Abstract
Nutmeg is a Traditional Chinese Medicine used to treat gastrointestinal diseases. Some reports have indicated that nutmeg has hepatoprotective activity. In this study, a thioacetamide (TAA)-induced acute liver injury model in mice was used to explore the mechanism of the protective effects of nutmeg extract (NME), including its major bioactive component myrislignan. The results indicated that NME could effectively protect TAA-induced liver damage as assessed by recovery of increased serumtransaminases, decrease in hepatic oxidative stress, and lower hepatic inflammation. Metabolomics analysis further revealed that treatment with NME led to the recovery of a series of lipids including lysophosphatidylcholines that were decreased and a lowering of acylcarnitines that were increased in mouse plasma and liver after TAA exposure. Gene expression analysis demonstrated that the hepatoprotective effect of NME was achieved by modulation of the peroxisome proliferator-activated receptor alpha (PPARα) as well as the decrease in oxidative stress. NME could not protect from TAA-induced liver injury in Ppara-null mice, suggesting that its protective effect was dependent on PPARα. Myrislignan, a representative neolignan in nutmeg, showed potent protective activity against TAA-induced liver toxicity. These data demonstrate that nutmeg alleviates TAA-induced liver injury through the modulation of PPARα and that the lignan compounds in nutmeg such as myrislignan partly contributed to this action.
Keywords: nutmeg, hepatoprotective activity, metabolomics, myrislignan, PPARα
Graphical Abstract
INTRODUCTION
Nutmeg, the seed of Myristica fragrans Houtt., is a Traditional Chinese Medicine (TCM) used for the treatment of gastrointestinal disorders.1 Nutmeg is also used for asthma, alleviation of rheumatic pain, and treatment of toothaches and infections.2 Additionally, nutmeg is a component in some combination TCMs, including Huangzhang Ruangan Granule,3 Changkang Granules,4 and Jiagasong Tang.5 Nutmeg contains numerous constituents, such as essential oil (monoterpenes, phenylpropanoids, myristicin, elemicin, safrole, eugenol, methylisoeugenol), lignans and neolignans, diphenylalkanes, phenylpropanediols, steroids, and cyclobutanones.1 Pharmacological studies revealed that nutmeg extract (NME) and some specific compounds in NME had various pharmacological activities. The aqueous extract of nutmeg can protect rats against hyperlipidaemia, hyperglycaemia, cardiac tissue damage, as well as hepatotoxicity induced by isoproterenol.6,7 The acetone extract of M. fragrans aril showed antibacterial activity.8 The ethanol extract of nutmeg exhibits hypolipidemic activity on experimentally induced hyperlipidemia in albino rabbits.9 The n-hexane extract of M. fragrans seeds had significant antidepressant activity in mice by interaction with the adrenergic, serotonergic, and dopaminergic systems10 and enhanced memory in mice.11 A recent study revealed that the supercritical CO2 extract of nutmeg showed therapeutic effects on colon cancer through its anti-inflammatory and antibacterial effects.12 Neolignans were identified as the bioactive components of nutmeg, which show similar pharmacological effects to nutmeg. In vitro assays indicated that two types of 8-O-4′ and benzofuran neolignans in nutmeg may have anti-inflammatory effects by inhibiting nitro oxide production.13 The antioxidant ability of M. fragrans may be associated with inhibition of the superoxide free radicals activity and lipid peroxidation in rat.14 It was found that nectandrin B could prevent tert-butylhydroperoxide-stimulated apoptosis in both HepG2 cells and primary mouse hepatocytes through the activation of NF-E2-related factor-2 (NRF2) and upregulation of expression of some antioxidant enzymes.15 Macelignan from M. fragrans can abrogate the cisplatin-induced phosphorylation of c-Jun N-terminal kinase 1/2 (JNK1/2) and extracellular signal-regulated kinase1/2 (ERK1/2), which may be associated with activation of the MAPK signaling pathway.16
In the present study, the hepatoprotective effect of NME was investigated using the thioacetamide (TAA)-induced acute liver injury in mice. TAA has been used widely for the study of acute liver injury in animal models because it was identified as a hepatotoxicant in 1948.17-19 Its bioactivation requires S-oxidation by CYP2E1 to TA sulfoxide (TASO) and the reactive metabolite sulfdioxide (TASO2), which modifies amine lipids and proteins leading to hepatic necrosis.20 Recently, metabolomics was employed to systematically profile the levels of endogenous metabolites in biological fluids, cells, tissues, or organs,21 and has been for mapping drug metabolism and identifying endogenous metabolic pathways affected by drugs and toxicants, and therefore this methodology can assist in the screening of therapeutic targets. Using this approach, the present study revealed changes in lipids upon TAA-induced liver injury, and demonstrated that NME can protect against liver injury via the modulation of the peroxisome proliferator-activated receptor alpha (PPARα). More importantly, myrislignan (MRL), a major 8-O-4′ neolignans in nutmeg, was found to be partly responsible for the hepatoprotective action of NME.
EXPERIMENTAL SECTION
Chemicals and Reagents
NME was prepared using CO2 supercritical extraction according to a previous report.12 MRL was obtained from Beta Biotechnology (Nanchang, China). TAA, formic acid, chlorpropamide, and sodium carboxymethylcellulose were purchased from Sigma-Aldrich (St. Louis, MO). Palmitoylcarnitine (16:0-carnitine), stearoyl-carnitine (18:0-carnitine), and oleoyl-lysophosphatidylcholine (18:1-LPC) were purchased from Sigma-Aldrich (St. Louis, MO), and stearoyl-lysophosphatidylcholine (18:0-LPC) was purchased from Shanghai Aladdin Bio-Chem Technology (Shanghai, China). Kits for testing of alanine aminotransferase (ALT), aspartate aminotransferase (AST) were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and the kits for measuring catalase (CAT) activity and hydrogen peroxide (H2O2) and malondialdehyde (MDA) levels were purchased from Suzhou Comin Biotechnology (Suzhou, China). All solvents and organic reagents were of the highest grade commercially available.
Animal Experiments
Wild-type C57BL/6J mice (6- to 7-week-old males, 20–22 g) were purchased from the Kunming Institute of Zoology, Chinese Academy of Sciences (Yunnan, China). Wild-type (Ppara+/+) and Ppara-null (Ppara−/−) mouse lines on the Sv/129 genetic background (6- to 7-week-old males, 20–23 g) were previously described.22 The mice were acclimatized for at least 1 week in cages at 23 ± 1 °C with a light/dark cycle of 12/12 h and humidity 50–60%. All procedures were conducted according to study protocols approved by the Institutional Animal Care and Use Committee of the Kunming Institute of Botany, Chinese Academy of Sciences. The dosages of NME and MRL were determined and optimized according to the Chinese Pharmacopoeia23 and previous studies. 12,24
Experiment 1:
To evaluate the hepatoprotective effects of NME, 15 C57BL/6J mice were used. The mice were randomly divided into three groups (n = 5). Group 1 (defined as TAA + NME group): NME was suspended in 0.5% sodium carboxymethylcellulose (CMC-Na). Mice were administered 250 mg/kg NME by intragastrical gavage three times at 24 h intervals before TAA treatment. One h after the last NME treatment, the mice were treated with 250 mg/kg TAA (TAA was dissolved in 0.9% NaCl solution) by intraperitoneal injection. Group 2 (defined as TAA group): Mice were administered with 0.5% CMC-Na by gavage as vehicle three times at 24 h intervals before TAA treatment. One h after the last treatment, 250 mg/kg of TAA was administered by intraperitoneal injection. Group 3 (defined as control group): Mice were treated with 0.5% CMC-Na by gavage as vehicle three times at 24 h intervals and received 0.9% NaCl by intraperitoneal injection 1 h after the last gavage. After 24 h of TAA treatment, whole blood was collected into an anti-coagulative tube by retro-orbital bleeding. Plasma was obtained by centrifugation of the blood at 2000g for 5 min at 4 °C. Mice were then killed by CO2 asphyxiation and the liver tissue was excised, weighted, and snap frozen in liquid nitrogen, then stored at −80 °C. Hepatic index was calculated according to the following formula: hepatic index = (liver weight/body weight) × 100. A portion of liver was excised and preserved in 10% buffered formalin for histological analysis, and the remaining liver was flash-frozen in liquid nitrogen.
