Summary
Long noncoding RNAs (lncRNAs) have emerged as critical regulators of tumorigenesis, and yet their mechanistic roles remain challenging to characterize. Here, we integrate functional proteomics with lncRNA-interactome profiling to characterize Urothelial Cancer Associated 1 (UCA1), a candidate driver of ovarian cancer development. Reverse phase protein array (RPPA) analysis indicates that UCA1 activates transcription coactivator YAP and its target genes. In vivo RNA antisense purification (iRAP) of UCA1 interacting proteins identified angiomotin (AMOT), a known YAP regulator, as a direct binding partner. Loss-of-function experiments show that AMOT mediates YAP activation by UCA1, as UCA1 enhances the AMOT-YAP interaction to promote YAP dephosphorylation and nuclear translocation. Together, we characterize UCA1 as a lncRNA regulator of Hippo-YAP signaling and highlight the UCA1-AMOT-YAP signaling axis in ovarian cancer development.
Subject Areas: Cancer Systems Biology, Omics, Proteomics, Systems Biology
Graphical Abstract
Highlights
-
•
A super-enhancer drives the expression of lncRNA UCA1 in EOC
-
•
Inactivation of UCA1 impairs tumor growth in vivo
-
•
UCA1 activates transcription coactivator YAP and its target genes
-
•
UCA1 promotes YAP dephosphorylation and nuclear translocation via AMOTp130
Cancer Systems Biology; Omics; Proteomics; Systems Biology
Introduction
Long noncoding RNAs (lncRNAs) are a class of noncoding transcripts over 200 nucleotides in length. They are expressed in a highly tissue- and disease-specific manner and play critical roles in development and disease (Derrien et al., 2012, Derrien et al., 2012, Guttman et al., 2010, Guttman et al., 2009, Kretz et al., 2012, Loewer et al., 2010). In cancer, dysregulated lncRNAs can function either as oncogenes (Calin et al., 2007, Gupta et al., 2010, Wang et al., 2008) or tumor suppressors (Kotake et al., 2011, Wang et al., 2012). LncRNAs have emerged as critical regulators of gene expression, with their activity largely governed by absolute expression levels, interacting partners, and subcellular localization (Guttman and Rinn, 2012).
Epithelial ovarian cancer (EOC) is the most lethal gynecological malignancy in the United States, with less than 50% of patients surviving more than 5 years (Siegel et al., 2016). This is largely due to lack of effective targeted therapies for treatment of EOC, which is often drug resistant when it recurs. So far, a handful of lncRNAs have been implicated in EOC. H19 and HOTAIR are expressed in ovarian tumors (Qiu et al., 2014, Tanos et al., 1999), and MEG3, DNM3OS, and MIAT are critical for epithelial-to-mesenchymal transition (Mitra et al., 2017). However, the underlying functional mechanisms of lncRNAs in EOC development remains poorly understood and their prospects as therapeutic targets for ovarian cancer unexplored.
For each tumor type there are typically thousands of dysregulated lncRNAs, but there is currently no consensus on the most efficient approach to identifying and characterizing the most critical players in disease development. Here, we implement a strategy for prioritization and characterization of lncRNAs implicated in cancer. The “LncRNA Interpreter” approach combines functional proteomics with interactome profiling to characterize the functional and biological role of a candidate lncRNA. We established and validated this strategy using lncRNA Urothelial Cancer Associated 1 (UCA1) as a proof of concept. We found UCA1 overexpression is a driver of ovarian cancer development. The reverse phase protein array (RPPA) profiling revealed deregulation of Hippo-YAP signaling when UCA1 is depleted. Characterization of UCA1 interacting proteins identified a mechanism of regulation of Hippo-YAP signaling via physical interactions between UCA1 and angiomotin (AMOT), a non-canonical RNA-binding protein. The LncRNA Interpreter approach can therefore efficiently provide critical mechanistic insights for characterization of candidate lncRNAs.
Results
LncRNA Interpreter, a Strategy for Mechanistic Analysis of LncRNAs
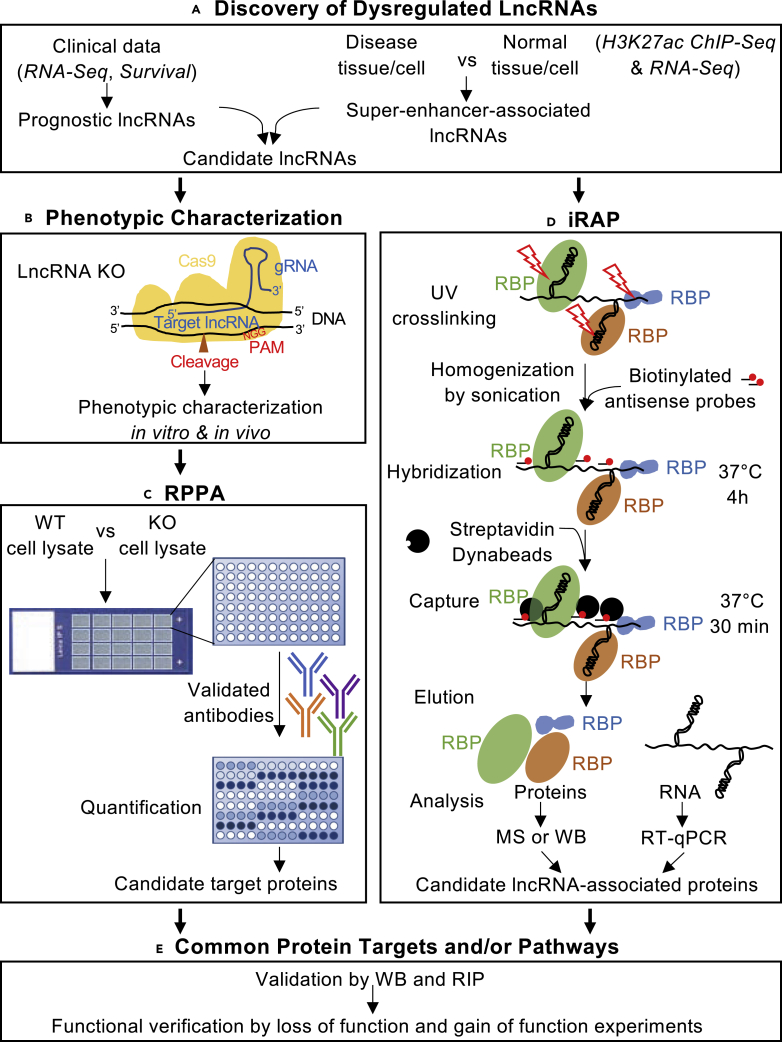
An overview of LncRNA Interpreter strategy is shown in Figure 1. Following lncRNA identification (e.g., from analysis of patient data or analyses of super-enhancer-associated lncRNAs), lncRNA knockout (KO) models are generated for disease-relevant phenotypic characterization. The RPPA is utilized to evaluate differentially expressed proteins and downstream pathways affected by disruption of lncRNAs. Most key cancer pathways are surveyed on the RPPA (Paweletz et al., 2001), which enables quantification of several protein post-translational modifications that are crucial for signaling cascades commonly deregulated in cancer (Charboneau et al., 2002). We developed in vivo RNA antisense purification (iRAP) from previously developed RNA-centric methods (Chu et al., 2015, McHugh et al., 2015, Minajigi et al., 2015) to catalog the interacting proteome of candidate lncRNAs. As protein-RNA interactions are essential for lncRNA functionality, intersection of proteins/pathways identified using RPPA with iRAP profiles provides an efficient approach for dissecting the underlying mechanism of candidate lncRNAs implicated in complex diseases such as cancer.
Figure 1.
