Abstract
Recent research indicates that weight suppression (WS: defined as the difference between highest lifetime and current weight) prospectively predicts illness trajectory across eating disorders characterized by binge eating, including AN binge-purge subtype (ANbp), bulimia nervosa (BN), and binge eating disorder (BED), collectively referred to as bulimic eating disorders. Through a series of studies, we have developed a model to explain the link between WS and illness trajectory in bulimic eating disorders. Our model posits that WS contributes to reduced circulating leptin, which leads to reduced postprandial glucagon-like peptide 1 (GLP-1) response. Diminished leptin and GLP-1 function contribute to alterations in two reward-related constructs in the Research Domain Criteria (RDoC): reward value/effort and reward satiation. Respectively, these changes increase drive/motivation to consume food and decrease ability for food consumption to lead to a state of satiation/satisfaction. Combined, these alterations increase risk for experiencing large, out-of-control binge-eating episodes. The following review presents evidence that contributed to the development of this model as well as preliminary findings from an on-going project funded to test this model.
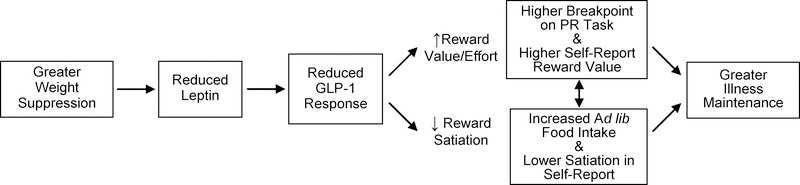
Weight suppression (WS) represents the difference between highest lifetime weight and current weight1. Recent research indicates that WS has relevance for understanding illness trajectory across bulimic eating disorders, including AN binge-purge subtype (ANbp), BN, and BED2. Through a series of studies and literature review, we have developed a model to explain the link between WS and illness trajectory in bulimic eating disorders. Our model (see Figure 1) posits that WS contributes to reduced circulating leptin, which leads to reduced postprandial glucagon-like peptide 1 (GLP-1) response. Diminished leptin and GLP-1 function contribute to alterations in two reward-related constructs in the Research Domain Criteria (RDoC): reward valuation/effort and reward satiation3. Respectively, these changes increase drive/motivation to consume food (valuation/effort) and decrease ability for food consumption to lead to a state of satiation/satisfaction. Combined, these alterations increase risk for experiencing large, out-of-control binge-eating episodes, explaining the link between WS and illness maintenance over time across bulimic eating disorders.
Figure 1.
Explanatory Model for Association between WS and Bulimic Eating Disorder Maintenance
Both AN and BN are characterized by weight and shape concerns and extreme efforts to achieve weight loss but differ in the extent to which these efforts result in objectively low weight. This difference is codified in the DSM-5 which defines AN by “significantly low body weight,” which is further defined as “a weight that is less than minimally normal or, for children and adolescents, less than that minimally expected” (p. 338)4. Because there is considerable symptom overlap between ANbp and BN, the DSM-5 goes on to require that BN cannot occur “exclusively during episodes of anorexia nervosa” (p. 345). Thus, a diagnosis of ANbp trumps a diagnosis of BN in the DSM-5 – which largely ensures that patients with BN are not underweight. In contrast, BED is not defined by body image disturbance in the DSM-5. Further, the DSM-5 precludes use of inappropriate compensatory behaviors, such as purging, fasting, or excessive exercise. Potentially reflecting these definitional differences, patients’ BMI range from normal weight to overweight or obese. However, when patients with BED are compared to BMI-matched non-eating disorder controls, they endorse greater body image concerns and greater efforts to lose weight4.
Eating disorder diagnosis predicts outcome, with ANbp demonstrating greater treatment utilization, lower treatment response, greater chronicity and mortality compared to BN, and BN demonstrating worse outcomes in these domains compared to BED5. While predictive validity contributed to the identification of these as three diagnostic categories in the DSM-54, we posit that the underlying dimension of WS contributes to these patterns, with ANbp demonstrating the greatest WS and worst outcomes, BN demonstrating intermediate and variable WS and intermediate and variable outcomes, and BED demonstrating the lowest WS and more favorable outcomes. Further, we posit that WS contributes to diagnostic migration commonly observed in large clinical samples of AN from the restricting subtype to the binge-purge subtype and weight gain that leads to migration to a diagnosis of BN6 or to remission in community-based samples7,8.
Conceptualizing diagnostic categories as residing along underlying biobehavioral dimensions is consistent with the NIMH’s Research Domain Criteria (RDoC) initiative. This approach seeks to identify core constructs that can be measured across multiple units of analysis (e.g., physiology, self-report, and behavior) that contribute to the emergence of mental illness. Mental illness is viewed on a continuum with normal behavior, such that the same processes that give rise to adaptive weight control and ingestive behavior contribute to maladaptive weight control and ingestive behavior. The RDoC matrix is divided into separate domains3. Among them, the Positive Valence Domain identifies core constructs thought to explain excessive or deficient engagement in rewarding behaviors that may contribute to patterns of comorbidity often seen in mental disorders. As such, the RDoC framework seeks to identify transdiagnostic biobehavioral mechanisms that account for psychopathology. Our model posits that WS contributes to alterations in two RDoC core constructs, reward valuation/effort and reward satiation, to explain the link between WS and eating disorder trajectory. In our model, trajectory refers to illness course, which can range from changes in symptom severity that result in worsening, maintenance, or remission, as well as migration from one symptom presentation to another. Although weight changes are only relevant to defining remission for ANbp, weight gain (or lack thereof) may impact illness trajectory for bulimic eating disorders more broadly.
Weight Suppression and Eating Disorder Trajectory
In addition to differing in BMI, eating disorders differ in WS, with greater mean WS in ANbp (>14 kg)9,10 than BN (> 7 kg)11–19, in BN than BED (>6 kg)20,21, and in all eating disorders than controls (<3 kg)22. In AN, WS is associated with illness severity and predicts bulimic symptom severity9. Although women with BN often have normal weight (e.g., 21 kg/m2); mean WS is 7.8 kg across studies11–19, indicating substantial weight loss is the norm. Three longitudinal studies, each in independent samples, support prospective associations between greater WS and BN maintenance, including BN patients from a randomized controlled trial11, naturalistic follow-up of a treatment-seeking BN sample13, and naturalistic follow-up of bulimic eating disorder cases identified in the community14. In addition to supporting that greater WS is a prospective risk factor for illness maintenance, multivariable models found significant effects for WS, controlling for age, BMI, body image disturbance, and dietary restraint14,19, suggesting that the effects of WS are not better accounted for by these other variables. Moreover, WS demonstrates very weak correlations with BMI in BN, with mean r=.0112,14,18,19,23, suggesting that it is weight relative to a prior maximum, rather than current weight, that matters. Other studies15–17,24 have not found an association between WS and eating disorder maintenance, potentially due to smaller samples15,16,24, restricted range of WS15–17, exclusion of participants who did not complete treatment 15,16,24, or inclusion of obese participants16,17. Individuals who are significantly overweight or obese are known to have other metabolic disturbances which may alter the association between WS and illness trajectory25,26. Our model may not extend across the BMI range.
Across studies examining the association between WS and illness maintenance, the mean weighted effect size is quite small (β=.07)11,13–17,24, suggesting the importance of larger samples to detect reliable associations. This small effect size may reflect limitations in how WS has been measured because the highest weight for height may have occurred before an individual reached their current height. Calculating WS as the difference between highest and current BMI percentile for age and sex permits evaluation of WS across development. This more developmentally appropriate measure of WS has demonstrated stronger associations with current eating disorder severity than the traditional approach to calculating WS27. In addition, even a very small effect (e.g., r=.05) for predicting a single event can have meaningful consequences over time28.
Finally, there is robust evidence that greater WS is a significant predictor of greater weight gain over follow-up9,10,12,15,23,24,29. For patients with AN, this means that WS may actually facilitate recovery. Weight gain reduces both WS and could reverse alterations in underlying mechanisms contributing to disorder maintenance, further accounting for null findings in some studies10,24,30. Moreover, the relative rarity of cross-over from AN to BN in community-based samples may reflect the high remission rate in these samples as recovery would signify both weight gain and reduction of WS7,8. Alternatively, recent data from a large longitudinal epidemiological study suggest that AN cases identified in the community have lower premorbid BMI percentile compared to those who do not develop eating disorders, and those who go on to develop BN or BED have higher premorbid BMI percentiles31. Thus, AN cases from community samples may not possess the same degree of WS observed in patients with AN drawn from clinical samples. Further, clinical samples of AN may represent cases in whom low weight and WS persist, increasing their likelihood of both seeking treatment32 and developing binge eating over time6 compared to community samples of AN7,8.
