Abstract
Synaptic plasticity, which is the neuronal substrate for many forms of hippocampus-dependent learning, is attenuated by GABA type A receptor (GABAAR)-mediated inhibition. The prevailing notion is that a synaptic or phasic form of GABAergic inhibition regulates synaptic plasticity; however, little is known about the role of GABAAR subtypes that generate a tonic or persistent inhibitory conductance. We studied the regulation of synaptic plasticity by α5 subunit-containing GABAARs (α5GABAARs), which generate a tonic inhibitory conductance in CA1 pyramidal neurons using electrophysiological recordings of field and whole-cell potentials in hippocampal slices from both wild-type and null mutant mice for the α5 subunit of the GABAAR (Gabra5−/− mice). In addition, the strength of fear-associated memory was studied. The results showed that α5GABAAR activity raises the threshold for induction of long-term potentiation in a highly specific band of stimulation frequencies (10–20 Hz) through mechanisms that are predominantly independent of inhibitory synaptic transmission. The deletion or pharmacological inhibition of α5GABAARs caused no change in baseline membrane potential or input resistance but increased depolarization during 10 Hz stimulation. The encoding of hippocampus-dependent memory was regulated by α5GABAARs but only under specific conditions that generate moderate but not robust forms of fear-associated learning. Thus, under specific conditions, α5GABAAR activity predominates over synaptic inhibition in modifying the strength of both synaptic plasticity in vitro and certain forms of memory in vivo.
Introduction
Information processing in the mammalian brain is governed by a dynamic interplay between excitatory and inhibitory neurotransmission. In the hippocampus, the encoding of episodic behavioral memory is highly dependent on the activation of glutamate receptors but can be attenuated by increasing GABAergic inhibition (Steckler et al., 1998). The neural correlate for learning and memory is thought to be long-term alterations in the efficacy of excitatory postsynaptic neurotransmission (Malenka and Bear, 2004; Whitlock et al., 2006). At CA1 synapses in the hippocampus, the long-term potentiation (LTP) of excitatory potentials occurs after brief periods of high-frequency stimulation, whereas low-frequency stimulation typically causes long-term depression (LTD) (Collingridge et al., 1983; Dudek and Bear, 1992). Although considerable progress has been made in understanding the role of excitatory neurotransmission in LTP and LTD, little is known about the contribution of various forms of GABAergic inhibition. In this regard, the attenuation of synaptic plasticity by GABA type A receptors (GABAARs) in CA1 pyramidal neurons has been generally attributed to phasic inhibitory currents generated by synaptically expressed receptors (Wigström and Gustafsson, 1983; Chapman et al., 1998; Grover and Yan, 1999; Levkovitz et al., 1999; Lu et al., 2000; Yoshiike et al., 2008). The role of GABAARs, which generate a tonic inhibitory conductance, contain mainly α5 or δ subunits, and are activated by ambient GABA or spillover of transmitter from the synaptic cleft (Bai et al., 2001; Caraiscos et al., 2004; Semyanov et al., 2004; Glykys and Mody, 2007), remains poorly understood. Indeed, the potential importance of extrasynaptic GABAARs may have been primarily overlooked because of the challenge of resolving and selectively modifying the low-amplitude, persistent GABAergic conductance that they generate (Semyanov et al., 2004; Farrant and Nusser, 2005; Kullmann et al., 2005).
In the hippocampus, α5 subunit-containing GABAARs (α5GABAARs) primarily populate the extrasynaptic regions of CA1 pyramidal neurons (Sur et al., 1999; Pirker et al., 2000), in which they generate a tonic inhibitory conductance (Caraiscos et al., 2004; Scimemi et al., 2005; Glykys and Mody, 2006; Glykys et al., 2008). These receptors are targets for memory-blocking drugs (Cheng et al., 2006; Martin et al., 2009); however, their physiological relevance requires additional clarification. Notably, α5GABAAR null mutant mice (Gabra5−/−) exhibit a normal behavioral phenotype for fear-associated contextual learning (Cheng et al., 2006). Also, electrophysiological experiments in hippocampal slices from wild-type (WT) and Gabra5−/− mice have revealed no differences in LTP (Collinson et al., 2002), whereas an α5GABAAR-selective inverse agonist increases LTP and the power of gamma frequency oscillations (Towers et al., 2004; Atack et al., 2006a).
In the current study, we sought to determine whether α5GABAARs modify synaptic plasticity in CA1 pyramidal neurons in vitro, particularly when slices were stimulated in the theta frequency range, which is associated with exploratory and memory behavior (Buzsáki, 2005). Next, we asked whether α5GABAARs influence the induction or maintenance phase of plasticity and whether α5GABAAR activity regulates the membrane potential, before, during, and after the induction of plasticity. Finally, we investigated whether enhanced synaptic plasticity correlated with improved memory performance in vivo. The results show that genetic deletion or pharmacological inhibition of α5GABAARs markedly reduces the threshold for the induction of LTP via mechanisms that are predominantly independent of synaptic inhibition, and this action correlates with improved memory performance.
Materials and Methods
Experimental animals.
The Animal Care Committee of the University of Toronto approved all experiments. The generation of Gabra5−/− mice on a C57BL/6J and Sv129Ev background has been previously described (Collinson et al., 2002). These mice do not display an overt behavioral phenotype, they breed normally, and they have a normal lifespan. In addition, they exhibit normal motor coordination, with no overt compensatory changes in the expression of other GABAAR subunits. Male offspring from homozygous breeding pairs were used for most of the experiments. In some studies, male offspring from heterozygous breeding pairs were used to confirm that the phenotype resulted from deletion of the α5 subunit gene rather than differences in the genetic background of the inbred parental breeding strains (Nguyen et al., 2000a,b).
Electrophysiology in hippocampal tissue slices.
The LTP of field EPSPs (fEPSPs) was studied with hippocampal slices prepared from WT and Gabra5−/− mice that ranged in age from postnatal days 21–36. After administration of isoflurane anesthesia, the mice were decapitated, and their brains were quickly removed and placed in ice-cold, oxygenated (95% O2, 5% CO2) artificial CSF (aCSF) (composition in mmol/L: 124 NaCl, 3 KCl, 1.3 MgCl2, 2.6 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, and 10 d-glucose) with osmolarity adjusted to 300–310 mOsm. Brain slices (350 μm) containing transverse sections of the hippocampus were prepared with a VT1000E tissue slicer (Leica). After a recovery period of 1 h in the oxygenated aCSF, the slices were transferred to a submersion recording chamber. The CA1 region was isolated from the CA3 region by a surgical cut, and the slices were continually perfused with aCSF. The fEPSPs were recorded from CA1 stratum radiatum neurons using electrodes that contained aCSF. Synaptic responses were evoked by stimulating the Schaffer collateral pathway with 0.1 ms pulses delivered by concentric bipolar stimulating electrodes (Rhodes Medical Instruments). To ensure that the baseline recordings were stable, responses were evoked at 0.05 Hz at an intensity that yielded a half-maximal field potential slope and were monitored in each slice for 20 min. To examine the role of α5GABAARs in synaptic plasticity, the induction threshold for LTP was investigated by varying the degree of presynaptic stimulation, which was achieved by altering the frequency of tetanic stimulation. Slices were stimulated at frequencies of 1, 5, 10, 20, 50, and 100 Hz. The number of stimulation pulses was kept constant (600 pulses per stimulation frequency), with the exception of the protocol for 100 Hz, in which we stimulated each slice three times for a period of 1 s, separated by 20 s intervals. The potentiation or depression of fEPSPs remained stable and persisted over the duration of the recordings (70–80 min) and is reported as a percentage of the baseline response.
For the pharmacological studies, L-655,708 [ethyl (13aS)-7-methoxy-9-oxo-11,12,13,13a-tetrahydro-9H-imidazo[1,5-α]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylate], bicuculline (8 μm), or SR-95531 [2-(3-carboxypropyl)-3-amino-6-(4-methoxyphenyl) pyridazinium bromide] (5 μm) was used to inhibit α5GABAARs, all GABAARs, or synaptically expressed GABAARs, respectively. L-655,708 was used in the current study because it is the most extensively characterized selective inverse agonist for α5GABAARs. The affinity of L-655,708 for α5GABAARs is 50- to 100-fold greater than its affinity for α1, α2, and α3 subunit-containing GABAA receptors (Quirk et al., 1996). All available inverse agonists for α5GABAARs bind to other benzodiazepine-sensitive GABAA receptor subtypes, albeit at lower affinity, and modify receptor function at higher concentrations. The subtype selectivity of L-655,708 is attributed to a higher affinity for α5GABAARs, because the efficacy of this compound is similar at the other GABAA receptor subtypes to which it binds (Quirk et al., 1996). Consequently, to ascribe an effect of L-655,708 to α5GABAARs, a careful selection of the concentration is required. The concentrations of L-655,708 used in the current study were selected on the basis of in vivo binding data, pharmacokinetic analyses, and previous memory studies. It has been estimated that L-655,708 (10 nm) would produce a highly selective inhibition of α5GABAARs, whereas selectivity may be reduced at concentrations >50–100 nm (Atack et al., 2006a; Vargas-Caballero et al., 2010). The drugs were applied for at least 10 min before 10 or 100 Hz stimulation. In some experiments, L-655,708 was also applied immediately after 10 Hz stimulation, as described below. The concentration of bicuculline was selected to minimize epileptiform bursting in the CA1 region, which is known to influence LTP (Wigström and Gustafsson, 1983; Grover and Yan, 1999). In some recordings, THIP (4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol) (Tocris Bioscience) was applied 10 min before, during, and after 10 Hz stimulation.
Voltage-clamp recordings.
In a separate set of experiments, a tight seal (>1 GΩ) was formed on the cell body of each visually identified CA1 pyramidal neuron, and whole-cell voltage-clamp recordings were obtained after the membrane was ruptured with negative pressure. Recording electrodes with resistances of 3–5 MΩ were constructed from borosilicate glass (1.5 mm diameter; WPI) using a two-stage puller (PP83; Narishige). Only recordings with stable holding current and series resistance maintained below 25 MΩ were considered for analysis. EPSCs were recorded from WT and Gabra5−/− neurons at holding potentials of −80 mV (AMPA component) and +40 mV (NMDA component). The AMPA and NMDA currents were further isolated by application of (2R)-amino-5-phosphonovaleric acid (APV) (10 μm) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (20 μm), respectively, in the presence of bicuculline (10 μm). The stimulation intensity used to evoke AMPA and NMDA currents ranged from 5 to 10 V for each cell, with six sweeps recorded at each intensity. Current traces were recorded using a Multiclamp 700A amplifier (Molecular Devices), low-pass filtered at 2 kHz, and digitized at 10–20 kHz with Clamplex (version 9.2; Molecular Devices). Recording electrodes were filled with an internal solution that contained the following (in mmol/L): 132.5 Cs-gluconate, 17.5 KCl, 0.2 EGTA, 10 HEPES, 2 MgATP, 0.3 GTP, and 5 QX-314 [2(triethylamino)-N-(2,6-dimethylphenyl) acetamine], pH 7.2–7.3. The tonic current, miniature IPSCs (mIPSCs), and spontaneous IPSCs were recorded from Gabra5−/− neurons at a holding potential of −60 mV. Spontaneous IPSCs and mIPSCs were analyzed using Minianalysis software (version 6.0.3; Synaptosoft). The intracellular solution for these recordings consisted of the following (in mmol/L): 140 CsCl, 10 HEPES, 10 EGTA, 4 Mg-ATP, 1 CaCl2, and 5 QX-314, pH 7.2–7.3. These recordings were performed with CNQX (20 μm) and APV (10 μm) in the aCSF.