LncRNA Interpreter, a Strategy for Mechanistic Analysis of lncRNAs
The LncRNA Interpreter strategy consists of five major components. (A) Discovery of candidate lncRNAs is performed using patient specimens. (B) Phenotypic characterization of the candidate lncRNA is performed in vitro and in vivo using KO cancer cell models generated using CRISPR/Cas9 genome editing technique. (C) Proteins affected by candidate lncRNA are identified by functional proteomics using RPPA. (D) LncRNA interacting proteins are identified using iRAP. (E) RPPA and iRAP data are integrated to identify common protein targets. ChIP-seq, chromatin immunoprecipitation sequencing; RNA-Seq, RNA sequencing; RPPA, reverse phase protein array; iRAP, in vivo RNA antisense purification; WT, wild-type; KO, knockout; RBP, RNA-binding protein; RIP, RNA immunoprecipitation; MS, mass spectrometry; WB, western blotting; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
UCA1 Is a Driver of Ovarian Cancer Development and Outcome
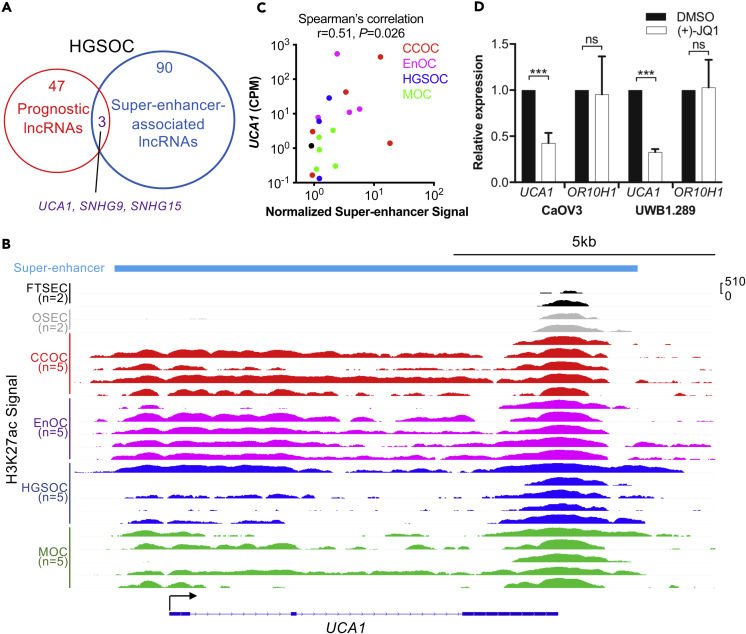
We evaluated data from The Cancer Genome Atlas (Cancer Genome Atlas Research Network, 2011) to identify candidate lncRNAs that drive the development and clinical outcome (survival) in high-grade serous EOC cases. We identified an lncRNA signature comprising 50 lncRNAs associated with patient prognosis (Figure 2A, Table S1). Since super-enhancers mark pivotal oncogenes in many tumor types (Jiang et al., 2018, Lovén et al., 2013, Peng et al., 2019, Xie et al., 2018), we integrated lncRNA expression data with genome-wide profiles of enhancer marks generated using chromatin immunoprecipitation sequencing (ChIP-seq) for H3K27ac in primary high-grade serous EOC tissues to identify super-enhancers that drive candidate oncogenic lncRNA expression. Ninety-three super-enhancers identified in high-grade serous EOC tissues were associated with lncRNA expression (Figure 2A, Table S2), of which three, UCA1, SNHG9, and SNHG15, were also associated with prognosis (Figure 2A, Table S1). UCA1 is of particular interest as this lncRNA has been implicated in the development of other cancers (Chen et al., 2016, Fang et al., 2014, Han et al., 2014, Hughes et al., 2015, Li et al., 2014, Li et al., 2016a, Li et al., 2016b, Na et al., 2015, Nie et al., 2016, Tian et al., 2014, Tuo et al., 2015, Wang et al., 2015, Zhang et al., 2016, Zhao et al., 2017, Zheng et al., 2015), although its functional targets remain elusive. The UCA1 locus is marked by a super-enhancer in all major histotypes of ovarian cancer—clear cell, endometrioid, high-grade serous, and mucinous—but not in precursor tissues—fallopian tube secretory epithelial cells (FTSECs) or ovarian surface epithelial cells (OSECs) (Figure 2B). UCA1 expression positively correlates with super-enhancer signal at this locus (r = 0.51, p = 0.026, Spearman's correlation) (Figure 2C), suggesting that this tumor-specific super-enhancer regulates UCA1 expression. Super-enhancer-associated genes are particularly responsive to treatment of (+)-JQ1 (Lovén et al., 2013), a potent inhibitor of BET family of bromodomain proteins including BRD4 (Filippakopoulos et al., 2010); we found that UCA1 expression was significantly downregulated in two ovarian cancer cell lines treated with (+)-JQ1 compared with dimethyl sulfoxide (DMSO)-treated control cells (Figure 2D). The expression of a neighboring gene OR10H1 (∼20 kb upstream), which has no super-enhancer at its locus (Figure S1A) and shows some evidence of correlation with the UCA1 super-enhancer (r = 0.47, p = 0.043, Spearman's correlation) (Figure S1B), was not affected by (+)-JQ1 treatment (Figure 2D). Together these analyses suggesting the super-enhancer at this locus drives the expression of UCA1 in ovarian cancer.
Figure 2.
UCA1 in Ovarian Cancer Development and Outcome
(A) In high-grade serous EOCs a signature of 50 lncRNAs was prognostic. Using tumor tissue H3K27ac data, 93 high-grade serous EOC super-enhancer-associated lncRNAs were identified. 3 lncRNAs were shared between the two analyses.
(B) UCA1-associated super-enhancer is present across the four major histological subtypes of EOC, but not normal precursor cells. H3K27ac ChIP-seq signal is shown in log scale. CCOC, clear cell ovarian cancer; EnOC, endometrioid ovarian cancer; HGSOC, high-grade serous ovarian cancer; MOC, mucinous ovarian cancer.
(C) Super-enhancer signal positively correlates with UCA1 expression (Spearman's correlation, r = 0.51, p = 0.026). Counts per million (CPM) of UCA1 from RNA-seq is shown. Super-enhancer signals were normalized using TMM (Robinson and Oshlack, 2010). We did not have sufficient tissue to do RNA-seq for one EnOC tumor (#3), and one HGSOC tumor (#4) does not express UCA1.
(D) UCA1 expression is sensitive to JQ1 treatment in HGSOC models. Two HGSOC cell lines (CaOV3, UWB1.289) were treated with (+)-JQ1 or DMSO. The expression of UCA1 and OR10H1 was measured by RT-qPCR.
Data shown here are mean ± SD from three independent experiments. ***p = 1×10−7 (CaOV3), ***p = 2×10−8 (UWB1.289), ns, not significant, Student's t test. See also Figure S1, Tables S1 and S2.
Deletion of UCA1 Impairs Ovarian Cancer Cell Growth
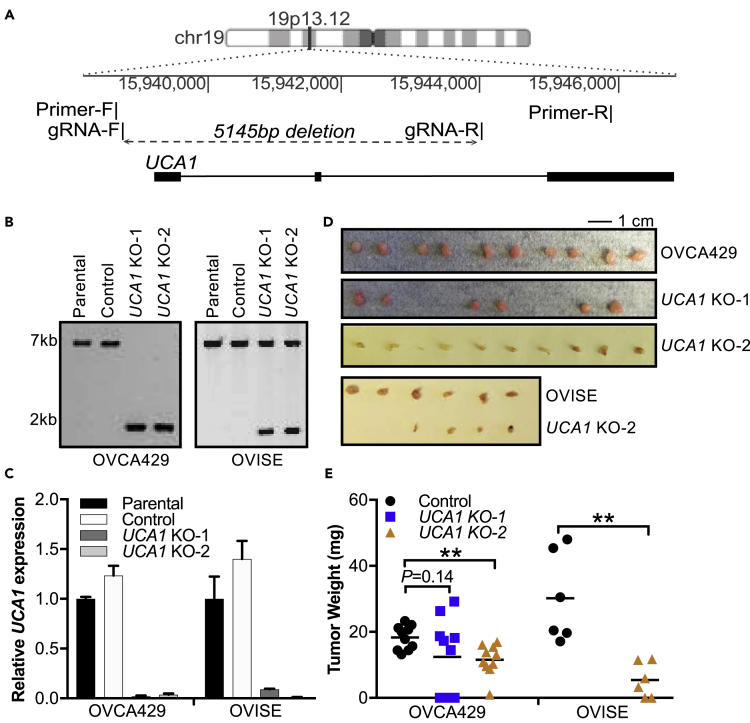
CRISPR/Cas9 genome editing was used to create stable UCA1 KO models in ovarian cancer cell lines OVCA429 and OVISE (Figures 3A and 3B). UCA1 expression was undetectable in all UCA1 KO models (Figure 3C). The effects of UCA1 KO on in vivo tumor cell growth were established after intra-peritoneal injection of 10 million wild-type (WT) UCA1 or UCA1 KO cells in nude mice. The mass of resulting tumors was significantly smaller in UCA1 KO models compared with those in mice injected with WT cells (OVCA429 p = 0.002; OVISE p = 0.002) (Figures 3D and 3E), indicating that UCA1 acts as a potent promoter of tumor seeding and/or growth in vivo.
