Abstract
CRISPR-Cas12a (Cpf1) proteins are RNA-guided DNA targeting enzymes that bind and cut DNA as components of bacterial adaptive immune systems. Like CRISPR-Cas9, Cas12a can be used as a powerful genome editing tool based on its ability to induce genetic changes in cells at sites of double-stranded DNA (dsDNA) cuts. Here we show that RNA-guided DNA binding unleashes robust, indiscriminate single-stranded DNA (ssDNA) cleavage activity in Cas12a sufficient to completely degrade both linear and circular ssDNA molecules. This target-activated non-specific ssDNase activity, catalyzed by the same active site responsible for site-specific dsDNA cutting, is also a fundamental property of other type V CRISPR-Cas12 enzymes. Activation of ssDNA cutting requires faithful recognition of a DNA target sequence matching the 20-nucleotide guide RNA sequence with specificity capable of distinguishing closely related DNA sequences. We combined target-dependent Cas12a ssDNase activation with isothermal amplification with target-dependent Cas12a ssDNase activation to create a method termed DNA Endonuclease Targeted CRISPR Trans Reporter (DETECTR), which achieves attomolar sensitivity for nucleic acid detection. We demonstrate that DETECTR enables rapid and specific detection of HPV in human patient samples, thereby providing a simple platform for nucleic acid-based, point-of-care diagnostics.
One Sentence Summary:
Cas12a (Cpf1) and related type V CRISPR interference proteins unleash non-specific, single-stranded DNase activity upon guide RNA-dependent DNA binding, which can be harnessed for rapid and specific nucleic acid detection.
CRISPR-Cas adaptive immunity in bacteria and archaea uses RNA-guided nucleases to target and degrade foreign nucleic acids (1–3). The CRISPR-Cas9 family of proteins has been widely deployed for gene editing applications (4, 5) based on the precision of double-stranded DNA (dsDNA) cleavage induced by two catalytic domains, RuvC and HNH, at sequences complementary to a guide RNA sequence (6, 7). A second family of enzymes, CRISPR-Cas12a (Cpf1), uses a single RuvC catalytic domain for guide RNA-directed dsDNA cleavage (8–13) (Fig. 1A). Distinct from Cas9, Cas12a enzymes recognize a T-rich protospacer adjacent motif (PAM) (8), catalyze their own guide RNA (crRNA) maturation (14) and generate a PAM-distal dsDNA break with staggered 5’ and 3’ ends (8), features that have attracted interest for gene editing applications (15–17). However, the substrate specificity and DNA cleavage mechanism of Cas12a remain to be fully elucidated.
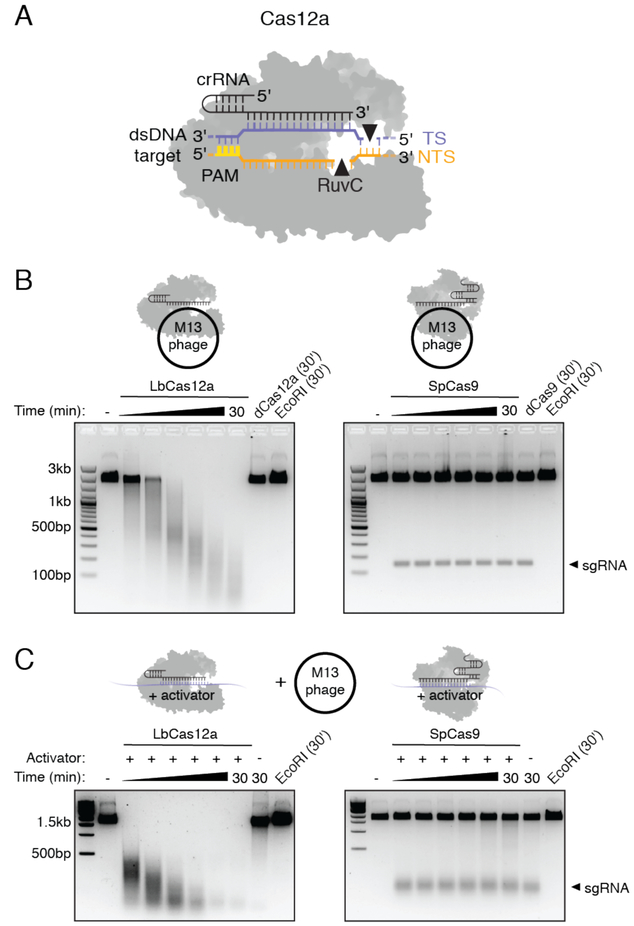
Fig. 1. Cas12a target recognition activates non-specific single-stranded DNA cleavage.