Current-clamp recordings.
Studies were performed to determine whether the GABAAR-mediated IPSPs were completely blocked by SR-95531 at the same concentration as used for field recordings (5 μm) and whether potentials were also blocked after 10 Hz stimulation. Whole-cell patch-clamp recordings were obtained from visually identified hippocampal CA1 pyramidal neurons. The GABAAR-mediated IPSPs were recorded in CA1 pyramidal neurons, and CNQX (20 μm), APV (10 μm), and CGP 55845 [(2S)-3-[(15)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl)(phenylmethyl)phosphinic acid] (10 μm), respectively, were used to block AMPA, NMDA, and GABAB responses. A stable GABAA-mediated IPSP baseline response was recorded, and SR-95531 was then added to the extracellular solution. Slices were stimulated at 10 Hz to determine whether the GABAA-mediated IPSPs remained blocked by SR-95531 during and after 10 Hz stimulation.
Current-clamp recordings were obtained to determine whether α5GABAAR activity modulated the input resistance and membrane potential of CA1 pyramidal neurons during LTP induced by 10 Hz stimulation. To monitor the input resistance of the cells, a small hyperpolarizing current pulse (10 pA intensity, 400 ms duration) was injected 500 ms before EPSPs were evoked from CA1 pyramidal neurons. EPSPs were evoked every 60 s to allow for recovery between sweeps. After a stable baseline period (in terms of both current pulse and EPSP amplitude), slices were stimulated with the same 10 Hz protocol as was used for the field recordings. EPSP amplitude, input resistance, and membrane potential were measured before and after 10 Hz stimulation. To calculate the input resistance, the steady-state membrane potential was divided by the amount of injected current (10 pA). In addition, the “depolarizing envelope” and membrane potential were analyzed before, during, and after the period of 10 Hz stimulation. The membrane potential was sampled for 15 ms every second before the stimulation artifact (60 samples in total). The depolarizing envelope was analyzed by measuring the total integrated area under the EPSPs for the entire 60 s of stimulation. For these experiments, SR-95531 (5 μm) and CGP 55845 (5 μm) were added to the bath solution to inhibit synaptically expressed GABAA and GABAB receptors, respectively. Patch pipettes had open tip resistances of 3–5 MΩ when filled with an intracellular solution that contained the following (in mmol/L): 132.5 K-gluconate, 10.3 KMeSO4, 7.2 KCl, 10 HEPES, 0.2 EGTA, 2 Mg-ATP, and 0.3 GTP, with the pH adjusted to 7.3 with KOH.
Paired-pulse facilitation protocol.
LTP may include a presynaptic as well as a postsynaptic component (Debanne et al., 1996). We sought to determine whether the genetic deletion of α5GABAARs altered a presynaptically mediated form of synaptic potentiation, which might have confounded the results. Paired-pulse facilitation (PPF), which involves eliciting two fEPSPs in quick succession, is taken to be a model of presynaptic plasticity. A single pulse was delivered to the Schaffer collateral pathway, and then, after a specified interstimulus interval, a second pulse was delivered; recordings were obtained from the stratum radiatum. The interstimulus intervals used for the current PPF experiment were 50, 100, 150, 200, and 300 ms.
Modified coastline index analysis.
To determine whether deletion of α5GABAARs increased excitability within the hippocampus, we attempted to quantify overall epileptiform activity. Population spikes were recorded in the CA1 pyramidal layer before and after application of bicuculline (8 μm). The degree of bursting was quantified using a modified coastline bursting index (Korn et al., 1987; Wang and Thompson, 2008). The coastline index was calculated as the sum of the point-to-point voltage differences measured during an epoch of the evoked response, beginning 5 ms after the stimulation artifact, until the trace returned to the baseline level. Because the coastline index depends on the initial stimulation intensity, the coastline response measured in bicuculline-treated slices was normalized by dividing the value by the control coastline value. Results are expressed as percentage of control. Minianalysis software (version 6.0.3; Synaptosoft) was used to analyze the coastline of the population spikes.
Preparation of protein samples, SDS-PAGE, and Western blot analysis.
Biochemical experiments were performed to determine whether the expression of glutamate receptors was different in WT and Gabra5−/− mice. Whole hippocampi (six per group) from 3-month-old male mice were isolated rapidly and placed in ice-cold PBS containing complete Mini protease inhibitors (Roche Diagnostics). Each group of hippocampi was homogenized separately in 0.75 ml of radioimmunoprecipitation assay buffer (150 mmol/L NaCl, 50 mmol/L Tris, pH 8.0, 5 mmol/L EDTA, 1% v/v Nonidet P-40, 0.5% w/v sodium deoxycholate, and 0.1% w/v SDS) containing 5 μg/ml aprotinin, 20 μg/ml leupeptin, and 1× Mini protease inhibitor cocktails using 1 ml Dounce glass–glass homogenizers. The homogenates were then centrifuged at 1000 × g for 5 min at 4°C to pellet any debris and nuclei. The supernatant was used for determining the protein concentration (BCA Protein Assay; Pierce Chemical). Tissue homogenates were then resolved on discontinuous 10% SDS-PAGE gels before transfer onto nitrocellulose membranes. Approximately 25–30 μg of protein was loaded per lane. The membranes were then washed in a mixture of Tris-buffered saline and 0.1% Tween 20 (TBS-T) and blocked for 1 h with 5% nonfat skim milk powder in TBS-T (BLOTTO) before overnight incubation at 4°C with the appropriate antibody: mouse anti-NR1, 1:1000 (BD Biosciences); rabbit anti-NR2A, 1:1000 (Covance); rabbit anti-NR2B, 1:1000 (Santa Cruz Biotechnology); rabbit anti-GluR1, 1:1000 (Millipore/Cedarlane Laboratories); rabbit anti-α5, 1:1000 (Millipore/Cedarlane Laboratories). The membranes were then washed in TBS-T and incubated for 1 h in HRP-conjugated anti-mouse (Santa Cruz Biotechnologies) or anti-rabbit (Invitrogen) secondary antibodies (diluted 1:5000 in BLOTTO) at room temperature. Membranes were taken through a final series of three to five washes in TBS-T before incubation in substrate. Size-specific bands were visualized by applying enzyme chemiluminescent substrates (SuperSignal West Pico ECL substrate; Pierce Chemical) and exposing the labeled membranes on a Kodak Image Station 2000R for 5–18 min. Specifically labeled bands at the appropriate molecular weights were analyzed for signal intensity using Kodak Image Station 2000R software and either the automated band label function or the edge-detection function to determine net band intensity. Specific band intensities from Gabra5−/− samples were normalized against control WT intensities. In some experiments, blots were stripped and probed with anti-β-actin antibody (1:5000; Sigma) to confirm equal loading of samples.
Fear conditioning.
We sought to determine whether the pharmacological inhibition of α5GABAARs influenced performance for fear-associated memory behavior. On day 1, individual animals were allowed to explore the test chamber for 180 s. An 800 Hz tone, created by a frequency generator, amplified to 70 dB, and lasting 20 s, was then presented. For cued fear conditioning, the last 2 s of each auditory tone was paired with an electric footshock (2 s, 0.7 mA); for trace fear learning, the auditory stimulus and footshock (2 s, 0.7 mA) were separated by 20 s. Each of these sequences was presented three times, separated by 60 s (for cued fear) or 240 s (for trace fear). For the drug studies, vehicle (10% dimethylsulfoxide) or L-655,708 (0.7 mg/kg) was administered intraperitoneally 30 min before or immediately after the conditioning trial. According to previous studies (Quirk et al., 1996; Chambers et al., 2004; Atack et al., 2006a; Dawson et al., 2006), it was estimated that, at 30 min after injection, L-655,708 (0.7 mg/kg, i.p.) would produce 60–70% occupancy of α5GABAARs in vivo with limited binding to α1, α2, and α3 subunit-containing GABAARs and no significant off-target behavioral effects such as sedation or motor impairment (Atack et al., 2006b).
On day 2, 24 h after the conditioning session, each mouse was assessed for a freezing response every 8 s for a total of 8 min (contextual fear). On day 3, the conditioning chamber was modified to measure the freezing response to the tone (either cued or trace fear learning), without any contextual influence. Mice were monitored for 180 s for a freezing response to the modified context, to rule out contextual influences. After the monitoring period, the auditory tone was presented continuously for 300 s, and the freezing response was recorded every 8 s.
Statistical analysis.
In the plots illustrating LTP and LTD, the data points (slope of fEPSP) were binned in 1 min increments to facilitate readability. The extent of LTP and LTD was quantified for statistical comparisons by averaging the slope of the fEPSPs during the final 5 min of each experiment and normalized to baseline values. Results are presented as percentage of the control response. Statistical comparisons were completed using unpaired Student's t tests with Bonferroni's correction or a one- or two-way ANOVA. Post hoc analyses, when required, were conducted using Tukey's honestly significant difference (HSD) test. For some of our datasets, testing for normality was problematic because of the small sample sizes (n < 5). For these datasets, we performed either a nonparametric Mann–Whitney U test or a nonparametric analysis using the Kruskal–Wallis rank sum test followed by the Bonferroni–Dunn test for multiple comparisons as indicated in Results.
