Abstract
Purpose
Associations between psychosocial factors and biomarkers are increasingly investigated in studies of cancer incidence and mortality. Documenting optimal data/biospecimen collection protocols and scale properties are fundamental for elucidating the impact of psychosocial factors on biologic systems and ultimately cancer development/progression.
Methods
Between 2013 and 2014, 233 Nurses’ Health Study II women (mean age: 60.6) participated in the Mind–Body Study. Participants completed a detailed online psychosocial assessment and provided hair, toenail, timed saliva over 1 day, urine and fasting blood twice, 1 year apart. Additionally, two separate microbiome collections for stool and saliva were conducted between the psychosocial assessments. We assessed correlations between various psychosocial measures and evaluated their 1-year reproducibility using intraclass correlations (ICC).
Results
Compliance with the protocols was high among participants. Psychosocial measures showed moderate-to-high reproducibility over 1 year (ICCs = 0.51–0.81). There was clear clustering of psychosocial factors according to whether they were querying positive (e.g., optimism, mastery, mindfulness) or negative (e.g., anxiety, depression, discrimination) emotion-related or social constructs.
Conclusion
Results suggest feasibility for self-administered collection of various biospecimens and moderate-to-high reproducibility of psychosocial factors. The Mind–Body Study provides a unique resource for assessing inter-relationships between psychosocial factors and biological processes linked with long-term health outcomes, including carcinogenesis.
Keywords: Biospecimen collection, Cancer biomarkers, Correlation study, Psychosocial factors, Reproducibility, Study design
Introduction
Stress-related phenomena are important determinants of health and potentially upstream modifiable factors for cancer prevention [1, 2]. A growing body of evidence suggests that psychosocial stressors and their related emotional responses are associated with increased risk and mortality of some cancers and relevant biologic processes (e.g., elevated inflammation) [3–10]. In parallel, emerging studies suggest health benefits of stress-reduction techniques (e.g., yoga, mindfulness strategies) and psychological well-being (e.g., optimism). These positive factors have been linked with improved physical and mental well-being in both healthy individuals and cancer patients [11–15], as well as healthier biological function, such as favorable inflammation and gene expression profiles [16, 17]. However, accurate assessment of such subjective positive and negative psychosocial factors is challenging in large population-based studies, and biologic mechanisms underlying the observed associations with cancer outcomes remain poorly understood. Identifying novel biomarkers related to psychosocial factors may help advance the field.
Psychosocial stressors and related emotional responses are common, but complex, experiences influenced by individual, social, and environmental factors. Existing epidemiologic studies have predominantly focused on either individual emotional functioning (e.g., depressive symptoms, optimism levels) or psychosocial stressors (e.g., discrimination, death of a spouse), as measured by self-reported psychometric scales. As there are inter-individual differences in perception of, response to, and coping with psychosocial stressors [18], assessment based on individual psychosocial scales may not fully explain biologic alterations (e.g., on the hypothalamic–pituitary–adrenal axis) that could influence carcinogenic processes. Furthermore, consideration of a single psychosocial factor (e.g., anxiety) may be insufficient, if other positive (e.g., social support) and negative (e.g., discrimination) psychosocial factors buffer or amplify the associations. Also, most prospective studies have relied on a one-time psychosocial assessment, which may introduce measurement error given the fluctuating nature of some psychosocial factors and the window of susceptibility during the long latency of cancer development [19]. Quantifying the stability of different psychosocial scales is needed to determine whether such factors assessed at a single time point can reasonably reflect exposure over a longer period.
Very few resources bring together multiple aspects of psychosocial factors (both positive and negative) and biologic specimens that can be used to measure activation of stress axes and downstream biologic markers. Thus, we conducted the Mind–Body Study (MBS) nested in the Nurses’ Health Study II (NHSII), collecting multiple subjective psychosocial scales and biospecimens, including samples suitable for microbiome assessment, twice over 1 year to allow assessment of within-person stability over time. This current paper describes the protocols used to implement the MBS and provide an initial examination of psychosocial scale characteristics. In future studies, the MBS data could be further used to evaluate the relationships of psychosocial scales assessing positive and negative constructs, in conjunction with behavioral correlates, with novel objective biomarkers (particularly leveraging omics platforms). The ultimate goal is to both understand the biologic correlates of various psychosocial factors and inform design of future epidemiologic studies investigating the role of positive and negative psychosocial factors in cancer etiology.
Methods
Study population
Women invited to the MBS were participants in NHSII, which is a large, prospective cohort study of US women with 116,429 registered nurses (25–42 years old) enrolled in 1989. All participants completed a baseline questionnaire, including basic demographic characteristics, and the cohort has been followed by biennially mailed questionnaires to update information on a variety of lifestyle and health-related factors and ascertain incident diseases. Follow-up on each questionnaire has been between 85 and 90% [20]. From 1996 to 1999, 29,611 NHSII participants (32–54 years old) provided blood and urine samples [21]; these women had a follow-up rate of 94.3% at the time of the MBS collection (2013–2014). The MBS was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health, and written informed consent was obtained from all participants.
MBS eligibility
Women were invited if they had a valid email address on file, had participated in the original blood/urine collection and had either (a) participated in the diet/physical activity validation study collecting multiple biospecimens from 2011 to 2012 [22] and were not part of another active ongoing substudy [23] or (b) given a second blood and urine sample between 2008 and 2011 and completed the 2011 biennial questionnaire [24]. This sampling rationale was used to enhance compliance to the biospecimen collection and to leverage previously collected specimens. Further, among the latter group, we randomly oversampled women who had reported childhood abuse (i.e., either childhood trauma or sexual abuse or both) on a 2001 questionnaire [25]. This sampling rationale was used to increase the likelihood that these women experienced psychosocial distress during adulthood [26, 27].
Recruitment and consent
Women were invited via email, with a reminder for nonresponders 2 weeks later, and were directed to a website with more study details. Interested participants were mailed a consent form and pointed to a website with additional details about the collection and frequently asked questions. Those who did not return the consent were sent an email reminder after 4 weeks and again 2 weeks later.
