Abstract
Macroscale biomaterials, such as preformed implantable scaffolds and injectable soft materials, possess powerful synergies with anti-cancer immunotherapies. Immunotherapies on their own typically have poor delivery properties, and often require repeated high-dose injections that result in serious off-tumor effects and/or limited efficacy. Rationally designed biomaterials allow for discrete localization and controlled release of immunotherapeutic agents, and have been shown in a large number of applications to improve outcomes in the treatment of cancers via immunotherapy. Among various strategies, macroscale biomaterial delivery systems can take the form of robust tablet-like scaffolds that are surgically implanted into a tumor resection site, releasing programmed immune cells or immunoregulatory agents. Alternatively they can be developed as soft gel-like materials that are injected into solid tumors or sites of resection to stimulate a potent anti-tumor immune response. Biomaterials synthesized from diverse components such as polymers and peptides can be combined with any immunotherapy in the modern toolbox, from checkpoint inhibitors and stimulatory adjuvants, to cancer antigens and adoptive T cells, resulting in unique synergies and improved therapeutic efficacy. The field is growing rapidly in size as publications continue to appear in the literature, and biomaterial-based immunotherapies are entering clinical trials and human patients. It is unarguably an exciting time for cancer immunotherapy and biomaterial researchers, and further work seeks to understand the most critical design considerations in the development of the next-generation of immunotherapeutic biomaterials. This review will discuss recent advances in the delivery of immunotherapies from localized biomaterials, focusing on macroscale implantable and injectable systems.
Keywords: Biomaterials, Immunotherapy, Cancer, Localized delivery
1. Introduction
Biomaterials have emerged as powerful methods of drug delivery and localization, allowing for both spatial and temporal control of loaded agents, as well as behavioral control of interacting cell populations [1,2]. Biomaterial research has progressed a long way since the field’s more humble beginnings in the mid-20th century, when war-time clinicians observed the surprising ‘biocompatibility’ of plastic aircraft shrapnel embedded in wounded pilots [3,4]. Now the term biomaterial encompasses a wide variety of systems that are much more dynamic, including lipid carriers [5–9], synthetic nanoparticles and microparticles [10–16], implantable or injectable scaffolds and hydrogels [17–25], or even microneedle arrays [26–30], which have each been used in a variety of synergistic therapeutic strategies. Representing a significant area of collaboration between the fields of chemistry, materials science, bioengineering, and medicine, the potential applications of biomaterials are enormous. This versatility has been directed to the field of immunotherapy, where materials can be designed to not only release immunomodulatory factors in a controlled fashion, but also to direct the host immune response and program immune cells trafficking to and from the material [22,31–33]. Much work therefore is being devoted to advancing the applications of biomaterials for immunomodulation, whether in infectious diseases [34–37], autoimmune disorders [38–41], regenerative medicine [42–44], or cancer [45–48].
Cancer represents a unique challenge as a disease, and remains one of the greatest threats to public health despite recent advances. In 2018, over 1.7 million new cancer cases and over 600,000 cancer deaths were projected to have occured in the United States alone [49]. One of the well-known hallmarks of cancer is a profound element of immunosuppression and avoidance of the natural immune system, which has inspired significant research and advances in the field of immunotherapy [50–55]. Immunotherapies have revolutionized the treatment of various cancers within the past few decades, with early clinical trials in the 1990 s leading to FDA approval of the first major immunotherapy drug, Sipuleucel-T, for prostate cancer in 2010 [56–59]. For example, thanks to recently approved checkpoint inhibitors such as CTLA-4 (ipilimumab) and PD-1 antibodies (nivolumab and pembrolizumab) which serve to reactivate tumor-suppressed immune cells [60–65], certain advanced disease states that were previously untreatable have shown remarkable susceptibility to immunotherapy drugs [66–71]. These remarkable strides led to the 2018 Nobel Prize in Physiology or Medicine being awarded to Drs. James Allison and Tasuku Honjo for their discoveries in checkpoint inhibitor immunotherapy [72]. Yet every discussion on the exciting prospects of immunotherapy includes the unfortunate disclaimer that current treatments still often don’t work, whether it is for certain patients who simply fail to respond or for certain cancers that are better able to avoid the immune system. Additionally, with repeated injections or infusions at high doses often required, many immunotherapy patients suffer from serious systemic side effects, resulting in increasing treatment costs both financially and physically [73–77]. Traditional immunotherapies therefore remain limited in scope and efficacy, driving the need to investigate alternate treatment strategies [78,79]. An ever-growing body of research has shown that the synergistic effects of biomaterials with various adjuvants and immunotherapies could rescue the field from some of its current limitations [80].
This review discusses the exciting prospects of using biomaterials for enhanced immunotherapy, and the newest work published in this growing field. Extensive research has been done on nanoparticle and microparticle biomaterials for encapsulated drug delivery and targeted therapies, which are ideal for use when tumors are prohibitively small or physically inaccessible, but these approaches will not be discussed here and the reader is directed to other publications on the subject [80–92]. A very recent review by Wang and Mooney highlighted many of the advances made in the past decade with cell-targeted, biomaterial-assisted cancer immunotherapy, including an extensive body of particle-based systems and their relevant ongoing clinical trials [93]. Furthermore, an elegant comprehensive review on the entire field of immunotherapeutic biomaterials was recently published by Bookstaver et al. [94]. This review will focus on the most recent advances made in the use of porous scaffolds, cryogels, and hydrogels to provide spatial and temporal control over therapy delivery. These localizable biomaterials show unique promise in delivering and presenting various bioactive factors to immune cells within a discrete, three-dimensional environment, allowing for not only local elimination of tumors, but also systemic disease immunity (Fig. 1).
Fig. 1.
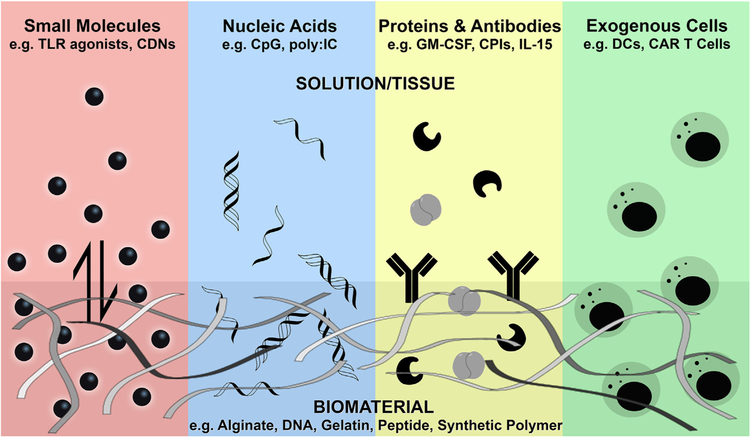
Biomaterials can be designed to control the release and presentation of a variety of bioactive immunotherapies, often creating an extended concentration gradient surrounding the material, which is often nanofibrous in structure. Loaded agents can include immunostimulatory small molecules, nucleic acid adjuvants, various proteins and antibodies, or even exogenous immune cells whose growth and expansion can be supported by the customized biomaterial scaffold.
2. Immunotherapy and biomaterials: A summary
In the following two sections, the broad topics of immunotherapies and biomaterials will be briefly introduced, providing a general overview of key terminology and the current strategies and designs used in these fields (Table 1).
Table 1.
Summary of key reviewed biomaterial immunotherapy strategies.
| Material Type | Immunotherapy Strategy | Results & Applications | Date & Reference | |
|---|---|---|---|---|
| Implantable Biomaterials | PLG polymer scaffold | GM-CSF recruitment factor, CpG danger signal, tumor lysate antigens, CPI mAbs | Locally recruits immune cells, activates DCs to enhance anti-tumor T cell response | 2009–2015 [19–21,95–99] |
| Alginate biopolymer implants | Exogenous T cells, stimulatory microparticles, IL-15 signal | Supports adoptive T cell viability and local expansion for tumor resection treatment | 2015 [24] | |
| CAR T cells, STING agonist stimulatory microparticles | Supports T cell action against solid tumors, eliminating heterogeneous cancers | 2017 [100] | ||
| Polyglyconate and porcine gelatin scaffold | CCL17 chemokine for CD8 + T cell engagement | Recruits T cells to site of implantation, reducing tumor size and inhibiting metastasis | 2017 [101] | |
| Collagen / hyaluronic acid scaffold | Gemcitabine for MDSC depletion, poly(I:C) agonist nanogels, tumor lysate antigens | Counters immune suppression, recruits and activates immune cells in resection site | 2018 [102] | |
| Hyaluronic acid hydrogel implant | TLR7/8 or STING agonists | Prevents cancer recurrence and metastasis post-surgery | 2018 [103] | |
| Injectable Biomaterials | Polymer hydrogel | DC-chemoattractants, immunostimulatory IL-10 siRNA, plasmid DNA antigens | Stimulates DC infiltration and promotes immune cell programming | 2009–2011 [17,18] |
| PEG-agarose microspheres/hydrogel | Activated macrophages to release natural cytokines | Delivers and confines activated immune cells to local site | 2009 [104] | |
| Alginate hydrogels | Exogenous DCs, IL-2 or CpG oligonucleotides | Facilitates DC proliferation and activity | 2008–2009 [105,106] | |
| Celecoxib and PD-1 CPIs | Local injection maintains high drug concentration and efficacy | 2015 [107] | ||
| GM-CSF, peptide antigens | Pore-forming gel accumulates enriched DC population, and increases antigen-presentation | 2015–2017 [108,109] | ||
| Alginate cryogel | GM-CSF, CpG, irradiated melanoma cell antigens | Promotes adhesion of irradiated cell antigens, and shows sustained immune response | 2012–2015 [110–112] | |
| Gelatin cryogel | GM-CSF | Supports immune cell attachment and proliferation | 2014 [113] | |
| Peptide nanofiber vaccines | Peptide antigen display, immune epitopes | Activates specific and controllable immune response | 2010–2017 [114–119] | |
| Mesoporous silica rods | GM-CSF, CpG-ODNs, model antigens or antigen pools | Self-assembles into an immune cell trafficking drug-depot | 2014–2018 [120–122] | |
| Structured DNA hydrogel | CpG dinucleotides, cationic antigens | Stimulates reaction against antigen-specific tumors | 2014–2015 [123,124] | |
| D-tetra-peptide hydrogel | Material immunogenicity, model antigens | Improved antigen uptake and potent anti-tumor activity | 2017 [125] | |
| Polypeptide-copolymer hydrogel | Tumor cell lysate antigens, poly(I:C) agonist | Recruits DCs and programs anti-tumor activity | 2018 [126] | |
| Self-assembling peptide hydrogels | Exogenous DCs, PD-1 CPIs, tumor antigens | Supports DC efficacy and prolongs survival | 2018 [127] | |
| Matrigel | Cyclic dinucleotide (CDN) STING agonist | Maintains high local drug concentration and generates potent tumor rejection | 2018 [128] | |
| CDN STING agonist | Localizes T cell activation and improves efficacy in resections | 2018 [129] | ||
| Other | Sprayable fibrin hydrogel | anti-CD47 antibodies | Inhibits tumor recurrence and metastatic spread | 2019 [130] |
2.1. Modern immunotherapies
Immunotherapy can be defined as any treatment that utilizes parts of a patient’s own immune system to combat cancer, or any other immune-susceptible disease. This is in contrast to traditional chemotherapies, which typically use strongly cytotoxic drugs to directly act against cancer cells. While chemotherapies can be effective at treating certain types of cancer, treatments often come with severe side effects and compromised quality of life, and disease recurrence is almost always a concern. While immunotherapies still have much room to improve, such treatments have shown impressive systemic tumor eradication resulting in durable immune memory and resistance. It is a field that is rapidly becoming an indispensable pillar of cancer treatment.
Many immunotherapy treatment strategies have arisen over the past few decades, seeking to enhance the immune system’s natural capability to recognize foreign or cancer-related antigens for selective elimination. In this section, some of the most promising current therapies will briefly be discussed, including checkpoint inhibitor antibodies, T cell-based therapies, signaling proteins, and immunostimulatory small molecules and agonists. As has already been mentioned, some of the major clinical advances in modern cancer immunotherapy have come through the development and regulatory approval of checkpoint inhibitor antibodies (CPIs). CPI antibodies function by binding to and blocking key inhibitory immune cell receptors, most significantly the CTLA-4 (cytotoxic T lymphocyte-associated antigen 4) and PD-1 (programmed cell death protein 1) receptors [60,131,132]. When activated by native protein agonists, these so called ‘checkpoint’ pathways negatively regulate T cell activation and function, effectively putting the brakes on the host’s immune system [133–135]. Cancer cells have evolved mechanisms to use these checkpoint receptors to avoid or inhibit detection and destruction by the immune system [136]. Many CPI antibodies seek to block the CTLA-4 and PD-1 receptors from being bound by deactivating checkpoint agonists, restoring T cell immune function against immunosuppressive cancers [70,137].
Cell-based therapies are also a large component of modern immunotherapeutic research, typically involving the reintroduction of amplified immune cells into a patient to facilitate an enhanced immune response, rather than trying to directly modulate a patient’s native immune cell population. This strategy is most commonly referred to as Adoptive T cell (ATC) immunotherapy, where cultured immune cells (often extracted from a patient and amplified ex vivo) are reintroduced into a subject in order to promote immune destruction of cancerous cells [138]. However, ATC immunotherapy often shows limited efficacy as well as systemic toxicity, because reintroduced immune cells can have compromised functionality or fail to expand and persist in the desired location [139–141]. Advanced ATC strategies include chimeric antigen receptor (CAR) T cell therapy which utilizes T cells that have been engineered to express recombinant CARs, both enhancing T cell activity and tumor antigen binding for improved selectivity [142,143]. Yet even CAR T cell therapy still shows poor lasting treatment efficacy against advanced solid tumors, due to the immunosuppressive tumor microenvironment and select cancer subpopulations better able to evade immune detection [48,144]. Different strategies (many of which are materials-based) can seek to support exogenous T cell viability and activity, as will later be discussed.
A variety of bioactive proteins are used in modern immunotherapeutic treatment strategies. GM-CSF (granulocyte–macrophage colony-stimulating factor) is an often-used cytokine that induces dendritic cell (DC) chemotaxis, differentiation, and proliferation [145–148]. As an inflammatory cytokine, it is ideally suited for use in immunotherapy applications to promote a localized immune response, and indeed many of the biomaterial studies reviewed here include GM-CSF in their strategic formulation. Other cytokines used for immunotherapy include CCL17 (Chemokine C–C motif ligand 17) or Thymus and Activation Regulated Chemokine (TARC), which regulates lymphocyte migration and can be used to enhance T cell targeting to a particular location [149–152], and IL-15 (Interleukin 15), which can be used in its native form or as a superagonist to support the expansion and survival of cytotoxic T cells and natural killer cells critical for effective immunotherapy [153–155].