Experiment 2:
The therapeutic effect of NME was also studied. In this study, a total of 20 C57BL/6J mice were used. The mice were randomly divided into four groups, with five mice per group. First, all animals were administered with 250 mg/kg TAA by intraperitoneal injection at zero time. Group 1 (TAA group): Mice were then treated with 0.5% CMC-Na by intragastrical gavage. Groups 2–4: Mice were administered 250 mg/kg of NME by intragastrical gavage after 1, 6, and 12 h of TAA treatment, respectively. Twenty-four h after TAA treatment, all mice were killed by CO2 asphyxiation and whole blood was collected. The preparation of plasma was the same as in Experiment 1.
Experiment 3:
To verify the therapeutic target of NME, wild-type (Ppara+/+) and Ppara-null (Ppara−/−) mice on the Sv/129 genetic background were used. The grouping and treatment methods were the same as in Experiment 1 (n = 5). After sampling, hepatic index and serum ALT and AST activities were determined.
Experiment 4:
For testing the hepatoprotective effect of MRL, another 15 C57BL/6J mice (6- to 7-week-old males, 20–22 g) were randomly divided into three groups (n = 5), and the groups were designated as TAA + MRL, TAA, and control. MRL was suspended in 0.5% CMC-Na. The TAA + MRL group was administered 200 mg/kg of MRL three times every 24 h by intragastrical gavage, and the TAA and control groups received only the 0.5% CMC-Na vehicle. One h after the last treatment, the TAA + MRL and TAA groups were intra-peritoneally injected with 250 mg/kg of TAA, and the control group was injected with 0.9% NaCl. Plasma samples were collected at the end of experiment for testing the ALT and AST activities.
Sample Preparation and UPLC-ESI-QTOFMS Analysis
Sample preparation for UPLC-ESI-QTOFMS analysis was carried out according to a previous report.25 Ten μL of plasma was added to 190 μL of 67% acetonitrile containing 5 μM of chlorpropamide (internal standard) and vortexed for 1 min. The mixture was then centrifuged at 18 000g for 20 min at 4 °C. For liver samples, ~20 mg of frozen liver tissue was homogenized with a 10-fold dilution of 50% aqueous acetonitrile (containing 5 μM of chlorpropamide) and shaken at room temperature for 20 min. The samples were then centrifuged at 18 000g for 20 min. Each liquid supernatant (150 μL) was transferred to a new tube and diluted with 150 μL of pure acetonitrile. The diluted solutions were centrifuged again under the same condition. The supernatants were prepared for testing. A 5 μL aliquot of each sample was injected into UPLC-ESI-QTOFMS (Agilent, Santa Clara, CA) for metabolomics analysis. The working conditions of UPLC-ESI-QTOFMS were the same as in a previous report.25
Plasma Chemistry Analysis and Histological Examination
The levels of ALT, AST, and CAT activities and H2O2 and MDA contents in plasma were tested by colorimetric method using manufactured kits according to the guidelines. Relative glutathione (GSH) contents were evaluated by measuring the abundance of its peak area in the plasma. The liver specimens were processed by soaking in increasing alcohol concentrations (75, 85, 95, and 100% ethanol), cleared in xylene, and embedded in paraffin. 5 μm thick sections were sliced and stained with hematoxylin and eosin (H&E). Tissue damage and inflammatory infiltration were observed under light microscopy (Eclipse E200, Nikon, Japan).
Multivariate Data Analysis and Biomarkers Identification
Chromatographic and spectral data were extracted by Mass-Hunter Workstation data Acquisition software (Agilent, Santa Clara, CA). Data alignment was performed by Mass Profiler Professional software (Agilent, Santa Clara, CA) and was used to generate a data matrix with peak areas corresponding to a sample ID, unique m/z, and retention time. The relative abundance of exported data was calculated based on the peak areas on ion counts after normalizing with the peak area of the internal standard. These normalized data matrices were further introduced to the SIMCA-P+13.0 software (Umetrics, Kinnelon, NJ) for partial least-squares discriminant analysis (PLS-DA). Potential endogenous metabolites were initially identified by analyzing the ions that contributed to the separation from each group in the loading scatter plot and further matched their molecular formula and MS/MS fragmentations in the online database, including HMDB and METLINE. Additionally, the changed metabolites were compared with the MS/MS fragmentation patterns of authentic compounds, as detailed in previous reports.25,26
Gene Expression Analysis
Real-time fluorogenic quantitative PCR was performed to quantify the levels of gene expression in the liver tissue. Total RNA was extracted using TRIzol reagent (Life Technologies, Carlsbad, CA) from the homogenized liver tissue on ice. After DNase digestion at 42 °C for 2 min, complementary DNA was generated from total RNA using Superscript II reverse transcriptase (TaKaRa, Dalian, China). Quantitative RT-PCR was conducted using SYBR green PCR master mix (TaKaRa, Dalian, China) in CFX Connect Real-Time System (Bio-Rad Laboratories, USA). Messenger RNA levels were normalized to those of Actb mRNA Thermal cycling condition was set as 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s, 55 °C for 30 s, and 72 °C for 40 s. QPCR primer sequences are listed in Supplementary Table 1.
Data Analysis
The experimental values were presented as mean ± SEM. Statistical analysis was performed by using Prism v. 6 (GraphPad Software, San Diego, CA). Significant differences were determined by p value < 0.05.
