Abstract
Purpose of review
Although checkpoint inhibitor blockade is now widely used clinically for cancer immunotherapy, the reverse process, (i.e. induction of checkpoints to slow autoimmunity) has not been extensively explored. CD8 T-cell exhaustion is a state of immune hyporesponsiveness that may be harnessed to treat autoimmunity.
Recent findings
We focus on the potential role of CD8 T-cell exhaustion as a mechanism of peripheral tolerance in T1D and its therapeutic implications.
Summary
CD8 T-cell exhaustion is a continuum in which cells change from precursor to terminally exhausted cells. Current thinking based on studies in cancer and chronic viral infection invokes a three-signal model for development of T-cell exhaustion, with persistent antigen, negative costimulatory signals and chronic inflammation comprising signals 1–3, respectively. Transcriptional signatures of CD8 T-cell exhaustion were associated with better prognosis across several autoimmune diseases, most profoundly in systemic diseases. In T1D, CD8 exhaustion was promoted by treatment with anti-CD3 therapy (teplizumab) and was more evident in islet-specific CD8 T cells of slow progressors, suggesting a beneficial role in T1D also. Thus, we apply this three-step process of exhaustion to discuss potential treatments to augment CD8 T-cell exhaustion in T1D.
Keywords: CD8 T cell, exhaustion, type 1 diabetes
INTRODUCTION
Autoimmunity is a complex and chronic disease setting involving immune-mediated destruction of cells expressing self-proteins. Self-reactive cells are regulated by immune tolerance mechanisms including deletion, inactivation (or hyporesponsiveness) and active regulation. Type 1 diabetes (T1D) is a prototypic autoimmune disease in which insulin secreting islet beta cells are destroyed by immune cells when tolerance mechanisms fail. Thus, it is important to identify therapies that deplete or inactivate harmful autoreactive cells in addition to enhancing immune tolerance that will prevent the resurgence of autoimmunity [1]. However, to date, no single treatment modality has been shown to persistently prevent progression of T1D in the majority of treated patients [2], most likely because of the failure to maintain enhanced peripheral tolerance.
Susceptibility to T1D is linked to both host genes and environment, and these linkages are thought to modulate immune responses including tolerance mechanisms. For example, active regulation by CD4+ regulatory T cells and thymic deletion have been shown to play a role in controlling T1D-associated autoimmunity [3,4], and these mechanisms are associated with host genetics (HLA and other T1D-associated risk alleles) [5]. Less is known about the role of the induction of immune hyporesponsiveness in controlling T1D susceptibility or pathogenesis. One mechanism for regulating T-cell responsiveness is induction of a hyporesponsive state, known as T-cell exhaustion, which contributes to immune dysfunction in cancer and limits natural antitumor immunity [6]. Blockade of inhibitory receptors contributing to T-cell exhaustion enables tumor-reactive T cells to overcome regulatory mechanisms (immune checkpoints) and mount an effective antitumor response [7–9]. Although checkpoint inhibitor blockade is now widely used clinically for cancer immunotherapy [10], the reverse process, (i.e. induction of checkpoints to slow autoimmunity) has not been extensively explored. Here, we focus on the potential role of CD8 T-cell exhaustion as a mechanism of peripheral tolerance in T1D and its therapeutic implications. As CD8 T-cell exhaustion has been extensively reviewed [6,11,12▪▪,13–15], we first provide highlights of the field that are relevant to the therapeutic application in autoimmunity, and then specifically discuss examples of CD8 T-cell exhaustion in T1D. Finally, we propose potential therapeutic strategies that may harness CD8 T-cell exhaustion.
Box 1.