Figure 3.
Inactivation of UCA1 Impairs Ovarian Cancer Cell Growth
(A) Schematic of CRISPR/Cas9 genome editing strategy for UCA1 knockout.
(B) Long-range PCR of parental (OVCA429 and OVISE) cells, control cells expressing the gRNA vector backbone, and two stable UCA1 knockout clones from each cell line (UCA1 KO-1, UCA1 KO-2).
(C) The expression of UCA1, measured by RT-qPCR, was normalized to the expression of GAPDH and β-actin in the UCA1 KO models, parental cells, and control cells. Data shown are mean ± SD from three independent experiments.
(D) Images of excised tumors from mouse xenograft model. Scale bar represents 1 cm.
(E) Tumor weight of xenografted UCA1 KO ovarian cancer cells and parental controls. **p < 0.01, Student's t test.
UCA1 Regulates Hippo-YAP Signaling in Ovarian Cancer
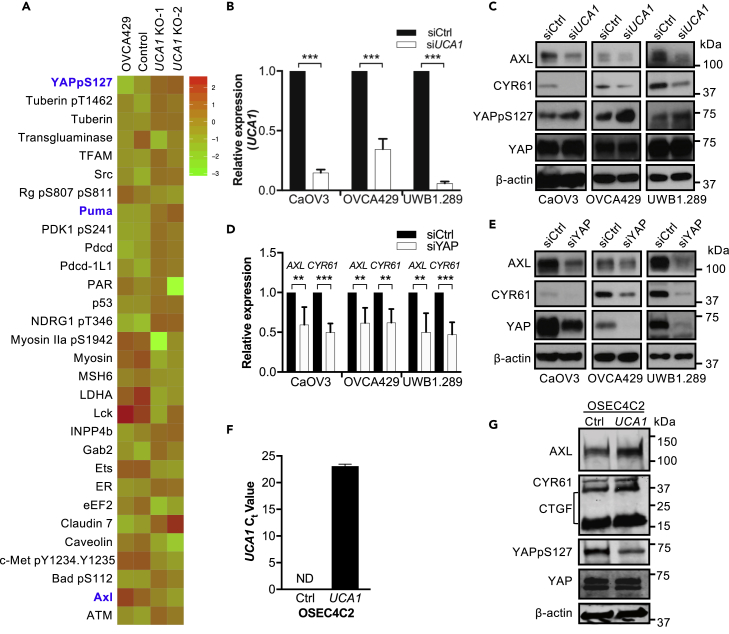
We used RPPAs to profile changes in protein abundance and phosphorylation following UCA1 KO. The most differentially expressed proteins between WT and UCA1 KO cells included phosphorylated YAP at Ser127 (YAPpS127, fold change [FC] = 1.3, p = 0.038), AXL (FC = 0.75, p = 0.031), and PUMA (FC = 1.18, p = 0.042), all of which belong to the Hippo-YAP signaling pathway (Figure 4A, Table S3). YAPpS127 is retained in the cytoplasm via binding to 14-3-3 protein, which leads to inhibition of downstream YAP signaling (Zhao et al., 2010). We observed an increased expression of YAPpS127 and pro-apoptotic YAP target gene PUMA (Matallanas et al., 2007) and decreased expression of the pro-proliferative YAP target gene AXL (Xu et al., 2011) in UCA1 KO compared with WT cells (Figure 4A), indicating that UCA1 activates Hippo-YAP signaling for cell survival and proliferation. Consistent with this, YAPpS127 protein expression is significantly lower in primary tumors with UCA1 overexpression or amplification (n = 105, average abundance −0.25) compared with tumors with no UCA1 overexpression (n = 477, average abundance 0.07, p = 2.8×10−4, Q = 0.019) (Figure S2A). There was no significant difference in total YAP protein expression between the two groups (Figure S2B), suggesting that UCA1 also activates YAP signaling in vivo.
Figure 4.
UCA1 Activates Hippo-YAP Signaling in Ovarian Cancer
(A) RPPA analyses of OVCA429 UCA1 KO cells and controls. All proteins with significant changes (FC > 1.2 or <0.9 and p ≤ 0.05) in abundance are shown. Proteins highlighted in blue are involved in Hippo-YAP signaling.
(B) The expression of UCA1 was measured by RT-qPCR and normalized to β-actin in both scramble control siRNA (siCtrl) and UCA1-specific siRNA (siUCA1)-transfected CaOV3, OVCA429, and UBW1.289 cells. Data shown are mean ± SD from three independent experiments.
(C) The expression of YAP, YAPpSer127, and YAP targets by western blotting in both siCtrl and siUCA1-transfected cells. Representative results from three independent experiments are shown. When UCA1 was knocked down, YAPpSer127 expression was elevated 90% (p < 0.01), 150% (p < 0.001), and 50% (p < 0.05) in CaOV3, OVCA429, and UWB1.289 cells, respectively. Student's t test.
(D) The expression of YAP targets AXL and CYR61 was measured by RT-qPCR in siCtrl and siYAP-transfected cells and normalized to β-actin. Data shown are mean ± SD from three independent experiments. **p < 0.01, ***p < 0.001, Student's t test.
(E) The expression of YAP and YAP targets by western blotting in CaOV3, OVCA429, and UBW1.289 cells transfected with siCtrl or siYAP.
(F) The Ct values of UCA1 lncRNA in both OSEC4C2 control cells and OSEC4C2 cells with UCA1 overexpression. ND, not detected. Data shown are mean ± SD from three independent experiments and normalized to 18S rRNA.
(G) The expression of YAP, YAPpSer127, and YAP targets by western blotting in both OSEC4C2 control cells and OSEC4C2 cells with UCA1 overexpression. β-Actin was used as loading control for western blotting.
Since the presence of a non-unique intronic sequence mandated the introduction of a >5 kb deletion to KO UCA1, it is plausible that noncoding elements within the UCA1 locus activate YAP target genes in cis or in trans. To test this possibility, loss-of-function experiments using small interfering RNAs (siRNAs) specific to UCA1 were conducted to validate the role of UCA1 in regulation of Hippo-YAP signaling. After UCA1 knockdown (KD) in high-grade serous ovarian cancer cell lines (Figure 4B), total YAP expression was unaltered, whereas YAPpS127 was elevated at least 50% in all three ovarian cancer cell lines (by ImageJ) (Figure 4C), consistent with RPPA results in UCA1 KO cells and primary tissues. YAP regulates the expression of AXL and CYR61 at both RNA (Figure 4D) and protein levels (Figure 4E), indicating that AXL and CYR61 are indeed YAP targets in ovarian cancer. The effect of UCA1 KD on the abundance of AXL and CYR61 largely phenocopied YAP KD (Figures 4C and 4E). Moreover, gain-of-function experiments performed by overexpressing UCA1 in TERT-immortalized, MYC-expressing normal ovarian epithelial cells (OSEC4C2) (Figure 4F) demonstrated that overexpression of UCA1 increased the expression of YAP target genes AXL, CYR61, as well as CTGF (Figure 4G). Consistent with the loss-of-function experiments, overexpression of UCA1 inhibits YAP phosphorylation but does not affect total YAP expression (Figure 4G). Taken together, these data indicate that UCA1 activates Hippo-YAP signaling in ovarian cancer.
