Abstract
In spite of significant strides in the treatment of sickle cell disease (SCD), SCD crises are still responsible for high morbidity and early mortality. While most patients initially seek care in the acute setting for a seemingly uncomplicated pain episode (pain crisis or vaso-occlusive crisis), this initial event is the primary risk factor for potentially life-threatening complications. The pathophysiological basis of these illnesses is end-organ ischemia and infarction combined with the downstream effects of hemolysis that results from red blood cell sickling. These pathological changes can occur acutely and lead to a dramatic clinical presentation, but are frequently superimposed over a milieu of chronic vasculopathy, immune dysregulation, and decreased functional reserve. In the lungs, acute chest syndrome is a particularly ominous lung injury syndrome with a complex pathogenesis and potentially devastating sequelae, but all organ systems can be affected. It is, therefore, critical to understand the SCD patients' susceptibility to acute complications and their risk factors so that they can be recognized promptly and managed effectively. Blood transfusions remain the mainstay of therapy for all severe acute crises. Recommendations and indications for the safest and most efficient implementation of transfusion strategies in the critical care setting are therefore presented and discussed, together with their pitfalls and potential future therapeutic alternatives. In particular, the importance of extended phenotypic red blood cell matching cannot be overemphasized, due to the high prevalence of severe complications from red cell alloimmunization in SCD.
Key Words: acute chest syndrome, red blood cells, sickle cell disease, transfusion
Abbreviations: ACS, acute chest syndrome; AIC, acute intrahepatic cholestasis; DHTR, delayed hemolytic transfusion reaction; HbS, hemoglobin S; MOFS, multiorgan failure syndrome; NO, nitric oxide; PRBCs, packed red blood cells; PRES, posterior reversible encephalopathy syndrome; SCD, sickle cell disease; TRV, tricuspid regurgitant jet velocity; TTP, thrombotic thrombocytopenic purpura; VOC, vaso-occlusive crisis
Sickle cell disease (SCD) is a term that denotes syndromes characterized by the presence of intraerythrocytic hemoglobin S (HbS), a hemoglobin tetramer composed of mutated βS-globin chains, and includes homozygous HbS disease (HbSS) and compound heterozygous HbSC, HbS/β-thalassemia, HbSD, HbSO, and HbSE disease. The fundamental, primary lesion of SCD is the polymerization of deoxygenated HbS resulting in the deformation of RBCs into elongated, pathognomonic sickle-shaped cells. RBC sickling causes structural membrane damage leading to premature clearing of the affected cells by the reticuloendothelial system, abnormal expression of surface adhesion molecules, and impaired blood rheology. These processes are compounded by intravascular sickle cell hemolysis with release of proinflammatory heme (the tetrapyrrolic iron-binding ring in hemoglobin) and cell-free plasma hemoglobin. Hemolysis and the alterations of blood rheology stemming from a high proportion of circulating sickle cells lead to a complex inflammatory cascade affecting all components of Virchow’s triad and resulting in endothelial dysfunction, hyperviscosity, and hemostatic activation.1, 2 While Virchow’s triad is traditionally invoked to explain thrombosis, a peculiar type of vascular occlusion ensues in SCD, characterized by adhesion of RBCs, leukocytes, and platelets to the endothelial wall of postcapillary venules and antegrade and retrograde vaso-occlusion.3, 4, 5, 6, 7, 8 This lesion is well documented in SCD animal models, where it is responsible for organ ischemia-reperfusion injury and infarction.9 Whereas RBC sickling is incessant and necessary for vaso-occlusion, vaso-occlusive crises (VOCs) are episodic, acute events that can be triggered by specific stressors in animal models and often have clear triggers in patients. Thus, patients with SCD suffer both from a chronic inflammatory vasculopathy and acute crises characterized by organ function loss. Herein, we focus on the most severe, acute manifestations of SCD that often require admission to an ICU.
The triggers of acute, life-threatening VOCs are protean and are discussed relative to the specific complications, but all arise from the chronic susceptibility of patients with SCD to illness. The major predisposing threats to homeostasis in SCD include immune dysfunction, chronic sterile inflammation, decreased cardiopulmonary reserve, and progressive renal deterioration. Immune dysfunction stems from functional asplenia, the result of autoinfarction of the spleen in the first years of life, and predisposes to episodes of overwhelming sepsis from encapsulated bacteria, the prime pediatric killer in SCD. Sterile inflammation has been amply documented by research showing that most proinflammatory cytokines and mediators are upregulated in SCD, leading to enhanced sensitivity to pathogenic triggers, such as respiratory allergens.10, 11, 12, 13 Biventricular chamber dilatation is an early cardiac manifestation of SCD, detectable echocardiographically14 and by magnetic resonance imaging15 in children. It suggests wall remodeling as a compensatory mechanism to chronic anemia and progresses through adulthood. The resulting baseline elevation in cardiac output, combined with the detrimental effects of intravascular hemolysis on endothelial function, cellular proliferation, and lung parenchymal damage, eventually lead to decreased cardiopulmonary reserve, diastolic dysfunction, and pulmonary hypertension. Thus, the cardiovascular system in SCD is frequently unable to compensate for sudden increases in oxygen demand as in acute sepsis or acute anemia, predisposing patients to hemodynamic collapse in these situations.16, 17 Finally, renal dysfunction is one of the first identifiable abnormalities in SCD and subtly progresses to overt kidney failure over time. A combination of glomerular hyperfiltration and tubular dysfunction, both contributing to maintain low serum creatinine levels and a relatively high estimated glomerular filtration rate in spite of progressive renal disease, lead to underdiagnosis and underestimation of renal dysfunction by medical providers until frank proteinuria and advanced kidney disease have developed.18 Hemolysis, as evidenced by hemoglobinuria, has been independently associated with chronic kidney disease and can have a pathogenic role.19
In the following sections we discuss the main SCD crises encountered in the ICU together with their respective triggers and predisposing factors. While we have strived to provide the most up-to-date therapeutic strategies to address these complications, we also wish to remind our readers of existing, recently published guidelines (Table 1). These include the National Heart, Lung, and Blood Institute guidelines,20 the British Committee for Standards in Haematology guidelines for acute chest syndrome (ACS),21 and the American Thoracic Society guidelines for pulmonary hypertension.22
Table 1.
Acute Crises in Sickle Cell Disease and Recommended Interventions
| Acute Crisis | Interventiona | Strength of Recommendation | Quality of Evidence |
|---|---|---|---|
| Acute chest syndrome | Antibiotic therapy with cephalosporin and macrolideb | Strong | Low |
Exchange transfusion
|
Strong | Low | |
Simple transfusionc
|
Weak | Low | |
| Multisystem organ failure syndrome | Simple or exchange transfusiond | Consensus | Panel expertise |
| Aplastic crisis | Simple transfusion Droplet isolation of patients |
Consensus | Panel expertise |
| Splenic sequestration | Simple transfusion | Strong | Low |
| Hyperhemolysis crisis | Reserve transfusion for hemodynamic compromise. Consider corticosteroids, immunoglobulins, erythropoietin | Not available | Not available |
| Thrombotic thrombocytopenic purpura-like syndrome | Consider plasma exchange in addition to exchange transfusion | Not available | Not available |
| Acute stroke | Exchange transfusion | Consensus | Panel expertise |
| Hepatic sequestration or acute intrahepatic cholestasis | Simple or exchange transfusione | Consensus | Panel expertise |
This table includes interventions recommended by the National Heart, Lung, and Blood Institute Expert Panel Report (2014).20 The strength of each recommendation and quality of evidence are reported according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework criteria.
It is critical that all packed RBC units administered for single or exchange transfusions be HbS negative; leukoreduced; and fully matched for C, E, and K antigens.
Fluoroquinolones represent an alternative antibiotic regimen for patients with penicillin or cephalosporin allergies.
The authors of this review favor an aggressive approach to transfuse all patients with SCD, with exchange transfusions reserved for patients with the most severe symptoms (see text).
The authors of this review favor exchange transfusion if it is available without delay.
The authors of this review favor exchange transfusion because of the risk of “reverse sequestration” (see text).
