Abstract
BACKGROUND:
Although rare in the United States, gallbladder cancer (GBCA) is a common cause of cancer death in some parts of the world. To investigate regional differences in pathogenesis and outcomes for GBCA, tumor mutations were analyzed from a sampling of specimens.
METHODS:
Primary tumors from patients with GBCA who were treated in Chile, Japan, and the United States between 1999 and 2016 underwent targeted sequencing of known cancer-associated genes. Fisher exact and Kruskal-Wallis tests assessed differences in clinicopathologic and genetic factors. Kaplan-Meier methods evaluated differences in overall survival from the time of surgery between mutations.
RESULTS:
A total of 81 patients were included. Japanese patients (11 patients) were older (median age, 72 years [range, 54–81 years]) compared with patients from Chile (21 patients; median age, 59 years [range, 32–73 years]) and the United States (49 patients; median age, 66 years [range, 46–87 years]) (P = .002) and had more well-differentiated tumors (46% vs 0% for Chile/United States; P < .001) and fewer gallstone-associated cancers (36% vs 67% for Chile and 69% for the United States; P = .13). Japanese patients had a median mutation burden of 6 (range, 1–23) compared with Chile (median mutation burden, 7 [range, 3–20]) and the United States (median mutation burden, 4 [range, 0–27]) (P = .006). Tumors from Japanese patients lacked AT-rich interaction domain 1A (ARID1A) and phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) mutations, whereas Chilean tumors lacked Erb-B2 receptor tyrosine kinase 3 (ERBB3) and AT-rich interaction domain 2 (APID2) mutations. SMAD family member 4 (SMAD4) was found to be mutated similarly across centers (38% in Chile, 36% in Japan, and 27% in the United States; P = .68) and was univariately associated with worse overall survival (median, 10 months vs 25 months; P = .039). At least one potentially actionable gene was found to be altered in 80% of tumors.
CONCLUSIONS:
Differences in clinicopathologic variables suggest the possibility of distinct GBCA pathogenesis in Japanese patients, which may be supported by differences in mutation pattern. Among all centers, SMAD4 mutations were detected in approximately one-third of patients and may represent a converging factor associated with worse survival. The majority of patients carried mutations in actionable gene targets, which may inform the design of future trials.
Keywords: cancer genes, gallbladder neoplasm, gene target, precision medicine, tumor mutation
INTRODUCTION
Gallbladder cancer (GBCA) is the most common biliary tract malignancy and often is rapidly fatal.1 Marked differences in global incidence have been noted, with the highest incidence observed in South America.2 Although the incidence and mortality from GBCA have been rising globally, epidemiologic studies have found that the clinical presentation and pathology of GBCA varies between regions.3–5 Of all countries, Chile has the highest GBCA-specific mortality rate, and GBCA is one of the most common causes of cancer death among Chilean women.6,7 In Japan, the incidence of GBCA is high, but it appears to have a lower associated mortality rate.8
Key differences in GBCA tumor biology have been identified across regions. Although the presence of gallstones, chronic inflammation, and an anomalous pancreatobiliary duct junction may predispose an individual to GBCA, the prevalence of these risk factors differs around the world.9 For example, gallstones are more commonly associated with GBCA in Chile and the United States, whereas an anomalous pancreatobiliary duct junction rarely is reported outside of Japan.10,11 It is interesting to note that KRAS mutations have been associated with this anomaly, suggesting that differences in clinical risk factors may be associated with differences in tumor biology.11 Studies of other genetic nuances specific to Japanese patients with GBCA also have demonstrated mutations in epidermal growth factor receptor family genes, the telomerase reverse transcriptase (TERT) promoter, cell cycle genes (eg, TP53 and RBI), AT-rich interaction domain 2 (ARID2), and PTEN and TSC1 inactivation.12 Although the mutational landscape of GBCA in Japanese patients has been described, to the best of our knowledge, little is known regarding the molecular profile of GBCA arising in other regions. Given the growing body of literature suggesting that tumor mutations in actionable gene targets may underpin response to therapy,13–20 the further molecular characterization of GBCA within a regional context is critical.
Our previous work suggested that differences exist with regard to clinical features and expression patterns of GBCA tumors among patients who were treated at the Arturo Lopez Perez Foundation Cancer Institute in Santiago, Chile; Yokohama City University in Yokohama, Japan; and the Memorial Sloan Kettering Cancer Center (MSKCC) in New York City.10,21 Collectively, these studies have suggested that there are differences in GBCA that are based on country of origin. In the current study, we build on this prior work by comparing the landscape of cancer-specific mutations in tumor samples from patients with GBCA who were treated in Chile, the United States, and Japan. We also assessed differences in clinicopathologic factors among these groups and examined how driver mutations influenced survival.
MATERIALS AND METHODS
Patients and Specimens
After institutional review board approval from all 3 centers, patients with GBCA who were treated between September 1999 and March 2016 and for whom primary tumor tissue from cholecystectomy or encompassing liver resection was available were included. Noting earlier work identifying important differences in the genomic profiles of patients with primary and metastatic tumors,22 a total of 14 patients for whom only metastatic tissue was available for genomic sequencing were excluded. All specimens were reviewed by pathologists experienced in the diagnosis, grading, and staging of GBCA to determine specimen adequacy for genomic analysis. Only specimens with adenocarcinoma and adenosquamous histologies were included. Six patients with other histologies were excluded, including 2 patients with neuroendocrine carcinoma, 1 patient with small cell carcinoma, 1 patient with sarcomatoid features, and 1 patient with cholangiocarcinoma. Information regarding clinical and pathologic variables, surgical history, perioperative outcome, histopathology and staging, follow-up, and survival were reported collectively and by treatment center. Tumor specimens collected by biopsy or resection of the primary gallbladder site and paired normal tissue were included in the analysis. For survival calculations, only those patients who underwent complete, potentially curative surgical resection were included.
Variables
Clinical and pathologic information was collected retrospectively. When GBCA was diagnosed after cholecystectomy for presumed benign disease, the case was classified as incidental. Patients were considered to have primary GBCA when the diagnosis was known or suspected preoperatively. TNM classification and staging of GBCA was performed according to the eighth edition of the American Joint Committee on Cancer.23 Tumor histology, tumor grade, lymph node status, and perineural and vascular invasion were identified from the pathology report. Curative resection was considered to be the removal of all tumor tissue with the goal of no tumor cells visible at the surgical resection margin. Any resection leaving behind tumor intentionally (for a palliative resection) or at a positive surgical margin was considered to be incomplete. Diagnostic procedures were performed percutaneously, laparoscopically, or during an aborted open resection. Resection of the gallbladder bed included segments IV and V. A hepatectomy involved resection of an entire lobe of the liver. An extended hepatectomy involved resection of an entire lobe and a portion of the contralateral liver.
