Abstract
Sulfur is an essential element in determining the productivity and quality of agricultural products. It is also an element associated with tolerance to biotic and abiotic stress in plants. In agricultural practice, sulfur has broad use in the form of sulfate fertilizers and, to a lesser extent, as sulfite biostimulants. When used in the form of bulk elemental sulfur, or micro- or nano-sulfur, applied both to the soil and to the canopy, the element undergoes a series of changes in its oxidation state, produced by various intermediaries that apparently act as biostimulants and promoters of stress tolerance. The final result is sulfate S+6, which is the source of sulfur that all soil organisms assimilate and that plants absorb by their root cells. The changes in the oxidation states of sulfur S0 to S+6 depend on the action of specific groups of edaphic bacteria. In plant cells, S+6 sulfate is reduced to S−2 and incorporated into biological molecules. S−2 is also absorbed by stomata from H2S, COS, and other atmospheric sources. S−2 is the precursor of inorganic polysulfides, organic polysulfanes, and H2S, the action of which has been described in cell signaling and biostimulation in plants. S−2 is also the basis of essential biological molecules in signaling, metabolism, and stress tolerance, such as reactive sulfur species (RSS), SAM, glutathione, and phytochelatins. The present review describes the dynamics of sulfur in soil and plants, considering elemental sulfur as the starting point, and, as a final point, the sulfur accumulated as S−2 in biological structures. The factors that modify the behavior of the different components of the sulfur cycle in the soil–plant–atmosphere system, and how these influences the productivity, quality, and stress tolerance of crops, are described. The internal and external factors that influence the cellular production of S−2 and polysulfides vs. other S species are also described. The impact of elemental sulfur is compared with that of sulfates, in the context of proper soil management. The conclusion is that the use of elemental sulfur is recommended over that of sulfates, since it is beneficial for the soil microbiome, for productivity and nutritional quality of crops, and also allows the increased tolerance of plants to environmental stresses.
Keywords: plant nutrition, sulfate, sulfite, plant health and nutrition, nutraceuticals, polysulfanes, polysulfides, soil microbiome
1. Introduction
Sulfur is one of the most abundant elements on Earth and is an essential element for living beings, of which constitutes on average 1% of dry weight. In plants, S content varies strongly between species, ranging from 0.1 to 6% of dry weight (0.03 to 2 mmol g−1 dry weight) [1]. S belongs to the VIA group of the periodic system, where it is found together with O, Se, Te, and Po; naturally, S is a mixture of four isotopes, 32S, 33S, 34S, and 35S. The natural abundance of each is 95.1%, 0.74%, 4.2%, and 0.016%, respectively. Sulfur exists in oxidation states ranging from +6 to −2 (Table 1), with the most oxidized state in the form of sulfate (SO42−), which is the chemical form that plants absorb from the soil to feed themselves with S [2].
Table 1.
Representative sulfur compounds and their oxidation states.
| Oxidation State | Representative Compound and Formula | Oxidation State | Representative Compound and Formula |
|---|---|---|---|
| +6 | Sulfate, SO42− | 0 | S0, elemental sulfur. Sulfoxide (R-S(-O)-R such as dimethyl sulfoxide (DMSO). Oxidized derivatives of sulfide and sulfenic acid (RSOH). |
| +6 and −2 | Thiosulfate, S2O32− | −1 | Disulfide (R-S-S-R) is a persulfide found in the linkages between two cysteine residues in proteins. RSSH denotes persulfides (or hydrosulfides) obtained by the action of H2S on cysteine residues (R-SH). Thioethers and thiols can be oxidized to disulfides. Major products of decomposition of persulfides are polysulfanes. Thiyl-radical RS*. |
| +5 and −2 | Polythionates (−O3S-Sn-SO3−): Dithionate, S2O62−; Trithionate, S3O62−; Tetrathionate, S4O62− | −2 | Sulfide, S2−, polysulfides, S22−, S32−, S52−; carbon disulfide (CS2); FeS2; NaHS and Na2S are sources of S2− and of its conjugated acids SH− and H2S. Polysulfides (with Sn > 2) contain S0 atoms, which allows a diversity of oxidation states. |
| +4 | Sulfur dioxide, SO2; Sulfite, SO32−; Disulfite, S2O52−; Sulfone, OS(S) the oxidation product of sulfoxides | −2 | Hydrogen sulfide (H2S), disulfane (H2S2), and polysulfanes (RSSnSR, n > 2). Polysulfanes contain S0 atoms, which allows a diversity of oxidation states. |
| +3 | Dithionite, S2O42− | −2 | Thioethers (C-S-C) such as dimethyl sulfide (DMS), CH3-S-CH3 and dimethyl disulfide (DMDS), CH3-S-S-CH3. |
| +2 | Carbonyl sulfide (COS), OCS | −2 | Thiols (R-SH) such as glutathione (GSH) and methyl mercaptan, CH3-SH. Thiols are derived from the sulfhydryl group -SH of cysteine, which enables multiple oxidation states (−2 to +6). Thiolates are derivatives of thiols in which a metal or other cation replaces H. |
| 0 | Elementary sulfur (S0), mainly S8 (cycloocta-S) | −2 | Carbon disulfide, CS2. |
Biological molecules, which range from small molecules to proteins and other polymers, contain S in its more reduced states 0, −1, and −2. For example, it is known that approximately 40% of enzymes depend for their catalytic activity on the presence of sulfhydryl groups (-SH). These -SH groups participate in redox reactions, provide binding sites for toxic or physiologically important metals, and are related to the detoxification of various xenobiotics. It is also known that the tertiary and quaternary structure of many proteins is the result of the presence of disulfane bonds (-S-S-) formed by the oxidation of -SH groups of cysteine, a sulfur amino acid that, together with methionine, is a key factor in determining the nutritional value of plants, as well as a central element in the metabolism of S in all organisms [2].
For the above reasons, a close relationship between nitrogen and sulfur nutritional status has been found in plants [3,4]. Approximately 80% of nitrogen and sulfur incorporated in organic compounds of plants is found in proteins when both elements are in adequate proportions. The S/N balance of a plant, described by the organic S/N ratio, is in the range of 0.025 (legumes) to 0.032 (grasses) and is relatively constant from one species to another. Therefore, the amount of S required by a plant is strongly dependent on its N nutrition. The consequence is that the availability of S below the needs of the crops does not allow the adequate use of applied N [5].
Compounds as important as β-lactam antibiotics (penicillins, cephalosporins, and cephamycins) have an S atom derived from cysteine. The sulfur compound S-adenosyl-l-methionine (SAM) is the most crucial methylating agent known in all organisms; SAM-mediated transmethylation reactions are essential in the regulation of gene expression, the activity of various enzymes, the synthesis of compounds such as the osmolyte DMSP (dimethyl sulfoniopropionate) and DMS (dimethyl sulfide) gas, as well as in the production of antibiotics [2].
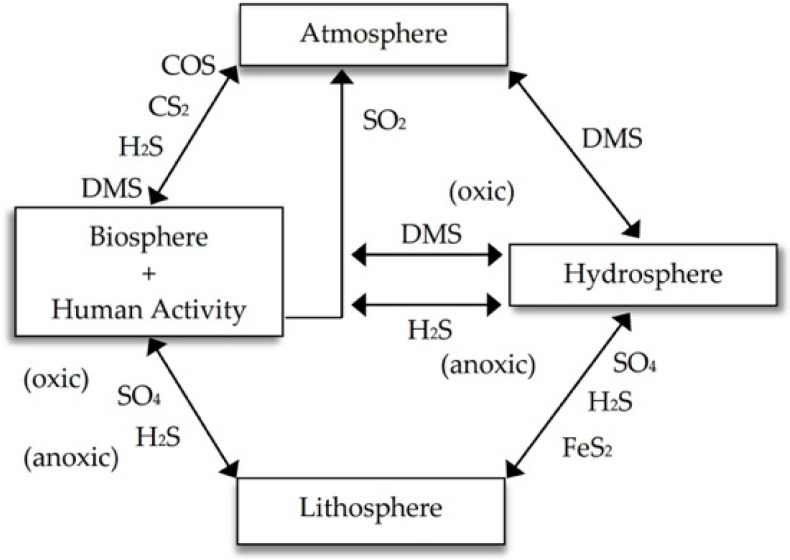
The Earth’s S stores are located in the lithosphere, hydrosphere, atmosphere, and biosphere. Human activities result in the extraction of S from the lithosphere (burning of fossil fuels, mining of elemental S and metals) and biosphere (oxidation of organic matter from the soil and burning of biomass). Anthropogenic S is incorporated into the global cycle mainly in the form of SO2 emitted into the atmosphere [6].