Results
α5GABAARs regulate synaptic plasticity
To characterize the influence of α5GABAARs on synaptic plasticity, the effects of delivering a wide range of input stimulation frequencies (1–100 Hz) on the fEPSPs were studied in slices from WT and Gabra5−/− mice. When slices were stimulated at a low frequency (1 or 5 Hz), LTD of excitatory synaptic transmission was observed in both genotypes (n = 8 slices per group, Student's t test, p = 0.43) (Fig. 1A). Conversely, after high-frequency stimulation (50 and 100 Hz), LTP was observed in WT and Gabra5−/− slices (n = 8 slices per group, Student's t test, p = 0.56) (Fig. 1B). After stimulation at submaximal frequency (10 Hz), the plasticity in WT and Gabra5−/− slices was opposite in polarity: LTD was observed in WT slices, whereas LTP was observed in Gabra5−/− slices (WT, 80.61 ± 6.08%; Gabra5−/−, 120.8 ± 8.26%; n = 8 slices per group; Student's t test, p = 0.01) (Fig. 1C). Accordingly, the frequency–response plot was shifted to the left for recordings from Gabra5−/− slices compared with recordings from WT slices, with the greatest differences observed at 10 and 20 Hz (two-way ANOVA, effect of genotype, F(1,60) = 6.95, p = 0.01; effect of stimulation frequency, F(5,60) = 54.22, p = 0.0001; effect of interaction, F(5,60) = 3.49, p = 0.007; Tukey's HSD, p < 0.01 for WT vs Gabra5−/− slices stimulated at 10 and 20 Hz) (Fig. 1D). It is noteworthy that the stimulation frequencies in which α5GABAAR activity had the greatest influence were those commonly associated with large-scale theta-frequency synchronization, which is coupled with some forms of hippocampus-dependent learning (Buzsáki, 2005).
Figure 1.
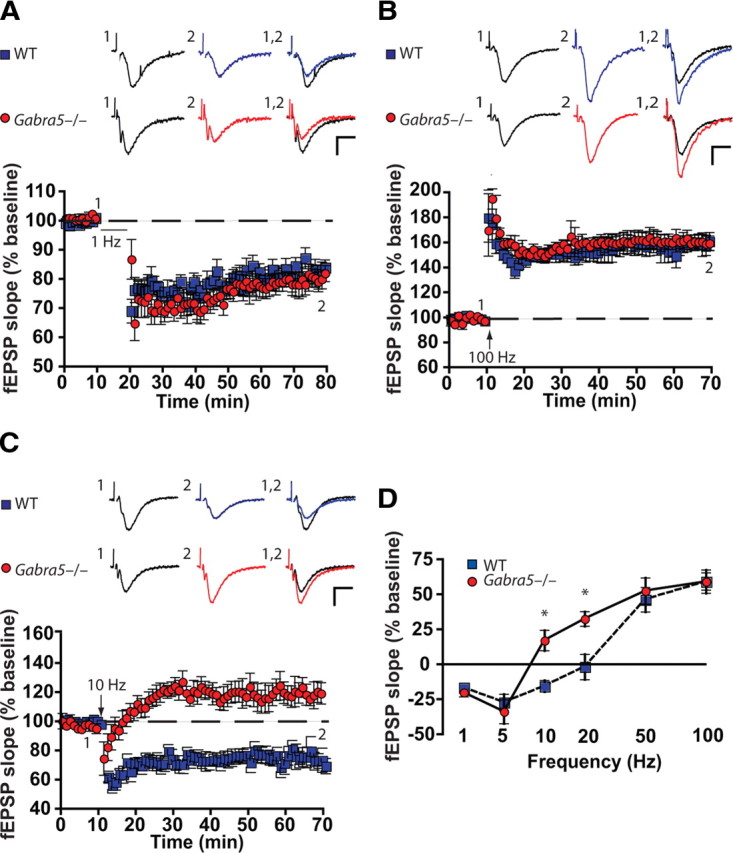
α5GABAARs critically regulate the threshold for LTP within a narrow range of stimulus frequencies. Genetic deletion of the α5 subunit of the GABAAR (Gabra5−/−) lowered the threshold for synaptic plasticity for slices subjected to moderate-frequency stimulation but not low- or high-frequency stimulation. The effects of stimulation at 1 Hz (A), 100 Hz (B), and 10 Hz (C) on the slope of the fEPSP (percentage of baseline) are shown. Sample traces are shown above each figure for the times indicated by the numbers. Calibration: 0.5 mV, 10 ms. D, Persistent changes in synaptic strength were significant only for stimulation at 10 and 20 Hz. *p < 0.001.
Because the genetic background of inbred parent strains can influence the strength of synaptic plasticity (Nguyen et al., 2000a,b) and the brain slices used for these experiments were prepared from the male offspring of homozygous parents, additional experiments were performed using offspring from heterozygous mating pairs. These results confirmed that LTD and LTP occurred after 10 Hz stimulation in slices from WT and Gabra5−/− littermates, respectively (WT, 83.43 ± 10.92% of baseline; Gabra5−/−, 122.95 ± 12.89% of baseline; n = 4 slices per group, Mann–Whitney U test, p = 0.03).
Synaptic transmission, excitability, and glutamate receptor expression is not altered in Gabra5−/− mice
Genetic deletion of α5GABAARs could be associated with unrecognized compensatory changes, such as alterations in the expression or function of other GABAARs, ion channels, or associated proteins in slices from Gabra5−/− mice (Brickley et al., 2001). To identify any nonspecific differences in excitatory synaptic transmission between WT and Gabra5−/− slices that might account for the observed differences in plasticity, the input–output relationships were compared. The stimulus intensity was varied systematically, and the presynaptic fiber volley versus the slope of the fEPSP was plotted as a scatter plot. When the data were fitted using a linear regression model, there was no observed difference between the slopes of the input–output relationship for WT and Gabra5−/− slices (effect of genotype, F(1,77) = 1.24, p = 0.27) (Fig. 2A,B). Additionally, PPF, which is considered to represent a presynaptic form of short-term plasticity, was similar between the genotypes (two-way ANOVA, effect of genotype, F(1,50) = 2.14, p = 0.14; effect of interpulse interval, F(4,50) = 16.61, p = 0.001; effect of interaction, F(4,50) = 0.12, p = 0.97) (Fig. 2C,D). Interestingly, our PPF results differed from those of Collinson et al. (2002), who reported a modest increase in PPF in Gabra5−/− slices stimulated with an interpulse interval of 100–300 ms. Those authors attributed the increase in PPF to an increase in the amplitude of the postsynaptic potential, because enhancement of fEPSP slopes over the same paired-pulse range was not affected (Collinson et al., 2002). We and others have not detected changes in the amplitude or time course of miniature or spontaneous IPSPs in CA1 pyramidal neurons from Gabra5−/− mice (Caraiscos et al., 2004; Cheng et al., 2006; Glykys and Mody, 2006).
Figure 2.

Normal synaptic transmission and excitability in α5 null mutant (Gabra5−/−) slices. There were no differences between the genotypes for any of the following characteristics. A, The traces showed no difference between WT and Gabra5−/− slices after stimulation at different intensities. B, Individual responses indicated that the input–output relationships were similar for WT and Gabra5−/− slices, which suggests normal baseline presynaptic function and synaptic efficacy in the Gabra5−/− mouse model. C, Sample traces show normal paired-pulse facilitation for each genotype. D, Grouped data show that facilitation of paired synaptic pulses (PSPs) with different interstimulus intervals (ISI) was the same for WT and Gabra5−/− slices. E, Traces showing enhanced excitability of population spikes after application of bicuculline.
It is also possible that deletion of the Gabra5 gene would increase the general intrinsic excitability of the slices, thus enhancing LTP. To test this possibility, population spikes were recorded from the CA1 stratum pyramidale, and the degree of excitation was quantified by measuring the coastline of the population spikes before and after application of the GABAAR antagonist bicuculline (8 μm) (Wang and Thompson, 2008). There was no difference in the coastline index between the genotypes as shown in Figure 2, E and F (WT, 482.61 ± 97.1%; Gabra5−/−, 471.05 ± 161.27%; n = 8 slices per group, Student's t test, p = 0.74). Thus, a nonspecific increase in excitability is unlikely to account for the enhancement of LTP observed at 10 Hz in Gabra5−/− slices.
Given that the threshold for activity-dependent plasticity is modified by altering the effectiveness of NMDA receptors (Herron et al., 1985), we also studied the properties of evoked glutamatergic EPSCs in WT and Gabra5−/− slices. Selective pharmacological blockers and various holding potentials were used to determine the ratio of the AMPA and NMDA components of the EPSCs. When slices were stimulated at intensities ranging from 5 to 10 V, the values of the ratios were similar for WT and Gabra5−/− slices (two-way ANOVA, effect of genotype, F(1,84) = 0.34, p = 0.56; effect of stimulation intensity, F(5,84) = 0.61, p = 0.69; effect of interaction, F(5,84) = 0.37, p = 0.87) (Fig. 3A,B). Additionally, there were no differences between the genotypes for the peak amplitudes of the AMPA currents (two-way ANOVA, effect of genotype, F(1,84) = 0.23, p = 0.63; effect of stimulation intensity, F(5,84) = 33.10, p = 0.001; effect of interaction, F(5,84) = 0.06, p = 0.99) (Fig. 3C) or NMDA currents (two-way ANOVA, effect of genotype, F(1,84) = 0.14, p = 0.71; effect of stimulation intensity, F(5,84) = 42.54, p = 0.001; effect of interaction, F(5,84) = 0.21, p = 0.92) (Fig. 3D). Furthermore, a Western immunoblot assay for ionotropic glutamate receptors indicated no differences between genotypes in the expression of AMPA and NMDA receptor subunits (Fig. 3E). These results indicate that upregulation of excitatory transmission does not account for the enhanced LTP observed in Gabra5−/− slices. Finally, to determine whether modification of LTP by α5GABAARs depended on conventional NMDA receptor-dependent pathways, the competitive NMDA receptor antagonist APV was applied to WT and Gabra5−/− slices, which were stimulated at 10 Hz (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). APV completely blocked plasticity in slices from both genotypes, which indicates that α5GABAARs modify plasticity through NMDA receptor-dependent mechanisms.
Figure 3.