Workup and Treatment
The approach to the diagnosis and management of patients with GBCA at each institution has been described previously.10,24–27 For patients with incidental GBCA, preoperative imaging most commonly consisted of ultrasound of the right upper quadrant. In some cases of incidental GBCA and the majority of cases of primary GBCA, contrast-enhanced computed tomography and/or magnetic resonance imaging of the abdomen and pelvis as well as computed tomography of the chest was performed. At the discretion of the medical oncologist or operating surgeon, [18F]fludeoxyglucose–positron emission tomography scans also were performed.
Staging laparoscopy typically was performed to exclude patients with metastatic disease when suspected. The degree of hepatic resection depended on the extent of disease and the optimal oncologic strategy to obtain negative surgical margins. Beyond resection of the liver, common bile duct excision was performed when it was not possible to obtain negative surgical margins from cystic duct resection, although this occasionally was performed to obtain better clearance of involved lymph nodes. Although vascular involvement (hepatic artery or portal vein) generally was considered to be advanced disease, vascular resection and reconstruction were performed selectively.
Systemic chemotherapy regimens varied and were determined by the medical oncologist based on guidelines and ongoing clinical trials. Adjuvant systemic chemotherapy pertained to therapy initiated after resection of GBCA. Preoperative systemic chemotherapy was initiated prior to GBCA resection.
Genomic Analyses
Paired DNA from tumor specimens and companion normal tissue was analyzed using the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) assay, a targeted next-generation sequencing platform designed to detect point mutations, copy number alterations, and select gene translocations or fusions in 341 to 410 cancer-associated genes.28 Mutational burden was adjusted based on megabase (mb) per IMPACT panel size: IMPACT-341 by 1.2 mb and IMPACT-410 by 1.38 mb based on a precedent from prior work.29 Analysis of the sequencing data was performed as described previously.28 Genomic data were stored and analyzed on a secure, institutionally licensed server for large-scale cancer genomics data (cBioPortal for Cancer Genomics).30,31 Actionable gene targets were defined by the MSKCC OncoKb database or reported in prior publications to be somatic alterations found to confer some heightened response or resistance to therapy relative to the wild-type configuration.19
Statistical Analysis
Patient and surgical characteristics were compared between centers using the Fisher exact test and the Kruskal-Wallis test when appropriate. Somatic alterations, including point mutations, copy number alterations, and fusions, were analyzed jointly. Gene mutations were tabulated and mutations that were found to be present in at least 6 patients were considered for formal statistical tests. Somatic alterations involved in common pathways, including cell cycle, chromatin remodeling, HIPPO, MYC, NOTCH, NRF2, phosphatidylinositide 3-kinases (PIK3), p53, receptor tyrosine kinase (RTK)-RAS, transforming growth factor β (TGF-β), and WNT, were described. Somatic alterations and mutation burden, as well as common pathways between centers, were compared using the Fisher exact test and the Kruskal-Wallis test when appropriate.
Associations between mutations and pathology characteristics (stage of disease, lymphadenopathy, vascular invasion, perineural invasion, and tumor grade) were analyzed using univariate logistic regression. Overall survival (OS) was calculated from the time of surgery until death for surgical patients as well as in the subset of patients who achieved complete resection. Patients who were alive at the time of last follow-up were censored. Mutations or pathway alterations that were present in at least 10 patients were included in analyses of survival outcomes. The log-rank test was used to assess the difference in survival based on the presence of somatic alterations. We explored the relationship between somatic alterations and OS while controlling for known confounders with multivariable Cox proportional hazards regression.
Mutual exclusivity was assessed with a 1-sided Fisher exact test and the effect size, when possible, was evaluated using the log odds ratio (log-OR). The 1-sided P value tested the alternative hypothesis that individual genes and gene pathways are mutually exclusive.
Correction for the false discovery rate was used to adjust P values with a separate correction for each outcome (ie, for site differences, for each pathology variable, and for each survival outcome) and separately for individual genes versus pathways. In the mutual exclusivity analyses, P values were adjusted overall for all combinations of genes. Two-sided adjusted P values (P-adj) < .05 were considered to be statistically significant. All analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc, Cary, North Carolina).
RESULTS
Patients
A total of 81 patients were included from all centers: 21 patients from Chile, 11 patients from Japan, and 49 patients from the United States (Table 1).Thedemographics of the Japanese patients were notably different from those of patients from the other centers. Specifically, Japanese patients on average were older (median age, 72 years [range, 54–81 years]) and were less often female (4 of 11 patients; 36%), especially in comparison with Chilean patients, who were younger (median age, 59 years [range, 32–73 years]) (P = .002) and more often female (17 of 21 patients; 81%) (P = .048). It is interesting to note that there were no patients of Japanese descent in the American cohort. Japanese patients accounted for all cases of well-differentiated disease (5 of 11 patients; 46% [P < .001]), and also were more likely to have had early-stage (stage I-III) disease (10 of 11 patients; 91%) when compared with patients from Chile (5 of 21 patients; 24%) or the United States (25 of 47 patients; 53%) (P = .001). In the current study, none of the Japanese patients had metastatic disease at the time of presentation, compared with 18% of the patients from the United States (9 of 49 patients) and 76% of the patients from Chile (16 of 21 patients) (P < .001).
TABLE 1.
