Abstract
Purpose:
Colorectal cancer (CRC) incidence has declined over the past two decades; however these declines have not occurred equally in all populations. To better understand the impact of CRC among Hispanics, we examined incidence trends by age and Hispanic ethnicity.
Methods:
Using data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program, we estimated CRC incidence rates during the period 2001 – 2014, and across all 50 U.S. states. We estimated incidence rates in younger (age <50 years) and older (age ≥50 years) adults by anatomic subsite and stage at diagnosis, separately for non-Hispanic whites and Hispanic whites.
Results:
CRC incidence rates declined among older (age ≥50 years) whites and Hispanics, but whites experienced a greater decline (31% vs. 27% relative decline among Hispanics). In contrast to older adults, there were continued increases in CRC incidence from 2001 to 2014 among younger (age 20–49 years) adults. The largest relative increases in incidence occurred in Hispanics aged 20–29 years (90% vs. 50% relative increase among whites).
Conclusions:
Opposing incidence trends in younger vs. older Hispanics may reflect generational differences in CRC risk by birth cohort, as well as environmental exposures and lifestyle-related risk factors associated with immigration and acculturation.
Keywords: Colorectal neoplasms, population-based, Hispanic Americans, incidence, registries
Introduction
Colorectal cancer (CRC) incidence and mortality in the U.S. have changed strikingly over the past two decades. Incidence declined annually by 3% from 2001 to 2010,1 with similar declines in mortality. The largest declines have occurred in older adults (age ≥50 years) and among non-Hispanic whites.2 These improvements are often attributed to increasing uptake of CRC screening,3,4 as well as declines in the prevalence of certain risk factors (e.g., smoking).5,6
However, declines in CRC incidence have not occurred equally in all populations. Compared to whites, blacks have experienced smaller declines in incidence and present more frequently with metastatic disease.7,8 Numerous studies have demonstrated black-white differences in CRC-related outcomes, including screening and early detection,9,10 follow-up of abnormal screening results,11 and receipt of curative treatment.12 By comparison, less is known about disparities between Hispanic and non-Hispanic populations. Information on cancer trends in Hispanics has historically been limited due to concerns about misclassification and cultural differences among ethnic subgroups.13 Because Hispanics are the fastest growing minority group in the U.S.,14 characterizing cancer incidence trends in this population has become increasingly important.
At the same time, CRC incidence is actually increasing in younger adults.15–17 Incidence has increased in this population (ages 20–49 years) from 8.6 per 100,000 in 1992 to 12.0 per 100,000 in 2014—a 40% relative increase.18 It is possible that the rising incidence of young-onset CRC may disproportionately burden Hispanics given differences in the age distribution relative to the total U.S. population. Nearly 60% of the U.S. Hispanic population are “millennials,” i.e., younger than 35 years, whereas only 40% of non-Hispanic whites belong to the same age group.19 Further, a higher proportion of young Hispanics were born in or have lived longer in the U.S. than older generations,20 which, given the associations between birthplace and other cancer types,21–23 may increase risk of CRC. To better understand the clinical and public health implications of CRC in Hispanics, particularly as it relates to young-onset disease, we examined incidence trends by age and Hispanic ethnicity using cancer registry data across all 50 U.S. states.
Methods
We derived incidence of invasive CRC from the National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) program during 2001 – 2014. Combined NPCR and SEER include cancer incidence and population data for all 50 states in the United States (including the District of Columbia), with demographic and tumor characteristics available for more than 22 million incident cancer cases.24
We estimated age-adjusted incidence (to the 2000 U.S. standard population) with SEER*Stat version 8.3.5 as incidence per 100,000 persons. Corresponding 95% confidence intervals were calculated as modified gamma intervals.25
To illustrate trends, we plotted CRC incidence rates across 10-year age groups (20–29 to 70–79, 80+) in each calendar year. We also calculated absolute change in incidence as the difference in rates from 2001 to 2014, and the relative change in incidence as the absolute change expressed as a percentage of the 2001 rate, for each 10-year age group.