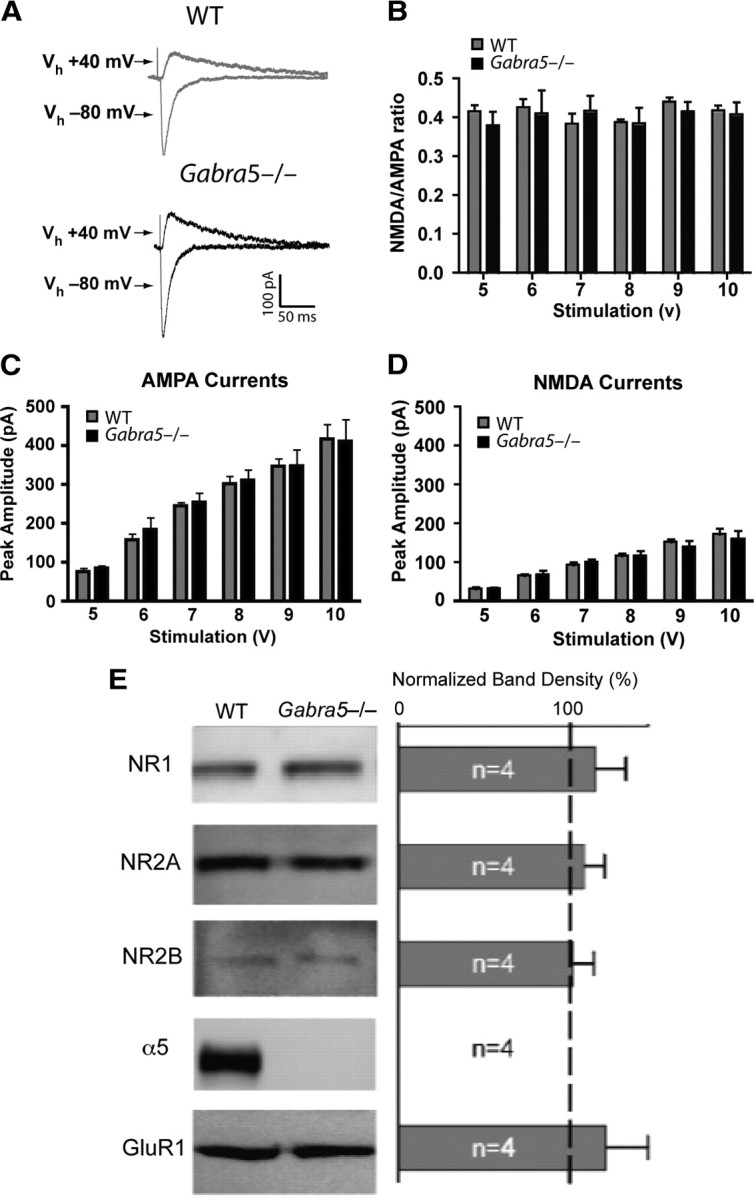
Glutamate receptor currents and expression were not altered in α5 null mutant (Gabra5−/−) neurons. A, AMPA (−80 mV) and NMDA (+40 mV) currents were recorded in WT and α5 null mutant (Gabra5−/−) neurons. B, There was no difference in the peak NMDA to AMPA ratio in WT (n = 7) and Gabra5−/− (n = 9) neurons for any of the stimulus intensities tested. C, AMPA currents, evoked at intensities ranging from 5 to 10 V, were the same in WT and Gabra5−/− neurons. D, There was no difference in the peak amplitude of NMDA currents evoked at intensities ranging from 5 to 10 V in WT and Gabra5−/− neurons. E, Left, Immunoblot assays for ionotropic glutamate receptor subtypes, including the NMDA receptor subunits NR1, NR2A, and NR2B and the AMPA receptor subunit GluR1, showed no difference in expression levels between WT and Gabra5−/− mice. Right, The change in the level of receptor expression was determined from the ratio of total protein expressed in Gabra5−/− tissue to total protein expressed in WT tissue. In the Gabra5−/− tissue, the total amount of protein expressed did not change for glutamatergic receptor subtypes, and the ratio for total α5 was 0 because Gabra5−/− tissue does not possess the α5 protein.
Tonic but not phasic inhibitory neurotransmission modifies submaximal LTP
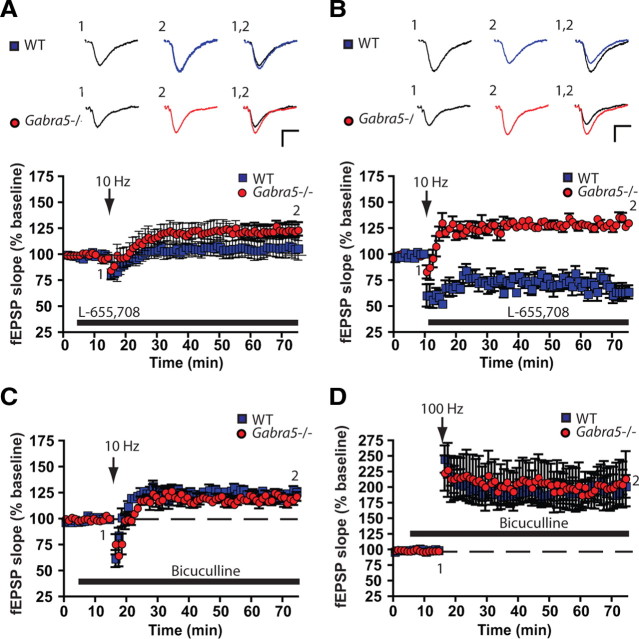
To determine whether a reduction in α5GABAAR activity accounted for the shift to the left of the activity-dependent plasticity curve, the effects of the selective inverse agonist L-655,708 in WT slices stimulated at 10 Hz were studied. L-655,708 (10 nm) altered the direction of plasticity in response to 10 Hz stimulation in WT slices, such that LTP rather than LTD was observed (baseline fEPSP, 98.73 ± 2.86%; fEPSP after 10 Hz stimulation with L-655,708, 107.91 ± 4.12%; n = 12 slices, Student's t test, p = 0.015). L-655,708 (100 nm) caused LTP in WT slices (Fig. 4A), which was similar to that produced by L-655,708 (10 nm) (Tukey's HSD, p > 0.05 for WT10 nm L-655,708 versus WT100 nm L-655,708). As predicted, L-655,708 (10 and 100 nm) had no effect on LTP in Gabra5−/− slices (Fig. 4A); moreover, the LTP in Gabra5−/− slices was not significantly different from that observed in L-655,708-treated WT slices [two-way ANOVA, effect of genotype, F(1,42) = 15.49, p = 0.001; effect of drug (vehicle or L-655,708 at 10 or 100 nm), F(2,42) = 9.54, p = 0.001; effect of interaction, F(2,42) = 3.57, p = 0.03; Tukey's HSD, p < 0.05 for WTvehicle vs WTL-655,708 10 nm, WTvehicle vs WTL-655,708 100 nm, WTvehicle vs Gabra5−/−vehicle, WTvehicle vs Gabra5−/−L-655,708 10 nm, and WTvehicle vs Gabra5−/−L-655,708 100 nm] (Fig. 4A).
Figure 4.
Pharmacologic studies confirmed that α5GABAARs are critical for the induction of LTP after moderate-frequency but not high-frequency stimulation. Recording of fEPSPs under control conditions was followed by application of L-655,708 for 10 min before (A) or immediately after (B) 10 Hz stimulation. Application of L-655,708 before but not after 10 Hz stimulation enhanced the fEPSPs of WT slices, which suggests that α5GABAARs are critical for the induction of threshold LTP but not for maintenance of the response. C, We next measured overall involvement of GABAARs in the LTP of submaximal stimulation by blocking these receptors with the competitive antagonist bicuculline. Application of bicuculline to WT slices that had been stimulated at 10 Hz produced LTP that was indistinguishable from that observed in Gabra5−/− slices but did not further enhance the LTP in Gabra5−/−slices. D, Bicuculline further potentiated LTP in WT and Gabra5−/− slices relative to drug-free conditions with 100 Hz stimulation, which suggests that GABAARs not containing the α5 subunit play an active role in LTP when the intensity of activation is increased. Black traces, Pretetanus baseline; blue and red traces, 60 min after tetanus in WT and Gabra5−/−slices, respectively. Calibration: 0.5 mV, 10 ms.
We next studied whether the ability of L-655,708 to increase LTP in WT slices depended on when the drug was applied relative to the time of stimulation. Notably, when L-655,708 (10 nm) was applied immediately after 10 Hz stimulation, no increase in synaptic strength was observed; indeed, LTD occurred rather than LTP (Student's t test, p = 0.02) (Fig. 4B). Thus, α5GABAAR activity regulated the threshold for the induction rather than the maintenance or expression phases of LTP.
We next asked whether α5GABAAR activity regulates synaptic plasticity induced by 10 Hz stimulation through mechanisms that are independent of other GABAARs subtypes. The nonselective competitive GABAAR antagonist bicuculline (8 μm) was applied to WT slices, which were then stimulated at 10 Hz. Bicuculline inhibits all GABAAR subtypes, with the exception of those containing the ρ subunit (Shimada et al., 1992), which are expressed at very low levels in the hippocampus (Enz et al., 1995; Alakuijala et al., 2005). Bicuculline caused LTP rather than LTD in WT slices (n = 10) stimulated at 10 Hz, whereas bicuculline had no effect on LTP in Gabra5−/− slices (two-way ANOVA, effect of genotype, F(1,40) = 7.53, p = 0.009; effect of bicuculline, F(3,40) = 4.36, p = 0.002; effect of interaction, F(3,40) = 3.48, p = 0.02; Tukey's HSD, p < 0.05 for WTcontrol vs WTbicuculline, WTcontrol vs Gabra5−/−bicuculline) (Fig. 4C). These results show that the activity of non-α5GABAAR subtypes plays a minor role in regulating the threshold for plasticity after 10 Hz stimulation. Interestingly, when WT and Gabra5−/− slices were stimulated at a high frequency (100 Hz), bicuculline enhanced plasticity equally in both genotypes (n = 7 slices per group) (Fig. 4D), suggesting that GABAARs other than the α5GABAARs play a predominate role in regulating plasticity under high-frequency stimulation.
To corroborate that a tonic conductance regulates plasticity, we asked whether an increase in the residual tonic current could prevent LTP in Gabra5−/−slices. The residual tonic current is mostly generated by δ subunit-containing GABAARs (Glykys and Mody, 2006). For these experiments, the δ subunit-containing GABAAR agonist THIP (Brown et al., 2002) was applied to Gabra5−/− slices. First, whole-cell currents were recorded to determine the concentration of THIP that produced a tonic conductance in Gabra5−/− CA1 neurons that was similar to that observed in WT neurons under baseline conditions. THIP (1 μm) consistently enhanced the tonic current by 10.6 ± 3.3 pA (n = 4) in slices from Gabra5−/− mice without increasing the time course of spontaneous IPSCs (supplemental Table 2, available at www.jneurosci.org as supplemental material). A higher concentration of THIP (3 μm) further increased the tonic current (54.4 ± 16.2 pA, n = 4) but also increased the baseline noise and obscured the synaptic currents. Higher concentrations of THIP also alter synaptic inhibition and were not studied further (Glykys and Mody, 2006; Krook-Magnuson et al., 2008). Field potential recordings showed that THIP (1 μm) attenuated LTP in Gabra5−/− slices (Gabra5−/−control 117.17 ± 1.96%, n = 7 vs Gabra5−/−THIP, 97.3 ± 1.3%; n = 7, Student's t test, p < 0.001) and resulted in no differences in plasticity between WT control slices and Gabra5−/− slices treated with THIP (Tukey's HSD, p > 0.05). THIP did not change the LTD in WT slices after 10 Hz stimulation (WTcontrol, 76.22 ± 8.92%, n = 4, vs WTTHIP, 83.78 ± 4.62%, n = 3; two-way ANOVA, effect of genotype, F(1,17) = 11.64, p = 0.003; effect of THIP, F(1,17) = 0.59, p = 0.45; effect of interaction, F(1,17) = 3.03, p = 0.1; Tukey's HSD, p < 0.05 for WTcontrol vs Gabra5−/−control and WTTHIP vs Gabra5−/−control).