Pulmonary Crises and Multiorgan Failure Syndrome
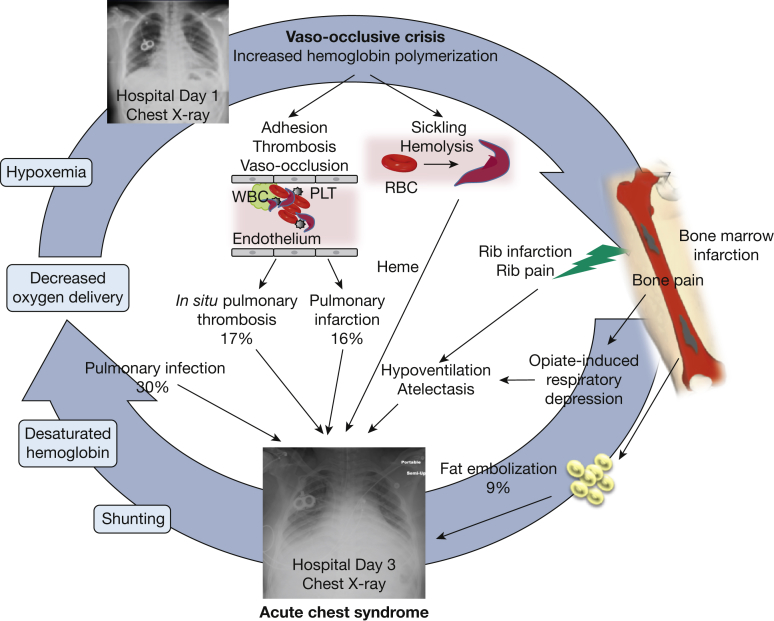
ACS is a lung injury syndrome that can complicate hospitalizations for acute VOC or develop de novo as a presenting admission diagnosis. It carries a high risk of respiratory failure (13% of patients requiring mechanical ventilation for a mean of 4.6 days) and a mortality of up to 9% in adults with SCD.23 ACS is also associated with prolonged hospitalization (on average, 10.5 days),23, 24 decreased long-term survival,25 and increased risk of developing chronic lung disease.26 Its presentation has a significant overlap with pneumonia because of the high prevalence of fever, dyspnea, and decreased oxygen saturation27 and because infection can both trigger or complicate a prior ACS. In its classic presentation (approximately 50% of cases), ACS develops in hospitalized patients, on average on hospital day 2.5, with more than 80% of patients having experienced a prior VOC (Fig 1).23, 27a Besides the aforementioned symptoms, chest pain is particularly common in ACS and can have a pleuritic component because of the presence of extensive lung infarction. Radiographically, multilobar involvement, affecting mostly the dependent lobes in adults and adolescents, is the norm (Fig 2)28 and because of its lower lobe predilection ACS is occasionally misdiagnosed as aspiration pneumonia. Patients who have new, complete lung segment consolidation on chest CT scan (approximately 60%) tend to have a more severe presentation with brisk leukocytosis and concurrent moderate to large pleural effusion and worse outcome (as indicated by the need for mechanical ventilation and extensive transfusion, and death).28 Several causes of ACS have been identified, although in most cases the direct insult is unknown.23 While infection is the leading offender in children,24 in adults the temporal association with VOC suggests the same mechanisms can underlie both syndromes. The finding of lipid-laden alveolar macrophages by oil red O stain in up to 44% of patients with ACS who have undergone bronchoalveolar lavage has substantiated a direct causal link to VOC.29 In these patients, it is hypothesized that bone marrow necrosis in the diaphysis of long bones leads to marrow fat embolization to the lungs and subsequent parenchymal ischemia and infarction. Most commonly, however, the finding of worse hemolysis in patients who develop ACS, as shown by rising lactate dehydrogenase and dropping hemoglobin levels, is indicative of the role of severe hemolysis in the causal path of ACS.30, 31 This role has been definitively demonstrated in animal models of ACS where hemin, the oxidized form of heme, is both necessary and sufficient to induce ACS in sickle mice, while scavenging of free plasma hemoglobin or heme released by hemolyzed RBCs has a protective effect.32 Another potential cause of ACS is hypoventilation from decreased respiratory effort, frequently resulting from either painful infarcted ribs33 or opiate-induced oversedation. The evidence from a small but significant clinical trial that incentive spirometry prevents ACS lends support to the potential role of hypoventilation and atelectasis in the pathogenesis of ACS.34 Finally, the finding that pulmonary thrombosis has a high prevalence in SCD35 and is a common incidental finding in ACS (17% of cases)36 has raised the question of whether thrombosis is a cause vs a complication of ACS. While patients with thrombosis had lower hemolysis and higher platelet counts at onset with higher peak counts,36 lower platelet counts at diagnosis (< 200,000/μL) have been long recognized to herald a worse clinical course with higher risk of respiratory failure, thereby suggesting that platelet consumption from hemolysis-induced hemostatic activation is also clinically relevant. Thus, physicians should take particular note of thrombosis, although at present the evidence is not sufficient to justify obtaining a CT angiogram in all patients with ACS. Well-established diagnostic algorithms to predict pulmonary embolism in other populations include lower extremity venous Doppler ultrasound and D-dimer measurements; it is important to recognize that these algorithms are usually not helpful in SCD as pulmonary thrombosis tends to be an in situ phenomenon (without accompanying evidence of lower extremity deep vein thrombosis)36 and D-dimer levels are elevated at baseline in SCD.37, 38, 39 The main causes and mechanisms of ACS are outlined in Figure 1.
Figure 1.
Causes and mechanisms of acute chest syndrome (ACS). Vaso-occlusive crises precede ACS in 80% of cases and are characterized by red blood cell sickling, cellular hyperadhesion, hemolysis, and vaso-occlusion. These processes are responsible for acute pain and bone marrow necrosis. ACS typically occurs 2.5 days after hospitalization for a vaso-occlusive episode, and radiographically presents as new infiltrates on a chest radiograph. Common causes include fat embolization from necrotic marrow (9% of cases), pulmonary infection (30% of cases), pulmonary infarction (16%), and hypoventilation. In situ pulmonary thrombosis has been identified in 17% of patients with ACS and may also be responsible for infarction. Animal models have shown that by-products of hemolysis, such as heme, cause experimental ACS. As a result of lung injury, ventilation-perfusion mismatches and shunting ensue, with subsequent hemoglobin desaturation and hypoxemia. Tissue hypoxia in turn triggers further sickling in a vicious cycle. The chest radiographs are those of a patient with SCD who received chronic exchange transfusions (note the presence of a double-lumen “port”) on hospital day 1 (top) and day 3 (bottom). By day 3 extensive infiltrates had developed in the patient, who required endotracheal intubation for respiratory failure. PLT = platelet.
Adapted with permission from Bope and Kellerman.27a
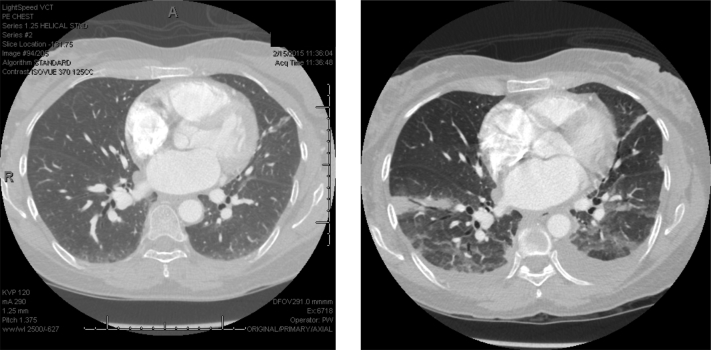
Figure 2.
CT scan of a patient with acute chest syndrome. A 53-year-old woman with sickle cell disease presented to the emergency department, complaining of pain typical of prior vaso-occlusive pain crises. A CT angiogram obtained on presentation to the emergency department revealed no infiltrate (left); however, extensive bilateral basilar airspace consolidations with small bilateral pleural effusions had developed on a repeat CT angiogram on day 3 (right).