Sample collection
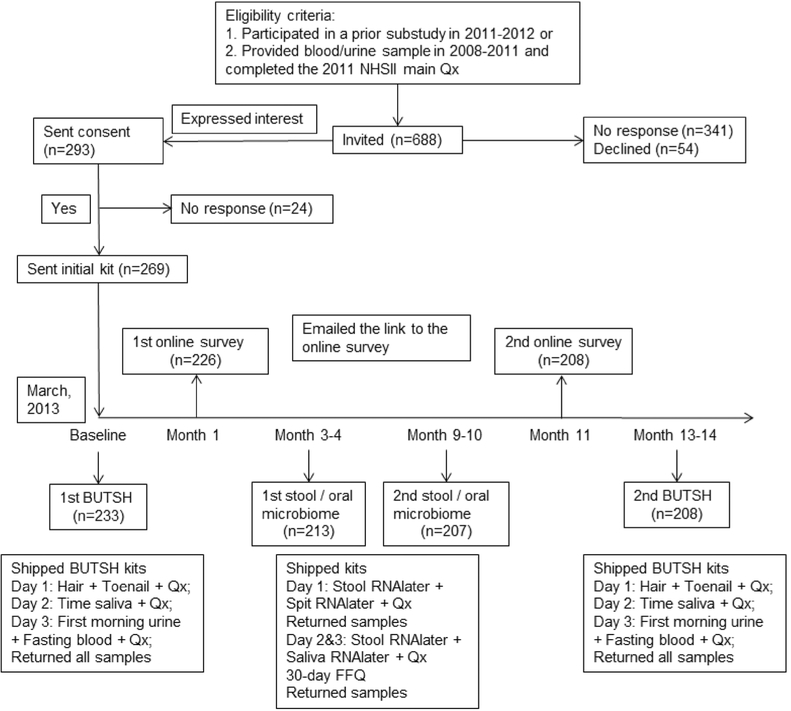
Women were asked to provide multiple biological specimens over a 1-year period in 4 collection waves (Fig. 1). The details for each collection are described in the Appendix. Briefly, at study entry (Collection 1) and 1 year postbaseline (Collection 4), women provided fasting blood, first morning urine, timed saliva [28], as well as hair and toenail samples (BUTSH kits). At approximately three (Collection 2) and 9 months (Collection 3) after study entry, women provided two stool and two saliva specimens collected 2–3 days apart. Collection kits, packed by Therapak (Buford, Georgia), contained all necessary supplies to conduct the collection along with detailed instructions (see Appendix) and shipping supplies/labels to return samples to our biorepository by overnight courier. The kit for Collection 1 was sent after the return of a signed consent form. For subsequent kits, we sent an email to tell the participant that the kit would be arriving in the next week and mailed the kit via ground FedEx shipping. Reminder emails were sent at 4 weeks, 8 weeks, and 10 weeks after kit delivery; continued nonresponders were called at 12–14 weeks after kit delivery. No monetary incentives were provided for participation. For women who completed all four collections, we sent a handwritten “thank you” note along with a free copy of the book “Healthy Women, Healthy Lives: A Guide to Preventing Disease, from the Landmark Nurses’ Health Study” [29].
Fig. 1.
Flowchart for the design of the Mind–Body Study in the Nurses’ Health Study II. Abbreviations: BUTSH = Blood, urine, toenail, timed saliva, and hair collection; Qx = biospecimen collection questionnaire
Psychosocial questionnaire
Within 1 month of completing the first collection kit, participants were emailed a link to an online questionnaire about psychosocial factors and medication use. This 45–60-min questionnaire was designed to capture information that overlapped the time of the biosample collections. Ten months later, women were emailed a link to the same questionnaire. At both assessments, reminder emails were sent 3 weeks later if needed.
Self-reported scales, most of which have been clinically or psychometrically validated, are described in Table 1. Briefly, psychological distress was queried using four scales: Kessler Psychological Distress Scale (K6), a screening measure for levels of non-specific distress that may be clinically significant [30]; Center for Epidemiological Study–Depression (CES-D) [31, 32] evaluating depressive symptoms; Generalized Anxiety Disorder–7 items (GAD-7) [33] and the Crown–Crisp Index (CCI) [34], which capture different dimensions of anxiety. To study psychological well-being, we used the Life Engagement Test (LET) [35] characterizing women’s sense of purpose in life; the Pearlin Mastery Scale (PMS) [36] capturing self-mastery and control over one’s life; and the Life Orientation Test–Revised (LOT-R) [37], which assesses optimism and the belief that positive outcomes will occur. We also included mindfulness, an attribute of consciousness, using items from the Freiburg Mindfulness Inventory (FMI) [38] and Mindful Attention Awareness Scale (MAAS) [39]. We administered the Emotion Regulation Questionnaire [40] to determine how participants adaptively or maladaptively manage positive and negative emotional experiences; cognitive reappraisal (ERQ-CR) and expressive suppression (ERQ-ES) were the two regulation strategies captured.
Table 1.