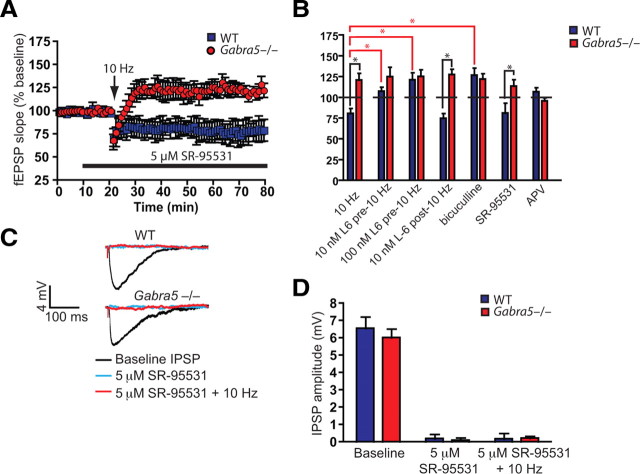
Next, to determine whether tonic GABAergic inhibition regulates plasticity via mechanisms that are predominantly independent of phasic inhibition, slices were treated with a selective GABAAR antagonist for synaptic currents, SR-95531 (5 μm). We have shown previously that this compound predominantly blocks IPSCs but not the tonic current in CA1 pyramidal neurons (Bai et al., 2001). First, we used whole-cell voltage-clamp recordings to confirm that SR-95531 blocks only the phasic conductance, that L-655,708 (10 nm) blocks the tonic but not the phasic conductance, and that bicuculline blocks both the phasic and tonic conductance (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). The portion of the tonic current blocked by bicuculline was significantly larger than that blocked by both L-655,708 and SR-95531, whereas the portion of the tonic current blocked by L-655,708 was significantly larger than that blocked by SR-95531 [one-way ANOVA, effect of drug (bicuculline, L-655,708 at 10 nm, SR-95531), F(2,17) = 39.45, p = 0.001; Tukey's HSD, p < 0.01 for all comparisons]. Additionally, the effect of L-655,708 on mIPSCs is summarized in supplemental Table 1 (available at www.jneurosci.org as supplemental material).
Inhibition of the IPSPs by SR-95531 did not enhance plasticity in WT slices (Student's t test, p = 0.43) (Fig. 5A), an effect that was clearly different from that of bicuculline as shown in Figure 4D. The relative changes in plasticity in response to the various GABAA blockers, at 10 Hz stimulation, are summarized in Figure 5B. To confirm that phasic IPSPs were completely abolished by SR-95531, both before and after 10 Hz stimulation, whole-cell current-clamp recordings were obtained. The GABAAR-mediated IPSPs were studied in the absence and presence of SR-95531, with pharmacological blockers for AMPA, NMDA, and GABAB receptors in the extracellular solution. SR-95531 completely blocked the IPSPs after 10 Hz stimulation (two-way ANOVA for IPSP amplitude, effect of genotype, F(1,18) = 3.04, p = 0.001; effect of SR-95531 application after 10 Hz stimulation, F(1,18) = 2843.5, p < 0.0001; effect of interaction, F(1,18) = 0.14, p = 0.71; Tukey's HSD, p < 0.01 for WTcontrol vs WTSR-95531 and Gabra5−/−control vs Gabra5−/−SR-95531) (Fig. 5C,D), which confirmed that fast phasic inhibition did not regulate synaptic plasticity at 10 Hz.
Figure 5.
Blockade of synaptic GABAARs does not enhance plasticity with 10 Hz stimulation. A, Selectively blocking synaptically expressed GABAARs with SR-95531 (5 μm) did not enhance synaptic potentiation in WT (n = 8) or α5 null mutant (Gabra5−/−) (n = 8) slices. B, A summary of changes in synaptic responses after 10 Hz stimulation. These data represent the average of the last 5 min of recording for each slice in each group. C, The IPSP (black) was blocked with SR-95531 before (light blue) and after (red) 10 Hz stimulation, which indicates that synaptic GABAARs could not account for the differences in plasticity at this frequency. D, Average data showing the raw amplitudes of the IPSP at baseline (without SR-95531), with SR-95531, and after 10 Hz stimulation for WT (n = 6) and Gabra5−/− (n = 6) cells. *p < 0.01.
α5GABAARs regulate membrane potential during 10 Hz stimulation
We next sought to determine whether the attenuation of plasticity by α5GABAARs was associated with changes in membrane potential and/or a reduction in membrane conductance (Staley and Mody, 1992; Brickley et al., 2001; Nusser and Mody, 2002; Wisden et al., 2002; Bonin et al., 2007). Whole-cell current-clamp techniques were used to record the resting membrane potential of pyramidal neurons before, during, and 30 min after 10 Hz stimulation. Small hyperpolarizing current pulses (10 pA intensity, 400 ms duration) were applied to measure input resistance before and 30 min after 10 Hz stimulation. For these experiments, SR-95531 and CGP 55845 were also added to the extracellular solution to inhibit GABAAR-mediated and GABABR-mediated IPSPs, respectively.
The biophysical properties of the WT and Gabra5−/− neurons were similar under baseline conditions (before 10 Hz stimulation): input resistance, 257.96 ± 14.17 MΩ for WT and 243.75 ± 28.32 MΩ for Gabra5−/−; resting membrane potential, −59.62 ± 0.91 mV for WT and −58.56 ± 0.81 mV for Gabra5−/− (n = 5 slices per group, Mann–Whitney U test, p > 0.05 for both). Also, the amplitude of the EPSPs was similar in the two groups before 10 Hz stimulation (WT, 6.77 ± 1.87 mV; Gabra5−/−, 6.93 ± 2.92 mV; n = 5, Mann–Whitney U test, p > 0.05). However, there was a marked difference in the amplitude of EPSPs and baseline membrane potential between genotypes during and after 10 Hz stimulation (Fig. 6A). During stimulation, there was robust depolarization in Gabra5−/− neurons (−54.39 ± 1.26 mV, Mann–Whitney U test, p < 0.05 compared with baseline resting membrane potential), whereas the membrane potential of WT neurons did not change (−60.29 ± 0.80 mV, Mann–Whitney U test, p < 0.05 compared with baseline resting membrane potential). Thirty minutes after 10 Hz stimulation, Gabra5−/− neurons returned to baseline resting membrane potential (−58.19 ± 0.61 mV, Mann–Whitney U test, p > 0.05 compared with baseline resting membrane potential) (Fig. 6A), whereas WT neurons were slightly hyperpolarized (−63.17 ± 0.85 mV, Mann–Whitney U test, p < 0.05 compared with baseline membrane potential). The depolarization and loss of membrane hyperpolarization observed in Gabra5−/− neurons after 10 Hz stimulation was mimicked in WT neurons by application of L-655,708 10 nm (−53.59 ± 2.42 mV during stimulation and −56.52 ± 1.24 mV after stimulation; n = 5). The input resistance, measured 30 min after 10 Hz stimulation, decreased significantly in untreated WT neurons (182.43 ± 21.97 MΩ, Mann–Whitney U test, p < 0.05 compared with baseline) (Fig. 6B), whereas there was no significant change in the average input resistance 30 min after 10 Hz stimulation for both Gabra5−/− neurons (239.19 ± 29.33 MΩ, Mann–Whitney U test, p > 0.05 compared with baseline) and WT neurons treated with L-655,708 (240.34 ± 24.31 MΩ, n = 5, Mann–Whitney U test, p > 0.05 compared with baseline) (Fig. 6B). The changes in membrane potential during stimulation were further analyzed by measuring the depolarizing envelope, which initially increased rapidly and then declined and was significantly greater in Gabra5−/− and WT neurons treated with L-655,708 than in WT control neurons (Kruskal–Wallis rank sum test followed by the Bonferroni–Dunn multiple-comparison test, p < 0.05) (Fig. 6C,D).
Figure 6.
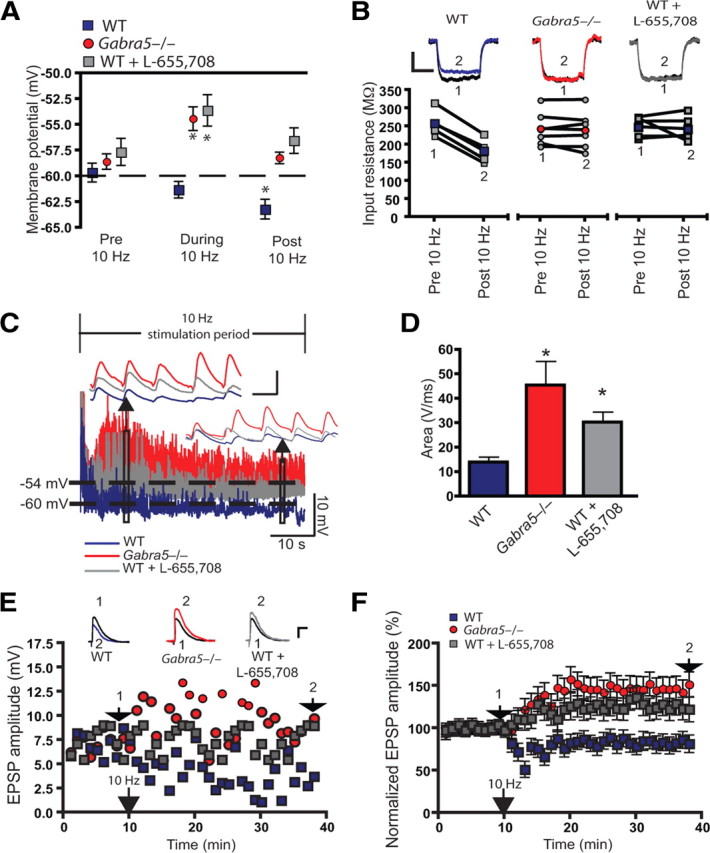
α5GABAARs regulate membrane properties and the depolarizing envelope to set the threshold for long-term potentiation within the 10 Hz stimulation range. A, The membrane potential was significantly depolarized in α5 null mutant (Gabra5−/−) and L-655,708-treated WT neurons during 10 Hz stimulation. The average membrane potential for the 10 min period after 10 Hz stimulation was significantly hyperpolarized from baseline values in WT. All recordings were conducted in the presence of SR-95531 and CGP 55845 to block synaptic GABAA and GABAB receptors, respectively. The membrane potential during and after 10 Hz stimulation were compared with baseline membrane potentials for the respective treatments. B, Sample traces show the membrane hyperpolarizing pulse for WT, Gabra5−/−, and L-655,708-treated WT neurons, before and 30 min after 10 Hz stimulation. The average input resistance was significantly decreased in WT but not Gabra5−/− or L-655,708-treated WT neurons. The individual changes in input resistance for each cell are shown in the “before” and “after” plots. Colored symbols represent the group means. 1, Baseline EPSP; 2, EPSP 30 min after 10 Hz stimulation. C, Sample traces show that, during 10 Hz stimulation, the EPSPs were significantly smaller, for the duration of the simulation period, in WT neurons than in Gabra5−/− and L-655,708-treated WT neurons. Insets show that the EPSPs were larger for Gabra5−/− and L-655,708-treated WT neurons near the beginning and at the end of the stimulation period. D, Pooled data show that the average depolarizing envelope was significantly larger in Gabra5−/− neurons and L-655,708-treated WT neurons than in WT control neurons. E, Examples of EPSP responses for single WT (blue), Gabra5−/− (red), and L-655,708-treated WT (gray) neurons. 1, Baseline EPSP; 2, EPSP 30 min after 10 Hz stimulation. Calibration: 4 mV, 10 ms. F, Summary of the EPSP changes after 10 Hz stimulation for all groups. *p < 0.05.
