Summary
Over recent decades, a multitude of studies have shown the ability of silicon (Si) to protect various plants against a range of microbial pathogens exhibiting different lifestyles and infection strategies. Despite this relative wealth of knowledge, an understanding of the action mechanism of Si is still in its infancy, which hinders its widespread application for agricultural purposes. In an attempt to further elucidate the molecular underpinnings of Si‐induced disease resistance, we studied the transcriptome of control and Si‐treated rice plants infected with the necrotrophic brown spot fungus Cochliobolus miyabeanus. Analysis of brown spot‐infected control plants suggested that C. miyabeanus represses plant photosynthetic processes and nitrate reduction in order to trigger premature senescence and cause disease. In Si‐treated plants, however, these pathogen‐induced metabolic alterations are strongly impaired, suggesting that Si alleviates stress imposed by the pathogen. Interestingly, Si also significantly increased photorespiration rates in brown spot‐infected plants. Although photorespiration is often considered as a wasteful process, recent studies have indicated that this metabolic bypass also enhances resistance during abiotic stress and pathogen attack by protecting the plant's photosynthetic machinery. In view of these findings, our results favour a scenario in which Si enhances brown spot resistance by counteracting C. miyabeanus‐induced senescence and cell death via increased photorespiration. Moreover, our results shed light onto the mechanistic basis of Si‐induced disease control and support the view that, in addition to activating plant immune responses, Si can also reduce disease severity by interfering with pathogen virulence strategies.
Keywords: Bipolaris oryzae, photorespiration, photosynthesis, plant immunity, plant–microbe interactions, rice, silicon
Introduction
The second most abundant element in the Earth's crust, silicon (Si), can comprise up to 70% of the soil mass in the form of silicate minerals and water‐soluble orthosilicic acid. Orthosilicic acid is readily taken up by plant roots and loaded into the xylem by a specific transporter system (Ma and Yamaji, 2006). Silicic acid is transported to the shoots via the xylem, where it is constantly polymerized, either as silica in the cell or as an insoluble, subcuticular silica layer outside the cell (Ma et al., 2011). To date, dozens of studies have documented the ability of Si to enhance plant growth and yield, and it is the only nutrient that is not detrimental when accumulated in excess (Epstein and Bloom, 2005). Moreover, Si‐treated plants often display enhanced resistance against a wide variety of biotic and abiotic stresses (Epstein, 2009; Fauteux et al., 2005; Guntzer et al., 2011; Ma and Yamaji, 2006; Van Bockhaven et al., 2013). As such, Si amendment is one of the only plant protection strategies that enables plants to maximize the efficiency of their response to the exact set of environmental conditions encountered, at the same time as conserving resources for growth and development.
Although much remains to be discovered about the underlying mechanisms, a number of recent microarray and proteome analyses have begun to elucidate the molecular basis and regulation of Si‐induced stress resistance (Brunings et al., 2009; Chain et al., 2009; Fauteux et al., 2005; Fleck et al., 2011; Ghareeb et al., 2011; Nwugo and Huerta, 2011). Emerging from these studies is the view that, rather than warding off pathogen attack through the formation of a physical silica barrier, Si mainly acts as a biological inducer of a wide variety of plant immune responses. Yet, Si does not appear to activate resistance per se, but rather intensifies and/or accelerates basal defence responses following stress perception. Moreover, several reports have documented the ability of Si to increase photosynthesis rates in plants subjected to either biotic or abiotic stresses, suggesting that Si also impinges on the primary metabolism of stressed plants (Chen et al., 2011; Dallagnol et al., 2013a; Farooq et al., 2013; Nwugo and Huerta, 2008; Perez et al., 2014; Resende et al., 2012; Shen et al., 2010).
In plants, primary metabolism encompasses both energy‐ and carbohydrate‐providing processes, such as photosynthesis, gluconeogenesis and respiration, as well as nitrogen‐associated reactions accounting for the incorporation of inorganic and organic nitrogen in amino acids (Rontein et al., 2002). Plant–pathogen interactions are generally determined by the pathogen's need for nutrients and carbohydrates, and the plant's requirement for energy to fuel defence mechanisms (Berger et al., 2007; Bolton, 2009). As plant primary metabolism is at the crossroads of these contrasting interests, central metabolic processes are believed to play a key role in moulding pathological outcomes (Rojas et al., 2014). Moreover, current concepts suggest that these infection‐induced metabolic alterations lie on a continuum between ‘endurance’ and ‘evasion’, two opposing physiological states during which plant cell death is suppressed or facilitated, respectively (Seifi et al., 2013). The endurance strategy is mostly effective against pathogens with a necrotrophic lifestyle, whereas evasion generally confers resistance towards biotrophic pathogens (Glazebrook, 2005; Mur et al., 2013; Seifi et al., 2013).
Interestingly, pathogens seem to have evolved sophisticated mechanisms to exploit these opposing metabolic states for their own benefit. For instance, archetypal necrotrophic pathogens, such as Botrytis cinerea and Sclerotinia sclerotiorum, are well known to trick the plant into activating certain types of programmed cell death, thus facilitating their own infection process (Govrin and Levine, 2000; Kabbage et al., 2013; Levine, 2007; Williams et al., 2011). Other necrotrophs have likewise been hypothesized to co‐opt plant apoptotic pathways in order to cause disease, including the rice fungal pathogen Cochliobolus miyabeanus (Xiao et al., 1991). Also known as Bipolaris oryzae (anamorph; Breda de Haan), C. miyabeanus is the causal agent of the devastating rice brown spot disease (Dela Paz et al., 2006). Consistent with plant cell death facilitating C. miyabeanus infection, brown spot is especially prevalent in rainfed ecosystems in which plants are prone to premature senescence as a result of suboptimal growth conditions (Leung et al., 2003; Ou, 1985; Zadoks, 2003). Although semi‐resistant rice cultivars are available, brown spot management still relies heavily on the application of hazardous fungicides (Castell‐Miller and Samac, 2012). In this context, the application of Si holds great potential for environmentally friendly, economically sound and sustainable control of brown spot disease. However, despite some recent progress (Dallagnol et al., 2009, 2011a), much remains to be learned about the precise mechanisms through which Si protects rice from brown spot attack.
Aiming to shed more light on the rice–C. miyabeanus interaction and the beneficial effect of Si on plant disease resistance, we performed a microarray experiment comparing the transcriptome of control and Si‐treated plants during the early stages of infection [12 h post‐inoculation (hpi)]. Our data support a central role of nitrogen‐ and photosynthesis‐related metabolic processes in shaping the outcome of rice–brown spot interactions. Moreover, we propose that Si protects rice from brown spot attack by preventing the pathogen from hijacking the plant's primary metabolism.
Results
Si amendment promotes growth and development of non‐stressed rice plants
To assess the impact of Si on the growth phenotype of rice (cultivar Nipponbare), we employed a hydroponic gnotobiotic system, wherein plants were continuously supplied with 2 mm silicic acid. Compared with control non‐treated plants, Si‐supplied plants not only accumulated more biomass, but also displayed early flowering and improved tillering and ear filling (Fig. 1A, B). Furthermore, leaf gas exchange measurements showed that, although the level of respiration (R d) was similar for both treatments, Si application increased net photosynthesis as well as the level of photorespiration (Fig. 1D and Table S1, see Supporting Information). Similarly, Si‐treated plants showed higher levels of photochemical quenching (q P), which is a measure of the amount of light energy used by the plants. In addition, and as suggested by the significant increase in photosystem II (PSII) efficiency (ΦPSII), Si‐treated plants also seemed to be able to channel more light energy into the reaction centre of PSII, a phenomenon which is probably explained by the increased chlorophyll content in these plants (Fig. 1C). Considering that net photosynthesis was improved without altering the transpiration rates, Si treatment also seemed to positively influence plant water‐use efficiency.
Figure 1.
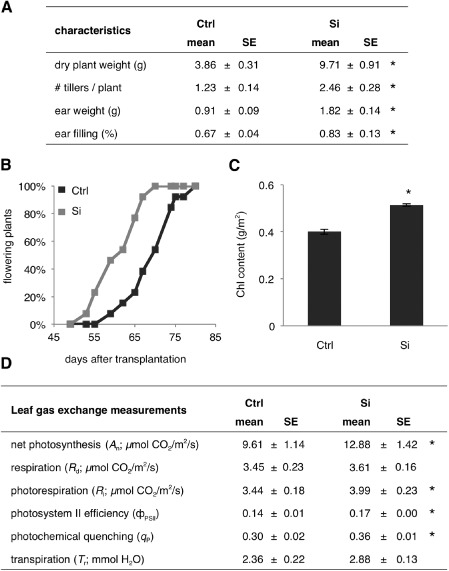
(A) Yield characteristics of control (Ctrl) and silicon (Si)‐treated rice plants. (B) Si treatment induces early flowering. (C) Impact of Si feeding on the plant chlorophyll (Chl) content. (D) Leaf gas exchange and chlorophyll a fluorescence measurements reveal a positive effect of Si on the photosynthetic abilities of treated plants. Data are means ± standard error (SE) of at least three independent biological repetitions. Asterisks indicate statistically significant differences compared with non‐treated controls (n ≥ 24, t‐test, α = 0.05).