Summary of psychosocial and other scales collected in the Mind–Body Study
| Scale [abbreviation] Construct measured (timeframe, if any) | Subscales | Number of items in the original version/in the MBS | Response options | Cutoff | Internal consistency in the MBS (Cronbach Alpha) | Example of items |
|---|---|---|---|---|---|---|
| Kessler Psychological Distress Scale [K6] Anxiety and depressive symptoms (past month) |
N/A | 6/6 | 0 “None of the time” to 4 “All the time” | N/A | 0.83 | “During the past month, about how often did you feel nervous?” |
| Center for Epidemiological Study-Depression [CES-D] Depression symptoms (past week) |
N/A | 10/10 | 1 “Rarely/none of the time” to 4 “All the time” | ≥8, 10 or 15 for clinical symptoms | 0.86 | “I was bothered by things that usually don’t bother me” |
| Generalized Anxiety Dis-order-7 items [GAD-7] Anxiety symptoms (past 2 weeks) |
[1] Anxiety symptoms; [2] Impact on functioning | 8/8 | Subscales [1] 0 “Not at all” to 3 “Nearly every day” (7 items); [2] 0 “Not difficult at all” to “Extremely difficult” (1 item) | ≥10 for cases of GAD; 5, 10, and 15 for mild, moderate, and severe levels of anxiety | 0.89 | “How often have you been bothered by… feeling nervous, anxious or on edge?” |
| Crown-Crisp Index [CCI] Anxiety symptoms | N/A | 8/8 | 0 “Never” to 2 “Often” | ≥4 | 0.63 | “Do you feel panicky in crowds?” |
| Life Engagement Test [LET] Purpose in life |
N/A | 6/6 | 1 “Strongly disagree” to 5 “Strongly agree” | N/A | 0.86 | “I have lots of reasons for living.” |
| Pearlin Mastery Scale [PMS] Self-mastery |
N/A | 7/7 | 1 “Strongly disagree” to 4 “Strongly agree”; missing for “Don’t know” | N/A | 0.82 | “What happens to me in the future mostly depends on me.” |
| Life Orientation Test-Revised [LOT-R] Optimism |
N/A | 6/6 | 0 “I disagree a lot” to 4 “I agree a lot” | N/A | 0.84 | “In uncertain times, I usually expect the best.” |
| Freiburg Mindfulness Inventory [FMI] Mindfulness |
N/A | 14/5 | 1 “Very rarely” to 5 “Very frequently | N/A | 0.72 | “In difficult situations, I can pause without immediately reacting.” |
| Mindful Attention Aware-ness Scale [MAAS] Mindfulness |
N/A | 15/5 | 1 “Almost always” to 6 “Very rarely” | N/A | 0.90 | “I find myself doing things without paying attention.” |
| Emotion Regulation Questionnaire [ERQ] Emotion regulation |
[1] Cognitive reappraisal; [2] Expressive suppression | 10/10 | Subscales [1] and [2]: 1 “Strongly disagree” to 7 “Strongly agree” | N/A | [1] 0.85 [2] 0.78 |
“I keep my emotions to myself.” |
| Everyday Discrimination Scale-short version [EDS] Life racial and non-racial discrimination (lifetime and day-to-day life) |
[1] Life events; [2] Everyday discrimination | 9/9 | Subscales 1) 1 “Yes”, 0 “No” (4 items); 2) 0 “Never” to 4 “At least once a week” (5 items) | N/A | 0.70 | [1] “For unfair reason, have you ever been fired from a job?” [2] “In your day-to-day life, how often have you been treated with less courtesy or respect than other people?” |
| Berkman–Syme Social Network Index [BSSNI] Social connections (past six months) | [1] Marital status; [2] Sociability; [3] Church group membership; [4] Membership in other community organizations | 4/4 | 0 or 1 to each category | N/A | 0.38 | “Apart from your children, how many relatives do you have with whom you feel close?” |
| Job Content Questionnaire [JCQ] Job characteristics (completed if women were employed in the last two years) |
[1] Psychological demands; [2] Decision latitude (control); [3] Supervisor and coworkers social support; [4] Physical job demands; [5] Job insecurity | 49/20 | Subscales [1] to [4]: From 1 “Strongly disagree” to 4 “Strongly agree” (5, 9, 4 and 1 items, respectively); [5] Various response options (4 items) | N/A | [1] 0.78 [2] 0.82 |
“My job requires that I learn new things |
| Pittsburgh Sleep Quality Index [PSQI] Sleep quality and disturbances (past month) |
[1] Subjective sleep quality; [2] Sleep latency; [3] Sleep duration; [4] Habitual sleep efficiency; [5] Sleep disturbances; [6] Use of sleeping medication; [7] Daytime function | 19/19 | Subscales [1] to [7]: weighted on a 0 to 3 scale | >5 | 0.70 | “During the past month, when have you usually gone to bed at night?” |
| Modified Yale Food Addiction Scale [MYFAS] Food addiction (past 6 months) |
[1] Addiction symptoms; [2] Related impairment or distress | 9/9 | Subscales [1] 0 “Never” to 4 “4 + times per week” (7 items); [2] “Yes”, “No”, “N/A” (2 items) | ≥3 (of 7) addiction symptoms + presence (“Yes”) of impairment or distress | 0.70 | “I find myself consuming certain foods even though I am no longer hungry.” |
| Lexington Attachment to Pets Scale [LAPS] Attachment of individuals to their pets |
[1] General attachment; [2] People substitution; [3] Animal rights and welfare | 23/6 | Subscales [1] to [3]: From 0 “Strongly disagree” to 3 “Strongly agree” (4, 1 and 1 items, respectively); missing for “Don’t know” | N/A | 0.92 | “I consider my pet a friend.” |
Social factors considered included racial and non-racial discrimination, measured with five scenarios of major-life discrimination [41] and the short-version Everyday Discrimination Scale (EDS) [42]; social integration via the Berkman–Syme Social Network Index (BSSNI) [43]; work-related demand and control via the Job Content Questionnaire (JCQ) [44, 45]; and caregiver stress (e.g., hours spent in caregiving outside of the employment as nurses to children, grandchildren, disabled/ill parent; self-perceived stress and reward from caregiving) [46]. We also asked participants whether they had experienced major stressful events in their lifetime (e.g., death of a child, serious physical assault, life-threatening illness/accident) and the number of stressful events experienced during the past 6 months (e.g., death of someone close, job loss, financial crisis, legal trouble, etc.) [47]. As other markers of psychosocial functioning, the Pittsburgh Sleep Quality Index (PSQI) examined multiple sleep dimensions (e.g., latency, duration, medication, daily functioning) [48], whereas food addiction was assessed with the Modified Yale Food Addiction Scale (MYFAS) [49]. Individuals’ attachment to their pets was studied with the Lexington Attachment to Pets Scale (LAPS) [50].
Of note, several scales have been collected earlier in time in NHSII, namely CCI (on the 2005 questionnaire), MYFAS (on the 2009 main questionnaire), CES-D and BSSNI (both from a 2008 substudy of posttraumatic stress disorder [51]). These data allowed assessment of the reproducibility of these psychosocial measures over a longer period of time (> 90% of MBS participants had these data).
Statistical methods
We calculated age-standardized means and standard deviations (SD) for continuous variables and percentages for binary variables to describe sample characteristics at baseline and 1-year follow-up. To estimate the reproducibility of psychosocial factors over 1 year, we computed intraclass correlation coefficients (ICC) using variance components from a random-effects mixed model. We conducted similar analyses of longer-term reproducibility for several psychosocial scales that were also assessed in 2005–2009. To assess the extent to which the diverse measures capture distinct psychosocial factors, Pearson correlation coefficients were calculated among and between positive and negative factors, using mean values for participants who completed both baseline and follow-up assessments. All analyses were conducted in SAS 9.4 (Cary, NC).