(A) Cas12a-crRNA complex binds a dsDNA substrate and generates a 5’ overhang staggered cut using a single RuvC nuclease. (B, C) Representative M13 ssDNA cleavage timecourses with purified LbCas12a (left) and SpCas9 (right) complexed with a (B) guide RNA complementary to M13 phage or (C) a guide RNA and complementary ssDNA activator with no sequence homology to M13 phage.
While investigating substrate requirements for Cas12a activation, we tested Lachnospiraceae bacterium ND2006 Cas12a (LbCas12a) for guide RNA-directed single-stranded DNA (ssDNA) cleavage, a capability of diverse CRISPR-Cas9 orthologs (18, 19). Purified LbCas12a or Streptococcus pyogenes Cas9 (SpCas9) proteins (fig. S1) were assembled with guide RNA sequences targeting a circular, single-stranded M13 DNA phage. In contrast to SpCas9, we were surprised to find that LbCas12a induced rapid and complete degradation of M13 by a cleavage mechanism that could not be explained by sequence-specific DNA cutting (Fig. 1B). This ssDNA shredding activity, not observed using an LbCas12a protein containing an inactivating mutation in the RuvC catalytic domain (D832A), raised the possibility that a target-bound LbCas12a could degrade any ssDNA, regardless of complementarity to the guide RNA. To test this idea, we assembled LbCas12a or SpCas9 with a different guide RNA and its complementary ssDNA that has no sequence homology to M13 phage genome sequence, and added single-stranded M13 DNA to the reaction. Remarkably, LbCas12a catalyzed M13 degradation only in the presence of this complementary ssDNA “activator”, an activity not observed for SpCas9 (Fig. 1C). These findings reveal that binding of the LbCas12a-crRNA complex to a guide-complementary ssDNA unleashes robust, non-specific ssDNA trans-cleavage activity.
We next investigated the requirements for LbCas12a-catalyzed trans-cleavage activity. Using a fluorophore quencher (FQ)-labeled reporter assay (20, 21), we assembled LbCas12a with its crRNA and either a complementary ssDNA, dsDNA or single-stranded RNA (ssRNA), and introduced an unrelated ssDNA- or ssRNA-FQ reporter in trans (fig. S2). Both the crRNA-complementary ssDNA or dsDNA (the activator) triggered LbCas12a to cleave the ssDNA-FQ reporter substrate (fig. S2A). However, ssRNA was neither capable of activating trans-cleavage nor susceptible to degradation by LbCas12a (fig. S2B), confirming that LbCas12a harbors a DNA-activated general DNase activity.
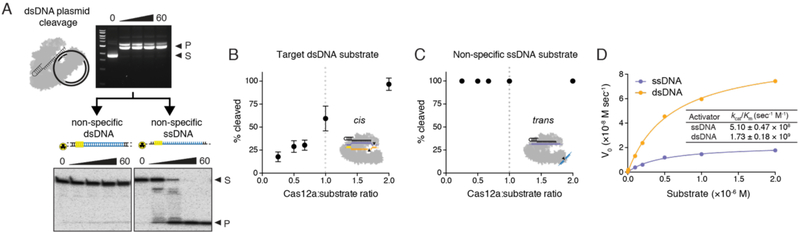
To determine how LbCas12a-catalyzed ssDNA cleavage activity relates to site-specific dsDNA cutting, we tested the length requirements of both the target strand (TS) and non-target strand (NTS) for LbCas12a activation using radiolabeled oligonucleotides. Although TS cutting occurred irrespective of the NTS length (fig. S3A), NTS cleavage occurred only when the TS contained at least 15 nucleotides (nt) of complementarity with the crRNA (fig. S3B). This showed that TS recognition is a prerequisite for NTS cutting. To test whether LbCas12a remains active for non-specific ssDNA cleavage after sequence-specific binding and cleavage of a dsDNA substrate, we first cut a dsDNA plasmid with an LbCas12a-crRNA complex, and then added an unrelated dsDNA or ssDNA to the reaction (Fig. 2A). Whereas the non-specific dsDNA substrate remained intact, the ssDNA was rapidly degraded in a RuvC-domain dependent manner (Fig. 2A; figs. S4, S5). Using truncated activators that are too short to be cleaved, we next determined that only target DNA binding is required to activate trans-ssDNA cleavage (fig. S6). Together, these results show that RNA-guided DNA binding activates LbCas12a for both site-specific dsDNA cutting and non-specific ssDNA trans-cleavage.
