Abstract
Nervous systems are built on synaptic connections, and our understanding of these complex compartments has deepened over the past quarter century as the diverse fields of genetics, molecular biology, physiology, and biochemistry each made significant in-roads into synaptic function. On the presynaptic side, an evolutionarily conserved core fusion apparatus constructed from a handful of proteins has emerged, with Unc13 serving as a hub that coordinates nearly every aspect of synaptic transmission. This review briefly highlights recent studies on diverse aspects of Unc13 function including roles in SN RE assembly and quality control, release site building, calcium channel proximity, and short-term synaptic plasticity.
Keywords: Munc13, Synapse, Synaptic Transmission, Synaptic Vesicle, Membrane Fusion, UNC-13, Evolution, Active Zone, SNARE, Syntaxin, Synaptic Plasticity
Introduction
The presynaptic vesicle fusion apparatus is a highly regulated chemomechanical machine that harnesses the energy of protein-protein and protein-lipid interactions as well as calcium binding to drive the merger of synaptic vesicle (SV) and plasma membranes on a remarkably fast time scale [1,2]. While the SNARE proteins are required for this and many other membrane fusion processes in eukaryotic cells, most synapses rely on members of the Unc13 protein family (Munc13s in mammals) to control SV fusion and endow synaptic transmission with multiple forms of regulation. widely adopted view of calcium-triggered SV fusion suggests that SVs are captured at specialized regions within the presynaptic active zone (AZ) termed release sites. Neuronal SNARE proteins on the plasma membrane (Syntaxin 1 and SNAP25 comprising the t-SNARE) bind to VAMP2 (also known as synaptobrevin) on the SV membrane and assemble into a metastable partially 2018zippered’ state poised to fuse upon local calcium elevation. This assembly is not intrinsically guaranteed to occur correctly and requires several additional proteins to guide and regulate SNARE complex formation [3–6]. Critical presynaptic proteins such as synaptotagmin 1 and complexin assist in preventing premature SV fusion (spontaneous fusion) while coordinating calcium binding with rapid completion of SNARE assembly and concomitant membrane fusion in response to voltage-gated calcium channel (VGCC) opening. Several excellent reviews cover these aspects of synaptic transmission in detail [1,3,7–11]. In addition to this minimal ‘parts list’ for the SV fusion apparatus, two classes of proteins have emerged as essential ingredients: the Unc13 and SM (Sec1/Munc18-like) protein families. Loss of either of these proteins effectively eliminates both spontaneous and calcium-triggered synaptic transmission in mouse (Munc13 and Munc18/STXBP1), fly (Unc13/Dunc13 and Rop), and worm (UNC-13 and UNC-18) [12–19]. Here, I focus on recent studies of Unc13 proteins and summarize current concepts ranging from the evolution of synaptic transmission, coordination of release site and SNARE complex assembly, presynaptic plasticity, and connections to priming and post-priming roles of Unc13.
Evolution of the Unc13 protein family
Unc13s are relatively large cytoplasmic proteins (typically ranging from 1100 – 3000 residues) and the canonical Unc13s comprise two or three C2 domains, one C1 domain, a CaM-binding motif, along with the ~700 residue SNARE-binding MUN domain shared by all Unc13 family members (Figure 1A). The MUN and C2 domains are also shared in more distant homologs such as CAPS and BAIAP3, but this review will focus solely on the Unc13 family [20,21]. What are the evolutionary origins of Unc13 and the SV fusion apparatus, and did this machinery evolve before or after the advent of a primitive nervous system?Asdescribedinmoredetailbelow,Unc13 plays a central role in the assembly of release sites, SV docking and priming, and in coupling calcium entry to fusion. Thus, the origin of Unc13 provides a clue regarding the emergence of fast and highly regulated secretion. Recent genome sequencing of representative early branching animals lacking neurons and muscle (such as the placozoan Trichoplax adhaerens and sponge Amphimedon queenslandica) as well as unicellular organisms closely related to animals such as the choanoflagellate Monosiga brevicollis reveal that a clear Unc13 homolog was present prior to the emergence of neurons and bona fide synapses [22,23]. Fungi harbor a homolog possessing either one or two C2 domains and a MUN domain that is split in two. The S. pombe homolog (Ync13) was recently described to play a role in cytokinesis and may represent an ancient demand for rapid regulated membrane fusion (e.g. creation of new membrane during septum formation in fission yeast ) in eukaryotes [24]. The 1100 residue core functional unit of Unc13 is reasonably approximated in choanoflagellates, and remarkably, both long and short isoforms of complete Unc13 exist in sponges despite the conspicuous lack of neurons, muscle, and synapses in these simple animals (Figure 1B). The -terminal C2A domain of Unc13 is sporadically lost across bilaterian evolution; it is absent in Drosophila (but present in many insects and other arthropods), while mammals and nematodes express Munc13 isoforms with and without the C2A domain (Figure 1C). As discussed later, this region may help couple VGCCs to the release site allowing for distinct release site architectures and properties. Based on the deep metazoan conservation of the complete Unc13 blueprint, together with the presence of other key presynaptic proteins such as synaptotagmin 1 and complexin in neuron-less placozoans and sponges, it is probable that a highly regulated form of calcium-triggered membrane fusion existed prior to the appearance of neurons and synapses [22,23,25–27]. Perhaps the emergence of more sophisticated forms of chemical transmission required a pre-existing ‘proto - synaptic’ protein complex.
Figure 1.