Si enhances resistance to C. miyabeanus
Consistent with previous results (Dallagnol et al., 2009, 2013a, b; Rezende et al., 2009; Silva et al., 2012), the application of Si resulted in a substantial reduction in brown spot disease severity in both intact plant and detached leaf assays (Fig. 2A–D). Si‐treated rice leaves not only displayed slower disease development, but also significantly smaller lesions and substantially less chlorosis and necrosis on brown spot infection compared with inoculated control plants. The prophylactic role of Si has been mechanistically explained through the combined effects of passive protection offered by the hard, subcuticular silica layer and Si‐mediated recruitment of active plant defence mechanisms. To investigate the importance of passive and active defences in Si‐inducible brown spot resistance and to assess the time span during which Si is effective, the influence of different Si treatments on brown spot resistance was investigated (Fig. 2E). All Si treatments were routinely compared with control plants that were grown in the absence of Si (Si–/Si–). Plants receiving Si were either continuously treated with Si throughout the course of the experiment (Si+/Si+), supplemented with Si up to 3 days before inoculation (Si+/Si–) or short term treated with Si starting 3 days prior to inoculation (Si–/Si+). Interestingly, depriving plants of Si for 3 days prior to inoculation (Si+/Si–) significantly attenuated the level of Si‐inducible brown spot resistance, despite having no discernible impact on the silicification of trichomes and the subcuticular silica layer of Si‐treated plants. In contrast, plants treated with Si for 3 days only (Si–/Si+) were as resistant as continuously treated plants (Si+/Si+). Considering that such a short time Si application did not lead to visible silicification of the plants, these results strongly suggest that active Si effects outweigh the importance of Si‐imposed physical barriers in the development of Si‐inducible brown spot resistance. Consistent with this hypothesis, microscopic analysis of the infection process in control and Si‐treated plants revealed no significant differences in the number of successful penetration events, indicating that Si does not impede pre‐penetration development of C. miyabeanus (Van Bockhaven et al., 2015).
Figure 2.
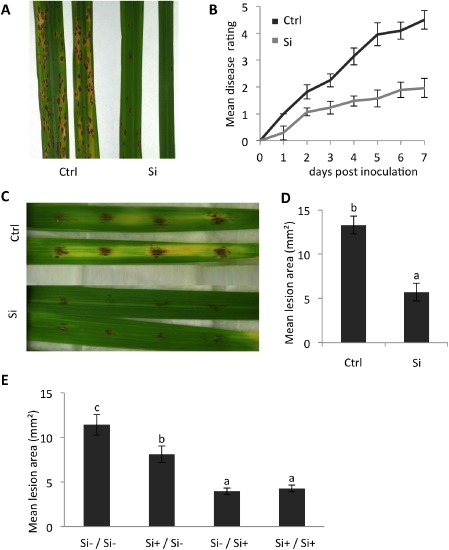
Silicon (Si) induces resistance against the rice brown spot fungus Cochliobolus miyabeanus. (A, B) Effect of Si feeding on symptom development following spray inoculation of intact plants. Disease was scored daily using a 1–5 disease severity scale as outlined in Experimental procedures. The photograph shown in (A) depicts the representative disease symptoms on control (Ctrl) and Si‐fed plants at 3 days post‐inoculation. (C, D) Effect of Si feeding on the mean lesion area 3 days after inoculation of detached leaf pieces with four 15‐μL droplets of spore solution (n = 20, Mann–Whitney, α = 0.05). (E) Impact of different Si treatments on the level of brown spot resistance at 3 days post‐C. miyabeanus infection. Plants were either continuously treated with Si (Si+/Si+), deprived of Si 3 days prior to inoculation (Si+/Si–) or short‐term treated with Si starting 3 days prior to inoculation (Si–/Si+). Plant non‐treated with Si (Si–/Si–) served as controls (n = 24, Mann–Whitney, α = 0.05). Different letters indicate statistically significant differences. All experiments were repeated at least twice with similar results.
Microarray experiment
To shed further light on the molecular basis underpinning Si‐induced resistance against C. miyabeanus, a microarray experiment was performed in which we compared the transcriptome of control and Si‐treated plants following pathogen infection (2 × 2 factorial design: control and Si‐treated; mock‐treated and infected). To maximize the chance of detecting causal resistance mechanisms, we chose to sample at 12 hpi, which is 6 h prior to the appearance of the first disease symptoms and 6 h post‐pathogen penetration. Of the 43 494 gene probes on the microarray, 2906 were significantly differentially expressed among the four comparisons (false discovery rate < 0.01; −2 > log2 fold change > 2) (Fig. 3A). The Volcano plots shown in Fig. 4 illustrate the distribution of the data points, the significance of the measured changes in gene expression levels and the number of differentially expressed genes for each treatment comparison. The dark grey dots represent genes with a low significance [–log10(P value) < 2], whereas the light grey dots are genes with a high significance [–log10(P value) > 2], but a fold change < 4. The black dots are the genes selected as differentially regulated [–log10(P value) > 2 and fold change > 4]. Using the Venny algorithm, we also constructed a Venn diagram to visualize the number of uniquely and commonly regulated genes for each pair of comparisons (Fig. 3B). Interestingly, Si had a fairly similar impact on uninfected (Si,m vs. Ctrl,m) and infected (Si,I vs. Ctrl,I) leaves, directing transcriptional reprogramming of 1822 and 2214 genes, respectively, most of which were down‐regulated (Fig. 3A and Tables S2 and S4, see Supporting Information). Brown spot infection, however, had a comparatively less pronounced effect, with 483 and 362 genes differentially expressed in control (Ctrl,I vs. Ctrl,m) and Si‐treated (Si,I vs. Si,m) leaves, respectively (Tables S3 and S5, see Supporting Information). Moreover, hierarchical clustering analysis (Fig. 3C) showed that the influence of Si in non‐inoculated leaves (Si,m vs. Ctrl,m) was similar to that in inoculated leaves (Si,I vs. Ctrl,I), whereas C. miyabeanus affected less genes in Si‐treated plants (Si,I vs. Si,m) than in non‐treated controls (Ctrl,I vs. Ctrl,m). Moreover, Si treatment appeared to reverse the effect of C. miyabeanus on the gene expression profile, whereby genes that were up‐regulated in inoculated control plants were now down‐regulated, and vice versa (Fig. 3C; comparison Ctrl,I vs. Ctrl,m and Si,I vs. Ctrl,I).
Figure 3.
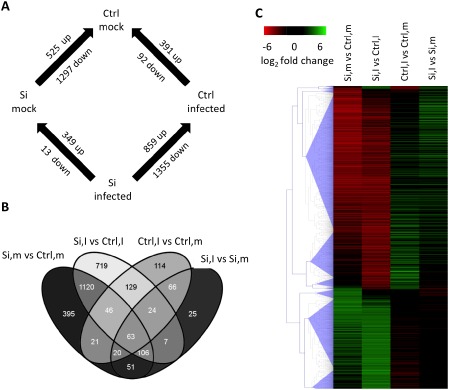
(A) Experimental design and the number of differentially expressed genes for each treatment comparison. (B) Venn diagram (Oliveros, 2007) showing genes differentially expressed for each comparison and all possible combinations. (C) Hierarchical clustering analysis identified similar expression patterns in Si‐treated vs. non‐treated rice leaves and in brown spot‐infected vs. uninfected samples (Euclidean distance, complete linkage clustering, false discovery rate < 0.01). Si, silicon; Ctrl, control; I, inoculated; m, mock‐inoculated.
Figure 4.
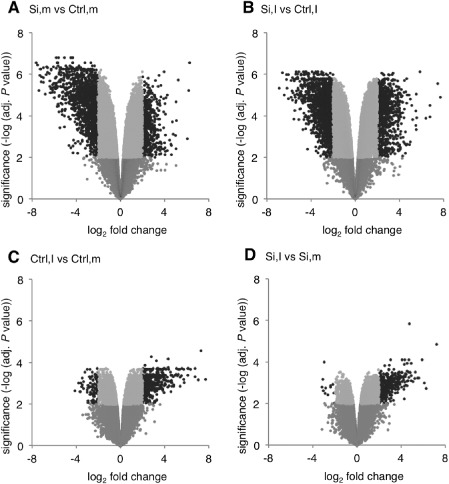
Volcano plots showing gene expression changes and associated significance values across the different treatment comparisons. Each array spot is represented by a single dot. Black dots represent differentially expressed genes [–2 > log2‐based signal ratio > 2 and −log10(P value) > 2]. Si, silicon; Ctrl, control; I, inoculated; m, mock‐inoculated.
To validate our microarray results, quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR) was performed on 15 genes that were differentially regulated on Si feeding. As shown in Table S6 and Fig. S1 (see Supporting Information), qRT‐PCR and microarray data were linearly correlated (R 2 = 0.91).
Gene ontology (GO) enrichment analysis
GO enrichment analysis using MapMan software (Usadel et al., 2009) identified a wide variety of metabolic processes (Fig. 5 and Table S7, see Supporting Information) and defence response pathways (Fig. 6 and Table S7) that were significantly influenced by brown spot infection and/or Si application.
Figure 5.
