Key Points
Question
Is obesity associated with multiple sclerosis risk and response to first-line therapy in a pediatric population in Germany?
Findings
In this single-center study of 453 German children with a multiple sclerosis diagnosis, obesity was associated with twofold greater odds of the disease and more frequent failure of first-line treatment with interferon beta-1a or 1b and glatiramer acetate, thereby increasing the number of patients on second-line treatment.
Meaning
Obesity appeared to be statistically significantly associated with increased risk of pediatric multiple sclerosis and with worse treatment response to first-line treatment; a healthy weight may potentially optimize treatment outcomes and reduce the disease burden and costs.
This study examines whether high body mass index in children and adolescents is a factor in the onset of multiple sclerosis and in treatment response.
Abstract
Importance
Obesity reportedly increases the risk of pediatric multiple sclerosis (MS), but little is known about its association with disease course.
Objective
To investigate the association of obesity with pediatric MS risk and with first-line therapy response among children with MS.
Design, Setting, and Participants
This single-center retrospective study used the medical records and database at the Center for MS in Childhood and Adolescence, Göttingen, Germany. The study included 453 patients with relapsing-remitting pediatric MS and body mass index (BMI) measurement taken within 6 months of diagnosis. Onset of the disease occurred between April 28, 1990, and June 26, 2016, and the mean disease duration was 38.4 months. Data were collected from July 14, 2016, to December 18, 2017.
Main Outcomes and Measures
Data on BMIs were stratified by sex and age using German BMI references and compared with the BMI data of 14 747 controls from a nationwide child health survey for odds ratio (OR) estimates. Baseline magnetic resonance imaging findings, intervals between first and second MS attacks, annualized relapse rates before and during treatment with interferon beta-1a or -1b and glatiramer acetate, frequency of second-line treatment, and Expanded Disability Status Scale (EDSS) scores were compared between nonoverweight (BMI≤90th percentile), overweight (BMI>90th-97th percentile), and obese (BMI>97th percentile) patients.
Results
In total, 453 patients with pediatric MS were included, of whom 306 (67.5%) were female, and the mean (SD) age at diagnosis was 13.7 (2.7) years. At diagnosis, 126 patients (27.8%) were overweight or obese, with obesity associated with statistically significant twofold odds of MS in both sexes (girls OR, 2.19; 95% CI, 1.5-3.1; P < .001 vs boys OR, 2.14; 95% CI, 1.3-3.5; P = .003). Obese patients, compared with nonoverweight patients, had statistically significantly more relapses on first-line treatment with interferon beta and glatiramer acetate (ARR, 1.29 vs 0.72; P < .001) and a higher rate of second-line treatment (21 [56.8%] of 37 vs 48 [38.7%] of 124; P = .06). Baseline neuroimaging, interval between first and second MS attacks, pretreatment relapses, and EDSS progression scores were not correlated with BMI.
Conclusions and Relevance
In this study, increased pediatric MS risk appeared to be associated with obesity, and obese patients did not respond well to first-line medications; altered pharmacokinetics appeared to be most likely factors in treatment response, suggesting that achieving healthy weight or adjusting the dose according to BMI could improve therapy response.
Introduction
The prevalence of overweight and obesity is on the rise worldwide, with the most substantial increase in the past 3 decades being observed among children and adolescents.1,2,3,4 This global phenomenon has attracted international attention as obesity has been consistently associated with increased mortality and several deleterious health outcomes, including greater susceptibility to inflammatory and autoimmune diseases.5,6,7,8
A plausible association between obesity and multiple sclerosis (MS) risk was first established in a 2009 study of the association between body size and MS in a cohort of more than 200 000 women in the United States.9 In that study, a more than twofold increased risk of developing adult-onset MS was associated with obesity (body mass index [BMI] of 30 or higher; calculated as weight in kilograms divided by height in meters squared) at age 18 years. These results have since been validated in other adult studies, with a growing body of evidence indicating that a high BMI in adolescence and possibly in early life increases MS risk.10,11,12,13,14,15,16,17,18 This association is evident even after controlling for established genetic and environmental risk factors and has been described in both sexes, although a stronger association has been repeatedly shown in females.16 The exact underlying pathologic mechanisms still require elucidation, but lower serum vitamin D levels, altered adipokine profiles favoring a proinflammatory state, deregulated gut microbiota, unfavorable interaction with HLA-risk genes in puberty, and earlier start of menses have all been suggested to play a role in obesity-associated MS risk.19,20,21,22,23,24,25
In the pediatric setting, to date, only a few studies on small cohorts have assessed the association between BMI and risk of pediatric MS; nevertheless, their findings also indicate substantially higher rates of overweight and obesity among children with MS compared with their healthy counterparts.14,25,26 At the Center for MS in Childhood and Adolescence in Göttingen, Germany, in which more than 150 pediatric patients with MS are followed up yearly, we have observed that many obese patients do not respond well to first-line therapy with interferon beta-1a or -1b and glatiramer acetate. This study was conducted to (1) ascertain whether obese patients have a worse response to first-line therapy, and (2) confirm increased pediatric MS risk associated with obesity in a large cohort.
Methods
This single-center analysis of MS cases (with onset between April 28, 1990, and June 26, 2016) received ethical approval from the University Medical Center Goettingen. All data were derived from database and medical record review and were deidentified; thus, informed consent was waived by the University Medical Center Goettingen. Data were collected from July 14, 2016, to December 18, 2017.