Additionally, there are a large number of natural and synthetic compounds commonly used as immune adjuvants or agonists. CpG (cytosine-guanine) oligonucleotides, while rare in vertebrate DNA, are common in bacteria such that the immune system has adapted to recognize CpG oligonucleotides as a danger signal for infection through Toll-Like Receptor 9 (TLR9) [156–159]. CpG has therefore long been recognized as a useful tool in immunotherapeutic applications to activate mammalian DCs [160]. Similarly, polyinosinic: polycytidylic acid (poly:IC) is a TLR3 agonist composed of inosine and cytidine RNA strands, and it has been shown to effectively mimic viral infection to induce an inflammatory immune response [159,161–163]. Other TLR7/8 agonists such as imidazoquinoline compounds are also used as immunotherapy adjuvants, inducing the expression of inflammatory cytokines and facilitating the maturation of DCs [159,164,165]. Finally, while this brief survey is not fully inclusive of all the therapeutic strategies employed by researchers, a discussion of modern immunotherapies is incomplete without including cyclic dinucleotides (CDNs). CDNs are a more recent class of compounds in the field, with ongoing clinical trials now evaluating their anti-tumor efficacy in human patients [166,167]. As potent activators of innate immunity through the STING (Stimulator of Interferon Genes) signaling pathway, CDNs are becoming increasingly used in the literature for anti-cancer therapy and systemic tumor elimination [168–171]. However, like other strongly immunostimulatory agents, systemic administration of CDNs can lead to excessive toxicity and inflammation [172–174]. CDN STING agonists have therefore been used in a variety of focused delivery systems, whether encapsulated in liposomes and other nanoparticles [172,175], or loaded in macroscale biomaterials as will soon be discussed in more detail [100,103,128].
2.2. Common macroscale biomaterials
A macroscale biomaterial can be defined as any biocompatible carrier material with a bulk size often greater than 1 mm3 that offers spatiotemporal control over loaded agents, regulating their release and presentation to the surrounding biological environment [1]. Such localizable bulk materials are by no means simple when compared to micro- or nano-scale strategies, and in many cases macroscale materials possess distinct and intentionally designed nanostructures that are key to their controlled release mechanisms. Biomaterials can be made from a wide host of natural or synthetic components, and bulk implantable or injectable systems have been developed from materials such as alginate, hyaluronic acid, collagen, nucleic acids, synthetic polymers, peptides, and more, representing an incredibly diverse set of tools in the bioengineer’s toolbox (Fig. 2) [176–183]. This section will attempt to provide a brief survey of some common macroscale biomaterial components and their properties, and examples of such materials will appear throughout this review as they are applied to immunotherapy.
Fig. 2.
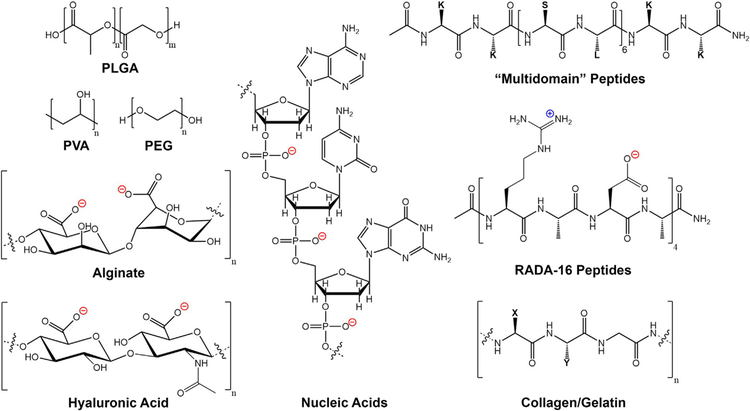
Common biomaterials used in immunotherapeutic applications can be synthesized from a wide variety of starting materials with unique chemical functionalities. Examples include synthetic polymers such as polyesters, polyethers, and polyalcohols (PLGA, PEG, PVA etc.), naturally derived polysaccharides such as alginate and hyaluronic acid, as well as natural protein matrices such as collagen or gelatin which are typically composed of variations of the repeating amino acid sequence XYG (where X is often Proline, and Y is often Hydroxyproline). Furthermore, materials can be created from nucleic acids (DNA, RNA), and rationally designed self-assembling peptides (e.g. Multidomain peptides, RADA-16 peptides).
One widely used polymer for biomaterial scaffolds is poly (lactide-co-glycolide) acid (PLG or PLGA). PLGA is an attractive polymer for biological applications for many reasons: it is easily degradable by hydrolysis to the biocompatible components lactic and glycolic acid, is easily customizable and tunable in the process of designing devices, and is an FDA approved material [184,185]. Materials made from PLGA polymers are typically relatively strong and stiff, and form large pores useful for bulk loading of payloads such as drugs, proteins, or even cells [186]. PLGA is also often used to encapsulate payloads into nanoparticles for improved delivery properties [187,188]. Other typical polymers used in biomaterials synthesis include poly(ethylene glycol) (PEG) [181,189], poly(vinyl alcohol) (PVA) [190], and composite systems containing more than one such material.
Another common class of biomaterials is based on collagen, which is a key component of the extracellular matrix (ECM) and the most abundant protein in the human body [191]. Collagen-based materials typically utilize Type-I collagen, and such materials have been used for decades as ECM-mimicking cell scaffolds for tissue regeneration and other applications [192–194]. Collagen hydrogels are biocompatible and easily support cell adhesion and proliferation, but often suffer from poor mechanical and handling properties, requiring further modification and customization [182,195]. Gelatin, a common food-additive protein derived from hydrolysis of natural collagen, is similarly used to create composite biomaterials with unique mechanical properties, though they are often sensitive to temperature changes [196,197]. Like collagen, hyaluronic acid (HA) is another example of a naturally derived, FDA approved material that is a key polysaccharide component of the ECM, giving it good biocompatibility and biodegradability [198]. HA has also been widely used to make various hydrogels and scaffolds with handling properties often more favorable than collagen, though chemical modification and covalent crosslinking is necessary to create such materials [178,199]. Collagen and HA have even been used in combination with each other to utilize the strengths of both components, generating composite materials which together effectively mimic the extracellular matrix [178].
One of the first biomaterials developed for wound treatment, fibrin gels are a common class of FDA approved materials formed from the polymerization of fibrinogen, a common protein found in blood plasma, and the protease thrombin that initiates the so called ‘clotting cascade’ [200,201]. The resulting fibrin hydrogels are very similar in structure and mechanical properties to a natural blood clot, and are therefore biodegradable and easily applicable to in vivo work [202]. Also on the FDA approved list of biomaterial components, alginate is a naturally occurring biocompatible polysaccharide which can act as a reservoir for loaded factors or cells [203,204]. Alginate is widely used as a biomaterial for its low cost to manufacture on a large scale, its low immunogenicity, and its inherently ionic chemical functionality and hydrophilicity. Alginate possesses regular carboxylic acid moieties from the sugar subunits that make up the polysaccharide, which allow for insolution electrostatic crosslinking with multivalent cations to form useful macroscale materials without covalent crosslinking [205,206].
Biomaterials can also be prepared from nucleic acids, such as crosslinked DNA or branched DNA hydrogels [207,208]. DNA biomaterials typically possess inherent immunogenicity, which can be directly enhanced or suppressed based on the choice of nucleic acid sequences. In many DNA-based materials, ligase enzymes are used to crosslink DNA branches and form robust interconnected networks. However, the process of ligation can result in residual enzyme contamination in the final material, and therefore alternative methods have been developed for ligation-free DNA hydrogels through complementary sticky-end self-assembly or pH control [123,209,210].
Finally, a large class of materials has been developed through the use of rationally designed peptides, often relying on supramolecular self-assembly to form useful biomaterials without harsh crosslinking reactions or the need for mixed material composites [211–213]. Peptide-based materials are highly customizable due to the modular nature of peptide synthesis, and such peptide materials are inherently biocompatible thanks to their fundamental amino acid composition. Examples of peptide designs that self-assemble into higher order nanostructures (and thus useful biomaterials) include the MAX β-hairpin hydrogels [214–216], the RADA-16 β-sheet hydrogels [217,218], Stupp’s peptide amphiphiles [219,220], and the family of Multidomain peptide (MDP) nanofibrous hydrogels [213,221,222]. Each of these peptide systems have been used in a variety of cell and drug delivery applications.
3. Implantable biomaterials: Porous scaffolds
3.1. Overview of pioneering work in immunotherapeutic scaffolds
Implantable porous scaffolds are biomaterials that can be functionalized or preloaded with various chemical agents, biologic factors, or even cells before being physically inserted into a living host, usually via a minor surgical procedure to place the implant (often the size and consistency of a small tablet or pill) into a subcutaneous or resected tissue space. From this implanted scaffold, bioactive agents can be released in a controlled fashion, often recruiting immune cells to traffic into the large porous matrix of the scaffold for further biological programming (see Fig. 3) [46,223].
Fig. 3.
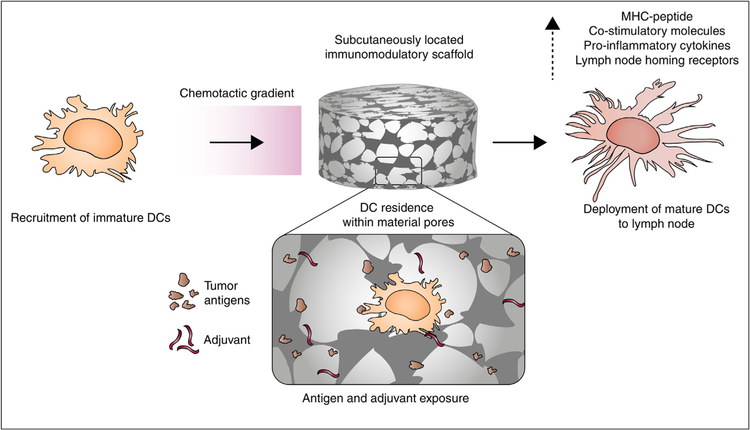
Porous scaffolds can be subcutaneously implanted and designed to release chemoattractants to recruit immature dendritic cells (DCs). Inside the scaffold, DCs are exposed to tumor antigens and adjuvants, resulting in activation and maturation, after which the activated immune cells can exit the scaffold and engage in anti-tumor activity. Reproduced from Koshy and Mooney 2016 [46].
Seminal work in the field of macroscale scaffolds for immunotherapy was done by Ali and Mooney in 2009, setting the stage for further innovation [20]. Ali et al. designed poly (lactide-co-glycolide) (PLG) polymer scaffolds that were loaded with GM-CSF as a recruitment factor, CpG oligonucleotide as a danger signal, and tumor cell lysate as an antigen source, resulting in a wholistic immunotherapy implant that could program specific populations of dendritic cells for anti-tumor activity [19,20]. It was also shown that the implanted scaffold had to remain in vivo for greater than 7 days in order to induce a sufficient immune response and result in tumor regression, and studies in a brain tumor model showed that tumor-treatment efficacy was directedly correlated with the implant’s ability to be in contact with tumor tissue and establish a GM-CSF gradient [95,96]. Further work from 2013 to 2014 by Ali et al. showed the adaptability of the PLG scaffold strategy, demonstrating that alternative immune adjuvants and recruitment factors could be incorporated into the 3-D scaffold and direct immune cell activity [21,97]. The authors continue to innovate and advance the design and applications of the PLG scaffold, describing its ability to load and deliver various agonists and be used in combination with checkpoint antibodies to enhance cytotoxic T cell activity [98,99]. Ali’s original, first-in-man PLG vaccine scaffold truly was a breakthrough in the field, and is currently being evaluated in a Phase I clinical trial for the treatment of stage IV melanoma with an expected completion in 2020 [224]. Fundamentally, these findings showed the exciting potential of macroscale biomaterial immunotherapies, inspiring much of the work that has followed.
3.2. Current work in implantable scaffolds
Advances in the field of implantable materials have continued in the Mooney lab and others. The Stephan lab in 2015 investigated a resorbable alginate biopolymer for surgical implantation post tumor resection, which was shown to significantly enhance adoptive T cell (ATC) immunotherapy [24]. As was briefly introduced earlier, usage of ATC immunotherapy often shows limited efficacy as well as systemic toxicity, for reintroduced immune cells can be functionally compromised or fail to thrive in the implanted location [139–141]. These problems suggest that a biomaterial able to support T cell viability and localize the desired immune response would be valuable. To this end, Stephan et al. developed castable porous scaffolds from polymerized alginate. In this study, the authors began with a calcium-crosslinked alginate scaffold and incorporated synthetic collagen-mimetic peptides (of sequence GFOGER [225]) to facilitate T cell adhesion and migration, integrated porous silica microparticles that displayed stimulatory antibodies for T cell programming, and released IL-15 superagonist from the loaded microparticles to promote cell expansion. This elegant scaffold design was shown to effectively increase T cell expansion and dispersion into tumor resection beds (approximately 100-fold compared to cells alone), promoting complete breast and ovarian tumor regression in 60% of treated mice compared to 0% survival in ATC-only treated mice.
A recent 2017 publication in the Stephan lab expanded on their previous work with these alginate scaffolds, demonstrating their ability to co-deliver programmed CAR T cells in conjunction with cyclic-dinucleotide (CDN) STING agonist danger signals to treat fully established solid tumors, rather than just treating tumor resection beds with only ~1% residual tumor tissue (see Fig. 4) [100]. In a murine pancreatic tumor model, the authors first showed that intravenous injections of pancreatic cancer-specific T cells alone failed to eradicate tumors due to the limitations of CAR T cell monotherapy. However, the authors then demonstrated that when combined in an alginate implant with a locally co-delivered CDN (cdGMP [226]), CAR T cell treatment efficacy could be significantly enhanced. Interestingly, in this study the authors observed that while T cell-loaded implants without CDN more than doubled the survival of treated mice vs. T cell treatment alone, such scaffolds consistently failed to completely clear the disease, demonstrating the necessity of using STING agonists to promote durable anti-tumor immunity [100]. The combined release of CAR T cells and STING agonist cdGMP from biomaterial implants eradicated tumors in 4 of 10 treated mice, with an average increase in survival of 37 days (4.6-fold higher than simple intratumoral co-injection of CAR T cells and STING agonist). Tumor rechallenge of the four surviving implant-treated mice showed they had developed complete immunity, with no subsequent pancreatic tumor growth. The same treatment strategy was also shown to be effective at eliminating melanoma tumors post-resection in 60% of treated mice.
Fig. 4.
