ABSTRACT
Observations that mistimed food intake may have adverse metabolic health effects have generated interest in personalizing food timing recommendations in interventional studies and public health strategies for the purpose of disease prevention and improving overall health. Small, controlled, and short-termed intervention studies suggest that food timing may be modified as it is presumed to be primarily regulated by choice. Identifying and evaluating social and biological factors that explain variability in food timing may determine whether changes in food timing in uncontrolled, free-living environments are sustainable in the long term, and may facilitate design of successful food timing-based interventions. Based on a comprehensive literature search, we summarize 1) cultural and environmental factors; 2) behavioral and personal preference factors; and 3) physiological factors that influence the time when people consume foods. Furthermore, we 1) highlight vulnerable populations who have been identified in experimental and epidemiological studies to be at risk of mistimed food intake and thus necessitating intervention; 2) identify currently used food timing assessment tools and their limitations; and 3) indicate other important considerations for the design of food timing interventions based on successful strategies that address timing of other lifestyle behaviors. Conclusions drawn from this overview may help design practical food timing interventions, develop feasible public health programs, and establish guidelines for effective lifestyle recommendations for prevention and treatment of adverse health outcomes attributed to mistimed food intake.
Keywords: food timing, chronobiology, fasting, dietary intake, cardiometabolic health
Introduction
Food timing is an important aspect of nutrition causally related to adverse metabolic outcomes
The timing of food intake is an emerging aspect of nutrition with increasing interest because of its influence on metabolic health (1, 2). Rodent studies have demonstrated that a high-fat diet consumed during the rest phase (light phase in nocturnal rodents) results in greater weight gain than when consumed during the active phase (3), that nocturnal light exposure increases body weight by shifting food timing (4), and that shifting food intake to the active phase prevents weight increase and reverts metabolic and rhythmic disturbances induced by shift work (5), highlighting the importance of appropriately timed food intake in animals. Consistent with those findings, in humans, we observed that the efficacy of a dietary weight-loss intervention was greater among participants who ate an earlier (before 1500) lunch, the main meal of the day in this Mediterranean population, than in those who ate a late lunch (after 1500) (6). These findings have been corroborated in bariatric surgery weight-loss interventions, whereby after 6 y of follow-up, the proportion of poor weight-loss responders was approximately double that of good weight-loss responders among patients eating lunch late (7). Additional adverse links between late eating and overall health including higher obesity and glucose intolerance in humans were detected in other observational studies (8, 9), highly controlled in-laboratory experiments (10, 11), and interventional studies (12, 13). Collectively, these studies support the notion that when we eat is relevant for energy balance (14), metabolism, and overall health (1).
Several plausible biological mechanisms have been described to explain the causal relations between late food timing and adverse cardiometabolic health, including changes in basal energy expenditure, diet-induced thermogenesis, glucose metabolism, wrist body temperature pattern, food intake concurrent with elevated melatonin signaling, and phase shifts of peripheral clocks including those in adipose tissue (1, 10, 11, 15–18) In a randomized, crossover trial, later food intake of the same meals was observed to induce several metabolic alterations including decreased energy expenditure, impaired glucose tolerance, and changes in the daily rhythms of cortisol, body temperature, and microbiota (19), similar to changes observed in obese and elderly women (20). In addition, mistimed food intake leads to increased peripheral inflammation in animals (21) and mistimed food and sleep leads to changes in blood pressure, inflammatory markers, and plasma proteins in humans (22–25). Food intake is also an external synchronizer of the endogenous biological clock, particularly peripheral clocks of tissues or organs related to metabolism, such as liver, gut, or adipose tissue (26). Accordingly, the timing of meals have been observed to synchronize, that is, entrain, the peripheral circadian rhythms in humans, but not the central clock (16). These recent human findings extend animal findings demonstrating that food can entrain peripheral tissues, such as the liver, heart, and pancreas, independent of the central clock (27–29). Conflicting timing between the central and peripheral clocks can lead to internal desynchrony, a state where clocks throughout the body are out of sync (30).
Although the described factors may explain the connections between food timing and cardiometabolic health, it remains unclear whether these metabolic alterations, particularly those detected in uncontrolled observational studies, are solely a result of changes in food timing or may further be attributed to other changes in dietary patterns including changes in total energy intake, energy and macronutrients intake distribution across the day, fasting duration, or food frequency, or to changes in timing of sleep. It has been shown that a low-energy breakfast and a high-energy dinner are associated with difficulties in weight loss and impaired glucose metabolism, consistent with those observed for late eating (9). Changes in meal timing can also affect the frequency of meals and higher meal frequency may lead to higher total energy intake (31). On the other hand, food timing may also influence sleep characteristics subsequently affecting metabolism (32). Thus, highly controlled intervention studies that allow the separation of these correlated factors are warranted to delineate the factor of most importance.
Food timing varies within and between populations and may be modified
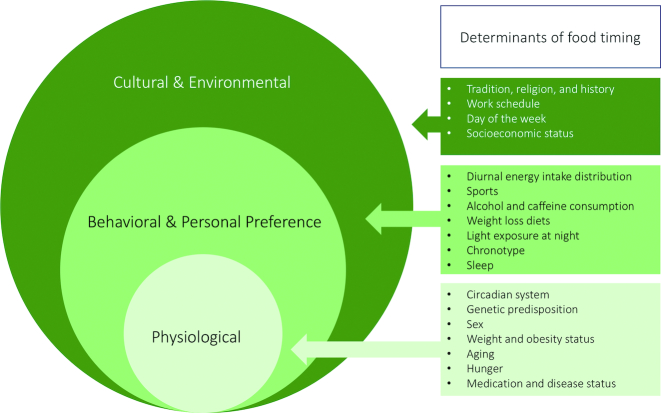
The compelling literature indicating that mistimed food intake causes adverse health effects has generated interest in personalizing food timing as a novel avenue to prevent diseases and improve health. Food timing recommendations are particularly relevant considering that the timing of food intake is a behavior that exhibits variation within and between populations, and within and between individuals (shown in Figure 1), and that current dietary guidelines lack explicit guidance on food timing (33). For example, in the United States, a large cluster analysis from NHANES from 1999 to 2004 including 7565 participants with one 24-h dietary recall indicated distinct temporal dietary patterns (34). The largest cluster reflected a 3-main-meal pattern, whereas the remaining clusters reflected greater intake either early or later in the day. Interestingly, the 3-main-meal pattern exhibited a higher quality diet, meanwhile, the cluster that reflected small, frequently peaked temporal dietary pattern, including frequent consumption at midnight, was associated with the lowest quality diet (35). The observed variability in food timing demonstrates that even within homogenous cultures, there may exist social and biological factors that influence when people eat (36). Furthermore, in a small cohort of young healthy volunteers, analysis of food timing with mobile technology indicated some irregular eating patterns across multiple days (37), indicating day-to-day variability in eating patterns.
FIGURE 1.
Determinants of food timing.
Food timing is suspected to be modifiable as it is presumed to be regulated by an individual's choice (20, 30). Small, controlled, short-term intervention studies indicate that food timing is indeed modifiable (15, 38). However, it is unknown whether these changes are sustainable in uncontrolled, free-living environments. Understanding both social and biological factors that explain variability in food timing will enable design of successful food timing-based interventions. Thus, in the present review, we provide an overview of currently known factors influencing at what time people consume foods, and summarize barriers for modifying food times. Conclusions drawn from this overview may help us design practical food timing interventions, develop feasible public health programs, and propose effective lifestyle recommendations for prevention and treatment of adverse health outcomes attributed to mistimed food, including obesity and metabolic diseases.
Determinants of Food Timing
We conducted a comprehensive PubMed search for literature on factors that influence timing of food intake. Our search strategy combined terms for food timing (e.g., “food timing,” “timing of food intake”). Because our strategy was focused on determinants rather than health consequences of food timing, manuscripts were included only if food timing was an outcome. Both epidemiological studies in free-living conditions and studies in controlled laboratory settings were included. Studies in children (aged <18 y) were excluded. We also cross-referenced recovered studies and relevant reviews to identify additional studies. Identified published studies for food timing determinants were categorized into 3 general categories: 1) cultural and environmental, 2) behavioral and personal preference, and 3) physiological (Figure 1). Studies included cross-sectional and longitudinal analyses of population-based cohort studies and experimental human studies.
Cultural and environmental
Tradition, religion, and history
Tradition and religion have played a major role in shaping when people eat. For example, over 2500 y of Traditional Chinese Medicine practice has led to the belief that the optimum time for carbohydrate-rich meals is between the hours of 0700 and 1100, whereas smaller meals are best later in the day as the body transitions from an active phase (“yang”) to a resting phase (“yin”) (39). This practice stemmed from the belief that energy-dense meals in the evening may be disruptive to sleep and various body functions. During the Islamic fasting month of Ramadan, food timing is restricted to the hours between dawn and sunset as Muslims refrain from drinking and eating during the daytime hours for a 1-mo period, and the fasting period may be as long as 18 h/d in the summer of temperate regions (40).
