ABSTRACT
The Cre/loxP system has been used extensively in mouse models with a limitation of one lineage at a time. Differences in function and other properties among populations of adult β-cells is termed β-cell heterogeneity, which was recently associated with diabetic phenotypes. Nevertheless, the presence of a developmentally derived β-cell heterogeneity is unclear. Here, we have developed a novel dual lineage-tracing technology, using a combination of two recombinase systems, Dre/RoxP and Cre/LoxP, to independently trace green fluorescent Pdx1-lineage cells and red fluorescent Ptf1a-lineage cells in the developing and adult mouse pancreas. We detected a few Pdx1+/Ptf1a− lineage cells in addition to the vast majority of Pdx1+/Ptf1a+ lineage cells in the pancreas. Moreover, Pdx1+/Ptf1a+ lineage β-cells had fewer Ki-67+ proliferating β-cells, and expressed higher mRNA levels of insulin, Glut2, Pdx1, MafA and Nkx6.1, but lower CCND1 and CDK4 levels, compared with Pdx1+/Ptf1a− lineage β-cells. Furthermore, more TSQ-high, SSC-high cells were detected in the Pdx1+Ptf1a+ lineage population than in the Pdx1+Ptf1a− lineage population. Together, these data suggest that differential activation of Ptf1a in the developing pancreas may correlate with this β-cell heterogeneity.
KEY WORDS: Dual lineage tracing, Cre/LoxP, Dre/RoxP, β-Cell heterogeneity, Pdx1, Ptf1a
Summary: Using a novel dual lineage-tracing technology (Dre/RoxP and Cre/LoxP), differential activation of Ptf1a in the developing pancreas is revealed to potentially correlate with β-cell heterogeneity.
INTRODUCTION
Diabetes is characterized by inadequate functional pancreatic β-cells, which are required for the maintenance of normal blood-glucose levels (Ackermann and Gannon, 2007). Although β-cells are typically regarded as a single homogeneous population, β-cell heterogeneity was identified as early as 50 years ago (Kiekens et al., 1992; Salomon and Meda, 1986; Van Schravendijk et al., 1992). β-Cell heterogeneity may affect the development of diabetes, as well as the outcome of different treatments (Pipeleers, 1992). β-Cell heterogeneity has been suggested to arise during pancreatic development, to stem from differences or changes in islet architecture, or to result from β-cell replication or dedifferentiation (Roscioni et al., 2016). Very recently, several new studies have made important advances in our understanding of β-cell heterogeneity (Pipeleers et al., 2017), by identifying new markers [e.g. Flattop (Bader et al., 2016); CD9 and ST8SIA1 (Dorrell et al., 2016)] for a small subpopulation of β-cells that are more proliferative. However, a developmental origin for β-cell heterogeneity has not been identified.
The morphogenesis and development of the pancreas require well-coordinated expression of a number of key transcription factors (Cleaver and Melton, 2003; Gittes, 2009; Murtaugh and Melton, 2003). Among these factors, pancreatic and duodenal homeobox factor 1 (Pdx1) (Gao et al., 2014; Jonsson et al., 1994; Kawaguchi et al., 2002; Kushner et al., 2002; Offield et al., 1996; Yang et al., 2011) and pancreas specific transcription factor 1a (Ptf1a/p48) (Afelik et al., 2006; Hoang et al., 2016; Kawaguchi et al., 2002; Krapp et al., 1998; Wiebe et al., 2007) play crucial roles in very early stages of pancreatic cell fate determination. Their co-expression in multipotent progenitor cells is necessary for normal development and proper function of exocrine and endocrine pancreatic cells (Burlison et al., 2008). However, little is known about how Pdx1 and Ptf1a may influence each other to make fate decisions that regulate the segregation of the multipotent progenitor cells into specific pancreatic lineages. Specifically, the relationship between Pdx1 and Ptf1a pancreatic lineages has been difficult to study because of the need for two separate lineage-tagging systems.
Site-specific recombinases (SSRs) have been widely used in DNA and genome engineering (Nagy et al., 2009). Cre recombinase from the coliphage P1 and FLP are the most commonly used SSRs. They function through a nucleophilic attack on the DNA phosphodiester backbone via a tyrosine hydroxyl group to produce a covalent protein-DNA intermediate complex during recombination between target sites (termed LoxP and FRT, respectively) (Nagy et al., 2009). The conditional Cre/LoxP system, which enables tissue-specific or cell-specific manipulation of gene expression, has been applied in numerous useful models (Magnuson and Osipovich, 2013). However, using the Cre/LoxP system to conditionally manipulate gene expression or track cells is limited to one lineage at a time, and the FRT system is a relatively weak system, which prevents its widespread application. Interestingly, another SSR called ‘Dre’ specifically recognizes a RoxP site that is distinct from the LoxP site for Cre (Sauer and McDermott, 2004). Importantly, Dre does not crossreact with the Cre/LoxP system, but has similar recombination efficiency (Sauer and McDermott, 2004). The Dre/RoxP system has been previously tested in some settings (Chuang et al., 2015), but not yet widely used in gene targeting studies to generate lineage manipulation in animals.
Here, we have developed a novel dual lineage tracing technology, using a combination of the Dre/RoxP system and the Cre/LoxP system to independently trace GFP1-expressing green fluorescent Pdx1-lineage cells and Tomato red fluorescent Ptf1a-lineage cells in the developing and adult mouse pancreas. We assessed the Pdx1+ and Ptf1a+ lineage pancreatic cells in dual-gene lineage-labelled mice and found the presence of two major subpopulations of β-cells: a Pdx1+Ptf1a+ lineage and a Pdx1+Ptf1a− lineage. The two subpopulations of β-cells were further characterized. The current study not only confirms the presence of β-cell heterogeneity in the adult mouse pancreas, but also indicates that differential activation of Ptf1a in the developing pancreas may contribute to this β-cell heterogeneity.
