Abstract
A recent push to provide more translationally relevant preclinical models for examination of pharmacological mechanisms underlying inhaled substances of abuse has resulted in the development of equipment and methods that allows exposure of freely moving rodents to aerosolized psychoactive drugs. In the present study, synthetic cannabinoids (CP55,940, ABCHMINACA, and AMB-FUBINACA) were administered intraperitoneally (i.p.) or aerosolized via a modified electronic cigarette device. Subsequently, the compounds were evaluated in adult male and female C57/Bl6 mice trained to discriminate i.p. 5.6 mg/kg Δ9-tetrahydrocannabinol (THC) for food reinforcement. When administered i.p., THC and AB-CHMINACA were equally potent at producing THC-like effects in both sexes, but CP55,940 and AMB-FUBINACA were more potent in males. Upon aerosol exposure, all compounds continued to produce THC-like effects in both sexes, with AMB-FUBINACA remaining the most potent. In contrast, aerosolized CP55,940 showed substantial decreases in potency in both sexes. Aerosolized nicotine did not substitute for THC in either sex. In females, aerosolized cumyl-4CN-BINACA produced concentration-dependent increases in responding on the THC-associated nosepoke. In addition, the effects of an active concentration of AMB-FUBINACA were reversed by rimonabant, suggesting CB1 receptor mediation. These results show that synthetic cannabinoids produce THC-like effects when injected i.p. or after aerosolization. This study adds to a growing literature suggesting that evaluation of abuse liability of substances via aerosol exposure is feasible and may provide a translationally relevant method that allows for investigation of factors important to the abuse of drugs which humans typically smoke or vape.
Keywords: drug discrimination, synthetic cannabinoids, tetrahydrocannabinol, vaping
1.0. Introduction
More than a decade after their initial synthesis for research purposes, synthetic cannabinoids such as the indole-derived compound JWH-018 began to appear on the recreational drug market, reaching worldwide scale of distribution in the early 2000s. Since then, design of the chemical structures of these compounds has evolved rapidly to evade drug control regulations, resulting in production and use of more potent second and third generation compounds for which available scientific data (especially in vivo data) are lacking. Not surprisingly, reports of aversive physical and psychological consequences up to and including death have increased with the advent of these newer synthetic cannabinoids (Adams et al., 2017; European Monitoring Centre for Drugs and Drug Addiction, 2018; Trecki et al., 2015). For example, AMB-FUBINACA (an indazole-derived cannabinoid) was linked to a cluster of overdose cases characterized by severe intoxication and incapacitation in New York City in July 2016 (Adams et al., 2017) and to at least 20 deaths in New Zealand (New Zealand Ministry of Health, 2018). AB-CHMINACA (an indazole-derived cannabinoid) was also linked to an overdose cluster in the spring of 2014 in Florida (Tyndall et al., 2015), as well as to sporadic reports of illness or death (Trecki et al., 2015).
Despite their variant chemical structure and toxicological profile, preclinical research suggests that synthetic cannabinoids represent a class of compounds that produce their cannabimimetic psychoactive effects through activation of CB1 receptors located within the brain’s endogenous cannabinoid system (Noble et al., 2018; Wiley et al., 1998; Wiley et al., 2015). Most of these compounds also activate CB2 cannabinoid receptors in the periphery (Huffman and Padgett, 2005; Manera et al., 2008; Noble et al., 2018). CB1 receptor affinities of abused synthetic cannabinoids are usually several-fold greater than Δ9-tetrahydrocannabinol (THC), the primary psychoactive constituent of cannabis, often resulting in enhanced potency compared to this phytocannabinoid (Noble et al., 2018; Wiley et al., 2017b). Further, synthetic cannabinoids tend to be full agonists at the CB1 receptor (Thomas et al., 2017) whereas THC is a partial agonist (Breivogel and Childers, 2000). Indeed, several recent compounds are reported to have greater CB1 receptor efficacy than other previously identified synthetic cannabinoids and have been referred to as “super agonists” (Thomas et al., 2017). In vivo, synthetic cannabinoids produce a profile of pharmacological effects in rodents that is characteristic of psychoactive cannabinoids such as THC: suppression of locomotor activity, antinociception, hypothermia and catalepsy (Wiley et al., 1998; Wiley et al., 2014b; Wiley et al., 2017b). They also produce THC-like effects in THC drug discrimination (reviewed in Wiley et al., 2018), a pharmacologically selective animal model of cannabis intoxication (Balster and Prescott, 1992). With only few exceptions (Wiley et al., 2017a; Wiley et al., 2011; Winsauer et al., 2012), the bulk of this research has been conducted in male rodents. Hence, one of the goals of the present study was to perform a side-by-side comparison of the discriminative stimulus effects of THC and selected synthetic cannabinoids in mice of both sexes trained under identical experimental conditions.
A second aim of the present study was to compare the discriminative stimulus effects of synthetic cannabinoids administered via intraperitoneal (i.p.) injection and aerosol exposure. In humans, synthetic cannabinoids are typically administered through smoking of infused plant material or vaping cannabinoid-containing e-liquids in tank-based electronic cigarettes (e-cigarettes). Recently, a push to provide a more translationally relevant preclinical model for examination of pharmacological mechanisms underlying inhaled substances of abuse has resulted in the development of equipment and methods that allows exposure of freely moving rodents to aerosolized psychoactive drugs (George et al., 2010; Lefever et al., 2017a; McLaughlin, 2018; Ponzoni et al., 2015; Smith et al., 2015). Using modified e-cigarette devices, these studies have shown that acute aerosol exposure to nicotine, stimulants, or cannabinoids produced pharmacological effects in rodents that are characteristic of their respective drug classes (Lefever et al., 2017a; Lefever et al., 2017b; Marusich et al., 2016; Nguyen et al., 2016a; Nguyen et al., 2016b). Further, for nicotine and THC, the observed in vivo effects are accompanied by increases in plasma and brain concentrations of the parent drug and/or its metabolites (Lefever et al., 2017a; Manwell et al., 2014a; Nguyen et al., 2016b). Here, we utilized these new methods to compare the effects of aerosolized e-liquids containing synthetic cannabinoids to injected doses of these compounds in mice trained to discriminate injected THC from vehicle in a standard two response procedure.
