Abstract
Lyme disease is the most prominent tick-borne disease in the United States. Co-infections with the tick-transmitted pathogens Babesia microti and Borrelia burgdorferi sensu stricto are becoming a serious health problem. B. burgdorferi is an extracellular spirochete that causes Lyme disease while B. microti is a protozoan that infects erythrocytes and causes babesiosis. Testing of donated blood for Babesia species is not currently mandatory due to unavailability of an FDA approved test. Transmission of this protozoan by blood transfusion often results in high morbidity and mortality in recipients. Infection of C3H/HeJ mice with B. burgdorferi and B. microti individually results in inflammatory Lyme disease and display of human babesiosis-like symptoms, respectively. Here we use this mouse model to provide a detailed investigation of the reciprocal influence of the two pathogens on each other during co-infection. We show that B. burgdorferi infection attenuates parasitemia in mice while B. microti subverts the splenic immune response, such that a marked decrease in splenic B and T cells, reduction in antibody levels and diminished functional humoral immunity, as determined by spirochete opsonophagocytosis, are observed in co-infected mice compared to only B. burgdorferi infected mice. Furthermore, immunosuppression by B. microti in co-infected mice showed an association with enhanced Lyme disease manifestations. This study demonstrates the effect of only simultaneous infection by B. burgdorferi and B. microti on each pathogen, immune response and on disease manifestations with respect to infection by the spirochete and the parasite. In our future studies, we will examine the overall effects of sequential infection by these pathogens on host immune responses and disease outcomes.
Keywords: Borrelia burgdorferi, Babesia microti, Lyme disease, babesiosis, co-infection, tick-borne co-infection, adaptive immune response
Introduction
The Centers of Disease Control and Prevention (CDC) estimates that ∼300,000 cases of Lyme disease and ∼2000 cases of human babesiosis occur in the United States annually, while ∼65,000 cases of Lyme disease are reported occur in Europe per year (Moore et al., 2016; Primus et al., 2018). However, projected case number in Germany alone was >200,000 per year emphasizing under-reporting of Lyme disease also in Europe (Muller et al., 2012). The Lyme disease causing spirochete Borrelia burgdorferi is an extracellular bacterial pathogen that may invade the skin, musculoskeletal system, heart, joints and neuronal system. In the United States, Lyme arthritis is the most common persistent manifestation while acrodermatitis and severe neuroborreliosis are more common in Europe (Jungnick et al., 2015; Steere et al., 2016). The protozoan parasites, Babesia microti and Babesia divergens are the major causes of human babesiosis in the United States and Europe, respectively. Babesiosis is generally asymptomatic in healthy individuals, which often results in establishment of a carrier state, such that donation of blood by infected, asymptomatic individuals can lead to transfusion-transmitted babesiosis (Krause et al., 1998, 2008), making this disease a serious health concern.
Concurrent infection with protozoan parasites and various bacterial pathogens occurs frequently (Cox, 2001). Ixodes species tick populations have been increasing in the endemic regions and beyond and these vectors can transmit both B. burgdorferi and B. microti (Piesman et al., 1986; Jaenson et al., 2012; Lommano et al., 2012; Rizzoli et al., 2014; Johnson et al., 2017, 2018; Hahn et al., 2018; Piedmonte et al., 2018). The rise in incidence of B. microti and B. burgdorferi co-infections in humans appears to be driven primarily by increased co-infection of their common vector, ticks of the Ixodes species, which are capable of transmitting both pathogens simultaneously (Schulze et al., 2013; Dunn et al., 2014; Hersh et al., 2014; Knapp and Rice, 2015; Diuk-Wasser et al., 2016; Moutailler et al., 2016; Edwards et al., 2019). Although overall tick-borne co-infection rates are not yet documented in the United States, incidence of Lyme spirochetes and B. microti co-infections were as high as 40% in studies conducted with patient samples in two states in the Eastern United States, New Jersey and Connecticut (Diuk-Wasser et al., 2016; Primus et al., 2018). B. microti-B. burgdorferi co-infected patients suffer from significantly more diverse and intense symptoms that persist longer than in patients infected with B. burgdorferi alone (Krause et al., 1996). Severe disease often requires patient hospitalization, and can even cause death due to multi-organ failure (Martinez-Balzano et al., 2015), emphasizing the need for a comprehensive evaluation of the effect of co-infections using susceptible animal models.
Two previous co-infection studies performed in mice reported contradictory results regarding the effect of concomitant B. microti infection on the severity of Lyme disease (Moro et al., 2002; Coleman et al., 2005). Neither study provided insight into the effect of B. burgdorferi (s.s.) infection on babesiosis. Our study was undertaken to provide the first description of the reciprocal interaction of the two pathogens, B. microti and B. burgdorferi sensu stricto (referred as B. burgdorferi hereafter), and the impact of co-infections on pathogenic mechanisms of the two diseases. We selected young C3H/HeJ mice for our experiments because they exhibit Lyme arthritis and carditis similar to humans (Barthold et al., 1990; Armstrong et al., 1992), and also display B. microti parasitemia, splenomegaly and anemia (Moro et al., 2002; Coleman et al., 2005). Splenic cells of B. burgdorferi infected C3H mice showed an increase in B and CD4+ lymphocytes, increased IFN-γ production and diminished IL-4 levels (Keane-Myers and Nickell, 1995; Anguita et al., 1996; Kang et al., 1997; Zeidner et al., 1997; Glickstein et al., 2001) suggesting that in addition to the innate immune response, humoral immunity as well as Th1 and Th2 responses are important for spirochetes clearance. The innate immune response, involving macrophage and NK cells, is also important in controlling protozoan infections including B. microti (Aguilar-Delfin et al., 2001; Hunter and Sibley, 2012; Basso and Marini, 2014). In C57BL/6 mice, it is critical for conferring resistance to highly infectious WA-1 strain of Babesia species (Aguilar-Delfin et al., 2003). In this study, we investigated the impact of splenic immune responses on the resolution of B. microti parasitemia at the acute phase of co-infection with B. burgdorferi. We also assessed the effect of changes in the adaptive immune response caused by infection with B. microti on the clearance of Lyme spirochetes. Thus, we show the effect of modulation of splenic immune response by B. microti on the persistence and severity of Lyme disease manifestations in co-infected mice even after resolution of parasitemia in mice. Our studies provide tools and an animal model to investigate the effects of a past or active infection with an undetectable B. microti parasitemia on Lyme disease.
Materials and Methods
Animal Studies Ethics Statement
The Institutional Animal Care and Use Committee (IACUC) members reviewed and approved the protocol number PROTO201702491 entitled, “Spirochetes and tick-borne pathogens,” of the corresponding author to conduct this study at Rutgers New Jersey Medical School following guidelines of the Animal Welfare Act, The Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals, and the Public Health Service Policy that are fully adopted at the Rutgers University.
B. burgdorferi and B. microti Culture, Maintenance and Infection of Mice
Bioluminescent B. burgdorferi N40 strain was grown in BSKII medium containing 6% rabbit serum at 33∘C until logarithmic phase. B. burgdorferi numbers were then adjusted to 104 spirochetes/ml of medium and 100 μl (103 spirochetes) injected subcutaneously (sc) in each mouse (Chan et al., 2015). Disseminated infection followed by colonization of organs and tissues by bioluminescent N40 was monitored weekly by live imaging, as described previously (Chan et al., 2015) using IVIS-200 (Perkin-Elmer). B. microti (ATCC30221) was first inoculated in C3H/SCID female mice to obtain inoculum for subsequent experiments and parasitemia determined using Giemsa-stained thin blood smears according to CLSI guidelines (Garcia et al., 2000). C3H/HeJ mice for the experiments were purchased from Jackson Laboratory. Mice were infected for experimental purpose with 104 B. microti infected RBCs as described previously (Djokic et al., 2018a, b). Female mice were used in all experiments to eliminate interference due to hormonal differences that sometimes affect parasitic infections. Young, 4-week-old mice were used because they display both Lyme disease and babesiosis disease manifestations. The mice were randomly divided into four experimental groups: (i) five uninfected, (ii) nine infected with N40 alone (one died during acclimatization period before infection), (iii) ten with N40 and B. microti together, and (iv) ten with B. microti alone. Mice infected with B. microti were monitored for parasitemia almost daily. To determine parasitemia at different days of infection, parasitized and total RBCs in 25 microscopic fields were counted in the stained blood smear from each infected mouse using oil immersion, 100× objective until parasitemia became undetectable by microscopic examination (21st day p.i.). Percent parasitemia was determined for each B. microti-infected and co-infected mouse throughout infection until euthanasia and is presented in Figure 1. Blood hemoglobin levels were also determined using a commercial kit (Hemocue® Hb 201+ analyzer) according to the manufacturer’s instructions. During the acute phase of infection (11th day p.i.), mice were euthanized when B. microti parasitemia was between 15 and 20% while the experiment was concluded at 21st day of infection to evaluate the impact on both diseases after parasitemia became undetectable by microscopy. Before euthanasia, heparinized blood was collected to recover plasma.
FIGURE 1.
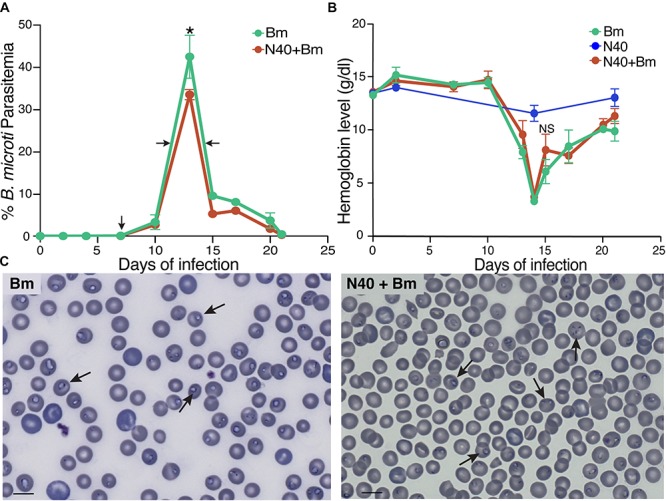
Effect of B. burgdorferi strain (N40) co-infection on B. microti (Bm) growth. (A) Percent of RBCs infected with B. microti in C3H mice infected either with B. microti alone, or with B. microti and N40, was determined over 21 days p.i. Each data point represents average parasitemia ± s.d. (n = 5). Both groups of mice exhibited peak parasitemia at 13th day p.i. with significantly high peak parasitemia in Bm-infected mice (*p < 0.05, df = 8, F = 1.72). (B) Hemoglobin levels were measured for 21 days p.i. N40-infected mice were used as controls. (C) Examination of blood smears showed lower erythrocytes density and higher parasitemia in Bm-infected mice (left) than co-infected mice (right). Bar represents 10 μm and arrows indicate parasitized RBCs.