Fig. 2. Kinetics of Cas12a ssDNA trans-cleavage.
(A) Sequence-specific plasmid DNA cleavage reactions by LbCas12a-crRNA (top) were introduce to a separate radiolabeled dsDNA or ssDNA substrate of unrelated sequence (bottom); timecourses represent minutes. (B) Target dsDNA or (C) non-specific ssDNA incubated with molar ratios of LbCas12a-crRNA as indicated. Each point represents the mean quantified percent cleavage after 30 minutes at 37°C, at which time the reaction was at completion. Error bars represent the mean ± s.d., where n = 3 replicates. (D) Representative Michaelis-Menten plot for LbCas12a-catalyzed ssDNA trans-cleavage using a dsDNA or ssDNA activator. Measured kcat/Km values report mean ± s.d., where n = 3 replicates.
The rapid degradation of a trans substrate suggested that the kinetics of LbCas12a-catalyzed site-specific dsDNA (cis-) cleavage and non-specific ssDNA (trans-) cleavage are fundamentally different. Stoichiometric titration experiments showed that cis-cleavage is single-turnover (22) (Fig. 2B), whereas trans-cleavage is multiple-turnover (Fig. 2C). Although the Cas12a-crRNA complex remains bound to the dsDNA target following cis-cleavage, the complex releases its PAM-distal cleavage products from the RuvC active site (22), enabling ssDNA substrate access and turnover. Using the FQ assay, we found that LbCas12a-crRNA bound to a ssDNA activator molecule catalyzed trans-ssDNA cleavage at a rate of ~250 per second and a catalytic efficiency (kcat/Km) of 5.1×108 s−1 M−1. When bound to a dsDNA activator, LbCas12a-crRNA catalyzed ~1250 turnovers per second with a catalytic efficiency approaching the rate of diffusion (23) with a kcat/Km of 1.7×109 s−1 M−1 (Fig. 2D; fig. S7). These differences suggest that the NTS of the dsDNA activator helps stabilize the Cas12a complex in an optimal conformation for trans-ssDNA cutting.
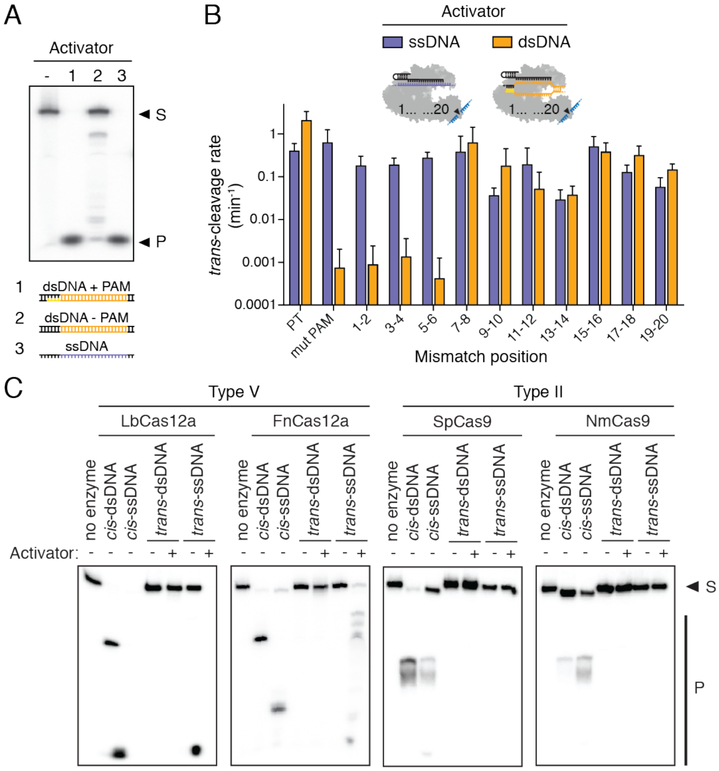
We next tested the specificity of trans-cleavage activation using either a ssDNA or dsDNA activator. We found that the PAM sequence required for dsDNA binding by CRISPRCas12a (22) is critical for catalytic activation by a crRNA-complementary dsDNA (9, 24), but not for a crRNA-complementary ssDNA (Fig. 3A). Two base-pair (bp) mismatches introduced along the crRNA-complementary sequence of either a ssDNA or dsDNA activator molecule slowed the trans-cleavage rate of a ssDNA-FQ reporter by up to ~100 fold, depending on the mismatch position. For