2.0. Materials and methods
2.1. Subjects
Twenty-seven adult male and female C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were singly housed in polycarbonate mouse cages in a temperature-controlled (20–22°C) colony room with a 12-hour light/dark cycle (lights on at 6:00 AM). After at least 7 days of acclimation with unrestricted food and water, mice were maintained at ~90% of free-feeding body weight with ad libitum access to water. All studies reported in this manuscript were carried out in accordance with guidelines published in the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and were approved by our Institutional Animal Care and Use Committee.
2.2. Apparatus
Mice were trained and tested in mouse operant chambers (Coulbourn Instruments, Whitehall, PA) housed within light- and sound-attenuating cubicles. Each chamber was outfitted with a house light, white noise generator, and two nose-poke apertures with stimulus lights over each aperture. A pellet feeder delivered 20 mg food pellets (Bioserv Inc., Frenchtown, NJ) into a pellet trough (with a light) centered between the two apertures. Chamber operations (i.e., illumination of house and stimulus lights, generation of white noise, delivery of food pellets, and recording of nose pokes) were controlled by a computer system (Coulbourn Instruments, Graphic State Software, v 3.03, Whitehall, PA).
With the exception of tests with cumyl-4CN-BINACA in female mice, aerosol was delivered from a modified e-cigarette device to mouse-sized chambers, as described previously (Lefever et al., 2017a; Lefever et al., 2017b; Marusich et al., 2016). Briefly, an iStick 30 W Variable Wattage personal vaporizer (ELeaf, Irvine, CA, USA) supplied power (7 W) to a CE5-S tank and bottom dual coil clearomizer (1.8Ω) (Aspire, Kent, WA, USA). Air was pumped through the tank at 1 L/min by an adjustable air pump (Pacific Coast Distributing, Phoenix, AZ) to generate aerosol, which was delivered via Tygon tubing (Fisher Scientific, Pittsburgh, PA, USA) directed by 3-way stopcocks (Grainger, Raleigh, NC, USA) into an EZ-177 Sure-Seal mouse induction anesthesia chamber (10 cm × 10 cm × 10 cm) (E-Z-Anesthesia, Palmer, PA, USA).
Cumyl-4CN-BINACA was evaluated in female mice in a commercially available aerosol exposure system, E-Vape (LJARI, La Jolla, CA). Mice were exposed using the same dosing chambers described above; however, airflow was constant (1L/min) and vapor was generated using the E-Vape system [TFV8 X-Baby tank and 0.25Ω V8 X-Baby M2 coils, SMOK, Shenzhen, China] set to 40W, delivering hits every 10 s for 2 min. Mice remained in the chamber for 1 additional min before returning to their home cage for a 5 min before being placed into the operant chambers.
2.3. Chemicals
CP55,940 [[(2)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl) cyclohexanol])] (National Institute on Drug Abuse [NIDA], Rockville, MD, USA), AB-CHMINACA [(N-[1-amino-3-methyl-oxobutan-2-yl]-1-[cyclohexylmethyl]-1H-indazole-3-carboxamide)] (Drug Enforcement Administration [DEA] Special Testing and Research Laboratory, Dulles, VA, USA), AMB-FUBINACA [methyl (2S)-2-[[1-[(4-fluorophenyl)methyl]indazole-3-carbonyl]amino]-3-methylbutanoate] (Cayman Chemical, Ann Arbor, MI), rimonabant (NIDA), and delta-9-THC (NIDA) were dissolved in 7.8% polysorbate 80 NF (Fisher Scientific, Pittsburgh, PA, USA) and 92.2% saline USP (Patterson Veterinary, Devens, MA, USA) for injection. CP55,940, AB-CHMINACA, AMB-FUBINACA, and cumyl-4CN-BINACA [1-(4-cyanobutyl)-N-(1-methyl-1-phenylethyl)-1H-indazole-3-carboxamide] (Cayman Chemical) were mixed with poly ethylene glycol 400 (PEG400) (Fisher Scientific, Pittsburgh, PA, USA) for aerosol administration. (−)-Nicotine hydrogen freebase (Sigma-Aldrich, St. Louis, MO) was mixed with a 50:50 propylene glycol and glycerin solution (Sigma-Aldrich) for aerosol administration. All injected compounds were administered at a volume of 10 mL/kg, with pretreatment intervals of 20 or 30 min (see procedures section), with the exception of rimonabant which was injected 30 min prior to the start of AMB-FUBINACA exposure. All cannabinoids were injected intraperitoneally (i.p.). Concentrations for aerosol administration are expressed as mg/mL in the e-cigarette tank and may not be representative of the actual amount of drug administered.
2.4. Procedure
Prior to the start of this study, mice (13 males and 14 females) had been trained to discriminate 5.6 mg/kg THC and had been tested with several cannabinoids. Briefly, mice were trained to respond on one of the two nose-poke apertures in the operant chamber following intraperitoneal (i.p.) administration of 5.6 mg/kg THC and to respond on the other aperture following i.p. vehicle administration according to a fixed ratio 10 (FR10) schedule of food reinforcement, under which 10 consecutive responses on the correct (injection-appropriate) lever resulted in delivery of a food pellet. Responses on the incorrect lever reset the ratio requirement on the correct lever. Daily injections were administered on a double alternation sequence of training drug and vehicle (e.g., THC, THC, vehicle, vehicle). Daily 15 min training sessions were held Monday-Friday.
