Abstract
Background and Objectives: Teeth decay and plaque are complicated problems created by oral pathogens. Tecoma stans (L.) and Cassia javanica (L.) are two ornamental evergreen plants widely distributed in Egypt. These plants are traditionally used for oral hygienic purposes. This study aims to elucidate the volatile oil constituents obtained from the flowers of these plants and evaluate the antimicrobial activity of these volatile oils against specific oral pathogens in comparison to chlorhexidine. Materials and Methods: The flowers obtained from both plants were extracted by n-hexane. GC-MS spectrometry was used to identify the constituents. Minimum inhibitory concentrations (MICs) were measured using tetrazolium salt (2,3-bis[2-methyloxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide) (XTT). Results: GC-MS analysis revealed the presence of 32 and 29 compounds, representing 100% of the volatile constituents of Tecoma stans and Cassia javanica, respectively. The GC-MS analysis showed more than 60% of the volatile oil constituents are represented in both plants with different proportions. Chlorhexidine exerted stronger activity than tested plants against all microorganisms. Cassia javanica flower extract was more active against all tested microorganisms than Tecoma stans. Of note was the effect on Streptococcus mutans, which was inhibited by 100% at 12.5 and 25 µg/mL of Cassia javanica and Tecoma stans, respectively. The growth of Lactobacillus acidophilus was also completely inhibited by 25 µg/mL of the Cassia javanica extract. MIC90 and MIC were also calculated, which revealed the superiority of Cassia javanica over Tecoma stans against all tested oral pathogens. Conclusion: Cassia javanica flower volatile oils showed a potential anti-oral pathogen activity at relatively low concentrations. Also, Cassia javanica and Tecoma stans demonstrated a strong activity against tooth decay’s notorious bacteria Streptococcus mutans. Both plants can be potential substituents to chlorhexidine. Formulating the constituents of these plants in toothpastes and mouthwashes as anti-oral pathogen preparations can be an interesting future plan.
Keywords: Cassia javanica, Tecoma stans, volatile oils, chlorhexidine, Streptococcus mutans, oral pathogens, antimicrobial activity
1. Introduction
Microorganisms are considered a major cause of disease induction in the world, in which the disease arises when a specific microorganism fits to the site where it can grow, colonize, and release its toxins [1]. Oral pathogens play a major role in the formation of dental plaque and, subsequently, dental caries and general periodontal diseases [2]. Health problems related to oral pathogens may extend outside the oral cavity to reach the cardiovascular system [3]. Also, oral care reduces the currency of influenza [4], pneumonia, and the mortality ratio in elderly individuals [5]. Plaque growth is regularly initiated due to bacterial colonization and adherence to the solid substratum, forming a microbial biofilm [6]. Bacterial colonies have the ability to ferment carbohydrates to produce acids. The produced acids are responsible for demineralization and cavities initiation in teeth [7,8]. Natural products, particularly plant-derived products, have been used for prophylactic and/or treatment of dental caries and plaques. For instance, the toothbrush tree “Salvadora persica” or Arak is the most well-known tree used in this regard [9,10,11]. Also, numerous plant extracts and their constituents have been examined for their effects on dental caries by inhibiting bacterial growth [12,13,14,15,16]. Chlorhexidine is highly effective against a wide range of oral pathogens such as Lactobacillus acidophilus and Streptococcus mutans. The long-term usage of chlorhexidine is accompanied by several side effects, such as tanning the tooth with yellowish brown discoloration. In addition, irritation and/or inflammation may arise due to chlorhexidine mouthwashes, which may be exaggerated to damage of mouth mucosa [17,18].
Cassia javanica L. is an ornamental tree that belongs to the Fabaceae family and produces a mass of beautiful pink flowers, so it is commonly known as the pink shower [19]. The plant is traditionally used in the treatment of fever, cold, gastric pain, constipation, diabetes mellitus, malaria [20], and also used for oral hygiene to stop bad breath [21]. There are various phytochemical classes in the plant, such as anthraquinone glycosides [21], flavonoids [22], alkaloids, sterols, tannins, saponins [20], and volatile oil [23]. Cassia javanica has many pharmacological activities, such as antimicrobial [23], anti-herpes simplex virus type 2 (anti-HSV-2) [24], antioxidant, anti-inflammatory [25], and antidiabetic [26] activities. In addition, its honey has antifungal and anticancer activities [27].
Tecoma stans (L.) (Family: Bignoniaceae) is an ornamental tree with gorgeous yellow bell-shaped flowers. Its fast growth and propagation rates cause it to be regarded as an invasive tree like those in South Africa and Namibia [28]. It is used in traditional medicine as a remedy for diabetes mellitus, digestive problems, stomach pain, intestinal worms, and snake bite [29]. Tecoma stans contains alkaloids, flavonoids, tannins, terpenes, phytosterols [30], and irridoids [31]. Many biological activities were confirmed, especially those based on folk use as a hypoglycemic and hypolipidemic agent for diabetes mellitus [32]. In addition, Tecoma stans has wound-healing [33], antioxidant, antimicrobial [34], analgesic, anti-inflammatory [35], nephroprotective [36], and cardioprotective activities [29].