no caption available
CHARACTERISTICS OF CD8 T-CELL EXHAUSTION
Much of what we know about T-cell exhaustion comes from studies using lymphocytic choriomeningitis virus (LCMV) in mice, a widely used experimental system in immunology. Initial studies by Moskophidis et al.[16] first demonstrated impaired cytotoxic functions during viral persistence in murine models. Although acute infection of adult mice with the noncytopathic LCMV normally induces a protective cytotoxic T-cell response that also causes immunopathology, some LCMV strains tend to persist chronically after acute infection of adult mice without causing lethal immunopathological disease. LCMV strains that persist induce a large antiviral CD8 cytotoxic T-cell response that nearly disappears within a few days, and thus neither eliminates the virus nor causes the lethal immunopathology seen during acute infection. Subsequent tetramer-staining studies [17,18] showed that CD8 T cells responding to chronic LCMV infection were not deleted and remained detectable throughout infection, but were unable to efficiently perform effector functions. Since these early studies, it has become apparent that exhausted CD8 T (Tex) cells are found in humans as well as mice, and play a role in many chronic viral infections, including HIV, hepatitis C virus (HCV), hepatitis B virus (HBV) and others [6,19–21]. Furthermore, the extent of exhaustion has been linked to the amount of antigen present irrespective of the type of antigen-presenting cell utilized [22,23]. More recently, T-cell exhaustion has been associated with immune dysfunction in multiple human cancers [6,20–22], and reversal of T-cell exhaustion with checkpoint inhibitor therapy can be therapeutically beneficial [24–29]. Finally, T-cell exhaustion has been associated with autoimmunity, with favorable prognosis or response to therapy linked to increased T-cell exhaustion [30–32,33▪▪]. Taken together, these early data established the role of T-cell exhaustion in regulation of T-cell responsiveness during chronic diseases and cancer. Current thinking invokes a three-signal model for development of T-cell exhaustion, with persistent antigen, negative costimulatory signals and chronic inflammation comprising signals 1–3, respectively [12▪▪].
Although multiple cell types may undergo exhaustion [34–38], CD8 Tex have been more thoroughly studied. CD8 Tex are characterized by several cellular and molecular features including:
-
(1)
Sequential loss of T-cell effector functions: T-cell dysfunction during exhaustion proceeds in a hierarchical manner and involves progressive reduction in the capacity to produce IL-2, and other cytokines [18,38].
-
(2)
Altered cytokine responses: In addition to the loss of ability to produce IL2, other cytokine responses are also altered, as illustrated by an inverse correlation between IL-7 receptor expression and CD8 T-cell exhaustion [39].
-
(3)
Altered metabolic programs: Another alteration during development of CD8 exhaustion during chronic LCMV expression is altered T-cell bioenergetics. The initial observation supporting altered bioenergetics was from gene-expression studies of dysfunctional LCMV-specific CD8 T cells, which revealed expression changes in genes involved in metabolism, including the citric acid cycle [40]. Subsequent studies showed that Tex cells display metabolic derangements, including restricted glucose uptake and use [41].
-
(4)
Altered gene expression programs: Comparison of the gene-expression profiles of dysfunctional LCMV-specific CD8 T cells from chronic infection to functional LCMV-specific effector and memory CD8 T cells generated distinct transcriptome signatures [40]. These and other studies showed [12▪▪,42] that a hallmark of CD8 Tex cells is overexpression of multiple inhibitory receptors, including PDCD1, KLRG1, TIGIT, HAVCR2 (TIM3), LAG3, CTLA4, CD160, and CD244. Combined RNA and protein expression studies at the single cell led to identification of a module of co-inhibitory receptors that are co-expressed in both CD4+ and CD8+ T cells [43▪▪]. This module is part of a larger co-inhibitory gene program shared by nonresponsive T cells and driven by the immunoregulatory cytokine IL-27 and specific transcription factors [43▪▪]. Other Tex alterations identified by transcriptome profiling include: alterations in T-cell receptor and cytokine-signaling pathways; differential expression of genes involved in chemotaxis, adhesion, and migration; and expression of a distinct set of transcription factors. Subsequent network studies identified further differences between exhausted and memory CD8 T cells including differential connectivity for transcription factors TBX21 and EOMES [44].
-
(5)
Altered epigenetic landscape: The epigenetic landscape directly influences transcriptional regulation during cellular development, differentiation and therapeutic intervention. Considerable effort has gone into defining the epigenetic landscape of Tex, including studies of methylation [45–47], histone modification [48], accessible chromatin regions [20,49,50] and epigenetic-guided mass cytometry [51▪]. Together, these studies demonstrate that Tex represent a distinct T-cell lineage, exhibiting approximately as many differences (∼6000) in chromatin accessibility with memory (Tmem) or effector (Teff) CD8 T cells as monocytes have with B cells [12▪▪].