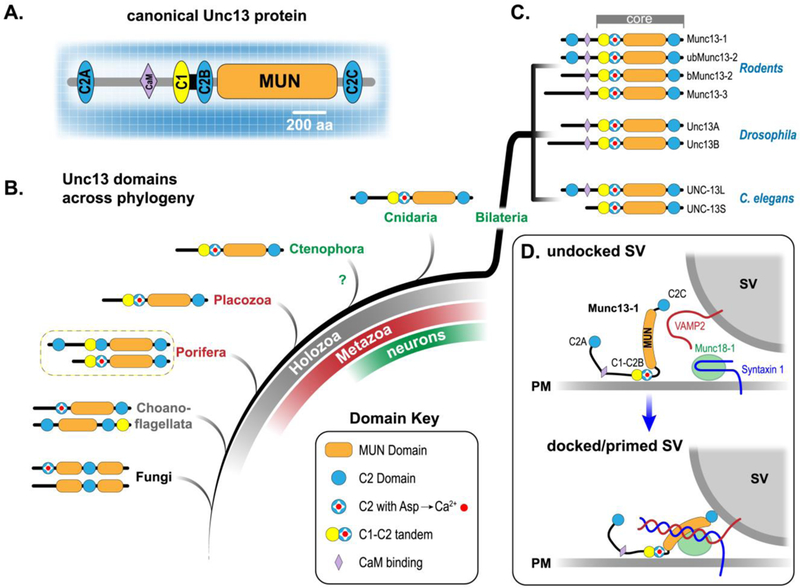
Domain organization and evolution of Unc13 proteins. A. Cartoon of the canonical Unc13 protein family member (e.g. mouse Munc13–1, worm UNC-13L, or human Unc13A). Scale bar indicates relative number of residues comprising each of the protein domains. B. Heuristic evolutionary organization of representative Unc13 homologs from several metazoan phyla as well as unicellular organisms. Unicellular organisms in gray/black, early branching animals lacking true neurons and muscle in red, and animals possessing neurons in green. Holozoa encompass metazoa and their closest unicellular relatives such as choanoflagellates. This Unc13 distribution across Holozoa suggests that the ancestral protein arose prior to the emergence of nervous systems. The relative phylogenetic position of Ctenophora is not clear [80]. Here, Ctenophora are grouped with other animals possessing nervous systems (green). C. Several isoforms of neuronal Unc13 are shown for mouse (top), fly (middle), and worm (bottom). D. Cartoon depicting a possible role for Munc13–1 and Munc18–1 in catalyzing SNARE assembly and synaptic vesicle (SV) docking and priming. Note that several critical presynaptic proteins such as SNAP25, Synaptotagmin 1, Rab3, and numerous AZ proteins have been omitted for clarity.
Munc13–1, Munc18–1 and SNARE assembly
The founding Unc13 family member was discovered by Sidney Brenner in his landmark C. elegans screen initiated in the late1960s [28]. Brenner originally isolated about 70 mutants displaying locomotion defects (uncoordinated or ‘unc’ mutants) and unc-13 along with unc-18 were two of only eight severely paralyzed mutants. Subsequent studies on the biochemistry and function of Unc13 and SM orthologs in worm, mouse and fly over the past 25 years have placed these proteins on a par with the SNAREs in terms of their essential role in synaptic transmission [1,3,7,10,17,18]. Recent work on mammalian SM proteins and their fungal homologs supports the concept that SM proteins evolved to channel SNARE assembly and fusion down an optimal pathway while excluding a myriad of unproductive or destructive off-path trajectories [4,29,30]. Indeed, the yeast SM proteins Sec1, Sly1, Vps33, and Vps45 may prevent promiscuous SNARE assembly along various trafficking and secretory pathways while catalyzing correct SNARE assembly only within the appropriate protein complexes [29,31]. How does Unc13 fit into this picture?
Disruption of Unc13 function typically leads to a large reduction in the number of fusion competent vesicles (the readily releasable pool or RRP) [12,13,18,32,33]. The core domain of Unc13 was originally proposed to catalyze the assembly of t- and v-SNAREs by stabilizing the ‘open’ state of syntaxin 1, thereby priming SVs for fusion (Figure 1D) [34]. Subsequent structural and functional studies have provided a detailed molecular model for the interaction of the Munc13–1 MUN domain and syntaxin 1 together with Munc18–1 [5,7,10,32,35,36]. Interestingly, in vitro SNARE proteoliposome fusion studies did not require Unc13 [37], suggesting that these proteins are not needed for SNARE assembly and membrane fusion despite their essential nature in vivo. Over the past few years, a more refined picture of SNARE assembly and its regulation has emerged in which Unc13 and an SM protein (Munc13–1 and Munc18–1 in mouse for instance) act together to coordinate and protect proper SNARE assembly in the face of constitutive SNARE disassembly driven by the ATPase NSF and SNAP (Figure 2) [3–5,10,36]. Given that SNAREs have the potential to assemble into numerous types of unproductive complexes, [6,38,39], ‘de-priming’ may serve as an effective proof-reading mechanism by selecting for correctly oriented and assembled SNARE complexes with optimal stoichiometry. In this scenario, the culling of mistaken and promiscuous SNARE complexes ultimately leads to the impressive performance of the SNARE fusion machinery when challenged with calcium. One recent study supports this picture by demonstrating that the severe synaptic transmission defects of Munc18–1 and Munc13–1 mouse mutants can be rescued to some extent by the impairment of NSF, perhaps reflecting less stringent disassembly conditions surrounding the release machinery and allowing suboptimal SNARE assembly and SV fusion [40]. Moreover, quantitative electron microscopy (EM) revealed a complete loss of morphologically docked SVs (within 3 nm of the plasma membrane) in AZs of cultured neurons lacking Munc13–1 [41]. A similar docked defect was observed using SNARE mutants, supporting the concept that morphologically docked and primed SVs are tethered by partially assembled SNAREs resulting from the combined actions of Munc13–1 and Munc18–1.Thus,afailuretoassembleSNAREsmay account for much of the RRP defects observed when Unc13 function is impaired.
Figure 2.