Given that WS reflects a history of greater weight relative to current weight and increases risk for weight gain, WS may heighten underlying cognitions that drive weight loss behaviors, and this could explain the link between WS and illness trajectory across eating disorders. Indeed, in a 20-year follow-up study, we found that greater WS prospectively predicted increases in drive for thinness, which prospectively predicted increased bulimic symptoms and that drive for thinness mediated the association between WS and bulimic symptom maintenance over 20-year follow-up33. However, the presence of a psychological explanation for the link between WS and illness trajectory does not rule out the presence of a biological explanation. Weight loss has biological consequences that promote weight gain, including defensive increases in metabolic efficiency as well as alterations in ingestive behaviors34. These same behaviors would also influence risk for binge-eating episodes.
Weight Loss and WS Reduce Leptin and GLP-1 Release
Leptin is produced by the ob (Lep) gene of white adipose tissue, such that higher fat mass results in higher circulating leptin levels (r-values>.90 in humans)35. Importantly, weight loss and WS impact leptin independently of BMI35. In non-eating disordered women, a 5–10% weight loss caused significant (40–60%) declines in leptin35,36 (r=.61, reflecting a large effect size for change in weight and leptin)36. Thus, individual differences in WS should predict differences in leptin levels among individuals of apparently similar weight. Supporting our model, we recently published on the significant association between greater WS and lower leptin, controlling for BMI and percentage of body fat22. Specifically, compared to controls, women with BN demonstrated no significant differences in current BMI. However, they had significantly greater WS, and greater WS was associated significantly with lower leptin concentrations. In a separate sample, we demonstrated that WS predicted leptin while controlling for BMI and that leptin statistically mediated the association between WS and reported duration of illness in bulimic disorders37.
Importantly, although acute changes in leptin are observed with fasting34,38, neither WS nor leptin change dramatically throughout the day. So, changes in leptin do not directly influence initiation or cessation of food intake in a given meal or binge episode. To understand dynamic influences on food consumption during binge episodes, it is important to consider the role of meal-related signals, such as GLP-1. GLP-1 is released in response to food intake by L cells in the intestine and acts as a peripheral signal of acute changes in nutritional status. Postprandial increase in GLP-1 is a satiation signal that contributes the end of a meal in normal feeding. Peripheral GLP-1 release is potently stimulated by leptin via leptin receptors on L cells of the intestine39,40. Thus, individuals with higher leptin levels also demonstrate more robust GLP-1 responses to food intake41. These associations appear to reflect the influence of leptin on GLP-1 levels rather than the reverse as neither meal-induced increases in GLP-1 nor exogenous GLP-1 administration influence leptin levels in healthy volunteers42,43. Alterations in GLP-1 release observed peripherally may reflect central processes because leptin crosses the blood-brain barrier where it could impact central GLP-1 function.
Given that ANbp is defined by maintenance of low weight, one would expect hypoleptinemia in ANbp and reduced postprandial GLP-1 response. Given that mean WS in BN is 7.8 kg and ranges from 2.3 kg17 to 12.0 kg12 across studies, one would expect both reduced and variable leptin levels in BN with concomitant alterations in GLP-1 function. Given that BED is associated with obesity, one would expect hyperleptinemia, though, depending on level of WS, leptin may still be depressed relative to leptin receptor sensitivity 44 which may still result in blunted GLP-1 responses in BED. Literature reviewed below supports these expectations.
Leptin Varies Across Eating Disorders and GLP-1 Response is Reduced
Low leptin levels have been found consistently in AN45–49, with lower leptin in the binge-purge compared to the restricting subtype and a significant association between lower leptin and higher binge frequency50. According to our model, WS and resulting hypoleptinemia may explain diagnostic migration from restricting AN to bulimic eating disorders observed in a majority of AN patients6,51. Of note, in community-based samples, diagnostic cross-over is less likely; however, this may reflect the much higher likelihood of weight gain and remission in community-based samples of AN7,8. A small minority of AN patients maintain WS and low leptin levels and never binge, suggesting the presence of other key processes in the maintenance of restricting AN, including the possibility that reduced leptin levels may influence reinforcing value of excessive exercise. Moreover, we do not propose that a single set of mechanisms can explain all eating disorders, and this model clearly does not explain chronic presentations of AN restricting subtype.
Given that mean WS in BN is 7.8 kg and ranges from 2.3 kg17 to 12.0 kg12 across studies, one would expect both reduced leptin levels and considerable variability in levels in BN. Supporting this expectation, several investigators have reported significantly reduced leptin levels in BN compared to control participants despite no differences in BMI38,44,45,52,53. In addition, Monteleone44 noted large leptin variability among BN patients in their38,45 and others’ studies52 and found significant inverse associations between leptin levels in BN and both current symptom severity and reported illness duration. Women recovered from BN demonstrated similar leptin levels to controls but had higher percent expected body weight54.
Although obese women with BED have higher leptin than healthy-weight controls45,55, comparisons to obese controls have revealed lower leptin56, higher leptin57, and no significant differences58. All studies involved small samples, and none examined WS or associations between leptin and illness duration within BED. Collectively, studies provide compelling support for associations between leptin and maintenance of BN but limited data for a full BMI range of bulimic eating disorders. Moreover, studies did not assess WS and examined associations cross-sectionally, making it impossible to evaluate whether WS contributes to observed alterations in leptin or whether reduced leptin levels predict illness maintenance. Indeed, an alternative interpretation of cross-sectional associations is that longer illness duration leads to lower leptin levels. Recently, we examined this possibility in statistical mediation models. Analyses supported a model in which leptin mediated associations between WS and illness duration but did not support an alternative model in which illness duration mediated associations between WS and leptin37. While these findings provide preliminary support for our model, longitudinal data are needed to establish prospective associations between WS, reduced leptin, and illness maintenance over time.
Consistent with lower leptin observed in ANbp and BN and the influence of leptin on GLP-1 release, studies found significantly lower pre- and post-prandial GLP-1 levels in AN59 and BN60,61 compared to controls. Peak GLP-1 was lower in obese BED (10 pM/ml) compared to obese controls (15 pM/ml)62, but this did not reach statistical significance due to very small samples (n=10 BED; n=9 controls). Transdiagnostically, leptin may explain shorter illness duration in BED compared to BN and in BN compared to AN4 with differences in leptin function secondary to differences in BMI and WS across diagnostic groups. Further, alterations in GLP-1 response emerging from hypoleptinemia or leptin resistance44 may account for differences between those with eating disorders and controls.
Our transdiagnostic model is informed by extensive animal-based research regarding the biological and behavioral consequences of altered leptin and GLP-1 function. Findings from basic research informed both our hypotheses and methods for testing these hypotheses. Following the RDoC’s focus on translating findings from basic science to examinations of clinical populations, we review the relevant animal literature below.
Peripheral Leptin and GLP-1 Impact Reward Value/Effort and Satiation through Distinct Neural Circuits
Circulating leptin crosses the blood-brain barrier and binds to leptin receptors throughout several brain regions, including the ventral tegmental area (VTA)63 and the hypothalamus (discussed below)64. Dopamine (DA) projections from the VTA to the nucleus accumbens (NAc) are directly implicated in many aspects of reward value65,66, and contribute to greater effort in a progressive ratio (PR) task for reinforcers (e.g., food, sex, intracranial self-stimulation [ICSS])67–70. At the cellular level, peripheral leptin administration inhibits firing of DA neurons in the VTA63. To dissociate leptin’s effects in the VTA from its effects in the hypothalamus, neuroscientists have used site-specific alterations in leptin function. Leptin infusions in the VTA, but not in the arcuate nucleus of the hypothalamus (Arc), reduced the amount of work rats were willing to do for a given threshold of ICSS71. Further, using microinjections of a viral vector to knock down leptin receptors in the midbrain (where the VTA is located), but not the lateral hypothalamus, increased break point (BP) on a PR task for food; disabling leptin function in the midbrain increased reward value for food assessed through rats’ effort to obtain food72.
Leptin also binds to receptors throughout the hypothalamus, including the Arc, paraventricular nucleus (PVN), and ventromedial and lateral hypothalamus (VMH and LH), where it modulates neural responses to gut-derived satiation signals. In the Arc, leptin inhibits neurons containing neuropeptide Y and agouti-related protein (NPY/AgRP) and activates neurons containing pro-opiomelanocortin and cocaine and amphetamine-related transcript (POMC/CART)64. POMC/CART activation decreases food intake in animals and humans73. In contrast, NPY and AgRP stimulate increased food intake64. Thus, when an organism loses adipose tissue (a state ensured by WS), leptin levels decrease, activation of POMC/CART containing neurons decreases, and NPY/AgRP neurons remain active and drive a defensive increase in ad lib feeding to return the organism to a state of energy balance64. These effects have been replicated in numerous rodent experiments applying food restriction to various degrees and for periods ranging from acute (24–72 hours) to chronic (≥4 weeks)64,74–76. Leptin infusions in the Arc71 decrease food intake in rats, and selective deletion of leptin receptors in POMC and AgRP neurons increased meal size in mice77. Thus, lower leptin levels contribute to diminished responsiveness to satiating signals during food intake.
