Summary
Peptides and small molecules produced by both the plant pathogen P hytophthora and host plants in the apoplastic space mediate the relationship between the interplaying organisms. Various P hytophthora apoplastic effectors, including small cysteine‐rich (SCR) secretory proteins, have been identified, but their roles during interaction remain to be determined. Here, we identified an SCR effector encoded by scr96, one of three novel genes encoding SCR proteins in P . cactorum with similarity to the P . cactorum phytotoxic protein PcF. Together with the other two genes, scr96 was transcriptionally induced throughout the developmental and infection stages of the pathogen. These genes triggered plant cell death (PCD) in the Solanaceae, including N icotiana benthamiana and tomato. The scr96 gene did not show single nucleotide polymorphisms in a collection of P . cactorum isolates from different countries and host plants, suggesting that its role is essential and non‐redundant during infection. Homologues of SCR96 were identified only in oomycetes, but not in fungi and other organisms. A stable protoplast transformation protocol was adapted for P . cactorum using green fluorescent protein as a marker. The silencing of scr96 in P . cactorum caused gene‐silenced transformants to lose their pathogenicity on host plants and these transformants were significantly more sensitive to oxidative stress. Transient expression of scr96 partially recovered the virulence of gene‐silenced transformants on plants. Overall, our results indicate that the P . cactorum scr96 gene encodes an important virulence factor that not only causes PCD in host plants, but is also important for pathogenicity and oxidative stress tolerance.
Keywords: oxidative stress, pathogenicity, Phytophthora cactorum, small cysteine‐rich protein, stable protoplast transformation, transcriptional profile, transient expression
Introduction
Phytophthora species are notorious oomycete pathogens that attack a wide range of agriculturally and ornamentally important plants. The best known potato late blight disease, which is caused by P. infestans and was responsible for the Great Irish Famine in the 1840s, remains a serious problem worldwide (Kamoun et al., 2015). Phytophthora cactorum, a destructive and widespread soil‐borne pathogen, has been reported to affect an extremely wide range of hosts that span several plant families. It often causes root, collar and crown rots, as well as foliar and fruit infections, limiting the production of many economically important crops, such as strawberry, apple, pear, tomato and rhododendron, worldwide (Erwin and Ribeiro, 1996). Despite the importance of Phytophthora diseases in agriculture and ecosystems, it is difficult to combat them because of a lack of understanding of their mechanisms of pathogenesis.
Plant survival in the presence of pathogen threats requires both effective perception of, and appropriate responses to, pathogenic attack. To do so, plants have developed two distinct layers of immunity: pathogen‐ or microbe‐associated molecular pattern (PAMP/MAMP) and damage‐associated molecular pattern (DAMP)‐triggered immunity (PTI), and effector‐triggered immunity (ETI) (Dodds and Rathjen, 2010; Jones and Dangl, 2006). PTI, known as the first layer of plant defence, is often achieved through the recognition of PAMPs or DAMPs by membrane‐located pattern recognition receptors (Boller and Felix, 2009; Monaghan and Zipfel, 2012). PAMPs or DAMPs are found in molecules previously designated as ‘general elicitors’. Their recognition initiates the induction of various antimicrobial responses, including the reactive oxygen species (ROS) burst, cell wall reinforcement and the expression of defence genes, without signs of programmed cell death (Boller and Felix, 2009). Although PTI restricts the growth of the vast majority of potential pathogens, successful pathogens have evolved mechanisms to effectively suppress this basal defence by the secretion of an array of effectors (Bozkurt et al., 2012). Plants have, in turn, evolved nucleotide‐binding leucine‐rich repeat resistance proteins, which allow for the direct or indirect recognition of pathogen effectors. This secondary immunity, namely ETI, is often accompanied by the hypersensitive response (HR), corresponding to a localized programmed cell death, and by systemic acquired resistance (Dou and Zhou, 2012; Giraldo and Valent, 2013; Jones and Dangl, 2006).
So far, several types of necrosis‐inducing ‘general elicitor’ have been characterized in plant pathogens and, in particular, in Phytophthora pathogens (Hein et al., 2009). These include elicitins, NLPs (Nep1‐like proteins), CBEL (cellulose‐binding elicitor lectin) glycoproteins and small cysteine‐rich (SCR) proteins, such as P. cactorum PcF (Kamoun, 2006). Little is known about the roles of the hypersensitive‐like response induced by such necrosis‐inducing elicitors. They are predicted to be located at the interface between pathogens and hosts, and to execute functions outside of the host cell, and are therefore called apoplastic effectors (Kamoun, 2006). NLPs appear to function as phytotoxins to induce cell death as a result of their structural homology to pore‐forming toxins produced by sea anemones (i.e. actinoporins) (Ottmann et al., 2009). The recognition of elicitins and the inability of pathogens to inhibit this recognition could limit the host range of the pathogens (Kamoun et al., 1998). Plant receptor‐like kinase, such as Nicotiana benthamiana BAK1/SERK3, contributes to elicitin‐mediated necrosis, and kinase gene silencing leads to enhanced plant susceptibility to P. infestans (Chaparro‐Garcia et al., 2011). Furthermore, elicitin‐, NLP‐ and CBEL‐induced cell death can be suppressed by host‐translocated effectors, demonstrating that oomycetes have evolved delicate mechanisms to counteract the host cell death triggered by necrosis‐inducing elicitors (Bos et al., 2006; Chen et al., 2013, 2014; Gilroy et al., 2011; Liu et al., 2011). Based on these results, it has been suggested that responses to these proteins could overlap with ETI and play an important role in the outcome of Phytophthora–plant interactions (Thomma et al., 2011).
Mature PcF, secreted after N‐terminal signal processing, is an acidic 52‐amino‐acid protein comprising a 4‐hydroxyproline and six cysteine residues bridged intramolecularly (Orsomando et al., 2001). PcF can trigger HR symptoms of localized cell death on the leaves of both strawberry and tomato, and induction of the key defence‐related enzyme PAL21 and other pathogenesis‐related (PR) genes in tomato (Orsomando et al., 2001, 2003). It has been suggested that PcF may mimic the effects of a plant signalling protein because it seemingly has structural homology to the pollen protein Ole‐e6 with allergenic activity (Nicastro et al., 2009). Oomycetic SCR proteins can be related to PcF mainly on the basis of their conserved sequences, cysteine patterns and N‐terminal secretory signals (Bos et al., 2003; Orsomando et al., 2011). The expansion of PcF/SCR gene families in oomycetes has been noted in whole‐genome analyses and the size of the family in several species, such as P. infestans (n = 16), P. sojae (n = 19), P. ramorum (n = 4), Pythium ultimum (n = 3) and Hyaloperonospora arabidopsidis (n = 2), has been estimated through annotation (Baxter et al., 2010; Haas et al., 2009; Lévesque et al., 2010; Tyler et al., 2006). In our recent work, three SCR genes plus PcF were detected in the P. cactorum transcriptome (Chen et al., 2014). Comparative structural and functional characterization of PcF and several homologues from P. infestans highlighted a surface‐exposed conserved amino acid stretch SK(E/C)C as a possible structural determinant responsible for the differential phytotoxicity (Orsomando et al., 2011). Although a ‘PcF/SCR’ family has been proposed, it has been studied much less than other effectors (Stassen and Van den Ackerveken, 2011). To date, PcF remains the only characterized member within the family. The other family members include proteins encoded by polymorphic genes, and are often identified by the abbreviation ‘SCR’ followed by their residue number, such as SCR70, SCR74 and SCR91 from P. infestans (Bos et al., 2003; Liu et al., 2005). Although PcF/SCR proteins share with fungal avirulence proteins (e.g. Avr2 and Avr4 of Cladosporium fulvum) the feature that they are secreted and are SCR proteins, the oomycete counterparts are generally considered to function as extracellular toxins (Bos et al., 2003; Stergiopoulos and de Wit, 2009; Wawra et al., 2012). However, their molecular action and biological functions have not been identified to date.