T-CELL EXHAUSTION AND CANCER THERAPY
Tumor-infiltrating lymphocytes (TILs) are often dysfunctional, limiting antitumor immunity despite the neoantigen-rich environment. Studies of CD8 T-cell dysfunction in tumors have shown that these cells share features with Tex, including overexpression of inhibitory receptors [6,27]. Although mechanisms underlying dysfunction of TILs are not well understood, treatment of tumors with monoclonal antibodies targeting inhibitory receptors leads to tumor regression in animal models [52–54] and in humans [20,55–57]. These findings have triggered investigations into the role of Tex in regulating antitumor immunity. Barber et al.[58] showed that blockade of the PD-1/PD-L1 inhibitory pathway restored the ability of Tex to undergo proliferation, secrete cytokines, kill infected cells and decrease viral load (Tex reinvigoration). Further studies in humans demonstrated that an imbalance between T-cell reinvigoration and tumour burden was linked to clinical response to an anti-PD-1 monoclonal antibody (pembrolizumab) [24]. More recent studies by Miller et al.[59▪▪] demonstrated the existence of two classes of dysfunctional CD8 TILs, ‘progenitor exhausted“ and “terminally exhausted’ Tex. Progenitor Tex retain polyfunctionality, persist long-term, respond to anti-PD-1 therapy, and may also differentiate into ‘terminally exhausted’ Tex. In contrast, terminally Tex cells are unable to respond to anti-PD-1 therapy. These and other studies [12▪▪] establish the PD-1 pathway as a specific target for manipulating T-cell exhaustion for therapeutic benefit in cancer, and, perhaps, chronic infections.
Despite the clinical successes of immune checkpoint inhibitors (ICI) like anti-PD1, they are only effective in some patients, with a significant fraction of patients that show no objective response [60]. In addition, serious immune-related adverse events (irAE) have been associated with ICI therapy, including colitis, pneumonitis, neuropathies, endocrinopathies, nephritis, dermatitis and arthritis [61,62▪,63,64]. More specifically, there are reports linking ICI therapy to development of T1D [65,66]. Multiple reports have suggested that irAE are associated with favorable clinical responses of tumor shrinkage upon ICI [67–73]. When taken together, these findings support a relationship between reinvigorating Tex, development of irAE and ultimately, the efficacy of ICI therapy.
T-CELL EXHAUSTION IN AUTOIMMUNITY
Given that autoimmune inflammation is driven by recognition of self-antigen and CD8 T-cell exhaustion dependent on chronic antigen stimulation, one may hypothesize that CD8 T-cell exhaustion may play a pivotal role in controlling CD8 immune responses towards islet beta cells in T1D. In fact, multiple lines of evidence suggest a relationship between Tex and T1D progression, including: the general association between higher levels of Tex and better prognosis in autoimmune disease [30–32,33▪▪]; the link between favorable treatment response to anti-CD3 monoclonal antibody (mAb) and Tex induction [33▪▪,74]; and, conversely, the induction of T1D in cancer patients upon Tex invigoration following IR blockade [65,66]. More specifically, in antineutrophil cytoplasmic antibody-associated vasculitis (ANCA) and systemic lupus erythematosus (SLE), good prognosis was associated with a CD8 exhaustion and poor CD4 help signature [30,31,33▪▪]. The same poor CD4 help signature was also observed in a smaller cohort of autoantibody positive pre-T1D and T1D subjects. However, the CD8 exhaustion transcription signature was not significantly enhanced in cases of slow T1D progression; this may be because of increased variability across T1D subjects, differences in the localization of disease (ANCA and SLE are systemic, whereas CD8 immune effects in T1D can be localized to the pancreas [75]), or incomplete CD8 Tex transcriptional signature in T1D. Importantly, in unpublished data, we found that the phenotype of islet-specific CD8 T cells typically includes more than one distinct phenotype (as shown by others [76,77]), and subjects with a greater proportion of exhausted islet-specific CD8 T cells demonstrated slower progression of T1D. Although this same Tex signature could also be detected in cells not identified as islet-specific with tetramer reagents, the T1D polyclonal exhausted population was smaller than that seen in systemic autoimmune diseases [30–32] and bystander cells in robust mouse models of diabetes [78] and TILs [79]. Importantly, the exhausted phenotype of islet-specific cells in slow progressors resembled that of TIGIT+KLRG1+PD-1+ cells with high EOMES expression that expand with teplizumab (anti-CD3) therapy in responders [33▪▪]. Whether islet-specific cells in teplizumab-treated responders are also exhausted is currently being investigated. Together, these findings suggest that CD8 Tex do restrain autoimmunity, and their therapeutic augmentation and maintenance is clinically beneficial.