Study inclusion criteria were a confirmed diagnosis of relapsing-remitting MS, as defined by the revised McDonald criteria and the International Pediatric MS Study Group criteria27,28; an MS onset before 18 years of age; and a reliably documented height and weight measurement within 6 months of first clinical presentation to a medical institution. We performed a database search (W.S.) and validated those data by medical records review (B.H.). We standardized BMI for sex and age according to Kromeyer-Hauschild29 data, the recommended BMI references for German children. These data originated from a meta-analysis of 34 422 German children (aged <18 years) pooled from 17 different regional studies between 1985 and 1999.29
Patients were grouped by BMI category using the definitions recommended by the European Childhood Obesity Group.30 Nonoverweight was defined as BMI at or below the 90th percentile; overweight, BMI above the 90th to 97th percentile; obese, BMI above the 97th percentile; and extremely obese, BMI above the 99.5th percentile. These BMI cutoff points approximate the International Obesity Task Force definitions, which are based on percentiles that correspond to the World Health Organization adult (18 years of age) cutoffs for overweight (BMI of 25) and obesity (BMI of 30).31
For MS risk assessment, the outcome of increasing BMI trends over time was considered in choosing the control collective for odds ratio (OR) estimates. Patient BMIs were compared with the BMI data of 14 747 healthy children aged 3 to 17 years who participated in the 2003 to 2006 German Health Interview and Examination Survey for Children and Adolescents (KiGGS).32 The data in KiGGS were sex- and age-stratified according to the Kromeyer-Hauschild29 reference values and were presented in a 2007 Federal Health Bulletin,32 which reported a 50% increase in overweight and obesity in that period compared with the 2 previous decades. Studies33,34 have shown a stagnation in prevalence rates in Germany since the KiGGS survey; thus, the KiGGS data are still representative of current BMI trends among German children. Patients with MS onset before 2003 were not considered likely to falsely elevate ORs.
The Center for MS in Childhood and Adolescence in Göttingen is a tertiary referral center for pediatric MS. Referred patients are assessed every 6 months until age 18 years. Documented at these visits are new relapses (defined as the presence of new or worsening neurologic symptoms lasting at least 24 hours and occurring more than 30 days after a previous clinical event); Expanded Disability Status Scale (EDSS) score (range: 0-10, with the highest score indicating death from MS and the lowest score indicating normal neurological examination); laboratory findings; and results of ophthalmic examination, evoked potentials, nerve conduction studies, and brain magnetic resonance imaging (MRI).35 Spinal MRIs are done yearly unless indicated earlier. For comparison between weight categories, data on the following were collected: age at diagnosis, baseline MRI findings, interval between first and second MS attacks, time to initiation of a disease-modifying therapy (DMT), relapses before and during first-line treatment with interferon beta and glatiramer, initiation of second-line DMT (fingolimod, natalizumab, alemtuzumab, or rituximab), BMI at second-line DMT initiation, and EDSS score at 2 years and last consultation.
Annualized relapse rate (ARR), reported in person-years, was used to measure first-line therapy response; only patients with at least 6 months of treatment with interferon beta or glatiramer were included in the analysis. In general, patients were treated with doses according to the drug manufacturer’s recommendations. The standard doses for subcutaneous glatiramer, 20 mg daily, and for intramuscular interferon beta-1a, 30 μg weekly, were not reduced. Subcutaneous interferon beta-1a was dosed at 44 μg 3 times weekly, and subcutaneous interferon beta-1b was dosed at 250 μg every other day. In patients who developed intolerable adverse effects, subcutaneous interferon beta-1a was reduced to 22 μg 3 times weekly, and subcutaneous interferon beta-1b was reduced to 187.5 μg, 125 μg, or 62.5 μg every other day. If concerns of noncompliance (medication taken irregularly over a 6-month period) were documented at follow-up, the noncompliant patients were excluded.
Analysis of second-line therapy included only patients presenting from 2010 and with a minimum of 12 months of follow-up, unless escalated in less than 12 months. Patients recommended for second-line therapy at last consultation were counted as escalated. The year 2010 was chosen as the cutoff because the practice of escalating pediatric patients before this time was less common.
The variables used to define disease activity were baseline MRI findings, interval between first and second MS attacks, and EDSS score. A brain or spine MRI within 6 months of first presentation was considered a baseline MRI. T2 axial images as well as sagittal and coronal images, if available, were evaluated for T2 and gadolinium lesion counts. Magnetic resonance imaging data were acquired on a clinical 1.5-T or 3-T MRI scanner with the following parameters: axial T2-weighted turbo spin echo (T2w) sequences (echo time [TE], 80-132 milliseconds; repetition time [TR], 2111-6290 ms; slice thickness, 3-5 mm; gap, ≤1 mm) and axial T1-weighted sequences (T1w; TE, 2.1-25 milliseconds; TR, 150-873 milliseconds; slice thickness, 2-6 mm; gap, ≤1 mm) before and 5 minutes after standard single-dose gadolinium injection. Lesions were analyzed manually and independent of the analysis of the clinical history (by B.H. and H.H.) and were reevaluated in case of conflicting results (by P.H. and J.G.).
Statistical Analysis
We reported frequencies, including 95% Clopper-Pearson CIs for predefined categories of obesity and categorical outcomes. Comparisons between groups were performed with the Fisher exact test and reported with 95% CIs for the OR. For continuous and ordinal data, we reported mean, median, and 25% and 75% quantiles. Group comparisons were performed with unpaired, 2-tailed Welch t test or Wilcoxon rank sum test, as appropriate. For count data, including relapse rates and lesions, a negative binomial regression model was used, allowing for different follow-up times as offset. Adjusted relapse rates along with 95% CIs were thus estimated and comparisons between groups were tested. Two-sided P < .05 was considered statistically significant. Analyses were conducted with R Stat, version 3.4.3 (R Foundation for Statistical Computing).