Patient Characteristics by Treatment Center
| Characteristic | All N = 81 No. (%) |
Chile N = 21 No. (%) |
Japan N = 11 No. (%) |
United States N = 49 No. (%) |
Pa | |
|---|---|---|---|---|---|---|
| Age at diagnosis, y | Median (range) | 64.9 (32.2−87.1) | 59.0 (32.2−73.0) | 72.0(54.0−81.0) | 66.4 (46.0−87.1) | .002 |
| Sex | Male | 30 (37) | 4(19) | 7 (63.6) | 19 (38.8) | .048 |
| Female | 51 (63) | 17(81) | 4 (36.4) | 30 (61.2) | ||
| Race | White non-Hispanic | 41 (50.6) | 0(0) | 0(0) | 41 (83.7) | |
| Black | 3 (3.7) | 0(0) | 0(0) | 3 (6.1) | ||
| Asian | 16 (19.8) | 0(0) | 11 (100) | 5 (10.2) | ||
| Unknown | 21 (25.9) | 21 (100) | 0(0) | 0(0) | ||
| Incidental diagnosis | Incidental | 17 (21) | 6 (28.6) | 2 (18.2) | 9 (18.4) | .58 |
| Primary | 64 (79) | 15(71.4) | 9(81.8) | 40(81.6) | ||
| Histology | Adenocarcinoma | 75 (92.6) | 21 (100) | 11 (100) | 43 (87.8) | .20 |
| Adenosquamous | 6 (7.4) | 0(0) | 0(0) | 6 (12.2) | ||
| Grade | Well differentiated | 5 (6.2) | 0(0) | 5 (45.5) | 0(0) | <.001 |
| Moderately differentiated | 45 (55.6) | 16(76.2) | 2 (18.2) | 27 (55.1) | ||
| Poorly differentiated | 30 (37) | 5 (23.8) | 4 (36.4) | 21 (42.9) | ||
| Unknown | 1 (1.2) | 0(0) | 0(0) | 1 (2) | ||
| Gallstones | Yes | 52 (64.2) | 14 (66.7) | 4 (36.4) | 34 (69.4) | .13 |
| No | 29 (35.8) | 7 (33.3) | 7 (63.6) | 15 (30.6) | ||
| T classification | T1 | 1 (1.2) | 0(0) | 1 (9.1) | 0(0) | .046 |
| T2 | 18 (22.2) | 2(9.5) | 5 (45.5) | 11 (22.4) | ||
| T3 | 44 (54.3) | 9 (42.9) | 4 (36.4) | 31 (63.3) | ||
| T4 | 9 (11.1) | 6 (28.6) | 1 (9.1) | 2 (4.1) | ||
| Tx | 9 (11.1) | 4(19) | 0(0) | 5 (10.2) | ||
| N classification | N0 | 21 (25.9) | 2(9.5) | 6 (54.5) | 13 (26.5) | .11 |
| N1 | 31 (38.3) | 9 (42.9) | 5 (45.5) | 17 (34.7) | ||
| N2 | 13 (16) | 2(9.5) | 0(0) | 11 (22.4) | ||
| Nx | 16 (19.8) | 8 (38.1) | 0(0) | 8 (16.3) | ||
| M classification | M0 | 56 (69.1) | 5 (23.8) | 11 (100) | 40 (81.6) | <.001 |
| M1 | 25 (30.9) | 16(76.2) | 0(0) | 9 (18.4) | ||
| Overall TNM stage | I | 2 (2.5) | 0(0) | 1 (9.1) | 1 (2) | .001 |
| of disease | II | 5 (6.2) | 0(0) | 3 (27.3) | 2 (4.1) | |
| III | 33 (40.7) | 5 (23.8) | 6 (54.5) | 22 (44.9) | ||
| IV | 39 (48.1) | 16(76.2) | 1 (9.1) | 22 (44.9) | ||
| Unknown | 2 (2.5) | 0(0) | 0(0) | 2 (4.1) |
Bold type indicates statistical significance.
Table 2 describes the surgical details for the 71 patients who underwent surgical intervention. Approximately two-thirds of patients underwent curative resection (47 of 71 patients; 66%), whereas tumor tissue was collected from the remaining patients at the time of a diagnostic (8 of 71 patients; 11%) or palliative (16 of 71 patients; 23%) procedure. No significant differences were observed across the 3 institutions with regard to the frequency of lymph node status (P = .22), perineural invasion (P= .16), or vascular invasion (P = .08), although each was common (56%−66%). All surgical resections performed in the Japanese patients were complete resections.
TABLE 2.
Surgical Characteristics by Treatment Center
| Characteristic | All N = 71 No. (%) |
Chile N = 16 No. (%) |
Japan N = 11 No. (%) |
United States N = 44 No. (%) |
Pa | |
|---|---|---|---|---|---|---|
| Resection status | Incomplete | 24 (33.8) | 10 (62.5) | 0(0) | 14(31.8) | .002 |
| Complete | 47 (66.2) | 6 (37.5) | 11 (100) | 30 (68.2) | ||
| Resection type | Diagnostic | 8(11.3) | 6 (37.5) | 0(0) | 2 (4.5) | .001 |
| Palliative | 16 (22.5) | 4(25) | 0(0) | 12 (27.3) | ||
| Curative | 47 (66.2) | 6 (37.5) | 11 (100) | 30 (68.2) | ||
| Resection details | Nondefinitive | 24 (33.8) | 10 (62.5) | 0(0) | 14(31.8) | .010 |
| Cholecystectomy | 3 (4.2) | 0(0) | 2 (18.2) | 1 (2.3) | ||
| Hepatectomy | 4 (5.6) | 1 (6.3) | 1 (9.1) | 2 (4.5) | ||
| Extended hepatectomy | 6 (8.5) | 0(0) | 2 (18.2) | 4 (9.1) | ||
| Segments IV and V | 34 (47.9) | 5(31.3) | 6 (54.5) | 23 (52.3) | ||
| Bile duct resection | Yes | 17 (23.9) | 5(31.3) | 5 (45.5) | 7 (15.9) | .08 |
| No | 54 (76.1) | 11 (68.8) | 6 (54.5) | 37 (84.1) | ||
| Extrahepatic resection | Yes | 13 (18.3) | 2 (12.5) | 2 (18.2) | 9 (20.5) | .91 |
| No | 58(81.7) | 14 (87.5) | 9(81.8) | 35 (79.5) | ||
| Lymph node status | Positive | 40 (56.3) | 9 (56.3) | 5 (45.5) | 26 (59.1) | .22 |
| Negative | 20 (28.2) | 2 (12.5) | 6 (54.5) | 12 (27.3) | ||
| Unknown | 11 (15.5) | 5 (31.3) | 0(0) | 6 (13.6) | ||
| Perineural invasion | Positive | 44 (62) | 13 (81.3) | 5 (45.5) | 26 (59.1) | .16 |
| Negative | 24 (33.8) | 3 (18.8) | 6 (54.5) | 15 (34.1) | ||
| Unknown | 3 (4.2) | 0(0) | 0(0) | 3(6.8) | ||
| Vascular invasion | Positive | 47 (66.2) | 14 (87.5) | 5 (45.5) | 28 (63.6) | .08 |
| Negative | 22 (31) | 2 (12.5) | 6 (54.5) | 14(31.8) | ||
| Unknown | 2 (2.8) | 0(0) | 0(0) | 2 (4.5) |
Bold type indicates statistical significance.