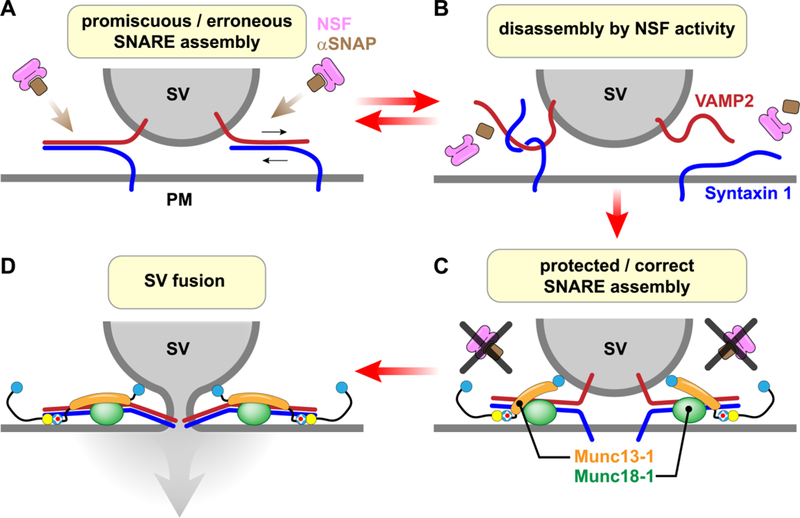
Munc13 and Munc18 protect SNARE complexes from disassembly by NSF. A & B. Inappropriate SNARE complexes (e.g. two Syntaxins with one SNAP25 or VAMP2 assembling in an antiparallel orientation with syntaxin 1) are rapidly disassembled by NSF (pink) and SNAP (brown). Note that SNAP25 has been omitted for simplicity. C. SNARE complexes assembled while bound to Munc13–1 (orange) and Munc18–1 (green) are protected from NSF and are correctly oriented. D. Vesicle fusion proceeds efficiently upon calcium elevation when all SNARE complexes have assembled appropriately as guided by Munc13–1 and Munc18–1.
Munc13–1 as a hub for release site assembly
Is the opening of syntaxin the only function of Unc13? The answer is almost certainly no: studies examining syntaxin mutants that bypass the requirement for Unc13 opening function still reported profound defects in synaptic transmission and nervous system function [4,34,42]. There is not a lot of Unc13 within a presynaptic terminal: an estimated 50–60 copies reside in the active zone of a hippocampal presynaptic terminal [43]. quantitative mass spectrometry study discerned its half-life (~ 1.3 days in cultured neurons) to be considerably shorter than many of its fellow presynaptic proteins such as SNAREs, Synaptotagmin 1, Munc18–1, VGCCs, and the AZ proteins Bassoon and Piccolo [44]. Moreover, several groups have identified Unc13 degradation as a critical determinant of synaptic strength and suggest that the regulation of its turnover can serve as a form of synaptic plasticity [45–47]. Thus, Unc13 may be a limiting reagent for some aspect of synaptic transmission beyond its catalytic role in SNARE assembly. Of particular interest, a fascinating recent study utilized super-resolution imaging and quantal analysis approaches to ascertain a quantitative relationship between the number of SVs available to fuse upon calcium entry (the readily releasable pool or RRP), the number of release sites, and the nano-scale organization of Munc13–1 in AZs of cultured hippocampal neurons [43]. At a typical presynaptic boutons examined in this study, Munc13–1 is organized into 5–6 clusters (about 45 nanometers in diameter), each containing about nine copies of Munc13–1. These Munc13–1 nanoassemblies correspond to individual release sites as well as the RRP. Even when expressed together with syntaxin 1 in non-neuronal cell lines and artificially recruited to the plasma membrane, Munc13–1 spontaneously assembled into similar clusters together with syntaxin. Syntaxin has a tendency to cluster with plasma membrane PI(4,5)P2, and Munc13–1 also interacts with PIP2 via its highly conserved C2B domain along with its previously mentioned syntaxin interactions [48–51]. Perhaps these three molecules provide the minimal ingredients of a self-assembling macromolecular complex that can then nucleate a proper release site (Figure 3A&B). In this scenario, Unc13 serves as the focal point for recruitment of plasma membrane, vesicular, and cytoplasmic components of the release site, befitting its ancient and essential role in regulated secretion.
Figure 3.
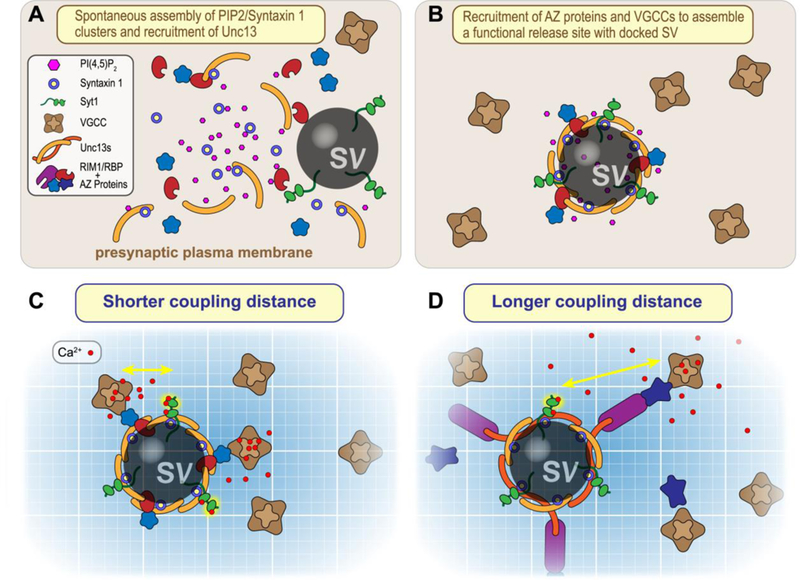
Nucleation of release site assembly by Unc13, PIP2>, and Syntaxin. A. Plasma membrane Syntaxin 1 (blue/yellow) and PI(4,5)P2 (pink) tend to coalesce into a nanodomain that can recruit Unc13 (orange) via PIP2 interactions with its C2B domain as well as via SNARE binding to the MUN domain. B. Unc13 nanoassemblies recruit other AZ proteins (blue and red) to create a functional release site. C & . Distinct Unc13 isoforms together with AZ proteins couple voltage-gated calcium channels (VGCCs) at different distances from the calcium sensor Synaptotagmin 1 (Syt1, green). Calcium ions entering the presynaptic terminal indicated in red.