Peripheral GLP-1 binds to GLP-1 receptors on the vagus nerve causing stimulation of vagal afferents, which activate several neurons projecting from the nucleus solitary tract (NTS), including preproglucagon cells that release GLP-1 centrally78. Similar to the effect of leptin on GLP-1 release in the periphery, central leptin administration enhances GLP-1 release from preproglucagon neurons of the NTS 79,80. GLP-1 neurons of the NTS project to multiple brain regions, including the VTA and NAc where GLP-1 influences reward value/effort39,80–83. In the VTA, 50% of DA neurons express GLP-1 receptors81 and 30% of GLP-1 neurons in the NTS project to the VTA84 and 40% project to the NAc82,84, making GLP-1 a prime candidate for examining how acute changes in food intake influence reward pathways in the brain. Infusion of GLP-1 to the NAc core reduced food intake, while its receptor antagonist increased food intake82. Injection of a potent GLP-1 agonist, Exendin 4, in the VTA or NAc decreased palatable food intake, and a GLP-1 receptor antagonist decreased intake84. Infusion of Exendin 4 in the VTA and NAc reduced the BP on a PR task for food85, and Exendin 4 diminished conditioned place preference for cocaine86, a function which implicates GLP-1 signaling in reducing activation of the mesolimbic DA pathway for a non-food, non-caloric reinforcer. In healthy volunteers, a meal pattern that increased GLP-1 levels reduced willingness to work for food rewards, with a very large effect size (d=2.29)87. Thus, similar to leptin, GLP-1 appears to reduce reward value.
GLP-1 projections from the NTS to the Arc and PVN contribute to satiation39,83. ICV administration of Exendin 4 increased activation of POMC/NPY neurons in the Arc, similar to leptin’s effects88, and central GLP-1 administration reduces food intake in rats89,90. In humans, peripheral GLP-1 infusion increased satiation and decreased food intake91, and a meal pattern that increased GLP-1 response was associated with a 10% reduction in food intake during a subsequent ad lib test meal, reflecting a large effect size (d=.80)87.
In summary, leptin and GLP-1 reduce reward value through inhibitory effects in the mesolimbic DA pathway and increase satiation through a combination of inhibitory and excitatory actions in the hypothalamus. Thus, lower leptin and GLP-1 response observed in eating disorders should contribute to both increased reward value/effort and decreased satiation. These behavioral consequences translate into increased risk for weight gain and increased risk for experiencing large, out-of-control binge-eating episodes. Research supports that disorders characterized by binge-eating episodes are characterized by increased reward value/effort and decreased satiation.
Increased Reward Value/Effort and Decreased Satiation in Eating Disorders
By definition, individuals with AN binge-eating/purging type, BN, or BED experience a loss of control over eating and consume an excessive amount of food when they binge. Because food is a primary reinforcer, investigators have posited that this reflects increased food reward value92,93. As a measure of reward value/effort, the PR task has many advantages, including high test-retest reliability, associations between BP on a PR task and food consumption in an ad lib test meal, and ability to use PR tasks with minimal modification in animals and humans, such that careful experimental manipulations of physiological factors in animal models may be translated into clinical phenomena observed in humans94. Despite these advantages, surprisingly few studies have employed PR tasks to measure reward value in eating disorders. These studies support increased reward value for food22,95–98 and cigarettes95 in BN compared to controls, specifically in a fed vs. fasted condition95,96. In a fed state, effect sizes have ranged from moderate (d=.50)95 to large (d=1.52)96 for responses elicited in a PR task for food relative to a non-food reinforcer. Notably, most studies95–97 have employed an adapted PR task to measure relative reinforcing food value by presenting participants with a choice between working for two rewards simultaneously (e.g., food vs. money95), which is not sensitive to how individual differences in central leptin or GLP-1 function might alter reward value across both food and non-food rewards. In eating disorders, as in most mental disorders, comorbidity is the rule rather than the exception 99, and our model would predict that alterations in reward value and satiation may explain why women with eating disorders are at increased risk for substance use disorders.
Schebendach and colleagues98 compared women with BN (n=10) to healthy control participants (n=10) using a PR task to evaluate absolute reinforcing value of food, which is most relevant to our model. Indeed, we adapted their PR task for our current research and review their methods here to provide the context for our approach. Participants in their study began a computerized PR task at 2 pm in which they pressed a computer key to earn strawberry yogurt shake portions. Across 12 trials, the amount of work required to receive a 175 ml portion increased in increments of 200 (trial 1=50, trial 2=250, … trial 12=2250 presses). As participants completed trials, the computer screen displayed an image of a pitcher filling up with shake, and an actual pitcher filled with yogurt shake next to the computer. Participants could earn up to 2.1 liters of shake and were free to discontinue as soon as they had earned as much shake as they felt they could consume per instructions. After completing the PR task, participants waited until 3 pm, at which time they were given 30 min to consume the amount of shake they had earned. Under instructions to work for the amount “you can binge on”/”you can overeat,” women with BN had a significantly higher BP than controls with a large effect size (d=1.24). Importantly, controls did not actually consume the total amount of shake earned under, resulting in a weak association between BP and shake consumption in controls (r=0.05). This suggests that controls’ motivation for shake may have declined more precipitously while consuming the shake than was reflected in their BP. In contrast, BN participants consumed almost all shake earned (r=0.98 for the association between what was earned and what was consumed). Given that binge instructions would map on to processes in which patients feel a loss of control over their eating, the BP difference between controls and BN participants likely reflects greater reward value for food in BN. However, results do not capture how reward value dynamically changes during food consumption because no food was consumed during the PR task. According to our model, controls consumed less of the shake than they earned because their intact GLP-1 response to food both reduced reward value and increased satiation during food consumption compared to BN participants.
Although animal-based studies of reward value/effort permit animals to consume rewards earned during the PR task, no prior study using PR tasks in BN 95–98 permitted participants to consume rewards earned during the PR task. Although this approach prevents the potential satiating effects of food from compromising assessment of approach motivation, it precludes evaluation of how individual differences in post-prandial GLP-1 response may alter reinforcing value of food during consumption. To measure willingness to work for food during food consumption, we adapted the approach used by Schebendach et al. Our machine shop created an M&M’s® dispenser, connected to a computer on which participants complete the PR task schedule used by Schebendach et al.98. Participants were told they could earn M&M’s® by pressing a computer key, that the task consisted of 10 trials, and that at the end of each trial they would receive and consume 10 M&M’s®. Participants were instructed to work for the amount they wanted, that they could press the key as little or as much as they chose, could stop at anytime, and there were no right or wrong answers. Participants were then left alone and asked to notify the experimenter when they completed all 10 trials or decided to stop. Each time the participant reached the criterion for a trial, the dispenser distributed 10 M&M’s® for consumption. Unlike Schebendach et al.98, we did not instruct participants to work for the amount of food they “can binge on” and did not provide enough food to replicate a binge. This ensured that the total amount of food that could be earned (100 M&M’s®; 426 kcal in 3 oz.) was consumable by all participants. Thus, differences in response primarily reflect differences in reward value for M&M’s®. According to our model, individual differences in leptin concentration should contribute to differences in BP for M&M’s®. Individual differences in GLP-1 response during consumption of the M&M’s® should further enhance differences in reward value, such that the rate at which M&M’s® become less rewarding (and willingness to work declines) should be less robust in those with lower GLP-1 responses. Individual differences in leptin and GLP-1 response also may contribute to individual differences in the extent to which participants begin to feel satiated. However, we wanted our PR task for food to most closely reflect processes that drive food intake during a binge, which include sufficient quantities of food to elicit individual differences in GLP-1 response61. BP, keyboard presses, key strokes/sec by trial, and M&M’s® consumed were recorded. We used real-time digital video monitoring to ensure that participants followed instructions. We examined test-retest reliability by having participants complete the task twice under the same conditions over a one-week interval to ensure that we captured stable individual differences in reward valuation/effort. Test-retest reliability (r=.95) and the correlation between BP and M&M’s® consumed (r=.99) were high, suggesting reliable and valid indicators of individual differences in approach motivation for food that translate into actual food consumption.
