Summary
Xanthomonas spp. reduce crop yields and quality worldwide. During infection of their plant hosts, many strains secrete transcription activator‐like (TAL) effectors, which enter the host cell nucleus and activate specific corresponding host genes at effector binding elements (EBEs) in the promoter. TAL effectors may contribute to disease by activating the expression of susceptibility genes or trigger resistance associated with the hypersensitive reaction (HR) by activating an executor resistance (R) gene. The rice bacterial leaf streak pathogen X. oryzae pv. oryzicola (Xoc) is known to suppress host resistance, and no host R gene has been identified against it, despite considerable effort. To further investigate Xoc suppression of host resistance, we conducted a screen of effectors from BLS256 and identified Tal2a as an HR elicitor in rice when delivered heterologously by a strain of the closely related rice bacterial blight pathogen X. oryzae pv. oryzae (Xoo) or by the soybean pathogen X. axonopodis pv. glycines. The HR required the Tal2a activation domain, suggesting an executor R gene. Tal2a activity was differentially distributed among geographically diverse Xoc isolates, being largely conserved among Asian isolates. We identified four genes induced by Tal2a in next‐generation RNA sequencing experiments and confirmed them using quantitative real‐time reverse transcription‐polymerase chain reaction (qPCR). However, neither individual nor collective activation of these genes by designer TAL effectors resulted in HR. A tal2a knockout mutant of BLS256 showed virulence comparable with the wild‐type, but plasmid‐based overexpression of tal2a at different levels in the wild‐type reduced virulence in a directly corresponding way. Overall, the results reveal that host resistance suppression by Xoc plays a critical role in pathogenesis. Further, the dose‐dependent avirulence activity of Tal2a and the apparent lack of a single canonical target that accounts for HR point to a novel, activation domain‐dependent mode of action, which might involve, for example, a non‐coding gene or a specific pattern of activation across multiple targets.
Keywords: bacterial leaf streak of rice, resistance, TAL effector, Xanthomonas
Introduction
Xanthomonas is a large group of bacteria that collectively infect a variety of important crop species, including soybean, cassava, cotton, banana, wheat and rice, several of which are staple foods in the developing world. The rice (Oryza sativa) pathogens Xanthomonas oryzae pv. oryzicola (Xoc) and Xanthomonas oryzae pv. oryzae (Xoo) cause bacterial leaf streak and bacterial blight by colonizing the leaf mesophyll and xylem, respectively, and cause yield losses as high as 30% and 50%, respectively (Niño‐Liu et al., 2006). Several Xanthomonas species and pathovars, including Xoc and Xoo, rely on transcription activator‐like (TAL) effectors to promote disease (Al‐Saadi et al., 2007; Antony et al., 2010; Athinuwat et al., 2009; Cernadas et al., 2014; Kay et al., 2007; Sugio et al., 2007; Swarup et al., 1991; Wichmann and Bergelson, 2004; Yang and White, 2004; Yang et al., 2006, 1994; Yu et al., 2011).
Functioning as heterologous transcription factors, TAL effectors (Yang et al., 2006) are delivered via the bacterial type III secretion system into host cells (Szurek et al., 2002), where C‐terminal nuclear localization signals direct them to the nucleus (Gurlebeck et al., 2005; Szurek et al., 2001, 2002; Van den Ackerveken et al., 1996; Yang and Gabriel, 1995). There, a central domain of highly conserved, 33–35‐amino‐acid repeats, each containing hypervariable residues at positions 12 and 13 (also called the repeat‐variable diresidue or RVD), directs the recognition of specific host gene promoter sequences called effector binding elements (EBEs) (Boch et al., 2009; Moscou and Bogdanove, 2009). Each TAL effector wraps the DNA in a right‐handed superhelix, positioning the second residue of each RVD into the major groove, where it contacts an individual nucleotide in the forward strand (Deng et al., 2012; Mak et al., 2012). Collectively, these interactions define, in a predictable way, the number and identity of adjacent nucleotides that constitute the EBE. A C‐terminal acidic activation domain (AD) then activates or enhances transcription, presumably by directly engaging the host RNA polymerase complex (Gu et al., 2005; Kay et al., 2007; Marois et al., 2002; Römer et al., 2007, 2009b, 2010; Sugio et al., 2007; Szurek et al., 2001; Yang et al., 2000, 2006; Zhu et al., 1998, 1999).
TAL effectors promote disease by inducing host susceptibility (S) genes, which produce conditions favourable for the pathogen or otherwise contribute to symptom development (Antony et al., 2010; Cernadas et al., 2014; Cohn et al., 2014; Hu et al., 2014; Streubel et al., 2013; Sugio et al., 2007; Yang et al., 2006; Yu et al., 2011). The modular mechanism by which TAL effectors recognize specific DNA sequences facilitates the identification of such genes by enabling not only the prediction of EBEs in host genomes, but also the design of artificial repeat arrays to bind user‐defined DNA sequences (Boch et al., 2009; Christian et al., 2010; Morbitzer et al., 2010; Moscou and Bogdanove, 2009). Using any of several rapid assembly methods for designer TAL effectors (dTALEs) (Cermak et al., 2011; Li et al., 2011; Morbitzer et al., 2011; Weber et al., 2011; Zhang et al., 2011), it is possible to target novel sites in the promoter of a gene up‐regulated by a TAL effector to activate the gene in isolation from other targets of that effector and assess whether the gene is important for disease susceptibility. This approach has been used for bacterial leaf streak and bacterial blight in rice, canker in citrus and bacterial blight in cassava to distinguish important host S genes up‐regulated by the major virulence TAL effectors of each pathogen (Cernadas et al., 2014; Cohn et al., 2014; Hu et al., 2014; Streubel et al., 2013).
Plants resist attack by TAL effector‐wielding pathogens in some cases with executor resistance (R) genes (Bogdanove et al., 2010). Like S genes, these are directly and specifically transcriptionally activated by TAL effectors, but they encode proteins that cause the hypersensitive reaction (HR) and arrest disease progression (Gu et al., 2005; Römer et al., 2007). Because executor genes depend on TAL effector function, the requirement for a specific sequence of RVDs and a functional AD in the effector is a hallmark of this resistance mechanism. The five characterized executor genes, Bs3 and Bs4c from pepper (Capsicum annuum), and Xa27, Xa10 and Xa23 from rice, recognize the activity of AvrBs3 and AvrBs4 of Xanthomonas campestris pv. vesicatoria (Xcv) and AvrXa27, AvrXa10 and AvrXa23 of Xoo, respectively (Gu et al., 2005; Römer et al., 2007; Strauss et al., 2012; Tian et al., 2014; Wang et al., 2015). None has similarity to other plant R genes, although Bs3 is homologous to the flavin‐dependent monooxygenases (Gu et al., 2005; Römer et al., 2007; Strauss et al., 2012) and Xa10 has low similarity to bacterial Orai and Na+/Ca2+ antiporters (Tian et al., 2014). Another rice bacterial blight R gene, Xa7, depends for recognition on the specific repeat region and AD of the cognate TAL effector, AvrXa7 (Yang et al., 2000; Zhu et al., 1998, 1999), and is therefore probably an executor R gene, but has yet to be cloned.
It is noteworthy that no R gene effective against Xoc has been discovered in rice, despite substantial effort and in contrast with the more than 30 R genes that have been identified against Xoo. This could be a result of an observed ability of Xoc to suppress resistance. The Xoo effectors AvrXa7 and AvrXa10 expressed heterologously in Xoc strain BLS303 from a low‐copy plasmid failed to confer avirulence on rice plants with the corresponding R gene, despite confirmed delivery (Makino et al., 2006). However, when the Avr gene was expressed from a high‐copy plasmid, the HR was observed, indicating that resistance suppression by Xoc is quantitative (Makino et al., 2006). Consistent with this finding, the maize Rxo1 gene, a member of the nucleotide‐binding site leucine‐rich repeat class of R genes, as a transgene in rice, provided HR‐associated immunity against strains of Xoc expressing the non‐TAL effector AvrRxo1 (Zhao et al., 2004, 2005). Also, Xa27 engineered to contain EBEs for multiple Xoc TAL effectors in its promoter, again as a transgene, conferred sufficiently strong resistance to be effective (Hummel et al., 2012).