RESULTS
Hepatoprotective Effects of Nutmeg Extract on Thioacetamide-Induced Acute Liver Injury
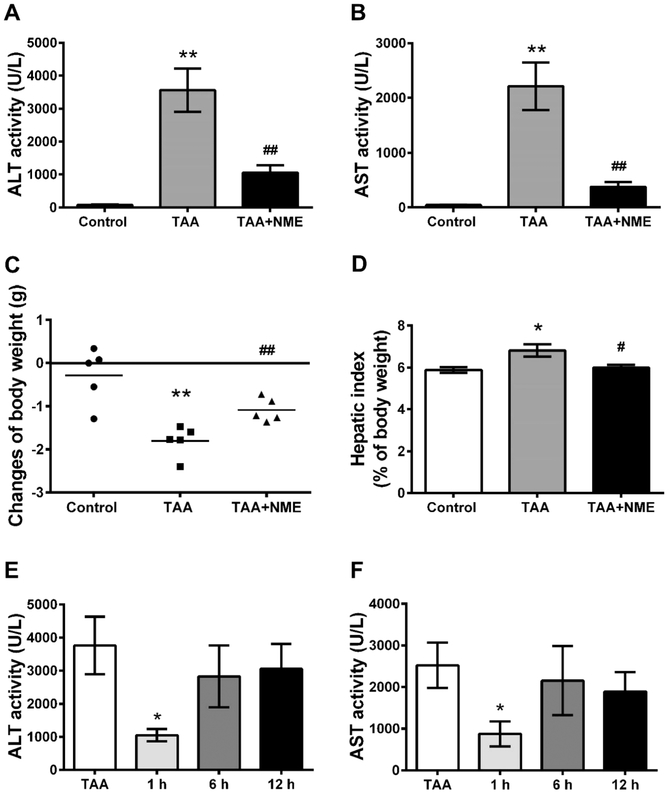
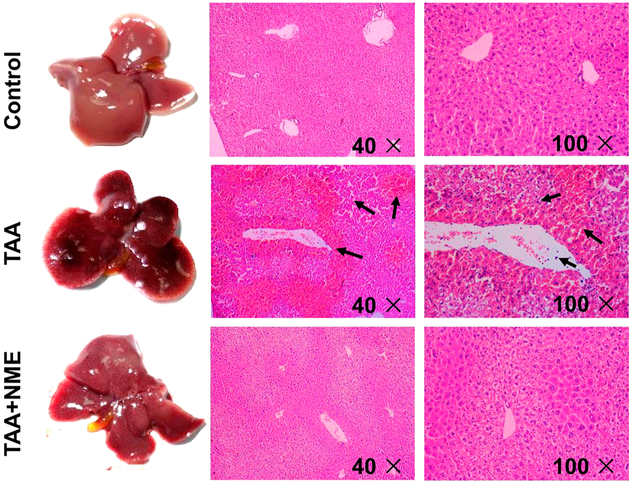
TAA has long been used as a classical experimental hepatotoxicant that can induce acute and chronic liver injury.17-19 In this study, plasma ALT and AST activities in the control group were 80 and 57 U/L, respectively. A single dose of TAA exposure significantly increased the activities of both transaminases to 3560 and 2210 U/L, respectively, indicating severe hepatotoxicity induced by TAA. NME remarkably decreased ALT and AST activities to 1050 and 377 U/L, respectively (p < 0.01) (Figure 1A,B). In addition, body weights of mice were reduced after 24 h of TAA treatment, and hepatic index was elevated (Figure 1C,D) in the TAA group. NME remarkably decreased these changes in the TAA + NME group. In addition, the therapeutic role of NME was investigated in the TAA-induced acute liver injury. After 1 h of TAA treatment, NME could inhibit the increase in transaminases (ALT and AST) in TAA-induced mouse plasma (Figure 1E,F). However, there were no effects after 6 and 12 h. The tissue color of TAA group mice was dark, whereas the NME-pretreated mouse liver was similar to that of control animals (Figure 2). Histological analysis by H&E staining revealed that livers from the TAA-treated mice exhibited extensive focal necrosis, cell membrane damage, irregularly arranged bile duct, and large inflammatory cell infiltration around the bile ducts, while NME pretreatment alleviated these symptoms (Figure 2). These results demonstrated that NME could protect TAA-induced acute liver injury.
Figure 1.
Effects of NME on TAA-induced liver injury after pre- and post-treatment in C57BL/6J mice. (A,B) Plasma ALT and AST activities in pretreatment status. (C) Changes of body weight during the last 24 h treatment period and (D) hepatic index (percentage of body weight) of animals in pretreatment status. (E,F) Plasma ALT and AST activities in post-treatment status. Mice were received NME after 1, 6, or 12 h of TAA administration. In TAA-treated group, ALT and AST activities in plasma and hepatic index increased significantly, and pretreatment with NME obviously inhibited the activities of the two transaminases and decreased hepatic index. Reduction in body weight was alleviated by NME. NME protected transaminases within 1 h after TAA administration. *p < 0.05 as compared with control group, **p < 0.01 as compared with control group, #p < 0.05 as compared with TAA-treated group, and ##p < 0.01 as with TAA-treated group.
Figure 2.
Histological examination. Livers from mice administered a single dose of TAA showed dark color and larger cholecyst; pretreatment with NME before TAA administration resembles that of normal control. In TAA-treated mouse liver sections, the arrows indicate necrocytosis and neutrophil infiltration. NME treatment protected the histomorphology of liver and reduced inflammatory cells infiltration.
Thioacetamide-Induced Decreased Lysophosphatidylcholine Metabolism in Liver Injury Was Recovered by Nutmeg Extract Treatment
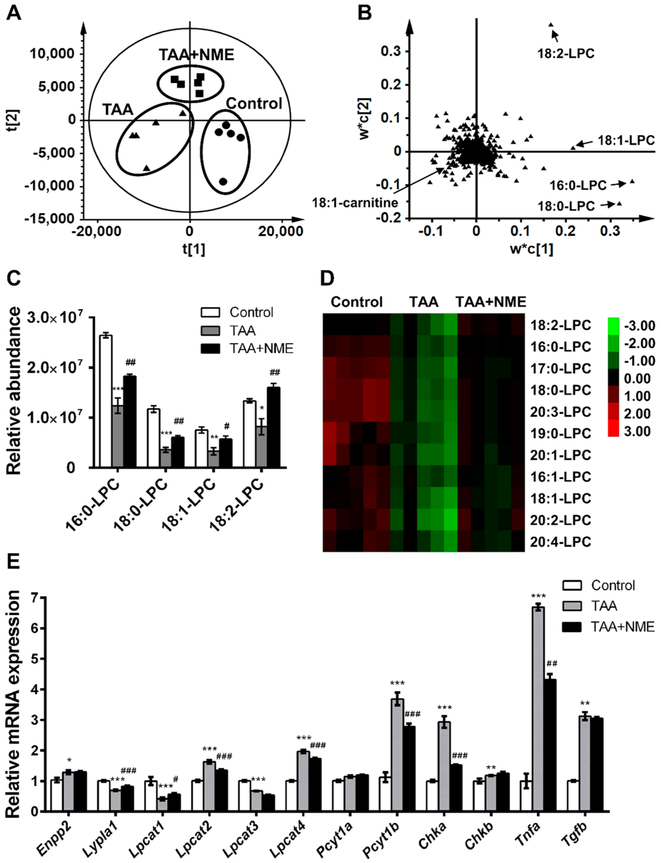
To better understand the effect of NME on TAA-induced liver injury, metabolomics was performed using UPLC-ESI-QTOFMS to discover potential NME therapeutic biomarkers. LC–MS-based metabolomics is a powerful tool to investigate multiple diseases such as hyperlipidemia,27 inflammatory bowel disease,28 and diabetes.29 In this study, PLS-DA was applied to analyze the plasma and hepatic data sets from control, TAA, and TAA + NME groups. The metabolite differences contributing to the separation of the three groups could be determined from the loading scatter plot of the PLS-DA model. The clustering of the three groups discriminated them from each other in the positive ESI mode (Figure 3A). In addition, four ions, 496.3395+, 524.3713+, 522.3568+, and 520.3403+, were separated from the ion cloud in the loading scatter plot (Figure 3B). The separated ions were identified as palmitoyl-lysophosphatidylcholine (16:0-LPC), stearoyl-lysophosphatidylcholine (18:0-LPC), oleoyl-lysophosphatidylcholine (18:1-LPC), and linoleoyl-lysophosphatidylcholine (18:2-LPC) by searching the HMDB and METLIN databases, and the MS/MS data of metabolites were confirmed as described in a previous study.25,26 In the TAA-induced group, these LPCs were significantly decreased as compared with the control group, whereas NME inhibited the decrease in these compounds (Figure 3C).