Results
We invited 688 women (238 from the diet/physical activity validation study) to participate (Fig. 1). Of these, 293 women (42% of invited) responded that they were willing to participate, 54 said they were not willing, and the remaining did not respond. Consents were returned by 269 women (91% of those willing to participate), with 233 (85%) returning a completed kit with at least one eligible biospecimen (fasting blood: 226, first morning urine: 233, timed saliva: 233, toenails: 228, hair: 212). Of these 233 women, 226 completed the first online psychosocial assessment. The Collection 2 kit was mailed to 233 women. Usable stool and saliva was obtained from 209 (90%) and 213 (91%) women, respectively. Eight women withdrew from the MBS (but not the main NHSII cohort) after Collection 2, citing time constraints. Among 225 women receiving the Collection 3 kit, 202 (90%) had usable stool and 207 (92%) had usable saliva samples. Finally, 208 (92%) completed the second online questionnaire and 215 (95%) returned the Collection 4 kit (fasting blood: 198, first morning urine: 202, timed saliva: 201, toenails: 209, hair: 196). Participants who provided biospecimens followed, overall, the collection instructions. For example, 184 of 226 (81.4%) provided a fasting blood sample (> 8 h since last meal), 226 of 233 (97.0%) provided a first morning urine, 214 of 233 (91.8%) provided all 5 timed saliva samples, and of 224 women providing the first saliva sample, all (100.0%) followed the instruction by collecting the sample without getting out of bed.
At baseline, women were on average 60.6 years old (range 49–67; SD: 4.0; all postmenopausal) and predominantly white (96%), with a mean BMI of 26.7 kg/m2 (SD: 6.1); 80% were employed in the past 2 years (Table 2). The prevalence was 19% for clinically meaningful depressive symptoms (CES-D ≥ 10), 21% for mild-to-severe anxiety symptoms (GAD-7 ≥ 5), 30% for experiencing major-life discrimination, and 5% for food addiction (MYFAS ≥ 3 plus-related symptoms). The distribution of psychosocial factors was similar among women who completed the 1-year assessment. Further, compared to women from the larger NHSII cohort who completed the 2013 main questionnaire (Supplemental Table 1), MBS participants were more likely to have childhood abuse experience (by design, 33% vs 14%) and take psychotropic medications, but less likely to have chronic conditions such as diabetes and hypertension.
Table 2.
Age-adjusted sample characteristics at baseline and 1-year follow-up
| Baseline | Follow-up | |
|---|---|---|
| N | 226 | 208 |
| Demographic factors | ||
| Age, years | 60.6 (4.0) | 61.4 (4.1) |
| Non-white, % | 4 | 5 |
| Employed in the past 2 years, % | 80 | 71 |
| Medical conditions/medication | ||
| History of hypertension, % | 34 | 33 |
| History of type 2 diabetes, % | 5 | 6 |
| Current antidepressant use, % | 25 | 26 |
| Current beta-blocker use, % | 13 | 13 |
| Current minor tranquilizer use, % | 8 | 7 |
| Complementary and alternative medicine practices, % | 44 | 49 |
| Psychological distress | ||
| Center for Epidemiologic Studies-Depression (CES-D) | 5.9 (4.8) | 5.5 (4.3) |
| Elevated depressive symptoms (CES-D ≥ 10), % | 19 | 17 |
| Generalized Anxiety Disorder 7-item (GAD-7) | 2.7 (3.5) | 2.7 (3.4) |
| Elevated anxiety symptoms (GAD-7 ≥ 5), % | 21 | 22 |
| Crown-Crisp Index (CCI) | 3.3 (2.3) | 3.4 (2.3) |
| Kessler Psychological Distress Scale (K6) | 2.8 (3.1) | 2.6 (2.8) |
| Psychological well-being | ||
| Life Engagement Test (LET) | 25.4 (4.3) | 25.9 (3.7) |
| Freiburg Mindfulness Inventory (FMI) | 14.8 (2.8) | 14.6 (2.7) |
| Mindful Attention Awareness Scale (MAAS) | 23.8 (3.8) | 23.7 (3.8) |
| Pearlin Mastery Scale (PMS) | 22.8 (3.5) | 22.8 (3.6) |
| Life Orientation Test-Revised (LOT-R) | 19.2 (4.7) | 19.2 (4.9) |
| Emotion Regulation Questionnaire | ||
| Cognitive reappraisal (ERQ-CR) | 30.7 (5.7) | 30.8 (6.2) |
| Expressive suppression (ERQ-ES) | 12.2 (4.7) | 11.9 (4.6) |
| Social factors | ||
| Childhood abuse, % | 33 | 32 |
| Experiencing major lifetime stressful events, % | 36 | 35 |
| Number of recent stressful events | 1.2 (1.6) | 1.2 (1.5) |
| Job characteristics1 | ||
| Job demand | 11.7 (3.0) | 11.8 (3.0) |
| Job control | 27.1 (4.0) | 27.0 (4.0) |
| Major-life discrimination, % | 30 | 29 |
| Everyday Discrimination Scale (EDS) | 2.4 (2.1) | 2.4 (2.3) |
| Berkman-Syme Social Network Index (BSSNI) | 2.9 (1.0) | 3.0 (1.0) |
| Caregiver, % | 31 | 35 |
| Total caregiving time, hours/week2 | 20.3 (29.1) | 24.1 (30.7) |
| Other relevant factors | ||
| Body mass index (BMI), kg/m2 | 26.7 (6.1) | 26.8 (6.2) |
| Pittsburg Sleep Quality Index (PSQI) | 6.6 (3.5) | 6.5 (3.2) |
| Poor habitual sleep quality (PSQI > 5) | 60 | 59 |
| Modified Yale Food Addiction Scale (MYFAS) | 0.6 (1.1) | 0.4 (1.1) |
| Food addition (MYFAS ≥ 3 plus related symptoms), % | 5 | 3 |
| Pet ownership, % | 62 | 64 |
| Lexington Attachment to Pet Scale (LAPS)2 | 15.3 (3.6) | 15.2 (3.9) |
| Frequent relaxation activities, % | 81 | 81 |
Values are means (SD) or percentages
Among recently employed participant
Among pet owners
Among caregivers
Reproducibility of psychosocial factors over 1 year was excellent (Table 3). In general, ICCs ranged from 0.51 (95% CI 0.40, 0.63) for MYFAS to 0.81 (95% CI 0.76, 0.85) for BSSNI, with the exception of an ICC of 0.34 (95% CI 0.24, 0.47) for the number of stressful events during the past 6 months. More specifically regarding commonly used measures of psychological distress, the 1-year ICC was 0.62 for CES-D, 0.55 for GAD-7, 0.74 for CCI and 0.63 for K6. Further, the longer-term ICC (95% CI) was 0.61 (0.53, 0.69) for CES-D over 6 years, 0.51 (0.41, 0.61) for CCI over 9 years, 0.52 (0.43, 0.62) for MYFAS over 5 years, and 0.76 (0.70, 0.82) for BSSNI over 6 years.