Unc13 as a calcium channel coupler
Are the nucleation of release sites and syntaxin opening the only functions of Unc13? Probably not. A defining feature of synaptic transmission is the tight coupling between calcium entry via voltage-gated calcium channels (VGCCs) and the subsequent exocytosis triggered by calcium binding to sensors such as Synaptotagmin 1 (Syt1) [2,52]. Although the molecular mechanisms that coordinate release sites and VGCC co-localization are not yet understood, several lines of evidence suggest that Unc13 plays a role in the organization and regulation of calcium coupling. One of the well-studied binding partners of Munc13–1 (via its C2A domain) is the AZ protein RIM1 [53]. RIM1 serves as a release-site tether for VGCCs, and RIM-VGCC interactions depend on which splice variant of av2.1 (P/Q type) is expressed [54,55]. This transcriptional decision may tune calcium coupling [56,57]. In addition, RIM1 binds the channel-interacting proteins known as RIM-binding proteins (RBPs), and these proteins also serve an important organizational role in calcium coupling [58–60]. Recent work at the fly neuromuscular junction (NMJ) demonstrated two distinct VGCC arrangements specified by fly Unc13A and Unc13B isoforms, differing only in their N-terminal domains [60–62]. Depending on which Unc13 isoform was present, VGCCs were estimated to be either 70 or 120 nanometers from the release site, and this topography relied on either Bruchpilot and RBP or Syd-1 and Liprin- . Functionally, the two arrangements generated distinct short-term synaptic plasticities, demonstrating the importance of detailed VGCC positioning near the sites of fusion as well as the potential significance of variable Unc13 N-terminal domains across paralogs and across species (Figure 3C&D). Comparisons of AZ proteins and EM studies of the AZ across several model organisms indicate a deep organizational conservation across metazoa [60]. Consistent with this picture, work in C. elegans on the two major isoforms of UNC-13 differing in their N-terminal domains (Figure 1C) demonstrated that these isoforms subserved distinct calcium-coupling roles at the neuromuscular junction [63,64]. Finally, one study recently described a novel interaction between the C2B domain of Munc13–1 and the synprint domain of VGCCs at hippocampal synapses, and this interaction modulated channel gating, underscoring an intriguing possibility that the fusion apparatus itself may regulate the VGCCs driving fusion [65]. This sort of feedback can generate novel forms of use-dependent plasticity and may provide a source of functional diversity across synapses. Thus, while we currently lack a detailed picture of the release site architecture and its variation across synapses, Unc13 will likely serve as a keystone in the coupling of calcium influx to neurotransmitter release.
Unc13 roles in priming and post-priming, and plasticity
Are calcium-coupling, release-site nucleating, and syntaxin-opening the only functions of Unc13? Unlikely. Disruption in either release-site nucleation or syntaxin opening will decrease the RRP, but several mutations in Unc13 have been associated with changes in the probability that a vesicle fuses (Pv) independent of the RRP size [32,66,67]. Moreover, one of the original defining features of the Unc13 family is the presence of a phorbol ester-binding C1 domain positioned next to a C2B domain that binds PIP2 and calcium (Figure 4) [66,68]. Studies on synaptic modulation by phorbol esters as well as recordings from neurons harboring Munc13–1 with a mutated C1 domain suggested that Munc13 and phorbol esters could alter Pv separately from the RRP [66,67,69]. Outstanding recent structural and functional work by the Rizo and Rosenmund labs and colleagues recently delineated this region of Munc13–1, revealing an interaction between the 1– 2B module and the neighboring MUN domain [49,70]. Moreover, two recent functional explorations of this interface have helped develop a new picture of calcium and lipid regulation of Unc13 [71,72]. In this model, C1-C2B module exerts an inhibitory effect on some aspect of Unc13 function (affecting Pv rather than the docking/priming function of Unc13). Elevations in calcium and DAG release this inhibitory control and boost release probability through an as yet unknown mechanism, perhaps reminiscent of conventional PKCs (Figure 4) [71,73]. Of note, a point mutation in human Unc13A at a critical and deeply conserved proline in this C1-C2B interface with the MUN domain was found to be associated with severe autism and motor impairment, underscoring the importance of this regulatory process in synapse function [72].
Figure 4.
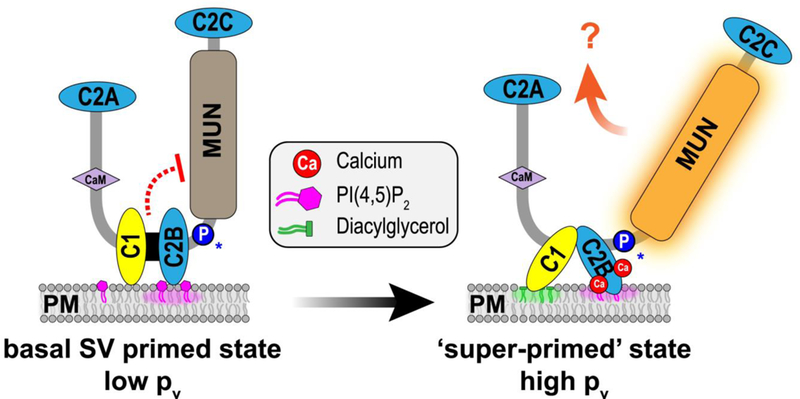
Model for relief of autoinhibition by calcium and DAG. Cartoon of the canonical Unc13 with C1-C2B module interacting with the plasma membrane (PM) via C2B-PIP2 interactions. This module also interacts with the MUN domain, stabilizing an inhibited state that corresponds to low release probability (Pv). Elevated calcium and DAG drive membrane translocation of the C1-C2B module thereby relieving autoinhibition and enhancing release probability. This may correspond to a super-primed state. Substitution of a conserved proline in the C2B-MUN Linker (blue “P”) destabilizes the inhibited state and has been associated with severe neurological disorders [71,72].