To date, these examples of CD8 Tex association with T1D progression have been the exception, not the rule. We offer several reasons why this may be the case. First, the exhausted state is a complex phenotype, which requires multidimensional analyses to precisely define. However, in T1D, this phenotype is most evident in antigen-specific T cells, which are rare and difficult to study. Technologies have only now advanced sufficiently to identify phenotype-rare, autoantigen-specific T cells in a multidimensional manner. In addition, a three-step process is required for terminal development of Tex, any one of which may be lacking in autoimmunity. For example, it is possible that epitope-spreading, episodic as opposed to chronic antigen exposure, a pro-inflammatory cytokine environment, or enhanced co-stimulation may result in incomplete or unstable CD8 T-cell exhaustion in autoimmune and autoimmune prone subjects.
CONCLUSION
Together, these observations suggest that enhancing Tex would be of therapeutic benefit in autoimmune disease, in general and in T1D, specifically. The three-signal model of Tex induction offers a useful way of thinking about how to approach this possibility clinically (Fig. 1). One of the most obvious approaches would be to enhance signal 1 by TCR triggering in the absence of costimulation, as has been reported for anti-TCR monoclonal antibodies [33▪▪]. Another option may be to use islet antigen peptides to trigger TCRs more specifically in islet antigen-reactive T cells. Given the clinical tractability of blockade of inhibitory receptor–ligand interactions for ICI therapy, it may be worth considering whether soluble inhibitory receptor agonists or mAbs could be used to enhance signal 2. Another approach to enhancing signal 2, perhaps in combination with TCR agonists that trigger signal 1, would be to block positive costimulation through CD28. CD8 T-cell rescue via ICI therapy is CD28-dependant [80,81], suggesting an important role for the CD28/B7 pathway in PD-1 therapy of cancer patents. Importantly, abatacept, a blocker of CD28 costimulation, has shown some success in treating new-onset T1D subjects [82]. Finally, cytokine agonists or antagonists that promote an immunosuppressive environment might be used to trigger signal 3. Such effects are complex, but in general, could be selected to act in the opposite direction from agents that reverse T-cell exhaustion (i.e. select cytokine agonists to trigger exhaustion in cases where antagonists reverse exhaustion, and vice versa) [12▪▪]. Thus, although anti-IL10 has been reported to enhance checkpoint inhibitor blockade [83], IL-10 supplementation may be effective at enhancing Tex, perhaps in combination with signal 1 or 2 agonists. Within the framework of this three-signal model, some pathways may be implicated because of their association with both Tex and autoimmunity. For example, some cytokines that mediate amplification or resolution of chronic inflammation and exhaustion are also associated with some T1D subjects (e.g. IFN signature [84], reduced IL-2 pathway [85], enhanced IL-6 pathway [86]). Likewise, there are T1D-associated SNPs in some inhibitory receptors (e.g. PDCD1, CTLA4). Thus, T1D and other autoimmune diseases may result, in part, from impaired CD8 T-cell exhaustion, and therapeutic benefit may result from restoration of faulty checkpoints. Overall, augmentation and expansion of Tex may benefit T1D, and ultimately all autoimmune subjects.
FIGURE 1.