Somatic Alterations
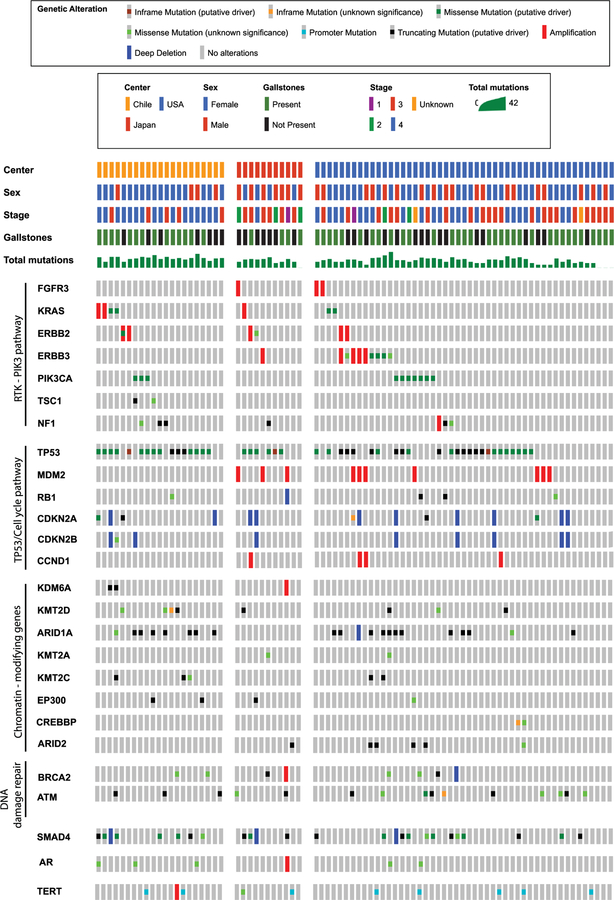
Figure 1 illustrates a heat map of the most common mutations stratified by country of origin. TP53 was the most commonly mutated gene across the cohort (47 of 81 patients; 58%) followed by SMAD family member 4 (SMAD4) (25 of 81 patients; 31%) and AT-rich interaction domain 1A (ARID1A) (20 of 81 patients; 25%). The majority of somatic alterations observed were point mutations. Table 3 breaks down the mutation data overall and across treatment sites. In comparison with earlier reports, approximately 93% of mutations detected herein were found to be associated with GBCA previously.12,32–38 Chilean patients had the highest mutation burden, with a median of 7 (range, 3–20), compared with 6 in the Japanese patients (range, 1–23) and 4 in the American patients (range, 0–27) (P-adj = .006). Mutation burden was not found to be significantly associated with stage of disease (P= .17). It is interesting to note that none of the 11 Japanese patients (0%) had an ARID1A mutation, compared with 38% of the Chilean (8 of 21 patients) and 25% of the American (12 of 49 patients) patients (P- adj = .35). Similarly, phosphatidyIinositoI-4,5-bis-phosphate 3-kinase catalytic subunit alpha (PIK3CA) mutations were identified exclusively in the Chilean (3 of 21 patients; 14%) and American (7 of 49 patients; 14%) patients (P-adj = .68). Furthermore, Erb-B2 receptor tyrosine kinase 3 (ERBB3) (P-adj = .45) and ARID2 (P-adj = .51) mutations were not identified in the Chilean cohort, but both were present in 9% (1 of 11 patients) and 9% (1 of 11 patients), respectively, of the Japanese patients, and 12% (6 of 49 patients) and 10% (5 of 49 patients), respectively, of the American patients. TP53 mutations were more common in Chilean patients (16 of 21 patients; 76%) compared with the Japanese (6 of 11 patients; 55%) and American (25 of 49 patients; 51%) cohorts, but this difference was not statistically significant (P-adj = .45). Across all centers, 80% of patients had at least 1 mutation in an actionable gene target.
Figure 1.
Heat map of common mutations stratified by country of origin.
TABLE 3.
Associations Between Common Mutations, Pathways, and Treatment Center
| Gene | All N = 81 No. (%) |
Chile N = 21 No. (%) |
Japan N = 11 No. (%) |
United States N = 49 No. (%) |
Unadjusted Pa | Adjusted Pa |
|---|---|---|---|---|---|---|
| Mutation burden (range)bb | 5 (0−27) | 7 (3−20) | 6 (1−23) | 4 (0−27) | <.001 | 0.006 |
| TP53 | 47 (58) | 16 (76.2) | 6 (54.5) | 25 (51) | .15 | 0.45 |
| SMAD4 | 25 (30.9) | 8 (38.1) | 4 (36.4) | 13 (26.5) | .58 | 0.68 |
| ARID1A | 20 (24.7) | 8 (38.1) | 0(0) | 12 (24.5) | .045 | 0.35 |
| ATM | 15 (18.5) | 3 (14.3) | 2 (18.2) | 10(20.4) | .92 | 0.92 |
| CDKN2A | 12 (14.8) | 4(19) | 2 (18.2) | 6 (12.2) | .60 | 0.68 |
| PIK3CA | 10 (12.3) | 3 (14.3) | 0(0) | 7(14.3) | .56 | 0.68 |
| TERT | 10 (12.3) | 3 (14.3) | 2 (18.2) | 5(10.2) | .70 | 0.75 |
| BRCA2 | 8 (9.9) | 2 (9.5) | 2 (18.2) | 4 (8.2) | .50 | 0.68 |
| KMT2D | 8 (9.9) | 4(19) | 1 (9.1) | 3 (6.1) | .21 | 0.45 |
| ERBB3 | 7 (8.6) | 0(0) | 1 (9.1) | 6 (12.2) | .24 | 0.45 |
| KRAS | 7 (8.6) | 4(19) | 1 (9.1) | 2 (4.1) | .09 | 0.35 |
| NF1 | 7 (8.6) | 4(19) | 1 (9.1) | 2 (4.1) | .09 | 0.35 |
| AR | 6 (7.4) | 3 (14.3) | 1 (9.1) | 2 (4.1) | .25 | 0.45 |
| ARID2 | 6 (7.4) | 0(0) | 1 (9.1) | 5 (10.2) | .32 | 0.51 |
| CDKN2B | 6 (7.4) | 3 (14.3) | 1 (9.1) | 2 (4.1) | .25 | 0.45 |
| All | Chile | Japan | United States | Unadjusted | Adjusted | |
| Pathway | (n = 81) | (n = 21) | (n = 11) | (n = 49) | p-value | p-value |
| Cell cycle | 23 (28.4) | 9 (42.9) | 3 (27.3) | 11 (22.4) | .18 | .62 |
| Chromatin Remodeling | 30 (37) | 11 (52.4) | 2 (18.2) | 17 (34.7) | .17 | .62 |
| NOTCH | 20 (24.7) | 8 (38.1) | 2 (18.2) | 10 (20.4) | .28 | .62 |