Although certain beliefs and traditions have kept food timing constant in certain parts of the world, food timing, overall, has slowly shifted over the years as a result of changing social classes, political and economic climate, and because of technological advancements, particularly the invention of artificial lighting (41). For example, dinner in medieval England, the primary meal of the day, was consumed in the middle of the day around noon or 1300 (41), whereas supper, a smaller meal, was consumed closer to sunset (between 1600 and 1800). More widespread use of artificial lighting (i.e., oil lamps) caused all human activities to shift towards later times, including dinner, which was pushed towards 1600 and 1700 in the late 18th century. Lunch was later introduced as a consequence of extended fasting periods between breakfast and dinner. During the Industrial Revolution, later dinner times were also necessary among middle-class and lower-class men who were bounded by work. For working men, lunch constituted a quick meal at noon, splitting the work day in half (41). These shifts quickly spread through mainland Europe, including France in the 1800s, and later the United States in the 1900s. Countries that have been less impacted by the Industrial Revolution continue to have large midday meals, that is, modern lunch, including Spain and Italy. Other world events played a role in establishing meal times, including World War II when Spain's General Francisco Franco decided in 1940 to advance Spain's clocks ahead by 1 h to align with Nazi Germany (42). Because the timing of behaviors remained constant, Spain is notable for its late lunches (∼1500) and dinners (∼2200).
Indeed, marked differences in meal timing, caloric distribution, and snacking behaviors are observed across Mediterranean and central/northern European countries (43). An analysis of 36,020 respondents aged 35–74 y spanning 10 European countries indicated a trend for fewer eating occasions in Mediterranean countries than in central/northern European countries (43). In addition, lunch provided ∼40% of daily energy intake in Mediterranean countries, but only ∼20% in central/northern European countries. Furthermore, a general south-north gradient was found for daily energy intake from snacks, with ∼15% of energy intake from snacks in Mediterranean countries but ∼25–30% in central/northern European countries. Other studies including 2600 elderly participants from 12 European countries assessed in 1988–1989 also corroborated the negative relation found between energy intake at the midday meal and geographical latitude in Europe (44). In addition, a higher frequency of intake was observed in northern/central Europe, with participants in the United Kingdom and the Netherlands having a mean of 6–7 eating occasions/d, but 5 eating occasions in other European countries (43). Another analysis including 559 men and women aged 44–65 y from 5 European countries extended the previously mentioned snacking findings and further indicated that mean overnight fasting was shortest in the Netherlands (9.2 h) and longest in the Czech Republic (10.9 h) (45). Lastly, in the 10 y from mid-1990 to 2007–2008, meal patterns did not change substantially in France, Norway, and the Netherlands.
In the United States, analyses from NHANES indicated that dinner and after-dinner snacks were the largest contributor of calories and provide nearly 45% of total energy (46). Over a 40-y period from 1971–1974 to 2007–2010, the timing of breakfast and lunch were generally delayed, whereas the timing of dinner remained stable (46). In the most recent analysis from NHANES including 15,341 adults from the 2009–2014 NHANES cycle, the average dinnertime was 1824, and the average time of the last eating episode was 2018. Furthermore, duration of the ingestive period was estimated at 12.6 h in men and 12.0 h in women, and main meals were estimated to provide 77.7% of total energy intake with the rest consumed as snacks (47).
Work schedule and day of the week
Weekday-weekend variability in food timing is suspected to be a result of the influence of the workplace and weekend social obligations. An analysis of the Nationwide Food Consumption Survey conducted by the USDA in 1977–1978, including 13,215 adults aged 23–74 y observed that the numbers of meals and snacks reported on weekends were significantly lower than those reported on weekdays (48). Whereas no differences were observed between weekday and weekend morning meals among adults aged 23–64 y, the percentages eating midday or evening meals were lower on weekends than on weekdays. More recently from the NHANES 2003–2012 cycles, an analysis of 11,646 adults aged ≥18 y indicated that weekend consumption, particularly Saturdays, was associated with overall increased calorie intake and poorer diet quality (49). Furthermore, and consistent with the earlier Nationwide Food Consumption Survey, fewer meals and snacks were consumed on weekends than on weekdays. However, total energy, fat, and protein consumed was greater on weekends. In addition, there was a general shift from evening main meals on weekdays to earlier midday main meals on weekends in both adults and younger adults. Indeed, fewer respondents reported their most energy-dense meal in the evening on weekends than on weekdays (49). Interestingly, weekend-weekday differences in intakes are consistent between employed and unemployed participants, suggesting that in addition to the workplace, other cultural and social expectations influence intakes. This is supported by a lack of weekday-weekend variation in dietary intakes in a study of rural populations in Nepal, where human activities did not vary by the day of the week (50).
Smaller studies in the United States further support a trend towards later morning meals, earlier evening meals, and less frequent but larger eating episodes on weekends compared to weekdays (51, 52). For example, a study in the United States, which used a smartphone application to estimate food timing, observed that while breakfast was generally delayed ∼1 h on weekends (1026) compared with weekdays (0921), the mean last caloric intake time was more commonly advanced (37). Other weekday-weekend differences were observed in Germany (53), with a lower number of eating episodes on weekends (similar to the United States) consisting of fewer mid-morning eating episodes but more common afternoon eating episodes. Shifts in food timing based on day of the week (i.e., work day compared with nonwork day) contribute to nutritional jetlag and may result in metabolic burden (54).
A growing working population relevant for food timing is nightshift workers, who comprise 14% of the workforce and eat during the biological night (55). A systematic review and meta-analysis did not identify differences in total energy intake between dayshift and nightshift schedules, so other factors, particularly food timing, are suspected to explain the higher prevalence of obesity and cardiometabolic diseases among nightshift workers (56). The hypothesized shift towards later food timing is supported by analyses from NHANES, which indicated more prevalent late-night eating (35) and breakfast skipping (57) among nightshift workers. A small study comparing the eating patterns of 9 nightshift nurses to 8 dayshift nurses observed that on work days, dayshift nurses consumed most of their calories during the day (0900–2100), whereas nightshift nurses redistributed their food intake to the nighttime (58). Eating patterns were similar on nonwork days between these groups. In addition, no differences were observed in total energy intake between the 2 groups, and consistent with earlier findings, both groups exhibited higher overall caloric intake on nonwork days than on work days. Other studies have also observed that nightshift workers tend to increase their meal frequencies and snack intake at night, yet these findings appear to vary by occupation [as reviewed in (59)].
Socioeconomic status
Throughout history, social class has impacted the timing of food intake. Middle-class tradesmen and merchants, for example, had to eat meals later in the day as they were bound by work (41). Americans with lower income, an indicator of socioeconomic status (SES), were more likely to be breakfast skippers (57) and in cycles of NHANES before the 2003–2008 cycle, have been consistently correlated with less frequent eating occasions (60). It remains unclear whether breakfast skipping and infrequent intake contribute to later eating, and whether these links may be a consequence of work obligations, so further studies should consider living farther away from home, or other time constraints, and any relation between SES and food timing.
Behavioral and personal preference
Diurnal energy intake distribution
It has long been suspected that there exists linkage across meals, whereby the content and timing from 1 meal influences the content and timing of subsequent meals (61). Indeed, an analysis from NHANES indicated that on days when breakfast was consumed, lunch, but not dinner, was less commonly consumed (68% compared with 80% lunch consumed) and snacks provided less energy (47). Furthermore, on days when breakfast was consumed, lunch had a 30% lower energy content and was consumed over 35 min later. In the Health Professionals Follow-Up Study, compared to breakfast consumers, breakfast skippers tended to eat before lunch and eat after dinner (62). These findings suggest a general shift towards later food times when breakfast is skipped, and provide further evidence that food intake in the morning is particularly satiating (63). In addition, an analysis in 1993 encompassing 8 towns across Europe indicated that the percentage of energy intake at the midday meal was negatively correlated with energy intake from snacks (44), supporting that meals may attenuate snacking habits or vice versa.
Sports, caffeine, alcohol, and weight-loss diets
To optimize performance and recovery, the timing of nutrient consumption relative to resistance and endurance training has been an area of major focus. A position paper by the International Society of Sports Nutrition has summarized the importance of the timely delivery of nutrients pre-, during-, and post-exercise (64). For example, consumption of carbohydrate and protein 30 min before exercise has been shown to stimulate muscle glycogen re-synthesis. In addition, delivery of an hourly high-glycemic, high-carbohydrate diet, is essential to extend endogenous glycogen stores to sustain endurance exercise beyond the 90-min to 3-h limit. Lastly, post-exercise intake of protein and carbohydrates within the first 3 h of exercise may stimulate increases in muscle protein synthesis. Trials continue to investigate the best ways to optimize body composition and adaptations to exercise through the timing of protein supplements closer to bedtime during the body's recovery phase (NCT01830946) as proteins ingested before sleep are likely digested and absorbed efficiently during nocturnal recovery (65).
In addition to the caloric contribution of sports drinks, the caffeine content of sports drinks may influence food timing. Upon consuming 1 mg/kg of caffeine in the morning, participants in a controlled laboratory study had a 10% transient reduction in caloric intake at breakfast, which was compensated at a later time (66). Among overweight and obese participants in another study, moderate coffee intake resulted in lower energy intake in the subsequent meal as well as daily intake (67). Caffeine's effect on appetite may thus exert, at a minimum, transient effects on timing and composition of subsequent meals. The effects of caffeinated sports drinks and other caffeinated beverages on the timing of meals over the course of the entire day remain unexplored.