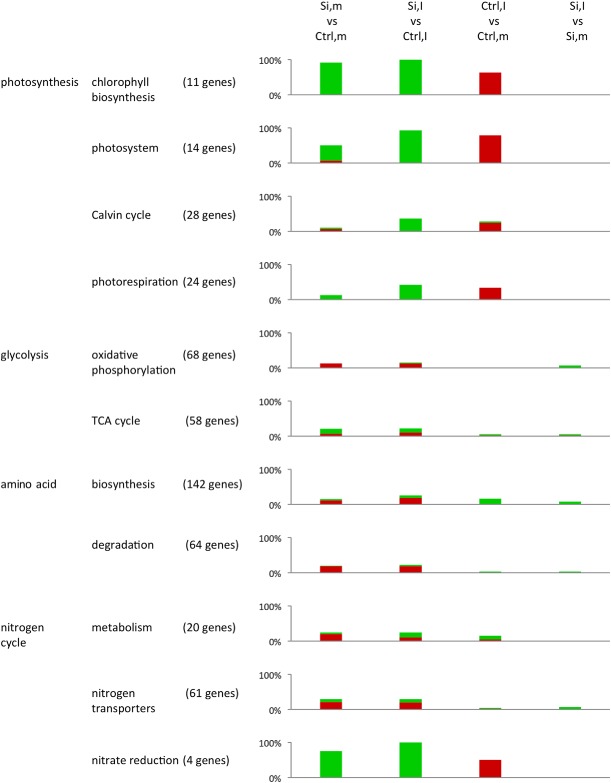
Gene ontology (GO) enrichment analysis demonstrating the impact of silicon treatment and brown spot infection on primary metabolic processes in rice (LIMMA; false discovery rate < 0.01). For each GO term, the total number of genes represented on the microarray are shown, whereas the coloured bars indicate the relative number of genes showing a significant difference in expression across the different treatment comparisons (green bars, up‐regulation, log2 fold change > 2; red bars, down‐regulation, log2 fold change < −2). GO terms were derived from the MapMan database (Usadel et al., 2009). Si, silicon; Ctrl, control; I, inoculated; m, mock‐inoculated.
Figure 6.
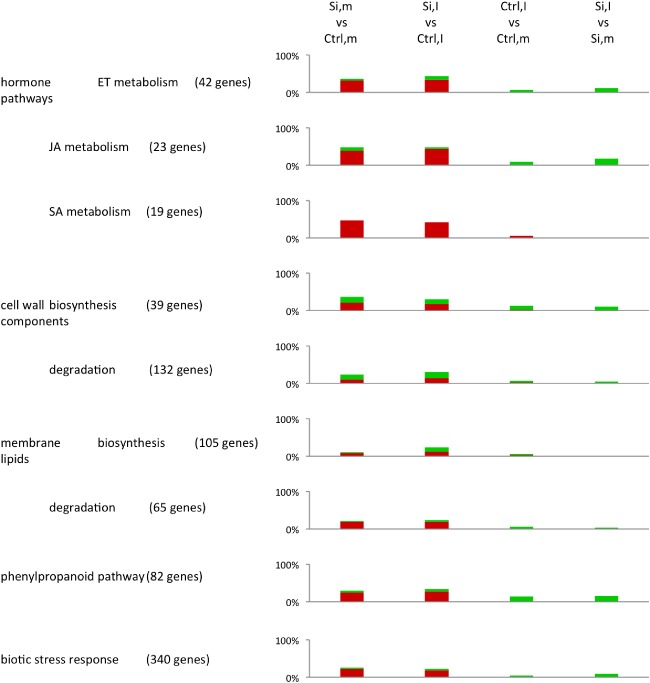
Enrichment analysis of plant defence‐related gene ontologies (GOs) (LIMMA; false discovery rate < 0.01). For each GO term, the total number of genes represented on the microarray are shown, whereas the coloured bars indicate the relative number of genes showing a significant difference in expression across the different treatment comparisons (green bars, up‐regulation, log2 fold change > 2; red bars, down‐regulation, log2 fold change < −2). GO terms were derived from the MapMan database (Usadel et al., 2009). Si, silicon; Ctrl, control; I, inoculated; m, mock‐inoculated.
Impact of Si on non‐inoculated leaves (Si,m vs. Ctrl,m)
The application of Si led to an increased expression of genes that mediate chlorophyll biosynthesis, light‐dependent reactions and nitrate reduction. Genes involved in glycolysis, cell wall biosynthesis and degradation, and nitrogen metabolism and transport were differentially expressed. Moreover, Si application down‐regulated amino acid metabolism‐related and a number of defence‐associated genes involved in lipid desaturation, the phenylpropanoid pathway, the biotic stress response and the metabolism of the defence hormones ethylene (ET), jasmonic acid (JA) and salicylic acid (SA).
Impact of C. miyabeanus (Ctrl,I vs. Ctrl,m)
One of the major influences of C. miyabeanus on primary metabolic processes was the down‐regulation of genes involved in photosynthetic processes, such as chlorophyll biosynthesis, light‐dependent reactions, the Calvin cycle and photorespiration. In addition, brown spot infection also weakly up‐regulated amino acid biosynthesis, whereas enzymes involved in nitrate reduction were down‐regulated. With respect to defence‐related pathways, brown spot infection resulted in the up‐regulation of genes involved in JA metabolism, cell wall biosynthesis and degradation, and the phenylpropanoid pathway. Finally, consistent with ET functioning as a virulence factor of C. miyabeanus (De Vleesschauwer et al., 2010), infected plants also displayed increased expression of various genes related to ET metabolism.
Impact of Si on infected leaves (Si,I vs. Ctrl,I)
The influence of Si on infected leaves almost completely mirrored its effect in non‐inoculated plants, the only notable difference being the higher number of differentially expressed genes associated with light‐dependent reactions, the Calvin cycle, photorespiration and nitrate reduction in infected tissues. Conspicuously, Si treatment induced relatively few defence‐related genes in infected leaves, suggesting that Si‐inducible brown spot resistance is not associated with widespread activation of plant resistance responses.
Impact of C. miyabeanus on Si‐treated leaves (Si,I vs. Si,m)
Brown spot infection did not significantly affect the defence‐related transcriptome of Si‐treated leaves, except for a slight increase in the number of genes implicated in defence‐related processes, such as the phenylpropanoid pathway, cell wall biosynthesis, JA and ET metabolism and biotic stress responses. Moreover, contrary to its strong effect on the primary metabolism of control inoculated leaves, C. miyabeanus failed to down‐regulate photosynthesis‐associated reactions and nitrate reduction in Si‐treated leaves.
Detailed analysis of the influence of C. miyabeanus and Si on the expression of central metabolic genes
Brown spot‐infected leaves are hallmarked by the formation of necrotic lesions surrounded by a chlorotic halo, whereas Si‐treated leaves demonstrated smaller lesions and less chlorosis (Fig. 2A, C). Linking these phenotypes to the results of the GO enrichment analysis (Figs 5 and 6; Table S7) strongly suggests that central metabolism plays a central role in shaping the outcome of rice–C. miyabeanus interactions. In the following sections, the impact of Si and/or C. miyabeanus on a subset of primary metabolism genes is discussed in more detail.
Impact of Si on central metabolism in non‐inoculated leaves (Si,m vs. Ctrl,m)
As shown in Fig. 7A and Tables S2 and S7, Si application increased the expression of photosynthetic genes that mediate light harvesting and the Calvin cycle, which corroborates the observed increase in photosynthesis in Si‐treated plants (Fig. 1D). Interestingly, the increased photosynthetic ability of Si‐treated plants coincided with a down‐regulation of genes involved in mitochondrial ATP synthesis. As Si‐treated plants are more efficient in using light energy (Fig. 1D), it is tempting to hypothesize that chloroplastic ATP synthesis may be sufficient for the plant's energy requirements, resulting in decreased ATP synthesis in the mitochondria. However, further proof is clearly needed. Meanwhile, Si feeding also led to an up‐regulated expression of several nitrate and nitrite reductases, as well as the nitrate transporters NRT1.1C, NRT1.4 and CLC1. As AtNRT1.1, the homologue of OsNRT1.1C, has been reported to act as a nitrate receptor/transporter (Gojon et al., 2011), and AtCLCa, the homologue of OsCLC1, accounts for the transport of nitrate from the vacuole to the cytosol (De Angeli et al., 2006; Geelen et al., 2000), these findings may point towards increased cytosolic nitrate concentrations that could fuel the predicted nitrate reduction in Si‐treated leaves. In addition, the ammonium transporters AMT2.2 and AMT3.3 were down‐regulated by Si treatment in infected leaves. The localization of most ammonium and nitrate transporters in rice leaves remains to be elucidated (Gaur et al., 2012; Martinoia et al., 2007; Suenaga et al., 2003), but their differential expression suggests that Si may induce a shift in nitrogen transport.
Figure 7.
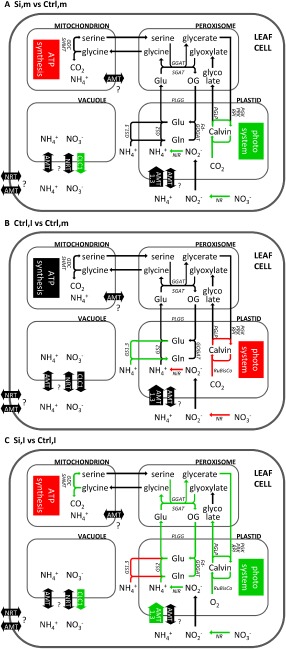
Influence of silicon (Si) application (A), Cochliobolus miyabeanus infection (B) and combined Si and C. miyabeanus treatment (C) on the expression of genes involved in central carbon/nitrogen (C/N) metabolism. Arrows in red and green indicate down‐ and up‐regulation of gene expression, respectively. AMT, ammonium transporter; CLC1, nitrate transporter; NRT, nitrate transporter; GDC, glycine dehydrogenase; GGAT, glutamate:glyoxylate aminotransferase; GOX, glycolate oxidase; GS, glutamine synthetase; NADH‐ or Fd‐GOGAT, NADH‐ or Ferredoxin‐dependent glutamate synthase; NiR, nitrite reductase; NR, nitrate reductase; PGK, phosphoglycerate kinase; PGLP, phosphoglycolate phosphatase; PLGG, plastidic glycolate/glycerate translocator; PRK, phosphoribulokinase; RPI, ribose‐5‐phosphate isomerase; RuBisCo, ribulose‐1,5‐bisphosphate carboxylase/oxygenase; SGAT, serine:glyoxylate aminotransferase; SHMT, serine hydroxymethyltransferase.