Cassia javanica (L.) and Tecoma stans (L.) Juss. are common trees in Egyptian gardens and are covered by numerous flowers in the blooming season. The availability of these trees and their enormous flower production gave the idea of finding an applicable biological activity. Accordingly, we aimed here to identify the flower volatile oil constituents obtained from these plants. In addition, the antimicrobial activity of the constituents of these plants against oral pathogens was evaluated, considering that these plants are used in folk medicine for oral hygiene.
2. Materials and Methods
2.1. Plant Collection and Method of Extraction
Cassia javanica (L.) and Tecoma stans (L.) Juss. flowers were collected in September 2018 from different gardens in Cairo, Egypt. The plants were kindly identified as Cassia javanica (L.) and Tecoma stans (L.) Juss. flowers by Dr. Mohamed El Gebali and plant engineer Therese Labib (Herbarium Section, Al-Orman Garden, Giza, Egypt) before shade drying in open air at room temperature. Accurately, 500 g of the grinded dried flowers from both plants were sonicated with n-hexane solvent using a sonicator for 24 h (ultrasonic, Branson 5800). The n-hexane extracts were vacuum dried under reduced pressure using a Rotavapor (BUCHI R-300) and the dried residues were stored in at −20 °C.
2.2. Gas Chromatography-Mass Spectroscopy (GC-MS) Analysis
The GC-MS analysis was performed on a Shimadzu GCMS-QP 2010 (Koyoto, Japan) equipped with an Rtx-5MS capillary column (30 m × 0.25 mm inner diameter (i.d). × 0.25 µm film thickness) (Restek, Bellefonte, PA, USA). The oven temperature was kept at 50 °C for 3 min (isothermal), programmed to 300 °C at 5 °C/min, and kept constant at 300 °C for 10 min (isothermal); the injector temperature was 280 °C. Helium was used as a carrier gas with a constant flow rate set at 1.37 mL/min. Diluted samples (1% v/v) were injected with a split ratio of 15:1 and the injected volume was 1 μL. The MS operating parameters were as follows: interface temperature, 280 °C; ion source temperature, 220 °C; EI mode, 70 eV; scan range, 35–500 amu. For compound identification, components were identified based on their retention indices and by matching their mass spectra with the National Institute of Standards and Technology (NIST-11), the Wiley library database, as well as published data in the literature (Adams 2004). Retention indices (RI) were calculated relative to a homologous series of n-alkanes (C8-C40) injected under the same conditions.
2.3. Identifications of the Cassia javanica and Tecoma stans Constituents
The flower constituents extracted by n-hexane from both plants were identified based on the experimental retention index (RI) calculated in comparison to a series of n-alkenes (C8 to C40) and retention indices obtained from the literature under similar GC experimental conditions. The identifications of the compounds were carried based on retention time, mass fragmentation pattern, and according to mass spectral libraries (NIST-11 and Wiley library database). Relative percentages of the constituents were calculated from the area under the peaks obtained from GC chromatograms.
2.4. Microorganisms
Oral pathogens; Streptococcus mutans ATCC 35668, Porphyromonas gingivalis ATCC 33277, Lactobacillus acidophilus ATCC 4356, and Candida albicans ATCC90028 obtained from the American Type Culture Collection were used in the antimicrobial assay.
2.5. Determination of the Minimal Inhibitory Concentration (MIC) Using XTT Assay
The MICs were determined using the micro-dilution method described by Tunney et al., 2004 [37]. The microbial inoculates were prepared, and the suspensions were adjusted to 106 CFU/mL. The n-hexane extracts and the standard drug (chlorhexidine) were prepared in dimethyl sulfoxide (DMSO). Subsequently, twofold dilutions were performed in a 96 well plate. Each well of the microplate included 40 μL of the growth medium brain heart infusion (BHI), 10 μL of inoculum, and 50 μL of the diluted extracts and standard compounds. DMSO was used as a negative control. The plates were incubated at 37 °C for 24 h. After that, 40 μL of tetrazolium salt (2,3-bis[2-methyloxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide) (XTT) was added to the wells. The plates were incubated in the dark place for 1 h at 37 °C, after which colorimetric change in the XTT reduction assay was measured using a microtiter plate reader (Tecan Sunrise absorbance reader; Tecan UK, Reading, United Kingdom) at 492 nm. The percentage of inhibition was calculated using the following equation:
Standard error means were calculated from three independent assays.
3. Results
The flower part of Cassia javanica and Tecoma stans was extracted by n-hexane to measure the antimicrobial effect of these plants against common oral pathogens in accordance with their traditional uses [38,39].
The constituents of n-hexane extracts obtained from both plants were investigated by GC and GC-MS spectrometry. The compounds identities were based on the retention index (RI), retention time (RT), and mass fragments obtained from the GC-MS chromatogram (GC chromatograms are shown in the Supplementary Materials), which were compared to the literature data as well as data from NIST-11 and the Wiley library database. In accordance with the aforementioned data, the results obtained from the GC-MS analysis ensured that the flower part of these plants contains 20 similar compounds. However, these compounds were represented in these plants by different concentrations (Table 1). For instance, decane and 2-methyldecane were represented by 6.06 and 2.57% in Tecoma stans and by 0.89 and 1.10% in Cassia javanica, respectively. Furthermore, some of the constituents were only represented in one of the plants while they were absent in the other. For example, p-cymen-8-ol was represented only in Tecoma stans with 23.73% while α-terpineol and 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl) ester were represented in Cassia javanica with 29.32 and 6.71%, respectively. Table 1 shows the results for the volatile oil analysis of Tecoma stans and Cassia javanica, which indicate that monoterpene hydrocarbons and monoterpene alcohols represent the majority of the volatile constituents in these plants.
