Abstract
It is imperative that research interrogating the biological pathways linking stress processes to health continue to translate the results of basic, preclinical experimental research to diverse and under-represented populations, particularly those at elevated risk for morbidity and mortality. Conducting research within these populations and in community settings carries considerable benefit while at the same time involves a number of challenges that ultimately contribute to their rarity and uneven quality in the scientific literature. In this review, we summarize the experiences and insights of members of an expert panel on this topic held at the 2018 meeting of the International Society of Psychoneuroendocrinology in Newport Beach, CA. The goals of the session were to identify challenges and share strategies for testing plausible biopsychosocial models within diverse community samples in order to encourage others and improve future research. The present paper is organized into three themes: 1) Recruitment and retention, 2) Collecting biological samples outside of the laboratory, 3) Data analysis, interpretation, and dissemination. Our goal in composing this overview of the conference session was to share within the field of psychoneuroendocrinology the challenges inherent in translating basic research to community populations.
Keywords: translational science, diversity, health disparities, biological mechanisms, community research
1. Introduction
During the past 50 years, our collective understanding of the ways in which psychological and social factors influence health and disease has grown exponentially. For example, early adversity, sleep disturbances, and loneliness have all been linked to all-cause mortality (Cappuccio et al., 2010; Chen et al., 2016; Holt-Lunstad et al., 2015). Knowledge such as this has come from the parallel efforts of basic, translational, and clinical scientists conducting experimental, observational, and intervention studies in both animals and humans. If the goal of this work is to ultimately understand, prevent, and treat human disease more effectively, then our findings need to be both widely generalizable, and well-characterized in high-risk populations.
Translational science takes knowledge creation from “bench to bedside” by translating findings from non-human experiments to humans, and experimental findings from laboratories into clinical and community populations and settings (Rubio et al., 2010; Trochim et al., 2011; Waldman and Terzic, 2010). Translating well-characterized causal models related to health outcomes in biopsychosocial research requires us to establish generalizability of our knowledge across populations. Yet biopsychosocial research is most commonly conducted with convenience samples who represent a limited slice of the human experience (e.g., college-aged, predominantly non-Hispanic White, above average socioeconomic status, from industrialized countries) (George et al., 2013; Henrich et al., 2010; Oh et al., 2015). As a consequence, people of the global majority -- an inclusive term for groups historically referred to as racial and ethnic minorities (Baumgratz, 1995) -- and individuals experiencing economic and educational disadvantage have been traditionally under-represented in research (Yancey et al., 2006). This is an important omission in biopsychosocial health-related research given the disproportionately higher rates of chronic diseases and health disparities in traditionally under-represented populations (Adler and Rehkopf, 2008).
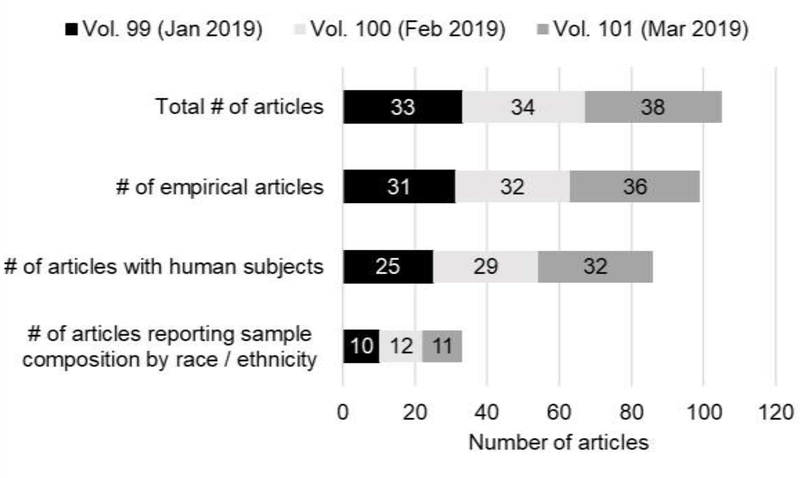
To address this limitation, the National Institutes of Health (NIH) have issued guidelines and policies aimed at increasing the recruitment and outreach of racial and ethnic minority communities in health research (“National Institutes of Health Revitalization Act of 1993,” 1993, “Notice of the ‘NIH Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research.,’” 1994; Pèrez-Stable and Collins, 2019). These guidelines require that the NIH provide support and resources to principal investigators to enhance the recruitment and retention of women and minorities in their research studies. Yet, significant deficiencies remain in enrolling racial and ethnic minorities in studies and subsequent reporting of that enrollment in publications. For example, there were 105 articles published in Psychoneuroendocrinology in the first three issues of the journal this year. Yet, only 38.4% of the empirical articles reporting data collected from human subjects reported their sample characteristics by race / ethnicity. Figure 1 illustrates the proportion of human subjects studies published in Psychoneuroendocrinology in January, February, and March of this year that have reported their sample composition by race / ethnicity. The failure to report these types of demographic details in publications is unfortunate because the work is actually quite diverse; these 86 publications using human subjects were conducted across Asia, Europe, North America, and South America and the publications that reported their sample’s ethnic composition ranged from 0 to 100% White. More broadly, it is estimated that 20 to 40% of published health research studies do not report sample sizes by race and ethnicity (Geller et al., 2011; Walsh and Ross, 2003), and up to 64% of clinical trials do not report intervention effects by race and ethnicity (Geller et al., 2011). Accordingly, when the ethnic and racial composition of samples is reported, significant disparities exist, particularly within clinical trials (Braunstein et al., 2008; Murthy et al., 2004; Stewart et al., 2007). Under-representation of individuals with elevated health risks may have major implications for our understanding of the links between stress and health. Indeed, in a systematic review of studies examining the link between childhood adversity and either HPA axis or inflammatory responses to acute stress (n = 27), only 14 (51.9%) reported the ethnic/racial composition of their sample (Zhuo et al., 2018). Importantly, studies that observed that childhood adversity was associated with lower or attenuated inflammatory responses had a smaller proportion of White participants, d=.71 (Nakamura, J. et al., 2018). Thus, disparities in reporting and enrolling participants from diverse backgrounds and from at-risk groups leave an inherent, field-wide limitation to our knowledge of underserved populations with the greatest need.
Figure 1.
Reporting of sample composition by race / ethnicity in recent issues of Psychoneuroendocrinology
Importantly, concerted efforts in the past 15 years have been made to encourage and aid researchers to use recruitment and retention strategies aimed at enhancing representativeness and inclusion in research study populations (Bonevski et al., 2014; Muñoz and Mendelson, 2005; Nicholson et al., 2015; Nueces et al., 2012; Yancey et al., 2006). All of this has bearing on the potential impact of psychoneuroendocrinology research which is uniquely positioned to address these important issues.
1.1. Scope of this review
The purpose of this review was to provide a summary of a panel discussion at the 48th annual meeting of the International Society for Psychoneuroendocrinology in Newport Beach, California. The panelists brought different perspectives from working for example, in Latin American communities in the U.S., studying ethnically and racially diverse, and low-income samples across U.S. urban and rural areas, conducting research with families in their homes in Los Angeles, and LMIC country collaborations. We organized this summary into three broad sections, each reflecting challenges faced by researchers attempting to increase representativeness in biopsychosocial research, along with potential solutions. The three sections are: 1) Recruitment and retention, 2) Collecting biological samples outside the laboratory, and 3) Data analysis, interpretation, and dissemination.
