Abstract
Introduction:
Type 1 diabetes (T1D) is characterized by autoimmune-induced dysfunction and destruction of the pancreatic beta cells. Unfortunately, this process is poorly understood, and the current best treatment for type 1 diabetes is administration of exogenous insulin. To better understand these mechanisms and to develop new therapies, there is an urgent need for biomarkers that can reliably predict disease stage.
Areas covered:
Mass spectrometry (MS)-based proteomics and complementary techniques play an important role in understanding the autoimmune response, inflammation and beta-cell death. MS is also a leading technology for the identification of biomarkers. This, and the technical difficulties and new technologies that provide opportunities to characterize small amounts of sample in great depth and to analyze large sample cohorts will be discussed in this review.
Expert opinion:
Understanding disease mechanisms and the discovery of disease-associated biomarkers are highly interconnected goals. Ideal biomarkers would be molecules specific to the different stages of the disease process that are released from beta cells to the bloodstream. However, such molecules are likely present in trace amounts in the blood due to the small number of pancreatic beta cells in the human body and the heterogeneity of the target organ and disease process.
Keywords: proteomics, liquid chromatography-mass spectrometry, pancreatic islets, beta cells, ion mobility spectrometry, type 1 diabetes, nanoPOTS, post-translational modifications, neoantigens
1.0. Introduction
Type 1 diabetes (T1D) is caused by immune-mediated dysfunction and destruction of insulin-producing β cells, resulting in chronic hyperglycemia and the lifelong need for exogenous insulin therapy. Histological analysis of donor pancreases from T1D patients, together with measurements of serum C-peptide in clinical cohorts, suggests that many patients with clinical T1D still have a sub-population of surviving but non-functional β cells [1]. T1D develops from a complex “dialogue” that is established between invading immune cells, which release a variety of chemokines and cytokines, and putative immunogenic signals released by injured or dying β cells [2]. This dialogue is shaped by a number of factors, including host genetic architecture, key clinical/demographic features such as age and the “exposome” [3], which includes viral infections, dietary components, environmental exposures, and others [4]. In susceptible individuals, the integration of these factors leads to triggering of an autoimmune assault against the pancreatic β cells that provokes local inflammation (insulitis) and progressive β-cell loss mostly due to apoptosis [2,4,5].
The prevalence of T1D is doubling every 25 years in children in many places around the globe [6,7], and persons affected by the disease lose around 12 years of life expectancy [8,9]. The Centers for Disease Control and Prevention (CDC) reports that T1D is the third most prevalent chronic disease of childhood in the USA, behind asthma and obesity (CDC, 2018). For the purposes of clinical trials and immune intervention, a staging classification system has been introduced that defines Stage 1 as a state of normoglycemia in the presence of β-cell autoimmunity (two or more autoantibodies), Stage 2 as a state of dysglycemia (but not overt diabetes) in the presence of β-cell autoimmunity, and Stage 3 as overt, symptomatic T1D [10]. Most of the past clinical trials have been initiated at the onset of Stage 3 T1D, which is a time associated with substantial loss of functional β-cell mass. To date, 6 different drugs have shown efficacy in delaying the loss of C-peptide secretion, when administered at Stage 3 onset. These include: 1) a monoclonal antibody against the B cell CD20 receptor (rituximab); 2) a monoclonal antibody against the T cell CD3 receptor (teplizumab); 3) CTLA4-lg-mediated co-stimulatory blockade with abatacept, 4) alefacept, which is a fusion protein that binds CD2 and targets CD4+ and CD8+ effector memory T cells [11,12], 5) imatinib, a tyrosine kinase inhibitor; and 6) low-dose anti-thymocyte globulin (ATG), which is a polyclonal IgG against T cells [13]. While these trials met their primary endpoint, defined as an improvement in insulin secretion in response to a nutrient challenge, the vast majority of treated individuals still required insulin therapy. In part, this could be because drugs were initiated too late in the disease process. Indeed, a recently completed trial of teplizumab in Stage 2 disease supported this concept and showed that teplizumab treatment was able to delay the onset of Stage 3 disease on average by 2 years [14].
Despite promising results of these clinical trials, no drug has yet been FDA-approved as a disease-modifying therapy for T1D. This gap in translating trial results into clinical practice is multifactorial and includes: 1) a continued lack of understanding of the heterogeneous molecular mechanisms that trigger and drive the autoimmune destruction of human β cells and 2) a lack of sufficiently accurate genetic or molecular biomarkers of disease progression that could enable the introduction of immunomodulatory therapies at a time prior to the irreversible damage of β cells and that can serve as “sentinels” to the fate of these β cells during the course of these interventions. In this context, mass spectrometry (MS)-based proteomics is playing a central role in elucidating pathogenic mechanisms of autoimmune destruction of β cells and in identifying new biomarkers of the disease. This review will focus on MS-based, bottom-up proteomics workflows, which typically consist of several steps, including protein extraction, enzymatic digestion, separations (e.g. strong cation exchange (SCX) or reversed-phase (RP) chromatography) at protein or peptide levels, liquid chromatography–tandem mass spectrometry (LC-MS/MS) analyses, and bioinformatics data analysis and interpretation. Such approaches have enabled deep profiling of proteomes (e.g., quantification of > 10,000 proteins) with great dynamic range [15]. Metabolic labeling strategies such as stable isotope labeling by amino acids in cell culture (SILAC) [16] or isobaric labeling methods such as tandem mass tags (TMT) [17] can be incorporated into this workflow to facilitate quantitative analysis. Besides isobaric labeling, label-free approaches have also been broadly utilized in MS-based proteomics [18]. A summary of the key techniques used in the field is listed in Table 1.
Table 1.
Key proteomics techniques used for studying type 1 diabetes.
| Technique | Brief description | Applications | References |
|---|---|---|---|
| Global shotgun proteomics | In global shotgun proteomics, samples are digested with proteases, most often trypsin, and the resulting peptides are analyzed by one- or two-dimensional LC-MS/MS. Peptides are identified through database search algorithms and the proteins are then inferred. | This technique is used to determine the global landscape of expressed proteins. It can be used to obtain semi-quantitative information of expressed proteins, peptides and post-translational modifications. | [15] |
| Isotopic labeling | Proteins and peptides can be labeled with amino acids or chemical groups containing stable, heavy isotopes. This labeling can be done in cell culture (SILAC – stable isotope labeling of amino acids in cell culture), in vivo or during sample processing. | The incorporation of heavy isotopes provides a mass shift, without altering other physical-chemical properties of peptides. Therefore, heavy labeled peptides have the same ionization efficiency and signal in the mass spectrometer, allowing samples to be multiplexed for quantitative proteomic analysis. | [16,133] |
| Isobaric chemical labeling | In isobaric chemical labeling, proteins or peptides are derivatized with chemical reagents (tandem mass tags – TMT, or isobaric tags for relative and absolute quantitation – iTRAQ) which incorporate a combination of heavy isotopes that provide the same intact mass. Upon tandem mass fragmentation of the labeled peptides, reporter ions of different masses are generated and facilitate peptide quantification. | Isobaric labeling is used for quantitative analysis. Commercially available kits allow to label and multiplex up to 11 samples into single analysis. | [17] |
| Targeted proteomics | In targeted proteomics, specific peptides are measured by selected-reaction monitoring using triple quadrupole mass spectrometers. Peptides of interest are selected in the first quadrupole, fragmented in the second and specific fragments are filtered for detection in the third quadrupole. This procedure drastically reduces the chemical background, allowing to detect trace amounts of samples. Targets are usually compared against heavy isotope-labeled peptides used as internal standards, resulting in accurate measurements of the analytes. | Precise quantification of specific proteins, peptides or post-translational modifications. This technique is especially powerful for validating targets identified by global proteomics. | [134] |
| Immunopeptidomics or HLA ligandome | In this approach peptides that are being presented by major histocompatibility molecules (MHC) are captured by immunoaffinity purification and analyzed by liquid chromatography-tandem mass spectrometry. | Determine the pool of antigens being presented by the organism to the immune system. | [19,20] |
| Immunodepletion | Highly abundant proteins from biofluids captured from samples with immunoaffinity columns. This reduces the overwhelming signals of the highly abundant proteins in the mass spectrometer, improving the detection of low abundant proteins. | Immunodepletion is a key step for deep proteomic analysis of samples, such as human blood plasma, in which the top 12 proteins represents approximately 95% of the protein mass. | [75,135] |
| Laser-capture microdissection | Regions of sliced tissues are precisely cut with laser. | Proteomic analysis of specific regions of tissues, such as the islets of Langerhans. | [136] |
| Nanoproteomics | Proteomic analysis performed in nanoscale (nanoliters of volume) to prevent sample loss. | Proteomic analysis of small samples, such as sorted cell populations or single cells. | [110,117] |
| Ion mobility spectrometry | Ion mobility spectrometry is a technique used to separate ionized molecules based on their mobility in an inert buffer gas under an electric field. In this technique molecules are separated by charge, size and shape. | This technique can separate isobaric molecules, allowing to characterize isomers. The separation also decreases the chemical background, enhancing the detection of analytes. Due to its separation speed, it allows samples to be analyzed in seconds, enabling analysis of thousands of samples in a single day. | [120] |
| Mass cytometry | In mass cytometry, cells or tissues are stained with metal-labeled antibodies, which are detected by inductively coupled plasma mass spectrometry. | Mass cytometry coupled to flow cytometry is especially powerful to determine subpopulations of cells. Imaging mass cytometry provides spatial resolution, allowing to determine the distribution of different cells in tissues. | [125,126] |
Here, we provide a summary of the current knowledge on the etiology and pathophysiology of T1D, recent work in biomarker development, and the potential of MS-based proteomics and other complementary technologies for advancing the understanding of β-cell dysfunction and the discovery of T1D biomarkers.