RESULTS
Generation of Pdx1-Dre knock-in mice and Dre-reporter mice
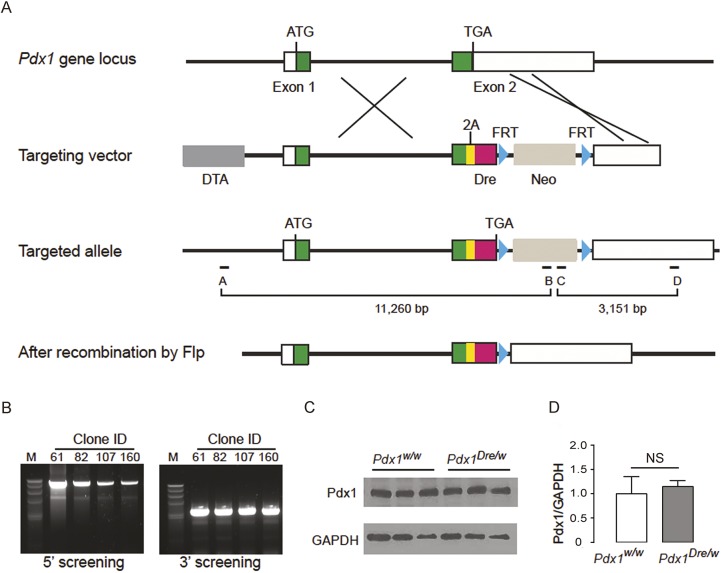
First, we generated Pdx1-Dre knock-in mice. To avoid Pdx1 haploinsufficiency and ensure maintenance of endogenous Pdx1 expression in this Pdx1-Dre knock-in strain, we used the viral 2A peptide for a bicistronic expression of Dre from the endogenous Pdx1 locus (Fig. 1A). Positive clones were obtained by PCR screening of targeted ES cells (Fig. 1B). One positive clone was used to produce Pdx1-Dre knock-in mice. Pdx1 expression in isolated islets was assessed by western blotting. We found that the islet Pdx1 levels were unaltered in Pdx1-Dre heterozygous mice, compared with wild-type mice, shown by representative blots (Fig. 1C) and by quantification (Fig. 1D). Moreover, we detected only one band for islet Pdx1 in Pdx1-Dre heterozygous mice, as in control wild-type mice, suggesting that the 2A peptide functions as expected to produce separate Pdx1 and Dre proteins.
Fig. 1.
Generation of Pdx1-Dre knock-in mice. (A) Diagrams showing the targeting strategy for Dre knock-in into the Pdx1 locus. Green and white boxes represent Pdx1-coding regions and UTRs, respectively. PCR primer binding sites (labeled A-D) are indicated by black dashes under the targeted allele. (B) PCR screening of targeted ES cells using the primers A-D shown in A. Four positive clones are shown (M, λ/Hind III DNA size marker). (C,D) Western blotting for Pdx1 in isolated islets from wild-type (Pdx1w/w) and Pdx1-Dre heterozygous (Pdx1Dre/w) mice, shown by representative blots (C) and by quantification (normalized to GAPDH) (D). NS, non-significant.
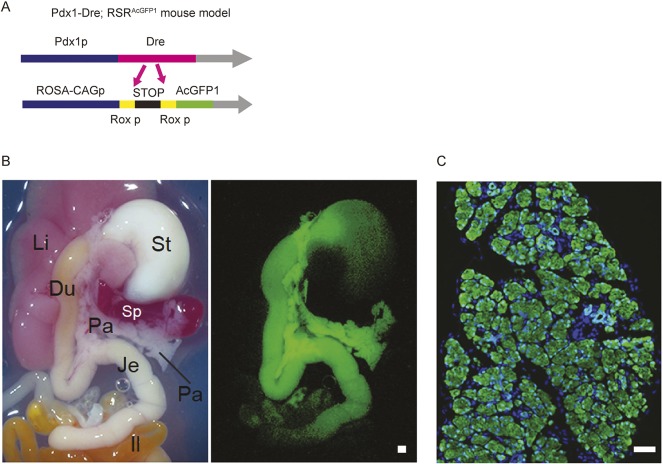
Next, we generated a Dre reporter mouse line (RSRAcGFP1), in which a Rosa26-CAG-RSR-AcGFP1 construct was electroporated into ES cells for gene targeting to the Rosa26 locus. The male Pdx1-Dre knock-in mice were then bred with the female AcGFP1-Dre-reporter mice, to avoid maternal effects on the expression of the Dre recombinase (Fig. 2A). The embryonic development of Pdx1-Dre/RSRAcGFP1 mice was monitored, showing AcGFP1 expression exclusively in Pdx1-positive distribution, similar to previously published Pdx1-Cre/reporter mice (Fig. 2B). Moreover, immunohistochemistry for GFP confirms that nearly all pancreatic cells, namely exocrine cells, endocrine, including α cells, and duct cells, were positively lineage tagged, whereas stromal cells, such as endothelium and smooth muscle cells, were not (Fig. 2C). These results suggest that Dre expression in the Pdx1-Dre mice is specific to the Pdx1-positive cell lineage, and that the expressed Dre recombines the RoxP sites efficiently.
Fig. 2.
Generation of Pdx1-Dre/RSRAcGFP1 mice. (A) Schematic representation of a Dre reporter mouse line (RSRAcGFP1). AcGFP1 is expressed under the control of the CAG promoter after Dre deletes the Rox-flanked stop cassette. (B) Bright-field image (left) and AcGFP1 fluorescent image (right) of Pdx1-Dre/RSRAcGFP1 double-mutant mice at postnatal day 5. AcGFP1 expression is seen in pancreas, antrum of stomach, duodenum and jejunum. Du, duodenum; Il, ileum; Je, jejunum; Li, liver; Pa, pancreas; Sp, spleen; St, stomach. (C) Histological image of Pdx1-Dre/RSRAcGFP1 double mutant mouse pancreas. The section was stained using anti-GFP antibody (green) and a nuclear marker HO (blue). Scale bar: 200 μm.