Table 3.
Stability of psychosocial measures over 1-year period
| ICC (95% CI) | |
|---|---|
| Psychosocial distress | |
| Center for Epidemiologic Studies-Depression | 0.62 (0.53, 0.70) |
| Generalized Anxiety Disorder 7-item | 0.55 (0.45, 0.64) |
| Crown-Crisp Index | 0.74 (0.67, 0.80) |
| Kessler Psychological Distress Scale | 0.63 (0.55, 0.71) |
| Psychological well-being | |
| Life Engagement Test | 0.68 (0.60, 0.75) |
| Freiburg Mindfulness Inventory | 0.71 (0.63, 0.77) |
| Mindful Attention Awareness Scale | 0.64 (0.56, 0.72) |
| Pearlin Mastery Scale | 0.65 (0.56, 0.73) |
| Life Orientation Test-Revised | 0.77 (0.70, 0.82) |
| Emotion Regulation Questionnaire | |
| Cognitive reappraisal | 0.57 (0.47, 0.66) |
| Expressive suppression | 0.65 (0.56, 0.72) |
| Social factors | |
| Number of recent stressful events | 0.34 (0.24, 0.47) |
| Everyday Discrimination Scale | 0.68 (0.60, 0.75) |
| Berkman-Syme Social Network Index | 0.81 (0.76, 0.85) |
| Job characteristics | |
| Job demand | 0.58 (0.46, 0.69) |
| Job control | 0.70 (0.61, 0.78) |
| Other relevant factors | |
| Pittsburgh Sleep Quality Index | 0.64 (0.55, 0.71) |
| Modified Yale Food Addiction Scale | 0.51 (0.40, 0.63) |
| Lexington Attachment to Pet Scale | 0.58 (0.46, 0.70) |
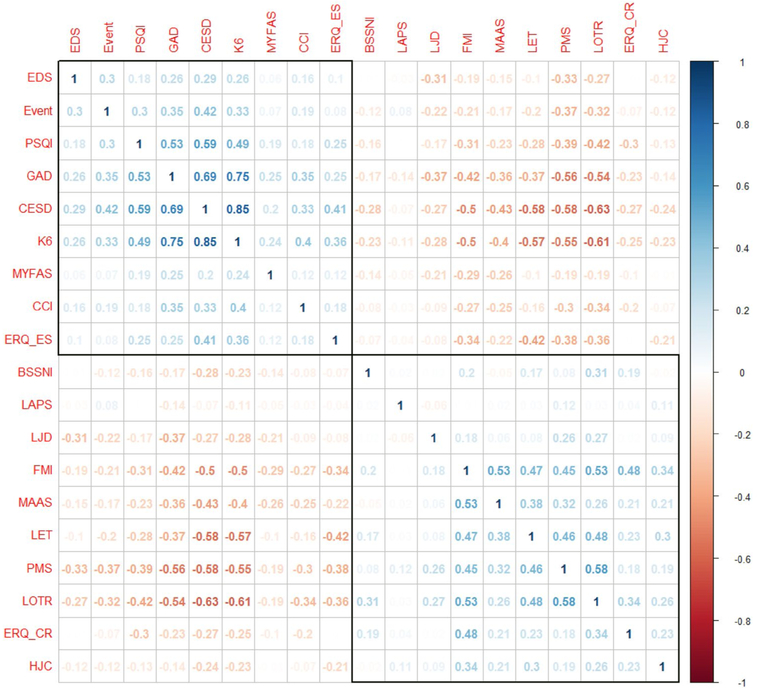
Scores obtained from psychosocial measures were associated with one another in expected directions, with a clear distinction between positive and negative factors (Fig. 2). Particularly, moderate-to-strong positive correlations were observed between most markers of distress (e.g., depression [CES-D], anxiety [GAD-7], and general distress [K6], r = 0.69–0.85). CES-D, GAD-7 and K6 were also positively correlated with PSQI (r = 0.49–0.59). Similarly, there were moderate positive correlations among many positive psychological factors (e.g., mindfulness [FMI], purpose in life [LET], optimism [LOT-R], mastery [PMS], and cognitive reappraisal [ERQ-CR], r = 0.45–0.58). Further, moderate correlations were obtained between social factors (e.g., stressful events and discrimination [EDS], r = 0.30). By contrast, inverse correlations were evident between each pair of negative and positive factors. LAPS, capturing attachment to one’s pet, was not associated with the other psychosocial factors.
Fig. 2.
Pearson correlations between scores of psychosocial scales. Abbreviations: BBSNI = Berkman–Syme Social Network Index; CCI = Crown–Crisp Index; CES-D = Center for Epidemiologic Studies-Depression scale; Events = number of recent stressful events; EDS = Everyday Discrimination Scale; ERQ-ES/CR = Emotion Regulation Questionnaire-Expressive Suppression/Cognitive Reappraisal; FMI = Freiburg Mindfulness Inventory; GAD-7 = Generalized Anxiety Disorder 7-item; HJC = High Job Control; K6 = Kessler Psychological Distress Scale,6 items version; LAPS = Lexington Pet Attachment Scale; LET = Life Engagement Test; LJD = Low Job Demand; LOT-R = Life Orientation Test-Revised; MAAS = Mindful Attention Awareness Scale; MYFAS = Modified Yale Food Addiction Scale; PSQI = Pittsburgh Sleep Quality Index; PMS = Pearlin Mastery Scale
Discussion
This study showed that self-administered collection of various biospecimens and psychosocial measures can be successfully performed in adult women. Further, most self-reported psychosocial scales had excellent reproducibility over 1 year (and longer for scales that were queried earlier in the larger parent cohort), suggesting that these constructs can be leveraged in epidemiologic studies. The extensive data collection, coupled with the rigorously developed and well-planned study design, provides a unique opportunity to examine the complex relationships between psychosocial factors and cancer-related biomarkers, with potential important implications fundamental to the design and conduct of future larger-scale studies [52].
