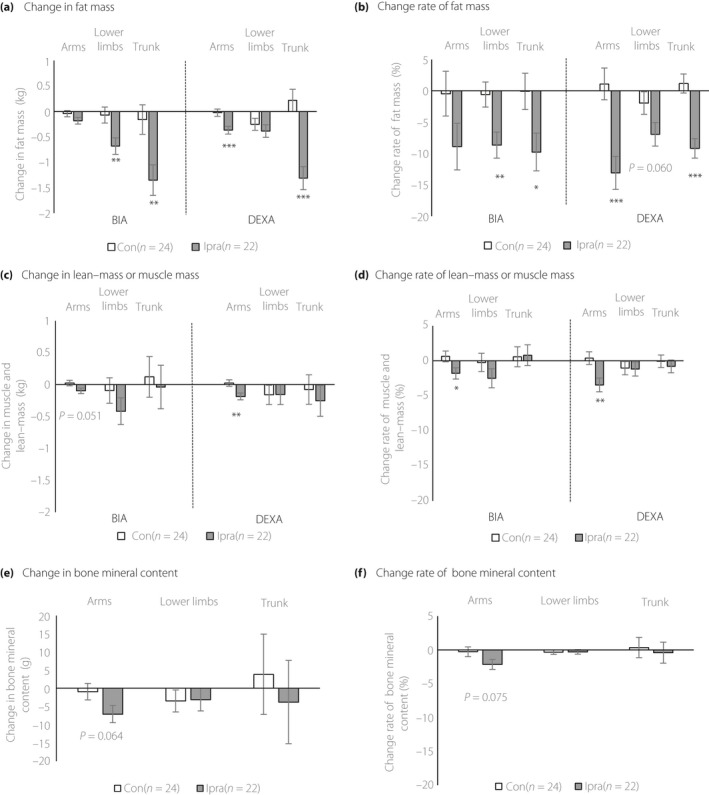
Changes in segmental body composition. (a) Change in fat mass measured with dual‐energy X‐ray absorptiometry (DEXA) after 24 weeks of intervention (right panel). Change in fat mass measured with BIA after 24 weeks of intervention (left panel). (b) Percentage change in fat mass measured with DEXA after intervention (right panel). Percentage change in fat mass measured with BIA after intervention (left panel). (c) Percentage change in lean mass measured with DEXA after 24 weeks of intervention (right panel). Percentage change in muscle mass measured with BIA after 24 weeks of intervention (left panel). (d) Change in lean mass measured with DEXA after 24 weeks of intervention (right panel). Change in muscle mass measured with BIA after 24 weeks of intervention (left panel). (e) Change in bone mineral content measured with DEXA after 24 weeks of intervention. (f) Percentage change in bone mineral content measured with DEXA after 24 weeks of intervention. Values are least square mean ± standard error for change. The P‐values show comparisons of changes between the group receiving add‐on ipragliflozin (Ipra) and the group receiving no additional treatment (Control) using ancova adjusted for sex. *P < 0.05, **P < 0.01, or ***P < 0.001.
As with DEXA, the absolute change in muscle mass in the arms measured with BIA tended to be larger in the Ipra group than the Control group (−0.10 kg vs 0.02 kg, respectively; P = 0.051), whereas no significant changes were observed in the lower limbs or trunk (Figure 2c). The percentage change in lean mass and muscle in the arms was significantly larger in the Ipra group than in the Control group; there was no significant difference in the percentage change in the lower limbs or trunk between groups (−3.46% vs 0.37%, P < 0.01; Figure 2d, DEXA; −1.81% vs 0.62%, P < 0.05; Figure 2d, BIA). Finally, the change in bone mineral content tended to be larger in the Ipra group than in the Control group only in the arms (−7.08 vs −0.93 g, P = 0.064; Figure 2e). The percentage change in bone mineral content showed a similar trend (−2.15% vs −0.27%, P = 0.075; Figure 2f).
Discussion
The present study has two major findings. First, ipragliflozin add‐on treatment reduced BW in type 2 diabetes patients receiving insulin therapy. Second, ipragliflozin preferentially decreased body fat, with no significant reduction in muscle mass or bone mineral content after 24 weeks of treatment.
