Summary
Introduction
The causal agents of Verticillium wilts are globally distributed pathogens that cause significant crop losses every year. Most Verticillium wilts are caused by V. dahliae, which is pathogenic on a broad range of plant hosts, whereas other pathogenic Verticillium species have more restricted host ranges. In contrast, V. longisporum appears to prefer brassicaceous plants and poses an increasing problem to oilseed rape production.
Taxonomy
Kingdom Fungi; Phylum Ascomycota; Class Sordariomycetes; Subclass Hypocreomycetida; Family Plectosphaerellaceae; genus Verticillium.
Disease symptoms
Dark unilateral stripes appear on the stems of apparently healthy looking oilseed rape plants at the end of the growing season. Microsclerotia are subsequently formed in the stem cortex beneath the epidermis.
Genome
Verticillium longisporum is the only non‐haploid species in the Verticillium genus, as it is an amphidiploid hybrid that carries almost twice as much genetic material as the other Verticillium species as a result of interspecific hybridization.
Disease management
There is no effective fungicide treatment to control Verticillium diseases, and resistance breeding is the preferred strategy for disease management. However, only a few Verticillium wilt resistance genes have been identified, and monogenic resistance against V. longisporum has not yet been found. Quantitative resistance exists mainly in the Brassica C‐genome of parental cabbage lines and may be introgressed in oilseed rape breeding lines.
Common name
Oilseed rape colonized by V. longisporum does not develop wilting symptoms, and therefore the common name of Verticillium wilt is unsuitable for this crop. Therefore, we propose ‘Verticillium stem striping’ as the common name for Verticillium infections of oilseed rape.
Keywords: amphidiploid, Arabidopsis, Brassica, host range, pathogenicity, disease management, vascular wilt
Introduction
Verticillium is a relatively small genus of ascomycete fungi that currently comprises 10 species (Inderbitzin et al., 2011a). All presently recognized Verticillium species are soil‐borne fungi, and several cause wilt disease on a variety of plant hosts across the world (Pegg and Brady, 2002). Although symptoms may vary considerably between plant hosts, the most frequently observed disease symptoms of Verticillium wilt include wilting, stunting, chlorosis, vascular discoloration and early senescence (Fradin and Thomma, 2006). The economic impact of Verticillium diseases can be severe, with an estimated annual loss of €3 billion worldwide in the 20 most affected hosts (M. Siebold and A. V. Tiedemann, unpublished data). Verticillium dahliae is the most economically important species of the Verticillium genus, and has the ability to infect more than 200 plant host species (Inderbitzin et al., 2011a; Pegg and Brady, 2002). Verticillium albo‐atrum, V. alfalfae, V. non‐alfalfae and V. longisporum are also vascular pathogens, albeit with a more restricted host range. Members of the genus reproduce asexually and a sexual stage has not yet been described for any Verticillium species (Short et al., 2014).
Taxonomy and Morphology
Verticillium belongs to the family Plectosphaerellaceae (Zare et al., 2007) in the subclass Hypocreomycetidae of the class Sordariomycetes, which is part of the phylum Ascomycota (Zhang et al., 2006). Verticillium is subdivided into two major groups: Clade Flavexudans and Clade Flavnonexudans (Inderbitzin et al., 2011a). Verticillium longisporum is a member of the Flavnonexudans lineage and thus lacks the ability to produce yellow hyphal pigmentation. The taxonomic history of Verticillium, including V. longisporum, is complicated as a result of name changes and taxonomic disagreements. Verticillium longisporum was first described as a variety of V. dahliae, as V. dahliae var. longisporum (Stark, 1961), and was then elevated to species rank 37 years later (Karapapa et al., 1997). Although first contested, the name V. longisporum is now widely adopted (Inderbitzin and Subbarao, 2014). The evolutionary history of V. longisporum is unique among Verticillium species, as V. longisporum is an amphidiploid hybrid that evolved repeatedly by hybridization among four different ancestors (Inderbitzin et al., 2011b; Ingram, 1968).
The differentiation of V. longisporum from related species may be based on morphological and cultural features (Table 1, Fig. 1), but these may not consistently discriminate V. longisporum from V. dahliae. In general, V. longisporum conidia are longer than those of its close relative V. dahliae (Karapapa et al., 1997; Stark, 1961) and, with respect to V. dahliae, V. longisporum has been reported to have elongated microsclerotia and a tendency towards the presence of three phialides in each whorl (Karapapa et al., 1997). However, for some V. longisporum strains, the conidial size ranges overlap with those of V. dahliae, microsclerotia are rounded and there are more than three phialides per whorl (Inderbitzin et al., 2011a). In addition, no morphological characters allow for the differentiation of the different hybrid lineages in V. longisporum. Therefore, the identity of V. longisporum strains and other Verticillium species should be confirmed using molecular techniques, such as DNA amplification with species‐specific primers (Inderbitzin et al., 2013) or DNA sequence determination of species‐specific gene regions (Inderbitzin et al., 2011a).
Table 1.
Non‐molecular criteria for the taxonomic discrimination of Verticillium longisporum and V. dahliae.
| Parameter | V. dahliae | V. longisporum |
|---|---|---|
| Microsclerotial shape*,‡,** | Mostly rounded or spherical | Mostly elongate |
| Conidial size *,¶,** | Mostly short (3.5–5.5 μm) | Mostly long (7.1–8.8 μm) |
| Extracellular polyphenol oxidase activity *,‡,¶ | Mostly strong | Mostly none |
| Culture filtrate fluorescence* | No | Yes |
| Host range *,†,§ | Broad (vegetables, trees, legumes, ornamental crops) | Mainly restricted to Brassicaceae |
Figure 1.
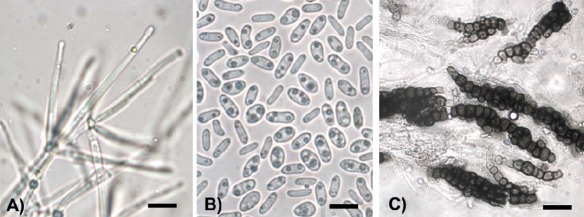
Microscopic appearance of Verticillium longisporum in vitro. (A) Verticillate conidiophores (bar, 20 µm). (B) Conidia (bar, 10 µm). (C) Young microsclerotia (bar, 25 µm).
Evolutionary History, Genomics and Pathogenicity
The typical life cycle of ascomycete fungi is dominated by the haploid state, whereas V. longisporum is amphidiploid as a result of hybridization between two haploid ancestors. Phylogenetic analysis separates V. longisporum isolates into three lineages with different ancestors (Inderbitzin et al., 2011b). Four parental lines are known that belong to three different Verticillium species. The parents include two V. dahliae genotypes, the V. dahliae lineage D2 and V. dahliae lineage D3, and two unknown species that were provisionally called Species A1 and Species D1. Based on ribosomal internal transcribed spacer (ITS) sequences and intron‐rich portions of the five protein‐encoding genes actin (ACT), elongation factor 1‐alpha (EF), glyceraldehyde‐3‐phosphate dehydrogenase (GPD), mitochondrial oxaloacetate transport protein (OX) and tryptophan synthase (TS), it was determined that all characterized V. longisporum isolates contain alleles derived from the Species A1 parent, in combination with Species D1, V. dahliae lineage D2 or V. dahliae lineage D3 alleles, to form the A1 × D1, A1 × D2 and A1 × D3 hybrids, respectively (Fig. 2).
Figure 2.
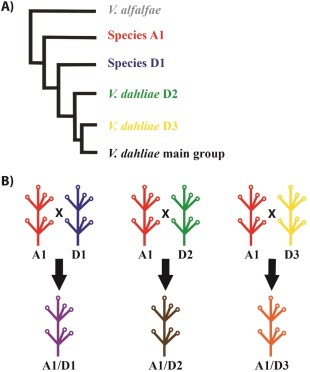
The genetic constitution of the three lineages of Verticillium longisporum. (A) Phylogenetic relationship between the parents of V. longisporum (adjusted from Inderbitzin and Subbarao, 2014). (B) The three hybridization events that resulted in the hybrid species V. longisporum. A1 and D1 progenitors are unknown and provisionally named haploid Verticillium species, whereas progenitors D2 and D3 are both V. dahliae lineages. A1 is a parent of all three V. longisporum lineages, as it hybridized with D1, D2 and D3, resulting in the three V. longisporum lineages A1/D1, A1/D2 and A1/D3, respectively.
Verticillium longisporum has also been referred to as a ‘near‐diploid’ as its nuclear DNA content is ±1.7–1.8 times that of V. dahliae, depending on the isolate, although the amount may vary considerably between isolates (Collins et al., 2003; Karapapa et al., 1997; Steventon et al., 2002). The DNA content of some isolates can be more than double the amount of others (Steventon et al., 2002). This difference in genome size may be a result of variation in DNA content between ancestors, may reflect the genomic plasticity of fungi (Zolan, 1995) or may indicate DNA loss associated with hybridization, as in the endophyte Neotyphodium uncinatum (Craven et al., 2001; Moon et al., 2004). However, two copies have so far been found for all nuclear genes examined in V. longisporum (Inderbitzin et al., 2011b; Tran et al., 2013), with the exception of the nuclear ribosomal region (rDNA), for which only one type was detected in each lineage (Inderbitzin et al., 2011b, Tran et al., 2013). The V. longisporum lineage A1/D3 rDNA region was derived from V. dahliae, whereas the V. longisporum lineage A1/D1 and A1/D2 rDNA regions were derived from Species A1. In addition to DNA loss, concerted evolution could also account for the loss of one rDNA type in each of the V. longisporum lineages.
Several suggestions have been made with regard to the origin of V. longisporum. Parasexual recombination has been proposed as the underlying mechanism (Karapapa et al., 1997), although parasexual processes generally end with chromosome loss to regain a haploid state after fusion of hyphae and nuclei (Caten, 1981). Thus, the stability of V. longisporum as a hybrid makes the hypothesis of interspecific hyphal fusion followed by nuclear fusion more plausible than parasexual processes (Inderbitzin et al., 2011b).