Determination of Tissue Colonization Levels and Disease Pathology
In each experiment, live spirochetes were recovered by culture using the skin at the injection site, ear, blood and urinary bladder in Borrelia medium while right joint and heart were processed for histological examination. For the acute phase of infection (11th day p.i.), blood was collected from the heart by cardiac puncture and two mice from each set were perfused after deep anesthesia to examine spirochetal colonization of brain (streamlined in Figure 2A). DNA was isolated from the left joint of mice in each experiment, from the ear only in the experiment concluded at 3 weeks of infection (not shown), and brain and heart in all other experiments. Burden of spirochetes was determined by employing our previously described duplex qPCR assay (Chan et al., 2013) and using CFX-96 Real-time system (Bio-Rad). Aseptically removed spleens were weighed, and splenocytes collected for flow cytometry as described previously (Djokic et al., 2018a, b). Sections of joints, spleen and hearts were mounted on slides and stained with hematoxylin and eosin (H&E) and used for histopathological examination. Sections of the heart and joints at 3 weeks of infection were evaluated by a pathologist board-certified by the American College of Veterinary Pathologists (KK) blinded to infection status according to established criteria. Two graduates of veterinary medicine (LA and VD) evaluated sections of spleen, and scored heart and joint samples at the acute phase of infection independently in a blinded manner and consensus results are presented here.
FIGURE 2.
Outline of animal experiments to determine host response and disease manifestations after infection with B. burgdorferi N40 strain and/or B. microti (Bm). (A) Experimental plan and samples analyses at the acute phase (11th day p.i.) of infection of C3H/HeJ female mice. (B) Evaluation of the impact of infection on the host and pathogens when the parasitemia becomes undetectable microscopically (21st day p.i.).
For immunostaining of B. burgdorferi in brains of infected mice, we used 1:100 dilution of FITC-Labeled BacTrace® Anti-B. burgdorferi Antibody (Seracare) and 1:150 dilution of PE conjugated anti-mouse CD31 antibodies (Biolegend) for endothelial cell staining and employed our previously optimized procedures for in vitro assays or for spleen sections (Chan et al., 2016; Djokic et al., 2018a). Images were either acquired with a motorized Nikon Ti2 microscope using a 60× Plan APO, NA- 1.4 objective lens or were captured using the Nikon Eclipse Ti A1 scanning confocal microscope controlled by NIS-Elements software for image acquisition, processing, and analysis. The sections examined using Nikon Ti2 microscope were illuminated using a Lumencor Spectra X light engine and images were captured with a Hamamatsu ORCA Flash4.0 V3 sCMOS camera and Nikon NIS Elements software.
Analyses of Splenic Cells by Flow Cytometry
Aseptically harvested spleens from infected mice were weighed, single cell suspensions of the splenocytes prepared, washed with PBS, and used for fluorescence-activated cell sorting (FACS) analyses using antibodies from Biolegend as previously described (Djokic et al., 2018a, b) with some modifications. Briefly, after counting live cells, splenocytes from each mouse were labeled with 1:50 dilution of anti-mouse CD45 coupled with PE (Biolegend) antibodies diluted in FACS buffer (PBS +5%FBS). Cell suspensions were incubated on ice in the dark for 30 min for staining. After washing three times with the buffer by centrifugation at 350 × g for 5 min each, cell pellets were suspended in 1 ml buffer and 5 samples from each mouse group pooled. Cell sorting was done using BD FACS AREA II (BD Biosciences) by first gating for appropriate cell size, then for DAPI negative live cells, followed by gating for PE positive cells. Then, CD45+ cells were stained for B cells with Brilliant violet 421 conjugated anti-mouse CD19 antibodies, T cells with PE/Cy7 conjugated anti-mouse CD3 antibodies, T helper cells with FITC conjugated anti-mouse CD4 antibodies and cytotoxic T cells with Alexafluor-700 conjugated anti-mouse CD8a antibodies, and macrophages with APC.Cy7 conjugated anti-mouse F4/80 antibodies (Biolegend) followed by FACS. Flow cytometry was conducted using BD LSRFortessaTM X-20 (BD Biosciences) driven by software FACS DiVa (BD Biosciences). Acquired data was analyzed using FlowJo, Version 10.3 software.
Humoral Response Determination
N40 culture was centrifuged at 4,000 × g for 15 min when density reached to 1–2 × 108 spirochetes/ml and washed with PBS three times. The bacterial pellet was suspended in 0.1% B-per detergent (Thermo Fisher Scientific) containing PBS followed by sonication to lyse. After complete lysis was observed microscopically, total cell extract was passed through a 0.22 microfilter and the antigen preparation was stored at –20∘C until used for ELISA. ELISA plates were coated with 50 μl of B. burgdorferi N40 lysate (concentration adjusted to 0.3 mg/ml) and incubated at 37∘C overnight. Wells without protein coating (buffer only) were included as “No antigen” controls. Plasma samples recovered from uninfected and B. burgdorferi infected mice diluted at 1:5,000 were incubated with spirochetal antigen-coated wells for 1 h at room temperature. Eight replicates for each sample were used to ensure reproducibility. After incubation and washing three times for 5 min each with 0.5% Tween-20 containing PBS (PBST), plates were incubated with 50 μl of anti-mouse HRP conjugate for 1 h. The plates were then washed and bound antibodies detected using TMB substrate (KPL SureBlue). Absorbance was measured at OD620 using a SpectraMax M2 plate reader (Molecular Devices).
Opsonophagocytosis of B. burgdorferi
To determine the changes in functional humoral immunity against B. burgdorferi N40 strain on co-infection with B. microti, 108 spirochetes were suspended in 500 μl of binding medium containing 1:2 ratios of BSK-H (Sigma) and GHS (10 mM glucose+50 mM HEPES pH 7.0+10 mM NaCl). After preincubation of spirochetes with respective plasma samples diluted at 1:100 in J774A.1 macrophage medium (DMEM medium supplemented with 10% FBS), opsonophagocytosis was conducted as previously described (Chan et al., 2016). Thus, images of green extracellular and red internalized spirochetes after 2 h incubation to allow phagocytosis together with blue macrophages, labeled with wheat agglutinin lectin conjugated with Alexa fluor 647, were captured using the Nikon Eclipse Ti A1 scanning confocal microscope controlled by NIS-Elements software. Video of phagocytosed B. burgdorferi by N40 infected mouse plasma (Supplementary Video S1) was obtained using Leica TCS SP8 scanning confocal microscope with the system controlled by LAS X software for image acquisition, processing, and analysis.
Statistical Analysis
All collected data were analyzed by Prism version 8.0 for Mac, GraphPad Software and comparisons made between groups using ANOVA and a two tailed unpaired student t-tests for unequal variance. Differences between paired groups with p < 0.05 were considered significant for a paired group comparison at 95% confidence interval.
Results
Effect of B. burgdorferi Strain N40 Co-infection on B. microti
In our experiments, mice infected with B. microti alone, and those co-infected with B. microti and N40, exhibited similar temporal patterns of parasitemia. In both cohorts, peak parasitemia was reached at 13th day post-infection (p.i.). Peak parasitemia levels were significantly higher in mice infected with B. microti (42.5 ± 5%) compared to co-infected mice (33.5 ± 1%) (Figure 1A). Increased parasitemia appears to have facilitated lysis of infected red blood cells (RBCs) and diminished hemoglobin levels in both sets of B. microti infected mice. Despite the lower peak parasitemia in co-infected mice, there was no statistically significant difference in hemoglobin levels compared to B. microti infected mice (Figure 1B). In both sets of mice, severe anemia was temporary and normal hemoglobin levels were restored within a few days of post-peak parasitemia. Higher parasitemia in B. microti infected mice was coupled with decreased erythrocyte density in blood compared to co-infected mice (Figure 1C, left versus right panel). Normal numbers of RBCs were restored in both sets of infected mice within 2 days of peak parasitemia (data not shown).
Effect of B. burgdorferi and B. microti on Spleen and Splenocytes at Acute Phase of Infection
Experimental plan to determine the effect of infections at acute phase of infection is outlined in Figure 2A. As a major organ in the reticuloendothelial system, the spleen is involved in clearance of old, damaged or parasitized erythrocytes facilitating both blood filtration and resolution of parasitic diseases, including babesiosis (White et al., 1998). The spleen is a hematopoietic organ involved in homing of the lymphocytes and is also a reservoir of RBCs and monocytes. To determine the effect of co-infection on different organs, immune response and pathogenesis during the acute phase, infected mice were sacrificed on day 11 p.i. (Figure 3A). At this stage, infection with N40 alone caused a relatively small increase in the spleen weight while infection with B. microti either alone or together with N40 caused significant splenomegaly (Figure 3B). Analysis of total splenic CD45+ leukocytes during early infection by flow cytometry (shown for a representative mouse in Figure 3C) indicated the most significant increase in the F4/80 positive macrophages in B. microti-infected (p < 0.05, df = 8, F = 1.91) and co-infected (p < 0.01, df = 8, F = 1.72) mice compared to naïve mice, which suggests the role of macrophages is important in clearance of B. microti at the acute phase of infection. In contrast, macrophage number was not affected significantly in N40-infected mice (Figure 3D).
FIGURE 3.
Babesia microti (Bm) infection caused splenic enlargement in C3H mice at the acute phase of infection and differentially increase splenocytes compared to N40-infected mice. (A) Overall experimental plan and samples analyses after infection of C3H/HeJ female mice. (B) Splenic weights were determined on day 11 p.i. Spleens of Bm infected and co-infected mice were significantly heavier than spleens from N40 infected mice, which showed moderate increase in weight compared to naïve mice. (C) Gating scheme used for FACS analysis of splenic CD45+ cells is shown. (D) Quantitative analyses of splenic B cells, T cells, CD4+ and CD8a+ cells, and macrophages in each group of mice. Each cell type is represented as percentage of total CD45+ splenic leukocytes (mean ± s.d., n = 5). Two-tailed unpaired student t-test for unequal variance between the paired groups was used to determine significance (NS, not significant, *p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ****p < 0.0001).