Ipragliflozin add‐on treatment reduced BW in type 2 diabetes patients receiving insulin therapy. This finding is in agreement with results from a previous study that reported reduced BW in type 2 diabetes mellitus patients treated with SGLT2 inhibitors10. Studies of insulin‐treated patients have shown a 2.8‐kg greater BW reduction after 48 weeks of dapagliflozin treatment11, and a 2.8‐kg greater BW reduction after 52 weeks of canagliflozin treatment, compared with placebo controls12. In those studies, BW reduction was near 3%, whereas in the present study it was 4%. This difference might have resulted from differences in the inhibitor used, but is more likely attributable to the difference in basal BW in the study populations (94 kg vs 74 kg). Another possible factor is the study setting. Most previous studies of ipragliflozin have been third‐phase clinical studies or single‐arm in a fourth‐phase clinical study. Our protocol aimed to elucidate the effect of ipragliflozin on BW in the clinical setting. The present study clarified that ipragliflozin treatment of diabetes patients reduced BW by approximately 2.5 kg.
Ipragliflozin treatment preferentially decreased body fat, with no significant reduction in muscle mass after 24 weeks of treatment (Table 2). Previous studies using DEXA showed that the BW reduction resulting from SGLT2 inhibitor treatment largely resulted from loss of fat mass, accounting for approximately 70% of weight loss in Caucasian patients4, 13. In the present study, 80% of BW reduction resulted from fat mass reduction (Figure S2). In the previous studies based on Japanese populations, 71–85% of BW reduction achieved with SGLT2 inhibitors resulted from fat loss as assessed with BIA14, 15, 16, 17, and the reduction rates seemed to be dependent on the “treatment period.” The studies16, 17 showed a trend toward a greater reduction rate at 6 or 12 months than that at 3 months. That would be another reason for the minor difference in the fat reduction rate in the same population. A recent study pointed out the possible importance of dietary therapy on ipragliflozin's effect on visceral fat14. In the present study, we carried out a dietary preference survey before and after intervention, and found no significant difference between the groups in changes in hunger or dietary preferences (Table S2).
Muscle mass atrophy is a major concern with the use of SGLT2 inhibitors, especially in elderly and lean patients. Because the present participants were relatively old, marginally overweight (30 > body mass index > 23) and had a typical Asian body composition, we combined several methods to analyze body composition. In our study, there were no significant changes in muscle mass after 24 weeks of treatment, as assessed with DEXA and BIA (Table 2). This result was supported by the unchanged area of the iliopsoas muscle on MRI. However, this analysis was underpowered because of the low number of participants (Table 2). To examine the effect of insulin dosage on our results, we added sensitivity analysis with adjustment for the insulin dose at enrollment, at the start of intervention and at the end of intervention, and changes during intervention. No effect of these adjustments was observed on changes in BW, fat mass change, lean mass change or muscle mass (Table S3).
Reduced muscle mass commonly occurs with BW reduction after various interventions. A previous study of obese patients who underwent bariatric surgery reported that 20% of BW reduction was attributable to lean‐mass reduction and 80% resulted from fat‐mass reduction18. A very low‐calorie diet caused a similar percentage change in lean mass in obese patients19. In patients with type 2 diabetes, a glucagon‐like peptide 1 analog also reduced BW, of which 20% resulted from lean‐mass reduction20. These studies suggest that muscle mass reduction occurs in conjunction with weight reduction, probably because of reduced weight‐bearing effects, especially in the antigravity muscles. A recent study of calorie restriction and exercise in healthy individuals showed reduced muscle mass in the arms21, similar to the findings of the current study (Figure 2d), but no change in grip power. The reasons for greater muscle loss in the upper arms compared with the lower limbs and trunk in the present study remain unknown. Muscle fiber types differ depending on the muscle. The upper arm muscles contain more type 2 fibers than the lower limbs and trunk22. It has been reported that less insulin signal transduction and subsequent glucose oxidation is observed in type 2 fibers, especially in patients with type 2 diabetes mellitus23. We speculate that the difference in the composition of fiber types might explain the discrepancy, and that trunk and lower limb muscles were relatively preserved in the present study. The quality of muscle performance is an important factor to be tested in future clinical trials.
The present study had three primary strengths. First, comprehensive analysis was used to measure body composition, including DEXA, BIA and MRI. Because BIA is non‐invasive, we could measure body composition at multiple time points. Second, these measurements have rarely been included in randomized controlled trials. A single‐arm study with ipragliflozin showed a significant reduction in total muscle mass compared with baseline, as assessed with BIA15, 24. Third, the present patients were relatively older15 and leaner than those in previous studies4, 10, 11, 12, 13, 24. Recent clinical trials showed the benefits of SGLT2 inhibitors for cardiovascular and renal outcomes, including heart failure12, 25. In subgroup analysis of the EMPA Outcome trial, body mass index <30 kg/m2 and age >65 years showed beneficial effects in these participants25. Because heart failure often occurs in older patients, it is expected that prescription of SGLT2 inhibitors to older and leaner patients might increase in the near future. In the present study, patients tolerated ipragliflozin treatment and maintained muscle mass, suggesting that muscle reduction might not be a large effect for at least 24 weeks in patients with these characteristics.