To confirm that the changes in membrane potential and input resistance were associated with changes in plasticity, the amplitude of the EPSPs was measured 30 min after 10 Hz stimulation. The amplitude of EPSPs declined in WT neurons but increased in Gabra5−/− and WT neurons treated with L-655,708 (Kruskal–Wallis rank sum test followed by the Bonferroni–Dunn multiple-comparison test, p < 0.05) (Fig. 6E,F). Sample responses for individual cells are shown in Figure 6E, and the summed data are presented in Figure 6F. These whole-cell changes in the amplitude of EPSPs are consistent with the change in fEPSPs shown in Figures 1 and 4. We also attempted to mimic the Gabra5−/− phenotype in WT neurons by introducing a step depolarization to −54 mV during tetanus. Under these conditions, LTD was induced in WT neurons (60 ± 17.63%; n = 7, Student's t test, p < 0.001). Clamping the membrane potential of Gabra5−/− neurons to −60 mV during 10 Hz stimulation did not alter the strength or polarity of synaptic plasticity measured 30 min after stimulation (125.37 ± 12.89%, n = 5, Student's t test, p = 0.43). These latter findings suggest that the mechanisms underlying the frequency-dependent regulation of plasticity by α5GABAARs are more complex than simply a change in baseline membrane potential during tetanus.
α5GABAARs control learning and memory for certain hippocampus-dependent tasks
It was anticipated that the alteration of plasticity by α5GABAARs in CA1 pyramidal neurons would be associated with changes in hippocampus-dependent associative learning and memory during fear conditioning, as was observed previously for spatial navigational memory (Collinson et al., 2002). In support of this postulate, an increase in α5GABAAR activity by the anesthetic etomidate inhibited LTP and robustly impaired fear memory (Cheng et al., 2006; Martin et al., 2009). However, no memory phenotype for contextual fear-associated memory has been demonstrated for Gabra5−/− mice in the absence of pharmacological agents (Cheng et al., 2006). Therefore, we undertook a series of stringent behavioral studies to determine whether α5GABAARs regulate the acquisition, retention, or recall of memory. WT and Gabra5−/− mice were studied in a strong contextual fear-conditioning task (three 0.5-mA footshocks of 2 s each, separated by 60 s intervals) and an equally stringent auditory cued fear-conditioning task (three tone–shock pairings: 20 s, 70 dB tone paired with a 2 s, 0.5 mA footshock, separated by 60 s intervals), with the shocks delivered during the last 2 s of the tone (Fig. 7A). Contextual fear-conditioning responses were similar in WT and Gabra5−/− mice, as indicated by high freezing scores (>75% freezing), after treatment with vehicle or L-655,708 (0.7 mg/kg) (two-way ANOVA, effect of genotype, F(1,38) = 1.32, p = 0.31; effect of L-655,708, F(1,38) = 1.16, p = 0.42; effect of interaction, F(1,38) = 0.92, p = 0.64) (Fig. 7B). It is unlikely that the ineffectiveness of L-655,708 in WT mice was attributable to a lack of functionality of the α5GABAARs in the hippocampus subregions involved in contextual fear acquisition because we showed previously that selectively enhancing the activity of α5GABAARs can impair memory performance for contextual fear memory (Cheng et al., 2006). Next, as a negative control, an amygdala-dependent cued fear-conditioning task was studied (Fanselow, 1980), because the expression of α5GABAARs in the amygdala is relatively low (Sur et al., 1999; Pirker et al., 2000). As anticipated, the freezing scores for cued fear conditioning were similar for WT and Gabra5−/− mice (Student's t test, p = 0.53) (Fig. 7C).
Figure 7.
α5GABAARs physiologically regulate the acquisition of weak hippocampus-dependent associative fear memory tasks. A, A schematic representation showing the timing for all three fear-conditioning protocols. In all protocols, a baseline activity period of 3 min preceded the conditioning procedure. Three 2 s, 0.5 mA footshocks, separated by 60 s intervals, were used for contextual fear conditioning. Three tone–shock pairings (20 s, 70 dB tone paired with a 2 s, 0.5 mA footshock), separated by 60 s intervals, were used for auditory cued fear conditioning. The procedure for trace fear conditioning was similar to that for cued fear conditioning (three tone–shock pairings, separated by 240 s intervals), except that an empty trace interval of 20 s was interposed between the tone and the footshock. B, There was no difference between WT (n = 9) and α5 null mutant (Gabra5−/−) (n = 11) mice for contextual fear conditioning, which forms strong hippocampus-dependent memories; furthermore, L-655,708 had no effect on the freezing response (WT, n = 10; Gabra5−/−, n = 11). C, The WT (n = 9) and Gabra5−/− (n = 11) mice had similar scores during the amygdala-dependent cued fear-conditioning task. D, The performance of Gabra5−/− (n = 12) mice was enhanced in trace fear conditioning (a weak associative task), relative to the effect in naive (n = 12) and vehicle-treated (n = 13) WT mice; in addition, inhibiting α5GABAARs with L-655,708 improved the performance of WT mice (n = 13) to the level observed in Gabra5−/− mice. E, The performance of WT mice (n = 9) was not enhanced with L-655,708 injections immediately after training in the trace fear-conditioning protocol, relative to Gabra5−/− mice (n = 8). *p < 0.05, significantly different from the control group.
It is plausible that the contribution of α5GABAARs to the regulation of learning and memory depends on the demands of the cognitive task. To investigate this hypothesis, a trace fear-conditioning paradigm was used, because rodents do not rapidly or efficiently learn this task (Misane et al., 2005). The strength of classical conditioning can be reduced by introducing a time interval (or “trace”) between the conditioned stimulus and the unconditioned stimulus. The procedure for trace fear conditioning was similar to that for cued fear conditioning (three tone–shock pairings: 20 s, 70 dB tone paired with a 2 s, 0.5 mA footshock, separated by intervals of 240 s), except that an empty trace interval of 20 s was interposed between the tone and the footshock (Fig. 7A). Trace fear conditioning depends strongly on the dorsal hippocampus, and the relative activity of α5GABAARs should correlate with the performance of mice in hippocampus-dependent tasks (Collinson et al., 2002, 2006). The naive and vehicle-treated Gabra5−/− mice significantly outperformed WT mice, as indicated by higher freezing scores. To confirm that the difference between the genotypes was attributable to a reduction in α5GABAAR activity, L-655,708 was administered before the fear-conditioning protocol and was shown to improve the performance of WT but not Gabra5−/− mice in trace fear conditioning (two-way ANOVA, effect of genotype, F(1,55) = 12.93, p = 0.003; effect of L-655,708 before training, F(1,55) = 11.84, p = 0.005; effect of interaction, F(1,55) = 14.82, p = 0.001; Tukey's HSD, p < 0.01 for WTnaive vs WTL-655,708, WTnaive vs Gabra5−/−naive, WTnaive vs Gabra5−/−vehicle, WTnaive vs Gabra5−/−L-655,708, WTvehicle vs WTL-655,708, WTvehicle vs Gabra5−/−naive, WTvehicle vs Gabra5−/−vehicle, and WTvehicle vs Gabra5−/−L-655,708) (Fig. 7D). Notably, the freezing scores of WT mice treated with L-655,708 and of vehicle-treated Gabra5−/− mice were similar (Tukey's HSD, p > 0.05), which suggests a possible ceiling effect that could not be further enhanced by complete deletion of α5GABAARs.
Because the electrophysiological studies showed that inhibiting α5GABAARs altered the induction but not the maintenance phase of synaptic plasticity, we next sought to determine whether the timing of α5GABAAR inhibition by L-655,708 influenced memory performance. Administration of L-655,708 immediately after training produced an effect that was similar to vehicle control in WT and Gabra5−/− mice (two-way ANOVA, effect of genotype, F(1,29) = 93.04, p < 0.0001; effect of L-655,708 after training, F(1,29) = 2.0, p = 0.23; effect of interaction, F(1,29) = 2.26, p = 0.14; Tukey's HSD, p < 0.05 for WTvehicle vs Gabra5−/−vehicle) (Fig. 7E). Together, these results show that α5GABAAR activity enhances the acquisition but not the consolidation of weakly acquired but not strongly acquired fear memory.
Finally, because breeding strategies can influence memory behavior, we compared the performance of male offspring from heterozygous and homozygous mating pairs for trace fear learning. A total of 125 male mice were used in the current study and a previous study (Martin et al., 2009). From this combined sample, 24 WT mice and 25 Gabra5−/− mice were from homozygous mating pairs. In addition, five wild-type mice and five Gabra5−/− mice offspring were from heterozygous mating pairs. There were no differences in freezing scores after trace fear conditioning between WT offspring from homozygous or heterozygous breeding pairs (37.39 ± 6.71 vs 43.59 ± 5.10%, respectively, Tukey's HSD, p > 0.05) or Gabra5−/− mice from homozygous or heterozygous breeding pairs (81.76 ± 15.68 vs 69.96 ± 16.75%, respectively, Tukey's HSD, p > 0.05). Also, the differences in trace fear conditioning between WT and Gabra5−/− offspring from heterozygous mating pairs paralleled the effect originally shown for WT and Gabra5−/− offspring from homozygous mice. Thus, regardless of breeding conditions, Gabra5−/− mice froze significantly more than WT mice.
