Abstract
Background and Purpose:
The effect of leptomeningeal collaterals for acute ischemic stroke patients with large vessel occlusion in the late window (>6 hours from last known normal) remains unknown. We sought to determine if collateral status on baseline CT angiography (CTA) impacted neurologic outcome, ischemic core growth, and moderated the effect of endovascular thrombectomy (EVT) in the late window.
Methods:
This is a prespecified analysis of Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3). We included patients with CTA as their baseline imaging and rated collateral status using the validated scales described by Tan and Maas. The primary outcome is functional independence (modified Rankin scale ≤2). Additional outcomes include the full range of the modified Rankin scale, baseline ischemic core volume, change from baseline in the ischemic core volume at 24 hours, and death at 90 days.
Results:
Of the 130 patients in our cohort, 33 (25%) had poor collaterals and 97 (75%) had good collaterals. There was no difference in the rate of functional independence with good versus poor collaterals in unadjusted analysis (30% vs. 39%, p=0.3) or after adjustment for treatment arm [OR (95% CI), 0.61 (0.26–1.45)]. Good collaterals were associated with significantly smaller ischemic core volume and less ischemic core growth. The difference in the treatment effect of EVT was not significant (p=0.8). Collateral status also did not affect the rate of stroke-related death [n (%), good vs. poor collaterals, 18/97 (19%) vs. 8/33 (24%), p=0.5].
Conclusions:
In DEFUSE 3 patients, good leptomeningeal collaterals on single phase CTA were not predictive of functional independence or death, and did not impact the treatment effect of EVT. These unexpected findings require further study to confirm their validity and to better understand the role of collaterals for stroke patients with anterior circulation large vessel occlusion in the late therapeutic window.
Clinical Trial Registration Information:
Keywords: Stroke, large vessel occlusion, leptomeningeal collaterals, endovascular thrombectomy, ischemic stroke, revascularization, CT
Introduction
Collateral blood vessels can provide crucial blood flow for patients with large vessel occlusions.1,2 The main collateral pathways in the brain include Circle of Willis arteries and the leptomeningeal collaterals, which are anastomotic connections between middle, anterior, and posterior cerebral artery branches. Prior research has shown that acute ischemic stroke patients with good leptomeningeal collaterals have a superior response to intravenous thrombolysis, endovascular thrombectomy (EVT), smaller final ischemic core volume and improved neurologic outcome.3–5 Poor collaterals have been reported to predispose to hemorrhagic complications and death following EVT, although some studies have also shown that patients with poor collaterals can have a favorable outcome.6–8 Collaterals vary widely between patients for demographic, genetic and metabolic reasons.9–11
The majority of research on collaterals has focused on stroke patients receiving intravenous thrombolytics within 4.5 hours from last known well or undergoing EVT within 6 hours from last known well. To evaluate the importance of collaterals in the late therapeutic window after stroke (>6 hours from last known well) we performed a prespecified analysis of the Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke (DEFUSE 3) randomized controlled trial. DEFUSE 3 provided evidence that ischemic stroke patients with occlusion of the cervical or intracranial internal carotid artery or the proximal middle cerebral artery, and salvageable brain tissue on perfusion imaging, benefit from EVT as opposed to medical therapy alone, 6 to 16 hours after last known well.12 The National Institutes of Health funded DEFUSE 3 through the StrokeNet, which contributed 38 hospitals who enrolled 182 patients before the trial was stopped when an interim analysis showed that the efficacy boundary had been exceeded. Our hypothesis is that good collaterals on baseline single phase CT angiography (CTA) will correlate with better neurologic outcome and less ischemic core growth, and will beneficially moderate the effect of EVT in the late window. Although multiphase CTA has been shown to very accurately measure collateral status,13 the DEFUSE 3 cohort has single phase CTA, which has also been validated as a reliable modality for collateral measurement and is widely used clinically.