Because of the uncertainty over the pathophysiology of ACS and the difficulty in identifying an exact trigger, treatment should target both infection and vaso-occlusion and necessarily include broad-spectrum antibiotic coverage and transfusion in all patients. Antibiotics should cover Streptococcus pneumoniae, taking into account the local antibiotic resistance patterns, and atypical microorganisms such as Chlamydia pneumoniae and Mycoplasma pneumoniae, as these rank highly among the commonly identified pathogens (>20% of all isolates).23 H1N1 pandemic influenza has also been responsible for increased morbidity and mortality in SCD, with ACS developing in 34% of patients, 17% receiving ICU care, and 10% undergoing mechanical ventilation,40 thus mandating early empiric treatment with oseltamivir during seasonal outbreaks. RBC transfusions aimed at increasing oxygen delivery and reducing the percentage of HbS should be administered as simple or automated exchange transfusions (erythrocytapheresis). Automated exchange transfusions are usually required in patients with the most severe symptoms, when rapid dilution of HbS is warranted, and require placement of a double-lumen catheter and rapid mobilization of blood banking resources. They are performed by dedicated nursing staff and involve cross-matching of several units of packed red blood cells (PRBCs). An alveolar-arterial oxygen gradient > 30 mm Hg was found to be associated with worse severity score and higher need for transfusion in children,41 but as a rule of thumb, patients with multilobar involvement, in respiratory distress, or who require ICU care should be aggressively treated with exchange transfusion. It is important to note, however, that simple and exchange transfusion strategies have never been directly compared in relation to ACS outcomes in a randomized trial. Potential risks of transfusion strategies in SCD are discussed in the “Hematologic Crises” section of this review. Particular care should also be afforded to patients who have compromised cardiopulmonary reserve. Approximately one-third of patients with SCD have elevated tricuspid regurgitant jet velocity (TRV) by transthoracic echocardiography at baseline, a finding that in patients with the highest elevations (> 3.0 m/s) is highly suggestive of pulmonary hypertension, and in those with more modest elevations (2.5-2.9 m/s) is linked to an increased risk for short-term mortality (rate ratio, 10.1).42 In a study of patients admitted to the ICU for severe ACS the pulmonary artery systolic pressure, as estimated from the TRV, rose sharply during ACS and returned to pre-ACS values on resolution of the illness. Sudden increases in pulmonary pressures carry an increased risk of pulmonary failure and sudden death and warrant close monitoring: in the aforementioned study cor pulmonale (36% of the cases), need for invasive mechanical ventilation (16%), and hospital death (12%) occurred only in the group with TRV elevation > 3 m/s during ACS.43 Furthermore, although volume expansion with crystalloids is important in SCD, it should be delivered at a cautious rate in these hemodynamically vulnerable patients, while RBC transfusions may need to be followed by diuretic therapy. Because of the established link between pulmonary thrombosis and ACS, DVT prophylaxis should be instituted routinely in patients with ACS, and clinical research is ongoing to establish whether systemic therapeutic dose anticoagulation can have a beneficial effect both in preventing thrombosis and improving the ACS disease course. Finally, although hydroxyurea has no role in the treatment of acute complications of SCD, it has been effective at preventing ACS in both children44 and adults45 and should be strongly recommended to any patient with SCD and a history of vaso-occlusive complications.
While transfusions have significantly improved the prognosis of ACS,41, 46 a small proportion of patients progresses to multiorgan failure syndrome (MOFS), an ominous complication of VOC (with or without concurrent ACS) with high mortality rates.47 MOFS typically occurs in patients with the most brisk hemolysis and is characterized by hemodynamic compromise and acute deterioration of at least two of three major organs.48 Fat embolism syndrome is a recognized cause of MOFS, although pathological confirmation is typically only obtained postmortem, with findings of widespread bone marrow necrosis leading to showers of fat emboli to the microvasculature. It has been hypothesized that sudden death during VOC may be due to fat embolization that has not yet resulted in MOFS.25 Acute respiratory distress syndrome physiology can complicate MOFS in one-quarter of cases and require intubation and mechanical ventilation with standard lung-protective strategies (ie, low tidal volume and plateau airway pressure less than 30 cm H2O).48 As for other patients with MOFS, care is supportive, although it is intuitive that minimizing HbS should be beneficial. This can be achieved by transfusing to keep the HbS level as low as possible (< 10%) although strong evidence to support this stringent threshold is not available. There are multiple reports of patients with ACS or MOFS who develop worrisome findings of thrombotic thrombocytopenic purpura (TTP),49, 50, 51, 52, 53, 54 a microangiopathic hemolytic anemia characterized by RBC fragments (schistocytes), thrombocytopenia, and microthrombosis, and classically due to deficiency or inhibition of ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), a protein responsible for cleavage of thrombogenic high molecular weight von Willebrand factor multimers. The mechanisms driving a TTP-like syndrome in SCD are unknown, although alterations in von Willebrand factor and ADAMTS13 homeostasis have been reported.55, 56, 57 Plasma exchange has been successfully used in conjunction with exchange transfusion in cases of TTP complicating ACS or MOFS,52 under the rationale of correcting the coagulopathy with fresh frozen plasma and removing any potential ADAMTS13 inhibitor.
The routine use of systemic corticosteroids has been proposed to prevent deterioration of patients with newly diagnosed ACS and to hasten the resolution of ACS-related acute respiratory distress syndrome. Lending support to corticosteroid use is the known association between pediatric bronchial hyperreactivity and ACS58, 59, 60 and the high prevalence of decreased FEV1 in adult patients with SCD.61 While intravenous corticosteroids prevented deterioration and reduced the need for transfusion in ACS,62 higher readmission rates among the treated patients62, 63 have, unfortunately, dampened enthusiasm for the routine use of these drugs. In these cases, it has been hypothesized that rebound adhesion of demarginated leukocytes after rapid corticosteroid withdrawal may have contributed to VOC. Finally, the role of noninvasive positive-pressure ventilation in preventing and treating ACS has not been definitively investigated but holds promise.64
Hematologic Crises
Acute or chronic anemia in SCD should be thoroughly investigated because it is frequently caused by a severe underlying illness. Any workup should begin with an assessment of the reticulocyte count to determine whether the acute anemia stems from decreased RBC production or increased loss through hemolysis or bleeding. In children with SCD, aplastic crises rank high in the differential diagnosis and should be suspected whenever the absolute reticulocyte count is < 100,000/μL. They are most commonly caused by infection with parvovirus B19, which has exquisite tropism to erythroid precursors and leads to transient red cell aplasia.65 While aplastic crises managed with supportive therapy generally resolve spontaneously with excellent outcomes, there has been a worrisome report of a high prevalence of acute parvovirus B19 infection complicating fat embolism syndrome, suggesting a possible link between the two conditions.66 Patients who should receive heightened surveillance for parvovirus B19 infection also include pregnant women, particularly before the 20th week of gestation, during which maternofetal transmission of the infection can lead to fetal hydrops and demise. Another major pediatric cause of acute anemia in SCD is splenic sequestration, which, contrary to aplastic crisis, causes increased reticulocytosis as RBCs are acutely sequestered within splenic tissue that has not yet atrophied and sclerosed. Rarely, splenic sequestration can occur in adult patients with persistent splenic tissue (most commonly those with HbSC disease), with the average nadir in hemoglobin (42% drop) and platelet count (62%) reached by hospital day 2.9.67 In both aplastic and splenic sequestration crises, the resulting anemia can be life-threatening and transfusions are the mainstay of treatment.