The main objective of this study was to investigate the roles of newly identified P. cactorum PcF/SCR effectors (hereafter referred to as ‘SCR’ followed by their residue number) in pathogen–plant interactions using transcriptional profiles, transient expression, sequence analysis and gene silencing. Overall, the results show that these genes differentially expressed during the infection phase can trigger plant cell death (PCD) in N. benthamiana and tomato, and one of them, SCR96, is important for the pathogenicity and oxidative stress tolerance of P. cactorum. This study demonstrates that PCD caused by these small molecules is not a PTI response and that these effectors are probably virulence determinants. The work described herein provides a crucial foundation for further determination of the roles of PcF/SCR effectors in the virulence of Phytophthora pathogens.
Results
PcF/SCR gene expression profiling of P . cactorum
Recently, we have assembled the transcriptome of P. cactorum life cycle stages containing 21 662 unique genes (Chen et al., 2014). About 620 effectors, including PcF and three SCR proteins (SCR96, SCR99 and SCR121), were identified (Chen et al., 2014) (Table 1). To understand the transcriptional profiles of these unique genes during life cycle stages, three cDNA libraries representing mycelia (MY), swimming zoospores (ZO) and germinating cysts with germ tubes (GC) were separately constructed and sequenced in this study using the Illumina Genome Analyzer IIx platform (Illumina, San Diego, CA, USA). Each sequenced sample yielded 100‐bp reads from single‐end sequencing of cDNA fragments. Most of the distinct reads were matched to P. cactorum genes (Table S1, see Supporting Information), and the transcript level of each expressed gene was calculated and normalized to the reads per kilobase of exon model per million mapped reads (RPKM) (Mortazavi et al., 2008). RPKM values showed that, among the effector genes, PcF and three SCR genes were differentially expressed during these developmental stages (Fig. 1a). scr96 and scr121 were highly expressed in MY versus GC and ZO, whereas the other two genes (scr99 and PcF) showed increased expression in GC versus MY and ZO (Fig. 1a).
Table 1.
P hytophthora cactorum PcF/small cysteine‐rich (SCR) effectors analysed in this study
| Gene* | GenBank accession no. | Gene length (bp) | SignalP HMM # | SignalP NN # | SignalP length (amino acids) # | Number of cysteine residues† |
|---|---|---|---|---|---|---|
| PcF | KT215392 | 222 | 0.999 | 0.940 | 21 | 6 |
| scr96 | KT215393 | 291 | 0.999 | 0.851 | 23 | 8 |
| scr99 | KT215394 | 300 | 1.00 | 0.964 | 19 | 6 |
| scr121 | KT215395 | 366 | 0.994 | 0.916 | 22 | 6 |
*The gene sequences were amplified from the isolate 10 300 (Chen et al., 2014).†Only cysteine residues in the mature peptide sequences were counted.
#Hidden Markov model (HMM) probability, Neural Network (NN) mean S score, and signal peptide length were predicted using SignalP v3.0.
Figure 1.
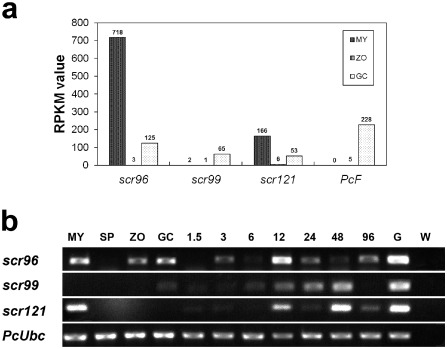
Expression profiles of P hytophthora cactorum PcF/small cysteine‐rich (SCR) effector‐encoding genes. (a) The gene expression level was determined by calculating the number of reads for each gene and then normalizing this to the reads per kilobase of exon model per million mapped reads (RPKM). The RPKM values of scr96, scr99, scr121 and PcF were calculated in this study using the RNA‐Seq data (GenBank SRA accession no. SRP059996). (b) The expression of scr96, scr99 and scr121 was profiled using reverse transcription‐polymerase chain reaction (RT‐PCR) throughout the P . cactorum life cycle, including mycelia (MY), sporangium (SP), zoospore (ZO) and germinating cyst (GC), and during infection stages, including 1.5, 3, 6, 12, 24, 48 and 96 h post‐inoculation (hpi) of N icotiana benthamiana. W, water used as PCR template; G, P . cactorum genomic DNA; PcU bc, internal control PcU bc gene of P . cactorum. Three independent biological experiments were performed and yielded similar results.
To determine whether SCR genes were induced during plant invasion by P. cactorum, we inoculated N. benthamiana with zoospores of the pathogen and investigated their expression using semi‐quantitative reverse transcription‐polymerase chain reaction (RT‐PCR). All the tested genes were differentially expressed during plant invasion (Fig. 1b). In particular, scr96 was highly expressed during the very early infection stage (3 h post‐inoculation, hpi) (Fig. 1b). The expression patterns during the life cycles profiled by RT‐PCR are consistent with the results obtained by RNA‐Seq analysis (Fig. 1).
Normally, Phytophthora pathogens invade plants by mycelia or germinating cysts, which develop into mycelia during the penetration process. As shown in Fig. 1, the transcript up‐regulation suggests that SCR proteins play important roles during the pre‐infection (cyst germination) and infection stages.
SCR96 and other SCR proteins trigger cell death in solanaceous plants
To investigate the biological function of effectors, transient expression assays were performed on solanaceous plants using the infiltration of Agrobacterium tumefaciens cells with Potato virus X (PVX) carrying scr96 or other homologous genes. At 6 days post‐treatment, negative control leaves (pGR107‐gfp and 10 mm MgCl2) remained green and healthy, but infiltration with SCR96 conferred symptoms of necrosis as vigorously as the positive control INF1 on N. benthamiana (Fig. 2a). No visible symptoms were observed from the transient expression of scr99, scr121 and PcF during the tests (Fig. 2b–d). We then performed similar experiments on Solanum lycopersicum and found that the expression of scr96, scr121 and scr99 resulted in leaf cell death at 4 days post‐treatment (Fig. 2e–g). No visible symptoms were observed when inf1 and PcF were transiently expressed in tomato leaves (Fig. 2h). Similar experiments were also conducted on N. tabacum, but no changes were observed with the tested PcF/SCR proteins, except INF1 (Fig. 2i). Notably, we cloned PcF from the isolate 10300 and performed parallel tests. Like the negative controls, PcF cannot induce PCD in these three tested plant species (Fig. 2d,h,i).