Table 1.
Chemical constituents of the n-hexane extracts of Cassia javanica and Tecoma stans flowers.
| No | Compound Name | RT | RI exp | RI rep | %R Tecoma stans | %R Cassia javanica |
|---|---|---|---|---|---|---|
| 1 | Decane | 9.161 | 989 | 891 | 6.06 | 0.89 |
| 2 | 3-Carene | 9.870 | 1012 | 1012 | 1.21 | 0.36 |
| 3 | m-Cymene | 10.160 | 1021 | 1021 | 0.62 | - |
| 4 | (Z)-β-Ocimene | 10.890 | 1044 | 1043 | 0.93 | - |
| 5 | Heptyl acetate | 10.980 | 1047 | 1047 | 0.92 | - |
| 6 | (Z)-β-Ocimene | 11.075 | 1050 | 1050 | 0.99 | 0.35 |
| 7 | 2-Methyldecane | 11.190 | 1053 | 1051 | 2.57 | 1.10 |
| 8 | γ-Terpinene | 11.395 | 1060 | 1059 | 0.96 | 0.45 |
| 9 | 2,9-Dimethyldecane | 12.350 | 1090 | 1086 | 15.87 | 11.46 |
| 10 | Nonanal | 12.875 | 1106 | 1107 | 1.62 | - |
| 11 | Myrcenol | 13.180 | 1116 | 1116 | 1.98 | - |
| 12 | Fenchol | 13.205 | 1117 | 1117 | - | 0.98 |
| 13 | trans-Pinene hydrate | 13.420 | 1124 | 1123 | 1.33 | 0.80 |
| 14 | neo-Isopulegol | 14.025 | 1143 | 1143 | 1.37 | - |
| 15 | trans-Verbenol | 14.070 | 1144 | 1144 | 1.41 | 1.73 |
| 16 | Z-Tagetone | 14.190 | 1148 | 1147 | 1.64 | 1.33 |
| 17 | Citronellal | 14.325 | 1152 | 1153 | 5.09 | 3.79 |
| 18 | β-Pinene oxide | 14.525 | 1159 | 1158 | 2.26 | 1.28 |
| 19 | Artemisyl acetate | 15.035 | 1175 | 1174 | - | 0.90 |
| 20 | Terpinen-4-ol | 15.176 | 1179 | 1177 | - | 0.50 |
| 21 | p-Cymen-8-ol | 15.455 | 1188 | 1188 | 23.73 | - |
| 22 | α-Terpineol | 15.490 | 1189 | 1189 | - | 29.32 |
| 23 | cis-Dihydrocarvone | 15.845 | 1200 | 1198 | 3.60 | 3.11 |
| 24 | Pulegone | 16.600 | 1226 | 1227 | 0.75 | - |
| 25 | Citronellol | 16.634 | 1228 | 1228 | - | 0.59 |
| 26 | Cumin aldehyde | 16.985 | 1240 | 1239 | 0.69 | - |
| 27 | Neral | 17.045 | 1242 | 1242 | 0.67 | - |
| 28 | Carvone | 17.180 | 1247 | 1248 | 0.88 | 0.55 |
| 29 | Piperitone | 17.320 | 1252 | 1253 | 2.77 | 2.42 |
| 30 | Methyl citronellate | 17.510 | 1258 | 1258 | 1.59 | 1.39 |
| 31 | Linalyl acetate | 17.580 | 1261 | 1261 | 2.95 | 2.58 |
| 32 | Geranial | 17.795 | 1268 | 1268 | - | 0.27 |
| 33 | Limonen-10-ol | 18.375 | 1288 | 1289 | 0.55 | 0.60 |
| 34 | Palmitic acid | 34.465 | 1951 | 1953 | 1.32 | - |
| 35 | 4-Hexadecyl hexanoate | 42.185 | 2367 | 2362 | 0.57 | |
| 36 | Pentacosane | 44.860 | 2533 | 2521 | 1.78 | |
| 37 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | 44.895 | 2535 | 2521 | - | 6.71 |
| 38 | Heptacosane | 46.980 | 2669 | 2666 | 6.44 | 6.80 |
| 39 | Octacosane | 48.450 | 2764 | 2738 | - | 1.34 |
| 40 | Squalene | 49.850 | 2854 | 2831 | 4.76 | 10.51 |
| 41 | Stigmastan-3,5-diene | 52.560 | 3028 | 3040 | 0.67 | 7.33 |
| Total percentage | 100 | 100 | ||||
| Monoterpene hydrocarbons | 29.12 | 14.61 | ||||
| Monoterpene alcohols | 30.37 | 34.02 | ||||
| Esters | 5.46 | 12.15 | ||||
| Aldehydes | 8.07 | 4.06 | ||||
| Ketones | 9.64 | 7.41 | ||||
| Long chain hydrocarbons | 13.65 | 25.98 | ||||
Exp Experimental retention index (RI) using a series of n-alkanes (C10–C40) calculated under identical experimental conditions. Rep Reported retention index according to the National Institute of Standards and Technology (NIST) library and published literature data and calculated under identical experimental conditions as the references [40,41,42,43,44,45,46,47,48,49]. %R The relative percentage of individual volatile components according to the peak area and calculated from the GC (Gas chromatography) chromatogram; RT = retention time.