To account for differences in incidence by age, we estimated incidence rates in younger (age 20–49 years) and older (age ≥50 years) adults by anatomic subsite and stage at diagnosis, separately for non-Hispanic whites (whites) and Hispanic whites (Hispanics). Anatomic subsites included proximal colon (cecum, ascending colon, hepatic flexure, transverse colon), descending colon (splenic flexure, descending colon, sigmoid colon), and rectum (rectosigmoid junction, rectum) according to the International Classification of Disease for Oncology, 3rd edition. We defined stage at diagnosis using SEER summary staging, where localized disease is limited to the large bowel, regional is limited to nearby lymph nodes or other organs, and distant is systematic metastasis. Hispanics are identified in cancer registry data by the NAACCR Hispanic/Latino Identification Algorithm (version 2.2.1), which uses Spanish/Hispanic origin, last name, maiden name, birthplace, and race to indirectly and directly assign ethnicity.26
We conducted sensitivity analyses examining incidence trends by Hispanic subgroup. Because country of ancestry/origin is only reported for cases (i.e., not population denominators), we categorized states by majority Hispanic subgroup (Mexican, Puerto Rican, Cuban, Central American, South American, Dominican) according to 2010 U.S. Census.27 As above, we plotted CRC incidence rates by 10-year age group across calendar years, separately for each Hispanic subgroup.
Results
A total of 1,565,527 incident CRC cases were diagnosed in whites, and 126,728 in Hispanics during the period 2001 – 2014. Across all age groups, incidence was 62.6 per 100,000 and 54.5 per 100,000 among whites and Hispanics, respectively. Detailed incidence rates by Hispanic ethnicity, age (+/− 50 years), anatomic subsite, and stage at diagnosis are available in Supplementary Tables 1 and 2.
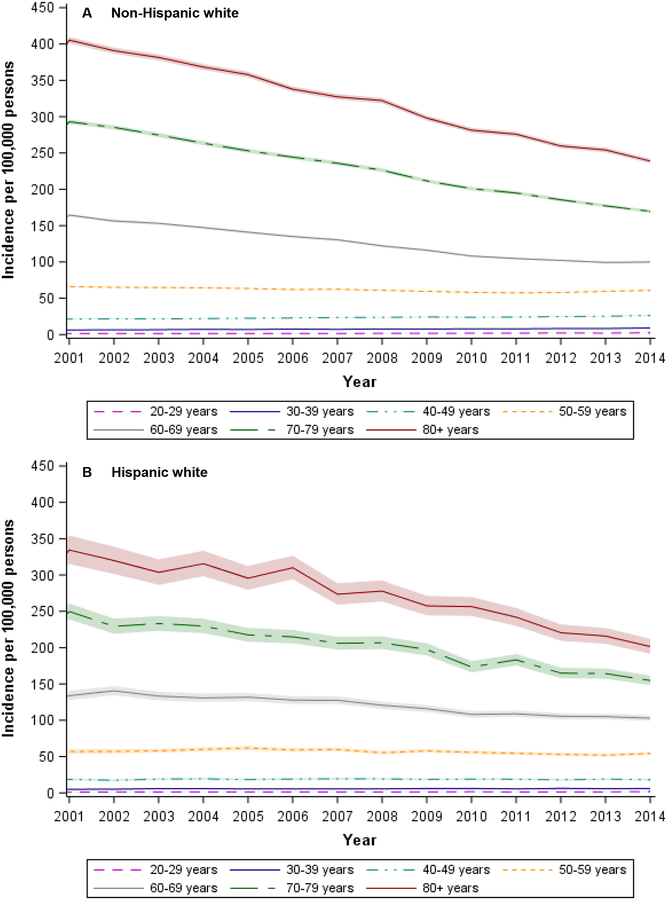
CRC incidence (all ages combined) declined from 2001 to 2014 (Table 1). In both whites and Hispanics, declines in incidence were limited to older adults (age ≥50 years). Whites experienced a greater decline: 31% (76.4 to 52.9 per 100,000) compared to 27% among Hispanics (64 to 46.7 per 100,000). In contrast to adults over the age of 50 years, there were continued increases in CRC incidence from 2001 to 2014 among younger adults (Figure 1). Among those age 20–49 years, incidence rates increased from 9.8 per 100,000 in 2001 to 12.2 per 100,000 in 2014, a 24% relative increase. Although we observed increases in young-onset CRC among both whites and Hispanics, we noted some differences between the two groups when stratified by 10-year age group. For example, among persons aged 20–29 years, incidence increased by 90% among Hispanics (from 1.0 to 1.9 per 100,000) versus 50% among whites (from 1.6 to 2.4 per 100,000). In contrast, incidence increased by 24% (from 21.2 to 26.3 per 100,000) among whites aged 40–49 years but remained stable (18.6 to 18.1 per 100,000) among Hispanics in the same age group (Table 1).