Results
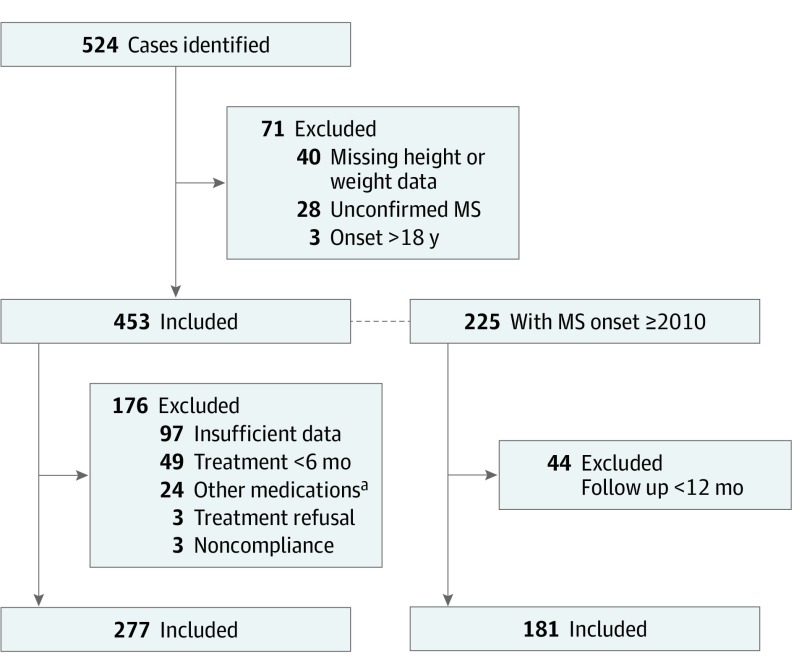
Of the 524 patients identified, 71 (13.5%) were excluded and 453 (86.5%) were included (Figure). Among the 453 patients, 306 (67.5%) were female and the mean (SD) age at diagnosis was 13.7 (2.7) years (Table 1).
Figure. Patient Selection.
MS indicates multiple sclerosis.
aOther medications were azathioprine sodium (n = 10), dimethyfumarate (n = 2), and only second-line therapy (n = 12).
Table 1. General Characteristics of the Cohort .
| Variable | All Patients (N = 453) | BMI | ||
|---|---|---|---|---|
| ≤90th Percentile (n = 327) | >90th-97th Percentile (n = 59) | >97th Percentile (n = 67) | ||
| Age at diagnosis, y | ||||
| Mean (SD) | 13.7 (2.7) | 13.6 (2.8) | 13.2 (2.6) | 14.2 (2.0) |
| Median (range) | 14.3 (2.2-17.8) | 14.4 (2.2-17.8) | 13.7 (5.5-16.7) | 14.4 (9.1-17.7) |
| P value | NA | 1 [Reference]a | .27 | .06 |
| Female, No./total No (%) | ||||
| Total cohort | 306/453 (67.5) | 222/327 (67.9) | 38/59 (64.4) | 46/67 (68.7) |
| P value | NA | 1 [Reference] | .65 | >.99 |
| <11 y at MS onset | 33/62 (53.2) | 25/44 (56.8) | 6/12 (50.0) | 2/6 (33.3) |
| P value | NA | 1 [Reference] | .75 | .39 |
| ≥11 y at MS onset | 273/391 (69.8) | 197/283 (69.6) | 32/47 (68.1) | 44/61 (72.1) |
| P value | NA | 1 [Reference] | .86 | .76 |
| Interval first attack to DMT, mo | ||||
| Mean | 11.7 | 11.9 | 11.8 | 10.6 |
| Median | 6.2 | 6.0 | 7.0 | 6.0 |
| No. | 352 | 258 | 40 | 54 |
| P value | NA | 1 [Reference] | .97 | .48 |
| Follow-up duration, mo | ||||
| Mean | 38.4 | 39.1 | 36.8 | 36.4 |
| Median (range) | 31 (1-158) | 31 (1-158) | 31 (1-139) | 30 (2-100) |
| No. | 453 | 327 | 59 | 67 |
| P value | NA | 1 [Reference] | .62 | .39 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DMT, disease-modifying therapy; NA, not applicable.
1 [Reference], reference group for P value.
The onset of MS occurred from April 28, 1990, to June 26, 2016, with 400 cases (88.3%) manifesting after 2000. Sixty-two patients (13.7%) were younger than 11 years at MS onset, a group with a lower female preponderance compared with the group of children 11 years or older (33 [53.2%] vs 273 [69.8%]). The mean (SD) age at BMI measurement was 13.9 (2.7) years. In total, 126 patients (27.8%) had a BMI greater than the 90th percentile, 59 (13.0%) of these patients had a BMI greater than the 90th to 97th percentile (grouped as overweight), and 67 (14.8%) had a BMI greater than the 97th percentile (grouped as obese). Mean (SD) follow-up was 38.4 (28.9) months, ARR excluding first attack was 0.85 (1239 relapses; 17 504 months; n = 452), and mean (SD) EDSS score was 0.9 (1.2).
High BMI was associated with statistically significantly increased odds of pediatric MS in both sexes (obese girls OR, 2.19; 95% CI, 1.5-3.1; P < .001 vs obese boys OR, 2.14; 95% CI, 1.3-3.5; P = .003). This association was dose dependent and had an OR of 1.37 (95% CI, 1.0-1.8; P = .03) in overweight participants and rose to 2.2 (95% CI, 1.7-2.9; P < .001) in those with obesity, an outcome seen equally in boys and girls. Higher rates of overweight and obesity were seen among both younger (7-10 years) and older (11-17 years) children with MS compared with controls, although statistical significance was not achieved in all age categories. Boys aged 7 to 10 years had the highest rate of overweight and obesity (10/25 [40.0%]) (Table 2). To control for secular changes in BMI, patients with MS onset between 2003 and 2006 (n = 56) were analyzed; 8 (14.3%) of these patients were obese (BMI>97th percentile). This figure was consistent with the findings for the whole cohort (14.8%).