Methods
Patient Selection and Outcomes
DEFUSE 3 was approved by the StrokeNet central institutional review board and the Food and Drug Administration (FDA) for an investigational device exemption (IDE G150028). The data that support the findings of this study are available from the corresponding author upon reasonable request. Enrolled patients or their surrogates provided written informed consent. To standardize our cohort, we only included DEFUSE 3 patients who had a baseline CT angiogram (CTA). The primary outcome is functional independence (defined as modified Rankin scale ≤2) at day 90. The secondary outcome is the ordinal score on the modified Rankin scale [range, 0 (no symptoms) to 6 (death)] at day 90. We performed analyses of neuroimaging outcomes (growth of the ischemic core between the baseline and 24-hour follow-up imaging and absolute volume of the ischemic core on the 24-hour follow-up imaging). Further details regarding the blinded determination of the 90 day mRS and volumetric neuroimaging outcomes have been published.14 Additional outcomes include stroke-related death at 90 days and successful recanalization after EVT, defined as a centrally adjudicated postprocedural conventional angiography grade of 2b or 3 on the modified Thrombolysis in Cerebral Infarction scale. We evaluated the outcomes of symptomatic intracranial hemorrhage at 36 hours from symptom onset (defined as a ≥ 4 point worsening of immediate pre‐deterioration NIH stroke scale neurological status vs. post deterioration and associated with brain hemorrhage) and the incidence of early neurologic deterioration prior to discharge (defined as ≥ 4 point worsening of the immediate pre‐deterioration NIHSS neurological status vs. post deterioration and not attributed to sedation). We also stratified patients by the outcome of “reperfused/recanalized,” defined as a greater than 90% reduction in the region of perfusion delay (Tmax of >6 seconds) between baseline and 24 hours and/or complete recanalization on the 24-hour CT or MR angiogram.
Collateral Assessment
The main predictor of outcome was leptomeningeal collateral status determined on the baseline CTA. To ensure reliable contrast transit into collaterals, we only included patients with CTAs that had contrast opacification of the major dural venous sinuses. The collateral assessment was performed by experienced neuroradiologists (JH, MMa) in the DEFUSE 3 core laboratory, who were blinded to patient treatment arm and outcome. All disagreement on collateral rating was resolved by consensus. Collaterals were rated with the binary modified Tan collateral scale as previously described.15,16 On CTA axial maximum intensity projection images, “good collaterals” filled >50% of the vascular territory distal to the occluded MCA and “poor collaterals” filled ≤50%. (Supplementary Figure I) Although the predictive ability of a binary collateral scale is comparable to multicategory ordinal scales,17 for a confirmatory analysis we graded collaterals using the ordinal scale developed by Maas et al.,18 which is scored: 1, absent; 2, less than the contralateral normal side; 3, equal to the contralateral normal side; 4, greater than the contralateral normal side; and 5, exuberant. Based on cut points proposed by Maas et al., we also made the scale binary, with poor collaterals defined as Maas 1–2 and good collaterals as Maas 3–5. The secondary predictor of outcome was the treatment arm assignment in DEFUSE 3 (EVT versus medical).
Statistical Methods
We compared demographics, clinical variables, and neuroimaging data between patients with good and poor collaterals using the χ2 and Wilcoxon rank-sum tests. To test the association between collateral status and neurologic outcome in DEFUSE 3, we fit logistic regression models to the primary outcome and ordinal logistic regression models to the secondary outcome, with the primary predictor of collateral status. We fit two models, 1) adjusted for treatment arm in DEFUSE 3 and 2) fully adjusted for treatment arm and additional covariates that were independently associated with the primary outcome: patient age, NIH stroke scale and serum glucose at enrollment, and time from last known normal to randomization. For ordinal logistic regression models, we verified that the proportional-odds assumption was met (p>0.05). To test the hypothesis that patients with good collaterals would respond better to the treatment effect of late window EVT, we included an interaction term (treatment arm*collateral status) in the model, derived odds ratios for treatment effect by collateral status, and assessed if they were significantly different. We defined an α value of less than 0.05 as statistical significance and report two-sided results. Statistical analysis was done using SAS 9.4.