The impact of Si on the transcriptome and metabolism of non‐stressed rice plants has been investigated in a number of previous studies. In agreement with our findings, Brunings et al. (2009) found Si to significantly alter the basal expression level of more than 220 rice genes, which leaves open the possibility that Si is essential for rice. In contrast, Watanabe et al. (2004) found only 20 genes to be differentially expressed on Si treatment, despite using a similar hydroponic rice‐growing system. Although differences in rice cultivars, microarray platforms and statistical settings used in the different studies cannot be excluded, none of these factors justifies a 10‐ to 100‐fold difference in the number of Si‐responsive genes. Another confounding factor, however, involves the plant growth conditions and, especially, the light conditions. Agarie et al. (1992) found that, under suboptimal light conditions, Si treatment leads to a stronger growth increase than under optimal light conditions. Considering that all our assays were performed under growth chamber conditions, it is not inconceivable that the relatively low light intensities to which our plants were exposed amplified the impact of Si on the plant's basal transcriptome.
Impact of C. miyabeanus on central metabolism (Ctrl,I vs. Ctrl,m)
The transcriptional changes in central metabolic genes after brown spot infection (Fig. 7B) indicated a down‐regulation of photosynthetic genes involved in light‐dependent reactions and the Calvin cycle (Tables S3 and S7). Brown spot infection in rice leaves is characterized by the occurrence of large necrotic lesions surrounded by a chlorotic halo (Ahn et al., 2005; Dallagnol et al., 2011b). Depending on the aggressiveness of the pathogen strain and the intrinsic level of resistance of the rice cultivar used, disease symptoms start to appear from 18 hpi onwards. Interestingly, we found C. miyabeanus to down‐regulate rice photosynthetic genes at 12 hpi, a time point at which disease lesions are not yet visible. Rather than being the consequence of symptom development, such photosynthetic impairment may thus be causally involved in the C. miyabeanus infection process.
Interestingly, photosynthetic inhibition could promote fungal pathogenesis in different ways. First, inhibition of photosynthesis could deprive invaded rice leaves from the ATP necessary to fuel energy‐demanding defence mechanisms. Furthermore, delinking light capture and the Calvin cycle results in an over‐reduction of electron transfer and oxidative damage to chloroplast membranes, which is well known to promote susceptibility to necrotrophic pathogens (Kangasjärvi et al., 2012). Finally, a decrease in photosynthesis may also induce metabolic changes that cause enhanced nutrient leakage, thus favouring C. miyabeanus pathogenicity. In line with this notion, our findings showed a strong up‐regulation of cytosolic glutamine synthetases (GS1.3) and down‐regulation of several nitrate and nitrite reductases in brown spot‐infected leaves, which is suggestive of senescence‐linked nitrogen remobilization (Masclaux et al., 2001; Pageau et al., 2006). Moreover, in keeping with our transcriptional data, a recent proteomics study has shown that C. miyabeanus also induces various enzymes involved in amino acid biosynthesis (Kim et al., 2014). Therefore, it is tempting to speculate that C. miyabeanus disrupts the plant's photosynthetic apparatus to induce host senescence and nitrogen remobilization, thereby securing access to nutrients throughout the course of infection.
Interestingly, brown spot infection also seemed to attenuate photorespiration, as evidenced by the down‐regulation of several photorespiratory marker genes, including several orthologues of ribulose‐1,5‐bisphosphate carboxylase/oxygenase (RuBisCo). Although RuBisCo plays an essential role in carboxylation during photosynthesis, transient alterations in the expression of RuBisCo genes are often attributed to photorespiration during which RuBisCo fixes oxygen rather than carbon dioxide (Lakshmanan et al., 2013; Wang et al., 2012). Previously, various groups have shown that victorin, a toxin produced by Cochliobolus victoriae, induces susceptibility in oat plants by inhibiting the photorespiratory enzyme glycine decarboxylase (Navarre and Wolpert, 1995, 1999; Tada et al., 2005). Moreover, we have demonstrated previously that C. miyabeanus is able to produce ET in planta, resulting in stomatal opening and reduced photorespiration activity (J. Van Bockhaven, unpublished data). Although further proof is clearly needed, these findings raise the possibility that, in addition to reducing photosynthesis rates, C. miyabeanus may also interfere with photorespiratory processes in order to cause disease.
Impact of Si on central metabolism of infected leaves (Si,I vs. Ctrl,I)
One of the most peculiar findings in this study was the observation that Si application does not result in a major activation of plant defence responses, such as the phenylpropanoid pathway or SA‐, JA‐ and ET‐dependent defences. Together with the apparent down‐regulation of mitochondrial ATP synthesis in Si‐treated plants, these observations strongly suggest that Si may not confer brown spot resistance via the activation of classic immune mechanisms, but rather may impinge on the central metabolism of treated rice plants (Figs 5, 6 and 7C). Most conspicuously, Si application seemed to reverse most metabolic processes that are associated with C. miyabeanus infection. First, the decreased expression of the ammonium transporters AMT2.2 and AMT3.3 and the up‐regulation of several nitrate and nitrite reductases, as well as the nitrate transporter genes NRT1.1C, NRT1.4 and CLC1, suggest that Si counteracts brown spot‐induced nitrogen remobilization. In addition, Si treatment protected infected rice leaves from C. miyabeanus‐mediated down‐regulation of photosynthetic genes. Safeguarding the photosynthesis machinery appears to be a common aspect of Si‐induced broad‐spectrum stress tolerance, as many reports have shown unanimously that Si protects and promotes photosynthesis during stress situations (Chen et al., 2011; Dallagnol et al., 2013a; Nwugo and Huerta, 2008, 2011; Perez et al., 2014).
Although the underlying mechanisms remain elusive, the Si‐induced up‐regulation of several photorespiratory marker genes, including glutamate:glyoxylate aminotransferase (GGAT), phosphoglycolate phosphatase (PGLP) and plastidic glycolate/glycerate translocator (PLGG), suggests that increased photorespiration rates may be one of the driving forces behind the protective role of Si on photosynthesis. Often considered to be a wasteful process, evidence is accumulating that photorespiration also prevents the over‐reduction of photosynthetic electron transport and subsequent damage to the photosynthetic apparatus during abiotic stress (Bauwe et al., 2012; Cai et al., 2011; Cheng et al., 2007; Hoshida et al., 2000; Rivero et al., 2009; Wang et al., 2012). In addition, photorespiration is increasingly being implicated in pathogen defence, an effect which is mostly linked to the photorespiration‐induced production of reactive oxygen species (ROS) and associated hypersensitive response (HR)‐like cell death (Kangasjärvi et al., 2012; Rojas et al., 2014; Sørhagen et al., 2013; Wingler et al., 2000). However, considering that ROS formation and HR are largely ineffective against C. miyabeanus (Ahn et al., 2005), the potential role of photorespiration in Si‐induced resistance against C. miyabeanus is probably caused by safeguarding of the photosynthetic apparatus.
Intriguingly, increased photorespiration rates may also provide a mechanistic framework for the increased nitrate reduction and chloroplastic ammonium transport and re‐assimilation seen in Si‐treated plants (Fig. 5; Bloom et al., 2010; Foyer et al., 2009; Naik, 2006; Rachmilevitch et al., 2004). Photorespiration is a complex and energy‐consuming interorganellar process that is usually initiated by high temperatures and stomatal closure resulting in low CO2 and/or high O2 levels in planta (Bauwe et al., 2012; Foyer et al., 2009). In C3 plants, such as rice, these conditions give rise to the addition of oxygen rather than CO2 to ribulose‐1,5‐bisphosphate by RuBisCo. The resulting glycolate is converted in the peroxisomes to glycine, which is then catalysed in the mitochondria with the resultant release of CO2 and NH3. Photorespiratory CO2 feeds back into the Calvin cycle, whereas excess NH3 is re‐assimilated in the cellular nitrogen cycle (Linka and Weber, 2005; Nunes‐Nesi et al., 2010; Wingler et al., 2000). Interestingly, application of Si not only up‐regulated the ammonium transporter AMT1.3 (Foyer et al., 2009; Li et al., 2012; Tanaka et al., 2004), but also chloroplastic glutamine synthetase 2 (GS2) and Ferredoxin‐dependent glutamate synthase (Fd‐GOGAT). Both enzymes play an essential role in nitrogen metabolism by catalysing the conversion of ammonium and glutamate to form glutamine (Lam et al., 1996; Lea and Miflin, 2010). In the light of the above‐mentioned induction of photorespiratory genes, these data may be interpreted to suggest that the Si‐induced increase in nitrate reduction and photorespiratory ammonium transport results in enhanced ammonium levels in the chloroplast, which are subsequently re‐assimilated by GS2 and Fd‐GOGAT.
Si‐induced broad‐spectrum resistance: a case of disarming the enemy?