Between the terrestrial and marine masses, there is a constant flow of S via the atmosphere through the gaseous forms of the element (SO2, COS, H2S, DMS, and CS2) [6] and aerosols (mainly SO42− from the oxidation of sulfur gases, and <10% of organosulfates) [7], or by runoff from terrestrial to oceanic regions (Figure 1). The constant mobilization of S causes changes in the sulfur species that move from one terrestrial compartment to another. Under oxic conditions, the predominant inorganic form of S is SO42−, resulting from atmospheric deposition or oxidation of reduced forms of S. In the soil, continuous land tillage that oxidizes soil organic matter and repeated extractions for crops cause the decrease of S stores; for this reason, the regular application of S with the fertilizers is recommended [8].
Figure 1.
Simplified biogeochemical sulfur cycle. Human activities, fauna, vegetation, and soil microorganisms can be visualized as an interface (as source and sink) to accelerate the transfer of sulfur species between the lithosphere, atmosphere, and hydrosphere.
In soils, most S is found in organic forms; the inorganic forms are elemental sulfur (S0) or SO42−, the latter can be found as gypsum or be adsorbed in the inorganic exchange matrix. The SO42− adsorbed in the soil is in dynamic equilibrium with the soil solution, and the adsorption/desorption quotient inversely depends on the pH value of the soil and the cations present in the exchange matrix, showing higher affinity for Al3+ > Ca2+ > K+ [9,10].
In soil, SO42− is subject to dissimilatory and assimilatory reduction. Dissimilatory reduction occurs when SO42− is used as a final acceptor of electrons in the anaerobic metabolism of microorganisms, producing H2S that is reoxidized in the presence of O2 or volatilized into the atmosphere. Assimilatory reduction is used by prokaryotes, algae, plants, and fungi for the biosynthesis of organic compounds, e.g., amino acids. Animals and protists cannot perform assimilatory reduction of SO42−; therefore, they depend on the organic sulfur compounds synthesized by other organisms [6]. In many crop species, sulfur is an element associated with nutritional quality and density of mineral nutrients, tolerance to stress, and the management of certain pests and pathogens [11,12,13].
In agricultural soils, SO42− used by crop plants comes mainly from the contribution of fertilizers with sulfates, such as ammonium sulfate, gypsum, potassium sulfate, magnesium sulfate, single superphosphate, ammonium phosphate sulfate, potassium magnesium sulfate, and sulfates of micronutrients [8,14], as well as the oxidation of S0, and of S2− contained in organic fertilizers. Another part of the S of crops is obtained from SO42− and aerosols coming from precipitation, as well as the absorption by soil and plants of aerosols and gases such as H2S, COS, and DMS. When S is added to the soil in the form of SO42−, plants and aerobic prokaryotes absorb it and incorporate it into a reductive metabolism that produces sulfide (S2−). On the other hand, when S is supplied as S0 or in the form of organic fertilizers (S2−), it must be oxidized to SO42− by the action of soil prokaryotes to be available to plants [2,6,8].
The aim of the present review is to describe the dynamics of sulfur in soil and plants, considering elemental sulfur as the starting point and, as a final point, the sulfur accumulated as S−2 in biomolecules and biological structures, transformed into myriad sulfur compounds and returned to atmosphere and hydrosphere as H2S and other gaseous molecules. The factors that modify the behavior of the different components of the sulfur flow in the soil–plant–atmosphere system are described, along with how these influences the productivity, quality and stress tolerance of crops.
2. Transformations of Elemental Sulfur in Soil
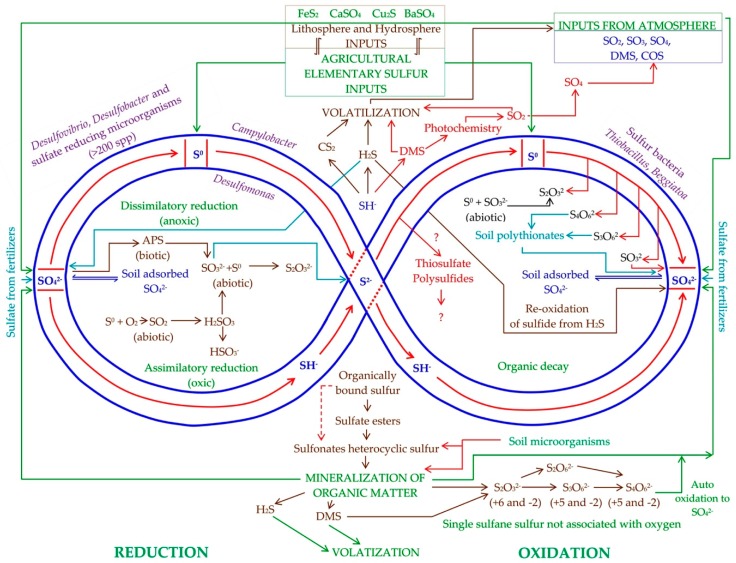
The S available for plants in agricultural ecosystems is in dynamic storage (Figure 2). It comes from gaseous forms and aerosols of S from the atmosphere, from dissolved S (mostly SO42−) in rain and snow precipitation, and from SO42−, which is obtained from the oxidation of S of soil organic matter and S0. Sulfates can be fixed in the soil exchange matrix or leached to the subsoil [10]. In arid regions, SO42− can be stored in large quantities as gypsum in the subsoil, but in areas with higher water availability, leached SO42− is mobilized to lower horizons and to the subsoil [15].
Figure 2.
Schematic representation of the flow of sulfur in soil. APS = adenosine 5′-phosphosulfate. Oxidation states of sulfur in the different molecules are: SO42− (+6); S2O62− (+5 and −2); S4O62− (+5 and −2); S3O62− (+5 and −2); SO32− (+4); SO2 (+4); S2O32− (+6 and −2); COS (+2); S0 (0); SH− (−2); S2− (−2); DMS (−2); CS2 (−2).
In the anoxic zones of the soil, S0 and SO42− are transformed to H2S that is volatilized or is reoxidized to S0 y and sulfate in the oxic zone. Plants and microorganisms take the SO42− and reduce it to S2− to incorporate it into a huge variety of organic compounds. Subsequently, these same plants and microorganisms transform a part of the sulfur to H2S, DMS, and CS2 [8,16]. The above volatile molecules have been associated with detoxification metabolism, stress tolerance, and signaling in plants and prokaryotes [17,18]. As with iodine [19], soil organic matter can transform the S to volatile forms by means of abiotic reactions, but the rate of transformation is very low in comparison with biotic metabolism of S [16].
Since there are several access ways by which S can enter the agricultural ecosystem, it is not possible to mark a specific starting point. Therefore, arbitrarily, the assumption of an application of S0 to the soil is taken, and the transformations that this material experiences up to SO42− are described. Once in the form of SO42−, it is assimilated into plant cells in the form of myriad organic compounds. The final part of the flow of S from soil to plants ends with the production of volatile compounds by plant cells, or in the transformation of the S contained in plant waste (Figure 2).
S atoms tend to avoid double bonds, therefore, in the S0, instead of forming molecules of S2 (S=S) the S atoms are grouped in the form of cyclic allotropes (cyclosulfur) or as long chains Sn (catena sulfur) [20]. The S0 used to apply to soil consists mainly of molecules of S8 (cycloocta-S) that are grouped, forming polymers of variable size; S8 is the most stable form from a thermodynamic point of view. S8 is a very electrophilic Lewis acid, so it reacts with nucleophilic anions or Lewis bases such as OH−, sulfides (S2−), thiols (R-SH), thiolates (RS−), I−, CN−, and SO32− [21,22].
S0 is applied to the soil or substrate in quantities ranging from 20 to 250 kg ha−1 yr−1, the last figure being equivalent to 200 mg S0 kg−1 soil. Once in the soil or substrate, S0 begins to transform into other chemical forms, mainly through biotic processes, and, to a lesser extent, by abiotic processes. The transformation rate is inversely proportional to the particle size and directly proportional to the temperature (Q10 = 4.0), humidity availability, and abundance of edaphic microorganisms [8,23,24].
Any factor that decreases bacterial activity, such as temperatures <10 °C or >40 °C and lack of humidity in the soil, will reduce the transformation of S. Flooded or compact soils will have anoxic conditions that induce high rates of conversion of S0 and SO42− into gaseous forms of sulfur [8,24]. The metabolism of S in soils can modify other processes, as in rice paddies, where the use of gypsum amendment has been shown to decrease greenhouse methane emissions [25]. In alkaline soils, it has been observed that the use of S0 induces acidification (by H2SO4), which increases the bioavailability of elements such as P [26].