Alcohol, on the other hand, may act as an appetite stimulant acutely influencing the timing of subsequent eating occasions (68). In a small study of 282 college students, students who drank more frequently also reported being more likely to eat later at night (69). In another study of Japanese adults with diabetes, no difference was observed in alcohol intake frequency between those who habitually consumed early (before 2000) and late (after 2000) dinner (70). Future investigations are warranted on the acute and chronic influence of alcohol intake on food timing in both younger and older adults.
Lastly, modern weight-loss diets promote intermittent fasting, or time-restricted feeding, focusing on restricted dietary intake during a specific time period (i.e., 1000–1800) for the purpose of reducing overall energy intake (71, 72). Because these diets influence food timing, they are particularly important to recognize for successful food timing interventions.
Light exposure at night
In preindustrial societies, sleep onset generally occurs 3.3 h after sunset and sleep offset before sunrise, where sleep timing is determined predominantly by environmental and endogenous cues (73). However the introduction of artificial lighting facilitated later human activities, including delayed (73) and inconsistent (74) bedtimes and later food consumption (41). Recent studies in nocturnal rodents indicated that light at night (LAN) induces an increase in relative daytime (rest phase) food intake (56% of nighttime food intake with LAN compared with 37% without LAN) and leads to excess weight gain (4). Thus, restricting prolonged nighttime exposure to artificial light, computer use, and television viewing, may prevent extended eating periods into the night.
Chronotype
A handful of cross-sectional studies have examined chronotype in relation to the timing of dietary intake. Chronotype is the behavioral manifestation of the timing of the circadian system and its entrainment by the light-dark cycle, although it is also influenced by sleep homeostatic, other environmental, social, and personal influences. It is a continuum of morningness to eveningness preference, and allows people to be classified as early, intermediate, and late chronotypes (75). In alignment with their chronotype, not surprisingly, a general trend is observed across studies indicating that foods are temporally shifted later in the day among later chronotypes (more evening types), whereby individuals eat later in the day, and consume more calories towards the end of the day. This relation was first investigated in a small study of 52 adult participants in the United States, which observed that later chronotypes as estimated with use of 7 d of wrist actigraphy, had later breakfast, lunch, dinner, and greater caloric intake at dinner and after 2000, which persisted even after 2300 (76). Similar patterns were consistently observed in larger studies and on both work days (i.e., weekdays) and nonwork days (i.e., weekends), the latter of which may be less influenced by societal pressures such as work and school. For example, an analysis of 119 obese, sleep-deprived participants from the Sleep Extension Study observed that on work days, later chronotypes ate breakfast ∼1 h and 20 min later than early chronotypes, and consumed twice as many calories after 2000 (51). A larger population-based investigation including 1845 participants from the National FINRISK 2007 and FINDIET 2007 studies consistently observed that late chronotypes had lower caloric intake by 1000 and higher caloric intake after 2000, and also intakes of breakfast, lunch, and dinner about an hour later than early chronotypes (52). Similar patterns are evident on nonwork days (51, 52). Interestingly, on nonwork days, later chronotypes also had more frequent intakes than did earlier chronotypes, with the largest intake during a meal at 1900 (as opposed to ∼2100 on work days), whereas calories were more evenly distributed among 3 meals for earlier chronotypes (52). Despite these differences, and regardless of chronotype, intakes before 1000 and after 2000 are lower across all chronotypes (52). Also, these trends may not hold true for individuals across all BMI groups (77).
Collectively, these results point to chronotype being an important determinant of food timing. Subgroup analyses from these studies further highlight the complexity of this relation, particularly on weekends and varying BMI. A recent analysis of the American Time Use Survey, with data from 53,689 participants in the United States revealed a near-normal distribution for chronotype in the overall population with a wide range of up to 10 h (78). In addition, chronotype is observed to become later in both sexes throughout adolescence, with a peak in later chronotype at 18 y and 19 y for females and males, respectively, followed by a steady advance thereafter (78). It is therefore possible that people of all age groups may be driven to eat around the clock, particularly adolescents given their later chronotype. Assessing chronotype may help identify those at risk of later dietary intake.
Sleep
Being awake at night because of social obligations may provide additional opportunities to eat. Indeed, epidemiological and experimental studies have indicated that sleep influences dietary choices and hunger (79–81), and suggest that shorter sleep leading to longer awake times may facilitate later eating times and frequent intake by providing more opportunities to eat. A longer sleep duration was observed to be correlated with earlier last meals in observational analysis of healthy adults (14). In addition, another study of middle-aged, obese Japanese men indicated that short sleep duration was associated with late dinner time (after 2100) (82). In a partial sleep restriction trial including 44 healthy adults, during a 5-d 4-h per night sleep restriction protocol, participants consumed a mean of 533 (standard deviation = 296 calories) during late-night hours (2200–0359), and consumed fewer calories from 2000 to 1459 than during baseline (83). Similarly, in a study of 16 lean adults, a 5-d partial sleep restriction of 5 h/night led to 0.8 kg weight gain, partly attributed to a smaller breakfast intake and an increase in nighttime food intake, predominantly a 42% increase in post dinner calories from carbohydrate, protein, and fiber (84). Whereas sleep extension trials have observed that prolonging sleep duration reduces the consumption of free sugar (80), whether extending sleep may cause earlier timing of food intake is plausible and needs to be examined systematically in future studies.
Physiological
Circadian system
The endogenous circadian system may play a key role in determining timing of food intake, partially through chronotype, as described above, and more directly through a circadian rhythm of hunger. During a 13-d laboratory study, 12 healthy, nonobese adults lived on a fixed 20-h sleep/wake, fasting/feeding, and rest/activity cycle under dim light conditions for 12 cycles (forced desynchrony protocol), a protocol designed to uncover the influence of the endogenous circadian system from those of the behavioral and environmental cycles. During this protocol, the participants rated their hunger, appetite, and food preference through use of visual analog scales (85). A circadian rhythm was observed for hunger, whereby a trough in hunger was observed during the biological morning (0800) and a peak during the biological evening (2000). These hunger rhythms are stable and remained the same even upon changing meal timing (16) and under conditions of chronic insufficient sleep (86). These rhythms in hunger and appetite relate to the rhythms of the appetite hormone ghrelin. Acylated ghrelin (the active form) has higher concentrations in the biological evening than in the biological morning, both in the fasted and postprandial states, consistent with hunger (86, 87). The hunger peak at 2000 also coincides with the average timing of the last eating episodes in the United States at 2018, and the hunger trough at 0800 may also partly explain variability in breakfast preference (47). The endogenous circadian rhythm in hunger and appetite, with a peak in the biological evening and a trough in the biological morning, has been proposed to potentially act as a counter regulatory mechanism to support a positive energy balance towards the end of the waking and eating phase, thereby facilitating the extended overnight fast. Furthermore, this circadian rhythm in hunger may decrease the chance of arousal from sleep because of hunger towards the end of the sleep episode (85).
In addition, energy expenditure is observed to be under endogenous circadian control (88, 89). For example, resting energy expenditure is determined to be lowest during the late biological night and highest during the biological afternoon and evening (89). The relation between food timing and energy expenditure, however, has not been extensively studied. In a weight-loss trial, no significant differences were observed in estimates of energy expenditure from self-reported physical activity between early and late lunch eaters despite apparent differences in weight loss (6). Understanding the likely complex link between energy expenditure and food timing is an aim of the ongoing Big Breakfast Study (88). Of interest, for the reversed effect, that is, the effect of food timing on energy expenditure, the endogenous circadian system causes diet-induced thermogenesis to be substantially lower in the evening than in the morning in response to identical test meals.
Genetic predisposition
Eating behavior has long been recognized as a complex trait with underlying genetic and environmental factors (90). Indeed, genetics have been estimated to account for variation in food timing (91), meal frequency, and average meal size (90). Two twin studies have investigated the genetic attribution to variation in meal timing (91, 92). A study of 265 identical and fraternal adult twin pairs who maintained 7-d food diaries, estimated 24% heritability for the timing of breakfast, and lower heritability estimates for lunch and dinner timing ranging from 18% to 22% (91). Similarly, in a Spanish twin study of 52 twin pairs with 7-d food and sleep diaries, we observed that the estimated heritability of food timing varied by meal, and ranged from 56% for breakfast, 38% for lunch, to being undetectable for dinner (92). In addition, bivariate analyses from the same studies suggested shared genetic architecture between food timing and chronotype, offering preliminary insight into specific genetic variants that may influence food timing. Indeed, analysis of the Circadian Locomotor Output Cycles Kaput (CLOCK) gene rs4580704 variant indicated that carrying the minor G allele was associated with significantly later lunch than carrying major alleles (1444 compared with 1436) among obese participants (6). In addition, the obesity-related Perilipin 1 (PLIN1) gene was also observed to influence the relation between food timing and weight loss, whereby among rs1052700 AA carriers, eating late was associated with less weight loss, whereas time of eating did not influence weight loss among TT carriers (93, 94). The first genome-wide association study for breakfast skipping, a phenotype related to food timing, with modest sample size (n = 2006) and incomplete genetic coverage (283,744 genotyped variants) in the TwinUK study did not identify any variants that surpassed the genome-wide significance threshold (95). Elucidating specific genes that alter food timing may reveal biological processes regulating food timing, enable testing of causal relations between food timing and other traits and diseases through Mendelian randomization approaches (96), identify participants at risk of mistimed food intake or more resistant to food timing interventions, and also inform the development of personalized nutrition recommendations based on genetic preferences for food timing.