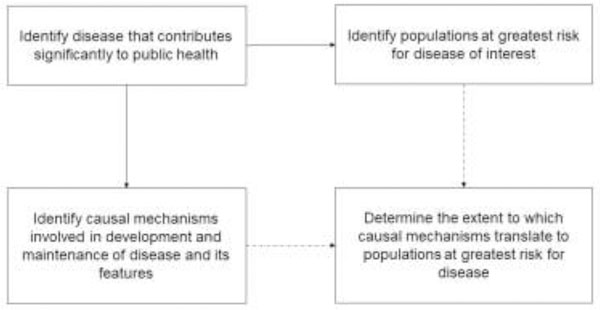
There are many methodological challenges involved in extending the results of highly-controlled experiments using both animals and humans; although these experiments produce elegant causal observations that inform our understanding of health and disease, the hypotheses and findings must also be tested in the communities at the highest risk for the relevant health outcomes. The need for convergence of public health initiatives with translational science in our field is illustrated in Figure 2. When faced with these challenges, many investigators opt for the participants they can recruit (e.g., convenience sampling), or resign themselves to small samples with high rates of non-compliance, missing data, and low retention. The goal of this summary is to inspire optimism by offering some solutions to these common challenges and increase the potential impact of our field on global health.
Figure 2.
Characterizing the pathogenesis of a disease and its maintenance requires translation of observations related to causal disease mechanisms to populations at greatest risk for the disease of interest
The authors of this review are all actively engaged in research involving low- and middle-income families, longitudinal cohorts of pregnant women, women living in shelters following domestic violence, refugees, predominantly Latino or Spanish-speaking communities, foster children, and African-American mothers. Accordingly, recommendations in this paper are drawn from the authors’ collective experiences applying previous knowledge, theory, and conceptual frameworks, as well as input and feedback from experts in the field. We recognize that our own experiences are also limited; for instance, most of our experience involves working with under-represented racial and ethnic groups within the United States of America. While under-representation as a function of race and ethnicity is a major issue as described above, we recognize that other identities and statuses are also under-represented. Ultimately, our goal is to highlight the challenges inherent in conducting inclusive, and thus truly translational, research and describe ways to meet those challenges.
2. Recruitment and retention
2.1. Challenges
There are several significant challenges faced by both health researchers and community members that impede participation of under-represented groups in research. These include factors that reduce an individual’s motivation or ability to participate, as well as standard practices on the part of researchers that inadvertently impede effective recruitment.
Common factors that reduce the motivation or ability of target communities to participate in studies include: lack of trust in the research process, lack of transportation or childcare to enable participation, and a mismatch in the language of recruitment or study materials and the preferred language of the prospective participants. It is well-documented that community members are hesitant to work with researchers from universities and other research-intensive institutions due to a long history of experienced racism, mistreatment of vulnerable populations by researchers, marginalization of specific racial/ethnic groups by health care systems, and research findings traditionally not being used to serve the needs of the community (see Horowitz et al., 2009 for review). In particular, many health researchers are perceived to engage in ‘helicopter research’ or ‘drive-by research’, collecting data from these communities and leaving once data collection is completed without any intention of sharing their study results, implementing community-serving programs, or facilitating policy changes to address the health needs of that community (Horowitz et al., 2009). Building and demonstrating trust with community leaders is particularly salient in biopsychosocial studies because the collection of biological specimens requires greater transparency by researchers in communicating how specimens are going to be used and confidentiality will be maintained.
Another important barrier to participation in research that disproportionately affects lower income families includes transportation to the research site and availability of childcare during participation. Research sites are often located far from the communities in which target participants live and work. This distance may serve as a major barrier for community members who do not have transportation, cannot incur the costs of participating (i.e., time away from work and childcare), or who do not feel comfortable leaving their community to participate in research where biological samples are collected. Finally, language barriers may also preclude participation if recruitment, assessment, and intervention materials are not provided in multiple languages or the research team does not reflect the diversity of the population being studied (Nicholson et al., 2015). Similarly, literacy issues may also be a barrier, depending on how research materials are designed for individuals with different reading levels.
There is also an inadvertent tendency for researchers to design studies largely from the perspective of what is feasible and cost-effective for the research team as opposed to what is convenient and comfortable for community members. For example, indirect or passive methods of recruitment are often used (e.g., mass mailings, newspaper/electronic advertisements, fliers), despite not being as effective in recruiting racial/ethnic minorities as more direct and active methods (e.g., face-to-face interaction in community settings). This phenomenon is understandable given the significant additional resources (e.g., staff time) and recruiter training involved in active recruitment methods (Nicholson et al., 2015), but also come at a cost to inclusion and generalizability. Eligibility criteria have also been identified as a barrier to study participation, with a recent review showing that medical comorbidities are one reason why racial and ethnic minorities are excluded from clinical trials (Nicholson et al., 2015). However, racial and ethnic minorities are also approached less often for study participation despite not differing in their willingness to participate in research compared to their White counterparts (Nicholson et al., 2015). While some eligibility criteria are related to exclusion of individuals with medical comorbidities or those taking certain medications, other criteria often include the requirement that participants travel to the institution where the research team is located. More broadly, requiring participants to travel to research institutions assumes that participants have the resources to meet this study requirement and can also take a participant’s comfort for granted by requiring long research visits where participants are often asked to abstain from eating and drinking. Taken together, each of these common practices contribute incrementally to the underrepresentation of key demographics in biopsychosocial health research.
2.2. Solutions
Many of the factors that contribute to underrepresentation of minorities in biopsychosocial research can be addressed by adopting a community-based participatory research (CBPR) framework (BeLue et al., 2014; Christopher et al., 2008; Israel et al., 1998; Shalowitz et al., 2009). CBPR involves creating a collaborative network of research and community stakeholders (e.g., research team, directors of community organizations, community leaders, community members) who work together to design, implement, evaluate, and disseminate research to address a community health need (Horowitz et al., 2009). An important component of CBPR is recognizing the strengths that each stakeholder contributes to the research process. CBPR has a long history in public health research and was adopted in the mid-1980s by the World Health Organization as a novel approach to health promotion in underserved communities (Minkler and Wallerstein, 2005). A key role for health researchers utilizing a CBPR framework is to help empower communities to be actively involved in social change to address health disparities.
Another major challenge for researchers is developing trust within the community with whom they want to conduct research. With this goal in mind, the first step in the CBPR process is often to discuss the pressing health issues and gaps in resources identified by community leaders from local hospitals, health organizations, departments of health, schools, and churches. For example, in one of our community-based studies, these discussions brought to our attention that funding for staff salaries in community clinics are often cut substantially and with little notice. As a result, salary support for clinic staff was included in the project budget (Thornburgh et al., 2017). This was mutually beneficial to the community and the academic partner as it guaranteed staff availability to oversee study outreach and facilitate proposed community health interventions while also assured support of clinic staff for an extended period of time.