Table 1.
Age-specific incidence rates of colorectal cancer by Hispanic ethnicity and 10-year age group, National Program of Cancer Registries, 2001–2014
| Non-Hispanic white (n=1,565,527 cases) | Hispanic white (n=126,728 cases) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | ||
| 2001 | 1.6 | 6.2 | 21.2 | 66.0 | 164.4 | 293.0 | 405.4 | 1.0 | 5.0 | 18.6 | 57.1 | 133.9 | 249.7 | 334.3 | |
| 2002 | 1.6 | 6.5 | 21.6 | 65.1 | 156.3 | 285.2 | 390.8 | 1.3 | 5.2 | 17.5 | 57.2 | 140.6 | 229.3 | 319.9 | |
| 2003 | 1.6 | 6.6 | 21.5 | 64.8 | 153.1 | 274.7 | 381.5 | 1.3 | 5.9 | 18.9 | 57.9 | 133.2 | 233.2 | 303.6 | |
| 2004 | 1.5 | 7.1 | 22.0 | 64.4 | 147.2 | 263.5 | 368.3 | 1.3 | 5.8 | 19.3 | 60.0 | 130.8 | 229.7 | 315.6 | |
| 2005 | 1.6 | 6.9 | 22.5 | 63.4 | 140.9 | 253.0 | 357.8 | 1.3 | 5.6 | 18.4 | 61.8 | 131.8 | 217.5 | 295.6 | |
| 2006 | 1.6 | 7.5 | 22.8 | 62.0 | 135.0 | 244.3 | 337.8 | 1.4 | 5.5 | 19.1 | 59.1 | 127.7 | 214.9 | 310.0 | |
| 2007 | 1.6 | 7.1 | 23.6 | 62.4 | 130.5 | 235.9 | 327.3 | 1.4 | 5.5 | 19.4 | 59.8 | 127.5 | 206.0 | 273.5 | |
| 2008 | 1.7 | 7.7 | 23.8 | 60.9 | 122.1 | 226.3 | 322.1 | 1.3 | 5.6 | 19.2 | 55.3 | 120.7 | 206.7 | 277.8 | |
| 2009 | 1.7 | 7.6 | 24.2 | 59.5 | 116.2 | 211.5 | 298.0 | 1.3 | 6.0 | 18.6 | 57.9 | 116.0 | 197.4 | 257.5 | |
| 2010 | 1.9 | 7.9 | 23.8 | 58.1 | 108.1 | 200.9 | 281.3 | 1.7 | 6.0 | 18.9 | 55.9 | 108.1 | 173.4 | 256.5 | |
| 2011 | 1.9 | 7.9 | 24.1 | 57.5 | 104.6 | 194.9 | 275.8 | 1.3 | 5.6 | 18.8 | 54.5 | 108.9 | 183.2 | 242.0 | |
| 2012 | 2.2 | 8.3 | 24.8 | 57.9 | 102.1 | 185.6 | 259.6 | 1.3 | 6.2 | 18.0 | 53.1 | 105.4 | 164.9 | 220.5 | |
| 2013 | 2.0 | 8.2 | 25.2 | 59.5 | 99.3 | 177.2 | 254.1 | 1.4 | 5.9 | 19.0 | 52.0 | 105.0 | 164.2 | 215.9 | |
| 2014 | 2.4 | 9.0 | 26.3 | 60.8 | 100.0 | 169.6 | 238.9 | 1.9 | 6.1 | 18.1 | 54.3 | 103.0 | 154.7 | 201.5 | |
| Total (all years) | 1.8 | 7.4 | 23.3 | 61.3 | 123.9 | 229 | 318.5 | 1.4 | 5.7 | 18.7 | 56.4 | 118.2 | 197.3 | 263.0 | |
| Absolute change1 | 0.8 | 2.8 | 5.1 | −5.2 | −64.4 | −123.4 | −166.5 | 0.9 | 1.1 | −0.5 | −2.8 | −30.9 | −95.0 | −132.8 | |
| Relative change2 | 50.0 | 45.2 | 24.1 | −7.9 | −39.2 | −42.1 | −41.1 | 90.0 | 22.0 | −2.7 | −4.9 | −23.1 | −38.0 | −39.7 | |
Absolute change = difference in incidence rates from 2001 to 2014 (i.e., 2014 incidence rate – 2001 incidence rate)
Relative change = absolute change expressed as a percentage of the 2001 incidence rate (i.e., [2014 incidence rate −2001 incidence rate]/2001 incidence rate)
Figure 1.