What is the physiological significance of Ca/DAG enhancement of a post-priming Unc13 function other than accounting for much of the phorbol ester effect at many synapses? Perhaps the enhanced Unc13 state is the molecular correlate of a long-studied form of presynaptic short-term plasticity (post-tetanic potentiation or PTP) [74] Several groups have proposed to distinguish low and high probability subsets of vesicles as primed and ‘super-primed’ respectively [75,76]. Parallels between PTP, super-priming and phorbol ester enhancement suggest that disinhibited Unc13 may correspond to the super-primed state. The connection between changes in initial release probability and short-term plasticity in these cases is not yet understood, but Unc13 will likely provide a useful molecular handle on this class of synaptic plasticity. Finally, work on the calmodulin-binding domains of Munc13–1/2 also indicate a role for Munc13 in a distinct form of use-dependent plasticity (affecting RRP replenishment rather than Pv in this case) [77]. As is the case with VGCC coupling, distinct variants of Unc13 have been associated with different forms of short-term plasticity and neuromodulation [77]. Together, these examples demonstrate how modulatory domains (such as the CaM-interacting domain and C1-C2B) register prior synaptic activity and neuromodulation to adjust vesicle priming and release probability, thereby endowing the synapse with numerous forms of short-term plasticity.
Concluding Remarks
Over the past several years, studies from an assortment of approaches and perspectives have placed Unc13 in a pivotal role at the synapse as briefly summarized above. Is Unc13 function absolutely synonymous with synaptic transmission? Not entirely - while Unc13s trace back to the dawn of animals, there are examples even in mammals of highly specialized synapses that appear to function independent of Unc13s. For example, auditory hair cells secrete neurotransmitter in a highly regulated calcium-dependent manner with no reliance on Munc13 [78], while deletion of ubMunc13–2 in photoreceptor and bipolar cell ribbon synapses (the only Munc13 isoform present in those cells) has surprisingly little impact on synaptic transmission [79]. These may be cases of independent synapse evolution where conventional and ribbon synapses do not share a common origin. Perhaps the exceptions prove the rule: the cellular role of Unc13 is overwhelmingly associated with the emergence of exquisitely regulated chemical synapses that wire together complex nervous systems. And while the present to-do list of Unc13 functions seems extensive thus far, it may prove short-sighted to conclude that all Unc13 functions have been cataloged in this review.
Highlights.
Nearly all forms of synaptic transmission rely on Unc13 function
Unc13 likely arose prior to the emergence of neurons and synapses
Unc13 functions together with SM proteins to guide proper SNARE assembly
Release site construction and calcium channel coupling are both controlled by Unc13
Unc13 endows synapses with several forms of short-term plasticity
Acknowledgements
This work was supported by the NIH (R01GM095674) as well as the Hirschl Scholar and Fleming Research Scholar Awards. I would like to thank Drs. Tim Ryan, Pawel Burkhardt, David DiGregorio and Murugesh Padmanarayana for valuable discussions and comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
Papers of particular interest, published with the period of review have been highlighted as:
• of special interest
•• of outstanding interest
References
- 1.Sudhof TC: Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 2013, 80:675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dittman JS, Ryan TA: The control of release probability at nerve terminals. Nature Reviews Neuroscience 2019, 20. [DOI] [PubMed] [Google Scholar]
- 3.Rizo J: Mechanism of neurotransmitter release coming into focus. Protein Sci 2018, 27:1364–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.•.Lai Y, Choi UB, Leitz J, Rhee HJ, Lee C, Altas B, Zhao M, Pfuetzner RA, Wang AL, Brose N, et al. : Molecular Mechanisms of Synaptic Vesicle Priming byMunc13andMunc18. Neuron 2017,95:591–607.e510. [DOI] [PMC free article] [PubMed] [Google Scholar]; In vitro proteoliposome fusion and smFRET SNARE assembly assays revealed specific roles for Munc13–1 and Munc18–1 in controlling correctly oriented assembled SNARE complexes. Munc13–1 was found to have a specific role in promoting the parallel assembly of the synaptobrevin-2 syntaxin-1A subconfiguration independent of Munc18–1. In addition, syntaxin-1A(LE), which bypasses the need for Munc13 opening function, did not fully restore several aspects of synaptic transmission in Munc13–1/2 DKO hippocampal neurons.
- 5.Ma C, Su L, Seven AB, Xu Y, Rizo J: Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science 2013, 339:421–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weninger K, Bowen ME, Choi UB, Chu S, Brunger AT: Accessory proteins stabilize the acceptor complex for synaptobrevin, the 1:1 syntaxin/SNAP-25 complex. Structure 2008, 16:308–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizo J, Xu J: The Synaptic Vesicle Release Machinery. Annu Rev Biophys 2015, 44:339–367. [DOI] [PubMed] [Google Scholar]
- 8.Jahn R, Fasshauer D: Molecular machines governing exocytosis of synaptic vesicles. Nature 2012, 490:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneggenburger R, Rosenmund C: Molecular mechanisms governing Ca(2+) regulation of evoked and spontaneous release. Nat Neurosci 2015, 18:935–941. [DOI] [PubMed] [Google Scholar]
- 10.Brunger AT, Choi UB, Lai Y, Leitz J, Zhou Q: Molecular Mechanisms of Fast Neurotransmitter Release. Annu Rev Biophys 2018, 47:469–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman ER: A calcium sensor for exocytosis. Trends Neurosci 2018, 41:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Augustin I, Rosenmund C, Sudhof TC, Brose N: Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 1999, 400:457–461. [DOI] [PubMed] [Google Scholar]
- 13.Richmond JE, Davis WS, Jorgensen EM: UNC-13 is required for synaptic vesicle fusion in . elegans. Nat Neurosci 1999, 2:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K: Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci 1999, 2:965–971. [DOI] [PubMed] [Google Scholar]
- 15.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. : Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 2000, 287:864–869. [DOI] [PubMed] [Google Scholar]
- 16.Weimer RM, Richmond JE, Davis WS, Hadwiger G, Nonet ML, Jorgensen EM: Defects in synaptic vesicle docking in unc-18 mutants. Nat Neurosci 2003, 6:1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richmond JE, Broadie KS: The synaptic vesicle cycle: exocytosis and endocytosis in Drosophila and C. elegans. Curr Opin Neurobiol 2002, 12:499–507. [DOI] [PubMed] [Google Scholar]
- 18.Brose N, Rosenmund C, Rettig J: Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol 2000, 10:303–311. [DOI] [PubMed] [Google Scholar]
- 19.Sassa T, Harada S, Ogawa H, Rand JB, Maruyama IN, Hosono R: Regulation of the UNC-18-Caenorhabditis elegans syntaxin complex by UNC-13. J Neurosci 1999, 19:4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.James DJ, Martin TF: CAPS and Munc13: CATCHRs that SNARE Vesicles. Front Endocrinol (Lausanne) 2013, 4:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Jiang S, Mitok KA, Li L, Attie AD, Martin TFJ: BAIAP3, a C2 domain-containing Munc13 protein, controls the fate of dense-core vesicles in neuroendocrine cells. J Cell Biol 2017, 216:2151–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varoqueaux F, Fasshauer D: Getting Nervous: An Evolutionary Overhaul for Communication. Annu Rev Genet 2017, 51:455–476. [DOI] [PubMed] [Google Scholar]
- 23.Burkhardt P, Sprecher SG: Evolutionary origin of synapses and neurons - Bridging the gap. Bioessays 2017, 39. [DOI] [PubMed] [Google Scholar]
- 24.•.Zhu YH, Hyun J, Pan YZ, Hopper JE, Rizo J, Wu JQ: Roles of the fission yeast UNC-13/Munc13 protein Ync13 in late stages of cytokinesis. Mol Biol Cell 2018, 29:2259–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors identify an Unc13 homolog (Ync13) in S. pompe and describe its role in cytokinesis. Ync13 mutants affect the localization of membrane trafficking machinery resulting in defective septation.