After acquisition, stimulus substitution tests were conducted in place of training sessions, with baseline discrimination training continuing between stimulus substitution test days. During the 15 min stimulus substitution tests, 10 consecutive responses on either aperture delivered reinforcement. If a mouse responded on the other aperture prior to completing 10 responses on an aperture, the ratio requirement on the original aperture was reset. To be eligible for a stimulus substitution test, mice must have completed a training session the previous day in which three criteria had been met: (1) the first completed FR10 was on the correct lever, (2) ≥ 80% of the total responding occurred on the correct lever, and (3) response rate must have been ≥ 0.1 responses/s. In addition, the mouse must have met these same criteria during the most recent training session with the alternate training compound (training drug or vehicle). Prior to testing with injected and aerosolized synthetic cannabinoids, a dose-effect curve for THC was determined. THC was injected 30 min pre-session.
To allow for the possibility that aerosolized cannabinoids might have a more rapid onset than injected cannabinoids, the pre-session injection interval was changed from 30 min to 20 min after completion of the initial THC dose-effect curve. Hence, all drugs except for THC were administered 20 min prior to the start of the drug discrimination session, regardless of route of administration. For exposure to aerosolized synthetic cannabinoids, mice were placed individually into closed anesthesia chambers, aerosol was generated for 10 s, and mice were held in the chamber for a 5-min exposure period. After the exposure period, mice were moved to their home cages for 15 additional minutes and then placed in the operant chambers for a drug discrimination test session. The same exposure timing and duration was used for nicotine; however, mice were placed immediately into the operant chamber after the 5-min exposure period. For cumyl-4CN-BINACA (females only), aerosol was generated for 3 s every 10 s for 2 min for a total of 10 3-s exposures over the 3-min time in the chamber. Mice were subsequently placed in their home cage for 5 min prior to being placed into the operant chamber for discrimination testing. In females, rimonabant was injected i.p. 30 min prior to 5-min exposure to aerosolized vehicle or 2.4 mg/mL AMB-FUBINACA and 15-min wait before the start of the drug discrimination session. During substitution tests with injected synthetic cannabinoids, CP55,940, AB-CHMINACA and AMB-FUBINACA, mice were injected i.p. and immediately placed into the closed anesthesia chamber for 5-min exposure to vehicle followed by the 15-min wait in their home cage.
2.5. Data analysis
For each drug discrimination session, percentage of responses on the drug-associated aperture and response rate (responses/s) were calculated. ED50 values were calculated on the linear part of the drug manipulandum selection dose-response curve for each drug that fully substituted (i.e., > 80% drug-associated responding) using least squares linear regression analysis, followed by calculation of 95% confidence intervals (CI). Response-rate data for injected drugs were analyzed using split-plot ANOVA, with sex as the between subject factor and dose as the within subject factor. Because dose could not be calculated for aerosolized drug (and, therefore, could not be equalized across sex), response rate data for aerosolized drug were analyzed separately in each sex through the use of one-way ANOVAs across concentration. Data from sessions in which a mouse did not earn food reinforcement (i.e., fewer than 10 consecutive responses were registered on either aperture) were excluded from analysis of percentage of drug response selection and from the corresponding figure, but response rate data were included. For all dose-effect curves, Tukey post hoc tests (α=0.05) were used, as appropriate, to determine differences in response rates between individual means. NCSS 11 Statistical Software (2016; NCSS, LLC. Kaysville, Utah, USA, ncss.com/software/ncss) was used for all analyses.
3.0. Results
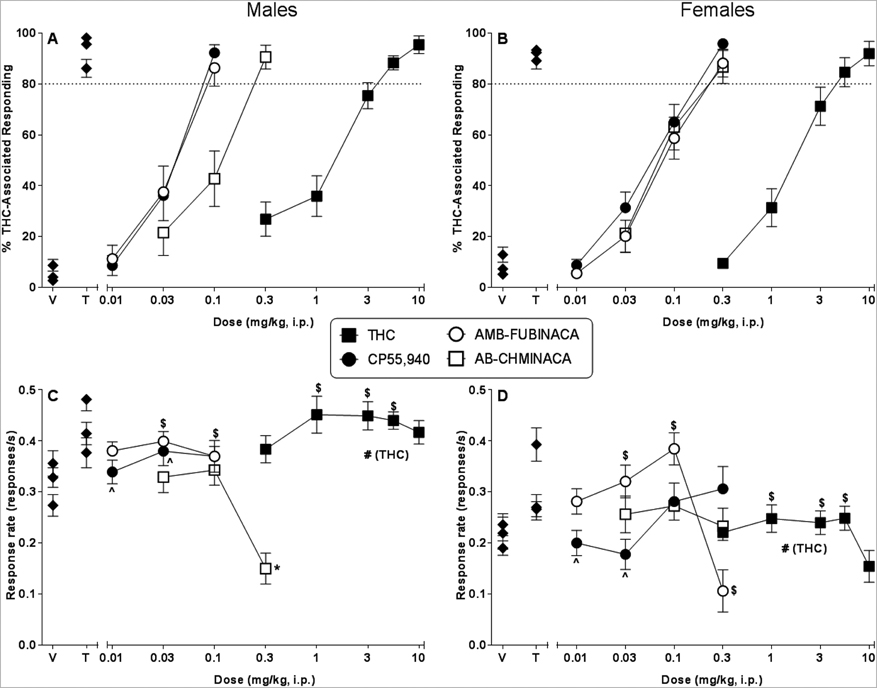
Mice of both sexes exhibited full dose-dependent substitution for the THC training dose when injected i.p. with THC (Figure 1, panels A and B for male and female mice, respectively). Further, potency was not substantially different across sex (Table 1). Similarly, the indazole-based synthetic cannabinoids, AB-CHMINACA and AMB-FUBINACA, and the bicyclic cannabinoid CP55,940 also fully and dose-dependently substituted for THC in both sexes following injection (Figure 1, panels A and B for male and female mice, respectively). AB-CHMINACA was of similar potency in both sexes; however, CP55,940 and AMB-FUBINACA were 2- and 1.75-fold less potent, respectively, in females than in males (Table 1). Further, full substitution occurred with 0.1 mg/kg CP55,940 and AMB-FUBINACA in male mice (Figure 1, panel A) whereas the lowest dose of either compound that produced full substitution in female mice was 0.3 mg/kg (Figure 1, panel B). In males, CP55,940 and AMB-FUBINACA were more potent than ABCHMINACA (non-overlapping 95% confidence limits) whereas CP55,940, AB-CHMINACA and AMB-FUBINACA were similar in potency in females (Table 1).