Figure 2.
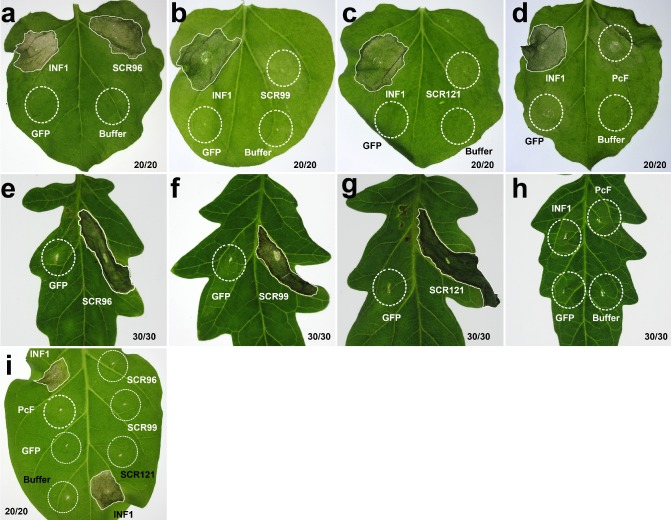
Transient assays of P hytophthora cactorum PcF/small cysteine‐rich (SCR) effectors in Solanaceae plants. The leaves of three species of Solanaceae plants, N icotiana benthamiana (a–d), tomato (e–h) and N . tabacum (i), were infiltrated with A grobacterium tumefaciens (strain GV3101) cells to express the effectors. The expression of scr96 triggered plant cell death (PCD) in both N . benthamiana (a) and tomato (e), whereas scr99 (f) and scr121 (g) induced PCD only in tomato. SCR99 (b) and SCR121 (c) did not induce PCD in N . benthamiana. Together with SCR96, these three cannot trigger PCD in N . tabacum (i). The P . cactorum protein PcF cannot cause PCD in any type of plant (d, h, i). The P . infestans elicitin INF1 served as a positive control in both N . benthamiana and N . tabacum (a–d, i), but not in tomato (h). The marker gene gfp and 10 mm MgCl2 buffer were used as negative controls in all tested plants. The broken circles indicate the agroinfiltration areas without PCD, whereas irregular shaped areas are tissues in which PCD occurs. Photographs were taken 6 days (a–d) or 4 days (e–i) after infiltration.
The sequences of scr96 were subsequently examined in a limited collection of P. cactorum isolates from different countries and host plants (Table S2, see Supporting Information) to determine whether sequence polymorphisms may exist. However, it was found that full‐length DNA sequences of scr96 were identical among all the tested P. cactorum isolates.
Homologues of SCR96 exist only in oomycetes, but not in fungi
The aforementioned SCR genes plus PcF were successfully full length cloned and sequenced, yielding the same sequences as transcriptomic data (Chen et al., 2014). These genes do not contain any introns and are typically short in length, ranging from 222 to 366 bp. SignalP analysis identified a putative signal peptide at the N‐terminus of each protein. Conserved domain analysis revealed nothing but an even number of cysteine residues in the mature peptide sequences (Table 1; Fig. S1, see Supporting Information).
As a result of its strong expression in MY, GC and early infection stages, and its PCD‐triggering activity in solanaceous plants, we investigated SCR96 in more detail. To determine the presence of its homologues in other organisms, we performed a blastx search of the FungiDB and genome databases for various oomycetes. Homologues of SCR96 were identified only in oomycetes, including P. capsici, P. parasitica, P. sojae, P. cinnamomi, H. arabidopsidis and Pythium spp., but not in fungi or other organisms. Notably, two homologues (PHYCA_130237 and PHYCA_511621) of SCR96 were identified in P. capsici. The alignment of SCR96 homologues demonstrated a high conservation of SCR96 among different species of oomycete (Fig. 3a). Phylogenetic analysis revealed that SCR96 and all of its homologues, except PcF, from other Phytophthora spp. form a clade distinct from that of other oomycetes, with the homologue from Pythium vexans as the closest relative. Intriguingly, like one of the secretory SCR proteins of C. fulvum, AVR2 (GenBank accession no. AIZ11404), P. cactorum PcF forms a clade distant from that of other oomycete SCRs (Fig. 3b).
Figure 3.
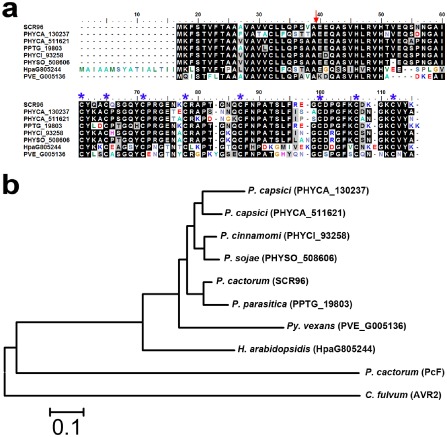
Protein sequence alignment and phylogenetic analysis of P hytophthora cactorum SCR96 and its homologues in different oomycete species. (a) Protein sequences were aligned and shaded for consensus (50% threshold for shading) using BioEdit. The homologues of SCR96 compared here include PHYCA_130237 and PHYCA_511621 from P . capsici, PPTG_19803 from P . parasitica, PHYCI_93258 from P . cinnamomi, PHYSO_508606 from P . sojae, HpaG805244 from H yaloperonospora arabidopsidis and PVE_G005136 from P ythium vexans. The predicted cleavage site for signal peptides is indicated by a red arrow. The conserved cysteine sites are marked at the top by blue asterisks for visualization. (b) Phylogeny of SCR96 and homologues from different species. The tree was constructed by the neighbour‐joining (NJ) algorithm implemented in MEGA 5 with 1000 bootstrap replicates. The IDs, except for SCR96, PcF and AVR2, in parentheses after the species names are from the FungiDB database. C ladosporium fulvum AVR2 served as an outgroup in the NJ tree.
Protoplast transformation and gfp (green fluorescent protein) expression in P . cactorum
It has been shown that the transformation of Phytophthora species with gene constructs results in gene silencing of both the transgenes and the endogenous gene (van West et al., 1999). As stable transformation of P. cactorum has not been attempted, to analyse the gene functions, the standard transformation protocol using protoplasts and a polyethylene glycol/calcium chloride (PEG/CaCl2)‐mediated method was adapted from the literature (Mcleod et al., 2008). The main modification is that the geneticin (G418) concentration used for transformant selection was 17.5 μg/mL based on a geneticin sensitivity test of the wild‐type (WT) isolate 10 300 (Fig. S2, see Supporting Information).