- 25.Smith CL, Varoqueaux F, Kittelmann M, Azzam RN, Cooper B, Winters CA, Eitel M, Fasshauer D, Reese TS: Novel Cell Types, Neurosecretory Cells, andBodyPlanoftheEarly-DivergingMetazoan Trichoplax adhaerens. Curr Biol 2014, 24:1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burkhardt P, Stegmann CM, Cooper B, Kloepper TH, Imig C, Varoqueaux F, Wahl MC, Fasshauer D: Primordial neurosecretory apparatus identified in the choanoflagellate Monosiga brevicollis. Proc Natl Acad Sci U S A 2011, 108:15264–15269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Pei J, Kaeser-Woo YJ, Bacaj T, Grishin NV, Sudhof TC: Evolutionary conservation of complexins: from choanoflagellates to mice. EMBO Rep 2015, 16:1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner S: The genetics of Caenorhabditis elegans. Genetics 1974, 77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker RW, Hughson FM: Chaperoning SNARE assembly and disassembly. Nat Rev Mol Cell Biol 2016, 17:465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakhanwal S, Lee CT, Urlaub H, Jahn R: An activated Q-SNARE/SM protein complex as a possible intermediate in SNARE assembly. EMBO J 2017, 36:1788–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.••.Baker RW, Jeffrey PD, Zick M, Phillips BP, Wickner WT, Hughson FM: A direct role for the Sec1/Munc18-family protein Vps33 as a template for SN RE assembly. Science 2015, 349:1111–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]; A new structure of an SM family member (Vps33) complexed with an R-SNARE (Nyv1) suggests a novel role for SM proteins such as Munc18 in catalyzing SNARE assembly by providing a simultaneous binding template for two SN REs held in proper register and orientation.
- 32.Madison JM, Nurrish S, Kaplan JM: UNC-13 interaction with syntaxin is required for synaptic transmission. Curr Biol 2005, 15:2236–2242. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Cooper B, Kalla S, Varoqueaux F, Young Jr.: The Munc13 proteins differentially regulate Readily releasablepooldynamicsandcalcium -dependent recovery at a central synapse. J Neurosci 2013, 33:8336–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richmond JE, Weimer RM, Jorgensen EM: An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature 2001, 412:338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S, Choi UB, Gong J, Yang X, Li Y, Wang AL, Brunger AT, Ma C: Conformational change of syntaxin linker region induced by Munc13s initiates SNARE complex formation in synaptic exocytosis. EMBO J 2017, 36:816–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang X, Wang S, Sheng Y, Zhang M, Zou W, Wu L, Kang L, Rizo J, Zhang R, Xu T, et al. : Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming. Nat Struct Mol Biol 2015, 22:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE: SNAREpins: minimal machinery for membrane fusion. Cell 1998, 92:759–772. [DOI] [PubMed] [Google Scholar]
- 38.Fasshauer D, Margittai M: transient N-terminal interaction of SNAP-25 and syntaxin nucleates SN RE assembly. J Biol Chem 2004, 279:7613–7621. [DOI] [PubMed] [Google Scholar]
- 39.Bethani I, Lang T, Geumann U, Sieber JJ, Jahn R, Rizzoli SO: The specificity of SNARE pairing in biological membranes is mediated by both proof-reading and spatial segregation. EMBO J 2007, 26:3981–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.••.He E, Wierda K, van Westen R, Broeke JH, Toonen RF, Cornelisse LN, Verhage M: Munc13–1 and Munc18–1 together prevent NSF-dependent de-priming of synaptic vesicles. Nat Commun 2017, 8:15915. [DOI] [PMC free article] [PubMed] [Google Scholar]; He et al found that severe impairment of neurotransmitter release caused by either loss of Munc13–1 or by swapping Munc18 isoforms could be rescued by disrupting NSF function with NEM or interfering peptides. The authors conclude that consitutive SNARE disassembly and vesicle de-priming is driven by NSF activity while Munc13 and Munc18 protect assembled SNAREs and stabilize the vesicle pool from this de-priming activity of NSF.
