SUMMARY
Recent adverse event reports have raised the question of increased angioedema risk associated with exposure to levetiracetam. To help address this question, the Observational Health Data Sciences and Informatics research network conducted a retrospective observational new-user cohort study of seizure patients exposed to levetiracetam (n = 276,665) across 10 databases. With phenytoin users (n = 74,682) as a comparator group, propensity score-matching was conducted and hazard ratios computed for angioedema events by per-protocol and intent-to-treat analyses. Angioedema events were rare in both the levetiracetam and phenytoin groups (54 vs. 71 in per-protocol and 248 vs. 435 in intent-to-treat). No significant increase in angioedema risk with levetiracetam was seen in any individual database (hazard ratios ranging from 0.43 to 1.31). Meta-analysis showed a summary hazard ratio of 0.72 (95% confidence interval [CI] 0.39–1.31) and 0.64 (95% CI 0.52–0.79) for the per-protocol and intent-to-treat analyses, respectively. The results suggest that levetiracetam has the same or lower risk for angioedema than phenytoin, which does not currently carry a labeled warning for angioedema. Further studies are warranted to evaluate angioedema risk across all antiepileptic drugs.
Keywords: Angioedema, Levetiracetam, Anticonvulsant hypersensitivity syndrome, Pharmacovigilance, Observational research, Adverse drug reactions
In late 2015, the U.S. Food and Drug Administration (FDA) noted a potential safety signal in the Federal Adverse Event Reporting System (FAERS1) associating the anticonvulsant levetiracetam with increased risk for angioedema.2 Anticonvulsant medications are known to be associated with a wide variety of cutaneous reactions, ranging from mild rash to severe hypersensitivity syndromes.3,4 Angioedema is a rare but potentially fatal adverse reaction manifested by a rapid swelling of the face, mouth, tongue, and throat.5 Angioedema may be accompanied by limb or intestinal swelling as well as severe respiratory compromise. Although most anticonvulsants have at least a few sporadic reports of angioedema in the FAERS system,6 currently only five anticonvulsants have demonstrated sufficient evidence to carry a labeled warning for angioedema (lamotrigine, gabapentin, pregabalin, brivaracetam, and lacosamide).7
In the FDA’s announcement regarding levetiracetam and angioedema, the agency stated that it was evaluating the evidence to determine the possible need for regulatory action. Given the rarity of angioedema, determining its association with medication exposures is challenging and requires a large number of patients to reliably assess. The Observational Health Data Sciences and Informatics (OHDSI) collaborative is a research network with federated data on >600 million patients harmonized to a common data model.8,9 One of OHDSI’s primary activities is pharmacovigilance, which is the science relating to the detection, assessment, and prevention of adverse drug effects.10 Using its large data network, OHDSI researchers sought to evaluate angioedema risk in patients exposed to levetiracetam and to provide additional insight to clinicians and regulators.
METHODS
Population
A retrospective, observational study was conducted looking at angioedema risk in 276,665 levetiracetam patients. Data were extracted from 10 clinical datasets—5 electronic medical records (EMRs) and 5 claims sets—across 2 countries (9 U.S. and one French). EMRs were from Columbia University Medical Center/New York-Presbyterian Hospital (4.5 million patients), IMS Ambulatory EMR (25 M), IMS French EMR (2.2 M), Stanford Clinical Data Ware-house (2 M), and the University of Texas Cerner Health Facts Database (2.4 M). Claims datasets were OptumIn-sight’s Clinformatics Datamart (40.7 M), IMS Pharmetrics Plus (105 M), Truven MarketScan Commercial Claims and Encounters (CCAE) (122 M), Truven MarketScan Multistate Medicaid (MDCD) (17.3 M), and Truven MarketScan Medicare Supplemental Beneficiaries (MDCR) (9.3 M).
Study design
We conducted a new-user cohort study comparing first-time users of levetiracetam with first-time users of phenytoin (n = 74,682) using the open-source OHDSI CohortMethod package11 with large-scale analytics achieved by Cyclops.12 Phenytoin was selected as a comparator drug because it is commonly used as a first-line anti-seizure treatment, has no labeled warning for angioedema after 64 years on the market,13 and has a proportional reporting ratio (PRR) <1 for angioedema in FAERS,14 indicating that angioedema reports associated with phenytoin occur at a frequency similar to the baseline frequency for angioedema reports across all drugs.