To investigate the importance of suppression of host resistance by Xoc in bacterial leaf streak, we initiated a screen (in rice cv. Nipponbare plants) of full‐length TAL effectors of strain BLS256 for resistance‐eliciting activity when delivered heterologously by strain EB08 of the soybean (Glycine max) pathogen Xanthomonas axonopodis pv. glycines (Xag) (Hummel et al., 2012), which causes no symptoms and no HR in rice, or by strain POX99A of Xoo. This screen identified Tal2a (and none of 16 other TAL effectors tested) as an elicitor of the rice HR, suggesting a role in avirulence for this TAL effector that is masked by suppression of host resistance during the natural infection process. HR required the Tal2a AD. However, assays of Tal2a avirulence activity in Xoc by knockout and overexpression and characterization of Tal2a targets using dTALEs revealed that Tal2a avirulence activity is quantitative, and suggested a novel mode of action distinct from canonical activation of a single, executor R gene.
Results
A Xoc TAL effector delivered heterologously elicits an AD‐dependent HR
Xag strain EB08 expressing tal2a of Xoc strain BLS256 (Bogdanove et al., 2011), carried on a plasmid, elicited HR within 48 h after inoculation by syringe infiltration into rice (cv. Nipponbare) leaves. Delivery of Tal2a by Xoo PXO99A elicited a similar phenotype, although the HR was visibly weaker than that elicited by Xag delivering Tal2a. A truncated Tal2a lacking the AD (Tal2aΔAD), expressed in either Xag or Xoo, did not trigger the HR (Fig. 1), suggesting that the response depends on gene activation by Tal2a.
Figure 1.

Xanthomonas axonopodis pv. glycines EB08 (Xag) and Xanthomonas oryzae pv. oryzae PXO99A (Xoo) delivering Tal2a induce activation domain‐dependent hypersensitive reaction (HR) in rice. Leaves were cleared in ethanol 72 h after syringe infiltration of bacteria transformed to express Tal2a or Tal2a missing its activation domain (Tal2aΔAD) from plasmid pKEB31 (see text). HR is visible as browning. Experiments were repeated independently a minimum of three times and showed consistent results.
Tal2a targets a ubiquitin carboxy‐terminal hydrolase gene, but activation is insufficient for HR
To better understand the apparent suppression of Tal2a‐triggered HR by Xoc, and because the activation domain dependence of the HR to Tal2a is characteristic of an executor R gene, we sought to identify the activated target(s) of Tal2a. We predicted Tal2a EBEs in the rice promoterome, which we defined as the sequences 1000 bp upstream of every annotated gene (Doyle et al., 2012; gene models from the MSU Rice Genome Annotation Project, version 6.1, http://rice.plantbiology.msu.edu), and cross‐referenced them with genes significantly induced by Xoc BLS256 in a previous microarray‐based experiment (Cernadas et al., 2014). Two of the Xoc‐induced genes contained a predicted EBE for Tal2a (Table S1, see Supporting Information), a putative ubiquitin carboxy‐terminal hydrolase gene (UCH; Os02g43760; q = 0.005) and a putative phosphatidylinositol‐4‐phosphate 5‐kinase gene (PPK; Os06g14750; q = 0.196).
To determine whether UCH and PPK are true targets of Tal2a, we analysed their expression patterns by quantitative real‐time reverse transcription‐polymerase chain reaction (qPCR) during infection of rice leaves with PXO99A delivering Tal2a or Tal2aΔAD. Relative to mock‐inoculated tissue, PPK transcript was induced significantly in tissue inoculated with PXO99A delivering Tal2a, but induction in tissue inoculated with PXO99A delivering Tal2aΔAD was similar (Fig. S1, see Supporting Information), suggesting that activation is not dependent on Tal2a activity and may be a non‐specific response to X. oryzae infection. In contrast, UCH mRNA was significantly more abundant in leaves infiltrated with PXO99A expressing Tal2a compared with Tal2aΔAD (Fig. 2a), suggesting that Tal2a activity is necessary for its induction. To confirm Tal2a as the sole activator of UCH in BLS256, we generated a BLS256 knockout mutant of tal2a (strain M169) and found that it did not activate the UCH gene. Re‐introduction of tal2a on a plasmid restored UCH activation (Fig. 2b), confirming that Tal2a is responsible for UCH induction by Xoc BLS256.
Figure 2.
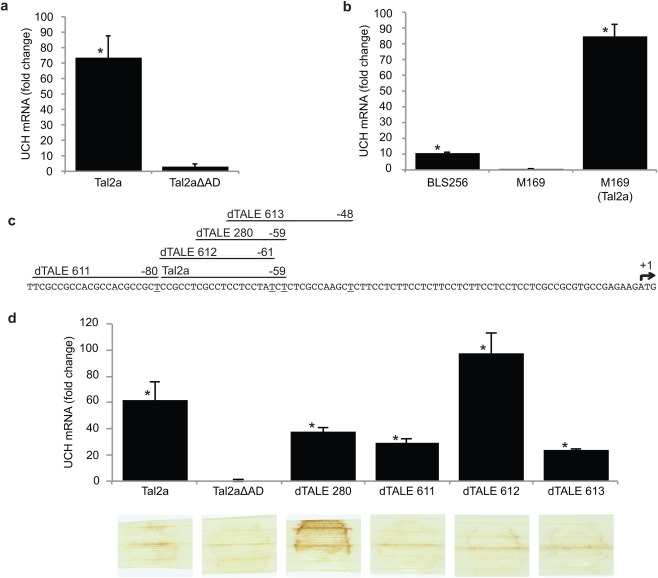
A ubiquitin carboxy‐terminal hydrolase (UCH) gene is activated by Tal2a, but is insufficient for the hypersensitive reaction (HR). (a) Quantitative real‐time reverse transcription‐polymerase chain reaction (qPCR) assay of expression of the UCH gene in response to Tal2a or Tal2aΔAD delivered by Xanthomonas oryzae pv. oryzae PXO99A (Xoo), relative to mock‐inoculated tissue. Tal2a was expressed from plasmid pKEB31. Tissue was sampled at 48 h after syringe infiltration of bacterial suspensions. Each value shown is the average of two technical replicates of three biological replicates. Capped vertical lines show the standard deviation. The relative fold change was calculated by the 2–ΔΔCt method. Asterisks indicate values significantly different (P ≤ 0.05) from mock as determined by two‐tailed, heteroscedastic t‐tests. This experiment was repeated three times with similar results. (b) qPCR assay of UCH expression as in (a) in response to wild‐type X. oryzae pv. oryzicola BLS256, tal2a mutant M169 and M169 carrying the tal2a gene in plasmid pKEB31. Independent experiments were repeated twice with similar results. (c) The 101 nucleotides of the UCH gene upstream of the translational start site showing the predicted effector‐binding element (EBE) for Tal2a and EBEs targeted by each of four designer transcription activator‐like effectors (dTALEs) designed to activate transcription of the gene. Numbers indicate the position of the last (3′) nucleotide of each EBE (underlined) relative to the translational start (+1, arrow). (d) qPCR results as in (a) showing the activation of the UCH gene by each of the four dTALEs delivered by Xoo and, below, the response of rice leaves (as in Fig. 1) to each. Each dTALE activated the UCH gene, but, of these, only dTALE 280, the EBE of which is contained entirely within the Tal2a EBE, triggered an HR, and this HR was stronger than that induced by Tal2a. Experiments were repeated at least twice with similar results.
To assess whether UCH activation is responsible for the HR elicited by Tal2a, we designed (Doyle et al., 2012) and constructed (Cermak et al., 2011) four dTALEs – 280, 611, 612 and 613 – targeted to the UCH promoter, overlapping or proximal to the predicted Tal2a EBE (Fig. 2c and Table S2, see Supporting Information). Although qPCR indicated that all four dTALEs specifically activated UCH compared with mock‐inoculated tissue (Fig. 2d), only dTALE 280, which was targeted to the last 15 nucleotides of the Tal2a EBE, triggered the HR (Fig. 2d). This HR was visibly stronger than that elicited by Tal2a (Fig. 1 and Fig. 2d). Because dTALEs 611, 612 and 613 each activated UCH to a similar extent as dTALE 280 without triggering the HR, UCH activation alone is not sufficient to cause HR. Instead, a different gene or set of genes activated by Tal2a and dTALE 280, but not by the other dTALEs, probably contributes to or causes the phenotype.