Unfortunately, transfusional therapy carries its own risks, increasingly evident as patients with SCD age. While hemosiderosis from transfusional iron overload is a chronic complication beyond the scope of this review, RBC alloimmunization is occasionally responsible for severe delayed hemolytic transfusion reactions (DHTRs) and rare but life-threatening hyperhemolytic crises. Patients with SCD are at particular risk for RBC alloimmunization because of the disparate expression of RBC antigens between the largely white donor pool and the overwhelmingly black SCD population, whose distinct pattern of expression of RBC antigens has been driven by the genomic pressure of the malaria parasite.68, 69 Thus, the risk of DHTR increases with the number of PRBC units received, although there is emerging evidence of a genetic predisposition to alloimmunization.70, 71 Unlike major RBC incompatibility reactions, DHTRs occur 10 to 14 days after exposure to the alloantigens. RBC alloimmunization can remain undiagnosed in asymptomatic patients unless routine screening by indirect Coombs test for development of new alloantibodies is conducted periodically. Because of the risk for DHTR, all major blood banks perform extended phenotypic RBC matching in addition to routine ABO and Rh (D) matching of blood units destined for patients with SCD. A common strategy, although there is no universal guideline, is to match for Rh (C, c, E, e) and Kell in all cases and to extend the matching to the Kidd and Duffy antigens for patients already alloimmunized. Even more stringent criteria include the MNS system and Rh genotyping, a practice motivated by the finding of clinically relevant, unexpected Rh antibodies in patients who have undergone Rh phenotyping.72 When in doubt, and particularly for patients with SCD who are not known by the local blood banks, the authors urge providers to specifically order extended phenotypic matching, in addition to leukoreduction and HbS-negative RBC products. In spite of extensive phenotypic matching, however, bouts of severe hyperhemolysis following blood transfusions develop in a minority of patients with alloimmunization and DHTR.73, 74, 75, 76, 77 During these life-threatening episodes, the hemoglobin drops to pretransfusion levels because of bystander RBC hemolysis likely resulting from an autoimmune process. In contrast to uncomplicated DHTR, the reticulocyte count is frequently suppressed in hyperhemolytic crises because of immune targeting of RBC precursors, and the direct antiglobulin test (Coombs test) may be negative if antibody-coated cells are rapidly cleared from the circulation. A search for culprit alloantigens is often futile, so that future transfusions continue to carry the same risk. Severe complications, including ACS, pancreatitis, congestive heart failure, and acute kidney injury, have ensued in patients undergoing hyperhemolysis crisis.74 Successful treatment has been reported anecdotally and is aimed at suppressing the immune response and stimulating erythropoiesis. Strategies have included systemic corticosteroids, daily intravenous erythropoietin, and intravenous immunoglobulins,78 although large studies validating these approaches are lacking. If repeat transfusion is absolutely needed, as in the case of hemodynamically unstable patients or in acute stroke, it should be administered while the patient is receiving immunosuppressive therapy.
Neurological Crises
The prevention of pediatric ischemic stroke in SCD has been a success story in hematology, and the prevalence of this complication has decreased dramatically from 11% to 1% after the implementation of screening and prophylactic transfusion for children with homozygous HbSS SCD.79, 80 Aging patients with SCD and children with SCD in settings with limited resources, poor access to state-of-the-art care, or contraindications to preventive treatments, however, continue to experience a high incidence of both ischemic and hemorrhagic stroke. The pathophysiology of stroke in SCD has not been fully elucidated, but a subset of children experience remodeling of large and medium-size cerebral arteries with intima medial hypertrophy, narrowing of the lumen, and increasing tortuosity.81, 82 These anatomical changes are accompanied by increased resting cerebral blood flow and oxygen extraction to meet the particularly high metabolic demands of the developing brain.83, 84 In situations of acute stress, such as severe acute anemia or ACS, two well-established risk factors,80, 85 the brain vasculature of children with compromised anatomy has no functional reserve to adapt with resulting acute territorial ischemia, particularly in the border zone regions of the brain. The manifestations of this pathological process range from subclinical silent infarcts detectable by MRI to overt stroke with a dramatic presentation and significant neurological sequelae. Thus, stroke in SCD has a distinct pathophysiology, and should be treated accordingly. Specifically, treatment in the acute care setting must rely heavily on prompt transfusion of PRBCs, preferably achieved by emergency exchange transfusion, both to improve the anemia and oxygenation and to dilute the HbS below 30%.86 If exchange transfusion cannot be rapidly performed, simple transfusion is the second-best strategy, with caution not to transfuse to a hemoglobin level > 10 g/dL to avoid the risk of hyperviscosity.87, 88 Supplemental oxygen to maintain oxygen saturation at > 95% should be delivered, and all care should be ideally provided in a neurological ICU with input from neurology and hematology consultants. It is unfortunate that with the exception of transfusion therapy, most other therapeutic interventions routinely considered for patients with ischemic stroke without SCD are a matter of contention. One major area where evidence is urgently needed is that of thrombolytic use in patients with SCD with acute stroke, particularly for patients with HbSC disease, who experience longer survival and higher exposure to traditional cardiovascular risk factors; caution is particularly warranted with these drugs as their therapeutic index may be extremely narrow in patients with SCD because of the patients’ increasing age-related risk of hemorrhagic stroke, which can occur concurrently with or complicate ischemic stroke. Hemorrhagic stroke is the most common acute neurological complication of SCD in adults.80 As in the case of ischemic stroke, anemia and recent ACS are risk factors, but most patients with hemorrhagic stroke have underlying anatomical abnormalities, including cerebral aneurysms, which can cause subarachnoid hemorrhages, or an abnormal pattern of cerebral vascularization known as moyamoya.89, 90, 91 This term denotes the radiological appearance of prominent cerebral vascular collateral networks arising to bypass stenosed arteries. No effective preventive strategies for hemorrhagic stroke are available, and care in the acute setting should follow the guidelines for the general population with spontaneous intracranial hemorrhage.
MRI scans are essential in the acute management of stroke as they allow quantification of the ischemic area, as well as aid in the differential diagnosis of other acute neurological conditions. Of these, posterior reversible encephalopathy syndrome (PRES) can have a similar presentation to stroke. PRES has, however, a better prognosis, since the pathogenic lesion is vasogenic edema, which is frequently reversible.89, 92, 93 Hypertension and fluid overload are often associated with PRES and should be aggressively treated. Since PRES can complicate severe ACS requiring endotracheal intubation, MRI surveillance is particularly indicated in these patients.93
Hepatic Crises
Liver involvement during acute VOCs is common in SCD but frequently limited to transient mild elevations of liver injury test results and mild conjugated hyperbilirubinemia. These laboratory abnormalities are the result of sinusoidal vaso-occlusion and modest hepatocellular injury. More ominous syndromes may develop in some patients, however, typically those with HbSS disease; these syndromes are characterized by tender hepatopathy/right upper quadrant pain, acute hepatomegaly, acute anemia, and more profound elevations of injury and cholestatic markers. There are no clear diagnostic criteria for hepatic crises, and the nomenclature has been ambiguous because of the overlap between hepatic sequestration and acute intrahepatic cholestasis (AIC). Thus, the commonly reported prevalence of 10% among hospitalized patients with SCD may not be correct.94 These syndromes are probably the result of the same vaso-occlusive process occurring along a continuum of severity. Hepatic sequestration, similar to splenic sequestration, is due to liver congestion with sickle cells during VOCs, which may lead to acute hepatomegaly (hence the need to monitor the liver span by physical examination during VOC) and severe anemia with a brisk reticulocyte response. The finding in case reports of sudden rises in hematocrit on resolution of hepatic sequestration crises suggests that not all trapped cells are phagocytized by the liver reticuloendothelial system and are subsequently available to reenter the systemic circulation, a phenomenon known as reverse sequestration.95 Care should therefore be taken when treating this complication with simple transfusions because of the risk of hyperviscosity that ensues after transfusion and release of the trapped cells from the liver. AIC is a complication at the extreme end of the spectrum of liver VOC and is characterized by severe direct hyperbilirubinemia. Although the initial transaminitis is usually mild to moderate, it can progress to shock liver and fulminant liver failure with complete arrest of the liver synthetic function and MOFS. Hepatic crises should invariably be treated by volume expansion, and exchange transfusion should not be delayed in patients with AIC, together with other supportive measures and correction of any acquired coagulopathy.94
Infectious Complications
Sepsis from encapsulated bacteria was the leading cause of mortality in pediatric SCD until a landmark clinical trial demonstrated the effectiveness of penicillin prophylaxis.96 Universal neonatal screening programs have allowed widespread adoption of this intervention early during infancy, in combination with seven-valent conjugate pneumococcal and Haemophilus influenzae type B vaccination, leading to a dramatic reduction of sepsis incidence97 and related mortality.98 The epidemiology of SCD sepsis has, therefore, changed. Most cases are now being attributed to contamination of totally implanted venous catheters (ports) routinely used for the ever-increasingly adopted chronic transfusion programs.99, 100, 101, 102 In these cases, empiric antibiotic coverage for oxacillin-resistant Staphylococcus aureus should be instituted pending the antibiogram results. In patients with recurrent sepsis and invasive infections the host susceptibility factors should be explored and, if possible, corrected. Hydroxyurea can predispose to worse sepsis outcomes by depressing the immune response, and should be discontinued in patients with active or frequent invasive infections. Iron chelation, another common therapeutic intervention in SCD, has been associated with an increased risk of sepsis from ferrophilic organisms such as Yersinia enterocolitica103, 104, 105, 106, 107 and Vibrio vulnificus, but its impact on acute bacteremia and endotoxemia overall has been less clear in other patient populations.108 In patients with SCD residing in or with a history of travel to a Plasmodium falciparum malaria endemic region, malarial parasitemia is associated with an increased risk for death during hospitalization (OR, 4.9),109, 110 suggesting that while HbS inheritance partially protects from infection, once acute malaria develops, it portends a more ominous course.