Figure 1.
Effects of i.p. THC (filled squares), CP55,940 (filled circles), AB-CHMINACA (unfilled squares), and AMB-FUBINACA (unfilled circles) on percentage of responses that occurred on the THC-associated aperture in male (panel A) and female (panel B) C57/Bl6J mice trained to discriminate 5.6 mg/kg THC (i.p.) from vehicle. Response rates for male (panel C) and female (panel D) mice for each dose are also shown. Points (filled diamonds) at the left side of each panel represent results of control tests with injected (i.p.) vehicle (V) and 5.6 mg/kg THC (T). Each point represents the mean (± SEM) of data for 12–13 male mice (panels A and C) or 12–14 female mice (panels B and D). Pound sign (#) indicates significant main effect of sex (p<0.05). Dollar sign ($) indicates significant main effect of dose or concentration (p<0.05) compared to overall vehicle condition. Asterisk (*) indicates significant interaction term and post hoc difference (p<0.05) compared to respective vehicle. Caret (^) indicates significant interaction term and post hoc difference (p<0.05) compared to other sex at indicated dose.
Table 1.
Effects of injected and aerosolized synthetic cannabinoids in mice trained to discriminate 5.6 mg/kg THC from vehicle
| Intraperitoneal Injection | Aerosol | |||
|---|---|---|---|---|
| ED50 (mg/kg) (± 95% CI) |
EC50 (mg/ml) (± 95% CI) |
|||
| Test substance CB1 receptor affinitya |
Males | Females | Males | Females |
|
THC CB1 Ki = 50 nMb |
1.50 (1.20 – 1.89) |
1.55 (1.23 – 1.94) |
Not tested | Not tested |
|
CP55,940 CB1 Ki = 1.74 nMc |
0.03 (0.03 – 0.04) |
0.06 (0.05 – 0.07) |
6.00 (3.92 – 9.17) |
7.32 (5.63 – 9.51) |
|
AB-CHMINACA CB1 Ki = 0.78 nMd |
0.09 (0.07 – 0.13) |
0.08 (0.06 – 0.10) |
3.67 (3.38 – 3.99) |
5.62 (4.99 – 6.32) |
|
AMB-FUBINACA CB1 Ki = 2 nMb |
0.04 (0.03 – 0.05) |
0.07 (0.06 – 0.09) |
0.39 (0.32 – 0.46) |
0.94 (0.74 – 1.20) |
|
Cumyl-4CN-BINACA CB1 Ki = 8.58 nMb |
Not tested | Not tested | Not tested | 1.86 (1.21 – 2.84) |
[3H]CP55,940 was used as ligand for all CB1 binding assays.
Personal communication (Michelle Glass, University of Otago, New Zealand). Binding affinities were obtained using methods described in (Banister et al., 2019).
Response rates were also affected by i.p. drug administration (Figure 1, panels C and D, males and females, respectively). THC increased responding in both sexes over a dose range of 1–5.6 mg/kg [main effect of dose: F(5,125)=4.70, p<0.05], but overall response rate in females was less at baseline and across all THC doses [main effect of sex: F(1,25)=93.41, p<0.05]. CP55,940 administration also resulted in significantly lower response rates in females than in males at lower doses (0.01 and 0.03 mg/kg) [interaction effect: F(4,87)=2.52, p<0.05], although it did not significantly decrease responding at any dose in either sex compared to their respective vehicles. In contrast, AB-CHMINACA significantly decreased response rates (compared to vehicle) at 0.3 mg/kg in male mice, but did not affect response rates in female mice at any dose [interaction effect: F(3,75)=5.75, p<0.05]. AMB-FUBINACA produced a biphasic effect on response rates: whereas it increased overall responding at lower (0.03 and 0.1 mg/kg) doses, decreased response rates were observed in females at 0.3 mg/kg AMB-FUBINACA, a dose that was not tested in males [main effect of dose: F(4,83)=8.74, p<0.05].