When it is desired that S0 produces SO42− rapidly available for crops, an S0 source with a small particle size (<150 μm or 100 mesh) should be chosen. Contrarily, if a long-term impact (two or more consecutive crops) is sought, it is desirable to use S0 sources with a larger particle diameter, or even granular forms such as S0 prills or S0-fortified N-P-K and DAP fertilizers [27,28]. At a temperature of 14 °C, it was found that, in 51 weeks, 51% of S0 with particle diameter 41 μm (300 mesh) was oxidized, compared to 18% of S0 with 125 μm (120 mesh). In soils with low temperatures, S0 sized 41 μm will oxidize at a rate equivalent to S0 sized 125 μm in soils with higher temperatures [23]. In another experiment, applying 50 kg ha−1 of S0, it was found that 80–90% of S0 with particles <150 μm was oxidized over a period of 340 days [29].
On the other hand, it has been found that repeated applications of S0 to soil increase the population and the activity of oxidizing bacteria of S0 [24]. Accompanying the increase in S0 oxidant bacteria was a reduction in the number of fungi and protists, while bacterial and actinomycete populations remained stable [30]. Other authors reported a decrease in biomass and bacterial metabolism by applying S0 annually for five years [31].
When S0 is in micronized form (<177 μm, <80 mesh) it is used for the control of mites and some fungi [32,33]. The reactivity of micronized S0 is a consequence of the high quotient surface/volume of the particles, estimated to be 1300 to 1940 cm2 g−1 for S0 of 125 and 41 μm, respectively [23]. Micronized S0 can be applied through the foliar route or even by using pressurized irrigation systems to incorporate it into the soil [34,35]. When applied by irrigation system, the problems associated with the application of micronized S0 (because it is a flammable and irritant material) by dusting machines are reduced [24].
The use of S nanoparticles for the control of pathogens in plants has also been described [36,37]. Taking into account the high value of the surface/volume ratio of S nanoparticles, furthermore being a source of S for rapid assimilation by plants and microorganisms, it is possible that they function as biostimulants [38], and that they provide highly reactive S0 that works as a tolerance-inducing factor against pathogenic fungi [32,39].
To be available for plants, S0 applied to the soil or substrate must be oxidized to SO42−. The change in the oxidation state of sulfur from 0 to +6 allows reduction equivalents to be obtained (8H+ + 6e−). The oxidation is carried out by most soil microorganisms, highlighting Thiobacillus, Beggiatoa, Desulfomicrobium, and Desulfovibrio, as well as other heterotrophic aerobics S-oxidizing bacteria such as Bacillus, Pseudomonas, and Arthrobacter [2,40]. Two metabolic pathways have been described that allow the oxidation of inorganic S to SO42−: the Kelly–Friedrich pathway, which does not involve the production of intermediates such as polythionates, and the Kelly–Trudinger pathway, which includes as an intermediate output tetrathionate (S4O62−) and other polythionates [16]. The existence of two different routes and the large number of taxa that carry out the oxidation of S0 allow a high redundancy, and capacity to tolerate extensive changes in pH and salinity in soils [41,42].
In Figure 2, the oxidation activity from S0 to SO42− is presented on the right side, and shows the Kelly–Trudinger pathway with the production of polythionates such as S4O62− and S3O62− (as well as S2O32− and SO32−), which serve as a source of reducing potential and possibly act as inducers of stress tolerance in plants, perhaps by containing a single sulfane sulfur not associated with oxygen [16]. In this regard, Li et al. [43] described polythionates as agents with antibiotic action, the efficacy of which is variable according to the pH. Additionally, the abiotic oxidation of S2− in the presence of S0 produces polysulfides [44], which have been described as agents associated with stress tolerance in animal cells [45]. Polysulfides possibly fulfill a similar stress-protection function in plants [46]. It is possible that the presence of S0 and S2− in polysulfides [44] explains their ability to induce stress tolerance. The production of polythionates and polysulfides represents an additional advantage of the use of S0 as a source of sulfur for crops.
Under anoxic conditions, S0 is produced as part of the dissimilatory reduction of SO42−. Later, the S0 can be assimilated into S2− that will be part of the biomolecules, or it will be volatilized in the case of excess S (see the central section of Figure 2). At the left and right ends of Figure 2, in the central part, the SO42− from fertilizers, precipitation, and mineralization of organic matter is represented. A portion of this SO42− forms a soil adsorbed sulfate storage, which will be in dynamic equilibrium with SO42− dissolved in the soil solution. Under oxic conditions, SO42− is assimilated in S2− by assimilatory reduction and then transformed back into SO42− during the organic decay and mineralization of organic matter [42].
As part of the processes of organic decay, mineralization of organic matter, and sulfate reduction, both the soil, through abiotic reactions, and micro-organisms and plants can be source or sink of volatile forms of S, such as H2S, DMS, COS, CS2, and SO2 (Figure 2). Generally, under anoxic conditions, the oxidized forms of S are reduced by the soil microbiome to H2S, CS2, COS, DMDS, methyl mercaptan, and COS [8]. These gaseous molecules are believed to be part of a mechanism of dissipation of excess S, although participation in other processes is not ruled out [16,47].
In terms of reductive and oxidative microbial reactions, the most abundant forms of sulfur in the soil and the edaphic microbiome are S2−, R-SH, RSSH, polysulfides (RSn2−), S2O32−, SO32−, SO42− and polythionates [16]. SO42− applied as fertilizer or obtained through the processes described above is the form of S that plants assimilate through their roots [40].
At best growth conditions, a plant’s sulfur requirement ranges from 2 to 10 μmol g−1 plant fresh weight day−1 [1]. As the flow of S is a dynamic process where the ecosystem receives S from the atmosphere, precipitation, subsoil water, and fertilizers, and loses S through the process of volatilization of S by soil and plants and by leaching, it is difficult to estimate the actual amount of S that a plant surface absorbs, assimilates, leaches, and volatilizes. As an exercise, let us suppose a single sampling point for a field of maize, for example before the harvest. In one hectare, there may be 78,000 kg of fresh weight ha−1, which would be equivalent to 25 kg of sulfur contained in the plants. However, the 25 kg ha−1 accumulated in the plant tissues at that specific sampling time does not include the S volatilized by the plant itself, or that leached, assimilated, or volatilized in the soil and by the microorganisms.
The point to highlight with the data of the previous paragraph is that the S of the soil is in constant exchange and extraction by the crops, atmosphere, and soil water. Therefore, a continuous supply of S is required, which is recommended to be applied in the form of S0 (40–60 kg ha−1) every one or two years, to maintain the edaphic store.
3. Absorption and Assimilation of Sulfur in Plants
3.1. Sulfur Absorption and Transport
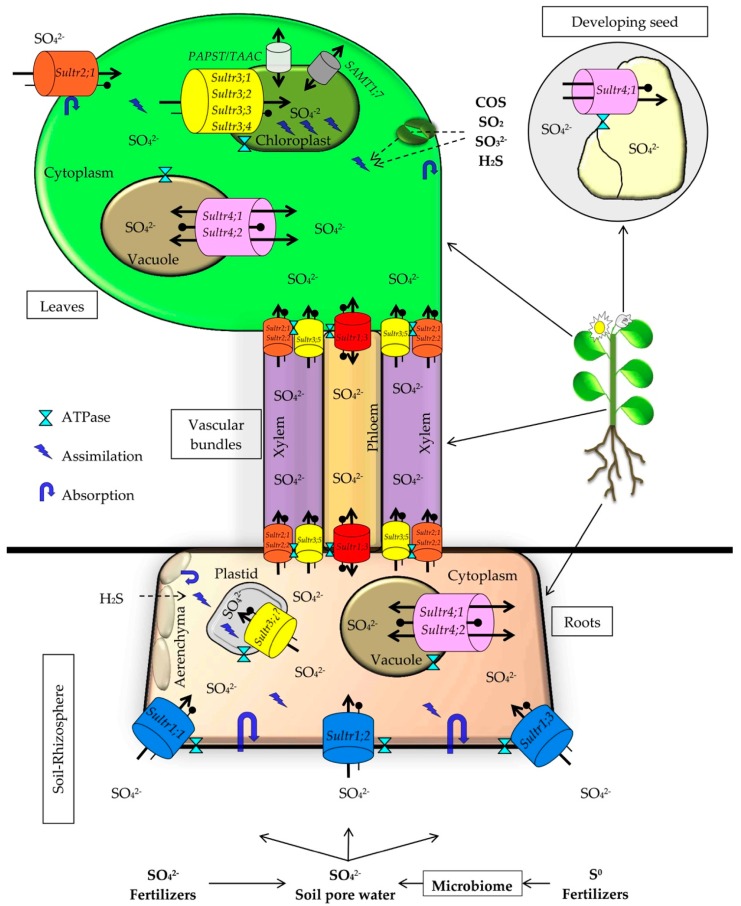
The absorption of S from atmospheric sources such as COS, SO2, DMS, and H2S, can represent a valuable contribution of sulfur for many plants. However, most of the S taken by the plants comes from SO42− dissolved in the soil solution [40,47].