Conversations with community leaders should also lead to the development of a memorandum of understanding (MOU) whenever possible. An MOU is a contract often used to formalize community-academic partnerships (Israel et al., 1998; Ross et al., 2010). The content of MOUs typically include the mission, mutual goals, responsibilities, and deliverables of each key stakeholder. Table 1 provides a summary of the common elements of an effective MOU. Additionally, we have included a template for developing an MOU for mutually beneficial research with community partners that was published by the Engagement Lab at Emerson College in our supplementary material (See supplement 1; Gordon and Racin, 2018). An example MOU from one of our authors’ past projects is also included (See Supplement 2; Urizar et al., 2019). The Engagement Lab template was designed to be completed during a conversation between all members of the collaboration to identify mutually beneficial goals and project outcomes. An effective MOU can be invaluable if there is turnover in leadership within community organizations, to provide clarification in roles and responsibilities during project implementation, and to orient new individuals who enter an established partnership. For example, the clinic director may leave unexpectedly for another position in the middle of an ongoing clinical trial. With an MOU in place, the new leadership is more likely to honor the commitment to the research project’s success and completion in the ways stated in the MOU.
Table 1.
Summary of essential elements of an effective memorandum of understanding
| Element | Description |
|---|---|
| Timeframe | Date MOU was established and timeframe for the collaborative relationship |
| Partners | Names, contact information, and brief descriptions of the partners participating in the collaborative relationship |
| Purpose | A description of the mutually agreed upon purpose of the collaboration and MOU |
| Planned activities | An itemized list of roles and responsibilities each partner will be accountable for while the MOU is active (e.g., planned meetings, allocation of resources, protection and maintenance of data/information, agreed upon procedures for managing conflict, likely barriers to recruitment/enrollment/retention and how they can be addressed by each party). |
| Financial responsibilities | A clear statement of which partner(s) will be responsible for any costs associated with the work conducted as part of the collaboration |
| Execution of agreement | Names, titles, signatures, and dates that authorized individuals within each partner organization agreed to the terms of the MOU |
Importantly, the process of creating an MOU can go a long way in helping build the community’s trust of the research team while achieving the project’s goals. Some issues that can emerge from these conversations are common barriers to appointment attendance in the target community, determinations of what language(s) should be used for recruitment and study materials, and identifying overarching values and norms within the population, all of which can be addressed in an MOU.
As noted previously, certain study logistics (e.g., traveling to the research institution) may serve as barriers to study participation. Recruitment and retention in studies can be aided considerably by providing reimbursements or vouchers for transportation to and from the research visits. Reimbursing participants for the costs of transportation incurred by participating in the project may also serve as a mutual benefit to the individual and the research team. In particular, if the study is being conducted in a community clinic and occurring in conjunction with regular or scheduled healthcare visits, remediation of transportation costs may improve continuity of care for the individual and the clinic. Similarly, a common barrier to study participation is the need for childcare during study visits which can require some creativity to overcome. To address the barrier of childcare, one study developed and provided an age-appropriate educational program for African-American children that paralleled the intervention program that was being taught to the mothers (Thornburgh et al., 2017). In this case, not only were the mothers available to participate in the intervention study, they were motivated to have their child benefit from engagement in the childcare provided. If your study staff is not capable of offering child care to participants during study visits, many hospitals and clinics have child care centers whose participation in child care services that support this ongoing study could be included in the MOU.
Strong community partners may even identify research spaces that are more convenient for community members. For example, in a study by one of the authors, partnering with the director of a large, public prenatal clinic resulted in access to several spaces in the clinic for recruitment, holding focus groups with patients (in Spanish and in English) to receive feedback from community members on study materials, protocols, and procedures (including biological collection procedures), and teaching a stress-management intervention (Urizar et al., 2016, 2019). Given the central location of the clinic and the volume of patients they serve, recruitment goals were achieved, serving an ethnically-diverse population of low-income women and their infants (69% Latina, 18% African-American) with high retention rates of 88% to 96% up to one-year following their baseline assessment (Urizar et al., 2019). Other sites that may suit community members best are community health centers, public libraries, and churches which some of us have used in studies in Los Angeles.
Another creative example of how barriers to enrollment and participation in research studies can be mitigated is by eliminating the need for travel altogether, such as by using a mobile laboratory. Mobile laboratories have been embraced in medicine and other physical sciences with great success. Equipping a converted van, tractor-trailer, or recreational vehicle (RV) with everything needed to conduct your research means that researchers can actively recruit members of the community at fairs, festivals, and other local events that are more likely to be representative of the community than a passively recruited sample. Part of the goal of a CBPR framework would be to help the research team learn how to tailor recruitment efforts specifically to the target community.
The solutions to language and literacy barriers are somewhat self-evident. If the research materials are only available in one language and that language is not the participant’s first language, the participant may struggle through the consent and questionnaires at the expense of their understanding and, ultimately, the data they provide. Conversations when developing the community partnership may elucidate the target population’s literacy and competencies in different languages and guide the research team to translate important materials into one or more languages.
Further, community partners are likely to point out whether the proposed eligibility criteria are too exclusionary, the research protocol is too long or questions too invasive for prospective participants, and they may have suggestions for improvement. It is common for researchers to have standard exclusion criteria for their studies without consideration for which of these exclusion criteria may inadvertently bias their sample and their results. Instead of adopting such a conservative approach to exclusion, particularly for comorbid conditions, each eligibility and exclusion criterion should be carefully evaluated for its prevalence in the target community and whether it can be adjusted for statistically with an appropriately-powered sample. In fact, it may be timely for our field to develop consensus guidelines regarding which factors can be accounted for statistically and which are necessary to exclude from a sample altogether (e.g., O’Connor et al., 2009). Finally, community stakeholders may identify whether long protocols should include breaks, refreshments, or modification of invasive procedures which will contribute positively to retention rates and recruitment through word-of-mouth. As demonstrated by these examples, developing strong and long-lasting research partnerships in the target community, such as using the CBPR framework, can lead to successful implementation and testing of biopsychosocial models in underserved populations.
3.0. Collecting biological samples in the community
There has never been a better time to be conducting field research on biological mechanisms of health and disease. Biomarkers are not only collected in smaller scale field and lab research, but also in population studies (Adam and Kumari, 2009; McDade et al., 2007). Scientists and laypersons are somewhat more accepting of collecting personal data in participants’ day-to-day lives because of widespread use of mobile and “smart home” technologies. Greater public acceptability has increased feasibility of collecting biological data at home and work such as accelerometry, ambulatory blood pressure and heart rate, and home sampling of biological specimens. Among the samples now commonly collected are saliva, urine, hair, and fecal samples. Furthermore, guidelines now exist for measuring many neuroendocrine parameters in daily life (Adam and Kumari, 2009; Kuhlman et al., 2019; Saxbe, 2008; Stalder et al., 2016). Written standardized protocols are a good starting point for achieving reliability and precision, comparability across research groups, and external validity. However, collecting biological samples from people in the community also requires minimizing participant burden and maximizing participant trust in the researchers and the broader research enterprise. Nonetheless, there are many challenges involved in collecting samples outside the laboratory and in participants’ daily lives, and, fortunately, there is also a growing number of potential solutions to these challenges.