Discussion
The major aim of this study was to determine whether α5GABAARs regulate synaptic plasticity in vitro and whether such regulation correlates with alterations in memory performance for hippocampus-dependent memory tasks. The most striking observation was that α5GABAARs controlled the induction of synaptic plasticity within a narrow window of stimulation frequencies. Genetic deletion and pharmacological inhibition of α5GABAARs modified the extent and directionality of plasticity but only when slices were stimulated at frequencies within the theta range (7–12 Hz) (O'Keefe, 1993; Buzsáki, 2005). α5GABAAR activity attenuated the excitatory synaptic potentials during 10 Hz stimulation and hyperpolarized the membrane through mechanisms that were predominantly independent of synaptic GABAergic inhibition. This latter finding challenges the widely held notion that fast synaptic inhibition accounts for the modulation of hippocampus-dependent learning by GABAARs, at least under certain conditions.
The regulation of plasticity by α5GABAARs after low- but not high-frequency stimulation could result from several factors, including the recruitment of low- versus high-affinity GABAA receptor subtypes and/or the frequency-dependent activation of different interneuron populations. Others have shown that the extent of GABAergic inhibition in the hippocampus depends on the frequency of network stimulation, possibly because the release of GABA increases during high-frequency stimulation (Chapman et al., 1998). At higher stimulation frequencies, it is likely that a broader range of GABAARs, including lower-affinity non-α5GABAARs, is activated (Wigström and Gustafsson, 1983). Extrasynaptic GABAARs are relatively high-affinity receptors that are activated by low ambient concentrations of GABA (Brickley et al., 1996) spilling over from the synaptic cleft (Semyanov and Kullmann, 2000). Because Gabra5−/− slices lack these relatively high-affinity GABAARs (Burgard et al., 1996; Yeung et al., 2003; Caraiscos et al., 2004), they may be insensitive to the effects of the low ambient concentrations of GABA that are released when slices are simulated within the low 10–20 Hz range.
Alternatively, specific stimulation frequencies may preferentially recruit distinct interneurons, which in turn, target subregions of CA1 pyramidal neurons in which α5GABAARs are expressed (Klausberger and Somogyi, 2008). The distribution of α5GABAARs in pyramidal neurons is non-uniform, with the highest expression levels found in the dendritic regions of the hippocampal subfields, a region that is targeted by dendritic-targeting bistratified interneurons (Thomson et al., 2000; Klausberger and Somogyi, 2008). Interestingly, the application of THIP to Gabra5−/− slices increased the residual tonic conductance and blocked LTP but failed to mimic the LTD seen in WT slices. The δGABAA and α5GABAA receptors, which generate the tonic conductances, may not spatially localize to the same dendritic compartments or perisomatic regions (Klausberger and Somogyi, 2008). Also, α5GABAARs may reside in proximity to different ion channels, signaling pathways, or structural proteins that also influence plasticity, as occurs with glutamate channel subtypes (Wang and Salter, 1994; Migaud et al., 1998; Huang et al., 2001; Ikegaya et al., 2002). Although THIP produced a comparable tonic conductance under resting conditions, the tonic conductance may not be equivalent during or after 10 Hz stimulation. Finally, although α5GABAARs are thought to predominantly generate a “tonic” form of inhibition, high-frequency trains of stimuli may recruit these receptors through spillover of GABA from the synaptic cleft and activation of a limited number of synaptic α5GABAARs (Christie and de Blas, 2002; Serwanski et al., 2006; Zarnowska et al., 2009). In the mouse hippocampus, α5GABAARs contribute to a small subset of large-amplitude evoked IPSCs that have been referred to as slow IPSCs (Zarnowska et al., 2009; Vargas-Caballero et al., 2010).
The reduced expression and function of α5GABAARs produced no changes in resting membrane potential, input resistance, baseline expression of glutamate receptors, baseline excitatory neurotransmission, or presynaptic transmitter release. However, current-clamp recordings showed that α5GABAARs strongly regulated the membrane potential and amplitude of EPSPs during and after 10 Hz stimulation. The large-amplitude EPSPs seen in Gabra5−/− slices were absent from WT slices. The application of L-655,708 to WT slices mimicked the effects of genetic deletion of α5GABAAR on membrane potential, input resistance, EPSPs, and the depolarizing envelope. Such changes in membrane potential could influence the initial events that trigger plasticity (Voglis and Tavernarakis, 2006). Depolarization of the postsynaptic membrane is associated with increased plasticity through a variety of mechanisms, including the voltage-dependent relief of Mg2+ blockade of NMDA receptors and activation of voltage-gated cation channels (Castellani et al., 2001; Cormier et al., 2001; Nolan et al., 2004). In addition, the reduction in input resistance could attenuate the influence of excitatory glutamatergic inhibition through a shunting mechanism (Mitchell and Silver, 2003). In hippocampal pyramidal neurons, the tonic conductance has been shown to alter the input–output function predominantly by altering neuronal excitability at spiking threshold (Pavlov et al., 2009).
Not surprisingly, attempts to mimic the LTP phenotype of Gabra5−/− slices in WT slices by simply introducing a depolarizing current step at the soma were unsuccessful. The depolarization of Gabra5−/− neurons during 10 Hz stimulation probably reflects the combined effect of several conductances triggered during tetanic stimulation of the network. Moreover, introducing a depolarizing or hyperpolarizing current to the soma may not regulate membrane potential in the large dendritic trees in which the α5GABAARs are expressed (Sur et al., 1998, 1999). Dynamic clamp experiments would be of interest to determine the influence of shunting inhibition on plasticity but would require an accurate estimate of the α5GABAAR conductance before, during, and after 10 Hz stimulation. It may also be necessary to mimic the conductance in proximity to other populations of receptors that regulate plasticity.
The regulation of plasticity by α5GABAARs correlated with changes in memory performance during trace but not contextual fear conditioning. In trace fear learning, Gabra5−/− mice exhibited higher freezing scores than WT mice. Consistent with this observation, L-655,708 increased freezing scores in the WT mice to the level observed in Gabra5−/− mice. Unidentified compensatory changes are unlikely to contribute to the behavioral phenotype of Gabra5−/− mice, because genetic deletion and pharmacological inhibition of α5GABAAR activity produced nearly identical effects. The greater freezing scores observed for Gabra5−/− mice are consistent with results from a second genetic model of α5GABAAR deficiency (Crestani et al., 2002). A histidine–arginine point mutation introduced at position 105 in the α5 subunit gene (α5H105R) causes a 30–40% reduction in the total number of α5GABAARs in the hippocampus, for as-yet-unknown reasons (Crestani et al., 2002). Consistent with our results, the α5H105R mice showed no difference in baseline contextual fear conditioning yet performed better in trace fear-conditioning studies than WT mice. Also in accord with our results, the synaptic plasticity of hippocampal slices induced by high-frequency stimulation was similar in α5H105R and WT mice (Crestani et al., 2002). The α5H105R mice exhibited several interesting memory phenotypes, including better trace fear conditioning, resistance to extinction of learning (Crestani et al., 2002; Yee et al., 2004), and altered latent inhibition (Gerdjikov et al., 2008).
The results presented here will be of potential therapeutic interest because they suggest a cellular mechanism to account for the memory-enhancing properties of inhibitors of α5GABAARs (Dawson et al., 2006; Ballard et al., 2009). Indiscriminant reduction in GABAAR-mediated inhibition by nonselective antagonists facilitates synaptic plasticity (Wigström and Gustafsson, 1983), increases vigilance (Ferraro et al., 1999), and improves memory performance (Zarrindast et al., 2002); however, these nonselective drugs have no therapeutic utility because of their proconvulsant and anxiogenic properties (Ben-Ari et al., 2007). Inverse agonists selective for α5GABAARs facilitate some forms of learning and enhance synaptic potentiation without a proconvulsant effect (Atack et al., 2006a). The desirable drug profile may be attributed to the restricted distribution of α5GABAARs, which represent <2% of all GABAARs in the brain yet are strongly expressed in the CA1 region of the hippocampus, in which they constitute 25% of GABAARs (Pirker et al., 2000). L-655,708 enhances LTP (Atack et al., 2006a) and promotes spontaneous gamma oscillations in the CA3 region of the hippocampus (Glykys et al., 2008). In a previous study, the inverse agonist α5IA reversed alcohol-induced memory impairment in human volunteers, although it had no effect on baseline memory performance (Nutt et al., 2007).
Many important questions remain, including whether the regulatory properties of α5GABAARs result from a unique pattern of cellular distribution, the amount of inhibitory charge, or proximity of the receptors to signaling pathways or other ion channels. For example, some NMDA receptors, much like the α5GABAARs, occur extrasynaptically (Köhr, 2006). Extrasynaptic NMDA receptors are thought to preferentially contain the NR2B subunit of the NMDA receptor (Tovar and Westbrook, 1999), and these receptors have been shown to participate in LTP (Bartlett et al., 2007). The close proximity of α5GABAARs to NMDA receptor subtypes may, through shunting inhibition and membrane hyperpolarization, limit the efficacy of the excitatory input, which would in turn inhibit plasticity and learning. Also, it will also be of interest to determine whether other forms of tonic inhibition, such as that generated by δ subunit-containing GABAARs, serve a similar frequency-dependent role in regulating plasticity in other hippocampal subfields and brain regions (Nusser and Mody, 2002; Stell et al., 2003; Lindquist and Birnir, 2006).
Footnotes
L.J.M. was supported by a Canadian Institutes of Health Research (CIHR)/Canada Graduate Scholarships–Doctoral scholarship. A.A.Z. is supported by a Natural Sciences and Engineering Research Council/Postgraduate Scholarships–Masters scholarship. B.A.O. is supported by CIHR Operating Grants MOP-38028 and MOP-79428 and holds a Canada Research Chair. We thank Drs. Paul Frankland and Michael Salter for their comments on previous versions of this manuscript and Ella Czerwinska and Dr. William Ju for their assistance with the biochemical experiments.