2.0. Autoantigens and the autoimmune response in T1D
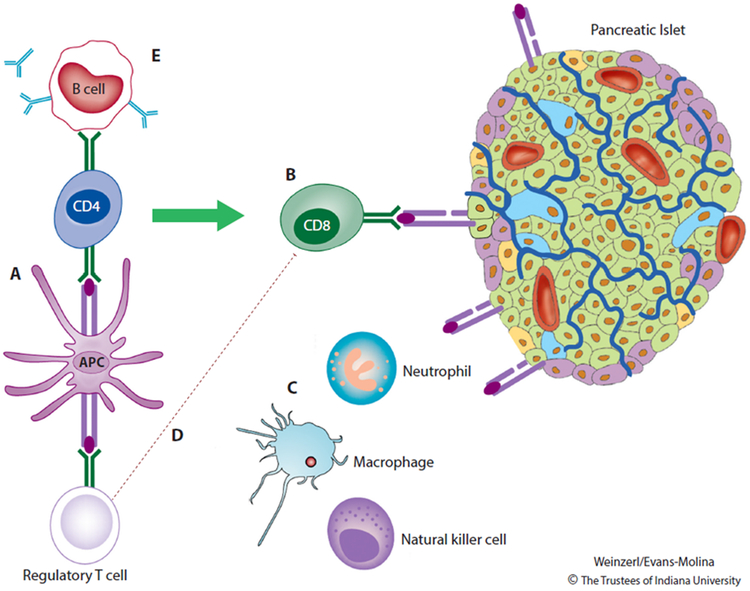
Immune activation in type 1 diabetes is thought to be first initiated by the presentation of β-cell peptides by antigen presenting cells (APCs) (Figure 1), but the initiating event(s) in this process remain to be discovered. These APCs migrate to the pancreatic lymph nodes, where they interact with autoreactive CD4+ T cells. CD4+ T cells mediate the activation of autoreactive CD8+ T lymphocytes cells, which are ultimately responsible for the lysis of β cells expressing immunogenic self-antigens on MHC class I surface molecules. Autoreactive CD4+ T cells within the pancreatic lymph node also stimulate B lymphocytes to produce autoantibodies against β-cell proteins. These autoantibodies are considered to be defining biomarkers of T1D [4].
Figure 1. Type 1 Diabetes Pathogenesis.
During the development of Type 1 diabetes, antigen-presenting cells (APCs) residing within the pancreas process and present β-cell peptides on MHC class I molecules. APCs bearing these peptides travel to the pancreatic lymph nodes, where they initiate an immune response that includes activation of autoreactive CD8 T cells and autoantibody production by B lymphocytes. Pancreatic β cells expressing immunogenic peptides on MHC class I surface molecules are targeted for destruction by autoreactive CD8 T cells. Cells of the innate immune system (macrophages, natural killer cells, and neutrophils) exacerbate β-cell death and inflammation through local release of cytokines, chemokines, and reactive oxygen species. β cells participate in this process by releasing chemokines and by up-regulating MHC class I molecules and the associated machinery for antigen presentation. The immune reaction is amplified by impairments in the ability of T regulatory cells to effectively suppress autoimmunity. Autoantibodies against β cells are important markers of immune activation but are not known to play a direct role in disease pathogenesis. Reprinted from The Lancet, Vol. 391, DiMeglio LA, Evans-Molina C, Oram RA, Type 1 diabetes, Pages 2449-2462, Copyright (2018) [4], with permission from Elsevier.
How immune tolerance is overcome and by which processes novel antigens (neoantigens) appear are still not well understood. Proteomics approaches can be instrumental in identifying key neoantigens, although these have been underutilized in T1D research. Peptides presented by cells can be co-purified by immunoprecipation of the MHC molecules (i.e. the “HLA ligandome”) and analyzed by LC-MS/MS in an approach known as “immunopeptidomics” [19,20]. Immunopeptidomics has been demonstrated in cell cultures, human islets and thymic and splenic tissues of non-obese diabetic mice [21,22]. Importantly, these studies showed that the peptides presented can change substantially when cells are stimulated with pro-inflammatory cytokines [21,22], which could be a source of neoantigen formation. Neoantigens can result from alternative splicing (AS) [23], formation of hybrid peptides [24], or via protein post-translational modifications (PTMs) [25,26]. AS is a complex post-transcriptional mechanism that regulates gene expression and generates protein diversity. By modulating the inclusion of different exon combinations into the pre-mRNA, AS allows individual genes to produce structurally distinct mRNA and protein isoforms with different biological functions [23]. Alternative exons can also introduce premature stop codons, regulating mRNA levels through degradation by the nonsense-mediated decay [27]. More than 90% of human genes undergo AS [28], with an enormous variation between cell types, developmental stage, stimuli and disease. Peptides derived from AS can be detected in human islets and they were shown to be reactive to CD8+ T cells, reinforcing the idea that AS is an important process that leads to autoimmunity [22].
Hybrid peptides are also known as spliced peptides and are formed by transpeptidation reactions (ligation of peptides from different proteins) by the proteasome [29]. A large immunopeptidomics survey has shown that these hybrid peptides comprise approximately 30% of all peptides presented by MHC class I molecules [30]. Hybrid peptides have also been detected in human and murine pancreatic islets by MS-based proteomics analysis [22,31]. Hybrid insulin peptides are indeed recognized by pathogenic CD4+ T cells, suggesting that this could be a mechanism leading to immunotolerance breakage [24,31].
PTMs are also an increasingly recognized mechanism to produce potential β-cell-specific neoantigens [25,26,32]. The inflammation or stress in the islet microenvironment could create unique conditions for β cells to produce various PTMs. For example, increased levels of reactive oxygen species (ROS), calcium dyshomeostasis, activation of inducible nitric oxide synthase (iNOS), as well as activation of tissue transglutaminase (tTG) and peptidylarginine deiminase (PAD), have all been reported in islets during inflammation [33-35]. Such conditions lead to the production of tissue specific PTMs. For instance, deamination of arginine residues into citrulline on glucose-regulated protein 78 (GRP78/Bip) is induced by PAD upon treatment of rat INS-1E β-cell line in a proteomics experiment. Importantly, citrullinated GRP78 is recognized by effector T cells, showing that this PTM is also a possible candidate for inducing autoimmune response [36,37].
3.0. Role of inflammation in the pathogenesis of β-cell death in T1D
During early immune cell infiltration into the pancreatic islet (insulitis), inflammation contributes both to the primary induction and secondary amplification of the immune assault, and inflammatory mediators trigger functional suppression and apoptosis of β cells (Figure 2) [2]. This inflammation takes place as part of the dialogue between invading immune cells and the target β cells. This dialogue is mediated by cytokines/chemokines released by β cells and immune cells and by putative immunogenic signals delivered by dying or “altered” β cells. Progressive loss of β-cell mass is the key central feature of T1D, and immune cells contribute to β-cell apoptosis by cell-to-cell interactions, via the Fas-FasL and perforin-granzyme systems, and by local release of pro-inflammatory cytokines [2]. Different cytokines affect β cells at different stages of the disease. For example, interferon-α (IFN- α) plays a crucial role during early stages of insulitis, and signaling downstream of IFN-α induces long-lasting HLA class I expression, endoplasmic reticulum (ER) stress (see below) and chemokine production [38,39], all potential contributors to insulitis and β-cell damage. On the other hand, IFN-α also induces the expression of key β-cell defense mechanisms against the immune attack, particularly PDL1 expression [40]. Interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ) and interleukin-17 (IL-17) are released at later stages of the autoimmune assault by the infiltrating macrophages and T-cells, contributing to β-cell dysfunction and death [2]. Cytokine-induced β-cell apoptosis is highly dependent on the activation of complex gene networks regulated by transcription factors (TFs) such as NF-κB [41,42] and STAT-1 [42].
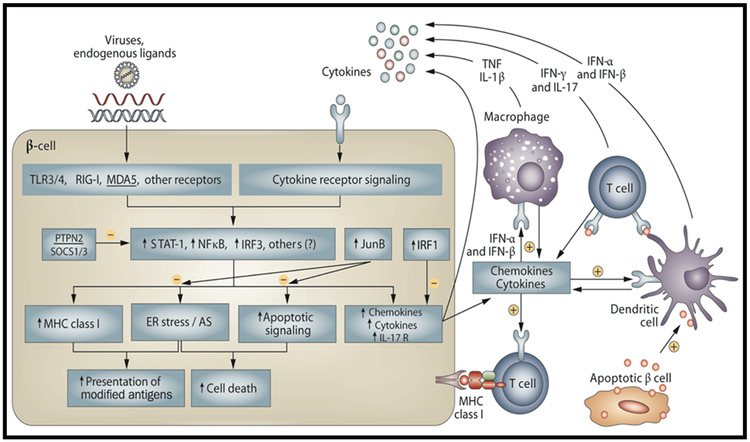
Figure 2. The dialogue between pancreatic β cells and the immune system that leads to induction and amplification of insulitis and the transition between innate and adaptive immune responses.