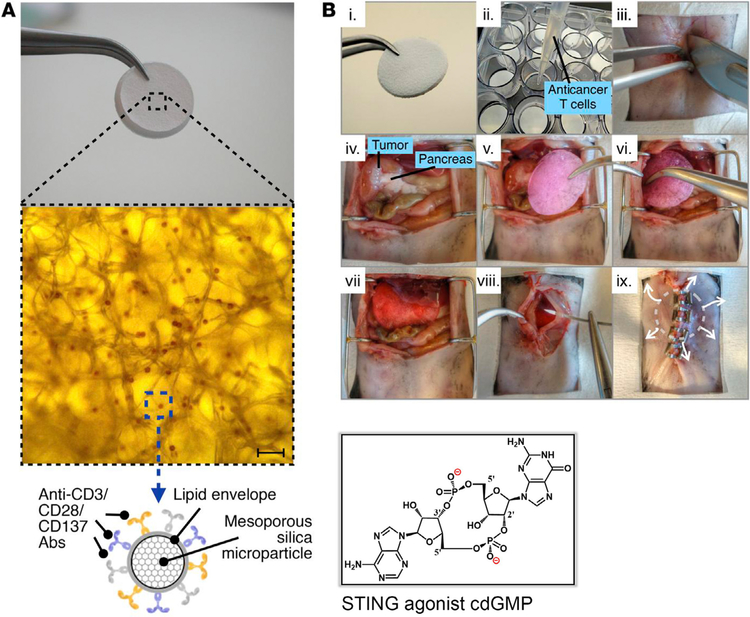
Alginate biomaterial implant placed on established pancreatic tumors as a delivery platform for CAR-programmed T cells and STING agonist cdGMP. (A) Bright-field microscopy image showing silica microparticles incorporated into the alginate polymer scaffold, followed by a schematic of the stimulatory particle design loaded internally with cdGMP. Scale bar = 70 μm. (B) Image series showing surgical methodology: (i) alginate polymer scaffold; (ii) seeding of tumor-reactive T cells into the implant; (iii) incision; (iv) orthotopic pancreatic tumor; (v-vi) implantation of a T cell-loaded device; (vii-viii) wound closure; (ix) extended release of STING agonist and tumor-reactive T cells. Adapted with permission from Smith et al. (2017) [100].
Other current examples of implantable immunotherapy systems include the work of Verma et al. in 2016, who developed a fibrin-based implantable scaffold to deliver activated dendritic cells to primary tumor sites and tumor resection beds [227]. Zhan et al. in 2017 used an electrospun polyglyconate and porcine gelatin design to create a fibrous scaffold, in which was loaded the chemokine CCL17 as a T cell recruitment factor [101]. With surgical co-implantation of the drug-eluting scaffold and pancreatic tumor tissue into mice, the authors observed increased T cell recruitment and tumor mass reduction, as well decreased metastasis [101]. One of the most recent reports in this field was published in May 2018 by Hathaichanok et al., describing the development of a collagen and hyaluronic acid (HA) scaffold vaccine for postoperative immunotherapeutic tumor treatment [102]. In an effort to reduce potential cancer relapse following surgical removal of tumors, the authors designed an implantable porous scaffold based on cross-linked collagen and hyaluronic acid (HA) to support cell migration and proliferation. The scaffold was loaded with the chemotherapy agent Gemcitabine (GEM) for both its anti-cancer properties and ability to deplete myeloid-derived suppressor cell (MDSC) populations [228,229], and the material also incorporated tumor lysate as an antigen source and cationic nanogels loaded with anionic poly(I:C) agonists for immunostimulation. MDSCs are known to accumulate in the tumor environment and suppress normal T cell immune function. Therefore the authors argued that suppressor cell depletion by sustained local release of GEM would result in enhanced immune activity and reduced tumor metastasis when done in conjunction with local antigen and agonist presentation [230,231]. The authors showed that their material resulted in sustained release of GEM over the first 7 days post implantation (during the key period of early tumor regrowth), and increased immune cell uptake of the negatively charged poly(I:C) stimulatory adjuvant due to encapsulation in amine terminated nanogels. Loaded scaffolds showed significantly increased dendritic cell infiltration and activation compared to blank collagen/HA scaffolds, and treated tumors exhibited a marked reduction in MDSCs as desired. In a mouse 4 T1 breast cancer resection model where approximately 90% of established tumor volume was removed before treatment, loaded scaffold implantation resulted in 25% of mice showing complete remission, and 100% showing greatly improved survival compared to controls, with significantly reduced tumor metastasis. Mice treated with scaffolds loaded with GEM alone or antigens/adjuvants alone showed no cases of full tumor remission and only marginally improved survival, demonstrating the efficacy of the author’s multicomponent materials-based immunotherapy strategy.
In summary, implantable biomaterial scaffolds remain a significant area of research for enhancing the efficacy of anti-cancer immunotherapies, whether by recruiting and supporting immune cell growth, programming and enhancing immune function, or even counteracting tumor immune suppression. Implantable scaffolds possess unique strengths in carrying out these functions due to their inherent ability to persist in a desired location for a long period of time, continuously releasing bioactive factors and allowing for facile cell infiltration into an often highly porous 3-D matrix. Such durable scaffolds have shown impressive results in various animal tumor models, and current human clinical trials are underway to evaluate their translatable efficacy.
4. Injectable biomaterials: Cryogels and hydrogels
4.1. Overview of pioneering work in injectable immunotherapeutic biomaterials
Before launching into published work on injectable biomaterials, it is useful to consider the relative advantages and disadvantages of injectable and implantable systems. As has been stated, implantable biomaterial scaffolds are useful as durable depots for loaded agents or cells, remaining in their implanted location for extended periods of time to maintain antigen presentation, control cell trafficking, and perform various other functions. However, implantable scaffolds also possess a few district drawbacks. Scaffolds such as PLG-based polymer vaccines suffer from stiffness and brittleness, being easily breakable and requiring individual surgical procedures to implant into the subcutaneous space. Pre-fabricated alginate scaffolds, while resorbable and thus not suffering from issues of brittleness, still require invasive surgery to implant in a tumor resection bed or near a solid tumor. These material characteristics limit the ability of implantable scaffolds to be easily directed to a desired location, and they often must remain in their implanted locations for a significant period to work. Thus, the literature on implanted scaffolds often focuses on tumor resection bed treatment or peritumoral insertion. Such scaffolds by definition cannot be placed in surgically inaccessible locations or volume-sensitive areas, and their persistent presence can potentially compromise normal organ function. For example, data on alginate implants for treating pancreatic tumors suggested some impairment of pancreatic activity compared to untreated controls without scaffold [100].
These limitations have led to the development of injectable immunotherapeutic biomaterials, which possess certain strengths when compared to implantable materials. Injectable biomaterials include hydrogels, cryogels, and other in situ self-assembling systems that can be derived from various natural or synthetic components [176–180,183]. Such materials have been widely used in the literature, for they possess a few distinct advantages. First, they can be localized anywhere a needle can reach, a simpler and less invasive procedure compared to surgical implantation. This avoids unnecessary tissue damage and the complications associated with an inflammatory wound response, and an injection often requires much less technical expertise to properly administer to animal or human subjects compared to a surgical procedure [113]. Second, with their viscoelastic properties, injectable materials can move and flow, conforming to any available space before forming into a persisting implant [232,233]. Thus, they can easily interface with living systems, occupying discrete places in the subcutaneous space or filling natural cavities and pockets in and around organs and other biological tissues [234]. However, injectable materials also come with disadvantages, namely that the chosen biomaterial must, by definition, form either a liquid or a gel that will allow it to pass through a needle, heavily limiting the types of materials and components that can be included. Many useful and desirable materials simply do not have the mechanical properties necessary for injectability, thus limiting the design space and prohibiting the use of more complex three-dimensional structures that are available to implantable scaffolds. Fundamentally, it is the goals of the project that determine the choice of biomaterial design, whether implantable or injectable, for they each have unique strengths when used for the proper applications.
In returning to a discussion of the literature, pioneering work in injectable biomaterials for the delivery of immunotherapies was reported by Singh et al., Wang et al., and Hori et al. in 2008–2009. Singh et al. developed an injectable Michael addition polymer hydrogel vaccine to act as an immune priming center, loading the hydrogel with chemo-attractants and immunomodulatory agents that promoted dendric cell infiltration and immune programming [17,18]. Such injectable therapies improved survival up to 2-fold in a weakly immunogenic A20 B cell lymphoma model [18]. Wang et al. developed a dual-layered hydrogel/microsphere composite as a delivery system for exogenous immunocytes, demonstrating the feasibility of localized cell-based immunotherapy on tumor cells in vitro [104]. Hori et al. reported on an injectable alginate-based system that formed a localizable hydrogel in situ, first reporting on its ability to load and carry exogenous dendritic cells as an injectable immunotherapy, and subsequently exploring the self-gelling system’s ability to deliver immunostimulatory molecules such as interleukin-2 or CpG oligonucleotides via bulk encapsulation or electrostatic anchoring [105,106].
4.2. Current work in injectable biomaterials
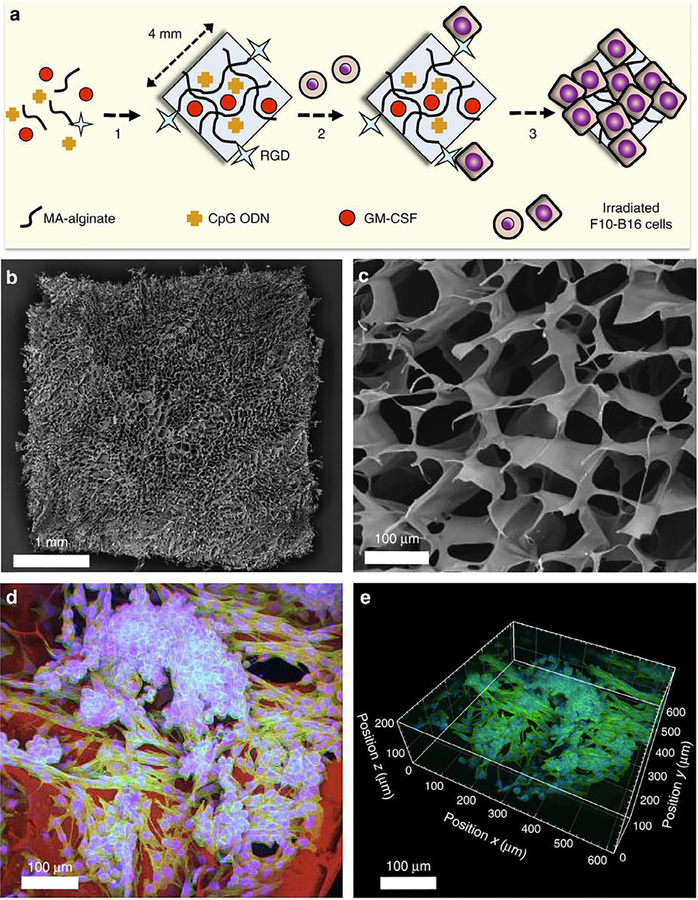
More recent injectable material designs include the work of Bencherif et al., who investigated an alginate-based cryogel biomaterial as an injectable cancer vaccine in 2012–2015, using GM-CSF for cell recruitment, CpG as a danger signal, and irradiated melanoma cells as an antigen source in a method similar to Ali’s original implantable PLG scaffold (Fig. 5) [110–112]. Bencherif’s cryogel incorporated RGD peptides to promote adhesion of the irradiated melanoma cells, and showed sustained release of loaded immunotherapeutic factors for over 1 month to result in potent tumor-treatment efficacy in mice [111]. Koshy et al. reported in 2014 on injectable gelatin cryogels derived from natural collagen, which were shown to be easily administered without a surgical procedure and fully biocompatible, promoting infiltration and proliferation of immune cells with controlled release of GM-CSF [113]. Verbeke et al. utilized an alginate hydrogel system in 2015 that formed larger pores than some of the more standard nanoporous alginate systems, whose small pores normally inhibit cell migration and infiltration [108,235,236]. These in situ large pore-forming alginate hydrogels showed greatly enhanced cell infiltration, and when loaded with GM-CSF, the injected material recruited a highly enriched population of millions of immature dendritic cells [108]. Follow-up work by Verbeke in 2017 showed that the same pore-forming alginate hydrogel loaded with GM-CSF could be used to deliver microparticle-encapsulated or directly-conjugated peptide antigens, resulting in the recruitment and programming of antigen-specific T cells, a natural progression from immature dendritic cell accumulation [109]. Other injectable strategies have also been reported in immune cell delivery and expansion applications [237].
Fig. 5.
(A) Preparation scheme for injectable alginate cryogel cancer vaccine. (B) Scanning electron microscopic (SEM) image of the macroporous cryogel. (C) Cross-sectional SEM image of cryogel showing porous structure. (D) 2D confocal image showing seeded melanoma cells on the cryogel. Cell nuclei are blue, intracellular actin is green, and cryogel polymer is red. (E) Reconstructed 3D confocal image showing spreading of melanoma cells 6 h post seeding. Reproduced from Bencherif et al. 2015 [111]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
In 2010 Rudra and Collier developed a system of injectable self-assembling peptide vaccines (based on the peptide termed Q11) that display synthetic antigenic peptides as immunotherapeutic epitopes along the surface of a nanofiber [114]. This unique method of epitope presentation resulted in specific and extended immune responses without supplemental adjuvants [114]. These nanofibrous Q11 peptides and their derivatives have been used to display antigenic sequences in order to specify a precise immune response [116,117], have been conjugated with active immunotherapy epitopes derived from immune cells [118,119], and have been used in other applications not related to immunotherapy [115]. The breadth of work reported using these peptide vaccines has not only demonstrated the promising efficacy of this system, but also the impressive customizable nature of peptide-based immunotherapies. Such studies also suggest that the nanostructured environment that antigens are presented in can result in a dramatically altered immune response, an exciting approach that has not yet been fully explored in the literature.