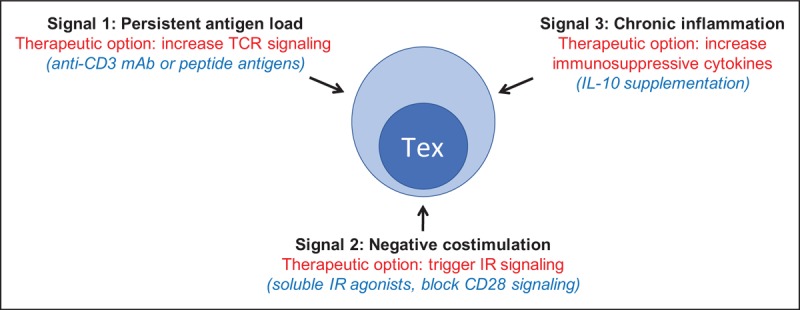
Therapeutic options for Tex in type 1 diabetes. Therapeutic options for T1D based on the hypothesis that Tex induction can be therapeutic for T1D. Black, signals constituting the three-signal model of Tex induction adapted from [12▪▪]; red, broad therapeutic options for triggering Tex and blocking T1D; and blue, more specific therapeutic options. T1D, type 1 diabetes.
Acknowledgements
We thank Dr Hannah DeBerg for critical reading of this review.
Financial support and sponsorship
The authors are supported, in part, by the NIH and JDRF to study CD8 T-cell exhaustion in T1D, the topic of this review.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Ehlers MR. Strategies for clinical trials in type 1 diabetes. J Autoimmun 2016; 71:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenbaum C, VanBuecken D, Lord S. Disease-modifying therapies in type 1 diabetes: a look into the future of diabetes practice. Drugs 2019; 79:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu H, Paiva R, Flavell RA. Harnessing the power of regulatory T-cells to control autoimmune diabetes: overview and perspective. Immunology 2018; 153:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tisch R, Wang B. Dysrulation of T cell peripheral tolerance in type 1 diabetes. Adv Immunol 2008; 100:125–149. [DOI] [PubMed] [Google Scholar]
- 5.Redondo MJ, Steck AK, Pugliese A. Genetics of type 1 diabetes. Pediatr Diabetes 2018; 19:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 2014; 35:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015; 348:56–61. [DOI] [PubMed] [Google Scholar]
- 8.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015; 27:450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018; 8:1069–1086. [DOI] [PubMed] [Google Scholar]
- 11.Crespo J, Sun H, Welling TH, et al. T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment. Curr Opin Immunol 2013; 25:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪▪.McLane LM, Abdel-Hakeem MS, Wherry EJ. CD8 T cell exhaustion during chronic viral infection and cancer. Annu Rev Immunol 2019; 37:457–495. [DOI] [PubMed] [Google Scholar]; This is an exhaustive review of the current state of knowledge regarding molecular, cellular and clinical aspects of T-cell exhaustion.
- 13.Pauken KE, Wherry EJ. SnapShot: T-cell exhaustion. Cell 2015; 163:1038.e1–1038.e1. [DOI] [PubMed] [Google Scholar]
- 14.Zehn D, Wherry EJ. Immune memory and exhaustion: clinically relevant lessons from the LCMV Model. Adv Exp Med Biol 2015; 850:137–152. [DOI] [PubMed] [Google Scholar]
- 15.Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492–499. [DOI] [PubMed] [Google Scholar]
- 16.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature 1993; 362:758–761. [DOI] [PubMed] [Google Scholar]
- 17.Zajac AJ, Blattman JN, Murali-Krishna K, et al. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med 1998; 188:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallimore A, Glithero A, Godkin A, et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med 1998; 187:1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015; 15:486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol 2015; 36:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeidi A, Zandi K, Cheok YY, et al. T-cell exhaustion in chronic infections: reversing the state of exhaustion and reinvigorating optimal protective immune responses. Front Immunol 2018; 9:2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richter K, Brocker T, Oxenius A. Antigen amount dictates CD8+ T-cell exhaustion during chronic viral infection irrespective of the type of antigen presenting cell. Eur J Immunol 2012; 42:2290–2304. [DOI] [PubMed] [Google Scholar]
- 23.Zuniga EI, Harker JA. T cell exhaustion due to persistent antigen: Quantity not quality? Eur J Immunol 2012; 42:2285–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017; 545:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee PP, Yee C, Savage PA, et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med 1999; 5:677–685. [DOI] [PubMed] [Google Scholar]
- 26.Baitsch L, Baumgaertner P, Devêvre E, et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J Clin Invest 2011; 121:2350–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114:1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Huang S, Gong D, et al. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human nonsmall cell lung cancer. Cell Mol Immunol 2010; 7:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother CII 2007; 56:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKinney EF, Lee JC, Jayne DRW, et al. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015; 523:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKinney EF, Smith KG. T cell exhaustion and immune-mediated disease-the potential for therapeutic exhaustion. Curr Opin Immunol 2016; 43:74–80. [DOI] [PubMed] [Google Scholar]
- 32.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007; 8:239–245. [DOI] [PubMed] [Google Scholar]
- 33▪▪.Long SA, Thorpe J, DeBerg HA, et al. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci Immunol 2016; 1: pii: eaai7793. [DOI] [PMC free article] [PubMed] [Google Scholar]; The first study linking T-cell exhaustion and therapeutic benefit in human autoimmune disease.