| NRF2 | 6 (7.4) | 1 (4.8) | 1 (9.1) | 4 (8.2) | >.95 | >.95 |
| p53 | 59 (72.8) | 18 (85.7) | 8 (72.7) | 33 (67.3) | .31 | .62 |
| PIK3 | 26 (32.1) | 9 (42.9) | 2 (18.2) | 15 (30.6) | .40 | .62 |
| RTK-RAS | 36 (44.4) | 11 (52.4) | 6 (54.5) | 19 (38.8) | .48 | .62 |
| TGF-β | 31 (38.3) | 10 (47.6) | 4 (36.4) | 17 (34.7) | .64 | .72 |
| WNT | 18 (22.2) | 4(19) | 4 (36.4) | 10 (20.4) | .44 | .62 |
Abbreviations: AR, androgen receptor; ARID1A, AT-rich interaction domain 1A; ARID2, AT-rich interaction domain 2; ATM, ATM serine/threonine kinase; CDKN2A, cyclin-dependent kinase inhibitor 2A; CDKN2B, cyclin-dependent kinase inhibitor 2b; ERBB3, Erb-B2 receptor tyrosine kinase 3; NF1, neurofibromin 1; NRF2, nuclear factor (erythraid-derived 2)-like 2; PIK3, phosphatidylinositide 3-kinases; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; RTK-RAS, receptor tyrosine kinase-RAS, SMAD4, SMAD family member 4; TERT, telomerase reverse transcriptase; TGF-β, transforming growth factor β.
Bold type indicates statistical significance.
Mutation burden includes point mutations, copy number alterations (amplifications and deletions), and fusions.
As shown in Table 3, no significant differences were noted across the 3 patient cohorts when mutations were grouped into gene pathways (P-adj = .62 to > .95). Mutations in the p53 (59 of 81 patients; 73%) and RTK-RAS (36 of 81 patients; 44%) pathways were the most common overall. No significant relationship was found between any somatic mutation or gene pathway with regard to tumor grade (mutation: P-adj = .86 and pathway: P-adj = .31 to .91), lymphatic invasion (mutation: P-adj = .67 to >.95 and pathway: P-adj = .35 to >.95), perineural invasion (mutation: P-adj = .50 to .84 and pathway: P-adj = .33 to .91), vascular invasion (mutation: P-adj = 0.25 to >.95 and pathway: P-adj = .35 to >.95), or stage of disease (mutation: P-adj = .30 to>.95 and pathway: P-adj = .17 to >.95). In addition, although gallstones were observed in a minority of Japanese patients (36% vs 67% for Chile and 69% for the United States [P = .13]), no mutations (P = .34 to .92) or gene pathways (P-adj = .25 to >.95) were found to be linked to gallstone-associated GBCA.
Survival
The median survival of patients undergoing complete resection (46 patients) was 19.6 months (95% confidence interval [95% CI],14.5–28.8 months) and the 5-year survival rate was 18% (95% CI, 8%−31%). Patients with a SMAD4 mutation had worse OS (median, 10.4 months; 95% CI, 5.4–23.8 months) compared with patients with wild-type SMAD4 (median, 24.8 months; 95% CI, 15.236.1 months) (P-adj = .039). This difference remained true for the subset of 46 patients who underwent complete resection (median, 15.6 months [95% CI, 5.4–24.8 months] vs median, 35.8 months [95% Cl, 22.3–78.4 months]; P-adj = .030) as shown in Figure 2. Furthermore, an exploratory multivariable model was built controlling for stage of disease, presence of gallstones, and sex; in this model, SMAD4 remained significantly associated with OS (hazard ratio, 2.01; 95% CI, 1.02–3.94 [P = .043]). In addition, patients with the PIK3CA mutation were found to have a trend toward worse survival with a median of 9.9 months (95% CI, 0.4–16.7 months) compared with those with wild-type PIK3CA (median, 24.5 months; 95% CI, 15.2–32.2 months) (P-adj = .09), as did patients with mutated cyclin-dependent kinase inhibitor 2A (CDKN2A) (median, 8.5 months; 95% CI, 3.8 to not reached) compared with wild-type CDKN2A (median, 23.8 months; 95% CI, 15.6–32.2 months) (P-adj = .09). No significant differences were found for TP53, ARID1A, or ATM serine/threonine kinase (ATM) with respect to OS (P-adj = .74).
Figure 2.
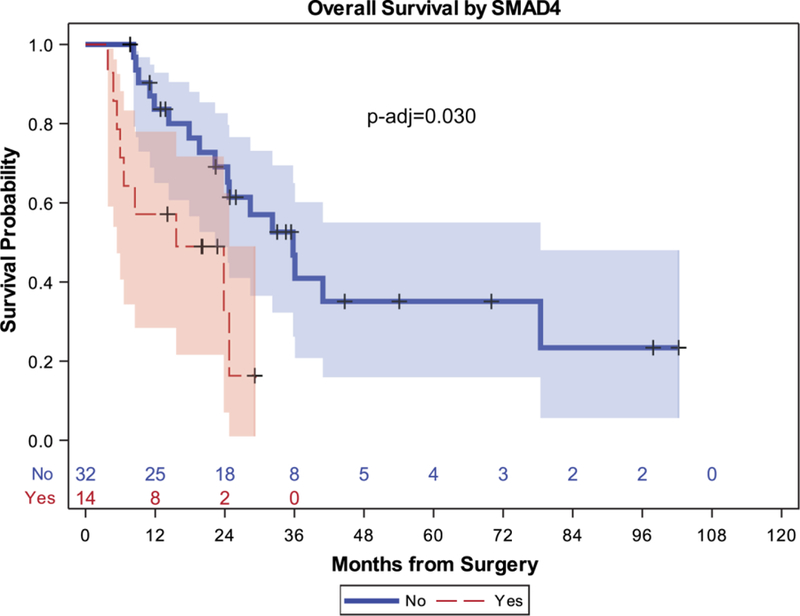
Overall survival after complete resection by SMAD family member 4 (SMAD4) mutation status. p-adj indicates adjusted P value.