only the dsDNA activator, alterations to the PAM sequence or mismatches between the crRNA and PAM-adjacent “seed region” also had large inhibitory effects on trans-ssDNA cleavage activity (Fig. 3B; fig. S8), similar to the mismatch tolerance pattern observed in Cas12a off-target studies (25–27). Together, these data are consistent with PAM-mediated dsDNA target binding and the role of base pairing between the crRNA and the target strand to activate trans-ssDNA cutting.
Fig. 3. Specificity and conservation of trans-cleavage activation.
(A) LbCas12a-crRNA in the absence or presence of indicated activator, incubated with a radiolabeled non-specific ssDNA substrate (S) for 30 min at 37°C; products (P) resolved by denaturing PAGE. (B) Observed trans-cleavage rates for LbCas12a using a ssDNA or dsDNA activator with indicated mismatches; rates represent the average of three different targets measured in triplicate, and error bars represent mean ± s.d., where n = 9 (three replicates for three independent targets). (C) Radiolabeled cis (complementary) or trans (non-complementary) substrates were incubated with Cas12a-crRNA or Cas9-sgRNA in the presence or absence of a ssDNA activator for 30 min at 37°C; a cis-dsDNA substrate was used in the “no enzyme” lanes. Substrate (S) and nucleotide products (P) were resolved by denaturing PAGE.
We wondered if this trans-ssDNA cutting activity might be a property shared by other Cas12a enzymes, and perhaps more evolutionarily distinct type V CRISPR effector proteins, considering that all type V effectors contain a single RuvC nuclease domain (3, 28). Consistent with this possibility, purified Cas12a orthologs from Acidaminococcus sp. (AsCas12a) and Francisella novicida (FnCas12a), as well as a Cas12b protein from Alicyclobacillus acidoterrestris (AaCas12b), all catalyzed non-specific ssDNase cleavage when assembled with crRNA and a complementary ssDNA activator (Fig. 3C; fig. S9). In contrast, none of the type II CRISPR-Cas9 proteins tested showed evidence for trans-ssDNA cleavage (Fig. 3C; fig. S9), suggesting that target-dependent activation of non-specific ssDNA cleavage is a fundamental feature of all type V CRISPR-Cas12 proteins. These results reveal the unexpected functional convergence of Cas12 enzymes with the type III CRISPR-Csm/Cmr and type VI CRISPR-Cas13 effectors, which also exhibit target-activated, non-specific ssDNase or ssRNase activity, respectively (29, 30).