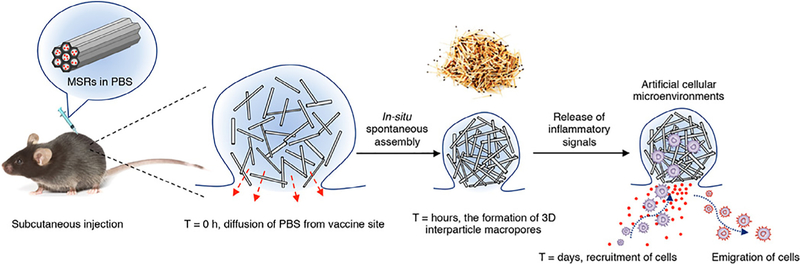
A fascinating strategy for an injectable cancer vaccine scaffold was developed in 2014 by Kim et al. and is based on mesoporous silica rods (MSRs). This system, which is an elegant compromise between implantable scaffolds and injectable biomaterials, self-assembles in situ into a distinct pocket-like depot when injected subcutaneously, allowing for extended release of loaded factors (Fig. 6) [120]. The spontaneously assembled MSR structures, which the authors liken to a pile of thrown matchsticks, retained sufficient porosity to allow cells to traffic into and out of the scaffold-like material. When pre-loaded with GM-CSF, CpG, and model antigens akin to previous systems, the injected MSR implant created an immunotherapeutic depot able to recruit and influence local immune cell populations. Further work studied the effects of surface modification on immune cell infiltration and response, experimenting with modifications such as Poly(ethylene–glycol) (PEG) and integrin-binding ligand Arg-Gly-Asp (RGD) on injectable MSR scaffolds [121]. Work published as recently as March of 2018 by Li et al. reported on a novel cationic polyethyleneimine-MSR system’s ability to easily adsorb peptide antigens and enhance antigen immunogenicity [122]. This system showed broad treatment efficacy on a wide range of tumor models, and impressively resulted in complete eradication of large established PC-1 tumors in 80% of treated mice and two-fold enhanced survival compared to the standard MSR vaccine system [122].
Fig. 6.
Scheme of MSR assembly and cell recruitment in vivo. MSRs are prepared in PBS and then subcutaneously injected to form a pocket. PBS quickly diffuses from the pocket leading to in situ spontaneous assembly of MSRs, which can be compared to the random assembly of a pile of thrown matchsticks. This assembly forms large interparticle spaces where immune cells can enter the MSR pocket and interact with the immunotherapy payload, after which mature immune cells can migrate from the implant and perform a desired function. Reproduced from Kim et al. (2014) [120].
In 2014–2015 Umeki and Nishikawa reported on the use of an injectable immunomodulatory DNA-based hydrogel for controlled release of cationic antigens [123,124]. The authors’ polypod-like structured DNA hydrogel was designed to possess intrinsic general immunogenicity due to the incorporation of CpG dinucleotides into the DNA sequences that made up the gel structure [238], but initial studies showed unfortunately fast release kinetics of loaded antigens that were meant to direct a specific immunotherapeutic response [123]. Further work demonstrated that extended release of such immunogenic antigens from the DNA-based hydrogel could be achieved by peptide or protein antigen cationization [124]. This elegant strategy took advantage of electrostatic interactions between the positively charged modified antigens and the inherently negatively charged DNA hydrogel, resulting in a greatly reduced release rate (unmodified antigen release had a half-life of less than 1 h, cationized antigen had a half-life of greater than 24 h). When injected intratumorally, the DNA hydrogel loaded with cationized ovalbumin (OVA) antigens significantly improved mouse survival in EG7-OVA tumors displaying the same gel-loaded antigen, where 4 out of 6 mice survived with complete tumor regression when treated with cationized antigen + DNA hydrogel, compared to only 1 out of 6 mice surviving when treated with native antigen + DNA hydrogel. The authors thus demonstrated the ability to significantly enhance response to immunotherapy through improved delivery methods, underlying one of the inherent strengths of biomaterial-based treatment strategies. Other biomaterial approaches and alternative release strategies have also been reported [239–242].
Li et al. reported in 2015 on an alginate hydrogel combination therapy used to locally deliver both celecoxib and PD-1 checkpoint inhibitor intratumorally [107]. The authors’ rationale was that the use of celecoxib, with its anti-inflammatory properties and inherent anti-tumor activity [243,244], might enhance the efficacy of PD-1 immunotherapy by offsetting deleterious PD-1-induced chronic inflammation [245,246]. The authors first demonstrated in a B16-F10 melanoma model that individual delivery of celecoxib or PD-1 mAb from a subcutaneously injected alginate hydrogel resulted in superior tumor growth inhibition compared to drug injections alone, showing that the hydrogel maintained high local drug concentrations and sustained delivery [107]. Furthermore, dual delivery of both celecoxib and PD-1 mAb showed significantly improved tumor treatment efficacy, not only strikingly reducing tumor size compared to celecoxib or PD-1 hydrogels alone, but also resulting in 5 out of 9 mice showing complete tumor regression compared to only 1 out of 9 mice surviving when treated with celecoxib and PD-1 without hydrogel. Further experiments showed that the combination hydrogel therapy, while effectively increasing anti-tumor immunity, also abolished the increase in inflammatory molecules caused by PD-1 blockade when celecoxib was co-administered, supporting the authors’ initial hypothesis.
Interesting PEG-based composite systems have also been used in the literature for immunotherapy strategies. For example, Tsao et al. used a PEG-g-chitosan hydrogel to delivery T cell immunotherapy in 2014, demonstrating their thermoreversible hydrogel’s ability to load T lymphocytes with applications in local delivery to glioblastoma tumors [247]. Wang et al. developed a thermo-sensitive PLGA-PEG hydrogel in 2017, using it as an immunotherapeutic vaccine delivery system for intramuscular injections of model antigens [248].
Other recent efforts in the field of injectable biomaterial-based immunotherapies include the work of Luo et al. in 2017, where a self-assembling D-tetra-peptide hydrogel was used for model antigen encapsulation and enhanced anti-tumor vaccine immunogenicity [125]. Song et al. in early 2018 used a relatively stiff polypeptide hydrogel assembled from PEGylated poly(L-valine) copolymers to load and deliver tumor cell lysate antigens and poly(I:C) agonist, resulting in dendritic cell recruitment, programming, and improved anti-tumor activity against B16 melanoma [126]. Furthermore, in 2018 Yang et al. showed that a RADA-16 peptide hydrogel (also known commercially as ‘‘PuraMatrix”) could be used as a carrier material for exogenous dendritic cells and PD-1 checkpoint inhibitors to create an effective immunotherapy vaccine [127].
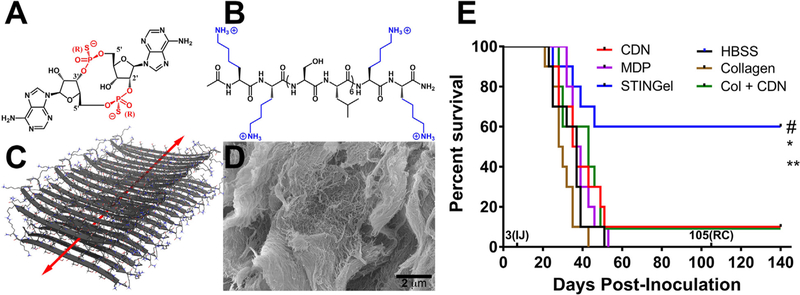
Leach et al. recently published in 2018 on the use of a self-assembling, nanofibrous peptide hydrogel for intratumoral immunotherapy delivery, greatly enhancing the tumor-treatment efficacy of the cyclic-dinucleotide (CDN) ML RR-S2 CDA in a challenging model of head and neck squamous cell carcinoma (HNSCC) [128]. Currently in clinical trials, ML RR-S2 CDA (Fig. 7a) is a powerful STING agonist able to activate the immune system against a wide variety of cancers, but its efficacy currently remains limited due to the common need for multiple drug injections at high doses to maintain sufficient activity [166,249,250]. Indeed, other clinical trials on CDNs (such as Merck’s STING agonist MK-1454) have also shown poor tumor treatment efficacy with high dose CDN monotherapy, suggesting the need for new method development or combination therapies [167,251]. In the study by Leach et al., a biomaterial delivery approach inspired by much of the previous work in the field was developed, utilizing an inherently cationic and biocompatible Multidomain peptide (MDP) hydrogel synthesized in-house [213,221] to encapsulate and control the release of anionic ML RR-S2 CDA (Fig. 7) [128]. The resulting favorable electrostatic charge-pairing between the negative thiophosphate linkages of the CDN drug molecules and the positive amine termini of the lysine-based nanofibrous hydrogel allowed for controlled release, showing 8-fold slower release compared to an off-the-shelf collagen control hydrogel. When the CDN-loaded hydrogel was injected subcutaneously, the maintenance of a high local CDN drug concentration was observed that stimulated a massive host immune response. The efficacy of intratumoral injections into the oral cavity of mice bearing aggressive MOC2-E6E7 tumors was then studied, and the material termed ‘STINGel’ (STING agonist loaded within peptide hydrogel) resulted in complete tumor elimination in 60% of treated mice after only a single treatment injection, compared to only 10% survival in CDN-alone treated groups or CDN released from the control collagen hydrogel. These results demonstrated the importance of using the rationally designed cationic MDP hydrogel. The use of a localized collagen hydrogel that lacked favorable interactions with the CDN drug molecules showed no improvement in treatment efficacy, likely due to burst release behavior and swift loss of STING agonist from the tumor site. Furthermore, all mice that were successfully treated demonstrated complete durable immunity, showing no tumor growth upon rechallenge with the same oral cancer cell line.
Fig. 7.
Chemical structures of (A) ML RR-S2 CDA STING agonist (CDN), and (B) K2(SL)6K2 Multidomain peptide (MDP), showing charge-pair complementarity. (C) Model of anti-parallel β-sheet nanofiber formed by the MDP in solution. The arrow indicates the axis of the nanofiber and orientation of hydrogen bonding. (D) SEM image of the MDP gel showing a wide field image of the self-assembled nanofibers. (E) Survival of the different experimental groups based on euthanasia timepoints resulting from excessive tumor burden. The total experimental period was 140 days post tumor cell inoculation. The 3(IJ) on the x-axis refers to timepoint for intratumoral injection, and 105(RC) refers to timepoint for survivor rechallenge. Whereas 60% of the STINGel treated C57BL/6 mice survived until the endpoint of the study, all control group (HBSS buffer, MDP gel, and collagen gel) mice were euthanized prior to reaching the endpoint due to excessive tumor burden. Only 10% of CDN alone and collagen + CDN treated mice survived (lines overlaid on plot). *p < 0.0282 vs. CDN, **p < 0.0064 vs. MDP gel, #p < 0.0498 vs. collagen + CDN. Adapted from Leach et al. 2018 [128].
Closely following Leach et al., Park et al. similarly reported on the use of a degradable hyaluronic acid (HA) hydrogel implant to locally deliver adjuvants for the suppression of tumor regrowth following surgical resection [103]. While this is not an injectable system, it is included in this section of the review for ease of comparison to other related treatment strategies. In this study, the authors sought to apply the same principle of focusing delivery of immunotherapies such as TLR7/8 agonists and STING agonist ML RR-S2 CDA to the site of tumors, in this case treating the post-operative cavity with an agonist-loaded hydrogel scaffold after removing the primary tumor with its immunosuppressive microenvironment. Their strategy used a cross-linked HA hydrogel that was specifically optimized to enhance its stiffness and form a robust scaffold for insertion into the post-operative cavity. Drug release studies showed that the material resulted in moderately extended release of TLR7/8 and STING agonists on the order of 3–6 h, likely representing a physical drug entrapment mechanism. Upon administration of the agonist-loaded hydrogel scaffold into 4 T1-Luc2 breast tumor resection cavities, the prevention of tumor recurrence and eradication of existing metastases was significantly enhanced, resulting in approximately 70% survival in hydrogel + agonist groups (whether TLR7/8 agonist or CDN) compared to only 30% or less survival when treated with weekly injections of agonist alone or agonist administered locally [103]. Baird et al. also recently published on a STING agonist loaded hydrogel therapy in late 2018 for localized tumor resection bed treatment [129]. Using commercially available Matrigel (composed primarily of laminin and collagen) for the carrier material, the authors’ localization strategy also showed promising results in preventing tumor recurrence and treating post-surgery tumor margins.
The work by Leach et al., Park et al., and Baird et al. each demonstrate the exciting potential of the combination of localized hydrogels with powerful adjuvants such as CDNs. Each of these studies saw significant improvement in tumor growth inhibition and overall survival when a CDN was delivered in a controlled fashion from a hydrogel biomaterial, whether by direct intratumoral injection into oral tumors, or local placement at a tumor resection site. These studies highlight the need for further research in the growing area of controlled STING agonist delivery.
In summary, injectable and localizable biomaterials represent a wide array of delivery systems in the literature, for such systems are immensely customizable to the chemical or biological needs of various drug or cell-based therapies. Biomaterials like cryogels and hydrogels can be synthesized from a large number of differing starting materials, each imbuing the overall matrix with unique bulk properties, and they can be modified or derivatized with alternative functional groups, signaling molecules, or anything else attachable by covalent bonds or supramolecular forces. Injectable materials provide a useful complement to pre-formed implantable scaffolds, and both strategies have shown impressive treatment results with relevant applications in different clinical settings. Other elegant studies have also shown the potential benefits of delivering cancer chemotherapies from biomaterial scaffolds whether alone or in combination with immunotherapies [252–255]. For example, an excellent recent publication by Brudno et al. reported on the development of an injectable and refillable hydrogel drug-depot, a system that could be repeatedly reloaded in vivo by systemic administration of a selectively reactive pro-drug [256]. Utilizing biorthogonal click chemistry to unite the functionalized hydrogel with modified anti-cancer pro-drug agents in situ, this single-injected, replenishable system had a theoretical maximum of 111 refills, a significant achievement [256]. While Brudno’s system used a chemotherapy agent, this could certainly be adapted to immunotherapeutic drug applications and expand the utility of materials-based immunotherapies. Furthermore, the ever-changing publication landscape is now not limited to only syringe injectable or surgically implantable biomaterials (though these methods are by far the most common). Indeed, within the realm of immunotherapeutic hydrogels, an excellent recent publication by Chen et al. in January 2019 utilized a system that was neither injected nor implanted, but was sprayed [130]. The authors’ in situ forming bioresponsive fibrin hydrogel was sprayed from a dual chamber device containing equal amounts of polymerizing fibrinogen and thrombin, and the resulting particle-loaded hydrogels allowed for controlled delivery of immunotherapeutic antibodies to treated post-surgery tumor sites. With the dynamic body of ongoing work in this area of research, it is with great anticipation and growing curiosity that we await further publications in the burgeoning field of immunotherapeutic biomaterials.
5. Conclusions and outlook
The united fields of immunotherapy and biomaterials represent an ever-growing body of research that promises to break scientific barriers and overcome clinical limitations that currently restrict cancer immunotherapy. The fields of biomaterial development and cancer immunotherapy are each advancing at impressive rates, with new leads and clinical trials emerging each year. New biomaterial delivery designs are regularly being published, demonstrating our maturing ability to intelligently regulate and target therapies [257,258]. It is advances like those explored in this review that show the exciting potential of biomaterial delivery systems, whether implantable, injectable, or even sprayable, and the potent synergies that can exist with the growing spectrum of small molecule, protein, or cell-based immunotherapies.