Generation of the Pdx1− and Ptf1a− dual lineage-tracing mouse model
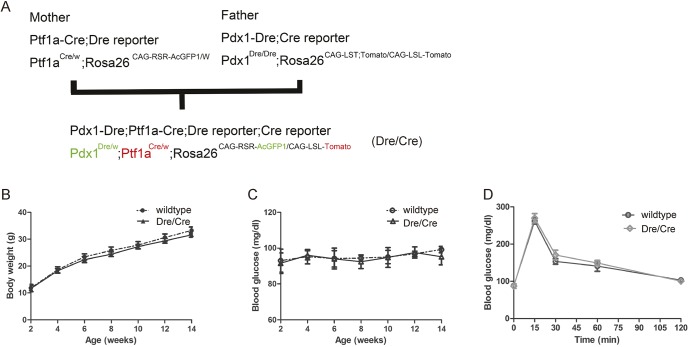
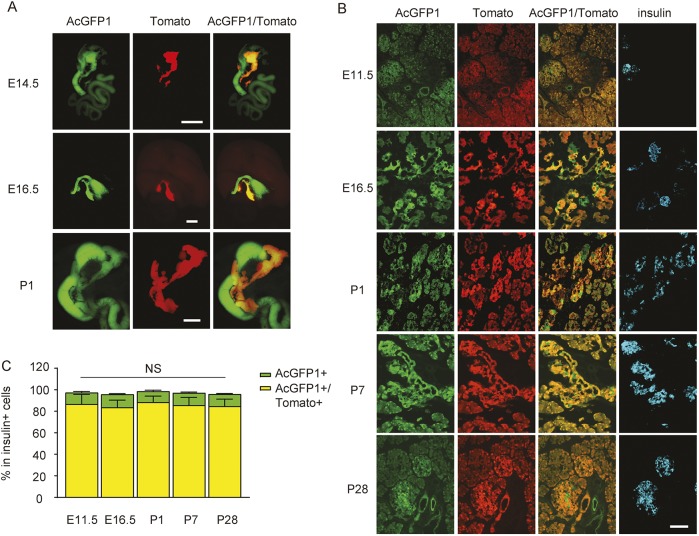
In order to study the differential effects of Pdx1 and Ptf1a activation on pancreatic cell fate, we generated Pdx1Dre/w; Ptf1aCre/w; Rosa26CAG-RSR-AcGFP1; Rosa26CAG-LSL-Tomato (overall mouse simply called Dre/Cre) mice (Fig. 3A). In these mice, all Pdx1 lineage cells are green fluorescent, whereas all Ptf1a lineage cells are red fluorescent. Dre/Cre mice appeared healthy after birth and maintained unaltered body weight gain (Fig. 3B), fasting blood glucose (Fig. 3C) and glucose tolerance (Fig. 3D), compared with wild-type mice. At E14.5, E16.5 or P1, green fluorescence was detected in the mouse pancreas, gastric antrum and duodenum, while Tomato fluorescence was detected only in the pancreas (Fig. 4A), consistent with the expression pattern of Pdx1 and Ptf1a in previous studies (Afelik et al., 2006; Gao et al., 2014; Hoang et al., 2016; Kawaguchi et al., 2002; Kushner et al., 2002; Wiebe et al., 2007; Yang et al., 2011). Immunostaining for insulin, amylase or DBA on the pancreas sections was performed, showing that the majority of pancreatic cells were positive for both green and red fluorescence, and suggesting that they were derived from progenitor cells that had activated both Pdx1 and Ptf1a (Fig. 4B-C, Fig. S1). Of note, we also examined green versus red fluorescent insulin-producing cells at E11.5, and we did not find higher numbers of green fluorescent insulin-positive cells, suggesting that this population is not likely to be derived from ‘early born’ endocrine cells. Although no red fluorescent-only cells were detected in the pancreas, a few islet cells (Fig. 4B,C) and duct cells, but not acinar cells (Fig. S1), were found to be green fluorescent only, suggesting that some pancreatic cells were derived from progenitor cells that activated only Pdx1, not Ptf1a.
Fig. 3.
Generation of Pdx1 and Ptf1a dual lineage-tracing mouse model. (A) Experimental schematic of breeding strategy. (B) Body weight. (C) Fasting blood glucose levels. (D) Glucose levels after an intraperitoneal glucose tolerance test at 14 weeks of age. n=5.
Fig. 4.
Fluorescence imaging of Pdx1 and Ptf1a dual lineage-tracing mice. (A) Fluorescence was assessed at E14.5, E16.5 or P1 in gross mouse viscera from Pdx1 and Ptf1a dual lineage-tracing mice. (B,C) Pancreas sections were immunostained for insulin and assessed for direct green/red fluorescence at E11.5, E16.5, P1, P7 and P28, shown by representative images (B), and by quantification of green-only and green/red double-positive cells (C). NS, not significant. Scale bar: 1 mm in A; 50 μm in B.
Purification of Pdx1+ Ptf1a+ lineage versus Pdx1+ Ptf1a− lineage islet cells
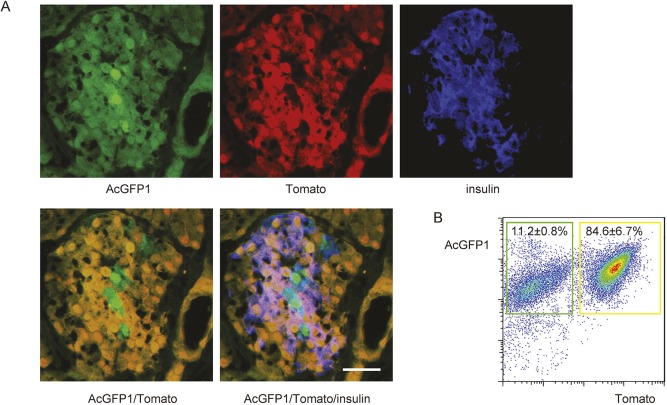
In mouse islets at 28 days of age, both green-only and yellow (red and green) β-cells were observed (Fig. 5A). To investigate a possible developmental origin of adult β-cell heterogeneity, we compared Pdx1+Ptf1a+ lineage versus Pdx1+Ptf1a− lineage β-cells. Mouse islets from Dre/Cre mice at 28 days of age were isolated and dissociated into single cells, after which the single-cell fraction of islets cells was sorted by flow cytometry based on fluorescence (Fig. 5B). The green islet cells represent Pdx1+Ptf1a− lineage islet cells (11.2±0.8% of total islet cells), while the yellow islet cells represent Pdx1+Ptf1a+ lineage islet cells (84.6±6.7% of total islet cells). These two subpopulations of islet cells, the majority of which are β-cells, were subjected to further characterization.
Fig. 5.
Purification of Pdx1+Ptf1a+ lineage versus Pdx1+Ptf1a− lineage islet cells. (A) A representative immunohistochemistry image showing a mouse islet at 28 days of age. Insulin was immunostained, shown as blue fluorescence. Green-only and yellow fluorescence represent Pdx1+Ptf1a− lineage cells versus Pdx1+Ptf1a+ lineage cells, respectively. (B) Mouse islets from the Pdx1 and Ptf1a dual lineage-tracing mice (Dre/Cre mice) at 28 days of age were isolated, dissociated into single cells and sorted based on green or yellow fluorescence by flow cytometry. Scale bar: 20 μm.