Using these procedures, the binary transformation vector pGFPN, containing both the gfp and selectable marker (neomycin phosphotransferase II, nptII) gene expression cassettes, was transformed into P. cactorum WT isolate 10 300 and 25 G418‐resistant transformants were obtained. After screening visually for fluorescence using an epifluorescent Olympus BX53 microscope (Tokyo, Japan) and later single zoospore isolation, the brightest transformants (GFP1 and GFP2) were retained. The transformants fluoresced green in the life stages including mycelia, sporangia, germinating cysts and oospores (Fig. S3, see Supporting Information). The WT isolate did not fluoresce at all. There were no significant differences in the tested characteristics (mycelia growth rate, yields of sporangia and oospores, and pathogenicity) between transgenic GFP isolates and the WT (data not shown).
SCR96 is important for the virulence of P . cactorum
To determine whether SCR96 was required for P. cactorum pathogenicity, stable scr96‐silenced strains of P. cactorum (10 300) were generated using the above established transformation‐mediated gene silencing method. We obtained three scr96‐silenced lines, S3, S10 and S21, in which scr96 expression was reduced by 65%, 80% and 95%, respectively, compared with the WT strain, whereas scr96 expression was not affected in the transformation control strain (gfp‐expressing transformant GFP1) (Fig. 4a). Inoculation assays were performed by dropping zoospore suspensions of the WT and the silenced transformants onto healthy leaves of 5‐week‐old N. benthamiana plants. At 2 days post‐inoculation (dpi), the leaves inoculated with the WT isolate zoospores showed typical disease symptoms and water‐soaked lesions (Fig. 4b,c). In contrast, the silenced transformants (S10 and S21 shown here) produced no visible lesions at all (Fig. 4b,c). S3, the third transformant, behaved overall in a similar manner to S10 and S21. We then tested whether transient expression of scr96 in planta could recover Phytophthora virulence on N. benthamiana plants. At 48 h after infiltration of A. tumefaciens cells harbouring scr96, the agroinfiltration zone was inoculated with scr96‐silenced transformants (S10 and S21). Intriguingly, transient expression of scr96 partially compensated for the virulence of gene‐silenced transformants on N. benthamiana (Fig. 4d–f). Together, these results indicate that SCR96 is required for Phytophthora virulence.
Figure 4.
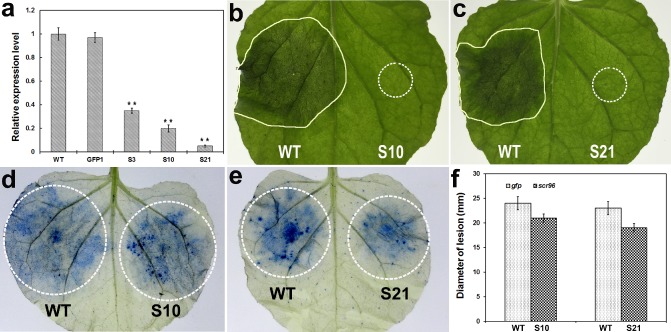
The role of P hytophthora cactorum scr96 in the infection of N icotiana benthamiana. (a) Silencing of scr96. The gene scr96 mRNA abundances in three stably silenced P . cactorum transformants (S3, S10 and S21) were determined by quantitative polymerase chain reaction (PCR) 3 days after mycelial growth in V8 medium relative to PcU bc mRNA abundances, and then normalized to the wild‐type isolate 10 300 (WT). GFP1 is a transformation control expressing green fluorescent protein. Standard errors from three technical replicates are shown. Statistical significance was analysed using Student's t‐test between the WT/GFP1 and WT/transformant comparisons (**P < 0.01). (b, c) Two representative gene‐silenced transformants (S10 and S21, respectively) show a loss in virulence on N . benthamiana compared with the WT. Photographs were taken at 48 h post‐inoculation (hpi). The irregular shaped areas are necrotic tissues caused by the WT isolate, whereas broken circles indicate the gene‐silenced transformant inoculation sites without visible necrosis. (d–f) Transient expression of scr96 partially recovered the virulence of gene‐silenced transformants on N . benthamiana. At 48 h after agroinfiltration, infiltration sites in each leaf expressing both gfp (left) and scr96 (right) were challenged with the WT isolate and gene‐silenced transformants [S10 (d) or S21 (e)]. Photographs were taken 48 h after pathogen inoculation. Leaves were stained by trypan blue and then destained by ethanol. The broken circles indicate the infiltration and inoculation sites. (f) The diameters of the lesions from (d) and (e) were measured and analysed. Error bars represent standard errors from technical replicates. There was no significant difference between left and right treatments (Student's t‐test).
SCR96 is related to oxidative stress tolerance
To gain further insight into the role of SCR96 in the infection process, two scr96‐silenced lines (S10 and S21), the WT strain 10 300 and the transformation control GFP1 were cultured on Plich's agar medium supplemented with different concentrations of H2O2. The scr96‐silenced lines showed no significant reduction in mycelial growth on Plich's agar medium in the absence of H2O2 when compared with the WT and control transformant GFP1 (Fig. 5). In contrast, treatment with 4 or 6 mm H2O2 completely suppressed the mycelial growth of scr96‐silenced transformants, whereas the WT and GFP1 lines still proliferated (Fig. 5). At 8 mm H2O2, all the strains were unable to grow (data not shown). Together, these results indicate that SCR96 is essential for oxidative stress tolerance of P. cactorum.
Figure 5.
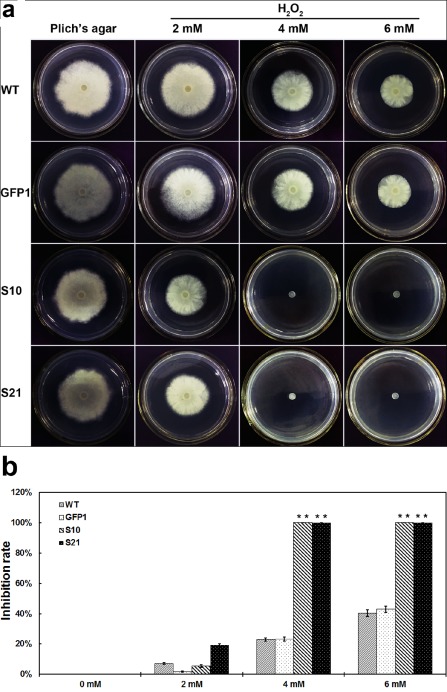
Mycelial growth assay of P hytophthora cactorum scr96‐silenced transformants under the oxidative stress condition. (a) Assay of mycelial growth of the wild‐type (WT), transformation control (GFP1) and scr96‐silenced transformants (S10 and S21 shown here) on Plich's agar medium only or supplemented with 2, 4 or 6 mm H2O 2. (b) Rates of growth inhibition were calculated for each treatment relative to growth on Plich's medium only. Colony diameters were measured in each independent biological experiment after 5 days of growth. Three independent biological experiments were performed with three replicates each time. Error bars represent the standard errors and asterisks indicate significant differences between the scr96‐silenced transformants and the WT (**P < 0.01).