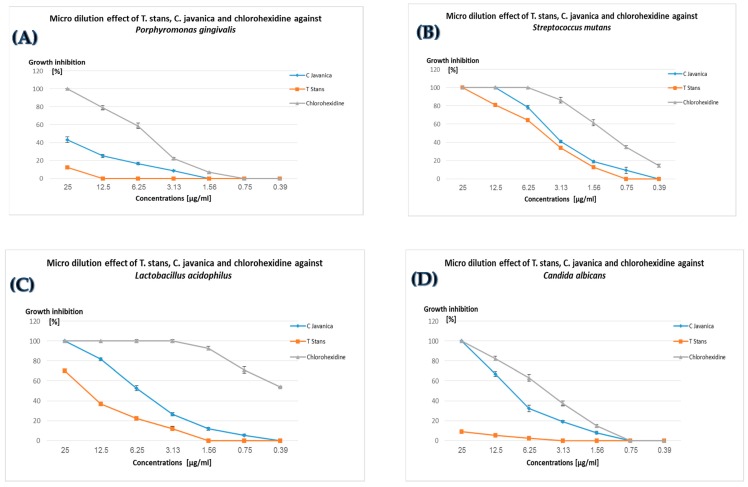
The antimicrobial assay was conducted by the rapid determination method of tetrazolium salt (2,3-bis[2-methyloxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide) (XTT) color reduction percentage, which is inversely proportional to the bacterial cell viability [37]. The results shown in Figure 1A–D for the n-hexane extracts of Cassia javanica and Tecoma stans in addition to those of the standard oral disinfectant “chlorhexidine” [50] against oral microbial pathogens indicate that the n-hexane extract of Cassia javanica was more active against all pathogens compared to the extract of Tecoma stans. For instance, Cassia javanica at a concentration of 3.13 µg/mL inhibit the growth of Porphyromonas gingivalis, Lactobacillus acidophilus, and Candida ablicans by 8.75, 26.47, and 19.16%, respectively, as compared to 0, 12, and 0% inhibition by Tecoma stans extract at the same concentration. The results in Figure 1 also reveal that the standard chlorhexidine was 2 to 10 times more active than n-hexane extracts from both plants, which were exaggerated—especially in the case of Porphyromonas gingivalis, which was inhibited by 12.7, 43.25, and 100% at 25 µg/mL of Tecoma stans, Cassia javanica, and chlorhexidine, respectively. In addition, the standard chlorhexidine inhibited the growth of Lactobacillus acidophilus at 0.39 µg/mL by 54% compared to 0% inhibition by Tecoma stans and Cassia javanica at the same concentration.
Figure 1.
Percentage of microbial growth inhibition at different concentrations of Cassia javanica, Tecoma stans, and chlorhexidine against Porphyromonas gingivalis (A), Streptococcus mutans (B), Lactobacillus acidophilus (C), and Candida albicans (D). The results are expressed as the mean ± standard deviation (SD) obtained from three independent experiments.
The anti-oral pathogenic effect of n-hexane extracts from Cassia javanica and Tecoma stans in addition to the positive control, chlorhexidine, was displayed in the form of MIC and MIC90. These represent the minimal concentration required to kill 100 and 90% of the microorganism, respectively (Table 2). The results summarized in Table 2 ensure the superiority of Cassia javanica over Tecoma stans n-hexane extract in the activity against tested oral pathogens. The MIC and MIC90 values were not recorded for Tecoma stans against the growth of Porphyromonas gingivalis and Candida albicans at the maximal experimental concentration (100 µg/mL).
Table 2.
The minimal inhibitory concentrations required to kill 50, 90, and 100% of the oral pathogens from Cassia javanica, Tecoma stans, and chlorhexidine.
| Pathogen | Cassia javanica | Tecoma stans | Chlorhexidine | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MIC | MIC50 | MIC90 | MIC | MIC50 | MIC90 | MIC | |
| Porphyromonas gingivalis | 30.13 ± 1.48 | 79.03 ± 0.78 | 100 | 96.50 ± 2.2 | nd | nd | 5.51 ± 0.17 | 16.25 ± 0.57 | 25 |
| Streptococcus mutans | 3.89 ± 0.07 | 9.57 ± 0.21 | 12.5 | 4.77 ± 0.02 | 18.49 ± 0.39 | 25 | 0.31 ± 0.01 | 0.99 ± 0.11 | 1.56 |
| Lactobacillus acidophilus | 5.93 ± 0.22 | 21.2 ± 0.23 | 25 | 17.39 ± 0.33 | 47.6 ± 1.8 | 100 | 0.36 ± 0.01 | 1.5 ± 0.03 | 3.13 |
| Candida albicans | 9.43 ± 0.41 | 17.5 ± 0.23 | 25 | nd | nd | nd | 1.16 ± 0.03 | 4.47 ± 0.18 | 6.25 |
nd = MIC and MIC90 were not detected until 100 µg/mL of the extract was employed; Minimum inhibitory concentration (MIC).