Tal2a activity is differentially distributed across geographically diverse isolates and these reveal three more specific rice targets
To determine whether another gene, potentially missed in the predictions based on the microarray data, might underlie the HR elicited by Tal2a, we examined RNAseq‐based global gene expression profiles in rice leaves inoculated individually with 11 different field isolates (Wilkins et al., 2015 and Gene Expression Omnibus accession GSE67588). Taking UCH activation as a marker for the presence of Tal2a, we classified the 11 field isolates into ‘Tal2a+’ and ‘Tal2a–’ groups. This revealed broad distribution among Asian isolates, with the Tal2a+ group including all of three Chinese isolates, an isolate from Malaysia and two of three isolates from the Philippines. The Tal2a– group included the remaining Philippines strain, an Indian strain and all of three African strains.
In addition to UCH, five other genes were activated by the six Tal2a+ strains that were not activated by strains in the Tal2a– group (Table S3, see Supporting Information). To assess Tal2a dependence of activation for these genes, we generated and cross‐referenced RNAseq data for rice leaves treated with PXO99A expressing Tal2a or dTALE 280. Compared with leaves treated with PXO99A expressing Tal11b, another BLS256 effector, 347 annotated genes were significantly up‐regulated at least 2.0‐fold (q < 0.05) in response to both Tal2a and dTALE 280 (Gene Expression Omnibus accession GSE67958). Two of the five genes that were up‐regulated in addition to UCH by the Tal2a+ field isolates of Xoc were absent from this list, leaving a total of four genes (including UCH) uniquely activated by the Xoc strains and by Xoo carrying Tal2a (or dTALE 280). The four genes actually comprise two pairs of adjacent genes, UCH and Os02g43770, oriented towards each other, and Os10g40130 and Os10g40120, oriented in the same direction (5′ to 3′; Fig. 3a). Only one member of each pair, UCH and Os10g40130, contains a predicted EBE for Tal2a and dTALE 280 (Doyle et al., 2012), raising the intriguing possibility that the others are activated by an enhancer function originating from TAL effector activity at that EBE.
Figure 3.
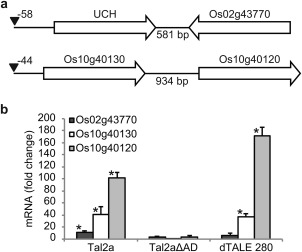
Three additional genes activated in common by strains that activate the ubiquitin carboxy‐terminal hydrolase (UCH) gene are activated by Tal2a. (a) Schematic diagram of the four genes activated in common by Xanthomonas oryzae strains that activate the UCH gene (Table S2). Genes are represented as block arrows oriented 5′ to 3′ and are not to scale. Introns are not depicted. Candidate effector‐binding elements (EBEs) for Tal2a are labelled with black triangles with the position indicated as in Fig. 2. (b) Expression of the genes in response to Tal2a or Tal2a missing its activation domain (Tal2aΔAD) assayed by quantitative real‐time reverse transcription‐polymerase chain reaction (qPCR), as described for Fig. 2. Experiments were repeated twice with similar results.
To confirm whether Os02g43770 (the Tal2a‐activated gene lacking a predicted EBE but opposite UCH) was expressed from the sense strand, and to account for any possible non‐annotated targets of Tal2a that may have been missed during transcript assembly, we analysed the RNAseq data again using Cufflinks with novel transcript discovery (Trapnell et al., 2010), Nearly all reads mapping to Os02g43770 indeed corresponded to the sense strand, and we found no additional sequences up‐regulated by PXO99A expressing Tal2a and by all Tal2a+ Xoc strains.
To confirm the Tal2a dependence of the activation of Os02g43770, Os10g40120 and Os10g40130, we carried out qPCR and found that all three were specifically activated in rice leaves inoculated with BLS256 relative to M169 (Fig. S2, see Supporting Information), and also by the PXO99A strain expressing Tal2a compared with mock‐inoculated leaves (Fig. 3b).
The four identified Tal2a targets activated individually or collectively are insufficient for HR
To determine whether any of the three additional Tal2a‐responsive genes is responsible for the HR elicited by Tal2a, we individually induced each with dTALEs (612 for UCH, 1066 for Os02g43770, 990 for Os10g40120 and 1170 for Os10g40130; Table S2) delivered by Xoo PX099A. Although qPCR indicated that each dTALE uniquely activated its intended target in comparison with mock‐inoculated tissue, none was sufficient to elicit the HR (Fig. 4).
Figure 4.
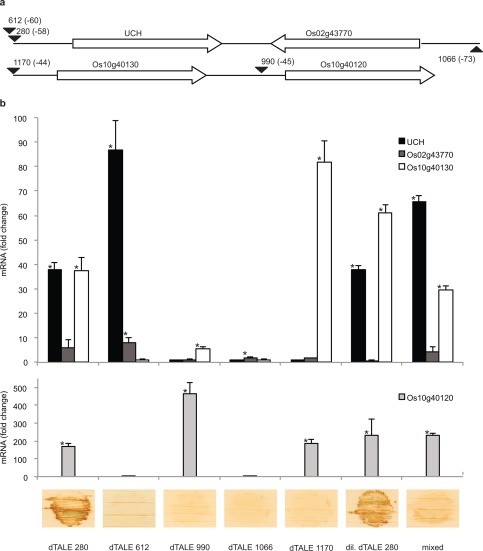
The four identified Tal2a‐activated genes activated in subsets or collectively are insufficient for HR. (a) Schematic of the Tal2a‐activated genes, as in Fig. 3, showing the locations (parentheses) of effector‐binding elements (EBEs) targeted by designer transcription activator‐like effectors (dTALEs) 280, 612, 990, 1066 and 1170. dTALE 1170, which binds an EBE with the same 3′ end as the Tal2a EBE but a longer 5′ end, is not predicted to bind at the Tal2a EBE upstream of the ubiquitin carboxy‐terminal hydrolase (UCH) gene. (b) Quantitative real‐time reverse transcription‐polymerase chain reaction (qPCR) results, as in Fig. 2, showing the expression of the genes in response to each of the dTALEs delivered by Xanthomonas oryzae pv. oryzae PXO99A (Xoo), and (below) the response of rice leaves (as in Fig. 1). All experiments were repeated at least twice with similar results; ‘mixed’ indicates co‐inoculation with the strains expressing dTALEs 612, 990, 1066 and 1170; ‘dil. dTALE 280’ indicates inoculation with the dTALE 280 strain diluted to the same titer as each of the strains included in the ‘mixed’ suspension.
To assess whether any of the genes act in concert to elicit the HR, we inoculated a mixture of the four strains each delivering one of the dTALEs. Despite the activation of all four genes, this also failed to result in the HR. As a control, the strain expressing dTALE 280 diluted to the same titre as an individual strain in the mixture elicited a strong HR (Fig. 4b).
Tal2a exhibits dose‐dependent avirulence activity
As our target characterization did not reveal an executor R gene, we chose to further characterize the Tal2a‐triggered resistance mechanism in rice and the apparent ability of Xoc to overcome it by examining the virulence of the pathogen expressing different levels of Tal2a. We compared wild‐type with M169 and with M169 expressing Tal2a or dTALE 280 from a low‐ or a high‐copy plasmid. We measured virulence using a previously described lesion length assay (Wang et al., 2007), and assessed the activity of Tal2a or dTALE 280 by measuring the activation of UCH using qPCR (Fig. 5). The average lesion length for M169 with no plasmid was slightly longer than that for the wild‐type strain, but this was significant in only one experiment (P = 0.031), suggesting, at most, a nominal negative effect of Tal2a on BLS256 virulence when expressed from the chromosome. Tal2a or dTAL280 in M169 expressed from the low‐copy plasmid resulted in a 1.79‐ or 2.28‐fold higher UCH induction, respectively, than did Tal2a expressed from the chromosome in the wild‐type (calculated from two independent qPCR experiments). When the TAL effectors were expressed from the high‐copy plasmid, UCH fold inductions were higher still (6.07‐ and 7.75‐fold higher than the wild‐type). Relative to the wild‐type, M169 expressing Tal2a from the low‐copy plasmid showed no significant difference in virulence. However, M169 with Tal2a expressed from the high‐copy plasmid showed a 30% reduction. M169 expressing dTALE 280 from the low‐copy vector showed roughly a 10% reduction in virulence relative to the wild‐type in two of four independent experiments conducted (one of these two is shown in Fig. 5; all four are shown in Fig. S3, see Supporting Information). M169 expressing dTALE 280 from the high‐copy plasmid showed a 90% reduction in lesion length relative to the wild‐type. The greater reductions in virulence resulting from dTALE 280 relative to Tal2a are consistent with the visibly stronger HR elicited by this effector compared with Tal2a when either is expressed from Xag or Xoo. Finally, the virulence of M169 expressing Tal2aΔAD from either vector was the same as the wild‐type (and UCH was not induced). The data overall show a quantitative, inverse correlation between Tal2a activity and virulence.