References
- Alakuijala A, Palgi M, Wegelius K, Schmidt M, Enz R, Paulin L, Saarma M, Pasternack M. GABA receptor ρ subunit expression in the developing rat brain. Brain Res Dev Brain Res. 2005;154:15–23. doi: 10.1016/j.devbrainres.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for α5-containing GABAA receptors. Neuropharmacology. 2006a;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Atack JR, Pike A, Clarke A, Cook SM, Sohal B, McKernan RM, Dawson GR. Rat pharmacokinetics and pharmacodynamics of a sustained release formulation of the GABAA α5-selective compound L-655,708. Drug Metab Dispos. 2006b;34:887–893. doi: 10.1124/dmd.105.006973. [DOI] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, Gasser R, Moreau JL, Wettstein JG, Buettelmann B, Knust H, Thomas AW, Trube G, Hernandez MC. RO4938581, a novel cognitive enhancer acting at GABAA α5 subunit-containing receptors. Psychopharmacology (Berl) 2009;202:207–223. doi: 10.1007/s00213-008-1357-7. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Martin LJ, MacDonald JF, Orser BA. α5GABAA receptors regulate the intrinsic excitability of mouse hippocampal pyramidal neurons. J Neurophysiol. 2007;98:2244–2254. doi: 10.1152/jn.00482.2007. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard EC, Tietz EI, Neelands TR, Macdonald RL. Properties of recombinant γ-aminobutyric acid A receptor isoforms containing the α5 subunit subtype. Mol Pharmacol. 1996;50:119–127. [PubMed] [Google Scholar]
- Buzsáki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani GC, Quinlan EM, Cooper LN, Shouval HZ. A biophysical model of bidirectional synaptic plasticity: dependence on AMPA and NMDA receptors. Proc Natl Acad Sci U S A. 2001;98:12772–12777. doi: 10.1073/pnas.201404598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, Ferris P, Hobbs SC, O'Connor D, Marshall G, Rycroft W, Macleod AM. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABAA α5 receptors with cognition enhancing properties. J Med Chem. 2004;47:5829–5832. doi: 10.1021/jm040863t. [DOI] [PubMed] [Google Scholar]
- Chapman CA, Perez Y, Lacaille JC. Effects of GABAA inhibition on the expression of long-term potentiation in CA1 pyramidal cells are dependent on tetanization parameters. Hippocampus. 1998;8:289–298. doi: 10.1002/(SICI)1098-1063(1998)8:3<289::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA. α5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, de Blas AL. α5 Subunit-containing GABAA receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport. 2002;13:2355–2358. doi: 10.1097/00001756-200212030-00037. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN. An inverse agonist selective for α5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology. 2006;188:619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Cormier RJ, Greenwood AC, Connor JA. Bidirectional synaptic plasticity correlated with the magnitude of dendritic calcium transients above a threshold. J Neurophysiol. 2001;85:399–406. doi: 10.1152/jn.2001.85.1.399. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal α5GABAA receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR. An inverse agonist selective for α5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-d-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R, Brandstätter JH, Hartveit E, Wässle H, Bormann J. Expression of GABA receptor ρ1 and ρ2 subunits in the retina and brain of the rat. Eur J Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Ferraro L, Antonelli T, Tanganelli S, O'Connor WT, Perez de la Mora M, Mendez-Franco J, Rambert FA, Fuxe K. The vigilance promoting drug modafinil increases extracellular glutamate levels in the medial preoptic area and the posterior hypothalamus of the conscious rat: prevention by local GABAA receptor blockade. Neuropsychopharmacology. 1999;20:346–356. doi: 10.1016/S0893-133X(98)00085-2. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Rudolph U, Keist R, Möhler H, Feldon J, Yee BK. Hippocampal α5 subunit-containing GABAA receptors are involved in the development of the latent inhibition effect. Neurobiol Learn Mem. 2008;89:87–94. doi: 10.1016/j.nlm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit-deficient mice. J Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover LM, Yan C. Blockade of GABAA receptors facilitates induction of NMDA receptor-independent long-term potentiation. J Neurophysiol. 1999;81:2814–2822. doi: 10.1152/jn.1999.81.6.2814. [DOI] [PubMed] [Google Scholar]
- Herron CE, Lester RA, Coan EJ, Collingridge GL. Intracellular demonstration of an N-methyl-d-aspartate receptor mediated component of synaptic transmission in the rat hippocampus. Neurosci Lett. 1985;60:19–23. doi: 10.1016/0304-3940(85)90375-1. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF. CAKβ/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Ishizaka Y, Matsuki N. BDNF attenuates hippocampal LTD via activation of phospholipase C: implications for a vertical shift in the frequency-response curve of synaptic plasticity. Eur J Neurosci. 2002;16:145–148. doi: 10.1046/j.1460-9568.2002.02051.x. [DOI] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhr G. NMDA receptor function: subunit composition versus spatial distribution. Cell Tissue Res. 2006;326:439–446. doi: 10.1007/s00441-006-0273-6. [DOI] [PubMed] [Google Scholar]
- Korn SJ, Giacchino JL, Chamberlin NL, Dingledine R. Epileptiform burst activity induced by potassium in the hippocampus and its regulation by GABA-mediated inhibition. J Neurophysiol. 1987;57:325–340. doi: 10.1152/jn.1987.57.1.325. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson EI, Li P, Paluszkiewicz SM, Huntsman MM. Tonically active inhibition selectively controls feedforward circuits in mouse barrel cortex. J Neurophysiol. 2008;100:932–944. doi: 10.1152/jn.01360.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitz Y, Avignone E, Groner Y, Segal M. Upregulation of GABA neurotransmission suppresses hippocampal excitability and prevents long-term potentiation in transgenic superoxide dismutase-overexpressing mice. J Neurosci. 1999;19:10977–10984. doi: 10.1523/JNEUROSCI.19-24-10977.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist CE, Birnir B. Graded response to GABA by native extrasynaptic GABA receptors. J Neurochem. 2006;97:1349–1356. doi: 10.1111/j.1471-4159.2006.03811.x. [DOI] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Oh GH, Orser BA. Etomidate targets α5GABAA receptors to regulate synaptic plasticity and memory blockade. Anesthesiology. 2009;111:1025–1035. doi: 10.1097/ALN.0b013e3181bbc961. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O'Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Duffy SN, Young JZ. Differential maintenance and frequency-dependent tuning of LTP at hippocampal synapses of specific strains of inbred mice. J Neurophysiol. 2000a;84:2484–2493. doi: 10.1152/jn.2000.84.5.2484. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000b;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsáki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Besson M, Wilson SJ, Dawson GR, Lingford-Hughes AR. Blockade of alcohol's amnestic activity in humans by an α5 subtype benzodiazepine receptor inverse agonist. Neuropharmacology. 2007;53:810–820. doi: 10.1016/j.neuropharm.2007.08.008. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. Hippocampus, theta, and spatial memory. Curr Opin Neurobiol. 1993;3:917–924. doi: 10.1016/0959-4388(93)90163-s. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAΑ receptors in pyramidal cells modulate neuronal offset, not gain. J Neurosci. 2009;29:15341–15350. doi: 10.1523/JNEUROSCI.2747-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the α5 subunit. Neuropharmacology. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Kullmann DM. Modulation of GABAergic signaling among interneurons by metabotropic glutamate receptors. Neuron. 2000;25:663–672. doi: 10.1016/s0896-6273(00)81068-5. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the α5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada S, Cutting G, Uhl GR. γ-Aminobutyric acid A or C receptor? γ-Aminobutyric acid ρ1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive gamma-aminobutyric acid responses in Xenopus oocytes. Mol Pharmacol. 1992;41:683–687. [PubMed] [Google Scholar]
- Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J Neurophysiol. 1992;68:197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- Steckler T, Sahgal A, Aggleton JP, Drinkenburg WH. Recognition memory in rats. III. Neurochemical substrates. Prog Neurobiol. 1998;54:333–348. doi: 10.1016/s0301-0082(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and human hippocampal α5 subunit-containing γ-aminobutyric acidA receptors have α5 β3 γ2 pharmacological characteristics. Mol Pharmacol. 1998;54:928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- Sur C, Fresu L, Howell O, McKernan RM, Atack JR. Autoradiographic localization of α5 subunit-containing GABAA receptors in rat brain. Brain Res. 1999;822:265–270. doi: 10.1016/s0006-8993(99)01152-x. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP, Hughes DI, Pawelzik H. Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur J Neurosci. 2000;12:425–436. doi: 10.1046/j.1460-9568.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers SK, Gloveli T, Traub RD, Driver JE, Engel D, Fradley R, Rosahl TW, Maubach K, Buhl EH, Whittington MA. α5 subunit-containing GABAA receptors affect the dynamic range of mouse hippocampal kainate-induced gamma frequency oscillations in vitro. J Physiol. 2004;559:721–728. doi: 10.1113/jphysiol.2004.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Caballero M, Martin LJ, Salter MW, Orser BA, Paulsen O. α5 Subunit-containing GABAA receptors mediate a slowly decaying inhibitory synaptic current in CA1 pyramidal neurons following Schaffer collateral activation. Neuropharmacology. 2010;58:668–675. doi: 10.1016/j.neuropharm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. The role of synaptic ion channels in synaptic plasticity. EMBO Rep. 2006;7:1104–1110. doi: 10.1038/sj.embor.7400830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Thompson SM. Maladaptive homeostatic plasticity in a rodent model of central pain syndrome: thalamic hyperexcitability after spinothalamic tract lesions. J Neurosci. 2008;28:11959–11969. doi: 10.1523/JNEUROSCI.3296-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Wigström H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- Wisden W, Cope D, Klausberger T, Hauer B, Sinkkonen ST, Tretter V, Lujan R, Jones A, Korpi ER, Mody I, Sieghart W, Somogyi P. Ectopic expression of the GABAA receptor α6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology. 2002;43:530–549. doi: 10.1016/s0028-3908(02)00151-x. [DOI] [PubMed] [Google Scholar]
- Yee BK, Hauser J, Dolgov VV, Keist R, Möhler H, Rudolph U, Feldon J. GABA receptors containing the α5 subunit mediate the trace effect in aversive and appetitive conditioning and extinction of conditioned fear. Eur J Neurosci. 2004;20:1928–1936. doi: 10.1111/j.1460-9568.2004.03642.x. [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- Yoshiike Y, Kimura T, Yamashita S, Furudate H, Mizoroki T, Murayama M, Takashima A. GABAA receptor-mediated acceleration of aging-associated memory decline in APP/PS1 mice and its pharmacological treatment by picrotoxin. PLoS ONE. 2008;3:e3029. doi: 10.1371/journal.pone.0003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor α5 subunits contribute to GABAA, slow synaptic inhibition in mouse hippocampus. J Neurophysiol. 2009;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Bakhsha A, Rostami P, Shafaghi B. Effects of intrahippocampal injection of GABAergic drugs on memory retention of passive avoidance learning in rats. J Psychopharmacol. 2002;16:313–319. doi: 10.1177/026988110201600405. [DOI] [PubMed] [Google Scholar]