Over the past few years, various other transcriptomic and proteomic studies have been conducted to explain the protective effects of Si in a number of pathosystems (Chain et al., 2009; Fauteux et al., 2006; Ghareeb et al., 2011; Nwugo and Huerta, 2011; Watanabe et al., 2004; Zargar et al., 2010). One of the most salient results of these studies is that Si negates many of the transcriptional changes induced by pathogen inoculation. For instance, in one of the first studies aimed at the investigation of the molecular basis of Si‐induced plant resistance, Fauteux et al. (2006) found that the inoculation of Arabidopsis with the powdery mildew fungus Erysiphe cichoracearum resulted in transcriptional reprogramming of an extensive gene set of nearly 4000 genes. Remarkably, comparing control and Si‐treated plants, no major changes were found in either the number or expression level of up‐regulated genes. In contrast, many of the down‐regulated genes were less severely affected when plants were treated with Si. Similar findings were obtained by Chain et al. (2009) when studying the effect of Si application on the transcriptome of wheat plants inoculated with the biotrophic pathogen Blumeria graminis f. sp. tritici. In this case, Si‐treated plants displayed an almost perfect mirror image of the expression changes seen in inoculated control plants. Moreover, contrary to the nearly 900 genes responding to Blumeria infection in control leaves, very few genes were regulated by the pathogen in Si‐treated wheat plants, indicating that Si almost eradicated the stress imposed by the pathogen (Chain et al., 2009). In rice, the study by Brunings et al. (2009) and our data on the effect of Si on rice blast and brown spot resistance, respectively, similarly show that the impact of pathogen infection on the plant's transcriptome is diminished by Si treatment. Therefore, rather than creating resistance by directing massive transcriptional reprogramming of defence‐related genes, Si appears to nullify the impact of pathogen inoculation on the plant's transcriptome. One interesting extrapolation is that Si may condition plant disease resistance by preventing the exploitation of pathogen virulence factors. Whether such impairment of pathogen virulence is a result of Si boosting the plant perception of and/or response to so‐called pathogen‐associated molecular patterns or, alternatively, results from the inhibition of the production and/or delivery of specific virulence factors remains to be elucidated.
Conclusions
In summary, our data have provided novel insights into the myriad cellular responses that enable rice to fend off C. miyabeanus attack, and support a model whereby the pathogen suppresses photosynthesis and nitrate reduction in order to trigger premature senescence in infected tissues and induce a state of susceptibility. Si application, however, may confer brown spot resistance, at least in part, by redirecting the plant's central metabolism in a photorespiration‐dependent manner. Moreover, in conjunction with other microarray studies on Si‐induced disease resistance, our data suggest that, rather than acting as an initiator of induced defence responses, Si may confer disease resistance by interfering with microbial virulence factors.
Experimental Procedures
Plant material and growth conditions
All rice plants (Oryza sativa L.) were japonica cultivar Nipponbare. Rice seeds were surface sterilized with 70% ethanol for 1 min and 1% sodium hypochlorite solution for 10 min, rinsed three times with sterile distilled water and germinated at 28 °C for 5 days on wet sterile filter paper in Petri dishes sealed with parafilm (≥92% relative humidity). The seedlings were transplanted on vermiculite in half‐strength modified Hoagland solution (Hewitt and Smith, 1975). Five days later, the plantlets were transferred to a gnotobiotic hydroponic growing system in full‐strength modified Hoagland solution [pH 6.5; 1 mm KNO3, 0.25 mm NH4H2PO4, 0.1 mm NH4Cl, 0.5 mm MgSO4, 1 mm Ca(NO3)2, 0.025 mm FeSO4, 0.025 mm ethylenediaminetetraacetic acid (EDTA)‐bisodium salt, 0.0003 mm CuSO4, 0.00033 mm ZnSO4, 0.01150 mm H3BO3, 0.0035 mm MnCl2, 0.0001 mm (NH4)6Mo7O24, 0.01 mm MnSO4]. Silicic acid was prepared from potassium silicate by means of a cation exchanger (Amberlite IR120, Acros organics, Geel, Antwerpen, Belgium) and added to the nutrient solution to a final density of 2 mm. The Hoagland solution was replaced every 7 days. Control and Si‐treated rice plants were grown together in a completely randomized design under growth chamber conditions (28 °C; 12 h/12 h light regime; light intensity, 600 μmol/m2/s) for 5 weeks until they reached the seven‐leaf stage.
Pathogen inoculation and disease rating
Cochliobolus miyabeanus isolate Cm988 (De Vleesschauwer et al., 2010) was routinely grown on potato dextrose agar (PDA, Difco (Becton, Dickinson and Company), Franklin Lakes, NJ, USA) at 28 °C in darkness. To induce sporulation, 7‐day‐old mycelium was exposed to blue light (12 h photoperiod; Philips TLD 18W/08 and Philips TLD 18W/33, Philips, Amsterdam, Noord‐Holland, the Netherlands) for 3 days. Conidia were harvested as described by Thuan et al. (2006) and suspended in 0.5% gelatin (type B from bovine skin; Sigma‐Aldrich, Diegem, Vlaams‐Brabant, Belgium G‐6650) to a final density of 1 × 104 conidia/mL. For inoculation, leaves of 5‐week‐old plants (seven‐leaf stage) were misted with conidial suspension (1 mL per plant) using a compressor‐powered airbrush gun. Control plants were sprayed evenly with a 0.5% gelatin solution. Inoculated control and Si‐treated plants were kept together in a humid and warm infection chamber (28 ± 4 °C, 92% or greater relative humidity) for 18 h to promote fungal penetration, and thereafter transposed to growth chamber conditions (28 °C, 12 h photoperiod) for disease development. Disease severity was assessed at 3 days post‐inoculation (dpi) by calculating the diseased leaf area using APS assess 2.0 imaging software (APS, St Paul, MN, USA). Alternatively, disease was scored using a 1–5 disease severity scale following De Vleesschauwer et al. (2010): 1, no infection or less than 2% of the leaf area infected with small brown specks of less than 1 mm in diameter; 2, less than 10% of the leaf area infected with brown spot lesions with grey to white centres, about 1–3 mm in diameter; 3, average of about 25% of the leaf area infected with brown spot lesions with grey to white centres, about 1–3 mm in diameter; 4, average of about 50% of the leaf area infected with typical spindle‐shaped lesions, 3 mm or longer, with necrotic grey centres and water‐soaked or reddish brown margins, with little or no coalescence of lesions; 5, more than 75% of the leaf area infected with coalescing spindle‐shaped lesions.
For detached leaf assays, the two youngest fully developed leaves of 5‐week‐old plants were detached, cut into 7‐cm segments, and floated overnight on sterile distilled water to eliminate residual wounding stress. The next day, leaf segments were placed in square Petri dishes lined with moist paper towels and drop inoculated with four 15‐μL droplets of suspension (1 × 104 conidia/mL in 0.25% gelatin). After 24 h, the droplets were removed with a laboratory tissue, and resistance was quantified at 72 hpi by measuring the mean lesion area using APS assess 2.0 imaging software (APS). All infection trials were repeated at least three times with similar results.
Plant phenotyping
All plants were phenotyped using a LI‐6400XT leaf gas exchange/chlorophyll fluorescence instrument fitted with a 6400‐40 Leaf Chamber Fluorometer (Li‐Cor Biosciences, Lincoln, NE, USA). The LI‐6400XT cuvette temperature was set to match the temperature in the growth chamber at the start of the measurement. The cuvette CO2 concentration was maintained at 400 ppm. Dark respiration was measured pre‐dawn and in the dark, with the light source of the LI‐6400XT disabled. Light‐acclimated gas exchange measurements were performed after turning on the light in the growth chamber, and allowing the plants to acclimate for 30 min. During measurements, the light source of the cuvette was maintained at 1500 μmol photosynthetically active radiation (PAR)/m2/s (0.1:0.9 mix of 460 nm and 640 nm).
For chlorophyll fluorescence measurements, dark‐adapted chlorophyll a fluorescence [before (F 0) and after (F m) a 1‐s 7000 μmol/m2/s saturating light flash] was quantified immediately after recording dark respiration. Light‐acclimated PSII efficiency (F v’/F m’ = 1 − F 0’/F m’; Valentini et al., 1995; Maxwell and Johnson, 2000), effective PSII quantum yield (ΦPSII = 1 − F s/F m’) and photochemical quenching, which relates F v’/F m’ to ΦPSII and is a non‐linear measure for the proportion of open PSII centres (q P = [F m’ − F s]/[F m’ − F 0’]), were calculated from steady‐state fluorescence (F s), maximum fluorescence after a saturating light flash (F m’ after 1 s at 7000 μmol/m2/s) and minimum fluorescence after a far‐red pulse (F 0’ after 3 s at 9 μmol/m2/s peaking at 740 nm).
Microarray analysis and data processing
Rice plants (cultivar Nipponbare) were grown and infected with C. miyabeanus isolate Cm988 as described previously. Samples from mock‐infected and brown spot‐inoculated plants were taken at 12 hpi. Three independent biological repetitions were included in the analysis, each repetition representing a pool from at least six individual plants. Total RNA was extracted using the spectrum plant total RNA kit (Sigma‐Aldrich, Diegem, Vlaams‐Brabant, Belgium) and subsequently Turbo DNase treated according to the provided protocol (Ambion (Thermo Fisher Scientific Inc.), Waltham, MA, USA). First‐strand cDNA was synthesized from 2 mg of total RNA using Multiscribe reverse transcriptase (Applied Biosystems (Thermo Fisher Scientific Inc.), Waltham, MA, USA) and random primers following the manufacturer's instructions. For microarray analysis, a previously described two‐dye method, allowing direct comparison between two samples on the same microarray, was used (Satoh et al., 2010). In brief, cyanine 3‐ or cyanine 5‐labelled cRNA samples were synthesized from 850 ng of total RNA using a low‐input RNA labelling kit (Agilent Technologies, Santa Clara, CA, USA) and hybridized to commercially available 60‐mer 44 K Agilent rice arrays according to the manufacturer's protocols (Agilent Technologies, Santa Clara, CA, USA). A full list of the expressed sequence tags (ESTs) represented on the array can be found at http://ricexpro.dna.affrc.go.jp/rice‐44k‐microarray.html. Following washing, slide image files were generated using a DNA microarray scanner (G2505B; Agilent Technologies) and signal intensities were extracted and normalized within each array using Feature Extraction version 9.5 (Agilent Technologies). Signal intensities among all arrays were normalized according to the quantile method for standardization (global scaling) using EXPANDER 6 (Shamir et al., 2005). All data are available in the Gene Expression Omnibus (NCBI‐GEO; http://www.ncbi.nlm.nih.gov/geo) database under the reference GSE55330. Significance analysis was performed using a fixed linear model (LIMMA) implemented in Multiple Experiment Viewer (MeV; Saeed et al., 2003). Differentially expressed genes were defined as genes with a false discovery rate of less than 0.01 (Tamura et al., 2011) and a four‐fold difference in signal ratio. Hierarchical linkage clustering was performed (complete linkage, Euclidian distance) using MeV. GO analysis was performed with MapMan version 3.5.1 (Usadel et al., 2009).