- 41.Imig C, Min SW, Krinner S, Arancillo M, Rosenmund C, Sudhof TC, Rhee J, Brose N, Cooper BH: The morphological and molecular nature of synaptic vesicle priming at presynaptic active zones. Neuron 2014, 84:416–431. [DOI] [PubMed] [Google Scholar]
- 42.McEwen JM, Madison JM, Dybbs M, Kaplan JM: Antagonistic regulation of synaptic vesicle priming by Tomosyn and UNC-13. Neuron 2006, 51:303–315. [DOI] [PubMed] [Google Scholar]
- 43.••.Sakamoto H, Ariyoshi T, Kimpara N, Sugao K, Taiko I, Takikawa K, Asanuma D, Namiki S, Hirose K:Synaptic weight set by Munc13–1 supramolecular assemblies. Nat Neurosci 2018, 21:41–49. [DOI] [PubMed] [Google Scholar]; Sakamoto and colleagues use an optical approach to determine and P at individual hippocampal synapses along with a superresolution imaging method to determine the number and distribution of Munc13–1 proteins. They find that hippocampal synapses have about 5 release sites corresponding to 5 clusters of Munc13 (~ 45 nm in size containing 9–11 copies of Munc13–1). Moreover, Munc13–1 together with Syntaxin 1 could form similarly sized nanoassemblies in nonneuronal cells.
- 44.Cohen LD, Zuchman R, Sorokina O, Müller A, Dieterich DC,Armstrong JD,Ziv T,ZivNE:Metabolic turnover of synaptic proteins: kinetics, interdependencies and implications for synaptic maintenance. PLoS One 2013, 8:e63191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsieh MC, Ho YC, Lai CY, Chou D, Chen GD, Lin TB, Peng HY: Spinal TNF-α impedes Fbxo45-dependent Munc13–1 ubiquitination to mediate neuropathic allodynia in rats. Cell Death Dis 2018, 9:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang X, Litkowski PE, Taylor AA, Lin Y, Snider BJ, Moulder KL: role for the ubiquitin-proteasome system in activity-dependent presynaptic silencing. J Neurosci 2010, 30:1798–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tada H, Okano HJ, Takagi H, Shibata S, Yao I, Matsumoto M, Saiga T, Nakayama KI, Kashima H, Takahashi T, et al. : Fbxo45, a novel ubiquitin ligase, regulates synaptic activity. J Biol Chem 2010, 285:3840–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, et al. : Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol 2010, 17:280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.•.Liu X, Seven AB, Camacho M, Esser V, Xu J, Trimbuch T, Quade B, Su L, Ma C, Rosenmund C, et al. :Functional synergy between the Munc13 C-terminal C1 and C2 domains. Elife 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors utilize in vitro assays of SNARE-mediated proteoliposome fusion and liposome clustering along with Munc13–1 structure/function assays in hippocampal neurons to support a model of the core Unc13 protein (C1-C2B-MUN-C2C) functioning as a tether between the plasma membrane and synaptic vesicles. This function is required for proper vesicle priming.
- 50.•.Milovanovic D, Platen M, Junius M, Diederichsen U, Schaap IA, Honigmann A, Jahn R, van den Bogaart G:Calcium Promotes the Formation of Syntaxin 1 Mesoscale Domains through Phosphatidylinositol 4,5-Bisphosphate. J Biol Chem 2016, 291:7868–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]; Combining superresolution microscopy, FRET, and atomic force microscopy to explore the submicron organization of syntaxin 1 within the plasma membrane, this study revealed that syntaxin 1 forms ~100 nm clusters with PI(4,5)P2 and that calcium can promote cluster formation.
- 51.van den Bogaart G, Meyenberg K, Risselada HJ, Amin H, Willig KI, Hubrich BE, Dier M, Hell SW, Grubmüller H, Diederichsen U, et al. : Membrane protein sequestering by ionic protein-lipid interactions. Nature 2011, 479:552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eggermann E, Bucurenciu I, Goswami SP, Jonas P: Nanodomain coupling between Ca(2+) channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci 2012, 13:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camacho M, Basu J, Trimbuch T, Chang S, Pulido-Lozano C, Chang SS, Duluvova I, Abo-Rady M, Rizo J, Rosenmund C: Heterodimerization of Munc13 C2A domain with RIM regulates synaptic vesicle docking and priming. Nat Commun 2017, 8:15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC: RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 2011, 144:282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirano M, Takada Y, Wong CF, Yamaguchi K, Kotani H, Kurokawa T, Mori MX, Snutch TP, Ronjat M, De Waard M, et al. : -terminal splice variants of P/Q-type Ca channel Cav2.1 alpha1 subunits are differentially regulated by Rab3-interacting molecule proteins. J Biol Chem 2017, 292:9365–9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thalhammer A, Contestabile A, Ermolyuk YS, Ng T, Volynski KE, Soong TW, Goda Y, Cingolani LA: Alternative Splicing of P/Q-Type Ca Channels Shapes Presynaptic Plasticity. Cell Rep 2017, 20:333–343. [DOI] [PubMed] [Google Scholar]
- 57.Lübbert M, Goral RO, Satterfield R, Putzke T, van den Maagdenberg AM, Kamasawa N, Young SM: A novel region in the Cav2.1 alpha1 subunit C-terminus regulates fast synaptic vesicle fusion and vesicle docking at the mammalian presynaptic active zone. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Picher MM, Oprişoreanu AM, Jung S, Michel K, Schoch S, Moser T: Rab Interacting Molecules 2 and 3 Directly Interact with the Pore-Forming Ca. Front Cell Neurosci 2017, 11:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grauel MK, Maglione M, Reddy-Alla S, Willmes CG, Brockmann MM, Trimbuch T, Rosenmund T, Pangalos M, Vardar G, Stumpf A, et al. : RIM-binding protein 2 regulates release probability by fine-tuning calcium channel localization at murine hippocampal synapses. Proc Natl Acad Sci U S A 2016, 113:11615–11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Böhme MA, Grasskamp AT, Walter AM: Regulation of synaptic release-site Ca channel coupling as a mechanism to control release probability and short-term plasticity. FEBS Lett 2018, 592:3516–3531. [DOI] [PubMed] [Google Scholar]
- 61.••.Böhme MA, Beis C, Reddy-Alla S, Reynolds E, Mampell MM, Grasskamp AT, Lützkendorf J, Bergeron DD, Driller JH, Babikir H, et al. : Active zone scaffolds differentially accumulate Unc13 isoforms to tune Ca(2+) channel-vesicle coupling. Nat Neurosci 2016, 19:1311–1320. [DOI] [PubMed] [Google Scholar]; In this study, the authors find that two distinct isoforms of fly Unc13 (A and B) are associated with distinct release site arrangements and calcium coupling. This Unc13-active zone topography changes during synapse development (moving from Unc13B to Unc13A) resulting in tight coupling with calcium channels, consistent with both STED imaging and GTA sensitivity.