Table 2. Odds of Pediatric Multiple Sclerosis.
| Variablea | Controls With BMI >90th-97th Percentile, % (95% CI)b | Patients With MS With BMI >90th-97th Percentile | OR (95% CI) | P Value | Controls With BMI >97th Percentile, % (95% CI)a | Patients With MS With BMI >97th Percentile (95% CI) | OR (95% CI) | P Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | No./Total No. | % (95% CI) | No./Total No. | |||||||
| Age 7-10 y | ||||||||||
| Boys | 8.9 (7.6-10.4) | 24.0 (9.4-45.1) | 6/25 | 2.45 (0.8-6.3) | .06 | 7.0 (5.8-8.3) | 16.0 (4.5-36.1) | 4/25 | 2.11 (.5-6.3) | .10 |
| Girls | 9.0 (7.6-10.7) | 18.2 (5.2-40.3) | 4/22 | 1.83 (0.5-5.5) | .30 | 5.7 (4.7-6.9) | 9.1 (1.1-29.2) | 2/22 | 1.50 (.2-6.3) | .60 |
| Total | 9.0 (8.0-10) | 21.3 (10.7-35.7) | 10/47 | 2.3 (1.1-4.5) | .04 | 6.4 (5.6-7.3) | 12.8 (4.8-25.7) | 6/47 | 1.85 (.6-4.5) | .20 |
| Age 11-17 y | ||||||||||
| Boys | 9.1 (8.9-11.0) | 12.6 (7.2-19.9) | 15/119 | 1.14 (0.6-2.0) | .70 | 7.7 (6.8-8.6) | 14.3 (8.5-21.9) | 17/119 | 1.7 (.9-2.9) | .05 |
| Girls | 9.4 (8.4-10.4) | 12.0 (8.4-16.5) | 33/274 | 1.15 (0.8-1.7) | .50 | 8.2 (7.3-9.2) | 16.1 (11.9-21.0) | 44/274 | 1.79 (1.2-2.6) | .002 |
| Total | 9.7 (9.0-10.4) | 12.2 (9.1-15.9) | 48/393 | 1.13 (0.8-1.6) | .50 | 7.9 (7.3-8.6) | 15.5 (12.1-19.5) | 61/393 | 1.8 (1.3-2.4) | <.001 |
| Age 3-17 y | ||||||||||
| Boys | 8.8 (8.0-9.7) | 14.5 (9.2-21.3) | 21/145 | 1.49 (0.9-2.4) | .10 | 6.3 (5.6-7.0) | 14.5 (9.2-21.3) | 21/145 | 2.14 (1.3-3.5) | .003 |
| Girls | 8.5 (7.9-9.2) | 12.5 (9.0-16.7) | 38/305 | 1.33 (.9-1.9) | .10 | 6.4 (5.8-7.1) | 4615.1 (11.3-19.6) | 46/305 | 2.19 (1.5-3.1) | <.001 |
| Total | 8.7 (8.2-9.2) | 13.1 (10.1-16.6) | 59/450 | 1.37 (1.0-1.8) | .03 | 6.3 (5.8-6.9) | 14.9 (11.7-18.5) | 67/450 | 2.2 (1.7-2.9) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); OR, odds ratio.
Patient data for age category 3 to 6 years (n = 10) are not shown. This group contained only 1 overweight and 0 obese children; the findings were insignificant. Three patients were excluded from this analysis for being younger than 3 years of age at BMI measurement.
The control group consisted of 14 747 healthy German children aged 3 to 17 years from the KiGGS (German Health Interview and Examination Survey for Children and Adolescents) study.32
Of the 453 patients, 352 (77.7%) received a DMT. For 6 months or longer, 277 patients were treated with interferon beta (n = 249) and/or glatiramer (n = 51). Interferon beta-1a (Rebif; EMD Serono Inc), provided to 126 children, and interferon beta-1b (Betaferon; Bayer PLC), provided to 107, were the most commonly used medications, followed by glatiramer (Copaxone; Teva Neuroscience Inc), provided to 51 patients, and interferon beta-1a (Avonex; Biogen), provided to 27.
Seventy-seven (27.8%) of the 277 patients had a BMI higher than the 90th percentile at diagnosis. Time to initiation of a DMT and treatment duration while receiving therapy with interferon beta and glatiramer were not statistically significantly different between weight categories (Table 3). Although the relapse rate prior to treatment was similar between nonoverweight (ARR, 1.13; 95% CI, 0.69-1.31), overweight (ARR, 1.17; 95% CI, 0.39-1.61), and obese (ARR, 1.2; 95% CI, 0.5-1.5) patients, statistically significantly more relapses were recorded for obese patients during treatment with interferon beta and glatiramer compared with those recorded for their nonoverweight counterparts (ARR, 1.29 [95% CI, 1.1-1.6] vs 0.72 [95% CI, 0.6-0.8]; P < .001) (Table 3). This finding was statistically significant for both children younger than 11 years and adolescents aged 11 to 17 years. Extremely obese patients (BMI>99.5th percentile; n = 20) had the worst response to interferon beta and glatiramer therapy (ARR, 1.37; 95% CI, 1.0-1.9; P < .001).