Sex
Historically, sex differences in food timing have been attributed to gender differences in daily activity patterns, resulting in women eating a midday meal, that is lunch, while men were at work (41). This trend continues to be observed in rural communities in Nepal (50). An analysis of 321 rural Nepalese adults who have maintained traditional cultural norms, aged ≥20 y, suggested that men frequently skipped lunch and consumed a daytime snack instead (50). Sex differences in the timing of meals also continue to be observed in the United States. Indeed, in the United States, the mean reported times of the first eating episode and of breakfast were approximately 10 min earlier in men than in women, but the last eating episode of the day occurred later in men than in women (47). These differences may also be related to sex differences in chronotypes, as men tend to be more evening than morning chronotypes (78), which may be caused by the longer endogenous circadian period (cycle length) observed in men compared to women (97), or continue to be related to work schedules. However, the differences between the 2 sexes tend to be minor (all <10 min) overall.
Weight and obesity status
Weight and obesity status further associate with the timing and frequency of eating episodes. In a small study of 52 participants with 7-d actigraphy and food logs, higher BMI was observed to be associated with higher caloric consumption after 2000 (76). Using a newly developed meal pattern questionnaire to describe meal frequency, type, and time, an analysis of 83 obese and 94 nonobese women aged 37–60 y from a southwest region of Sweden observed a trend towards later eating among obese women (98). Obese women consumed a smaller fraction of their total meals during the morning period between 0600 and 0959, and more meals during nontraditional meal times in the afternoon, 1400 and 1559, and in the evening and night, 2000 and 2159 and 2200 and 2359. In addition, obese women had more frequent eating episodes than did nonobese women (6.1 compared to 5.2 eating episodes). Population-based cohort analyses have observed that overweight individuals were also more likely to skip breakfast (57) and eat more in the evening (53). However, other studies have identified no correlations between BMI and the timing of the last meal or eating frequency (14). Inconsistencies in observations across studies may be related to different assessment tools. Using picture-based technology to assess food timing, an analysis of 110 participants identified a positive association of BMI and adiposity with later times relative to the onset of the biological night (through use of dim light melatonin onset). Importantly, no significant relation was found through use of clock hours (99), suggesting that the time of food intake relative to the central circadian system may be more relevant than the “time on the wall.”
Aging
Studies have generally observed earlier and more structured eating patterns with older age. In population-based studies in the United States, breakfast skippers tended to be younger (i.e., 20–39 y) (57), and young adults also tend to have higher variability in energy intake with later meal times relative to older adults (53). These relations may be mediated, at least in part, by earlier chronotype resulting from older age (78), or other changes in school/work commitments, retirement, and social obligations. It is also possible that regular meal times may provide daily structure for older adults.
Disease conditions and medication use
A handful of disease conditions may impact the timing of food intake or have specific dietary recommendations related to food timing for adequate management of the disease. In the case of 2 eating disorders, night-eating syndrome and sleep-related eating disorder, patients exhibit morning anorexia and evening hyperphagia, resulting in nocturnal food ingestion (100). Thus, upon manifestation of the disorder, patients tend to have severe shifts in their food intake towards later times. In the case of patients with various glycogen storage diseases, such as liver cirrhosis, patients are recommended to consume small and frequent snacks and to supplement their diets with a carbohydrate-based late-evening snack necessary to spare hepatic glycogen depletion and early-onset breakdown of endogenous protein (101). Prolonged fasting duration among those patients is not recommended. Other diseases may not have specific guidelines pertaining to food timing; however, medications and supplements for those diseases may influence food timing choices as food intake relative to medication or supplement may influence the tolerability, absorption, pharmacokinetics, and systematic bioavailability (102), whereas others may lead to increased or decreased appetite, resulting in shifts in food timing. In addition, patient populations suffering from various disease conditions necessitating nutrition support, either enteral and parenteral feeding, are customarily fed, or recommended to be fed, for 12-h periods overnight (typically 2000–0800) for presumed convenience purposes, such as complete freedom of physical activity (103). Lastly, it is also possible that psychiatric and mental disorders that influence other aspects of dietary intake, such as appetite, composition, or impulsivity, may further influence food timing.
Recognizing Determinants of Food Timing and Other Recommendations for Future Interventions
Pilot studies to investigate food timing-based interventions have recently been described (15, 38, 104) and are currently ongoing, including food timing interventions for successful weight loss (NCT02204735), observational studies in meal timing, genetics and weight loss (ONTIME) (NCT02829619), and time-restricted feeding studies in overweight (NCT03365544) or obese adults (NCT03527290; NCT02633722). Here, we provide recommendations for future food timing intervention studies.
Personalized approach
Through personalization, previous pilot studies suggested that lifestyle habits could be modified successfully, at least in the short term (80, 81). To tailor food timing interventions, it is important to recognize an individual's ability, situational context, and other factors to determine modifiable factors and potential barriers to adopting food timing changes in free-living conditions. Based on the nature of the food timing determinants (see Figure 1) we identified from the literature, we predict that extrinsic (cultural and environmental factors) and intrinsic (physiological) factors may impede adherence to food timing interventions as they are, at least for the most part, not modifiable by an individual. For example, physiological factors that impact food timing such as 1) genetic predisposition to eating at certain times; 2) aging; 3) extreme early or extreme late chronotype; 4) health status and medication use that influence food timing; and 5) sleep disorders, to a large extent cannot be changed and may limit adherence to certain food timing recommendations. Likewise, cultural and environmental factors, such as tradition and work hours, may also be less conducive to food timing changes at an individual level as these are determined at an organizational level. Instead, factors that stem from an individual's choice and here are termed "behavioral and personal preference" (see Figure 1) are more likely to be targeted to modify food timing. As an example, considering the link across meal times, recognizing an individual's breakfast intake preference can help predict the timing of lunch intake as breakfast skipping leads to earlier lunch intake (57). We provide additional recommendations on likely modifiable food timing factors in Table 1. Thus, upon starting an intervention, participants should receive counseling on potential modifiable factors and recognize barriers in their lifestyle that may limit food timing changes. Understanding these factors may also explain lack of adherence. Future research delineating whether changes to these factors truly affect food timing is warranted.
TABLE 1.
Recommendations for likely modifiable behavioral and personal preference determinants to achieve earlier food timing
| Determinant | Recommendations |
|---|---|
| Diurnal energy intake distribution and weight-loss diets | Recommend breakfast and limiting late-night eating |
| Sports | Recommend earlier times for sports |
| Alcohol | Avoid late-night alcohol intake |
| Light exposure | Avoid nighttime bright light exposure |
| Chronotype | Adopt earlier chronotype, if possible |
| Sleep | Attain adequate sleep durations 7–9 h |
Vulnerable populations
Planned food timing intervention studies are warranted in vulnerable populations recognized to be prone to the detrimental effects of mistimed food intake and related diseases. Intervention trials in these vulnerable populations may identify countermeasures for the adverse effects of mistimed food intake, and provide data to develop evidence-based dietary guidelines based on food timing.
Among the most vulnerable are nightshift workers, who are prone to mistimed food intake possibly resulting from routine nighttime eating during their work hours (58). Because nightshift workers tend to have diets similar in composition to day workers (56), associations with elevated cardiometabolic diseases and mortality risk among nightshift workers may be attributed to their nighttime food intake (34, 57, 58). The ongoing “Shifting the Risk” crossover trial is examining the impact of changing food timing in a group of nightshift workers with abdominal obesity (38). The 4-wk intervention aims to restrict energy intake overnight (5 h between 0100 and 0600) during 8 nightshifts for the purpose of improving cardiometabolic health. Additional interventions targeting a range of nightshift schedules across different fields of work are warranted, particularly as nighttime eating is suspected to be only evident among night workers with work environments that are conducive to nighttime eating (59).
Individuals self-identifying as being “evening” types have been associated with higher cardiometabolic disease and have been consistently observed to have later times of food intake (51, 52, 76). However, a possibly delayed circadian phase among those individuals may be protective against the effect of night eating, as has been observed for cardiovascular disease risk (105). Evaluating whether or not earlier eating among those individuals is beneficial is important. Similarly, as very early eating may also coincide with the biological night among individuals with later chronotype (106), evaluating the biological consequences of breakfast intake among this population is important. In addition, drastic shifts in food timing based on day of the week (i.e., work day compared with nonwork day) resulting in social and nutritional jetlag may incur a metabolic burden (54). Investigating the effects of limiting shifts in food timing during the weekends, as has been conducted in sleep interventions (107), for the purpose of attaining regular and consistent food times is important. Also, as genetic background may exacerbate the detrimental effects of nighttime eating, food timing intervention focused on genetically vulnerable populations is important. This is the case for carriers of the common type 2 diabetes Melatonin Receptor 1B (MTNR1B) gene rs10830963 risk allele. Compared to noncarriers of the risk alleles, carriers of the common G risk allele, which constitute over 50% of the population of European or Asian ancestry, have previously displayed exacerbated glucose intolerance resulting from nighttime eating and greater intolerance when administered exogenous melatonin pills (12, 108). A similar case has been observed for carriers of the PLIN1 in terms of lunch timing (93).