The SO42− dissolved in the soil solution is absorbed by H+/sulfate cotransporters called SULTRs (Figure 3). Plant SULTRs are encoded by a multigene family. SULTRs include high-affinity transport proteins (HAST), low-affinity transport proteins (LAST), vacuole transporters, and plastid membranes and endosymbionts transporters [48,49]. The level of SO42− in the soil solution that induces high-affinity transporters is <10 mg L−1 (0.1 mM) [1]. In the soil solution of agricultural areas under strong fertilization management, values of 40–200 mg L−1 SO42− are found, while for non-agricultural fertile soils, concentrations of SO42− of 4.5–40.5 mg L−1 were reported in the soil solution [50]. The assimilation of S shows a high degree of control and coordination with the assimilation of C and N, and in root transporters there seems to be a relevant regulatory site [1,49].
Figure 3.
Schematic representation of the processes of absorption, transport, and storage of sulfate.
HAST Sultr1;1, Sultr1;2, and Sultr1;3 facilitate the absorption of SO42− in the root. The HAST of the epidermis and the cortex is accompanied by the LAST Sultr2;1, Sultr2;2, and Sultr3;5, with which they act synergistically. HAST are very abundant in the epidermis and cortex of the root, while LAST proliferate in the parenchyma adjacent to the xylem and phloem [49,51]. The SO42− absorbed is stored in the vacuoles thanks to the co-transporters Sultr4;1 and Sultr4;2 [49,51,52], or it is distributed to the rest of the plant, depending on the sink tissues’ demand. Translocation from the root to the stems and leaves through the xylem occurs through Sultr1;3, Sultr2;1, Sultr2;2, and Sultr3;5 [49,51,53,54,55]. In most species, the absorbed sulfate is assimilated largely into proteins and other biomolecules. However, in others, such as Brassica oleracea seedlings, it is possible to observe high amounts of SO42− in the plant tissues [1].
The discharge of SO42− from the xylem to the mesophyll cells of the leaf is mediated by HAST and LAST (Sultr1;3, Sultr2;1, Sultr2;2, and Sultr3;5). As occurs in the root, a part of the sulfate is stored in the vacuoles of stems and leaves by Sultr4;1, and Sultr4;2 [49,51,52], while a part is taken to the chloroplasts by the co-transporters Sultr3;1, Sultr3;2, Sultr3;3, and Sultr3;4 [49,51,55,56], where it will be reduced to S2− to be assimilated into biological molecules [56]. According to the plant’s needs, the sulfate stored in the vacuoles can be re-mobilized through the co-transporters Sultr4;1 and Sultr4;2 [51,52].
The capacity of sulfur absorption, assimilation, and volatilization responds to the nutritional status of the plant. In turn, the sulfur nutritional status of the latter depends on the growth rate and the interaction with the C and N levels of the plants. Biomolecules synthesized from C and N assimilated during photosynthesis are the primary sink for S, and probably constitute part of the signals that regulate SO42− absorption, transport, and assimilation [1]. It is assumed that an excess of S will trigger a higher accumulation of SO42− in the vacuoles, as well as an increase in the synthesis of volatile forms of S, such as H2S [16,47]. On the other hand, under S deprivation there will be a significant increase in the expression of sulfate transporters, which will increase the absorption and assimilation of SO42− [1].
Other environmental factors, which possibly modify the absorption of SO42−, also regulate the relative abundance of HAST and LAST. Among these factors are salinity, drought, and high temperature in maize [57], drought and salinity in Arabidopsis and Medicago truncatula [58], and heavy metals such as Cd in sorghum [59].
Considering now the absorption of gas molecules of S, atmospheric SO2 can be absorbed through the stomata. In the water film of the substomatal chamber, it is transformed into HSO3− and SO32−, which is incorporated into the sulfur reduction pathway to be reduced to S2−. Another alternative is that SO32− can be oxidized extra- and intracellularly to SO42− by peroxidases, or non-enzymatically by O2− radicals or metal ions. This new SO42− is again incorporated into sulfur reduction pathway or transferred into the vacuole. A high level of SO42− is typical in plants exposed to SO2 [1,60,61].
Similarly, by diffusion, H2S from the soil, vegetation, or atmosphere can be absorbed directly or dissolved in air H2O aerosols, through stomata [62,63,64]. In the mesophyll, H2S is assimilated by O-acetyl-serine (thiol)lyase for the biosynthesis of cysteine [60]. The exogenous application of H2S has been described as a factor that increases tolerance to water deficit directly through a higher content of cysteine, and indirectly through the synthesis of metabolites such as proline and glycine betaine, and upregulating antioxidant enzymes [65].
Additionally, a synergistic interplay between nitric oxide (NO) and H2S signaling has been shown during stress events [66]. In anoxic soils, the aerenchyma of the root intervenes in the fixation of the H2S produced by microorganisms [67], so that the H2S of the soil can also trigger adaptive responses to stress in the radical tissues. On the other hand, the absorption and assimilation of SO2 and H2S in the leaves is a factor that modifies the nutritional status of the sulfur in the plant and decreases the activity of the SO42− transporters in the root [60].
In the case of COS, this gas is produced by biotic synthesis or by the oxidation of CS2 and DMDS. In plants, the SCN− obtained from glucosinolate metabolism is hydrolyzed to COS and NH3. COS is the most stable and abundant gas form of S in the atmosphere (0.5 ppb). In the atmosphere, COS can be oxidized to sulfate to form aerosols, or be absorbed by plants and microorganisms that transform it into CO2 and H2S through carbonic anhydrase, RUBISCO, nitrogenase, and other metalloenzymes. In plants, COS can also be reduced to CS2, which is released back into the atmosphere [18]. The SO42− from the aerosols can be absorbed in the leaves by means of the co-transporter Sultr2;1 [62], located in the cells of the mesophyll.
3.2. Sulfur Assimilation and the Synthesis of H2S
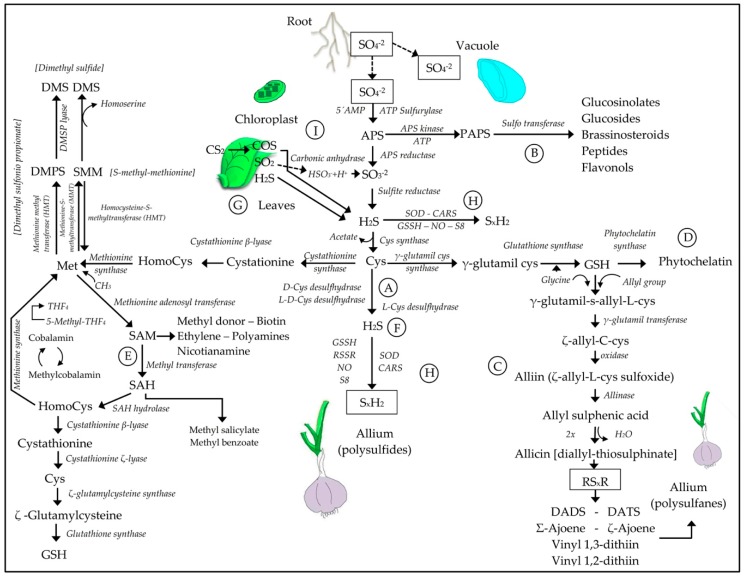
Once SO42− is available in the cells from root absorption and transport, or by absorption of gaseous forms of S by the stomata, (Figure 4G,I) it is used to assimilate S into biomolecules [68,69,70]. The first step is to activate SO42− by means of the enzyme ATP sulfurylase. The resulting compound, adenosine 5′-phosphosulfate (APS), is used as a bifurcation between two assimilation pathways, primary and secondary. In the primary pathway, the APS is reduced by APS reductase to SO32−, which in turn is reduced to S2−/H2S, which is assimilated into the amino acid cysteine (Figure 4A) [68,71]. Cysteine is the first product of the primary assimilation pathway of SO42−, and is used for the synthesis of methionine and proteins, or as a donor of S2− for the synthesis of a large number of metabolites, such as H2S (Figure 4F) [72], SAM, GSH, phytochelatins (Figure 4D,E) [48,68], SAM [68,73], polysulfides [72], and polysulfanes [74,75] (Figure 4C,H). In the secondary pathway (Figure 4B), SO42− is phosphorylated by APS kinase to 3′-phosphoadenosine 5′-phosphosulfate (PAPS) [48,68]. PAPS is the active sulfate donor for a variety of sulfation reactions in secondary metabolism. The reaction of sulfation is catalyzed by sulfotransferases, located mainly in the cytoplasm. To date, a large number of sulfated secondary compounds have been found to be involved in growth and stress signaling, and in the detoxification of environmental toxins [76,77].