The present study had some limitations. First, the number of participants was relatively small, although a sufficient sample size was calculated. Second, this study was an open‐label randomized study. The characteristics of BW reduction might be different with randomization. This is a limitation and a strength of this study in the clinical setting. Third, some inconsistent results were observed between DEXA and BIA in segmental muscle mass. These discrepancies probably resulted from technical difficulty in estimating muscle mass, especially segmental mass26, 27. Finally, the study participants were recruited at a single hospital. Therefore, any generalizations must be made with caution.
In conclusion, 24 weeks of ipragliflozin add‐on treatment effectively reduced BW mainly by reducing fat content in inadequately controlled type 2 diabetes patients receiving insulin therapy. Total muscle mass and bone mineral content were maintained, but arm muscle mass might have been affected by ipragliflozin treatment. Further investigation is necessary to confirm the long‐term effects of SGLT2 inhibitors on body composition.
Disclosure
HM received research support unrelated to this study from Astellas Pharma Inc., AstraZeneca K.K., Ono Pharmaceutical and Nippon Boehringer Ingelheim; and lecture fees and fees for serving on advisory boards from Astellas Pharma, AstraZeneca, Taisho Toyama Pharmaceutical, Ono Pharmaceutical, Novo Nordisk Pharma, Eli Lilly Japan, MSD, Daiichi Sankyo, Nippon Boehringer Ingelheim, Mitsubishi Tanabe Pharma, Sanofi, Kowa Pharmaceutical and Takeda Pharmaceutical. K Morino received grants from AstraZeneca and Ono Pharmaceutics unrelated to this study. HA received lecture fees from Bayer, Daiichi Sankyo, Fukuda Denshi and Takeda Pharmaceutical, and consultant fees from Kyowa Hakko Kirin. The other authors declare no conflict of interest.
Supporting information
Figure S1 Changes in bodyweight over the study period.
Figure S2 Changes in body composition measured with dual‐energy X‐ray absorptiometry.
Figure S3 Visceral and subcutaneous fat evaluated with abdominal bioelectrical impedance analysis.
Table S1 Major adverse events during the clinical trial.
Table S2 Dietary preferences according to visual analog scale.
Table S3 Changes in body composition adjusted for sex and insulin dose.
Appendix S1 Complete list of members of SUMS‐ADDIT‐1 Investigator.
Appendix S2 CONSORT checklist.
Acknowledgments
The authors thank the participants and the SUMS‐ADDIT‐1 investigators for their involvement in this study. A complete list of members of the Shiga University of Medical Science Anti‐Diabetic Drugs Intervention Trial‐1 (SUMS‐ADDIT‐1) investigators is provided in the Appendix S1. We also thank Keiko Kosaka, Keiko Asano, Megumi Matsuo and Naoko Yamanaka for their expert technical assistance. We thank Michal Bell, PhD, and Rebecca Tollefson, DVM, from Edanz Group (www.edanzediting.com/ac) for editing the English text of drafts of this manuscript. This study was funded by Astellas Pharma Inc. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
J Diabetes Investig 2019; 10: 1012–1021
Clinical Trial Registry
UMIN Clinical Trials Registry UMIN000018839
References
- 1. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 2. Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta‐analysis. Diabetes Obes Metab 2011; 13: 1008–1019. [DOI] [PubMed] [Google Scholar]
- 3. Vasilakou D, Karagiannis T, Athanasiadou E, et al Sodium‐glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta‐analysis. Ann Intern Med 2013; 159: 262–274. [DOI] [PubMed] [Google Scholar]
- 4. Bolinder J, Ljunggren Ö, Kullberg J, et al Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012; 97: 1020–1031. [DOI] [PubMed] [Google Scholar]
- 5. Hu X, Zhang L, Wang H, et al Malnutrition‐sarcopenia syndrome predicts mortality in hospitalized older patients. Sci Rep 2017; 7: 3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rizzoli R, Reginster JY, Arnal JF, et al Quality of life in sarcopenia and frailty. Calcif Tissue Int 2013; 93: 101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim TN, Park MS, Yang SJ, et al Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010; 33: 1497–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yale JF, Bakris G, Cariou B, et al Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 2014; 16: 1016–1027. [DOI] [PubMed] [Google Scholar]
- 9. Darin R, Patompong U, Jutarat S, et al Sodium‐glucose cotransporter 2 (SGLT2) inhibitors and fracture risk in patients with type 2 diabetes mellitus: a meta‐analysis. Diabetes Metab Res Rev 2017; 33: e2903. [DOI] [PubMed] [Google Scholar]