Discussion
Since the discovery of the PcF/SCR family, little has been determined about its biological functions and roles in the virulence of Phytophthora pathogens. Apart from PcF, all other members have not been characterized and their functions have not been verified experimentally. In this study, we demonstrated that a putative P. cactorum PcF/SCR protein, SCR96, is one of the ‘general elicitors’ triggering PCD in N. benthamiana and tomato, and silencing of its expression affects its pathogenicity on host plants and its tolerance to oxidative stress.
Our previous transcriptomic study detected three novel PcF/SCR effectors (SCR96, SCR121 and SCR99) and PcF from P. cactorum isolate 10 300 (Chen et al., 2014). Based on transcriptomic profiling and RT‐PCR data, it was found that these genes were transcribed differentially throughout the P. cactorum life cycle and infection phase on N. benthamiana (Fig. 1). Among them, scr96 was highly expressed during mycelium, cyst germination and early infection stages (Fig. 1a,b). Similarly, the expression of scr74 was significantly up‐regulated during P. infestans colonization stages of potato and tomato (Liu et al., 2005). The expression patterns of the PcF/SCR effector genes are consistent with their expected roles in assisting the colonization of plant tissues by the pathogen. To explore their predicted roles further, we tested whether the effectors could cause phenotypic reactions when expressed in host plants, and found that three effectors (SCR96, SCR121 and SCR99) could elicit PCD in N. benthamiana and/or tomato (Fig. 2). Purified PcF from culture filtrate induced leaf necrosis on tomato and strawberry (Orsomando et al., 2001). Two PcF homologues from P. infestans, SCR74 and SCR91, together with PcF, after recombinant expression in bacteria and purification, triggered leaf withering and phenylalanine ammonia lyase induction on tomato (Orsomando et al., 2011). PCD induced by phytotoxins is thought to play a central role in providing a source of nutrition for necrotrophic and hemibiotrophic pathogens (Dou and Zhou, 2012; Mott et al., 2014). However, such a response caused by different PcF/SCR effectors shows various degrees of effectiveness (Orsomando et al., 2011). In our case, we observed no changes visible to the naked eye when some of the PcF/SCRs, e.g. SCR96 and PcF, were expressed in leaves of solanaceous plants (Fig. 2). Whether such effectors are host species or even variety selective, or are required for infection on susceptible host plants that have the corresponding dominant receptor, requires further study. More work needs to be performed to determine whether these observations in transient expression assays are also a result of poor protein expression or stability.
As fungal pathogen virulence factors, phytotoxins contribute to successful infection by causing direct damage to cell membranes, alteration of gene expression, inhibition of plant protein function, mimicking of plant hormones and induction of cell death through the production of ROS (Möbius and Hertweck, 2009). In oomycetes, it is presumed that two families of toxic proteins act in an offensive manner by triggering host cell death that could favour the necrotrophic phase of development. NLPs have been suggested to be involved in the disruption of plant plasma membranes and subsequent cytolysis on the basis of their similarities to the pore‐forming toxins of sea anemones and from in planta experiments (Ottmann et al., 2009). In contrast, the nature of PcF/SCRs remains to be resolved. It has been assumed previously that they are possibly oomycete avirulence proteins because of the characteristics they share with fungal avirulence proteins, i.e. SCR secretory proteins (Bos et al., 2003). Unlike fungal avirulence proteins, such as Avr2 of C. fulvum (Rooney et al., 2005), however, plant targets of PcF/SCR effectors are still unknown. PcF/SCRs are assumed to function in the plant apoplast as phytotoxins (Stassen and Van den Ackerveken, 2011). It is within this space that the fate of many host–pathogen interactions is determined (Mott et al., 2014). We found that P. cactorum scr96‐silenced transformants lost their pathogenicity to N. benthamiana, indicating that this effector plays an important role in virulence during pathogen infection (Fig. 4). On detection of pathogen invasion through the recognition of PAMPs, one of the early reactions of plants is the production of toxic ROS. This production is classically associated with direct microbial killing (Peng and Kuc, 1992). To elucidate whether PcF/SCR effectors are involved in ROS responses, gene‐silenced transformants were tested on Plich's medium supplemented with H2O2. We found that scr96‐silenced transformants were more sensitive to H2O2 than the control isolates (Fig. 5). The results indicated that the PcF/SCR effectors contribute positively to the virulence of the pathogens, and loss of pathogenicity of the transformants may be caused by a compromised ability to degrade H2O2 and/or reductions in other stress tolerance capabilities during infection. Therefore, SCR96 is probably a virulence determinant and involved in ROS responses. On the basis of these results, we assume that SCR proteins may not act as toxins, or may be targeted by ROS. This speculation is partially supported by previous findings indicating that PcF shows structural similarity to Ole‐e6, a major allergen from olive tree pollen, by nuclear magnetic resonance (Nicastro et al., 2009), and PcF/SCR proteins share certain characters with fungal Avr proteins, such as being SCR secretory proteins. Nonetheless, the exact mode of action of PcF/SCR family proteins remains to be determined.
Studies have shown how the primary sequences of host‐translocated effectors have been shaped by positive selection, and how genes undergo accelerated birth and death evolution (Cui et al., 2012; Stassen and Van den Ackerveken, 2011; Win et al., 2007). In this study, sequence polymorphism was not observed for SCR96. This is possibly because only a few P. cactorum isolates were investigated for sequence polymorphism. Another possible reason may be that this effector is conserved in P. cactorum with slow rates of evolution. This effector is seemingly specific to oomycetes because homologues exist only in oomycetes, but not in fungi (Fig. 3). In contrast, a previous study has shown that positive selection probably caused the extensive polymorphism observed within the SCR74 family of P. infestans (Liu et al., 2005). This may suggest that distinct selective forces have shaped different PcF/SCR family members throughout the evolution of Phytophthora species.
This is the first study to report that PCD‐triggering PcF/SCR effectors are important for pathogenicity and oxidative stress tolerance in Phytophthora. However, their molecular mechanisms remain to be elucidated. On the basis of an ‘arms race’ model, all PcF/SCR effectors have been tentatively classified as ‘apoplastic effectors’, interacting with plant surface receptors (Bos et al., 2003; Liu et al., 2005; Tyler et al., 2006). Although PcF/SCR effectors have no known protein domains, they are rich in cysteine residues that can form disulfide bridges (Orsomando et al., 2003, 2011). The intramolecular S–S bridge is presumed to be important for the protection of such proteins in the harsh acidic and protease‐rich apoplast environment in which they are delivered during plant infection (Kamoun, 2006). In the near future, the nature of effectors and plant target proteins should be determined. Ultimately, the knowledge obtained from these studies will help us to establish functional connections between pathogen effectors and host defence processes, and to provide insights into novel disease control strategies.