Figure 4.
Schematic representation of the primary and secondary pathways of sulfur assimilation. In the primary assimilation pathway (A), APS is reduced to SO3 and subsequently to S2−/H2S, which are assimilated to form the amino acid cysteine [68,71]. In the secondary pathway (B), SO4 is phosphorylated and converted to 3′-phosphoadenosine 5′-phosphosulfate (PAPS) [48,68]. Cysteine is a central point for the synthesis of methionine or the production of polysulfanes (C), polysulfides (H), phytochelatins (D), SAM (E), and H2S (F) [48,68,72,73,74,75]. The absorption of sulfur in its gaseous forms is carried out by the stomatal route, directly incorporated into the primary pathway (SO2 and H2S) (G) [68], or through the action of carbonic anhydrase (COS) (I) [69,70].
As occurs in the metabolism of S in the soil (Figure 2), the great diversity of oxidation states of S allows the construction of a sophisticated and rich network of functional sulfur molecules for cell metabolism and signaling of plants. As in the soil, plants can exchange sulfur from the plant to the atmosphere and vice versa in the form of H2S, COS, and CS2, to incorporate the S in the assimilation pathways shown in Figure 4.
In recent years, both H2S and several of the derivative compounds (polysulfides and polysulfanes) or donors of this molecule have aroused great interest for their participation as oxidative stress reducers, in cellular signaling, and as post-translational modifiers. Because of its lipophilic nature, H2S is biologically reactive, since it can rapidly cross the membranes of cells without the intervention of channels. A possible H2S signaling mechanism is the formation of persulfides or hydrosulfides (RSSH) from the protein cysteine residues. It is assumed that H2S interacts in this way, with a great diversity of proteins such as channels, transcription factors, and enzymes [78,79].
H2S autooxidises in the presence of O2, forming polysulfanes, SO32−, S2O32−, and SO42− [70]; additionally, H2S is also a precursor of biological polysulfides [72]. Polysulfanes, polysulfides (with Sn > 2), and RSSH contain S0 atoms, which allows a diversity of oxidation states between the sulfur atoms and allows the molecules a dual character as oxidants and reducers. This diversity probably contributes to a multifunctionality character of the signaling of H2S and its derived compounds. Although H2S has a reactivity comparable to that of GSH against H2O2 and free radicals, it is believed that its value as a cellular antioxidant is limited because of its low concentration in vivo [78,79].
H2S donor compounds have been explored in the agricultural field for their possible applications in improving the productivity and quality of crops. It has been found that H2S mediates in signaling and in the increase in tolerance to different stresses such as heavy metals (Cd, Cr, Cu, Al, As), salinity, high temperature, and water deficit [17,65,80,81,82,83,84,85] Additionally, H2S and reactive sulfur species (RSS) interact with other relevant signaling molecules such as reactive oxygen (ROS) and reactive nitrogen (RNS) species [66,86], so the set of reactive chemical species could form a cellular network of redox signals [87]. These facts emphasize on the one hand the importance of adequate crop nutrition with S and, on the other hand, they highlight the advantages of the use of S0 applied to the soil and by dusting machine [33], because, presumably, S0 is a source of RSS as polysulfanes and polysulfides.
In addition to its relevance as a cell signaling and tolerance inducer, H2S is a source of RSS, a group of molecules of great biological importance that includes polysulfides and polysulfanes, SO2, S2O32−, allicin, diallyl disulfide (DADS), and diallyl trisulfane (DATS), shown in Figure 4C,H. RSS can also be formed by the oxidation of thiols (e.g., GSH oxidized by H2O2). RSS are sulfur species capable of initiating oxidation reactions by nucleophilic substitutions, and can be non-radical or radical, as the thiyl radical RS*. RSS are not inactivated by antioxidants such as vitamins C and E or NADPH; for the above, GSH is required [88].
Polysulfides are inorganic RSS of the general formula RSn2− (n > 2) such as H2S22−, H2S32−, H2S42−, and H2S52−. Polysulfides are produced metabolically by enzymatic catalysis, by partial oxidation of S2− of H2S to produce H2Sn, by reduction of H2S in the presence of polysulfanes, or by the reduction of polysulfanes in the presence of GSH. Polysulfides, depending on the molecule with which they interact, can behave as oxidants or reducers. It is considered that H2Sn polysulfides could be part of the signaling network and antioxidant impact currently attributed entirely to H2S [72]. The IUPAC [89] defines polysulfides as compounds R-[S]n-R, with a chain of S atoms n ≥ 2 and R ≠ H, however, in this manuscript we utilized the definition of Kharma et al. [72].
In soil, the abiotic synthesis of polysulfides occurs through the reaction between S0 and S2−, the oxidation of H2S by O2, H2O2, and possibly by iron oxides. Polysulfides are also produced by the bacterial oxidation of S2− and constitute an essential substrate for both aerobic and anaerobic microbial metabolism, for example during S0 metabolism. In fact, it has been proposed that polysulfides and polysulfanes represent a critical part of the sulfur flux in ecosystems [44].
Polysulfanes are organic RSS with the general formula RSnH (R ≠ H, n ≥ 2). Polysulfanes contain S2− and are very reactive with proteins and enzymes that contain cysteine; this characteristic possibly turns them into very versatile signaling agents [90].
The long-chain polysulfanes are characteristic molecules of the Allium species, with diallyl sulfanes in garlic and dipropyl sulfanes in onions [91,92]. The molecules frequently found in these species are allin (S-allyl-l-cysteine sulfoxide), diallyl trisulfane (DATS), diallyl tetrasulfane (DATTS), diallyl-pentasulfane (DAPS) or diallyl-hexasulfane, dimethyl-pentasulfane (DMPES), dipropyltrisulfane (DPTS), and dipropyl tetrasulfane (DPTTS), among others [90,91].
The compounds found in plants of the Allium genus have traditionally been used as plant protection products for both humans and crops [12]. This characteristic is believed to be related to the presence of polysulfides and polysulfanes in large quantities, 1.4% of fresh weight [93]. The reduced forms of polysulfanes can mitigate the impact of ROS such as superoxide and bind metal ions, decreasing oxidative stress in proteins and cell membranes [90]. The bioactivity of polysulfanes is enhanced by increasing the number of S atoms in the central functional groups of the molecule [94]. Pluth et al. [95] present in their review a large number of natural products that provide polysulfide-, polysulfane-, and H2S-releasing moieties.
The IUPAC [89] defines polysulfanes as having a chain > 2 of unbranched S atoms terminating in H:HSnH, however, this manuscript has utilized the definition of Kharma et al. [72].
4. Conclusions
The use of elemental sulfur (S0) as a sulfur source for plants has several advantages over the use of sulfate fertilizers. The long residence time in the soil, the activation of the soil microbiome, and the transformation of S0 into volatile sulfur species and reactive sulfur species that will induce higher tolerance to stress in plants were mentioned.
The process followed by sulfur was described, beginning with the application of S0 to the soil or foliage until it is transformed into sulfate, which is incorporated into the metabolism of sulfur in the plant through the primary and secondary pathways. Again, the application of S0 is associated with a great diversity of biomolecules that have a beneficial impact for plants, compared to the use of sulfur as sulfate.
The primary sulfate assimilation pathway was described as a process that gives rise to a great diversity of sulfur compounds, including H2S, polysulfides, and polysulfanes, which increase the nutritional quality of plants and increase tolerance to biotic and abiotic stresses.
The sulfur nutrition of plants, especially using S0 to cover all or part of the sulfur needs of both the soil and plants, should be explored with a higher intensity as a sustainable technique for the management and care of crops.