Figure 5.
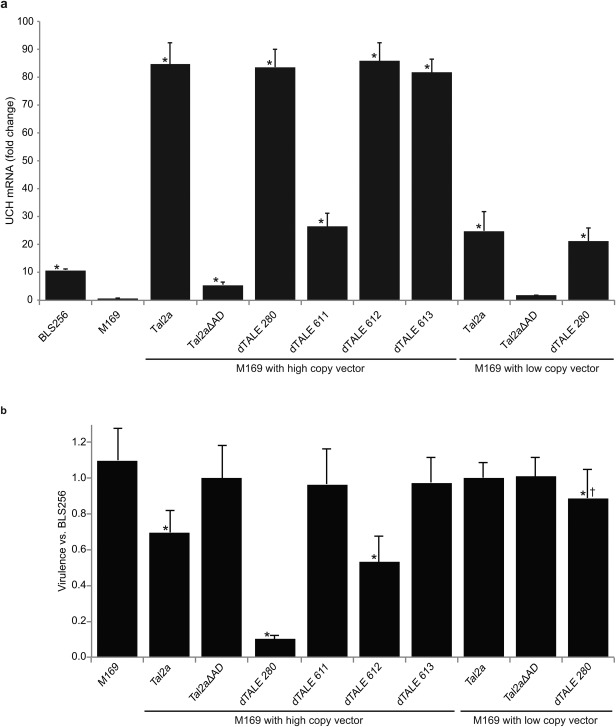
Tal2a inhibits the virulence of Xanthomonas oryzae pv. oryzicola (Xoc) in a dose‐dependent manner. (a) Quantitative real‐time reverse transcription‐polymerase chain reaction (qPCR) results, as in Fig. 2, showing the expression of the ubiquitin carboxy‐terminal hydrolase (UCH) gene in response to wild‐type Xoc strain BLS256, the Tal2a mutant derivative M169 and M169 transformed to express Tal2a, Tal2a missing its activation domain (Tal2aΔAD) or one of several designer transcription activator‐like effectors (dTALEs), with the TAL effector genes borne on a high‐copy (pKEB31) or low‐copy (pHM1) vector. Experiments were repeated twice with similar results. (b) Virulence of the BLS256‐derived strains in (a) relative to BLS256. Vertical bars represent ratios of lesion lengths resulting from side‐by‐side inoculation of the indicated strain and BLS256 at 10 days post‐inoculation (Cernadas et al., 2014). Each is the average of at least eight biological replications. Experiments were repeated at least twice with similar results. Capped vertical lines show the standard deviation. Asterisks indicate values significantly different (P ≤ 0.05) from 1.0 (no change). M169 expressing dTALE 280 from the low‐copy vector † resulted in an average lesion length ratio of <1.0 in each of the four independent experiments in which it was tested (Fig. S3), but this was statistically significant in only two of these experiments.
As an additional test for a role of UCH in the avirulence activity of Tal2a, although the gene was insufficient for HR, we included virulence assays for M169 expressing dTALEs 611, 612 or 613 from the high‐copy vector. No effect on virulence could be detected for dTALEs 611 or 613, despite robust activation of UCH by both effectors, providing further evidence that the UCH gene is not the relevant or sole relevant target of Tal2a. Unexpectedly, dTALE 612 caused a 47% reduction in the virulence of M169 when expressed from the high‐copy vector, although we did not observe a visible HR when it was delivered by PXO99A. dTALE 612 shares 13 of the 19 nucleotides in its UCH EBE with the UCH EBE for dTALE 280, and all of its EBE with the UCH EBE for Tal2a. Thus, it is possible that dTALE 612 triggers resistance at a level below the threshold required for the visible HR, but sufficiently high to impair virulence. Alternatively, or in addition, a distinct target of dTALE 612 might contribute to this avirulence effect.
Finally, we examined the influence of Tal2a and dTALE 280 on the virulence of PXO99A. Consistent with the results in M169, expression of Tal2a or dTALE 280 in PXO99A from the high‐copy vector resulted in reduced lesion lengths following leaf clip inoculation (Kauffman et al., 1973), relative to strains expressing Tal2aΔAD or dTALEs 611 or 613; dTALE 612, in contrast with its effect in M169, had no measurable effect on PXO99A virulence (Fig. S4, see Supporting Information).
Discussion
The lack of characterized major gene resistance to Xoc in rice is an increasingly serious problem as bacterial leaf streak becomes more widespread. A marked ability of Xoc to suppress host defence (Makino et al., 2006) may account for the failure of breeders and pathologists so far to identify an effective native R gene. Our discovery of the avirulence function of Tal2a and the apparent widespread distribution of this effector reveals that the suppression of effector‐triggered host resistance by Xoc plays a critical role during infection.
Our finding that elicitation of the HR by (heterologously expressed) Tal2a requires the AD suggests an executor R gene. However, dTALE‐driven activation of the four targets of Tal2a identified, individually or together, does not recapitulate the HR. A tal2a knockout mutant of BLS256 shows virulence comparable with the wild‐type, but plasmid‐based overexpression of tal2a at different levels in the wild‐type reduces virulence in a directly corresponding way. The apparent lack of a canonical target that can account for the HR to Tal2a and the AD and dose dependence of Tal2a avirulence activity point to a novel mechanism of defence elicitation distinct from the activation of a single, executor R gene.
Because TAL effectors typically act by binding to and activating target promoters, molecular cloning of an executor R gene that corresponds to an avirulence TAL effector can reasonably be approached by gene expression analysis and EBE prediction (Boch et al., 2014). For example, RNAseq of host genes in the presence and absence of the TAL effector AvrBs4 was used to identify the Bs4c executor R gene in pepper (Strauss et al., 2012). Our inability to pinpoint the source of Tal2a‐triggered resistance using this approach reveals its limitations. These limitations may stem from an oversimplified view of the effects of TAL effectors on the host transcriptome. In addition to canonical, activated genes, some targets might be directly down‐regulated by a TAL effector that binds in such a way as to block transcript elongation or otherwise prevent expression. TAL effectors may induce the expression of small RNAs that would not be captured in a polyA‐enriched cDNA library. The activation of long non‐coding RNAs or unannotated genes might also occur and would not be detected using annotation‐based methods of mapping RNAseq reads. To account for the possibility of novel transcripts, we performed a second analysis on the aligned RNAseq reads using Cufflinks (Trapnell et al., 2010), but found no sequences up‐regulated in common by all Tal2a+ strains.
Limitations might also stem from the specific parameters applied in a search, or the experimental design. For example, we applied a cutoff of a two‐fold minimum induction compared with controls. This leaves the formal possibility that a very low level of induction of the putative target, driven by weak binding to a highly imperfect EBE, is responsible for the HR, which would have caused us to overlook the source of resistance. Although unlikely, the possibility also exists that one or more required, but insufficient, mediators of the HR are activated by PXO99A and Xag EB08 independently of Tal2a. This scenario would have resulted in exclusion from consideration as a Tal2a target in our analysis. In future studies, an RNAseq‐based comparison of wild‐type BLS256 with M169, a mutant that we succeeded in isolating only after the RNAseq experiments presented here were completed, might better reveal all relevant targets of Tal2a. The dataset would be strengthened if rice varieties differentially responsive to Tal2a could be identified and included in the gene expression analysis. Nonetheless, partial redundancy of BLS256 TAL effectors for the activation of some Tal2a targets could yet confound the results.