Results
For the 182 patients enrolled in DEFUSE 3, 133 (73%) had a baseline CTA of which 130 (71%) were included in our final cohort. Three CTAs (2%) were excluded secondary to inadequate opacification of the dural venous sinuses. Of the 130 patients in our final cohort, 97 (75%) had good collaterals and 33 (25%) had poor collaterals on the binary Tan scale. The hypoperfusion intensity ratio (HIR) on CT perfusion has been previously shown to correlate well with collateral status on conventional angiography.19 The HIR in our cohort was significantly lower in patients with good collaterals, which is expected and provides validation of the collateral grading [median (IQR) HIR, good vs. poor collaterals, 0.28 (0.15–0.49) vs. 0.44 (0.26–0.58), p<0.001]. On the binary Maas scale, 61 patients (47%) had good collaterals and 70 (53%) had poor collaterals. The demographics of the entire cohort are shown in Table 1 with stratification by good versus poor collaterals on the Tan scale (see Supplementary Table I for stratification by binary Maas scale). In patients with poor collaterals, there was a significantly higher proportion of Hispanic ethnicity (21% versus 4% for patients with good collaterals, p = 0.006). Additional baseline clinical characteristics were not predictive of collateral status (Table 1).
Table 1:
Demographics of entire cohort, patients with good and poor collaterals and p-value for difference between Tan collateral status arms.
| Entire cohort (n=130) | Good collaterals (n=97) | Poor collaterals (n=33) | p-value | |
|---|---|---|---|---|
| Age | 71 (60–80) | 71 (59–78) | 73 (60–82) | 0.425 |
| Female | 63 (49) | 51 (53) | 12 (36) | 0.107 |
| Ethnicity Hispanic | 11 (9) | 4 (4) | 7 (21) | 0.006 |
| Race White | 111 (85) | 83 (86) | 28 (85) | 1.0 |
| Hypertension | 104 (80) | 78 (80) | 26 (79) | 0.840 |
| Hyperlipidemia | 63 (48) | 45 (46) | 18 (55) | 0.418 |
| Atrial fibrillation | 49 (38) | 38 (39) | 11 (33) | 0.550 |
| Diabetes | 38 (29) | 32 (33) | 6 (18) | 0.095 |
| Prior CVA | 21 (16) | 15 (15) | 6 (18) | 0.714 |
| Presentation NIHSS | 16 (12–21) | 16 (12–20) | 18 (11–21) | 0.592 |
| Treated with tPA | 8 (6) | 5 (5) | 3 (9) | 0.679 |
| MCA occlusion | 82 (63) | 58 (60) | 24 (73) | 0.184 |
| Ischemic core (mL) | 9.8 (1.7–23.4) | 7.8 (0–17.3) | 21.7 (6.1–42.9) | 0.001 |
| Perfusion lesion (mL) | 117.1 (76.3–162.3) | 110.5 (65.5–157.0) | 138.4 (108.1–181.5) | 0.008 |
| Mismatch volume (mL) | 104.8 (62.7–138.8) | 90.9 (60.0–139.5) | 108.1 (87.4–136.4) | 0.164 |
| Hypoperfusion intensity ratio | 0.35 (0.21–0.53) | 0.28 (0.15–0.49) | 0.44 (0.26–0.58) | <0.001 |
| Time from last known well to baseline imaging | 10:15 (8:20–11:47) | 10:16 (8:12–11:47) | 9:46 (8:32–11:36) | 0.900 |
| Time from last known well to femoral puncture | 11:35 (9:47–12:59) | 11:28 (9:37–13:03) | 11:48 (10:22–12:53) | 0.610 |
| Time from last known well to reperfusion | 12:34 (17:57–13:42) | 12:23 (9:48–13:42) | 12:39 (11:30–13:41) | 0.465 |
| ASPECTS | 8 (7–9) | 8 (7–9) | 8 (7–9) | 0.975 |
| Right hemisphere stroke | 60 (46) | 45 (46) | 15 (45) | 0.926 |
| Baseline SBP | 146 (134–161) | 147 (135–160) | 143 (128–160) | 0.487 |
| Creatinine | 0.9 (0.78–1.11) | 0.9 (0.78–1.1) | 0.9 (0.78–1.2) | 0.395 |
| Glucose | 124 (108–152) | 125 (108–151) | 124 (109–155) | 0.634 |
| WBC | 8.9 (6.9–10.9) | 9.2 (6.98–11.0) | 7.9 (6.9–10.4) | 0.394 |
| Platelets | 231 (170–262) | 215 (168–265) | 209 (174–240) | 0.385 |
| Hematocrit | 40.8 (36.9–43.3) | 40.4 (36.2–43.2) | 41 (39.7–44) | 0.148 |
| INR | 1.04 (1.0–1.11) | 1.0 (1.0–1.1) | 1.1 (1.0–1.2) | 0.051 |
Notes:
1) Ethnicity: N=96 & 33; Perfusion lesion: N=96 & 33; Time from stroke onset to femoral puncture: N=49 &16; Time from stroke onset to reperfusion: N=45 & 14; ASPECTS: N=86 &31; HIR: N=96 & 33.