Author Contributions
Conceptualization, A.B.-M.; All authors were responsible for processing and organizing information; S.G.-M., A.J.-M., L.O.F.L., and A.B.-M. were responsible for manuscript drafting; All authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.De Kok L.J., Castro A., Durenkamp M., Koralewska A., Posthumus F.S., Stuiver C.E.E., Yang L., Stulen I. Pathways of plant sulfur uptake and metabolism—An overview. In: Schnug E., de Kok L.J., editors. Proceedings of the 1st Sino-German Workshop on Aspects of Sulfur Nutrition of Plants; Shenyang, China. 23–27 May 2004; Braunschweig, Germany: FAL Agricultural Research; 2005. pp. 5–13. [Google Scholar]
- 2.Huxtable R.J. Biochemistry of Sulfur. Springer; Boston, MA, USA: 1986. [Google Scholar]
- 3.Rendig V.V., Oputa C., McComb E.A. Effects of sulfur deficiency on non-protein nitrogen, soluble sugars, and N/S ratios in young corn (Zea mays L.) plants. Plant Soil. 1976;44:423–437. doi: 10.1007/BF00015893. [DOI] [Google Scholar]
- 4.Reuveny Z., Dougall D.K., Trinity P.M. Regulatory coupling of nitrate and sulfate assimilation pathways in cultured tobacco cells. Proc. Natl. Acad. Sci. USA. 1980;77:6670–6672. doi: 10.1073/pnas.77.11.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rennenberg H. The fate of excess sulfur in higher plants. Annu. Rev. Plant Physiol. 1984;35:121–153. doi: 10.1146/annurev.pp.35.060184.001005. [DOI] [Google Scholar]
- 6.Andreae M.O. Ocean-atmosphere interactions in the global biogeochemical sulfur cycle. Mar. Chem. 1990;30:1–29. doi: 10.1016/0304-4203(90)90059-L. [DOI] [Google Scholar]
- 7.Tolocka M.P., Turpin B. Contribution of organosulfur compounds to organic aerosol mass. Environ. Sci. Technol. 2012;46:7978–7983. doi: 10.1021/es300651v. [DOI] [PubMed] [Google Scholar]
- 8.Mikkelsen R., Norton R. Soil and fertilizer sulfur. Better Crop. 2013;97:7–9. [Google Scholar]
- 9.Chao T.T., Harward M.E., Fang S.C. Cationic effects on sulfate adsorption by soils. Soil Sci. Soc. Am. J. 1963;27:35–38. doi: 10.2136/sssaj1963.03615995002700010015x. [DOI] [Google Scholar]
- 10.Chao T.T., Harward M.E., Fang S.C. Adsorption and desorption phenomena of sulfate ions in soils. Soil Sci. Soc. Am. J. 1962;26:234–237. doi: 10.2136/sssaj1962.03615995002600030014x. [DOI] [Google Scholar]
- 11.Przygocka-Cyna K., Biber M., Przygocka-Cyna K., Grzebisz W., Pluta M., Grzebisz W. Mineral density of onion bulbs as affected by fertilizers based on elemental sulfur. J. Elem. 2016;21:485–499. doi: 10.5601/jelem.2015.20.2.939. [DOI] [Google Scholar]
- 12.González-Morales S., Pérez-Labrada F., García-Enciso E.L., Leija-Martínez P., Medrano-Macías J., Dávila-Rangel I.E., Juárez-Maldonado A., Rivas-Martínez E.N., Benavides-Mendoza A. Selenium and sulfur to produce Allium functional crops. Molecules. 2017;22:558. doi: 10.3390/molecules22040558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tea I., Genter T., Naulet N., Boyer V., Lummerzheim M., Kleiber D. Effect of foliar sulfur and nitrogen fertilization on wheat storage protein composition and dough mixing properties. Cereal Chem. J. 2004;81:759–766. doi: 10.1094/CCHEM.2004.81.6.759. [DOI] [Google Scholar]
- 14.TSI Sulphur Fertilizer Types. [(accessed on 5 May 2019)]; Available online: https://www.sulphurinstitute.org/fertilizer/sulphate.cfm.
- 15.Johnson D.W., Cole D.W. Anion mobility in soils: Relevance to nutrient transport from forest ecosystems. Environ. Int. 1980;3:79–90. doi: 10.1016/0160-4120(80)90040-9. [DOI] [Google Scholar]
- 16.Hutt L.P. Taxonomy, Physiology and Biochemistry of the Sulfur Bacteria. Plymouth University; Plymouth, UK: 2017. [Google Scholar]
- 17.Montesinos-Pereira D., de la Torre-González A., Blasco B., Ruiz J.M. Hydrogen sulphide increase the tolerance to alkalinity stress in cabbage plants (Brassica oleracea L. ’Bronco’) Sci. Hortic. (Amsterdam) 2018;235:349–356. doi: 10.1016/j.scienta.2018.03.021. [DOI] [Google Scholar]
- 18.Steiger A.K., Zhao Y., Pluth M.D. Emerging roles of carbonyl sulfide in chemical biology: Sulfide transporter or gasotransmitter? Antioxid. Redox Signal. 2018;28:1516–1532. doi: 10.1089/ars.2017.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medrano-Macías J., Leija-Martínez P., González-Morales S., Juárez-Maldonado A., Benavides-Mendoza A. Use of iodine to biofortify and promote growth and stress tolerance in crops. Front. Plant Sci. 2016;7:1146. doi: 10.3389/fpls.2016.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong M.W. Quantum-chemical calculations of sulfur-rich compounds. Top. Curr. Chem. 2003;231:1–29. doi: 10.1007/b13180. [DOI] [Google Scholar]
- 21.Mayer R. Elemental sulfur and its reactions. In: Oae S., editor. Organic Chemistry of Sulfur. Plenum Press; New York, NY, USA: 1977. p. 681. [Google Scholar]
- 22.Reusch W. Nucleophilicity of Sulfur Compounds. [(accessed on 5 May 2019)]; Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Thiols_and_Sulfides/Nucleophilicity_of_Sulfur_Compounds.
- 23.Chapman S.J. Powdered elemental sulphur: Oxidation rate, temperature dependence and modelling. Nutr. Cycl. Agroecosyst. 1996;47:19–28. doi: 10.1007/BF01985715. [DOI] [Google Scholar]
- 24.Lucheta A.R., Lambais M.R. Sulfur in agriculture. Rev. Bras. Ciência do Solo. 2012;36:1369–1379. doi: 10.1590/S0100-06832012000500001. [DOI] [Google Scholar]
- 25.Wörner S., Zecchin S., Dan J., Todorova N.H., Loy A., Conrad R., Pester M. Gypsum amendment to rice paddy soil stimulated bacteria involved in sulfur cycling but largely preserved the phylogenetic composition of the total bacterial community. Environ. Microbiol. Rep. 2016;8:413–423. doi: 10.1111/1758-2229.12413. [DOI] [PubMed] [Google Scholar]
- 26.DeLuca T.H., Skogley E.O., Engel R.E. Band-applied elemental sulfur to enhance the phytoavailability of phosphorus in alkaline calcareous soils. Biol. Fertil. Soils. 1989;7:346–350. doi: 10.1007/BF00257831. [DOI] [Google Scholar]
- 27.Degryse F., Ajiboye B., Baird R., da Silva R.C., McLaughlin M.J. Oxidation of elemental sulfur in granular fertilizers depends on the soil-exposed surface area. Soil Sci. Soc. Am. J. 2016;80:294. doi: 10.2136/sssaj2015.06.0237. [DOI] [Google Scholar]
- 28.Zhao C., Degryse F., Gupta V., McLaughlin M.J. Low effective surface area explains slow oxidation of co-granulated elemental sulfur. Soil Sci. Soc. Am. J. 2016;80:911–918. doi: 10.2136/sssaj2015.09.0337. [DOI] [Google Scholar]
- 29.Lee A., Boswell C.C., Watkinson J.H. Effect of particle size on the oxidation of elemental sulphur, thiobacilli numbers, soil sulphate, and its availability to pasture. N. Z. J. Agric. Res. 1988;31:179–186. doi: 10.1080/00288233.1988.10417943. [DOI] [Google Scholar]
- 30.Haneklaus S., Bloem E., Schnug E. Sulfur interactions in crop ecosystems. In: Hawkesford M.J., De Kok L.J., editors. Sulfur in Plants An Ecological Perspective. Springer; Dordrecht, The Netherlands: 2007. pp. 16–58. [Google Scholar]
- 31.Gupta V.V.S.R., Lawrence J.R., Germida J.J. Impact of elemental sulfur fertilization on agricultural soils. I. Effects on microbial biomass and enzyme activities. Can. J. Soil Sci. 1988;68:463–473. doi: 10.4141/cjss88-045. [DOI] [Google Scholar]
- 32.Cooper R.M., Williams J.S. Elemental sulphur as an induced antifungal substance in plant defence. J. Exp. Bot. 2004;55:1947–1953. doi: 10.1093/jxb/erh179. [DOI] [PubMed] [Google Scholar]