On the PR task, BN participants (n=30) had a significantly higher BP (754±430) than controls (n=30) (498±380) (t(56)=2.39, p=.02; d=.63)22. Examining dimensional associations, we observed significant associations between greater WS and higher BP (r=.35, p<.01 in the full sample/r=.37 in BN), and, in BN, greater binge size (r=.47, p<.01), severity (r=.36, p=.02), and duration of illness (r=.44, p=.04). Consistent with a model in which hedonic and homeostatic regulation of food intake are integrated, we found a significant association between higher BP and lower self-reported satiation (r=−.31, p=.05/r=−.27), more severe loss of control over eating (r=.36, p=.04/r=.20), and, in BN, longer duration of illness (r=.34, p=.05). Although lower leptin was linked to higher BP, controlling for BMI and %body fat (β=−.13/β=−.11)22, the modest sample size constrained power to find a significant association for this small effect size, and we did not assess postprandial GLP-1 response in this study.
To further address influences of GLP-1 on approach motivation independently of its influences on satiation, we developed a novel PR task for a non-food reinforcer. Our biomedical engineers programmed a PR task that required participants to press a key on a keyboard to gain access to playing 1 min of Angry Birds per trial completed, using the same instructions, number of trials, and PR schedule used in our M&M’s® PR task. We developed this task because we wanted a non-food reinforcer that participants would find rewarding (popularity of Angry Birds supported this) and could consume during the PR task so that both the food and non-food tasks measure approach motivation for primary reinforcers, which most closely models animal-based research, and factors that could explain loss of control over a range of positively reinforced behaviors. We interpret play as a primary reinforcer because it produces pleasure and because the access to game play was not exchanged for any other reinforcer100. This task eliminates the influence of individual differences in GLP-1 on satiation as consumption of game play does not affect nutritional state. To infer whether GLP-1 response might influence approach motivation independently of its influence on satiation, we compared responses for the non-food reinforcer in the fed vs. fasted state. Thus, we are using a fixed meal to experimentally manipulate GLP-1 levels to examine the impact of physiological changes on approach motivation. If peripheral changes in GLP-1 reflect central changes in GLP-1 that reduce DA activation in the mesolimbic reward pathway, then we should observe lower BP for the non-food reinforcer in the fed vs. fasted state even though game play itself does not contribute to satiation.
Preliminary findings support these hypotheses. As part of an ongoing study, we have behavioral data on 108 women with bulimic disorders and 26 controls on reward value of a non-food reinforcer in the fed vs. fasted state. BP for Angry Birds was significantly higher in the fasted (824.63±456.48) compared to the fed (587.31±386.55) state (t(133)=7.17, p<.001). BP in the fasted condition was significantly correlated with BP in the fed condition (r(134)=.60, p<.001), supporting stability in individual differences in reward value for the non-food reinforcer. We found higher BP for the non-food reinforcer in our bulimic eating disorder participants compared to our control participants (t(133)=2.33, p=.02). In participants who have completed PR tasks for M&Ms and Angry Birds in the fasted and fed condition, we have found significant correlations between BP for M&Ms and Angry Birds in the fasted (r(133)=.44, p<.001) and fed conditions (r(132)=.42, p<.001), supporting our hypotheses that behavioral responses for food and non-food reinforcers reflect shared underlying processes. Importantly, we are currently completing assays of GLP-1 response to the fixed test meal used in the fed condition of the Angry Birds PR task, and analyses of associations GLP-1 response and changes in Angry Birds BP between the fasted and fed condition are planned to test this part of our model.
In contrast to the limited use of PR tasks to measure approach motivation in eating disorders, several studies have used ad lib test meal to measure satiation101. Across studies, women with bulimic disorders consumed significantly more food during ad lib test meals102–108 with moderate (d=.45)107 to very large (d=2.5)109 effect sizes, but reported either similar102,104,106 or lower103,110 levels of fullness following food intake compared to controls. In our lab, participants completed a single-item ad lib meal in the afternoon after consuming a standardized breakfast in the morning. Immediately before and after the ad lib meal, participants completed VAS items to assess subjective satiation. A one-quart (946 ml) serving of vanilla frozen yogurt (1.5 kcal/g) was served at an individual place setting. Participants were presented with the meal and instructions in print and on tape recorder to eat until they felt satiated, similar to prior studies104,111,112. Yogurt was weighed before and after the meal using a top-loading, self-calibrated electronic balance, and total intake was calculated in grams and kcal. BN and control participants did not differ in meal duration or subjective ratings of satiation after the ad lib meal (mean VAS rating=77 out of 100). However, to achieve the same level of satiation, mean intake was significantly greater in BN vs. controls (t(86)=3.36, p=.001; d=.70) 113. Thus, individuals with BN demonstrate decreased satiation in a behavioral ad lib test meal.
Within our ongoing study, we are using an ad lib test meal to measure reward satiation and its associations with WS and reward value/effort. Because behavioral tasks are completed over a series of visits to avoid fatigue, we are able to measure WS and changes in weight and their associations with study variables. First, those with greater WS at their first study visit report greater food intake in interviews of binge-eating (r(103)=.35, p<.001), consume larger amounts in the ad lib test meal to achieve satiation (r(132)=.24, p=.006), and demonstrate the greatest increase in body weight by their fourth study visit (r(134)=.28, p=.001). Additionally, consumption during the ad lib test meal is significantly associated with BP for the food reinforcer (r(130)=.53, p<.001) and the non-food reinforcer (r(132)=.25, p=.004). Importantly, these findings are preliminary as we have only collected data from less than half of our total target sample at baseline and have insufficient longitudinal data to examine differences in illness trajectory and insufficient biological data to test hypotheses related to leptin and GLP-1.
Complementary Future Directions for Alternative Methods and an Expanded Model
Our model examines peripheral release of leptin and GLP-1, but does not directly assess central processes. Other studies have used functional magnetic resonance imaging (fMRI) tasks to evaluate differences in brain activity that may explain binge eating. However, no neuroimaging studies have examined reward effort directly in individuals with bulimic eating disorders. One study114 examined a related construct, expected value (EV) which is related to the RDoC subconstruct of Probabilistic and Reinforcement Learning, in participants with restricting AN (n=28), participants recovered from AN (n=20), participants with BN (n=20) and healthy controls (n=43). EV was derived from a Pavlovian learning paradigm in which fractile images were paired probabilistically with receipt of a sweet solution, a neutral-tasting solution or nothing and then neural responses to these conditions and violation of expected reward receipt were measured. This approach is distinct from effort exerted to consume food or non-food rewards that we measure in our operant PR task. Across groups, BMI was negatively correlated with EV in the right anterior cingulate cortex (ACC), and this association extended to the analyses of a subgroup with normal to high normal weight, for whom EV in the left ACC was greatest in those with the lowest BMI. In addition, AN-ill had greater EV than all other groups, who did not differ from each other significantly. These results support an association between increased EV and reduced weight but do not speak to WS per se and do not support altered EV in BN. Moreover, these findings add to the work supporting increased prediction error as an index of increased Reward Learning and predictor of treatment outcome in AN (for review see, 115). In addition to this study, several studies have used neural responses to images or Pavlovian learning paradigms to infer elevated reward valuation of food using fMRI tasks with food-related stimuli. The majority of these studies have examined differences between individuals with and without eating disorders in neural response to food versus non-food (neutral) images 116,117 or high versus low calorie food images 118. Compared to controls, individuals with binge eating have demonstrated greater neural response to palatable food stimuli in the ventromedial prefrontal cortex 116, medial orbitofrontal cortex 117, insula 117,119, and (ACC) 117,118. However, one study noted decreased activation in the ACC in individuals with BN compared to healthy controls 120, and others have failed to demonstrate significant differences between those with and without bulimic eating disorders in reward region response to food images 121 or to anticipatory food cues 122. Thus, independent fMRI studies have supported that individuals with binge eating have elevated reward region responsiveness. However, neural correlates of approach motivation (reward effort) have yet to be examined. Moreover, to our knowledge, none of the above-mentioned studies compared responses during fasted and fed states that may better capture the construct of reward satiation. Thus, a complementary direction for our model would involve use of fMRI to examine neural activity in reward regions during PR tasks for food and non-food rewards in fed and fasted states in controls and individuals with bulimic eating disorders.