Table 3. Comparison of Treatment Responses .
| Variable | All Patients | BMI | ||
|---|---|---|---|---|
| ≤90th Percentile | >90th-97th Percentile | >97th Percentile | ||
| Months to start of DMT | ||||
| Mean | 11.7 | 11.9 | 11.8 | 10.6 |
| Median | 6.2 | 6.0 | 7.0 | 6.0 |
| No. | 352 | 258 | 40 | 54 |
| P value | NA | 1 [Reference]a | .97 | .48 |
| First-line therapyb | ||||
| Interferon beta-1a, 1b, and glatiramer acetate | ||||
| ARR (95% CI) | 0.81 (0.7-0.9) | 0.72 (0.6-0.8) | 0.84 (0.7-1.1) | 1.29 (1.1-1.6) |
| No. | 277 | 200 | 32 | 45 |
| P value | NA | 1 [Reference] | .24 | <.001 |
| Patients <11 y at MS onset | ||||
| ARR (95% CI) | 0.55 (0.4-0.7) | 0.49 (0.4-0.6) | 0.37 (0.2-0.7) | 1.22 (0.7-2.0) |
| No. | 43 | 32 | 6 | 5 |
| P value | NA | 1 [Reference] | .45 | <.001 |
| Patients ≥11 y at MS onset | ||||
| ARR (95% CI) | 0.90 (0.8-1.0) | 0.79 (0.7-0.9) | 1.04 (0.8-1.4) | 1.31 (1.1-1.6) |
| No. | 234 | 168 | 26 | 40 |
| P value | NA | 1 [Reference] | .07 | <.001 |
| Interferon beta-1a and 1b | ||||
| ARR (95% CI) | 0.79 (0.7-0.9) | 0.7 (0.6-0.8) | 0.89 (0.7-1.2) | 1.22 (1.0-1.5) |
| No. | 249 | 180 | 27 | 42 |
| P value | NA | 1 [Reference] | .12 | <.001 |
| Glatiramer acetate | ||||
| ARR (95% CI) | 0.89 (0.7-1.1) | 0.82 (0.6-1.1) | 0.47 (0.2-1.1) | 1.86 (1.1-3.0) |
| No. | 51 | 31 | 11 | 9 |
| P value | NA | 1 [Reference] | .17 | .004 |
| Mean duration of treatment with interferon beta and glatiramer acetate, y | 2.1 | 2.1 | 2.3 | 1.9 |
| P value | NA | 1 [Reference] | .63 | .25 |
| Frequency of second-line therapy, No./total no. (%)c | 78/181 (43.1) | 48/124 (38.7) | 9/20 (45.0) | 21/37 (56.8) |
| P value | NA | 1 [Reference] | .63 | .06 |
Abbreviations: ARR, annualized relapse rate (relapses per person-years); BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DMT, disease-modifying therapy; NA, not applicable.
1 [Reference], reference group for P value.
Only patients with 6 or more months treatment duration.
Only patients with first presentation in 2010 or later and at least 12 months of follow-up since first attack unless escalated in 12 months or less. Second-line medications: fingolimod, natalizumab, alemtuzumab, and rituximab.
Overall, 181 patients had MS onset in 2010 or later and had 12 months or more of follow-up. Mean (SD) follow-up for these patients was 35.4 (17.7) months with no statistically significant difference between weight categories. Seventy-eight patients (43.1%) either received or were recommended for second-line treatment. Fifty-three (29.3%) received natalizumab; 31 (17.1%), fingolimod; 2 (1.1%), alemtuzumab; and 2 (1.1%), rituximab. Ten patients (5.5%) received 2 or more second-line agents. Thirty (38.5%) of the 78 patients had a high BMI at diagnosis, and 21 (26.9%) were obese. The likelihood of receiving a second-line DMT was approximately 1.5 times higher among obese (21 [56.8%] of 37) and extremely obese (11 [61.1%] of 18) patients compared with nonoverweight patients (48 [38.7%] of 124). Body mass index data at the time of second-line DMT initiation were available for 76 of 78 cases. Most patients (69 of 76 [90.8%]) had not changed their weight categories. Twenty-seven (93.1%) of 29 patients who had displayed a high BMI at first clinical presentation were still displaying a high BMI at escalation. By the time of therapy escalation, none of the obese patients had a weight below the 90th percentile, whereas 2 initially overweight patients had a weight below the 90th percentile, and 4 nonoverweight patients had become overweight.
No BMI association was shown for disease activity on baseline brain or spine MRI, interval between first and second MS attacks, or EDSS score progression (Table 4). Mean disease duration was not statistically significantly different between weight classes.
Table 4. Comparison of Disease Severity.
| Variable | All Patients (n = 453) | BMI | ||
|---|---|---|---|---|
| ≤90th Percentile (n = 327) | >90th-97th Percentile (n = 59) | >97th Percentile (n = 67) | ||
| MRI ≤6 mo of first clinical presentation | ||||
| T2 Cranial lesions | ||||
| Median | 15.0 | 14.0 | 15.5 | 15.0 |
| No. | 385 | 278 | 50 | 57 |
| P value | NA | 1 [Reference]a | .93 | .32 |
| Gadolinium | ||||
| Median | 1.0 | 1.0 | 1.0 | 1.0 |
| No. | 366 | 259 | 49 | 58 |
| P value | NA | 1 [Reference] | .06 | .60 |
| T2 Spinal lesions | ||||
| Median | 1.0 | 1.0 | 1.0 | 1.0 |
| No. | 333 | 233 | 46 | 54 |
| P value | NA | 1 [Reference] | .26 | .34 |
| Gadolinium | ||||
| Median | 0.0 | 0.0 | 0.0 | 0.0 |
| No. | 295 | 206 | 40 | 49 |
| P value | NA | 1 [Reference] | .54 | .13 |
| Interval between 1st and 2nd attack, mo | ||||
| Mean | 9.2 | 9.1 | 7.9 | 10.9 |
| Median | 5.5 | 5.2 | 5.0 | 6.0 |
| No. | 361 | 254 | 45 | 62 |
| P value | NA | 1 [Reference] | .40 | .41 |
| EDSS at 2 y | ||||
| Mean | 0.8 | 0.8 | .70 | .80 |
| Median | 0.0 | 0.0 | 0.0 | 0.0 |
| No. | 308 | 220 | 36 | 52 |
| P value | NA | 1 [Reference] | .71 | .85 |
| EDSS at last consultation | ||||
| Mean | 0.9 | 0.9 | 0.7 | 1.0 |
| Median | 0.0 | 0.0 | 0.0 | 1.0 |
| No. | 433 | 318 | 53) | 62 |
| P value | NA | 1 [Reference] | .41 | .30 |
Abbreviations: EDSS, Expanded Disability Status scale (range: 0-10, with the highest score indicating death from MS and the lowest score indicating normal neurological examination); NA, not applicable.