Validation of microarray results
Real‐time qRT‐PCR analysis was performed on 15 genes that were differentially regulated on Si feeding. All PCR amplifications were conducted in optical 96‐well plates with the Mx3005P real‐time PCR detection system (Stratagene (Agilent Technologies), Santa Clara, CA, USA), using Sybr Green master mix (Fermentas (Thermo Fisher Scientific Inc.), Waltham, MA, USA) to monitor double‐stranded DNA synthesis. The expression of each gene was assayed in duplicate in a total volume of 25 μL including a passive reference dye (ROX) according to the manufacturer's instructions (Fermentas (Thermo Fisher Scientific Inc.), Waltham, MA, USA). The thermal profile used consisted of an initial denaturation step at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, 57 °C for 30 s and 72 °C for 30 s. To verify the amplification of one specific target cDNA, a melting curve analysis was included according to the thermal profile suggested by the manufacturer (Stratagene (Agilent Technologies), Santa Clara, CA, USA). The amount of plant RNA in each sample was normalized using elongation factor eIFα (LOC_Os03g08020) as internal control. All primer sequences are listed in Table S8 (see Supporting Information).
Supporting information
Fig. S1 Correlation of microarray (x axis) and real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) data (y axis) for a subset of 15 differentially expressed genes.
Table S1 Overview of physiological parameters analysed during leaf gas exchange measurements of control and silicon‐treated plants.
Table S2 List of differentially expressed genes in non‐inoculated silicon‐treated plants (Si,m vs. Ctrl,m).
Table S3 List of differentially expressed genes in Cochliobolus miyabeanus‐infected control plants (Ctrl,I vs. Ctrl,m).
Table S4 List of differentially expressed genes in Cochliobolus miyabeanus‐infected and silicon‐treated plants vs. infected control plants (Si,I vs. Ctrl,I).
Table S5 List of differentially expressed genes in silicon‐treated plants following Cochliobolus miyabeanus infection (Si,I vs. Si,m).
Table S6 Validation of microarray data by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR).
Table S7 Central metabolism‐associated genes differentially expressed after silicon (Si) application and/or Cochliobolus miyabeanus infection.
Table S8 Primers used for microarray validation.
Acknowledgements
This work was supported by a specialization fellowship of the Flemish Institute for the stimulation of Scientific–Technological Research in Industry (IWT, Belgium) given to JVB, grants from the Special Research Fund of Ghent University (GOA 01GB3013), the Research Foundation Flanders (FWO G.0833.12N) and a FWO postdoctoral fellowship to DDV.
References
- Agarie, S. , Agata, W. , Kubota, F. and Kaufman, P.B. (1992) Physiological roles of silicon in photosynthesis and dry matter production in rice plants. Jpn. J. Crop Sci. 64, 200–206. [Google Scholar]
- Ahn, I.P. , Kim, S. , Kang, S. , Suh, S.C. and Lee, Y.H. (2005) Rice defense mechanisms against Cochliobolus miyabeanus and Magnaporthe grisea are distinct. Phytopathology, 95, 1248–1255. [DOI] [PubMed] [Google Scholar]
- Bauwe, H. , Hagemann, M. , Kern, R. and Timm, S. (2012) Photorespiration has a dual origin and manifold links to central metabolism. Curr. Opin. Plant Biol. 15, 269–275. [DOI] [PubMed] [Google Scholar]
- Berger, S. , Sinha, A.K. and Roitsch, T. (2007) Plant physiology meets phytopathology: plant primary metabolism and plant–pathogen interactions. J. Exp. Bot. 58, 4019–4026. [DOI] [PubMed] [Google Scholar]
- Bloom, A.J. , Burger, M. , Rubio Asensio, J.S. and Cousins, A.B. (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science, 328, 899–903. [DOI] [PubMed] [Google Scholar]
- Bolton, M.D. (2009) Primary metabolism and plant defense: fuel for the fire. Mol. Plant–Microbe Interact. 22, 487–497. [DOI] [PubMed] [Google Scholar]
- Brunings, A. , Datnoff, L.E. , Ma, J.F. , Mitani, N. , Nagamura, Y. , Rathinasabapathi, B. and Kirst, M. (2009) Differential gene expression of rice in response to silicon and rice blast fungus Magnaporthe oryzae . Annu. Appl. Biol. 155, 161–170. [Google Scholar]
- Cai, Y. , Cao, F. , Wei, K. , Zhang, G. and Wu, F. (2011) Genotypic dependent effect of exogenous glutathione on Cd‐induced changes in proteins, ultrastructure and antioxidant defense enzymes in rice seedlings. J. Hazard. Mater. 192, 1056–1066. [DOI] [PubMed] [Google Scholar]
- Castell‐Miller, C.V. and Samac, D.A. (2012) Population genetic structure, gene flow and recombination of Cochliobolus miyabeanus on cultivated wildrice (Zizania palustris). Plant Pathol. 61, 903–914. [Google Scholar]
- Chain, F. , Côté‐Beaulieu, C. , Belzile, F. , Menzies, J.G. and Bélanger, R.R. (2009) A comprehensive transcriptomic analysis of the effect of silicon on wheat plants under control and pathogen stress conditions. Mol. Plant–Microbe Interact. 22, 1323–1330. [DOI] [PubMed] [Google Scholar]
- Chen, W. , Yao, X. , Cai, K. and Chen, J. (2011) Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 142, 67–76. [DOI] [PubMed] [Google Scholar]
- Cheng, C. , Yun, K.Y. , Ressom, H.W. , Mohanty, B. , Bajic, V.B. , Jia, Y. , Yun, S.J. and Reyes, B.G. (2007) An early response regulatory cluster induced by low temperature and hydrogen peroxide in seedlings of chilling‐tolerant japonica rice. BMC Genomics, 8, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallagnol, L.J. , Rodrigues, F.A. , Mielli, M.V.B. , Ma, J.F. and Datnoff, L.E. (2009) Defective active silicon uptake affects some components of rice resistance to brown spot. Phytopathology, 99, 116–121. [DOI] [PubMed] [Google Scholar]
- Dallagnol, L.J. , Rodrigues, F.A. , DaMatta, F.M. , Mielli, M.V.B. and Pereira, S.C. (2011a) Deficiency in silicon uptake affects cytological, physiological, and biochemical events in the rice–Bipolaris oryzae interaction. Phytopathology, 101, 92–104. [DOI] [PubMed] [Google Scholar]
- Dallagnol, L.J. , Rodrigues, F.A. , Martins, S.C.V. , Cavatte, P.C. and DaMatta, F.M. (2011b) Alterations in rice leaf physiology during infection by Bipolaris oryzae . Australas. Plant Pathol. 40, 360–365. [Google Scholar]
- Dallagnol, L.J. , Rodrigues, F.A. , Chaves, A.R.M. , Vale, F.X.R. and DaMatta, F.M. (2013a) Photosynthesis and sugar concentration are impaired by the defective active silicon uptake in rice plants infected with Bipolaris oryzae . Plant Pathol. 62, 120–129. [Google Scholar]
- Dallagnol, L.J. , Rodrigues, F.A. and Mielli, M.V.B. (2013b) Silicon improves the emergence and sanity of rice seedlings obtained from seeds infected with Bipolaris oryzae . Trop. Plant Pathol. 38, 478–484. [Google Scholar]
- De Angeli, A. , Monachello, D. , Ephritikhine, G. , Frachisse, J.M. , Thomine, S. , Gambale, F. and Barbier‐Brygoo, H. (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature, 442, 939–942. [DOI] [PubMed] [Google Scholar]
- Dela Paz, M.A.G. , Goodwin, P.H. , Raymundo, A.K. , Ardales, E.Y. and Cruz, C.M.V. (2006) Phylogenetic analysis based on ITS sequences and conditions affecting the type of conidial germination of Bipolaris oryzae . Plant Pathol. 55, 756–765. [Google Scholar]
- De Vleesschauwer, D. , Yang, Y. , Cruz, C.V. and Höfte, M. (2010) Abscisic acid‐induced resistance against the brown spot pathogen Cochliobolus miyabeanus in rice involves MAP kinase‐mediated repression of ethylene signaling. Plant Physiol. 152, 2036–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, E. (2009) Silicon: its manifold roles in plants. Ann. Appl. Biol. 155, 155–160. [Google Scholar]
- Epstein, E. and Bloom, A.J. (2005) Mineral nutrition of plants: principles and perspectives, Sunderland: Sinauer Associates.