- 33.Bloem E., Haneklaus S., Schnug E. Milestones in plant sulfur research on sulfur-induced-resistance (SIR) in Europe. Front. Plant Sci. 2015;5:779. doi: 10.3389/fpls.2014.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terán G.E., Benavides A., Hernández F., Quero E. New Technologies for Horticultural Crops. In: Struik P.C., Vredenberg W.J., Renkema J.A., Parlevliet J.E., editors. Plant Production on the Threshold of a New Century. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1994. pp. 375–380. [Google Scholar]
- 35.Almutairi K.F., Machado R.M.A., Bryla D.R., Strik B.C. Chemigation with micronized sulfur rapidly reduces soil pH in a new planting of northern highbush blueberry. HortScience. 2017;52:1413–1418. doi: 10.21273/HORTSCI12313-17. [DOI] [Google Scholar]
- 36.Roy Choudhury S., Ghosh M., Mandal A., Chakravorty D., Pal M., Pradhan S., Goswami A. Surface-modified sulfur nanoparticles: An effective antifungal agent against Aspergillus niger and Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2011;90:733–743. doi: 10.1007/s00253-011-3142-5. [DOI] [PubMed] [Google Scholar]
- 37.Rao K.J., Paria S. Use of sulfur nanoparticles as a green pesticide on Fusarium solani and Venturia inaequalis phytopathogens. RSC Adv. 2013;3:10471. doi: 10.1039/c3ra40500a. [DOI] [Google Scholar]
- 38.Juárez-Maldonado A., Ortega-Ortíz H., Morales-Díaz A.B., González-Morales S., Morelos-Moreno Á., Cabrera-De la Fuente M., Sandoval-Rangel A., Cadenas-Pliego G., Benavides-Mendoza A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019;20:162. doi: 10.3390/ijms20010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams J.S., Cooper R.M. Elemental sulphur is produced by diverse plant families as a component of defence against fungal and bacterial pathogens. Physiol. Mol. Plant Pathol. 2003;63:3–16. doi: 10.1016/j.pmpp.2003.08.003. [DOI] [Google Scholar]
- 40.Wainwright M. Sulfur oxidation in soils. Adv. Agron. 1984;37:349–396. doi: 10.1016/S0065-2113(08)60458-7. [DOI] [Google Scholar]
- 41.Zhao C., Gupta V.V.S.R., Degryse F., McLaughlin M.J. Effects of pH and ionic strength on elemental sulphur oxidation in soil. Biol. Fertil. Soils. 2017;53:247–256. doi: 10.1007/s00374-016-1170-0. [DOI] [Google Scholar]
- 42.Kumar U., Panneerselvam P., Gupta V.V.S.R., Manjunath M., Priyadarshinee P., Sahoo A., Dash S.R., Kaviraj M., Annapurna K. Diversity of sulfur-oxidizing and sulfur-reducing microbes in diverse ecosystems. In: Adhya T., Lal B., Mohapatra B., Paul D., Das S., editors. Advances in Soil Microbiology: Recent Trends and Future Prospects. Microorganisms for Sustainability. Volume 3. Springer; Singapore: 2018. pp. 65–89. [Google Scholar]
- 43.Li G., Zhao Y., Li P., Zhang F., Qu P., Li B., Gao Q., Wang S. Antibacterial activities of polythionates enhanced by carbonates. Medchemcomm. 2015;6:1643–1648. doi: 10.1039/C5MD00275C. [DOI] [Google Scholar]
- 44.Findlay A.J. Microbial impact on polysulfide dynamics in the environment. FEMS Microbiol. Lett. 2016;363:fnw103. doi: 10.1093/femsle/fnw103. [DOI] [PubMed] [Google Scholar]
- 45.Kimura H. Signaling molecules: Hydrogen sulfide and polysulfide. Antioxid. Redox Signal. 2015;22:362–376. doi: 10.1089/ars.2014.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calderwood A., Kopriva S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nitric Oxide. 2014;41:72–78. doi: 10.1016/j.niox.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Rennenberg H. Synthesis and emission of hydrogen sulfide by higher plants. In: Saltzman E.S., Cooper W.J., editors. Biogenic Sulfur in the Environment. American Chemical Society; Washington, DC, USA: 1989. pp. 44–57. [Google Scholar]
- 48.Gigolashvili T., Kopriva S. Transporters in plant sulfur metabolism. Front. Plant Sci. 2014;5:442. doi: 10.3389/fpls.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maruyama-Nakashita A. Metabolic changes sustain the plant life in low-sulfur environments. Curr. Opin. Plant Biol. 2017;39:144–151. doi: 10.1016/j.pbi.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 50.Janík R., Bublinec E., Dubová M. Sulphate concentration and S-SO42– flux in soil solutions in the West Carpathians Mountains on an example of submontane beech forest stand. J. For. Sci. 2012;58:35–44. doi: 10.17221/122/2010-JFS. [DOI] [Google Scholar]
- 51.Takahashi H., Kopriva S., Giordano M., Saito K., Hell R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011;62:157–184. doi: 10.1146/annurev-arplant-042110-103921. [DOI] [PubMed] [Google Scholar]
- 52.Kataoka T., Watanabe-Takahashi A., Hayashi N., Ohnishi M., Mimura T., Buchner P., Hawkesford M.J., Yamaya T., Takahashi H. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell. 2004;16:2693–2704. doi: 10.1105/tpc.104.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshimoto N., Inoue E., Saito K., Yamaya T., Takahashi H. Phloem-localizing sulfate transporter, Sultr1;3, mediates re-distribution of sulfur from source to sink organs in Arabidopsis. Plant Physiol. 2003;131:1511–1517. doi: 10.1104/pp.014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirschner S., Woodfield H., Prusko K., Koczor M., Gowik U., Hibberd J.M., Westhoff P. Expression of SULTR2;2, encoding a low-affinity sulphur transporter, in the Arabidopsis bundle sheath and vein cells is mediated by a positive regulator. J. Exp. Bot. 2018;69:4897–4906. doi: 10.1093/jxb/ery263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kataoka T. Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol. 2004;136:4198–4204. doi: 10.1104/pp.104.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao M.J., Wang Z., Wirtz M., Hell R., Oliver D.J., Xiang C. Bin SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana. Plant J. 2013;73:607–616. doi: 10.1111/tpj.12059. [DOI] [PubMed] [Google Scholar]
- 57.Huang Q., Wang M., Xia Z. The SULTR gene family in maize (Zea mays L.): Gene cloning and expression analyses under sulfate starvation and abiotic stress. J. Plant Physiol. 2018;220:24–33. doi: 10.1016/j.jplph.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 58.Gallardo K., Courty P.-E., Le Signor C., Wipf D., Vernoud V. Sulfate transporters in the plants response to drought and salinity: Regulation and possible functions. Front. Plant Sci. 2014;5:580. doi: 10.3389/fpls.2014.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akbudak M.A., Filiz E., Kontbay K. Genome-wide identification and cadmium induced expression profiling of sulfate transporter (SULTR) genes in sorghum (Sorghum bicolor L.) BioMetals. 2018;31:91–105. doi: 10.1007/s10534-017-0071-5. [DOI] [PubMed] [Google Scholar]
- 60.Aghajanzadeh T., Hawkesford M.J., De Kok L.J. Atmospheric H2S and SO2 as sulfur sources for Brassica juncea and Brassica rapa: Regulation of sulfur uptake and assimilation. Environ. Exp. Bot. 2016;124:1–10. doi: 10.1016/j.envexpbot.2015.12.001. [DOI] [Google Scholar]
- 61.Mazid M., Zeba H.K., Quddusi S., Khan T.A., Mohammad F. Significance of sulphur nutrition against metal induced oxidative stress in plants. J. Stress Physiol. Biochem. 2011;7:165–184. [Google Scholar]
- 62.Birke H., De Kok L.J., Wirtz M., Hell R. The Role of compartment-specific cysteine synthesis for sulfur homeostasis during H2S exposure in Arabidopsis. Plant Cell Physiol. 2015;56:358–367. doi: 10.1093/pcp/pcu166. [DOI] [PubMed] [Google Scholar]
- 63.Riemenschneider A., Wegele R., Schmidt A., Papenbrock J. Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS J. 2005;272:1291–1304. doi: 10.1111/j.1742-4658.2005.04567.x. [DOI] [PubMed] [Google Scholar]
- 64.Jing W.W., Li N., Li X.F., Li D.Q., Wang L.L. Exchange fluxes of VOSCs between rice paddy fields and the atmosphere in the oasis of arid area in Xinjiang, China. J. Atmos. Chem. 2018;75:17–32. doi: 10.1007/s10874-017-9360-1. [DOI] [Google Scholar]