Age-specific incidence rates of colorectal cancer by 10-year age group and Hispanic ethnicity, National Program of Cancer Registries, 2001 – 2014
NOTE: Shaded regions in figure denote 95% confidence intervals
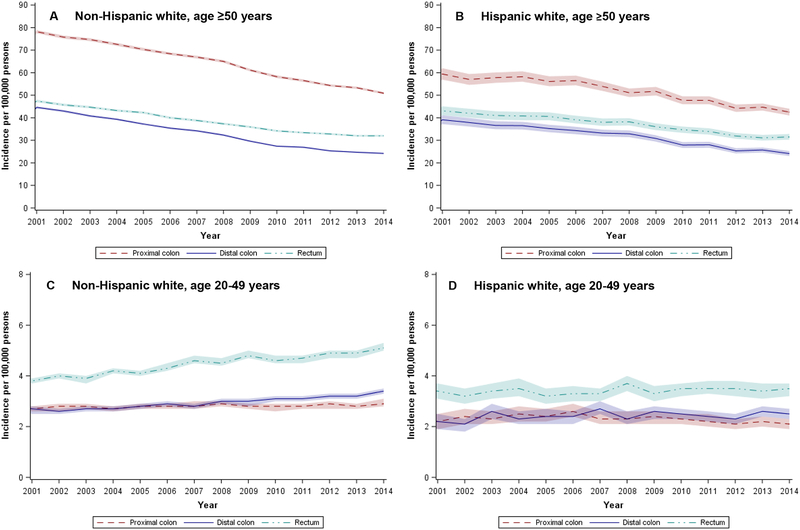
Incidence of proximal and distal colon cancer decreased from 2001 to 2014 for both older whites (Figure 2A) and Hispanics (Figure 2B), with smaller declines in the incidence of rectal cancer. Although whites consistently had higher overall (and age-specific) CRC rates than Hispanics, they had similar rates of distal colon and rectal cancers in more recent years. In younger whites, incidence of rectal cancer increased during the study period (34% increase, from 3.8 to 5.1 per 100,000), with smaller increases in proximal and distal colon cancers (Figure 2C). For young Hispanics (Figure 2D), the largest increases in incidence occurred in the distal colon (14% increase, from 2.2 to 2.5 per 100,000), and there was little to no change in the incidence of proximal colon or rectal cancer.
Figure 2.
Age-adjusted (2000 U.S. standard population) incidence of colorectal cancer by Hispanic ethnicity, age (+/− 50 years), and anatomic subsite, National Program of Cancer Registries, 2001 – 2014
NOTE: Proximal colon includes the cecum, ascending colon, hepatic flexure, and transverse colon; distal colon includes the splenic flexure, descending colon, and sigmoid colon; rectum includes the rectosigmoid junction and rectum. Shaded regions in figure denote 95% confidence intervals.
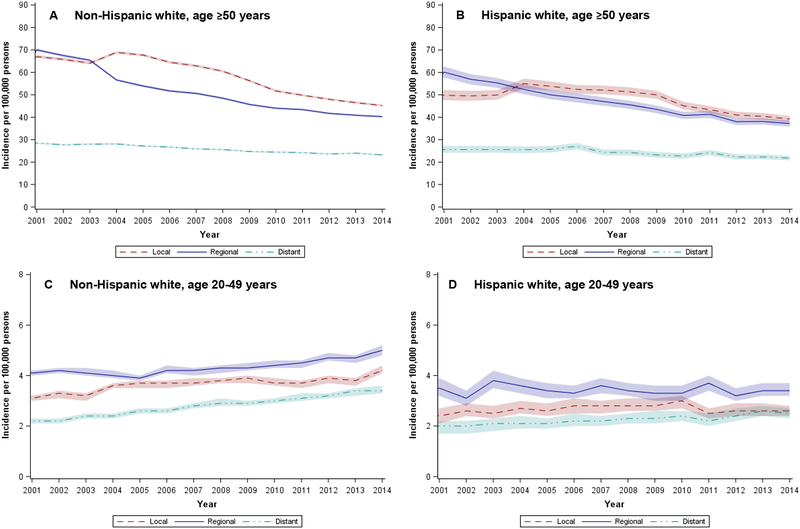
Among older (age ≥ 50 years) whites, incidence of local, regional, and distant disease decreased from 2001 to 2014 (18.5– 42.5% decrease, Figure 3A). Older Hispanics experienced similar declines in CRC incidence by stage at diagnosis, although of smaller relative magnitude (Figure 3B). In 2014, the most recent year with available data, older whites and Hispanics had similar rates of distant CRC (25.9 per 100,000 whites vs. 24.3 per 100,000 Hispanics). A higher incidence of local disease in older whites accounted for almost all of the difference in overall CRC rates between whites and Hispanics. In younger populations, incidence of local, regional, and distant disease increased in both whites (Figure 2C) and Hispanics (Figure 2D), with no appreciable differences by ethnicity.