iRAP Catalogs the UCA1 Interacting Proteome
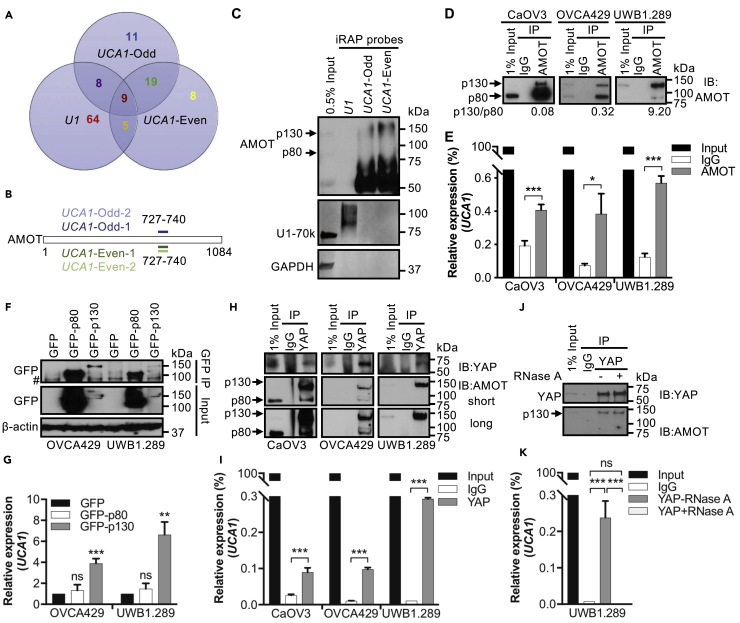
Proteins purified from two independent UCA1-iRAP experiments were profiled by mass spectrometry. Each iRAP experiment included two non-overlapping UCA1 probe sets (UCA1-odd, UCA1-even) for cross-validation and a U1 probe as positive control (Figure S3A, Table S4). The iRAP assay was highly specific: both UCA1 probe sets significantly enriched UCA1 but not U1 snRNA; conversely, the U1 probe enriched U1 snRNA but not UCA1. As expected, U6 snRNA was not enriched by any of the probes (Figure S3B). iRAP-MS recovered 86 U1-associated proteins (Figure 5A), including six known U1 direct binding proteins and another 35 U1-associated proteins (Figure S3C, Table S5) (Chu et al., 2015). These data indicate that iRAP efficiently identifies bona fide RNA-binding proteins associated with a specific target transcript. We isolated 47 and 42 proteins, respectively, using UCA1-odd and UCA1-even probe sets (Figure 5A). Thirty-eight proteins were only associated with UCA1, 19 of which were identified by both sets of probes (Figure 5A). In most instances the same peptide was retrieved by both UCA1 probe sets across the two experiments (Figures 5B and S3D, Table S5), suggesting that these 19 proteins are true UCA1-binding proteins.
Figure 5.
UCA1 Interacts Directly with Hippo-YAP Signaling Regulator AMOTp130
(A) iRAP followed by mass spectrometry to catalog the UCA1 interactome.
(B) The same AMOT peptides were reproducibly retrieved from UCA1 iRAP. Numbers indicate the amino acid position; protein not drawn to scale. Data shown are from two independent experiments.
(C) Western blotting validation of AMOT as a direct binding protein for UCA1 using iRAP samples. U1-70k, a known direct binding protein for U1 snRNA, is used as positive control for U1. As expected, GAPDH binds neither U1 nor UCA1.
(D and E) RIP validation of the AMOT-lncRNA UCA1 interaction. (D) Western blotting validation of AMOT pull-down, using an AMOT specific antibody, in three high-grade serous EOC cell lines. Isotype IgG was included as negative control. Numbers beneath the blots are the relative ratios for AMOTp130:AMOTp80. (E) Relative expression of UCA1 in AMOT immunoprecipitated RNA samples by RT-qPCR.
(F and G) RIP validation of AMOTp130-UCA1 interaction. (F) Western blotting validation of GFP-tagged AMOT pull-down with an anti-GFP antibody. Representative images from three independent experiments are shown. Control cells were transduced with a GFP expressing vector only. (G) Relative expression of UCA1 for GFP-RIP samples by RT-qPCR. # denotes a non-specific band observed with this GFP antibody. (H-I) RIP validation of YAP-AMOTp130-UCA1 complex.
(H) Western blotting validation of YAP pull-down and AMOT co-immunoprecipitated using a YAP specific antibody. Isotype IgG was included as negative control.
(I) RT-qPCR measurement of relative expression of UCA1 in YAP-RIP samples.
(J) RIP following RNase A treatment, UCA1 is not required for the YAP-AMOTp130 interaction. Western blotting validation of YAP pull-down and AMOT co-immunoprecipitated by YAP specific antibody, with or without RNase A treatment. Isotype IgG was included as negative control.
(K) Relative expression of UCA1 by RT-qPCR in YAP RIP samples, incubated with or without RNase A.
Data shown are mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, Student's t test. See also Figure S3, Tables S4 and S5.
UCA1 Interacts Directly with Hippo-YAP Signaling Regulator AMOTp130
We intersected the UCA1 interacting proteome with the pathways identified in the RPPA profiling described earlier. This highlighted AMOT, a known regulator of Hippo-YAP signaling (Moleirinho et al., 2017, Zhao et al., 2011). A peptide mapping to amino acids 727–740 of AMOT was identified by both UCA1 probe sets in two independent experiments (Figure 5B), suggesting this predicted coiled-coil domain of AMOT is responsible for the interaction with UCA1. AMOT exists as two isoforms, AMOTp130 and AMOTp80, a shorter isoform that lacks 409 amino acids at the N terminus due to alternative splicing of the AMOT gene between exon 2 and 3 (Ernkvist et al., 2006). Western blotting of iRAP lysates validated the AMOT-UCA1 interaction. We observed shifted AMOTp130 (to around 160 kDa) but not AMOTp80, suggesting that UCA1 associates specifically with AMOTp130 (Figure 5C). To further verify the AMOT-UCA1 interaction, we performed RNA immunoprecipitation (RIP) using an anti-AMOT antibody (Table S6). Western blotting for AMOT confirmed successful pull-down of both AMOTp80 and AMOTp130 isoforms (Figure 5D), and an ∼2- to 5-fold enrichment of UCA1 was detected in AMOT-RIP samples compared with corresponding control isotype IgG pulldowns (CaOV3, p = 7.4×10−6; OVCA429, p = 0.011; UWB1.289, p = 7.2×10−7) (Figure 5E). We noted that a higher ratio of AMOTp130/p80 correlates with a higher fold enrichment of UCA1 (Figures 5D and 5E), further suggesting that UCA1 preferentially associates with AMOTp130. To validate this, we overexpressed GFP-AMOTp130 or GFP-AMOTp80 in EOC cells and performed GFP-RIP. Western blotting validated successful pull-down of both GFP-AMOTp80 and GFP-AMOTp130 (Figure 5F) with significant enrichment of UCA1 observed for GFP-AMOTp130 but not GFP-AMOTp80 (Figure 5G), despite the markedly more efficient pull-down of AMOTp80 (Figure 5F). These data together indicate that UCA1 interacts directly with AMOTp130 but not AMOTp80.
AMOTp130 but not AMOTp80 was previously reported to interact with YAP and regulate its activity (Sowa et al., 2009, Zhao et al., 2011). We tested whether this interaction is intact in EOC. In YAP-RIP experiments, AMOTp130 was detected in YAP pull-downs (Figure 5H), confirming that YAP interacts with AMOTp130. AMOTp80 was also detected in YAP pull-downs for two of the three cell lines, which may be due to hetero-oligomerization (Ernkvist et al., 2008, Patrie, 2005, Zheng et al., 2009). Interestingly, a 4- to 29-fold enrichment of UCA1 was detected in YAP pulldowns compared with IgG isotype controls (Figure 5I). Since we did not identify YAP using iRAP (which preferentially detects direct binding proteins), these data suggest that YAP, AMOTp130, and UCA1 form a trimer complex with AMOTp130 bridging the interaction between YAP and UCA1. Once formed, the AMOTp130-YAP interaction is independent of UCA1 since the association still exists following RNase A treatment (Figures 5J and 5K).
