Abstract
Mucoepidermoid carcinoma (MEC) is the most common salivary gland malignancy, but categorization is complicated by variability in grading systems and uncertain prognostic significance of MAML2 rearrangement. The aims of this study were to determine the prognostic significance of MEC grading systems and MAML2 rearrangement status. Fifty-three carcinomas originally diagnosed as MEC (45 primary; 8 recurrent) of major and minor salivary glands were graded according to modified Healey, Brandwein, AFIP, and Katabi systems. Fluorescence in-situ hybridization for MAML2 rearrangement was performed. Clinical features and outcomes were recorded. Twenty-five (47%) carcinomas scored the same in all grading systems. The most common histologic feature leading to a diagnosis of intermediate grade was isolated solid growth. Brandwein assigned the highest percentage of high grade (29%) and AFIP the highest percentage of low grade (80%). MAML2 was rearranged in 37/46 (80%) cases. Forty-three (81%) were morphologically compatible with MEC, and these were more likely to be low-intermediate grade and MAML2-rearranged. Of primary carcinomas, 6 (13%) recurred. Statistically significant univariate risk factors for recurrence included non-MEC morphology, stage T4, and high Brandwein grade. Margin status, MAML2 rearrangement, and isolated solid growth were not predictive of recurrence. A binary grading system (Brandwein high versus low-plus-intermediate) could be considered to better reflect biologic behavior in MEC. Our study confirms that MAML2 wildtype tumors more likely represent high grade non-MECs, and prior studies demonstrating worse prognosis in MAML2-non-rearranged MECs may be diluted by high grade non-MECs.
Keywords: Salivary gland cancer, mucoepidermoid carcinoma, grade, MAML2, prognosis, recurrence
Introduction:
Salivary gland carcinomas represent approximately 11% of head and neck malignancies, the most common being mucoepidermoid carcinoma (MEC).1,2 Variety in clinical presentation, behavior, and histologic appearance has made these carcinomas challenging to stage and treat. In the initial description of “mucoepidermoid tumors,” Stewart et al. separated them into benign and malignant.3 Since then, all tumors have been determined to possess malignant potential4 (hence the unifying term mucoepidermoid carcinoma), and multiple histopathologic grading systems have been created in order to better stratify patients for prognostication and treatment, namely the modified Healey5,6, AFIP7,8, Brandwein9, and Katabi10. All grading systems (whether quantitative or qualitative) have three grades: low, intermediate, and high. One difficulty lies in the inconsistency of the grading systems: applying the different grading systems to the same tumor may result in different grades, with the Brandwein system tending to assign higher grades and the AFIP system lower grades. Furthermore, treatment for a histologically intermediate grade carcinoma is not straightforward. Low grade tumors generally require complete surgical excision and high grade tumors are treated with adjuvant radiotherapy with or without chemotherapy.11 Intermediate grade carcinomas may behave similarly to low grade carcinomas, and treatment with surgical excision alone has been considered.12,13 Lastly, presence of a CRTC1/3-MAML2 fusion has been suggested by some to correlate with lower histologic grade and to portend better prognosis.14–22 However, these results have not been uniformly reproducible, and MAML2 rearrangement can be present in high grade or high stage MEC and may not necessarily correlate to prognosis.23–29 Arguably, fusion-negative tumors may represent other high grade non-mucoepidermoid carcinomas.16,24,25,28,30 Clarification of the divergent grading systems, the significance of MAML2 rearrangement status, and the possible treatment protocols for intermediate grade tumors is warranted. The aims of this study are to compare mucoepidermoid carcinoma grading systems and to evaluate MAML2 rearrangement status in the context of patient outcomes in order to determine the prognostic significance of histologic grading systems and genetics.
Materials & Methods:
With Institutional Review Board approval, cases of primary head & neck mucoepidermoid carcinoma were identified in the diagnostic surgical pathology database, between years 1993 and 2018. Resection specimens of primary MECs of major and minor salivary glands were included. MECs occurring outside the head & neck (including trachea, lung, and breast) were excluded. Diagnostic hematoxylin and eosin (H&E) slides and formalin-fixed paraffin embedded (FFPE) blocks were retrieved from the diagnostic surgical pathology archives.
Histologic Review and Grading:
Diagnostic H&E slides were reviewed by two head & neck pathologists (NAC, ML) and each carcinoma was graded according to four published grading systems: 1) the qualitative modified Healey system of Batsakis and Luna5,6; 2) the quantitative AFIP system of Auclair, Goode, and Ellis7,8; 3) the quantitative system of Brandwein, et al9; and 4) the qualitative system of Katabi, et al10. [Table 1] The grade assigned by the original signout pathologist was recorded. Over the 25 year time period, the original signout pathologist varied. Whether the tumor was morphologically compatible with mucoepidermoid carcinoma (as determined by head & neck pathologists NAC & ML) was also recorded.
Table 1:
Comparison of Mucoepidermoid Carcinoma Histologic Grading Systems
| L: macro + micro cysts I: micro cysts + solid H: solid +/− micro cysts |
2 (<20%) | 2 (<25%) | L: predominantly cystic (>80%) I: predominantly solid H: any (usually solid) |
| H: present | 2 | 3 | n/a |
| n/a | 3 | 3 | L: absent I: absent H: present |
| L: rare I: few H: many |
3 (4/10 HPF) | 3 (5/10 HPF) | L: 0–1/10 HPF I: 2–3/10 HPF H: 4+/10 HPF |
| L: absent/minimal I: slight/moderate H: considerable (including nucleoli) |
4 | 2 | L: no significant I: no significant H: any |
| L: broad/circumscribed I: uncircumscribed H: soft tissue/perineural/vascular invasion |
n/a | 2 (small nests & islands) | L: well circumscribed I: well circumscribed or infiltrative H: any (usually infiltrative) |
| H: present | n/a | 3 | n/a |
| n/a | n/a | 3 | n/a |
| L: rare I: more common H: predominant |
n/a | n/a | n/a |
| L: extravasated mucin + fibrosis + CI I: fibrosis separating nests + CI H: desmoplasia, minimal CI |
n/a | n/a | n/a |
| L: daughter cysts from larger I: large duct less conspicuous H: variable architecture/cell morphology |
n/a | n/a | n/a |
Key: L=low grade, I=intermediate grade, H=high grade, n/a=not applicable, CI=chronic inflammation.