3.1. Challenges
Reliably collecting biological samples during everyday life requires recognition that participants have to change their behavior in some way to engage in data collection procedures and protocols. For example, having to take a saliva sample before getting out of bed or refrigerating infant diapers for microbiome samples are not usual behaviors in one’s routine. New procedures and skills must be learned, daily routines altered, and those changes remembered. Researchers often underestimate the size of this request to their participants. However, requesting that these changes be maintained over days or weeks requires significant motivation on the part of the participants. Theories of behavior change may help frame challenges and potential solutions particularly for studies requiring this sort of serial biological sampling at home over time (Sheeran et al., 2017). One framework that integrates across multiple theories, the behavior change wheel, proposes that behavior change is a function of motivation, capability, and opportunity (Michie et al., 2011). Although not all studies require this sort of major behavioral change, the application of this theory is a way to frame the problems and solutions in studies that do.
Here, we focus primarily on participants collecting samples from themselves. Regardless of self- or staff-collection, some populations are less likely to provide biological samples out of concern that samples could be used unethically or even maliciously. For example, fears that biological samples will be tested for substances that result either in the removal of benefits such as from sheltered living situations, or that scientific results could be subpoenaed in child custody proceedings. Such concerns are often shared by key stakeholders in affected communities who do not want to risk having their clients inadvertently harmed. In addition, some populations are wary of genetic testing done without their awareness. Indeed, African-American populations continue to experience such fears and mistrust in researchers due to historical precedents that occurred prior to the institutionalization of strong human subject research protections (see Horowitz et al., 2009 for review).
Above all else, participants must have the motivation to first participate and then comply with researchers’ instructions. This includes reflective motivation (conscious plans and evaluative beliefs about what is desirable or undesirable) and automatic motivation (implicit attitudes, biases, goals, and habits). When participants and the research team have a superficial and time-limited relationship, and when there is little participant investment or trust in the researchers’ motivations, the research process, or outcomes, motivation is further diminished. More broadly, when the study concepts, measures, or procedures are viewed as inappropriate by the participants’ culture, motivation may also decrease. This issue can be particularly salient at the community level, as described in the previous section on recruitment and retention. Of course, motivation can also be impeded by issues of capability and opportunity. Capability refers to the physical ability, knowledge, skills, and stamina to perform a behavior. Physical ability and stamina are typically a concern for specific populations, such as infants, young children, or individuals with physical disabilities. The major challenge is that all participants must acquire new knowledge and skills to collect samples in a reliable manner. One barrier can be differences in language and literacy between the research team and participants. In addition, default education and training methods often involve passive learning, in which participants are instructed on what to do but have no opportunity for specific feedback from the trainer. However, passive learning, in the context of STEM education, is related to poorer retention (Freeman et al., 2014).
Factors that influence opportunity involve the physical (including times, resources, locations, and cues) and social environment. Collecting daily samples can disrupt individuals’ physical environment and routines. For example, in a study by one of the authors, a family reported that collecting samples just before dinner disrupted their normal routine of eating outside the home because they wanted to avoid collecting samples in public. Social environments consist of immediate interpersonal influences like individuals living in the home, one’s social and occupational circles, and relationships with larger institutions and organizations (Sallis et al., 2015; Stokols, 1992). The cues and norms that are transmitted (explicitly and implicitly) in those social environments can be a barrier to reliable sample collection. For instance, a participant may delay taking a saliva sample to avoid embarrassment from drooling in front of others. Important high-status people (parents, teachers, supervisors, colleagues) who are not involved in the study may also disapprove of a participant deviating from their normal routine to collect samples.
3.2. Solutions
The first critical step toward increasing motivation to participate in research that involves biological sampling is to mitigate mistrust of the research process in the community. The composition of a particular research team can also be a factor in making or breaking trust with the community. Creating opportunities during the research process where study staff and participants can build relationships that are friendly and supportive, where both parties care about one another’s well-being, can increase motivation to participate in research and go a long way toward increasing participant retention and compliance with procedures. This can be accomplished by having research staff with experience with the target communities or even from those communities, and also by making hiring of research staff who have excellent interpersonal and teaching skills a priority. The opportunities for these friendly and supportive interactions between study staff and participants will also increase if active recruitment methods and interactive protocol training are employed.
Active engagement in local and social media is also a useful strategy for maintaining relationships between your research team and the community that may further reduce the mistrust communities have for studies requesting biological samples. For example, the research team can share results of their research with members of the community as a demonstration of how biological data is used and how those results can impact health in their community and beyond. Many labs also publish a newsletter with updates on their lab’s projects which can help to keep members of the community engaged. Another helpful strategy can be to provide more information to prospective participants about the safeguards in place to protect their privacy. This can include explaining the role and involvement of the institutional review board in protecting participants, as well as the explicit permissions the research team does and does not have for analyzing their data. Engaging directly in this conversation may help participants better appreciate the limitations placed on researchers when handling their sensitive information such as medical history and biological samples that can be used to extract their DNA.
To increase participant capability, education and training are a starting point. Important topics include: the degree of flexibility around sample timing and collection procedures, and the importance of honestly self-reporting sample collection date, time, and confounds. Importantly, instructions should be accessible in multiple formats (e.g., written, illustrated, video), and researchers should provide hands-on training on how to perform procedures (See Supplement 3 for an example of written saliva sampling instructions; Urizar et al., 2019). When these strategies are actively engaged, research teams report excellent fidelity with at-home collection of biological samples (Kuhlman et al., 2017, 2016; O’Campo et al., 2016). Researchers must also take steps to reduce communication and didactic barriers. Materials and didactic procedures must be clear and understandable with regard to participant language, reading ability, and culture. Training must promote active rather than passive learning. For instance, collaborative interactions between trainers and participants that involve specific feedback from the trainer are related to better educational outcomes, due to better retention, metacognition, and motivation.
Modifying physical and social environments can be a powerful way to increase opportunity for participants to reliably collect samples. Training in the contexts where collection will occur, such as the home, school, or workplace, combined with collaborating with participants to identify where to place materials increases convenience and more importantly, provides physical cues that sample collection must take place. Understanding a participants’ expected routines can facilitate collaborative efforts to conveniently and creatively insert sample collection into those routines, and create a system of written or electronic reminders. Researchers can also work with participants to identify and manage social environments. For instance, participants could indicate (after considering the risks and benefits of disclosure) if they are willing to disclose to family members or co-workers that they are participating in a study. To this end, collecting samples from social groups (i.e., studying families, work teams) can actually help facilitate compliance and precision in home biological sampling. For example, in one of our studies, family members reported reminding each other to collect samples on time when several family members were collecting samples at the same time.
4.0. Data analysis, interpretation, and dissemination
4.1. Challenges
The challenges associated with collecting biopsychosocial data in the community do not necessarily end with the completion of data collection but extend into data analysis, interpretation, and disseminating the results. One major challenge is related to sample size. The labor-intensive nature of many community-based studies relative to convenience sampling combined with the high cost of testing biological samples often results in relatively small samples. For example, a systematic review of more than 200 studies of biological and psychological predictors of postpartum depression contrasts the mean sample size of studies with a biological measure at 238 (range: 16 – 1,084) to a sample size of 960 (range: 35 – 15,389) for studies relying solely on psychosocial measures (Yim et al., 2015). These smaller sample sizes have implications for our growing knowledge base. Indeed, the average sample size for studies looking at neuroendocrine functioning via the cortisol awakening response (CAR) is 245 participants despite meta-analytic evidence that at least 617 participants may be needed in a cross-sectional study to reliably detect an effect of a psychosocial predictor on the CAR (Boggero et al., 2017). While achieving large sample sizes is possible, there are financial and time-demands to this type of study that disproportionally affect biopsychosocial work in the community.