Conclusions
SCD has protean acute complications affecting virtually every organ and leading to high morbidity and mortality. In addition to best supportive care in a center familiar with the management of this disease, SCD-specific therapy aimed at improving anemia, reducing hyperviscosity, and diluting HbS is the mainstay of therapy. As it will not have escaped our reader, SCD-specific therapy is currently limited to PRBC transfusions. Such a dearth of therapeutic options is worrisome and is responsible for persistent early mortality in adulthood.111 Potential new treatments have therefore been explored to target specific pathogenic lesions of SCD. While the list of putative agents is extensive, in the acute setting broad categories include interventions aimed at reducing inflammation and vascular adhesion and restoring vascular function. Promising new agents currently under investigation to reduce inflammation and hyperadhesion include intravenous immunoglobulins, shown to decrease neutrophil adhesion to endothelium and red blood cell-neutrophil interactions,112, 113 anti-natural killer cell molecules aimed at reducing natural killer cell-mediated inflammatory interleukin production,114 and anti-selectin compounds115 targeting molecules critical for cell adhesion to the endothelium. The discovery that hemolysis alters vascular function through multiple pathways involving nitric oxide (NO) depletion and inhibition, hemostatic activation, and sterile inflammation has spurred intense research into NO therapeutics and antihemolytic agents. While NO therapeutics such as inhaled NO,116, 117 arginine supplementation,118 and phosphodiesterase inhibitors119 have collectively been disappointing, more research is being conducted to determine which subgroups of patients may benefit most from these treatments and more effective delivery methods.120 For instance, while inhaled NO in patients with mild to moderate ACS did not improve the rate of treatment failure, it can be beneficial in patients with severe ACS and hypoxemia.116 Antihemolytic agents are currently being developed to reduce chronic and acute hemolysis and thus prevent the pathogenic effects of free heme and free plasma hemoglobin that have been demonstrated in SCD animal models, and therapies directly targeting heme and free plasma hemoglobin are on the horizon.121
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. T. G. has no conflicts pertaining directly to this manuscript, but in the interest of full disclosure of all potential perceived conflicts, discloses the following: Dr Gladwin is a co-inventor on a U.S. government patent (that has been licensed) for the use of nitrites for cardiovascular indications. He has served as a consultant/advisory board member to Bayer Corp. He is the co-author of a medical textbook for students, for which he receives royalties. He is a member of the Executive Steering Committee for the EPIC trial conducted by MAST therapeutics and receives funding for participation in this trial. None declared (E. N.).
References
- 1.Akinola N.O., Stevens S.M., Franklin I.M. Subclinical ischaemic episodes during the steady state of sickle cell anaemia. J Clin Pathol. 1992;45(10):902–906. doi: 10.1136/jcp.45.10.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinola N.O., Stevens S.M., Franklin I.M. Rheological changes in the prodromal and established phases of sickle cell vaso-occlusive crisis. Br J Haematol. 1992;81(4):598–602. doi: 10.1111/j.1365-2141.1992.tb02998.x. [DOI] [PubMed] [Google Scholar]
- 3.Hebbel R.P. Blockade of adhesion of sickle cells to endothelium by monoclonal antibodies. N Engl J Med. 2000;342(25):1910–1912. doi: 10.1056/NEJM200006223422512. [DOI] [PubMed] [Google Scholar]
- 4.Hebbel R.P., Boogaerts M.A., Eaton J.W. Erythrocyte adherence to endothelium in sickle-cell anemia: a possible determinant of disease severity. N Engl J Med. 1980;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 5.Hebbel R.P., Boogaerts M.A., Koresawa S. Erythrocyte adherence to endothelium as a determinant of vasocclusive severity in sickle cell disease. Trans Assoc Am Physicians. 1980;93:94–99. [PubMed] [Google Scholar]
- 6.Hebbel R.P., Yamada O., Moldow C.F. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaul D.K., Fabry M.E., Nagel R.L. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proc Natl Acad Sci U S A. 1989;86(9):3356–3360. doi: 10.1073/pnas.86.9.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaul D.K., Fabry M.E., Nagel R.L. Vaso-occlusion by sickle cells: evidence for selective trapping of dense red cells. Blood. 1986;68(5):1162–1166. [PubMed] [Google Scholar]
- 9.Kaul D.K., Hebbel R.P. Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice. J Clin Invest. 2000;106(3):411–420. doi: 10.1172/JCI9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ataga K.I., Moore C.G., Hillery C.A. Coagulation activation and inflammation in sickle cell disease-associated pulmonary hypertension. Haematologica. 2008;93(1):20–26. doi: 10.3324/haematol.11763. [DOI] [PubMed] [Google Scholar]
- 11.Belcher J.D., Bryant C.J., Nguyen J. Transgenic sickle mice have vascular inflammation. Blood. 2003;101(10):3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 12.Duits A.J., Pieters R.C., Saleh A.W. Enhanced levels of soluble VCAM-1 in sickle cell patients and their specific increment during vasoocclusive crisis. Clin Immunol Immunopathol. 1996;81(1):96–98. doi: 10.1006/clin.1996.0163. [DOI] [PubMed] [Google Scholar]
- 13.Platt O.S. Sickle cell anemia as an inflammatory disease. J Clin Invest. 2000;106(3):337–338. doi: 10.1172/JCI10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lester L.A., Sodt P.C., Hutcheon N. Cardiac abnormalities in children with sickle cell anemia. Chest. 1990;98(5):1169–1174. doi: 10.1378/chest.98.5.1169. [DOI] [PubMed] [Google Scholar]
- 15.Meloni A., Detterich J., Berdoukas V. Comparison of biventricular dimensions and function between pediatric sickle-cell disease and thalassemia major patients without cardiac iron. Am J Hematol. 2013;88(3):213–218. doi: 10.1002/ajh.23376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fitzhugh C.D., Lauder N., Jonassaint J.C. Cardiopulmonary complications leading to premature deaths in adult patients with sickle cell disease. Am J Hematol. 2010;85(1):36–40. doi: 10.1002/ajh.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladwin M.T., Sachdev V. Cardiovascular abnormalities in sickle cell disease. J Am Coll Cardiol. 2012;59(13):1123–1133. doi: 10.1016/j.jacc.2011.10.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson J., Reid M., Hambleton I. Albuminuria and renal function in homozygous sickle cell disease: observations from a cohort study. Arch Intern Med. 2007;167(7):701–708. doi: 10.1001/archinte.167.7.701. [DOI] [PubMed] [Google Scholar]
- 19.Saraf S.L., Zhang X., Kanias T. Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. Br J Haematol. 2014;164(5):729–739. doi: 10.1111/bjh.12690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yawn B.P., Buchanan G.R., Afenyi-Annan A.N. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. doi: 10.1001/jama.2014.10517. [DOI] [PubMed] [Google Scholar]
- 21.Howard J., Hart N., Roberts-Harewood M. Guideline on the management of acute chest syndrome in sickle cell disease. Br J Haematol. 2015;169(4):492–505. doi: 10.1111/bjh.13348. [DOI] [PubMed] [Google Scholar]