Figure 3.
Age-adjusted (2000 U.S. standard population) incidence of colorectal cancer by Hispanic ethnicity, age (+/− 50 years), and stage at diagnosis, National Program of Cancer Registries, 2001 – 2014
NOTE: Stage at diagnosis based on SEER summary staging, where local disease is limited to the large bowel, regional is limited to nearby lymph nodes or other organs, and distant is systematic metastasis. Shaded regions in figure denote 95% confidence intervals.
In subgroup analyses of CRC incidence trends by sex (male vs. female), incidence was consistently higher among men than women. However, younger Hispanic women had higher rates of distal colon cancer than Hispanic men. This difference declined from 2001 to 2014 because distal colon cancer rates remained stable among Hispanic women (at around 2.8 per 100,000) but increased by 35% among men (from 1.7 to 2.3 per 100,000) (Supplementary Tables 3 and 4).
Sensitivity analyses by Hispanic subgroup (Supplementary Figure 1) show higher age-specific incidence rates in states with majority Cuban and Puerto Rican populations and lower rates in states with a majority Mexican population.
Discussion
Across all 50 U.S. states, we observed smaller declines in CRC incidence among older Hispanics compared to whites. In contrast to older adults, CRC incidence increased from 2001 to 2014 in both younger whites and Hispanics, with particularly large relative increases among Hispanics age 20–29 years. Our findings support generational or birth cohort differences in cancer risk that may be due to acculturation and time in the U.S.28,29
Older Hispanics experienced smaller declines in CRC incidence over time compared to whites, perhaps due to differences in screening uptake. National data consistently show a lower proportion of age-eligible Hispanics are up-to-date with CRC screening than whites.30–34 In 2015, only 47% of Hispanics reported recent CRC screening compared to 64% of non-Hispanics, with even larger differences by period of U.S. residence (65% among U.S. born vs. 36% among those in the U.S. for less than 10 years).35 Other factors, such as low educational attainment, lack of insurance, and no usual source of health care,36 which are common among Hispanics, are also associated with CRC screening.31,37 There may also be differences in cultural and health beliefs contributing to screening, for example, Hispanics frequently report medical mistrust.38 Slower declines in CRC incidence, combined with low screening uptake, underscore the importance of screening outreach interventions targeted to Hispanic populations, such as those in community health centers39–41 and safety-net systems.42,43
We observed opposing CRC incidence trends in the oldest and youngest age groups, with larger relative increases in incidence among young Hispanics but lower age-specific rates in older Hispanics compared to whites. These opposing trends may reflect generational differences in CRC risk by birth cohort, as well as environmental exposures and lifestyle-related risk factors associated with immigration and acculturation.44 For example, CRC incidence rates in U.S. Hispanics are higher than those in most Latin American countries, suggesting that changes in risk factors that occur after migration to the U.S. may increase risk.28 The large relative increases in CRC incidence in younger Hispanics parallel the higher incidence of other gastrointestinal cancers, including gastric cancer,45 gallbladder cancer,46 and hepatocellular carcinoma,47,48 in this population relative to whites. Shared risk factors (e.g., obesity,49 type II diabetes50) may contribute to an excess disease burden, particularly among younger Hispanics or those born in the U.S. Exploring the synergistic effects of acculturation, birthplace, and time in the U.S., across both generations and time, may better identify mechanisms driving these differences.