UCA1 Activates YAP and Target Genes by Enhancing the AMOTp130-YAP Interaction to Promote YAP Dephosphorylation and Nuclear Translocation
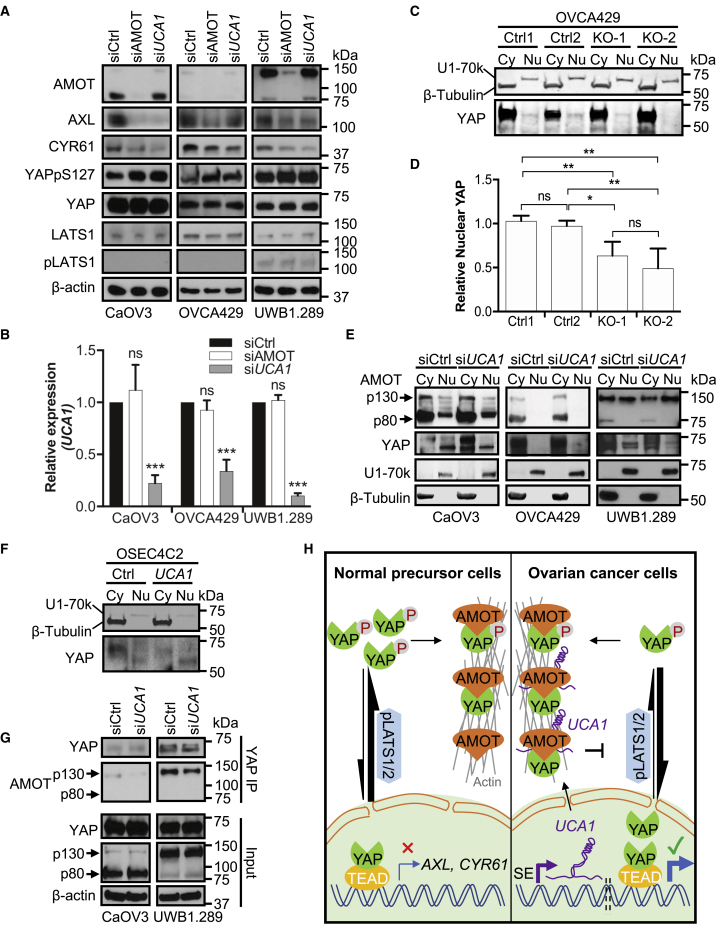
We next tested whether AMOT is required for activation of YAP target genes by UCA1. Western blotting and RT-qPCR validated the successful depletion of AMOT (Figure 6A) and UCA1 (Figure 6B), respectively. Both UCA1 and AMOT KD resulted in reduced expression of AXL and CYR61 and elevated the expression of YAPpS127 40%–100% but had no effect on total expression levels of YAP, YAP kinase LATS1, or active pLATS1 (Figure 6A). Although the expression of UCA1 was not affected by AMOT KD (Figure 6B), the activation of YAP target genes was dampened (Figure 6A), indicating that AMOT mediates the activation of YAP target genes by UCA1.
Figure 6.
UCA1 Activates YAP and Its Target Genes by Facilitating AMOTp130-YAP Interaction to Promote YAP Dephosphorylation and Nuclear Translocation
(A) AMOT mediates the activation of YAP and its target genes by UCA1. OC cells were transfected with siCtrl, siAMOT, or siUCA1 for 72 h. Whole-cell lysates were prepared and blotted with antibodies indicated. When UCA1 or AMOT is knocked down, YAPpSer127 expression is elevated 80% (p < 0.01), 100% (p < 0.01), and 40% (p < 0.01) in CaOV3, OVCA429, and UWB1.289 cells, respectively.
(B) Validation of siRNA knockdown efficiency for UCA1. Relative expression of UCA1 by RT-qPCR for samples is shown in (A).
(C) UCA1 KO in OVCA429 cells decreases nuclear YAP. UCA1 wild-type (Ctrl1, Ctrl2) or knockout (KO-1, KO-2) OVCA429 cells were fractionated into cytoplasmic (Cy) and nuclear (Nu) fractions and blotted for β-tubulin (a cytoplasmic marker), U1-70k (a nuclear marker), and YAP.
(D) Quantification of nuclear YAP using ImageJ.
(E) UCA1 KD decreases nuclear YAP. CaOV3, OVCA429, and UWB1.289 cells transfected with control siRNAs or siUCA1 were fractionated into cytoplasmic and nuclear fractions and blotted for β-tubulin, U1-70k, AMOT, and YAP. Nuclear YAP is reduced 50% (CaOV3, p < 0.05) and 70% (UWB1.289, p < 0.05) in cells transfected with siUCA1.
(F) UCA1 overexpression in OSEC4C2 cells increases nuclear YAP. OSEC4C2 control cells and OSEC4C2 cells with UCA1 overexpression were fractionated into Cy and Nu fractions and blotted for β-tubulin, U1-70k, and YAP. Nuclear YAP is elevated 30% (p < 0.05).
(G) UCA1 facilitates AMOTp130 binding to YAP. High-grade serous EOC cell lines were transfected with siCtrl or siUCA1 for 72 h. Co-immunoprecipitation experiments were performed using an anti-YAP antibody. Retrieval of AMOT is reduced 70% (CaOV3, p < 0.05) and 50% (UWB1.289, p < 0.05) in cells transfected with siUCA1.
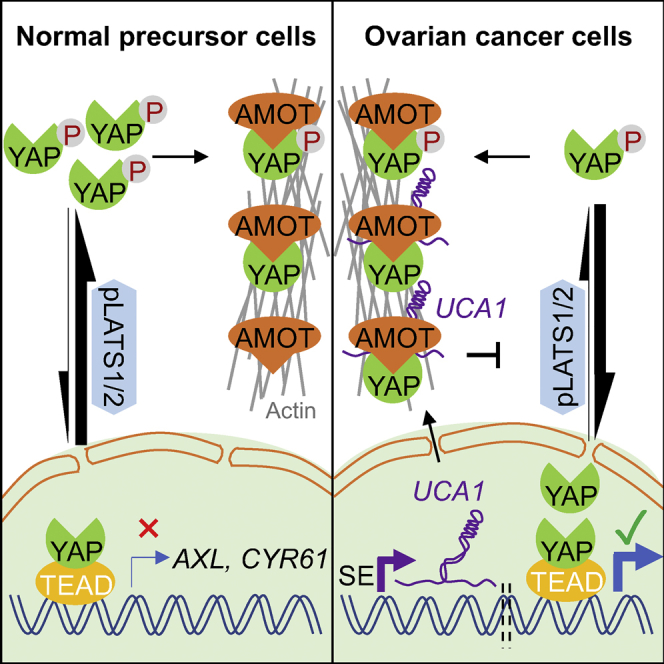
(H) Working model for AMOT-mediated activation of YAP and target genes by UCA1.
SE, super-enhancer. ImageJ was used for quantification of proteins in (A, C, E, F, and G) from three independent replicates. Data shown in (B and D) are mean ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ns, not significant, Student's t test. See also Figure S4.
When phosphorylated at Ser127, YAP protein is retained in the cytoplasm and downstream signaling is repressed (Zhao et al., 2010). When total YAP protein levels are constant, increased YAPpS127 should result in reduced nuclear YAP. We therefore compared nuclear YAP expression between control and UCA1 KO cells using cellular fractionation. Successful fractionation of the OVCA429 KO model was verified using cytoplasmic protein marker β-tubulin and nuclear protein marker U1-70k (Figure 6C) as well as cytoplasmic RNA marker 18S rRNA and nuclear RNA marker U6 snRNA (Figure S4A). These data show that UCA1 predominantly localizes to the cytoplasm (∼94% on average, Figure S4A). Nuclear YAP expression decreased ∼2-fold in UCA1 KO cells (KO-1 and KO-2) compared with WT cells (Ctrl 1 and Ctrl 2) (p = 3×10−4) (Figures 6C and 6D). In addition, fractionation experiments were performed in three ovarian cancer cell lines following UCA1 KD. Similarly, nuclear YAP was reduced 2-fold in UCA1 KD CaOV3 and 3-fold in UCA1 KD UWB1.289 cells compared with control siCtrl-treated cells (p = 0.014 and p = 0.006, respectively) (Figure 6E). Fractionation experiments were also conducted in OSEC4C2 cells overexpressing with control vector or UCA1 (Figures 6F and S4B). Predominant cytoplasmic expression UCA1 was confirmed in OSEC4C2 cells overexpressing UCA1 (Figure S4B) and nuclear YAP was increased (Figure 6F). These data indicate that cytoplasmic UCA1 activates YAP target genes via dephosphorylation and nuclear translocation of YAP.
Since UCA1 KD affects neither AMOT protein expression (Figure 6A) nor localization (Figure 6E), we tested whether UCA1 affects the AMOT-YAP interaction. Two-fold less AMOTp130 coimmunoprecipitated with YAP when UCA1 was depleted (Figure 6G), evidence that UCA1 promotes AMOTp130-YAP interaction. In summary, we propose a model in which cytoplasmic UCA1 binds to AMOT to facilitate an interaction between AMOT and YAP, which prevents the pLATS1/2-YAP interaction and YAP phosphorylation. The net result is a larger pool of active YAP, which relocates to the activate YAP target genes (Figure 6H).