MAML2 Rearrangement:
For all cases with available FFPE blocks, a tumor-rich block was selected. Fluorescence in-situ hybridization (FISH) for MAML2 was performed on paraffin-embedded tissue sections using locus specific, dual-color break apart probes consisting of a 680 Kb fragment corresponding to the 5’ MAML2 gene labelled in ZyGreen and a 370 Kb fragment corresponding to the 3’ MAML2 gene labelled in ZyOrange and (Zytovision, Bremerhaven, Germany). Briefly, slides were deparaffinized in CitriSolv (Decon Laboratories Inc, Kings of Prussia, PA) three times for 10 minutes each followed by two dehydration steps using 100% ethanol alcohol for 5 minutes each. Tissue sections were immediately transferred to 0.2N Hydrochloric acid (Thermo Fisher Scientific, Waltham, MA) for 30 minutes at room temperature followed by Sodium Thiocyanate (Sigma-Aldrich, St Louis, MO) for 30 minutes at 80°C. Tissue sections were digested in protease solution (Sigma-Aldrich, St Louis, MO) for six minutes followed by 2x SSC (Invitrogen, Carlsbad, CA), fixed in 10% buffered formalin for 10 minutes followed by 2x SSC twice for 5 minutes. Tissue sections were manually denatured at 80°C for 5 minutes in 70% Formamide (Thermo Fisher Scientific, Waltham, MA) followed by sequentially dehydrated in ethanol alcohol (70%, 85%, and 100%) and air-dried at room temperature. After applying probe, the slides were hybridized overnight in a humidified chamber at 37°C. Tissue sections were washed the next day in 0.3% NP40/2× SSC at 75°C for 2 minutes followed by 0.3% NP40/2× SSC at room temperature. Slides were mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) with 3 uL of DAPI 4′,6-diamidino-2-phenylindole (Sigma-Aldrich, St Louis, MO). A total of 50 tumor nuclei were manually evaluated for each case. FISH results were considered positive for a MAML2 rearrangement if >10% of tumor cells showed a separation of the orange and green signals by ≥2 signal widths, or if a deletion of the green signal was noted.
Clinical Outcomes & Statistical Analysis:
Patient data was collected from the institution’s electronic medical record. Data included: age, sex, date of primary surgical resection, site of primary tumor, size of primary tumor, margin status, pathologic stage, and treatment. Outcomes were recorded as date and site of recurrence (including locoregional versus distant recurrence) and date and status at last follow-up. Kaplan-Meier estimates of survival were compared using log-rank tests. Tests of concordance between categorical variables were performed using Fisher’s exact test. Statistical programming was completed in R (version 3.4.0), using packages “survival”, “ggplot2”, and “survminer”.
Results:
A total of 53 resection specimens with an original diagnosis of mucoepidermoid carcinoma from major and minor salivary glands of the head & neck were identified and present in the archives. Forty-two were primary resections and 3 were re-excisions for positive margins (of which primary resection was performed at an outside institution and slides were reviewed internally). As the re-excisions represent part of the primary surgical treatment of these patients, they will be considered along with the initial primary resections for a total of 45 primary resections. Eight were recurrences (of which primary resection was performed at an outside institution, slides were not available, and recurrence occurred 6–134 months following reported primary resection). The most frequent primary site was minor salivary gland (n=32: 14 palate, 5 tongue, 3 retromolar trigone, 2 oropharynx, 2 floor of mouth, 2 mandible, 2 buccal, 1 lip, and 1 external ear canal) followed by parotid (n=20) and submandibular gland (n=1). Average age of patients with primary carcinomas was 52.7 (range 9–85) and that of patients with recurrent carcinomas 49.6 (range 28–69). Overall, females (n=34) were more frequently affected than males (n=19). Average size of the primary tumors was 1.8 cm (range 0.3–6.8 cm). Of patients with primary tumors, pathologic stage was T1 in 28 patients (18 NX, 9 N0, 1 N2), T2 NX in 1 patient, T3 in 4 patients (3 N0, 1 N2b), T4a in 9 patients (4 NX, 4 N0, 1 N1), and TX in 3 patients (2 NX, 1 N2). Surgical margins of primary tumors were negative in 29 patients and positive in 16 (6 in which the separately submitted margins were negative). Ten primary patients received adjuvant radiotherapy with or without chemoradiotherapy: 4 patients with T4 disease (3 N0, 1 N1), 2 patients with T3 disease (1 N0, 1 N2b), 1 patient with N2 disease, 2 patients with intermediate to high grade disease and close or positive margins, and 1 patient with low grade disease and positive margins. [Table 2, Supplementary Figure 1]
Table 2:
Clinicopathologic Features of Mucoepidermoid Carcinoma Patients
| Morphologically-MEC (n=43) | Morphologically-Not-MEC (n=10) | |
|---|---|---|
| Specimen Type | ||
| - Excision of primary or initial re-excision | 40 | 5 |
| - Excision of recurrence | 3 | 5 |
| Clinical Features | ||
| Site, all | ||
| - Minor salivary gland | 26 | 6 |
| - Major salivary gland | 17 | 4 |
| Average Age (years) | ||
| - Primary | 52 | 58 |
| - Recurrent | 31 | 61 |
| Sex | ||
| - Female | 29 | 5 |
| - Male | 14 | 5 |
| Treatment, primary cases | ||
| - Surgery only | 32 | 3 |
| - Surgery + RT | 3 | 2 |
| - Surgery + CRT | 5 | 0 |
| Recurrence, primary cases | 4 | 2 |
| Average Follow-up (years), primary cases, non-recurrent | 4.0 | 1.6 |
| Pathologic Features | ||
| Average Size (cm), primary cases | 1.8 | 1.6 |
| Pathologic Stage, primary cases | 30 pT1–3, 8 pT4, 2 pTX | 3 pT1, 1 pT4, 1 pTX |
| Margins Involved, primary cases | 14 | 2 |
| Original Grade, primary cases | 3 HG, 10 IG, 27 LG | 2 HG, 2 IG, 1 LG |
| Reviewed Brandwein Grade, primary cases | 9 HG, 17 IG, 14 LG | 4 HG, 1 IG |
| Reviewed Brandwein Grade, recurrence cases | 2 HG, 1 LG | 5 HG |
| MAML2 Status | ||
| - Rearranged | 37 | 0 |
| - Not-Rearranged | 3 | 6 |
Key: MEC=mucoepidermoid, RT=radiotherapy, CRT=chemoradiotherapy, F/U=follow-up, HG=high grade, IG=intermediate grade, LG=low grade.
Histologic Review and Grading:
Upon contemporary review and re-grading of all cases, 25 (47%) scored the same in all grading systems (15 low and 10 high); the remaining 28 (53%) scored differently in in at least 2 systems.
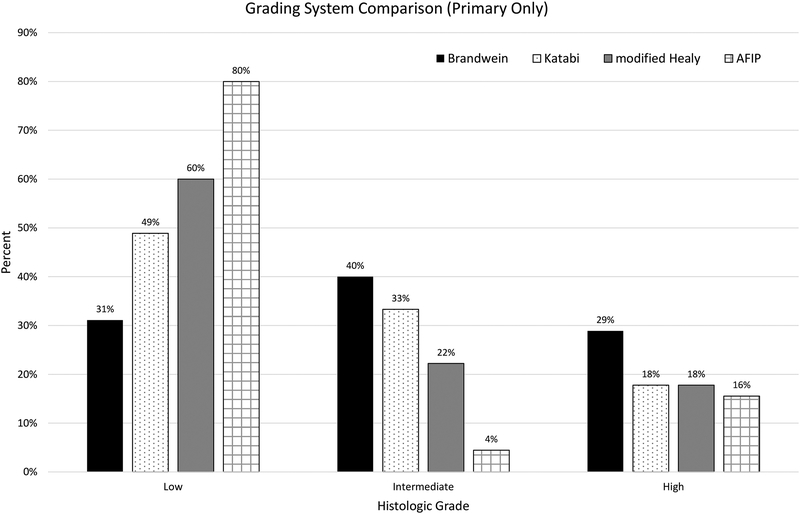
Of the 45 primary resections/re-excisions, the original signout grade was high in 5 cases, intermediate in 12, and low in 28. Upon re-review, 7 scored high grade in all 4 systems, 6 scored high grade in 1–2 systems, 18 scored at most intermediate grade in at least 1 system, and 14 scored low grade in all systems [Table 2]. Specifically, carcinomas scored as follows: using the Brandwein system, 13 (29%), 18 (40%), and 14 (31%) were high, intermediate, and low grade, respectively; using the Katabi system, 8 (18%), 15 (33%), and 22 (49%) were high, intermediate, and low grade, respectively; using the modified Healey system, 8 (18%), 10 (22%), and 27 (60%) were high, intermediate, and low grade, respectively; using the AFIP system, 7 (16%), 2 (4%), and 36 (80%) were high, intermediate, and low grade, respectively [Figure 1]. The distribution was similar when considering only the 40 primary cases that were morphologically compatible with MEC on contemporary re-review [Supplementary Figure 2].