Small sample sizes obviously affect statistical power and also limit the statistical methods that can be confidently applied to the data. As a result, the data-analytic approach is often limited to simple comparisons with few independent variables. Further, limiting data analyses to simple comparisons prevents a thoughtful, theory-driven consideration of the influence of confounding, moderating, and mediating variables that may be especially knowledge-generating in heterogeneous community samples. Moreover, because of the issues related to participant compliance with biological sampling (see section 3.1 above), missing data is more likely to occur in studies outside of the laboratory, particularly in longitudinal designs, further compounding concerns about adequate statistical power.
There can also be significant barriers to getting studies with small sample sizes published, particularly in high impact and mainstream journals. In part, this makes sense because studies with larger sample sizes allow for more rigorous statistical analyses that can control for confounds, rule out alternative explanations, and incorporate tests of mediating and moderating factors. Thus, larger samples sizes increase confidence in the internal validity of the findings reported. However, if the tradeoff for larger samples is that they are homogenous middle- to high-income, educated, and White, then external validity is sacrificed at the expense of sample size. Therefore, these concerns can hinder the generalizability and relevance of our research to difficult-to-recruit, often disadvantaged, and traditionally under-represented populations, such as abuse survivors, homeless individuals, or low-literacy populations (See Bonevski et al., 2014 for review).
We note that barriers to publishing work on under-represented community samples may present themselves at different stages of the review process. The peer review process in scientific journals is plagued by implicit biases (Kuehn, 2017; McNutt, 2016; Pinholster, 2016), such that papers may be more likely to be desk-rejected or triaged by the editorial team of a journal because of a perceived lower potential for impact, or a less favorable perception of the quality and rigor of the work in small samples with missing data.
When a paper passes this initial hurdle, studies conducted on under-represented community samples face additional challenges during peer review. For interdisciplinary, biopsychosocial community studies, normative reviewer biases (e.g., confirmatory bias, negative results bias, gender, and race orientation; Hojat et al., 2003) are compounded by nominating peer reviewers who may each be experts in one but not all disciplinary aspects of the study. In particular, peer reviewers may undervalue the integrative contribution of a study or be unaware of the conceptual and logistical challenges involved in conducting research with under-represented groups (Laudel, 2006). As a consequence, this type of study may be more likely to be rejected or eventually published in less visible, “niche” journals that neither have the same scientific impact nor attract the same degree of public attention. Ultimately, these challenges create small echo chambers, where there is little cross-talk between researchers who read and publish in mainstream journals and researchers who study disease processes in under-represented communities.
In sum, researchers who study under-represented communities face additional analytic and publishing challenges compared to scholars conducting research using convenience sampling of easily accessible populations. This has significant implications for a scholar’s career development which is determined, in large part, by the number and impact of their publications. Until we, as a scientific community, agree to more flexibly evaluate inclusive research, and find concrete ways in which to incentivize and reward researchers engaging in this type of research, we will not only systematically disadvantage researchers committed to inclusive research, but also systematically exclude the parts of our society with the greatest need from participation to the detriment of public health.
4.2. Solutions
Enacting the strategies summarized in Sections 2 and 3 of this review would mitigate some of the problems that plague research in under-represented communities at this stage of the research process. However, a range of statistical and methodological approaches to increasing power without increasing sample size have been suggested, including blocking, using designs with planned missingness, and borrowing information from larger data sets (Fritz et al., 2015). Bootstrapping techniques may also be helpful, in particular when assumptions of normality or homoscedasticity are violated (Erceg-Hurn and Mirosevich, 2008).
Another way for our field to begin combatting problems of small sample sizes would be to more aggressively engage in data sharing and data pooling such as through the Open Science Framework. Initiatives such as the Human Connectome Project (http://www.humanconnectomeproject.org/) and the ENIGMA (enigma.ini.usc.edu) have resulted in a burst of knowledge creation within neuroscience. With a similar initiative in psychoneuroendocrinology, labs and the field more broadly would be more likely to generate generalizable findings. For example, imagine what we might know about human stress reactivity if we could compile a dataset of HPA axis reactivity across all studies that have administered the Trier Social Stress Test. This may be particularly informative given the differences in HPA axis regulation observed in racial minority groups following acute stress (Hostinar et al., 2014). Much like neuroimaging research, the methodological nuances of psychoneuroendocrine research and variability in those methods across labs would be a limitation. Members of the International Society of Psychoneuroendocrinology are well-positioned to compose standardized protocols for sample collection, assays, and data processing that is so far monitored solely through the peer-review process after a study has been completed. In some ways, members of the society are already beginning to establish these standards through the publication of expert consensus guidelines (Stalder et al., 2016), yet many standards in the field remain poorly documented.
One strategy to mitigate publication barriers for small studies in diverse community samples is providing strong and detailed arguments in manuscripts for the contribution of interrogating established biopsychosocial processes in a target community within a translational science framework. Investigators can also more consistently provide demographic information on their study sample in order to adhere to the guidelines established by the NIH. Additionally, investigators can routinely address the strengths and limitations of their studies in the context of those sample characteristics. If your study was inclusive of under-represented groups, what are the implications of that for the broader field? If the study was not inclusive, what are the important next steps in this area? In composing this summary of our panel, there is simply a paucity of peer-reviewed documentation of many of these challenges and solutions to conducting biopsychosocial research within under-represented groups that can easily be addressed by the way we document our methods. For example, what recruitment methods were used, which of these methods were most effective, how many people were excluded based on each exclusion criteria. In other words, what wisdom can be passed to the field about working with this population? This approach may go a long way in educating interdisciplinary peer reviewers on the challenges inherent in conducting community research while also underscoring its importance to public health.
Scientists, editors, and reviewers across disciplines are increasingly aware of the necessity of conducting more research with traditionally under-represented populations which may help to solve some of the challenges in this regard with time (e.g., Pérez-Stable and Collins, 2019). In some ways, the field of psychoneuroendocrinology is better prepared to address this challenge because assessing biomarkers in large samples is often cost-prohibitive, placing financial limits on sample sizes. Thus, peer reviewers in our subfield are more likely to be familiar with the challenges and the value of studies with small sample sizes. On the other hand, the challenges of community research are not limited to a small sample size per se. Steps that can be taken by publishers, editors, and editorial boards would be to enforce the reporting of demographic characteristics in submitted manuscripts, and reflect upon the representation of community-based research on their editorial boards as internal advocates of the value of translating biological models of stress and health to under-represented communities. Similarly, monitoring the frequency of diverse samples represented within each issue and across each year would go a long way in highlighting the deficit of translation into under-represented and high-risk communities endemic in the field. Finally, sponsorship of special issues aimed directly at translating our existing animal and human models linking stress processes and health to different at-risk, and hard-to-reach communities would be a valuable next step. Again, the field of psychoneuroendocrinology may be particularly well positioned to accomplish this given that each issue boasts rigorous tests of links between the brain, hormones, and behavior in both animals and humans.