- 34.Bi J, Tian Z. NK cell exhaustion. Front Immunol 2017; 8:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moir S, Fauci AS. B-cell responses to HIV infection. Immunol Rev 2017; 275:33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crawford A, Angelosanto JM, Kao C, et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 2014; 40:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morou A, Palmer BE, Kaufmann DE. Distinctive features of CD4+ T cell dysfunction in chronic viral infections. Curr Opin HIV AIDS 2014; 9:446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wherry EJ, Blattman JN, Murali-Krishna K, et al. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 2003; 77:4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang KS, Recher M, Navarini AA, et al. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol 2005; 35:738–745. [DOI] [PubMed] [Google Scholar]
- 40.Wherry EJ, Ha S-J, Kaech SM, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007; 27:670–684. [DOI] [PubMed] [Google Scholar]
- 41.Bengsch B, Johnson AL, Kurachi M, et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8(+) T cell exhaustion. Immunity 2016; 45:358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McLane LM, Banerjee PP, Cosma GL, et al. Differential localization of T-bet and Eomes in CD8 T cell memory populations. J Immunol 19502013; 190:3207–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪▪.Chihara N, Madi A, Kondo T, et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 2018; 558:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification of a molecular circuit that underlies the co-expression of co-inhibitory receptors in T cells.
- 44.Doering TA, Crawford A, Angelosanto JM, et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 2012; 37:1130–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youngblood B, Noto A, Porichis F, et al. Cutting edge: prolonged exposure to HIV reinforces a poised epigenetic program for PD-1 expression in virus-specific CD8 T cells. J Immunol 2013; 191:540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn E, Youngblood B, Lee J, et al. Demethylation of the PD-1 promoter is imprinted during the effector phase of CD8 T cell exhaustion. J Virol 2016; 90:8934–8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Youngblood B, Oestreich KJ, Ha S-J, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity 2011; 35:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang F, Zhou X, DiSpirito JR, et al. Epigenetic manipulation restores functions of defective CD8+ T cells from chronic viral infection. Mol Ther J Am Soc Gene Ther 2014; 22:1698–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sen DR, Kaminski J, Barnitz RA, et al. The epigenetic landscape of T cell exhaustion. Science 2016; 354:1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pauken KE, Sammons MA, Odorizzi PM, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016; 354:1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51▪.Bengsch B, Ohtani T, Khan O, et al. Epigenomic-guided mass cytometry profiling reveals disease-specific features of exhausted CD8 T cells. Immunity 2018; 48:1029.e5–1045.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Provides a resource for investigating Tex cell biology, with implications for biomarkers and therapeutic targeting in chronic infections, autoimmunity, and cancer.