We next explored whether LbCas12a could be repurposed as a DNA detection platform for use in clinical specimens, based on its ability to induce a fluorescent readout in response to a specific dsDNA sequence. In particular, accurate and rapid identification of human papillomavirus (HPV) is critical for identification of those at risk of HPV-related pre-cancer and cancer, with types 16 (HPV16) and 18 (HPV18) accounting for the majority of precancerous lesions (31). To test if LbCas12a-catalyzed trans-ssDNA cleavage can distinguish between these two dsDNA viruses, we selected a 20 nt target sequence located next to a TTTA PAM that varied by only six base pairs between the two HPV genotypes (fig. S10). Plasmids containing a ~500 bp fragment of the HPV16 or HPV18 genome, including the target sequence, were incubated with the LbCas12a-crRNA complex targeting either the HPV16 or HPV18 fragment and a quenched-fluorescent ssDNA reporter. After one hour, LbCas12a produced a robust fluorescent signal only in the presence of the cognate HPV target, whose identity could be distinguished down to ~10 pM of plasmid (fig. S10). To enhance assay sensitivity, we coupled isothermal amplification by Recombinase Polymerase Amplification (RPA) with LbCas12a to develop a rapid one-pot detection method termed DNA Endonuclease Targeted CRISPR Trans Reporter (DETECTR) (fig. S11A). When programmed to recognize its cognate plasmid, DETECTR was able to identify targets with attomolar sensitivity (fig. S11B).
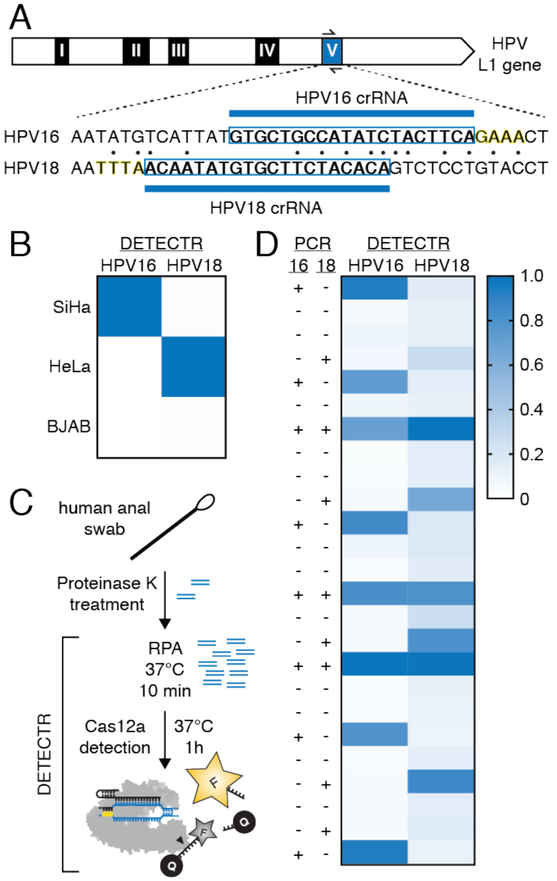
To assess whether we could detect HPV in more complex mixtures, DNA extracted from cultured human cells infected with HPV types 16 (SiHa), 18 (HeLa), or without HPV (BJAB) was added to LbCas12a complexed with a crRNA targeting the hypervariable loop V of the L1 gene within HPV16 or HPV18 (Fig. 4A) (32). Whereas LbCas12a-crRNA alone was not sensitive enough to detect HPV, DETECTR unambiguously identified HPV types 16 and 18 only in SiHa and HeLa cells, respectively (Fig. 4B; fig. S12A, B). To investigate the utility of DETECTR on patient samples, we tested crude DNA extractions from 25 human anal swabs previously analyzed by a PCR-based method for HPV infection (fig. S13) (33). Within one hour, DETECTR accurately identified the presence or absence of HPV16 (25/25 agreement) and HPV18 (23/25 agreement) in 25 patient samples containing a heterogeneous mixture of HPV types, with good correlation between the PCR-based intensity and DETECTR signal (Fig. 4C, D; figs. S12C, D; S13). Furthermore, the absence of fluorescence signal in specimens that were not infected with HPV types 16 or 18, but did contain other HPV types, was an indicator of good specificity by DETECTR. These results demonstrate a new platform for CRISPR-based diagnostics, and suggest that DETECTR could in principle be extended to rapidly detect any DNA sequence of interest with high sensitivity and specificity.
Fig. 4. Rapid identification of HPV types 16 and 18 in human samples by DETECTR.
(A) Diagram of HPV16 and HPV18 sequences within the hypervariable loop V of the L1 gene targeted by Cas12a; highlighted bases indicate 5’ PAM sequence. (B) Heatmap represents normalized mean fluorescence values of HPV types 16 and 18 detected in human cell lines by DETECTR; normalized scale represented in (D). (C) Schematic outlining DNA extraction from human anal samples to HPV identification by DETECTR. (D) Identification of HPV types 16 and 18 in 25 patient samples by PCR (left) and DETECTR (right); DETECTR heatmap represents normalized mean fluorescence values.