Mutual Exclusivity
At the time of formal testing for mutual exclusivity and co-occurrence among the most commonly mutated genes, mutations in ATM and TP53 appeared to be mutually exclusive, with a log-OR of 1.92, but this was not found to be statistically significant (P-adj = .13), as shown in Figure 3. There also appeared to be co-occurrence between SMAD4 and TP53, SMAD4 and KRAS, SMAD4 and cyclin-dependent kinase inhibitor 2b (CDKN2B), SMAD4 and CDKN2A, CDKN2A and CDKN2B, BRCA2 and AR, and BRCA2 and KMT2D, as well as ARID2 and TERT mutations; however, none of these pairs reached adjusted statistical significance. Other than CDKN2A and CDKN2B, which reside adjacently on chromosome 9, none of the other pairs share a chromosomal location.
Figure 3.
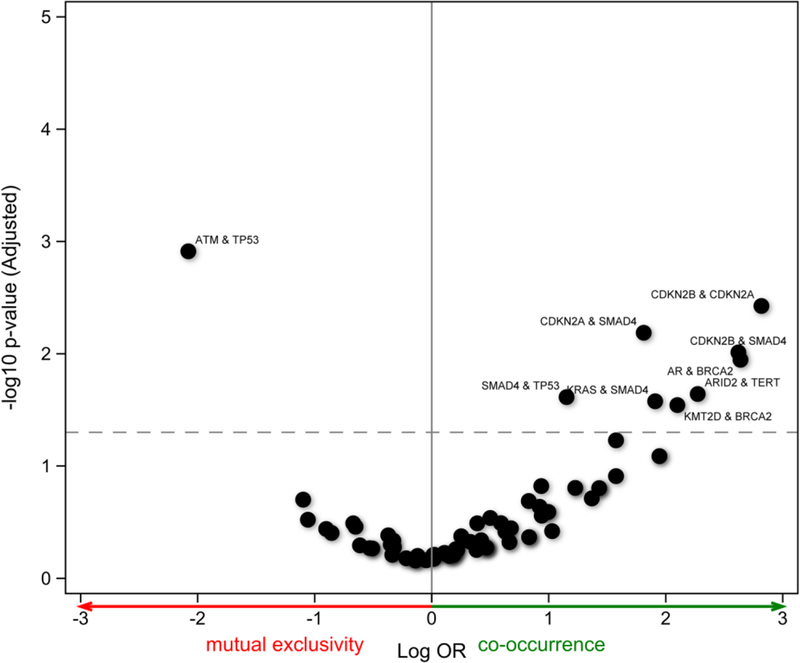
Mutual exclusivity with all pairs and the minimum one-sided test. OR indicates odds ratio.
DISCUSSION
GBCA is the most common biliary tract malignancy worldwide, and is associated with poor OS.1,39 The global incidence of GBCA varies strikingly, and there is support for a geographic influence on pathogenesis. For example, in a study of South Asian patients (a demographic known to have aggressive variants of GBCA with high mortality40), those who moved to the United Kingdom continued to demonstrate the same rate of GBCA-associated mortality but, interestingly, the mortality for subsequent generations diagnosed with GBCA mirrored that of native UK patients diagnosed with GBCA.5 A better understanding of GBCA locality is important not only for understanding the disease but also for the design of future clinical trials that may include international centers.
The findings of the current study not only support the possibility of regional differences in tumor biology but also suggest that different drivers of tumor biology may be identifiable. Although to the best of our knowledge the literature investigating the clinical implications of GBCA mutations is limited, studies in other cancers have shown that mutational patterns have important treatment ramifications, such as KRAS mutant-associated resistance to cetuximab therapy in patients with colorectal cancer.13 It is important to note that approximately 80% of patients in the current study had at least 1 mutation in a potentially actionable gene target.19 Therefore, the early identification of GBCA mutations has the potential to inform better, more tailored therapy.
Previous work from our group has described related differences in RNA expression from GBCA tumors arising in other patients from Chile, Japan, and the United States.21 Therein, we noted that the Japanese patient cohort completely lacked MDM2 expression. Although the MDM2 mutation was uncommon in this study (6%), it shares the same pathway as TP53 (occurs more frequently in Chileans) and ATM (found to be protective of survival in patients with intrahepatic cholangiocarcinoma; unpublished data). The detection of p53 was found to be lowest in Japanese patients in that study as well. Similar clinicopathologic differences also were demonstrated, including male predominance, less gallstone-associated disease, and less advanced tumor stage in Japanese patients compared with the patients from Chile and the United States.10,21
In the current study, the cohort of Japanese patients were older at the time of presentation, appeared to have less aggressive histology, and had a lower incidence of metastatic disease, suggesting a different tumor biology from that in the other 2 cohorts from Chile and the United States. Japanese patients were predominantly male, whereas patients from the other centers were for the most part female. The sex difference is most striking in comparison with Chile, where GBCA has the highest mortality rate and is a common cause of cancer death in women.6,7 In contrast, female sex is associated with a survival advantage in earlier reports of Japanese patients.8 Similar clinicopathologic differences were demonstrated in our previous work.10,21 It is interesting to note that pathologic findings such as lymph node status as well as perineural and vascular invasion did not appear to differ significantly among the cohorts. In the current study, the rate of gallstone-associated disease was lower in patients from Japan, but this difference was not found to be statistically significant, possibly due to the small sample size in the current study. In contrast, previous studies of clinical differences between patients from these countries demonstrated significantly more gallstone-associated GBCA in Chilean patients and less frequent incidental diagnoses of GBCA in Japanese patients.10
Differences in somatic alterations align with the aggressive nature of GBCA in the Chilean population, in whom the mutation burden was greatest among the cohorts. Although no specific somatic or gene pathway alteration was found to be significantly more prevalent among the cohorts, several mutations were notably absent in specific populations. No instances of ARID1A or PIK3CA mutations were observed in the Japanese patient cohort, which contrasts with one report identifying inactivating ARID1A mutations in Japanese patients.38 In contrast, other reports using sequencing assays failed to find ARID1A mutations among Asian patients.37,40,41 In addition, TP53 mutations were more prevalent in the Chilean cohort (76%), although this difference did not reach statistical significance. Prior reports have noted that TP53 mutations occur in 47% to 64% of patients.37,41 Moreover, Chilean patients lacked ARID2 and ERBB3 mutations.