While the collection of psychosocial factors is increasingly implemented in epidemiologic research, the feasibility of self-managed collection of various biospecimens in large epidemiologic cohorts is less well studied. Although our study was observational, these specimen protocols may also be applied to intervention studies, among other designs. Careful documentation of collection characteristics using specimen-specific questionnaires (e.g., antibiotics use for microbiome-related collections, physical activity for timed saliva collection) is critical to capture relevant sources of variability and ensure research quality at the assay and analytical stages [53]. Also, publishing specimen collection protocols can enhance standardized biospecimen practices in epidemiologic research, as recommended by the NCI Best Practices for Biospecimen Resources [54], which are key to promoting high-quality data harmonization. Although our participants were all registered nurses at baseline enrollment in 1989, collection of these biospecimens is mostly non-invasive, requiring minimal training (except for blood); therefore, the procedures may be applied to a broader population with appropriate illustrations and instructions. In fact, most of our participants had their blood drawn at a local clinic (as opposed to having a colleague collect the sample), which could be replicated in other populations. Providing telephone and online support to identify nearby clinics was crucial to improving compliance to the blood draw. Finally, as all samples were mailed, pilot testing of novel biomarkers in these samples is needed to demonstrate stability with delayed sample processing, as previously reported for a metabolomics assay [55]. Although we have not directly tested stability of markers in hair and toenails, prior work has shown stability of multiple markers over long periods of storage [56, 57], and we used RNALater for the microbiome collection to ensure preservation of the sample at the moment of collection (e.g., to prevent microbial overgrowth of certain species). Novel technologies to preserve specimens with delayed processing and freezing is a fast-growing area of development. The feasibility of hair and toenail collection is particularly important because of the low cost and ease of collecting these samples in large populations; interestingly, a recent study has suggested its potential utility among cancer patients [52].
Our analysis highlights that a wide array of psychosocial factors are moderately to highly stable over time. The 1-year ICCs for most factors are comparable with or superior to other well-validated biologic factors, such as blood pressure (ICC = 0.70–0.90 over days to weeks) [58], serum cholesterol (ICC = 0.65 over 1 year) [59], and plasma prolactin (ICC = 0.53 over 2–3 years) [60]. This suggests that a single assessment can reliably reflect psychosocial factors over 1 year. For several psychosocial scales (e.g., CES-D, BSSNI) linked to assessments that occurred 5–9 years earlier, the ICCs were remarkably similar, suggesting that the reliability and reproducibility of a single psychosocial assessment may potentially extend to a longer period of time and be used in prospective studies to evaluate its role in cancer development and progression.
We also observed consistent correlation patterns within and across negative and positive psychosocial factors. While such consistency may correspond to some common underlying constructs, their moderate correlations suggest that each measure captures distinct psychosocial factors. Therefore, to dissect the independent effect of one psychosocial factor, it may be important to consider potential confounding or effect modification by other factors. A joint analysis of several related psychosocial factors, with the integration of lifestyle constructs (e.g., smoking, physical activity), will further help elucidate their synergistic or antagonistic interactions on chronic disease outcomes, including cancer [61, 62]. However, few studies have addressed this question, possibly due to the fact that most epidemiological cohorts assessed only a few negative factors and had limited information on positive factors or biologic markers. Given that existing studies disproportionately focus on stressors and distress, more research is needed to understand the potential health benefits of positive psychosocial factors, including their roles in alleviating the adverse outcomes resulting from negative factors. Lastly, the two collections of various biospecimen types permit assessment of canonical stress hormones (diurnal cortisol rhythms [28], urinary catecholamines), as well as multiple omics (metabolomics [63], DNA methylation [64], proteomics [65], microbiome, etc.), leading to a sample of deeply phenotyped women. Through linkage with psychosocial factors and longitudinal lifestyle and co-morbidity data assessed in NHSII biennial questionnaires, there will be many opportunities to elucidate the biology relating psychosocial functioning and health.
One limitation is that the study sample was comprised of predominantly white female nurses, who agreed to provide extensive biological and psychosocial data over multiple repeated assessments voluntarily; while it increases the study internal validity, it limits the generalizability of the results to other populations. Characteristics of this sample (e.g., greater knowledge or motivation towards health) may also impact the results and should be considered in the interpretation. Therefore, additional studies are needed to confirm whether these findings can be directly extrapolated to studies of cancer incidence and survivorship in other populations.
Our results demonstrate feasibility of self-administered collection of various biospecimens in adults, as well as stability and consistency across different psychosocial measures. These results are important for designing and carrying out epidemiologic studies that aim to investigate the association of psychosocial factors with cancer incidence/mortality and to understand the underlying biologic processes involved in carcinogenesis. Incorporating such data at the individual level with other constructs, such as behavioral mechanisms (e.g., lifestyle) and macro data at the population level (e.g., environmental exposures linked to geographic information), will be instrumental to elucidating the impact of psychosocial factors in cancer etiology.
Supplementary Material
Acknowledgments
We thank the participants and the staff of the Nurses’ Health Study II for their valuable contributions. This work was supported by the National Institute of Health (R01 CA163451) as well as by the Department of Defense (W81XWH-13-1-0493). The Nurses’ Health Study II is supported by the National Institute of Health (UM1 CA176726). T.H. is a recipient of the American Heart Association postdoctoral fellowship (Founders Affiliate) award (16POST27480007). C.T.F. received a postdoctoral fellowship from the Fonds de Recherche du Québec - Santé. The authors do not have any potential conflicts of interest to report. The authors assume full responsibility for analyses and interpretation of these data.