Figure 1: Four Mucoepidermoid Carcinoma (MEC) Grading Systems Compared.
Forty-five primary carcinomas (of which 40 were morphologically compatible with MEC on contemporary re-review) were graded according to four published grading systems. Brandwein assigned the highest rate of high grade (29%), while AFIP the highest rate of low grade (80%). The distribution was similar after eliminating the 5 non-MEC cases [Supplementary Figure 2].
Forty percent (18/45) of primary carcinomas scored at most intermediate grade in at least one system. The most frequent histologic finding resulting in upgrade from low to intermediate grade was solid growth (11, 61%), followed by peripheral growth in nests and islands (4, 22%), and bony invasion (3, 17%) in palatal and mandibular tumors. Histologic features present only in the 13 tumors scoring high grade in at least one system included: anaplasia (7, 54%), necrosis (6, 46%), increased mitoses (5, 38%), perineural invasion (4, 31%), and lymphovascular invasion (2, 15%). Additionally, peripheral growth in nests and islands was present in 11 (85%), solid growth in 11 (85%), and bony invasion in 3 (23%).
Of the 8 recurrences, the original signout grade was high in 3 cases, intermediate in 3, and not specified in 2. Upon re-review, three scored high grade in all 4 systems, 4 scored high grade in 1–2 systems, and 1 scored low grade in all systems (primary ear canal carcinoma) [Table 2]. Specifically, carcinomas scored as follows: using the Brandwein system, 7 (88%) and 1 (13%) were high and low grade, respectively; using the Katabi and modified Healey systems, 5 (63%), 2 (25%), and 1 (13%) were high, intermediate, and low grade, respectively; using the AFIP system, 3 (38%), 3 (38%), and 2 (25%) were high, intermediate, and low grade, respectively.
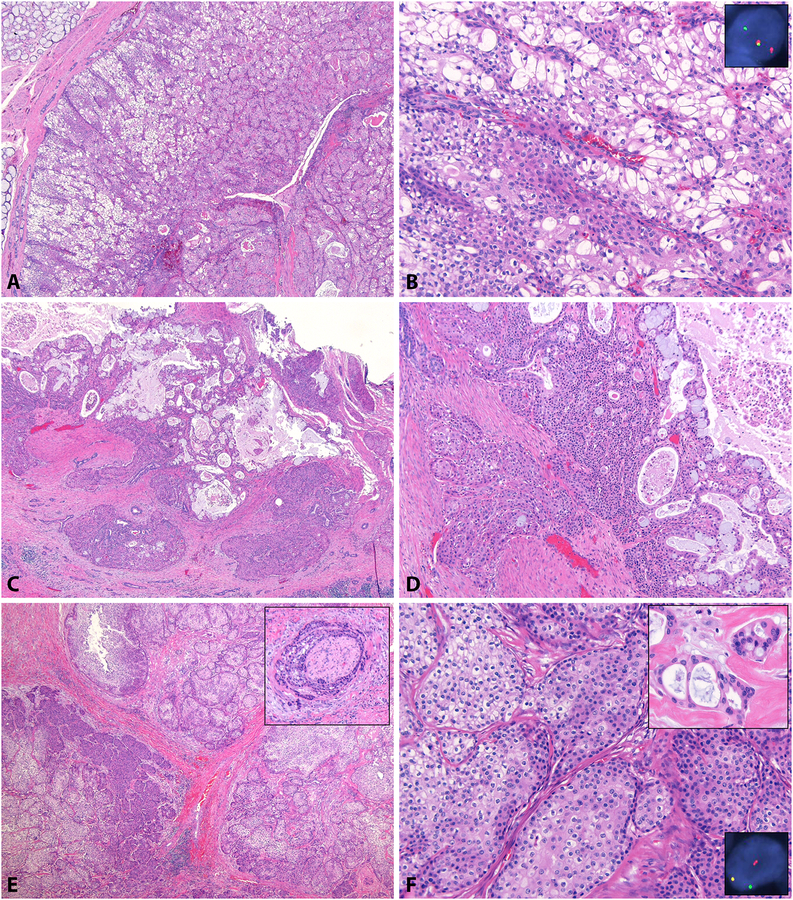
After histologic review, 43 (81%) cases were morphologically compatible with mucoepidermoid carcinoma [Figure 2]. Forty were primary carcinomas and 3 were recurrences. Ten (19%) cases were not morphologically compatible with MEC [Figure 3]. Five were primary carcinomas and 5 were recurrences. Of all 20 carcinomas scoring high grade in Brandwein, 11 (55%) were morphologically MEC; of all 33 carcinomas scoring low-intermediate grade in all systems, 32 (97%) were morphologically MEC (p=0.0003) [Table 3A]. Of the 13 primary carcinomas scoring high grade in Brandwein, 9 (69%) were morphologically MEC; of the 32 primary carcinomas scoring low-intermediate grade in all systems, 31 (97%) were morphologically MEC (p=0.0198) [Table 3B]. Of 42 primary cases with known pathologic T stage, 38 (90%) were morphologically MEC, which was not different among patients with T1-T3 (30/33, 91%) compared to T4 (8/9, 89%) disease (p=0.6344) [Table 3C].
Figure 2: Carcinomas with Mucoepidermoid Morphology.
Most (81%) carcinomas reviewed were morphologically compatible with MEC. Solid growth was the most common reason for assigning Brandwein intermediate grade, as seen in this well-circumscribed (A) mucoepidermoid carcinoma with intermediate cells, glycogenated cells (B), rare mucinous cells, and MAML2 rearrangement (B, inset). Occasional carcinomas morphologically consistent with MEC (C), showed intermediate cells, squamoid cells, and mucinous cells (D) but lacked MAML2 rearrangement. A true high grade mucoepidermoid carcinoma also showed appropriate cell types and high grade features, including perineural invasion (E, inset), rare mucinous cells (F, top inset), and MAML2 rearrangement (F, bottom inset).