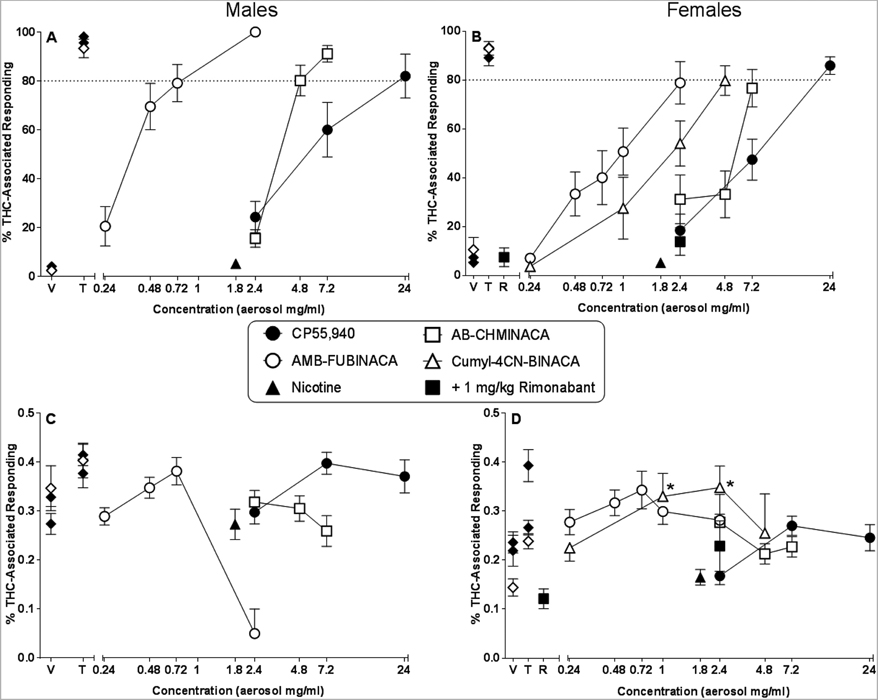
Figure 2 (panels A and B for male and female mice, respectively) shows results of substitution tests with aerosolized CP55,940, AB-CHMINACA, AMB-FUBINACA, and cumyl-4CNBINACA (females only, panel B). Whereas the first three of these compounds produced full concentration-dependent substitution for THC in male mice (panel A), only CP55,940 did so in female mice (panel B). The 80% criterion for full substitution was not quite reached in females, although concentration-dependent increases in THC-like responding were observed for ABCHMINACA, AMB-FUBINACA and for cumyl-4CN-BINACA. In females, the THC-like effects of the 2.4 mg/ml concentration of AMB-FUBINACA were reversed by co-administration of 1 mg/kg rimonabant (panel B), a dose that did not produce THC-aperture responding when administered alone (left side of panel B). In contrast with the synthetic cannabinoids, the 1.8 mg/mL concentration of nicotine did not engender responding on the THC-associated aperture in either sex.
Figure 2.
Effects of aerosolized CP55,940 (filled circles), AB-CHMINACA (unfilled squares), AMB-FUBINACA (unfilled circles), cumyl-4CN-BINACA (unfilled triangles; females only) and 1.8 mg/ml nicotine (filled triangles) on percentage of responses that occurred on the THC-associated aperture in male (panel A) and female (panel B) C57/Bl6J mice trained to discriminate 5.6 mg/kg THC (i.p.) from vehicle. Response rates for male (panel C) and female (panel D) mice for each dose are also shown. Also shown are results of control test with i.p. 1 mg/kg rimonabant alone (R; left side of panels B and D; filled squares) and in combination with 2.4 mg/ml aerosolized AMB-FUBINACA (right side of panels B and D; filled squares). Points (filled diamonds) at the left side of each panel represent results of control tests with vehicle (V) and 5.6 mg/kg THC (T) (filled diamonds = i.p. injection alone; unfilled diamonds = i.p. injection followed by vehicle aerosol exposure). At each concentration, 12–13 male mice (panels A and C) or 12–14 female mice (panels B and D) were evaluated for response rate data and for percentage THC-aperture responding, except n=6 and 7 male and female mice, respectively, for aerosol vehicle; n=3 male mice at the 2.4 mg/ml concentration of AMB-FUBINACA; n=8 female mice for tests with rimonabant (R) alone and in combination with 2.4 mg/ml AMB-FUBINACA; and n=6 female mice at each concentration of cumyl-4CN-BINACA. In addition, data for percentage THC aperture responding only (not for response rate) were excluded from mean calculations for mice that did not respond at least 10 times on either aperture (i.e., did not meet the criteria for choosing an aperture). Hence, for this measure, the mean percentage of THC aperture responding was comprised of data from 5 mice at the 4.8 mg/ml concentration of cumyl-4CN-BINACA, and n=1 and 11 male and female mice, respectively, at the 2.4 mg/ml concentration of AMB-FUBINACA. Each point represents the mean (± SEM) of included data, as specified above. Asterisk (*) indicates significant difference (p<0.05) compared to respective vehicle.
Sex differences in weight and the inability to calculate dose complicate between-sex comparisons of potency; however, relative potencies of the aerosolized compounds were the same for both sexes: AMB-FUBINACA > AB-CHMINACA > CP55,940, with cumyl-4CN-BINACA in between AMB-FUBINACA and AB-CHMINACA for the females. In males, relative potencies of the CP55,940 and AB-CHMINACA were reversed following aerosol exposure as compared to i.p. injection, with CP55,940 being 3-fold more potent than AB-CHMINACA when each compound was administered i.p. and 1.6-fold less potent than AB-CHMINACA when each was aerosolized. This effect was not pronounced in females. However, in mice of both sexes, aerosolized AMB-FUBINACA showed substantially greater relative potency than aerosolized CP55,940 and AB-CHMINACA whereas i.p. AMB-FUBINACA was of similar potency to i.p. CP55,940 (both sexes) and/or i.p. AB-CHMINACA (females only).
Figure 2 shows effects of aerosolized synthetic cannabinoids on response rates in male and female mice (panels C and D, respectively). CP55,940, AB-CHMINACA and AMB-FUBINACA did not significantly affect response rates (compared to aerosolized vehicle) in either sex. (Note: although the 2.4 mg/mL concentration of the latter decreased responding in males, the fact that only 3 males were evaluated at this concentration precluded inclusion of this concentration in statistical analysis.) In contrast, at concentrations of 1 and 2.4 mg/mL, aerosolized cumyl-4CN-BINACA increased responding (compared to aerosolized vehicle) in females [F(4,20)=4.19, p<0.05].
4.0. Discussion
Consistent with previous results, THC served as a robust discriminative stimulus in male mice (present study; Gamage et al., 2018; Wiley et al., 2015) and rats (Wiley et al., 2014a; Wiley et al., 1995). The present study also is one of the few reports of THC’s discriminative stimulus effects in female rodents (Wiley et al., 2018; Wiley et al., 2011; Winsauer et al., 2012). In mice, use of the same THC training dose (5.6 mg/kg) was effective in both sexes (as reported previously; Wiley et al., 2011), and potency for producing responding on the THC-associated aperture was similar, although overall response rates were lower at baseline and across all THC doses in females. In contrast, prominent sex differences in THC’s discriminative stimulus effects have been reported in Sprague-Dawley rats (Wiley et al., 2017a), suggesting the possibility of a species X sex interaction effect. Additional research with female rodents to determine the generality of this finding is needed and is likely to occur, given the 2016 United States National Institutes of Health mandated consideration of sex as a biological variable in the review of grant proposals using animal models (Clayton and Collins, 2014).