Recognition of endogenous and exogenous “danger signals” by PRRs (TRL3/4, RIG-1, MDA5) lead to activation of key transcription factors such as NF-κB, IRF1, IRF3 and STATs (stimulatory of inflammatory signaling) and JunB (inhibitory). Activation of these transcription factors will induce: 1. release of several chemokines promoting attraction and activation of immune cells; 2. increase β-cell expression of MHC class I which, associated with ER stress and changes in AS, lead to presentation of modified antigens to the immune cells; 3. activation of pro-apoptotic signals leading to β-cell death. Products of dying β cells are taken by professional antigen-presenting cells (dendritic cells) contributing for activation of auto-reactive T-cells. During this local inflammatory process, known as insulitis, pro-inflammatory cytokines such as IFN-α (early stage) or IL-1β, IL-17, TNF-α and IFN-γ (later stages) are released by the immune cells and induce transcription factors such as STAT-1, STAT-2 NF-κB and IRF-1 in the β cells contributing either for the maintenance and amplification of the networks described above, or, in some cases, to down-regulation of immunity via activation of PDL1 and other T-cell inhibitory signals. If insulitis is not resolved, it will result in a progressive and selective destruction of pancreatic β cells. Abbreviations: AS, alternative splicing; ER, endoplasmic reticulum; IFN, interferon; IL, interleukin; PRR, pattern-recognition receptor; TLR, Toll-like receptor; TNF, tumor necrosis factor. Reprinted by permission from: Springer Nature, Nature Reviews Endocrinology, The role of inflammation in insulitis and β-cell loss in type 1 diabetes, Eizirik DL, Colli ML, Ortis F, Copyright 2009 [2].
Proteomic analysis of a rat INS-1E cell line showed that IL-1β and IFN-γ regulate a large number of proteins related to insulin secretion, cytoskeleton organization, RNA metabolism, and ER and oxidative stress [43]. To better understand the mechanisms of cytokine-mediated regulation of β cells, Ramos-Rodriguez and colleagues performed a comprehensive multi-omics analysis of human islets and the human EndoC-βH1 cell line treated with the combination of IL-1β + IFN-γ, and integrated the resulting data. The measurements included proteomics, chromatin immunoprecipitation followed by DNA sequencing (ChIP-Seq), DNA methylation analysis, assay for transposase-accessible chromatin sequencing (ATAC-seq), transcriptomics (RNA-seq) and genome-wise association study (GWAS). Such combination of techniques enabled the investigation of how cytokines promote chemical and physical changes in the structure of the chromatin, and whether these changes are associated with genomic loci with higher risk of T1D and with consequent changes in mRNA and protein expression [44]. The study found two loci in genomic regions that are regulated by cytokines: rs78037977 in the region of the cytokine gene TNFSF18, and rs193778 in the region of the DEXI gene, which was shown to participate in β-cell death via cytokine signaling [45]. Importantly, the polymorphism in these loci was shown to have a strong effect in the expression of the respective genes [44].
Viral infections from Enteroviruses have been proposed as one mechanism through which autoimmunity is triggered [46]. A proteomics study of human islets infected in vitro with Coxsackievirus B4 showed a strong immune response associated with type I interferon signaling, which is consistent with the expression of cytokines and other antiviral response factors [47]. In agreement with these observations, transcriptomics analyses of human islets exposed to the pro-inflammatory cytokines IL-1β + IFN-γ, or infected with Coxsackievirus B5, showed similar expression patterns [48]. Despite similarities in the response to cytokines and viral infections in whole islets, α and β cells individually showed important differences in response to Coxsackievirus infections. Coxsackievirus 5 infection induced massive apoptosis in FACS-sorted rat β cells but not in α cells [48]. This may be explained by the higher expression of innate immunity/antiviral proteins in α cells compared to β cells [48], suggesting that α cells are better equipped to fight the viral infection, whereas β cells are unable clear the virus and eventually undergo apoptosis. These findings can provide an explanation on the selective death of β cells during T1D development.
PTMs, such as protein phosphorylation, acetylation, ubiquitination and methylation, are also of significant interest for gaining detailed mechanistic understanding of cellular processes during insulitis and cell death. Despite its importance, comprehensive PTM analyses by mass spectrometry have been poorly explored in studying β-cell death, primarily due to the limited sensitivity of typical PTM workflows. To date, most studies were carried out with cell lines or samples from mouse models. For example, Engholm-Keller et al. identified ~6,600 unique phosphopeptides from the insulin-secreting rat INS-cell line [49]. Several studies reported the phosphoproteome dynamics during glucose-stimulated insulin secretion (GSIS) using rat and mouse pancreatic islets by implementing TiO2 enrichment and LC-MS/MS [50-52]. In the case of β-cell death, Palmisano et al treated the rat insulinoma cell line NHI 6F Tu28 with the pro-inflammatory cytokines IL-1β + TNFα and performed phosphoproteomic and glycoproteomic analysis of extracellular vesicles secreted by these cells [53]. This analysis showed regulation of PTMs on proteins related to cell signaling. Furthermore, such modifications were upregulated in proteins related to cell death and downregulated in cell morphology proteins [53]. A major hurdle for a more systematic analysis of islet PTMs is the large amounts of material (typically milligrams of peptides) required for enriching modified peptides. More recently, Yi et al. reported a “boosting to amplify signal with isobaric labeling (BASIL)” strategy for quantitative phosphoproteome profiling in small cell populations [54]. They further demonstrated a pilot study of examining the phosphoproteome changes of human islets treated by IL-1β and IFN-γ for 24 h where a comprehensive coverage of ~25,000 phosphopeptides was achieved. The observed alterations of phosphorylation were involved in a number of previously reported pathways such as interferon and cytokine signaling, and antigen processing and presentation. The continual advances for of more sensitive techniques for PTM analysis should have an immediate impact in further elucidating the mechanisms underlining the insulitis and β-cell death processes.
4.0. Potential roles of endoplasmic reticulum stress in insulitis
One potentially important mechanism by which pro-inflammatory signaling contributes to β-cell death and amplification of inflammation in T1D is via induction of endoplasmic reticulum (ER) stress, and the consequent triggering of the Unfolded Protein Response (UPR) [55,56]. The UPR is mediated through activation of three ER transmembrane proteins: inositol-requiring protein 1α (IRE1α), protein kinase RNA-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6). These proteins sense the accumulation of unfolded proteins in the ER lumen and activate mechanisms to restore its homeostasis. In case of unresolved ER stress, persistent stimulation of the UPR triggers apoptosis via activation of C/EBP homologous protein (CHOP), c-jun N-terminal kinase (JNK), death protein 5 (DP5) and other pro-apoptotic signals [57]. Consistent with these observations, proteomic analysis of the rat INS-1E cell line treated with IL-1β + IFN-γ showed a regulation of ER stress response [43]. A subsequent study in INS-1E cells using the ER stress inducer cyclopiazonic acid showed that ER stress induces massive apoptosis [58], linking the cytokine-induced ER stress response and UPR to cell death [55,56]. The same study also showed that ER stress has a major impact in expression, processing and release of insulin [58]. A key component of the UPR system, GRP78 protein, was shown to be regulated in islets from non-obese diabetic mice (NOD), a mouse model of autoimmune diabetes, in the prediabetic stage [59], indicating that a similar ER stress response occurs in vivo. Moreover, a GWAS analysis showed that mutations on the GATA4 transcription factor resulted in increased expression of UPR proteins in association with T1D occurrence [60].
Importantly, in both NOD mice and in T1D individuals, the β cells express markers of ER stress [61-63], but eventually fail to sustain key components of the adaptive UPR [62]. This contributes to their eventual demise. Activation of ER stress may also lead to presentation of neoantigens through the generation of aberrant or modified proteins that escape central tolerance [26,36,64-66]. Upon ER stress induction, alternative initiation sites can be used during translation of insulin mRNA and generate highly immunogenic insulin gene-derived polypeptides targeted by T-cell autoreactivity in individuals with T1D [67]. Induction of the UPR was shown also to impact on calcium-dependent enzymes that induce post-translational changes, such as tTG2 and PAD [33,35], leading to the production of neoantigens as described above.
Despite the advances in knowledge described above, the precise mechanisms by which autoimmunity is triggered and aggravated in T1D remain poorly understood, and the regulatory networks and signaling events that promote β-cell dysfunction and death or lead to protection remain to be clarified.
5.0. Linking pathways of β-cell dysfunction to biomarker development
Most clinical trials in T1D have been initiated at the onset of Stage 3 T1D. As the success of this approach has been modest, an alternative is to intervene earlier in the disease process, at a time when greater β-cell mass remains. Whilst the presence of multiple autoantibodies signifies future risk of developing T1D, the time-frame of disease development is unclear based on the measurement of autoantibody status alone. Furthermore, determination of these autoantibodies neither allows evaluation of progressive cell death nor of the potential impact of novel therapies to protect them. Thus, the idea of developing biomarkers of β-cell stress and death is attractive and offers the potential of both targeting the individuals at highest risk and evaluating the effects of intervention therapies. However, a major challenge is to discriminate the signals emitted from insulitis and the dying β cells, as β cells comprise only 1-2% of the pancreas mass (1-2 g total in humans), from signals emitted by other tissues. Moreover, T1D poses a unique challenge owing to the relative inaccessibility of the target organ for interrogation by imaging or biopsy in living individuals.