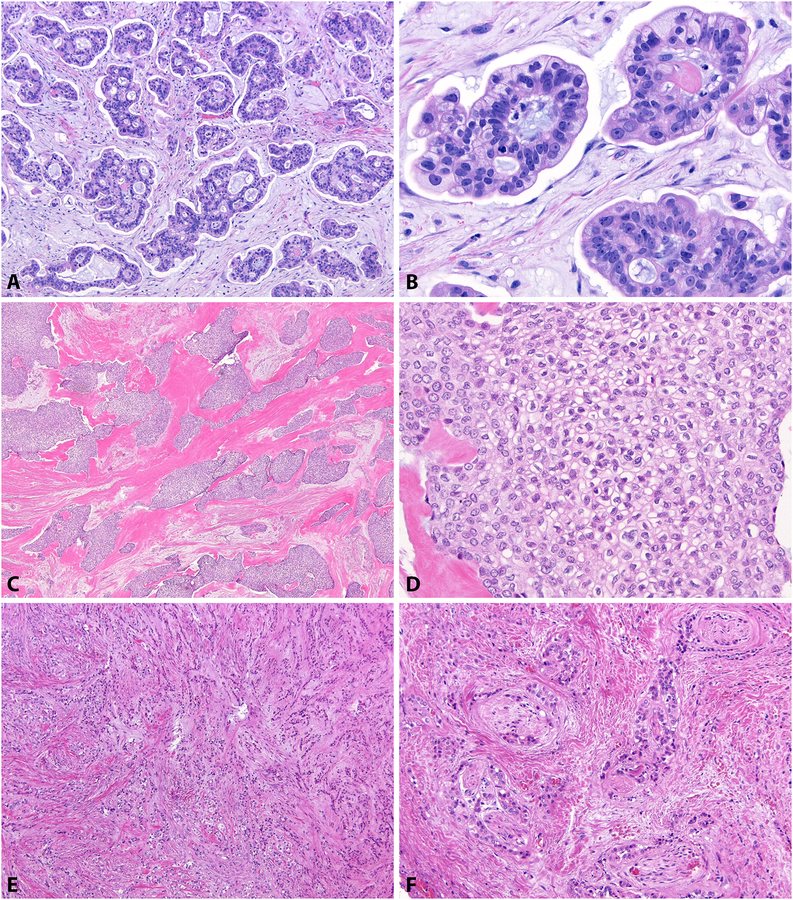
Figure 3: Carcinomas of Non-Mucoepidermoid Morphology.
Some carcinomas (19%) originally diagnosed as MEC by the signout pathologist were not compatible with MEC upon contemporary review. This primary palate carcinoma showed micropapillary architecture (A) with extracellular mucin pools (B) and was negative for MAML2 rearrangement, suggestive of the rare mucinous adenocarcinoma. A buccal carcinoma demonstrated abundant eosinophilic hyalinized stroma (C) and a single cell type with clear cytoplasm and wrinkled nuclei (D), consistent with clear cell carcinoma. Another palate carcinoma also showed a single cell type with single-file infiltration within fibrous stroma (E) and perineural invasion (F). The remaining non-MEC cases were not morphologically specific for other salivary type carcinomas.
Table 3:
Statistical Analyses of Mucoepidermoid Carcinoma Patients
| 1 | 9 | p=0.0003 | 1 | 4 | p=0.0198 |
| 32 | 11 | 31 | 9 | ||
| 3 | 1 | p=0.6344 | 6 | 0 | p=0.000008 |
| 30 | 8 | 3 | 37 | ||
| 2 | 28 | p=0.0049 | 2 | 27 | p=0.5297 |
| 7 | 9 | 1 | 7 | ||
| 4 | 27 | p=0.6102 | 2 | 27 | p=0.4417 |
| 1 | 5 | 1 | 5 | ||
| 3 | 2 | p=0.1248 | 3 | 7 | p=0.0017 |
| 36 | 4 | 36 | 7 | ||
| 26 | 3 | p=0.3585 | 25 | 1 | p=0.1145 |
| 13 | 3 | 11 | 3 | ||
| 5 | 0 | p=0.6548 | 5 | 4 | p=0.0867 |
| 31 | 3 | 31 | 6 | ||
| 32 | 1 | p=0.0050 | 29 | 1 | p=0.0237 |
| 5 | 4 | 5 | 3 | ||
| 32 | 0 | p=0.0002 | 31 | 0 | p=0.0014 |
| 7 | 6 | 5 | 4 | ||
Key: LG=low grade, IG=intermediate grade, HG=high grade, MEC=mucoepidermoid carcinoma, WT=wildtype.
MAML2 Rearrangement:
FISH was attempted on all 53 cases. Hybridization failed in 7, including 3 due to decalcification. Of the 46 successful cases, 37 (80%) were positive for MAML2 rearrangement and 9 (20%) were negative (2 of which showed polysomy). The positive cases included 3 clinically recurrent cases in which the primary was not reviewed; the remaining 34 positive cases were primary resections. All 37 positive cases had >48% rearranged cells (mean 90%, median 90%, range 48–100%). Two cases had <10% rearranged cells (2% and 4%); these cases were considered negative. One primary, high grade, morphologically MEC case had an unusual FISH pattern: 90% of cells had 2–3 single orange signals (3’ of the MAML2 gene), but no single green signals (5’ of the MAML2 gene). This pattern could represent an unbalanced rearrangement, gain of the region 3’ to MAML2, or deletion of the region 5’ to MAML2. Because it was suspicious for rearrangement, it was considered positive for the purposes of this study.
Of the 7 unsuccessful cases, 3 were morphologically compatible with mucoepidermoid carcinoma. Of the 37 fusion-positive and 9 negative cases, 37 (100%) and 3 (33%), respectively, were morphologically compatible with mucoepidermoid carcinoma (p=0.000008) [Table 3D]. Of the 37 fusion-positive and 9 negative cases, 9 (24%) and 7 (78%), respectively, were Brandwein high grade (p=0.0049) [Table 3E]. In other words, 93% (28/30) Brandwein low-intermediate grade were MAML2-rearranged, and 56% (9/16) Brandwein high grade were MAML2-rearranged. Considering only the primary cases morphologically compatible with MEC: of the 34 positive and 3 negative cases, 7 (21%) and 1 (33%), respectively, were Brandwein high grade (p=0.5297) [Table 3F]. Of primary cases with known pathologic T stage, 86% (32/37) were MAML2-rearranged, which was not different among patients with T1-T3 (27/31, 87%) compared to T4 (5/6, 83%) disease (p=0.6102) [Table 3G]. When considering only the primary cases morphologically compatible with MEC, MAML2-rearrangement was also not different among patients with T1-T3 (27/29, 93%) compared to T4 (5/6, 83%) disease (p=0.4417) [Table 3H].
Clinical Outcomes:
Of the 45 patients with primary resections/re-excisions, 6 (13%) recurred: patient 1 had a left parotid pT4 N0 carcinoma (morphologically MEC; high grade in all systems) who received adjuvant chemoradiation and recurred in the left submandibular area 9 months after resection; patient 2 had a palate T4 NX carcinoma (morphologically not MEC; high grade in Brandwein, low grade in AFIP, intermediate in Katabi and modified Healey) who was treated with surgery and had a rib metastasis 2 years after resection; patient 3 had a parotid T4 N1 carcinoma (morphologically MEC; high grade in Brandwein and Katabi, intermediate in modified Healey and AFIP) who received adjuvant chemoradiation, had a chest wall metastasis 1 year after resection, and died of diffuse metastasis 2.4 years after resection; patient 4 had a TX NX buccal carcinoma (morphologically not MEC; high grade in Brandwein, intermediate in Katabi and modified Healey, low in AFIP) who received adjuvant radiotherapy, recurred in the buccal region, and was alive with metastatic disease 22 years after surgery; patient 5 had a T4 NX palate carcinoma (morphologically MEC; high grade in Brandwein and modified Healey, intermediate in Katabi, low in AFIP) who was treated with surgery, had local recurrence in the neck 3 months after surgery, followed by radiation therapy and additional neck and orbital recurrences; patient 6 had a T3 N2b submandibular carcinoma (morphologically MEC; high grade in all systems) who received adjuvant chemoradiation, recurred in the floor of mouth 2 months after resection, developed pulmonary metastases 4 months after resection, and died of disease 10 months after resection. Of the remaining 39 patients, follow-up ranged from less than 1 month to 13.3 years (average 3.82 years) and none recurred.