The relative karyotypic stability of V. longisporum is unusual within Verticillium, as artificially induced hybrids between Verticillium species tend to be unstable (Fordyce and Green, 1964; Hastie, 1973; Typas and Heale, 1976) and undergo parasexual processes. In contrast, in a V. longisporum strain maintained in culture for 51 years, no gene loss was observed (Inderbitzin et al., 2011b). The amphidiploid nature of V. longisporum also explains why auxotrophic mutants for the study of vegetative compatibility groups could not be generated (Bhat and Subbarao, 1999; Joaquim and Rowe, 1990; Puhalla, 1979; Nagao et al., 1994; Subbarao et al., 1995; Zeise and Tiedemann, 2001).
Individual V. longisporum lineages are genetically homogeneous, as only a single substitution was found across seven nuclear loci. This suggests a recent origin of V. longisporum (Inderbitzin et al., 2011b). However, there are differences in pathogenicity and virulence between the lineages. Lineage A1/D1 is the most pathogenic lineage on oilseed rape, whereas lineage A1/D3 isolates are generally not pathogenic on this crop (Novakazi et al., 2015; Tran et al., 2013). Lineage A1/D2 is known only from horseradish in Illinois (USA) (Eastburn and Chang, 1994; Inderbitzin et al., 2011b), and was the most virulent lineage on this crop (Novakazi et al., 2015).
Disease Cycle
As far as details are available, the infection process of V. longisporum is highly similar to that of V. dahliae. Verticillium wilts are monocyclic diseases (Klosterman et al., 2011) (Fig. 3). Like V. dahliae, V. longisporum produces melanized microsclerotia (Stark, 1961) for survival to bridge the gap between hosts. Microsclerotia are clusters of melanized, thick‐walled fungal cells, which are derived from hyphal cells through lateral budding of the hyaline mycelium (Klebahn, 1913). In the absence of a host, V. dahliae microsclerotia remain dormant and viable in the soil for more than 10 years (Wilhelm, 1955), which may be similar for V. longisporum. Root exudates stimulate the germination of V. longisporum microsclerotia, after which hyphae grow towards the root of the plant (Berlanger and Powelson, 2000; Leino, 2006). Subsequently, hyphae colonize the surface of the root hairs and grow towards the root surface (Eynck et al., 2007; Zhou et al., 2006). On oilseed rape (Brassica napus), the fungus enters the root by direct penetration of rhizodermal cells of lateral roots or root hairs. Once inside the root, hyphae initially grow both intercellularly and intracellularly in the root cortex towards the central cylinder, where the pathogen enters the xylem (Eynck et al., 2007). Next, conidia may be produced that are carried upwards with the transpiration stream. Conidia that become trapped in pit membranes or at vessel end walls may germinate and penetrate into adjacent vessels (Garber and Houston, 1966). The colonization induces occlusions of the vessels, which may disturb the sap stream in the xylem (Kamble et al., 2013). Only during senescence does the pathogen grow out of the xylem vessels, invades the stem parenchyma and forms microsclerotia beneath the stem epidermis and in the stem pith. The microsclerotia are released into the soil during tissue decomposition (Heale and Karapapa, 1999). Clear evidence for a transmission of V. longisporum by seeds has not been provided so far.
Figure 3.
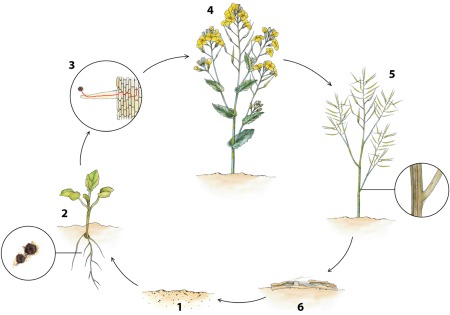
Disease cycle of Verticillium longisporum on oilseed rape. 1. Microsclerotia are persistent resting structures that reside in the soil and bridge the gap between hosts. 2. Triggered by root exudates, microsclerotia start to germinate and hyphae grow towards the root of the plant. 3. The fungus enters the root through wounds, or by direct penetration of epidermal cells of lateral roots or root hairs. In the root, hyphae grow intercellularly and intracellularly towards the central cylinder and enter the xylem. 4. No disease symptoms are observed during the major part of the growing season. 5. Dark unilateral striping develops on the stem of oilseed rape during the ripening of the crop. Ultimately, black microsclerotia are formed in the stem cortex. 6. Microsclerotia are released into the soil on decomposition of plant debris.
Susceptible Crops
The currently known V. longisporum isolates are mainly found on brassicaceous hosts, whereas V. dahliae infects this plant family relatively infrequently (Inderbitzin and Subbarao, 2014). The most likely first description of a V. longisporum infection on a brassicaceous host was from Brussels sprouts in England (Isaac, 1957). Oilseed rape was first reported as a host of V. longisporum in the west and south of Scania, southern Sweden, in 1969 (Kroeker, 1970). The hybridization events that resulted in the novel species V. longisporum may have facilitated this shift in host preference (Inderbitzin et al., 2011b; Mallet, 2007), as V. longisporum is more pathogenic on brassicaceous hosts than V. dahliae (Novakazi et al., 2015). However, clear host range segregation is not found between V. dahliae and V. longisporum, as examples of hosts to both species include oilseed rape (Steventon et al., 2002), horseradish (Armoracia rusticana) (Babadoost et al., 2004), sugar beet (Beta vulgaris) (Jackson and Heale, 1985) and the model plant Arabidopsis thaliana (Fradin et al., 2011; Yadeta et al., 2011).
Pathogenicity tests confirm that V. longisporum has the capacity to infect non‐brassicaceous plants (Bhat and Subbarao, 1999; Novakazi et al., 2015; Qin et al., 2006; Zeise and Tiedemann, 2002). Moreover, V. longisporum lineages can be more or equally virulent to V. dahliae on non‐brassicaceous hosts, such as eggplant, tomato, lettuce and watermelon (Novakazi et al., 2015). This suggests that either the natural host range of particular V. longisporum lineages comprises non‐brassicaceous plant species, or that natural infection of V. longisporum encounters a barrier that is not encountered in pathogenicity tests. The pathogenicity tests performed in these studies involved the inoculation of plants by root dipping in a conidial suspension, which may differ from natural infections which originate from microsclerotia.
Oilseed rape is economically the most important crop affected by V. longisporum. The oil from the seeds is used for human consumption and biodiesel, whereas byproducts become a protein source used in animal feed (Berry et al., 2012). Oilseed rape is the second most important arable oilseed crop, after soybean, and comprises spring and winter cultivars (Berry et al., 2012; Diepenbrock, 2000). Winter oilseed rape is sown between mid‐August and mid‐September in Northwest Europe and has a higher yield than spring oilseed rape, which is sown between March and April (Christen et al., 1999). In 2013, the worldwide annual production of oilseed rape was over 72 megatons, with China, Canada, India and Germany as the leading producers (FAOSTAT, 2015). Verticillium longisporum is one of the major pathogens of oilseed rape and is found in Europe (Gladders et al., 2011; Karapapa et al., 1997; Steventon et al., 2002; Zeise and Tiedemann, 2002), Russia (Pantou et al., 2005) and, recently, in Canada (CFIA, 2015).
Verticillium longisporum is also pathogenic on a broad range of brassicaceous horticultural crops. The pathogen has been found on several diseased Brassica oleracea species, including cauliflower (Debode et al., 2005a; Koike et al., 1994), cabbage (Inderbitzin et al., 2011b; Subbarao et al., 1995) and Brussels sprouts (Isaac, 1957; Karapapa and Typas, 2001; Karapapa et al., 1997). Verticillium longisporum has also been reported in Japan on other brassicaceous vegetables: Chinese cabbage (Brassica rapa var. pekinensis) (Narisawa et al., 2004; Watanabe et al., 1973), turnip (B. rapa var. rapa) (Carder and Barbara, 1994) and wild radish (Raphanus sativus var. hortensis f. raphinistroides) (Okoli et al., 1994).
Interestingly, not all Brassica crops are susceptible to V. longisporum. Typical Verticillium wilt symptoms are not observed on broccoli grown in infested soil. Verticillium longisporum is able to colonize the cortical surface of the roots of this crop, but does not progress into the vascular system (Njoroge et al., 2011; Shetty et al., 2000). However, resistance of broccoli to Verticillium wilt is not consistently observed if the plants are inoculated by root dipping in a conidiospore suspension (Zeise and Tiedemann, 2002). Nevertheless, cultivars from the USA demonstrated resistance against 15 isolates from different hosts using this root dipping inoculation method (Bhat and Subbarao, 2001).
Development of Disease Symptoms and Impact
Most field research on V. longisporum has been conducted on oilseed rape and cauliflower. Although both crops are Brassica species, the disease symptoms on these crops differ. Infected oilseed rape develops dark, unilateral striping on the stem late in the growing season, indicating the necrosis of cortical tissue (Heale and Karapapa, 1999) (Fig. 4). Symptom development coincides with increased pathogen colonization of root and shoot tissues (Dunker et al., 2008). In the final stages of the disease, the fungus forms black microsclerotia in the stem cortex. In contrast with the disease caused by this pathogen on other crops, conventional wilting symptoms are typically not observed on oilseed rape. Rather, the crop ripens prematurely, making disease symptoms difficult to distinguish from natural senescence. Strikingly, not much is known about the impact of Verticillium infection on the yield of oilseed rape under field conditions. Verticillium longisporum symptoms can be omnipresent with a disease incidence of up to 80% (Dixelius et al., 2005). Under practical conditions, yield losses caused by V. longisporum have been suggested to range between 10% and 50%, but this has not yet been experimentally verified (Dunker et al., 2008). One study showed no significant effect on the ‘thousand‐seed‐weight’ (TSW) yield or oil content of the crop after artificial inoculation of the soil (Dunker et al., 2008). Although there was no effect on the whole‐plot yield, disease symptoms of single plants in the field negatively correlated with yield.
Figure 4.

Typical disease symptoms caused by Verticillium longisporum on oilseed rape. Dark unilateral striping appears on the stems of apparently healthy looking plants at the end of the growing season (A), indicating the necrosis of cortical tissue. The necrosis develops further in a later stage of the disease, which may lead to stem cracks (B). Finally, microsclerotia are formed in the stem cortex beneath the epidermis (C).
Interestingly, disease development in oilseed rape on artificial inoculation differs from disease development under field conditions. Whereas symptoms in the field involve dark unilateral striping on the stem late in the growing season, on root dip inoculation in the seedling stage, oilseed rape plants exhibit chlorosis, vascular discoloration and stunting at an early stage (Eynck et al., 2007, 2009b; Floerl et al., 2008; Zeise and Tiedemann, 2002). Moreover, clear biomass reduction is observed, whereby roots are significantly more affected than shoots (Keunecke, 2009). Recent studies have demonstrated that extensively increased branching of the shoot occurs on inoculated plants (D. Lopisso and A. V. Tiedemann, unpublished data). It is currently not understood why these differences in disease development occur.