Eating behavior is determined by a complex interaction among various peptides123, including, but not limited to, leptin and GLP-1. Our current model focuses on two peptides implicated in both the homeostatic and hedonic regulation of eating that are dysregulated in the bulimic eating disorders. Future research of this model could examine peptides that are 1) altered in response to weight loss, 2) dysregulated across bulimic eating disorders, and 3) implicated in both homeostatic and hedonic regulation of eating. The strongest candidate may be ghrelin, an orexogenic peptide. Ghrelin increases in response to weight loss 124 and decreases in response to weight gain 125. Ghrelin levels are positively associated with binge-purge behavior in AN-bp and BN-p 126. Additionally, ghrelin levels tend to be elevated in both AN and BN relative to healthy controls; however, ghrelin levels tends to be lower in BED relative to controls 127. Although this maps onto findings for leptin, prior evidence that leptin is lower in obese BED compared to obese controls provides more support for leptin in our model. Finally, like leptin, ghrelin crosses the blood-brain barrier and is implicated in both the homeostatic and hedonic control of food intake 128,129. Thus, ghrelin may play an important role in mediating the link between WS and binge eating.
Other candidates for future work include insulin and brain-derived neurotrophic factor (BDNF). Insulin levels are correlated with body fat mass and implicated in reward function 130. The available data suggest that insulin levels and insulin response are lower in AN than healthy controls 126,131,132, whereas insulin levels and insulin response tends to be higher in BED compared to healthy controls 133. There is limited evidence for dysregulated insulin response in BN134,135; thus, the overall pattern of insulin dysregulation in bulimic eating disorders presents a less clear connection to the presence of binge eating transdiagnostically. Insulin’s role in homeostatic regulation of weight control and food intake is well characterized136. Additionally, animal data implicate insulin in the enhancement of dopaminergic signaling in the NAcc137 and indicate insulin administration in the VTA reduces the consumption of palatable food in a sated state138. However, intranasal insulin manipulations failed to support a link to hedonic eating in women139.
Turning to BDNF, serum levels are positively correlated with BMI, and studies of anorexia nervosa suggest that BDNF is reduced through weight loss55,140. Additionally, BDNF is reduced in BN relative to healthy controls, but does not appear to be dysregulated in BED55, creating some challenges for understanding its role transdiagnostically. Finally, BDNF has been implicated in both the homeostatic and hedonic regulation of food intake141. Taken together, investigating ghrelin, insulin, and BDNF in future work may further elucidate how WS contributes to binge eating in eating disorders. Further, the literature has and will continue to grow as we continue the current five-year study. It is inevitable that new findings will emerge that were not available at the time we developed our model, and these new findings will likely suggest both future and alternative directions.
One alternative direction may involve examining the interplay between premorbid weight and risk for bulimic eating disorders31. Our model posits that WS directly impacts reward valuation and satiation, but research related to weight gain and obesity suggests that overeating also may contribute to reward region responsiveness to palatable food cues (i.e., reward valuation)142. Thus, recent work supports dynamic relationships among weight, WS, reward valuation/effort, satiation, and binge eating that may contribute to illness maintenance.
Conclusion
WS in bulimic eating disorders prospectively predicts illness trajectory. WS is likely to contribute to decreased leptin levels due to loss of adipose tissue. Decreased leptin diminishes GLP-1 response to food intake, and reduced leptin and GLP-1 response contribute to greater reward value and decreased satiation. In particular, a less robust GLP-1 response during food intake would maintain greater drive to eat despite changes in nutritional status that should normally diminish this drive, and this combined with decreased ability to achieve satiation would contribute to both a sense of loss of control over eating and consumption of excessive amounts of food during – the defining features of binge episodes in eating disorders. Importantly, factors that influence illness maintenance may or may not be relevant to illness onset. Although Ancel Keys’ landmark study143 established that significant weight loss produced the onset of binge eating, this was observed in 30% of participants. This suggests that several other factors, including genetic risk, are important to understanding how WS may impact risk for binge eating. Moreover, the effect size for the association between WS and illness maintenance across studies is small, suggesting that it is one among many factors worthy of investigation.
We are currently in the third year of a five-year, NIMH-funded study to test our model in a longitudinal design of 320 participants. Our goal is to retain 260 participants through 6- and 12-month follow-up assessments to obtain reliable estimates of associations. If our model is supported, this work can significantly impact both conceptualization and clinical practice for eating disorders. Data regarding the predictive validity and clinical utility of distinguishing among three DSM-5 eating disorders may be reconceptualized as reflecting a single underlying dimension, such that three DSM-5 bulimic eating disorder categories are reframed as one bulimic eating disorder. Alternatively, findings may reveal new thresholds for distinguishing among these disorders. For example, if our model does not extend to the upper BMI range of bulimic eating disorders, differences between BN and BED may shift from emphasizing presence versus absence of compensatory behaviors to emphasizing BMI regardless of compensatory behaviors. In addition, results can impact assessment. Established assessments probe weight only for diagnosis of AN, ignoring weight for BN and BED, and none evaluates history of highest weight. Thus, there is no current standard for evaluating WS in eating disorders. If findings demonstrate that WS predicts illness maintenance transdiagnostically, then future assessments will incorporate simple, yet key questions for WS. Finally, data demonstrating that biological processes longitudinally mediate the association between WS and illness course will promote innovative treatment development for eating disorders characterized by binge eating, such as exploring the efficacy of GLP-1 agonists currently FDA-approved for Type 2 diabetes as a new intervention for eating disorders.
Highlights.
Weight suppression (WS) reflects the difference between lifetime highest adult weight and current weight.
WS predicts illness trajectory in bulimic eating disorders.
We review the literature and present new findings supporting that biological consequences of WS may alter responses to food to increase binge eating.
Acknowledgments
This work was funded by a grant from the NIMH (R01 MH111263).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lowe MR. The effects of dieting on eating behavior: a three-factor model. Psychol Bull. July 1993;114(1):100–121. [DOI] [PubMed] [Google Scholar]
- 2.Lowe MR, Piers AD, Benson L. Weight Suppression in Eating Disorders: a Research and Conceptual Update. Curr Psychiatry Rep. August 28 2018;20(10):80. [DOI] [PubMed] [Google Scholar]
- 3.National Institute of Mental Health. Definitions of the RDoC Domains and Constructs. 2018; https://www.nimh.nih.gov/research-priorities/rdoc/definitions-of-the-rdoc-domains-and-constructs.shtml#part_154189. Accessed November 11, 2018.