Complexity in the gene expression pattern required for resistance might have played a role in our inability to pinpoint the genetic source of the HR to Tal2a. Specifically, the phenotype might be the result not only of the activation of two or more genes by Tal2a (or dTALE 280), but of a certain proportional pattern of activation across the targets that we failed to recapitulate with our mixed dTALEs. Or, elicitation of the HR by Tal2a and dTALE 280 might depend on an alternative transcript for one or more targets that results from TAL effector‐dependent repositioning of the transcriptional start site, which can occur as a function of the site at which a TAL effector binds (Antony et al., 2010; Hummel et al., 2012; Kay et al., 2007, 2009; Römer et al., 2009a, 2009b). This mechanism would depend on the exact position of the activating TAL effector on the DNA and would be difficult to replicate by targeting dTALEs to different sequences in the promoter. It may be significant that the only two TAL effectors sufficient to elicit the HR in this study, Tal2a and dTALE 280, share EBEs that terminate at the same nucleotide in the UCH promoter.
Should any of these scenarios turn out to be true for Tal2a recognition, it would expand the current model of TAL effector‐mediated avirulence, i.e. activation of a single, protein‐coding, executor R gene by binding to the promoter of that R gene. Already, an example of target activation that may depart from the general model for TAL effectors was provided in this study by Os02g43770, which is downstream of UCH and inversely oriented (Fig. 3), and is activated by Tal2a despite having no predicted EBE in its promoter. The UCH promoter bound by Tal2a may act as an enhancer to drive the expression of Os02g43770. Another possible example is the Tal2a‐activated gene Os10g40120, which lacks a predicted Tal2a EBE and may be driven similarly by an enhancer effect of Tal2a binding at the neighbouring upstream gene Os10g40130.
We have reported recently complete genome sequences for all but one of the Xoc strains used in this study. These genome sequences confirmed our classification of the strains as Tal2a+ or Tal2a– (Wilkins et al., 2015). Given its avirulence function and apparent dispensability for virulence, why is Tal2a so broadly distributed among Asian isolates? Perhaps, in some rice genotypes, activation of one or more of the Tal2a targets identified here or other targets specific to these genotypes contributes to virulence. Although we did not assay for a virulence contribution of Tal2a in cultivars other than Nipponbare, we did observe that Tal2a delivered by Xag elicits a strong HR not only in Nipponbare, but also in japonica cv. Kitaake and indica cv. IR24 (data not shown). The cultivars indica and japonica represent two of the three major lineages of rice, suggesting broad conservation of Tal2a recognition among cultivated rice varieties. Therefore, persistence of Tal2a almost certainly depends on the ability of Tal2a‐expressing strains to suppress host resistance. Perhaps, without a detectable fitness cost for the level of expression of Tal2a that occurs with the endogenous, chromosomal gene, there is insufficient selective pressure to eliminate the gene, even without it conferring any selective advantage.
This study provides the first example in Tal2a of an Xoc TAL effector that functions as an avirulence factor. The quantitative nature of Tal2a activity sets it apart from the avirulence factors characterized to date. Although activity varies across avirulence factors, we are unaware of any with a dose‐dependent effect such as observed for Tal2a. One possible exception is AvrBs3, which triggers resistance mediated by the nucleotide‐binding, leucine‐rich repeat tomato R protein Bs4 only when the bacterial protein is expressed highly in plant cells via Agrobacterium‐mediated t‐DNA delivery (Schornack et al., 2005). In that case, presumably direct interaction of the effector and the R protein triggers the response. The fact that overexpression of AvrBs3 is necessary suggests that the interaction is relatively low affinity. The same may be true of Tal2a and its resistance‐mediating DNA target(s). This supposition is strengthened by our observation that dTALE 280, which, as described earlier, was designed to optimally target a sequence within the Tal2a EBE in the UCH promoter, exhibits avirulence activity that is overall stronger than that of Tal2a but similarly dose dependent. Future identification of the genetic source of the resistance response to Tal2a might afford the opportunity, through genome editing, to increase the affinity of the target(s) for Tal2a and increase the plant response beyond the capacity of Xoc to suppress it, providing a new resource for the management of bacterial leaf streak. As the rice response triggered by Tal2a expressed from a high‐copy vector in Xoo reduced bacterial blight symptoms as well, the targets might additionally be edited to trap a conserved Xoo TAL effector for control of that disease, or a set of TAL effectors for broad‐spectrum control of both diseases, in a manner similar to the recent modifications of Xa27 and Xa10 (Hummel et al., 2012; Zeng et al., 2015). Identification of the genetic basis for the Tal2a‐triggered HR will also be important to understand the quantitative nature of Tal2a function itself, and may help to further elucidate the reason for the lack of observed major gene resistance to bacterial leaf streak in rice.
Experimental Procedures
Plant growth, plant inoculations, virulence assays and qPCR
Rice (Oryza sativa L. cv. Nipponbare) was grown and inoculated as described by Hummel et al. (2012), except that, for Xoc virulence assays, virulence of M169 strains was expressed as the lesion length relative to that caused by wild‐type BLS256 inoculated on the opposite side of the leaf midrib in each case. Xoo PXO99A virulence assays were conducted as described by Hummel et al. (2012), except that virulence was expressed as the ratio of the lesion length to the total leaf length. qPCR was performed as described previously (Hummel et al., 2012; Livak and Schmittgen, 2001; Schmittgen and Livak, 2008), using gene‐specific primers (Table S4, see Supporting Information), except total reaction volumes were reduced to 25 µL, total RNA template was reduced to 50 ng and a minimum of two qPCR technical replicates was performed for each independent biological sample.
TAL effector clones, bacterial transformation and tal2a mutagenesis
The tal2a gene was cloned from Xoc BLS256 as described by Hummel et al. (2012). dTALEs were assembled, and then transferred via the Gateway LR II Clonase enzyme kit (Life Technologies, Grand Island, NY, USA) into the broad‐host‐range and moderately high‐copy plasmid pKEB31, as described by Cermak et al. (2011). For low copy, the pKEB31‐derived plasmids were digested with HindIII and the fragment containing the lac promoter and TAL effector gene was ligated into the HindIII site of pHM1 (Hopkins et al., 1992). The growth and transformation of Xanthomonas were performed as described by Hummel et al. (2012). To generate the tal2a knockout mutant, the suicide plasmid pAH412 was manufactured by replacing the first 19.5 repeats of the central repeat region of the tal2a clone with the KanR gene of the EZ‐Tn5 Transposon (Epicentre, Madison, WI, USA). This clone is in pBlueScript II (Agilent Technologies, Santa Clara, CA, USA), and flanking the modified central repeat region are 5′ and 3′ sequences from the Xoc TAL effector genes tal2a and tal1c (Hummel et al., 2012). M169 was generated by transformation of Xoc BLS256 by electroporation with pAH412, followed by selection on plates amended with kanamycin. In M169, the N‐terminal region of the knockout cassette was mapped to the tal2a genomic locus by PCR and sequencing. Knockout of tal2a was confirmed by qPCR showing loss of UCH induction by M169.
Next‐generation RNA sequencing
For Xoo strains expressing Tal2a and dTALE 280, rice leaves were inoculated and total RNA was isolated for qPCR as described by Hummel et al. (2012). Activation of UCH by Xoo PXO99A expressing Tal2a and dTALE 280, and not by Xoo PXO99A expressing Tal11b, was confirmed by qPCR. For each of the Xoc field isolates, plus a mock inoculum, eight leaves from four 15‐day‐old rice plants were syringe infiltrated and pooled, and total RNA was extracted for qPCR as described previously (Hummel et al., 2012; Wilkins et al., 2015). Independent experiments were repeated three times. The Iowa State University DNA Facility prepared mRNA libraries using the Illumina (San Diego, CA, USA) TruSeq RNA Sample Preparation Kit v2, according to the manufacturer's protocol. For the PXO99A experiment, two independent biological replicates of each treatment were indexed and multiplexed into a single flow cell lane; for the Xoc field isolate experiment, three independent biological replicates of each treatment were indexed and filled the remaining lanes of the flow cell.