CRC incidence rates increased by 90% among the youngest age group of Hispanics (20–29 years). Data from the Texas Cancer Registry similarly show incidence increased annually by up to 3% from 1995 through 2010 among Hispanics age 20–39 years (Texas is home to 20% of the U.S. population).51 Quickly rising incidence rates are concerning given the large absolute number of Hispanics falling into this age category – about 26.5 million.52 Although incidence rates are slightly lower in Hispanics than whites, CRC could disproportionately affect young Hispanics because of differences in the age distribution of each population. About 26% of U.S. Hispanics are between ages 18 and 33 years compared to 20% of whites.19 As the population of young Hispanics living in the U.S. continues to grow, understanding the distribution of CRC and other cancers in this population group may inform prevention strategies.
We also found ethnic differences in incidence by anatomic subsite in younger age groups. Among whites, increases in young-onset CRC have been primarily driven by increases in rectal cancer; however, among Hispanics, increases have been driven by higher rates of distal colon cancer. Differences in incidence by anatomic subsite make a strong case for teasing apart risk factors for colon vs. rectal cancer. Most etiologic studies group colon and rectal cancers together as a single disease, but very few have examined risk factors separately or how race/ethnicity may further modify associations by subsite. Variations in cancer registry reporting standards and misclassification may also explain these differences. Prior studies of CRC mortality have documented challenges distinguishing death from colon vs. rectal cancer.53,54
Prior studies have demonstrated differences in CRC incidence among Hispanics by country of origin, whereby Puerto Ricans and Cubans tend to have higher incidence than Mexicans.29,55 Data concerning country of origin/ancestry are only available in NPCR data for cases but not population denominators; however, our sensitivity analysis by Hispanic subgroup shows higher rates among Puerto Rican- and Cuban-majority states. Birthplace data are also frequently missing in cancer registries,56 and differences in cases with and without missing data may systematically bias estimates in minority populations.57,58 Further, undocumented Hispanic immigrant populations in the U.S. could potentially artificially inflate incidence rates because only permanent residents or citizens may be counted in population denominators.28 These challenges underscore the importance of continued work to characterize cancer risk and develop new methods to account for missing data.
An important strength of our study is using the entire U.S. population to estimate incidence. Combined NPCR and SEER data offer a number of advantages for conducting epidemiologic research because participating registries have a defined population, and the NPCR program includes registries with a high proportion of Hispanics (e.g., Texas, Florida, New York, Arizona) that do not participate in SEER. The large size and diversity of registry populations allowed us to estimate trends in Hispanics by age, stage at diagnosis, and anatomic subsite. To our knowledge, this is the first study of CRC incidence trends using these national data.
In summary, our study provides important evidence that CRC incidence has declined among older Hispanics, although at a slower rate than whites. Incidence rates have increased among younger whites and Hispanics, and the largest relative increases in incidence have occurred among the youngest (age 20–29 years) Hispanics. Understanding generational differences in CRC risk factors, as well as differences by immigration and acculturation status, may improve our understanding of cancer in this rapidly growing population.
Supplementary Material
Grant support:
This work was supported by the National Cancer Institute (P30CA142543) and National Center for Advancing Translational Sciences (KL2TR001103 to Dr. Murphy) at the National Institutes of Health.
Footnotes
Disclosures: The authors declare no conflicts of interest or financial disclosures.