Additional vulnerable populations to consider include: 1) populations, or even ethnic groups and cultures, in which late dinner is a societal norm, such as Spain, to provide robust data to guide public health strategies; 2) children, adolescents, younger adults, and adults who tend to skip breakfast and thus may be susceptible to late-night eating; 3) obese adults, to identify whether obesity is a cause or consequence of eating late; and 4) participants in weight-loss trials, to establish the causal relation between earlier food intake and greater success at weight loss previously observed in secondary analyses of weight-loss trials. In addition, understanding whether advancing the timing of nutrition in patients on nighttime cycled enteral or parental nutrition support improves cardiovascular health profile is important, and further examining the feasibility of food timing intervention in patients with night eating syndrome to delay rapid obesity onset resulting from disease.
Food timing assessment tools
A major limitation in the area of dietary pattern research is the lack of standard methodologies devised for the purpose of ascertaining food timing patterns. Earlier studies have relied on questionnaires, modifications of traditional dietary assessment tools, or picture-based smartphone applications (Table 2). Identifying appropriate tools to assess food timing pre, post, and during an intervention is necessary to evaluate compliance and intervention feasibility.
TABLE 2.
Emerging tools for assessing food timing
| Assessment method | Description/examples | Strength | Limitation |
|---|---|---|---|
| Single questionnaire items | “The timing of breakfast, lunch, or dinner” or “Time-of-day wherein majority of daily energy intake is consumed (morning, midday, evening/late)” | Single-questionCan be customized to meal of interest | Recall biasTypically does not assess for inter-meal intakeMay be interpreted differently by people, particularly across culturesDifficult to apply to nightshift workers |
| Modified traditional dietary assessment tools | Modified 24-h dietary recalls | Uses commonly used and recognized dietary assessment tools and modifies those by assessing for food timing | High participant burden |
| Modified food records | Allows for multiple day assessment (work days and nonwork days) | High analytical burdenScalabilityLikely same limitations of 24-h dietary recalls and food records apply, i.e. recall bias | |
| Food Timing Questionnaire | Meal Pattern Questionnaire (98) | Developed and recognized assessment tools | Does not assess weekly variability |
| Night Eating Syndrome questionnaire (111): “Do you have cravings or urges to eat snacks after supper, but before bedtime?” and “How much of your daily food intake do you consume after suppertime?” and “When you get up in the middle of the night, how often do you snack?” | Short | Not validated | |
| Picture-based smartphone applications | Using a camera phone, participants are instructed to record every item consumed (food, drink, water). If foods are not entirely consumed, then leftovers are recorded. Pictures are assessed for content and analyzed for time stamp and geolocation. Nonwater items are further annotated by research team by looking up Food and Nutrient Databases, such as NDSR1 | Prospective designMetadata such as time and locationAllows for multiple-day recording | High participant burdenHigh analytical burdenScalabilityPrivacy issues |
| Accelerometer-based detection | An automated approach to detect food timing from 3-dimensional wrist-worn accelerometers | Passive data collectionMetadata such as motion and light exposureAllows for multiple-day recordingScalability | High analytical burdenValidity |
NDSR, Nutrition Data System for Research.
Single questionnaire items
For secondary analyses of cohort studies, the particular approach chosen is often guided by the dietary assessment method (109). Thus, a variety of approaches to define meal patterns have been used in the literature based on various responses from the participant. These data may be limited to specific meals, that is, breakfast, lunch, or dinner, or may be related to any eating occasion, that is, any instance of ingestion of food or drink. For example, in the US Health Professionals Follow-up study, the authors used a question on “eating after going to bed” to determine late-night eating and assess relations between late-night eating and diet quality (110).
Modified traditional dietary assessment tools
Few studies have melded traditional dietary assessment tools, such as 24-h recalls of food records, with other areas of nutrition science, such as dietary pattern and food timing. For example, in NHANES, during 24-h dietary recalls, participants were further asked to report clock times of each eating occasion reported (47). Other studies have used two 24-h recalls and for each eating occasion, questions on time (1-h intervals) and place of consumption were further probed (45). This approach enables the continued use of familiar and validated dietary assessment tools supplemented with information for other aspects of nutrition. In an ongoing food timing intervention, weekly 24-h recalls are conducted during an intervention and control period via a phone call (38). Participants in that intervention will be asked to recall foods consumed over a 24-h period from 0600 to 0600.
Food timing questionnaires
Thus far, only 1 questionnaire has been developed specifically for food timing. The Meal Pattern Questionnaire (98) asks respondents to: “Describe how you eat during a 24-h period.” Respondents list usual times of food intake and for each time, indicate the corresponding meal type, that is “main meal,” “light meal/breakfast,” “snack meal,” or “drink, only.” The questionnaire has been tested in a Swedish population, and when readministered to the same population, resulted in largely consistent responses (correlation r2 = 0.70). Although an excellent questionnaire for its simplicity, it has yet to be validated against prospectively collected food timing data. Another questionnaire is the Night Eating Questionnaire that has been developed to measure the severity of Night Eating Syndrome (111). Although specifically developed as a diagnosis tool, 3 questions from that questionnaire may be useful to probe nighttime eating in a general population (Table 2).
Picture-based smartphone applications and accelerometry
With widespread use of smartphones and the modern food-picture culture, picture-based technologies are being used for dietary assessment (37, 99, 112). Many of these systems require active participation beyond picture taking, as users often must document the names and quantities of food items for the content of the images to be evaluated by trained nutritionists and nutrient values estimated. The time-stamped photos may further be used to analyze the temporal aspect of dietary intake. For example, the “myCircadianClock” phone application asks participants to take pictures of all foods and beverages with a smartphone (37). Picture and text entries are tagged with time-stamps and geolocation data. A major advantage of such technology is the metadata that can be acquired with a picture, allowing examination of various aspects of dietary constructs including timing, format, and context. In addition to smartphones, wrist-worn accelerometers can be used to passively determine food timing based on detecting motion features of eating and drinking activities (113, 114).
Considerations for future tools
To advance the area of dietary pattern research, existing methods used to collect dietary pattern data need to be further examined, validated, and refined, and newer methods need further development. These newer tools will likely provide the necessary platform to advance future food timing research. In addition, existing methods need to be further examined for various limitations that are well recognized for dietary assessment tools and refined. First, it remains unclear whether food timing is prone to misreporting or meal frequency prone to underreporting, which is possible considering that more frequent eating is positively related to energy intake (14, 98) and later food times tend to be stigmatized (109). Second, current methods do not differentiate between work day and nonwork day food patterns, such as the Meal Pattern Questionnaire, and this may be particularly relevant for night workers. Interrogating work days and nonwork days separately may also be necessary to effectively capture “nutritional jetlag.” Third, single 24-h recalls do not provide sufficient information on food timing regularity, and instead, multiple assessments are necessary to capture inter-daily variability. Fourth, although valuable, findings from older data sets need to be confirmed in more recent data whenever possible, as 15–20-y-old data may not reflect current temporal habits as food timing is continuously evolving (46).
Newer questionnaires and survey methods must also acknowledge other limitations. First, although theoretically simple to ascertain, the accuracy of recalled food timing has not been validated against prospectively collected data, such as multiple days of food logs. Second, early studies have relied on self-reported clock times for main meals (i.e., breakfast, lunch, and dinner), and in dealing with modern grazing behavior, few studies have further interrogated the times of inter-meal eating episodes. Understanding meal patterns, that is, distribution of calories across 24 h, of specific populations is necessary to determine ideal tools for food timing assessment. For some populations, such as French and Spanish, the ascertainment of timing for breakfast, lunch, and dinner may be sufficient as those populations continue to be dominated by a 3 meals/d meal pattern structure (53). However, other populations need to interrogate the timing of inter-meal eating occasions.
There is also a need for measures that are objective, inexpensive to administer, have low participant burden, and are scalable. These technologies could reliably capture various aspects of modern eating patterns. For example, future picture-based dietary assessments need an interface that is user-friendly and intuitive for nontechnical users, yet that meets the requirements imposed by the image analysis process. These technologies ideally would collect data passively, and therefore have very low participant burden. Various technologies are currently being developed for this purpose and need to be tested for scalability considering the volume of data collected. Data safety of picture-based methods must also be ensured. Lastly, assessment of food timing should be complemented with assessment of the onset of biological night. Recent findings have indicated that rather than clock time, circadian misalignment, that is, food timing relative to the central circadian pacemaker, is associated with body composition (99). Central circadian timing can be established by measuring melatonin rhythms (30). Serial sampling of melatonin measured in blood or saliva can be used to determine the time at which levels rise above baseline, termed the dim light melatonin onset. Although less accurate, this may also be made possible by attaining self-reported chronotype (105). Other methods to assess circadian timing but requiring fewer blood draws are also being developed (e.g., (115, 116)).