Figure 3.
Metabolomics analysis of phospholipids in mouse plasma. (A) PLS-DA model of plasma samples from control group, TAA-treated group, and NME protected group under ESI+ mode. These three groups were well separated from each other. (B) Loading scatter plot of PLS-DA model. Metabolites contributing to the separation of three groups were labeled. (C) NME significantly recovered the phospholipid homeostasis in mouse plasma. (D) Heat map analysis of the relative abundances of phospholipids. (E) QPCR analysis of mRNAs involved in LPC metabolism and PC synthesis. The t[1] and t[2] values express the scores of each sample in principal components 1 and 2, respectively. The w*c[1] and w*c[2] values express the contributing weights of each ion to principal components 1 and 2. *p < 0.05 as compared with control group, **p < 0.01 as compared with control group, ***p < 0.001 as compared with control group, #p < 0.05 as compared with TAA-treated group, ##p < 0.01 as compared with TAA-treated group, and ###p < 0.001 as compared with TAA-treated group.
A total of 11 LPCs were extracted and their relative abundance was normalized to internal standard (Figure 3D). Among the metabolites, nine LPCs were significantly increased (p < 0.05) by NME treatment in TAA-induced model, including 16:0-LPC, 17:0-LPC, 18:0-LPC, 18:1-LPC, 18:2-LPC, 19:0-LPC, 20:1-LPC, 20:2-LPC, and 20:3-LPC. In the NME-treated group, 16:1-LPC and 20:4-LPC were partially recovered. Similarly, among the ten extracted LPCs from the liver, seven were remarkably recovered by NME treatment (Figure S-1A). The identities of the changed LPCs are summarized in Table S-2 and the MS/MS fragmentation pattern of authentic 18:0-LPC and 18:1-LPC is shown in Figure S-2A,B. Because liver is the primary site for LPC biosynthesis, the expression levels of hepatic mRNAs encoding enzymes involved in LPC synthesis, metabolism, degradation, or hydrolysis including ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2), lysophospholipase A1 (LYPLA1), lysophosphatidylcholine acyltransferases 1–4 (LPCAT1–4), phosphate cytidylyltransferase 1a and b (PCYT1a and PCYT1b), and choline kinase A and B (CHKA/B) were measured. The expression of Enpp2, Lpcat2, Lpcat4, Pcyt1b, Chka, and Chkb mRNAs was significantly elevated by TAA, and the increases were reversed by NME, except Enpp2 and Chkb mRNAs. Decreased Lypla1, Lpcat1, and Lpcat3 mRNAs after TAA administration was observed, and NME could recover the expression of Lypla1 and Lpcat1 mRNAs, while the expression of Pcyt1a mRNA was not influenced by TAA and NME (Figure 3E).
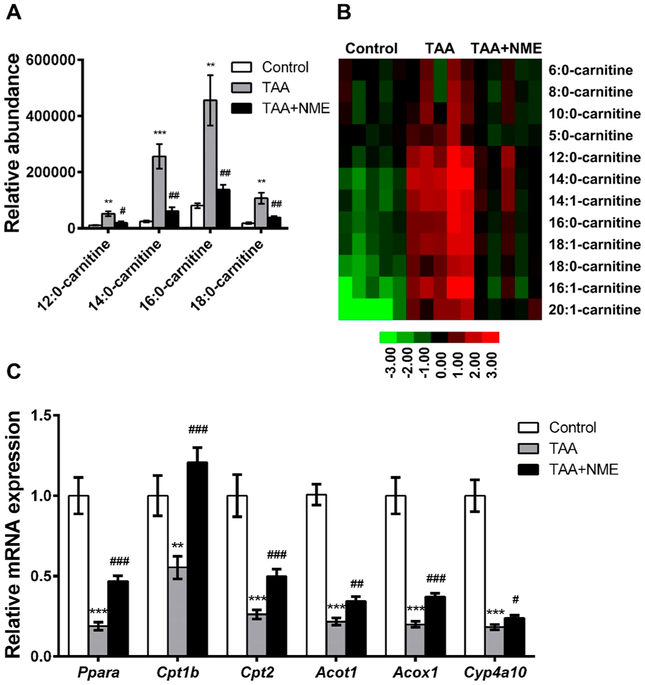
Thioacetamide-Induced Increases in Acylcarnitine Were Reduced by Nutmeg Extract Treatment
In addition to the decrease in LPCs in the loading scatter plot, the m/z 426.3758+ ion, identified as 18:1-carnitine, was increased in the TAA group (Figure 3B). Targeted metabolomics analysis revealed that 12 acylcarnitines were increased in mouse plasma of the TAA group, including 5:0-carnitine, 6:0-carnitine, 8:0-carnitine, 10:0-carnitine, 12:0-carnitine, 14:0-carnitine, 16:0-carnitine, 18:0-carnitine, 14:1-carnitine, 16:1-carnitine, 18:1-carnitine, and 20:1-carnitine. All increased acylcarnitines in plasma of the TAA group were lower in the TAA + NME-treated group (Figure 4A,B). Consistently, the acylcarnitine levels in livers of the TAA-induced group were also reduced by NME treatment (Figure S-1B). The identities of the acylcarnitines are listed in Table S-3 and the MS/MS fragmentation pattern of authentic 16:0-carnitine and 18:0-carnitine is shown in Figure S-2C,D. The mRNA expression of peroxisome proliferator-activated receptor alpha (Ppara) and its downstream target gene mRNAs, including carnitine palmitoyltransferase 1b and 2 (Cpt1b and Cpt2), acyl-CoA thioesterase 1 (Acot1), acyl-Co-A oxidase 1 (Acox1), and Cyp4a10, were measured, and, as expected, these mRNAs were decreased in the TAA group, while NME treatment elevated their expression (Figure 4C).
Figure 4.
Metabolomics analysis of acylcarnitines in mouse plasma. NME recovered the abnormal metabolism of acylcarnitine. (A) NME significantly recovered the acylcarnitines homeostasis in mouse plasma. (B) Heat map analysis of the relative abundances of acylcarnitines. (C) Expression of acylcarnitine transfer-related mRNAs. *p < 0.05 as compared with control group, **p < 0.01 as compared with control group, ***p < 0.001 as compared with control group, #p < 0.05 as compared with TAA-treated group, ##p < 0.01 as compared with TAA-treated group, and ###p < 0.001 as compared with TAA-treated group.