References
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64(2):104–117. [DOI] [PubMed] [Google Scholar]
- 2.Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in Incidence of Colorectal Cancer Among Individuals 50 Years or Older After Recommendations for Population-based Screening. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2017;15(6):903–909.e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koblinski J, Jandova J, Nfonsam V. Disparities in incidence of early-and late-onset colorectal cancer between Hispanics and Whites: A 10-year SEER database study. The American Journal of Surgery. 2017. [DOI] [PubMed] [Google Scholar]
- 4.Meester RG, Doubeni CA, Lansdorp-Vogelaar I, et al. Colorectal cancer deaths attributable to nonuse of screening in the United States. Annals of epidemiology. 2015;25(3):208–213.e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronin KA, Krebs-Smith SM, Feuer EJ, Troiano RP, Ballard-Barbash R. Evaluating the impact of population changes in diet, physical activity, and weight status on population risk for colon cancer (United States). Cancer causes & control: CCC. 2001;12(4):305–316. [DOI] [PubMed] [Google Scholar]
- 6.Vogelaar I, van Ballegooijen M, Schrag D, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer. 2006;107(7):1624–1633. [DOI] [PubMed] [Google Scholar]
- 7.Robbins AS, Siegel RL, Jemal A. Racial disparities in stage-specific colorectal cancer mortality rates from 1985 to 2008. J Clin Oncol. 2012;30(4):401–405. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA: a cancer journal for clinicians. 2017;67(3):177–193. [DOI] [PubMed] [Google Scholar]
- 9.Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, van Ballegooijen M, Zauber AG, Jemal A. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(5):728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doubeni CA, Field TS, Buist DS, et al. Racial differences in tumor stage and survival for colorectal cancer in an insured population. Cancer. 2007;109(3):612–620. [DOI] [PubMed] [Google Scholar]
- 11.Laiyemo AO, Doubeni C, Pinsky PF, et al. Race and colorectal cancer disparities: health-care utilization vs different cancer susceptibilities. Journal of the National Cancer Institute. 2010;102(8):538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy CC, Harlan LC, Warren JL, Geiger AM. Race and Insurance Differences in the Receipt of Adjuvant Chemotherapy Among Patients With Stage III Colon Cancer. J Clin Oncol. 2015;33(23):2530–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy CC, Sanoff HK, Stitzenberg KB, Baron JA, Lund JL, Sandler RS. Patterns of Sociodemographic and Clinicopathologic Characteristics of Stages II and III Colorectal Cancer Patients by Age: Examining Potential Mechanisms of Young-Onset Disease. Journal of cancer epidemiology. 2017;2017:4024580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among US Hispanic/Latino populations. Cancer. 2006;107(8):1711–1742. [DOI] [PubMed] [Google Scholar]
- 15.Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(6):1695–1698. [DOI] [PubMed] [Google Scholar]
- 16.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975–2010. JAMA surgery. 2015;150(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. Journal of the National Cancer Institute. 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1992–2015), National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission. [Google Scholar]
- 19.Patten E The Nation’s Latino Population is Defined by Its Youth. 2016; http://www.pewhispanic.org/2016/04/20/the-nations-latino-population-is-defined-by-its-youth/#fn-24238-1. Accessed April 19, 2018.
- 20.Flores A How the U.S. Hispanic population is changing. 2017; http://www.pewresearch.org/fact-tank/2017/09/18/how-the-u-s-hispanic-population-is-changing/. Accessed April 19, 2018.
- 21.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiology and Prevention Biomarkers. 2005;14(12):2905–2913. [DOI] [PubMed] [Google Scholar]
- 22.Siegel R, Naishadham D, Jemal A. Cancer statistics for hispanics/latinos, 2012. CA: a cancer journal for clinicians. 2012;62(5):283–298. [DOI] [PubMed] [Google Scholar]
- 23.Eschbach K, Mahnken JD, Goodwin JS. Neighborhood composition and incidence of cancer among Hispanics in the United States. Cancer. 2005;103(5):1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Control DoCPa. NPCR and SEER Incidence – USCS Public Use Databases. 2017; https://www.cdc.gov/cancer/npcr/public-use/index.htm. Accessed February 15, 2018, 2018. [Google Scholar]
- 25.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Statistical methods in medical research. 2006;15(6):547–569. [DOI] [PubMed] [Google Scholar]
- 26.Group NRaEW. NAACCR Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2.1]. In: Springfield (IL): North American Association of Central Cancer; RegistriesSeptember 2011. [Google Scholar]
- 27.Finder AF. U.S. 2010 Census Summary File 1, Table Qt-P10 Hipsanic or Latino by Type. 2018; https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=DEC_10_SF1_QTP10&prodType=table. Accessed July 20, 2018.