In contrast with oilseed rape, cauliflower displays typical wilting symptoms on infection with V. longisporum, which starts with chlorosis and necrosis of the lower leaves (Koike et al., 1994). At maturity, stunting of the plants and wilting can be observed. Furthermore, V. longisporum infection may lead to an increase in the number of cauliflower leaves (Subbarao et al., 1995), although this is not universally observed across cultivars (Debode et al., 2005b; Xiao and Subbarao, 1998).
The symptom development of the disease caused by V. longisporum in cauliflower and oilseed rape seems to be temperature dependent. Cauliflower grown as a winter crop in infested fields remains unaffected, whereas more disease is observed at higher temperatures (Koike et al., 1994). At the same time, increased temperatures may also be unconducive to V. longisporum infection, as infected cauliflower did not display any symptoms when grown in a glasshouse at temperatures in the range 27–35°C. More recently, studies in a soil heating facility demonstrated a significant increase in V. longisporum colonization of winter oilseed rape when soil temperatures were elevated by 1.6 or 3.2°C with respect to ambient temperature, indicating a higher vulnerability of spring‐sown crops growing into the warmer season (Siebold and Tiedemann, 2013).
The incidence and severity of disease caused by V. longisporum on cauliflower and oilseed rape is not always correlated with inoculum density, although symptoms on cauliflower can occur earlier at higher inoculum densities (França et al., 2013; Johansson et al., 2006a; Xiao and Subbarao, 1998). In contrast, in a study on oilseed rape, the disease incidence and severity were positively correlated to inoculum level (Dunker et al., 2008).
Plant Responses
Chemical and mechanical responses
The influence of plant secondary metabolites on the interaction between V. longisporum and its host plants has not been extensively explored to date, but glucosinolate concentrations in infected hosts have been investigated. Glucosinolates are constitutively expressed sulfur‐containing phytochemicals that are predominantly found in brassicaceous plants (Wittstock and Halkier, 2002). In general, they are grouped into aliphatic, aromatic and indole glucosinolates, depending on whether they originate from aliphatic amino acids, aromatic amino acids or tryptophan. Upon tissue damage, glucosinolates are hydrolysed with the formation of biologically active and sometimes toxic compounds. Interestingly, concentrations of aliphatic glucosinolates are generally higher in the roots of infected broccoli than in the shoots, whereas the opposite is observed in cauliflower, which may be implicated in the resistance of broccoli towards V. longisporum (Njoroge et al., 2011). Levels of glucosinolates in the roots of V. longisporum‐infected Arabidopsis plants are higher than in non‐inoculated plants. However, the increase in glucosinolates is not accompanied by an increase in glucosinolate breakdown products in the roots (Witzel et al., 2015). Verticillium longisporum infection induces the transcriptional activation of genes involved in tryptophan biosynthesis and tryptophan‐derived secondary metabolism. Furthermore, genetic disruption of tryptophan‐derived secondary metabolism leads to enhanced susceptibility. However, no increase in antifungal indole glucosinolate breakdown products is observed and the tryptophan‐derived phytoalexin, camalexin, does not contribute significantly to defence against V. longisporum in roots (Iven et al., 2012). This indicates that other, as yet unidentified, tryptophan‐derived metabolites play an important role in fungal defence in roots. In contrast with the protective role of the tryptophan‐derived secondary metabolites, monoterpenes produced by the monoterpene synthase TPS23/27 stimulate in vitro conidial germination and subsequent invasion of V. longisporum in Arabidopsis roots (Roos et al., 2015).
In leaf tissue of A. thaliana, soluble phenylpropanoids, rather than tryptophan‐derived metabolites, have been found to accumulate in response to V. longisporum infection. Mutant analysis and in vitro growth assays have revealed that sinapate glucose and coniferin are involved in restriction of the pathogen (König et al., 2014). In addition, the phenylpropanoid pathway is important for the defence of B. napus against V. longisporum, as more phenolic compounds were produced by a resistant line of B. napus on infection with V. longisporum when compared with a susceptible line (Eynck et al., 2009a). Moreover, concentrations of phenylpropanoids were correlated with V. longisporum resistance in B. napus (Obermeier et al., 2013).
The ability of V. longisporum to synthesize aromatic amino acids and the cross‐pathway control of amino acid biosynthesis are required for pathogenicity. Silencing mutants impaired in chorismate synthase or CPC1, the conserved transcription factor of cross‐pathway control, caused less disease and showed reduced growth in the hypocotyl of B. napus and Arabidopsis. Chorismate is essential for the biosynthesis of tryptophan, phenylalanine and tyrosine, whereas cross‐pathway control allows fungi to increase amino acid biosynthesis on amino acid starvation (Singh et al., 2010; Timpner et al., 2013). Brassica napus xylem sap contains only low concentrations of amino acids, and aromatic amino acids are especially scarce (Singh et al., 2010). An increased production of plant secondary metabolites in response to V. longisporum infection probably further depletes amino acid concentrations in the xylem. Hence, the fungus requires a functional cross‐pathway control to overcome the imbalance in amino acid supply in the xylem.
Drought stress tolerance increases when Arabidopsis plants are challenged with V. longisporum, which may be the result of pathogen‐induced reduction of stomatal apertures or de novo xylem formation (Reusche et al., 2012; Roos et al., 2014). Reduction in stomatal apertures may be linked to increased abscisic acid (ABA) levels in Arabidopsis leaves in response to V. longisporum, as ABA is a known central regulator of the stomatal apparatus (Acharya and Assmann, 2009; Roos et al., 2014). Furthermore, V. longisporum infection induces transdifferentiation of bundle sheath cells into functional xylem elements in A. thaliana and B. napus. Verticillium longisporum also causes the reinitiation of cambial activity and the transdifferentiation of xylem parenchyma in A. thaliana, resulting in xylem hyperplasia (Reusche et al., 2012, 2014).
MicroRNAs (miRNAs)
It has been demonstrated recently that V. longisporum interferes with plant miRNAs to reprogram plant gene expression. Sixty‐two miRNAs were responsive to V. longisporum infection in B. napus, the majority of which were down‐regulated. Important targets of down‐regulated miRNAs include auxin response factors (ARFs), which control the transcription of genes in response to auxin. The resulting increase in ARFs may suppress plant defence responses by enhancing auxin signalling. Another down‐regulated miRNA targets a positive regulator of leaf senescence. At early infection stages, the greatest suppression was observed for miR168, which interferes with Argonaute 1 (AGO1) (Shen et al., 2014). AGO1 is an RNA‐binding protein involved in RNA silencing that regulates diverse physiological processes, including a number of pathogen‐associated molecular pattern (PAMP)‐triggered immune responses (Li et al., 2010). AGO1 mutants were clearly more resistant to V. longisporum, suggesting a key role of AGO1 in the compatible interaction with V. longisporum (Shen et al., 2014).
Plant hormones
To date, the involvement of typical plant hormone signalling pathways in the interaction with V. longisporum remains unclear, and the role of the various plant hormones in the defence of A. thaliana and B. napus against V. longisporum appears to be different (Ratzinger et al., 2009). Verticillium longisporum infection increases the level of jasmonic acid (JA) in A. thaliana and activates the corresponding marker genes VSP2 and PDF1.2, but biosynthesis and signalling mutants do not show major differences in disease susceptibility when compared with wild‐type plants (Johansson et al., 2006b; Ralhan et al., 2012). This suggests that JA does not contribute to V. longisporum resistance in A. thaliana. However, the treatment of Arabidopsis plants with methyl jasmonate (MeJA) results in enhanced resistance towards V. longisporum (Johansson et al., 2006b). Moreover, V. longisporum requires JA‐independent CORONATINE INSENSITIVE1 (COI1) function in the roots to elicit disease symptoms in A. thaliana shoots (Ralhan et al., 2012). In oilseed rape, JA concentrations increase over time in both healthy and infected plants, which is probably caused by aging‐related processes, as JA acts in senescence (He et al., 2002; Ratzinger et al., 2009).
In Arabidopsis, metabolites of the salicylic acid (SA) pathway, salicylic acid glucoside (SAG) and dihydroxybenzoic acid, increase after V. longisporum infection, and the SA marker genes PR1 and PR2 are activated. However, mutants in the SA pathway (eds1‐1, NahG, npr1‐3, pad4‐1 and sid2‐1) do not exhibit enhanced susceptibility, indicating that SA signalling may not contribute to V. longisporum resistance in Arabidopsis (Johansson et al., 2006b; Ralhan et al., 2012). In contrast, SA appears to play a role in B. napus susceptibility to V. longisporum infection (Ratzinger et al., 2009). Concentrations of SA and SAG in the xylem sap of B. napus plants increase on V. longisporum infection and correlate with disease severity; a strong correlation between SAG levels in the shoot and the amount of V. longisporum DNA in hypocotyls was found. However, the exact role of the enhanced levels of SA and SAG in xylem sap after infection with V. longisporum is not clear (Ratzinger et al., 2009).
Ethylene (ET) production and the expression of ET‐dependent plant defences have been shown to be induced by V. longisporum in Arabidopsis. Moreover, pretreatment with the ET precursor 1‐aminocyclopropane‐1‐carboxylic acid (ACC) enhances host resistance to V. longisporum. The Arabidopsis mutants impaired in ET signalling, ein4‐1, ein2‐1 and ein6‐1, were more susceptible than the wild‐type to V. longisporum. In contrast, the Arabidopsis mutant etr1‐1 showed enhanced resistance and a higher chlorophyll content compared with the wild‐type, indicating that prolonged ET perception via ETR1 enhances susceptibility via the induction of senescence (Johansson et al., 2006b; Veronese et al., 2003). Reusche et al. (2013) found that V. longisporum triggers early senescence in Arabidopsis by actively decreasing cytokinin levels in the leaves. Senescing tissue may provide easy access to nutrients for the development of microsclerotia during the last phase of the life cycle of V. longisporum. Stabilization of cytokinin levels inhibits fungal growth and reduces disease symptom development (Reusche et al., 2013).