In discussing what questions will likely influence the direction of continuing research in this field, future work must enhance our understanding of the design criteria needed for the ‘ideal’ immunotherapeutic biomaterial, if such a material can be achieved. Among many factors, such as desired material persistence vs. degradation rates, the necessary release profiles of antigens and adjuvants is a complicated challenge. Release of adjuvants to the body’s immune system that is too fast could result in the development of immune tolerance, whereas release that is too slow could fail to properly align with antigen presentation, and therefore leave dendritic cells insufficiently activated [93]. Other complications include the innate immune response to an inherently foreign carrier material and its influence (or lack of) on immunotherapy treatment, whether obvious or subtle. Furthermore, growing evidence shows that the future lies in personalized medicine, and biomaterial-based therapies will also need to be tailored to fit the unique immune response profiles of individual patients. Thus, while current designs show that the field has much to offer in terms of enhancing therapy outcomes and reviving flagging drug classes, there are still many excellent questions and avenues of research that must be fully explored to bring these diverse biomaterial immunotherapies to the clinic.
Statement of significance.
Anti-cancer immunotherapies have shown exciting clinical results in the past few decades, yet they suffer from a few distinct limitations, such as poor delivery kinetics, narrow patient response profiles, and systemic side effects. Biomaterial systems are now being developed that can overcome many of these problems, allowing for localized adjuvant delivery, focused dose concentrations, and extended therapy presentation. The field of biocompatible carrier materials is uniquely suited to be combined with immunotherapy, promising to yield significant improvements in treatment outcomes and clinical care.
In this review, the first pioneering efforts and most recent advances in biomaterials for immunotherapeutic applications are explored, with a specific focus on implantable and injectable biomaterials such as porous scaffolds, cryogels, and hydrogels.
Acknowledgments
This work was supported by the National Institutes of Health (grants DE021798, DE023577, DE024173, and DE027794), the Oral and Maxillofacial Surgery Foundation (Research Support Grant), and the Welch Foundation (C1557). DGL was supported by the NSF Graduate Research Fellowship Program under Grant No. 1450681.
Footnotes
Conflicts of interest
The authors declare no conflict of interests.
References
- [1].Kearney CJ, Mooney DJ, Macroscale delivery systems for molecular and cellular payloads, Nat. Mater 12 (2013) 1004. [DOI] [PubMed] [Google Scholar]
- [2].Goldberg Michael S., Immunoengineering: how nanotechnology can enhance cancer immunotherapy, Cell 161 (2) (2015) 201–204. [DOI] [PubMed] [Google Scholar]
- [3].Ratner BD, Bryant SJ, Biomaterials: where we have been and where we are going, Annu. Rev. Biomed. Eng 6 (1) (2004) 41–75. [DOI] [PubMed] [Google Scholar]
- [4].Huebsch N, Mooney DJ, Inspiration and application in the evolution of biomaterials, Nature 462 (7272) (2009) 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kwong B, Liu H, Irvine DJ, Induction of potent anti-tumor responses while eliminating systemic side effects via liposome-anchored combinatorial immunotherapy, Biomaterials 32 (22) (2011) 5134–5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liao D, Liu Z, Wrasidlo WJ, Luo Y, Nguyen G, Chen T, Xiang R, Reisfeld RA, Targeted therapeutic remodeling of the tumor microenvironment improves an HER-2 DNA vaccine and prevents recurrence in a murine breast cancer model, Cancer Res 71 (17) (2011) 5688–5696. [DOI] [PubMed] [Google Scholar]
- [7].Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Limon PL, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM, Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy, Nat. Mater 11 (10) (2012) 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kwong B, Gai SA, Elkhader J, Wittrup KD, Irvine DJ, Localized immunotherapy via liposome-anchored anti-CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity, Cancer Res 73 (5) (2013) 1547–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bracho-Sanchez E, Xia CQ, Clare-Salzler MJ, Keselowsky BG, Micro and Nano Material Carriers for Immunomodulation, Am. J. Transpl 16 (12) (2016) 3362–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Singh M, Briones M, Ott G, O’Hagan D, Cationic microparticles: a potent delivery system for DNA vaccines, Proc. Natl. Acad. Sci 97 (2) (2000) 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li Y-P, Pei Y-Y, Zhou Z-H, Zhang X-Y, Gu Z-H, Ding J, Zhou J-J, Gao X-J, PEGylated polycyanoacrylate nanoparticles as tumor necrosis factor-α carriers, J. Control. Release 71 (3) (2001) 287–296. [DOI] [PubMed] [Google Scholar]
- [12].Kasturi SP, Sachaphibulkij K, Roy K, Covalent conjugation of polyethyleneimine on biodegradable microparticles for delivery of plasmid DNA vaccines, Biomaterials 26 (32) (2005) 6375–6385. [DOI] [PubMed] [Google Scholar]
- [13].O’Hagan DT, Singh M, Ulmer JB, Microparticle-based technologies for vaccines, Methods 40 (1) (2006) 10–19. [DOI] [PubMed] [Google Scholar]
- [14].Sánchez A, Tobío MA, González L, Fabra A, Alonso MAJ, Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-alpha, Eur. J. Pharm. Sci 18 (3) (2003) 221–229. [DOI] [PubMed] [Google Scholar]
- [15].Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, Swartz MA, Hubbell JA, Nanoparticle conjugation of antigen enhances cytotoxic T-cell responses in pulmonary vaccination, Proc. Natl. Acad. Sci 108 (44) (2011) E989–E997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pillai G, Nanomedicines for cancer therapy: an update of FDA approved and those under various stages of development, SOJ Pharm. Pharm. Sci 1 (2) (2014) 13. [Google Scholar]
- [17].Singh A, Suri S, Roy K, In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA–DNA carrying microparticles to dendritic cells, Biomaterials 30 (28) (2009) 5187–5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Singh A, Qin H, Fernandez I, Wei J, Lin J, Kwak LW, Roy K, An injectable synthetic immune-priming center mediates efficient T-cell class switching and T-helper 1 response against B cell lymphoma, J. Control. Release 155 (2) (2011) 184–192. [DOI] [PubMed] [Google Scholar]
- [19].Ali OA, Emerich D, Dranoff G, Mooney DJ, In situ regulation of DC subsets and T cells mediates tumor regression in mice, Sci. Transl. Med 1 (8) (2009) 8ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ, Infection-mimicking materials to program dendritic cells in situ, Nat. Mater 8 (2009) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ali OA, Tayalia P, Shvartsman D, Lewin S, Mooney DJ, Inflammatory cytokines presented from polymer matrices differentially generate and activate DCs in situ, Adv. Funct. Mater 23 (36) (2013) 4621–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ankur S, Peppas NA, Hydrogels and Scaffolds for Immunomodulation, Adv. Mater 26 (38) (2014) 6530–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bae KH, Wang LS, Kurisawa M, Injectable biodegradable hydrogels: progress and challenges, J. Mater. Chem. B 1 (40) (2013) 5371–5388. [DOI] [PubMed] [Google Scholar]
- [24].Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT, Biopolymer implants enhance the efficacy of adoptive T-cell therapy, Nat. Biotechnol 33 (2015) 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cezar CA, Kennedy SM, Mehta M, Weaver JC, Gu L, Vandenburgh H, Mooney DJ, Biphasic ferrogels for triggered drug and cell delivery, Adv. Healthcare Mater 3 (11) (2014) 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim Y-C, Park J-H, Prausnitz MR, Microneedles for drug and vaccine delivery, Adv. Drug Deliv. Rev 64 (14) (2012) 1547–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Indermun S, Luttge R, Choonara YE, Kumar P, du Toit LC, Modi G, Pillay V, Current advances in the fabrication of microneedles for transdermal delivery, J. Control. Release 185 (2014) 130–138. [DOI] [PubMed] [Google Scholar]
- [28].Zeng Q, Gammon JM, Tostanoski LH, Chiu Y-C, Jewell CM, In vivo expansion of melanoma-specific T cells using microneedle arrays coated with immune-polyelectrolyte multilayers, ACS Biomater. Sci. Eng 3 (2) (2017) 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z, Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody, Nano Lett 16 (4) (2016) 2334–2340. [DOI] [PubMed] [Google Scholar]
- [30].Chen M-C, Huang S-F, Lai K-Y, Ling M-H, Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination, Biomaterials 34 (12) (2013) 3077–3086. [DOI] [PubMed] [Google Scholar]
- [31].Hotaling NA, Tang L, Irvine DJ, Babensee JE, Biomaterial strategies for immunomodulation, Annu. Rev. Biomed. Eng 17 (2015) 317–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Purwada A, Roy K, Singh A, Engineering vaccines and niches for immune modulation, Acta Biomater 10 (4) (2014) 1728–1740. [DOI] [PubMed] [Google Scholar]
- [33].Mehta NK, Moynihan KD, Irvine DJ, Engineering New Approaches to Cancer Vaccines, Cancer Immunology Research 3 (8) (2015) 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, García-Sastre A, Compans R, Pulendran B, Programming the magnitude and persistence of antibody responses with innate immunity, Nature 470 (2011) 543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Karch CP, Li J, Kulangara C, Paulillo SM, Raman SK, Emadi S, Tan A, Helal ZH, Fan Q, Khan MI, Burkhard P, Vaccination with self-adjuvanted protein nanoparticles provides protection against lethal influenza challenge, Nanomed.: Nanotechnol. Biol. Med 13 (1) (2017) 241–251. [DOI] [PubMed] [Google Scholar]
- [36].Chahal JS, Fang T, Woodham AW, Khan OF, Ling J, Anderson DG, Ploegh HL, An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model, Sci. Rep 7 (1) (2017) 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE, Pechar M, Pola R, Gerner MY, Yamamoto A, Buechler CR, Quinn KM, Smelkinson MG, Vanek O, Cawood R, Hills T, Vasalatiy O, Kastenmüller K, Francica JR, Stutts L, Tom JK, Ryu KA, Esser-Kahn AP, Etrych T, Fisher KD, Seymour LW, Seder RA, In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity, Nat. Biotechnol 33 (2015) 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Graham JG, Zhang X, Goodman A, Pothoven K, Houlihan J, Wang S, Gower RM, Luo X, Shea LD, PLG scaffold delivered antigen-specific regulatory T cells induce systemic tolerance in autoimmune diabetes, Tissue Eng. Part A 19 (11–12) (2013) 1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Whitmire RE, Scott Wilson D, Singh A, Levenston ME, Murthy N, García AJ, Self-assembling nanoparticles for intra-articular delivery of anti-inflammatory proteins, Biomaterials 33 (30) (2012) 7665–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Verbeke CS, Gorda S, Schubert DA, Lewin SA, Desai RM, Dobbins J, Wucherpfennig KW, Mooney DJ, Multicomponent injectable hydrogels for antigen-specific tolerogenic immune modulation, Adv. Healthcare Mater 6 (6) (2017) 1600773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhang W, Gorantla VS, Campbell PG, Li Y, Yang Y, Komatsu C, Weiss LE, Zheng XX, Solari MG, Biopatterned CTLA4/Fc matrices facilitate local immunomodulation, engraftment, and glucose homeostasis after pancreatic islet transplantation, Diabetes 65 (12) (2016) 3660–3666. [DOI] [PubMed] [Google Scholar]
- [42].Andorko JI, Jewell CM, Designing biomaterials with immunomodulatory properties for tissue engineering and regenerative medicine, Bioeng. Transl. Med 2 (2) (2017) 139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fabiilli ML, Wilson CG, Padilla F, Martín-Saavedra FM, Fowlkes JB, Franceschi RT, Acoustic droplet–hydrogel composites for spatial and temporal control of growth factor delivery and scaffold stiffness, Acta Biomater 9 (7) (2013) 7399–7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH, Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells, Science 352 (6283) (2016) 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].DeMuth PC, Min Y, Huang B, Kramer JA, Miller AD, Barouch DH, Hammond PT, Irvine DJ, Polymer multilayer tattooing for enhanced DNA vaccination, Nat. Mater 12 (4) (2013) 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Koshy ST, Mooney DJ, Biomaterials for enhancing anti-cancer immunity, Curr. Opin. Biotechnol 40 (2016) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gammon JM, Dold NM, Jewell CM, Improving the clinical impact of biomaterials in cancer immunotherapy, Oncotarget 7 (13) (2016) 15421–15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gu L, Mooney DJ, Biomaterials and emerging anticancer therapeutics: engineering the microenvironment, Nat. Rev. Cancer 16 (1) (2016) 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Siegel RL, Miller KD, Jernal A, Cancer statistics, 2018, CA: A Cancer J. Clin 68 (1) (2018) 7–30. [DOI] [PubMed] [Google Scholar]
- [50].Hanahan D, Robert A Weinberg, Hallmarks of cancer: the next generation, Cell 144 (5) (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [51].Junttila MR, de Sauvage FJ, Influence of tumour micro-environment heterogeneity on therapeutic response, Nature 501 (2013) 346. [DOI] [PubMed] [Google Scholar]