Other considerations
Other important considerations for food timing interventions can be learned from interventions to change other complex human behaviors. Assessing compliance during the intervention is important for intervention success. Brief follow-up visits have been incorporated in the past to improve compliance during an intervention (81). In addition, automated reminders via short mobile messages to remind participants may improve compliance. In addition, food timing interventions should be implemented gradually and incrementally to maximize compliance and durability. In a sleep extension trail, prolonging time in bed by introducing earlier bedtimes for the purpose of extending sleep duration had an unintended negative consequence on sleep quality (80), whereas gradual and personalized sleep extension (i.e. 5-min advancements per day) had beneficial effects on sleep (107). In a recent time-restricted feeding pilot, participants reported that the intervention affected social eating/drinking opportunities in the evening, which may limit long-term compliance (104). Thus, to limit the chances of unintended consequences of earlier food times, such as time scarcity resulting in decrease in food preparation timing leading to the intake of lower quality diets and fast food intake, interventions should be gradual for sustained and positive behavior changes. Combining food timing interventions with healthy diets may also be useful, as was done for sleep hygiene, which focused on optimizing the sleep environment such as TV at night and caffeine intake close to bedtime, combined with sleep extension (107). It is therefore possible for food timing interventions to incorporate healthy food counseling.
It remains unclear as to the timing of which eating episode or meal of the day should be ascertained and modified. Studies have provided evidence for assessing the following eating episodes: 1) the last eating episode of the day [i.e., the timing of the last meal or snack, which may coincide with the biological night that has been observed to range from as early as 1700 to as late as 0200 (14)]; 2) any eating episode that coincides with the biological night (i.e., time between melatonin onset and bedtime, time between wake time and melatonin offset, and time between bedtime and wake time); 3) the most energy-dense meal of the day (i.e., dinner in the United States [providing 36% of 24-h energy (47)], but in some cases may be lunch); or, 4) the most commonly consumed meal of the day {i.e., dinner in the United States [consumed by 93% of Americans (47)], lunch in Spain, and breakfast and dinner in Germany (53)}. Deciding what eating occasion to target may also partly be determined by what timing of the day is more feasible and physiologically easier to manipulate, especially when considering the different genetic contributions across meals (92).
Alternatively, food timing interventions may focus on promoting fasting, by restricting intake during the biological night or delaying timing of intake of first meal of the day (1000) and earlier last meal of the day. In addition, food timing interventions may combat snacking behavior, which tends to prolong the ingestion period, increase the number of eating episodes per day, and contribute to positive energy balance (14, 98), or focus on healthy snack alternatives. Lastly, the duration and chronicity of an intervention remains unclear. Ongoing food timing interventions are 4 wk (38). Pilot interventions may provide the framework on the necessary duration of future interventions, which may also shine light on the importance of meal timing regularity and restricting day-to-day variability.
Conclusion
Here, we provided a comprehensive overview of determinants that may need to be considered for tailoring food timing recommendations. These factors may also be important to consider analytically, as they may be confounders and/or modifiers of relations between food timing and health outcomes. However, considering variability in assessing food timing across these investigations and overall few available studies in this field thus far, the relative contribution and importance of each determinant on food timing cannot be determined at this point. Furthermore, we: 1) highlight vulnerable populations who have been identified in experimental and epidemiological studies to be at risk of mistimed food intake and thus necessitating intervention; 2) list currently used food timing assessment tools and indicate limitations; and 3) indicate other important considerations based on successful interventions addressing timing of other behaviors. Furthermore, controlling the diurnal distribution of food can affect the rhythmicity and regularity of parts of the circadian system and of other behaviors.
There exists a great opportunity to develop the evidence base for necessary food timing guidelines for vulnerable populations and public health initiatives to shift societal norms across cultures with very late eating habits (e.g., Spain's return to the correct time zone), frequent and erratic eating habits (e.g., United States and Netherlands), or simply lunch hours at the workplace or educational setting to be conducive to healthier eating times. In addition to guidelines related to timing, these studies may provide data advocating for the importance of providing access to healthy foods around the clock, particularly for nightshift workers and later chronotypes, who tend to have greater consumption of convenience foods or unhealthy foods, and lower intakes of fruits and vegetables (76) as their meals are unmatched with those of their family and friends (117). Furthermore, these findings will help dissect the role of breakfast and whether it should be re-introduced in future dietary guidelines. Finally, these studies may provide critical insight into the fundamental mechanisms related to circadian coordination with the feeding/fasting cycle, energy balance, and metabolic control.
Feasibility studies are required, and pilot trials are currently being implemented. However, to address the universality of food timing findings, intervention studies should be extended to populations across diverse geographical regions, cultures, health status, work schedules (e.g., shift-workers), and age groups. Limitations and controversies should be addressed, including consensus on a standard, robust method for food timing research and identifying the critical eating episode or meal of the day that should be targeted. In addition, monitoring of food timing, currently and longitudinally over time, and across various cultures and populations, including isolated societies, such as hunter-gatherer societies, could provide critical context to research efforts to characterize relations between dietary intake and health.
Acknowledgments
The authors’ responsibilities were as follows—HSD and MG: designed the study; HSD, FAJLS, RS, and MG: participated in acquisition and interpretation of data; HSD, FAJLS, RS, and MG: wrote the manuscript; and all authors: have read and approved the final paper.
Notes
FAJLS was supported in part by National Institutes of Health grants R01HL094806, R01HL118601, R01DK099512, R01DK102696, and R01DK105072. RS was supported by National Institutes of Health grants R01DK102696, R01 DK107859 and R01DK105072, and the Phyllis and Jerome Lyle Rappaport MGH Research Scholar Award. MG was supported by the Spanish Government of Investigation, Development and Innovation (SAF2017-84135-R) including FEDER co-funding, and National Institutes of Health grant NIDDK R01DK105072 granted to MG.
Author disclosures: HSD, RS, and MG, no conflicts of interest. FAJLS received speaker fees from Bayer Healthcare, Sentara Healthcare, Philips, Kellogg Company, Vanda Pharmaceuticals, and Pfizer Pharmaceuticals.
References
- 1. Garaulet M, Gómez-Abellán P.. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. [DOI] [PubMed] [Google Scholar]
- 2. Pérez-Martínez P, Mikhailidis DP, Athyros VG, Bullo M, Couture P, Covas MI, de Koning L, Delgado-Lista J, Díaz-López A, Drevon CA et al.. Lifestyle recommendations for the prevention and management of metabolic syndrome: an international panel recommendation. Nutr Rev. 2017;75:307–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17:2100–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A. 2010;107:18664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–29. [DOI] [PubMed] [Google Scholar]
- 6. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee Y-C, Ordovás JM, Scheer FAJL. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond). 2013;37:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruiz-Lozano T, Vidal J, de Hollanda A, Scheer FAJL, Garaulet M, Izquierdo-Pulido M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clin Nutr. 2016;35:1308–14. [DOI] [PubMed] [Google Scholar]
- 8. Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2014;27:(Suppl 2):255–62. [DOI] [PubMed] [Google Scholar]
- 9. Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring). 2013;21:2504–12. [DOI] [PubMed] [Google Scholar]
- 10. Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FAJL. The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity (Silver Spring). 2015;23:2053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, Buxton OM, Shea SA, Scheer FAJL. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015;112:E2225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez-Minguez J, Saxena R, Bandín C, Scheer FA, Garaulet M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: a randomized, cross-over study. Clin Nutr. 2018;37:1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bandín C, Scheer FAJL, Luque AJ, Ávila-Gandía V, Zamora S, Madrid JA, Gómez-Abellán P, Garaulet M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int J Obes (Lond). 2015;39:828–33. [DOI] [PubMed] [Google Scholar]