ABA levels increase after infection with V. longisporum in Arabidopsis (Ralhan et al., 2012; Roos et al., 2014). The ABA‐deficient mutant aba2‐1 is susceptible to V. longisporum and accumulates less anthocyanin than wild‐type plants, whereas ABA‐insensitive mutants do not show enhanced susceptibility (Johansson et al., 2006b; Veronese et al., 2003). In B. napus, however, ABA concentrations in xylem sap are not affected by V. longisporum infection (Ratzinger et al., 2009).
Disease Management
Chemical control, heat treatments and solarization
The management of Verticillium wilts is challenging, as current disease control strategies do not provide appropriate protection. Consequently, a combination of management techniques is necessary to contain the disease. Protective or curative control by conventional fungicides is not an option for V. longisporum. Soil fumigation is a successful strategy to reduce the inoculum density of V. dahliae in the soil (Powelson and Carter, 1973), but is no longer available for agricultural use because of its detrimental effects on stratospheric ozone (Subbarao, 2002). Although heat treatment of the soil can similarly reduce the viability of microsclerotia, steam‐mediated heat treatment and most other heat treatment methods are energy consuming and not cost‐effective in most commercial field production systems (Pullman et al., 1981). However, soil solarization by heating the soil under a tarpaulin may be economically feasible and effective, depending on the temperature and duration of treatment (Pullman et al., 1981), and is currently only commercially practised in Mediterranean, desert and tropical climates (Stapleton, 2000), because these climates allow the accumulation of adequate heat units to neutralize the pathogen.
Weed management and crop rotation
The more confined host range of V. longisporum, in comparison with V. dahliae, theoretically facilitates the use of crop rotation as a disease management strategy. However, the persistence of microsclerotia, potential non‐brassicaceous reservoir plants (Johansson et al., 2006a) and inadequate weed management may jeopardize the effects of crop rotation. Brassicaceous weeds may act as a reservoir for V. longisporum, as Verticillium isolates have been recovered from B. rapa spp. campestris, shepherd's purse (Capsella bursa‐pastoris), annual wall‐rocket (Diplotaxis muralis), clasping pepperweed (Lepidium perfoliatum), tumble mustard (Sisymbrium altissimum), Descurainia hartwegiana, field pennycress (Thlaspi arvense) and charlock (Sinapis arvensis) (Johansson et al., 2006a; Vargas‐Machuca et al., 1987; Woolliams, 1966). In addition to weed management, the prevention of Brassica volunteers in subsequent crops is also important; oilseed rape is particularly prone to volunteers as a result of high seed losses before and during harvest (Price et al., 1996).
Few crop rotation studies have been conducted with V. longisporum, and more long‐term research is needed to determine whether crop rotation could be an effective management strategy. Hitherto, only studies on the impact of fallow treatment in cauliflower fields have been conducted. These suggest that fallow treatment does not reduce microsclerotial accumulation in the soil (França et al., 2013; Subbarao and Hubbard, 1999). The microsclerotial density in the soil after 2 years of consecutive cauliflower crops is not significantly higher than when a cauliflower crop is followed by a fallow treatment the year after (Subbarao and Hubbard, 1999). Moreover, even a 4‐year fallow period after a long history of cauliflower cropping did not reduce the microsclerotial density in the soil (França et al., 2013). The long‐term release of microsclerotia from the plant debris of the previous crop may be the reason why fallow did not lead to a significant reduction in the study of Subbarao and Hubbard (1999), as the microsclerotial density continued to increase during the fallow period. Therefore, a more extended fallow period may be more effective in decreasing microsclerotial densities in the soil. In contrast, França et al. (2013) did not report an increase in microsclerotial density in the soil of fallow‐treated plots, but reported a fluctuation with a seasonal pattern. Interestingly, similar patterns and inoculum levels occur in the soil of plots with continuous cauliflower cropping. Possibly, the amount of microsclerotia formed in the cauliflower debris may be affected by the incomplete inflorescence development of cauliflower as the generative phase of the plant is interrupted by the harvest of the curd (França et al., 2013). However, this hypothesis is not in line with the increasing microsclerotial density observed previously (Subbarao and Hubbard, 1999; Xiao et al., 1998).
Bio‐control agents and organic soil amendments
Several microorganisms, including bacteria and fungi, have the ability to reduce the colonization by, and deleterious effects of, V. longisporum, and can thus potentially serve as biological control agents (BCAs), provided that an ecologically fit and effective agent is developed. Specific, non‐pathogenic Verticillium isolates, such as the V. isaacii isolate Vt305, are able to suppress disease symptoms caused by pathogenic isolates. The strain Vt305 was isolated from a Verticillium wilt‐suppressive cauliflower field in Belgium (França et al., 2013). Vt305 appears to be an endophyte of cauliflower and shows effective biological control capacities under controlled conditions (Tyvaert et al., 2014). Inoculation of Vt305, 1 week prior to V. longisporum inoculation, reduced symptom development and the colonization of plant tissue by V. longisporum. However, the mechanism by which Vt305 protects cauliflower against Verticillium wilt is unknown, although it has been suggested that competition for infection sites and induced resistance responses are the two most likely possibilities (Tyvaert et al., 2014).
Microsphaeropsis ochracea is another ascomycete and BCA of V. longisporum (Stadler and Tiedemann, 2014). The effectiveness of M. ochracea as a BCA has been proven in vitro and under sterile soil conditions, as it causes high rates of microsclerotial mortality. Nevertheless, M. ochracea appears not to have sufficient microbial competitiveness to control V. longisporum under field conditions (Stadler and Tiedemann, 2014).
A large‐scale screening for root‐colonizing and endophytic fungi with BCA capacities has led to the isolation of two Phialocephala fortinii isolates, one Heteroconium chaetospira isolate and one Meliniomyces variabilis isolate (Narisawa et al., 1998, 2000, 2004; Ohtaka and Narisawa, 2008). All isolates reduced the symptoms of V. longisporum on in vitro‐grown Chinese cabbage when the colonization of the plant by the BCA preceded V. longisporum infection. Only the H. chaetospira and M. variabilis isolates were able to reduce the disease severity and incidence of Verticillium wilt in Chinese cabbage under field conditions.
In addition to fungal BCAs, the plant beneficial bacterium Serratia plymuthica HRO‐C48 also reduces Verticillium symptoms in oilseed rape (Müller and Berg, 2008). A biological product based on Serratia plymuthica HRO‐C48 has been developed, called RhizoStar® (E‐nema, Raisdorf, Germany). Furthermore, certain Bacillus amyloliquefaciens strains are BCAs towards several fungal pathogens of oilseed rape, including V. longisporum (Danielsson et al., 2007). Bacillus amyloliquefaciens ssp. plantarum UCMB5113 is the most effective strain against V. longisporum and also shows plant growth‐promoting activity. UCMB5113 produces antibiotic compounds and bio‐surfactants, which are probably involved in the bio‐control properties of the bacterium (Danielsson et al., 2007; Niazi et al., 2014.). However, its BCA capacities can also act indirectly and be caused by plant defence priming (Sarosh et al., 2009). The latter hypothesis is supported by the observation that the soil‐borne isolate UCMB5113 confers resistance to airborne pathogens, where the spatial separation of BCA and the pathogen prevents direct interaction between the two (Sarosh et al., 2009).
Amendments of organic material to soils can suppress soil‐borne fungal diseases (Bonanomi et al., 2007). Several crop residues are able to reduce microsclerotium viability in naturally infested cauliflower fields (Debode et al., 2005a; França et al., 2013). The incorporation of ryegrass and corn residues is more effective than brassicaceous plant material. However, the reduction of primary inoculum does not reduce the incidence or severity of the disease. Other publications have reported a Verticillium wilt‐suppressive effect by broccoli residues (Subbarao and Hubbard, 1996, 1999). The broccoli amendments reduce disease abundance and microsclerotium viability in naturally infested soils (Xiao et al., 1998). Moreover, broccoli residues may even inhibit the cauliflower root colonization ability of surviving microsclerotia (Njoroge et al., 2011; Shetty et al., 2000). The lignin content of the incorporated crop residues appears to be a key determinant for the effectiveness of Verticillium control. The ‘lignin–melanin hypothesis’ proposes that enzymes involved in lignin biodegradation also degrade fungal melanin (Butler and Day, 1998; Debode et al., 2005a; Shetty et al., 2000). Melanin protects microsclerotia against biotic and abiotic stress during the period between hosts (Bell and Wheeler, 1986). Therefore, soil amendments with relatively high lignin content may stimulate microbial organisms that decompose lignin and that simultaneously reduce microsclerotial viability.
Resistance breeding
Resistance breeding is the most favoured means of Verticillium disease management, and several crops with polygenic V. longisporum resistance have been reported (Fradin and Thomma, 2006; Kemmochi et al., 2000; Rygulla et al., 2008). Unfortunately, a genuine resistance (R) gene against V. longisporum has not yet been found, and Ve1 presently remains the only R gene that has been described against Verticillium wilts (Fradin et al., 2009, 2011). Although Ve1 was initially identified in tomato (Kawchuk et al., 2001), functional Ve1 homologues have also been identified in other plant species, such as Nicotiana glutinosa (Zhang et al., 2013), lettuce (Hayes et al., 2011) and cotton (Zhang et al., 2012, 2011). Tomato Ve1 confers resistance against race 1 isolates of V. dahliae and V. albo‐atrum (presently V. alfalfae) which contain the Ave1 gene (de Jonge et al., 2012). Ave1 encodes an effector protein that activates Ve1‐mediated resistance, but Ave1 contributes to fungal virulence in susceptible plants that lack Ve1. Thus far, functionality of Ve1‐mediated resistance has not been demonstrated against V. longisporum, which has been attributed to the observation that the currently investigated isolates do not carry the Ave1 gene (Fradin et al., 2011). However, there are genetic resources that may be used to reduce the susceptibility of brassicaceous plants to V. longisporum. For instance, constitutive expression of the Enhancer of vascular Wilt Resistance 1 (EWR1) gene enhances the resistance against Verticillium wilt caused by V. albo‐atrum (presently V. alfalfae), V. dahliae and V. longisporum in Arabidopsis (Yadeta et al., 2011, 2014). EWR1 encodes a putatively secreted protein of unknown function and has homologues that are only found within the Brassicaceae family (Yadeta et al., 2014). EWR1 homologues facilitate enhanced Verticillium resistance in transformed A. thaliana. Interestingly, the brassicaceous‐specific EWR1 homologues can also be used to increase resistance against Verticillium wilts in non‐brassicaceous plants, as Nicotiana benthamiana displays resistance against V. dahliae when Brassicaceae EWR1 homologues are over‐expressed.