In contrast, the SMAD4 mutation was found to be present in approximately one-third of patients in the current study and was associated with worse survival. This may suggest that although some gene mutations (eg, PIK3CA, ARID1A, ARID2, and ERBB3) are region-specific drivers, SMAD4 may represent a converging pathway to poor prognosis. Although SMAD4 is involved in TGF-β signaling, somatic alterations in this overall gene pathway were not found to be associated with a survival difference in the current study. In light of the poor survival of Chilean women with GBCA, there is interesting evidence to suggest that estrogen contributes to tumorigenesis through the TGF-β signaling pathway.7 In a mouse model, an inactivating mutation in a nuclear receptor subunit involved in TGF-β signaling, liver X receptor beta (LXR-beta), was associated with the formation of preneoplastic lesions in female gallbladders.42 It is interesting to note that this effect disappeared after oophorectomy, thereby suggesting an estrogen-dependent mutation driver.
The current study has several limitations. First, the small sample size, although large in comparison with other published studies of this type, limits the power to make definitive conclusions. The low incidence of GBCA restricts the number of patients who can be enrolled in these studies.1,4 Second, the retrospective nature of the current study introduces inherent limitations, such as selection biases and heterogeneous samples. We reduced some of this heterogeneity by limiting the current study to primary tumor tissue samples and common histologies. However, the inclusion of metastatic tumor specimens in future studies may reveal nuances in the genomic profile not captured from analysis of the primary tumor. Furthermore, although approximately 70% of gallbladder cancers are diagnosed incidentally,43 only 21% of patients in the current study had incidental tumors. This low percentage of incidental diagnoses is expected because these cases often do not have enough tumor specimen available for genomic testing. Third, although several differences were identified between the sample cohorts, it is uncertain whether these reflect the respective national populations of the participating centers or selection bias resulting from nonconsecutive patient enrollment. As noted, earlier reports have identified several mutations associated with GBCA, including TP53, SMAD4, ARID1A, PIK3CA, TERT, KRAS, ERBB3, ARID2, CDKN2A, and CDKN2B,12,33,36,37 Approximately 93% of patients in the current study had at least 1 mutation in a gene previously reported, suggesting that the genomic spectrum reported herein is reflective of prior work. Fourth, comparison of gene pathways may be best accomplished with expression assay data rather than genomic sequencing because we cannot determine whether compensatory pathways dampen the impact of observed mutations. Despite these limitations, the data presented in the current study suggest the possibility of regional differences in disease biology.
Clinicopathologic dissimilarities among patients with GBCA from different regions underscore the possibility of a unique pathogenesis potentially related to differences in mutational pattern. The absence of certain mutations (ARID1A and PIK3CA in Japanese patients and ERBB3 and ARID2 in Chilean patients) and the varying burden of tumor mutations may explain differences related to GBCA pathogenesis between regions around the world. With the majority of tumors harboring mutations in potentially actionable gene targets, investigation into differences in response to therapy within the context of tumor mutations may yield strategies for better outcomes with tailored therapy. Somatic profiling can highlight targeted therapeutic approaches. In addition, the SMAD4 mutation may be a similarly prevalent and convergent predictor of poor OS in patients from all 3 regions.3,21 Several studies to date have described concurring demographic and clinical features associated with GBCA across different regions in the world. Definitive conclusions must await confirmation in larger series.
Acknowledgments
FUNDING SUPPORT
Supported in part by the National Institutes of Health/National Cancer Institute CI P30 CA008748 Cancer Center Support Grant.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Ghassan Abou-Alfa has received grants from Acta Biologica, Agios, Array, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Casi Pharmaceuticals, Celgene, Exelixis, Genentech, Halozyme, Incyte, Lilly, MabVax Therapeutics, Novartis, OncoQuest Inc, Polaris Puma, QED, and Roche, and has acted as a paid consultant for 3DMedcare, Agios, Alignmed, Amgen, Antengene, Aptus, Aslan, Astellas, AstraZeneca, Bayer, BeiGene, Bioline, Bristol-Myers Squibb, Boston Scientific, BridgeBio, CARsgen, Celgene, Casi Pharmaceuticals, Cipla, CytomX, Daiichi, Debio, Delcath, Eisai, Exelixis, Genoscience Pharma, Gilead, Halozyme, Hengrui Therapeutics, Inovio, Ipsen, Jazz, Jansen, Kyowa Kirin, LAM, Lilly, Loxo, Merck, Mina, NewLink Genetics, Novella, Onxeo, PCI Biotech, Pfizer, PharmaCyte Biotech Inc, Pharmacyclics, Pier is, QED, Redhill, Sanofi, Servier, Silenseed, Sillajen, Sobi, Targovax, Tekmira, twoXAR, Vicus, Yakult, and Yiviva for work performed outside of the current study.