Several studies have identified markers of β-cell dysfunction and new onset of T1D in human urine and blood plasma or serum [68-71]. A proteomic analysis of urine from children with T1D showed a consistent increase of several lysosomal enzymes [72], which was validated in a second cohort [73]. Release of lysosomal enzymes is a process associated with inflammation, which in this case could be a consequence of the damage to the renal tubular epithelial cells caused by chronic hyperglycemia [72,73]. Proteins related to inflammation and other innate immune response processes are commonly found as blood biomarkers of islet autoimmunity and T1D onset [68-71]. While a number of biomarker candidates have been reported from recent literature [74], most of them are present in plasma in mid to high abundances, but not necessarily being directly derived from β cells. The extremely low abundance of proteins specific to pancreatic islet cells and the heterogeneity of disease make the discovery of new biomarkers more challenging. To aggravate this scenario, blood plasma is one of the most challenging sample types for proteomics analysis due to the presence of highly abundant proteins, with 12 of the most abundant proteins comprising more than 95% of the total mass [75]. LC-MS/MS analysis of whole, normal human plasma typically leads to the detection of less than 400 proteins [76,77]. However, this number can be increased to ~2,000 with immunodepletion of the most abundant proteins together with peptide fractionation prior to the LC-MS/MS analysis [78]. The challenge in this case is the significant labor and instrument time required which typically prevents the analysis of large cohorts of samples. One way to circumvent this issue is to design the study in two phases, a “discovery” phase where deep proteomic analysis on a limited number of samples is performed, followed by a “validation” phase using a targeted proteomics approach. Targeted proteomics is limited in the number of proteins that can be measured in a single measurement - up to ~200 proteins - but it can be used to analyze large sample cohorts with high sensitivity (as low as 10 pg/mL range), since, depending on the concentration of protein targets, immunodepletion may not be required, and the overall analytical analysis time can be significantly shortened [79]. This discovery/validation approach was applied to analyze plasma from patients with new onset T1D and led to the discovery of platelet basic protein and C1 inhibitor as biomarkers [68]. The recent advances in mass spectrometry and proteomics technologies, increasing the sensitivity and throughput of measurements (see below), will have a major role to enable precise quantification of islet-derived proteins in plasma from large sample cohorts.
Measurement of serum insulin and C-peptide can provide insight into T1D risk [80,81] and impaired insulin secretion may be present as early as five years before disease onset [82]. However, measurement of C-peptide is typically performed in the context of glucose or mixed-meal stimulation, which requires fasting and multiple blood draws after nutrient ingestion. An indicator of β-cell dysfunction and ER stress is the delayed conversion of proinsulin to insulin [61], measurable as the proinsulin to C-peptide or insulin ratio. In this regard, fasting proinsulin/C-peptide ratios were found to be elevated 12 months in advance of diabetes diagnosis and predicted T1D risk [83]. Recently, proinsulin secretion was found to persist in persons with long-standing T1D, even in those who were functionally C-peptide negative [84]. A second secreted prohormone, β cell-derived pro-islet amyloid polypeptide (pro-IAPP) relative to total IAPP, was found to be similarly elevated in children with longstanding type 1 diabetes [85], suggesting that impaired prohormone processing may be an important component of T1D pathophysiology as well as a potential source of novel biomarkers.
MicroRNAs (miRNAs) are small non-coding RNAs that have been found to play a central role in the regulation of gene expression. Along these lines, several miRNAs have been shown to play a role in T1D pathogenesis [86-88]. Compared to mRNA species, miRNAs are highly stable in biological fluids, where they have been detected in high abundance in blood, urine, saliva, cerebrospinal fluid, milk, seminal fluid, and amniotic fluid [89]. Analysis of sera from individuals with established T1D or autoantibody-positivity has identified a number of differentially expressed miRNAs, several of which were correlated with indices of β-cell function [90,91]. Although miRNAs are of intracellular origin, they may also be packaged into microvesicles or exosomes and release of vesicle-associated miRNAs may increase under disease conditions. A recent study showed that T lymphocyte derived miRNAs can be transferred to the β cell in models of T1D, where these miRNAs were found to impact β-cell survival [92]. Pro-inflammatory cytokines increased both β cell miR-21-5p expression and its packaging into exosomes. Moreover, exosomal-associated miR-21-5p was increased in sera of pediatric subjects with recent onset T1D as compared to non-diabetic controls [93]. Similarly, differences in exosome-derived miRNA signatures have also been demonstrated in long-duration T1D [94].
Exosomes may also serve as a source of protein biomarkers in T1D. Proteomics and immunoblot analysis identified several autoantigens in human islet and rat-islet derived exosomes, including proinsulin, GAD65, and IA-2. Furthermore, cytokine treatment was found to increase markers of ER stress in exosomes [95]. Indeed, glypican 1-containing exosomes has been described as a potential biomarker for pancreatic cancer [96]. Therefore, exosomes have the potential to lead to the discovery of biomarkers for T1D as well. A key challenge in translating these ex vivo findings is the development of techniques that allow for the isolation and analysis of β cell-derived exosomes found in trace amounts in serum or plasma, for instance by “fishing” them based on the expression of cell [97,98] or islet specific proteins [97]. Furthermore, new advances in microfluidic exosome isolation [99,100] coupled to nanoscale proteomics sample preparation (see below) may provide sufficient sensitivity and specificity, opening new opportunities to explore exosomes as sources of T1D biomarkers.
Other nucleic acid species have also been proposed to serve as biomarkers of early β-cell stress and death. Cell-free DNA in the circulation is thought to arise largely from dead or dying cells, since release of cellular DNA, unlike miRNAs, does not occur routinely in living cells. The premise of interrogating cell-free DNA is based on the concept that stable, epigenetic modifications of DNA (such as cytosine methylation) can vary from one cell type to another within a given organism. Some genes expressed largely or exclusively in the β cell, such as the gene encoding insulin (INS), are unmethylated at cytosines, whereas they are methylated in virtually every other cell type. Using bisulfite chemistry and PCR, several groups have demonstrated increased unmethylated INS DNA in the circulation of NOD mice just prior to T1D development and in humans at the onset of T1D [101-103]. Notably, it has been demonstrated that unmethylated INS in the circulation can be detected in individuals prior to the onset of overt T1D, suggesting that β-cell death precedes the onset of T1D [104]. Other β cell-specific genes have been similarly developed as biomarkers, including IAPP and GCK [105,106]. Whereas cell-free DNAs may represent very sensitive biomarkers owing to the high sensitivity of PCR methodologies, they nevertheless pose other significant limitations, including lack of cell-type specificity and their inability to report on cell stress (only death).
6.0. Advanced MS technologies provide new perspectives on studying T1D progression
Particular challenges associated with proteomics studies of pancreatic β cells, islets, or clinical samples (e.g. plasma), are the need for high sensitivity in the analytical approaches due to the limited amounts of material available, especially for primary mouse or human tissues or cells; the need for a high measurement dynamic range in order to accurately quantify both high and low abundance proteins, the latter of which may be among the most interesting biomarker candidates; and the need for high throughput in order to have the capacity for screening samples from large study cohorts. High measurement sensitivity can also enable direct proteome characterization of clinical specimens such as islet cells isolated by laser-capture microdissection (LCM) at near single-cell level.
An important area of recent advance is nanoscale proteomics for effective analyses of small populations of cells (e.g., <5000 cells). One of the first related reports was the analysis of freshly harvested single mouse pancreatic islets containing 2,000–4,000 cells by Waanders et al [107]. Although proteomics of fixed tissue is challenging, due to the cross-linking process, the analysis of 13,000-30,000 cells laser-capture microdissected from human islets led to the identification of 1,100-2,000 proteins [108,109]. More recently, it was described an online processing system described as a simplified nano-proteomics platform (SNaPP), which consisted of an online immobilized enzyme reactor digestion system and a solid phase extraction (SPE) column coupled with a nanoLC separation system [110]. SNaPP was applied for proteome profiling for as few as 500 mouse lung alveolar cells isolated by LCM [111]. Several other single tube-based approaches were also reported for analyzing small populations of cells, such as the trifluoroethanol-based approach [112], focused ultrasonication [113], and the integrated proteome analysis device (iPAD) [114].
Despite these advances, it remains challenging to achieve robust analysis of extremely small cell populations (e.g., <500 cells). Li and colleagues used a microfluidic reaction chamber for proteomics analysis of a single HeLa cell, identifying 51 proteins [115], while Budnik et al. recently used a carrier cell approach to minimize the non-specific sample losses that frequently occur in analyses of single cells due to binding to tubes and columns during sample preparation [116]. It has been recently reported the development of the nanoPOTS (Nanodroplet Processing in One-pot for Trace Samples) platform for enabling robust proteomics measurements of 10-100 cells [117]. By coupling this with laser microdissection, this novel platform enabled quantitative proteome profiling of single human islet sections (10 μm in thickness, ~100 cells), resulting in the quantitative detection of ~2,400 proteins (Figure 3). Significant differences in abundances of 304 proteins were observed between T1D and control islets based on single islet proteomics, including drastic reduction of the abundances of β cell-specific proteins such as insulin and PCSK1, and increased abundances of immune-responsive proteins such as β−2-microglobulin and HLA-class I antigens (Figure 3). Importantly, nanoPOTS opens opportunities for investigating the underlying molecular mechanisms of islet inter- and intra-individual heterogeneity [118] by performing measurements at single islet levels. Furthermore, the nanoPOTS platform also enabled deep proteome profiling for <1000 cells or ~10 islet sections from an AAb+ donor with >6,300 islet proteins being identified, including low abundance transcription factors such as PDX1 and NKX 2.2 [119].