Sequencing was performed on a HiSeq 2000 (Illumina) by the Iowa State University DNA Facility, yielding 178.8 million and 912.4 million high‐quality reads for the Xoo and Xoc experiments, respectively; 177.2 million and 901.4 million reads, respectively, passed all filtering steps. Before the reads were aligned, adapter sequences were removed using the Trimmomatic (Lohse et al., 2012) IlluminaClip trimming step with the following settings: seedMismatches = 2, palindromeClipThreshold = 40 and simpleClipThreshold = 15. Low‐quality read ends (bases with a phred quality score of less than 20) were then removed using BRAT (Harris et al., 2010) with default settings, because independent evaluation suggests that this step improves alignment quality (Yu et al., 2012). Alignment and splice site identification were completed using TopHat (Kim et al., 2013; Trapnell et al., 2009) with a maximum intron length of 10 000 and default settings otherwise. Reads were aligned to the Os‐Nipponbare‐Reference‐IRGSP‐1.0 rice genome using the MSU Rice Genome Annotation Project version 7.0 as a reference annotation (Kawahara et al., 2013). The aligned reads were assembled into a minimal set of transcripts using Cufflinks (Trapnell et al., 2010) with the option ‘–multi‐read‐correct’, the same reference annotation used for alignment and default settings otherwise.
On a first run, the reference annotation was passed to Cufflinks using the ‘–G’ flag, which requires that Cufflinks ignore reads that are incompatible with existing transcripts. When none of the previously annotated genes was confirmed as the Tal2a target, this step was repeated using the ‘–g’ flag instead, allowing Cufflinks to assemble novel transcripts to explain the observed reads. In order to extract non‐normalized read counts for each of the resulting transcripts, the HTSeq function htseq‐count (http://www-huber.embl.de/users/anders/HTSeq/doc/overview.html) was used with default settings for non‐stranded reads. Differentially expressed genes were then identified usinq Quasiseq (Lund et al., 2012). Genes were considered to be differentially expressed if they had a q value of <0.05 and at least a two‐fold change in expression. The analysis described above was performed separately for the RNAseq reads obtained from rice inoculated with Xoo PXO99A and the reads obtained from rice inoculated with the Xoc isolates.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Similar activation of a phosphatidylinositol‐4‐phosphate 5‐kinase gene (PPK) predicted to be a target of Tal2a by Xanthomonas oryzae pv. oryzae (Xoo) expressing Tal2a or Tal2a lacking its activation domain.
Fig. S2 Tal2a‐dependent activation of three additional rice genes by Xanthomonas oryzae pv. oryzicola (Xoc).
Fig. S3 Virulence of tal2a mutant M169 expressing dTALE 280 from the low‐copy vector pHM1.
Fig. S4 Inhibition of Xanthomonas oryzae pv. oryzae (Xoo) virulence by Tal2a.
Table S1 Targets of Tal2a predicted using microarray data of Cernadas et al. (2014).
Table S2 Repeat‐variable diresidue (RVD) and effector‐binding element (EBE) sequences for Tal2a and designer transcription activator‐like effectors (dTALEs) that activate Tal2a‐responsive genes.
Table S3 Genes up‐regulated by all Tal2a+ field isolates of Xanthomonas oryzae pv. oryzicola (Xoc).
Table S4 Gene‐specific primers used for quantitative real‐time reverse transcription‐polymerase chain reaction (qPCR).
Acknowledgements
The authors thank C. Schmidt for cloning tal2a and E. Doyle and W. Wierson for other technical assistance. This work was supported by a grant from the US National Science Foundation, http://www.nsf.gov/ (Plant Genome Research Program Award IOS 1238189).
References
- Al‐Saadi, A. , Reddy, J.D. , Duan, Y.P. , Brunings, A.M. , Yuan, Q. and Gabriel, D.W. (2007) All five host‐range variants of Xanthomonas citri carry one pthA homolog with 17.5 repeats that determines pathogenicity on Citrus, but none determine host‐range variation. Mol. Plant–Microbe Interact. 20, 934–943. [DOI] [PubMed] [Google Scholar]
- Antony, G. , Zhou, J. , Huang, S. , Li, T. , Liu, B. , White, F. and Yang, B. (2010) Rice xa13 recessive resistance to bacterial blight is defeated by induction of the disease susceptibility gene Os‐11N3 . Plant Cell, 22, 3864–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athinuwat, D. , Prathuangwong, S. , Cursino, L. and Burr, T. (2009) Xanthomonas axonopodis pv. glycines soybean cultivar virulence specificity is determined by avrBs3 homolog avrXg1 . Phytopathology, 99, 996–1004. [DOI] [PubMed] [Google Scholar]
- Boch, J. , Scholze, H. , Schornack, S. , Landgraf, A. , Hahn, S. , Kay, S. , Lahaye, T. , Nickstadt, A. and Bonas, U. (2009) Breaking the code of DNA‐binding specificity of TAL‐type III effectors. Science, 326, 1509–1512. [DOI] [PubMed] [Google Scholar]
- Boch, J. , Bonas, U. and Lahaye, T. (2014) TAL effectors—pathogen strategies and plant resistance engineering. New Phytol. 204, 823–832. [DOI] [PubMed] [Google Scholar]
- Bogdanove, A.J. , Schornack, S. and Lahaye T. (2010) TAL effectors: finding plant genes for disease and defense. Curr. Opin. Plant Biol. 13, 394–401. [DOI] [PubMed] [Google Scholar]
- Bogdanove, A.J. , Koebnik, R. , Lu, H. , Furutani, A. , Angiuoli, S.V. , Patil, P.B. , Van Sluys, M.A. , Ryan, R.P. , Meyer, D.F. , Han, S.W. , Aparna, G. , Rajaram, M. , Delcher, A,L. , Phillippy, A.M. , Puiu, D. , Schatz, M.C. , Shumway, M. , Sommer, D.D. , Trapnell, C. , Benahmed, F. , Dimitrov, G. , Madupu, R. , Radune, D. , Sullivan, S. , Jha, G. , Ishihara, H. , Lee, S.W. , Pandey, A. , Sharma, V. , Sriariyanun, M. , Szurek, B. , Vera‐Cruz, C.M. , Dorman, K.S. , Ronald, P.C. , Verdier, V. , Dow, J.M. , Sonti, R.V. , Tsuge, S. , Brendel, V.P. , Rabinowicz, P.D. , Leach, J.E. , White, F.F. and Salzberg, S.L. (2011) Two new complete genome sequences offer insight into host and tissue specificity of plant pathogenic Xanthomonas spp. J. Bacteriol. 193, 5450–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak, T. , Doyle, E.L. , Christian, M. , Wang, L. , Zhang, Y. , Schmidt, C. , Baller, J.A. , Somia, N.V. , Bogdanove, A.J. and Voytas, D.F. (2011) Efficient design and assembly of custom TALEN and other TAL effector‐based constructs for DNA targeting. Nucleic Acids Res. 39, 7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernadas, R.A. , Doyle, E.L. , Nino‐Liu, D.O. , Wilkins, K.E. , Bancroft, T. , Wang, L. , Schmidt, C.L. , Caldo, R. , Yang, B. , White, F.F. , Nettleton, D. , Wise, R.P. and Bogdanove, A.J. (2014) Code‐assisted discovery of TAL effector targets in bacterial leaf streak of rice reveals contrast with bacterial blight and a novel susceptibility gene. PLoS Pathog. 10, e1003972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian, M. , Cermak, T. , Doyle, E.L. , Schmidt, C. , Zhang, F. , Hummel, A. , Bogdanove, A.J. and Voytas, D.F. (2010) Targeting DNA double‐strand breaks with TAL effector nucleases. Genetics, 186, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn, M. , Bart, R.S. , Shybut, M. , Dahlbeck, D. , Gomez, M. , Morbitzer, R. , Hou, B.H. , Frommer, W.B. , Lahaye, T. and Staskawicz, B. (2014) Xanthomonas axonopodis virulence is promoted by a transcription activator‐like effector‐mediated induction of a SWEET sugar transporter in cassava. Mol. Plant–Microbe Interact. 27, 1186–1198. [DOI] [PubMed] [Google Scholar]