Hepatoprotective Effects of Nutmeg Extract Were Dependent on PPARα
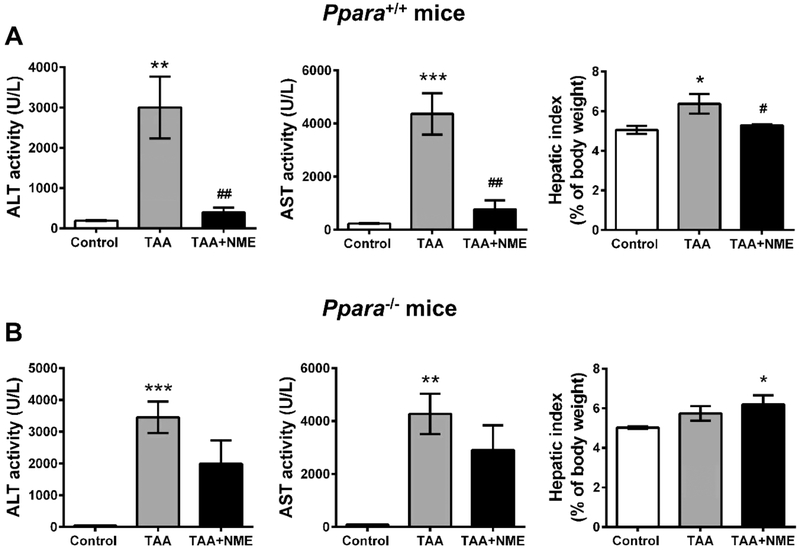
Both metabolomic data and the genetic data indicated that the effects of NME might be dependent on PPARα. To demonstrate this hypothesis, the hepatoprotective effects of NME on wild-type (Ppara+/+) and Ppara-null (Ppara−/−) mice were examined. ALT and AST activities were significantly increased in both Ppara+/+ and Ppara−/− mice, indicating that both mouse lines were sensitive to TAA (Figure 5A,B). NME inhibited the increased ALT and AST activities in Ppara+/+ mice (Figure 5A) and only slightly decreased the activities in Ppara−/− mice compared with the TAA-treated group (p > 0.05) (Figure 5B). The hepatic index was also protected by NME in Ppara+/+ mice but not affected in Ppara−/− mice. These results demonstrated that PPARα is an important target in the hepatoprotective effect of NME in the TAA-induced acute liver injury model.
Figure 5.
NME could protect TAA-induced liver injury via PPARα. Male Sv/129 wild-type (Ppara+/+) and Ppara-null (Ppara−/−) mice were treated with vehicle (control group) or TAA (TAA group) or pretreated with NME before TAA-induced (TAA + NME group). (A) Plasma ALT, AST activities, and hepatic index of Ppara+/+ mice. (B) Plasma ALT, AST activities, and hepatic index of Ppara−/− mice. NME remarkably protected TAA-induced liver injury in Ppara+/+ mice but showed slight protective effects in Ppara−/− mice without significant difference as compared with TAA-treated group. *p < 0.05 as compared with control group, **p < 0.01 as compared with control group, ***p < 0.001 as compared with control group, #p < 0.05 as compared with TAA-treated group, and ##p < 0.01 as compared with TAA-treated group.
Nutmeg Extract Attenuated Oxidative Stress in Liver Injury
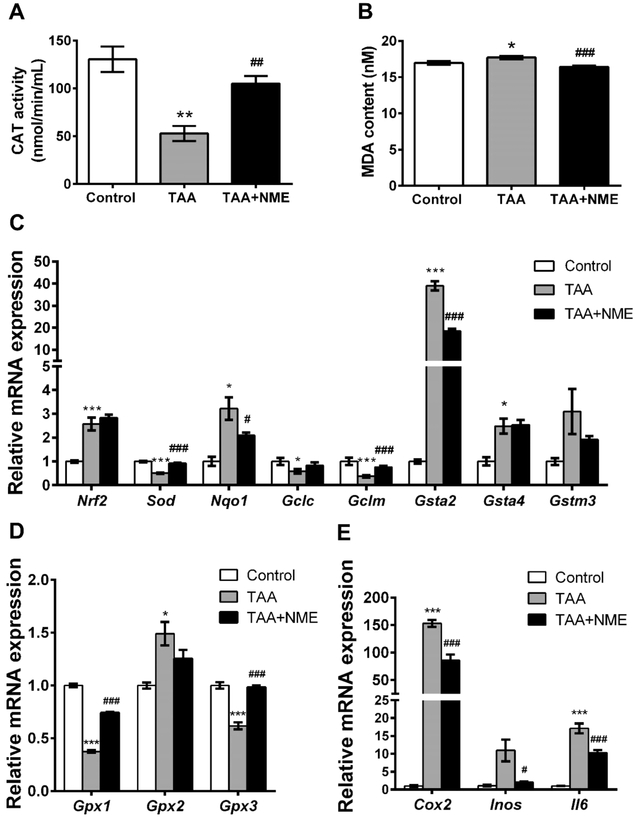
TASO2, the primary metabolite of TAA, has been considered as a toxic metabolite that can adduct to biomacromolecules and can cause oxidative stress.20,30 In the present study, CAT activity in the TAA-treated group mouse plasma was inhibited and was increased by NME treatment (Figure 6A). MDA, the lipid peroxide product, was significantly increased (p < 0.05) in plasma of the TAA group mice but markedly decreased by NME treatment (Figure 6B). In addition, the content of H2O2 in plasma was slightly increased in the TAA-treated group (without statistical significance), and the reduced GSH level was decreased, respectively. Although their levels were partly recovered by NME treatment, there were no significant differences (Figure S-3A,B). These results demonstrated that the oxidative stress induced by TAA was attenuated by NME.
Figure 6.
NME eliminated oxidative stress and inflammation in C57BL/6J mice. (A) CAT activity and (B) MDA content in mouse plasma. (C) QPCR analysis of the expression of NRF2 target genes. (D) QPCR analysis of the gene expression of Gpx’s. (E) QPCR analysis of Cox2, Inos, and Il6 mRNAs. The represented fold changes were normalized to control. *p < 0.05 as compared with control group, **p < 0.01 as compared with control group, ***p < 0.001 as compared with control group, #p < 0.05 as compared with TAA-treated group, ##p < 0.01 as compared with TAA-treated group, and ###p < 0.001 as compared with TAA-treated group.
Subsequently, the mRNA expression levels of some antioxidative genes were measured. NF-E2-related factor 2 (Nrf2) mRNA was significantly upregulated by TAA treatment (Figure 6C), indicating the presence of oxidative stress. Elevated Nqo1 mRNA expression in the TAA group was inhibited by NME, demonstrating that increased oxidative stress was alleviated. The expression level of superoxide dismutase (Sod) mRNA was decreased in the TAA-treated group, and its level was recovered in the NME-treated group. Similarly, the glutathione peroxidase1/3 (Gpx1/3) mRNAs decreased by TAA were significantly recovered by NME (Figure 6D). The transcription factor NRF2 is sensitive to oxidative stress and plays an important role in GSH synthesis, antioxidant defense system, and detoxifying in cell through the antioxidant response element (ARE) and activating its target genes.31 Therefore, the expression levels of the NRF2 target gene mRNAs, including Gclc, Gclm, and Gst’s, were measured. As expected, the Gclc and Gclm mRNAs encoding enzymes involved in GSH synthesis were inhibited by TAA, and NME treatment enhances their levels.
Nutmeg Extract Alleviated Hepatic Inflammation Induced by Thioacetamide
Cyclooxygenase 2 (COX2) and inducible nitric oxide synthase (iNOS) are very important enzymes that modulate the inflammatory response through the pro-inflammatory mediators prostaglandin E2 (PGE2) and nitric oxide (NO), respectively. 32 The expression levels of hepatic Cox2 and Inos mRNAs in the TAA-induced group were increased 150- and 15-fold compared with the control group, respectively (Figure 6E). The levels of both mRNAs were suppressed by NME. Furthermore, Tnfa (Figure 3E) and Il6 mRNAs encoding proinflammatory cytokines were significantly suppressed by NME. These results revealed that NME alleviated liver inflammation induced by TAA.