Discussion
UCA1 is conserved in primates and expressed highly in the early embryo but not in most adult tissues (Wang et al., 2008). Originally UCA1 was found to be overexpressed in bladder carcinoma (Wang et al., 2006) and was later shown to be upregulated in many other malignancies (Chen et al., 2016, Fang et al., 2014, Han et al., 2014, Hughes et al., 2015, Li et al., 2014, Li et al., 2016a, Li et al., 2016b, Na et al., 2015, Nie et al., 2016, Tian et al., 2014, Tuo et al., 2015, Wang et al., 2015, Zhang et al., 2016, Zhao et al., 2017, Zheng et al., 2015). Multiple underlying mechanisms have been proposed to explain the pro-oncogenic effects of UCA1, including inhibition of p27 (Han et al., 2014), activation of Wnt/β-Catenin signaling (Yang et al., 2016), promotion of KLF4-KRT6/13 signaling (Na et al., 2015), or function as miRNA sponge (Bian et al., 2016, Fang et al., 2017, He et al., 2017, Li et al., 2015, Li et al., 2017, Li et al., 2018, Lu et al., 2017, Nie et al., 2016, Sun et al., 2018, Tian et al., 2018, Tuo et al., 2015, Wang et al., 2015, Wu and Zhou, 2018, Xiao et al., 2017, Xue et al., 2016, Zhang et al., 2017, Zhou et al., 2018, Zhou et al., 2017, Zhu et al., 2018). However, the mechanistic pathways directly regulated by UCA1 are unclear.
In our study, we found that UCA1 overexpression drives ovarian cancer development. Our functional proteomic analyses of UCA1 KO models revealed that UCA1 activates YAP and its target genes, which may account for UCA1's tumor growth promotion phenotype. To dissect the underlying mechanism of how UCA1 activates YAP and its target gens, iRAP was performed and revealed that UCA1 directly interacts with AMOTp130. AMOT was initially reported as a negative regulator of YAP via cytoplasmic retention of YAP protein (Zhao et al., 2011). However, more recent evidence suggests that AMOT can both negatively (Chan et al., 2011, Dai et al., 2013, Paramasivam et al., 2011, Wang et al., 2011) and positively regulate (Lv et al., 2016, Yi et al., 2013) YAP. In the present study, we demonstrated that AMOT is a positive regulator of YAP and its targets genes in ovarian cancer. In the cytoplasm of EOC cells, overexpressed UCA1 enhances the interaction between AMOTp130 and YAP. This may be due to a conformational change of AMOTp130 induced by UCA1 binding; alternatively, UCA1 may affect the phosphorylation of AMOTp130, which in turn regulates AMOTp130-YAP interaction and subcellular localization of the complex (Dai et al., 2013, Moleirinho et al., 2017). However, the latter situation is unlikely as UCA1 does not affect AMOT localization in EOC (Figure 6E). The enhanced AMOTp130-YAP interaction is therefore more likely to antagonize the pLATS1/2-YAP interaction that leads to phosphorylation of YAP (Yi et al., 2013) since overlapping regions of YAP bind to AMOT or LATS1/2 (Hao et al., 2008, Zhao et al., 2011). The indirect impact of UCA1 is therefore dephosphorylation of YAP, which in turn promotes nuclear translocation of YAP and facilitates its binding to TEAD to activate expression of a pro-oncogenic gene signature.
In the current study, we develop a strategy LncRNA Interpreter to prioritize and then functionally dissect candidate lncRNAs in cancer. Using this approach, we reveal UCA1 as a super-enhancer-regulated lncRNA in EOC and an unconventional lncRNA regulator of Hippo-YAP signaling and characterize a critical function for UCA1 in the activation of YAP and its target genes in EOC. Our data implicate the UCA1-AMOTp130-YAP signaling axis in the development of EOC that may represent a potential target for therapeutic intervention. More broadly, this proof-of-concept study demonstrates that the protein-centric LncRNA Interpreter strategy can be readily applied to efficiently elucidate the functional role of other lncRNAs implicated in complex diseases.
Limitations of the Study
In this study, we demonstrated that UCA1 activates the transcription coactivator YAP and its target genes in both gain-of-function and loss-of-function experiments. It is plausible that UCA1 prevents YAP phosphorylation by pLATS1/2, but direct evidence for this is missing owing to the low expression of LATS1/2 in our models. Future studies evaluating YAP and AMOTp130 post-transcriptional modifications would likely yield a deeper mechanistic insight into the regulation of Hippo-YAP signaling pathway by UCA1.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by a K99/R00 grant from the National Cancer Institute (NCI) (Grant number 1K99CA184415-01) to K.L., a Pilot Award from the Southern California Clinical and Translational Science Institute to S.A.G. and K.L., an Ann and Sol Schreiber Mentored Investigator Award (Grant number 458799) from Ovarian Cancer Research Alliance (OCRA) to X.L., and an R01 grant (Grant number 5R01CA20745602) from the NCI to T.A.S., S.A.G, and K.L. We thank Charles Nicolet at the Epigenome Center Core, University of Southern California for RNA-Seq services, the team at the MD Anderson RPPA Core for the protein profiling, Drs. Wei Yang and Bo Zhou at the Biomarker Discovery Platform Core, Cedars-Sinai Medical Center for mass spectrometry analyses, Drs. Karst and Drapkin at University of Pennsylvania for their generous provision of immortalized fallopian tube epithelial cells, and Dr. Colleen McHugh at University of California, San Diego for help with iRAP protocol.
Author Contributions
Conceptualization, X.L. and K.L.; Data Curation, X.L. and K.L.; Investigation, X.L., T.J.S., and J.M.L.; Formal Analysis, X.L., T.J.S., M.A. de Souza Fonseca, R.I.C., F.S.D., L.L., H.W.L., and H.N.; Writing – Original Draft, X.L. and K.L.; Writing – Review & Editing, X.L., T.A.S., M.L.F., S.A.G., and K.L.; Funding Acquisition, X.L., T.A.S., S.A.G., and K.L.; Resources, B.Y.K., J.-H.S., M.L.F., and S.A.G.; Supervision, K.L.
Declaration of Interests
The authors declare no competing interests.
Published: July 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.06.025.