Similar to THC, i.p. administration of a bicyclic cannabinoid (CP55,940) and the two indazole-derived synthetic cannabinoids (AB-CHMINACA and AMB-FUBINACA) resulted in full, dose-dependent substitution for THC in both female and male mice, as has been shown previously in male rodents (Gamage et al., 2018; Gatch and Forster, 2019; Gold et al., 1992; Wiley et al., 2015). Rank order potencies were identical across sex (i.e., CP55,940 > AMB-FUBINACA > ABCHMINACA > THC). Based upon the previously observed significant correlation between in vivo potency in THC discrimination and CB1 receptor binding affinity (Compton et al., 1993; Wiley et al., 1998), rank order affinity would be expected to similar. Interestingly, however, AMB-FUBINACA did not conform to this prediction, as it did not have the highest affinity for the CB1 receptor. As shown in Table 1, the CB1 affinity for AMB-FUBINACA is approximately equal to CP55,940 and 2.6-fold less than AB-CHMINACA. Yet, AMB-FUBINACA was of similar or greater potency than AB-CHMINACA in female and male mice, respectively. Although the reason for this apparent discrepancy is unknown, one possibility may be formation of an active and more potent metabolite. Like many synthetic cannabinoids, AMB-FUBINACA undergoes extensive metabolism. For example, the parent compound was not detected in any of the serum/blood samples from patients in the New York City 2016 overdose cluster (Adams et al., 2017); rather, all samples contained its de-esterified acid metabolite, 2-(1-(4-fluorobenzyl)-1H-indazole-3-carboxamido)-3-methylbutanoic acid (Adams et al., 2017). Neither this compound, nor other potential metabolites (Banister et al., 2016), have been evaluated for their activity at cannabinoid receptors.
While the same rank order potency was observed in female and male mice, absolute potencies were similar across sex only for AB-CHMINACA and THC. In contrast, CP55,940 and AMBFUBINACA were more potent in males than in females. These results are consistent with a previous study which found that two synthetic cannabinoids (CP47,497 and WIN55,212–2) were more potent at producing THC-like discriminative stimulus effects in male rats than females; however, this interpretation was complicated by unequal THC training doses, with males trained at a higher THC dose (Wiley et al., 2017a). Indeed, with THC training dose equal across sex, the greater potency of synthetic cannabinoids in male mice (vs females) in the current study, albeit small in magnitude, is opposite of the greater sensitivity of female rats to THC’s discriminative stimulus effects (Wiley et al., 2017a).
When route of administration was changed from i.p. injection to aerosol exposure, relative potency of the synthetic cannabinoids tested here shifted. While aerosolized AMB-FUBINACA remained the most potent compound in both sexes, aerosolized CP55,940 became the least potent. These results represent one of the first demonstrations of an abuse-related effect of aerosolized synthetic cannabinoids. Previously, Marshell and colleagues (2014) showed that aerosolization of first generation synthetic cannabinoids (JWH-073 and JWH-018) resulted in partial or full substitution for THC in drug discrimination in rats, although these effects were accompanied by response rate decreases. Vaporized THC also produced conditioned place preference in another study (Manwell et al., 2014a). In addition, aerosolized cannabinoids have been shown to produce characteristic cannabinoid effects in rodents such as suppression of locomotor activity and hypothermia (Javadi-Paydar et al., 2018; Lefever et al., 2017b; Manwell et al., 2014b; Nguyen et al., 2016b). Together, this research supports the feasibility of this experimental approach to characterize vaporization-based formulations of synthetic cannabinoids. Further, alteration in relative potencies among the compounds suggests that route of administration has potentially important translational implications, although the reason for the shift in CP55,940’s relative potency remains undetermined.
Two other findings of this study are worth noting. First, the THC-like effects of aerosolized AMB-FUBINACA in THC-trained female mice were reversed by pre-injection with the CB1 receptor antagonist rimonabant. Although not conclusive, these results suggest that these effects were likely mediated via CB1 receptor activation. This interpretation is consistent with the results of several other studies which have reported that the THC-like discriminative stimulus effects of injected psychoactive synthetic cannabinoids were antagonized by rimonabant or other CB1 receptor antagonists in male rodents (Järbe et al., 2006; Wiley et al., 1995). Another noteworthy finding is that aerosolized cumyl-4CN-BINACA produced concentration-dependent increases in responding on the THC-associated aperture in female mice to a maximum just shy of full substitution. This pilot experiment is important for two reasons: a) it is the first in vivo examination of cumyl-4CN-BINACA and b) it offers confirmation that similar results with aerosolized synthetic cannabinoids may be obtained with two distinct exposure systems, adding to the convergent validity of this translational model. The concentration-dependent increases in THC-like responding observed with cumyl-4CN-BINACA support a prediction that this compound would produce cannabimimetic effects in humans.