4. Discussion
Natural products, particularly plant-derived secondary metabolites, have contributed to health promotions and enhanced humans’ life quality. These secondary metabolites include essential oils, different types of glycosides, alkaloids, and so forth. Regarding essential oils, they are famous antimicrobial agents against a broad spectrum of bacterial and fungal strains [51,52].
Exploring nature to find an effective treatment for oral health with minimal or no side effects caused by synthetic compounds is a great challenge. Chlorhexidine has many local side effects, the most common one being brown discoloration of the teeth, some restorative materials, the oral mucosa, and notably the tongue dorsum, which is an intraoral cosmetic problem [17,18,53].
The present work represents the volatile constituents of Cassia javanica and Tecoma stans extracted by n-hexane solvent. Cassia javanica and Tecoma stans are traditionally used for oral hygienic purposes due to their activity as antimicrobial agents [38,39]. The spectrometric analysis of Cassia javanica and Tecoma stans n-hexane extracts revealed that more than 60% of the volatile oil constituents of these plants are similar. Specifically, the identities of 20 compounds were quite similar out of the 29 and 32 identified compounds in Cassia javanica and Tecoma stans, respectively (Table 1). Meanwhile, large differences were recorded in the proportions of these compounds in the plants, as apparent in Table 1 for the proportions of decane, 3-carene, squalene, and stigmastan-3,5-diene.
The determination of antimicrobial activity revealed that the standard oral disinfectant chlorhexidine was more active against all oral pathogens than the n-hexane extract of tested plants. Also, the antimicrobial assay ensured that Cassia javanica was more active than Tecoma stans against all tested oral pathogens, which may be related to the presence of the monoterpene alcohols α-terpineol and 1,2-benzenedicarboxylic acid, mono(2-ethylhexyl) ester in relatively high concentrations in Cassia javanica. 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester, which was identified only in Cassia javanica (6.71%), was reported in a similar concentration (6.55%) in Muscodor tigerii, which inhibits the growth of a broad spectrum of bacterial and fungal microorganisms [54]. Furthermore, α-terpineol, which was reported for its strong activity as an antimicrobial agent against oral pathogens and used as a component in toothpastes [55], was identified as a major compound in Cassia javanica (29.32%) while it was absent in Tecoma stans. Furthermore, Cassia javanica n-hexane extract at 12.5 µg/mL inhibits the growth of Streptococcus mutans, which plays a major role in dental decay, by 100% [56]. Also, a 100% inhibition in the growth of Candida albicans and Lactobacillus acidophilus was achieved by 25 µg/mL of Cassia javanica n-hexane extract. The potentiated activity for Tecoma stans was observed against Streptococcus mutans (100% inhibition) and Lactobacillus acidophilus (70% inhibition) at a concentration of 25µg/mL. This potential antimicrobial activity of Tecoma stans may be attributed to the high proportion of monoterpene alcohols (30.37%), particularly p-cymen-8-ol, which represents 23.73% of the total Tecoma stans volatile constituents. It is worth mentioning that p-cymen-8-ol is represented in the anti-oral pathogenic plant Cyperus articulates [57].
5. Conclusions
The phytochemical constituents of n-hexane extracts obtained from flowers of Cassia javanica and Tecoma stans indicate major similarities in identity of these plant volatile oils, including more than 60% of the constituents. Meanwhile, major differences in the proportion of these constituents were recorded. The antimicrobial record in this study ensured that Cassia javanica flower n-hexane extract might play a major role in the antimicrobial activity of this plant against oral pathogens, as also indicated by its use in folk medicine for oral hygiene. Tecoma stans flower extract exerted some activity, especially against tooth decay’s notorious bacteria Streptococcus mutans. Although Cassia javanica and Tecoma stans were less effective against oral pathogens than chlorhexidine, these plants might be safer for human use because they are naturally derived sources. Accordingly, these plants can be potential substitutes for chlorhexidine. Our future plan is to formulate the constituents of these plants in toothpastes and mouthwashes for anti-oral pathogens.
Acknowledgments
The authors greatly appreciate the facilities kindly provided by the Pharmacognosy Department, Faculty of Pharmacy, Al-Azhar University, with special acknowledgement of Lutfy Diab (Dean of the College) and Atef Ahmed El-hila (Head of the Department).
Supplementary Materials
The following are available online at https://www.mdpi.com/1010-660X/55/6/301/s1, Figure S1: Gas chromatography chromatogram of Tecoma stans flowers volatile oils, Figure S2: Gas chromatography chromatogram of Cassia javanica flowers volatile oils.