2) Ischemic core = relative cerebral blood flow <30%; perfusion lesion = Tmax <6 seconds; mismatch volume = perfusion lesion-ischemic core.
3) Continuous variables: median (IQR); categorical data: n(%).
The baseline Alberta Stroke Program Early CT Score (ASPECTS) did not differ between the collateral groups [median (IQR), good vs. poor collaterals, 8 (7–9) vs. 8 (7–9), p=0.975] and there was no difference in the time from last known normal to baseline imaging (Table 1). In patients randomized to EVT (n=65), collateral status did not determine successful recanalization [n (%), mTICI 2b/3, good vs. poor collaterals, 35/49 (71%) vs. 14/16 (88%), p=0.318]. In the whole cohort, there was no difference in the rates of recanalization, reperfusion, or symptomatic intracerebral hemorrhage between patients with good versus poor collaterals (Table 2, Supplementary Table II). On the baseline neuroimaging, patients with good collaterals had smaller core ischemic regions, but also smaller perfusion lesion volumes (Table 1, Supplementary Figure II). As a result, the baseline mismatch volume was not different. Patients with good collaterals had less growth of the ischemic core between the baseline and 24-hour follow-up imaging, [median (IQR), good vs. poor collaterals, 26.9mL (13.1–54.5) vs. 43.1mL (13.6–131.3), p=0.031] and a smaller absolute ischemic core volume at 24 hours (Table 2). After dividing the cohort by reperfusion/recanalization, it was evident that patients with poor collaterals have a significantly larger ischemic core volume and growth on the 24-hour follow-up imaging if not reperfused/recanalized (Figure 1, Supplementary Figure III).
Table 2:
Selected outcomes in DEFUSE 3 for the entire cohort, patients with good and poor collaterals and p-value for difference between Tan collateral status arms.
| Entire cohort (n=130) | Good collaterals (n=97) | Poor collaterals (n=33) | p-value | |
|---|---|---|---|---|
| Early neurologic deterioration | 13 (10) | 12 (12) | 1 (3) | 0.182 |
| Ischemic core volume (mL) at 24 hours | 39.3 (23.9–107.1) | 32.9 (17.5–68.7) | 65.7 (36.2–164.5) | 0.002 |
| Ischemic core growth (mL) from baseline to 24 hours | 29.7 (13.2–74.9) | 26.9 (13.1–54.5) | 43.1 (13.6–131.3) | 0.031 |
| Perfusion lesion (mL) at 24 hours | 6.8 (0–63.5) | 4.4 (0–47.4) | 29.8 (0–76.8) | 0.071 |
| Mismatch volume (mL) at 24 hours | −26.2 (−59.3 - −2.5) | −26.4 (−54.6 - −4.6) | −36.2 (−126.8 - −0.7) | 0.139 |
| Endovascular therapy randomization | 65 (50) | 49 (51) | 16 (48) | 0.840 |
| Reperfusion >90%* | 47 (48) | 38 (51) | 9 (39) | 0.333 |
| Complete recanalization on CTA/MRA | 56 (50) | 44 (52) | 12 (43) | 0.414 |
| Reperfusion and/or recanalization | 54 (47) | 42 (48) | 12 (41) | 0.519 |
| Symptomatic ICH | 6 (5) | 5 (5) | 1 (3) | 0.693 |
| Parenchymal hematoma 2 (ECASS II) | 8 (6) | 6 (6) | 2 (6) | 1.0 |
| mRS 0–2 at 90 days | 42 (32) | 29 (30) | 13 (39) | 0.314 |
| Stroke-related death at 90 days | 26 (20) | 18 (19) | 8 (24) | 0.481 |
Notes:
1) Ischemic core volume at 24 hours: N=96 & 33; Ischemic core growth: N=96 & 33; reperfusion: N=75 & 23; recanalization: N=85 & 28; reperfusion and/or recanalization: N=87 & 29.