- Farooq, M.A. , Ali, S. , Hameed, A. , Ishaque, W. , Mahmood, K. and Iqbal, Z. (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes; suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol. Environ. Saf. 96, 242–249. [DOI] [PubMed] [Google Scholar]
- Fauteux, F. , Rémus‐Borel, W. , Menzies, J.G. and Bélanger, R.R. (2005) Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 249, 1–6. doi: 10.1016/j.femsle.2005.06.034. [DOI] [PubMed] [Google Scholar]
- Fauteux, F. , Chain, F. , Belzile, F. , Menzies, J.G. and Bélanger, R.R. (2006) The protective role of silicon in the Arabidopsis‐powdery mildew pathosystem. Proc. Natl. Acad. Sci. USA, 103, 17 554–17 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck, A.T. , Nye, T. , Repenning, C. , Stahl, F. , Zahn, M. and Schenk, M.K. (2011) Silicon enhances suberization and lignification in roots of rice (Oryza sativa). J. Exp. Bot. 62, 2001–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer, C.H. , Bloom, A.J. , Queval, G. and Noctor, G. (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 60, 455–484. [DOI] [PubMed] [Google Scholar]
- Gaur, V.S. , Singh, U.S. , Gupta, A.K. and Kumar, A. (2012) Understanding the differential nitrogen sensing mechanism in rice genotypes through expression analysis of high and low affinity ammonium transporter genes. Mol. Biol. Rep. 39, 2233–2241. [DOI] [PubMed] [Google Scholar]
- Geelen, D. , Lurin, C. , Bouchez, D. , Frachisse, J.M. , Lelièvre, F. , Courtial, B. , Barbier‐Brygoo, H. and Maurel, C. (2000) Disruption of putative anion channel gene AtCLCa in Arabidopsis suggests a role in the regulation of nitrate content. Plant J. 21, 259–267. [DOI] [PubMed] [Google Scholar]
- Ghareeb, H. , Bozsó, Z. , Ott, P.G. , Repenning, C. , Stahl, F. and Wydra, K. (2011) Transcriptome of silicon‐induced resistance against Ralstonia solanacearum in the silicon non‐accumulator tomato implicates priming effect. Physiol. Mol. Plant Pathol. 75, 83–89. [Google Scholar]
- Glazebrook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Gojon, A. , Krouk, G. , Perrine‐Walker, F. and Laugier, E. (2011) Nitrate transceptor(s) in plants. J. Exp. Bot. 62, 2299–2308. [DOI] [PubMed] [Google Scholar]
- Govrin, E.M. and Levine, A. (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea . Curr. Biol. 10, 751–757. [DOI] [PubMed] [Google Scholar]
- Guntzer, F. , Keller, C. and Meunier, J.‐D. (2011) Benefits of plant silicon for crops: a review. Agron. Sustain. Dev. 32, 201–213. [Google Scholar]
- Hewitt, E.J. and Smith, T.A. (1975) Plant mineral nutrition In: Experimental Methods for the Investigation of Plant Nutrient Requirements (Hewitt E.J. and Smith T.A., eds), pp. 176–222. London: English Universities Press Ltd. [Google Scholar]
- Hoshida, H. , Tanaka, Y. , Hibino, T. , Hayashi, Y. , Tanaka, A. and Takabe, T. (2000) Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol. Biol. 43, 103–111. [DOI] [PubMed] [Google Scholar]
- Kabbage, M. , Williams, B. and Dickman, M.B. (2013) Cell death control: the interplay of apoptosis and autophagy in the pathogenicity of Sclerotinia sclerotiorum. PLoS Path. 9, e1003287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangasjärvi, S. , Neukermans, J. , Li, S. , Aro, E.M. and Noctor, G. (2012) Photosynthesis, photorespiration, and light signalling in defence responses. J. Exp. Bot. 63, 1619–1636. [DOI] [PubMed] [Google Scholar]
- Kim, J.Y. , Wu, J. , Kwon, S.J. , Oh, H. , Lee, S.E. , Kim, S.G. , Wang, Y. , Agrawal, G.K. , Rakwal, R. , Kang, K.Y. , Ahn, I.P. , Kim, B.G. and Kim, S.T. (2014) Proteomics of rice and Cochliobolus miyabeanus fungal interaction: Insight into proteins at intracellular and extracellular spaces. Prot. 14, 2307–2318. [DOI] [PubMed] [Google Scholar]
- Lakshmanan, M. , Mohanty, B. and Lee, D.Y. (2013) Identifying essential genes/reactions of the rice photorespiration by in silico model‐based analysis. Rice (N.Y.), 6, 1–5. doi: 10.1186/1939-8433-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, H.M. , Coschigano, K.T. , Oliveira, I.C. , Melo‐Oliveira, R. and Coruzzi, G.M. (1996) The molecular genetics of nitrogen assimilation into amino acids in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 569–593. [DOI] [PubMed] [Google Scholar]
- Lea, P.J. and Miflin, B.J. (2010) Nitrogen assimilation and its relevance to crop improvement In Annual Plant Reviews Volume 42: Nitrogen Metabolism in Plants in the Post‐Genomic Era (Foyer C.H. and Zhang H., eds), doi: 10.1002/9781444328608.ch1. Oxford: Wiley‐Blackwell. [DOI] [Google Scholar]
- Leung, H. , Zhu, Y. , Revilla‐Molina, I. , Fan, J.X. , Chen, H. , Pangga, I. , Cruz, C.V. and Mew, T.W. (2003) Using genetic diversity to achieve sustainable rice disease management. Plant Dis. 87, 1156–1169. [DOI] [PubMed] [Google Scholar]
- Levine, B. (2007) Cell biology: autophagy and cancer. Nature, 446, 745–747. [DOI] [PubMed] [Google Scholar]
- Li, S. , Li, B. and Shi, W. (2012) Expression patterns of nine ammonium transporters in rice in response to N status. Pedosph. 22, 860–869. [Google Scholar]
- Linka, M. and Weber, A.P.M. (2005) Shuffling ammonia between mitochondria and plastids during photorespiration. Trends Plant Sci. 10, 461–465. [DOI] [PubMed] [Google Scholar]
- Ma, J.F. and Yamaji, N. (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci. 11, 392–397. [DOI] [PubMed] [Google Scholar]
- Ma, J.F. , Yamaji, N. and Mitani‐Ueno, N. (2011) Transport of silicon from roots to panicles in plants. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 87, 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia, E. , Maeshima, M. and Neuhaus, H.E. (2007) Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 58, 83–102. [DOI] [PubMed] [Google Scholar]
- Masclaux, B.C. , Quilleré, I. , Gallais, A. and Hirel, B. (2001) The challenge of remobilisation in plant nitrogen economy. A survey of physio‐agronomic and molecular approaches. Ann. Appl. Biol. 138, 69–81. [Google Scholar]
- Maxwell, K. and Johnson, G.N. (2000) Chlorophyll fluorescence: a practical guide. J. Exp. Bot. 51, 659–668. [DOI] [PubMed] [Google Scholar]
- Mur, L.A.J. , Simpson, C. , Gay, A. , Smith, J.A. , Paveley, N. , Sánchez‐Martin, J. and Prats, E. (2013) Stomatal lock‐up following pathogenic challenge: source or symptom of costs of resistance in crops? Plant Pathol. 62, 72–82. [Google Scholar]
- Naik, R.M. (2006) Dependence of in vivo nitrate reduction on photorespiration and mitochondrial respiration in leaves of Nicotiana sylvestris . J. Plant Biochem. Biotechnol. 15, 59–61. [Google Scholar]
- Navarre, D.A. and Wolpert, T.J. (1995) Inhibition of the glycine decarboxylase multienzyme complex by the host‐selective toxin victorin. Plant Cell, 7, 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarre, D.A. and Wolpert, T.J. (1999) Victorin induction of an apoptotic/senescence‐like response in oats. Plant Cell, 11, 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes‐Nesi, A. , Fernie, A.R. and Stitt, M. (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol. Plant. 3, 973–996. [DOI] [PubMed] [Google Scholar]
- Nwugo, C.C. and Huerta, A.J. (2008) Silicon‐induced cadmium resistance in rice (Oryza sativa). J. Plant Nutr. Soil Sci. 171, 841–848. [Google Scholar]
- Nwugo, C.C. and Huerta, A.J. (2011) The effect of silicon on the leaf proteome of rice (Oryza sativa L.) plants under cadmium‐stress. J. Proteome Res. 10, 518–528. [DOI] [PubMed] [Google Scholar]
- Oliveros, J. (2007) VENNY. An interactive tool for comparing lists with Venn diagrams. BioinfoGP, CNB‐CSIC . Available at http://bioinfogp.cnb.csic.es/tools/venny/index.html
- Ou, S.H. (1985) Rice Diseases. Kew: Commonwealth Mycological Institute. [Google Scholar]
- Pageau, K. , Reisdorf‐Cren, M. , Morot‐Gaudry, J.F. and Masclaux‐Daubresse, C. (2006) The two senescence‐related markers, GS1 (cytosolic glutamine synthetase) and GDH (glutamate dehydrogenase), involved in nitrogen mobilization, are differentially regulated during pathogen attack and by stress hormones and reactive oxygen species in Nicotiana tabacum ana tabacum L. leaves. J. Exp. Bot. 57, 547–557. [DOI] [PubMed] [Google Scholar]
- Perez, C.E.A. , Rodrigues, F. , Moreira, W.R. and Damatta, F.M. (2014) Leaf gas exchange and chlorophyll a fluorescence in wheat plants supplied with silicon and infected with Pyricularia oryzae . Phytopathology, 104, 143–149. [DOI] [PubMed] [Google Scholar]