For entry into the study, patients were required to have an exposure to levetiracetam or phenytoin as well as a diagnosis of seizure disorder (code list at https://goo.gl/n2zXZe) prior to the exposure date. Patients were required to have continuous observation for at least 6 months prior to drug exposure in the database and those with a previous diagnosis of angioedema were excluded from the study.
Patient records were assessed for the occurrence of angioedema (code list at https://goo.gl/XOg1kE). The time of risk was defined in two ways: first, as all time on the drug following initial drug exposure until either the drug was stopped or the patient was no longer observable in the data-base (“per-protocol”); and second, as all time from drug initiation until the patient was no longer observable in the database (“intention to treat”). Multiple prescriptions for a drug were treated as a continuous exposure if the gap between prescriptions was <30 days.
Cox proportional hazard models were used to assess the hazard ratios (HRs) between the two exposure cohorts. Adjustment for baseline confounders was performed by fitting a propensity model and using the resultant propensity scores to match the treatment and comparator cohorts using variable ratio matching.15 The proportional hazards out-come models were conditioned on the matched sets of strata. To identify potential residual bias in HR estimates, 100 negative control outcomes (i.e., not believed to be caused by either levetiracetam or phenytoin) were included in our study.16 HRs were computed for these negative controls and used to compute calibrated p-values17 for angioedema in each dataset. If within-database residual bias is minor according to our negative control analysis and the heterogeneity between databases is low (I2 < 0.25), a meta-analysis for random effects will be performed to combine the estimates across databases. We set a nominal type 1 error rate of 5% without adjusting for multiple testing.
RESULTS
In the per-protocol analysis, 59,367 levetiracetam users were matched with 74,550 phenytoin users for cumulative follow-up of 11,199,152 and 10,597,206 days, respectively. The most important and consistent propensity score model covariates were patient age and year of study entry, followed by race, gender, and number of visits. Angioedema was rare, with 54 events in the levetiracetam cohort and 71 events in the phenytoin cohort (Table I). In 4 of the 10 data-bases, the number of events was too low to compute a unique HR estimate. In the remaining six databases, no statistically significant difference in hazard rate was seen between the levetiracetam and phenytoin groups. Figure 1 shows the forest plot of the per-database estimates for the per-protocol analysis as well as the summary estimate computed using a random-effects model. The I2 for the analysis was 0, indicating heterogeneity low enough to combine estimates across databases. The summary hazard ratio for the per-protocol analysis was 0.72 (0.39–1.31).