There was significant increase in percentage of the CD19+ B cells in B. burgdorferi infected mice relative to naïve mice at this early stage of infection demonstrating that induced expansion of these cells had started in these mice. However, increase in the percentage of CD19+ cells in B. microti infected (∼56%) and co-infected mice (∼51%) were even higher than in N40-infected (∼49%) and naïve (∼36%) mice (Figures 3C,D). B. microti infection also resulted in a significant increase in the T cell population in B. microti infected and co-infected mice, as reflected by the increase in the percent of CD3+ cells, the CD8a+ cells and CD4+ T cells (Figure 3D). This increase is consistent with previous observations in other parasitic diseases (Sponaas et al., 2006; Abel et al., 2012; Li et al., 2015). Significant increase in the T cells during B. microti infection from ∼17% in naïve to ∼29% in B. microti infection (p < 0.001, df = 6, F = 6.4) and ∼33% during co-infection (p < 0.01, df = 6, F = 9.99) compared to ∼22% in N40 infected mice emphasizes the role of these cells specifically during early stage of B. microti infection.
Lyme Disease at Acute Phase of Infection
At day 11 p.i., live imaging was used to detect dissemination of N40 from the site of infection. Bioluminescence was observed in the joints and head regions of all N40 infected and co-infected mice (not shown). Live spirochetes were recovered from the injection site, ear and bladder of all N40-infected and co-infected mice confirming a disseminated infection by B. burgdorferi (Table 1). To accurately quantify spirochetes burden in different tissues, N40 recA copy numbers were normalized to 105 mouse nidogen copies in the duplex qPCR assay (Figure 4A). There was a high spirochete burden (>106 recA copies/105 nidogen copy number) in joints and brain of N40 infected or co-infected mice. High B. burgdorferi burden (>106 spirochete recA copies/105 mouse nidogen copies) is likely because the adaptive immune response was still not fully developed in these mice. N40 infected mice displayed inflammation in the tibiotarsus and their joints had significant infiltration of leukocytes compared to B. microti infected control mice (Figure 4B). Co-infection led to more pronounced inflammation with 2/5 co-infected mice displaying maximum (+++) arthritic severity and 3/5 displaying moderate (++) inflammatory arthritis. In the N40 infected group 4/5 mice showed moderate (++) and 1/5 mice displayed minimal (+) inflammation (Figure 4B and Table 2). None of the N40-infected or co-infected mice displayed any apparent carditis (data not shown).
TABLE 1.
Cultivation of B. burgdorferi from different organs of the infected mice in BSKII medium containing 6% rabbit serum.
|
N40 |
N40+Bm |
|||||
| Infection stage | Ear | Injection Site | Bladder | Ear | Injection Site | Bladder |
| 11 days | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| 21 days | 9/9 | 9/9 | 9/9 | 10/10 | 10/10 | 10/10 |
FIGURE 4.

Effect of B. microti (Bm) and N40 co-infection on brain and joint colonization by B. burgdorferi, and their effect on joint inflammation during the acute phase of infection. (A) High numbers of spirochetes were observed in joints and brains of B. burgdorferi infected mice, while the N40 burden was significantly higher in joints of co-infected mice as determined by a two-tailed unpaired student t-tests for unequal variance between the paired groups (∗∗p < 0.01, df = 8, F = 3.60). (B) B. burgdorferi infection caused only mild joints inflammation during the acute phase of disease as indicated by change in synovial space (arrow 1), synovial hyperplasia and erosion of cartilage (arrow 2), and lymphocytic infiltration (arrow 3) while respective markers show higher inflammation in co-infected mice and no inflammation in Bm-infected mice. Bar represents 100 μm. (C) Images of the head region (left dorsal, right ventral) of N40-infected and co-infected mice captured by live imaging using IVIS-200 after i.p. injection of D-luciferin substrate showed an increase in bioluminescence, particularly in the frontal region of brain of co-infected mice. (D) Mice were deeply anesthetized, perfused with PBS and fixative before euthanasia. Brain sections were labeled with anti-B. burgdorferi antibodies conjugated to FITC and nuclei of host cells stained with DAPI. Endothelial cells were labeled using anti-CD31 antibodies tagged with PE (red) and are marked in the figure by arrowheads. Green spirochetes were detected in brain sections from N40 infected and co-infected mice (arrows) when the sections were examined using Nikon Ti2 microscope. B. microti infected mice used as a negative control did not show any spirochetes. Bar represents 100 μm.
TABLE 2.
Histopathological scoring of joints of infected mice at two points of infection.
|
No of mice with each histological score |
||||||||
| Experimental groups |
Knee |
Tibiotarsus* |
||||||
| Score | − | ± | + | − | ± | + | ++ | +++ |
| Day 11th N40 | 1 | 2 | 2 | 0 | 0 | 1 | 4 | 0 |
| N40+Bm | 1 | 0 | 4 | 0 | 0 | 0 | 3 | 2 |
| Bm | 5 | 0 | 0 | 5 | 0 | 0 | 0 | 0 |
| Day 21st N40 | 2 | 2 | 5 | 0 | 1 | 3 | 5 | 0 |
| N40+Bm | 2 | 1 | 7 | 0 | 0 | 2 | 4 | 4 |
| Bm | 9 | 1 | 0 | 9 | 1 | 0 | 0 | 0 |
*Scoring of severity of arthritic manifestation of knee was negative (−), equivocal (±) or positive (+). Tibiotarsus inflammation ranged from negative (−) to severe (+++) in B. burgdorferi infected/co-infected mice based upon; (i) synovial hyperplasia (ii) erosion of cartilage, (iii) increase in lymphocytic infiltration and (iv) change in synovial space compared to the naïve mice or mice infected with B. microti alone.
Earlier studies that reported dissemination of N40 to the mouse brain did not conduct a thorough investigation of brain colonization. Anecdotally, many researchers do not now believe that B. burgdorferi can invade mouse brain despite this being reported by Barthold et al. (1992). Light emission by our bioluminescent N40 focused in the head region by IVIS (Figure 4C) indicated that live spirochetes were likely present in brain. After removing frontal brain region for immunohistology, we confirmed colonization in remaining part of the brain by N40 by qPCR (Figure 4A). B. burgdorferi uses blood as transient conduit for dissemination. To minimize the presence of spirochetes in the vasculature, deeply anesthetized mice were perfused with PBS and fixative before euthanasia. We removed a small frontal section of brain (6–8 mm in thickness from chiasma opticum) from perfused mice for immunostaining to detect B. burgdorferi. Fixed brain sections from frontal region of N40-infected and co-infected perfused mice were immunostained with B. burgdorferi specific antibodies conjugated to FITC at the acute phase of infection, i.e., 11th day p.i. (Figure 4D). In both; N40 infected and co-infected groups of mice, green spirochetes were detected in brain tissue in the frontal region, similar to that shown previously in parenchyma of B. burgdorferi infected rhesus macaque brain sections (Ramesh et al., 2008, 2009). The presence of N40 was not restricted to vasculature, as demonstrated by spirochetes location in brain that is distant from red-labeled CD31, a marker for endothelial cells (Figure 4D). B. microti infected mice used as negative controls showed no green spirochetes in brain section, as expected (Figure 4D). Thus, in addition to live imaging and qPCR results, we examined several forebrain sections (frontal lobe) from perfused animals to confirm B. burgdorferi presence in the brains beyond vasculature and to show brain parenchyma colonization.
Impact of B. microti Infection on Splenomegaly and Splenocytes Post-parasitemia
Outline of the experiment and samples analyses post-parasitemia (day 21 p.i.) is provided in Figure 2B. Splenocytes proliferation has been reported to occur in response to B. burgdorferi infection but spleens of N40 infected mice were only slightly larger than naive mice while B. microti infected mice consistently demonstrated pronounced splenomegaly at 21st day p.i. (Figures 5A,B). More than 3-fold increase in spleen weight was observed in B. microti infected (p < 0.001, df = 5, F = 1.93) and co-infected mice (p < 0.0001, df = 7, F = 3.01) compared to N40 infected mice. The enlargement of spleens from B. microti-infected mice could be attributed to the increased hematopoiesis support it provides, while its dark coloration could result from B. microti-mediated RBCs lysis and erythrophagocytosis by macrophages. Histopathological examination of spleen sections demonstrated a clear demarcation between the red and white pulp regions, the marginal zone and trabeculae in N40-infected mice that was similar to naive mice (Figures 5C,D). In spleens from B. microti-infected and co-infected mice, the demarcation zone between red and white pulp was indistinguishable (Figures 5E,F). Co-infected animals demonstrated cellular proliferation, general infiltration of white cells and expansion of the red pulp (Figure 5E) while B. microti-infected mice displayed overall enlargement of the white pulp (Figure 5F).
FIGURE 5.

Babesia microti (Bm) infection caused pronounced splenomegaly affecting splenic architecture in C3H mice at day 21 p.i. (A,B) A significant but moderate increase in spleen size is observed in N40 infected mice (2) compared to naïve mice (1) while very pronounced splenomegaly is observed in Bm infected (3) mice (∗∗∗p < 0.001, df = 3, F = NA) and co-infected (4) mice (∗∗∗p < 0.001, df = 5, F = NA). (C,D) H&E stained spleen sections displayed normal architecture with a clear demarcation between the white and red pulp (arrows 1 and 3, respectively) and marginal zone (arrow 2), in uninfected (C), and N40-infected (D) mice. (E,F) Demarcation between red and white pulp was indistinguishable in spleens of co-infected (E), and B. microti infected (F) mice. Bar in microscopic images represents 100 μm.
Effect of B. microti on Splenic Immunity After Parasitemia Resolution
To determine longer-term effects of B. microti infection on the spleen, using the gating scheme presented in Figure 3C, we examined changes in splenic leukocyte sub-populations after resolution of parasitemia at day 21 p.i. The percentage of macrophages remained significantly higher in co-infected mice (∼13%, p < 0.0001, df = 12, F = 2.69) and in B. microti infected mice (∼12%, p < 0.0001, df = 12, F = 2.68) relative to naïve mice (∼4.5%) even at this stage of infection while their percentage in N40 infected mice (∼5%) was not significantly different from controls (Figure 6). The percentage of CD19+ B cells was significantly higher at this stage of infection primarily in mice infected with B. burgdorferi (∼48%, p < 0.01, df = 12, F = 1.38) compared to naïve mice. In B. microti infected mice, percentage of B cells was reduced significantly (∼25%, p < 0.001, df = 13, F = 1.54) compared to naïve mice (39%). A marked reduction in B cells in co-infected mice was also observed relative to naïve (∼20%, <0.0001, df = 13, F = 1.69) and N40-infected (p < 0.0001, df = 17, F = 2.33) mice (Figure 6 and Table 3) at day 21 p.i. Thus, percentage of B cells in spleen appeared to be consistently lower in co-infected mice compared to B. microti infected mice.