1 [Reference], reference group for P value.
Discussion
Many of the obese patients who were treated at the Center for MS in Childhood and Adolescence have not responded to first-line medications. Although obesity has been previously associated with increased risk of pediatric MS, its association with treatment response has not been studied to date. A great advantage of studies in the pediatric population is that MS onset and the association with BMI can be more reliably addressed because of more reliable data collection and reduced recall bias (unreliable data collection and recall bias are common problems in adult investigations).
In the first part of this study, aimed at confirming the association between obesity and increased pediatric MS risk, we analyzed 453 patients, which, to our knowledge, was the largest cohort to date. We found that the rate of overweight and obesity among pediatric patients with MS was statistically significantly higher than among healthy controls, with obesity increasing MS susceptibility twofold. The outcome was dose dependent, with MS odds rising from 1.37 in overweight children to 2.2 in obese children. The magnitude of MS risk associated with obesity was similar for boys and girls. Findings for female patients matched those in the literature, which indicated that obesity has consistently been associated with a 1.6 to 2.4 increase in MS risk, but the degree of male risk has been disputed.9,10,11,14,16,26 Most adult studies have shown either no risk or only attenuated risk in males.10,11,13,16,36 Pediatric findings are scarce, but a study has also reported statistically significant male risk (n = 59; BMI>85th percentile; OR, 1.43; P = .01).14 This finding may be specific to pediatric cohorts or may reflect more reliable data collection owing to reduced recall bias. Another possibility is the implication of increasing BMI trends over time given that adult MS studies most likely reflect BMI data from an earlier generation of men with lower obesity rates. Two previous pediatric MS studies did not show any relevant association between BMI and MS risk in prepubertal children (aged 2-11 years), but we found a high rate of overweight or obesity among younger patients with MS (aged 7-10 years), particularly boys.14,26
In the second part of the study, we analyzed the association of obesity with response to first-line therapy in 277 patients treated with interferon beta or glatiramer. Supporting our observation, patients who were obese at diagnosis experienced nearly twice as many relapses while being treated with these medications, compared with nonoverweight patients. Consistent with this finding, the switch rate to a second-line DMT was approximately 50% higher. All children who were obese at diagnosis were still displaying high BMIs at therapy escalation. To our knowledge, the association between BMI and treatment response in pediatric MS has never been investigated before. Yamamoto et al,37 however, did observe higher relapse rates, in general, among overweight and obese children in a study describing 60 pediatric patients with MS, but treatment response was not investigated. An association between high BMI and worse therapy response to interferon beta has been described in a small adult cohort of 86 patients.38 In that study, Kvistad et al38 found that obese patients were substantially less likely to reach no evidence of disease activity under interferon beta treatment. Kvistad et al38 postulated that obesity-associated proinflammatory outcomes promote a state of chronic low-grade systemic inflammation, thereby leading to greater disease activity in these patients. We considered this possibility and extended this study to analyze disease activity in the total cohort of 453 patients.
To exclude the confounders of treatment, we assessed MRI activity at diagnosis, interval between first and second MS attacks, relapse pretreatment, and disability progression as measures of disease activity. Contrary to our expectation, we found no evidence that a high BMI was associated with a more active inflammatory process. The association between obesity and disease activity in pediatric MS has, to our knowledge, been described in only one study with a small cohort (n = 50), which analyzed ARR and new lesions on MRI.39 Consistent with our findings, BMI at diagnosis was not correlated with disease activity. Kvistad et al38 also did not find an association between BMI and MRI disease activity or EDSS score prior to interferon beta treatment. Without evidence of higher disease activity, the suboptimal treatment response observed in this study may be associated with altered drug pharmacokinetics in obese patients. No pharmacokinetic data are available on the absorption of interferon beta and glatiramer after subcutaneous application in obese patients with MS, but studies of other medications such as enoxaparin sodium and insulin have shown slower but complete absorption in obese patients.40,41 Increased adipose tissue mass and lean body mass as well as the physiochemical properties of the drug, such as lipophilicity, also alter drug distribution.42 To our knowledge, this distribution has not been studied for interferon beta or glatiramer, but a reduction of interferon alfa serum concentrations in obese patients has been described before.43 Studying the association of obesity with the pharmacokinetics of first-line DMTs may improve the understanding of treatment response in obese patients and possibly even enable the development of BMI-adjusted dosing recommendations.
Strengths and Limitations
A strength of this study was the large cohort size. All previous BMI studies of children with MS had considerably smaller sample sizes, particularly with respect to the number of male and prepubertal children.