Differential expression of Ptf1a may contribute to the developmental origin of β-cell heterogeneity
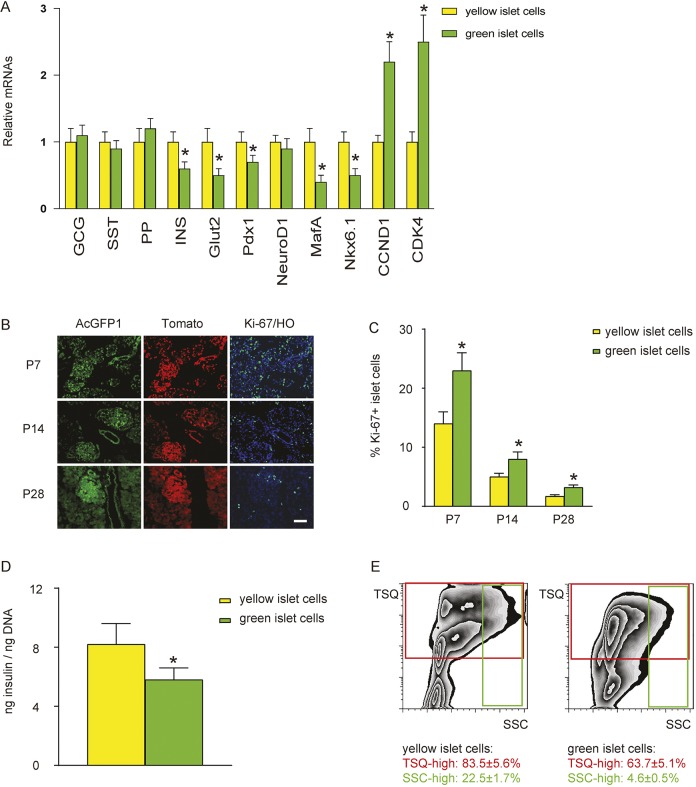
Gene profiling was performed on the sorted Pdx1+Ptf1a− lineage islet cells compared with the Pdx1+Ptf1a+ lineage islet cells. We detected no difference in levels of glucagon (GCG), somatostatin (SST) or pancreatic polypeptide (PP) mRNAs between the two populations (Fig. 6A). Moreover, there was no difference in the percentage of β-cells, α-cells, δ-cells or PP-cells in the Pdx1+/Ptf1a+ (yellow) and Pdx1+/Ptf1a− (green) lineages (Fig. S2). Thus, the differences seen in the expression of β-cell-specific genes (Fig. 6A) should predominantly result from changes in mRNA levels in β-cells, rather than from changes in the percentage of β-cells in the islets. We found that the Pdx1+/Ptf1a+ lineage islet cells expressed higher levels of mRNAs for insulin, Glut2, Pdx1, MafA and Nkx6.1, but lower for CCND1 and CDK4, compared with Pdx1+/Ptf1a− islet cells (Fig. 6A). A similar mRNA expression pattern was detected in mouse pancreas at a time point as early as E17/P1 (Fig. S3). For quantification of β-cell proliferation, we examined Ki-67 in islet cells. As AcGFP1, Tomato, Ki-67 and HO already take four channels, the insulin staining was carried out on consecutive slides to help determine the insulin-positive cells. As it is possible to use the consecutive slide to determine the islet area, and thus the islet cells, but is difficult to determine exactly the individual β-cell, we assessed Ki-67+ islet cells using this method. Fewer Ki-67+ proliferating islet cells (Fig. 6B,C), and a higher insulin protein content per cell (Fig. 6D), were detected in Pdx1+Ptf1a+ lineage islet cells compared with Pdx1+Ptf1a− lineage islet cells. Mature β-cells are known to be highly granulated, showing high sideward scatter (SSC high) and heavy staining with 6-methoxy-8-p-toluenesulphonamido-quinoline (TSQ), a zinc-chelating fluorophore (Xu et al., 2008). We found that Pdx1+Ptf1a+ lineage islet cells contained more TSQ-high and SSC-high cells compared with Pdx1+Ptf1a− lineage islet cells (Fig. 6E). Together, these data not only confirm the presence of β-cell heterogeneity in the adult mouse pancreas, but also indicate that differential cellular activation of Ptf1a in the developing pancreas may contribute to this β-cell heterogeneity.
Fig. 6.
Differential expression of Ptf1a may contribute to a developmental origin of β-cell heterogeneity. (A) RT-qPCR for glucagon (GCG), somatostatin (SST), pancreatic polypeptide (PP), insulin (INS), Glut2, Pdx1, MafA, Nkx6.1, CCND1 and CDK4 in sorted Pdx1+Ptf1a− lineage islet cells (green-only) compared with Pdx1+Ptf1a+ lineage islet cells (yellow). (B) Ki-67 staining was carried out. (C) Percentage of Ki-67+ islet cells at P7, P14 and P28 in Pdx1+Ptf1a− lineage islet cells versus Pdx1+Ptf1a+ lineage islet cells. (D) Insulin protein content per cell was determined by ELISA, normalized to genomic DNA content. (E) TSQ and SSC analysis on sorted Pdx1+Ptf1a− lineage islet cells versus Pdx1+Ptf1a+ lineage islet cells. n=5. *P<0.05. Scale bar: 50 μm.
DISCUSSION
Expression of a set of key transcriptional regulators is tightly controlled to guarantee the proper development of multiple tissue and organs during embryogenesis. In the fate determination and organogenesis of the mouse pancreas, Pdx1 (Gao et al., 2014; Kawaguchi et al., 2002; Kushner et al., 2002; Yang et al., 2011) and Ptf1a (Afelik et al., 2006; Hoang et al., 2016; Kawaguchi et al., 2002; Wiebe et al., 2007) appear to play pivotal roles. Previous approaches have focused on the coordination and interactions between Pdx1 and Ptf1a during pancreas development, showing that a pool of multipotent progenitor cells that co-express Pdx1 and Ptf1a contribute to the majority of pancreatic cells (Burlison et al., 2008). In that study, Magnuson et al. elegantly showed that Pdx1 is required prior to Ptf1a for the specification of pancreatic multipotent progenitor cells, whereas Ptf1a is not required for endocrine cell specification or β-cell maturation (Burlison et al., 2008). However, the technology used in this study, as well as in other previous studies, does not allow analysis of the contribution of a small subpopulation of progenitor cells, especially when this contribution itself is also small. A dual lineage-tracing system would seem to be required for this purpose.