Supplemental Information
References
- Bian Z., Jin L., Zhang J., Yin Y., Quan C., Hu Y., Feng Y., Liu H., Fei B., Mao Y. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci. Rep. 2016;6:23892. doi: 10.1038/srep23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin G.A., Liu C.G., Ferracin M., Hyslop T., Spizzo R., Sevignani C., Fabbri M., Cimmino A., Lee E.J., Wojcik S.E. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–229. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S.W., Lim C.J., Chong Y.F., Pobbati A.V., Huang C., Hong W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J. Biol. Chem. 2011;286:7018–7026. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charboneau L., Tory H., Chen T., Winters M., Petricoin E.F., 3rd, Liotta L.A., Paweletz C.P. Utility of reverse phase protein arrays: applications to signalling pathways and human body arrays. Brief. Funct. Genomic. Proteomic. 2002;1:305–315. doi: 10.1093/bfgp/1.3.305. [DOI] [PubMed] [Google Scholar]
- Chen P., Wan D., Zheng D., Zheng Q., Wu F., Zhi Q. Long non-coding RNA UCA1 promotes the tumorigenesis in pancreatic cancer. Biomed. Pharmacother. 2016;83:1220–1226. doi: 10.1016/j.biopha.2016.08.041. [DOI] [PubMed] [Google Scholar]
- Chu C., Zhang Q.C., da Rocha S.T., Flynn R.A., Bharadwaj M., Calabrese J.M., Magnuson T., Heard E., Chang H.Y. Systematic discovery of Xist RNA binding proteins. Cell. 2015;161:404–416. doi: 10.1016/j.cell.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., She P., Chi F., Feng Y., Liu H., Jin D., Zhao Y., Guo X., Jiang D., Guan K.L. Phosphorylation of angiomotin by Lats1/2 kinases inhibits F-actin binding, cell migration, and angiogenesis. J. Biol. Chem. 2013;288:34041–34051. doi: 10.1074/jbc.M113.518019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H., Guernec G., Martin D., Merkel A., Knowles D.G. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernkvist M., Aase K., Ukomadu C., Wohlschlegel J., Blackman R., Veitonmaki N., Bratt A., Dutta A., Holmgren L. p130-angiomotin associates to actin and controls endothelial cell shape. FEBS J. 2006;273:2000–2011. doi: 10.1111/j.1742-4658.2006.05216.x. [DOI] [PubMed] [Google Scholar]
- Ernkvist M., Birot O., Sinha I., Veitonmaki N., Nystrom S., Aase K., Holmgren L. Differential roles of p80- and p130-angiomotin in the switch between migration and stabilization of endothelial cells. Biochim. Biophys. Acta. 2008;1783:429–437. doi: 10.1016/j.bbamcr.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Fang Z., Wu L., Wang L., Yang Y., Meng Y., Yang H. Increased expression of the long non-coding RNA UCA1 in tongue squamous cell carcinomas: a possible correlation with cancer metastasis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014;117:89–95. doi: 10.1016/j.oooo.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Fang Z., Zhao J., Xie W., Sun Q., Wang H., Qiao B. LncRNA UCA1 promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by suppressing miR-184 expression. Cancer Med. 2017;6:2897–2908. doi: 10.1002/cam4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W.B., Fedorov O., Morse E.M., Keates T., Hickman T.T., Felletar I. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.A., Shah N., Wang K.C., Kim J., Horlings H.M., Wong D.J., Tsai M.C., Hung T., Argani P., Rinn J.L. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Amit I., Garber M., French C., Lin M.F., Feldser D., Huarte M., Zuk O., Carey B.W., Cassady J.P. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–227. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Garber M., Levin J.Z., Donaghey J., Robinson J., Adiconis X., Fan L., Koziol M.J., Gnirke A., Nusbaum C. Ab initio reconstruction of cell type-specific transcriptomes in mouse reveals the conserved multi-exonic structure of lincRNAs. Nat. Biotechnol. 2010;28:503–510. doi: 10.1038/nbt.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Yang Y.N., Yuan H.H., Zhang T.T., Sui H., Wei X.L., Liu L., Huang P., Zhang W.J., Bai Y.X. UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology. 2014;46:396–401. doi: 10.1097/PAT.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Hao Y., Chun A., Cheung K., Rashidi B., Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- He Z., Wang Y., Huang G., Wang Q., Zhao D., Chen L. The lncRNA UCA1 interacts with miR-182 to modulate glioma proliferation and migration by targeting iASPP. Arch. Biochem. Biophys. 2017;623–624:1–8. doi: 10.1016/j.abb.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Hughes J.M., Legnini I., Salvatori B., Masciarelli S., Marchioni M., Fazi F., Morlando M., Bozzoni I., Fatica A. C/EBPalpha-p30 protein induces expression of the oncogenic long non-coding RNA UCA1 in acute myeloid leukemia. Oncotarget. 2015;6:18534–18544. doi: 10.18632/oncotarget.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Jiang Y.-Y., Xie J.-J., Mayakonda A., Hazawa M., Chen L., Xiao J.-F., Li C.-Q., Huang M.-L., Ding L.-W. Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2 promotes squamous cancer progression. Nat. Commun. 2018;9:3619. doi: 10.1038/s41467-018-06081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake Y., Nakagawa T., Kitagawa K., Suzuki S., Liu N., Kitagawa M., Xiong Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30:1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz M., Webster D.E., Flockhart R.J., Lee C.S., Zehnder A., Lopez-Pajares V., Qu K., Zheng G.X., Chow J., Kim G.E. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev. 2012;26:338–343. doi: 10.1101/gad.182121.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Li H., Yang Y., Kang L. Long noncoding RNA urothelial carcinoma associated 1 promotes the proliferation and metastasis of human lung tumor cells by regulating MicroRNA-144. Oncol. Res. 2018 doi: 10.3727/096504017X15009792179602. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li H.J., Li X., Pang H., Pan J.J., Xie X.J., Chen W. Long non-coding RNA UCA1 promotes glutamine metabolism by targeting miR-16 in human bladder cancer. Jpn. J. Clin. Oncol. 2015;45:1055–1063. doi: 10.1093/jjco/hyv132. [DOI] [PubMed] [Google Scholar]
- Li H.J., Sun X.M., Li Z.K., Yin Q.W., Pang H., Pan J.J., Li X., Chen W. LncRNA UCA1 promotes mitochondrial function of bladder cancer via the MiR-195/ARL2 signaling pathway. Cell. Physiol. Biochem. 2017;43:2548–2561. doi: 10.1159/000484507. [DOI] [PubMed] [Google Scholar]
- Li J.Y., Ma X., Zhang C.B. Overexpression of long non-coding RNA UCA1 predicts a poor prognosis in patients with esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2014;7:7938–7944. [PMC free article] [PubMed] [Google Scholar]
- Li W., Xie P., Ruan W.H. Overexpression of lncRNA UCA1 promotes osteosarcoma progression and correlates with poor prognosis. J. Bone Oncol. 2016;5:80–85. doi: 10.1016/j.jbo.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang T., Li Y., Chen D., Yu Z., Jin L., Ni L., Yang S., Mao X., Gui Y., Lai Y. Identification of long-non coding RNA UCA1 as an oncogene in renal cell carcinoma. Mol. Med. Rep. 2016;13:3326–3334. doi: 10.3892/mmr.2016.4894. [DOI] [PubMed] [Google Scholar]
- Loewer S., Cabili M.N., Guttman M., Loh Y.H., Thomas K., Park I.H., Garber M., Curran M., Onder T., Agarwal S. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat. Genet. 2010;42:1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovén J., Hoke H.A., Lin C.Y., Lau A., Orlando D.A., Vakoc C.R., Bradner J.E., Lee T.I., Young R.A. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153:320–334. doi: 10.1016/j.cell.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Liu W.G., Lu J.H., Liu Z.J., Li H.B., Liu G.J., She H.Y., Li G.Y., Shi X.H. LncRNA UCA1 promotes renal cell carcinoma proliferation through epigenetically repressing p21 expression and negatively regulating miR-495. Tumour Biol. 2017;39 doi: 10.1177/1010428317701632. 1010428317701632. [DOI] [PubMed] [Google Scholar]
- Lv M., Li S., Luo C., Zhang X., Shen Y., Sui Y.X., Wang F., Wang X., Yang J., Liu P., Yang J. Angiomotin promotes renal epithelial and carcinoma cell proliferation by retaining the nuclear YAP. Oncotarget. 2016;7:12393–12403. doi: 10.18632/oncotarget.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matallanas D., Romano D., Yee K., Meissl K., Kucerova L., Piazzolla D., Baccarini M., Vass J.K., Kolch W., O’Neill E. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol. Cell. 2007;27:962–975. doi: 10.1016/j.molcel.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh C.A., Chen C.K., Chow A., Surka C.F., Tran C., McDonel P., Pandya-Jones A., Blanco M., Burghard C., Moradian A. The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature. 2015;521:232–236. doi: 10.1038/nature14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minajigi A., Froberg J., Wei C., Sunwoo H., Kesner B., Colognori D., Lessing D., Payer B., Boukhali M., Haas W., Lee J.T. Chromosomes. A comprehensive Xist interactome reveals cohesin repulsion and an RNA-directed chromosome conformation. Science. 2015;349 doi: 10.1126/science.aab2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R., Chen X., Greenawalt E.J., Maulik U., Jiang W., Zhao Z., Eischen C.M. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat. Commun. 2017;8:1604. doi: 10.1038/s41467-017-01781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moleirinho S., Hoxha S., Mandati V., Curtale G., Troutman S., Ehmer U., Kissil J.L. Regulation of localization and function of the transcriptional co-activator YAP by angiomotin. Elife. 2017;6 doi: 10.7554/eLife.23966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na X.Y., Liu Z.Y., Ren P.P., Yu R., Shang X.S. Long non-coding RNA UCA1 contributes to the progression of prostate cancer and regulates proliferation through KLF4-KRT6/13 signaling pathway. Int. J. Clin. Exp. Med. 2015;8:12609–12616. [PMC free article] [PubMed] [Google Scholar]