Myrislignan from Nutmeg Protected against Thioacetamide-Induced Liver Injury
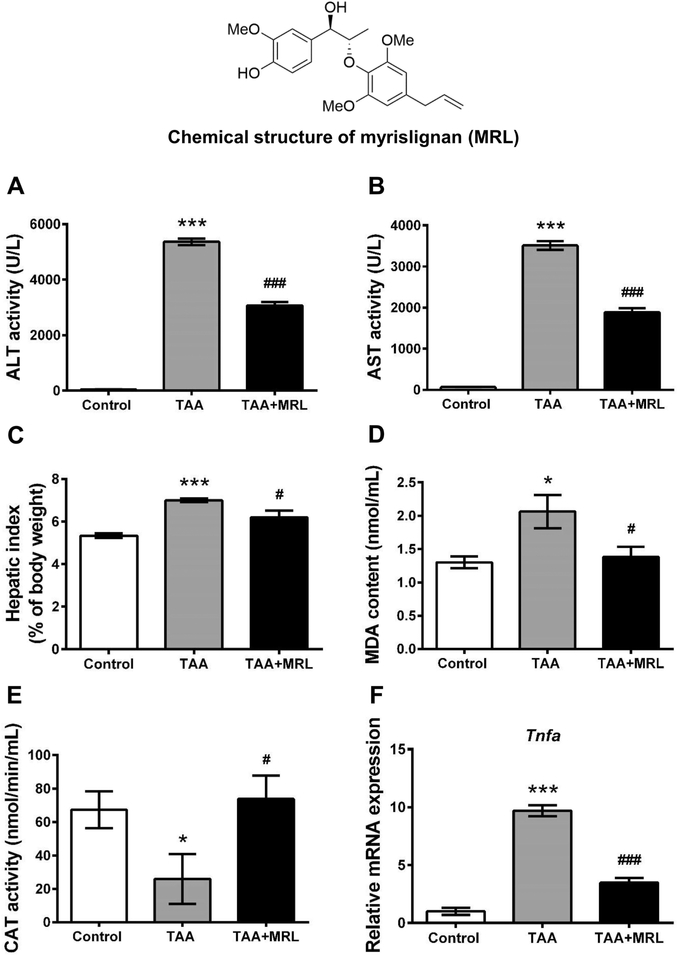
To clarify which constituent was responsible for the role of NME, the hepatoprotective effect of MRL, a representative 8-O-4′ neolignan from nutmeg, was tested in C57BL/6J mice. MRL decreased the ALT and AST activities induced by TAA (Figure 7A,B). The hepatic index was also reduced by MRL after TAA exposure (Figure 7C). The oxidative stress caused by TAA was attenuated by MRL, as indicated by the decreased MDA content and increased CAT activity in the TAA + MRL group (Figure 7D,E). Additionally, the increased expression level of Tnfa mRNA was also decreased by MRL (Figure 7F).
Figure 7.
Protective effects of MRL on TAA-induced liver injury. (A) ALT activity in plasma. (B) AST activity in plasma. (C) Hepatic index. (D) MDA content in plasma. (E) CAT activity in plasma. (F) Expression of Tnfa mRNA. ALT and AST activities in plasma increased significantly in TAA-treated group; treatment with MRL remarkably reduced the activities of the two amino transaminases. Oxidative stress mediated by TAA was inhibited by MRL; the expression of Tnfa was also decreased by MRL. *p < 0.05 as compared with control group, ***p < 0.001 as compared with control group, #p < 0.05 as compared with TAA-treated group, ###p < 0.001 as compared with TAA-treated group.
DISCUSSION
The hepatoprotective effects of NME were investigated in the present study, revealing that NME could protect mouse liver against TAA-induced acute injury in both pretreated and 1 h post-treated models. However, after 6 h of TAA treatment, NME did not show a hepatoprotective effect. The pharmaco-kinetics of TAA was been previously studied, and the half life of TAA was 1 to 1.5 h.33 The toxicant TASO in plasma was readily detected as early as 5 min, and the plasma level of TASO reached peak values at 180 min after a 300 mg/kg dose in rat.34 Post-treatments of NME at 6 and 12 h after TAA-induced were not able to protect liver injury, perhaps because of the rapid metabolism of TAA.
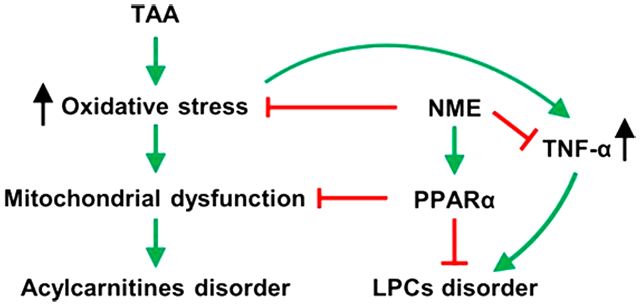
Recently, LC–MS-based metabolomics was used to explore the potential mechanisms of liver diseases by determining changes in endogenous metabolites.35 The discovery of biomarkers in NME therapy could help to profile valuable metabolic pathway data and determine the potential therapeutic targets so that the underlying mechanisms of hepatoprotective effect of NME could be further discovered. In this study, a disorder of LPCs was clearly observed after TAA exposure, and NME recovered the levels of LPCs. The decrease in LPCs was also observed in many other liver diseases, including NASH, chemical-induced liver injury, and tumorigenesis-induced liver injury. Tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) could induce the expression of LPCAT2/4 (lysophosphatidylecholine acyltransferase 2/4), which converts LPC to PC, resulting in the decline of LPCs in nonalcoholic steatohepatitis (NASH).36 The reduced LPC levels and increased expression of Lpcat2/4 mRNA noted in this study indicated that the change of LPC was caused by alteration of genes involved in LPC synthesis, which was reversed by NME treatment, demonstrating the protective effect of NME. Previous studies demonstrated that the increased content of TNF-α and TGF-β was correlated with LPCs depletion.35,36 In agreement with these observations, Tnfa mRNA was increased in liver after TAA exposure, and NME treatment decreased its mRNA levels in TAA-induced liver. However, the level of Tgfb mRNA increased in TAA group but was not significantly changed by NME. These results suggest that the alterations of Lpcat2/4 were mainly caused by TNF-α.
The increase in acylcarnitines in blood indicates mitochondrial dysfunction and the presence of intracellular oxidative stress.37 CPT1A and CPT2 are responsible for the transport of fatty acid into mitochondria for β-oxidation, which are regulated by PPARα.38 CPT1 (including CPT1A, CPT1B, and CPT1C) was located in the outer mitochondrial membrane, which catalyzes the transfer of the acyl group of long-chain fatty acyl-CoA to carnitine forming acylcarnitine. CPT2 is connected to the inner surface of mitochondrial membrane, which transfers acyl group form acylcarnitine to form acyl-CoA in the mitochondrial matrix.39 The present study revealed that NME treatment elevated the Ppara mRNA expression and its target genes, suggesting that the mitochondrial dysfunction was recovered by NME partly through modulating PPARα. Furthermore, the disorder of LPCs was also partly influenced by PPARα;40 these results demonstrated that NME possessed recovery effects on LPC and acylcarnitine levels, perhaps in part through modulation of PPARα.