- 10. Cai X, Ji L, Chen Y, et al Comparisons of weight changes between sodium‐glucose cotransporter 2 inhibitors treatment and glucagon‐like peptide‐1 analogs treatment in type 2 diabetes patients: a meta‐analysis. J Diabetes Investig 2017; 8: 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilding JP, Woo V, Rohwedder K, et al Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab 2014; 16: 124–136. [DOI] [PubMed] [Google Scholar]
- 12. Neal B, Perkovic V, de Zeeuw D, et al Efficacy and safety of canagliflozin, an inhibitor of sodium‐glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care 2015; 38: 403–411. [DOI] [PubMed] [Google Scholar]
- 13. Bolinder J, Ljunggren Ö, Johansson L, et al Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014; 16: 159–169. [DOI] [PubMed] [Google Scholar]
- 14. Yamamoto C, Miyoshi H, Ono K, et al Ipragliflozin effectively reduced visceral fat in Japanese patients with type 2 diabetes under adequate diet therapy. Endocr J 2016; 63: 589–596. [DOI] [PubMed] [Google Scholar]
- 15. Kawata T, Iizuka T, Iemitsu K, et al Ipragliflozin improves glycemic control and decreases body fat in patients with type 2 diabetes mellitus. J Clin Med Res 2017; 9: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seino T, Yabe D, Sasaki T, et al Sodium‐glucose cotransporter‐2 inhibitor luseogliflozin added to glucagon‐like peptide 1 receptor agonist liraglutide improves glycemic control with bodyweight and fat mass reductions in Japanese patients with type 2 diabetes: a 52‐week, open‐label, single‐arm study. J Diabetes Investig 2018; 9: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Osonoi T, Nakamoto S, Saito M, et al Efficacy of ipragliflozin as monotherapy or as add‐on therapy with other oral antidiabetic medications for treating type 2 diabetes in Japanese patients with inadequate glycemic control: a subgroup analysis based on patient characteristics. J Diabetes Investig 2018; 9: 341–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one‐year follow‐up. Obes Surg 2006; 16: 1602–1608. [DOI] [PubMed] [Google Scholar]
- 19. Gomez‐Arbelaez D, Bellido D, Castro AI, et al Body composition changes after very‐low‐calorie ketogenic diet in obesity evaluated by 3 standardized methods. J Clin Endocrinol Metab 2017; 102: 488–498. [DOI] [PubMed] [Google Scholar]
- 20. Jendle J, Nauck MA, Matthews DR, et al Weight loss with liraglutide, a once‐daily human glucagon‐like peptide‐1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab 2009; 11: 1163–1172. [DOI] [PubMed] [Google Scholar]
- 21. Kim B, Tsujimoto T, So R, et al Weight reduction does not induce an undesirable decrease in muscle mass, muscle strength, or physical performance in men with obesity: a pilot study. J Exerc Nutrition Biochem 2017; 21: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson MA, Polgar J, Weightman D, et al Data on the distribution of fibre types in thirty‐six human muscles: an autopsy study. J Neurol Sci 1973; 18: 111–129. [DOI] [PubMed] [Google Scholar]
- 23. Albers P, Pedersen A, Birk J, et al Human muscle fiber type‐specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes 2015; 64: 485–497. [DOI] [PubMed] [Google Scholar]
- 24. Nomiyama T, Shimono D, Horikawa T, et al Efficacy and safety of sodium–glucose cotransporter 2 inhibitor ipragliflozin on glycemic control and cardiovascular parameters in Japanese patients with type 2 diabetes mellitus; Fukuoka Study of Ipragliflozin (FUSION). Endocr J 2018; 65: 859–867. [DOI] [PubMed] [Google Scholar]
- 25. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 26. Achamrah N, Colange G, Delay J, et al Comparison of body composition assessment by DXA and BIA according to the body mass index: a retrospective study on 3655 measures. PLoS ONE 2018; 13: e0200465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Buckinx F, Reginster J‐Y, Dardenne N, et al Concordance between muscle mass assessed by bioelectrical impedance analysis and by dual energy X‐ray absorptiometry: a cross‐sectional study. BMC Musculoskelet Disord 2015; 16: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Changes in bodyweight over the study period.
Figure S2 Changes in body composition measured with dual‐energy X‐ray absorptiometry.
Figure S3 Visceral and subcutaneous fat evaluated with abdominal bioelectrical impedance analysis.
Table S1 Major adverse events during the clinical trial.
Table S2 Dietary preferences according to visual analog scale.
Table S3 Changes in body composition adjusted for sex and insulin dose.
Appendix S1 Complete list of members of SUMS‐ADDIT‐1 Investigator.
Appendix S2 CONSORT checklist.