- [52].Piersma SJ, Immunosuppressive tumor microenvironment in cervical cancer patients, Cancer Microenviron 4 (3) (2011) 361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tong CCL, Kao J, Sikora AG, Recognizing and reversing the immunosuppressive tumor microenvironment of head and neck cancer, Immunol. Res 54 (1) (2012) 266–274. [DOI] [PubMed] [Google Scholar]
- [54].Mellman I, Coukos G, Dranoff G, Cancer immunotherapy comes of age, Nature 480 (7378) (2011) 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Chen Daniel S., Mellman I, Oncology meets immunology: the cancer-immunity cycle, Immunity 39 (1) (2013) 1–10. [DOI] [PubMed] [Google Scholar]
- [56].Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC, Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity, Proc. Natl. Acad. Sci 90 (8) (1993) 3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mukherji B, Chakraborty NG, Yamasaki S, Okino T, Yamase H, Sporn JR, Kurtzman SK, Ergin MT, Ozols J, Meehan J, Induction of antigen-specific cytolytic T cells in situ in human melanoma by immunization with synthetic peptide-pulsed autologous antigen presenting cells, Proc. Natl. Acad. Sci 92 (17) (1995) 8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC, Progress on new vaccine strategies for the immunotherapy and prevention of cancer, J. Clin. Investig 113 (11) (2004) 1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM, Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer, J. Clin. Oncol 24 (19) (2006) 3089–3094. [DOI] [PubMed] [Google Scholar]
- [60].Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL, CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms, Mol. Cell. Biol 25 (21) (2005) 9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Leach DR, Krummel MF, Allison JP, Enhancement of antitumor immunity by CTLA-4 blockade, Science 271 (5256) (1996) 1734–1736. [DOI] [PubMed] [Google Scholar]
- [62].Yang Y-F, Zou J-P, Mu J, Wijesuriya R, Ono S, Walunas T, Bluestone J, Fujiwara H, Hamaoka T, Enhanced induction of antitumor T-cell responses by cytotoxic T lymphocyte-associated molecule-4 blockade: the effect is manifested only at the restricted tumor-bearing stages, Cancer Res 57 (18) (1997) 4036–4041. [PubMed] [Google Scholar]
- [63].Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF, PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T Cells, Cancer Res 64 (3) (2004) 1140–1145. [DOI] [PubMed] [Google Scholar]
- [64].Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA, Tumor antigen–specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired, Blood 114 (8) (2009) 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Peggs KS, Quezada SA, Allison JP, Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy, Immunol. Rev 224 (1) (2008) 141–165. [DOI] [PubMed] [Google Scholar]
- [66].Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJM, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ, Improved survival with ipilimumab in patients with metastatic melanoma, N. Engl. J. Med 363 (8) (2010) 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ, CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma, Clin. Cancer Res 18 (7) (2012) 2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, Weber JS, Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs, Int. Immunol 19 (10) (2007) 1223–1234. [DOI] [PubMed] [Google Scholar]
- [69].Iwai Y, Terawaki S, Honjo T, PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells, Int. Immunol 17 (2) (2005) 133–144. [DOI] [PubMed] [Google Scholar]
- [70].Pardoll DM, The blockade of immune checkpoints in cancer immunotherapy, Nat. Rev. Cancer 12 (2012) 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS, Nivolumab and ipilimumab versus ipilimumab in untreated melanoma, N. Engl. J. Med 372 (21) (2015) 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Press release: The Nobel Prize in Physiology or Medicine 2018, Nobel Media AB, NobelPrize.org, 2018. [Google Scholar]
- [73].Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, Kammula US, Topalian SL, Sherry RM, Kleiner D, Quezado M, Lowy I, Yellin M, Rosenberg SA, Yang JC, Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte–associated antigen 4, J. Clin. Oncol 24 (15) (2006) 2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Aranda F, Vacchelli E, Eggermont A, Galon J, Fridman WH, Zitvogel L, Kroemer G, Galluzzi L, Trial watch, OncoImmunology 3 (2) (2014) e27297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Mirsoian A, Bouchlaka MN, Sckisel GD, Chen M, Pai C-CS, Maverakis E, Spencer RG, Fishbein KW, Siddiqui S, Monjazeb AM, Martin B, Maudsley S, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Taub DD, Murphy WJ, Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice, J. Exp. Med 211 (12) (2014) 2373–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bouchlaka MN, Sckisel GD, Chen M, Mirsoian A, Zamora AE, Maverakis E, Wilkins DEC, Alderson KL, Hsiao H-H, Weiss JM, Monjazeb AM, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Redelman D, Taub DD, Murphy WJ, Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy, J. Exp. Med 210 (11) (2013) 2223–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Weber JS, Kähler KC, Hauschild A, Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab, J. Clin. Oncol 30 (21) (2012) 2691–2697. [DOI] [PubMed] [Google Scholar]
- [78].Vanneman M, Dranoff G, Combining immunotherapy and targeted therapies in cancer treatment, Nat. Rev. Cancer 12 (2012) 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ignery FH, Krammer PH, Immune escape of tumors: apoptosis resistance and tumor counterattack, J. Leukocyte Biol 71 (6) (2002) 907–920. [PubMed] [Google Scholar]
- [80].Cheung AS, Mooney DJ, Engineered materials for cancer immunotherapy, Nano Today 10 (4) (2015) 511–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zamboni WC, Torchilin V, Patri AK, Hrkach J, Stern S, Lee R, Nel A, Panaro NJ, Grodzinski P, Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance, Clin. Cancer Res 18 (12) (2012) 3229–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Albanese A, Tang PS, Chan WCW, The effect of nanoparticle size, shape, and surface chemistry on biological systems, Annu. Rev. Biomed. Eng 14 (1) (2012) 1–16. [DOI] [PubMed] [Google Scholar]
- [83].Wang AZ, Langer R, Farokhzad OC, Nanoparticle delivery of cancer drugs, Annu. Rev. Med 63 (1) (2012) 185–198. [DOI] [PubMed] [Google Scholar]
- [84].Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P, Nanoparticle-Based Immunotherapy for Cancer, ACS Nano 9 (1) (2015) 16–30. [DOI] [PubMed] [Google Scholar]
- [85].Fan Y, Moon JJ, Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy, Vaccines 3 (3) (2015) 662–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Irvine DJ, Hanson MC, Rakhra K, Tokatlian T, Synthetic nanoparticles for vaccines and immunotherapy, Chem. Rev 115 (19) (2015) 11109–11146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kawai M, Nakamura T, Miura N, Maeta M, Tanaka H, Ueda K, Higashi K, Moribe K, Tange K, Nakai Y, Yoshioka H, Harashima H, Akita H, DNA-loaded nano-adjuvant formed with a vitamin E-scaffold intracellular environmentally-responsive lipid-like material for cancer immunotherapy, Nanomed. Nanotechnol. Biol. Med (2018). [DOI] [PubMed]
- [88].Perica K, De León Medero A, Durai M, Chiu YL, Bieler JG, Sibener L, Niemöller M, Assenmacher M, Richter A, Edidin M, Oelke M, Schneck J, Nanoscale artificial antigen presenting cells for T cell immunotherapy, Nanomed. Nanotechnol. Biol. Med 10 (1) (2014) 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Qian Y, Jin H, Qiao S, Dai Y, Huang C, Lu L, Luo Q, Zhang Z, Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy, Biomaterials 98 (2016) 171–183. [DOI] [PubMed] [Google Scholar]
- [90].Sexton A, Whitney PG, Chong S-F, Zelikin AN, Johnston APR, De Rose R, Brooks AG, Caruso F, Kent SJ, A protective vaccine delivery system for in vivo T cell stimulation using nanoengineered polymer hydrogel capsules, ACS Nano 3 (11) (2009) 3391–3400. [DOI] [PubMed] [Google Scholar]
- [91].Rahimian S, Fransen MF, Kleinovink JW, Amidi M, Ossendorp F, Hennink WE, Polymeric microparticles for sustained and local delivery of antiCD40 and antiCTLA-4 in immunotherapy of cancer, Biomaterials 61 (2015) 33–40. [DOI] [PubMed] [Google Scholar]
- [92].Meir R, Shamalov K, Sadan T, Motiei M, Yaari G, Cohen CJ, Popovtzer R, Fast Image-Guided Stratification Using Anti-Programmed Death Ligand 1 Gold Nanoparticles for Cancer Immunotherapy, ACS Nano 11 (11) (2017) 11127–11134. [DOI] [PubMed] [Google Scholar]
- [93].Wang H, Mooney DJ, Biomaterial-assisted targeted modulation of immune cells in cancer treatment, Nat. Mater (2018). [DOI] [PubMed]
- [94].Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM, Improving vaccine and immunotherapy design using biomaterials, Trends Immunol 39 (2) (2018) 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Ali OA, Doherty E, Bell WJ, Fradet T, Hudak J, Laliberte M-T, Mooney DJ, Emerich DF, The efficacy of intracranial PLG-based vaccines is dependent on direct implantation into brain tissue, J. Control. Release 154 (3) (2011) 249–257. [DOI] [PubMed] [Google Scholar]
- [96].Ali OA, Doherty E, Mooney DJ, Emerich D, Relationship of vaccine efficacy to the kinetics of DC and T-cell responses induced by PLG-based cancer vaccines, Biomatter 1 (1) (2011) 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ali OA, Verbeke C, Johnson C, Sands RW, Lewin SA, White D, Doherty E, Dranoff G, Mooney DJ, Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants, Cancer Res 74 (6) (2014) 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ali OA, Lewin SA, Dranoff G, Mooney DJ, Vaccines combined with immune checkpoint antibodies promote cytotoxic T cell activity and tumor eradication, Cancer Immunol. Res (2015). [DOI] [PMC free article] [PubMed]
- [99].Ali OA, Dranoff, Glenn, Mooney, David J, Controlled Delivery of TLR Agonists in Structural Polymeric Devices, in: U.P. Office (Ed.) President and Fellows of Harvard College (Cambridge, MA, US), Dana-Farber Cancer Institute, Inc. (Boston, MA, US), United States, 2018. [Google Scholar]
- [100].Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang X, Pillarisetty VG, Pillai SPS, Wittrup KD, Stephan MT, Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors, J. Clin. Investig 127 (6) (2017) 2176–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhan Q, Shen B, Fang Y, Deng X, Chen H, Jin J, Peng C, Li H, Drug-eluting scaffold inhibited in vivo pancreatic tumorigenesis by engaging murine CCR4+CD8+ T cells, Colloids Surf., B 158 (2017) 469–473. [DOI] [PubMed] [Google Scholar]
- [102].Hathaichanok P, Chanyoung S, Ho US, Taik LY, Implantable synthetic immune niche for spatiotemporal modulation of tumor-derived immunosuppression and systemic antitumor immunity: postoperative immunotherapy, Adv. Mater 30 (18) (2018) 1706719. [DOI] [PubMed] [Google Scholar]
- [103].Park CG, Hartl CA, Schmid D, Carmona EM, Kim H-J, Goldberg MS, Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases, Sci. Transl. Med 10 (433) (2018). [DOI] [PubMed] [Google Scholar]
- [104].Wang C, Adrianus GN, Sheng N, Toh S, Gong Y, Wang D-A, In vitro performance of an injectable hydrogel/microsphere based immunocyte delivery system for localised anti-tumour activity, Biomaterials 30 (36) (2009) 6986–6995. [DOI] [PubMed] [Google Scholar]
- [105].Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ, Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy, Biomaterials 29 (27) (2008) 3671–3682. [DOI] [PubMed] [Google Scholar]
- [106].Hori Y, Winans AM, Irvine DJ, Modular injectable matrices based on alginate solution/microsphere mixtures that gel in situ and co-deliver immunomodulatory factors, Acta Biomater 5 (4) (2009) 969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Li Y, Fang M, Zhang J, Wang J, Song Y, Shi J, Li W, Wu G, Ren J, Wang Z, Zou W, Wang L, Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity, OncoImmunology 5 (2) (2015) e1074374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Verbeke CS, Mooney DJ, Paulson JA, injectable, pore-forming hydrogels for in vivo enrichment of immature dendritic cells, Adv. Healthcare Mater 4 (17) (2015) 2677–2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Verbeke CS, Gordo S, Schubert DA, Lewin SA, Desai RM, Dobbins J, Wucherpfennig KW, Mooney DJ, Multicomponent injectable hydrogels for antigen-specific tolerogenic immune modulation, Adv. Healthcare Mater 6 (6) (2017) 1600773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, Mooney DJ, Injectable preformed scaffolds with shape-memory properties, PNAS 109 (48) (2012) 19590–19595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Bencherif SA, Sands RW, Ali OA, Li WA, Lewin SA, Braschler TM, Shih T-YS, Verbeke CS, Bhatta D, Dranoff G, Mooney DJ, Injectable cryogel-based whole cell cancer vaccines, Nat. Commun 6 (2015) 7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112]. S.A.D. Bencherif, MA, US), Sands, Roger Warren (Chicago, IL), Koshy, Sandeep T. (Boston, MA, US), Mooney, David J. (Sudbury, MA, US), Injectable Cryogel Vaccine Devices and Methods of Use Thereof, President and Fellows of Harvard College (Cambridge, MA, US), United States, 2018.