Very recently, β-cell heterogeneity has been re-investigated. Minor subpopulations of β-cells have been identified in the adult islet that may become important during β-cell loss or in the development of diabetes. Certain cells seem to be more able to proliferate and self-renew (Bader et al., 2016; Dorrell et al., 2016). Of note, this latter subpopulation of β-cells may be associated with β-cell dedifferentiation (Efrat, 2016; Talchai et al., 2012; Wang et al., 2014; Weinberg et al., 2007; Weir et al., 2013), which may be protective in an inflamed milieu (Efrat, 2016; Talchai et al., 2012; Wang et al., 2014; Weinberg et al., 2007; Weir et al., 2013), and also may be necessary for them to enter an active cell cycle to proliferate (El-Gohary et al., 2014; Gunasekaran et al., 2012; Shirakawa and Kulkarni, 2016; Song et al., 2016; Xiao et al., 2016).
Here, we have used a novel Dre/RoxP system in combination with a Cre/LoxP system to create a dual lineage-tracing system for both Pdx1 lineage and Ptf1a lineage cells in the same animal. To avoid leaky expression of the fluorescent protein, we bred Dre/LoxP reporter mice with Cre/RoxP reporter mice. The fluorescence expression pattern in the chimeric mice demonstrated a fairly high labeling efficiency for the Dre system. Although the potential differences in the recombination efficiency between the Cre and Dre system here may affect the interpretation of the data, we expect that this effect should be minimal, for several reasons. First, according to previous reports, the Dre recombinase is a highly efficient site-specific recombinase like Cre recombinase (Anastassiadis et al., 2009) and no important association of biological function or identity with absence of PTF1a-cre lineage-tagging has been reported (Burlison et al., 2008; Fukuda et al., 2008; Kawaguchi et al., 2002; Willet et al., 2014). Second, here, nearly all pancreatic cells appeared to be of the Pdx1 lineage, which suggests high recombination by Dre/Roxp-AcGFP1. Third, the recombination efficiency in Pdx1 and Ptf1a lineages should be primarily determined by the insert size between the two LoxPs or between the two RoxPs. As the insert sizes are very similar, Tomato and GFP reporter gene expression should correlate with Pdx1 and Ptf1a expression levels. Finally, here, we found a significant phenotype in the green-only cells, which could not be explained by the random lack of tagging of some endocrine cells in the pancreas.
In addition to the majority of the pancreatic cells, which were positive for both Pdx1+ and Ptf1a+ lineages, we also detected a smaller population of islet cells that were a Pdx1+Ptf1a− lineage. As Pdx1 is required for activation of Ptf1a (Burlison et al., 2008), it is possible that these cells may be derived from Pdx1-low expressing cells, in which Ptf1a is not successfully activated. Interestingly, a small population of Pdx1+Ptf1a− lineage cells was detected in the islets and in the ducts, but not among acinar cells, which suggests that some duct-originated progenitor cells may activate Pdx1, but not activate (or minimally) Ptf1a. Of note, these Pdx1+Ptf1a− lineage β-cells appeared to be less mature than Pdx1+Ptf1a+ lineage β-cells, as they expressed lower levels of mRNA for insulin, Glut2, Pdx1, MafA and Nkx6.1. As these cells also express higher mRNA levels of CCND1 and CDK4, two crucial cell-cycle regulators for the G0/G1 transition, these Pdx1+Ptf1a− lineage β-cells appear to be more proliferative, as confirmed by Ki-67-labeling. Although this subpopulation of β-cells persists in the adult mouse pancreas, the fraction of the total β-cells did not seem to increase with age, possibly owing to their higher susceptibility to apoptosis or senescence. However, as β-cell heterogeneity is now thought to play a more important role in pathological states, e.g. during β-cell loss in diabetes, it may be interesting in the future to assess the relative contribution of this smaller subpopulation of β-cells in diabetes or in other conditions involving postnatal β-cell growth, e.g. in pregnant mice.
To summarize, in the current study, we present a novel dual lineage-tracing/gene manipulation system, which could be widely used in medical research. Specifically, here we have used this system to provide evidence for a possible developmental origin of β-cell heterogeneity, which merits further investigation.
MATERIALS AND METHODS
Gene targeting
A gene targeting vector for bicistronic expression of Pdx1 and Dre from the Pdx1 gene locus in mice was constructed by a galK-based BAC recombineering technique (Warming et al., 2005) (Fig. 1). Briefly, exon 2 of the Pdx1 gene in a BAC clone RP23-59N19 (Empire Genomics) was replaced with a modified exon 2 in which a 2A peptide sequence followed by the Dre sequence was inserted immediately before the stop codon of the Pdx1-coding sequence. In this way, Pdx1 and Dre are translated as two individual proteins from a single mRNA. Afterwards, the FRT-flanked neomycin selection cassette (neo) was placed at the beginning of the 3′ untranslated sequence. The modified exon 2 was retrieved into the PGKdtabpA plasmid (a gift from Philippe Soriano, Addgene plasmid #13440) (Soriano, 1997) along with a 8.6 kb 5′ arm and a 2.4 kb 3′ arm to generate the Pdx1-Dre targeting vector. Gene targeting in G4 ES cells (George et al., 2007) and blastocyst injection of ES cells were performed in the Transgenic and Molecular Core at Magee-Women Research Institute in Pittsburgh, PA, USA. Screening of targeted ES cells was performed by long-range PCR using the LA PCR kit (Takara Bio), in which 33 positive clones out of 240 neomycin-resistant clones were obtained. One of the clones was used to produce chimeric mice, which were then bred to Rosa26Flpo mice (The Jackson Laboratory) to remove the neo cassette.
To generate a Dre reporter mouse line, LoxP and ZsGreen DNA sequences in the Ai6 plasmid (a gift from Hongkui Zeng, Addgene plasmid #22498) (Madisen et al., 2010), which is Rosa26 knock-in targeting vector for ZsGreen Cre reporter, were replaced with Rox and AcGFP1 sequences, respectively. The resulting construct, Rosa26-CAG-Froxed-AcGFP1, was electroporated into G4 ES cells for gene targeting to the Rosa26 locus. Twenty targeted clones were obtained from 24 neomycin-resistant clones by PCR analysis. Morula aggregation was performed in-house to produce chimeric mice.