Abbreviations
- BBSNI
Berkman–Syme Social Network Index
- CCI
Crown–Crisp Index
- CES-D
Center for Epidemiologic Studies-Depression Scale
- Events
Number of recent stressful events
- EDS
Everyday Discrimination Scale
- ERQ-ES/CR
Emotion Regulation Questionnaire-Expressive Suppression/Cognitive Reappraisal
- FMI
Freiburg Mindfulness Inventory
- GAD-7
Generalized Anxiety Disorder 7-item
- HJC
High job control
- ICC
Intraclass correlation coefficients
- K6
Kessler Psychological Distress Scale, 6 items version
- LAPS
Lexington Pet Attachment Scale
- LET
Life Engagement Test
- LJD
Low Job Demand
- LOT-R
Life Orientation Test-Revised
- MAAS
Mindful Attention Awareness Scale
- MBS
Mind–Body Study
- MYFAS
Modified Yale Food Addiction Scale
- NHSII
Nurses’ Health Study II
- PSQI
Pittsburgh Sleep Quality Index
- PMS
Pearlin Mastery Scale
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10552–019-01176–0) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferrer RA, Green PA, Barrett LF (2015) Affective science perspectives on cancer control: strategically crafting a mutually beneficial research agenda. Perspect Psychol Sci. 10:328–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green McDonald P, O’Connell M, Suls J (2015) Cancer control falls squarely within the province of the psychological sciences. Am Psychol 70:61–74 [DOI] [PubMed] [Google Scholar]
- 3.Trudel-Fitzgerald C, Chen Y, Singh A, Okereke OI, Kubzansky LD (2016) Psychiatric, psychological, and social determinants of health in the Nurses’ Health Study cohorts. Am J Public Health 106:1644–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chida Y, Hamer M, Wardle J, Steptoe A (2008) Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol 5:466–475 [DOI] [PubMed] [Google Scholar]
- 5.Steptoe A, Hamer M, Chida Y (2007) The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 21:901–912 [DOI] [PubMed] [Google Scholar]
- 6.Batty GD, Russ TC, Stamatakis E, Kivimaki M (2017) Psychological distress in relation to site specific cancer mortality: pooling of unpublished data from 16 prospective cohort studies. BMJ 356:j108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer A, Ziogas A, Anton-Culver H (2018) Perception matters: stressful life events increase breast cancer risk. J Psychosom Res 110:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang T, Poole EM, Okereke OI et al. (2015) Depression and risk of epithelial ovarian cancer: results from two large prospective cohort studies. Gynecol Oncol 139:481–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuevas AG, Trudel-Fitzgerald C, Cofie L, Zaitsu M, Allen J, Williams DR (2019) Placing prostate cancer disparities within a psychosocial context: challenges and opportunities for future research. Cancer Causes Control 30:443–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trudel-Fitzgerald C, Zhou ES, Poole EM et al. (2017) Sleep and survival among women with breast cancer: 30 years of follow-up within the Nurses’ Health Study. Br J Cancer 116:1239–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C, Collet JP, Lau J (2004) The effect of Tai Chi on health outcomes in patients with chronic conditions: a systematic review. Arch Intern Med 164:493–501 [DOI] [PubMed] [Google Scholar]
- 12.Kim ES, Hagan KA, Grodstein F, DeMeo DL, De Vivo I, Kubzansky LD (2017) Optimism and cause-specific mortality: a prospective cohort study. Am J Epidemiol 185:21–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cramer H, Lange S, Klose P, Paul A, Dobos G (2012) Yoga for breast cancer patients and survivors: a systematic review and meta-analysis. BMC Cancer 12:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allison PJ, Guichard C, Fung K, Gilain L (2003) Dispositional optimism predicts survival status 1 year after diagnosis in head and neck cancer patients. J Clin Oncol 21:543–548 [DOI] [PubMed] [Google Scholar]
- 15.Kubzansky LD, Huffman JC, Boehm JK et al. (2018) Positive psychological well-being and cardiovascular disease: JACC health promotion series. J Am Coll Cardiol 72:1382–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harkess KN, Ryan J, Delfabbro PH, Cohen-Woods S (2016) Preliminary indications of the effect of a brief yoga intervention on markers of inflammation and DNA methylation in chronically stressed women. Transl Psychiatry 6:e965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Creswell JD, Irwin MR, Burklund LJ et al. (2012) Mindfulness-based stress reduction training reduces loneliness and pro-inflammatory gene expression in older adults: a small randomized controlled trial. Brain Behav Immun 26:1095–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stamps JA (2016) Individual differences in behavioural plasticities. Biol Rev Camb Philos Soc 91:534–567 [DOI] [PubMed] [Google Scholar]
- 19.Barlow DH (2014) Clinical handbook of psychological disorders: a step-by-step treatment manual. Guilford, New York [Google Scholar]
- 20.Bao Y, Bertoia ML, Lenart EB et al. (2016) Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health 106:1573–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tworoger SS, Sluss P, Hankinson SE (2006) Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women. Cancer Res 66:2476–2482 [DOI] [PubMed] [Google Scholar]
- 22.Yuan C, Spiegelman D, Rimm EB et al. (2017) Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 185:570–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr J, Marinac CR, Ellis K et al. (2017) Comparison of accelerometry methods for estimating physical activity. Med Sci Sports Exerc 49:617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang T, Tobias DK, Hruby A, Rifai N, Tworoger SS, Hu FB (2016) An increase in dietary quality is associated with favorable plasma biomarkers of the brain-adipose axis in apparently healthy US women. J Nutr 146:1101–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN et al. (2010) Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med 39:529–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz AV, Widom CS, McLaughlin J, White HR (2001) The impact of childhood abuse and neglect on adult mental health: a prospective study. J Health Soc Behav 42:184–201 [PubMed] [Google Scholar]
- 27.Anda RF, Felitti VJ, Bremner JD et al. (2006) The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci 256:174–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang T, Poole EM, Vetter C et al. (2017) Habitual sleep quality and diurnal rhythms of salivary cortisol and dehydroepiandrosterone in postmenopausal women. Psychoneuroendocrinology 84:172–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hankinson SE, Manson JE, Colditz GA (2002) Healthy women, healthy lives: a guide to preventing disease, from the landmark Nurses’ Health Study. Simon and Schuster, New Yok [Google Scholar]