- 4.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) Washington, DC: American Psychiatric Publishing, Incorporated; 2013. [Google Scholar]
- 5.Keel PK, Brown TA, Holland LA, Bodell LP. Empirical classification of eating disorders. Annu Rev Clin Psychol. 2012;8:381–404. [DOI] [PubMed] [Google Scholar]
- 6.Eddy KT, Dorer DJ, Franko DL, Tahilani K, Thompson-Brenner H, Herzog DB. Diagnostic crossover in anorexia nervosa and bulimia nervosa: implications for DSM-V. Am J Psychiatry. February 2008;165(2):245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen KL, Byrne SM, Oddy WH, Crosby RD. DSM-IV-TR and DSM-5 eating disorders in adolescents: prevalence, stability, and psychosocial correlates in a population-based sample of male and female adolescents. J Abnorm Psychol. August 2013;122(3):720–732. [DOI] [PubMed] [Google Scholar]
- 8.Stice E, Marti CN, Rohde P. Prevalence, incidence, impairment, and course of the proposed DSM-5 eating disorder diagnoses in an 8-year prospective community study of young women. J Abnorm Psychol. May 2013;122(2):445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildes JE, Marcus MD. Weight suppression as a predictor of weight gain and response to intensive behavioral treatment in patients with anorexia nervosa. Behav Res Ther. April 2012;50(4):266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter FA, Boden JM, Jordan J, McIntosh VV, Bulik CM, Joyce PR. Weight suppression predicts total weight gain and rate of weight gain in outpatients with anorexia nervosa. Int J Eat Disord. November 2015;48(7):912–918. [DOI] [PubMed] [Google Scholar]
- 11.Butryn ML, Lowe MR, Safer DL, Agras WS. Weight suppression is a robust predictor of outcome in the cognitive-behavioral treatment of bulimia nervosa. Journal of abnormal psychology. February 2006;115(1):62–67. [DOI] [PubMed] [Google Scholar]
- 12.Lowe MR, Davis W, Lucks D, Annunziato R, Butryn M. Weight suppression predicts weight gain during inpatient treatment of bulimia nervosa. Physiology & Behavior. March 30 2006;87(3):487–492. [DOI] [PubMed] [Google Scholar]
- 13.Lowe MR, Berner LA, Swanson SA, et al. Weight suppression predicts time to remission from bulimia nervosa. J Consult Clin Psychol. December 2011;79(6):772–776. [DOI] [PubMed] [Google Scholar]
- 14.Keel PK, Heatherton TF. Weight suppression predicts maintenance and onset of bulimic syndromes at 10-Year follow-up. Journal of Abnormal Psychology May 2010;119(2):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter FA, McIntosh VVW, Joyce PR, Bulik CM. Weight suppression predicts weight gain over treatment but not treatment completion or outcome in bulimia nervosa. Journal of abnormal psychology. November 2008;117(4):936–940. [DOI] [PubMed] [Google Scholar]
- 16.Dawkins H, Watson HJ, Egan SJ, Kane RT. Weight suppression in bulimia nervosa: Relationship with cognitive behavioral therapy outcome. Int J Eat Disord. April 20 2013. [DOI] [PubMed] [Google Scholar]
- 17.Zunker C, Crosby RD, Mitchell JE, Wonderlich SA, Peterson CB, Crow SJ. Weight suppression as a predictor variable in treatment trials of bulimia nervosa and binge eating disorder. Int J Eat Disorder. December 2011;44(8):727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Son GE, van der Meer PA, Van Furth EF Correlates and associations between weight suppression and binge eating symptomatology in a population-based sample. Eating Behaviors. 2013;14(2):102–106. [DOI] [PubMed] [Google Scholar]
- 19.Butryn ML, Juarascio A, Lowe MR. The relation of weight suppression and BMI to bulimic symptoms. Int J Eat Disord. November 2011;44(7):612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook BJ, Steffen KJ, Mitchell JE, et al. A pilot study examining diagnostic differences among exercise and weight suppression in bulimia nervosa and binge eating disorder. Eur Eat Disord Rev. May 2015;23(3):241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roehrig M, Masheb RM, White MA, Grilo CM. Dieting frequency in obese patients with binge eating disorder: behavioral and metabolic correlates. Obesity (Silver Spring). April 2009;17(4):689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodell LP, Keel PK. Weight suppression in bulimia nervosa: Associations with biology and behavior. J Abnorm Psychol. November 2015;124(4):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzog DB, Thomas JG, Kass AE, Eddy KT, Franko DL, Lowe MR. Weight suppression predicts weight change over 5 years in bulimia nervosa. Psychiatry Res. May 30 2010;177(3):330–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solmi M, Gallicchio D, Collantoni E, et al. The impact of weight suppression and weight loss speed on baseline clinical characteristics and response to treatment. Int J Eat Disord. June 2018;51(6):542–548. [DOI] [PubMed] [Google Scholar]
- 25.Myers MG Jr., Leibel RL, Seeley RJ, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. November 2010;21(11):643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiology & Behavior. June 30 2006;88(3):249–256. [DOI] [PubMed] [Google Scholar]
- 27.Lowe MR. Damned if they do and damned if they don’t: Weight suppression as a maintenance factor in eating disorders. Society for Biological Psychiatry 74th Annual Scientific Convention Chicago 2019. [Google Scholar]
- 28.Funder DC, Ozer DJ. Evaluating effect size in psychological research: Sense and nonsense. Advances in Methods and Practices in Psychological Science. In Press. [Google Scholar]
- 29.Stice E, Durant S, Burger KS, Schoeller DA. Weight suppression and risk of future increases in body mass: effects of suppressed resting metabolic rate and energy expenditure. Am J Clin Nutr. July 2011;94(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witt AA, Berkowitz SA, Gillberg C, Lowe MR, Rastam M, Wentz E. Weight suppression and body mass index interact to predict long-term weight outcomes in adolescent-onset anorexia nervosa. J Consult Clin Psychol. December 2014;82(6):1207–1211. [DOI] [PubMed] [Google Scholar]
- 31.Yilmaz Z, Gottfredson NC, Zerwas SC, Bulik CM, Micali N. Developmental Premorbid Body Mass Index Trajectories of Adolescents With Eating Disorders in a Longitudinal Population Cohort. J Am Acad Child Adolesc Psychiatry. February 2019;58(2):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keel PK, Dorer DJ, Eddy KT, et al. Predictors of treatment utilization among women with anorexia and bulimia nervosa. Am J Psychiatry. January 2002;159(1):140–142. [DOI] [PubMed] [Google Scholar]
- 33.Bodell LP, Brown TA, Keel PK. Weight suppression predicts bulimic symptoms at 20-year follow-up: The mediating role of drive for thinness. J Abnorm Psychol. January 2017;126(1):32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenbaum M, Leibel RL. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. October 2014;223(1):T83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. November 1997;82(11):3647–3654. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe BE, Jimerson DC, Orlova C, Mantzoros CS. Effect of dieting on plasma leptin, soluble leptin receptor, adiponectin and resistin levels in healthy volunteers. Clin Endocrinol. September 2004;61(3):332–338. [DOI] [PubMed] [Google Scholar]
- 37.Keel PK, Bodell LP, Haedt-Matt AA, Williams DL, Appelbaum J. Weight suppression and bulimic syndrome maintenance: Preliminary findings for the mediating role of leptin. Int J Eat Disord. December 2017;50(12):1432–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monteleone P, Bortolotti F, Fabrazzo M, La Rocca A, Fuschino A, Maj M. Plasma leptin response to acute fasting and refeeding in untreated women with bulimia nervosa. J Clin Endocr Metab. July 2000;85(7):2499–2503. [DOI] [PubMed] [Google Scholar]
- 39.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. October 2012;15(10):1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. February 2003;52(2):252–259. [DOI] [PubMed] [Google Scholar]
- 41.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocr Metab. August 2006;91(8):2913–2919. [DOI] [PubMed] [Google Scholar]
- 42.Drewes C, Nauck MA, Horn R, Holst J, Schmiegel W, Brabant G. A liquid mixed meal or exogenous glucagon-like peptide 1 (GLP-1) do not alter plasma leptin concentrations in healthy volunteers. Acta Diabetologica. October 1997;34(3):230–234. [DOI] [PubMed] [Google Scholar]
- 43.Shalev A, Vosmeer S, Keller U. Absence of short-term effects of glucagon-like peptide-1 and of hyperglycemia on plasma leptin levels in man. Metabolism-Clinical and Experimental. July 1997;46(7):723–725. [DOI] [PubMed] [Google Scholar]
- 44.Monteleone P, Fabrazzo M, Tortorella A, Fuschino A, Maj M. Opposite modifications in circulating leptin and soluble leptin receptor across the eating disorder spectrum. Molecular Psychiatry. 2002;7(6):641–646. [DOI] [PubMed] [Google Scholar]
- 45.Monteleone P, Di Lieto A, Tortorella A, Longobardi N, Maj M. Circulating leptin in patients with anorexia nervosa, bulimia nervosa or binge-eating disorder: relationship to body weight, eating patterns, psychopathology and endocrine changes. Psychiat Res. May 15 2000;94(2):121–129. [DOI] [PubMed] [Google Scholar]