Mouse strains and husbandry
All mouse experiments were approved by the Animal Research and Care Committee at the Children's Hospital of Pittsburgh and the University of Pittsburgh IACUC, and were carried out in accordance with the approved guidelines. Pdx1/Ptf1a dual-lineage reporter animals were obtained by crossing Pdx1-Dre; Rosa26CAG-LSL-Tomato/CAG-LSL-Tomato mice with Ptf1a-Cre; Rosa26CAG-RSR-AcGFP1/w mice. Bacterial artificial-chromosome (BAC) transgenic Ptf1a promoter Cre reporter (Ptf1a-Cre) mice have a C57BL/6 background, as has been described before (Kawaguchi et al., 2002; Xiao et al., 2017a,c). C57BL/6 mice were purchased from Jackson Lab. Dual transgenic offspring (Pdx1Dre/w; Ptf1aCre/w; Rosa26CAG-RSR-AcGFP1/CAG-LSL-Tomato) were then identified by polymerase chain reaction of genomic DNA following a tail biopsy using the following primers: AcGFP1 sequence, 5′-TGA AGT TCA TCT GCA CCA CCG and 5′-GGC GGA TCT TGA AGT TCA CCT; Tomato sequence, 5′-ACC GTG ACC CAG GAC TCCT and 5′- ATG CCC CAG GAA CAG GTG GT; Cre sequence, 5′-ACC TGA AGA TGT TCG CGA TTA TCT and 5′-ACC GTC AGT ACG TGA GAT ATC TT; and Dre sequence, 5′-CAA CTC TAG GCT GAT GGA CTC C and 5′-GAT TGG AAG CGC TCT CTT TG. Body weight measurement, fasting blood glucose and intraperitoneal glucose tolerance tests (IPGTT) were performed as previously described (Xiao et al., 2013a,b, 2016, 2017b, 2014, 2018).
Pancreatic digestion and FACS
Pancreatic ductal perfusion with digestive enzyme, islet isolation and islet dissociation were performed as described previously (Xiao et al., 2013a,b, 2016, 2017b, 2014, 2018). Purification of Pdx1+Ptf1a+ lineage islet cells versus Pdx1+Ptf1a− lineage islet cells was based on yellow versus green fluorescence by fluorescence-activated cell sorting (FACS) using a FACSAria (Becton-Dickinson Biosciences), as has been described previously (Xiao et al., 2013a). TSQ analysis was carried out as previously described (Jindal et al., 1993; Martens et al., 2018; Xu et al., 2008). Briefly, cell debris was gated out based on forward scatter (FCS) and a cytometric selecting window for TSQ positivity was set up to allow more than 95% of living cells to be readily selected. For specific gating parameters, operator-dependent thresholds were applied, based on baseline use of single high/low TSQ cells for sorting separately by the gating parameters that best distinguished TSQ-high from TSQ-low populations. Flow cytometry data were analyzed and presented using FlowJo software (version 10).
RNA extraction and RT-qPCR
RNA extraction and RT-qPCR have been described previously (Xiao et al., 2013a,b, 2016, 2017b, 2014, 2018). Primers were all purchased from Qiagen (Hilden, Germany). They are GAPDH (QT01658692), Glut2 (QT00116186), CCND1 (QT00154595), CDK4 (QT00170618), Pdx1 (QT00102235), NeuroD1 (QT00251265), MafA (QT01037638), Nkx6.1 (QT00143318), insulin (INS; QT00114289), glucagon (GCG; QT00124033), somatostatin (SST; QT01046528) and pancreatic polypeptide (PP; QT00103999). RT-qPCR values were normalized against GAPDH, which was proven to be stable across the samples.
Immunohistochemistry and western blotting
Immunohistochemistry was performed as described previously (Xiao et al., 2013a,b, 2016, 2017b, 2014, 2018). AcGFP1 and Tomato were detected by direct fluorescence. Nuclear staining was performed with Hoechst solution (HO; Sigma-Aldrich). Quantification of Ki-67+ insulin+ cells was performed as previously described (Xiao et al., 2014, 2013c). Western blotting was carried out as previously described (Song et al., 2016; Xiao et al., 2016, 2017b, 2018). Primary antibodies used were: guinea pig polyclonal anti-insulin (immunohistochemistry, 1:500, A0564, Dako), rat anti-Ki-67 (immunohistochemistry, 1:300, 14-5698-82, lot number 2002315, Invitrogen), rabbit anti-amylase (immunohistochemistry, 1:100, A8273-1VL, lot number 017M4793v, Sigma-Aldrich), biotin-DBA (immunohistochemistry, 1:200, B-1035, lot number ZA0417, Vector Labs), goat anti-Pdx1 (western blotting, 1:1000, Ab-47383, lot number GR201765-1, Abcam) and anti-GAPDH (western blotting, 1:1000, CS-2118, lot number 10, Cell Signaling). Secondary antibodies used were Cy5-, AMCA- or streptoavidin-conjugated goat-, rabbit- or guinea pig-specific antibodies (Jackson ImmunoResearch Labs).
ELISA for insulin
Measurement of cellular insulin levels was performed using a mouse insulin ELISA kit (ALPCO). The insulin level per cell was obtained by normalizing the insulin content to genomic DNA levels of the analyzed cells (Qiagen).
Data analysis
The data were statistically analyzed with GraphPad Prism (version 7). All values are given as mean±s.d. Five repeats were analyzed for each condition. All data were statistically analyzed by one-way ANOVA with a Bonferroni correction, followed by Fisher's exact test to compare two groups. Results were considered to be significant when P<0.05.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: X.X., G.K.G.; Methodology: C.S., X.X.; Validation: C.S.; Formal analysis: C.C., C.S.; Investigation: C.C., C.S., G.A., D.R., Y.J., J.M., K.P., X.X.; Data curation: C.S.; Writing - original draft: X.X.; Writing - review & editing: X.X., G.K.G.; Supervision: X.X., G.K.G.; Funding acquisition: X.X., G.K.G.