In conclusion, the results presented here demonstrate that abused synthetic cannabinoids produce THC-like discriminative stimulus effects in mice of both sexes via two routes of administration, traditional parenteral injection and aerosol exposure. Further, this study adds to a growing literature suggesting that evaluation of abuse liability of substances via aerosol exposure is feasible and may provide a translationally relevant method that allows for investigation of factors important to the abuse of drugs which humans typically smoke or vape. While exact determination of dose is still not possible in currently available aerosol exposure systems, this issue is not unique to this model, as it is also faced by researchers who evaluate the pharmacological effects of inhaled drugs in humans. Despite this limitation, aerosol exposure has at least two distinct translational advantages over injection in characterization of synthetic cannabinoids. First, human exposure to synthetic cannabinoids almost always involves heating or burning, thermolytic processes that may result in chemical transformation with resultant exposure to modified chemicals not necessarily contained in the bulk product (Bell and Nida, 2015; Thomas et al., 2017). In addition, biotransformation of the chemicals may vary with route of administration. The aerosol exposure paradigm described here will facilitate investigation of these issues. Second, aerosol exposure will allow investigation of the effects of flavors, olfactory factors, and other chemical constituents related to use of cannabis or synthetic cannabinoids (e.g., terpenoids), including factors that previously have been shown to be important to vaping of tobacco products (Litt et al., 2016; Zare et al., 2018). Combined with the findings reported herein, these potential advantages of aerosol exposure support continued use and development of this novel translational model.
Highlights.
In humans, synthetic cannabinoids (SC) are typically smoked or vaped.
We compared the effects of injected and vaped SC in THC discrimination in mice.
Vaped and intraperitoneal SC produced THC-like discriminative stimulus effects.
THC-like effects of SC occurred in mice of both sexes.
Results support feasibility of evaluation of abuse liability with aerosol exposure.
Acknowledgements
The authors express appreciation to Daniel Barrus and Nikita Pulley for excellent technical assistance in the conduct of this research. Research was supported by U.S. National Institutes of Health / National Institute on Drug Abuse grants DA-003672, DA-045003, and DA-040460. NIDA had no further role in the writing of the manuscript or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest.
References
- Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R, 2017. “Zombie” outbreak caused by the synthetic cannabinoid AMB-FUBINACA in New York. N Engl J Med 376, 235–242. [DOI] [PubMed] [Google Scholar]
- Balster RL, Prescott WR, 1992. Δ9-Tetrahydrocannabinol discrimination in rats as a model for cannabis intoxication. Neurosci Biobehav Rev 16, 55–62. [DOI] [PubMed] [Google Scholar]
- Banister SD, Kevin RC, Martin L, Adams A, Macdonald C, Manning JJ, Boyd R, Cunningham M, Stevens MY, McGregor IS, Glass M, Connor M, Gerona RR, 2019. The chemistry and pharmacology of putative synthetic cannabinoid receptor agonist (SCRA) new psychoactive substances (NPS) 5F-PY-PICA, 5F-PY-PINACA, and their analogues. Drug Test Anal, doi: 10.1002/dta.2583 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, Mack JB, Glass M, McGregor IS, Connor M, Kassiou M, 2016. Pharmacology of valinate and tert-leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues. ACS Chem Neurosci 7, 1241–1254. [DOI] [PubMed] [Google Scholar]
- Bell S, Nida C, 2015. Pyrolysis of drugs of abuse: a comprehensive review. Drug Test Anal 7, 445–456. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, 2000. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther 295, 328–336. [PubMed] [Google Scholar]
- Clayton JA, Collins FS, 2014. Policy: NIH to balance sex in cell and animal studies. Nature 509, 282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, Martin BR, 1993. Cannabinoid structure-activity relationships: Correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther 265, 218–226. [PubMed] [Google Scholar]
- European Monitoring Centre for Drugs and Drug Addiction, 2018. Fentanils and synthetic cannabinoids: driving greater complexity into the drug situation An update from the EU Early Warning System (June 2018). Publications Office of the European Union, Luxembourg. [Google Scholar]
- Finlay DB, Cawston EE, Grimsey NL, Hunter MR, Korde A, Vemuri VK, Makriyannis A, Glass M, 2017. Gαs signalling of the CB1 receptor and the influence of receptor number. Br J Pharmacol 174, 2545–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamage TF, Farquhar CE, Lefever TW, Marusich JA, Kevin RC, McGregor IS, Wiley JL, Thomas BF, 2018. Molecular and behavioral pharmacological characterization of abused synthetic cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA. J Pharmacol Exp Ther 365, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, 2019. Cannabinoid-like effects of five novel carboxamide synthetic cannabinoids. Neurotoxicology 70, 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Grieder TE, Cole M, Koob GF, 2010. Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacol Biochem Behav 96, 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LH, Balster RL, Barrett RL, Britt DT, Martin BR, 1992. A comparison of the discriminative stimulus properties of delta 9-tetrahydrocannabinol and CP 55,940 in rats and rhesus monkeys. J Pharmacol Exp Ther 262, 479–486. [PubMed] [Google Scholar]
- Huffman JW, Padgett LW, 2005. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes. Curr Med Chem 12, 1395–1411. [DOI] [PubMed] [Google Scholar]
- Järbe TU, Liu Q, Makriyannis A, 2006. Antagonism of discriminative stimulus effects of delta(9)-THC and (R)-methanandamide in rats. Psychopharmacology (Berl) 184, 36–45. [DOI] [PubMed] [Google Scholar]
- Javadi-Paydar M, Nguyen JD, Kerr TM, Grant Y, Vandewater SA, Cole M, Taffe MA, 2018. Effects of Delta9-THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology (Berl) 235, 2541–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever TW, Lee YO, Kovach AL, Silinski MA, Marusich JA, Thomas BF, Wiley JL, 2017a. Delivery of nicotine aerosol to mice via a modified electronic cigarette device. Drug Alcohol Depend 172, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Thomas BF, Barrus DG, Peiper NC, Kevin RC, Wiley JL, 2017b. Vaping synthetic cannabinoids: A novel preclinical model of e-cigarette use in mice. Subst Abuse Res Treat 11, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Duffy V, Oncken C, 2016. Cigarette smoking and electronic cigarette vaping patterns as a function of e-cigarette flavourings. Tob Control 25, ii67–ii72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manera C, Tuccinardi T, Martinelli A, 2008. Indoles and related compounds as cannabinoid ligands. Mini Rev Med Chem 8, 370–387. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Charchoglyan A, Brewer D, Matthews BA, Heipel H, Mallet PE, 2014a. A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part I: development and validation of a pulmonary cannabinoid route of exposure for experimental pharmacology studies in rodents. J Pharmacol Toxicol Methods 70, 120–127. [DOI] [PubMed] [Google Scholar]
- Manwell LA, Ford B, Matthews BA, Heipel H, Mallet PE, 2014b. A vapourized Delta(9)-tetrahydrocannabinol (Delta(9)-THC) delivery system part II: comparison of behavioural effects of pulmonary versus parenteral cannabinoid exposure in rodents. J Pharmacol Toxicol Methods 70, 112–119. [DOI] [PubMed] [Google Scholar]
- Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL, Fantegrossi WE, 2014. In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Δ9-THC in mice: Inhalation versus intraperitoneal injection. Pharmacol Biochem Behav 124, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Lefever TW, Blough BE, Thomas BF, Wiley JL, 2016. Pharmacological effects of methamphetamine and alpha-PVP vapor and injection. Neurotoxicology 55, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin RJ, 2018. Toward a translationally relevant preclinical model of cannabis use. Neuropsychopharmacology 43, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the care and use of laboratory animals. National Academies Press, Washington, D.C. [Google Scholar]
- New Zealand Ministry of Health, 2018. AB-FUBINACA and AMB FUBINACA: Report to the Expert Advisory Committee on Drugs New Zealand, https://www.health.govt.nz/system/files/documents/pages/ab-fubinaca_and_amb_fubinaca_eacd_report.docx.