- Nie W., Ge H.J., Yang X.Q., Sun X., Huang H., Tao X., Chen W.S., Li B. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371:99–106. doi: 10.1016/j.canlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Paramasivam M., Sarkeshik A., Yates J.R., 3rd, Fernandes M.J., McCollum D. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol. Biol. Cell. 2011;22:3725–3733. doi: 10.1091/mbc.E11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrie K.M. Identification and characterization of a novel tight junction-associated family of proteins that interacts with a WW domain of MAGI-1. Biochim. Biophys. Acta. 2005;1745:131–144. doi: 10.1016/j.bbamcr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Paweletz C.P., Charboneau L., Bichsel V.E., Simone N.L., Chen T., Gillespie J.W., Emmert-Buck M.R., Roth M.J., Petricoin I.E., Liotta L.A. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20:1981–1989. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- Peng L., Jiang B., Yuan X., Qiu Y., Peng J., Huang Y., Zhang C., Zhang Y., Lin Z., Li J. Super-enhancer-associated long noncoding RNA HCCL5 is activated by ZEB1 and promotes the malignancy of hepatocellular carcinoma. Cancer Res. 2019;79:572–584. doi: 10.1158/0008-5472.CAN-18-0367. [DOI] [PubMed] [Google Scholar]
- Qiu J.J., Lin Y.Y., Ye L.C., Ding J.X., Feng W.W., Jin H.Y., Zhang Y., Li Q., Hua K.Q. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol. Oncol. 2014;134:121–128. doi: 10.1016/j.ygyno.2014.03.556. [DOI] [PubMed] [Google Scholar]
- Robinson M.D., Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11:R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Sowa M.E., Bennett E.J., Gygi S.P., Harper J.W. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M.D., Zheng Y.Q., Wang L.P., Zhao H.T., Yang S. Long noncoding RNA UCA1 promotes cell proliferation, migration and invasion of human leukemia cells via sponging miR-126. Eur. Rev. Med. Pharmacol. Sci. 2018;22:2233–2245. doi: 10.26355/eurrev_201804_14809. [DOI] [PubMed] [Google Scholar]
- Tanos V., Prus D., Ayesh S., Weinstein D., Tykocinski M.L., De-Groot N., Hochberg A., Ariel I. Expression of the imprinted H19 oncofetal RNA in epithelial ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999;85:7–11. doi: 10.1016/s0301-2115(98)00275-9. [DOI] [PubMed] [Google Scholar]
- Tian S., Yuan Y., Li Z., Gao M., Lu Y., Gao H. LncRNA UCA1 sponges miR-26a to regulate the migration and proliferation of vascular smooth muscle cells. Gene. 2018 doi: 10.1016/j.gene.2018.06.031. [DOI] [PubMed] [Google Scholar]
- Tian Y., Zhang X., Hao Y., Fang Z., He Y. Potential roles of abnormally expressed long noncoding RNA UCA1 and Malat-1 in metastasis of melanoma. Melanoma Res. 2014;24:335–341. doi: 10.1097/CMR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- Tuo Y.L., Li X.M., Luo J. Long noncoding RNA UCA1 modulates breast cancer cell growth and apoptosis through decreasing tumor suppressive miR-143. Eur. Rev. Med. Pharmacol. Sci. 2015;19:3403–3411. [PubMed] [Google Scholar]
- Wang F., Li X., Xie X., Zhao L., Chen W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett. 2008;582:1919–1927. doi: 10.1016/j.febslet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Wang F., Ying H.Q., He B.S., Pan Y.Q., Deng Q.W., Sun H.L., Chen J., Liu X., Wang S.K. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget. 2015;6:7899–7917. doi: 10.18632/oncotarget.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Ren Z., Sun P. Overexpression of the long non-coding RNA MEG3 impairs in vitro glioma cell proliferation. J. Cell Biochem. 2012;113:1868–1874. doi: 10.1002/jcb.24055. [DOI] [PubMed] [Google Scholar]
- Wang W., Huang J., Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J. Biol. Chem. 2011;286:4364–4370. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.S., Zhang Z., Wang H.C., Cai J.L., Xu Q.W., Li M.Q., Chen Y.C., Qian X.P., Lu T.J., Yu L.Z. Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin. Cancer Res. 2006;12:4851–4858. doi: 10.1158/1078-0432.CCR-06-0134. [DOI] [PubMed] [Google Scholar]
- Wu H., Zhou C. Long non-coding RNA UCA1 promotes lung cancer cell proliferation and migration via microRNA-193a/HMGB1 axis. Biochem. Biophys. Res. Commun. 2018;496:738–745. doi: 10.1016/j.bbrc.2018.01.097. [DOI] [PubMed] [Google Scholar]
- Xiao J.N., Yan T.H., Yu R.M., Gao Y., Zeng W.L., Lu S.W., Que H.X., Liu Z.P., Jiang J.H. Long non-coding RNA UCA1 regulates the expression of Snail2 by miR-203 to promote hepatocellular carcinoma progression. J. Cancer Res. Clin. Oncol. 2017;143:981–990. doi: 10.1007/s00432-017-2370-1. [DOI] [PubMed] [Google Scholar]
- Xie J.-J., Jiang Y.-Y., Jiang Y., Li C.-Q., Lim M.-C., An O., Mayakonda A., Ding L.-W., Long L., Sun C. Super-enhancer-Driven long non-coding RNA LINC01503, regulated by TP63, is over-expressed and oncogenic in squamous cell carcinoma. Gastroenterology. 2018;154:2137–2151.e1. doi: 10.1053/j.gastro.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Xu M.Z., Chan S.W., Liu A.M., Wong K.F., Fan S.T., Chen J., Poon R.T., Zender L., Lowe S.W., Hong W., Luk J.M. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30:1229–1240. doi: 10.1038/onc.2010.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Pang H., Li X., Li H., Pan J., Chen W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016;107:18–27. doi: 10.1111/cas.12844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.T., Wang Y.F., Lai J.Y., Shen S.Y., Wang F., Kong J., Zhang W., Yang H.Y. Long non-coding RNA UCA1 contributes to the progression of oral squamous cell carcinoma by regulating the WNT/beta-catenin signaling pathway. Cancer Sci. 2016;107:1581–1589. doi: 10.1111/cas.13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C., Shen Z., Stemmer-Rachamimov A., Dawany N., Troutman S., Showe L.C., Liu Q., Shimono A., Sudol M., Holmgren L. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci. Signal. 2013;6:ra77. doi: 10.1126/scisignal.2004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Cao X., Zhang L., Zhang X., Sheng H., Tao K. UCA1 overexpression predicts clinical outcome of patients with ovarian cancer receiving adjuvant chemotherapy. Cancer Chemother. Pharmacol. 2016;77:629–634. doi: 10.1007/s00280-016-2963-4. [DOI] [PubMed] [Google Scholar]
- Zhang X., Gao F., Zhou L., Wang H., Shi G., Tan X. UCA1 regulates the growth and metastasis of pancreatic cancer by sponging miR-135a. Oncol. Res. 2017;25:1529–1541. doi: 10.3727/096504017X14888987683152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Lu Q., Wang L.H., Liu C.Y., Lei Q., Guan K.L. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Tumaneng K., Wang C.Y., Guan K.L. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Sun C., Cui Z. A long noncoding RNA UCA1 promotes proliferation and predicts poor prognosis in glioma. Clin. Transl Oncol. 2017;19:735–741. doi: 10.1007/s12094-016-1597-7. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Wu F., Dai W.Y., Zheng D.C., Zheng C., Ye H., Zhou B., Chen J.J., Chen P. Aberrant expression of UCA1 in gastric cancer and its clinical significance. Clin. Transl Oncol. 2015;17:640–646. doi: 10.1007/s12094-015-1290-2. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Vertuani S., Nystrom S., Audebert S., Meijer I., Tegnebratt T., Borg J.P., Uhlen P., Majumdar A., Holmgren L. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ. Res. 2009;105:260–270. doi: 10.1161/CIRCRESAHA.109.195156. [DOI] [PubMed] [Google Scholar]
- Zhou G., Li C., Feng J., Zhang J., Fang Y. lncRNA UCA1 is a novel regulator in cardiomyocyte hypertrophy through targeting the miR-184/HOXA9 Axis. Cardiorenal Med. 2018;8:130–139. doi: 10.1159/000487204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Wang X., Zhang J., He A., Wang Y.L., Han K., Su Y., Yin J., Lv X., Hu H. Artesunate suppresses the viability and mobility of prostate cancer cells through UCA1, the sponge of miR-184. Oncotarget. 2017;8:18260–18270. doi: 10.18632/oncotarget.15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H.Y., Bai W.D., Ye X.M., Yang A.G., Jia L.T. Long non-coding RNA UCA1 desensitizes breast cancer cells to trastuzumab by impeding miR-18a repression of Yes-associated protein 1. Biochem. Biophys. Res. Commun. 2018;496:1308–1313. doi: 10.1016/j.bbrc.2018.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.