Experimental Procedures
Growth of plant materials and P hytophthora strains
Nicotiana benthamiana, N. tabacum (cv. Samsun NN) and S. lycopersicum (cv. Maofen‐802) were grown in Styrofoam cups containing sterile soil at 25 °C under a 16‐h/8‐h light/dark regime.
The strains (Table S2) of P. cactorum were routinely cultured on 10% V8 agar medium at 25 °C in the dark (Erwin and Ribeiro, 1996). To test for stress sensitivity, culture plugs (5 mm × 5 mm) were placed onto freshly prepared Plich's agar plates supplemented with H2O2 (2–8 mm) and cultured in the dark at 25 °C.
Nucleic acid isolation and mRNA sequencing
Genomic DNA was isolated from Phytophthora mycelia as described by Zelaya‐Molina et al. (2011). Total RNA from different life stages of P. cactorum and from inoculated plant samples was prepared as described by Chen et al. (2014). Total RNA was isolated using RNAiso Plus reagents (TaKaRa Biotechnology, Dalian, China) according to the manufacturer's instructions.
Three P. cactorum RNA‐Seq libraries (MY, SP and GC) were constructed according to the manufacturer's instructions using an Illumina Gene Expression Preparation Kit. DNA fragments were single‐end sequenced on the Illumina Cluster Station and Illumina Genome Analyzer IIx sequencing platform. The clear reads were mapped to P. cactorum transcriptome reference sequences (Chen et al., 2014) using the blast‐Like Alignment Tool (BLAT) (Kent, 2002) with all default parameters, except –tileSize = 9 and –stepSize = 6. The datasets are available at the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database through the project accession no. SRP059996.
RT‐PCR and quantitative RT‐PCR analysis
mRNA was reverse transcribed into cDNA using M‐MLV reverse transcriptase (RNase H Minus) and random hexamer primers (TaKaRa Biotechnology) following the manufacturer's protocol. PCRs were run on an LS‐96G thermocycler (Thermofisher, Dubuque, IA, USA) with the following program: 94 °C for 5 min, followed by 29 cycles (40 cycles for quantitative PCR) of 95 °C for 15 s, 46–60 °C for 30 s and 72 °C for 1 min, and, finally, melting curve analysis. For quantitative PCR, SYBR Green dye (Applied Biosystems, Foster City, CA, USA) was used according to the manufacturer's instructions. In each case, the housekeeping gene encoding ubiquitin‐conjugating enzyme (PcUbc) (GenBank accession no. KM068098) was used as the internal control (Chen et al., 2014). For quantitative RT‐PCR, expression data were analysed by Student's t‐test (P < 0.01). When gene expression was monitored in infected N. benthamiana, cDNA from mock‐inoculated plants was used as an additional negative control for each primer pair to exclude the possibility that the amplification was caused by homologous plant genes. The primers used in the reactions are listed in Table S3 (see Supporting Information). All RT‐PCRs were performed three times using independently isolated RNA samples.
Molecular cloning and sequence analysis
For transient expression in solanaceous plants, full‐length effector genes amplified from genomic DNA using high‐fidelity DNA polymerase (PrimeSTAR® HS DNA Polymerase, TaKaRa Biotechnology) and primer pairs (Table S3) were cloned into the SmaI site of the binary PVX vector pGR107 or ClaI‐SalI sites of pGR106 (Jones et al., 1999). For Phytophthora transformation, a full‐length scr96 gene fragment was amplified from genomic DNA using high‐fidelity DNA polymerase and primer pairs (SCR96‐ClaIF/BsiWIR; Table S3), digested with ClaI and BsiwI, and ligated with equally digested pTORmRFP4 (Whisson et al., 2007) to yield the plasmid pTOR‐scr96.
blastx (E ≤ 1e‐5) was used for homology searches of the FungiDB database (http://fungidb.org) and genome websites for several oomycetes. The homologues are arbitrarily defined here to have a predicted signal peptide, a mature length of ≤200 amino acid residues, at least four cysteines and a total cysteine content of ≥5%. Alignment of SCR96 and its homologues from other organisms was performed with BioEdit (v7.2.0). Phylogenetic trees were generated by the neighbour‐joining algorithm implemented in MEGA (v5) with default parameters. Tree node support was evaluated by bootstrapping with 1000 pseudoreplicate datasets.
High‐fidelity PCR amplification of polymorphism analysis for scr96 was conducted as described by Chen et al. (2014). Briefly, the primer pair (SCR96snp‐F/R; Table S3) was designed to amplify a 392‐bp fragment containing the entire open reading frame (ORF) of the gene. The genomic DNA from each of eight P. cactorum isolates (Table S2) was used as PCR template. The fragments were cloned into a TA vector pMD‐19T (TaKaRa Biotechnology) and 30 randomly selected clones for each fragment were sequenced using the universal M13 primers (Sangon Biotech, Shanghai, China).
Agroinfiltration assay
The culture of A. tumefaciens strain GV3101 carrying pGR107‐ or pGR106‐derived constructs and infiltration into leaves of N. benthamiana, N. tabacum and S. lycopersicum were performed as described by Chen et al. (2014). Each assay consisted of at least five plants inoculated on four leaves (total of 20 leaves). Symptom development was monitored visually 3–15 days after infiltration. The experiments were repeated at least three times on independent occasions, producing similar results in each case.
Phytophthora transformation
Stable transformation of P. cactorum was performed using a PEG/CaCl2‐mediated protoplast transformation protocol (Mcleod et al., 2008) with minor modifications. Briefly, isolate 10 300 was grown on nutrient pea broth (NPB) agar plates (Mcleod et al., 2008) for 4 days before being transferred into NPB broth for 2–3 days of cultivation. Transformants were selected on pea broth‐mannitol (PM) agar medium (Mcleod et al., 2008) containing 17.5 μg/mL G418 (geneticin) (Sangon Biotech). The plasmid pGFPN containing both the gfp gene driven by the promoter of the Bremia lactucae Ham34 gene and the nptII gene driven by the promoter of the B. lactucae Hsp70 gene (Ah‐Fong and Judelson, 2011) was introduced into P. cactorum using the above transformation strategy, and green fluorescence of the different cell types was observed using an Olympus BX53 fluorescence microscope at excitation and emission wavelengths of 488 nm and 495–530 nm, respectively.
For gene silencing, the plasmid pTOR‐scr96 was introduced into P. cactorum using the same protoplast transformation protocol, and putative SCR gene‐silenced transformants were screened by quantitative RT‐PCR using gene‐specific primer, with the PcUbc gene (Table S3) serving as a constitutively expressed endogenous control.