- 14. Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res. 2014;34:930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–21..e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, Skene DJ, Johnston JD. Meal timing regulates the human circadian system. Curr Biol. 2017;27:1768–75.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qian J, Dalla Man C, Morris CJ, Cobelli C, Scheer FAJL. Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes Metab. 2018;20(10):2481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garaulet M, Gómez-Abellán P, Rubio-Sastre P, Madrid JA, Saxena R, Scheer FAJL. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism. 2015;64:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collado MC, Engen PA, Bandín C, Cabrera-Rubio R, Voigt RM, Green SJ, Naqib A, Keshavarzian A, Scheer FAJL, Garaulet M. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB J. 2018;32:2060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leung GKW, Huggins CE, Bonham MP. Effect of meal timing on postprandial glucose responses to a low glycemic index meal: a crossover trial in healthy volunteers. Clin Nutr. 2017; Available from: http://linkinghub.elsevier.com/retrieve/pii/S0261561417314085. [DOI] [PubMed] [Google Scholar]
- 21. Fonken LK, Lieberman RA, Weil ZM, Nelson RJ. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology. 2013;154:3817–25. [DOI] [PubMed] [Google Scholar]
- 22. Scheer FAJL, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010;107:20541–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grimaldi D, Carter JR, Van Cauter E, Leproult R. Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertens (Dallas, Tex 1979). 2016;68:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris CJ, Purvis TE, Hu K, Scheer FAJL. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016;113:E1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Depner CM, Melanson EL, McHill AW, Wright KP. Mistimed food intake and sleep alters 24-hour time-of-day patterns of the human plasma proteome. Proc Natl Acad Sci U S A. 2018;115(23):E5390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oosterman JE, Kalsbeek A, la Fleur SE, Belsham DD. Impact of nutrients on circadian rhythmicity. Am J Physiol Integr Comp Physiol. 2015;308:R337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stokkan K-A, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–3. [DOI] [PubMed] [Google Scholar]
- 28. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gamble KL, Young ME.. Metabolism as an integral cog in the mammalian circadian clockwork. Crit Rev Biochem Mol Biol. 2013;48:317–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vetter C, Scheer FAJL. Circadian biology: uncoupling human body clocks by food timing. Curr Biol. 2017;27:R656–8. [DOI] [PubMed] [Google Scholar]
- 31. Bellisle F. Impact of the daily meal pattern on energy balance. Scand J Nutr. 2004;48:114–8. [Google Scholar]
- 32. St-Onge M-P, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr. 2016;7:938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DeSalvo KB, Olson R, Casavale KO. Dietary guidelines for Americans. JAMA. 2016;315:457. [DOI] [PubMed] [Google Scholar]
- 34. Khanna N, Eicher-Miller HA, Boushey CJ, Gelfand SB, Delp EJ. Temporal dietary patterns using kernel k-means clustering. ISM. 2011;2011:375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eicher-Miller HA, Khanna N, Boushey CJ, Gelfand SB, Delp EJ. Temporal dietary patterns derived among the adult participants of the National Health and Nutrition Examination Survey 1999–2004 are associated with diet quality. J Acad Nutr Diet. 2016;116:283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Almoosawi S, Vingeliene S, Karagounis LG, Pot GK. Chrono-nutrition: a review of current evidence from observational studies on global trends in time-of-day of energy intake and its association with obesity. Proc Nutr Soc. 2016;75:487–500. [DOI] [PubMed] [Google Scholar]
- 37. Gill S, Panda S.. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22:789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bonham MP, Leung GKW, Davis R, Sletten TL, Murgia C, Young MJ, Eikelis N, Lambert EA, Huggins CE. Does modifying the timing of meal intake improve cardiovascular risk factors? Protocol of an Australian pilot intervention in night shift workers with abdominal obesity. BMJ Open. 2018;8:e020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Drok A. Eating with the Chinese body clock[Internet]. Chinese Medicine Melbourne; 2016. [Google Scholar]
- 40. Shariatpanahi ZV, Shariatpanahi MV, Shahbazi S, Hossaini A, Abadi A. Effect of Ramadan fasting on some indices of insulin resistance and components of the metabolic syndrome in healthy male adults. Br J Nutr. 2008;100:147–51. [DOI] [PubMed] [Google Scholar]
- 41. McMillan S. What time is dinner? History Magazine[Internet]. 2001. Available from: https://www.history-magazine.com/dinner2.html [Google Scholar]
- 42. Atienza B. Gobierno De La Nacion. Boletin Oficial Del Estado 1940. [Google Scholar]
- 43. Huseinovic E, Winkvist A, Slimani N, Park MK, Freisling H, Boeing H, Buckland G, Schwingshackl L, Weiderpass E, Rostgaard-Hansen AL et al.. Meal patterns across ten European countries—results from the European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study. Public Health Nutr. 2016;19:2769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schlettwein-Gsell D, Decarli B, de Groot L. Meal patterns in the SENECA study of nutrition and the elderly in Europe: assessment method and preliminary results on the role of the midday meal. Appetite. 1999;32:15–22. [DOI] [PubMed] [Google Scholar]
- 45. Park MK, Freisling H, Huseinovic E, Winkvist A, Huybrechts I, Crispim SP, de Vries JHM, Geelen A, Niekerk M, van Rossum C et al.. Comparison of meal patterns across five European countries using standardized 24-h recall (GloboDiet) data from the EFCOVAL project. Eur J Nutr. 2018;57:1045–57. [DOI] [PubMed] [Google Scholar]
- 46. Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet. 2015;115:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kant AK. Eating patterns of US adults: meals, snacks, and time of eating. Physiol Behav. 2018;193(Part B):270–8. [DOI] [PubMed] [Google Scholar]
- 48. Thompson FE, Larkin FA, Brown MB. Weekend-weekday differences in reported dietary intake: the Nationwide Food Consumption Survey, 1977–78. Nutr Res. 1986;6:647–62. [Google Scholar]
- 49. An R. Weekend-weekday differences in diet among U.S. adults, 2003–2012. Ann Epidemiol. 2016;26:57–65. [DOI] [PubMed] [Google Scholar]
- 50. Sudo N, Sekiyama M, Ohtsuka R, Maharjan M. Gender differences in “luxury food intake” owing to temporal distribution of eating occasions among adults of Hindu communities in lowland Nepal. Asia Pac J Clin Nutr. 2009;18:441–6. [PubMed] [Google Scholar]
- 51. Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge L, Csako G, Cizza G; Sleep Extension Study Group. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS One. 2013;8:e56519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Maukonen M, Kanerva N, Partonen T, Kronholm E, Tapanainen H, Kontto J, Männistö S. Chronotype differences in timing of energy and macronutrient intakes: a population-based study in adults. Obesity (Silver Spring). 2017;25:608–15. [DOI] [PubMed] [Google Scholar]
- 53. Winkler G, Döring A, Keil U. Meal patterns in middle-aged men in Southern Germany: results from the MONICA Augsburg Dietary Survey 1984/85. Appetite. 1999;32:33–7. [DOI] [PubMed] [Google Scholar]
- 54. Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–43. [DOI] [PubMed] [Google Scholar]
- 55. National Center for Health Statistics. National Health Interview Survey, 2011. [Internet]. Hyattsville (MD); 2012. Available from: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/NHIS/2010/samadult_freq.pdf. [Google Scholar]
- 56. Bonham MP, Bonnell EK, Huggins CE. Energy intake of shift workers compared to fixed day workers: a systematic review and meta-analysis. Chronobiol Int. 2016;33:1086–100. [DOI] [PubMed] [Google Scholar]
- 57. Kant AK, Graubard BI.. Within-person comparison of eating behaviors, time of eating, and dietary intake on days with and without breakfast: NHANES 2005–2010. Am J Clin Nutr. 2015;102:661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Molzof HE, Wirth MD, Burch JB, Shivappa N, Hebert JR, Johnson RL, Gamble KL. The impact of meal timing on cardiometabolic syndrome indicators in shift workers. Chronobiol Int. 2017;34:337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lowden A, Moreno C, Holmbäck U, Lennernäs M, Tucker P. Eating and shift work—effects on habits, metabolism and performance. Scand J Work Environ Health. 2010;36:150–62. [DOI] [PubMed] [Google Scholar]
- 60. Kant AK, Graubard BI.. Family income and education were related with 30-year time trends in dietary and meal behaviors of American children and adolescents. J Nutr. 2013;143:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Castro JM. Macronutrient relationships with meal patterns and mood in the spontaneous feeding behavior of humans. Physiol Behav. 1987;39:561–9. [DOI] [PubMed] [Google Scholar]
- 62. Mekary RA, Giovannucci E, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in men: breakfast omission, eating frequency, and snacking. Am J Clin Nutr. 2012;95:1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Castro JM. The time of day of food intake influences overall intake in humans. J Nutr. 2004;134:104–11. [DOI] [PubMed] [Google Scholar]
- 64. Kerksick CM, Arent S, Schoenfeld BJ, Stout JR, Campbell B, Wilborn CD, Taylor L, Kalman D, Smith-Ryan AE, Kreider RB et al.. International Society of Sports Nutrition position stand: nutrient timing. J Int Soc Sports Nutr. 2017;14:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trommelen J, van Loon LJC. Pre-sleep protein ingestion to improve the skeletal muscle adaptive response to exercise training. Nutrients. 2016;8:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Panek-Shirley LM, DeNysschen C, O'Brien E, Temple JL. Caffeine transiently affects food intake at breakfast. J Acad Nutr Diet. 2018;118:1832–43. [DOI] [PubMed] [Google Scholar]
- 67. Gavrieli A, Karfopoulou E, Kardatou E, Spyreli E, Fragopoulou E, Mantzoros CS, Yannakoulia M. Effect of different amounts of coffee on dietary intake and appetite of normal-weight and overweight/obese individuals. Obesity (Silver Spring). 2013;21:1127–32. [DOI] [PubMed] [Google Scholar]
- 68. Caton SJ, Ball M, Ahern A, Hetherington MM. Dose-dependent effects of alcohol on appetite and food intake. Physiol Behav. 2004;81:51–8. [DOI] [PubMed] [Google Scholar]