Follow-up of the 8 recurrent patients ranged from 0.8–26.9 years after recurrence (average 8.4 years). Three had additional loco-regional recurrences, one had distant metastasis, one had no additional recurrences, one had unexplained dysphagia and weight loss, one died at home of unknown causes, and one was lost to follow-up.
Risk Factors for Recurrence in Primary Carcinomas:
Morphology:
Of the 40 carcinomas morphologically compatible with MEC, 4 (10%) recurred. Of the 5 morphologically non-MECs, 2 (40%) recurred. Morphology did not correlate to recurrence in primary carcinomas (p=0.1248) [Table 3I]. However, when considering all 53 carcinomas (including non-primary), those morphologically non-MEC were more likely to recur (7/10, 70%) compared to MEC (7/43, 16%) (p=0.0017) [Table 3J].
Margins:
Of the 16 primary carcinomas with positive surgical margins, 3 (19%) recurred, all of which were Brandwein high grade, 2 of which were treated with adjuvant therapy. Of the remaining 13 with positive margins that did not recur, 5 (38%) were treated with adjuvant therapy, 7 (54%) with surgery only, and one was lost to follow-up. Of the 29 primary carcinomas with negative surgical margins, 3 (10%) recurred. One was treated with adjuvant radiation for close margins, one with adjuvant chemoradiation for N2b disease, and one with surgery only. Of the remaining 26 with negative margins that did not recur, one was treated with adjuvant radiation for skeletal muscle invasion (T3) and 25 were treated with surgery alone. Margin status did not correlate to recurrence (p=0.3585) [Table 3K]. Considering only primary carcinomas that were morphologically MEC (n=40), margin status still did not correlate to recurrence (p=0.1145) [Table 3L].
FISH:
Of the 34 MAML2-rearranged cases, 3 (9%) recurred. Of the 5 non-rearranged cases, 0 (0%) recurred. FISH status did not correlate to recurrence (p=0.6548) [Table 3M]. Considering all carcinomas (including non-primary), FISH status also did not correlate to recurrence (p=0.0867) [Table 3N]. Of the 7 carcinomas in which hybridization failed, 4 (57%) recurred.
Stage:
Of the 9 T4 carcinomas, 4 (44%) recurred. Of the 33 T1-T3 carcinomas, 1 (3%) recurred. T4 carcinomas were more likely to recur (p=0.0050) [Table 3O]. Considering only primary carcinomas that were morphologically MEC (n=38), T4 carcinomas were still more likely to recur (p=0.0237) [Table 3P].
Grade:
Recurrence rates based on grade were highly dependent on the grading system used. Of the 13 primary carcinomas that scored high grade in the Brandwein system, 6 (46%) recurred; of the remaining 32 primary carcinomas that scored low-intermediate grade in all systems, none recurred (p=0.0002) [Table 3Q]. Considering only primary carcinomas that were morphologically MEC (n=40), Brandwein high grade also correlated to recurrence (p=0.0014) [Table 3R]. Of the 11 patients with intermediate grade due to isolated solid growth, all received surgery only and none recurred after an average of 4.4 years (median 3.8, range 0.5–11.7 years).
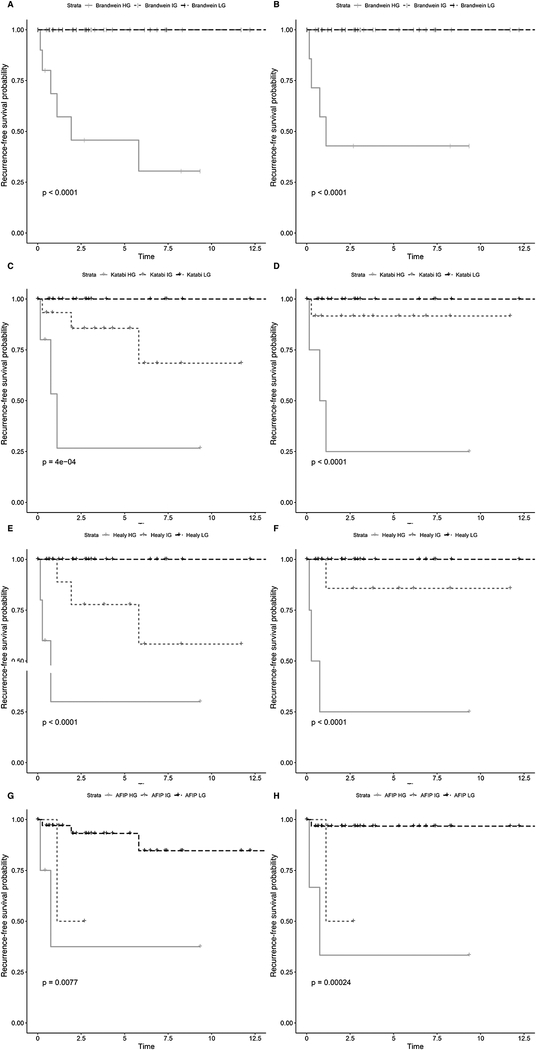
Of the 45 primary cases graded in the Brandwein system, 0/32 (0%) low or intermediate grade and 6/13 (46%) high grade carcinomas recurred (p<0.0001) [Figure 4A, Supplementary Figure 3A]. Of these cases graded in the Katabi system, 0/22 (0%) low grade, 3/15 (20%) intermediate grade, and 3/8 (38%) high grade carcinomas recurred (p=0.0004) [Figure 4C]. Of these cases graded in the modified Healey system, 0/27 (0%) low grade, 3/10 (30%) intermediate grade, and 3/8 (38%) high grade carcinomas recurred (p=<0.0001) [Figure 4E]. Of these cases graded in the AFIP system, 3/36 (8%) low grade, 1/2 (50%) intermediate grade, and 2/7 (29%) high grade carcinomas recurred (p=0.0077) [Figure 4G, Supplementary Figure 3C].
Figure 4: Kaplan-Meier Recurrence Curves Based on Ternary Grading of Primary Cases.
Considering all 45 primary cases, Brandwein intermediate grade carcinomas behaved similarly to low grade, with no recurrences in either group. Recurrences only occurred in the high Brandwein grade group (A). A binary Brandwein system could be employed as an improved predictor of recurrence [Supplementary Figure 3A]. In the Katabi (C) and modified Healy (E) systems, low, intermediate, and high grade carcinomas had different recurrence-free survival rates, including no recurrences in the low grade groups. AFIP low and intermediate grade carcinomas had worse recurrence-free survival rates compared to other systems, suggesting that this system undergrades carcinomas (G). Additionally, AFIP intermediate grade carcinomas behaved similarly to high grade [Supplementary Figure 3C]. Considering only the 40 primary carcinomas morphologically compatible with mucoepidermoid, the findings are very similar (B, D, F, H) [Supplementary Figure 3B, 3D].