- [113].Koshy ST, Ferrante TC, Lewin SA, Mooney DJ, Injectable, porous, and cell-responsive gelatin cryogels, Biomaterials 35 (8) (2014) 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Rudra JS, Tian YF, Jung JP, Collier JH, A self-assembling peptide acting as an immune adjuvant, Proc. Natl. Acad. Sci 107 (2) (2010) 622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Jung JP, Moyano JV, Collier JH, Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices, Integrative Biol.: Quantit. Biosci. Nano to Macro 3 (3) (2011) 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Rudra JS, Sun T, Bird KC, Daniels MD, Gasiorowski JZ, Chong AS, Collier JH, Modulating adaptive immune responses to peptide self-assemblies, ACS Nano 6 (2) (2012) 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Chen J, Pompano RR, Santiago FW, Maillat L, Sciammas R, Sun T, Han H, Topham DJ, Chong AS, Collier JH, The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation, Biomaterials 34 (34) (2013) 8776–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Pompano RR, Chen J, Verbus EA, Han H, Fridman A, McNeely T, Collier JH, Chong AS, Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs, Adv. Healthcare Mater 3 (11) (2014) 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Mora-Solano C, Wen Y, Han H, Chen J, Chong AS, Miller ML, Pompano RR, Collier JH, Active immunotherapy for TNF-mediated inflammation using self-assembled peptide nanofibers, Biomaterials 149 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, Mooney DJ, Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy, Nat. Biotechnol 33 (2014) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Li WA, Lu BY, Gu L, Choi Y, Kim J, Mooney DJ, The effect of surface modification of mesoporous silica micro-rod scaffold on immune cell activation and infiltration, Biomaterials 83 (2016) 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Li AW, Sobral MC, Badrinath S, Choi Y, Graveline A, Stafford AG, Weaver JC, Dellacherie MO, Shih T-Y, Ali OA, Kim J, Wucherpfennig KW, Mooney DJ, A facile approach to enhance antigen response for personalized cancer vaccination, Nat. Mater 17 (6) (2018) 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Nishikawa M, Ogawa K, Umeki Y, Mohri K, Kawasaki Y, Watanabe H, Takahashi N, Kusuki E, Takahashi R, Takahashi Y, Takakura Y, Injectable, self-gelling, biodegradable, and immunomodulatory DNA hydrogel for antigen delivery, J. Control. Release 180 (2014) 25–32. [DOI] [PubMed] [Google Scholar]
- [124].Yuka U, Kohta M, Yohji K, Hiroshi W, Rei T, Yuki T, Yoshinobu T, Makiya N, Induction of potent antitumor immunity by sustained release of cationic antigen from a DNA-based hydrogel with adjuvant activity, Adv. Funct. Mater 25 (36) (2015) 5758–5767. [Google Scholar]
- [125].Luo Z, Wu Q, Yang C, Wang H, He T, Wang Y, Wang Z, Chen H, Li X, Gong C, Yang Z, A powerful CD8+ T-Cell stimulating D-tetra-peptide hydrogel as a very promising vaccine adjuvant, Adv. Mater 29 (5) (2017) 1601776. [DOI] [PubMed] [Google Scholar]
- [126].Song H, Huang P, Niu J, Shi G, Zhang C, Kong D, Wang W, Injectable polypeptide hydrogel for dual-delivery of antigen and TLR3 agonist to modulate dendritic cells in vivo and enhance potent cytotoxic T-lymphocyte response against melanoma, Biomaterials 159 (2018) 119–129. [DOI] [PubMed] [Google Scholar]
- [127].Yang P, Song H, Qin Y, Huang P, Zhang C, Kong D, Wang W, Engineering dendritic-cell-based vaccines and PD-1 blockade in self-assembled peptide nanofibrous hydrogel to amplify antitumor T-cell immunity, Nano Lett 18 (7) (2018) 4377–4385. [DOI] [PubMed] [Google Scholar]
- [128].Leach DG, Dharmaraj N, Piotrowski SL, Lopez-Silva TL, Lei YL, Sikora AG, Young S, Hartgerink JD, STINGel: controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy, Biomaterials 163 (2018) 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Baird JR, Bell RB, Troesch V, Friedman DJ, Bambina S, Kramer G, Blair T, Medler TR, Wu Y, Sun Z, de Gruijl TD, van de Ven R, Leidner R, Crittenden MR, Gough MJ, Evaluation of explant responses to STING ligands: personalized immunosurgical therapy for head and neck squamous cell carcinoma, Cancer Res (2018). [DOI] [PubMed]
- [130].Chen Q, Wang C, Zhang X, Chen G, Hu Q, Li H, Wang J, Wen D, Zhang Y, Lu Y, Yang G, Jiang C, Wang J, Dotti G, Gu Z, In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment, Nat. Nanotechnol 14 (1) (2019) 89–97. [DOI] [PubMed] [Google Scholar]
- [131].Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA, Cancer, regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma, Proc. Natl. Acad. Sci 100(14) (2003) 8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubat T, Yagita H, Honjo T, Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes, Int. Immunol 8 (5) (1996) 765–772. [DOI] [PubMed] [Google Scholar]
- [133].Walunas TL, Bakker CY, Bluestone JA, CTLA-4 ligation blocks CD28-dependent T cell activation, J. Exp. Med 183 (6) (1996) 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Greenwald RJ, Oosterwegel MA, van der Woude D, Kubal A, Mandelbrot DA, Boussiotis VA, Sharpe AH, CTLA-4 regulates cell cycle progression during a primary immune response, Eur. J. Immunol 32 (2) (2002) 366–373. [DOI] [PubMed] [Google Scholar]
- [135].Bennett F, Luxenberg D, Ling V, Wang I-M, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, Collins M, Carreno BM, Program Death-1 Engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, But Not CD28, IL-7, and IL-15 responses, J. Immunol 170 (2) (2003) 711–718. [DOI] [PubMed] [Google Scholar]
- [136].Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N, Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade, Proc. Natl. Acad. Sci 99 (19) (2002) 12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Korman A, Yellin M, Keler T, Tumor immunotherapy: preclinical and clinical activity of anti-CTLA4 antibodies, Curr. Opin. Invest. Drugs 6 (6) (2005) 582–591. [PubMed] [Google Scholar]
- [138].Baruch EN, Berg AL, Besser MJ, Schachter J, Markel G, Adoptive T cell therapy: An overview of obstacles and opportunities, Cancer 123 (S11) (2017) 2154–2162. [DOI] [PubMed] [Google Scholar]
- [139].Jin C, Yu D, Hillerdal V, Wallgren A, Karlsson-Parra A, Essand M, Allogeneic lymphocyte-licensed DCs expand T cells with improved antitumor activity and resistance to oxidative stress and immunosuppressive factors, Mol. Ther. Methods Clin. Dev 1 (2014) 14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Zeng W, Su M, Anderson KS, Sasada T, Artificial antigen-presenting cells expressing CD80, CD70, and 4–1BB ligand efficiently expand functional T cells specific to tumor-associated antigens, Immunobiology 219 (8) (2014) 583–592. [DOI] [PubMed] [Google Scholar]
- [141].Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA, Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma, J. Clin. Oncol 23 (10) (2005) 2346–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Sadelain M, Brentjens R, Rivière I, The basic principles of chimeric antigen receptor design, Cancer Discovery 3 (4) (2013) 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Barrett DM, Grupp SA, June CH, Chimeric antigen receptor– and TCR-modified T cells enter main street and wall street, J. Immunol 195 (3) (2015) 755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Motz Greg T., Coukos G, Deciphering and reversing tumor immune suppression, Immunity 39 (1) (2013) 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G, Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand, Cancer Res 60 (12) (2000) 3239–3246. [PubMed] [Google Scholar]
- [146].Dranoff G, GM-CSF based cancer vaccines, Cancer Immunity Archive 5 (Suppl 1) (2005). [Google Scholar]
- [147].Dobbs NA, Zhou X, Pulse M, Hodge LM, Schoeb TR, Simecka JW, Antigen-pulsed bone marrow derived and pulmonary dendritic cells promote Th2 cell responses and immunopathology in lungs during the pathogenesis of murine mycoplasma pneumonia, J. Immunol. (Baltimore, Md. : 1950) 193 (3) (2014) 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yan W-L, Shen K-Y, Tien C-Y, Chen Y-A, Liu S-J, Recent progress in GM-CSF-based cancer immunotherapy, Immunotherapy 9 (4) (2017) 347–360. [DOI] [PubMed] [Google Scholar]
- [149].Geginat J, Lanzavecchia A, Sallusto F, Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines, Blood 101 (11) (2003) 4260–4266. [DOI] [PubMed] [Google Scholar]
- [150].Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A, Two subsets of memory T lymphocytes with distinct homing potentials and effector functions, Nature 401 (1999) 708. [DOI] [PubMed] [Google Scholar]
- [151].Saeki H, Tamaki K, Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases, J. Dermatol. Sci 43 (2) (2006) 75–84. [DOI] [PubMed] [Google Scholar]
- [152].Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H, Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation, Allergy 64 (7) (2009) 995–1002. [DOI] [PubMed] [Google Scholar]
- [153].Rubinstein MP, Kovar M, Purton JF, Cho J-H, Boyman O, Surh CD, Sprent J, Converting IL-15 to a superagonist by binding to soluble IL-15Ra, Proc. Natl. Acad. Sci 103 (24) (2006) 9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Judge AD, Zhang X, Fujii H, Surh CD, Sprent J, Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8+ T Cells, J. Exp. Med 196 (7) (2002) 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JCL, Joyce S, Peschon JJ, Reversible defects in natural killer and memory Cd8 T cell lineages in interleukin 15–deficient mice, J. Exp. Med 191 (5) (2000) 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Weiner GJ, Liu H-M, Wooldridge JE, Dahle CE, Krieg AM, Immunostimulatory oligodeoxynucleotides containing the CpG motif are effective as immune adjuvants in tumor antigen immunization, PNAS 94 (20) (1997) 10833–10837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Ahmad-Nejad P, Häcker H, Rutz M, Bauer S, Vabulas RM, Wagner H, Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments, Eur. J. Immunol 32 (7) (2002) 1958–1968. [DOI] [PubMed] [Google Scholar]
- [158].Rothenfusser S, Tuma E, Endres S, Hartmann G, Plasmacytoid dendritic cells: the key to CpG11The authors dedicate this article to Prof. K. Lennert, M. D., Department of Hematology and Oncology, University of Kiel, Germany, who was the first to describe plasmacytoid dendritic cells histologically in 1958, Human Immunol 63 (12) (2002) 1111–1119. [DOI] [PubMed] [Google Scholar]
- [159].Loré K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, Roederer M, Seder RA, Koup RA, Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T Cell responses, J. Immunol 171 (8) (2003) 4320–4328. [DOI] [PubMed] [Google Scholar]
- [160].Klinman DM, Immunotherapeutic uses of CpG oligodeoxynucleotides, Nat. Rev. Immunol 4 (2004) 249. [DOI] [PubMed] [Google Scholar]
- [161].Fortier M-E, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN, The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism, Am. J. Physiol.-Regulat., Integr. Comparative Physiol 287 (4) (2004) R759–R766. [DOI] [PubMed] [Google Scholar]
- [162].Manetti R, Annunziato F, Tomasevic L, Giannò V, Parronchi P, Romagnani S, Maggi E, Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-α and interleukin-12, Eur. J. Immunol 25 (9) (1995) 2656–2660. [DOI] [PubMed] [Google Scholar]
- [163].Alexopoulou L, Holt AC, Medzhitov R, Flavell RA, Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3, Nature 413 (2001) 732. [DOI] [PubMed] [Google Scholar]
- [164].Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, Ozaki Y, Tomizawa H, Akira S, Fukuhara S, Interferon-α and interleukin-12 are induced differentially by toll-like receptor 7 ligands in human blood dendritic cell subsets, J. Exp. Med 195 (11) (2002) 1507–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S, Small anti-viral compounds activate immune cells via the TLR7 MyD88–dependent signaling pathway, Nat. Immunol 3 (2002) 196. [DOI] [PubMed] [Google Scholar]
- [166].AduroBiotech, NCT02675439: Study of the Safety and Efficacy of MIW815 (ADU-S100) in Patients With Advanced/Metastatic Solid Tumors or Lymphomas, National Institute of Health, ClinicalTrails.gov, 2017. [Google Scholar]
- [167].Merck, NCT03010176: Study of MK-1454 Alone or in Combination With Pembrolizumab in Participants With Advanced/Metastatic Solid Tumors or Lymphomas (MK-1454–001), National Institute of Health, ClinicalTrails.gov, 2017. [Google Scholar]
- [168].Dubensky TW, Kanne DB, Leong ML, Rationale, progress and development of vaccines utilizing STING-activating cyclic dinucleotide adjuvants, Therapeutic Adv. Vaccines 1 (4) (2013) 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Barber GN, STING infection, inflammation and cancer, Nat. Rev. Immunol 15 (12) (2015) 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Burdette DL, Vance RE, STING and the innate immune response to nucleic acids in the cytosol, Nat. Immunol 14 (1) (2013) 19–26. [DOI] [PubMed] [Google Scholar]
- [171].Barber GN, STING-dependent cytosolic DNA sensing pathways, Trends Immunol 35 (2) (2014) 88–93. [DOI] [PubMed] [Google Scholar]
- [172].Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ, Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants, J. Clin. Investig 125 (6) (2015) 2532–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Gray PM, Forrest G, Wisniewski T, Porter G, Freed DC, DeMartino JA, Zaller DM, Guo Z, Leone J, Fu T-M, Vora KA, Evidence for cyclic diguanylate as a vaccine adjuvant with novel immunostimulatory activities, Cell. Immunol 278 (1) (2012) 113–119. [DOI] [PubMed] [Google Scholar]
- [174].Chen W, KuoLee R, Yan H, The potential of 3ʹ,5ʹ -cyclic diguanylic acid (c-di-GMP) as an effective vaccine adjuvant, Vaccine 28 (18) (2010) 3080–3085. [DOI] [PubMed] [Google Scholar]
- [175].Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ, Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy, Nanomedicine: Nanotechnology, Biol. Med 14 (2) (2018) 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Hoare TR, Kohane DS, Hydrogels in drug delivery: Progress and challenges, Polymer 49 (8) (2008) 1993–2007. [Google Scholar]
- [177].Yu L, Ding J, Injectable hydrogels as unique biomedical materials, Chem. Soc. Rev 37 (8) (2008) 1473–1481. [DOI] [PubMed] [Google Scholar]
- [178].Burdick JA, Prestwich GD, Hyaluronic Acid Hydrogels for Biomedical Applications, Adv. Mater 23 (12) (2011) H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Ungerleider JL, Christman KL, Concise review: injectable biomaterials for the treatment of myocardial infarction and peripheral artery disease: translational challenges and progress, Stem Cells Transl. Med 3 (9) (2014) 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Hixon KR, Lu T, Sell SA, A comprehensive review of cryogels and their roles in tissue engineering applications, Acta Biomater 62 (2017) 29–41. [DOI] [PubMed] [Google Scholar]
- [181].Alexander A, Ajazuddin J, Khan S, Saraf Saraf S, Poly(ethylene glycol)–poly (lactic-co-glycolic acid) based thermosensitive injectable hydrogels for biomedical applications, J. Control. Release 172 (3) (2013) 715–729. [DOI] [PubMed] [Google Scholar]
- [182].Wang F, Li Z, Khan M, Tamama K, Kuppusamy P, Wagner WR, Sen CK, Guan J, Injectable, rapid gelling and highly flexible hydrogel composites as growth factor and cell carriers, Acta Biomater 6 (6) (2010) 1978–1991. [DOI] [PubMed] [Google Scholar]
- [183].Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, Li S, Deng Y, He N, Injectable hydrogels for cartilage and bone tissue engineering, Bone Res 5 (2017) 17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Makadia HK, Siegel SJ, Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier, Polymers 3 (3) (2011) 1377–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Holderegger C, Schmidlin PR, Weber FE, Mohn D, Preclinical in vivo performance of novel biodegradable, electrospun poly(lactic acid) and Poly (lactic-co-glycolic acid) nanocomposites: a review, Materials 8 (8) (2015) 4912–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Jain RA, The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices, Biomaterials 21 (23) (2000) 2475–2490. [DOI] [PubMed] [Google Scholar]