Funding
This work was supported by an Assistant Professor Startup grant to X.X. from the Pediatric Division of the Children's Hospital of Pittsburgh, and by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK111460 and R01DK112836 to G.K.G.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.164913.supplemental
References
- Ackermann A. M. and Gannon M. (2007). Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 38, 193-206. 10.1677/JME-06-0053 [DOI] [PubMed] [Google Scholar]
- Afelik S., Chen Y. and Pieler T. (2006). Combined ectopic expression of Pdx1 and Ptf1a/p48 results in the stable conversion of posterior endoderm into endocrine and exocrine pancreatic tissue. Genes Dev. 20, 1441-1446. 10.1101/gad.378706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiadis K., Fu J., Patsch C., Hu S., Weidlich S., Duerschke K., Buchholz F., Edenhofer F. and Stewart A. F. (2009). Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis. Model. Mech. 2, 508-515. 10.1242/dmm.003087 [DOI] [PubMed] [Google Scholar]
- Bader E., Migliorini A., Gegg M., Moruzzi N., Gerdes J., Roscioni S. S., Bakhti M., Brandl E., Irmler M., Beckers J. et al. (2016). Identification of proliferative and mature beta-cells in the islets of Langerhans. Nature 535, 430-434. 10.1038/nature18624 [DOI] [PubMed] [Google Scholar]
- Burlison J. S., Long Q., Fujitani Y., Wright C. V. E. and Magnuson M. A. (2008). Pdx-1 and Ptf1a concurrently determine fate specification of pancreatic multipotent progenitor cells. Dev. Biol. 316, 74-86. 10.1016/j.ydbio.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang K., Nguyen E., Sergeev Y. and Badea T. C. (2015). Novel heterotypic Rox sites for combinatorial dre recombination strategies. G3 (Bethesda) 6, 559-571. 10.1534/g3.115.025841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O. and Melton D. A. (2003). Endothelial signaling during development. Nat. Med. 9, 661-668. 10.1038/nm0603-661 [DOI] [PubMed] [Google Scholar]
- Dorrell C., Schug J., Canaday P. S., Russ H. A., Tarlow B. D., Grompe M. T., Horton T., Hebrok M., Streeter P. R., Kaestner K. H. et al. (2016). Human islets contain four distinct subtypes of beta cells. Nat. Commun. 7, 11756 10.1038/ncomms11756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S. (2016). Mechanisms of adult human beta-cell in vitro dedifferentiation and redifferentiation. Diabetes Obes. Metab. 18 Suppl. 1, 97-101. 10.1111/dom.12724 [DOI] [PubMed] [Google Scholar]
- El-Gohary Y., Tulachan S., Wiersch J., Guo P., Welsh C., Prasadan K., Paredes J., Shiota C., Xiao X., Wada Y. et al. (2014). A smad signaling network regulates islet cell proliferation. Diabetes 63, 224-236. 10.2337/db13-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A., Kawaguchi Y., Furuyama K., Kodama S., Horiguchi M., Kuhara T., Kawaguchi M., Terao M., Doi R., Wright C. V. E. et al. (2008). Reduction of Ptf1a gene dosage causes pancreatic hypoplasia and diabetes in mice. Diabetes 57, 2421-2431. 10.2337/db07-1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., McKenna B., Li C., Reichert M., Nguyen J., Singh T., Yang C., Pannikar A., Doliba N., Zhang T. et al. (2014). Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab. 19, 259-271. 10.1016/j.cmet.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S. H., Gertsenstein M., Vintersten K., Korets-Smith E., Murphy J., Stevens M. E., Haigh J. J. and Nagy A. (2007). Developmental and adult phenotyping directly from mutant embryonic stem cells. Proc. Natl. Acad. Sci. USA 104, 4455-4460. 10.1073/pnas.0609277104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes G. K. (2009). Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 326, 4-35. 10.1016/j.ydbio.2008.10.024 [DOI] [PubMed] [Google Scholar]
- Gunasekaran U., Hudgens C. W., Wright B. T., Maulis M. F. and Gannon M. (2012). Differential regulation of embryonic and adult beta cell replication. Cell Cycle 11, 2431-2442. 10.4161/cc.20545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang C. Q., Hale M. A., Azevedo-Pouly A. C., Elsässer H. P., Deering T. G., Willet S. G., Pan F. C., Magnuson M. A., Wright C. V. E., Swift G. H. et al. (2016). Transcriptional maintenance of pancreatic acinar identity, differentiation, and homeostasis by PTF1A. Mol. Cell. Biol. 36, 3033-3047. 10.1128/MCB.00358-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal R. M., Gray D. W. and Morris P. J. (1993). The use of TSQ as an islet-specific stain for purification of islets by fluorescence-activated sorting. Transplantation 56, 1282-1284. 10.1097/00007890-199311000-00050 [DOI] [PubMed] [Google Scholar]
- Jonsson J., Carlsson L., Edlund T. and Edlund H. (1994). Insulin-promoter-factor 1 is required for pancreas development in mice. Nature 371, 606-609. 10.1038/371606a0 [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Cooper B., Gannon M., Ray M., MacDonald R. J. and Wright C. V. (2002). The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 32, 128-134. 10.1038/ng959 [DOI] [PubMed] [Google Scholar]
- Kiekens R., In ‘t Veld P., Mahler T., Schuit F., Van De Winkel M. and Pipeleers D. (1992). Differences in glucose recognition by individual rat pancreatic B cells are associated with intercellular differences in glucose-induced biosynthetic activity. J. Clin. Invest. 89, 117-125. 10.1172/JCI115551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A., Knofler M., Ledermann B., Burki K., Berney C., Zoerkler N., Hagenbuchle O. and Wellauer P. K. (1998). The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 12, 3752-3763. 10.1101/gad.12.23.3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner J. A., Ye J., Schubert M., Burks D. J., Dow M. A., Flint C. L., Dutta S., Wright C. V., Montminy M. R. and White M. F. (2002). Pdx1 restores beta cell function in Irs2 knockout mice. J. Clin. Invest. 109, 1193-1201. 10.1172/JCI0214439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R. et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133-140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson M. A. and Osipovich A. B. (2013). Pancreas-specific cre driver lines and considerations for their prudent use. Cell Metab. 18, 9-20. 10.1016/j.cmet.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens G. A., De Punt V. and Stange G. (2018). CD99 as surface anchor for human islet endocrine cell purification. J. Tissue Eng. Regen. Med. 12, e171-e176. 10.1002/term.2329 [DOI] [PubMed] [Google Scholar]
- Murtaugh L. C. and Melton D. A. (2003). Genes, signals, and lineages in pancreas development. Annu. Rev. Cell Dev. Biol. 19, 71-89. 10.1146/annurev.cellbio.19.111301.144752 [DOI] [PubMed] [Google Scholar]
- Nagy A., Mar L. and Watts G. (2009). Creation and use of a cre recombinase transgenic database. Methods Mol. Biol. 530, 365-378. 10.1007/978-1-59745-471-1_19 [DOI] [PubMed] [Google Scholar]