FIGURE 6.
Flow cytometry analysis of splenic leukocytes from infected mice at day 21 p.i. Percentage of each cell type in each mouse is calculated using total CD45+ cells and data presented as mean ± s.d. Increase in F4/80+ macrophage percentage remained significantly higher only in co-infected mice (n = 10) compared to the naïve, uninfected mice (n = 5). Significant but only moderate increase in T and B cells was observed in Bm infected mice (n = 10). Increase in CD19+B cells, total CD3+ T cells, and CD8a+ cells in N40 infected (n = 9) and Bm infected mice was observed at this stage while significant reduction in co-infected mice occurred compared to respective cells in mice infected with each pathogen individually. Increases in CD4+ T cells relative to naïve mice in Bm-infected and N40 infected mice were higher than in co-infected mice. Each bar represents the mean ± s.d. (NS, not significant, *p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ****p < 0.0001).
TABLE 3.
Analyses of splenic macrophage, B, and T cells by flow cytometry at 11th day and 21st day post-infection.
|
11th day p.i. |
21st day p.i. |
|||||||
| Splenocytes | *Total No. × 102 | Std. Dev. | Splenocytes | *Total No. × 102 | Std. Dev. | |||
| N40 | CD45 | 500 | 0 | CD45 | 500 | 0 | ||
| F4/80 macrophages | 22.2 | 1.9 | F4/80 macrophages | 26.1 | 6.8 | |||
| CD19 B cells | 642.0 | 40.8 | CD19 B cells | 237.9 | 25.4 | |||
| CD3 T cells | 108.8 | 9.3 | CD3 T cells | 102.7 | 6.9 | |||
| CD8a | 7.6 | 1.1 | CD8a | 10.9 | 2.9 | |||
| CD4 | 21.4 | 2.1 | CD4 | 18.8 | 2.9 | |||
| N40+Bm | CD45 | 500 | 0 | CD45 | 500 | 0 | ||
| F4/80 macrophages | 27.7 | 1.9 | F4/80 macrophages | 66.2 | 7.5 | |||
| CD19 B cells | 254.4 | 8.6 | CD19 B cells | 101.5 | 16.6 | |||
| CD3 T cells | 166.5 | 19.8 | CD3 T cells | 71.8 | 7.7 | |||
| CD8a | 15.4 | 1.2 | CD8a | 2.8 | 1.0 | |||
| CD4 | 49.6 | 3.5 | CD4 | 15.8 | 1.2 | |||
| Bm | CD45 | 500 | 0 | CD45 | 500 | 0 | ||
| F4/80 macrophages | 25.9 | 1.9 | F4/80 macrophages | 60.0 | 7.5 | |||
| CD19 B cells | 279.1 | 10.7 | CD19 B cells | 125.9 | 26.8 | |||
| CD3 T cells | 142.5 | 6.7 | CD3 T cells | 85.9 | 11.6 | |||
| CD8a | 113.9 | 1.4 | CD8a | 4.8 | 2.8 | |||
| CD4 | 49.2 | 3.0 | CD4 | 18.8 | 1.8 | |||
| Naïve | CD45 | 500 | 0 | CD45 | 500 | 0 | ||
| F4/80 macrophages | 22.7 | 2.4 | F4/80 macrophages | 22.7 | 4.6 | |||
| CD19 B cells | 181.5 | 27.1 | CD19 B cells | 196.1 | 21.6 | |||
| CD3 T cells | 92.2 | 6.6 | CD3 T cells | 87.1 | 8.4 | |||
| CD8a | 3.9 | 1.5 | CD8a | 3.2 | 2.2 | |||
| CD4 | 4.9 | 1.8 | CD4 | 4.9 | 1.8 | |||
*Average of estimated cell numbers for five in naïve mice and in each infection group.
We found that percentage of total T (CD3+) cells in B. microti infected mouse spleens remained comparable to naïve mice (∼17% each), increase in percentage of total T cells, CD8+ cells, and CD4+ cells was noted to be high in response to infection with B. burgdorferi alone similar to that observed for B cells (Figure 5). Thus, percentage of the CD3+ T cell was significantly lower in co-infected mice (p < 0.0001, df = 17, F = 1.23) with an average of ∼14% T cells compared to ∼21% in N40 infected mice despite pronounced splenomegaly observed in the B. microti infected and co-infected mice (Figure 6). Overall, percentage of total splenic B and T cells were significantly lower in B. microti infected relative to N40 infected mice in multiple experiments indicating that B. microti infection (with or without B. burgdorferi infection) stimulated proliferation of splenic B and T cells at lower levels than that by N40 infection at a time point when adaptive immune response is usually established (21st day p.i.). In fact, B. microti appeared to suppress adaptive immune response (Figure 6) since percentage of the splenic CD8a+ cells also diminished in co-infected mice (∼0.6%) compared to N40 infected mice (∼2%) and only increased slightly in B. microti infected (∼1%) relative to naïve mice (∼0.8%). There was a significant increase in percentage of CD4+ cells in all three groups of infected mice compared with naïve mice (∼1%) such that their levels were comparable in N40 infected and B. microti infected mice (∼3.8% each) while percentage of these cells was significantly lower (∼3.2%) in co-infected mice compared to N40 infected (p < 0.01, df = 17, F = 6.23) and B. microti infected (p < 0.001, df = 18, F = 2.41) mice (Figure 6).
Immunomodulation of Humoral Response by B. microti
The significant decrease in the percentage of B and T cells (p < 0.0001) in spleen of co-infected mice compared to N40-infected mice as well as naïve mice (Figure 6) suggests that B. microti infection either leads to depletion of T and B cells or inhibits their proliferation. We further determined if B. microti-mediated depletion in B and T helper cells affected the antibody response against B. burgdorferi. We used ELISA to quantify antibody responses, in co-infected and N40-infected mice, using total B. burgdorferi protein extract as antigen. The antibody response in co-infected mice was significantly attenuated compared to N40-infected mice (Figure 7). The decreased antibody response in co-infected mice is consistent with diminished levels of the B cells in these mice.
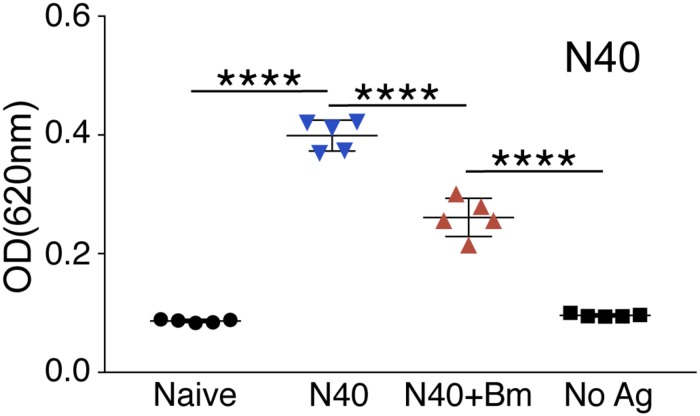
FIGURE 7.
Determination of the specific antibody response in N40 infected and co-infected mice at day 21 p.i. ELISA analysis using N40 protein extract probed with pooled plasma of either N40 infected or co-infected mice indicated a significant reduction (****p < 0.0001, df = 8, F = 1.55) in the specific antibodies against B. burgdorferi in co-infected mice. No reactivity was observed in the negative control (No Ag) or using naïve mice plasma.
B. microti Enhances B. burgdorferi Survival and Increases Lyme Disease Severity
Increased bioluminescence in co-infected mice suggested a higher bacterial burden in this group compared to N40-infected mice at 2 weeks of infection (Figure 8A). The increased bacterial burden in co-infected mice was also observed at day 21 p.i. (Figure 8B). Live spirochetes could be recovered from all tissues of N40 infected and co-infected mice (Table 1). For further examination, brain, heart and joint tissues were collected from mice at day 21 p.i. Spirochete burden in different tissues was determined by qPCR and histopathological evaluations of joint and heart sections were also conducted. Significantly higher levels of N40 burden in the brains and joints of co-infected mice compared to N40-infected mice (Figure 8C) confirmed the live-imaging results. Although the colonization level in hearts was slightly higher in co-infected mice, the difference was not statistically significant (Figure 8C). Inflammatory arthritic manifestations in the tibiotarsus were scored to be significantly higher (p = 0.045) in co-infected mice at day 21 p.i., as depicted by synovial membrane hyperplasia, erosion of articular cartilage, lymphocytic infiltration in synovial membranes, and widening of synovial space in co-infected mice (Figure 8D). Indeed, 8/10 co-infected mice showed moderate to severe (++ to +++) arthritis while none of the N40 infected mice showed severe arthritis and only 5/9 showed moderate (++) inflammatory arthritis (Table 2). Hence, increased colonization of joints by N40 in co-infected mice resulted in more pronounced inflammatory Lyme arthritis. Cardiac inflammation determined by histological scoring was comparable in N40 infected and co-infected mice indicating correlation of inflammatory disease with spirochete burden in heart (data not shown). Lower B. burgdorferi numbers at day 21 p.i. compared to day 11 p.i. (Figure 4A versus Figure 8C) likely represented partial clearance of spirochetes by an adaptive immune response. Overall, severity of inflammatory Lyme disease correlated with the N40 load in the respective tissues and both spirochetes burden and inflammatory responses were amplified by co-infection with B. microti.
FIGURE 8.