Of the 40 primary cases that were morphologically MEC, the findings were similar: Of cases graded in the Brandwein system, 0/31 (0%) low or intermediate grade and 4/9 (44%) high grade carcinomas recurred (p<0.0001) [Figure 4B, Supplementary Figure 3B]. Of these cases graded in the Katabi system, 0/22 (0%) low grade, 1/12 (8%) intermediate grade, and 3/6 (50%) high grade carcinomas recurred (p<0.0001) [Figure 4D]. Of these cases graded in the modified Healey system, 0/26 (0%) low grade, 1/8 (13%) intermediate grade, and 3/6 (50%) high grade carcinomas recurred (p<0.0001) [Figure 4F]. Of these cases graded in the AFIP system, 1/33 (3%) low grade, 1/2 (50%) intermediate grade, and 2/5 (40%) high grade carcinomas recurred (p=0.00024) [Figure 4H, Supplementary Figure 3D].
Discussion:
In this evaluation of 53 historically-diagnosed mucoepidermoid carcinomas of the head & neck, a binary grading system (Brandwein high versus low-intermediate) best stratified patients into groups with differing risks of recurrence [Table 4]. Additionally, absence of MAML2 rearrangement was not associated with recurrence, but non-mucoepidermoid morphology was.
Table 4:
Clinicopathologic Features of Primary Morphologically Mucoepidermoid Carcinomas Using Proposed Binary Grading System
| Parameter (n=40) | Brandwein Low-Intermediate Grade (n=31) | Brandwein High Grade (n=9) | p-value |
|---|---|---|---|
| Site (Minor Salivary Gland) | 21 (67.7%) | 3 (33.3%) | 0.1198 |
| Average Age, Years | 50.8 (range 9–75) | 56.7 (28–85) | 0.3782 |
| Sex (Female) | 21 (67.7%) | 6 (66.7%) | 1 |
| Average Tumor Size, cm | 1.4 (0.3–3.8) | 3.4 (1.2–6.8) | <0.0001 |
| Margins Positive | 9 (29.0%) | 5 (55.6%) | 0.2338 |
| Stage (pT4) | 4 (12.9%) | 4 (44.4%) | 0.0594 |
| Treatment (Surgery Alone) | 28 (90.3%) | 3 (33.3%) | 0.0014 |
| Recurrence | 0 | 4 (44.4%) | 0.0014 |
| MAML2-rearranged | 27/29 (93.1%) | 7/8 (87.5%) | 1 |
These findings agree with prior studies reporting that the Brandwein system assigns higher grades to carcinomas and the AFIP system lower grades. The Katabi and modified Healey systems appear to grade tumors similarly and in an intermediate fashion between Brandwein and AFIP. Given the rate of recurrence of primary carcinomas that scored high grade in the Brandwein system (46% of all high grade carcinomas and 44% of morphologically mucoepidermoid high grade carcinomas), Brandwein high grade tumors could be considered biologically high grade, perhaps necessitating adjuvant therapy. Additionally, given the lack of recurrence in primary carcinomas that scored low-intermediate grade in the Brandwein system (0%), both Brandwein intermediate and low grade tumors could be considered biologically low grade, in which adjuvant therapy may not be necessary. This finding is consistent with other studies which have suggested that low and intermediate grade carcinomas behave similarly.12,13,31 McHugh et al, using cystic versus solid growth and cellular anaplasia as main grading criteria in a cohort of 125 patients, found no differences in disease-free or overall survival in low compared to intermediate grade cases.13 Recurrence occurred in 11% of low grade, 9% of intermediate grade, and 57% of high grade cases. Nance et al, using the Brandwein system in 50 patients, also found no differences in disease-free or overall survival in low compared to intermediate grade.12 Guzzo et al, graded 108 patients using the AFIP system, found no case that scored intermediate grade, and did find a significant difference in recurrence between low and high grade cases.31
In this study, 40% of primary carcinomas scored intermediate grade in at least one system, most frequently due to isolated solid growth (61%). None of these patients recurred after an average of 4.4 years. In light of these findings, a tumor that has only solid growth could be designated low grade. Conversely, perineural invasion, lymphovascular invasion, necrosis, increased mitoses, and anaplasia were only present in tumors scoring high grade in at least one system. A tumor that has any one of these features should be designated high grade. The features of peripheral invasion in nests and islands as well as bony invasion were present in both intermediate and high grade tumors, and may only helpful be in conjunction with other high grade features. While Katabi and modified Healey systems do stratify patients into three distinct grades with different recurrence rates, up to 30% of primary carcinomas scoring intermediate grade recurred. Management of these patients can therefore be uncertain. Of the 6 patients scoring high in Brandwein but intermediate in Katabi or modified Healey, 4 recurred. Brandwein grade (high versus low-or-intermediate) was a better independent predictor of recurrence. Finally, the AFIP system was the least accurate at predicting recurrence and should be avoided. For these reasons, a binary grading system for mucoepidermoid carcinoma should be considered using the Brandwein system: histologically high grade should be considered biologically aggressive and histologically low-or-intermediate grade should be considered biologically indolent or low grade. Only two other studies have compared histologic grading systems in mucoepidermoid carcinomas. Qannam et al compared 19 MECs across all 4 systems and found overall low (32%) agreement, similar to that in our study (43%). They found that the AFIP and Katabi systems yield lower grades while Brandwein and modified Healey assign higher grades.32 Their findings agree with ours, however, they did not correlate grade to recurrence rates. Bai et al compared 79 MECs using AFIP and Brandwein systems and found overall 41% of cases were upgraded from AFIP to Brandwein.33 They also did not compare the predictive performance of the grading systems.
Additional clinicopathologic features predictive of recurrence were evaluated in our study. In primary carcinomas, pathologic stage T4 was associated with recurrence but margin status was not. Stage T4 was not associated with MEC morphology or MAML2 rearrangement. Evaluating all carcinomas, presence of MAML2 rearrangement was not associated with recurrence, but morphology was: carcinomas morphologically compatible with MEC were less likely to recur. These findings are likely explained by a few confounding factors: tumors that are low grade are more likely to be accurately classified by morphology as true MEC (97% Brandwein low-intermediate grade compared to 55% high grade were morphologically MEC and 93% Brandwein low-intermediate grade compared to 56% high grade were MAML2 rearranged). Tumors that are high grade may be given a diagnosis of MEC in the absence of ideal histologic features or MAML2 gene rearrangement, simply for lack of a more specific diagnosis [Figure 3]. Morphologic features compatible with MEC (regardless of grade) included abundant intermediate cells, multifocal presence of cells with true intracytoplasmic mucin, and usually combined solid and cystic growth (either macro or microcysts). Squamoid cells were the least in abundance. The application of a mucicarmine or PAS/PAS-diastase stain could facilitate in the identification of true intracytoplasmic mucin rather than clearing due to glycogen. Additionally, carcinomas with architectural high grade features (perineural invasion lymphovascular invasion, necrosis) were occasionally cytologically bland. Morphologic features compatible with MEC were strongly associated with presence of MAML2 gene rearrangement, such that all 37 MAML2-rearranged carcinomas were morphologically MEC. Only 3 cases that were morphologically MEC were not rearranged, and all had intermediate cells and intracellular mucin [Figure 2]. In these cases, absence of MAML2 rearrangement could be explained by inherent limitations of FISH to detect a rearrangement or absence of MAML2 rearrangement in a small subset of mucoepidermoid carcinomas. Recent whole-exome sequencing has demonstrated TP53 mutations in some MAML2 wildtype MECs.34
The prevalence and prognostic significance of CRTC1-MAML2 or CRTC3-MAML2 rearrangement in MEC has been studied with varying results. Prevalence rates ranging from 34% to 82% have been reported, with CRTC1 being the more common partner.14–22,24–29,35 Better prognosis in MAML2-rearranged carcinomas has been shown, mostly in studies with low rearrangement rates.14–16,18–22 Five of these eight studies had MAML2 rearrangement rates ≤55%. Other studies showing similar prognosis between MAML2-rearranged and non-rearranged carcinomas had rates between 56% and 75%.24,25,28,29 In this study, MAML2 rearrangement was present in 93% of cases morphologically compatible with MEC, and rearrangement did not correlate with recurrence. It has been suggested that non-rearranged tumors may represent non-mucoepidermoid carcinomas, some of which may be EWSR-rearranged clear cell carcinomas. High grade carcinomas of uncertain morphology may have a higher tendency to be called MEC even in the absence of the combined features of intracellular mucin, intermediate cells, and squamoid cells.16,24,25,28,30,36 Therefore, when restricting the diagnosis of MEC to those with certain morphology, the prognostic significance of MAML2 rearrangement diminishes. Mucoepidermoid carcinomas with MAML2-rearrangement may also behave aggressively, and some have suggested that MAML2-rearrangement in the presence of a complex genome26, HMGA2 expression17, or CDKN2A loss23 portends worse prognosis. Further characterization of the grading systems in the presence of genomic information is warranted.