- [187].Dalpiaz A, Contado C, Mari L, Perrone D, Pavan B, Paganetto G, Hanuskovà M, Vighi E, Leo E, Development and characterization of PLGA nanoparticles as delivery systems of a prodrug of zidovudine obtained by its conjugation with ursodeoxycholic acid, Drug Delivery 21 (3) (2014) 221–232. [DOI] [PubMed] [Google Scholar]
- [188].Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V, PLGA-based nanoparticles: an overview of biomedical applications, J. Control. Release 161 (2) (2012) 505–522. [DOI] [PubMed] [Google Scholar]
- [189].Zhu J, Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering, Biomaterials 31 (17) (2010) 4639–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Ossipov DA, Piskounova S, Hilborn J, Poly(vinyl alcohol) cross-linkers for in vivo injectable hydrogels, Macromolecules 41 (11) (2008) 3971–3982. [Google Scholar]
- [191].Silver FH, Pins G, Cell growth on collagen: a review of tissue engineering using scaffolds containing extracellular matrix, J. Long Term Eff. Med. Implants 2 (1) (1992) 67–80. [PubMed] [Google Scholar]
- [192].Malcor J-D, Juskaite V, Gavriilidou D, Hunter EJ, Davidenko N, Hamaia S, Sinha S, Cameron RE, Best SM, Leitinger B, Farndale RW, Coupling of a specific photoreactive triple-helical peptide to crosslinked collagen films restores binding and activation of DDR2 and VWF, Biomaterials 182 (2018) 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Ashworth JC, Best SM, Cameron RE, Quantitative architectural description of tissue engineering scaffolds, Mater. Technol 29 (5) (2014) 281–295. [Google Scholar]
- [194].Chattopadhyay S, Raines RT, Review collagen-based biomaterials for wound healing, Biopolymers 101 (8) (2014) 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Antoine EE, Vlachos PP, Rylander MN, Review of Collagen I Hydrogels for Bioengineered Tissue Microenvironments: Characterization of Mechanics, Structure, and Transport, Tissue Engineering, Part B, Reviews 20 (6) (2014) 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Mari CE, Laura Saenz del B, Jose LP, Gorka O, Gelatin as Biomaterial for Tissue Engineering, Curr. Pharm. Des 23 (24) (2017) 3567–3584. [DOI] [PubMed] [Google Scholar]
- [197].Su K, Wang C, Recent advances in the use of gelatin in biomedical research, Biotechnol. Lett 37 (11) (2015) 2139–2145. [DOI] [PubMed] [Google Scholar]
- [198].Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia X, Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks, Soft Matter 8 (12) (2012) 3280–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Allison DD, Grande-Allen KJ, Review. Hyaluronan: a powerful tissue engineering tool, Tissue Eng 12 (8) (2006) 2131–2140. [DOI] [PubMed] [Google Scholar]
- [200].Janmey PA, Winer JP, Weisel JW, Fibrin gels and their clinical and bioengineering applications, J. R. Soc. Interface 6 (30) (2009) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Janmey PA, Kinetics of formation of fibrin oligomers. I. Theory, Biopolymers 21 (11) (1982) 2253–2264. [DOI] [PubMed] [Google Scholar]
- [202].Weisel JW, The mechanical properties of fibrin for basic scientists and clinicians, Biophys. Chem 112 (2) (2004) 267–276. [DOI] [PubMed] [Google Scholar]
- [203].Sun J, Tan H, Alginate-based biomaterials for regenerative medicine applications, Materials 6 (4) (2013) 1285–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Augst AD, Kong HJ, Mooney DJ, Alginate hydrogels as biomaterials, Macromol. Biosci 6 (8) (2006) 623–633. [DOI] [PubMed] [Google Scholar]
- [205].Baldwin AD, Kiick KL, Polysaccharide-modified synthetic polymeric biomaterials, Biopolymers 94 (1) (2010) 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Augst AD, Kong HJ, Mooney DJ, Alginate hydrogels as biomaterials, Macromol. Biosci 6 (8) (2006) 623–633. [DOI] [PubMed] [Google Scholar]
- [207].Um SH, Lee JB, Park N, Kwon SY, Umbach CC, Luo D, Enzyme-catalysed assembly of DNA hydrogel, Nat. Mater 5 (2006) 797. [DOI] [PubMed] [Google Scholar]
- [208].Lin DC, Yurke B, Langrana NA, Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel, J. Biomech. Eng 126 (1) (2004) 104–110. [DOI] [PubMed] [Google Scholar]
- [209].Cheng E, Xing Y, Chen P, Yang Y, Sun Y, Zhou D, Xu L, Fan Q, Liu D, A pH-triggered, fast-responding DNA Hydrogel, Angew. Chem. Int. Ed 48 (41) (2009) 7660–7663. [DOI] [PubMed] [Google Scholar]
- [210].Xing Y, Cheng E, Yang Y, Chen P, Zhang T, Sun Y, Yang Z, Liu D, Self-assembled DNA hydrogels with designable thermal and enzymatic responsiveness, Adv. Mater 23 (9) (2010) 1117–1121. [DOI] [PubMed] [Google Scholar]
- [211].Kyle S, Aggeli A, Ingham E, McPherson MJ, Production of self-assembling biomaterials for tissue engineering, Trends Biotechnol 27 (7) (2009) 423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Sun L, Zheng C, Webster TJ, Self-assembled peptide nanomaterials for biomedical applications: promises and pitfalls, Int. J. Nanomed 12 (2017) 73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Moore AN, Hartgerink JD, Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration, Acc. Chem. Res 50 (4) (2017) 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J, Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide, J. Am. Chem. Soc 124 (50) (2002) 15030–15037. [DOI] [PubMed] [Google Scholar]
- [215].Salick DA, Kretsinger JK, Pochan DJ, Schneider JP, Inherent antibacterial activity of a peptide-based β-hairpin hydrogel, J. Am. Chem. Soc 129 (47) (2007) 14793–14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, Lamm MS, Pochan DJ, Schneider JP, Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells, Proc. Natl. Acad. Sci 104 (19) (2007) 7791–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Sun Y, Li W, Wu X, Zhang N, Zhang Y, Ouyang S, Song X, Fang X, Seeram R, Xue W, He L, Wu W, Functional self-assembling peptide nanofiber hydrogels designed for nerve degeneration, ACS Appl. Mater. Interfaces 8 (3) (2016) 2348–2359. [DOI] [PubMed] [Google Scholar]
- [218].Nagai Y, Unsworth LD, Koutsopoulos S, Zhang S, Slow release of molecules in self-assembling peptide nanofiber scaffold, J. Control. Release 115 (1) (2006) 18–25. [DOI] [PubMed] [Google Scholar]
- [219].Cui H, Webber MJ, Stupp SI, Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials, Pept. Sci 94 (1) (2010) 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Hendricks MP, Sato K, Palmer LC, Stupp SI, Supramolecular assembly of peptide amphiphiles, Acc. Chem. Res 50 (10) (2017) 2440–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Aulisa L, Dong H, Hartgerink JD, Self-assembly of multidomain peptides: sequence variation allows control over cross-linking and viscoelasticity, Biomacromolecules 10 (9) (2009) 2694–2698. [DOI] [PubMed] [Google Scholar]
- [222].Kumar VA, Shi S, Wang BK, Li I-C, Jalan AA, Sarkar B, Wickremasinghe NC, Hartgerink JD, Drug-triggered and cross-linked self-assembling nanofibrous hydrogels, J. Am. Chem. Soc 137 (14) (2015) 4823–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Leifer CA, Dendritic cells in host response to biologic scaffolds, Semin. Immunol 29 (2017) 41–48. [DOI] [PubMed] [Google Scholar]
- [224].D.-F.C. Institute, NCT01753089: Dendritic Cell Activating Scaffold in Melanoma, National Institute of Health, ClinicalTrails.gov, 2012. [Google Scholar]
- [225].Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, Guldberg RE, García AJ, Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair, Biomaterials 31 (9) (2010) 2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE, STING is a direct innate immune sensor of cyclic di-GMP, Nature 478 (7370) (2011) 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Verma V, Kim Y, Lee M-C, Lee J-T, Cho S, Park I-K, Min JJ, Lee JJ, Lee SE, Rhee JH, Activated dendritic cells delivered in tissue compatible biomatrices induce in-situ anti-tumor CTL responses leading to tumor regression, Oncotarget 7 (26) (2016) 39894–39906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Cappuzzo F, Mazzoni F, Gennari A, Donati S, Salvadori B, Orlandini C, Cetto GL, Molino A, Galligioni E, Mansutti M, Tumolo S, Lucentini A, Valduga F, Bartolini S, Crinò L, Conte PF, Multicentric phase II trial of gemcitabine plus epirubicin plus paclitaxel as first-line chemotherapy in metastatic breast cancer, Br. J. Cancer 90 (1) (2004) 31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM, Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity, Clin. Cancer Res 11 (18) (2005) 6713–6721. [DOI] [PubMed] [Google Scholar]
- [230].Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE, Myeloid-derived suppressor cells in breast cancer, Breast Cancer Res. Treat 140 (1) (2013) 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Kiew L-V, Cheong S-K, Sidik K, Chung L-Y, Improved plasma stability and sustained release profile of gemcitabine via polypeptide conjugation, Int. J. Pharm 391 (1) (2010) 212–220. [DOI] [PubMed] [Google Scholar]
- [232].Guvendiren M, Lu HD, Burdick JA, Shear-thinning hydrogels for biomedical applications, Soft Matter 8 (2) (2012) 260–272. [Google Scholar]
- [233].Chen MH, Wang LL, Chung JJ, Kim Y-H, Atluri P, Burdick JA, Methods to assess shear-thinning hydrogels for application as injectable biomaterials, ACS Biomater. Sci. Eng 3 (12) (2017) 3146–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Myron S, Teck Chuan L, Injectable biomaterials: a perspective on the next wave of injectable therapeutics, Biomed. Mater 11(1) (2016) 014110. [DOI] [PubMed] [Google Scholar]
- [235].Hill E, Boontheekul T, Mooney DJ, Regulating activation of transplanted cells controls tissue regeneration, PNAS 103 (8) (2006) 2494–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Huebsch N, Lippens E, Lee K, Mehta M, Koshy Sandeep T., Darnell Max C., Desai RM, Madl Christopher M., Xu M, Zhao X, Chaudhuri O, Verbeke C, Kim Woo S., Alim K, Mammoto A, Ingber Donald E., Duda Georg N., Mooney David J. , Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation, Nat. Mater 14 (2015) 1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Monette A, Ceccaldi C, Assaad E, Lerouge S, Lapointe R, Chitosan thermogels for local expansion and delivery of tumor-specific T lymphocytes towards enhanced cancer immunotherapies, Biomaterials 75 (2016) 237–249. [DOI] [PubMed] [Google Scholar]
- [238].Barchet W, Wimmenauer V, Schlee M, Hartmann G, Accessing the therapeutic potential of immunostimulatory nucleic acids, Curr. Opin. Immunol 20 (4) (2008) 389–395. [DOI] [PubMed] [Google Scholar]
- [239].Koutsopoulos S, Zhang S, Two-layered injectable self-assembling peptide scaffold hydrogels for long-term sustained release of human antibodies, J. Control. Release 160 (3) (2012) 451–458. [DOI] [PubMed] [Google Scholar]
- [240].Xu K, Lee F, Gao SJ, Chung JE, Yano H, Kurisawa M, Injectable hyaluronic acid-tyramine hydrogels incorporating interferon-α2a for liver cancer therapy, J. Control. Release 166 (3) (2013) 203–210. [DOI] [PubMed] [Google Scholar]
- [241].Ueda K, Akiba J, Ogasawara S, Todoroki K, Nakayama M, Sumi A, Kusano H, Sanada S, Suekane S, Xu K, Bae KH, Kurisawa M, Igawa T, Yano H, Growth inhibitory effect of an injectable hyaluronic acid–tyramine hydrogels incorporating human natural interferon-α and sorafenib on renal cell carcinoma cells, Acta Biomater 29 (2016) 103–111. [DOI] [PubMed] [Google Scholar]
- [242].Han L, Xue J, Wang L, Peng K, Zhang Z, Gong T, Sun X, An injectable, low-toxicity phospholipid-based phase separation gel that induces strong and persistent immune responses in mice, Biomaterials 105 (2016) 185–194. [DOI] [PubMed] [Google Scholar]
- [243].Kawamori T, Rao CV, Seibert K, Reddy BS, Chemopreventive Activity of Celecoxib, a Specific Cyclooxygenase-2 Inhibitor, against Colon Carcinogenesis, Cancer Res 58 (3) (1998) 409–412. [PubMed] [Google Scholar]
- [244].Barlow M, Edelman M, Glick RD, Steinberg BM, Soffer SZ, Celecoxib inhibits invasion and metastasis via a cyclooxygenase 2–independent mechanism in an in vitro model of Ewing sarcoma, J. Pediatr. Surg 47 (6) (2012) 1223–1227. [DOI] [PubMed] [Google Scholar]
- [245].Kanterman J, Sade-Feldman M, Baniyash M, New insights into chronic inflammation-induced immunosuppression, Semin. Cancer Biol 22 (4) (2012) 307–318. [DOI] [PubMed] [Google Scholar]
- [246].Dirks J, Egli A, Sester U, Sester M, Hirsch HH, Blockade of programmed death receptor-1 signaling restores expression of mostly proinflammatory cytokines in anergic cytomegalovirus-specific T cells, Transplant Infectious Disease 15 (1) (2013) 79–89. [DOI] [PubMed] [Google Scholar]
- [247].Tsao C-T, Kievit FM, Ravanpay A, Erickson AE, Jensen MC, Ellenbogen RG, Zhang M, Thermoreversible poly(ethylene glycol)-g-chitosan hydrogel as a therapeutic T lymphocyte depot for localized glioblastoma immunotherapy, Biomacromolecules 15 (7) (2014) 2656–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Wang X, Zhang Y, Xue W, Wang H, Qiu X, Liu Z, Thermo-sensitive hydrogel PLGA-PEG-PLGA as a vaccine delivery system for intramuscular immunization, J. Biomater. Appl 31 (6) (2017) 923–932. [DOI] [PubMed] [Google Scholar]
- [249].Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, Woo S-R, Lemmens E, Banda T, Leong JJ, Metchette K, Dubensky TW, Gajewski TF, Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity, Cell Reports 11 (7) (2015) 1018–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Moore EC, Clavijo PE, Davis R, Cash H, Van Waes C, Kim Y, Allen C, Established T cell-inflamed tumors rejected after adaptive resistance was reversed by combination STING activation and PD-1 pathway blockade, Cancer Immunol. Res 4 (12) (2016) 1061–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Wire B, First Presentation of Early Data for Merck’s Investigational STING Agonist (MK-1454) in Patients with Advanced Solid Tumors or Lymphomas at ESMO 2018 Congress, Berkshire Hathaway, Kenilworth, NJ, 2018. [Google Scholar]
- [252].Janani I, Lakra R, Kiran MS, Korrapati PS, Selectivity and sensitivity of molybdenum oxide-polycaprolactone nanofiber composites on skin cancer: preliminary in-vitro and in-vivo implications, J. Trace Elem. Med Biol 49 (2018) 60–71. [DOI] [PubMed] [Google Scholar]
- [253].Yavuz B, Zeki J, Coburn JM, Ikegaki N, Levitin D, Kaplan DL, Chiu B, In vitro and in vivo evaluation of etoposide - silk wafers for neuroblastoma treatment, J. Control. Release 285 (2018) 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, Ye Y, Bomba H, Hu X, Liu Z, Dotti G, Gu Z, In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy, Sci. Transl. Med 10 (429) (2018). [DOI] [PubMed] [Google Scholar]
- [255].Sarika C, Praveen G, Snima KS, Shantikumar VN, Pavithran K, Krishnaprasad C, Vinoth-Kumar L, Potential use of drug loaded nano composite pectin scaffolds for the treatment of ovarian cancer, Curr. Drug Deliv 10 (3) (2013) 326–335. [DOI] [PubMed] [Google Scholar]
- [256].Brudno Y, Pezone MJ, Snyder TK, Uzun O, Moody CT, Aizenberg M, Mooney DJ, Replenishable drug depot to combat post-resection cancer recurrence, Biomaterials 178 (2018) 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Kennedy S, Roco C, Déléris A, Spoerri P, Cezar C, Weaver J, Vandenburgh H, Mooney D, Improved magnetic regulation of delivery profiles from ferrogels, Biomaterials 161 (2018) 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Ishihara J, Fukunaga K, Ishihara A, Larsson HM, Potin L, Hosseinchi P, Galliverti G, Swartz MA, Hubbell JA, Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events, Sci. Transl. Med 9 (415) (2017). [DOI] [PubMed] [Google Scholar]