Current European oilseed rape cultivars possess a low level of Verticillium resistance, and the availability of novel germplasm for resistance breeding is limited because of the narrow genetic basis of currently used cultivars (Cowling, 2007; Seyis et al., 2003). However, three quantitative trait loci (QTLs) that significantly correlate with V. longisporum resistance have been identified, one on the C1 and two on the C5 chromosome, of the partly resistant oilseed rape cultivar Express 617 (Obermeier et al., 2013). These QTLs indicate sources of quantitative resistance available in the C‐genome derived from oilseed rape parental cabbage lines. Interestingly, the QTLs co‐localize with loci for two soluble phenylpropanoids that are negatively correlated with disease severity during V. longisporum infection, whereas a positive correlation exists with precursors of cell wall‐bound phenols related to lignin. This is in agreement with the higher constitutive and induced levels of cell wall‐bound phenols in roots and hypocotyls of resistant rapeseed genotypes. The resistance genotypes were identified in V. longisporum resistance screenings of B. napus accessions (Eynck et al., 2009b). One of the identified accessions with quantitative resistance to V. longisporum tolerates root invasion, but hinders the pathogen from colonizing the shoot by means of vascular occlusions, and strongly enhances the accumulation of phenols in the xylem parenchyma at the hypocotyl interface (Eynck et al., 2009a). In spite of these specific observations, the genuine mechanism of quantitative resistance is not entirely clear.
Resistance traits in B. oleracea and B. rapa can be applied in oilseed rape resistance breeding, as B. napus is an interspecific hybrid between these two plant species (Eynck et al., 2009b; Happstadius et al., 2003; Obermeier et al., 2013; Rygulla et al., 2007a, b,2008). Cultivars of B. oleracea crops have been screened for V. longisporum susceptibility, with differences in susceptibility found among cauliflower cultivars (Debode et al., 2005b) and dominant polygenic resistance occurring in cabbage (Kemmochi et al., 2000).
In addition to breeding, resistance sources from outside the Brassicaceae may improve the resistance of current V. longisporum hosts. These include sugar beet, whose BvGLP‐1 gene reduces V. longisporum disease symptoms in Arabidopsis. BvGLP‐1 has high sequence homology to a set of plant germin‐like proteins, and is highly induced after nematode (Heterodera schachtii) infection of resistant sugar beet plants containing the single dominant resistance gene Hs1pro‐1 (Knecht et al., 2010).
Conclusion
Verticillium longisporum is becoming a global problem in oilseed rape production. Recently, the disease has been reported outside continental Europe in two important oilseed rape production areas: the UK in 2011 (Gladders et al., 2011) and Canada in 2015 (CFIA, 2015). To improve the management of the V. longisporum disease of oilseed rape and other crops, several steps could be implemented. First, the search for sources of resistance should be intensified. Host resistance is generally considered to be the most desirable control strategy, but R genes, such as Ve1 in tomato against V. dahliae, are unknown for V. longisporum. Therefore, more B. napus, B. oleracea and B. rapa germplasm should be screened for resistance traits for introgression into new, improved cultivars. R genes should be deployed cautiously and combined with other resistance traits and management measures to improve the durability of resistance. Second, phytosanitary measures should be expanded to prevent the spread of V. longisporum with contaminated soil and equipment to new areas. Verticillium longisporum is a soil‐borne pathogen and cannot move autonomously over great distances. Therefore, international trade and travel are likely to be responsible for V. longisporum's continually expanding geographical range. Finally, accurate assessments of the impact of V. longisporum infections on crop quality and yield under field conditions are required. These would determine the economic relevance of the pathogen, and provide a solid economic basis for disease management decisions.
Verticillium longisporum on oilseed rape is not a causal agent of wilt, and therefore the use of ‘Verticillium wilt’ as the common name of V. longisporum on oilseed rape is incorrect. Perhaps, V. longisporum mainly has an endophytic lifestyle in oilseed rape, which causes symptoms on the stem that do not result in reduced crop quality or yield losses. Therefore, we propose ‘Verticillium stem striping’ as the common name to describe V. longisporum on oilseed rape.
Acknowledgements
The authors would like to thank the Marie Curie Actions programme of the European Commission that financially supports the research investigating the threat of V. longisporum to British oilseed rape production. Work in the laboratory of B.P.H.J.T. is supported by the Research Council Earth and Life Sciences (ALW) of the Netherlands Organization of Scientific Research (NWO). We greatly appreciate the drawing of the disease cycle diagram of V. longisporum on oilseed rape (Fig. 3) by Hannah R. Pritchard. The authors declare no conflicts of interest.
References
- Acharya, B.R. and Assmann, S.M. (2009) Hormone interactions in stomatal function. Plant Mol. Biol. 69, 451–462. [DOI] [PubMed] [Google Scholar]
- Babadoost, M. , Chen, W. , Bratsch, A.D. and Eastman, C.E. (2004) Verticillium longisporum and Fusarium solani: two new species in the complex of internal discoloration of horseradish roots. Plant Pathol. 53, 669–676. [Google Scholar]
- Bell, A.A. and Wheeler, M.H. (1986) Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 24, 411–451. [Google Scholar]
- Berlanger, I. and Powelson, M.L. (2000) Verticillium wilt. The Plant Health Instructor. Available at: http://www.apsnet.org/edcenter/intropp/lessons/fungi/ascomycetes/Pages/VerticilliumWilt.aspx. doi: 10.1094/PHI-I-PHI-1-2000-0801-01. Updated 2005. [DOI]
- Berry, P. , Cook, S. , Ellis, S. , Gladders, P. and Roques, S. (2012) HGCA Oilseed rape guide (Boys E., ed.). Stoneleigh Park, Kenilworth, Warwickshire: HGCA Publications. [Google Scholar]
- Bhat, R.G. and Subbarao, K.V. (1999) Host range specificity in Verticillium dahliae . Phytopathology, 89, 1218–1225. [DOI] [PubMed] [Google Scholar]
- Bhat, R.G. and Subbarao, K.V. (2001) Reaction of broccoli to isolates of Verticillium dahliae from various hosts. Plant Dis. 85, 141–146. [DOI] [PubMed] [Google Scholar]
- Bonanomi, G. , Antignani, V. , Pane, C. and Scala, F. (2007) Suppression of soilborne fungal diseases with organic amendments. J. Plant Pathol. 89, 311–324. [Google Scholar]
- Butler, M.J. and Day, A.W. (1998) Destruction of fungal melanins by ligninases of Phanerochaete chrysosporium and other white rot fungi. Int. J. Plant Sci. 159, 989–995. [Google Scholar]
- Carder, J.H. and Barbara, D.J. (1994) Molecular variation within some Japanese isolates of Verticillium dahliae . Plant Pathol. 43, 947–950. [Google Scholar]
- Caten, C.E. (1981) Parasexual processes in fungi In: The Fungal Nucleus (Gull K. and Oliver S.G., eds), pp. 191–214. Cambridge University Press, Cambridge. [Google Scholar]
- CFIA (2015) Verticillium wilt –Verticillium longisporum. Canadian Food Inspection Agency. Available at: http://www.inspection.gc.ca/plants/plant-pests-invasive-species/diseases/verticillim-wilt/eng/1420746212959/1420746213803. Accessed: 22 February 2016.
- Christen, O. , Evans, E. , Nielsson, C. and Haldrup, C. (1999) Oilseed rape cropping systems in NW Europe. In: Proceedings of the 10th International Rapeseed Congress, Canberra, Australia.
- Collins, A. , Ada, C. , Okoli, N. , Morton, A. , Parry, D. , Edwards, S.G. and Barbara, D.J. (2003) Isolates of Verticillium dahliae pathogenic to crucifers are of at least three distinct molecular types. Phytopathology, 93, 364–376. [DOI] [PubMed] [Google Scholar]
- Cowling, W.A. (2007) Genetic diversity in Australian canola and implications for crop breeding for changing future environments. Field Crop Res. 104, 103–111. [Google Scholar]
- Craven, K.D. , Blankenship, J.D. , Leuchtmann, A. , Hignight, K. and Schardl, C.L. (2001) Hybrid fungal endophytes symbiotic with the grass Lolium pratense . Sydowia, 53, 44–73. [Google Scholar]
- Danielsson, J. , Reva, O. and Meijer, J. (2007) Protection of oilseed rape (Brassica napus) toward fungal pathogens by strains of plant‐associated Bacillus amyloliquefaciens . Microbial Ecol. 54, 134–140. [DOI] [PubMed] [Google Scholar]
- Debode, J. , Clewes, E. , De Backer, G. and Höfte, M. (2005a) Lignin is involved in the reduction of Verticillium dahliae var. longisporum inoculum in soil by crop residue incorporation. Soil Biol. Biochem. 37, 301–309. [Google Scholar]
- Debode, J. , Declercq, B. and Höfte, M. (2005b) Identification of cauliflower cultivars that differ in susceptibility to Verticillium longisporum using different inoculation methods. J. Phytopathol. 153, 257–263. [Google Scholar]
- Diepenbrock, W. (2000) Yield analysis of winter oilseed rape (Brassica napus L.): a review. Field Crop Res. 67, 35–49. [Google Scholar]
- Dixelius, C. , Happstadius, I. and Berg G. (2005) Verticillium wilt on Brassica oilseed crops – a Swedish perspective. J. Swed. Seed Assoc. 115, 36–48. [Google Scholar]
- Dunker, S. , Keunecke, H. , Steinbach, P. and Tiedemann, A.V. (2008) Impact of Verticillium longisporum on yield and morphology of winter oilseed rape (Brassica napus) in relation to systemic spread in the plant. J. Phytopathol. 156, 698–707. [Google Scholar]
- Eastburn, D.M. and Chang, R.J. 1994. Verticillium dahliae: a causal agent of root discoloration of horseradish in Illinois. Plant Dis. 78, 496–498. [Google Scholar]
- Eynck, C. , Koopmann, B. , Grunewaldt‐Stoecker, G. , Karlovsky, P. and Tiedemann, A.V. (2007) Differential interactions of Verticillium longisporum and V. dahliae with Brassica napus detected with molecular and histological techniques. Eur. J. Plant Pathol. 118, 259–274. [Google Scholar]
- Eynck, C. , Koopmann, B. , Karlovsky, P. and Tiedemann A.V. (2009a) Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum . Phytopathology, 99, 802–811. [DOI] [PubMed] [Google Scholar]
- Eynck, C. , Koopmann, B. and Tiedemann, A.V. (2009b) Identification of Brassica accessions with enhanced resistance to Verticillium longisporum under controlled and field conditions. Z. Pflanzenk. Pflanzen. 116, 63–72. [Google Scholar]
- FAOSTAT (2015) Statistical database. Food and Agriculture Organization of the United Nations: Statistics Division. Available at: http://faostat3.fao.org/browse/Q/QC/E. Accessed: 22 February 2016.