Pathogenicity test
Zoospores of the WT isolate, transformation control and SCR gene‐silenced transformants of P. cactorum were used to inoculate plants. The abaxial sides of detached leaves of N. benthamiana plants were inoculated with 1000 zoospores of each strain. Inoculated leaves were kept in 80% humidity and darkness at 25 °C, and inspected every 2 h for disease symptoms.
Trypan blue staining
Trypan blue staining was performed to detect cell death in N. benthamiana leaves. Leaves were soaked in Farmer's solution (95% ethanol–chloroform–glacial acetic acid, 6 : 3 : 1) for 30 s and then submerged in a 0.05% trypan blue mixture (Sigma‐Aldrich, St. Louis, MO, USA) for 8 h in the dark. The leaves were rinsed with deionized water and then destained using multiple changes of boiling 95% ethanol. The infected areas of the leaves were then compared with those of the uninoculated controls and photographed.
Supporting information
Fig. S1 Protein sequence alignment of SCR96, SCR99, SCR121 and PcF identified in this study. The gene sequences of scr96, scr99, scr121 and PcF were cloned from Phytophthora cactorum isolate 10 300. The deduced protein sequences were aligned and shaded for consensus (50% threshold for shading) using BioEdit.
Fig. S2 The growth of Phytophthora cactorum wild‐type isolate 10 300 on nutrient pea broth (NPB) medium (Mcleod et al., 2008) supplemented with geneticin (G418) at different concentrations. Photographs were taken at 4 days post‐inoculation (dpi).
Fig. S3 Phytophthora cactorum transformant GFP1 expressing green fluorescent protein. (a) Mycelia. (b) Sporangia. (c) A germinating cyst. (d) Oospores.
Table S1 Summary of Illumina reads mapping to Phytophthora cactorum reference transcriptome by blast‐Like Alignment Tool (BLAT) analysis.
Table S2 Phytophthora cactorum isolates used in this study.
Table S3 List of primers used in this study.
Acknowledgements
This work was supported by grants to Xiao‐Ren Chen from the National Natural Science Foundation of China (31101395), Natural Science Foundation of the Higher Education Institutions of Jiangsu Province of China (13KJB210009), Special Fund for Agro‐scientific Research in the Public Interest of China (201303018) and Postgraduate Research and Innovation Projects of Colleges and Universities of Jiangsu Province of China (KYLX_1354). The authors declare no conflicts of interest.
References
- Ah‐Fong, A.M. and Judelson, H.S. (2011) Vectors for fluorescent protein tagging in Phytophthora: tools for functional genomics and cell biology. Fungal Biol. 115, 882–890. [DOI] [PubMed] [Google Scholar]
- Baxter, L. , Tripathy, S. , Ishaque, N. , Boot, N. , Cabral, A. , Kemen, E. , Thines, M. , Ah‐Fong, A. , Anderson, R. , Badejoko, W. , Bittner‐Eddy, P. , Boore, J.L. , Chibucos, M.C. , Coates, M. , Dehal, P. , Delehaunty, K. , Dong, S. , Downton, P. , Dumas, B. , Fabro, G. , Fronick, C. , Fuerstenberg, S.I. , Fulton, L. , Gaulin, E. , Govers, F. , Hughes, L. , Humphray, S. , Jiang, R.H. , Judelson, H. , Kamoun, S. , Kyung, K. , Meijer, H. , Minx, P. , Morris, P. , Nelson, J. , Phuntumart, V. , Qutob, D. , Rehmany, A. , Rougon‐Cardoso, A. , Ryden, P. , Torto‐Alalibo, T. , Studholme, D. , Wang, Y. , Win, J. , Wood, J. , Clifton, S.W. , Rogers, J. , Van den Ackerveken, G. , Jones, J.D. , McDowell, J.M. , Beynon, J. and Tyler, B.M. (2010) Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science, 330, 1549–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller, T. and Felix, G. (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. [DOI] [PubMed] [Google Scholar]
- Bos, J.I.B. , Armstrong, M. , Whisson, S.C. , Torto, T.A. , Ochwo, M. , Birch, P.R.J. and Kamoun, S. (2003) Intraspecific comparative genomics to identify avirulence genes from Phytophthora . New Phytol. 159, 63–72. [DOI] [PubMed] [Google Scholar]
- Bos, J.I.B. , Kanneganti, T.‐D. , Young, C. , Cakir, C. , Huitema, E. , Win, J. , Armstrong, M.R. , Birch, P.R.J. and Kamoun, S. (2006) The C‐terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a‐mediated hypersensitivity and suppress INF1‐induced cell death in Nicotiana benthamiana . Plant J. 48, 165–176. [DOI] [PubMed] [Google Scholar]
- Bozkurt, T.O. , Schornack, S. , Banfield, M.J. and Kamoun, S. (2012) Oomycetes, effectors, and all that jazz. Curr. Opin. Plant Biol. 15, 483–492. [DOI] [PubMed] [Google Scholar]
- Chaparro‐Garcia, A. , Wilkinson, R.C. , Gimenez‐Ibanez, S. , Findlay, K. , Coffey, M.D. , Zipfel, C. , Rathjen, J.P. , Kamoun, S. and Schornack, S. (2011) The receptor‐like kinase SERK3/BAK1 is required for basal resistance against the late blight pathogen Phytophthora infestans in Nicotiana benthamiana . PLoS ONE, 6, e16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X.‐R. , Xing, Y.‐P. , Li, Y.‐P. , Tong, Y.‐H. and Xu, J.‐Y. (2013) RNA‐Seq reveals infection‐related gene expression changes in Phytophthora capsici . PLoS ONE, 8, e74588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X.‐R. , Zhang, B.‐Y. , Xing, Y.‐P. , Li, Q.‐Y. , Li, Y.‐P. , Tong, Y.‐H. and Xu, J.‐Y. (2014) Transcriptomic analysis of the phytopathogenic oomycete Phytophthora cactorum provides insights into infection‐related effectors. BMC Genomics, 15, 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, L. , Yin, W. , Dong, S. and Wang, Y. (2012) Analysis of polymorphism and transcription of the effector gene Avr1b in Phytophthora sojae isolates from China virulent to Rps1b . Mol. Plant Pathol. 13, 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds, P.N. and Rathjen, J.P. (2010) Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Dou, D. and Zhou, J.‐M. (2012) Phytopathogen effectors subverting host immunity: different foes, similar battleground. Cell Host Microbe, 12, 484–495. [DOI] [PubMed] [Google Scholar]