This study has several limitations. First, because the Center for MS in Childhood and Adolescence is a tertiary referral center for pediatric MS, it may have attracted a higher number of patients with less benign disease, which in turn may have changed the outcome measures. Furthermore, some relapses were treated in local hospitals and therefore not verified at the center. Second, patient BMI measurements were taken within 6 months of diagnosis and thus may not truly reflect pre-disease BMI or changes in status over the study period. Factors against a radical change from pre-disease BMI status in our patients were that the mean time between MS diagnosis and height and weight measurement was only 2.4 months; the steroid exposure was not prolonged; and the EDSS score was low, indicating minimal disability. Subgroup analysis of BMI at initiation of second-line therapy also indicated little change in BMI status over the study period. Moreover, 2 adult studies have shown no association between MS and BMI status, with a baseline EDSS score also not a predictor of BMI change in one of these studies.12,44 In the analysis of MS risk, the implications of recognized MS risk factors such as pre-disease levels of serum Vitamin D, exposure to Epstein-Barr virus, or genetic factors were not considered; however, a causal relationship between BMI and onset of adult and pediatric MS has been confirmed even after controlling for these variables.16,25
Disease activity assessment was limited by 2 factors. First, MRI data were based on lesion count and not lesion volume; thus, disease activity was underrepresented in patients with large confluent lesions yet low overall lesion count, whereas a degree of inaccuracy was possible in patients with high lesion counts. Second, follow-up was short and EDSS scores generally were low in all patients, a common finding in pediatric MS that limits the value of this variable. Because only German children were evaluated, the findings may not be reflective of children of other race/ethnicity or geographic location.
Conclusions
This study’s findings appear to have confirmed increased pediatric MS risk association with obesity as well as a statistically significantly worse response to current first-line medications interferon beta and glatiramer and greater likelihood of switch to second-line agents in obese patients. The findings do not indicate that obesity promotes greater disease activity, but pharmacokinetic factors are more likely associated with treatment response. This suggestion may have relevant management implications given that a healthy weight may potentially optimize treatment outcomes and reduce disease-related burden and health care costs. These findings also imply that understanding obesity-associated pharmacokinetics of first-line DMTs may increase their value by enabling BMI-adjusted doses.
References
- 1.Stevens GA, Singh GM, Lu Y, et al. ; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) . National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10(1):22. doi: 10.1186/1478-7954-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766-781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92(5):1257-1264. doi: 10.3945/ajcn.2010.29786 [DOI] [PubMed] [Google Scholar]
- 4.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627-2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861-1867. doi: 10.1001/jama.293.15.1861 [DOI] [PubMed] [Google Scholar]
- 6.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13(9):981-1000. doi: 10.1016/j.autrev.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 7.Khalili H, Ananthakrishnan AN, Konijeti GG, et al. Measures of obesity and risk of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21(2):361-368. doi: 10.1097/MIB.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harpsøe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol. 2014;43(3):843-855. doi: 10.1093/ije/dyu045 [DOI] [PubMed] [Google Scholar]
- 9.Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology. 2009;73(19):1543-1550. doi: 10.1212/WNL.0b013e3181c0d6e0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munger KL, Bentzen J, Laursen B, et al. Childhood body mass index and multiple sclerosis risk: a long-term cohort study. Mult Scler. 2013;19(10):1323-1329. doi: 10.1177/1352458513483889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Zhang TT, Yu J, et al. Excess body weight during childhood and adolescence is associated with the risk of multiple sclerosis: a meta-analysis. Neuroepidemiology. 2016;47(2):103-108. doi: 10.1159/000450854 [DOI] [PubMed] [Google Scholar]
- 12.Mokry LE, Ross S, Timpson NJ, Sawcer S, Davey Smith G, Richards JB. Obesity and multiple sclerosis: a Mendelian randomization study. PLoS Med. 2016;13(6):e1002053. doi: 10.1371/journal.pmed.1002053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wesnes K, Riise T, Casetta I, et al. Body size and the risk of multiple sclerosis in Norway and Italy: the EnvIMS study. Mult Scler. 2015;21(4):388-395. doi: 10.1177/1352458514546785 [DOI] [PubMed] [Google Scholar]
- 14.Chitnis T, Graves J, Weinstock-Guttman B, et al. ; U.S. Network of Pediatric MS Centers . Distinct effects of obesity and puberty on risk and age at onset of pediatric MS. Ann Clin Transl Neurol. 2016;3(12):897-907. doi: 10.1002/acn3.365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cortese M, Riise T, Bjørnevik K, Myhr KM; Multiple Sclerosis Conscript Service Database Study Group . Body size and physical exercise, and the risk of multiple sclerosis. Mult Scler. 2018;24(3):270-278. doi: 10.1177/1352458517699289 [DOI] [PubMed] [Google Scholar]
- 16.Gianfrancesco MA, Acuna B, Shen L, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes Res Clin Pract. 2014;8(5):e435-e447. doi: 10.1016/j.orcp.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedström AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler. 2012;18(9):1334-1336. doi: 10.1177/1352458512436596 [DOI] [PubMed] [Google Scholar]
- 18.Hedström AK, Olsson T, Alfredsson L. Body mass index during adolescence, rather than childhood, is critical in determining MS risk. Mult Scler. 2016;22(7):878-883. doi: 10.1177/1352458515603798 [DOI] [PubMed] [Google Scholar]