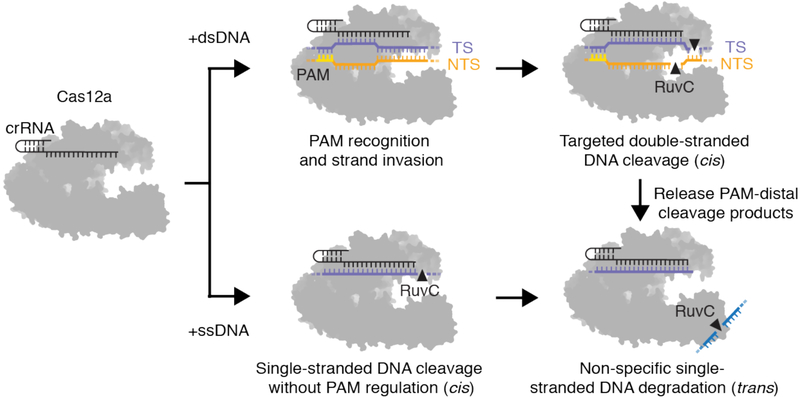
Together, these findings support a unifying mechanism of target interference that begins with the Cas12a-guide RNA complex binding to a complementary DNA sequence in a PAM-dependent (dsDNA) or PAM-independent (ssDNA) manner (Fig. 5). Within a host bacterium, such enzyme activation could provide simultaneous protection from both dsDNA and ssDNA phages, and could also target ssDNA sequences that arise temporarily during phage replication or transcription (34). In a genome-editing context, target-activated ssDNA cutting by Cas12a may be a rare event, but it has the potential to cleave transiently exposed ssDNA at replication forks (35), R-loops (36) and transcription bubbles (37), or ssDNA templates used for homology-directed repair (38). Finally, unleashing the ssDNase activity of Cas12 proteins offers a new strategy to improve the speed, sensitivity and specificity of nucleic acid detection for point-of-care diagnostic applications.
Fig. 5. Model for PAM-dependent and PAM-independent activation of cis and trans-cleavage by Cas12a.
The Cas12a-crRNA complex binds to a complementary dsDNA in a PAM-dependent manner (top) or ssDNA in a PAM-independent manner (bottom), which is sufficient to unleash indiscriminate ssDNase activity by the RuvC nuclease. Cas12a can also release its PAM-distal cleavage products, which exposes the RuvC active site for multiple rounds of non-specific ssDNA degradation.
Supplementary Material
Acknowledgments:
We thank O. Mavrothalassitis, D. Burstein, G. Knott, A. Wright, J. Cofsky, D. Lee and members of the Doudna laboratory for comments and discussions, and we thank the patients for their generosity. This research was supported in part by the Allen Distinguished Investigator Program, through The Paul G. Allen Frontiers Group, and the National Science Foundation (MCB-1244557 to J.A.D.). J.S.C. and L.B.H. are supported by National Science Foundation Graduate Research Fellowships. J.M.P is a Professor of Medicine at the University of California, San Francisco, and a scientific advisor to Antiva Biosciences, Agenovir and Merck and Co. J.A.D. is an Investigator of the Howard Hughes Medical Institute and executive director of the Innovative Genomics Institute at the University of California, Berkeley and the University of California, San Francisco. J.A.D. is a co-founder of Editas Medicine, Intellia Therapeutics, and Caribou Biosciences and a scientific adviser to Caribou, Intellia, eFFECTOR Therapeutics and Driver. The Regents of the University of California have patents pending for CRISPR technologies on which the authors are inventors.