Table I.
Angioedema events in propensity score-matched levetiracetam and phenytoin exposed patients using per-protocol analysis and intent-to-treat analysis
| Levetiracetam | Phenytoin | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Patients | Days treated | Events | Patients | Days treated | Events | Hazard ratio (Cl) | p-Value (calibrated) | |
| Per protocol | IMS P-Plus | 6,893 | 351,090 | 2 | 7,745 | 398,827 | 2 | 1.41 (0.05–36.73) | 0.83 (0.79) |
| Optum Clinformatics | 10,819 | 3,150,504 | 14 | 14,115 | 3,030,739 | 19 | 0.69 (0.18–2.34) | 0.57 (0.62) | |
| Truven CCAE | 13,088 | 3,549,812 | 13 | 16,234 | 2,962,530 | 14 | 0.59 (0.15–1.93) | 0.41 (0.45) | |
| Truven MDCD | 8,227 | 1,883,518 | 15 | 9,969 | 1,666,857 | 19 | 0.65 (0.20–1.91) | 0.45 (0.55) | |
| Truven MDCR | 4,592 | 1,400,797 | 8 | 6,433 | 1,564,355 | 14 | 0.96 (0.28–3.1 1) | 0.94 (0.94) | |
| IMS Ambulatory | 8,762 | 618,757 | 1 | 10,732 | 730,158 | 3 | 0.00 (0.00–1.41) | 0.23 (0.23) | |
| Cerner Health Facts (UT) | 5,584 | 54,852 | 1 | 7,624 | 93,543 | 0 | – | – | |
| Columbia | 501 | 111,307 | 0 | 603 | 68,251 | 0 | – | – | |
| IMS French EMR | 7 | 552 | 0 | 37 | 2,463 | 0 | – | – | |
| Stanford EMR | 404 | 12,313 | 0 | 460 | 13,525 | 0 | – | – | |
| lntent-to-treat | IMS P-Plus | 18,213 | 16,233,093 | 78 | 21,631 | 18,398,218 | 117 | 0.61 (0.42–0.87) | 0.01 (0.008) |
| Optum Clinformatics | 10,890 | 9,101,161 | 31 | 14,254 | 10,818,306 | 65 | 0.73 (0.39–1.33) | 0.31 (0.36) | |
| Truven CCAE | 13,434 | 11,347,801 | 41 | 16,886 | 13,390,9 IS | 61 | 0.87 (0.49–1.52) | 0.63 (0.57) | |
| Truven MDCD | 8,536 | 7,328,658 | 41 | 11,688 | 9,603,677 | 96 | 0.43 (0.25–0.72) | 0.00 (0.002) | |
| Truven MDCR | 4,656 | 4,317,982 | 15 | 6,534 | 5,516,323 | 37 | 0.54 (0.23–1.18) | 0.14 (0.19) | |
| IMS Ambulatory | 8,762 | 9,978,497 | 19 | 10,732 | 12,739,915 | 28 | 0.65 (0.31–1.31) | 0.25 (0.25) | |
| Cerner Health Facts (UT) | 9,094 | 5,842,344 | 22 | 12,076 | 8,229,377 | 29 | 0.95 (0.46–1.94) | 0.90 (0.69) | |
| Columbia | 553 | 523,215 | 1 | 686 | 643,655 | 1 | – | – | |
| IMS French EMR | 7 | 5,542 | 0 | 37 | 45,559 | 0 | – | – | |
| Stanford EMR | 404 | 342,136 | 0 | 460 | 41 1,371 | 0 | – | – | |
Figure 1.
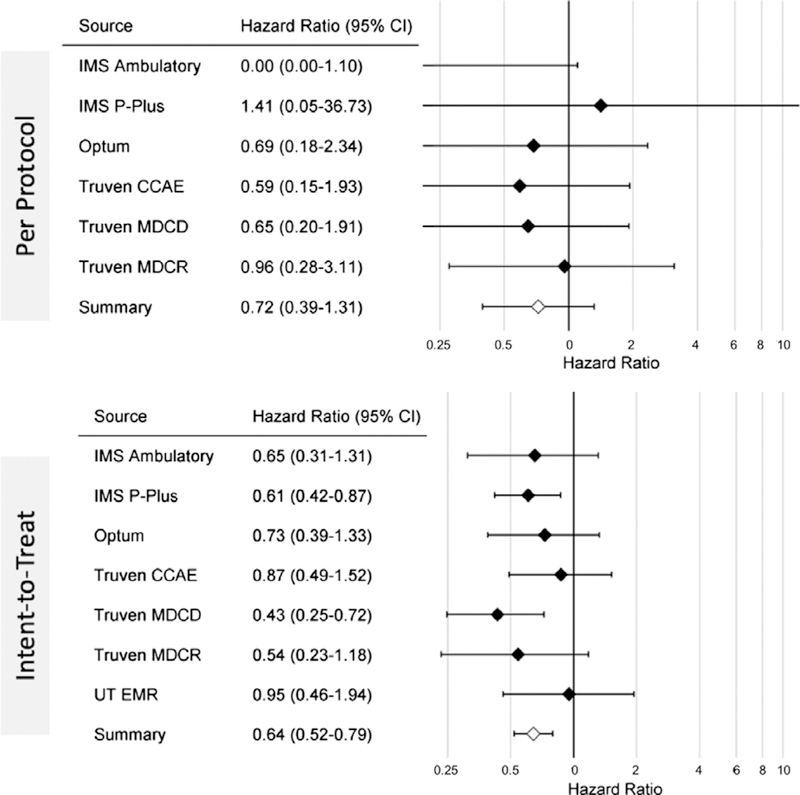
Forest plots showing the HRs for angioedema with exposure to levetiracetam compared with phenytoin in patients with seizure disorder across multiple databases, as well as combined estimate, for the per-protocol and intent-to-treat analyses.