- 46.Monteleone P, Maj M. Dysfunctions of leptin, ghrelin, BDNF and endocannabinoids in eating disorders: beyond the homeostatic control of food intake. Psychoneuroendocrinology. March 2013;38(3):312–330. [DOI] [PubMed] [Google Scholar]
- 47.Grinspoon S, Gulick T, Askari H, et al. Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab. November 1996;81(11):3861–3863. [DOI] [PubMed] [Google Scholar]
- 48.Hebebrand J, Blum WF, Barth N, et al. Leptin levels in patients with anorexia nervosa are reduced in the acute stage and elevated upon short-term weight restoration. Molecular Psychiatry July 1997;2(4):330–334. [DOI] [PubMed] [Google Scholar]
- 49.Eckert ED, Pomeroy C, Raymond N, Kohler PF, Thuras P, Bowers CY. Leptin in anorexia nervosa. J Clin Endocrinol Metab. March 1998;83(3):791–795. [DOI] [PubMed] [Google Scholar]
- 50.Eddy KT, Lawson EA, Meade C, et al. Appetite regulatory hormones in women with anorexia nervosa: binge-eating/purging versus restricting type. J Clin Psychiatry January 2015;76(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eddy KT, Keel PK, Dorer DJ, Delinsky SS, Franko DL, Herzog DB. Longitudinal comparison of anorexia nervosa subtypes. Int J Eat Disord. March 2002;31(2):191–201. [DOI] [PubMed] [Google Scholar]
- 52.Brewerton TD, Lesem MD, Kennedy A, Garvey WT. Reduced plasma leptin concentrations in bulimia nervosa. Psychoneuroendocrinology. October 2000;25(7):649–658. [DOI] [PubMed] [Google Scholar]
- 53.Jimerson DC, Wolfe BE, Carroll DP, Keel PK. Psychobiology of purging disorder: reduction in circulating leptin levels in purging disorder in comparison with controls. Int J Eat Disord. November 1 2010;43(7):584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gendall KA, Kaye WH, Altemus M, McConaha CW, La Via MC. Leptin, neuropeptide Y, and peptide YY in long-term recovered eating disorder patients. Biol Psychiat. July 15 1999;46(2):292–299. [DOI] [PubMed] [Google Scholar]
- 55.Monteleone P, Fabrazzo M, Martiadis V, Serritella C, Pannuto M, Maj M. Circulating brain-derived neurotrophic factor is decreased in women with anorexia and bulimia nervosa but not in women with binge-eating disorder: relationships to co-morbid depression, psychopathology and hormonal variables. Psychol Med. June 2005;35(6):897–905. [DOI] [PubMed] [Google Scholar]
- 56.Brandao PP, Garcia-Souza EP, Neves FA, et al. Leptin/adiponectin ratio in obese women with and without binge eating disorder. Neuro Endocrinol Lett. 2010;31(3):353–358. [PubMed] [Google Scholar]
- 57.Adami GF, Campostano A, Cella F, Scopinaro N. Serum leptin concentration in obese patients with binge eating disorder. Int J Obes Relat Metab Disord. August 2002;26(8):1125–1128. [DOI] [PubMed] [Google Scholar]
- 58.Geliebter A, Gluck ME, Hashim SA. Plasma ghrelin concentrations are lower in binge-eating disorder. J Nutr. May 2005;135(5):1326–1330. [DOI] [PubMed] [Google Scholar]
- 59.Tomasik PJ, Sztefko K, Malek A. GLP-1 as a satiety factor in children with eating disorders. Hormone and Metabolic Research. February 2002;34(2):77–80. [DOI] [PubMed] [Google Scholar]
- 60.Dossat AM, Bodell LP, Williams DL, Eckel LA, Keel PK. Preliminary examination of glucagon-like peptide-1 levels in women with purging disorder and bulimia nervosa. Int J Eat Disord. March 2015;48(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naessen S, Carlstrom K, Holst JJ, Hellstrom PM, Hirschberg AL. Women with bulimia nervosa exhibit attenuated secretion of glucagon-like peptide 1, pancreatic polypeptide, and insulin in response to a meal. American Journal of Clinical Nutrition. October 2011;94(4):967–972. [DOI] [PubMed] [Google Scholar]
- 62.Geliebter A, Hashim SA, Gluck ME. Appetite-related gut peptides, ghrelin, PYY, and GLP-1 in obese women with and without binge eating disorder (BED). Physiology & Behavior. August 6 2008;94(5):696–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hommel JD, Trinko R, Sears RM, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. September 21 2006;51(6):801–810. [DOI] [PubMed] [Google Scholar]
- 64.Saper CB, Chou TC, Elmquist JK. The need to feed: Homeostatic and hedonic control of eating. Neuron. October 10 2002;36(2):199–211. [DOI] [PubMed] [Google Scholar]
- 65.van Zessen R, van der Plasse G, Adan RA. Contribution of the mesolimbic dopamine system in mediating the effects of leptin and ghrelin on feeding. Proc Nutr Soc. November 2012;71(4):435–445. [DOI] [PubMed] [Google Scholar]
- 66.National Institute of Mental Health. Positive Valence Systems: Workshop Proceedings. 2011; http://www.nimh.nih.gov/research-funding/rdoc/positive-valence-systems-workshop-proceedings.shtml. Accessed June 4, 2013.
- 67.Wise RA, Hoffman DC. Localization of drug reward mechanisms by intracranial injections. Synapse March 1992;10(3):247–263. [DOI] [PubMed] [Google Scholar]
- 68.Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012;4(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ranaldi R, Wise RA. Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: Possible role for dendritically released dopamine. Journal of Neuroscience. August 1 2001;21(15):5841–5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mcgregor A, Roberts DCS. Dopaminergic antagonism within the nucleus accumbens or the amygdala produces differential effects on intravenous cocaine self-administration under fixed and progressive ratio schedules of reinforcement. Brain Research. October 8 1993;624(1–2):245–252. [DOI] [PubMed] [Google Scholar]
- 71.Bruijnzeel AW, Corrie LW, Rogers JA, Yamada H. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav Brain Res. June 1 2011;219(2):254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davis JF, Choi DL, Schurdak JD, et al. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol Psychiatry. April 1 2011;69(7):668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. October 22 1998;395(6704):763–770. [DOI] [PubMed] [Google Scholar]
- 74.Johansson A, Fredriksson R, Winnergren S, Hulting AL, Schioth HB, Lindblom J. The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides. September 2008;29(9):1588–1595. [DOI] [PubMed] [Google Scholar]
- 75.Kinzig KP, Hargrave SL, Tao EE. Central and peripheral effects of chronic food restriction and weight restoration in the rat. Am J Physiol Endocrinol Metab. February 2009;296(2):E282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu Y, Deng C, Huang XF. Obese reversal by a chronic energy restricted diet leaves an increased Arc NPY/AgRP, but no alteration in POMC/CART, mRNA expression in diet-induced obese mice. Behav Brain Res. December 14 2009;205(1):50–56. [DOI] [PubMed] [Google Scholar]
- 77.van de Wall E, Leshan R, Xu AW, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. April 2008;149(4):1773–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. June 2009;150(6):2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hisadome K, Reimann F, Gribble FM, Trapp S. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes August 2010;59(8):1890–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldstone AP, Morgan I, Mercer JG, et al. Effect of leptin on hypothalamic GLP-1 peptide and brain-stem pre-proglucagon mRNA. Biochem Biophys Res Commun. March 16 2000;269(2):331–335. [DOI] [PubMed] [Google Scholar]
- 81.Toth K, Abraham H, Hajnal A. Glucagon-like peptide-1 (GLP-1) receptors in the ventral tegmental area of the rat: neuronal distribution and in vivo electrophysiological effects. Society of Neuroscience Abstracts. 2011;37:285–202. [Google Scholar]
- 82.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. October 12 2011;31(41):14453–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. January 11 1999;403(2):261–280. [DOI] [PubMed] [Google Scholar]
- 84.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. February 2012;153(2):647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. April 4 2012;32(14):4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Graham DL, Erreger K, Galli A, Stanwood GD. GLP-1 analog attenuates cocaine reward. Mol Psychiatry. October 23 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lemmens SG, Martens EA, Born JM, Martens MJ, Westerterp-Plantenga MS. Staggered meal consumption facilitates appetite control without affecting postprandial energy intake. J Nutr. March 2011;141(3):482–488. [DOI] [PubMed] [Google Scholar]
- 88.Dalvi PS, Nazarians-Armavil A, Purser MJ, Belsham DD. Glucagon-like peptide-1 receptor agonist, exendin-4, regulates feeding-associated neuropeptides in hypothalamic neurons in vivo and in vitro. Endocrinology. May 2012;153(5):2208–2222. [DOI] [PubMed] [Google Scholar]
- 89.Tang-Christensen M, Larsen PJ, Goke R, et al. Central administration of GLP-1-(7–36) amide inhibits food and water intake in rats. Am J Physiol. October 1996;271(4 Pt 2):R848–856. [DOI] [PubMed] [Google Scholar]
- 90.Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. January 4 1996;379(6560):69–72. [DOI] [PubMed] [Google Scholar]
- 91.Edholm T, Degerblad M, Gryback P, et al. Differential incretin effects of GIP and GLP-1 on gastric emptying, appetite, and insulin-glucose homeostasis. Neurogastroenterol Motil. November 2010;22(11):1191–1200, e1315. [DOI] [PubMed] [Google Scholar]