Author Contributions
Conceptualization, M.M.H. and H.A.M.; methodology, M.M.H., H.A.M., and M.M.A.-A.; software, H.A.M.; validation, H.A.M. and M.M.A.-A.; formal analysis, M.M.H. and H.A.M.; investigation, M.M.H. and M.M.A.-A.; resources, M.M.H., H.A.M., and M.M.A.-A.; data curation, H.A.M.; writing—original draft preparation, H.A.M.; writing—review and editing, M.M.H., H.A.M., and M.M.A.-A.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Kleinberg I. A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: An alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit. Rev. Oral Biol. Med. 2002;13:108–125. doi: 10.1177/154411130201300202. [DOI] [PubMed] [Google Scholar]
- 2.Koo H., Gomes B., Rosalen P.L., Ambrosano G.M.B., Park Y.K., Cury J.A. In vitro antimicrobial activity of propolis and Arnica montana against oral pathogens. Arch. Oral Biol. 2000;45:141–148. doi: 10.1016/S0003-9969(99)00117-X. [DOI] [PubMed] [Google Scholar]
- 3.Meyer D.H., Fives-Taylor P.M. Oral pathogens: From dental plaque to cardiac disease. Curr. Opin. Microbiol. 1998;1:88–95. doi: 10.1016/S1369-5274(98)80147-1. [DOI] [PubMed] [Google Scholar]
- 4.Abe S., Ishihara K., Adachi M., Sasaki H., Tanaka K., Okuda K. Professional oral care reduces influenza infection in elderly. Arch. Gerontol. Geriatr. 2006;43:157–164. doi: 10.1016/j.archger.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Ichinose-Tsuno A., Aoki A., Takeuchi Y., Kirikae T., Shimbo T., Yoshino F., Maruoka Y., Itoh T., Ishikawa I., Izumi Y. Antimicrobial photodynamic therapy suppresses dental plaque formation in healthy adults: A randomized controlled clinical trial. BMC Oral Health. 2014;14:152. doi: 10.1186/1472-6831-14-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sbordone L., Bortolaia C. Oral microbial biofilms and plaque-related diseases: Microbial communities and their role in the shift from oral health to disease. Clin. Oral Investig. 2003;7:181–188. doi: 10.1007/s00784-003-0236-1. [DOI] [PubMed] [Google Scholar]
- 7.Miller W.D. Microorganisms of the Human Mouth. The SS White Dental Mfg; Philadelphia, PA, USA: 1890. [Google Scholar]
- 8.Imfeld T.N. Identification of low caries risk dietary components. Monogr. Oral Sci. 1983;11:1–198. [PubMed] [Google Scholar]
- 9.Akhtar J., Siddique K.M., Bi S., Mujeeb M. A review on phytochemical and pharmacological investigations of miswak (Salvadora persica Linn.) J. Pharm. Bioallied Sci. 2011;3:113. doi: 10.4103/0975-7406.76488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alali F., Al-Lafi T. GC-MS analysis and bioactivity testing of the volatile oil from the leaves of the toothbrush tree Salvadora persica L. Nat. Prod. Res. 2003;17:189–194. doi: 10.1080/1057563021000040790. [DOI] [PubMed] [Google Scholar]
- 11.Halawany H.S. A review on miswak (Salvadora persica) and its effect on various aspects of oral health. Saudi Dent. J. 2012;24:63–69. doi: 10.1016/j.sdentj.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayacibara M.F., Koo H., Rosalen P.L., Duarte S., Franco E.M., Bowen W.H., Ikegaki M., Cury J.A. In vitro and in vivo effects of isolated fractions of Brazilian propolis on caries development. J. Ethnopharmacol. 2005;101:110–115. doi: 10.1016/j.jep.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Chung J.Y., Choo J.H., Lee M.H., Hwang J.K. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine. 2006;13:261–266. doi: 10.1016/j.phymed.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Tichy J., Novak J. Extraction, assay, and analysis of antimicrobials from plants with activity against dental pathogens (Streptococcus sp.) J. Altern. Complement. Med. 1998;4:39–45. doi: 10.1089/acm.1998.4.1-39. [DOI] [PubMed] [Google Scholar]
- 15.Smullen J., Koutsou G.A., Foster H.A., Zumbé A., Storey D.M. The antibacterial activity of plant extracts containing polyphenols against Streptococcus mutans. Caries Res. 2007;41:342–349. doi: 10.1159/000104791. [DOI] [PubMed] [Google Scholar]
- 16.Badria F.A., Zidan O.A. Natural products for dental caries prevention. J. Med. Food. 2004;7:381–384. doi: 10.1089/jmf.2004.7.381. [DOI] [PubMed] [Google Scholar]
- 17.Flötra L., Gjermo P.E.R., Rölla G., Waerhaug J. Side effects of chlorhexidine mouth washes. Eur. J. Oral Sci. 1971;79:119–125. doi: 10.1111/j.1600-0722.1971.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 18.Pemberton M.N., Gibson J. Chlorhexidine and hypersensitivity reactions in dentistry. Br. Dent. J. 2012;213:547. doi: 10.1038/sj.bdj.2012.1086. [DOI] [PubMed] [Google Scholar]