- Rachmilevitch, S. , Cousins, A.B. and Bloom, A.J. (2004) Nitrate assimilation in plant shoots depends on photorespiration. Proc. Natl. Acad. Sci. USA, 101, 11 506–11 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende, R.S. , Rodrigues, F. , Cavatte, P.C. , Martins, S.C.V. , Moreira, W.R. , Chaves, A.R.M. and Damatta, F.M. (2012) Leaf gas exchange and oxidative stress in sorghum plants supplied with silicon and infected by Colletotrichum sublineolum . Phytopathology, 102, 892–898. [DOI] [PubMed] [Google Scholar]
- Rezende, D.C. , Rodrigues, F.Á. , Carré‐Missio, V. , Schurt, D.A. , Kawamura, I.K. and Korndörfer, G.H. (2009) Effect of root and foliar applications of silicon on brown spot development in rice. Australas. Plant Pathol. 38, 67–73. [Google Scholar]
- Rivero, R.M. , Shulaev, V. and Blumwald, E. (2009) Cytokinin‐dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol. 150, 1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas, C.M. , Senthil‐Kumar, M. , Tzin, V. and Mysore, K.S. (2014) Regulation of primary plant metabolism during plant–pathogen interactions and its contribution to plant defense. Front. Plant Sci. 5, 1–7. doi: 10.3389/fpls.2014.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rontein, D. , Dieuaide‐Noubhani, M. , Dufourc, E.J. , Raymond, P. and Rolin, D. (2002) The metabolic architecture of plant cells. Stability of central metabolism and flexibility of anabolic pathways during the growth cycle of tomato cells. J. Biol. Chem. 277, 43 948–43 960. [DOI] [PubMed] [Google Scholar]
- Saeed, A.I. , Sharov, V. , White, J. , Li, J. , Liang, W. , Bhagabati, N. , Braisted, J. , Klapa, M. , Currier, T. , Thiagarajan, M. , Sturn, A. , Snuffin, M. , Rezantsev, A. , Popov, D. , Ryltsov, A. , Kostukovich, E. , Borisovsky, I. , Liu, Z. , Vinsavich, A. , Trush, V. and Quackenbush, J. (2003) TM4: a free, open‐source system for microarray data management and analysis. Biotechniques, 34, 374–378. [DOI] [PubMed] [Google Scholar]
- Satoh, K. , Kondoh, H. , Sasaya, T. , Shimizu, T. , Choi, I.R. , Omura, T. and Kikuchi, S. (2010) Selective modification of rice (Oryza sativa) gene expression by rice stripe virus infection. J. Gen. Virol. 91, 294–305. [DOI] [PubMed] [Google Scholar]
- Seifi, H.S. , Van Bockhaven, J. , Angenon, G. and Höfte, M. (2013) Glutamate metabolism in plant disease and defense: friend or foe? Mol. Plant–Microbe. Interact. 26, 475–485. [DOI] [PubMed] [Google Scholar]
- Shamir, R. , Maron‐Katz, A. , Tanay, A. , Linhart, C. , Steinfeld, I. , Sharan, R. , Shiloh, Y. and Elkon, R. (2005) EXPANDER–an integrative program suite for microarray data analysis. BMC Bioinformatics, 6, 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, X. , Zhou, Y. , Duan, L. , Li, Z. , Eneji, A.E. and Li, J. (2010) Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet‐B radiation. J. Plant Physiol. 167, 1248–1252. [DOI] [PubMed] [Google Scholar]
- Silva, M.R.J. , Pereira, S.C. , Rodrigues, F.A. , Antônio, L. , Júnior, Z. , Fontes, L.F. and Oliveira, M.G.A. (2012) Silicon and manganese on the activity of enzymes involved in rice resistance against brown spot. Trop. Plant Pathol. 37, 339–345. [Google Scholar]
- Sørhagen, K. , Laxa, M. , Peterhänsel, C. and Reumann, S. (2013) The emerging role of photorespiration and non‐photorespiratory peroxisomal metabolism in pathogen defence. Plant Biol. (Stuttg), 15, 723–736. [DOI] [PubMed] [Google Scholar]
- Suenaga, A. , Moriya, K. , Sonoda, Y. , Ikeda, A. , Wirén, N.V. , Yamaguchi, J. and Yamaya, T. (2003) Constitutive expression of a novel‐type ammonium transporter OsAMT2 in rice plants. Plant Cell Physiol. 44, 206–211. [DOI] [PubMed] [Google Scholar]
- Tada, Y. , Kusaka, K. , Betsuyaku, S. , Shinogi, T. , Sakamoto, M. , Ohura, Y. , Hata, S. , Mori, T. , Tosa, Y. and Mayama, S. (2005) Victorin triggers programmed cell death and the defense response via interaction with a cell surface mediator. Plant Cell Physiol. 46, 1787–1798. [DOI] [PubMed] [Google Scholar]
- Tamura, K. , Peterson, D. , Peterson, N. , Stecher, G. , Nei, M. and Kumar, S. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, N. , Fujita, M. , Handa, H. , Murayama, S. , Uemura, M. , Kawamura, Y. , Mitsui, T. , Mikami, S. , Tozawa, Y. , Yoshinaga, T. and Komatsu, S. (2004) Proteomics of the rice cell?: systematic identification of the protein populations in subcellular compartments. Mol. Genet. Genomics, 271, 566–576. [DOI] [PubMed] [Google Scholar]
- Thuan, N.T.N. , Bigirimana, J. , Roumen, E. , Straeten, D. and Höfte, M. (2006) Molecular and pathotype analysis of the rice blast fungus in North Vietnam. Eur. J. Plant Pathol. 114, 381–396. [Google Scholar]
- Usadel, B. , Poree, F. , Nagel, A. , Lohse, M. , Czedik‐Eysenberg, A. and Stitt, M. (2009) A guide to using MapMan to visualize and compare omics data in plants: a case study in the crop species, maize. Plant Cell Environ. 32, 1211–1229. [DOI] [PubMed] [Google Scholar]
- Valentini, R. , Epron, D. , Angelis, P. , Matteucci, G. and Dreyer, E. (1995) In situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in Turkey oak (Q. cerris L.) leaves: diurnal cycles under different levels of water supply. Plant, Cell Environ. 18, 631–640. [Google Scholar]
- Van Bockhaven, J. , De Vleesschauwer, D. and Höfte, M. (2013) Towards establishing broad‐spectrum disease resistance in plants: silicon leads the way. J. Exp. Bot. 64, 1281–1293. [DOI] [PubMed] [Google Scholar]
- Van Bockhaven, J. , Spichal, L. , Novak, O. , Strnad, M. , Asano, T. , Kikuchi, S. , Höfte, M. and De Vleesschauwer, D. (2015) Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanus by preventing the pathogen from hijacking the rice ethylene pathway. New Phytol. In press. doi: 10.1111/nph.13270. [DOI] [PubMed] [Google Scholar]
- Wang, H. , Zhang, M. , Guo, R. , Shi, D. , Liu, B. , Lin, X. and Yang, C. (2012) Effects of salt stress on ion balance and nitrogen metabolism of old and young leaves in rice (Oryza sativa L.). BMC Plant Biol. 12, 1–11. doi: 10.1186/1471-2229-12-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, S. , Shimoid, E. , Ohkamab, N. , Hayashid, H. , Yoneyamad, T. , Yazakie, Y. , Fujiie, F. , Shinboe, K. , Yamamotoe, K. , Sakatae, K. , Sasakie, T. , Kishimotoe, N. , Kikuchi, S. and Fujiwara, T. (2004) Identification of several rice genes regulated by Si nutrition. Soil Sci. Plant Nutr. 50, 1273–1276. [Google Scholar]
- Williams, B. , Kabbage, M. , Kim, H.J. , Britt, R. and Dickman, M.B. (2011) Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 7, e1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler, A. , Lea, P.J. , Quick, W.P. and Leegood, R.C. (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 355, 1517–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, J.Z. , Tsuda, M. , Doke, N. and Nishimura, S. (1991) Phytotoxins produced by germinating spores of Bipolaris oryzae . Phytopathology, 81, 58–64. [Google Scholar]
- Zadoks, J.C. (2003) Fifty years of crop protection, 1950–2000. NIAS Wageningen J. Life Sci. 50, 181–193. [Google Scholar]
- Zargar, S.M. , Nazir, M. , Agrawal, G.K. , Kim, D.W. and Rakwal, R. (2010) Silicon in plant tolerance against environmental stressors: towards crop improvement using omics approaches. Curr. Proteomics, 7, 135–143. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Correlation of microarray (x axis) and real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) data (y axis) for a subset of 15 differentially expressed genes.
Table S1 Overview of physiological parameters analysed during leaf gas exchange measurements of control and silicon‐treated plants.
Table S2 List of differentially expressed genes in non‐inoculated silicon‐treated plants (Si,m vs. Ctrl,m).
Table S3 List of differentially expressed genes in Cochliobolus miyabeanus‐infected control plants (Ctrl,I vs. Ctrl,m).
Table S4 List of differentially expressed genes in Cochliobolus miyabeanus‐infected and silicon‐treated plants vs. infected control plants (Si,I vs. Ctrl,I).
Table S5 List of differentially expressed genes in silicon‐treated plants following Cochliobolus miyabeanus infection (Si,I vs. Si,m).
Table S6 Validation of microarray data by quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR).
Table S7 Central metabolism‐associated genes differentially expressed after silicon (Si) application and/or Cochliobolus miyabeanus infection.
Table S8 Primers used for microarray validation.