- Floerl, S. , Druebert, C. , Majcherczyk, A. , Karlovsky, P. , Kües, U. and Polle, A. (2008) Defence reactions in the apoplastic proteome of oilseed rape (Brassica napus var. napus) attenuate Verticillium longisporum growth but not disease symptoms. BMC Plant Biol. 8, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce, C. and Green R.J. (1964) Mechanisms of variation in Verticillium albo‐atrum . Phytopathology, 54, 795–798. [Google Scholar]
- Fradin, E.F. and Thomma, B.P.H.J. (2006) Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo‐atrum . Mol. Plant Pathol. 7, 71–86. [DOI] [PubMed] [Google Scholar]
- Fradin, E.F. , Zhang, Z. , Ayala, J.C.J. , Castroverde, C.D.M. , Nazar, R.N. , Robb, J. , Liu, C.M. and Thomma, B.P.H.J. (2009) Genetic dissection of Verticillium wilt resistance mediated by tomato Ve1. Plant Physiol. 150, 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin, E.F. , Abd‐El‐Haliem, A. , Masini, L. , van den Berg, G.C.M. , Joosten, M.H.A.J. and Thomma, B.P.H.J. (2011) Interfamily transfer of tomato Ve1 mediates Verticillium resistance in Arabidopsis . Plant Physiol. 156, 2255–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França, S.C. , Spiessens, K. , Pollet, S. , Debode, J. , De Rooster, L. , Callens, D. and Höfte, M. (2013) Population dynamics of Verticillium species in cauliflower fields: influence of crop rotation, debris removal and ryegrass incorporation. Crop Prot. 54, 134–141. [Google Scholar]
- Garber, R.H. and Houston, B.R. (1966) Penetration and development of Verticillium albo‐atrum in the cotton plant. Phytopathology, 56, 1121–1126. [Google Scholar]
- Gladders, P. , Smith, J.A. , Kirkpatrick, L. , Clewes, E. , Grant, C. , Barbara, D. , Barnes, A.V. and Lane, C.R. (2011) First record of Verticillium wilt (Verticillium longisporum) in winter oilseed rape in the UK. New Dis. Rep. 23, 8. [Google Scholar]
- Happstadius, I. , Ljungberg, A. , Kristiansson, B. and Dixelius, C. (2003) Identification of Brassica oleracea germplasm with improved resistance to Verticillium wilt. Plant Breed. 122, 30–34. [Google Scholar]
- Hastie, A.C. (1973) Hybridization of Verticillium albo‐atrum and Verticillium dahliae . Trans. Br. Mycol. Soc. 60, 511–523. [Google Scholar]
- Hayes, R.J. , Truco, M.J. , Vallad, G.E. , McHale, L.K. , Ochoa, O.E. , Michelmore, R.W. , Klosterman, S.J. , Maruthachalam, K. and Subbarao, K.V. (2011) The inheritance of resistance to race 1 isolates of Verticillium dahliae in the lettuce cultivar La Brillante. Theor. Appl. Genet. 123, 509–517. [DOI] [PubMed] [Google Scholar]
- He, Y. , Fukushige, H. , Hildebrand, D.F. and Gan, S. (2002) Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 128, 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heale, J.B. and Karapapa, V.K. (1999) The Verticillium threat to Canada's major oilseed crop: canola. Can . J. Plant. Pathol. 21, 1–7. [Google Scholar]
- Inderbitzin, P. and Subbarao, K.V. (2014) Verticillium systematics and evolution: how confusion impedes Verticillium wilt management and how to resolve it. Phytopathology, 104, 564–574. [DOI] [PubMed] [Google Scholar]
- Inderbitzin, P. , Bostock, R.M. , Davis, R.M. , Usami, T. , Platt, H.W. and Subbarao, K.V. (2011a) Phylogenetics and taxonomy of the fungal vascular wilt pathogen Verticillium, with the descriptions of five new species. PLoS One, 6, e28341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderbitzin, P. , Davis, R.M. , Bostock, R.M. and Subbarao, K.V. (2011b) The ascomycete Verticillium longisporum is a hybrid and a plant pathogen with an expanded host range. PLoS One, 6, e18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderbitzin, P. , Davis, R.M. , Bostock, R.M. and Subbarao, K.V. (2013) Identification and differentiation of Verticillium species and V. longisporum lineages by simplex and multiplex PCR assays. PLoS One, 8, e65990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram, R. (1968) Verticillium dahliae var. longisporum, a stable diploid. Trans. Br. Mycol. Soc. 51, 339–341. [Google Scholar]
- Isaac, I. (1957) Verticillium wilt of Brussels sprout. Ann. Appl. Biol. 45, 276–283. [DOI] [PubMed] [Google Scholar]
- Iven, T. , König, S. , Singh, S. , Braus‐Stromeyer, S.A. , Bischoff, M. , Tietze, L.F. , Braus, G.H. , Lipka, V. , Feussner, I. and Dröge‐Laser, W. (2012) Transcriptional activation and production of tryptophan‐derived secondary metabolites in Arabidopsis roots contributes to the defense against the fungal vascular pathogen Verticillium longisporum . Mol. Plant. 5, 1389–1402. [DOI] [PubMed] [Google Scholar]
- Jackson, C.W. and Heale, J.B. (1985) Relationship between DNA content and spore volume in sixteen isolates of Verticillium lecanii and two new diploids of V. dahliae (=V. dahliae var. longisporum Stark). J. Gen. Microbiol. 131, 3229–3236. [Google Scholar]
- Joaquim, T.R. and Rowe, R.C. (1990) Reassessment of vegetative compatibility relationships among strains of Verticillium dahliae using nitrate‐nonutilizing mutants. Phytopathology, 80, 1160–1166. [Google Scholar]
- Johansson, A. , Goud, J.K.C. and Dixelius, C. (2006a) Plant host range of Verticillium longisporum and microsclerotia density in Swedish soils. Eur. J. Plant Pathol. 114, 139–149. [Google Scholar]
- Johansson, A. , Staal, J. and Dixelius, C. (2006b) Early responses in the Arabidopsis–Verticillium longisporum pathosystem are dependent on NDR1, JA‐ and ET‐associated signals via cytosolic NPR1 and RFO1 Mol. Plant–Microbe Interact 19, 958–969. [DOI] [PubMed] [Google Scholar]
- de Jonge, R. , van Esse, H.P. , Maruthachalam, K. , Bolton, M.D. , Santhanam, P. , Saber, M.K. , Zhang, Z. , Usami, T. , Lievens, B. , Subbarao, K.V. and Thomma, B.P.H.J. (2012) Tomato immune receptor Ve1 recognizes effector of multiple fungal pathogens uncovered by genome and RNA sequencing. Proc. Natl Acad. Sci. USA, 109, 5110–5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamble, A. , Koopmann, B. and Tiedemann, A.V. (2013) Induced resistance to Verticillium longisporum in Brassica napus by β‐aminobutyric acid. Plant Pathol. 62, 552–561. [Google Scholar]
- Karapapa, V.K. and Typas, M.A. (2001) Molecular characterization of the host‐adapted pathogen Verticillium longisporum on the basis of a group‐I intron found in the nuclear SSU‐rRNA gene. Curr. Microbiol. 42, 217–224. [DOI] [PubMed] [Google Scholar]
- Karapapa, V.K. , Bainbridge, B.W. and Heale, J.B. (1997) Morphological and molecular characterization of Verticillium longisporum comb. nov., pathogenic to oilseed rape. Mycol. Res. 101, 1281–1294. [Google Scholar]
- Kawchuk, L.M. , Hachey, J. , Lynch, D.R. , Kulcsar, F. , van Rooijen, G. , Waterer, D.R. , Robertson, A. , Kokko, E. , Byers, R. , Howard, R.J. , Fischer, R. and Prüfer, D. (2001) Tomato Ve disease resistance genes encode cell surface‐like receptors. Proc. Natl Acad. Sci. USA, 98, 6511–6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmochi, I. , Kobayashi, I. , Tsuchiya, M. , Sakai, H. and Shimizu, M. (2000) Breeding materials for resistance to Verticillium wilt in Japanese cabbage. J. Jpn. Soc. Hort. Sci. 69, 483–491. [Google Scholar]
- Keunecke, H. (2009) Einfluss von Kohlfliegenbefall auf die Infektion und Schadwirkung von Verticillium longisporum und Phoma lingam an Raps. PhD thesis, University of Göttingen (in German).
- Klebahn, H. (1913) Beiträge zur Kenntnis der Fungi imperfecti. 1. Eine Verticillium‐Krankheit auf Dahlien. Mycol. Centbl. 3, 49–66. [Google Scholar]
- Klosterman, S.J. , Subbarao, K.V. , Kang, S. , Veronese, P. , Gold, S.E. , Thomma, B.P.H.J. , Chen, Z. , Henrissat, B. , Lee, Y.H. , Park, J. , Garcia‐Pedrajas, M.D. , Barbara, D.J. , Anchieta, A. , de Jonge, R. , Santhanam, P. , Maruthachalam, K. , Atallah, Z. , Amyotte, S.G. , Paz, Z. , Inderbitzin, P. , Hayes, R.J. , Heiman, D.I. , Young, S. , Zeng, Q. , Engels, R. , Galagan, J. , Cuomo, C.A. , Dobinson, K.F. and Ma, L.J. (2011) Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog. 7, e1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht, K. , Seyffarth, M. , Desel, C. , Thurau, T. , Sherameti, I. , Lou, B. , Oelmüller, R. and Cai, D. (2010) Expression of BvGLP‐1 encoding a germin‐like protein from sugar beet in Arabidopsis thaliana leads to resistance against phytopathogenic fungi. Mol. Plant–Microbe Interact. 23, 446–457. [DOI] [PubMed] [Google Scholar]
- Koike, S.T. , Subbarao, K.V. , Davis, R.M. , Gordon, T.R. and Hubbard, J.C. (1994) Verticillium wilt of cauliflower in California. Plant Dis. 78, 1116–1121. [Google Scholar]
- König, S. , Feussner, K. , Kaever, A. , Landesfeind, M. , Thurow, C. , Karlovsky, P. , Gatz, C. , Polle, A. and Feussner, I. (2014) Soluble phenylpropanoids are involved in the defense response of Arabidopsis against Verticillium longisporum . New Phytol. 202, 823–837. [DOI] [PubMed] [Google Scholar]
- Kroeker, G. (1970) Vissnesjuka på rabs och rybs i Skåne orsakad av Verticillium. [Verticillium on oilseed rape and turnip rape in Scania caused by Verticillium.] Svensk Frötidning. 19, 10–13. [Google Scholar]
- Leino, M. (2006) Fungal Diseases on Oilseed Rape and Turnip Rape. Norrköping: Jordbruksverket.