- Deng, D. , Yan, C. , Pan, X. , Mahfouz, M. , Wang, J. , Zhu, J.K. , Shi, Y. and Yan, N. (2012) Structural basis for sequence‐specific recognition of DNA by TAL effectors. Science, 335, 720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, E.L. , Booher, N.J. , Standage, D.S. , Voytas, D.F. , Brendel, V.P. , Vandyk, J.K. and Bogdanove, A.J. (2012) TAL Effector‐Nucleotide Targeter (TALE‐NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 40, W117–W122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, K.Y. , Yang, B. , Tian, D.S. , Wu, L.F. , Wang, D.J. , Sreekala, C. , Yang, F. , Chu, Z. , Wang, G.L. , White, F.F. and Yin, Z. (2005) R gene expression induced by a type‐III effector triggers disease resistance in rice. Nature, 435, 1122–1125. [DOI] [PubMed] [Google Scholar]
- Gurlebeck, D. , Szurek, B. and Bonas, U. (2005) Dimerization of the bacterial effector protein AvrBs3 in the plant cell cytoplasm prior to nuclear import. Plant J. 42, 175–187. [DOI] [PubMed] [Google Scholar]
- Harris, E.Y. , Ponts, N. , Levchuk, A. , Roch, K.L. and Lonardi, S. (2010) BRAT: bisulfite‐treated reads analysis tool. Bioinformatics, 26, 572–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, C.M. , White, F.F. , Choi, S.H. , Guo, A. and Leach, J.E. (1992) Identification of a family of avirulence genes from Xanthomonas oryzae pv. oryzae. Mol. Plant–Microbe Interact. 5, 451–459. [DOI] [PubMed] [Google Scholar]
- Hu, Y. , Zhang, J. , Jia, H. , Sosso, D. , Li, T. , Frommer, W.B. , Yang, B. , White, F.F. , Wang, N. and Jones, J.B. (2014) Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proc. Natl. Acad. Sci. USA, 111, E521–E529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel, A.W. , Doyle, E.L. and Bogdanove, A.J. (2012) Addition of transcription activator‐like effector binding sites to a pathogen strain‐specific rice bacterial blight resistance gene makes it effective against additional strains and against bacterial leaf streak. New Phytol. 195, 883–893. [DOI] [PubMed] [Google Scholar]
- Kauffman, H.E. , Reddy, A.P.K. , Hsieh, S.P.Y. and Merca, S.D. (1973) An improved technique for evaluating resistance of rice varieties to Xanthomonas oryzae . Plant Dis. Rep. 57, 537–541. [Google Scholar]
- Kawahara, Y. , de la Bastide, M. , Hamilton, J.P. , Kanamori, H. , McCombie, W.R. , Ouyang, S. , Schwartz, D.C. , Tanaka, T. , Wu, J. , Zhou, S. , Childs, K.L. , Davidson, R.M. , Lin, H. , Quesada‐Ocampo, L. , Vaillancourt, B. , Sakai, H. , Lee, S.S. , Kim, J. , Numa, H. , Itoh, T. , Buell, C.R. and Matsumoto, T. (2013) Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice, 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay, S. , Hahn, S. , Marois, E. , Hause, G. and Bonas, U. (2007) A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science, 318, 648–651. [DOI] [PubMed] [Google Scholar]
- Kay, S. , Hahn, S. , Marois, E. , Wieduwild, R. and Bonas, U. (2009) Detailed analysis of the DNA recognition motifs of the Xanthomonas type III effectors AvrBs3 and AvrBs3Δrep16. Plant J. 59, 859–871. [DOI] [PubMed] [Google Scholar]
- Kim, D. , Pertea, G. , Trapnell, C. , Pimentel, H. , Kelley, R. and Salzberg, S. (2013) TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T. , Huang, S. , Zhao, X. , Wright, D.A. , Carpenter, S. , Spalding, M.H. , Weeks, D.P. and Yang, B. (2011) Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res. 39, 6315–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2–ΔΔCt method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lohse, M. , Bolger, A.M. , Nagel, A. , Fernie, A.R. , Lunn, J.E. , Stitt, M. and Usadel, B. (2012) RobiNA: a user‐friendly, integrated software solution for RNA‐Seq‐based transcriptomics. Nucleic Acids Res. 40, W622–W627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund, S.P. , Nettleton, D. , McCarthy, D.J. and Smyth, G.K. (2012) Detecting differential expression in RNA‐sequence data using quasi‐likelihood with shrunken dispersion estimates. Stat. Appl. Genet. Mol. Biol. 11, doi 10.1515/1544-6115.1826. [DOI] [PubMed] [Google Scholar]
- Mak, A.N. , Bradley, P. , Cernadas, R.A. , Bogdanove, A.J. and Stoddard, B.L. (2012) The crystal structure of TAL effector PthXo1 bound to its DNA target. Science, 335, 716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino, S. , Sugio, A. , White, F. and Bogdanove, A.J. (2006) Inhibition of resistance gene‐mediated defense in rice by Xanthomonas oryzae pv. oryzicola. Mol. Plant–Microbe Interact. 19, 240–249. [DOI] [PubMed] [Google Scholar]
- Marois, E. , Van den Ackerveken, G. and Bonas, U. (2002) The Xanthomonas type III effector protein AvrBs3 modulates plant gene expression and induces cell hypertrophy in the susceptible host. Mol. Plant–Microbe Interact. 15, 637–646. [DOI] [PubMed] [Google Scholar]
- Morbitzer, R. , Römer, P. , Boch, J. and Lahaye, T. (2010) Regulation of selected genome loci using de novo‐engineered transcription activator‐like effector (TALE)‐type transcription factors. Proc. Natl. Acad. Sci. USA, 107, 21 617–21 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morbitzer, R. , Elsaesser, J. , Hausner, J. and Lahaye, T. (2011) Assembly of custom TALE‐type DNA binding domains by modular cloning. Nucleic Acids Res. 39, 5790–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou, M.J. and Bogdanove, A.J. (2009) A simple cipher governs DNA recognition by TAL effectors. Science, 326, 1501. [DOI] [PubMed] [Google Scholar]
- Niño‐Liu, D.O. , Ronald, P.C. and Bogdanove, A.J. (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol. Plant Pathol. 7, 303–324. [DOI] [PubMed] [Google Scholar]
- Römer, P. , Hahn, S. , Jordan, T. , Strauss, T. , Bonas, U. and Lahaye, T. (2007) Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science, 318, 645–648. [DOI] [PubMed] [Google Scholar]
- Römer, P. , Recht, S. and Lahaye, T. (2009a) A single plant resistance gene promoter engineered to recognize multiple TAL effectors from disparate pathogens. Proc. Natl. Acad. Sci. USA, 106, 20 526–20 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer, P. , Strauss, T. , Hahn, S. , Scholze, H. , Morbitzer, R. , Grau, J. , Bonas, U. and Lahaye, T. (2009b) Recognition of AvrBs3‐like proteins is mediated by specific binding to promoters of matching pepper Bs3 alleles. Plant Physiol. 150, 1697–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer, P. , Recht, S. , Strauss, T. , Elsaesser, J. , Schornack, S. , Boch, J. , Wang, S. and Lahaye, T. (2010) Promoter elements of rice susceptibility genes are bound and activated by specific TAL effectors from the bacterial blight pathogen, Xanthomonas oryzae pv. oryzae. New Phytol. 187, 1048–1057. [DOI] [PubMed] [Google Scholar]
- Schmittgen, T.D. and Livak, K.J. (2008) Analyzing real‐time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Schornack, S. , Peter, K. , Bonas, U. and Lahaye, T. (2005) Expression levels of avrBs3‐like genes affect recognition specificity in tomato Bs4‐ but not in pepper Bs3‐mediated perception. Mol. Plant–Microbe Interact. 18, 1215–1225. [DOI] [PubMed] [Google Scholar]
- Strauss, T. , van Poecke, R.M. , Strauss, A. , Römer, P. , Minsavage, G.V. , Singh, S. , Wolf, C. , Strauss, A. , Kim, S. , Lee, H.A. , Yeom, S.I. , Parniske, M. , Stall, R.E. , Jones, J.B. , Choi, D. , Prins, M. and Lahaye, T. (2012) RNA seq pinpoints a Xanthomonas TAL effector activated resistance gene in a large crop genome. Proc. Natl. Acad. Sci. USA, 109, 19 480–19 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel, J. , Pesce, C. , Hutin, M. , Koebnik, R. , Boch, J. and Szurek, B. (2013) Five phylogenetically close rice SWEET genes confer TAL effector‐mediated susceptibility to Xanthomonas oryzae pv. oryzae. New Phytol. 200, 808–819. [DOI] [PubMed] [Google Scholar]