- Offield M. F., Jetton T. L., Labosky P. A., Ray M., Stein R. W., Magnuson M. A., Hogan B. L. and Wright C. V. (1996). PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development 122, 983-995. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G. (1992). Heterogeneity in pancreatic beta-cell population. Diabetes 41, 777-781. 10.2337/diab.41.7.777 [DOI] [PubMed] [Google Scholar]
- Pipeleers D., De Mesmaeker I., Robert T. and Van Hulle F. (2017). Heterogeneity in the beta-cell population: a guided search into its significance in pancreas and in implants. Curr. Diab Rep. 17, 86 10.1007/s11892-017-0925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscioni S. S., Migliorini A., Gegg M. and Lickert H. (2016). Impact of islet architecture on beta-cell heterogeneity, plasticity and function. Nat. Rev. Endocrinol. 12, 695-709. 10.1038/nrendo.2016.147 [DOI] [PubMed] [Google Scholar]
- Salomon D. and Meda P. (1986). Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp. Cell Res. 162, 507-520. 10.1016/0014-4827(86)90354-X [DOI] [PubMed] [Google Scholar]
- Sauer B. and McDermott J. (2004). DNA recombination with a heterospecific Cre homolog identified from comparison of the pac-c1 regions of P1-related phages. Nucleic Acids Res. 32, 6086-6095. 10.1093/nar/gkh941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa J. and Kulkarni R. N. (2016). Novel factors modulating human beta-cell proliferation. Diabetes Obes. Metab. 18, 71-77. 10.1111/dom.12731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Fusco J., Zimmerman R., Fischbach S., Chen C., Ricks D. M., Prasadan K., Shiota C., Xiao X. and Gittes G. K. (2016). Epidermal growth factor receptor signaling regulates beta cell proliferation in adult mice. J. Biol. Chem. 291, 22630-22637. 10.1074/jbc.M116.747840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. (1997). The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 124, 2691-2700. [DOI] [PubMed] [Google Scholar]
- Talchai C., Xuan S., Lin H. V., Sussel L. and Accili D. (2012). Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 150, 1223-1234. 10.1016/j.cell.2012.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schravendijk C. F., Kiekens R. and Pipeleers D. G. (1992). Pancreatic beta cell heterogeneity in glucose-induced insulin secretion. J. Biol. Chem. 267, 21344-21348. [PubMed] [Google Scholar]
- Wang Z., York N. W., Nichols C. G. and Remedi M. S. (2014). Pancreatic beta cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab. 19, 872-882. 10.1016/j.cmet.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming S., Costantino N., Court D. L., Jenkins N. A. and Copeland N. G. (2005). Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33, e36 10.1093/nar/gni035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg N., Ouziel-Yahalom L., Knoller S., Efrat S. and Dor Y. (2007). Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic beta-cells. Diabetes 56, 1299-1304. 10.2337/db06-1654 [DOI] [PubMed] [Google Scholar]
- Weir G. C., Aguayo-Mazzucato C. and Bonner-Weir S. (2013). beta-cell dedifferentiation in diabetes is important, but what is it? Islets 5, 233-237. 10.4161/isl.27494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe P. O., Kormish J. D., Roper V. T., Fujitani Y., Alston N. I., Zaret K. S., Wright C. V. E., Stein R. W. and Gannon M. (2007). Ptf1a binds to and activates area III, a highly conserved region of the Pdx1 promoter that mediates early pancreas-wide Pdx1 expression. Mol. Cell. Biol. 27, 4093-4104. 10.1128/MCB.01978-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willet S. G., Hale M. A., Grapin-Botton A., Magnuson M. A., MacDonald R. J. and Wright C. V. E. (2014). Dominant and context-specific control of endodermal organ allocation by Ptf1a. Development 141, 4385-4394. 10.1242/dev.114165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Chen Z., Shiota C., Prasadan K., Guo P., El-Gohary Y., Paredes J., Welsh C., Wiersch J. and Gittes G. K. (2013a). No evidence for beta cell neogenesis in murine adult pancreas. J. Clin. Invest. 123, 2207-2217. 10.1172/JCI66323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Guo P., Chen Z., El-Gohary Y., Wiersch J., Gaffar I., Prasadan K., Shiota C. and Gittes G. K. (2013b). Hypoglycemia reduces vascular endothelial growth factor a production by pancreatic Beta cells as a regulator of Beta cell mass. J. Biol. Chem. 288, 8636-8646. 10.1074/jbc.M112.422949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Wiersch J., El-Gohary Y., Guo P., Prasadan K., Paredes J., Welsh C., Shiota C. and Gittes G. K. (2013c). TGFbeta receptor signaling is essential for inflammation-induced but not beta-cell workload-induced beta-cell proliferation. Diabetes 62, 1217-1226. 10.2337/db12-1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Gaffar I., Guo P., Wiersch J., Fischbach S., Peirish L., Song Z., El-Gohary Y., Prasadan K., Shiota C. et al. (2014). M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc. Natl. Acad. Sci. USA 111, E1211-E1220. 10.1073/pnas.1321347111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Fischbach S., Song Z., Gaffar I., Zimmerman R., Wiersch J., Prasadan K., Shiota C., Guo P., Ramachandran S. et al. (2016). Transient suppression of TGFbeta receptor signaling facilitates human islet transplantation. Endocrinology 157, 1348-1356. 10.1210/en.2015-1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Chen C., Guo P., Zhang T., Fischbach S., Fusco J., Shiota C., Prasadan K., Dong H. and Gittes G. K. (2017a). Forkhead box protein 1 (FoxO1) inhibits accelerated beta cell aging in pancreas-specific SMAD7 mutant mice. J. Biol. Chem. 292, 3456-3465. 10.1074/jbc.M116.770032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Fischbach S., Zhang T., Chen C., Sheng Q., Zimmerman R., Patnaik S., Fusco J., Ming Y., Guo P. et al. (2017b). SMAD3/Stat3 signaling mediates beta-cell epithelial-mesenchymal transition in chronic pancreatitis-related diabetes. Diabetes 66, 2646-2658. 10.2337/db17-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Guo P., Shiota C., Zhang T., Coudriet G. M., Fischbach S., Prasadan K., Fusco J., Ramachandran S., Witkowski P. et al. (2018). Endogenous reprogramming of alpha cells into beta cells, induced by viral gene therapy, reverses autoimmune diabetes. Cell Stem Cell 22, 78-90.e74. 10.1016/j.stem.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., D'Hoker J., Stangé G., Bonné S., De Leu N., Xiao X., Van de Casteele M., Mellitzer G., Ling Z., Pipeleers D. et al. (2008). Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197-207. 10.1016/j.cell.2007.12.015 [DOI] [PubMed] [Google Scholar]
- Yang Y.-P., Thorel F., Boyer D. F., Herrera P. L. and Wright C. V. E. (2011). Context-specific alpha- to-beta-cell reprogramming by forced Pdx1 expression. Genes Dev. 25, 1680-1685. 10.1101/gad.16875711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.