- 22.Klings E.S., Machado R.F., Barst R.J. An official American Thoracic Society clinical practice guideline: diagnosis, risk stratification, and management of pulmonary hypertension of sickle cell disease. Am J Respir Crit Care Med. 2014;189(6):727–740. doi: 10.1164/rccm.201401-0065ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vichinsky E.P., Neumayr L.D., Earles A.N., and the National Acute Chest Syndrome Study Group Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342(25):1855–1865. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 24.Vichinsky E.P., Styles L.A., Colangelo L.H. Acute chest syndrome in sickle cell disease: clinical presentation and course: Cooperative Study of Sickle Cell Disease. Blood. 1997;89(5):1787–1792. [PubMed] [Google Scholar]
- 25.Platt O.S., Brambilla D.J., Rosse W.F. Mortality in sickle cell disease: life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 26.Knight-Madden J.M., Forrester T.S., Lewis N.A. The impact of recurrent acute chest syndrome on the lung function of young adults with sickle cell disease. Lung. 2010;188(6):499–504. doi: 10.1007/s00408-010-9255-2. [DOI] [PubMed] [Google Scholar]
- 27.Charache S., Scott J.C., Charache P. “Acute chest syndrome” in adults with sickle cell anemia: microbiology, treatment, and prevention. Arch Intern Med. 1979;139(1):67–69. [PubMed] [Google Scholar]
- Bope E.T., Kellerman RD. Elsevier; Philadelphia, PA: 2016. Conn's Current Therapy 2016. [Google Scholar]
- 28.Mekontso Dessap A., Deux J.F., Habibi A. Lung imaging during acute chest syndrome in sickle cell disease: computed tomography patterns and diagnostic accuracy of bedside chest radiograph. Thorax. 2014;69(2):144–151. doi: 10.1136/thoraxjnl-2013-203775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vichinsky E., Williams R., Das M. Pulmonary fat embolism: a distinct cause of severe acute chest syndrome in sickle cell anemia. Blood. 1994;83(11):3107–3112. [PubMed] [Google Scholar]
- 30.Adisa O.A., Hu Y., Ghosh S. Association between plasma free haem and incidence of vaso-occlusive episodes and acute chest syndrome in children with sickle cell disease. Br J Haematol. 2013;162(5):702–705. doi: 10.1111/bjh.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stankovic Stojanovic K., Steichen O., Lefevre G. High lactate dehydrogenase levels at admission for painful vaso-occlusive crisis is associated with severe outcome in adult SCD patients. Clin Biochem. 2012;45(18):1578–1582. doi: 10.1016/j.clinbiochem.2012.07.114. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S., Adisa O.A., Chappa P. Extracellular hemin crisis triggers acute chest syndrome in sickle mice. J Clin Invest. 2013;123(11):4809–4820. doi: 10.1172/JCI64578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rucknagel D.L. The role of rib infarcts in the acute chest syndrome of sickle cell diseases. Pediatr Pathol Mol Med. 2001;20(2):137–154. [PubMed] [Google Scholar]
- 34.Bellet P.S., Kalinyak K.A., Shukla R. Incentive spirometry to prevent acute pulmonary complications in sickle cell diseases. N Engl J Med. 1995;333(11):699–703. doi: 10.1056/NEJM199509143331104. [DOI] [PubMed] [Google Scholar]
- 35.Novelli E.M., Huynh C., Gladwin M.T. Pulmonary embolism in sickle cell disease: a case-control study. J Thromb Haemost. 2012;10(5):760–766. doi: 10.1111/j.1538-7836.2012.04697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mekontso Dessap A., Deux J.F., Abidi N. Pulmonary artery thrombosis during acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2011;184(9):1022–1029. doi: 10.1164/rccm.201105-0783OC. [DOI] [PubMed] [Google Scholar]
- 37.Hagger D., Wolff S., Owen J. Changes in coagulation and fibrinolysis in patients with sickle cell disease compared with healthy black controls. Blood Coagul Fibrinolysis. 1995;6(2):93–99. doi: 10.1097/00001721-199504000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Tomer A., Harker L.A., Kasey S. Thrombogenesis in sickle cell disease. J Lab Clin Med. 2001;137(6):398–407. doi: 10.1067/mlc.2001.115450. [DOI] [PubMed] [Google Scholar]
- 39.Wang A., Liu F., Dong N. Thrombospondin-1 and ADAMTS13 competitively bind to VWF A2 and A3 domains in vitro. Thromb Res. 2010;126(4):e260–e265. doi: 10.1016/j.thromres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Strouse J.J., Reller M.E., Bundy D.G. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood. 2010;116(18):3431–3434. doi: 10.1182/blood-2010-05-282194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emre U., Miller S.T., Gutierez M. Effect of transfusion in acute chest syndrome of sickle cell disease. J Pediatr. 1995;127(6):901–904. doi: 10.1016/s0022-3476(95)70025-0. [DOI] [PubMed] [Google Scholar]
- 42.Gladwin M.T., Sachdev V., Jison M.L. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350(9):886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 43.Mekontso Dessap A., Leon R., Habibi A. Pulmonary hypertension and cor pulmonale during severe acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 2008;177(6):646–653. doi: 10.1164/rccm.200710-1606OC. [DOI] [PubMed] [Google Scholar]
- 44.Wang W.C., Ware R.E., Miller S.T. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Charache S., Terrin M.L., Moore R.D., and the Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 46.Mallouh A.A., Asha M. Beneficial effect of blood transfusion in children with sickle cell chest syndrome. Am J Dis Child. 1988;142(2):178–182. doi: 10.1001/archpedi.1988.02150020080034. [DOI] [PubMed] [Google Scholar]
- 47.Castro O. Systemic fat embolism and pulmonary hypertension in sickle cell disease. Hematol Oncol Clin North Am. 1996;10(6):1289–1303. doi: 10.1016/s0889-8588(05)70401-9. [DOI] [PubMed] [Google Scholar]
- 48.Hassell K.L., Eckman J.R., Lane P.A. Acute multiorgan failure syndrome: a potentially catastrophic complication of severe sickle cell pain episodes. Am J Med. 1994;96(2):155–162. doi: 10.1016/0002-9343(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 49.Vlachaki E., Agapidou A., Neokleous N. Thrombotic thrombocytopenic purpura or immune thrombocytopenia in a sickle cell/β+-thalassemia patient: a rare and challenging condition. Transfus Apher Sci. 2014;51(2):175–177. doi: 10.1016/j.transci.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Venkata Sasidhar M., Tripathy A.K., Viswanathan K. Thrombotic thrombocytopenic purpura and multiorgan system failure in a child with sickle cell-hemoglobin C disease. Clin Pediatr (Phila) 2010;49(10):992–996. doi: 10.1177/0009922809338314. [DOI] [PubMed] [Google Scholar]
- 51.Shelat S.G. Thrombotic thrombocytopenic purpura and sickle cell crisis. Clin Appl Thromb Hemost. 2010;16(2):224–227. doi: 10.1177/1076029608323804. [DOI] [PubMed] [Google Scholar]
- 52.Lee H.E., Marder V.J., Logan L.J. Life-threatening thrombotic thrombocytopenic purpura (TTP) in a patient with sickle cell-hemoglobin C disease. Ann Hematol. 2003;82(11):702–704. doi: 10.1007/s00277-003-0715-0. [DOI] [PubMed] [Google Scholar]
- 53.Bolanos-Meade J., Keung Y.K., Lopez-Arvizu C. Thrombotic thrombocytopenic purpura in a patient with sickle cell crisis. Ann Hematol. 1999;78(12):558–559. doi: 10.1007/s002770050558. [DOI] [PubMed] [Google Scholar]
- 54.Chinowsky M.S. Thrombotic thrombocytopenic purpura associated with sickle cell-hemoglobin C disease. South Med J. 1994;87(11):1168–1171. doi: 10.1097/00007611-199411000-00025. [DOI] [PubMed] [Google Scholar]