Figure 3. nanoPOTS-enabled proteomics analysis of single human pancreatic islet sections.
a) Overview of the nanoPOTs workflow (scale bar is 500 μm), b) reproducibility of protein abundances in nine human islet sections from a non-diabetic donor as represented by pairwise correlations, c) islet protein expression differences between a non-diabetic donor and a donor with T1D, based on nanoPOTS-based proteomics measurements of single islets. Reproduced from Zhu et al. [117] under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
To address current analytical limitations in measurement dynamic range and overall study throughput for MS-based proteomics, ion mobility (IM) separations are increasingly incorporated into laboratory workflows. IM instruments separate molecules in the gas-phase based on their charge state, size and shape, as influenced by their chemical makeup and spatial structure of the molecule. IM separations typically occur on the scale of milliseconds, allowing them to be “nested” between LC (minutes to hours) and MS (microseconds) dimensions [120]. Thus, integrating IM into LC-MS-based proteomics workflows can potentially provide greater coverage of sample proteomes by increasing the dynamic range of the measurement through addition of an additional dimension of separation, or can allow for decreasing LC separation times while maintaining the same measurement coverage and thereby increasing overall analysis throughput [120].
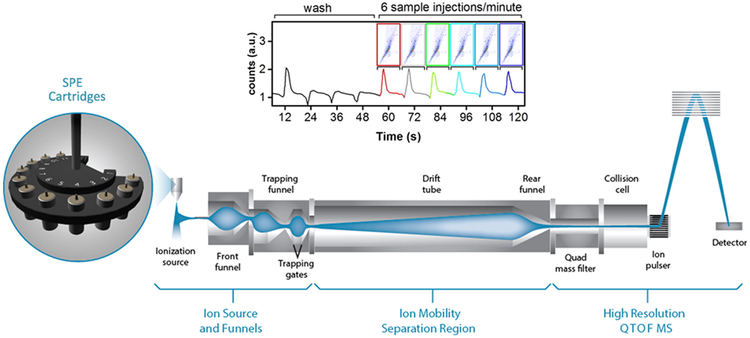
Although the incorporation of IM measurements into routine molecular analysis workflows is still emerging, there have been several very promising developments recently that should facilitate protein biomarker discovery and validation efforts. Zhang et al. reported on the development of an automated SPE-IM-MS platform for analysis of metabolites that required less than 1 minute per sample and that allowed a theoretical throughput of 8,000 analyses per day (Figure 4) [121]. The implementation of this platform in high throughput proteomics analyses of large clinical cohorts would provide a long sought after solution for population-scale proteomics analyses. Meier and colleagues recently developed a data acquisition method called parallel accumulation - serial fragmentation (PASEF), using trapped-IM integrated with LC-MS. The PASEF approach enabled an unprecedented 600,000 fragmentation spectra to be collected using a standard 120-minute LC separation, identifying >6,000 proteins in a single analysis of a HeLa cell protein digest. In terms of sensitivity, PASEF resulted in the identification of >2,500 proteins in a 30-minute analysis of 10 ng of HeLa digest [122]. A next generation IM device – structures for lossless ion manipulations (SLIM) – has been developed and should provide resolving powers much higher than traditional IM instruments, comparable to those provided by traditional LC separations [123]. In a recent, intriguing study, Dou et al. coupled nanoPOTS with SLIM in analysis of a HeLa protein digest; the combined LC-SLIM IM separation peak capacity was ~3600 or over ~18-fold higher than that for LC-MS alone [124].
Figure 4. Ultra-high throughput molecular analyses using SPE-IM-MS.
Samples are bulk separated using SPE, followed by analysis using IM-MS. Each SPE-IM-MS analysis requires just 10-seconds. Reproduced from Zhang et al. [121] under a Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/).
Mass cytometry is another recently developed technique for high throughput analysis of samples in single cell resolution. In mass cytometry, proteins/markers of interest are targeted by antibodies labeled with metals. These metals are then detected by highly sensitive inductively coupled plasma-mass spectrometry (ICP-MS). Unlike fluorophores that usually have a broad spectrum of emission, metals have discrete masses, consequently increasing the resolution and avoiding overlapping readings. This allows to track more than 35 cellular markers simultaneously, enabling the resolution of cell type subpopulations [125]. Mass cytometry can be either coupled to flow cytometry to measure cell populations or used to obtain images of sliced tissue [125,126]. Both techniques have been applied to characterize human islets. Mass cytometry coupled to flow cytometry has been applied to characterize different populations of endocrine cells in the islets of healthy, non-diabetic individuals [127]. The results showed that the β-cell population is on average 65% of the islets in children, but it is reduced to approximately 42% in adults. This analysis also showed that there were two subpopulations of proliferating β cells that combined represented 4% of the total population in an 18-day old baby but were reduced to almost zero in adults [127]. The very low proliferation rate of β cells observed in older children and adults is in line with previous observations based on other methods [128] and highlights the importance of developing novel approaches to prevent β-cell loss in T1D. Mass cytometry has also been used to track immune cells in individuals with insulitis, which identified subpopulations of regulatory T cells and natural killer cells that are increased during islet autoimmunity [129]. Besides those cell types, some aberrant lymphocytes containing characteristics of both B and T cells, have been very recently identified in T1D patients [130]. This novel immune cell type might have a key role in autoimmunity, but these intriguing observations must be confirmed by additional studies.
Compared to flow cytometry, imaging mass cytometry has the advantage of providing spatial resolution, which allows to study intra-individual variability between islets and to determine immune cell populations infiltrated in islets. Recent studies also showed recruitment of cytotoxic and helper T cells into the islets in individuals with T1D [131,132]. These papers also showed intra-individual variation in endocrine cell population in islets when comparing the head and tail of the pancreas [131,132]. Previous analyses of pancreata from individuals with T1D using traditional histologic approaches have shown geographic variability in the persistence of β cells (reviewed in [1]). These results highlight a potential heterogeneity in the susceptibility of some populations of β cells to immune-mediated destruction. Overall, novel techniques such as nanoPOTS, IM-MS and mass cytometry have tremendously enhanced the sensitivity and throughput of MS-based omics measurements. These technologies are likely to play a major role in obtaining a better understanding of the molecular mechanisms behind T1D development and the identification of associated protein biomarkers.
7.0. Conclusions
MS-based proteomics is playing a central role in elucidating mechanisms of autoimmune response, insulitis, and β-cell death, leading to the identification of new biomarkers for T1D. We are presently facing a situation where T1D can be predicted with good accuracy via determination of circulating autoantibodies and where understanding of the disease is improving. However, this has not yet translated into novel therapies that can prevent or revert the disease. The recent developments in MS-based proteomics techniques and instrumentation have resulted in significant improvements in the sensitivity and throughput of sample analysis, which opens new opportunities for better understanding the T1D pathogenesis and for discovering and validating novel biomarkers.
8.0. Expert opinion
Understanding the pathways that lead to β-cell dysfunction and death in T1D are crucial for the discovery of circulating biomarkers that can be monitored during the initiation and amplification of insulitis and the progressive -cell death. For this purpose, it will be necessary to measure both information that “spills” from stressed/dying cells and released factors (i.e., exosomes, cytokines and hormones) into the circulation as a result of the above-described dialog between the immune system and cells. It is indeed a major challenge to discriminate the signals emitted from insulitis and the dying cells – cells comprise only 1-2% of the pancreas volume and an average total weight of 1-2 g – as compared to other autoimmune diseases with strong inflammatory components that affect much larger amounts of tissue, such as rheumatoid arthritis or inflammatory bowel disease. To achieve this goal, we must keep in mind the question “what is unique to T1D”? The answer is the specific destruction of pancreatic cells by the immune system. We must thus focus first and foremost on the mechanisms leading to autoimmunity, insulitis and -cell apoptosis, identify locally produced/released biomarkers, and then search for them in the circulation.
Article Highlights.
Biomarkers that can accurately predict the early stages of type 1 diabetes are urgently needed to improve our knowledge about the disease mechanism and to develop new therapies.
Proteomics analysis has provided insights to islet autoimmunity and inflammation, as well as signaling events and regulated processes in type 1 diabetes.
Immunopeptidomics analyses contributed to the discovery of auto-reactive neoantigens present in type 1 diabetes.
Post-translationally modified and hybrid peptides are targets of the autoimmune response during type 1 diabetes development.
Nanoproteomics and mass cytometry analyses have enabled to study in great detail the intra- and inter-individual islet variability and immune cell infiltration in islets during insulitis.
Emerging technologies, such as ion mobility spectrometry, will have a major role in identifying new biomarkers and better understanding of the disease.