It was reported that nutmeg possessed potent antioxidant activity.8,41 The transcription factor NRF2 plays a crucial role in cellular antioxidant signaling and sensitivity to oxidative stress. NRF2 is inhibited by KEAP-1 in the cytoplasm under unstressed conditions.42 However, it responds to oxidative stress through dissociation from KEAP-1 and is translocated to the nucleus. Subsequently, the increased nuclear NRF2 binds to the ARE and leas to transcription series antioxidant genes. The NRF2 target genes include glutathione peroxidases (Gpx’s), glutathione S-transferases (Gsta1–4, Gstm2, Gstm3, etc.), glutamate cysteine ligase (Gclc and Gclm), NAD(P)H:quinone oxidoreductase-1 (Nqo1), and superoxide dismutase (Sod).43,44,31 SOD converts superoxides to peroxides,45 GPXs and CAT reduce peroxides to H2O,46 and GCLC and GCLM are the catalytic and modifier subunits of glutamate cysteine ligase,31 respectively. The present study indicated that Gpx2 and Gsta2 mRNAs were increased in the TAA-induced group, suggesting enhanced oxidative stress induced by TAA. After NME treatment, Sod, Gpx1, Gpx3, Gclc, and Gclm mRNAs were increased. The Gst’s encoding glutathione S-transferases that catalyze the conjugation of the reduced form of GSH to xenobiotic substrates perform detoxication. TAA stimulated the expression of Gst’s, and NME downregulated the expression of Gsta2 and Gstm3. These results demonstrated that the oxidative stress levels in the TAA-induced group were alleviated by NME.
Previous reports revealed that MRL exhibited various bioactivity, including antioxidant activity and decrease in lipopolysaccharide-induced nitric oxide production,13 inhibition of vascular smooth muscle contraction,47 and antifungal properties.48 The present study demonstrated that MRL exhibited a protective effect on TAA-induced liver injury, suggesting that MRL is an active component in nutmeg. However, the hepatoprotective effect of MRL was weaker than NME, indicating that other substances in NME may possess a more significant hepatoprotective role. A previous study reported that nectandrin B, a lignan compound from nutmeg, protected hepatocytes from tert-butylhydroperoxide-induced oxidative stress through NRF2 activation and AMPK-dependent inhibition of GSK-3β.15 Macelignan is another lignan compound isolated from nutmeg and exhibited a protective effect on cisplatin-induced hepatotoxicity associated with JNK activation.16 These data suggest that the lignan compounds are responsible for the hepatoprotective effects of NME. However, the mechanism of hepatoprotective activity of lignans in nutmeg remains to be further investigated.
In summary, the present study demonstrated that NME possessed hepatoprotective action through PPARα modulation and increase in antioxidant capacity associated with NRF2. A neolignan MRL from nutmeg also showed protective effects on TAA-induced liver damage. These data suggest that the lignan compounds are the bioactive ingredients of nutmeg.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the open fund of state key laboratory of Pharmaceutical Biotechnology, Nan-jing University (KF-GN-201705), Postdoctoral fund from Yunnan Province, National Key Research and Development Program of China (2017YFC1700906, 2017YFC0906903), the U.S.-China Program for Biomedical Collaborative Research between F.J.G. and X.-N.Y. (81161120429), State Key Laboratory of Phytochemistry and Plant Resources in West China (52Y67A9211Z1), and Thousand Young Talents Program of China.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jproteome.7b00901.
Table S-1. Sequences of the qPCR primers. Table S-2. Identities of LPCs in mice. Table S-3. Identities of acylcarnitines in mice. Figure S-1. NME recovered the lysophosphatidylcholines and acylcarnitines metabolism in mouse liver of TAA-induced toxicity. Figure S-2. Tandem MS spectrum of authentic compounds. Figure S-3. Hydrogen peroxide (H2O2) content in plasma and relative GSH abundance in plasma. (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Abourashed EA; El-Alfy AT Chemical diversity and pharmacological significance of the secondary metabolites of nutmeg (Myristica fragrans Houtt.). Phytochem. Rev. 2016, 15 (6), 1035–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Saxena R; Patil P Phytochemical studies on Myristica fragrance essential oil. Flavour Fragrance J. 2012, 4 (2), 62–64. [Google Scholar]
- (3).Sun Z.-w; Huang Y.-p.; Ju J.-m. Quality standard of compound Huangzhang Ruangan Granule. China Pharm. 2011, 22 (11), 1024–1026. [Google Scholar]
- (4).Zhang D.-r.; Zhao C.-s.; Jing F.-b.; Wang L.-l. Study on quality criterition of Changkang Granules. Chin. J. Mod. Appl. Pharm. 2003, 3, 213–215. [Google Scholar]
- (5).Xiong T; Li H Study on protective effect of Jiagasong Tang for liver injury animal model and cell oxidative stress model in vitro. Mol. Med. Rep. 2013, 29 (1), 132–135. [Google Scholar]
- (6).Kareem MA; Krushna SG; Hussain SA; Devi KL Effect of aqueous extract of nutmeg on hyperglycaemia, hyperlipidaemia and cardiac histology associated with isoproterenol-induced myocardial infarction in rats. Trop. J. Pharm. Res. 2009, 8 (4), 337–344. [Google Scholar]
- (7).Kareem MA; Gadhamsetty SK; Shaik AH; Prasad EM; Kodidhela LD Protective effect of nutmeg aqueous extract against experimentally-induced hepatotoxicity and oxidative stress in rats. J. Ayurveda Integr Med. 2013, 4 (4), 216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Singh G; Marimuthu P; de Heluani CS; Catalan C Antimicrobial and antioxidant potentials of essential oil and acetone extract of Myristica fragrans Houtt. (Aril part). J. Food Sci. 2005, 70 (2), 141–148. [Google Scholar]
- (9).Ram A; Lauria P; Gupta R; Sharma VN Hypolipidaemic effect of Myristica fragrans fruit extract in rabbits. J. Ethnopharmacol. 1996, 55, 49–53. [DOI] [PubMed] [Google Scholar]
- (10).Dhingra D; Sharma A Antidepressant-like activity of n-hexane extract of nutmeg (Myristica fragrans) seeds in mice. J. Med. Food 2006, 9 (1), 84–89. [DOI] [PubMed] [Google Scholar]
- (11).Parle M; Dhingra D; Kulkarni SK Improvement of mouse memory by Myristica fragrans seeds. J. Med. Food 2004, 7 (2), 157–161. [DOI] [PubMed] [Google Scholar]
- (12).Li F; Yang XW; Krausz KW; Nichols RG; Xu W; Patterson AD; Gonzalez FJ Modulation of colon cancer by nutmeg. J. Proteome Res. 2015, 14 (4), 1937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Cao GY; Xu W; Yang XW; Gonzalez FJ; Li F New neolignans from the seeds of Myristica fragrans that inhibit nitric oxide production. Food Chem. 2015, 173, 231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Yadav AS; Bhatnagar D Modulatory effect of spice extracts on iron-induced lipid peroxidation in rat liver. BioFactors 2007, 29 (2–3), 147–157. [DOI] [PubMed] [Google Scholar]
- (15).Song JS; Kim EK; Choi YW; Oh WK; Kim YM Hepatocyte-protective effect of nectandrin B, a nutmeg lignan, against oxidative stress: Role of Nrf2 activation through ERK phosphorylation and AMPK-dependent inhibition of GSK-3beta. Toxicol. Appl. Pharmacol. 2016, 307, 138–149. [DOI] [PubMed] [Google Scholar]