References:
- 1.Barrangou R et al. , CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Marraffini LA, Sontheimer EJ, CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843–1845 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koonin EV, Makarova KS, Zhang F, Diversity, classification and evolution of CRISPR-Cas systems. Curr Opin Microbiol 37, 67–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doudna JA, Charpentier E, Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Barrangou R, Horvath P, A decade of discovery: CRISPR functions and applications. Nat Microbiol 2, 17092 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Jinek M et al. , A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JS, Doudna JA, The chemistry of Cas9 and its CRISPR colleagues. Nat Rev Chem 1, (2017). [Google Scholar]
- 8.Zetsche B et al. , Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759–771 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swarts DC, van der Oost J, Jinek M, Structural Basis for Guide RNA Processing and Seed-Dependent DNA Targeting by CRISPR-Cas12a. Mol Cell 66, 221–233 e224 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong D et al. , The crystal structure of Cpf1 in complex with CRISPR RNA. Nature 532, 522–526 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Gao P, Yang H, Rajashankar KR, Huang Z, Patel DJ, Type V CRISPR-Cas Cpf1 endonuclease employs a unique mechanism for crRNA-mediated target DNA recognition. Cell Res 26, 901–913 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stella S, Alcon P, Montoya G, Structure of the Cpf1 endonuclease R-loop complex after target DNA cleavage. Nature 546, 559–563 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Yamano T et al. , Crystal Structure of Cpf1 in Complex with Guide RNA and Target DNA. Cell 165, 949–962 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fonfara I, Richter H, Bratovic M, Le Rhun A, Charpentier E, The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 532, 517–521 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Zetsche B et al. , Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat Biotechnol 35, 31–34 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang X et al. , A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat Plants 3, 17018 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Tak YE et al. , Inducible and multiplex gene regulation using CRISPR-Cpf1-based transcription factors. Nat Methods, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Rajan R, Seifert HS, Mondragon A, Sontheimer EJ, DNase H Activity of Neisseria meningitidis Cas9. Mol Cell 60, 242–255 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma E, Harrington LB, O’Connell MR, Zhou K, Doudna JA, Single-Stranded DNA Cleavage by Divergent CRISPR-Cas9 Enzymes. Mol Cell 60, 398–407 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.East-Seletsky A et al. , Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 538, 270–273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gootenberg JS et al. , Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 356, 438–442 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh D et al. , Real-time observation of DNA target interrogation and product release by the RNA-guided endonuclease CRISPR Cpf1. bioRxiv, (2017). doi: 10.1101/205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alberty RA, Hammes GG, Application of the Theory of Diffusion-Controlled Reactions to Enzyme Kinetics. J Phys Chem-Us 62, 154–159 (1958). [Google Scholar]
- 24.Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA, DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature 507, 62–67 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D et al. , Genome-wide analysis reveals specificities of Cpf1 endonucleases in human cells. Nat Biotechnol 34, 863–868 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Kleinstiver BP et al. , Genome-wide specificities of CRISPR-Cas Cpf1 nucleases in human cells. Nat Biotechnol 34, 869–874 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HK et al. , In vivo high-throughput profiling of CRISPR-Cpf1 activity. Nat Methods 14, 153–159 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Shmakov S et al. , Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol 15, 169–182 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmore JR et al. , Bipartite recognition of target RNAs activates DNA cleavage by the Type III-B CRISPR-Cas system. Genes Dev 30, 447–459 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abudayyeh OO et al. , C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science 353, aaf5573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodman CB, Collins SI, Young LS, The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer 7, 11–22 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Seaman WT et al. , Detection and quantitation of HPV in genital and oral tissues and fluids by real time PCR. Virol J 7, 194 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palefsky JM, Holly EA, Ralston ML, Jay N, Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis 177, 361–367 (1998). [DOI] [PubMed] [Google Scholar]
- 34.Samai P et al. , Co-transcriptional DNA and RNA Cleavage during Type III CRISPR-Cas Immunity. Cell 161, 1164–1174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sogo JM, Lopes M, Foiani M, Fork reversal and ssDNA accumulation at stalled replication forks owing to checkpoint defects. Science 297, 599–602 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Bhatia V, Herrera-Moyano E, Aguilera A, Gomez-Gonzalez B, The Role of Replication-Associated Repair Factors on R-Loops. Genes-Basel 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y et al. , Structural basis of transcription initiation. Science 338, 1076–1080 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson CD, Ray GJ, DeWitt MA, Curie GL, Corn JE, Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nature Biotechnology 34, 339-+ (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.