Acknowledgments
Funding
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants UC4 DK104166 (to R.G.M, C.E. M., D.L.E, and T.O.M.), UC4 DK104167 and DP3 DK110844 (to W.J.Q), R01 DK60581 and R01 DK 105588 (to R.G.M.) and R01 DK093954 (to C.E.M); VA Merit Award I01BX001733 (to C.E.M.); Fonds National de la Recherche Scientifique (FNRS), Welbio CR-2015A-06, Belgium (to D.L.E.); a JDRF Strategic Research Agreement (to C.E.M and R.G.M.) and gifts from the Sigma Beta Sorority, the Ball Brothers Foundation, the George and Frances Ball Foundation, and the Holiday Management Foundation (to C.E.M and R.G.M.). D.L.E. also received funds from Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115797 (INNODIA), which is supported by the Union’s Horizon 2020 research and innovation program and EFPIA, JDRF and The Leona M. and Harry B. Helmsley Charitable Trust.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Oram RA, Sims EK, Evans-Molina C. Beta cells in type 1 diabetes: mass and function; sleeping or dead? Diabetologia, 62(4), 567–577 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol, 5(4), 219–226 (2009). [DOI] [PubMed] [Google Scholar]
- 3.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev, 14(8), 1847–1850 (2005). [DOI] [PubMed] [Google Scholar]
- 4.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet, 391(10138), 2449–2462 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todd JA. Etiology of type 1 diabetes. Immunity, 32(4), 457–467 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Pociot F, Lernmark A. Genetic risk factors for type 1 diabetes. Lancet, 387(10035), 2331–2339 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Patterson CC, Harjutsalo V, Rosenbauer J et al. Trends and cyclical variation in the incidence of childhood type 1 diabetes in 26 European centres in the 25 year period 1989-2013: a multicentre prospective registration study. Diabetologia, 62(3), 408–417 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Livingstone SJ, Levin D, Looker HC et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA, 313(1), 37–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huo L, Harding JL, Peeters A, Shaw JE, Magliano DJ. Life expectancy of type 1 diabetic patients during 1997-2010: a national Australian registry-based cohort study. Diabetologia, 59(6), 1177–1185 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Insel RA, Dunne JL, Atkinson MA et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care, 38(10), 1964–1974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rigby MR, DiMeglio LA, Rendell MS et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. The lancet. Diabetes & endocrinology, 1(4), 284–294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigby MR, Harris KM, Pinckney A et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J Clin Invest, 125(8), 3285–3296 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haller MJ, Schatz DA, Skyler JS et al. Low-Dose Anti-Thymocyte Globulin (ATG) Preserves β-Cell Function and Improves HbA. Diabetes Care, 41(9), 1917–1925 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. *.Herold KC, Bundy BN, Long SA et al. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med, in press (2019).This article represents a proof-of-concept that interventions can be developed to prevent the onset of type 1 diabetes.
- 15.Aebersold R, Mann M. Mass-spectrometric exploration of proteome structure and function. Nature, 537(7620), 347–355 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Ong SE, Blagoev B, Kratchmarova I et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell Proteomics, 1(5), 376–386 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Rauniyar N, Yates JR. Isobaric Labeling-Based Relative Quantification in Shotgun Proteomics. Journal of Proteome Research, 13(12), 5293–5309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahnsen S, Bielow C, Reinert K, Kohlbacher O. Tools for label-free peptide quantification. Mol Cell Proteomics, 12(3), 549–556 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt DF, Henderson RA, Shabanowitz J et al. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science, 255(5049), 1261–1263 (1992). [DOI] [PubMed] [Google Scholar]
- 20.Marino F, Chong C, Michaux J, Bassani-Sternberg M. High-Throughput, Fast, and Sensitive Immunopeptidomics Sample Processing for Mass Spectrometry. Methods Mol Biol, 1913, 67–79 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Dudek NL, Tan CT, Gorasia DG, Croft NP, Illing PT, Purcell AW. Constitutive and inflammatory immunopeptidome of pancreatic beta-cells. Diabetes, 61(11), 3018–3025 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. **.Gonzalez-Duque S, Azoury ME, Colli ML et al. Conventional and Neo-antigenic Peptides Presented by beta Cells Are Targeted by Circulating Naive CD8+ T Cells in Type 1 Diabetic and Healthy Donors. Cell Metab, 28(6), 946–960 e946 (2018).Gonzales-Duque et al. showed that neo-antigens comprised of alternative splicing and hybrid peptides are presented by β cells and are recognized by the lymphocytes during autommine response and type 1 diabetes.
- 23.Alvelos MI, Juan-Mateu J, Colli ML, Turatsinze JV, Eizirik DL. When one becomes many-Alternative splicing in beta-cell function and failure. Diabetes Obes Metab, 20 Suppl 2, 77–87 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. **.Delong T, Wiles TA, Baker RL et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science, 351(6274), 711–714 (2016).Seminal paper that showed that hybrid peptide neoantigens are recognized by CD4 T lymphocytes in type 1 diabetes.
- 25.Roep BO, Kracht MJ, van Lummel M, Zaldumbide A. A roadmap of the generation of neoantigens as targets of the immune system in type 1 diabetes. Curr Opin Immunol, 43, 67–73 (2016). [DOI] [PubMed] [Google Scholar]
- 26.van Lummel M, Duinkerken G, van Veelen PA et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes, 63(1), 237–247 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Yan Q, Weyn-Vanhentenryck SM, Wu J et al. Systematic discovery of regulated and conserved alternative exons in the mammalian brain reveals NMD modulating chromatin regulators. Proc Natl Acad Sci U S A, 112(11), 3445–3450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang ET, Sandberg R, Luo S et al. Alternative isoform regulation in human tissue transcriptomes. Nature, 456(7221), 470–476 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vigneron N, Ferrari V, Stroobant V, Abi Habib J, Van den Eynde BJ. Peptide splicing by the proteasome. J Biol Chem, 292(51), 21170–21179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liepe J, Marino F, Sidney J et al. A large fraction of HLA class I ligands are proteasome-generated spliced peptides. Science, 354(6310), 354–358 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Aaron Wiles T, Powell R, Michel CR et al. Identification of Hybrid Insulin Peptides (HIPs) in Mouse and Human Islets by Mass Spectrometry. J Proteome Res, 18(3), 814–825 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Storling J, Overgaard AJ, Brorsson CA et al. Do post-translational beta cell protein modifications trigger type 1 diabetes? Diabetologia, 56(11), 2347–2354 (2013). [DOI] [PubMed] [Google Scholar]
- 33.McLaughlin RJ, de Haan A, Zaldumbide A et al. Human islets and dendritic cells generate post-translationally modified islet autoantigens. Clinical and experimental immunology, 185(2), 133–140 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays, 25(11), 1106–1118 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Marre ML, McGinty JW, Chow IT et al. Modifying Enzymes Are Elicited by ER Stress, Generating Epitopes That Are Selectively Recognized by CD4(+) T Cells in Patients With Type 1 Diabetes. Diabetes, 67(7), 1356–1368 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. *.Rondas D, Crevecoeur I, D’Hertog W et al. Citrullinated glucose-regulated protein 78 is an autoantigen in type 1 diabetes. Diabetes, 64(2), 573–586 (2015).This article shows that post-translationally modified peptides can be neoantigens and be recognized by the immune system in type 1 diabetes.
- 37.Buitinga M, Callebaut A, Marques Camara Sodre F et al. Inflammation-Induced Citrullinated Glucose-Regulated Protein 78 Elicits Immune Responses in Human Type 1 Diabetes. Diabetes, 67(11), 2337–2348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marroqui L, Dos Santos RS, Op de Beeck A et al. Interferon-alpha mediates human beta cell HLA class I overexpression, endoplasmic reticulum stress and apoptosis, three hallmarks of early human type 1 diabetes. Diabetologia, 60(4), 656–667 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Coomans de Brachene A, Dos Santos RS, Marroqui L et al. IFN-alpha induces a preferential long-lasting expression of MHC class I in human pancreatic beta cells. Diabetologia, 61(3), 636–640 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colli ML, Hill JLE, Marroqui L et al. PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-alpha and-gamma via IRF1 induction. EBioMedicine, 36, 367–375 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardozo AK, Heimberg H, Heremans Y et al. A comprehensive analysis of cytokine-induced and nuclear factor-kappa B-dependent genes in primary rat pancreatic beta-cells. J Biol Chem, 276(52), 48879–48886 (2001). [DOI] [PubMed] [Google Scholar]
- 42.Eldor R, Yeffet A, Baum K et al. Conditional and specific NF-kappaB blockade protects pancreatic beta cells from diabetogenic agents. Proc Natl Acad Sci U S A, 103(13), 5072–5077 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Hertog W, Overbergh L, Lage K et al. Proteomics analysis of cytokine-induced dysfunction and death in insulin-producing INS-1E cells: new insights into the pathways involved. Mol Cell Proteomics, 6(12), 2180–2199 (2007). [DOI] [PubMed] [Google Scholar]
- 44. **.Ramos-Rodríguez M, Raurell-Villa H, Colli ML et al. The impact of pro-inflammatory cytokines on the β-cell regulatory landscape provides new insights into the genetics of type 1 diabetes. bioRxiv, 560193 (2019).This paper is an excellent example on integrating multiple omics measurements as a powerful approach to understand the immunoregulation in type 1 diabetes.