- (16).Sohn JH; Han KL; Kim JH; Rukayadi Y; Hwang JK Protective effects of macelignan on cisplatin-induced hepatotoxicity is associated with JNK activation. Biol. Pharm. Bull. 2008, 31 (2), 273–277. [DOI] [PubMed] [Google Scholar]
- (17).Fitzhugh OG; Nelson AA liver tumors in rats fed thiourea or thioacetamide. Science 1948, 108 (2814), 626–628. [DOI] [PubMed] [Google Scholar]
- (18).Fontana L; Moreira E; Torres MI; Fernández MI; Ríos A; Sánchez de Medina F; Gil A Serum amino acid changes in rats with thioacetamide-induced liver cirrhosis. Toxicology 1996, 106 (1–3), 197–206. [DOI] [PubMed] [Google Scholar]
- (19).Fernandez-Martinez A; Callejas NA; Casado M; Bosca L; Martin-Sanz P Thioacetamide-induced liver regeneration involves the expression of cyclooxygenase 2 and nitric oxide synthase 2 in hepatocytes. J. Hepatol. 2004, 40 (6), 963–70. [DOI] [PubMed] [Google Scholar]
- (20).Hajovsky H; Hu G; Koen Y; Sarma D; Cui W; Moore DS; Staudinger JL; Hanzlik RP Metabolism and toxicity of thioacetamide and thioacetamide S-oxide in rat hepatocytes. Chem. Res. Toxicol. 2012, 25 (9), 1955–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Idle JR; Gonzalez FJ Metabolomics. Cell Metab. 2007, 6 (5), 348–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Lee SS; Pineau T; Drago J; Lee EJ; Owens JW; Kroetz DL; Fernandez-Salguero PM; Westphal H; Gonzalez FJ Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 1995, 15, 3012–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia (Part One); Medical Science and Technology Press: Beijing, China, 2015; pp 136. [Google Scholar]
- (24).Wang Y; Yang XW Quantitative Determination of Neolignanoids in the Seeds of Myristica fragrans. Mod. Chin. Med 2008, 10 (2), 10–13. [Google Scholar]
- (25).Zhao Q; Yang R; Wang J; Hu DD; Li F PPARα activation protects against cholestatic liver injury. Sci. Rep. 2017, 7 (1), 9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Matsubara T; Tanaka N; Patterson AD; Cho JY; Krausz KW; Gonzalez FJ Lithocholic acid disrupts phospholipid and sphingolipid homeostasis leading to cholestasis in mice. Hepatology 2011, 53 (4), 1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wu Q; Zhang H; Dong X; Chen X-F; Zhu Z-Y; Hong Z-Y; Chai Y-F UPLC-Q-TOF/MS based metabolomic profiling of serum and urine of hyperlipidemic rats induced by high fat diet. J. Pharm. Anal. 2014, 4 (6), 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Jansson J; Willing B; Lucio M; Fekete A; Dicksved J; Halfvarson J; Tysk C; Schmitt-Kopplin P Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS One 2009, 4 (7), e6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Suhre K; Meisinger C; Doring A; Altmaier E; Belcredi P; Gieger C; Chang D; Milburn MV; Gall WE; Weinberger KM; Mewes HW; Hrabe de Angelis M; Wichmann HE; Kronenberg F; Adamski J; Illig T Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One 2010, 5 (11), e13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kang JS; Wanibuchi H; Morimura K; Wongpoomchai R; Chusiri Y; Gonzalez FJ; Fukushima S Role of CYP2E1 in thioacetamide-induced mouse hepatotoxicity. Toxicol. Appl. Pharmacol. 2008, 228 (3), 295–300. [DOI] [PubMed] [Google Scholar]
- (31).Fan X; Jiang Y; Wang Y; Tan H; Zeng H; Wang Y; Chen P; Qu A; Gonzalez FJ; Huang M; Bi H Wuzhi tablet (Schisandra Sphenanthera extract) protects against acetaminophen-induced hepatotoxicity by inhibition of CYP-mediated bioactivation and regulation of NRF2-ARE and p53/p21 pathways. Drug Metab. Dispos. 2014, 42 (12), 1982–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tomlinson A; Appleton I; Moore AR; Gilroy DW; Willis D; Mitchell JA; Willoughby DA Cyclo-oxygenase and nitric oxide synthase isoforms in rat carrageenin-induced pleurisy. Br. J. Pharmacol. 1994, 113 (3), 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Porter WR; Gudzinowicz MJ; Neal RA Thioacetamide-induced hepatic necrosis. II. Pharmacokinetics of thioacetamide and thioacetamide-S-oxide in the rat. J. Pharmacol. Exp. Ther. 1979, 208 (3), 386–391. [PubMed] [Google Scholar]
- (34).Chilakapati J; Shankar K; Korrapati MC; Hill RA; Mehendale HM Saturation toxicokinetics of thioacetamide: role in initiation of liver injury. Drug Metab. Dispos. 2005, 33 (12), 1877–85. [DOI] [PubMed] [Google Scholar]
- (35).Beyoglu D; Idle JR The metabolomic window into hepatobiliary disease. J. Hepatol. 2013, 59 (4), 842–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Tanaka N; Matsubara T; Krausz KW; Patterson AD; Gonzalez FJ Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology 2012, 56 (1), 118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).McGill MR; Li F; Sharpe MR; Williams CD; Curry SC; Ma X; Jaeschke H Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch. Toxicol. 2014, 88 (2), 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Mandard S; Muller M; Kersten S Peroxisome proliferator-activated receptor alpha target genes. Cell. Mol. Life Sci. 2004, 61 (4), 393–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Khan HA; Alhomida AS Single nucleotide polymorphism in CPT1B and CPT2 genes and its association with blood carnitine levels in acute myocardial infarction patients. Gene 2013, 523 (1), 76–81. [DOI] [PubMed] [Google Scholar]
- (40).Liu A; Krausz KW; Fang Z-Z; Brocker C; Qu A; Gonzalez FJ Gemfibrozil disrupts lysophosphatidylcholine and bile acid homeostasis via PPARα and its relevance to hepatotoxicity. Arch. Toxicol. 2014, 88 (4), 983–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Olaleye MT; Akinmoladun AC; Akindahunsi AA Antioxidant properties of Myristica fragrans (Houtt) and its effect on selected organs of albino rats. Afr. J. Biotechnol 2006, 5 (13), 1274–1278. [Google Scholar]
- (42).Nguyen T; Nioi P; Pickett CB The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284 (20), 13291–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Singh A; Rangasamy T; Thimmulappa RK; Lee H; Osburn WO; Brigelius-Flohe R; Kensler TW; Yamamoto M; Biswal S Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am. J. Respir. Cell Mol. Biol. 2006, 35 (6), 639–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Chanas SA; Jiang Q; McMahon M; McWalter GK; McLellan LI; Elcombe CR; Henderson CJ; Wolf CR; Moffat GJ; Itoh K; Yamamoto M; Hayes JD Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1 Gsta2 Gstm1 Gstm2 Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem. J. 2002, 365 (12), 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Hassan HM Superoxide Dismutases In Biological Roles of Copper; Ciba Foundation Symposium; Excerpta Medica: Amsterdam, 1980; Vol. 79, pp 125–142. [DOI] [PubMed] [Google Scholar]
- (46).Saint-Denis M; Labrot F; Narbonne JF; Ribera D Glutathione, glutathione-related enzymes, and catalase activities in the earthworm Eisenia fetida andrei. Arch. Environ. Contam. Toxicol. 1998, 35 (4), 602–614. [DOI] [PubMed] [Google Scholar]
- (47).Nakajima K; Yamazaki T; Kawashima K; Shinho Y; Kurashige T; Nohara T; Nishimura M, Vascular smooth muscle contraction inhibitors from Myristica. Jpn. Kokai Tokkyo Koho 1999, 8. [Google Scholar]
- (48).Miyazawa M; Kasahara H; Kameoka H Antifungal activities of 8-O-4′-neolignans from Myristica Fragrans. Nat. Prod. Lett. 1996, 8 (4), 271–273. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.