- 52.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996; 271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 53.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002; 99:12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793–800. [DOI] [PubMed] [Google Scholar]
- 55.Le Mercier I, Lines JL, Noelle RJ. Beyond CTLA-4 and PD-1, the generation Z of negative checkpoint regulators. Front Immunol 2015; 6:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Couzin-Frankel J. Cancer immunotherapy. Science 2013; 342:1432–1433. [DOI] [PubMed] [Google Scholar]
- 57.Wolchok J. Putting the immunologic brakes on cancer. Cell 2018; 175:1452–1454. [DOI] [PubMed] [Google Scholar]
- 58.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682–687. [DOI] [PubMed] [Google Scholar]
- 59▪▪.Miller BC, Sen DR, Al Abosy R, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 2019; 20:326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]; Molecular identification functionally distinct subsets of Tex.
- 60.Alexander W. The checkpoint immunotherapy revolution. Pharm Ther 2016; 41:185–191. [PMC free article] [PubMed] [Google Scholar]
- 61.Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016; 13:473–486. [DOI] [PubMed] [Google Scholar]
- 62▪.Clotman K, Janssens K, Specenier P, et al. Programmed cell death-1 inhibitor-induced type 1 diabetes mellitus. J Clin Endocrinol Metab 2018; 103:3144–3154. [DOI] [PubMed] [Google Scholar]; Demonstration that development of T1D may be an immune-related adverse event of checkpoint inhibitor therapy.
- 63.Le Burel S, Champiat S, Mateus C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-programmed cell death 1/anti-programmed cell death-ligand 1 agents: a single-centre pharmacovigilance database analysis. Eur J Cancer 2017; 82:34–44. [DOI] [PubMed] [Google Scholar]
- 64.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018; 4:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018; 67:1471–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes J, Vudattu N, Sznol M, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 2015; 38:e55–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2017; 4:374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yuan J, Adamow M, Ginsberg BA, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A 2011; 108:16723–16728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol 2015; 151:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: a multiinstitutional retrospective study. J Dermatol 2017; 44:117–122. [DOI] [PubMed] [Google Scholar]
- 71.Hua C, Boussemart L, Mateus C, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016; 152:45–51. [DOI] [PubMed] [Google Scholar]
- 72.Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 2016; 22:886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Teulings H-E, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015; 33:773–781. [DOI] [PubMed] [Google Scholar]
- 74.Perdigoto AL, Preston-Hurlburt P, Clark P, et al. Immune Tolerance Network. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia 2019; 62:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Culina S, Lalanne AI, Afonso G, et al. ImMaDiab Study Group. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol 2018; 3: pii: eaao4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ogura H, Preston-Hurlburt P, Perdigoto AL, et al. Identification and analysis of islet antigen-specific CD8+ T cells with T cell libraries. J Immunol 2018; 201:1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laban S, Suwandi JS, van Unen V, et al. Heterogeneity of circulating CD8 T-cells specific to islet, neo-antigen and virus in patients with type 1 diabetes mellitus. PloS One 2018; 13:e0200818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Christoffersson G, Chodaczek G, Ratliff SS, et al. Suppression of diabetes by accumulation of nonislet-specific CD8+ effector T cells in pancreatic islets. Sci Immunol 2018; 3: pii: eaam6533. [DOI] [PubMed] [Google Scholar]
- 79.Simoni Y, Becht E, Fehlings M, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018; 557:575–579. [DOI] [PubMed] [Google Scholar]
- 80.Hui E, Cheung J, Zhu J, et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 2017; 355:1428–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kamphorst AO, Wieland A, Nasti T, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017; 355:1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Orban T, Bundy B, Becker DJ, et al. Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 2011; 378:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brooks DG, Ha S-J, Elsaesser H, et al. IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A 2008; 105:20428–20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lombardi A, Tsomos E, Hammerstad SS, Tomer Y. Interferon alpha: the key trigger of type 1 diabetes. J Autoimmun 2018; 94:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosenzwajg M, Churlaud G, Hartemann A, Klatzmann D. Interleukin 2 in the pathogenesis and therapy of type 1 diabetes. Curr Diab Rep 2014; 14:553. [DOI] [PubMed] [Google Scholar]
- 86.Jones BE, Maerz MD, Buckner JH. IL-6: a cytokine at the crossroads of autoimmunity. Curr Opin Immunol 2018; 55:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