In the intent-to-treat analysis, 75,056 levetiracetam patients were matched with 95,598 phenytoin patients for cumulative observation periods of 80,164,173 and 96,182,651 days, respectively. Overall, 248 angioedema occurrences were identified in levetiracetam users and 435 events in phenytoin users (Table I). Two of 10 datasets (IMS Pharmetrics and MDCD) yielded a statistically significant lower rate of angioedema in levetiracetam compared with phenytoin. The remaining datasets showed no significant difference between groups. Figure 1 (lower) shows the forest plot of the per-database estimates for the intent-to-treat analysis as well as the computed summary estimate (I2 was again 0). The summary hazard ratio for the intent-to-treat analysis was 0.64 (0.52–0.79).
Shown in Figure 2 are the Kaplan-Meier curves from the four databases where the event count was greater than five in the per-protocol analysis. The curves highlight the lack of significant difference in angioedema outcomes over a 5-year horizon. The shaded areas (representing the 95% confidence interval [CI]) suggest higher confidence in this nondifference during the early postexposure period (e.g., <500 days).
Figure 2.
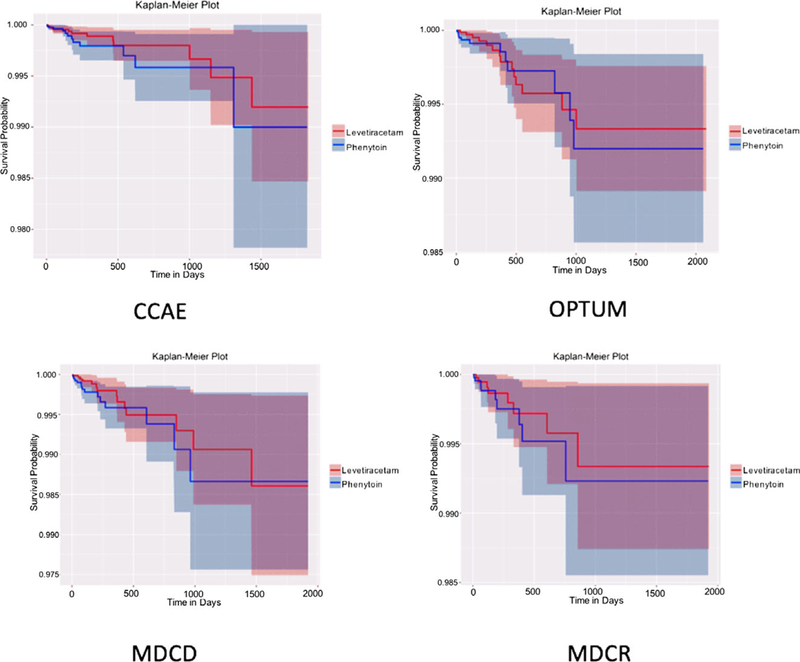
Kaplan-Meier plots showing angioedema events in levetiracetam (red) and phenytoin (blue) cohorts from four claims datasets in the per-protocol analysis. Shaded areas indicate 95% CI.
DISCUSSION
This large retrospective observational study of patients with seizure disorder showed no evidence of increased angioedema risk with levetiracetam use compared with phenytoin use. These results were consistent across 10 data-sets including both claims and EMR data as well as U.S. and European sources. These results will hopefully provide useful evidence to the FDA in its regulatory assessments as well as to clinicians making prescribing decisions regarding levetiracetam.
Of interest, our study found an increased risk of angioedema associated with phenytoin use compared with levetiracetam use in the intent-to-treat analysis. Although phenytoin, like other anticonvulsants, has been associated with hypersensitivity syndromes, it currently carries no labeled warning for angioedema. The threshold for assignation of labeled warnings may have changed over the decades since phenytoin’s approval. However, it would be worth conducting further studies to clarify phenytoin’s risk profile regarding angioedema in large-scale populations.
Several methodologic aspects of this study are worth note. First, the study was conducted across a global research network in which a common data model allows the rapid execution of observational research studies including large numbers of patients. Second, all protocols and study codes are available publicly for detailed review and to encourage reproducibility in research. Finally, recognizing that bias can be a major concern in studies using claims and EMR data, extensive effort has been made to account for such biases including the use of negative control outcomes. Our 100 negative controls showed little residual bias in the study, as reflected in the minor differences between calibrated and uncalibrated p-values. This finding also helps to limit remaining concern that propensity score matching may introduce bias when imbalance in baseline covariates remains between matched pairs.18
A limitation of this analysis is the rarity of angioedema events. Several datasets did not have sufficient numbers of cases to calculate HRs. Because of the paucity of events, CIs grew very large at time windows distant from exposure (e.g., 3–5 years). Thus the assertion of no increased risk with levetiracetam can be most reliably made for the short- to medium-term and would require much larger volumes of patient data to confidently assess over the long term.