- 28.Haile RW, John EM, Levine AJ, et al. A review of cancer in U.S. Hispanic populations. Cancer Prev Res (Phila). 2012;5(2):150–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinheiro PS, Sherman RL, Trapido EJ, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18(8):2162–2169. [DOI] [PubMed] [Google Scholar]
- 30.Klabunde CN, Cronin KA, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(8):1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(2):389–394. [DOI] [PubMed] [Google Scholar]
- 32.Sabatino SA, White MC, Thompson TD, Klabunde CN. Cancer screening test use - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(17):464–468. [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21(6):895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17(7):1623–1630. [DOI] [PubMed] [Google Scholar]
- 35.White A, Thompson TD, White MC, et al. Cancer Screening Test Use - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liss DT, Baker DW. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med. 2014;46(3):228–236. [DOI] [PubMed] [Google Scholar]
- 37.Valdovinos C, Penedo FJ, Isasi CR, et al. Perceived discrimination and cancer screening behaviors in us Hispanics: the Hispanic community health study/study of Latinos sociocultural ancillary study. Cancer Causes & Control. 2016;27(1):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jandorf L, Ellison J, Villagra C, et al. Understanding the barriers and facilitators of colorectal cancer screening among low income immigrant hispanics. Journal of immigrant and minority health. 2010;12(4):462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller DP Jr., Denizard-Thompson N, Weaver KE, et al. Effect of a Digital Health Intervention on Receipt of Colorectal Cancer Screening in Vulnerable Patients: A Randomized Controlled Trial. Ann Intern Med. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reuland DS, Brenner AT, Hoffman R, et al. Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population: A Randomized Clinical Trial. JAMA Intern Med. 2017;177(7):967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldman SN, Liss DT, Brown T, et al. Comparative Effectiveness of Multifaceted Outreach to Initiate Colorectal Cancer Screening in Community Health Centers: A Randomized Controlled Trial. J Gen Intern Med. 2015;30(8):1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singal AG, Gupta S, Skinner CS, et al. Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: A Randomized Clinical Trial. JAMA: the journal of the American Medical Association. 2017;318(9):806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA internal medicine. 2013;173(18):1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gushulak BD, MacPherson DW. The basic principles of migration health: population mobility and gaps in disease prevalence. Emerg Themes Epidemiol. 2006;3(3):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X, Chen VW, Andrews PA, Ruiz B, Correa P. Incidence of esophageal and gastric cancers among Hispanics, non-Hispanic whites and non-Hispanic blacks in the United States: subsite and histology differences. Cancer Causes Control. 2007;18(6):585–593. [DOI] [PubMed] [Google Scholar]
- 46.Trapido EJ, Burciaga RV, Obeso JL, Strickman-Stein N, Rotger A, Perez-Stable EJ. Epidemiology of cancer among Hispanics in the United States. Journal of the National Cancer Institute Monographs. 1995(18):17–28. [PubMed] [Google Scholar]
- 47.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. Journal of clinical oncology. 2009;27(9):1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Archives of internal medicine. 2007;167(18):1983–1989. [DOI] [PubMed] [Google Scholar]
- 49.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. Jama. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mainous IIIAG, Majeed A, Koopman RJ, et al. Acculturation and diabetes among Hispanics: evidence from the 1999–2002 National Health and Nutrition Examination Survey. Public health reports. 2006;121(1):60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang DY, Thrift AP, Zarrin-Khameh N, et al. Rising incidence of colorectal cancer among young Hispanics in Texas. J Clin Gastroenterol. 2017;51(1):34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.FactFinder USCBA. B01001I: Sex by Age [Hispanic or Latino Only]. http://factfinder2.census.gov. Accessed April 19, 2018.
- 53.Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139(4):1128–1137. [DOI] [PubMed] [Google Scholar]
- 54.Yin D, Morris CR, Bates JH, German RR. Effect of misclassified underlying cause of death on survival estimates of colon and rectal cancer. J Natl Cancer Inst. 2011;103(14):1130–1133. [DOI] [PubMed] [Google Scholar]
- 55.Soto-Salgado M, Suarez E, Calo W, Cruz-Correa M, Figueroa-Valles NR, Ortiz AP. Incidence and mortality rates for colorectal cancer in Puerto Rico and among Hispanics, non-Hispanic whites, and non-Hispanic blacks in the United States, 1998–2002. Cancer. 2009;115(13):3016–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez SL, Glaser SL. Quality of cancer registry birthplace data for Hispanics living in the United States. Cancer Causes Control. 2005;16(6):713–723. [DOI] [PubMed] [Google Scholar]
- 57.Lin SS, O’Malley CD, Lui SW. Factors associated with missing birthplace information in a population-based cancer registry. Ethnicity & disease. 2001;11(4):598–605. [PubMed] [Google Scholar]
- 58.Gomez SL, Le GM, West DW, Satariano WA, O’Connor L. Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace. American journal of public health. 2003;93(10):1685–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.