- Nguyen JD, Aarde SM, Cole M, Vandewater SA, Grant Y, Taffe MA, 2016a. Locomotor stimulant and rewarding effects of inhaling methamphetamine, MDPV, and mephedrone via electronic cigarette-type technology. Neuropsychopharmacology 41, 2759–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA, 2016b. Inhaled delivery of Delta(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropsychopharmacology 109, 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble C, Cannaert A, Linnet K, Stove CP, 2018. Application of an activity-based receptor bioassay to investigate the in vitro activity of selected indole- and indazole-3-carboxamide-based synthetic cannabinoids at CB1 and CB2 receptors. Drug Test Anal 11, 501–511. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C, Braida D, 2015. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur Neuropsychopharmacol 25, 1775–1786. [DOI] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, Chen G, Lazarus P, Collaco JM, McGrath-Morrow SA, 2015. Adult behavior in male mice exposed to e-cigarette nicotine vapors during late prenatal and early postnatal life. PLoS One 10, e0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BF, Lefever TW, Cortes RA, Grabenauer M, Kovach AL, Cox AO, Patel PR, Pollard GT, Marusich JA, Kevin RC, Gamage TF, Wiley JL, 2017. Thermolytic degradation of synthetic cannabinoids: Chemical exposures and pharmacological consequences. J Pharmacol Exp Ther 361, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trecki J, Gerona RR, Schwartz MD, 2015. Synthetic cannabinoid-related illnesses and deaths. N Engl J Med 373, 103–107. [DOI] [PubMed] [Google Scholar]
- Tyndall JA, Gerona R, De Portu G, Trecki J, Elie MC, Lucas J, Slish J, Rand K, Bazydlo L, Holder M, Ryan MF, Myers P, Iovine N, Plourde M, Weeks E, Hanley JR, Endres G, St Germaine D, Dobrowolski PJ, Schwartz M, 2015. An outbreak of acute delirium from exposure to the synthetic cannabinoid AB-CHMINACA. Clin Toxicol (Phila) 53, 950–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Compton DR, Dai D, Lainton JA, Phillips M, Huffman JW, Martin BR, 1998. Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J Pharmacol Exp Ther 285, 995–1004. [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Cortes RA, Marusich JA, 2014a. Cross-substitution of Delta9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol Biochem Behav 124, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lefever TW, Marusich JA, Craft RM, 2017a. Comparison of the discriminative stimulus and response rate effects of Delta9-tetrahydrocannabinol and synthetic cannabinoids in female and male rats. Drug Alcohol Depend 172, 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, Martin BR, 1995. Antagonism of the discriminative stimulus effects of delta 9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther 275, 1–6. [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Huffman JW, 2014b. Moving around the molecule: Relationship between chemical structure and in vivo activity of synthetic cannabinoids. Life Sci 97, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, Patel PR, Grabenauer M, Moore KN, Thomas BF, 2015. AB-CHMINACA, AB-PINACA, and FUBIMINA: Affinity and potency of novel synthetic cannabinoids in producing delta9-tetrahydrocannabinol-like effects in mice. J Pharmacol Exp Ther 354, 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Thomas BF, 2017b. Combination chemistry: Structure-activity relationships of novel psychoactive cannabinoids. Curr Top Behav Neurosci 32, 231–248. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Owens RA, Lichtman AH, 2018. Discriminative stimulus properties of phytocannabinoids, endocannabinoids, and synthetic cannabinoids. Curr Top Behav Neurosci 39, 153–173. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Walentiny DM, Vann RE, Baskfield CY, 2011. Dissimilar cannabinoid substitution patterns in mice trained to discriminate Delta(9)-tetrahydrocannabinol or methanandamide from vehicle. Behav Pharmacol 22, 480–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Filipeanu CM, Bailey EM, Hulst JL, Sutton JL, 2012. Ovarian hormones and chronic administration during adolescence modify the discriminative stimulus effects of delta-9-tetrahydrocannabinol (Delta(9)-THC) in adult female rats. Pharmacol Biochem Behav 102, 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare S, Nemati M, Zheng Y, 2018. A systematic review of consumer preference for e-cigarette attributes: Flavor, nicotine strength, and type. PLoS ONE 13, e0194145. [DOI] [PMC free article] [PubMed] [Google Scholar]