- 69. Lloyd-Richardson EE, Lucero ML, Dibello JR, Jacobson AE, Wing RR. The relationship between alcohol use, eating habits and weight change in college freshmen. Eat Behav. 2008;9:504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sakai R, Hashimoto Y, Ushigome E, Miki A, Okamura T, Matsugasumi M, Fukuda T, Majima S, Matsumoto S, Senmaru T et al.. Late-night-dinner is associated with poor glycemic control in people with type 2 diabetes: The KAMOGAWA-DM cohort study. Endocr J. 2018;65:395–402. [DOI] [PubMed] [Google Scholar]
- 71. Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 2015;73:661–74. [DOI] [PubMed] [Google Scholar]
- 72. Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci. 2018;7:e22. [Google Scholar]
- 73. Yetish G, Kaplan H, Gurven M, Wood B, Pontzer H, Manger PR, Wilson C, McGregor R, Siegel JM. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015;25:2862–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wright KP, McHill AW, Birks BR, Griffin BR, Rusterholz T, Chinoy ED. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23:1554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vera B, Dashti HS, Gómez-Abellán P, Hernández-Martínez AM, Esteban A, Scheer FAJL, Saxena R, Garaulet M. Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci Rep. 2018;8:945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19:1374–81. [DOI] [PubMed] [Google Scholar]
- 77. Muñoz JSG, Cañavate R, Hernández CM, Cara-Salmerón V, Morante JJH. The association among chronotype, timing of food intake and food preferences depends on body mass status. Eur J Clin Nutr. 2017;71:736–42. [DOI] [PubMed] [Google Scholar]
- 78. Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US—influence of age and sex. Tosini G, editor PLoS One. 2017;12:e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6:648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Al Khatib HK, Hall WL, Creedon A, Ooi E, Masri T, McGowan L, Harding SV, Darzi J, Pot GK. Sleep extension is a feasible lifestyle intervention in free-living adults who are habitually short sleepers: a potential strategy for decreasing intake of free sugars? A randomized controlled pilot study. Am J Clin Nutr. 2018;107:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tasali E, Chapotot F, Wroblewski K, Schoeller D. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: a home-based intervention. Appetite. 2014;80:220–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hsieh SD, Muto T, Murase T, Tsuji H, Arase Y. Association of short sleep duration with obesity, diabetes, fatty liver and behavioral factors in Japanese men. Intern Med. 2011;50:2499–502. [DOI] [PubMed] [Google Scholar]
- 83. Spaeth AM, Dinges DF, Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr. 2014;100:559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci. 2013;110:5695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Scheer FAJL, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring). 2013;21:421–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McHill AW, Hull JT, McMullan CJ, Klerman EB. Chronic insufficient sleep has a limited impact on circadian rhythmicity of subjective hunger and awakening fasted metabolic hormones. Front Endocrinol (Lausanne). 2018;9:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Qian J, Morris CJ, Caputo R, Garaulet M, Scheer FAJL. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int J Obes (Lond). 2018. 10.1038/s41366-018-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ruddick-Collins LC, Johnston JD, Morgan PJ, Johnstone AM. The Big Breakfast Study: chrono-nutrition influence on energy expenditure and bodyweight. Nutr Bull. 2018;43:174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zitting K-M, Vujovic N, Yuan RK, Isherwood CM, Medina JE, Wang W, Buxton OM, Williams JS, Czeisler CA, Duffy JF. Human resting energy expenditure varies with circadian phase. Curr Biol. 2018;28:3685–90..e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. de Castro JM. Behavioral genetics of food intake regulation in free-living humans. Nutrition. 1999;15:550–4. [DOI] [PubMed] [Google Scholar]
- 91. de Castro JM. Heritability of diurnal changes in food intake in free-living humans. Nutrition. 2001;17:713–20. [DOI] [PubMed] [Google Scholar]
- 92. Lopez-Minguez J, Dashti HS, Madrid-Valero JJ, Madrid JA, Saxena R, Scheer FA, Ordoñana JR, Garaulet M. Heritability of the timing of food intake. Clin Nutr. 2018. pii: S0261-5614(18)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Garaulet M, Vera B, Bonnet-Rubio G, Gómez-Abellán P, Lee Y-C, Ordovás JM. Lunch eating predicts weight-loss effectiveness in carriers of the common allele at PERILIPIN1: the ONTIME (Obesity, Nutrigenetics, Timing, Mediterranean) study. Am J Clin Nutr. 2016;104:1160–6. [DOI] [PubMed] [Google Scholar]
- 94. Lopez-Minguez J, Gómez-Abellán P, Garaulet M. Circadian rhythms, food timing and obesity. Proc Nutr Soc. 2016;75:501–11. [DOI] [PubMed] [Google Scholar]
- 95. Boraska V, Davis OSP, Cherkas LF, Helder SG, Harris J, Krug I, Pei-Chi Liao T, Treasure J, Ntalla I, Karhunen L et al.. Genome-wide association analysis of eating disorder-related symptoms, behaviors, and personality traits. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Duffy JF, Cain SW, Chang A-M, Phillips AJK, Münch MY, Gronfier C, Wyatt JK, Dijk D-J, Wright KP, Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A. 2011;108:(Suppl 3):15602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bertéus Forslund H, Lindroos AK, Sjöström L, Lissner L. Meal patterns and obesity in Swedish women—a simple instrument describing usual meal types, frequency and temporal distribution. Eur J Clin Nutr. 2002;56:740–7. [DOI] [PubMed] [Google Scholar]
- 99. McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, Garaulet M, Scheer FA, Klerman EB. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106(5):1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. O'Reardon JP, Peshek A, Allison KC. Night eating syndrome: diagnosis, epidemiology and management. CNS Drugs. 2005;19:997–1008. [DOI] [PubMed] [Google Scholar]
- 101. Yamanaka-Okumura H, Nakamura T, Takeuchi H, Miyake H, Katayama T, Arai H, Taketani Y, Fujii M, Shimada M, Takeda E. Effect of late evening snack with rice ball on energy metabolism in liver cirrhosis. Eur J Clin Nutr. 2006;60:1067–72. [DOI] [PubMed] [Google Scholar]
- 102. Shimada H, Nilsson C, Noda Y, Kim H, Lundström T, Yajima T. Effects of food on the pharmacokinetics of omega-3-carboxylic acids in healthy Japanese male subjects: a phase I, randomized, open-label, three-period, crossover trial. J Atheroscler Thromb. 2017;24:980–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Matuchansky C, Fabre J, Guillard O, Morichau-Beauchant M, Reinberg A. Effects of cyclic (nocturnal) total parenteral nutrition and continuous enteral nutrition on circadian rhythms of blood lipids, lipoproteins and apolipoproteins in humans. Am J Clin Nutr. 1985;41:727–34. [DOI] [PubMed] [Google Scholar]
- 104. Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci. 2018;7:e22. [Google Scholar]
- 105. Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, Rosner B, Stampfer MJ, Schernhammer ES. Association between rotating night shift work and risk of coronary heart disease among women. JAMA. 2016;315:1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Eckel RH, Depner CM, Perreault L, Markwald RR, Smith MR, McHill AW, Higgins J, Melanson EL, Wright KP. Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Curr Biol. 2015;25:3004–10. [DOI] [PubMed] [Google Scholar]
- 107. Dewald-Kaufmann JF, Oort FJ, Meijer AM. The effects of sleep extension and sleep hygiene advice on sleep and depressive symptoms in adolescents: a randomized controlled trial. J Child Psychol Psychiatry. 2014;55:273–83. [DOI] [PubMed] [Google Scholar]
- 108. Garaulet M, Gómez-Abellán P, Rubio-Sastre P, Madrid JA, Saxena R, Scheer FAJL. Common type 2 diabetes risk variant in MTNR1B worsens the deleterious effect of melatonin on glucose tolerance in humans. Metabolism. 2015;64:1650–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Leech RM, Worsley A, Timperio A, McNaughton SA. Understanding meal patterns: definitions, methodology and impact on nutrient intake and diet quality. Nutr Res Rev. 2015;28:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mekary RA, Giovannucci E, Cahill L, Willett WC, van Dam RM, Hu FB. Eating patterns and type 2 diabetes risk in older women: breakfast consumption and eating frequency. Am J Clin Nutr. 2013;98:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Allison KC, Lundgren JD, O'Reardon JP, Martino NS, Sarwer DB, Wadden TA, Crosby RD, Engel SG, Stunkard AJ. The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the Night Eating Syndrome. Eat Behav. 2008;9:62–72. [DOI] [PubMed] [Google Scholar]
- 112. Gupta NJ, Kumar V, Panda S. A camera-phone based study reveals erratic eating pattern and disrupted daily eating-fasting cycle among adults in India. PLoS One. 2017;12:e0172852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Thomaz E, Essa I, Abowd GD. A practical approach for recognizing eating moments with wrist-mounted inertial sensing. Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing—UbiComp ’15. New York, (NY), USA: ACM Press; 2015. pp. 1029–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhang S, Ang MH, Xiao W, Tham CK. Detection of activities by wireless sensors for daily life surveillance: eating and drinking. Sensors (Basel). 2009;9:1499–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Laing EE, Möller-Levet CS, Poh N, Santhi N, Archer SN, Dijk D-J. Blood transcriptome based biomarkers for human circadian phase. Elife. 2017;6:e20214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Wittenbrink N, Ananthasubramaniam B, Münch M, Koller B, Maier B, Weschke C, Bes F, de Zeeuw J, Nowozin C, Wahnschaffe A et al.. High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest. 2018;128(9):3826–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Baron KG, Reid KJ, Horn L Van, Zee PC. Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite. 2013;60:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]