- Li, Y. , Zhang, Q. , Zhang, J. , Wu, L. , Qi, Y. and Zhou, J.M. (2010) Identification of microRNAs involved in pathogen‐associated molecular pattern‐triggered plant innate immunity. Plant Physiol. 152, 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet, J. (2007) Hybrid speciation. Nature, 446, 279–283. [DOI] [PubMed] [Google Scholar]
- Moon, C.D. , Craven, K.D. , Leuchtmann, A. , Clement, S.L. and Schardl, C.L. (2004) Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol. Ecol. 13, 1455–1467. [DOI] [PubMed] [Google Scholar]
- Müller, H. and Berg, G. (2008) Impact of formulation procedures on the effect of the biocontrol agent Serratia plymuthica HRO‐C48 on Verticillium wilt in oilseed rape. BioControl, 53, 905–916. [Google Scholar]
- Nagao, H. , Wakatabe, D. and Iijima, T. (1994) Difficulty to establish vegetative compatibility of Japanese isolates of Verticillium dahliae Kleb. using melanin‐synthesis deficient mutants. J. Gen. Appl. Microbiol. 40, 277–285. [Google Scholar]
- Narisawa, K. , Tokumasu, S. and Hashiba, T. (1998) Suppression of clubroot formation in Chinese cabbage by the root endophytic fungus, Heteroconium chaetospira . Plant Pathol. 47, 206–210. [Google Scholar]
- Narisawa, K. , Ohki, K.T. and Hashiba, T. (2000) Suppression of clubroot and Verticillium yellows in Chinese cabbage in the field by the root endophytic fungus, Heteroconium chaetospira . Plant Pathol. 49, 141–146. [Google Scholar]
- Narisawa, K. , Usuki, F. and Hashiba, T. (2004) Control of Verticillium yellows in Chinese cabbage by the dark septate endophytic fungus LtVB3. Phytopathology, 94, 412–418. [DOI] [PubMed] [Google Scholar]
- Niazi, A. , Manzoor, S. , Asari, S. , Bejai, S. , Meijer, J. and Bongcam‐Rudloff, E. (2014) Genome analysis of Bacillus amyloliquefaciens subsp. plantarum UCMB5113: a rhizobacterium that improves plant growth and stress management. PLoS One, 9, e104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njoroge, S.M.C. , Vallad, G.E. , Park, S.Y. , Kang, S. , Koike, S.T. , Bolda, M. , Burman, P. , Polonik, W. and Subbarao K.V. (2011) Phenological and phytochemical changes correlate with differential interactions of Verticillium dahliae with broccoli and cauliflower. Phytopathology, 101, 523–534. [DOI] [PubMed] [Google Scholar]
- Novakazi, F. , Inderbitzin, P. , Sandoya, G. , Hayes, R.J. , Tiedemann, A.V. and Subbarao, K.V. (2015) The three lineages of the diploid hybrid Verticillium longisporum differ in virulence and pathogenicity. Phytopathology, 105, 662–673. [DOI] [PubMed] [Google Scholar]
- Obermeier, C. , Hossain, M.A. , Snowdon, R. , Knüfer, J. , Tiedemann, A.V. and Friedt, W. (2013) Genetic analysis of phenylpropanoid metabolites associated with resistance against Verticillium longisporum in Brassica napus . Mol. Breed. 31, 347–361. [Google Scholar]
- Ohtaka, N. and Narisawa, K. (2008) Molecular and endophytic nature of the root‐associated fungus Meliniomyces variabilis (LtVB3). J. Gen. Plant Pathol. 74, 24–31. [Google Scholar]
- Okoli, C.A.N. , Carder, J.H. and Barbara, D.J. (1994) Restriction fragment length polymorphisms (RFLPs) and the relationships of some host‐adapted isolates of Verticillium dahliae . Plant Pathol. 43, 33–40. [Google Scholar]
- Pantou, M.P. , Strunnikova, O.K. , Shakhnazarova, V.Y. , Vishnevskaya, N.A. , Papalouka, V.G. and Typas, M.A. (2005) Molecular and immunochemical phylogeny of Verticillium species. Mycol. Res. 109, 889–902. [DOI] [PubMed] [Google Scholar]
- Pegg, G.F. and Brady, B.L. (2002) Verticillium Wilts. Wallingford, Oxfordshire: CABI Publishing. [Google Scholar]
- Powelson, R.L. and Carter, G.E. (1973) Efficacy of soil fumigants for control of Verticillium wilt of potatoes. Am. Potato J. 50, 162–167. [Google Scholar]
- Price, J.S. , Hobson, R.N. , Neale M.A. and Bruce, D.M. (1996) Seed losses in commercial harvesting of oilseed rape. J. Agric. Eng. Res. 65, 183–191. [Google Scholar]
- Puhalla, J.E. (1979) Classification of isolates of Verticillium dahliae based on heterokaryon incompatibility. Phytopathology, 69, 1186–1189. [Google Scholar]
- Pullman, G.S. , DeVay, J.E. and Garber, R.H. (1981) Soil solarization and thermal death: a logarithmic relationship between time and temperature for four soilborne plant pathogens. Phytopathology, 71, 959–964. [Google Scholar]
- Qin, Q.M. , Vallad, G.E. , Wu, B.M. and Subbarao, K.V. (2006) Phylogenetic analyses of phytopathogenic isolates of Verticillium spp. Phytopathology, 96, 582–592. [DOI] [PubMed] [Google Scholar]
- Ralhan, A. , Schöttle, S. , Thurow, C. , Iven, T. , Feussner, I. , Polle, A. and Gatz, C. (2012) The vascular pathogen Verticillium longisporum requires a jasmonic acid‐independent COI1 function in roots to elicit disease symptoms in Arabidopsis shoots. Plant Physiol. 159, 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzinger, A. , Riediger, N. , Tiedemann, A.V. and Karlovsky, P. (2009) Salicylic acid and salicylic acid glucoside in xylem sap of Brassica napus infected with Verticillium longisporum . J. Plant Res. 122, 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusche, M. , Thole, K. , Janz, D. , Truskina, J. , Rindfleisch, S. , Drübert, C. , Polle, A. , Lipka, V. and Teichmann, T. (2012) Verticillium infection triggers VASCULAR‐RELATED NAC DOMAIN7‐dependent de novo xylem formation and enhances drought tolerance in Arabidopsis . Plant Cell, 24, 3823–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusche, M. , Klásková, J. , Thole, K. , Truskina, J. , Novák, O. , Janz, D. , Strnad, M. , Spíchal, L. , Lipka, V. and Teichmann, T. (2013) Stabilization of cytokinin levels enhances Arabidopsis resistance against Verticillium longisporum . Mol. Plant–Microbe Interact. 26, 850–860. [DOI] [PubMed] [Google Scholar]
- Reusche, M. , Truskina, J. , Thole, K. , Nagel, L. , Rindfleisch, S. , Tran, V.T. , Braus‐Stromeyer, S.A. , Braus, G.H. , Teichmann, T. and Lipka, V. (2014) Infections with the vascular pathogens Verticillium longisporum and Verticillium dahliae induce distinct disease symptoms and differentially affect drought stress tolerance of Arabidopsis thaliana . Environ. Exp. Bot. 108, 23–37. [Google Scholar]
- Roos, J. , Bejai, S. , Oide, S. and Dixelius, C. (2014) RabGAP22 is required for defense to the vascular pathogen Verticillium longisporum and contributes to stomata immunity. PLoS One, 9, e88187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos, J. , Bejai, S. , Mozūraitis, R. and Dixelius, C. (2015) Susceptibility to Verticillium longisporum is linked to monoterpene production by TPS23/27 in Arabidopsis . Plant J. 81, 572–585. [DOI] [PubMed] [Google Scholar]
- Rygulla, W. , Friedt, W. , Seyis, F. , Lühs, W. , Eynck, C. , Tiedemann, A.V. and Snowdon, R.J. (2007a) Combination of resistance to Verticillium longisporum from zero erucic acid Brassica oleracea and oilseed Brassica rapa genotypes in resynthesized rapeseed (Brassica napus) lines. Plant Breed. 126, 596–602. [Google Scholar]
- Rygulla, W. , Snowdon, R.J. , Eynck, C. , Koopmann, B. , Tiedemann, A.V. , Lühs, W. and Friedt, W. (2007b) Broadening the genetic basis of Verticillium longisporum resistance in Brassica napus by interspecific hybridization. Phytopathology, 97, 1391–1396. [DOI] [PubMed] [Google Scholar]
- Rygulla, W. , Snowdon, R.J. , Friedt, W. , Happstadius, I. , Cheung, W.Y. and Chen, D. (2008) Identification of quantitative trait loci for resistance against Verticillium longisporum in oilseed rape (Brassica napus). Phytopathology, 98, 215–221. [DOI] [PubMed] [Google Scholar]
- Sarosh, B.R. , Danielsson, J. and Meijer, J. (2009) Transcript profiling of oilseed rape (Brassica napus) primed for biocontrol differentiates genes involved in microbial interactions with beneficial Bacillus amyloliquefaciens from pathogenic Botrytis cinerea . Plant Mol. Biol. 70, 31–45. [DOI] [PubMed] [Google Scholar]
- Seyis, F. , Snowdon, R.J. , Lühs, W. and Friedt, W. (2003) Molecular characterization of novel resynthesized rapeseed (Brassica napus) lines and analysis of their genetic diversity in comparison with spring rapeseed cultivars. Plant Breed. 122, 473–478. [Google Scholar]
- Shen, D. , Suhrkamp, I. , Wang, Y. , Liu, S. , Menkhaus, J. , Verreet, J.A. , Fan, L. and Cai, D. (2014) Identification and characterization of microRNAs in oilseed rape (Brassica napus) responsive to infection with the pathogenic fungus Verticillium longisporum using Brassica AA (Brassica rapa) and CC (Brassica oleracea) as reference genomes. New Phytol. 204, 577–594. [DOI] [PubMed] [Google Scholar]