2) Continuous variables: median (IQR); categorical data: n(%).
Figure 1.
(A) Box and whisker plot showing absolute ischemic core volume (mL) on 24-hour follow-up imaging, with patients stratified by poor versus good collaterals and further divided by reperfused/recanalized (red, N=54) and not reperfused/recanalized (blue, n=62). Collateral status was associated with a significant difference in ischemic core volume for the “not reperfused/recanalized” patients (p=0.003), but not in reperfused/recanalized patients (p=0.423). (B) Box and whisker plot showing ischemic core growth (mL) between the baseline and 24-hour follow-up imaging in the same cohort. Collateral status was associated with a significant difference in ischemic core growth for the “not reperfused/recanalized” patients (p=0.014), but not in reperfused/recanalized patients (p=0.827).
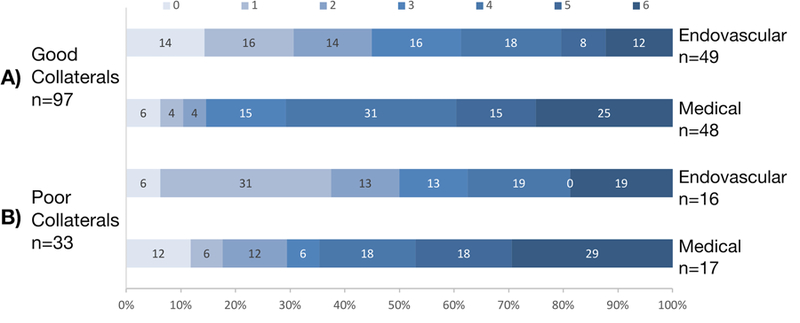
Despite the expected imaging findings based on collateral status, there was no association between collateral status and neurologic outcome (Figure 2). There was no difference in the number of patients who achieved functional independence [n (%), good vs. poor collaterals, 29/97 (30%) vs. 13/33 (39%), p=0.314]. After adjustment for treatment arm, collateral status still did not predict functional independence [OR (95% CI), 0.61 (0.26–1.45)]. The difference in the treatment effect of EVT between patients with good versus poor collaterals was not significant (p=0.845 for difference between odds ratios). When we ran the same analysis with the binary Maas scale, there was no difference in rate of functional independence (Supplementary Table II) or the EVT treatment effect between good versus poor collaterals (p=0.528).
Figure 2.

Modified Rankin scale values at 90 day follow-up. (A) Ordinal modified Rankin scale shift in patients with good collaterals (endovascular versus medical arm). (B) Ordinal modified Rankin scale shift in patients with poor collaterals (endovascular versus medical arm). There was not a significant difference in the treatment effect of endovascular versus medical therapy in patients with good versus poor collaterals (p=0.437). In fact, the Grotta bars for the poor collateral patients show an increase in the percentage of patients with a modified Rankin scale of 0–2, but this was not statistically significant.
In the ordinal logistic regression fit to the individual values of the 90-day modified Rankin scale and adjusted for treatment arm, good collaterals were not predictive of modified Rankin scale [OR (95% CI), 1.05 (0.52–2.11)]. In the fully adjusted model, collaterals remained unassociated with neurologic outcome (Table 3). Identical results were seen with the binary Maas scale, both when adjusted for treatment arm and in the fully adjusted model. Finally, there was no difference in the rate of early neurologic deterioration [n (%), good vs. poor collaterals, 12/97 (12%) vs. 1/33 (3%), p=0.182] or stroke-related death [n (%), good vs. poor collaterals, 18/97 (19%) vs. 8/33 (24%), p=0.481].
Table 3.
Odds of a better value on the ordinal modified Rankin scale for DEFUSE 3 patients with CT angiogram as the baseline imaging (n=130). The confidence interval for collateral status spans 1 (0.66–2.80), showing it is not a significant predictor of neurologic outcome.
| OR* | 95% CI | p value | |
|---|---|---|---|
| EVT versus medical treatment arm | 3.89 | 2.00–7.56 | <0.001 |
| Patient age (years) | 0.94 | 0.92–0.97 | <0.001 |
| NIH stroke scale | 0.85 | 0.80–0.90 | <0.001 |
| Serum glucose (mg/dL) | 0.99 | 0.99–1.00 | 0.017 |
| Time to randomization (minutes) | 0.92 | 0.81–1.05 | 0.220 |
| Poor versus good collaterals | 1.36 | 0.66–2.80 | 0.405 |
ORs are for fully adjusted model that includes all listed variables.