- 30.Kessler RC, Andrews G, Colpe LJ et al. (2002) Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med 32:959–976 [DOI] [PubMed] [Google Scholar]
- 31.Irwin M, Artin KH, Oxman MN (1999) Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D). Arch Intern Med 159:1701–1704 [DOI] [PubMed] [Google Scholar]
- 32.Bjorgvinsson T, Kertz SJ, Bigda-Peyton JS, McCoy KL, Aderka IM (2013) Psychometric properties of the CES-D-10 in a psychiatric sample. Assessment 20:429–436 [DOI] [PubMed] [Google Scholar]
- 33.Spitzer RL, Kroenke K, Williams JB, Lowe B (2006) A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 166:1092–1097 [DOI] [PubMed] [Google Scholar]
- 34.Crown S, Crisp AH (1966) A short clinical diagnostic self-rating scale for psychoneurotic patients: the Middlesex Hospital Questionnaire (M.H.Q.). Br J Psychiatry 112:917–923 [DOI] [PubMed] [Google Scholar]
- 35.Scheier MF, Wrosch C, Baum A et al. (2006) The life engagement test: assessing purpose in life. J Behav Med 29:291–298 [DOI] [PubMed] [Google Scholar]
- 36.Pearlin LI, Schooler C (1978) The structure of coping. J Health Soc Behav 19:2–21 [PubMed] [Google Scholar]
- 37.Scheier MF, Carver CS, Bridges MW (1994) Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the life orientation test. J Pers Soc Psychol 67:1063–1078 [DOI] [PubMed] [Google Scholar]
- 38.Walach H, Buchheld N, Buttenmüller V, Kleinknecht N, Schmidt S (2006) Measuring mindfulness: the Freiburg mindfulness inventory (FMI). Pers Individ Dif 40:1543–1555 [Google Scholar]
- 39.Brown KW, Ryan RM (2003) The benefits of being present: mindfulness and its role in psychological well-being. J Pers Soc Psychol 84:822–848 [DOI] [PubMed] [Google Scholar]
- 40.Gross JJ, John OP (2003) Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol 85:348–362 [DOI] [PubMed] [Google Scholar]
- 41.Harnois CE, Ifatunji M (2011) Gendered measures, gendered models: Toward an intersectional analysis of interpersonal racial discrimination. Ethnic and Racial Studies. 34:1006–1028 [Google Scholar]
- 42.Sternthal MJ, Slopen N, Williams DR (2011) Racial disparities in health: how much does stress really matter? Du Bois Rev 8:95–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berkman LF, Syme SL (1979) Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda county residents. Am J Epidemiol 109:186–204 [DOI] [PubMed] [Google Scholar]
- 44.Karasek R, Brisson C, Kawakami N, Houtman I, Bongers P, Amick B (1998) The Job Content Questionnaire (JCQ): an instrument for internationally comparative assessments of psychosocial job characteristics. J Occup Health Psychol 3:322–355 [DOI] [PubMed] [Google Scholar]
- 45.Karasek R, Theorell T (1990) Healthy work: Atress, productivity and the reconstruction of working life. Basic Books, New York [Google Scholar]
- 46.Kroenke CH, Hankinson SE, Schernhammer ES, Colditz GA, Kawachi I, Holmes MD (2004) Caregiving stress, endogenous sex steroid hormone levels, and breast cancer incidence. Am J Epidemiol 159:1019–1027 [DOI] [PubMed] [Google Scholar]
- 47.Sternthal MJ, Slopen N, Williams DR (2011) Racial disparities in health: how much does stress really matter? Du Bois Rev 8:95–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213 [DOI] [PubMed] [Google Scholar]
- 49.Flint AJ, Gearhardt AN, Corbin WR, Brownell KD, Field AE, Rimm EB (2014) Food-addiction scale measurement in 2 cohorts of middle-aged and older women. Am J Clin Nutr 99:578–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnson TP, Garrity TF, Stallones L (1992) Psychometric evaluation of the Lexington attachment to pets scale (LAPS). Anthrozoös 5:160–175 [Google Scholar]
- 51.Koenen KC, De Vivo I, Rich-Edwards J, Smoller JW, Wright RJ, Purcell SM (2009) Protocol for investigating genetic determinants of posttraumatic stress disorder in women from the Nurses’ Health Study II. BMC Psychiatry 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fruge AD, Cases MG, Howell CR et al. (2018) Fingernail and toenail clippings as a non-invasive measure of chronic cortisol levels in adult cancer survivors. Cancer Causes Control 29:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tworoger SS, Hankinson SE (2006) Use of biomarkers in epidemio-logic studies: minimizing the influence of measurement error in the study design and analysis. Cancer Causes Control 17:889–899 [DOI] [PubMed] [Google Scholar]
- 54.Vaught J, Rogers J, Myers K et al. (2011) An NCI perspective on creating sustainable biospecimen resources. J Natl Cancer Inst Monogr 2011:1–7 [DOI] [PubMed] [Google Scholar]
- 55.Townsend MK, Clish CB, Kraft P et al. (2013) Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem 59:1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Izawa S, Miki K, Tsuchiya M et al. (2015) Cortisol level measurements in fingernails as a retrospective index of hormone production. Psychoneuroendocrinology 54:24–30 [DOI] [PubMed] [Google Scholar]
- 57.Russell E, Koren G, Rieder M, Van Uum S (2012) Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37:589–601 [DOI] [PubMed] [Google Scholar]
- 58.Stanforth PR, Gagnon J, Rice T et al. (2000) Reproducibility of resting blood pressure and heart rate measurements. The HERITAGE Family Study. Ann Epidemiol. 10:271–277 [DOI] [PubMed] [Google Scholar]
- 59.Shekelle RB, Shryock AM, Paul O et al. (1981) Diet, serum cholesterol, and death from coronary heart disease. The Western Electric study. N Engl J Med 304:65–70 [DOI] [PubMed] [Google Scholar]
- 60.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE (1995) Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol Biomarkers Prev 4:649–654 [PubMed] [Google Scholar]
- 61.Brumpton BM, Leivseth L, Romundstad PR et al. (2013) The joint association of anxiety, depression and obesity with incident asthma in adults: the HUNT study. Int J Epidemiol 42:1455–1463 [DOI] [PubMed] [Google Scholar]
- 62.Trudel-Fitzgerald C, Qureshi F, Appleton AA, Kubzansky LD (2017) A healthy mix of emotions: underlying biological pathways linking emotions to physical health. Curr Opin Behav Sci 15:16–21 [Google Scholar]
- 63.Huang T, Zeleznik OA, Poole EM, et al. (2018) Habitual sleep quality, plasma metabolites and risk of coronary heart disease in post-menopausal women. Int J Epidemiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaimi I, Pei D, Koestler DC et al. (2018) Variation in DNA methylation of human blood over a 1-year period using the illumina methylation EPIC array. Epigenetics 13:1056–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim CH, Tworoger SS, Stampfer MJ et al. (2018) Stability and reproducibility of proteomic profiles measured with an aptamer-based platform. Sci Rep 8:8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.