- 19.Hedström AK, Lima Bomfim I, Barcellos L, et al. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology. 2014;82(10):865-872. doi: 10.1212/WNL.0000000000000203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parikh SJ, Edelman M, Uwaifo GI, et al. The relationship between obesity and serum 1,25-dihydroxy vitamin D concentrations in healthy adults. J Clin Endocrinol Metab. 2004;89(3):1196-1199. doi: 10.1210/jc.2003-031398 [DOI] [PubMed] [Google Scholar]
- 21.Smotkin-Tangorra M, Purushothaman R, Gupta A, Nejati G, Anhalt H, Ten S. Prevalence of vitamin D insufficiency in obese children and adolescents. J Pediatr Endocrinol Metab. 2007;20(7):817-823. doi: 10.1515/JPEM.2007.20.7.817 [DOI] [PubMed] [Google Scholar]
- 22.Ramagopalan SV, Valdar W, Criscuoli M, et al. ; Canadian Collaborative Study Group . Age of puberty and the risk of multiple sclerosis: a population based study. Eur J Neurol. 2009;16(3):342-347. doi: 10.1111/j.1468-1331.2008.02431.x [DOI] [PubMed] [Google Scholar]
- 23.Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(suppl 3):S5-S78. doi: 10.1017/S0007114511005460 [DOI] [PubMed] [Google Scholar]
- 24.Bhargava P, Mowry EM. Gut microbiome and multiple sclerosis. Curr Neurol Neurosci Rep. 2014;14(10):492. doi: 10.1007/s11910-014-0492-2 [DOI] [PubMed] [Google Scholar]
- 25.Gianfrancesco MA, Stridh P, Rhead B, et al. ; Network of Pediatric Multiple Sclerosis Centers . Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology. 2017;88(17):1623-1629. doi: 10.1212/WNL.0000000000003849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langer-Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology. 2013;80(6):548-552. doi: 10.1212/WNL.0b013e31828154f3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krupp LB, Banwell B, Tenembaum S; International Pediatric MS Study Group . Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. 2007;68(16)(suppl 2):S7-S12. doi: 10.1212/01.wnl.0000259422.44235.a8 [DOI] [PubMed] [Google Scholar]
- 28.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292-302. doi: 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kromeyer-Hauschild K. Perzenzile für den body-mass-index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben [in German]. Monatsschr Kinderheilkd. 2001;149:807-818. doi: 10.1007/s001120170107 [DOI] [Google Scholar]
- 30.Poskitt EM. Defining childhood obesity: the relative body mass index (BMI). European Childhood Obesity group. Acta Paediatr. 1995;84(8):961-963. doi: 10.1111/j.1651-2227.1995.tb13806.x [DOI] [PubMed] [Google Scholar]
- 31.Wabitsch M, Kunze D Konsensbasierte (S2) leitlinie zur diagnostik, therapie und prävention von Übergewicht und adipositas im kindes-und jugendalter [in German]. https://www.adipositas-gesellschaft.de/fileadmin/PDF/Leitlinien/AGA_S2_Leitlinie.pdf. Accessed July 14, 2016
- 32.Kurth BM, Schaffrath Rosario A. [The prevalence of overweight and obese children and adolescents living in Germany. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2007;50(5-6):736-743. doi: 10.1007/s00103-007-0235-5 [DOI] [PubMed] [Google Scholar]
- 33.Brettschneider AK, Schaffrath Rosario A, Kuhnert R, et al. Updated prevalence rates of overweight and obesity in 11- to 17-year-old adolescents in Germany. Results from the telephone-based KiGGS Wave 1 after correction for bias in self-reports[published correction appears in BMC Public Health. 2016;16:247]. BMC Public Health. 2015;15:1101. doi: 10.1186/s12889-015-2467-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brettschneider AK, Schienkiewitz A, Schmidt S, Ellert U, Kurth BM. Updated prevalence rates of overweight and obesity in 4- to 10-year-old children in Germany. Results from the telephone-based KiGGS Wave 1 after correction for bias in parental reports. Eur J Pediatr. 2017;176(4):547-551. doi: 10.1007/s00431-017-2861-8 [DOI] [PubMed] [Google Scholar]
- 35.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452. doi: 10.1212/WNL.33.11.1444 [DOI] [PubMed] [Google Scholar]
- 36.Gunnarsson M, Udumyan R, Bahmanyar S, Nilsagård Y, Montgomery S. Characteristics in childhood and adolescence associated with future multiple sclerosis risk in men: cohort study. Eur J Neurol. 2015;22(7):1131-1137. doi: 10.1111/ene.12718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto E, Ginsberg M, Rensel M, Moodley M. Pediatric-onset multiple sclerosis: a single center study. J Child Neurol. 2018;33(1):98-105. doi: 10.1177/0883073817739789 [DOI] [PubMed] [Google Scholar]
- 38.Kvistad SS, Myhr KM, Holmøy T, et al. Body mass index influence interferon-beta treatment response in multiple sclerosis. J Neuroimmunol. 2015;288:92-97. doi: 10.1016/j.jneuroim.2015.09.008 [DOI] [PubMed] [Google Scholar]
- 39.Krysko K, Yeh EA, Hanwell H, Cohen A, Rotstein D. Obesity and disease activity in pediatric-onset multiple sclerosis (P1.376). Neurology. 2016;86(16 suppl):376. [Google Scholar]
- 40.Sanderink GJ, Le Liboux A, Jariwala N, et al. The pharmacokinetics and pharmacodynamics of enoxaparin in obese volunteers. Clin Pharmacol Ther. 2002;72(3):308-318. doi: 10.1067/mcp.2002.127114 [DOI] [PubMed] [Google Scholar]
- 41.Clauson PG, Linde B. Absorption of rapid-acting insulin in obese and nonobese NIDDM patients. Diabetes Care. 1995;18(7):986-991. doi: 10.2337/diacare.18.7.986 [DOI] [PubMed] [Google Scholar]
- 42.Jain R, Chung SM, Jain L, et al. Implications of obesity for drug therapy: limitations and challenges. Clin Pharmacol Ther. 2011;90(1):77-89. doi: 10.1038/clpt.2011.104 [DOI] [PubMed] [Google Scholar]
- 43.Lam NP, Pitrak D, Speralakis R, Lau AH, Wiley TE, Layden TJ. Effect of obesity on pharmacokinetics and biologic effect of interferon-alpha in hepatitis C. Dig Dis Sci. 1997;42(1):178-185. doi: 10.1023/A:1018865928308 [DOI] [PubMed] [Google Scholar]
- 44.Bove R, Musallam A, Xia Z, et al. Longitudinal BMI trajectories in multiple sclerosis: sex differences in association with disease severity. Mult Scler Relat Disord. 2016;8:136-140. doi: 10.1016/j.msard.2016.05.019 [DOI] [PubMed] [Google Scholar]