- 19.Murugesan K., Sahadevan R. Optimization of nonedible oil extraction from Cassia javanica seeds. Energy Sources Part A Recover. Util. Environ. Eff. 2017;39:1140–1146. doi: 10.1080/15567036.2017.1299259. [DOI] [Google Scholar]
- 20.Sharma A., Ahmad S., Harikumar S.L. Pharmacognosy, phytochemistry & pharmacology of Cassia javanica Linn.: A Review. Int. J. Pharma Res. Rev. 2014;3:101–105. [Google Scholar]
- 21.Tiwari R.D., Singh J. Anthraquinone rhamnosides from Cassia javanica root bark. Phytochemistry. 1979;18:906. doi: 10.1016/0031-9422(79)80051-5. [DOI] [Google Scholar]
- 22.Tiwari R.D., Yadava O.P. The flavonoids of Cassia javanica flowers. Phytochemistry. 1971;10:2256–2263. doi: 10.1016/S0031-9422(00)97242-X. [DOI] [Google Scholar]
- 23.Bhuvaneswari R., Gobalakrishnan R. Antimicrobial potential and structural elucidation of bioactive compounds from flower extract of Cassia javanica L. Indian J. Nat. Prod. Resour. (IJNPR) 2015;5:34–39. [Google Scholar]
- 24.Cheng H.-Y., Yang C.-M., Lin T.-C., Shieh D.-E., Lin C.-C. ent-Epiafzelechin-(4α→8)-epiafzelechin extracted from Cassia javanica inhibits herpes simplex virus type 2 replication. J. Med. Microbiol. 2006;55:201–206. doi: 10.1099/jmm.0.46110-0. [DOI] [PubMed] [Google Scholar]
- 25.Mehta J.P., Parmar P.H., Vadia S.H., Patel M.K., Tripathi C.B. In-vitro antioxidant and in-vivo anti-inflammatory activities of aerial parts of Cassia species. Arab. J. Chem. 2017;10:S1654–S1662. doi: 10.1016/j.arabjc.2013.06.010. [DOI] [Google Scholar]
- 26.Sundaramoorthy S., Gunasekaran S., Arunachalam S., Sathiavelu M. A phytopharmacological review on cassia species. J. Pharm. Sci. Res. 2016;8:260. [Google Scholar]
- 27.El-Gendy M.M.A. In vitro, evaluation of medicinal activity of Egyptian honey from different floral sources as anticancer and antimycotic infective agents. J. Microb. Biochem. Technol. 2010;2:1000035. doi: 10.4172/1948-5948.1000035. [DOI] [Google Scholar]
- 28.Cunningham P.L. Tecoma stans a potential invasive alien in Namibia. Dinteria. 2008:33–39. [Google Scholar]
- 29.Ittagi S., Merugumolu V.K., Siddamsetty R.S. Cardioprotective effect of hydroalcoholic extract of Tecoma stans flowers against isoproterenol induced myocardial infarction in rats. Asian Pac. J. Trop. Dis. 2014;4:S378–S384. doi: 10.1016/S2222-1808(14)60474-6. [DOI] [Google Scholar]
- 30.Anburaj G., Marimuthu M., Rajasudha V., Manikandan R. Phytochemical screening and GC-MS analysis of ethanolic extract of Tecoma stans (Family: Bignoniaceae) Yellow Bell Flowers. J. Pharm. Phytochem. 2016;5:172. [Google Scholar]
- 31.Bianco A., Massa M., Oguakwa J.U., Passacantilli P. 5-deoxystansioside, an iridoid glucoside from Tecoma stans. Phytochemistry. 1981;20:1871–1872. doi: 10.1016/0031-9422(81)84024-1. [DOI] [Google Scholar]
- 32.Aguilar-Santamaría L., Ramírez G., Nicasio P., Alegría-Reyes C., Herrera-Arellano A. Antidiabetic activities of Tecoma stans (L.) Juss. ex Kunth. J. Ethnopharmacol. 2009;124:284–288. doi: 10.1016/j.jep.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 33.Kameshwaran S., Senthilkumar R., Thenmozhi S., Dhanalakshmi M. Wound healing potential of ethanolic extract of tecoma stans flowers in rats. Pharmacologia. 2014;5:215–221. doi: 10.5567/pharmacologia.2014.215.221. [DOI] [Google Scholar]
- 34.Rajamurugan R., Thirunavukkarasu C., Sakthivel V., Sivashanmugam M., Raghavan C.M. Phytochemical screening, antioxidant and antimicrobial activities of ethanolic extract of Tecoma stans flowers. Int. J. Pharma Bio Sci. 2013;4:P124–P130. [Google Scholar]
- 35.Kameshwaran S., Suresh V., Arunachalam G., Frank P.R., Manikandan V. Evaluation of antinociceptive and anti-inflammatory potential of flower extract Tecoma stans. Indian J. Pharm. 2012;44:543. doi: 10.4103/0253-7613.99352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raju S., Kavimani S., Reddy K.S., Kumar G.V. Floral extract of Tecoma stans: A potent inhibitor of gentamicin-induced nephrotoxicity in vivo. Asian Pac. J. Trop. Med. 2011;4:680–685. doi: 10.1016/S1995-7645(11)60173-9. [DOI] [PubMed] [Google Scholar]
- 37.Tunney M.M., Ramage G., Field T.R., Moriarty T.F., Storey D.G. Rapid colorimetric assay for antimicrobial susceptibility testing of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2004;48:1879–1881. doi: 10.1128/AAC.48.5.1879-1881.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganesan S. Traditional Oral Care Medicinal Plants Survey of Tamil Nadu. CSIR; New Delhi, India: 2008. pp. 166–172. [Google Scholar]