- 45.Dos Santos RS, Marroqui L, Velayos T et al. DEXI, a candidate gene for type 1 diabetes, modulates rat and human pancreatic beta cell inflammation via regulation of the type I IFN/STAT signalling pathway. Diabetologia, 62(3), 459–472 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Op de Beeck A, Eizirik DL. Viral infections in type 1 diabetes mellitus--why the beta cells? Nat Rev Endocrinol, 12(5), 263–273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nyalwidhe JO, Gallagher GR, Glenn LM et al. Coxsackievirus-Induced Proteomic Alterations in Primary Human Islets Provide Insights for the Etiology of Diabetes. J Endocr Soc, 1(10), 1272–1286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marroqui L, Lopes M, dos Santos RS et al. Differential cell autonomous responses determine the outcome of coxsackievirus infections in murine pancreatic alpha and beta cells. Elife, 4, e06990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engholm-Keller K, Birck P, Storling J, Pociot F, Mandrup-Poulsen T, Larsen MR. TiSH--a robust and sensitive global phosphoproteomics strategy employing a combination of TiO2, SIMAC, and HILIC. J Proteomics, 75(18), 5749–5761 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Li J, Li Q, Tang J, Xia F, Wu J, Zeng R. Quantitative Phosphoproteomics Revealed Glucose-Stimulated Responses of Islet Associated with Insulin Secretion. J Proteome Res, 14(11), 4635–4646 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Kang T, Jensen P, Huang H, Lund Christensen G, Billestrup N, Larsen MR. Characterization of the Molecular Mechanisms Underlying Glucose Stimulated Insulin Secretion from Isolated Pancreatic beta-cells Using Post-translational Modification Specific Proteomics (PTMomics). Mol Cell Proteomics, 17(1), 95–110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sacco F, Humphrey SJ, Cox J et al. Glucose-regulated and drug-perturbed phosphoproteome reveals molecular mechanisms controlling insulin secretion. Nat Commun, 7, 13250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmisano G, Jensen SS, Le Bihan MC et al. Characterization of membrane-shed microvesicles from cytokine-stimulated beta-cells using proteomics strategies. Mol Cell Proteomics, 11(8), 230–243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. *.Yi L, Tsai C-F, Dirice E et al. A Boosting to Amplify Signal with Isobaric Labeling (BASIL) Strategy for Comprehensive Quantitative Phosphoproteomic Characterization of Small Populations of Cells. Analytical Chemistry, (2019).Yi et al. developed a strategy using a boosting sample to obtain a very deep coverage of the phosphoproteome from a limited number of human islets.
- 55.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev, 29(1), 42–61 (2008). [DOI] [PubMed] [Google Scholar]
- 56.Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia, 56(2), 234–241 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Eizirik DL, Cnop M. ER stress in pancreatic beta cells: the thin red line between adaptation and failure. Sci Signal, 3(110), pe7 (2010). [DOI] [PubMed] [Google Scholar]
- 58.D’Hertog W, Maris M, Ferreira GB et al. Novel insights into the global proteome responses of insulin-producing INS-1E cells to different degrees of endoplasmic reticulum stress. J Proteome Res, 9(10), 5142–5152 (2010). [DOI] [PubMed] [Google Scholar]
- 59.Crevecoeur I, Gudmundsdottir V, Vig S et al. Early differences in islets from prediabetic NOD mice: combined microarray and proteomic analysis. Diabetologia, 60(3), 475–489 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Sartori DJ, Wilbur CJ, Long SY et al. GATA factors promote ER integrity and beta-cell survival and contribute to type 1 diabetes risk. Mol Endocrinol, 28(1), 28–39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tersey SA, Nishiki Y, Templin AT et al. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes, 61(4), 818–827 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Engin F, Yermalovich A, Nguyen T et al. Restoration of the unfolded protein response in pancreatic beta cells protects mice against type 1 diabetes. Sci Transl Med, 5(211), 211ra156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marhfour I, Lopez XM, Lefkaditis D et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia, 55(9), 2417–2420 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Khan MW, Sherwani S, Khan WA, Ali R. Characterization of hydroxyl radical modified GAD65: a potential autoantigen in type 1 diabetes. Autoimmunity, 42(2), 150–158 (2009). [DOI] [PubMed] [Google Scholar]
- 65.Mannering SI, Harrison LC, Williamson NA et al. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. The Journal of experimental medicine, 202(9), 1191–1197 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGinty JW, Chow IT, Greenbaum C, Odegard J, Kwok WW, James EA. Recognition of posttranslationally modified GAD65 epitopes in subjects with type 1 diabetes. Diabetes, 63(9), 3033–3040 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kracht MJ, van Lummel M, Nikolic T et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nature medicine, 23(4), 501–507 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Zhang Q, Fillmore TL, Schepmoes AA et al. Serum proteomics reveals systemic dysregulation of innate immunity in type 1 diabetes. The Journal of experimental medicine, 210(1), 191–203 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhi W, Sharma A, Purohit S et al. Discovery and validation of serum protein changes in type 1 diabetes patients using high throughput two dimensional liquid chromatography-mass spectrometry and immunoassays. Mol Cell Proteomics, 10(11), M111 012203 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Toerne C, Laimighofer M, Achenbach P et al. Peptide serum markers in islet autoantibody-positive children. Diabetologia, 60(2), 287–295 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Moulder R, Bhosale SD, Erkkila T et al. Serum proteomes distinguish children developing type 1 diabetes in a cohort with HLA-conferred susceptibility. Diabetes, 64(6), 2265–2278 (2015). [DOI] [PubMed] [Google Scholar]
- 72.Suh MJ, Tovchigrechko A, Thovarai V et al. Quantitative Differences in the Urinary Proteome of Siblings Discordant for Type 1 Diabetes Include Lysosomal Enzymes. J Proteome Res, 14(8), 3123–3135 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Singh H, Yu Y, Suh MJ et al. Type 1 Diabetes: Urinary Proteomics and Protein Network Analysis Support Perturbation of Lysosomal Function. Theranostics, 7(10), 2704–2717 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yi L, Swensen AC, Qian WJ. Serum biomarkers for diagnosis and prediction of type 1 diabetes. Transl Res, 201, 13–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hortin GL, Sviridov D, Anderson NL. High-abundance polypeptides of the human plasma proteome comprising the top 4 logs of polypeptide abundance. Clin Chem, 54(10), 1608–1616 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Buil A, Collins BC et al. Quantitative variability of 342 plasma proteins in a human twin population. Mol Syst Biol, 11(1), 786 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geyer PE, Kulak NA, Pichler G, Holdt LM, Teupser D, Mann M. Plasma Proteome Profiling to Assess Human Health and Disease. Cell Syst, 2(3), 185–195 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Liu CW, Bramer L, Webb-Robertson BJ, Waugh K, Rewers MJ, Zhang Q. Temporal expression profiling of plasma proteins reveals oxidative stress in early stages of Type 1 Diabetes progression. J Proteomics, 172, 100–110 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nie S, Shi T, Fillmore TL et al. Deep-Dive Targeted Quantification for Ultrasensitive Analysis of Proteins in Nondepleted Human Blood Plasma/Serum and Tissues. Anal Chem, 89(17), 9139–9146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferrannini E, Mari A, Nofrate V, Sosenko JM, Skyler JS. Progression to diabetes in relatives of type 1 diabetic patients: mechanisms and mode of onset. Diabetes, 59(3), 679–685 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sosenko JM, Palmer JP, Greenbaum CJ et al. Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care, 29(3), 643–649 (2006). [DOI] [PubMed] [Google Scholar]
- 82.Evans-Molina C, Sims EK, DiMeglio LA et al. β cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight, in press (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sims EK, Chaudhry Z, Watkins R et al. Elevations in the Fasting Serum Proinsulin-to-C-Peptide Ratio Precede the Onset of Type 1 Diabetes. Diabetes Care, 39(9), 1519–1526 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sims E, Bahnson H, Nyalwidhe J et al. Proinsulin Secretion Is a Persistent Feature of Type 1 Diabetes. Diabetes Care, in press (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Courtade JA, Klimek-Abercrombie AM, Chen YC et al. Measurement of Pro-Islet Amyloid Polypeptide (1-48) in Diabetes and Islet Transplants. J Clin Endocrinol Metab, 102(7), 2595–2603 (2017). [DOI] [PubMed] [Google Scholar]
- 86.Guay C, Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol, 9(9), 513–521 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Sims EK, Lakhter A, Anderson-Baucum E, Kono T, Tong X, Evans-Molina C. MicroRNA 21 Targets B Cell Lymphoma 2 (BCL2) mRNA to Increase Beta Cell Apoptosis. Diabetologia, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grieco FA, Sebastiani G, Juan-Mateu J et al. MicroRNAs miR-23a-3p, miR-23b-3p, and miR-149-5p Regulate the Expression of Proapoptotic BH3-Only Proteins DP5 and PUMA in Human Pancreatic beta-Cells. Diabetes, 66(1), 100–112 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weber JA, Baxter DH, Zhang S et al. The microRNA spectrum in 12 body fluids. Clin Chem, 56(11), 1733–1741 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Erener S, Marwaha A, Tan R, Panagiotopoulos C, Kieffer TJ. Profiling of circulating microRNAs in children with recent onset of type 1 diabetes. JCI Insight, 2(4), e89656 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Snowhite IV, Allende G, Sosenko J, Pastori RL, Messinger Cayetano S, Pugliese A. Association of serum microRNAs with islet autoimmunity, disease progression and metabolic impairment in relatives at risk of type 1 diabetes. Diabetologia, 60(8), 1409–1422 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guay C, Kruit JK, Rome S et al. Lymphocyte-Derived Exosomal MicroRNAs Promote Pancreatic beta Cell Death and May Contribute to Type 1 Diabetes Development. Cell Metab, 29(2), 348–361 e346 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Lakhter AJ, Pratt RE, Moore RE et al. Beta cell extracellular vesicle miR-21-5p cargo is increased in response to inflammatory cytokines and serves as a biomarker of type 1 diabetes. Diabetologia, 61(5), 1124–1134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garcia-Contreras M, Shah SH, Tamayo A et al. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci Rep, 7(1), 5998 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cianciaruso C, Phelps EA, Pasquier M et al. Primary Human and Rat beta-Cells Release the Intracellular Autoantigens GAD65, IA-2, and Proinsulin in Exosomes Together With Cytokine-Induced Enhancers of Immunity. Diabetes, 66(2), 460–473 (2017). [DOI] [PubMed] [Google Scholar]