Discussion
We did not find that collaterals had the expected effect on acute ischemic stroke patients with anterior circulation large vessel occlusion and Target Mismatch in the late therapeutic window. For example, we failed to find an association between collaterals and several baseline variables that have previously been associated with collateral status, including patient age, gender, pre-morbid hypertension, baseline systolic blood pressure, creatinine, time from stroke onset to baseline imaging, NIH stroke scale, or ASPECTS,.9,11,20 These baseline variables were correlated with collateral status prior to any study procedures, reducing the role of possible confounding. We did find that patients with Hispanic ethnicity were more likely to have poor collaterals, which has not been reported in prior studies and requires validation in a larger cohort. We also did not find that collaterals influenced the rate of early neurologic deterioration, successful recanalization after EVT, functional independence at 90 days, death, or symptomatic intracranial hemorrhage. Our findings are contrary to prior studies that showed good collaterals were associated with better neurologic and radiographic outcomes for acute ischemic stroke patients in earlier time windows.7
The central paradox of this study is that good collateral status did not affect neurologic outcome but did reduce the size and growth of the ischemic core, a reliable biomarker of neurologic outcome.21 There are several possible explanations. The collateral circulation ultimately fails in the majority of stroke patients with large vessel occlusion who are not recanalized.22 The ischemic core of the DEFUSE 3 patients with good collaterals, particularly those who were not recanalized, may have continued to grow after the 24-hour follow-up imaging and, ultimately, be comparable to patients with poor collaterals.23 An additional imaging study at 72 or 96 hours after randomization would help answer this question and also provide valuable information on collateral evolution over time, which is dependent on dynamic factors, such as intravascular volume, adrenergic tone, blood pressure, brain edema, core temperature, and medications being administered.20 Until we better understand collateral evolution, we will not fully appreciate their effect on neurologic outcome or comprehend how to therapeutically modify that effect.
Another possibility is that we were unable to accurately measure collaterals. There is a low percentage of DEFUSE 3 patients with poor collaterals on the Tan scale (33/130, 25%), compared to 45% with poor collaterals in a recent meta-analysis of 2,004 acute ischemic stroke patients who had EVT in the standard time window.7 This was expected because of the inclusion criteria of a Target Mismatch. When using the binary Maas scale, we found a higher percentage with poor collaterals (70/130, 53%), but only 2 of those 70 patients were in category 1 (absent collaterals), which is lower than previous studies in the 6 hour window.24 However, the unexpected presence of patients with poor collaterals and a Target Mismatch in the late window introduces the possibility that we were not able to fully visualize their collaterals. On CTA we infer collaterals from retrograde blood flow into the distal branches of the MCA, but cannot visualize the more numerous pial arteries and parenchymal arterioles that allow retrograde blood flow.25,26 Some patients in our cohort may have poor collaterals on single phase CTA while still perfusing through pial arteries and parenchymal arterioles. Prior studies have shown that time-resolved multiphase CTA or tissue-specific measures of blood flow derived from perfusion imaging, such as the HIR, can be independent predictors of clinical outcome because they portray a more comprehensive picture of collateral flow.19 While we did find that the HIR in our cohort showed the expected difference between patients with good versus poor collaterals, it is possible that with multiphase CTA we would have found delayed filling of collaterals in patients who appeared to have poor collaterals on single phase CTA.