- Sugio, A. , Yang, Bing, Zhu, Tong and White, Frank, F. (2007) Two type III effector genes of Xanthomonas oryzae pv. oryzae control the induction of the host genes OsTFIIAg1 and OsTFX1 during bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 104, 10 720–10 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup, S. , De Feyter, R. , Brlansky, R.H. and Gabriel, D.W. (1991) A pathogenicity locus from Xanthomonas citri enables strains from several pathovars of X. campestris to elicit cankerlike lesions on Citrus . Phytopathology, 81, 802–809. [Google Scholar]
- Szurek, B. , Marois, E. , Bonas, U. and Van den Ackerveken, G. (2001) Eukaryotic features of the Xanthomonas type III effector AvrBs3: protein domains involved in transcriptional activation and the interaction with nuclear import receptors from pepper. Plant J. 26, 523–534. [DOI] [PubMed] [Google Scholar]
- Szurek, B. , Rossier, O. , Hause, G. and Bonas, U. (2002) Type III‐dependent translocation of the Xanthomonas AvrBs3 protein into the plant cell. Mol. Microbiol. 46, 13–23. [DOI] [PubMed] [Google Scholar]
- Tian, D. , Wang, J. , Zeng, X. , Gu, K. , Qiu, C. , Yang, X. , Zhou, Z. , Goh, M. , Luo, Y. , Murata‐Hori, M. , White, F.F. and Yin, Z. (2014) The rice TAL effector‐dependent resistance protein XA10 triggers cell death and calcium depletion in the endoplasmic reticulum. Plant Cell, 26, 497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell, C. , Pachter, L. and Salzberg, S.L. (2009) TopHat: discovering splice junctions with RNA‐Seq. Bioinformatics, 25, 1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell, C. , Williams, B.A. , Pertea, G. , Mortazavi, A. , Kwan, G. , van Baren, M.J. , Salzberg, S.L. , Wold, B.J. and Pachter, L. (2010) Transcript assembly and quantification by RNA‐Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ackerveken, G. , Marois, E. and Bonas, U. (1996) Recognition of the bacterial avirulence protein AvrBs3 occurs inside the host plant cell. Cell, 87, 1307–1316. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Zhang, X. , Fan, Y. , Gao, Y. , Zhu, Q. , Zheng, C. , Qin, T. , Li, Y. , Che, J. , Zhang, M. , Yang, B. , Liu, Y. and Zhao, K. (2015) XA23 is an executor R protein and confers broad‐spectrum disease resistance in rice. Mol. Plant, 8, 290–302. [DOI] [PubMed] [Google Scholar]
- Wang, L. , Makino, S. , Subedee, A. and Bogdanove, A.J. (2007) Novel candidate virulence factors in rice pathogen Xanthomonas oryzae pv. oryzicola as revealed by mutational amalysis. Appl. Environ. Microbiol. 73, 8023–8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, E. , Gruetzner, R. , Werner, S. , Engler, C. and Marillonnet, S. (2011) Assembly of designer TAL effectors by golden gate cloning. PLoS One, 6, e19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann, G. and Bergelson, J. (2004) Effector genes of Xanthomonas axonopodis pv. vesicatoria promote transmission and enhance other fitness traits in the field. Genetics, 166, 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins, K.E. , Booher, N.J. , Wang, L. and Bogdanove, A.J. (2015) TAL effectors and activation of predicted host targets distinguish Asian from African strains of the rice pathogen Xanthomonas oryzae pv. oryzicola while strict conservation suggests universal importance of five TAL effectors. Front. Plant Sci. 6, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. and White, F.F. (2004) Diverse members of the AvrBs3/PthA family of type III effectors are major virulence determinants in bacterial blight disease of rice. Mol. Plant–Microbe Interact. 17, 1192–1200. [DOI] [PubMed] [Google Scholar]
- Yang, B. , Zhu, W. , Johnson, L.B. and White, F.F. (2000) The virulence factor AvrXa7 of Xanthomonas oryzae pv. oryzae is a type III secretion pathway‐dependent nuclear‐localized double‐stranded DNA‐binding protein. Proc. Natl. Acad. Sci. USA, 97, 9807–9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Sugio, A. and White, F.F. (2006) Os8N3 is a host disease‐susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 103, 10 503–10 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. and Gabriel, D.W. (1995) Xanthomonas avirulence/pathogenicity gene family encodes functional plant nuclear targeting signals. Mol. Plant–Microbe Interact. 8, 627–631. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , De Feyter, R. and Gabriel, D.W. (1994) Host‐specific symptoms and increased release of Xanthomonas citri and X. campestris pv. malvacearum from leaves are determined by the 102‐bp tandem repeats of PthA and Avrb6, respectively. Mol. Plant–Microbe Interact. 7, 345–355. [Google Scholar]
- Yu, X. , Guda, K. , Willis, J. , Veigl, M. , Wang, Z. , Markowitz, S. , Adams, M.D. and Sun, S. (2012) How do alignment programs perform on sequencing data with varying qualities and from repetitive regions? BioData Min. 5, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Streubel, J. , Balzergue, S. , Champion, A. , Boch, J. , Koebnik, R. , Feng, J. , Verdier, V. and Szurek, B. (2011) Colonization of rice leaf blades by an African strain of Xanthomonas oryzae pv. oryzae depends on a new TAL effector that induces the rice Nodulin‐3 Os11N3 gene. Mol. Plant–Microbe Interact. 24, 1102–1113. [DOI] [PubMed] [Google Scholar]
- Zeng, X. , Tian, D. , Gu, K. , Zhou, Z. , Yang, X. , Luo, Y. , White, F.F. and Yin, Z. (2015) Genetic engineering of the Xa10 promoter for broad‐spectrum and durable resistance to Xanthomonas oryzae pv. oryzae. Plant Biotechnol. J. 13, 993–1001. [DOI] [PubMed] [Google Scholar]
- Zhang, F. , Cong, L. , Lodato, S. , Kosuri, S. , Church, G.M. and Arlotta, P. (2011) Efficient construction of sequence‐specific TAL effectors for modulating mammalian transcription. Nat. Biotechnol. 29, 149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, B. , Ardales, E.Y. , Raymundo, A. , Bai, J. , Trick, H.N. , Leach, J.E. and Hulbert, S.H. (2004) The avrRxo1 gene from the rice pathogen Xanthomonas oryzae pv. oryzicola confers a nonhost defense reaction on maize with resistance gene Rxo1 . Mol. Plant–Microbe Interact. 17, 771–779. [DOI] [PubMed] [Google Scholar]
- Zhao, B.Y. , Lin, X.H. , Poland, J. , Trick, H. , Leach, J. and Hulbert, S. (2005) A maize resistance gene functions against bacterial streak disease in rice. Proc. Natl. Acad. Sci. USA, 102, 15 383–15 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, W. , Yang, B. , Chittoor, J.M. , Johnson, L.B. and White, F.F. (1998) AvrXa10 contains an acidic transcriptional activation domain in the functionally conserved C terminus. Mol. Plant–Microbe Interact. 11, 824–832. [DOI] [PubMed] [Google Scholar]
- Zhu, W. , Yang, B. , Wills, N. , Johnson, L.B. and White, F.F. (1999) The C terminus of AvrXa10 can be replaced by the transcriptional activation domain of VP16 from the herpes simplex virus. Plant Cell, 11, 1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website:
Fig. S1 Similar activation of a phosphatidylinositol‐4‐phosphate 5‐kinase gene (PPK) predicted to be a target of Tal2a by Xanthomonas oryzae pv. oryzae (Xoo) expressing Tal2a or Tal2a lacking its activation domain.
Fig. S2 Tal2a‐dependent activation of three additional rice genes by Xanthomonas oryzae pv. oryzicola (Xoc).
Fig. S3 Virulence of tal2a mutant M169 expressing dTALE 280 from the low‐copy vector pHM1.
Fig. S4 Inhibition of Xanthomonas oryzae pv. oryzae (Xoo) virulence by Tal2a.
Table S1 Targets of Tal2a predicted using microarray data of Cernadas et al. (2014).
Table S2 Repeat‐variable diresidue (RVD) and effector‐binding element (EBE) sequences for Tal2a and designer transcription activator‐like effectors (dTALEs) that activate Tal2a‐responsive genes.
Table S3 Genes up‐regulated by all Tal2a+ field isolates of Xanthomonas oryzae pv. oryzicola (Xoc).
Table S4 Gene‐specific primers used for quantitative real‐time reverse transcription‐polymerase chain reaction (qPCR).