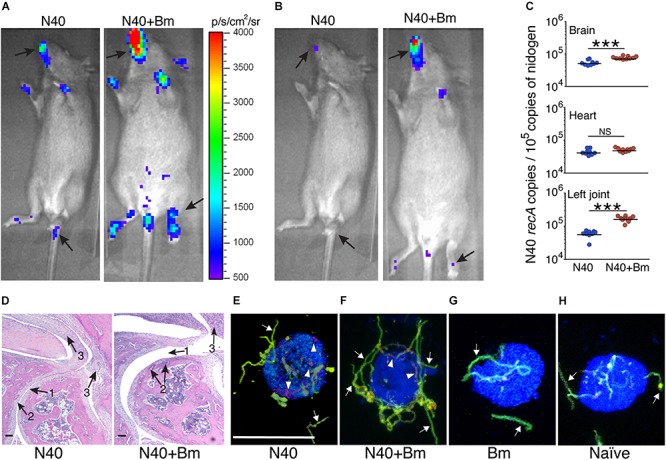
Increased colonization of organs by N40 and exacerbation of Lyme disease in mice by co-infection with B. microti (Bm). (A) Representative real-time images using IVIS-200 displaying higher spirochetal burden in co-infected than N40-infected mice at 2 weeks, and (B) 3 weeks of infection. (C) Burden of N40 in tissues determined using duplex qPCR assay with horizontal lines representing the mean N40 recA copy number. Joint and brain of co-infected mice showed significantly (∗∗∗p < 0.001) higher spirochete burden as compared to mice infected with N40 alone while B. burgdorferi burden in hearts was not significantly different (NS). (D) More severe arthritis manifested by change in synovial space (arrow 1), synovial hyperplasia and erosion of cartilage (arrow 2), and higher lymphocytic infiltration (arrow 3) were observed in co-infected mice as compared to the N40-infected mice. (E) Opsonophagocytosis of N40 by mouse J774.1 macrophages was observed after 2 h of co-incubation of plasma from N40-infected mice with spirochetes such that macrophages showed significant phagocytosis detected as red, internalized bacteria (arrowheads) and some green extracellular spirochetes (arrows). The macrophages are marked blue. (F) Although phagocytosis occurred after opsonization of N40 with plasma from co-infected mice, it showed significantly lower internalized spirochetes compared to those using plasma from N40-infected mice (E). (G) Incubation of B. burgdorferi using plasma from B. microti infected mice, and (H) uninfected, naïve mice showed no phagocytosis of the spirochetes after 2 h of co-incubation of B. burgdorferi with macrophages. Bar represents 100 μm in Figures 7E–H.
To determine the role of N40-specific antibodies in functional immunity, we further conducted phagocytosis following opsonization with pooled plasma from N40 infected and co-infected mice (Figures 8E,F). Pooled plasma from the naïve mice and B. microti infected mice served as the negative controls (Figures 8G,H). When opsonized with pooled plasma from N40 infected mice, all macrophages (100%) showed red phagocytosed B. burgdorferi (Figure 8E and Supplementary Video S1). Opsonization with plasma from co-infected mice led to phagocytosis by 60% of macrophages. Only extracellular spirochetes were observed attached to the remaining (40%) macrophages (Figure 8F and data not shown) indicating diminished inducible functional immunity in these mice. No opsonophagocytosis was observed in the negative controls (Figures 8G,H) indicating that this assay determined the specific functional immune response in infected/co-infected mice.
Finally, similar to that observed during the acute phase of infection, we examined brain sections of mice perfused with PBS before euthanasia at day 21 p.i. after staining for host nuclei with DAPI, embedded spirochetes with anti-B. burgdorferi FITC conjugate, and endothelial cells with PE conjugated anti-mouse CD31 antibodies (Figure 9). N40 spirochetes were either detected as clumps (Figure 9A) or individually at 3 weeks p.i. (Figure 9B).
FIGURE 9.
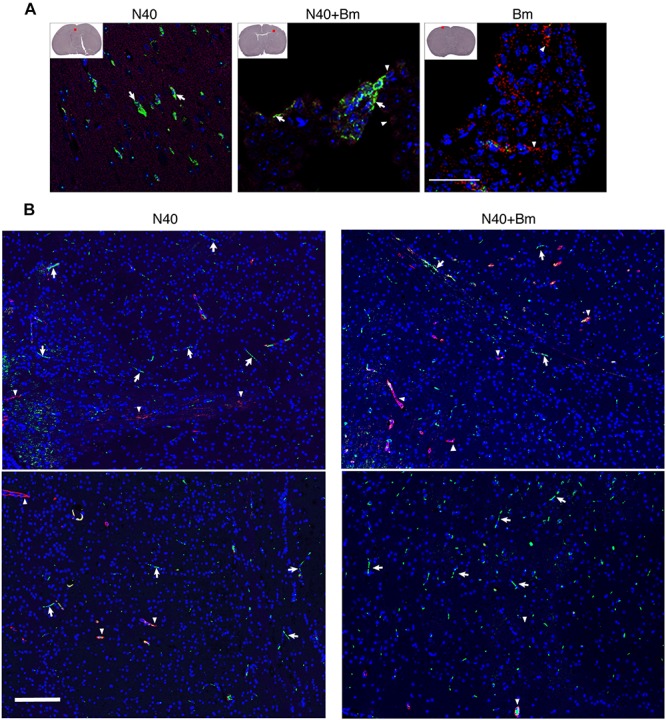
Borrelia burgdorferi N40 strain infection with or without B. microti co-infection resulted in colonization of the forebrain at day 21 p.i., as shown in multiple sections by immunostaining. (A) Deeply anesthetized mice were perfused with PBS and fixative before euthanasia. Brain sections were labeled with anti-B. burgdorferi antibodies conjugated to FITC (white arrows) and endothelial cells using anti-CD31 antibodies tagged with PE (red), marked by arrowheads. The nuclei of the host cells are stained blue by DAPI. Aggregates of green spirochetes (arrows) were detected in brain sections from N40 infected and co-infected mice when examined by Nikon Eclipse Ti A1 scanning confocal microscope. Control B. microti infected mice brain did not show any spirochetes. (B) Additional sections of brains of mice infection with N40 alone or co-infected with B. microti also showed presence of the spirochetes when the sections were examined using Nikon Ti2 microscope illuminated using a Lumencor Spectra X light engine and images captured with a Hamamatsu ORCA Flash4.0 V3 sCMOS camera and Nikon NIS Elements software. Arrows mark green spirochetes in the image while arrowheads depict red endothelial cells. Bar represents 100 μm.
Discussion
Our study demonstrates the interactions and effects of two tick-borne pathogens on each other and on the susceptible host. We examined B. burgdorferi and B. microti infection at the acute phase of infection (before peak parasitemia and development of adaptive immune response) and immediately following resolution of B. microti parasitemia. We observed a lower peak parasitemia during co-infection (Figure 1). Previously, higher B. microti parasitemia was observed after tick-transmission in outbred, Peromyscus leucopus mice when animals were co-infected with another strain of B. burgdorferi, B348, compared to the animals infected by B. microti alone (Dunn et al., 2014). B348 strain causes disseminated infection in humans (Hanincova et al., 2013) but is a slow disseminator in mice (Hanincova et al., 2008). Differences in our results and outcomes in studies by Dunn et al. (2014) could be either due to the mode of infection (tick versus needle), genotypes of pathogens, or genetic differences between Peromyscus versus Mus musculus C3H mouse strain used. Supporting this premise, stimulation of the innate immune response against invasive N40 strain could possibly resolve babesiosis while it is likely not sufficient against the less fit B. burgdorferi B348 strain in P. leucopus, thus resulting in higher Babesia parasitemia in co-infected than B. microti infected animals (Dunn et al., 2014). In any case, higher parasitemia in P. leucopus could facilitate acquisition of B. microti from this co-infected animal-reservoir host in nature by tick vector (Diuk-Wasser et al., 2016). High parasitemia with Apicomplexan protozoan that infect RBCs, such as malaria causing Plasmodium species, is followed by lysis of erythrocytes causing anemia (Shabani et al., 2017). Hematologic abnormalities, such as anemia and thrombocytopenia are also associated with babesiosis in humans, often requiring blood transfusion and even hospitalization (White et al., 1998; Hatcher et al., 2001; Joseph et al., 2011). B. microti infection in mice in this study (Figure 1C) and previously reported (Coleman et al., 2005), and infection of gerbils by B. divergens (Dkhil et al., 2010) resulted in overall reduction of erythrocytes in blood. Thus, our results here reflect the effect of Babesia infection in the susceptible hosts.
The spleen is suggested to be an important lymphoid organ that produces plasma cells, which are the major producer of antibodies during protozoan infection (Bermejo et al., 2011). Previous studies have shown that humoral immune response against parasites causing Chagas disease and malaria are delayed or abrogated due to splenic B cell apoptosis and depletion (Muxel et al., 2011; Obishakin et al., 2014). Furthermore, severe babesiosis in splenectomized patients results in high morbidity and even mortality indicating a critical role of the spleen in resolution of Babesia infection. Thus, splenomegaly and alteration in spleen architecture in B. microti infected mice as observed in our study are consistent with reports on other parasitic diseases (Kafetzis, 2003; Dkhil et al., 2010; Wilson et al., 2011; Kuna et al., 2015). Movement of activated marginal zone B cells and dendritic cells to the T cell zone help presentation of antigen directly and therefore, activation of T cells followed by their migration to the edge of the follicles (Vannier and Krause, 2012). These changes can possibly obliterate the demarcation between the red and white pulp, as we observed (Figure 5). In humans, babesiosis can be a life-threatening disease in asplenic individuals, further emphasizing the importance of the spleen in babesiosis resolution (Krause et al., 2008; Raffalli and Wormser, 2016). Even after elimination of the parasite, recovery of internal organs including spleen could lag behind, prolonging illness. Unlike humans, death has not been reported in mice due to B. microti infection. We also did not observe any visual differences in vitality of B. microti infected versus co-infected mice.
Innate immunity was reported to be critical for determining the fate of Babesia infection in mice (Aguilar-Delfin et al., 2001). In mice, the spleen is a major reservoir of undifferentiated monocytes that can be differentiated into macrophages and dendritic cells in vitro. It is conceivable that infection with B. microti stimulates these cells to develop into macrophages, which then facilitate clearance of the infected erythrocytes as we showed previously (Djokic et al., 2018a, b). Depletion of macrophages using drugs at different stages of B. microti infection resulted in significant increases in parasitemia and caused mortality in the mice (Terkawi et al., 2015). Despite development of high B. microti parasitemia levels, anti-inflammatory response could prevent death in our experiments unlike that reported for highly infectious B. duncani WA-1 strain in mice and hamsters (Dao and Eberhard, 1996; Hemmer et al., 2000). Mortality due to WA-1 strain is associated with the high levels of IFN-γ and TNF-α in spleen and lungs, heavy intravascular hemolysis, and pronounced vascular stasis with multi-organ failure (Dao and Eberhard, 1996; Hemmer et al., 2000). Our findings suggest that unlike B. duncani WA-1 strain, proinflammatory immunological response to B. microti is more subdued, protecting animals from death.