Histologic grading across all four MEC grading systems was inconsistent. However, Brandwein high grade is likely the best predictor of aggressive behavior. A binary grading system is proposed: cases scoring low or intermediate grade in the Brandwein system could be considered biologically low grade; cases scoring Brandwein high grade as biologically high grade. The most likely histologic finding for an increase to intermediate grade was solid growth, but behavior in these patients compared to those with low grade disease did not change. In the setting of isolated solid growth, upgrading to intermediate may not be warranted. Lastly, presence of MAML2 rearrangement was highly correlated to MEC morphology but not to recurrence. High grade non-rearranged carcinomas are likely morphologic mimics of MEC, and prior studies demonstrating worse prognosis in MAML2-non-rearranged MECs were likely diluted by high grade non-MECs. Strict histologic criteria should be used when rendering a diagnosis of MEC.
Supplementary Material
Flow Chart of Included Cases
Four Mucoepidermoid Carcinoma Grading Schemes Compared Forty primary carcinomas that were morphologically compatible with mucoepidermoid on contemporary re-review were graded according to four published grading systems. Brandwein assigned the highest rate of high grade (23%), while AFIP the highest rate of low grade (83%). The distribution was similar when including all 45 primary carcinomas, 5 of which were non-mucoepidermoid [Figure 1].
Kaplan-Meier Recurrence Curves Based on Binary Grading of Primary Cases Of all 45 primary carcinomas (A) and 40 primary carcinomas that were morphologically mucoepidermoid on re-review (B), a Brandwein binary grading system could be used as a predictor of recurrence, as neither low nor intermediate grade carcinomas recurred. In these same groups (C, D), AFIP intermediate and high grade cases clustered together with higher rates of recurrence compared to low grade. Overall, AFIP undergraded carcinomas.
Acknowledgments:
The authors would like to thank Dr. Julia A. Bridge for consultation regarding interpretation of a case with unusual MAML2 FISH pattern.
Footnotes
Disclosures: The authors have no conflicts of interest or funding to disclose.
Contributor Information
Nicole A. Cipriani, Department of Pathology, The University of Chicago Medicine, 5841 S. Maryland Ave, Chicago, IL 60637, USA.
Jonathan J. Lusardi, Otolaryngologist, Northwest Otolaryngology, 12277 DePaul Drive, Suite 502S, Bridgeton, MO 63044, USA.
James McElherne, Constitutional Cytogenetics and Cytogenomics, Department of Pathology, The University of Chicago Medicine, 5841 S. Maryland Ave, Chicago, IL 60637, USA.
Alexander T. Pearson, Section of Hematology / Oncology, Department of Internal Medicine, The University of Chicago Medicine, 5841 S. Maryland Ave, Chicago, IL 60637, USA.
Andrea D. Olivas, Department of Pathology, The University of Chicago Medicine, 5841 S. Maryland Ave, Chicago, IL 60637, USA.
Carrie Fitzpatrick, Constitutional Cytogenetics and Cytogenomics, Department of Pathology, The University of Chicago Medicine, 5841 S. Maryland Ave, Chicago, IL 60637, USA.
Mark W. Lingen, Department of Pathology, The University of Chicago Medicine, 5841 S. Maryland Ave, Chicago, IL 60637, USA.
Elizabeth A. Blair, Section of Otolaryngology Head and Neck Surgery, Department of Surgery, The University of Chicago Medicine, 5841 S. Maryland Ave, Chicago, IL 60637, USA.
References:
- 1.Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2015, National Cancer Institute 2018:1–33. Available at: https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018.
- 2.Boukheris H, Curtis RE, Land CE, et al. Incidence of carcinoma of the major salivary glands according to the WHO classification, 1992 to 2006: a population-based study in the United States. Cancer Epidemiol. Biomarkers Prev 2009;18:2899–2906. doi: 10.1158/1055-9965.EPI-09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart FW, Foote FW, Becker WF. Muco-Epidermoid Tumors of Salivary Glands. Ann. Surg 1945;122:820–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foote FW, Frazell EL. Tumors of the major salivary glands. Cancer 1953;6:1065–1133. [DOI] [PubMed] [Google Scholar]
- 5.Healey WV, Perzin KH, Smith L. Mucoepidermoid carcinoma of salivary gland origin. Classification, clinical-pathologic correlation, and results of treatment. Cancer 1970;26:368–388. [DOI] [PubMed] [Google Scholar]
- 6.Batsakis JG, Luna MA. Histopathologic grading of salivary gland neoplasms: I. Mucoepidermoid carcinomas. Ann. Otol. Rhinol. Laryngol 1990;99:835–838. doi: 10.1177/000348949009901015. [DOI] [PubMed] [Google Scholar]
- 7.Auclair PL, Goode RK, Ellis GL. Mucoepidermoid carcinoma of intraoral salivary glands. Evaluation and application of grading criteria in 143 cases. Cancer 1992;69:2021–2030. [DOI] [PubMed] [Google Scholar]
- 8.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998;82:1217–1224. [DOI] [PubMed] [Google Scholar]
- 9.Brandwein MS, Ivanov K, Wallace DI, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001;25:835–845. [DOI] [PubMed] [Google Scholar]
- 10.Katabi N, Ghossein R, Ali S, et al. Prognostic features in mucoepidermoid carcinoma of major salivary glands with emphasis on tumour histologic grading. Histopathology 2014;65:793–804. doi: 10.1111/his.12488. [DOI] [PubMed] [Google Scholar]
- 11.Luna MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol 2006;13:293–307. doi: 10.1097/01.pap.0000213058.74509.d3. [DOI] [PubMed] [Google Scholar]
- 12.Nance MA, Seethala RR, Wang Y, et al. Treatment and survival outcomes based on histologic grading in patients with head and neck mucoepidermoid carcinoma. Cancer 2008;113:2082–2089. doi: 10.1002/cncr.23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McHugh CH, Roberts DB, El-Naggar AK, et al. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer 2012;118:3928–3936. doi: 10.1002/cncr.26697. [DOI] [PubMed] [Google Scholar]
- 14.Behboudi A, Enlund F, Winnes M, et al. Molecular classification of mucoepidermoid carcinomas-prognostic significance of the MECT1-MAML2 fusion oncogene. Genes Chromosom. Cancer 2006;45:470–481. doi: 10.1002/gcc.20306. [DOI] [PubMed] [Google Scholar]