REFERENCES
- 1.Rahman R, Simoes EJ, Schmaltz C, Jackson CS, Ibdah JA. Trend analysis and survival of primary gallbladder cancer in the United States: a 1973–2009 population-based study. Cancer Med 2017;6:874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazcano-Ponce EC, Miquel JF, Munoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001;51:349–364. [DOI] [PubMed] [Google Scholar]
- 3.Are C, Ahmad H, Ravipati A, et al. Global epidemiological trends and variations in the burden of gallbladder cancer. J Surg Oncol 2017;115:580–590. [DOI] [PubMed] [Google Scholar]
- 4.Sharma A, Sharma KL, Gupta A, Yadav A, Kumar A. Gallbladder cancer epidemiology, pathogenesis and molecular genetics: recent update. World J Gastroenterol 2017;23:3978–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangtani P, Maringe C, Rachet B, Coleman MP, dos Santos Silva I. Cancer mortality in ethnic South Asian migrants in England and Wales (1993–2003): patterns in the overall population and in first and subsequent generations. Br J Cancer 2010;102: 1438–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randi G, Malvezzi M, Levi F, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol 2009;20:146–159. [DOI] [PubMed] [Google Scholar]
- 7.Andia KM, Gederlini GA, Ferreccio RC. Gallbladder cancer: trend and risk distribution in Chile. Rev Med Chil 2006;134:565–574. [DOI] [PubMed] [Google Scholar]
- 8.Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer 2007; 110: 572–580. [DOI] [PubMed] [Google Scholar]
- 9.Wolpin BM, Mayer RJ. A step forward in the treatment of advanced biliary tract cancer. N Engl J Med 2010;362:1335–1337. [DOI] [PubMed] [Google Scholar]
- 10.Butte JM, Matsuo K, Gonen M, et al. Gallbladder cancer: differences in presentation, surgical treatment, and survival in patients treated at centers in three countries. J Am Coll Surg 2011;212:50–61. [DOI] [PubMed] [Google Scholar]
- 11.Kayahara M, Nagakawa T, Nakagawara H, Kitagawa H, Ohta T. Prognostic factors for gallbladder cancer in Japan. Ann Surg 2008;248:807–814. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003–1010. [DOI] [PubMed] [Google Scholar]
- 13.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757–1765. [DOI] [PubMed] [Google Scholar]
- 14.Dancea HC, Shareef MM, Ahmed MM. Role of radiation-induced TGF-beta signaling in cancer therapy. Mol Cell Pharmacol 2009;1:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sartore-Bianchi A, Martini M, Molinari F, et al. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res 2009;69:1851–1857. [DOI] [PubMed] [Google Scholar]
- 16.Gao K, Li G, Qu Y, et al. TERT promoter mutations and long telomere length predict poor survival and radiotherapy resistance in gliomas. Oncotarget 2016;7:8712–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeLeon TT, Ahn DH, Bogenberger JM, et al. Novel targeted therapy strategies for biliary tract cancers and hepatocellular carcinoma. Future Oncol 2018;14:553–566. [DOI] [PubMed] [Google Scholar]
- 18.Perkhofer L, Schmitt A, Romero Carrasco MC, et al. ATM deficiency generating genomic instability sensitizes pancreatic ductal adenocarcinoma cells to therapy-induced DNA damage. Cancer Res 2017;77:5576–5590. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol 2017;2017. [DOI] [PMC free article] [PubMed]
- 20.Lin YF, Tseng IJ, Kuo CJ, Lin HY, Chiu IJ, Chiu HW. High-level expression of ARID 1A predicts a favourable outcome in triple-negative breast cancer patients receiving paclitaxel-based chemotherapy. J Cell Mol Med 2018;22:2458–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butte JM, Torres J, Veras EF, et al. Regional differences in gallbladder cancer pathogenesis: insights from a comparison of cell cycle-regulatory, PI3K, and pro-angiogenic protein expression. Ann Surg Oncol 2013;20:1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical sequencing defines the genomic landscape of metastatic colorectal cancer. Cancer Cell 2018;33:125–136.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu AX, Pawlik TM, Kooby DA, Schefter TE, Vauthey JN. AJCC Cancer Staging Manual 8th ed. Chicago: Springer; 2017. [Google Scholar]
- 24.Butte JM, Gonen M, Allen PJ, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB (Oxford) 2011;13:463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Angelica M, Dalai KM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Analysis of the extent of resection for adenocarcinoma of the gallbladder. Ann Surg Oncol 2009;16:806–816. [DOI] [PubMed] [Google Scholar]
- 26.Shimada H, Endo I, Fujii Y, et al. Appraisal of surgical resection of gallbladder cancer with special reference to lymph node dissection. Langenbecks Arch Surg 2000;385:509–514. [DOI] [PubMed] [Google Scholar]
- 27.Butte JM, Redondo F, Waugh E, et al. The role of PET-CT in patients with incidental gallbladder cancer. HPB (Oxford) 2009;11:585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 2015;17:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stadler ZK, Battaglin F, Middha S, et al. Reliable detection of mismatch repair deficiency in colorectal cancers using mutational load in next-generation sequencing panels. J Clin Oncol 2016;34:2141–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:p11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borger DR, Tanabe KK, Fan KC, et al. Frequent mutation of isocitrate dehydrogenase (IDH)l and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist 2012;17:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao Y, Pawlik TM, Anders RA, et al. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID 1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 2013;45:1470–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simbolo M, Fassan M, Ruzzenente A, et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 2014;5:2839–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumari N, Corless CL, Warrick A, et al. Mutation profiling in gallbladder cancer in Indian population. Indian J Pathol Microbiol 2014;57:9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Javle M, Rashid A, Churi C, et al. Molecular characterization of gallbladder cancer using somatic mutation profiling. Hum Pathol 2014;45:701–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, Zhang Z, Li X, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet 2014;46:872–876. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki M, Nitta T, Sato Y, Nakanuma Y. Loss of ARID1A expression presents a novel pathway of carcinogenesis in biliary carcinomas. Am J Clin Pathol 2016;145:815–825. [DOI] [PubMed] [Google Scholar]
- 39.Coburn NG, Cleary SP, Tan JC, Law CH. Surgery for gallbladder cancer: a population-based analysis. J Am Coll Surg 2008;207:371–382. [DOI] [PubMed] [Google Scholar]
- 40.Yadav S DE Sarkar N, Kumari N, Krishnani N, Kumar A, Mittal B. Targeted gene sequencing of gallbladder carcinoma identifies high-impact somatic and rare germline mutations. Cancer Genomics Proteomics 2017;14:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noguchi R, Yamaguchi K, Ikenoue T, et al. Genetic alterations in Japanese extrahepatic biliary tract cancer. Oncol Lett 2017;14:877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabbi C, Kim HJ, Barros R, Korach-Andre M, Warner M, Gustafsson JA. Estrogen-dependent gallbladder carcinogenesis in LXRbeta−/− female mice. Proc Natl Acad Sci USA 2010;107:14763–14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pawlik TM, Gleisner AL, Vigano L, et al. Incidence of finding residual disease for incidental gallbladder carcinoma: implications for re-resection. J Gastrointest Surg 2007;11:1478–1486; discussion 1486–1487. [DOI] [PubMed] [Google Scholar]