Our results agree with the previous report that young C3H mice show pronounced inflammatory Lyme arthritis manifestations (Barthold et al., 1990). Severity of Lyme arthritis and carditis in C3H mice correlates with the B. burgdorferi burden (Yang et al., 1994; Ma et al., 1998; Brown et al., 2001; Thomas et al., 2001; Parveen et al., 2006; Sahay et al., 2011; Schlachter et al., 2018). Several host factors contribute to inflammatory disease. Previous histopathological examination of B. burgdorferi-infected mice showed infiltration of innate immune cells, predominantly neutrophils, at sites of inflammation in the joints (Barthold et al., 1990, 1992; Ruderman et al., 1995; Sahay et al., 2011). In addition, depletion of CD8+ cells using antibodies helped resolution of ankle swelling in C3H/HeJ mice, indicating that these cells exacerbate inflammatory Lyme arthritis (Lasky et al., 2016). Other factors, such as the increase in proinflammatory cytokines production, also contribute to inflammatory Lyme disease. For example, reduction in B. burgdorferi-specific proinflammatory cytokine production in infected C3Hgld mice, due to the presence of a non-functional mutation in Fas ligand (FasL), caused diminished inflammatory response and less severe Lyme arthritis even though spirochete burden in C3Hgld mice was similar to C3H mice (Shi et al., 2006). Fas is reported to be expressed at high levels in macrophages, dendritic cells, fibroblasts, and lymphocytes present in inflamed synovium, while FasL is expressed in macrophages and γδ T cells of synovium (Perlman et al., 1999; Roessner et al., 2003; Ma et al., 2004; Shi et al., 2006). Although we did not use mice defective in a particular cell type or immuno-depleted our mice for any particular cell type, we observed a high level of infiltration of leukocytes in the inflamed joints of B. burgdorferi infected and co-infected mice (Figure 8D).
Unlike a previous report that B. microti and B. burgdorferi have independent courses of infection in co-infected mice (Coleman et al., 2005), we observed that B. microti infection has a significant impact on increasing B. burgdorferi survival and tissue colonization. B cells are important professional antigen presenting cells, display regulatory functions through cytokine production and are critical for humoral immunity due to their production of protective antibodies. Subversion of different B-cell subsets during parasitic and viral infections was reviewed recently (Borhis and Richard, 2015). Significant reduction in total B and T cells was also reported after infection with malaria parasite, P. falciparum in patients compared to uninfected controls (Kassa et al., 2006). Significantly lower numbers of splenic B and T cells after B. microti co-infection in our study agrees with these findings. The impact of destabilization of B cell numbers by B. microti is also reflected in the attenuated antibody response against B. burgdorferi during co-infections (Figure 7). Antibodies play an important role in clearance of extracellular B. burgdorferi by engaging different effector mechanisms, such as complement activation, neutralization, and opsonization, which results in phagocytosis facilitated by interaction of the Fc-region of antibodies and Fc-receptors on the professional phagocytes. In fact, adaptive immune responses involving both B and T cells have been implicated in resolution of inflammatory Lyme disease in mice (Barthold et al., 1992; McKisic and Barthold, 2000; McKisic et al., 2000; Bockenstedt et al., 2001). Both splenic B cell populations and serum immunoglobulin levels are elevated in response to B. burgdorferi infection. Immunological memory persists for a long period after antibody maturation. Therefore, B. burgdorferi-specific antibodies are important for clearance of the spirochetes in animals by opsonophagocytosis (Belperron et al., 2014). However, residual spirochetes remain in various organs of mice (Barthold et al., 1990). Overall, diminished functional humoral immunity due to B. microti infection specifically against B. burgdorferi, as determined by opsonophagocytosis, could prolong survival of Lyme spirochetes in the co-infected mice (Figures 4, 8). Alternatively, induction and preferential expression of the specific genes in N40 during co-infections could facilitate survival and persistence of spirochetes in tissues. Reported changes in essential gene expression in pathogens during co-infections support this hypothesis (Steere et al., 2011; Wu et al., 2015; Ibberson et al., 2017).
Borrelia burgdorferi stimulates splenic B and T development at 3 weeks of infection which is suppressed by B. microti and results in overall reduction in the humoral immunity, increases tissue colonization by B. burgdorferi and facilitates persistence of inflammatory Lyme arthritis in co-infected mice. Somewhat higher levels of infiltration of leukocytes in the co-infected mice could also contribute to increased joints inflammation (Barthold et al., 1990, 1992; Ruderman et al., 1995; Lasky et al., 2016). The effect of infection with N40 on B. microti was subtle, but we consistently observed diminished peak parasitemia in the co-infected mice. Our results here reflect outcome of simultaneous co-infections with two tick-borne pathogens and may differ when infection with B. microti and B. burgdorferi occurs in sequential manner. It is of great interest to us to examine the impact of B. microti prior presence in animals on a follow up infection by B. burgdorferi and vice versa on the host. This will be focus of our future studies. Despite some differences observed in severity of diseases in mice and humans during co-infection with B. burgdorferi and B. microti, our results indicate that a thorough investigation using susceptible mice can provide insights into their respective pathogenesis. In addition, a better understanding of pathogenesis also requires a careful examination of the mechanisms involved in the development and stimulation of splenic B and different T cell populations at different stages of infection using the susceptible C3H animal model system developed here. Furthermore, mechanisms involved in reduction of B and to some extent T cells need to be determined to fully understand the impact of co-infection with B. burgdorferi and B. microti. Our future studies will address these questions.
Conclusion
Our studies indicated that during co-infection of susceptible C3H mice with tick-borne pathogens, potential stimulation of the innate immune response by B. burgdorferi attenuate B. microti parasitemia while changes in symptoms of babesiosis were not discernible. However, in our model, B. microti suppressed adaptive immune response triggered by B. burgdorferi infection such that diminished splenic B and T cells populations were reflected by overall reduction in the specific functional humoral immunity against both pathogens. As a consequence, B. burgdorferi persists at higher levels in tissues causing more severe Lyme disease in the susceptible C3H mice.
Data Availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Information File).
Ethics Statement
The Institutional Animal Care and Use Committee (IACUC) members reviewed and approved the protocol number PROTO201702491 entitled, “Spirochetes and tick-borne pathogens,” of the corresponding author to conduct this study at Rutgers New Jersey Medical School following guidelines of the Animal Welfare Act, The Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals, and the Public Health Service Policy that are fully adopted at the Rutgers University.
Author Contributions
NP conceived and designed the experiments. PB provided training to LA in microscopy. VD, LA, SP, and SS performed the experiments. LA and VD conducted imaging of H&E stained organs sections, and performed analysis of heart and joints (at acute phase), and spleen and liver independently at three weeks post-infection. KK performed histopathological analysis and inflammation scoring of heart and joints in a blinded manner at three weeks of infection.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We greatly appreciate Dr. Linda Bockenstedt of Yale School of Medicine, who suggested examination of perfused brain sections for spirochetes presence. We also acknowledge valuable assistance provided by the technical Director, Sukhwinder Singh of the Flow Cytometry Core Laboratory of Rutgers New Jersey Medical School, and Luke Fritzky and Joel Pierre for assistance in organ samples preparation, sectioning and H&E staining for the histopathological examination.
Footnotes
Funding. This work was supported by the National Institutes of Health (R01AI089921) and New Jersey Health Foundation grant to NP.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.01596/full#supplementary-material
3D visualization of the phagocytosis of B. burgdorferi using pooled N40-infected mice plasma for opsonization. Rotating angle view in the space demonstrates surface labeling of J774.1 macrophages (blue), extracellular B. burgdorferi (green), and B. burgdorferi internalized by the macrophages, i.e., phagocytosed spirochetes (red).