- 55.Chen J., Hobbs W.E., Le J. The rate of hemolysis in sickle cell disease correlates with the quantity of active von Willebrand factor in the plasma. Blood. 2011;117(13):3680–3683. doi: 10.1182/blood-2010-08-302539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novelli E.M., Kato G.J., Hildesheim M.E. Thrombospondin-1 inhibits ADAMTS13 activity in sickle cell disease. Haematologica. 2013;98(11):e132–e134. doi: 10.3324/haematol.2013.092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnog J.J., Hovinga J.A., Krieg S. ADAMTS13 activity in sickle cell disease. Am J Hematol. 2006;81(7):492–498. doi: 10.1002/ajh.20653. [DOI] [PubMed] [Google Scholar]
- 58.Boyd J.H., Macklin E.A., Strunk R.C. Asthma is associated with acute chest syndrome and pain in children with sickle cell anemia. Blood. 2006;108(9):2923–2927. doi: 10.1182/blood-2006-01-011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knight-Madden J.M., Forrester T.S., Lewis N.A. Asthma in children with sickle cell disease and its association with acute chest syndrome. Thorax. 2005;60(3):206–210. doi: 10.1136/thx.2004.029165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Field J.J., Stocks J., Kirkham F.J. Airway hyperresponsiveness in children with sickle cell anemia. Chest. 2011;139(3):563–568. doi: 10.1378/chest.10-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Field J.J., Glassberg J., Gilmore A. Longitudinal analysis of pulmonary function in adults with sickle cell disease. Am J Hematol. 2008;83(7):574–576. doi: 10.1002/ajh.21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bernini J.C., Rogers Z.R., Sandler E.S. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92(9):3082–3089. [PubMed] [Google Scholar]
- 63.Griffin T.C., McIntire D., Buchanan G.R. High-dose intravenous methylprednisolone therapy for pain in children and adolescents with sickle cell disease. N Engl J Med. 1994;330(11):733–737. doi: 10.1056/NEJM199403173301101. [DOI] [PubMed] [Google Scholar]
- 64.Fartoukh M., Lefort Y., Habibi A. Early intermittent noninvasive ventilation for acute chest syndrome in adults with sickle cell disease: a pilot study. Intensive Care Med. 2010;36(18):1355–1362. doi: 10.1007/s00134-010-1907-4. [DOI] [PubMed] [Google Scholar]
- 65.Saarinen U.M., Chorba T.L., Tattersall P. Human parvovirus B19-induced epidemic acute red cell aplasia in patients with hereditary hemolytic anemia. Blood. 1986;67(5):1411–1417. [PubMed] [Google Scholar]
- 66.Tsitsikas D.A., Gallinella G., Patel S. Bone marrow necrosis and fat embolism syndrome in sickle cell disease: increased susceptibility of patients with non-SS genotypes and a possible association with human parvovirus B19 infection. Blood Rev. 2014;28(1):23–30. doi: 10.1016/j.blre.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Naymagon L., Pendurti G., Billett H.H. Acute splenic sequestration crisis in adult sickle cell disease: a report of 16 cases. Hemoglobin. 2015;39(6):375–379. doi: 10.3109/03630269.2015.1072550. [DOI] [PubMed] [Google Scholar]
- 68.Rosse W.F., Gallagher D., Kinney T.R. Transfusion and alloimmunization in sickle cell disease: the Cooperative Study of Sickle Cell Disease. Blood. 1990;76(7):1431–1437. [PubMed] [Google Scholar]
- 69.Vichinsky E.P., Earles A., Johnson R.A. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood. N Engl J Med. 1990;322(23):1617–1621. doi: 10.1056/NEJM199006073222301. [DOI] [PubMed] [Google Scholar]
- 70.Alarif L., Castro O., Ofosu M. HLA-B35 is associated with red cell alloimmunization in sickle cell disease. Clin Immunol Immunopathol. 1986;38(2):178–183. doi: 10.1016/0090-1229(86)90136-4. [DOI] [PubMed] [Google Scholar]
- 71.Higgins J.M., Sloan S.R. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112(6):2546–2553. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 72.Chou S.T., Jackson T., Vege S. High prevalence of red blood cell alloimmunization in sickle cell disease despite transfusion from Rh-matched minority donors. Blood. 2013;122(6):1062–1071. doi: 10.1182/blood-2013-03-490623. [DOI] [PubMed] [Google Scholar]
- 73.King K.E., Shirey R.S., Lankiewicz M.W. Delayed hemolytic transfusion reactions in sickle cell disease: simultaneous destruction of recipients’ red cells. Transfusion. 1997;37(4):376–381. doi: 10.1046/j.1537-2995.1997.37497265337.x. [DOI] [PubMed] [Google Scholar]
- 74.Talano J.A., Hillery C.A., Gottschall J.L. Delayed hemolytic transfusion reaction/hyperhemolysis syndrome in children with sickle cell disease. Pediatrics. 2003;111(6 Pt 1):e661–e665. doi: 10.1542/peds.111.6.e661. [DOI] [PubMed] [Google Scholar]
- 75.Win N., Doughty H., Telfer P. Hyperhemolytic transfusion reaction in sickle cell disease. Transfusion. 2001;41(3):323–328. doi: 10.1046/j.1537-2995.2001.41030323.x. [DOI] [PubMed] [Google Scholar]
- 76.Win N., New H., Lee E. Hyperhemolysis syndrome in sickle cell disease: case report (recurrent episode) and literature review. Transfusion. 2008;48(6):1231–1238. doi: 10.1111/j.1537-2995.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- 77.Sirchia G., Morelati F., Rebulla P. The sickle cell hemolytic transfusion reaction syndrome. Transfusion. 1997;37(10):1098–1099. doi: 10.1046/j.1537-2995.1997.371098016453.x. [DOI] [PubMed] [Google Scholar]
- 78.Win N., Yeghen T., Needs M. Use of intravenous immunoglobulin and intravenous methylprednisolone in hyperhaemolysis syndrome in sickle cell disease. Hematology. 2004;9(5-6):433–436. doi: 10.1080/10245330400001926. [DOI] [PubMed] [Google Scholar]
- 79.Adams R.J., McKie V.C., Hsu L. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 80.Ohene-Frempong K., Weiner S.J., Sleeper L.A. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 81.Belizna C., Loufrani L., Ghali A. Arterial stiffness and stroke in sickle cell disease. Stroke. 2012;43(4):1129–1130. doi: 10.1161/STROKEAHA.111.635383. [DOI] [PubMed] [Google Scholar]
- 82.Merkel K.H., Ginsberg P.L., Parker J.C., Jr. Cerebrovascular disease in sickle cell anemia: a clinical, pathological and radiological correlation. Stroke. 1978;9(1):45–52. doi: 10.1161/01.str.9.1.45. [DOI] [PubMed] [Google Scholar]
- 83.Kugler S., Anderson B., Cross D. Abnormal cranial magnetic resonance imaging scans in sickle-cell disease. Neurological correlates and clinical implications. Arch Neurol. 1993;50(6):629–635. doi: 10.1001/archneur.1993.00540060059019. [DOI] [PubMed] [Google Scholar]
- 84.Strouse J.J., Cox C.S., Melhem E.R. Inverse correlation between cerebral blood flow measured by continuous arterial spin-labeling (CASL) MRI and neurocognitive function in children with sickle cell anemia (SCA) Blood. 2006;108(1):379–381. doi: 10.1182/blood-2005-10-4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.DeBaun M.R., Sarnaik S.A., Rodeghier M.J. Associated risk factors for silent cerebral infarcts in sickle cell anemia: low baseline hemoglobin, sex, and relative high systolic blood pressure. Blood. 2012;119(16):3684–3690. doi: 10.1182/blood-2011-05-349621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kirkham F.J., DeBaun M.R. Stroke in children with sickle cell disease. Curr Treat Options Neurol. 2004;6(5):357–375. doi: 10.1007/s11940-996-0028-4. [DOI] [PubMed] [Google Scholar]
- 87.Chien S., Kaperonis A.A., King R.G. Rheology of sickle cells and its role in microcirculatory dynamics. Prog Clin Biol Res. 1987;240:151–165. [PubMed] [Google Scholar]
- 88.Schmalzer E.A., Lee J.O., Brown A.K. Viscosity of mixtures of sickle and normal red cells at varying hematocrit levels: implications for transfusion. Transfusion. 1987;27(3):228–233. doi: 10.1046/j.1537-2995.1987.27387235626.x. [DOI] [PubMed] [Google Scholar]