- 39.Bever B.O. Oral hypoglycaemic plants in West Africa. J. Ethnopharmacol. 1980;2:119–127. doi: 10.1016/0378-8741(80)90005-7. [DOI] [PubMed] [Google Scholar]
- 40.Sobeh M., Braun M.S., Krstin S., Youssef F.S., Ashour M.L., Wink M. Chemical profiling of the essential oils of Syzygium aqueum, Syzygium samarangense and Eugenia uniflora and their discrimination using chemometric analysis. Chem. Biodivers. 2016;13:1537–1550. doi: 10.1002/cbdv.201600089. [DOI] [PubMed] [Google Scholar]
- 41.Nadaf M., Nasrabadi M., Halimi M. GC-MS analysis of n-hexane extract from aerial parts of Salvia nemorosa. Middle-East. J. Sci Res. 2012;11:1127–1130. [Google Scholar]
- 42.Mostafa N.M. β-Amyrin rich Bombax ceiba leaf extract with potential neuroprotective activity against scopolamine-induced memory impairment in rats. Rec. Nat. Prod. 2018;12:480. doi: 10.25135/rnp.47.17.10.062. [DOI] [Google Scholar]
- 43.Mohamed S.H., Mohamed M.S.M., Khalil M.S., Azmy M., Mabrouk M.I. Combination of essential oil and ciprofloxacin to inhibit/eradicate biofilms in multidrug-resistant Klebsiella pneumoniae. J. Appl. Microbiol. 2018;125:84–95. doi: 10.1111/jam.13755. [DOI] [PubMed] [Google Scholar]
- 44.Hudaib M., Bellardi M.G., Rubies-Autonell C., Fiori J., Cavrini V. Chromatographic (GC-MS, HPLC) and virological evaluations of Salvia sclarea infected by BBWV-I. Farmaco. 2001;56:219–227. doi: 10.1016/S0014-827X(01)01038-2. [DOI] [PubMed] [Google Scholar]
- 45.Hudaib M.M., Aburjai T.A. Composition of the essential oil from Artemisia herba-alba grown in Jordan. J. Essent. Oil Res. 2006;18:301–304. doi: 10.1080/10412905.2006.9699096. [DOI] [Google Scholar]
- 46.Hanamanthagouda M.S., Kakkalameli S.B., Naik P.M., Nagella P., Seetharamareddy H.R., Murthy H.N. Essential oils of Lavandula bipinnata and their antimicrobial activities. Food Chem. 2010;118:836–839. doi: 10.1016/j.foodchem.2009.05.032. [DOI] [Google Scholar]
- 47.Hamdan D.I., Mohamed M.E., Abdulla R.H., Mohamed S.M., El-Shazly A.M. Anti-inflammatory, insecticidal and antimicrobial activities and chemical composition of the essential oils of different plant organs from navel orange (Citrus sinensis (L.) Osbeck var. Malesy) grown in Egypt. J. Med. Plants Res. 2013;7:1204–1215. [Google Scholar]
- 48.Hamdan D.I., Abdulla R.H., Mohamed M.E., El-Shazly A.M. Chemical composition and biological activity of essential oils of Cleopatra mandarin (Citrus reshni) cultivated in Egypt. J. Pharm. Phyther. 2013;5:83–90. [Google Scholar]
- 49.Do N., Fontes J.E., Ferraz R.P.C., Britto A.C.S., Carvalho A.A., Moraes M.O., Pessoa C., Costa E.V., Bezerra D.P. Antitumor effect of the essential oil from leaves of Guatteria pogonopus (Annonaceae) Chem. Biodivers. 2013;10:722–729. doi: 10.1002/cbdv.201200304. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues M.E., Henriques M., Silva S. Fungal Biofilms and Related Infections. Springer; Cham, Switzerland: 2016. Disinfectants to fight oral candida biofilms; pp. 83–93. [DOI] [PubMed] [Google Scholar]
- 51.Mohammed H.A., Al-Omar M.S., Aly M.S.A., Hegazy M.M. Essential oil constituents and biological activities of the halophytic plants, Suaeda vermiculata Forssk and Salsola cyclophylla Bakera growing in Saudi Arabia. J. Essent. Oil Bear. Plants. 2019:1–12. doi: 10.1080/0972060X.2019.1574611. [DOI] [Google Scholar]
- 52.Reyes-Jurado F., Navarro-Cruz A.R., Ochoa-Velasco C.E., Palou E., López-Malo A., Ávila-Sosa R. Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. Nutr. 2019;15:1–10. doi: 10.1080/10408398.2019.1586641. [DOI] [PubMed] [Google Scholar]
- 53.Imfeld T. Chlorhexidine-containing chewing gum. Schweizer Monatsschrift fur Zahnmedizin. 2006;116:476. [PubMed] [Google Scholar]
- 54.Saxena S., Meshram V., Kapoor N. Muscodor tigerii sp. nov.-Volatile antibiotic producing endophytic fungus from the Northeastern Himalayas. Ann. Microbiol. 2015;65:47–57. doi: 10.1007/s13213-014-0834-y. [DOI] [Google Scholar]
- 55.Park S.-N., Lim Y.K., Freire M.O., Cho E., Jin D., Kook J.-K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe. 2012;18:369–372. doi: 10.1016/j.anaerobe.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Loesche W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986;50:353. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bersan S.M., Galvão L.C., Goes V.F., Sartoratto A., Figueira G.M., Rehder V.L., Alencar S.M., Duarte R.M., Rosalen P.L., Duarte M.C. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. BMC Complement. Altern. Med. 2014;14:451. doi: 10.1186/1472-6882-14-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.