- Shetty, K.G. , Subbarao, K.V. , Huisman, O.C. and Hubbard, J.C. (2000) Mechanism of broccoli‐mediated Verticillium wilt reduction in cauliflower. Phytopathology, 90, 305–310. [DOI] [PubMed] [Google Scholar]
- Short, D.P.G , Gurung, S. , Hu, X. , Inderbitzin, P. and Subbarao, K.V. (2014) Maintenance of sex‐related genes and the co‐occurrence of both mating types in Verticillium dahliae . PLoS One, 9, e112145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebold, M. and Tiedemann A.V. (2013) Effects of experimental warming on fungal disease progress in oilseed rape. Glob. Chang. Biol. 19, 1736–1747. [DOI] [PubMed] [Google Scholar]
- Singh, S. , Braus‐Stromeyer, S.A. , Timpner, C. , Tran, V.T. , Lohaus, G. , Reusche, M. , Knüfer, J. , Teichmann, T. , Tiedemann, A.V. and Braus, G.H. (2010) Silencing of Vlaro2 for chorismate synthase revealed that the phytopathogen Verticillium longisporum induces the cross‐pathway control in the xylem. Appl. Microbiol. Biotechnol. 85, 1961–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler, M. and Tiedemann, A.V. (2014) Biocontrol potential of Microsphaeropsis ochracea on microsclerotia of Verticillium longisporum in environments differing in microbial complexity. BioControl, 59, 449–460. [Google Scholar]
- Stapleton, J.J. (2000) Soil solarization in various agricultural production systems. Crop Prot. 19, 837–841. [Google Scholar]
- Stark, C. (1961) Das Auftreten der Verticillium‐Tracheomykosen in Hamburger Gartenbaukulturen. Gartenbauwissenschaf. 2, 493–528. [Google Scholar]
- Steventon, L.A. , Fahleson, J. , Hu, Q. and Dixelius, C. (2002) Identification of the causal agent of Verticillium wilt of winter oilseed rape in Sweden, V. longisporum . Mycol. Res. 106, 570–578. [Google Scholar]
- Subbarao, K.V. (2002) Methyl bromide alternatives: meeting the deadlines – Introduction. Phytopathology, 92, 1334–1336. [DOI] [PubMed] [Google Scholar]
- Subbarao, K.V. and Hubbard, J.C. (1996) Interactive effects of broccoli residue and temperature on Verticillium dahliae microsclerotia in soil and on wilt in cauliflower. Phytopathology, 86, 1303–1310. [Google Scholar]
- Subbarao, K.V. and Hubbard, J.C. (1999) Evaluation of broccoli residue incorporation into field soil for Verticillium wilt control in cauliflower. Plant Dis. 83, 124–129. [DOI] [PubMed] [Google Scholar]
- Subbarao, K.V. , Chassot, A. , Gordon, T.R. , Hubbard, J.C. , Bonello, P. , Mullin, R. , Okamoto, D. , Davis, R.M. and Koike S.T. (1995) Genetic relationships and cross pathogenicities of Verticillium dahliae isolates from cauliflower and other crops. Phytopathology, 85, 1105–1112. [Google Scholar]
- Timpner, C. , Braus‐Stromeyer, S.A. , Tran, V.T. and Braus, G.H. (2013) The Cpc1 regulator of the cross‐pathway control of amino acid biosynthesis is required for pathogenicity of the vascular pathogen Verticillium longisporum . Mol. Plant–Microbe Interact. 26, 1312–1324. [DOI] [PubMed] [Google Scholar]
- Tran, V.T. , Braus‐Stromeyer, S.A. , Timpner, C. and Braus, G.H. (2013) Molecular diagnosis to discriminate pathogen and a pathogen species of the hybrid Verticillium longisporum on the oilseed crop Brassica napus . Appl. Microbiol. Biotechnol. 97, 4467–4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Typas, M.A. and Heale, J.B. (1976) Heterokaryosis and role of cytoplasmic inheritance in dark resting structure formation in Verticillium spp. Mol. Gen. Genet. 146, 17–26. [Google Scholar]
- Tyvaert, L. , França, S.C. , Debode, J. and Höfte, M. (2014) The endophyte Verticillium Vt305 protects cauliflower against Verticillium wilt. J. Appl. Microbiol. 116, 1563–1571. [DOI] [PubMed] [Google Scholar]
- Vargas‐Machuca, R. , Martin, C. and Galindez, W. (1987) Recovery of Verticillium dahliae from weed plants in farmers’ fields in Peru. Plant Dis. 71, 756–758. [Google Scholar]
- Veronese, P. , Narasimhan, M.L. , Stevenson, R.A. , Zhu, J.K. , Weller, S.C. , Subbarao, K.V. and Bressan, R.A. (2003) Identification of a locus controlling Verticillium disease symptom response in Arabidopsis thaliana . Plant J. 35, 574–587. [DOI] [PubMed] [Google Scholar]
- Watanabe, T. , Ozawa, M. and Sakai, R. (1973) A new disease of Chinese cabbage caused by Verticillium albo‐atrum and some factors related to the incidence of the disease. Ann. Phytopathol. Soc. Jpn. 39, 344–349. [Google Scholar]
- Wilhelm, S. (1955) Longevity of Verticillium wilt fungus in the laboratory and field. Phytopathology, 45, 180–181. [Google Scholar]
- Wittstock, U. and Halkier B.A. (2002) Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 7, 263–270. [DOI] [PubMed] [Google Scholar]
- Witzel, K. , Hanschen, F.S. , Klopsch, R. , Ruppel, S. , Schreiner, M. and Grosch, R. (2015) Verticillium longisporum infection induces organ‐specific glucosinolate degradation in Arabidopsis thaliana . Front. Plant Sci. 6, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolliams, G.E. (1966) Host range and symptomatology of Verticillium dahliae in economic, weed, and native plants in interior British Columbia. Can. J. Plant Sci. 46, 661–669. [Google Scholar]
- Xiao, C.L. and Subbarao, K.V. (1998) Relationships between Verticillium dahliae inoculum density and wilt incidence, severity, and growth of cauliflower. Phytopathology, 88, 1108–1115. [DOI] [PubMed] [Google Scholar]
- Xiao, C.L. , Subbarao, K.V. , Schulbach, K.F. and Koike, S.T. (1998) Effects of crop rotation and irrigation on Verticillium dahliae microsclerotia in soil and wilt in cauliflower. Phytopathology, 88, 1046–1055. [DOI] [PubMed] [Google Scholar]
- Yadeta, K.A. , Hanemian, M. , Smit, P. , Hiemstra, J.A. , Pereira, A. , Marco, Y. and Thomma B.P.H.J. (2011) The Arabidopsis thaliana DNA‐binding protein AHL19 mediates Verticillium wilt resistance. Mol. Plant–Microbe Interact. 24, 1582–1591. [DOI] [PubMed] [Google Scholar]
- Yadeta, K.A. , Valkenburg, D.J. , Hanemian, M. , Marco, Y. and Thomma, B.P.H.J. (2014) The Brassicaceae‐specific EWR1 gene provides resistance to vascular wilt pathogens. PLoS One, 9, e88230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare, R. , Gams, W. , Starink‐Willemse, M. and Summerbell, R.C. (2007) Gibellulopsis, a suitable genus for Verticillium nigrescens, and Musicillium, a new genus for V. theobromae . Nova Hedwigia, 85, 463–489. [Google Scholar]
- Zeise, K. and Tiedemann, A.V. (2001) Morphological and physiological differentiation among vegetative compatibility groups of Verticillium dahliae in relation to V. longisporum . J. Phytopathol. 149, 469–475. [Google Scholar]
- Zeise, K. and Tiedemann, A.V. (2002) Host specialization among vegetative compatibility groups of Verticillium dahliae in relation to Verticillium longisporum . J. Phytopathol. 150, 112–119. [Google Scholar]
- Zhang, B. , Yang, Y. , Chen, T. , Yu, W. , Liu, T. , Li, H. , Fan, X. , Ren, Y. , Shen, D. , Liu, L. , Dou, D. and Chang, Y. (2012) Island cotton Gbve1 gene encoding a receptor‐like protein confers resistance to both defoliating and non‐defoliating isolates of Verticillium dahliae . PLoS One, 7, e51091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N. , Castlebury, L.A. , Miller, A.N. , Huhndorf, S.M. , Schoch, C.L. , Seifert, K.A. , Rossman, A.Y. , Rogers, J.D. , Kohlmeyer, J. , Volkmann‐Kohlmeyer, B. and Sung G.H. (2006) An overview of the systematics of the Sordariomycetes based on a four‐gene phylogeny. Mycologia, 98, 1076–1087. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, X. , Yang, S. , Chit, J. , Zhang, G. and Ma, Z. (2011) Cloning and characterization of a Verticillium wilt resistance gene from Gossypium barbadense and functional analysis in Arabidopsis thaliana . Plant Cell Rep. 30, 2085–2096. [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Fradin, E. , de Jonge, R. , van Esse, H.P. , Smit, P. , Liu, C.M. and Thomma, B.P.H.J. (2013) Optimized agroinfiltration and virus‐induced gene silencing to study Ve1‐mediated Verticillium resistance in tobacco. Mol. Plant–Microbe Interact. 26, 182–190. [DOI] [PubMed] [Google Scholar]
- Zhou, L. , Hu, Q. , Johansson, A. and Dixelius, C. (2006) Verticillium longisporum and V. dahliae: infection and disease in Brassica napus . Plant Pathol. 55, 137–144. [Google Scholar]
- Zolan, M.E. (1995) Chromosome‐length polymorphism in fungi. Microbiol. Rev. 59, 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]