Our study has additional limitations. Although this is a prespecified analysis, it is a selected cohort of patients from a clinical trial, which could create bias. To standardize our collateral assessment, we introduced a selection bias by only including patients with CTA as their baseline imaging, although that was >70% of patients enrolled in DEFUSE 3. Additional neuroimaging limitations include that we did not have full control of the CTA scan parameters, collateral assessment is inherently subjective, and we do not have more sensitive measures of collateral status, such as multiphase CTA or catheter angiography, for all patients in the study.13,17,27 A separate analysis of the CT perfusion source data will examine the role of collaterals visualized during the venous phase of image acquisition, while we focused on single phase CTA due to its widespread use in research and clinical care. The small subgroup of patients with poor collaterals may have reduced the precision of our point estimates. We do not have data on patients who failed to qualify for DEFUSE 3, which would be an important comparison group to understand the clinical spectrum of collateral status in the late window. DEFUSE 3 also had a large number of wake-up strokes, which is expected in the late window because awake patients with large vessel occlusion are typically treated early. However, the wake-up strokes may have presented earlier from true stroke onset than 6 hours. Despite these limitations, the strengths of our study are notable and include its unique patient population, rigorous adjudication of neurologic outcomes, use of two previously validated collateral scales with distinct methodology, high-quality neuroimaging, and serial measurements of ischemic core and ischemic penumbra with validated postprocessing software.
Conclusion
In DEFUSE 3, good collaterals on single phase CTA were associated with smaller ischemic core volume at baseline and reduced ischemic core growth, but not with improved neurologic outcome, success of endovascular therapy, hemorrhagic complications, or death. We also did not find an association between collaterals and many of the traditional demographic predictors of baseline collateral status. These unexpected findings require further study to confirm their validity and to better understand the role of collaterals for anterior circulation large vessel occlusion stroke patients in the late therapeutic window.
Supplementary Material
Acknowledgements:
The authors would like to thank the DEFUSE 3 Primary Investigators and the National Institute of Neurological Disorders and Stroke.
Sources of Funding: DEFUSE 3 was funded by the NIH-National Institute of Neurological Disorders and Stroke U01NS092076.
Footnotes
Disclosures: Dr. Kim-Tenser, Speaker’s Bureau for Chiesi. Dr. Albers, equity in and consultant fees from IschemaView. Dr. Broderick, steering committee fees for trials supported by Genentech. Dr. Christensen, equity in and consultant fees from IschemaView. The remaining authors have no disclosures.
Contributor Information
Adam de Havenon, Department of Neurology, University of Utah, 175 N. Medical Dr.Salt Lake City, UT 84132he the.
Michael Mlynash, Department of Neurology, Stanford University.
May A. Kim-Tenser, Department of Neurology, University of Southern California.
Maarten Lansberg, Department of Neurology, Stanford University.
Thalabe Leslie-Mazwi, Department of Neurology, Massachusetts General Hospital.
Soren Christensen, Department of Neurology, Stanford University.
Ryan McTaggart, Department of Radiology, Brown Medical School.
Matthew Alexander, Department of Radiology ,University of Utah.
Greg Albers, Department of Neurology, Stanford University.
Joseph Broderick, Department of Neurology, University of Cincinnati.
Michael P. Marks, Department of Radiology, Stanford University.
Jeremy J. Heit, Department of Radiology, Stanford University.
References
- 1.Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin. N. Am 2005;15:553–573, x. [DOI] [PubMed] [Google Scholar]
- 2.Brozici M, Zwan A van der, Hillen B. Anatomy and Functionality of Leptomeningeal Anastomoses: A Review. Stroke 2003;34:2750–2762. [DOI] [PubMed] [Google Scholar]
- 3.Martinon E, Lefevre PH, Thouant P, Osseby GV, Ricolfi F, Chavent A. Collateral circulation in acute stroke: Assessing methods and impact: A literature review. Journal of Neuroradiology 2014;41:97–107. [DOI] [PubMed] [Google Scholar]