- 65.Khan M.N., AlZuaibr F.M., Al-Huqail A.A., Siddiqui M.H., M. Ali H., Al-Muwayhi M.A., Al-Haque H.N. Hydrogen sulfide-mediated activation of O-Acetylserine (Thiol) Lyase and l/d-Cysteine Desulfhydrase enhance dehydration tolerance in Eruca sativa Mill. Int. J. Mol. Sci. 2018;19:3981. doi: 10.3390/ijms19123981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Da-Silva C.J., Modolo L.V. Hydrogen sulfide: A new endogenous player in an old mechanism of plant tolerance to high salinity. Acta Bot. Brasilica. 2018;32:150–160. doi: 10.1590/0102-33062017abb0229. [DOI] [Google Scholar]
- 67.Ausma T., Parmar S., Hawkesford M.J., De Kok L.J. Impact of atmospheric H2S, salinity and anoxia on sulfur metabolism in Zea mays. In: De Kok L.J., Hawkesford M.J., Haneklaus S.H., Schnug E., editors. Sulfur Metabolism in Higher Plants—Fundamental, Environmental and Agricultural Aspects. Springer International Publishing; Cham, Switzerland: 2017. pp. 93–101. [Google Scholar]
- 68.Saito K. Sulfur assimilatory metabolism. The long and smelling road. Plant Physiol. 2004;136:2443–2450. doi: 10.1104/pp.104.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stimler K., Montzka S.A., Berry J.A., Rudich Y., Yakir D. Relationships between carbonyl sulfide (COS) and CO2 during leaf gas exchange. New Phytol. 2010;186:869–878. doi: 10.1111/j.1469-8137.2010.03218.x. [DOI] [PubMed] [Google Scholar]
- 70.Stimler K., Berry J.A., Yakir D. Effects of carbonyl sulfide and carbonic anhydrase on stomatal conductance. Plant Physiol. 2012;158:524–530. doi: 10.1104/pp.111.185926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wirtz M., Droux M. Synthesis of the sulfur amino acids: Cysteine and methionine. Photosynth. Res. 2005;86:345–362. doi: 10.1007/s11120-005-8810-9. [DOI] [PubMed] [Google Scholar]
- 72.Kharma A., Grman M., Misak A., Domínguez-Álvarez E., Nasim M., Ondrias K., Chovanec M., Jacob C. Inorganic polysulfides and related reactive sulfur–selenium species from the perspective of chemistry. Molecules. 2019;24:1359. doi: 10.3390/molecules24071359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bullock H.A., Luo H., Whitman W.B. Evolution of dimethylsulfoniopropionate metabolism in marine phytoplankton and bacteria. Front. Microbiol. 2017;8:637. doi: 10.3389/fmicb.2017.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh V.K., Singh D.K. Pharmacological effects of garlic (Allium sativum L.) Annu. Rev. Biomed. Sci. 2008;10:6–26. doi: 10.5016/1806-8774.2008.v10p6. [DOI] [Google Scholar]
- 75.Yoshimoto N., Yabe A., Sugino Y., Murakami S., Sai-Ngam N., Sumi S.-I., Tsuneyoshi T., Saito K. Garlic γ-glutamyl transpeptidases that catalyze deglutamylation of biosynthetic intermediate of alliin. Front. Plant Sci. 2015;5:758. doi: 10.3389/fpls.2014.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rennenberg H., Herschbach C. A detailed view on sulphur metabolism at the cellular and whole-plant level illustrates challenges in metabolite flux analyses. J. Exp. Bot. 2014;65:5711–5724. doi: 10.1093/jxb/eru315. [DOI] [PubMed] [Google Scholar]
- 77.Kopriva S., Mugford S.G., Baraniecka P., Lee B.-R., Matthewman C.A., Koprivova A. Control of sulfur partitioning between primary and secondary metabolism in Arabidopsis. Front. Plant Sci. 2012;3:163. doi: 10.3389/fpls.2012.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Q., Lancaster J.R. Chemical foundations of hydrogen sulfide biology. Nitric Oxide. 2013;35:21–34. doi: 10.1016/j.niox.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Predmore B.L., Lefer D.J., Gojon G. Hydrogen sulfide in biochemistry and medicine. Antioxid. Redox Signal. 2012;17:119–140. doi: 10.1089/ars.2012.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin Z., Sun L., Yang G., Pei Y. Hydrogen sulfide regulates energy production to delay leaf senescence induced by drought stress in Arabidopsis. Front. Plant Sci. 2018;9:1722. doi: 10.3389/fpls.2018.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo H., Xiao T., Zhou H., Xie Y., Shen W. Hydrogen sulfide: A versatile regulator of environmental stress in plants. Acta Physiol. Plant. 2016;38:16. doi: 10.1007/s11738-015-2038-x. [DOI] [Google Scholar]
- 82.Chen J., Shang Y.-T., Wang W.-H., Chen X.-Y., He E.-M., Zheng H.-L., Shangguan Z. Hydrogen sulfide-mediated polyamines and sugar changes are involved in hydrogen sulfide-induced drought tolerance in Spinacia oleracea seedlings. Front. Plant Sci. 2016;7:1173. doi: 10.3389/fpls.2016.01173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H., Hu L.-Y., Hu K.-D., He Y.-D., Wang S.-H., Luo J.-P. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J. Integr. Plant Biol. 2008;50:1518–1529. doi: 10.1111/j.1744-7909.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- 84.Christou A., Manganaris G.A., Papadopoulos I., Fotopoulos V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013;64:1953–1966. doi: 10.1093/jxb/ert055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang H., Jiao H., Jiang C.-X., Wang S.-H., Wei Z.-J., Luo J.-P., Jones R.L. Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiol. Plant. 2010;32:849–857. doi: 10.1007/s11738-010-0469-y. [DOI] [Google Scholar]
- 86.Corpas F.J., González-Gordo S., Cañas A., Palma J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J. Exp. Bot. 2019 doi: 10.1093/jxb/erz031. [DOI] [PubMed] [Google Scholar]
- 87.Hancock J.T. Hydrogen sulfide and environmental stresses. Environ. Exp. Bot. 2019;161:50–56. doi: 10.1016/j.envexpbot.2018.08.034. [DOI] [Google Scholar]
- 88.Gruhlke M.C.H., Slusarenko A.J. The biology of reactive sulfur species (RSS) Plant Physiol. Biochem. 2012;59:98–107. doi: 10.1016/j.plaphy.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 89.IUPAC Compendium of Chemical Terminology Gold Book. [(accessed on 12 May 2019)]; Release 2.3.3b. Available online: http://goldbook.iupac.org/index.html.
- 90.Schneider T., Ba L.A., Khairan K., Zwergel C., Bach N.D., Bernhardt I., Brandt W., Wessjohann L., Diederich M., Jacob C. Interactions of polysulfanes with components of red blood cells. Medchemcomm. 2011;2:196–200. doi: 10.1039/c0md00203h. [DOI] [Google Scholar]
- 91.Grman M., Nasim M., Leontiev R., Misak A., Jakusova V., Ondrias K., Jacob C. Inorganic reactive sulfur-nitrogen species: Intricate release mechanisms or cacophony in yellow, blue and red? Antioxidants. 2017;6:14. doi: 10.3390/antiox6010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oosthuizen C., Arbach M., Meyer D., Hamilton C., Lall N. Diallyl polysulfides from Allium sativum as immunomodulators, hepatoprotectors, and antimycobacterial agents. J. Med. Food. 2017;20:685–690. doi: 10.1089/jmf.2016.0137. [DOI] [PubMed] [Google Scholar]
- 93.Anwar A., Gould E., Tinson R., Iqbal J., Hamilton C. Redox modulation at work: Natural phytoprotective polysulfanes from alliums based on redox-active sulfur. Curr. Pharmacol. Rep. 2018;4:397–407. doi: 10.1007/s40495-018-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Gara E.A., Hill D.J., Maslin D.J. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl. Environ. Microbiol. 2000;66:2269–2273. doi: 10.1128/AEM.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pluth M.D., Bailey T.S., Hammers M.D., Hartle M.D., Henthorn H.A., Steiger A.K. Natural products containing hydrogen sulfide releasing moieties. Synlett. 2015;26:2633–2643. doi: 10.1055/s-0035-1560638. [DOI] [Google Scholar]