- 15.Okabe M, Miyabe S, Nagatsuka H, et al. MECT1-MAML2 fusion transcript defines a favorable subset of mucoepidermoid carcinoma. Clin Cancer Res 2006;12:3902–3907. doi: 10.1158/1078-0432.CCR-05-2376. [DOI] [PubMed] [Google Scholar]
- 16.Tirado Y, Williams MD, Hanna EY, et al. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: implications for histogenesis and biologic behavior. Genes Chromosom. Cancer 2007;46:708–715. doi: 10.1002/gcc.20458. [DOI] [PubMed] [Google Scholar]
- 17.Fehr A, Meyer A, Heidorn K, et al. A link between the expression of the stem cell marker HMGA2, grading, and the fusion CRTC1-MAML2 in mucoepidermoid carcinoma. Genes Chromosom. Cancer 2009;48:777–785. doi: 10.1002/gcc.20682. [DOI] [PubMed] [Google Scholar]
- 18.Miyabe S, Okabe M, Nagatsuka H, et al. Prognostic significance of p27Kip1, Ki-67, and CRTC1-MAML2 fusion transcript in mucoepidermoid carcinoma: a molecular and clinicopathologic study of 101 cases. J. Oral Maxillofac. Surg 2009;67:1432–1441. doi: 10.1016/j.joms.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama T, Miyabe S, Okabe M, et al. Clinicopathological significance of the CRTC3-MAML2 fusion transcript in mucoepidermoid carcinoma. Modern Pathology 2009;22:1575–1581. doi: 10.1038/modpathol.2009.126. [DOI] [PubMed] [Google Scholar]
- 20.Okumura Y, Miyabe S, Nakayama T, et al. Impact of CRTC1/3-MAML2 fusions on histological classification and prognosis of mucoepidermoid carcinoma. Histopathology 2011;59:90–97. doi: 10.1111/j.1365-2559.2011.03890.x. [DOI] [PubMed] [Google Scholar]
- 21.Noda H, Okumura Y, Nakayama T, et al. Clinicopathological significance of MAML2 gene split in mucoepidermoid carcinoma. Cancer Sci 2013;104:85–92. doi: 10.1111/cas.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luk PP, Wykes J, Selinger CI, et al. Diagnostic and prognostic utility of Mastermind-like 2 (MAML2) gene rearrangement detection by fluorescent in situ hybridization (FISH) in mucoepidermoid carcinoma of the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;121:530–541. doi: 10.1016/j.oooo.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Anzick SL, Chen W-D, Park Y, et al. Unfavorable prognosis of CRTC1-MAML2 positive mucoepidermoid tumors with CDKN2A deletions. Genes Chromosom. Cancer 2010;49:59–69. doi: 10.1002/gcc.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seethala RR, Dacic S, Cieply K, et al. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol 2010;34:1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]
- 25.Chiosea SI, Dacic S, Nikiforova MN, et al. Prospective testing of mucoepidermoid carcinoma for the MAML2 translocation: clinical implications. The Laryngoscope 2012;122:1690–1694. doi: 10.1002/lary.22419. [DOI] [PubMed] [Google Scholar]
- 26.Jee KJ, Persson M, Heikinheimo K, et al. Genomic profiles and CRTC1-MAML2 fusion distinguish different subtypes of mucoepidermoid carcinoma. Modern Pathology 2013;26:213–222. doi: 10.1038/modpathol.2012.154. [DOI] [PubMed] [Google Scholar]
- 27.Nakano T, Yamamoto H, Hashimoto K, et al. HER2 and EGFR gene copy number alterations are predominant in high-grade salivary mucoepidermoid carcinoma irrespective of MAML2 fusion status. Histopathology 2013;63:378–392. doi: 10.1111/his.12183. [DOI] [PubMed] [Google Scholar]
- 28.Saade RE, Bell D, Garcia J, et al. Role of CRTC1/MAML2 Translocation in the Prognosis and Clinical Outcomes of Mucoepidermoid Carcinoma. JAMA Otolaryngol Head Neck Surg 2016;142:234–240. doi: 10.1001/jamaoto.2015.3270. [DOI] [PubMed] [Google Scholar]
- 29.Birkeland AC, Foltin SK, Michmerhuizen NL, et al. Correlation of Crtc1/3-Maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral Oncology 2017;68:5–8. doi: 10.1016/j.oraloncology.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chenevert J, Barnes LE, Chiosea SI. Mucoepidermoid carcinoma: a five-decade journey. Virchows Archiv 2011;458:133–140. doi: 10.1007/s00428-011-1040-y. [DOI] [PubMed] [Google Scholar]
- 31.Guzzo M, Andreola S, Sirizzotti G, et al. Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol 2002;9:688–695. [DOI] [PubMed] [Google Scholar]
- 32.Qannam A, Bello IO. Comparison of histological grading methods in mucoepidermoid carcinoma of minor salivary glands. Indian J Pathol Microbiol 2016;59:457–462. doi: 10.4103/0377-4929.191765. [DOI] [PubMed] [Google Scholar]
- 33.Bai S, Clubwala R, Adler E, et al. Salivary mucoepidermoid carcinoma: a multi-institutional review of 76 patients. Head and Neck Pathol 2013;7:105–112. doi: 10.1007/s12105-012-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang H, Tan M, Bishop JA, et al. Whole-Exome Sequencing of Salivary Gland Mucoepidermoid Carcinoma. Clin Cancer Res 2017;23:283–288. doi: 10.1158/1078-0432.CCR-16-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martins C, Cavaco B, Tonon G, et al. A study of MECT1-MAML2 in mucoepidermoid carcinoma and Warthin’s tumor of salivary glands. J Mol Diagn 2004;6:205–210. doi: 10.1016/S1525-1578(10)60511-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh M-S, Wang H, Lee Y-H, et al. Reevaluation of MAML2 fusion-negative mucoepidermoid carcinoma: a subgroup being actually hyalinizing clear cell carcinoma of the salivary gland with EWSR1 translocation. Human Pathology 2017;61:9–18. doi: 10.1016/j.humpath.2016.06.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow Chart of Included Cases
Four Mucoepidermoid Carcinoma Grading Schemes Compared Forty primary carcinomas that were morphologically compatible with mucoepidermoid on contemporary re-review were graded according to four published grading systems. Brandwein assigned the highest rate of high grade (23%), while AFIP the highest rate of low grade (83%). The distribution was similar when including all 45 primary carcinomas, 5 of which were non-mucoepidermoid [Figure 1].
Kaplan-Meier Recurrence Curves Based on Binary Grading of Primary Cases Of all 45 primary carcinomas (A) and 40 primary carcinomas that were morphologically mucoepidermoid on re-review (B), a Brandwein binary grading system could be used as a predictor of recurrence, as neither low nor intermediate grade carcinomas recurred. In these same groups (C, D), AFIP intermediate and high grade cases clustered together with higher rates of recurrence compared to low grade. Overall, AFIP undergraded carcinomas.