- 62.•.Reddy-Alla S, Böhme MA, Reynolds E, Beis C, Grasskamp AT, Mampell MM, Maglione M, Jusyte M, Rey U, Babikir H, et al. : Stable Positioning of Unc13 Restricts Synaptic Vesicle Fusion to Defined Release Sites to Promote Synchronous Neurotransmission. Neuron 2017, 95:1350–1364.e1312. [DOI] [PubMed] [Google Scholar]; The authors examine the active zone organization of fly neuromuscular junctions using superresolution imaging and find that different fragments of Unc13A selectively affect either release site formation (C-terminal fragment) or the specific location of a release site within the active zone (N-terminal fragment). Both perturbations affected synaptic transmission and short-term plasticity.
- 63.Hu Z, Tong XJ, Kaplan JM: UNC-13L, UNC-13S, and Tomosyn form a protein code for fast and slow neurotransmitter release in Caenorhabditis elegans. Elife 2013, 2:e00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou K, Stawicki TM, Goncharov A, Jin Y: Position of UNC-13 in the active zone regulates synaptic vesicle release probability and release kinetics. Elife 2013, 2:e01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calloway N, Gouzer G, Xue M, Ryan TA: The active-zone protein Munc13 controls the use-dependence of presynaptic voltage-gated calcium channels. Elife 2015, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, ugustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, et al. : Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell 2002, 108:121–133. [DOI] [PubMed] [Google Scholar]
- 67.Basu J, Betz A, Brose N, Rosenmund C: Munc13–1 C1 domain activation lowers the energy barrier for synaptic vesiclefusion. JNeurosci 2007,27:1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maruyama IN, Brenner S: A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc Natl Acad Sci U S A 1991, 88:5729–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lou X, Korogod N, Brose N, Schneggenburger R: Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci 2008, 28:8257–8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.••.Xu J, Camacho M, Xu Y, Esser V, Liu X, Trimbuch T, Pan YZ, Ma C, Tomchick DR, Rosenmund C, et al. : Mechanistic insights into neurotransmitter release and presynaptic plasticity from the crystal structure of Munc13–1 C1C2BMUN. life 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xu and colleagues solved the structure of most of the Munc13–1 protein core fragment and explored the impact of perturbing various inter-domain interfaces expressing mutated versions of Munc13–1 in hippocampal autaptic cultures.
- 71.•.Michelassi F, Liu H, Hu Z, Dittman JS: C1-C2 Module in Munc13 Inhibits Calcium-Dependent Neurotransmitter Release. Neuron 2017, 95:577–590.e575. [DOI] [PMC free article] [PubMed] [Google Scholar]; Michelassi et al identified a novel inhibitory function of the C1 and C2B domains of C. elegans UNC-13. Removal of the entire C2B domain enhanced calcium-triggered neurotransmitter release without increasing the readily releasable pool, suggesting that this inhibitory function operates at a post-priming step to regulate vesicular release probability.
- 72.••.Lipstein N, Verhoeven-Duif NM, Michelassi FE, Calloway N, van Hasselt PM, Pienkowska K, van Haaften G, van Haelst MM, van Empelen R, Cuppen I, et al. : Synaptic UNC13A protein variant causes increased neurotransmission and dyskinetic movement disorder. J Clin Invest 2017, 127:1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lipstein and colleagues investigate a point mutation in a highly conserved proline between the C2B and MUN domains of human UNC13A that was associated with a dyskinetic movement disorder and autism in a patient. Neurotransmitter release in hippocampal cultures expressing the mutated Munc13–1 was significantly enhanced. Similar phenotypes were observed in C. elegans expressing an equivalent mutation in UNC-13, consistent with a deep conservation of this negative regulatory function.
- 73.Igumenova TI: Dynamics and Membrane Interactions of Protein Kinase C. Biochemistry 2015, 54:4953–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Jong AP, Fioravante D: Translating neuronal activity at the synapse: presynaptic calcium sensors in short-term plasticity. Front Cell Neurosci 2014, 8:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schluter OM, Basu J, Sudhof TC, Rosenmund C: Rab3 superprimes synaptic vesicles for release: implications for short-term synaptic plasticity. J Neurosci 2006, 26:1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taschenberger H, Woehler A, Neher E: Superpriming of synaptic vesicles as a common basis for intersynapse variability and modulation of synaptic strength. Proc Natl Acad Sci U S A 2016, 113:E4548–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lipstein N, Schaks S, Dimova K, Kalkhof S, Ihling C, Kolbel K, Ashery U, Rhee J, Brose N, Sinz A, et al. : Nonconserved Ca(2+)/calmodulin binding sites in Munc13s differentially control synaptic short-term plasticity. Mol Cell Biol 2012, 32:4628–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vogl C, Cooper BH, Neef J, Wojcik SM, Reim K, Reisinger E, Brose N, Rhee JS, Moser T, Wichmann C: Unconventional molecular regulation of synaptic vesicle replenishment in cochlear inner hair cells. J Cell Sci 2015, 128:638–644. [DOI] [PubMed] [Google Scholar]
- 79.Cooper B, Hemmerlein M, Ammermüller J, Imig C, Reim K, Lipstein N, Kalla S, Kawabe H, Brose N, Brandstätter JH, et al. : Munc13-independent vesicle priming at mouse photoreceptor ribbon synapses. J Neurosci 2012, 32:8040–8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.King N, Rokas A: Embracing Uncertainty in Reconstructing Early Animal Evolution. Curr Biol 2017, 27:R1081–R1088. [DOI] [PMC free article] [PubMed] [Google Scholar]