References
- Abel S., Luckheide N., Westendorf A. M., Geffers R., Roers A., Muller W., et al. (2012). Strong impact of CD4+ Foxp3+ regulatory T cells and limited effect of T cell-derived IL-10 on pathogen clearance during Plasmodium yoelii infection. J. Immunol. 188 5467–5477. 10.4049/jimmunol.1102223 [DOI] [PubMed] [Google Scholar]
- Aguilar-Delfin I., Homer M. J., Wettstein P. J., Persing D. H. (2001). Innate resistance to Babesia infection is influenced by genetic background and gender. Infect. Immun. 69 7955–7958. 10.1128/iai.69.12.7955-7958.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Delfin I., Wettstein P. J., Persing D. H. (2003). Resistance to acute babesiosis is associated with interleukin-12- and gamma interferon-mediated responses and requires macrophages and natural killer cells. Infect. Immun. 71 2002–2008. 10.1128/iai.71.4.2002-2008.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguita J., Persing D. H., Rincon M., Barthold S. W., Fikrig E. (1996). Effect of anti-interleukin 12 treatment on murine lyme borreliosis. J. Clin. Invest. 97 1028–1034. 10.1172/jci118494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong A. L., Barthold S. W., Persing D. H., Beck D. S. (1992). Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 47 249–258. 10.4269/ajtmh.1992.47.249 [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Beck D. S., Hansen G. M., Terwilliger G. A., Moody K. D. (1990). Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162 133–138. 10.1093/infdis/162.1.133 [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Sidman C. L., Smith A. L. (1992). Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47 605–613. 10.4269/ajtmh.1992.47.605 [DOI] [PubMed] [Google Scholar]
- Basso B., Marini V. (2014). Experimental Chagas disease. Innate immune response in Balb/c mice previously vaccinated with Trypanosoma rangeli. I. The macrophage shows immunological memory: reality or fiction?. Immunobiology 219 275–284. 10.1016/j.imbio.2013.10.012 [DOI] [PubMed] [Google Scholar]
- Belperron A. A., Liu N., Booth C. J., Bockenstedt L. K. (2014). Dual role for Fcgamma receptors in host defense and disease in Borrelia burgdorferi-infected mice. Front. Cell. Infect. Microbiol. 4:75. 10.3389/fcimb.2014.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermejo D. A., Amezcua Vesely M. C., Khan M., Acosta Rodriguez E. V., Montes C. L., Merino M. C., et al. (2011). Trypanosoma cruzi infection induces a massive extrafollicular and follicular splenic B-cell response which is a high source of non-parasite-specific antibodies. Immunology 132 123–133. 10.1111/j.1365-2567.2010.03347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt L. K., Kang I., Chang C., Persing D., Hayday A., Barthold S. W. (2001). CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect. Immun. 69 5264–5269. 10.1128/iai.69.9.5264-5269.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borhis G., Richard Y. (2015). Subversion of the B-cell compartment during parasitic, bacterial, and viral infections. BMC Immunol. 16:15. 10.1186/s12865-015-0079-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. L., Wooten R. M., Johnson B. J., Iozzo R. V., Smith A., Dolan M. C., et al. (2001). Resistance to Lyme disease in decorin-deficient mice. J. Clin. Invest. 107 845–852. 10.1172/jci11692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Alter L., Barthold S. W., Parveen N. (2015). Disruption of bbe02 by insertion of a luciferase gene increases transformation efficiency of Borrelia burgdorferi and allows live imaging in Lyme disease susceptible C3H mice. PLoS One 10:e0129532. 10.1371/journal.pone.0129532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Marras S. A., Parveen N. (2013). Sensitive multiplex PCR assay to differentiate Lyme spirochetes and emerging pathogens Anaplasma phagocytophilum and Babesia microti. BMC Microbiol. 13:295. 10.1186/1471-2180-13-295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Nasereddin T., Alter L., Centurion-Lara A., Giacani L., Parveen N. (2016). Treponema pallidum lipoprotein TP0435 expressed in Borrelia burgdorferi produces multiple surface/periplasmic isoforms and mediates adherence. Sci. Rep. 6:25593. 10.1038/srep25593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Levine D., Thill C., Kuhlow C., Benach J. L. (2005). Babesia microti and Borrelia burgdorferi follow independent courses of infection in mice. J. Infect. Dis. 192 1634–1641. [DOI] [PubMed] [Google Scholar]
- Cox F. E. (2001). Concomitant infections, parasites and immune responses. Parasitology 122(Suppl.), S23–S38. [DOI] [PubMed] [Google Scholar]
- Dao A. H., Eberhard M. L. (1996). Pathology of acute fatal babesiosis in hamsters experimentally infected with the WA-1 strain of Babesia. Lab. Invest. 74 853–859. [PubMed] [Google Scholar]
- Diuk-Wasser M. A., Vannier E., Krause P. J. (2016). Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends Parasitol. 32 30–42. 10.1016/j.pt.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djokic V., Akoolo L., Parveen N. (2018a). Babesia microti infection changes host spleen architecture and is cleared by a Th1 immune response. Front. Microbiol. 9:85. 10.3389/fmicb.2018.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djokic V., Primus S., Akoolo L., Chakraborti M., Parveen N. (2018b). Age-related differential stimulation of immune response by Babesia microti and Borrelia burgdorferi during acute phase of infection affects disease severity. Front. Immunol. 9:2891. 10.3389/fimmu.2018.02891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhil M. A., Al-Quraishy S., Abdel-Baki A. S. (2010). Hepatic tissue damage induced in Meriones ungliculatus due to infection with Babesia divergens-infected erythrocytes. Saudi J. Biol. Sci. 17 129–132. 10.1016/j.sjbs.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. M., Krause P. J., Davis S., Vannier E. G., Fitzpatrick M. C., Rollend L., et al. (2014). Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PLoS One 9:e115494. 10.1371/journal.pone.0115494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. J., Russell J. C., Davidson E. N., Yanushefski T. J., Fleischman B. L., Heist R. O., et al. (2019). A 4-Yr survey of the range of ticks and tick-borne pathogens in the Lehigh Valley region of Eastern Pennsylvania. J. Med. Entomol. 56 1122–1134. 10.1093/jme/tjz043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. S. M., Bullock-Iacullo S. L., Fritsche T. R., Grady K. K., Healy G. R., Palmer J., et al. (2000). Laboratory Diagnosis of Blood-borne Parasitic Diseases; Approved Guideline. Wayne, PA: Clinical and Laboratory Standards Institute, 1–36. [Google Scholar]
- Glickstein L., Edelstein M., Dong J. Z. (2001). Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69 3737–3743. 10.1128/iai.69.6.3737-3743.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. B., Bjork J. K. H., Neitzel D. F., Dorr F. M., Whitemarsh T., Boegler K. A., et al. (2018). Evaluating acarological risk for exposure to Ixodes scapularis and Ixodes scapularis-borne pathogens in recreational and residential settings in Washington County, Minnesota. Ticks Tick Borne Dis. 9 340–348. 10.1016/j.ttbdis.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K., Mukherjee P., Ogden N. H., Margos G., Wormser G. P., Reed K. D., et al. (2013). Multilocus sequence typing of Borrelia burgdorferi suggests existence of lineages with differential pathogenic properties in humans. PLoS One 8:e73066. 10.1371/journal.pone.0073066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K., Ogden N. H., Diuk-Wasser M., Pappas C. J., Iyer R., Fish D., et al. (2008). Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl. Environ. Microbiol. 74 153–157. 10.1128/aem.01567-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher J. C., Greenberg P. D., Antique J., Jimenez-Lucho V. E. (2001). Severe babesiosis in long island: review of 34 cases and their complications. Clin. Infect. Dis. 32 1117–1125. 10.1086/319742 [DOI] [PubMed] [Google Scholar]
- Hemmer R. M., Ferrick D. A., Conrad P. A. (2000). Up-regulation of tumor necrosis factor-alpha and interferon-gamma expression in the spleen and lungs of mice infected with the human Babesia isolate WA1. Parasitol. Res. 86 121–128. 10.1007/s004360050021 [DOI] [PubMed] [Google Scholar]
- Hersh M. H., Ostfeld R. S., Mchenry D. J., Tibbetts M., Brunner J. L., Killilea M. E., et al. (2014). Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PLoS One 9:e99348. 10.1371/journal.pone.0099348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. A., Sibley L. D. (2012). Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10 766–778. 10.1038/nrmicro2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibberson C. B., Stacy A., Fleming D., Dees J. L., Rumbaugh K., Gilmore M. S., et al. (2017). Co-infecting microorganisms dramatically alter pathogen gene essentiality during polymicrobial infection. Nat. Microbiol. 2:17079. 10.1038/nmicrobiol.2017.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenson T. G., Jaenson D. G., Eisen L., Petersson E., Lindgren E. (2012). Changes in the geographical distribution and abundance of the tick Ixodes ricinus during the past 30 years in Sweden. Parasit. Vectors 5:8. 10.1186/1756-3305-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. L., Boegler K. A., Clark R. J., Delorey M. J., Bjork J. K. H., Dorr F. M., et al. (2018). An acarological risk model predicting the density and distribution of host-seeking Ixodes scapularis Nymphs in Minnesota. Am. J. Trop. Med. Hyg. 98 1671–1682. 10.4269/ajtmh.17-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. L., Graham C. B., Boegler K. A., Cherry C. C., Maes S. E., Pilgard M. A., et al. (2017). Prevalence and diversity of tick-borne pathogens in nymphal ixodes scapularis (Acari: Ixodidae) in Eastern National Parks. J. Med. Entomol. 54 742–751. 10.1093/jme/tjw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J. T., Roy S. S., Shams N., Visintainer P., Nadelman R. B., Hosur S., et al. (2011). Babesiosis in lower hudson valley, New York, USA. Emerg. Infect. Dis. 17 843–847. 10.3201/eid1705.101334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnick S., Margos G., Rieger M., Dzaferovic E., Bent S. J., Overzier E., et al. (2015). Borrelia burgdorferi sensu stricto and Borrelia afzelii: population structure and differential pathogenicity. Int. J. Med. Microbiol. 305 673–681. 10.1016/j.ijmm.2015.08.017 [DOI] [PubMed] [Google Scholar]
- Kafetzis D. A. (2003). An overview of paediatric leishmaniasis. J. Postgrad. Med. 49 31–38. [DOI] [PubMed] [Google Scholar]
- Kang I., Barthold S. W., Persing D. H., Bockenstedt L. K. (1997). T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect. Immun. 65 3107–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassa D., Petros B., Mesele T., Hailu E., Wolday D. (2006). Characterization of peripheral blood lymphocyte subsets in patients with acute Plasmodium falciparum and P. vivax malaria infections at Wonji Sugar Estate, Ethiopia. Clin. Vaccine Immunol. 13 376–379. 10.1128/cvi.13.3.376-379.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane-Myers A., Nickell S. P. (1995). Role of IL-4 and IFN-gamma in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 155 2020–2028. [PubMed] [Google Scholar]
- Knapp K. L., Rice N. A. (2015). Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J. Parasitol. Res. 2015:587131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause P. J., Gewurz B. E., Hill D., Marty F. M., Vannier E., Foppa I. M., et al. (2008). Persistent and relapsing babesiosis in immunocompromised patients. Clin. Infect. Dis. 46 370–376. 10.1086/525852 [DOI] [PubMed] [Google Scholar]
- Krause P. J., Spielman A., Telford S. R., III, Sikand V. K., Mckay K., Christianson D., et al. (1998). Persistent parasitemia after acute babesiosis. N. Engl. J. Med. 339 160–165. 10.1056/nejm199807163390304 [DOI] [PubMed] [Google Scholar]