Additional large-scale observational studies are warranted to compare angioedema risk across all antiepileptic medications. A broader class analysis will minimize bias associated with selection of comparator drugs and provide clearer insight into the relative risk of this rare but potentially fatal outcome associated with epilepsy treatment.
ACKNOWLEDGMENTS
No funding was received for the conduct of this study. One of the authors (MS) is supported in part through the National Science Foundation grant IIS 1251151.
Biography

Dr. Jon Duke is Director of the Center for Health Analytics and Informatics at the Georgia Tech Research Institute.
Footnotes
DISCLOSURE OF CONFLICT OF INTEREST
One of the authors (JD) changed institutions after the protocol had been completed and at his new institution joined an ongoing unrelated informatics project funded by Union Chimique Belge (UCB), a brand manufacturer of levetiracetam. The remaining authors have no conflicts of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
REFERENCES
- 1.FDA Adverse Event Reporting System (FAERS) (formerly AERS). Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. Accessed October 25,2013.
- 2.Research C for DE. FDA Adverse Events Reporting System (FAERS) - Potential Signals of Serious Risks/New Safety Information Identified by the FDA Adverse Event Reporting System (FAERS) between October–December 2015. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm491645.htm. Accessed February 12, 2017.
- 3.Vittorio CC, Muglia JJ. Anticonvulsant hypersensitivity syndrome. ArchInternMed 1995;155:2285. [PubMed] [Google Scholar]
- 4.Leyva L, Torres MJ, Posadas S, et al. Anticonvulsant-induced toxic epidermal necrolysis: monitoring the immunologic response. J Allergy Clin Immunol 2000;105:157–165. [DOI] [PubMed] [Google Scholar]
- 5.Lerch M Drug-induced angioedema. Chem Immunol Allergy 2012;97:98–105. [DOI] [PubMed] [Google Scholar]
- 6.Böhm R, von Hehn L, Herdegen T, et al. OpenVigil FDA – inspection of U.S. American adverse drug events pharmacovigilance data and novel clinical applications. PLoS ONE 2016;11:e0157753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DailyMed: About DailyMed. http://dailymed.nlm.nih.gov/dailymed/about.cfm. Accessed February 10, 2017.
- 8.Hripcsak G, Duke JD, Shah NH, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform 2015;216:574–578. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26262116. Accessed February 12, 2017. [PMC free article] [PubMed] [Google Scholar]
- 9.OHDSI Research Network Databases. Available at: http://www.ohdsi.org/web/wiki/doku.php?id=resources:data_network. Accessed April 27, 2017.
- 10.World Health Organization. The importance of pharmacovigilance. 2002. Available at: http://apps.who.int/iris/bitstream/10665/42493/1/a75646.pdf. Accessed April 27,2017.
- 11.Schuemie MJ, Suchard M, Ryan P. CohortMethod: New-user cohort method with large scale propensity and outcome models. 2015. Available at: http://www.github.com/ohdsi/CohortMethod. Accessed November 15, 2017.
- 12.Suchard MA, Simpson SE, Zorych I, et al. Massive parallelization of serial inference algorithms for complex generalized linear models. ACM Trans Model Comput Simul 2013;23:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DailyMed - DILANTIN- phenytoin sodium capsule, extended release. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8848de76-8d74-4620-bcc7-a86a596e5dd9. Accessed February 12, 2017.
- 14.KnowledgeBaseWeb. Available at: http://www.ohdsi.org/web/knowledgebaseweb/. Accessed February 12, 2017.
- 15.Rassen JA, Shelat AA, Myers J, et al. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 2012;21:69–80. [DOI] [PubMed] [Google Scholar]
- 16.Arnold BF, Ercumen A, Benjamin-Chung JCJJ. Negative controls to detect selection bias and measurement bias in epidemiologic studies. Epidemiology 2016;275:637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuemie MJ, Ryan PB, DuMouchel W, et al. Interpreting observational studies: why empirical calibration is needed to correct p-values. Stat Med 2014;33:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King G, Nielsen R. Why propensity scores should not be used for matching. 2016. Available at: http://j.mp/1sexgVw. Accessed April 27,2017.