- 89.Alkan O., Kizilkilic E., Kizilkilic O. Cranial involvement in sickle cell disease. Eur J Radiol. 2010;76(2):151–156. doi: 10.1016/j.ejrad.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 90.Dobson S.R., Holden K.R., Nietert P.J. Moyamoya syndrome in childhood sickle cell disease: a predictive factor for recurrent cerebrovascular events. Blood. 2002;99(9):3144–3150. doi: 10.1182/blood.v99.9.3144. [DOI] [PubMed] [Google Scholar]
- 91.Drew J.M., Scott J.A., Chua G.T. General case of the day: moyamoya syndrome in a child with sickle cell disease. Radiographics. 1993;13(2):483–484. doi: 10.1148/radiographics.13.2.8460234. [DOI] [PubMed] [Google Scholar]
- 92.Geevasinga N., Cole C., Herkes G.K. Sickle cell disease and posterior reversible leukoencephalopathy. J Clin Neurosci. 2014;21(8):1329–1332. doi: 10.1016/j.jocn.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 93.Henderson J.N., Noetzel M.J., McKinstry R.C. Reversible posterior leukoencephalopathy syndrome and silent cerebral infarcts are associated with severe acute chest syndrome in children with sickle cell disease. Blood. 2003;101(2):415–419. doi: 10.1182/blood-2002-04-1183. [DOI] [PubMed] [Google Scholar]
- 94.Banerjee S., Owen C., Chopra S. Sickle cell hepatopathy. Hepatology. 2001;33(5):1021–1028. doi: 10.1053/jhep.2001.24114. [DOI] [PubMed] [Google Scholar]
- 95.Lee E.S., Chu P.C. Reverse sequestration in a case of sickle crisis. Postgrad Med J. 1996;72(850):487–488. doi: 10.1136/pgmj.72.850.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaston M.H., Verter J.I., Woods G. Prophylaxis with oral penicillin in children with sickle cell anemia: a randomized trial. N Engl J Med. 1986;314(25):1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- 97.Baskin M.N., Goh X.L., Heeney M.M. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131(6):1035–1041. doi: 10.1542/peds.2012-2139. [DOI] [PubMed] [Google Scholar]
- 98.Quinn C.T., Rogers Z.R., McCavit T.L. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alkindi S., Matwani S., Al-Maawali A. Complications of PORT-A-CATH® in patients with sickle cell disease. J Infect Public Health. 2012;5(1):57–62. doi: 10.1016/j.jiph.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 100.Chulamokha L., Scholand S.J., Riggio J.M. Bloodstream infections in hospitalized adults with sickle cell disease: a retrospective analysis. Am J Hematol. 2006;81(10):723–728. doi: 10.1002/ajh.20692. [DOI] [PubMed] [Google Scholar]
- 101.Zarrouk V., Habibi A., Zahar J.R. Bloodstream infection in adults with sickle cell disease: association with venous catheters, Staphylococcus aureus, and bone-joint infections. Medicine (Baltimore) 2006;85(1):43–48. doi: 10.1097/01.md.0000197023.46846.1c. [DOI] [PubMed] [Google Scholar]
- 102.Wagner S.C., Eschelman D.J., Gonsalves C.F. Infectious complications of implantable venous access devices in patients with sickle cell disease. J Vasc Interv Radiol. 2004;15(4):375–378. doi: 10.1097/01.rvi.0000121410.46920.6e. [DOI] [PubMed] [Google Scholar]
- 103.Green N.S. Yersinia infections in patients with homozygous β-thalassemia associated with iron overload and its treatment. Pediatr Hematol Oncol. 1992;9(3):247–254. doi: 10.3109/08880019209016592. [DOI] [PubMed] [Google Scholar]
- 104.Abcarian P.W., Demas B.E. Systemic Yersinia enterocolitica infection associated with iron overload and deferoxamine therapy. AJR Am J Roentgenol. 1991;157(4):773–775. doi: 10.2214/ajr.157.4.1892033. [DOI] [PubMed] [Google Scholar]
- 105.Mazzoleni G., deSa D., Gately J. Yersinia enterocolitica infection with ileal perforation associated with iron overload and deferoxamine therapy. Dig Dis Sci. 1991;36(8):1154–1160. doi: 10.1007/BF01297465. [DOI] [PubMed] [Google Scholar]
- 106.Gallant T., Freedman M.H., Vellend H. Yersinia sepsis in patients with iron overload treated with deferoxamine. N Engl J Med. 1986;314(25):1643. doi: 10.1056/NEJM198606193142515. [DOI] [PubMed] [Google Scholar]
- 107.Robins-Browne R.M., Prpic J.K. Desferrioxamine and systemic yersiniosis. Lancet. 1983;2(8363):1372. doi: 10.1016/s0140-6736(83)91136-4. [DOI] [PubMed] [Google Scholar]
- 108.van Eijk L.T., Heemskerk S., van der Pluijm R.W. The effect of iron loading and iron chelation on the innate immune response and subclinical organ injury during human endotoxemia: a randomized trial. Haematologica. 2014;99(3):579–587. doi: 10.3324/haematol.2013.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McAuley C.F., Webb C., Makani J. High mortality from Plasmodium falciparum malaria in children living with sickle cell anemia on the coast of Kenya. Blood. 2010;116(10):1663–1668. doi: 10.1182/blood-2010-01-265249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Makani J., Komba A.N., Cox S.E. Malaria in patients with sickle cell anemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010;115(2):215–220. doi: 10.1182/blood-2009-07-233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hassell K.L. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(4 Suppl):S512–S521. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 112.Chang J., Shi P.A., Chiang E.Y. Intravenous immunoglobulins reverse acute vaso-occlusive crises in sickle cell mice through rapid inhibition of neutrophil adhesion. Blood. 2008;111(2):915–923. doi: 10.1182/blood-2007-04-084061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turhan A., Jenab P., Bruhns P. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood. 2004;103(6):2397–2400. doi: 10.1182/blood-2003-07-2209. [DOI] [PubMed] [Google Scholar]
- 114.Field J.J., Lin G., Okam M.M. Sickle cell vaso-occlusion causes activation of iNKT cells that is decreased by the adenosine A2A receptor agonist regadenoson. Blood. 2013;121(17):3329–3334. doi: 10.1182/blood-2012-11-465963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Telen M.J., Wun T., McCavit T.L. Randomized phase 2 study of GMI-1070 in SCD: reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood. 2015;125(17):2656–2664. doi: 10.1182/blood-2014-06-583351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maitre B., Djibre M., Katsahian S. Inhaled nitric oxide for acute chest syndrome in adult sickle cell patients: a randomized controlled study. Intensive Care Med. 2015;41(12):2121–2129. doi: 10.1007/s00134-015-4060-2. [DOI] [PubMed] [Google Scholar]
- 117.Gladwin M.T., Kato G.J., Weiner D. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA. 2011;305(9):893–902. doi: 10.1001/jama.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Morris C.R., Kuypers F.A., Lavrisha L. A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica. 2013;98(9):1375–1382. doi: 10.3324/haematol.2013.086637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Machado R.F., Barst R.J., Yovetich N.A. Hospitalization for pain in patients with sickle cell disease treated with sildenafil for elevated TRV and low exercise capacity. Blood. 2011;118(4):855–864. doi: 10.1182/blood-2010-09-306167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mack A.K., McGowan V.R., II, Tremonti C.K. Sodium nitrite promotes regional blood flow in patients with sickle cell disease: a phase I/II study. Br J Haematol. 2008;142(6):971–978. doi: 10.1111/j.1365-2141.2008.07259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schaer D.J., Buehler P.W., Alayash A.I. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]