With respect to career advancement, taking all of the aforementioned steps may help to mitigate the costs of conducting translational psychoneuroendocrinology in hard-to-recruit populations by increasing sample sizes, data quality, and the overall impact of translational community research. Further steps that can be taken by researchers include asserting the importance of community research within a translational science framework within both manuscripts and promotion materials. Clearly explaining why translating the results of basic research to specific populations is critical to evaluating the potential impact of observations in specific subpopulations. Secondary to asserting the value of observations, it can be helpful to describe the efforts taken to recruit and retain participants. Providing more detailed descriptions of these methods will benefit the field by documenting effective recruitment strategies, but also highlight the considerable efforts involved in developing effective partnerships with the community. This is also a place where use of data sharing and pooling may be beneficial to the field. Researchers concerned about the limitations to their data may disproportionately benefit from sharing and using standardized protocols so that their data may be pooled with that of other translational psychoneuroendocrinologists. Likewise, these same researchers may be able to increase the impact of their work by testing their research questions about how basic research translates to high-risk populations in large, publicly available datasets; which are currently underutilized. Ultimately, steps taken by individual researchers will pale in comparison to efforts to change the institutions and contexts within which our scholarly work is conducted and evaluated.
5. Conclusion
In this review, we have summarized the content of an expert panel on testing plausible biopsychosocial models in diverse community samples that occurred at the 2018 meeting of the International Society of Psychoenuroendocrinology. Three themes emerged. First, the difficulties in recruitment and retention of under-represented groups such as racial and ethnic minorities and low-income populations are often greater than in studies of higher income and less diverse samples who have been studied most often in biopsychosocial research. Second, difficulties in collecting biological samples outside of the laboratory in the understudied communities experiencing health disparities can introduce challenges that may undermine the rigor and generalizability of the data. Third, researchers conducting studies of biopsychosocial processes in traditionally under-represented samples may face additional challenges in knowledge dissemination. Among these are editorial and reviewer biases, smaller samples sizes that preclude the use of advanced statistical methods, and higher rates of missing data. Table 2 provides a summary of these challenges facing researchers who study stress and health within under-represented communities along with the solutions proposed by this panel. Solutions have been outlined and citations provided where they are available. Of note, the challenges and solutions summarized in this review are not exhaustive. Rather, they reflect a combination of the experiences of our expert panel, questions raised by the audience, and the ensuing discussion about the research process from recruitment to publication. It should also be noted that diversity in this panel largely focused on racial, ethnic, and socioeconomic diversity, which are only a few of the many dimensions of diversity to consider.
Table 2.
Summary of challenges faced by researchers studying stress and health mechanisms in diverse communities and proposed solutions
| Challenges | Solutions |
|---|---|
| Recruitment and Retention | |
| • Mistrust of researchers and research institutions especially in racial/ethnic minority communities | • Develop a written memorandum of understanding with local clinics and community centers to create continuity within the research relationship |
| • Create a way to show your investment in using your research findings to better meet community’s needs (e.g., present results of studies to the community with an emphasis on its contribution to our understanding) | |
| • Utilize data to help fund health initiatives needed by the community | |
| • Provide information to prospective participants about the role of the IRB and steps taken to assure the security of their information | |
| • Share results of research studies using biological samples with the community through local media and other sources trusted by the community as a demonstration of how data is used | |
| • Cost and inconvenience of travel for participants to and from the research institution | • Develop partnerships to conduct the work in community sites where target participants live and work |
| • Write salary support for local clinic/community center staff into grants to help recruit and conduct study | |
| • Reimburse participants for transportation costs to and from research visits or provide transportation vouchers | |
| • Develop complimentary programs to provide childcare during parent participation | |
| • Mismatch in language between research materials and preferred language of prospective participant | • Work with community partners or focus groups to anticipate any common language or literacy issues that may emerge with research materials and translate or edit as needed |
| • Commonly used and passive recruitment strategies such as mass mailings, newspaper and online advertisements, fliers that tend to under-recruit members of racial/ethnic minority participants | • Use active recruitment methods especially face-to-face interactions within community settings to recruit participants and community members as partners in recruitment |
| • Strict health-related inclusion criteria often exclude individuals from racial/ethnic minority groups | • Power studies to account for comorbidities rather than exclude them where possible |
| • Study procedures are or are perceived to be invasive, excessively long, or uncomfortable | • Design research procedures from the perspective of the participant’s comfort (e.g., build breaks into long protocols, offer snacks if possible) |
| Collecting biological samples | |
| • Collecting biological specimens inherently requires participants to change their behavior | • Use existing research on behavior change to identify ways to increase participant motivation, capability, and opportunity to participate and comply with the study protocol |
| • Adhering to sample collection protocols requires new skills and knowledge about how to correctly collect specimens and why adherence to collection protocols is important | • Engage participants in active rather than passive training (e.g., detailed in-person explanation and demonstration of sampling in the location where samples will be collected such as the participants’ homes) |
| • Share results of previous research using similar samples to demonstrate how samples will be used | |
| • Conduct focus groups to identify parts of protocol that are confusing or unlikely to be adhered to | |
| • Provide multiple forms of information including, in person demonstration, written, illustrated, and video training on sample collection to emphasize the importance of timing and adherence to protocols and honest reporting of collection times | |
| • Biological sample collection procedures interfere with participants’ daily routines | • Incorporate discussion of barriers to compliance in sample collection into in-home trainings and how to proactively minimize them (e.g., identify physical cues and set reminders) |
| • Interpersonal contexts may interfere with participant motivation to adhere to study protocols (e.g., expectation to collect samples before dinner even though not all family dinners are at home) | • Consider engaging social groups in sample collection (e.g., families, teams) |
| • Diminished participant engagement across time in longitudinal studies | • Hire enthusiastic, friendly, committed, and diverse staff (preferably that come from the target community) that develop rapport with the participants |
| • Create staff consistency for participants (e.g., assign one well-chosen staff person to participant contact or use the same person to maintain the participant connection throughout the project as much as possible) | |
| Data analysis, interpretation, and dissemination | |
| • Small study sample sizes with more limited options for data analysis | • Use blocking, planned missingness, and borrow from larger datasets when power is low |
| • Use bootstrapping techniques when assumptions of normality cannot be met | |
| • Contribute to data sharing / pooling initiatives that harness the strengths of diverse samples across multiple studies | |
| • Use large, publicly-available datasets to test research questions if possible | |
| • Rejection of papers from target journals | • Provide strong rationale in manuscripts about the importance of biopsychosocial processes in the target population including both basic and translational rationales where relevant |
| • Obstacles to tenure and promotion | • Assert importance of your research in target communities within a translational science framework, details on difficulty of the recruitment, retention, and procedural work involved in studying your target population, as well as the long-term benefits of working relationships with community partners to long-term projects in promotion materials |
This discussion is placed in the context of the current focus on translational science across many sub-disciplines for which one main objective is to reduce rates of disease including stress-related illness. The aim of the panel, and this summary, was to foster a formal discourse within the field on these topics and generate shared knowledge across research labs and disciplines in order to more effectively test plausible biopsychosocial mechanisms in the communities at the greatest risk of health disparities broadly, and stress-related diseases specifically.