- 92.Brown TA, Keel PK. What contributes to excessive diet soda intake in eating disorders: appetitive drive, weight concerns, or both? Eat Disord. 2013;21(3):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Wagner A, Bischoff-Grethe A. Does a shared neurobiology for foods and drugs of abuse contribute to extremes of food ingestion in anorexia and bulimia nervosa? Biol Psychiatry. May 1 2013;73(9):836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: A multilevel analysis. Psychological Bulletin. September 2007;133(5):884–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bulik CM, Brinded EC. The effect of food deprivation on the reinforcing value of food and smoking in bulimic and control women. Physiol Behav. April 1994;55(4):665–672. [DOI] [PubMed] [Google Scholar]
- 96.Nasser JA, Evans SM, Geliebter A, Pi-Sunyer FX, Foltin RW. Use of an operant task to estimate food reinforcement in adult humans with and without BED. Obesity. August 2008;16(8):1816–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goldfield GS, Adamo KB, Rutherford J, Legg C. Stress and the relative reinforcing value of food in female binge eaters. Physiol Behav. February 27 2008;93(3):579–587. [DOI] [PubMed] [Google Scholar]
- 98.Schebendach J, Broft A, Foltin RW, Walsh BT. Can the reinforcing value of food be measured in bulimia nervosa? Appetite. March 2013;62:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hudson JI, Hiripi E, Pope HG Jr., Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. February 1 2007;61(3):348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Westlund K Formal definitions of “primary” and “secondary” reinforcers promote more efficient animal training. Journal of Animal Behavior Technology. 2018;8:33–41. [Google Scholar]
- 101.Mitchell JE, Crow S, Peterson CB, Wonderlich S, Crosby RD. Feeding laboratory studies in patients with eating disorders: a review. Int J Eat Disord. September 1998;24(2):115–124. [DOI] [PubMed] [Google Scholar]
- 102.Geliebter A, Melton PM, McCray RS, Gallagher DR, Gage D, Hashim SA. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr. October 1992;56(4):656–661. [DOI] [PubMed] [Google Scholar]
- 103.Nakai Y, Kinoshita F, Koh T, Tsujii S, Tsukada T. Perception of hunger and satiety induced by 2-deoxy-D-glucose in anorexia nervosa and bulimia nervosa. Int J Eat Disorder. January 1987;6(1):49–57. [Google Scholar]
- 104.Rolls BJ, Andersen AE, Moran TH, McNelis AL, Baier HC, Fedoroff IC. Food intake, hunger, and satiety after preloads in women with eating disorders. Am J Clin Nutr. June 1992;55(6):1093–1103. [DOI] [PubMed] [Google Scholar]
- 105.Hadigan CM, Walsh BT, Devlin MJ, LaChaussee JL, Kissileff HR. Behavioral assessment of satiety in bulimia nervosa. Appetite. June 1992;18(3):233–241. [DOI] [PubMed] [Google Scholar]
- 106.Kissileff HR, Wentzlaff TH, Guss JL, Walsh BT, Devlin MJ, Thornton JC. A direct measure of satiety disturbance in patients with bulimia nervosa. Physiol Behav. October 1996;60(4):1077–1085. [DOI] [PubMed] [Google Scholar]
- 107.Galanti K, Gluck ME, Geliebter A. Test meal intake in obese binge eaters in relation to impulsivity and compulsivity. Int J Eat Disord. December 2007;40(8):727–732. [DOI] [PubMed] [Google Scholar]
- 108.Geliebter A, Hassid G, Hashim SA. Test meal intake in obese binge eaters in relation to mood and gender. Int J Eat Disord. May 2001;29(4):488–494. [DOI] [PubMed] [Google Scholar]
- 109.LaChaussee JL, Kissileff HR, Walsh BT, Hadigan CM. The single-item meal as a measure of binge-eating behavior in patients with bulimia nervosa. Physiol Behav March 1992;51(3):593–600. [DOI] [PubMed] [Google Scholar]
- 110.Walsh BT, Kissileff HR, Cassidy SM, Dantzic S. Eating behavior of women with bulimia. Arch Gen Psychiatry. January 1989;46(1):54–58. [DOI] [PubMed] [Google Scholar]
- 111.Hetherington MM, Rolls BJ. Eating behavior in eating disorders: response to preloads. Physiol Behav July 1991;50(1):101–108. [DOI] [PubMed] [Google Scholar]
- 112.Wolfe BE, Metzger ED, Jimerson DC. Serotonin and satiety in bulimia nervosa. Biol Psychiat. April 15 2002;51(8):12s–12s. [Google Scholar]
- 113.Keel PK, Haedt-Matt AA, Hildebrandt B, Bodell LP, Wolfe BE, Jimerson DC. Satiation deficits and binge eating: Probing differences between bulimia nervosa and purging disorder using an ad lib test meal. Appetite. August 1 2018;127:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olsavsky AK, Shott ME, DeGuzman MC, Frank GKW. Neural correlates of taste reward value across eating disorders. Psychiatry Res Neuroimaging August 18 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frank GKW, DeGuzman MC, Shott ME. Motivation to eat and not to eat – the psychobiological conflict in anorexia nervosa. Physiology & Behavior. 2019;206:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Uher R, Murphy T, Brammer MJ, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. July 2004;161(7):1238–1246. [DOI] [PubMed] [Google Scholar]
- 117.Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. April 15 2009;65(8):654–661. [DOI] [PubMed] [Google Scholar]
- 118.Geliebter A, Benson L, Pantazatos SP, Hirsch J, Carnell S. Greater anterior cingulate activation and connectivity in response to visual and auditory high-calorie food cues in binge eating: Preliminary findings. Appetite. January 1 2016;96:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim KR, Ku J, Lee JH, Lee H, Jung YC. Functional and effective connectivity of anterior insula in anorexia nervosa and bulimia nervosa. Neurosci Lett. July 19 2012;521(2):152–157. [DOI] [PubMed] [Google Scholar]
- 120.Joos AA, Saum B, Zeeck A, et al. Frontocingular dysfunction in bulimia nervosa when confronted with disease-specific stimuli. Eur Eat Disord Rev. Sep-Oct 2011;19(5):447–453. [DOI] [PubMed] [Google Scholar]
- 121.Brooks SJ, O’Daly OG, Uher R, et al. Differential neural responses to food images in women with bulimia versus anorexia nervosa. Plos One. 2011;6(7):e22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bohon C, Stice E. Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. Int J Eat Disord. November 2011;44(7):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr March 2009;139(3):629–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. May 23 2002;346(21):1623–1630. [DOI] [PubMed] [Google Scholar]
- 125.Tolle V, Kadem M, Bluet-Pajot MT, et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab. January 2003;88(1):109–116. [DOI] [PubMed] [Google Scholar]
- 126.Tanaka M, Naruo T, Nagai N, et al. Habitual binge/purge behavior influences circulating ghrelin levels in eating disorders. J Psychiatr Res. Jan-Feb 2003;37(1):17–22. [DOI] [PubMed] [Google Scholar]
- 127.Atalayer D, Gibson C, Konopacka A, Geliebter A. Ghrelin and eating disorders. Prog Neuropsychopharmacol Biol Psychiatry. January 10 2013;40:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. June 20 2011;340(1):80–87. [DOI] [PubMed] [Google Scholar]
- 129.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides. November 2005;26(11):2274–2279. [DOI] [PubMed] [Google Scholar]
- 130.Davis JF, Choi DL, Benoit SC. Insulin, leptin and reward. Trends Endocrinol Metab. February 2010;21(2):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. April 2005;90(4):2161–2168. [DOI] [PubMed] [Google Scholar]
- 132.Uhe AM, Szmukler GI, Collier GR, Hansky J, O’Dea K, Young GP. Potential regulators of feeding behavior in anorexia nervosa. Am J Clin Nutr. January 1992;55(1):28–32. [DOI] [PubMed] [Google Scholar]
- 133.Geliebter A, Yahav EK, Gluck ME, Hashim SA. Gastric capacity, test meal intake, and appetitive hormones in binge eating disorder. Physiol Behav. July 2004;81(5):735–740. [DOI] [PubMed] [Google Scholar]
- 134.Casper RC, Pandy GN, Jaspan JB, Rubenstein AH. Hormone and metabolite plasma levels after oral glucose in bulimia and healthy controls. Biol Psychiatry. October 1988;24(6):663–674. [DOI] [PubMed] [Google Scholar]
- 135.Kojima S, Nakahara T, Nagai N, et al. Altered ghrelin and peptide YY responses to meals in bulimia nervosa. Clin Endocrinol (Oxf). January 2005;62(1):74–78. [DOI] [PubMed] [Google Scholar]
- 136.Woods SC, Seeley RJ, Porte D Jr., Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. May 29 1998;280(5368):1378–1383. [DOI] [PubMed] [Google Scholar]
- 137.Stouffer MA, Woods CA, Patel JC, et al. Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun. October 27 2015;6:8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mebel DM, Wong JC, Dong YJ, Borgland SL. Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur J Neurosci. August 2012;36(3):2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab. April 2008;93(4):1339–1344. [DOI] [PubMed] [Google Scholar]
- 140.Monteleone P, Tortorella A, Martiadis V, Serritella C, Fuschino A, Maj M. Opposite changes in the serum brain-derived neurotrophic factor in anorexia nervosa and obesity. Psychosom Med. Sep-Oct 2004;66(5):744–748. [DOI] [PubMed] [Google Scholar]
- 141.Cordeira J, Rios M. Weighing in the role of BDNF in the central control of eating behavior. Mol Neurobiol. December 2011;44(3):441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stice E, Burger K. Neural vulnerability factors for obesity. Clin Psychol Rev December 19 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation: Volume 1 Minneapolis: University of Minnesota Press; 1950. [Google Scholar]