- Krause P. J., Telford S. R., III, Spielman A., Sikand V., Ryan R., Christianson D., et al. (1996). Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 275 1657–1660. 10.1001/jama.275.21.1657 [DOI] [PubMed] [Google Scholar]
- Kuna A., Gajewski M., Szostakowska B., Nahorski W. L., Myjak P., Stanczak J. (2015). Imported malaria in the material of the institute of maritime and tropical medicine: a review of 82 patients in the years 2002-2014. Biomed. Res. Int. 2015:941647. 10.1155/2015/941647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky C. E., Pratt C. L., Hilliard K. A., Jones J. L., Brown C. R. (2016). T cells exacerbate Lyme Borreliosis in TLR2-deficient mice. Front. Immunol. 7:468. 10.3389/fimmu.2016.00468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Ji P. Y., Song L. G., Lei J. X., Lv Z. Y., Wu Z. D., et al. (2015). The expression of molecule CD28 and CD38 on CD4(+)/CD8(+) T lymphocytes in thymus and spleen elicited by Schistosoma japonicum infection in mice model. Parasitol. Res. 114 3047–3058. 10.1007/s00436-015-4507-y [DOI] [PubMed] [Google Scholar]
- Lommano E., Bertaiola L., Dupasquier C., Gern L. (2012). Infections and coinfections of questing Ixodes ricinus ticks by emerging zoonotic pathogens in Western Switzerland. Appl. Environ. Microbiol. 78 4606–4612. 10.1128/AEM.07961-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Liu H., Tu-Rapp H., Thiesen H. J., Ibrahim S. M., Cole S. M., et al. (2004). Fas ligation on macrophages enhances IL-1R1-Toll-like receptor 4 signaling and promotes chronic inflammation. Nat. Immunol. 5 380–387. 10.1038/ni1054 [DOI] [PubMed] [Google Scholar]
- Ma Y., Seiler K. P., Eichwald E. J., Weis J. H., Teuscher C., Weis J. J. (1998). Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Balzano C., Hess M., Malhotra A., Lenox R. (2015). Severe babesiosis and Borrelia burgdorferi co-infection. QJM 108 141–143. [DOI] [PubMed] [Google Scholar]
- McKisic M. D., Barthold S. W. (2000). T-cell-independent responses to Borrelia burgdorferi are critical for protective immunity and resolution of lyme disease. Infect. Immun. 68 5190–5197. 10.1128/iai.68.9.5190-5197.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKisic M. D., Redmond W. L., Barthold S. W. (2000). Cutting edge: T cell-mediated pathology in murine Lyme borreliosis. J. Immunol. 164 6096–6099. 10.4049/jimmunol.164.12.6096 [DOI] [PubMed] [Google Scholar]
- Moore A., Nelson C., Molins C., Mead P., Schriefer M. (2016). Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of lyme disease, United States. Emerg. Infect. Dis. 22 1169–1177. 10.3201/eid2207.151694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro M. H., Zegarra-Moro O. L., Bjornsson J., Hofmeister E. K., Bruinsma E., Germer J. J., et al. (2002). Increased arthritis severity in mice coinfected with Borrelia burgdorferi and Babesia microti. J. Infect. Dis. 186 428–431. [DOI] [PubMed] [Google Scholar]
- Moutailler S., Valiente Moro C., Vaumourin E., Michelet L., Tran F. H., Devillers E., et al. (2016). Co-infection of ticks: the rule rather than the exception. PLoS Negl. Trop. Dis. 10:e0004539. 10.1371/journal.pntd.0004539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller I., Freitag M. H., Poggensee G., Scharnetzky E., Straube E., Schoerner C., et al. (2012). Evaluating frequency, diagnostic quality, and cost of Lyme borreliosis testing in Germany: a retrospective model analysis. Clin. Dev. Immunol. 2012:595427. 10.1155/2012/595427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muxel S. M., Freitas Do Rosario A. P., Zago C. A., Castillo-Mendez S. I., Sardinha L. R., Rodriguez-Malaga S. M., et al. (2011). The spleen CD4+ T cell response to blood-stage Plasmodium chabaudi malaria develops in two phases characterized by different properties. PLoS One 6:e22434. 10.1371/journal.pone.0022434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obishakin E., De Trez C., Magez S. (2014). Chronic Trypanosoma congolense infections in mice cause a sustained disruption of the B-cell homeostasis in the bone marrow and spleen. Parasite Immunol. 36 187–198. 10.1111/pim.12099 [DOI] [PubMed] [Google Scholar]
- Parveen N., Cornell K. A., Bono J. L., Chamberland C., Rosa P., Leong J. M. (2006). Bgp, a secreted GAG-binding protein of B. burgdorferi strain N40, displays nucleosidase activity and is not essential for infection of immunodeficient mice. Infect. Immun. 74 3016–3020. 10.1128/iai.74.5.3016-3020.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman H., Pagliari L. J., Georganas C., Mano T., Walsh K., Pope R. M. (1999). FLICE-inhibitory protein expression during macrophage differentiation confers resistance to fas-mediated apoptosis. J. Exp. Med. 190 1679–1688. 10.1084/jem.190.11.1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedmonte N. P., Shaw S. B., Prusinski M. A., Fierke M. K. (2018). Landscape features associated with blacklegged tick (Acari: Ixodidae) density and tick-borne pathogen prevalence at multiple spatial scales in central New York State. J. Med. Entomol. 55 1496–1508. 10.1093/jme/tjy111 [DOI] [PubMed] [Google Scholar]
- Piesman J., Mather T. N., Donahue J. G., Levine J., Campbell J. D., Karakashian S. J., et al. (1986). Comparative prevalence of Babesia microti and Borrelia burgdorferi in four populations of Ixodes dammini in eastern Massachusetts. Acta Trop. 43 263–270. [PubMed] [Google Scholar]
- Primus S., Akoolo L., Schlachter S., Gedroic K., Rojtman A. D., Parveen N. (2018). Efficient detection of symptomatic and asymptomatic patient samples for Babesia microti and Borrelia burgdorferi infection by multiplex qPCR. PLoS One 13:e0196748. 10.1371/journal.pone.0196748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffalli J., Wormser G. P. (2016). Persistence of babesiosis for > 2 years in a patient on rituximab for rheumatoid arthritis. Diagn. Microbiol. Infect. Dis. 85 231–232. 10.1016/j.diagmicrobio.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Ramesh G., Borda J. T., Dufour J., Kaushal D., Ramamoorthy R., Lackner A. A., et al. (2008). Interaction of the Lyme disease spirochete Borrelia burgdorferi with brain parenchyma elicits inflammatory mediators from glial cells as well as glial and neuronal apoptosis. Am. J. Pathol. 173 1415–1427. 10.2353/ajpath.2008.080483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G., Borda J. T., Gill A., Ribka E. P., Morici L. A., Mottram P., et al. (2009). Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. J. Neuroinflammation 6 23. 10.1186/1742-2094-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli A., Silaghi C., Obiegala A., Rudolf I., Hubalek Z., Foldvari G., et al. (2014). Ixodes ricinus and its transmitted pathogens in Urban and Peri-Urban Areas in Europe: new hazards and relevance for public health. Front. Public Health 2:251. 10.3389/fpubh.2014.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner K., Wolfe J., Shi C., Sigal L. H., Huber S., Budd R. C. (2003). High expression of Fas ligand by synovial fluid-derived gamma delta T cells in Lyme arthritis. J. Immunol. 170 2702–2710. 10.4049/jimmunol.170.5.2702 [DOI] [PubMed] [Google Scholar]
- Ruderman E. M., Kerr J. S., Telford S. R., Spielman A., Glimcher L. H., Gravallese E. M. (1995). Early murine Lyme carditis has a macrophage predominance and is independent of major histocompatibility complex class II-CD4+ T cell interactions. J. Infect. Dis. 171 362–370. 10.1093/infdis/171.2.362 [DOI] [PubMed] [Google Scholar]
- Sahay B., Singh A., Gnanamani A., Patsey R. L., Blalock J. E., Sellati T. J. (2011). CD14 signaling reciprocally controls collagen deposition and turnover to regulate the development of lyme arthritis. Am. J. Pathol. 178 724–734. 10.1016/j.ajpath.2010.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlachter S., Seshu J., Lin T., Norris S., Parveen N. (2018). The Borrelia burgdorferi Glycosaminoglycan binding protein Bgp in the B31 strain is not essential for infectivity despite facilitating adherence and tissue colonization. Infect. Immun. 86 e00667-17. 10.1128/IAI.00667-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze T. L., Jordan R. A., Healy S. P., Roegner V. E. (2013). Detection of Babesia microti and Borrelia burgdorferi in host-seeking Ixodes scapularis (Acari: Ixodidae) in Monmouth County, New Jersey. J. Med. Entomol. 50 379–383. 10.1603/me12088 [DOI] [PubMed] [Google Scholar]
- Shabani E., Hanisch B., Opoka R. O., Lavstsen T., John C. C. (2017). Plasmodium falciparum EPCR-binding PfEMP1 expression increases with malaria disease severity and is elevated in retinopathy negative cerebral malaria. BMC Med. 15:183. 10.1186/s12916-017-0945-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Wolfe J., Russell J. Q., Fortner K., Hardin N., Anguita J., et al. (2006). Fas ligand deficiency impairs host inflammatory response against infection with the spirochete Borrelia burgdorferi. Infect. Immun. 74 1156–1160. 10.1128/iai.74.2.1156-1160.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponaas A. M., Cadman E. T., Voisine C., Harrison V., Boonstra A., O’garra A., et al. (2006). Malaria infection changes the ability of splenic dendritic cell populations to stimulate antigen-specific T cells. J. Exp. Med. 203 1427–1433. 10.1084/jem.20052450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Drouin E. E., Glickstein L. J. (2011). Relationship between immunity to Borrelia burgdorferi outer-surface protein A (OspA) and Lyme arthritis. Clin. Infect. Dis. 52(Suppl. 3), s259–s265. 10.1093/cid/ciq117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Strle F., Wormser G. P., Hu L. T., Branda J. A., Hovius J. W., et al. (2016). Lyme borreliosis. Nat. Rev. Dis. Primers 2:16090. 10.1038/nrdp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkawi M. A., Cao S., Herbas M. S., Nishimura M., Li Y., Moumouni P. F., et al. (2015). Macrophages are the determinant of resistance to and outcome of nonlethal Babesia microti infection in mice. Infect. Immun. 83 8–16. 10.1128/IAI.02128-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas V., Anguita J., Barthold S. W., Fikrig E. (2001). Coinfection with Borrelia burgdorferi and the agent of human granulocytic ehrlichiosis alters murine immune responses, pathogen burden, and severity of Lyme arthritis. Infect. Immun. 69 3359–3371. 10.1128/iai.69.5.3359-3371.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannier E., Krause P. J. (2012). Human babesiosis. N. Engl. J. Med. 366 2397–2407. [DOI] [PubMed] [Google Scholar]
- White D. J., Talarico J., Chang H. G., Birkhead G. S., Heimberger T., Morse D. L. (1998). Human babesiosis in New York State: review of 139 hospitalized cases and analysis of prognostic factors. Arch. Intern. Med. 158 2149–2154. [DOI] [PubMed] [Google Scholar]
- Wilson S., Vennervald B. J., Dunne D. W. (2011). Chronic hepatosplenomegaly in African school children: a common but neglected morbidity associated with schistosomiasis and malaria. PLoS Negl. Trop. Dis. 5:e1149. 10.1371/journal.pntd.0001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Guan G., Liu Z., Li Y., Luo J., Yin H. (2015). RNA-Seq-based analysis of changes in Borrelia burgdorferi gene expression linked to pathogenicity. Parasit. Vectors 8:155. 10.1186/s13071-014-0623-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Weis J. H., Eichwald E., Kolbert C. P., Persing D. H., Weis J. J. (1994). Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect. Immun. 62 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidner N., Mbow M. L., Dolan M., Massung R., Baca E., Piesman J. (1997). Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect. Immun. 65 3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3D visualization of the phagocytosis of B. burgdorferi using pooled N40-infected mice plasma for opsonization. Rotating angle view in the space demonstrates surface labeling of J774.1 macrophages (blue), extracellular B. burgdorferi (green), and B. burgdorferi internalized by the macrophages, i.e., phagocytosed spirochetes (red).
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information File).