In conclusion, biopsychosocial health researchers, and those with whom they collaborate, must meet the challenge of studying all people especially populations at the greatest risk of disease. The challenges are notable and, like most methodological challenges, may seem insurmountable at times. Yet science will only progress for the public good if these methodological challenges lead to new strategies, techniques, creative solutions, and efforts to surmount them.
Supplementary Material
Highlights.
Biopsychosocial research traditionally underrepresents segments of the population
Numerous challenges contribute to underrepresentation of groups in research
Researchers have found effective strategies for recruiting minorities
Focus groups and active training can aid biological sampling in communities
Institutional shifts are needed to more effectively promote community translation
Acknowledgements and Role of the Funding Source
We would like to thank the International Society for Psychoneuroendocrinology for their support of this expert panel as a plenary event at the 2018 annual meeting, including the local organizing committee and the attendees of the session whose questions and comments fostered an engaging discussion. Composition of this manuscript was supported in part by the National Institute of Mental Health through a Mentored Clinical Scientist Career Development Award (K08MH112773) awarded to Dr. Kuhlman who was not involved in writing of the manuscript or the decision to submit the article for publication.
Footnotes
Conflict of interest
The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK, Kumari M, 2009. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 34, 1423–1436. 10.1016/j.psyneuen.2009.06.011 [DOI] [PubMed] [Google Scholar]
- Adler NE, Rehkopf DH, 2008. US disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health 29, 235–252. 10.1146/annurev.publhealth.29.020907.090852 [DOI] [PubMed] [Google Scholar]
- Baumgratz G, 1995. Language, culture and global competence: An essay on ambiguity. Eur. J. Educ 30, 437–447. 10.2307/1503516 [DOI] [Google Scholar]
- BeLue R, Schafer P, Chung B, Vance M, Lanzi R, O’Campo P, 2014. Evaluation of the Community Child Health Research Network (CCHN) Community-Academic Partnership. J. Health Disparities Res. Pract 7, 4. [Google Scholar]
- Boggero IA, Hostinar CE, Haak EA, Murphy MLM, Segerstrom SC, 2017. Psychosocial functioning and the cortisol awakening response: Meta-analysis, P-curve analysis, and evaluation of the evidential value in existing studies. Biol. Psychol 129, 207–230. 10.1016/j.biopsycho.2017.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonevski B, Randell M, Paul C, Chapman K, Twyman L, Bryant J, Brozek I, Hughes C, 2014. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med. Res. Methodol 14, 42 10.1186/1471-2288-14-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein JB, Sherber NS, Schulman SP, Ding EL, Powe NR, 2008. Race, medical researcher distrust, perceived harm, and willingness to participate in cardiovascular prevention trials. Medicine (Baltimore) 87, 1. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, D’Elia L, Strazzullo P, Miller MA, 2010. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 33, 585–592. 10.1093/sleep/33.5.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Turiano NA, Mroczek DK, Miller GE, 2016. Association of reports of childhood abuse and all-cause mortality rates in women. JAMA Psychiatry 73, 920–927. 10.1001/jamapsychiatry.2016.1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher S, Watts V, McCormick AKHG, Young S, 2008. Building and maintaining trust in a community-based participatory research partnership. Am. J. Public Health 98, 1398 10.2105/AJPH.2007.125757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erceg-Hurn DM, Mirosevich VM, 2008. Modern robust statistical methods: an easy way to maximize the accuracy and power of your research. Am. Psychol 63, 591 10.1037/0003-066X.63.7.591 [DOI] [PubMed] [Google Scholar]
- Freeman S, Eddy SL, McDonough M, Smith MK, Okoroafor N, Jordt H, Wenderoth MP, 2014. Active learning increases student performance in science, engineering, and mathematics. Proc. Natl. Acad. Sci 111, 8410–8415. 10.1073/pnas.1319030111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz MS, Cox MG, MacKinnon DP, 2015. Increasing statistical power in mediation models without increasing sample size. Eval. Health Prof 38, 343 10.1177/0163278713514250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller SE, Koch A, Pellettieri B, Carnes M, 2011. Inclusion, analysis, and reporting of sex and race/ethnicity in clinical trials: Have we made progress? J. Womens Health 10.1089/jwh.2010.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Duran N, Norris K, 2013. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am. J. Public Health 104, e16–e31. 10.2105/AJPH.2013.301706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E, Racin L, 2018. Memorandum of Understanding for Mutually Beneficial Research, in: Community Academic Research Partnerships in Digital Contexts. Engagement Lab @ Emerson College, pp. 15–23. [Google Scholar]
- Henrich J, Heine SJ, Norenzayan A, 2010. The weirdest people in the world? Behav. Brain Sci 33, 61–83. 10.1017/S0140525X0999152X [DOI] [PubMed] [Google Scholar]
- Hojat M, Gonnella JS, Caelleigh AS, 2003. Impartial judgment by the “gatekeepers” of science: Fallibility and accountability in the peer review process. Adv. Health Sci. Educ. Theory Pract 8, 75. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D, 2015. Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspect. Psychol. Sci 10, 227–237. 10.1177/1745691614568352 [DOI] [PubMed] [Google Scholar]
- Horowitz CR, Robinson M, Seifer S, 2009. Community-based participatory research from the margin to the mainstream: Are researchers prepared? Circulation 119, 2633–2642. 10.1161/CIRCULATIONAHA.107.729863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, McQuillan MT, Mirous HJ, Grant KE, Adam EK, 2014. Cortisol responses to a group public speaking task for adolescents: Variations by age, gender, and race. Psychoneuroendocrinology 50, 155–166. 10.1016/j.psyneuen.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel BA, Schulz AJ, Parker EA, Becker AB, 1998. Review of community-based research: assessing partnership approaches to improve public health. Annu. Rev. Public Health 19, 173. [DOI] [PubMed] [Google Scholar]
- Kuehn BM, 2017. Rooting out bias. eLife 6, e32014 10.7554/eLife.32014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Irwin MR, Ganz PA, Crespi CM, Petersen L, Asher A, Bower JE, 2017. Cortisol awakening response as a prospective risk factor for depressive symptoms in women after treatment for breast cancer. Psychosom. Med 79, 763–769. 10.1097/PSY.0000000000000499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Repetti RL, Reynolds BM, Robles TF, 2016. Change in parent-child conflict and the HPA-axis: Where should we be looking and for how long? Psychoneuroendocrinology 68, 74–81. 10.1016/j.psyneuen.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Robles TF, Dickenson L, Reynolds B, Repetti RL, 2019. Stability of diurnal cortisol measures across days, weeks, and years during middle childhood and early adolescence: Exploring the role of age, pubertal development, and sex. Psychoneuroendocrinology 100, 67–74. 10.1016/j.psyneuen.2018.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudel G, 2006. Conclave in the Tower of Babel: how peers review interdisciplinary research proposals. Res. Eval. 15, 57–68. 10.3152/147154406781776048 [DOI] [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ, 2007. What a drop can do: dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography 44, 899. [DOI] [PubMed] [Google Scholar]
- McNutt M, 2016. Implicit bias. Science 352, 1035–1035. 10.1126/science.aag1695 [DOI] [PubMed] [Google Scholar]
- Michie S, van Stralen MM, West R, 2011. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement. Sci. IS 6, 42 10.1186/1748-5908-6-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler M, Wallerstein N, 2005. Improving health through community organization. Community Organ. Community Build. Health 26–51. [Google Scholar]
- Muñoz RF, Mendelson T, 2005. Toward evidence-based interventions for diverse populations: The San Francisco General Hospital prevention and treatment manuals. J. Consult. Clin. Psychol 73, 790 10.1037/0022-006X.73.5.790 [DOI] [PubMed] [Google Scholar]
- Murthy VH, Krumholz HM, Gross CP, 2004. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA 291, 2720 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- Nakamura J, Koos E, Zhuo K, Sara A, Shin J, Kainth P, Kuhlman KR, 2018. A meta-analytic review of the literature on early life stress and inflammatory biomarkers. Assoc. Psychol. Sci. San Franc. CA. [Google Scholar]
- National Institutes of Health Revitalization Act of 1993, 1993.