- 4.Marks MP, Lansberg MG, Mlynash M, Olivot J-M, Straka M, Kemp S, et al. Effect of collateral blood flow on patients undergoing endovascular therapy for acute ischemic stroke. Stroke 2014;45:1035–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang OY, Saver JL, Buck BH, Alger JR, Starkman S, Ovbiagele B, et al. Impact of collateral flow on tissue fate in acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2008;79:625–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao Y, Yang D, Wang H, Zi W, Zhang M, Geng Y, et al. Predictors for Symptomatic Intracranial Hemorrhage After Endovascular Treatment of Acute Ischemic Stroke. Stroke 2017;48:1203–1209. [DOI] [PubMed] [Google Scholar]
- 7.Leng X, Fang H, Leung TWH, Mao C, Miao Z, Liu L, et al. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2016;87:537–544. [DOI] [PubMed] [Google Scholar]
- 8.Hwang Y-H, Kang D-H, Kim Y-W, Kim Y-S, Park S-P, Liebeskind DS. Impact of Time-to-Reperfusion on Outcome in Patients with Poor Collaterals. American Journal of Neuroradiology 2015;36:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol 2013;74:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab 2010;30:923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik N, Hou Q, Vagal A, Patrie J, Xin W, Michel P, et al. Demographic and Clinical Predictors of Leptomeningeal Collaterals in Stroke Patients. Journal of Stroke and Cerebrovascular Diseases 2014;23:2018–2022. [DOI] [PubMed] [Google Scholar]
- 12.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menon BK, d’Esterre CD, Qazi EM, Almekhlafi M, Hahn L, Demchuk AM, et al. Multiphase CT Angiography: A New Tool for the Imaging Triage of Patients with Acute Ischemic Stroke. Radiology 2015;275:510–520. [DOI] [PubMed] [Google Scholar]
- 14.Albers GW, Lansberg MG, Kemp S, Tsai JP, Lavori P, Christensen S, et al. A multicenter randomized controlled trial of endovascular therapy following imaging evaluation for ischemic stroke (DEFUSE 3). Int J Stroke 2017;12:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan IYL, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT Angiography Clot Burden Score and Collateral Score: Correlation with Clinical and Radiologic Outcomes in Acute Middle Cerebral Artery Infarct. American Journal of Neuroradiology 2009;30:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo LLL, Paliwal P, Teoh HL, Seet RC, Chan BP, Ting E, et al. Assessment of Intracranial Collaterals on CT Angiography in Anterior Circulation Acute Ischemic Stroke. American Journal of Neuroradiology 2015;36:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McVerry F, Liebeskind DS, Muir KW. Systematic review of methods for assessing leptomeningeal collateral flow. AJNR Am J Neuroradiol 2012;33:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral Vessels on CT Angiography Predict Outcome in Acute Ischemic. Stroke 2009;40:3001–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivot JM, Mlynash M, Inoue M, Marks MP, Wheeler HM, Kemp S, et al. Hypoperfusion intensity ratio predicts infarct progression and functional outcome in the DEFUSE 2 Cohort. Stroke 2014;45:1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocha M, Jovin TG. Fast Versus Slow Progressors of Infarct Growth in Large Vessel Occlusion Stroke: Clinical and Research Implications. Stroke 2017;48:2621–2627. [DOI] [PubMed] [Google Scholar]
- 21.Barber PA, Darby DG, Desmond PM, Yang Q, Gerraty RP, Jolley D, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology 1998;51:418–426. [DOI] [PubMed] [Google Scholar]
- 22.Lansberg MG, O’Brien MW, Tong DC, Moseley ME, Albers GW. Evolution of cerebral infarct volume assessed by diffusion-weighted magnetic resonance imaging. Arch. Neurol 2001;58:613–617. [DOI] [PubMed] [Google Scholar]
- 23.Albers GW. Late Window Paradox. Stroke 2018;STROKEAHA.117.020200. [DOI] [PubMed] [Google Scholar]
- 24.Menon BK, Qazi E, Nambiar V, Foster LD, Yeatts SD, Liebeskind D, et al. Differential effect of baseline CTA Collaterals on Clinical Outcome in patients enrolled in the IMS-III trial. Stroke 2015;46:1239–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cipolla MJ, Sweet JG, Chan S-L. Effect of hypertension and peroxynitrite decomposition with FeTMPyP on CBF and stroke outcome. J Cereb Blood Flow Metab 2017;37:1276–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan SL, Sweet JG, Bishop N, Cipolla MJ. Pial Collateral Reactivity During Hypertension and Aging: Understanding the Function of Collaterals for Stroke Therapy., Pial Collateral Reactivity During Hypertension and Aging: Understanding the Function of Collaterals for Stroke Therapy. Stroke 2016;47, 47:1618, 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Chen W, Tang H, Han Q, Yan S, Zhang X, et al. The Prognostic Value of a Four-Dimensional CT Angiography-Based Collateral Grading Scale for Reperfusion Therapy in Acute Ischemic Stroke Patients. PLoS ONE 2016;11:e0160502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