- 96.Melo SA, Luecke LB, Kahlert C et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature, 523(7559), 177–182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flamez D, Roland I, Berton A et al. A genomic-based approach identifies FXYD domain containing ion transport regulator 2 (FXYD2)gammaa as a pancreatic beta cell-specific biomarker. Diabetologia, 53(7), 1372–1383 (2010). [DOI] [PubMed] [Google Scholar]
- 98.Vallabhajosyula P, Korutla L, Habertheuer A et al. Tissue-specific exosome biomarkers for noninvasively monitoring immunologic rejection of transplanted tissue. J Clin Invest, 127(4), 1375–1391 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shao H, Chung J, Lee K et al. Chip-based analysis of exosomal mRNA mediating drug resistance in glioblastoma. Nat Commun, 6, 6999 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee K, Shao H, Weissleder R, Lee H. Acoustic purification of extracellular microvesicles. ACS Nano, 9(3), 2321–2327 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Akirav EM, Lebastchi J, Galvan EM et al. Detection of beta cell death in diabetes using differentially methylated circulating DNA. Proc Natl Acad Sci U S A, 108(47), 19018–19023 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fisher MM, Perez Chumbiauca CN, Mather KJ, Mirmira RG, Tersey SA. Detection of islet beta-cell death in vivo by multiplex PCR analysis of differentially methylated DNA. Endocrinology, 154(9), 3476–3481 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lehmann-Werman R, Neiman D, Zemmour H et al. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci U S A, 113(13), E1826–1834 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Herold KC, Usmani-Brown S, Ghazi T et al. beta cell death and dysfunction during type 1 diabetes development in at-risk individuals. J Clin Invest, 125(3), 1163–1173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Olsen JA, Kenna LA, Spelios MG, Hessner MJ, Akirav EM. Circulating Differentially Methylated Amylin DNA as a Biomarker of beta-Cell Loss in Type 1 Diabetes. PLoS One, 11(4), e0152662 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sklenarova J, Petruzelkova L, Kolouskova S, Lebl J, Sumnik Z, Cinek O. Glucokinase Gene May Be a More Suitable Target Than the Insulin Gene for Detection of beta Cell Death. Endocrinology, 158(7), 2058–2065 (2017). [DOI] [PubMed] [Google Scholar]
- 107.Waanders LF, Chwalek K, Monetti M, Kumar C, Lammert E, Mann M. Quantitative proteomic analysis of single pancreatic islets. Proc Natl Acad Sci U S A, 106(45), 18902–18907 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L, Lanzoni G, Battarra M, Inverardi L, Zhang Q. Proteomic profiling of human islets collected from frozen pancreata using laser capture microdissection. J Proteomics, 150, 149–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nyalwidhe JO, Grzesik WJ, Burch TC et al. Comparative quantitative proteomic analysis of disease stratified laser captured microdissected human islets identifies proteins and pathways potentially related to type 1 diabetes. PLoS One, 12(9), e0183908 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huang EL, Piehowski PD, Orton DJ et al. SNaPP: Simplified Nano-Proteomics Platform for reproducible global proteomic analysis of nanogram protein quantities. Endocrinology, en20151821 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clair G, Piehowski PD, Nicola T et al. Spatially-Resolved Proteomics: Rapid Quantitative Analysis of Laser Capture Microdissected Alveolar Tissue Samples. Sci Rep, 6, 39223 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang H, Qian WJ, Mottaz HM et al. Development and evaluation of a micro- and nanoscale proteomic sample preparation method. J Proteome Res, 4(6), 2397–2403 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li S, Plouffe BD, Belov AM et al. An Integrated Platform for Isolation, Processing, and Mass Spectrometry-based Proteomic Profiling of Rare Cells in Whole Blood. Mol Cell Proteomics, 14(6), 1672–1683 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen Q, Yan G, Gao M, Zhang X. Ultrasensitive Proteome Profiling for 100 Living Cells by Direct Cell Injection, Online Digestion and Nano-LC-MS/MS Analysis. Anal Chem, 87(13), 6674–6680 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Li ZY, Huang M, Wang XK et al. Nanoliter-Scale Oil-Air-Droplet Chip-Based Single Cell Proteomic Analysis. Anal Chem, 90(8), 5430–5438 (2018). [DOI] [PubMed] [Google Scholar]
- 116.Budnik B, Levy E, Harmange G, Slavov N. SCoPE-MS: mass spectrometry of single mammalian cells quantifies proteome heterogeneity during cell differentiation. Genome Biol, 19(1), 161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. **.Zhu Y, Piehowski PD, Zhao R et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10-100 mammalian cells. Nat Commun, 9(1), 882 (2018).Zhu et al showed that deep proteomics coverage can be obtained from a slice of laser-capture microdissected human islet.
- 118.Dybala MP, Hara M. Heterogeneity of the Human Pancreatic Islet. Diabetes, 68(6), 1230–1239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dou M, Zhu Y, Liyu A et al. Nanowell-mediated two-dimensional liquid chromatography enables deep proteome profiling of <1000 mammalian cells. Chemical Science, 9(34), 6944–6951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Metz TO, Baker ES, Schymanski EL et al. Integrating ion mobility spectrometry into mass spectrometry-based exposome measurements: what can it add and how far can it go? Bioanalysis, 9(1), 81–98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. *.Zhang X, Romm M, Zheng X et al. SPE-IMS-MS: An automated platform for sub-sixty second surveillance of endogenous metabolites and xenobiotics in biofluids. Clin Mass Spectrom, 2, 1–10 (2016).This article introduced the concept of solid phase extraction coupled to ion mobility mass spectrometry for fast analysis of samples, enabling to perform thousands of measurements a day.
- 122.Meier F, Brunner AD, Koch S et al. Online Parallel Accumulation-Serial Fragmentation (PASEF) with a Novel Trapped Ion Mobility Mass Spectrometer. Mol Cell Proteomics, 17(12), 2534–2545 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Webb IK, Garimella SV, Tolmachev AV et al. Experimental evaluation and optimization of structures for lossless ion manipulations for ion mobility spectrometry with time-of-flight mass spectrometry. Anal Chem, 86(18), 9169–9176 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dou M, Chouinard CD, Zhu Y et al. Nanowell-mediated multidimensional separations combining nanoLC with SLIM IM-MS for rapid, high-peak-capacity proteomic analyses. Anal Bioanal Chem, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bendall SC, Simonds EF, Qiu P et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science, 332(6030), 687–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bodenmiller B Multiplexed Epitope-Based Tissue Imaging for Discovery and Healthcare Applications. Cell Syst, 2(4), 225–238 (2016). [DOI] [PubMed] [Google Scholar]
- 127. **.Wang YJ, Golson ML, Schug J et al. Single-Cell Mass Cytometry Analysis of the Human Endocrine Pancreas. Cell Metab, 24(4), 616–626 (2016).Wang et al. used flow cytometry couple to mass cytometry for a deep characterization of the endocrine cell types of the islets of Langerhans.
- 128.Cnop M, Hughes SJ, Igoillo-Esteve M et al. The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia, 53(2), 321–330 (2010). [DOI] [PubMed] [Google Scholar]
- 129.Barcenilla H, Akerman L, Pihl M, Ludvigsson J, Casas R. Mass Cytometry Identifies Distinct Subsets of Regulatory T Cells and Natural Killer Cells Associated With High Risk for Type 1 Diabetes. Front Immunol, 10, 982 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ahmed R, Omidian Z, Giwa A et al. A Public BCR Present in a Unique Dual-Receptor-Expressing Lymphocyte from Type 1 Diabetes Patients Encodes a Potent T Cell Autoantigen. Cell, 177(6), 1583–1599 e1516 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. **.Wang YJ, Traum D, Schug J et al. Multiplexed In Situ Imaging Mass Cytometry Analysis of the Human Endocrine Pancreas and Immune System in Type 1 Diabetes. Cell Metab, 29(3), 769–783 e764 (2019).In a parallel publication with Damond et al., the authors use imaging mass cytometry to assess the intra-individual variability of islets and the dynamics of subpopulations of cells in islets in type 1 diabetes.
- 132. **.Damond N, Engler S, Zanotelli VRT et al. A Map of Human Type 1 Diabetes Progression by Imaging Mass Cytometry. Cell Metab, 29(3), 755–768 e755 (2019).In a parallel publication with Wang et al., the authors use imaging mass cytometry to assess the intra-individual variability of islets and the dynamics of subpopulations of cells in islets in type 1 diabetes.
- 133.Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B. Quantitative mass spectrometry in proteomics: a critical review. Anal Bioanal Chem, 389(4), 1017–1031 (2007). [DOI] [PubMed] [Google Scholar]
- 134.Shi T, Song E, Nie S et al. Advances in targeted proteomics and applications to biomedical research. Proteomics, 16(15-16), 2160–2182 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu C, Duan J, Liu T, Smith RD, Qian WJ. Contributions of immunoaffinity chromatography to deep proteome profiling of human biofluids. J Chromatogr B Analyt Technol Biomed Life Sci, 1021, 57–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu BJ. Combining laser capture microdissection and proteomics: methodologies and clinical applications. Proteomics Clin Appl, 4(2), 116–123 (2010). [DOI] [PubMed] [Google Scholar]