- Nicholson LM, Schwirian PM, Groner JA, 2015. Recruitment and retention strategies in clinical studies with low-income and minority populations: Progress from 2004–2014. Contemp. Clin. Trials 45, 34–40. 10.1016/j.cct.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Notice of the “NIH Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research,” 1994.
- Nueces DDL, Hacker K, DiGirolamo A, Hicks LS, 2012. A systematic review of community-based participatory research to enhance clinical trials in racial and ethnic minority groups. Health Serv. Res. 47, 1363–1386. 10.1111/j.1475-6773.2012.01386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Campo P, BeLue R, Borenstein H, Reed-Vance M, Lanzi RG, Schafer P, Jones L, Woolord R, 2016. Issues and solutions for collecting biological specimen in longitudinal studies: Experience from the Community Child Health Network Research Network. J. Health Care Poor Underserved 27, 339–351. 10.1353/hpu.2016.0021 [DOI] [PubMed] [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR, 2009. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain. Behav. Immun 23, 887–897. 10.1016/j.bbi.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SS, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, Bruin D.M. de, Greenblatt RM, Bibbins-Domingo K, Wu AHB, Borrell LN, Gunter C, Powe NR, Burchard EG, 2015. Diversity in clinical and biomedical research: A promise yet to be fulfilled. PLOS Med. 12, e1001918 10.1371/journal.pmed.1001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pèrez-Stable EJ, Collins FS, 2019. Science visioning in minority health and health disparities. Am. J. Public Health 109, S5–S5. 10.2105/AJPH.2019.304962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinholster G, 2016. Journals and funders confront implicit bias in peer review. Science 352, 1067–1068. 10.1126/science.352.6289.1067 [DOI] [Google Scholar]
- Ross LF, Loup A, Nelson RM, Botkin JR, Kost R, Smith GR Jr, Gehlert S, 2010. The challenges of collaboration for academic and community partners in a research partnership: Points to consider. J. Empir. Res. Hum. Res. Ethics JERHRE 5, 19 10.1525/jer.2010.5.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio DM, Schoenbaum EE, Lee LS, Schteingart DE, Marantz PR, Anderson KE, Platt LD, Baez A, Esposito K, 2010. Defining translational research: Implications for training. Acad. Med. J. Assoc. Am. Med. Coll 85, 470–475. 10.1097/ACM.0b013e3181ccd618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis JF, Owen N, Fisher E, 2015. Ecological models of health behavior, in: Glanz K, Rimer BK, Viswanath K (Eds.), Health Behavior: Theory, Research, and Practice. John Wiley & Sons, San Francisco, pp. 43–64. [Google Scholar]
- Saxbe DE, 2008. A field (researcher’s) guide to cortisol: tracking HPA axis functioning in everyday life. Health Psychol. Rev. 2, 163–190. 10.1080/17437190802530812 [DOI] [Google Scholar]
- Shalowitz MU, Isacco A, Barquin N, Clark-Kauffman E, Delger P, Nelson D, Quinn A, Wagenaar KA, 2009. Community-based participatory research: A review of the literature with strategies for community engagement. J. Dev. Behav. Pediatr. JDBP 30, 350 10.1097/DBP.0b013e3181b0ef14 [DOI] [PubMed] [Google Scholar]
- Sheeran P, Klein WM, Rothman AJ, 2017. Health Behavior Change: Moving from observation to intervention. Annu. Rev. Psychol 68, 573 10.1146/annurev-psych-010416-044007 [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, Dockray S, Smyth N, Evans P, Hellhammer DH, 2016. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 63, 414–432. 10.1016/j.psyneuen.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP, 2007. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann. Surg. Oncol 14, 3328. [DOI] [PubMed] [Google Scholar]
- Stokols D, 1992. Establishing and maintaining healthy environments. Toward a social ecology of health promotion. Am. Psychol 47, 6. [DOI] [PubMed] [Google Scholar]
- Thornburgh GM, Dunne L, Brown C, Urizar G Jr, 2017. The effects of a stress management program on self-efficacy, stress, and mood among low-income African-American mothers, in: Annals of Behavioral Medicine. pp. S343–S343. [Google Scholar]
- Trochim W, Kane C, Graham MJ, Pincus HA, 2011. Evaluating translational research: A process marker model. Clin. Transl. Sci. 4, 153–162. 10.1111/j.1752-8062.2011.00291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urizar G, Rodriguez A, Yim I, Schetter CD, 2016. CBSM effects on stress outcomes among low-income mothers: Role of ethnicity and prenatal anxiety, in: Psychosomatic Medicine. pp. A38–A38. [Google Scholar]
- Urizar GG, Yim IS, Rodriguez A, Schetter CD, 2019. The SMART Moms Program: A Randomized Trial of the Impact of Stress Management on Perceived Stress and Cortisol in Low-Income Pregnant Women. Psychoneuroendocrinology 104, 174–184. 10.1016/j.psyneuen.2019.02.022 [DOI] [PubMed] [Google Scholar]
- Waldman SA, Terzic A, 2010. Clinical and translational science: From bench-bedside to global village. Clin. Transl. Sci. 3, 254–257. 10.1111/j.1752-8062.2010.00227.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, Ross LF, 2003. Are minority children under-or overrepresented in pediatric research? Pediatrics 112, 890. [DOI] [PubMed] [Google Scholar]
- Yancey AK, Ortega AN, Kumanyika SK, 2006. Effective recruitment and retention of minority research participants. Annu. Rev. Public Health 27, 1–28. 10.1146/annurev.publhealth.27.021405.102113 [DOI] [PubMed] [Google Scholar]
- Yim IS, Stapleton LRT, Guardino CM, Hahn-Holbrook J, Schetter CD, 2015. Biological and psychosocial predictors of postpartum depression: Systematic review and call for integration. Annu. Rev. Clin. Psychol 11, 99 10.1146/annurev-clinpsy-101414-020426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo K, Koos E, Nakamura J, Sara A, Shin J, Kainth P, Kuhlman KR, 2018. Low childhood SES and physiological responses to stress across the lifespan: A meta-analytic review. Assoc. Psychol. Sci. San Franc. CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.