- Erwin, D.C. and Ribeiro, O.K. (1996) Phytophthora Diseases Worldwide. St. Paul, MN.: American Phytopathological Society. [Google Scholar]
- Gilroy, E.M. , Taylor, R.M. , Hein, I. , Boevink, P. , Sadanandom, A. and Birch, P.R.J. (2011) CMPG1‐dependent cell death follows perception of diverse pathogen elicitors at the host plasma membrane and is suppressed by Phytophthora infestans RXLR effector AVR3a. New Phytol. 190, 653–666. [DOI] [PubMed] [Google Scholar]
- Giraldo, M.C. and Valent, B. (2013) Filamentous plant pathogen effectors in action. Nat. Rev. Microbiol. 11, 800–814. [DOI] [PubMed] [Google Scholar]
- Haas, B.J. , Kamoun, S. , Zody, M.C. , Jiang, R.H. , Handsaker, R.E. , Cano, L.M. , Grabherr, M. , Kodira, C.D. , Raffaele, S. , Torto‐Alalibo, T. , Bozkurt, T.O. , Ah‐Fong, A.M. , Alvarado, L. , Anderson, V.L. , Armstrong, M.R. , Avrova, A. , Baxter, L. , Beynon, J. , Boevink, P.C. , Bollmann, S.R. , Bos, J.I. , Bulone, V. , Cai, G. , Cakir, C. , Carrington, J.C. , Chawner, M. , Conti, L. , Costanzo, S. , Ewan, R. , Fahlgren, N. , Fischbach, M.A. , Fugelstad, J. , Gilroy, E.M. , Gnerre, S. , Green, P.J. , Grenville‐Briggs, L.J. , Griffith, J. , Grünwald, N.J. , Horn, K. , Horner, N.R. , Hu, C.H. , Huitema, E. , Jeong, D.H. , Jones, A.M. , Jones, J.D. , Jones, R.W. , Karlsson, E.K. , Kunjeti, S.G. , Lamour, K. , Liu, Z. , Ma, L. , Maclean, D. , Chibucos, M.C. , McDonald, H. , McWalters, J. , Meijer, H.J. , Morgan, W. , Morris, P.F. , Munro, C.A. , O'Neill, K. , Ospina‐Giraldo, M. , Pinzón, A. , Pritchard, L. , Ramsahoye, B. , Ren, Q. , Restrepo, S. , Roy, S. , Sadanandom, A. , Savidor, A. , Schornack, S. , Schwartz, D.C. , Schumann, U.D. , Schwessinger, B. , Seyer, L. , Sharpe, T. , Silvar, C. , Song, J. , Studholme, D.J. , Sykes, S. , Thines, M. , van de Vondervoort, P.J. , Phuntumart, V. , Wawra, S. , Weide, R. , Win, J. , Young, C. , Zhou, S. , Fry, W. , Meyers, B.C. , van West, P. , Ristaino, J. , Govers, F. , Birch, P.R. , Whisson, S.C. , Judelson, H.S. and Nusbaum, C. (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans . Nature, 461, 393–398. [DOI] [PubMed] [Google Scholar]
- Hein, I. , Gilroy, E.M. , Armstrong, M.R. and Birch, P.R.J. (2009) The zig–zag–zig in oomycete–plant interactions. Mol. Plant Pathol. 10, 547–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Jones, L. , Hamilton, A.J. , Voinnet, O. , Thomas, C.L. , Maule, A.J. and Baulcombe, D.C. (1999) RNA–DNA interactions and DNA methylation in post‐transcriptional gene silencing. Plant Cell, 11, 2291–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun, S. (2006) A catalogue of the effector secretome of plant pathogenic oomycetes. Annu. Rev. Phytopathol. 44, 41–60. [DOI] [PubMed] [Google Scholar]
- Kamoun, S. , van West, P. , Vleeshouwers, V.G. , de Groot, K.E. and Govers, F. (1998) Resistance of Nicotiana benthamiana to Phytophthora infestans is mediated by the recognition of the elicitor protein INF1. Plant Cell, 10, 1413–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun, S. , Furzer, O. , Jones, J.D. , Judelson, H.S. , Ali, G.S. , Dalio, R.J. , Roy, S.G. , Schena, L. , Zambounis, A. , Panabières, F. , Cahill, D. , Ruocco, M. , Figueiredo, A. , Chen, X.R. , Hulvey, J. , Stam, R. , Lamour, K. , Gijzen, M. , Tyler, B.M. , Grünwald, N.J. , Mukhtar, M.S. , Tomé, D.F. , Tör, M. , Van Den Ackerveken, G. , McDowell, J. , Daayf, F. , Fry, W.E. , Lindqvist‐Kreuze, H. , Meijer, H.J. , Petre, B. , Ristaino, J. , Yoshida, K. , Birch, P.R. and Govers, F. (2015) The Top 10 oomycete pathogens in molecular plant pathology. Mol. Plant Pathol. 16, 413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent, W.J. (2002) BLAT—the BLAST‐like alignment tool. Genome Res. 12, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque, C.A. , Brouwer, H. , Cano, L. , Hamilton, J.P. , Holt, C. , Huitema, E. , Raffaele, S. , Robideau, G.P. , Thines, M. , Win, J. , Zerillo, M.M. , Beakes, G.W. , Boore, J.L. , Busam, D. , Dumas, B. , Ferriera, S. , Fuerstenberg, S.I. , Gachon, C.M. , Gaulin, E. , Govers, F. , Grenville‐Briggs, L. , Horner, N. , Hostetler, J. , Jiang, R.H. , Johnson, J. , Krajaejun, T. , Lin, H. , Meijer, H.J. , Moore, B. , Morris, P. , Phuntmart, V. , Puiu, D. , Shetty, J. , Stajich, J.E. , Tripathy, S. , Wawra, S. , van West, P. , Whitty, B.R. , Coutinho, P.M. , Henrissat, B. , Martin, F. , Thomas, P.D. , Tyler, B.M. , De Vries, R.P. , Kamoun, S. , Yandell, M. , Tisserat, N. and Buell, C.R. (2010) Genome sequence of the necrotrophic plant pathogen Pythium ultimum reveals original pathogenicity mechanisms and effector repertoire. Genome Biol. 11, R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, T. , Ye, W. , Ru, Y. , Yang, X. , Gu, B. , Tao, K. , Lu, S. , Dong, S. , Zheng, X. , Shan, W. , Wang, Y. and Dou, D. (2011) Two host cytoplasmic effectors are required for pathogenesis of Phytophthora sojae by suppression of host defenses. Plant Physiol. 155, 490–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Bos, J.I. , Armstrong, M. , Whisson, S.C. , da Cunha, L. , Torto‐Alalibo, T. , Win, J. , Avrova, A.O. , Wright, F. , Birch, P.R. and Kamoun, S. (2005) Patterns of diversifying selection in the phytotoxin‐like scr74 gene family of Phytophthora infestans . Mol. Biol. Evol. 22, 659–672. [DOI] [PubMed] [Google Scholar]
- McLeod, A. , Fry, B.A. , Zuluaga, A.P. , Myers, K.L. and Fry, W.E. (2008) Toward improvements of oomycete transformation protocols. J. Eukaryot. Microbiol. 55, 103–109. [DOI] [PubMed] [Google Scholar]
- Monaghan, J. and Zipfel, C. (2012) Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 15, 349–357. [DOI] [PubMed] [Google Scholar]
- Mortazavi, A. , Williams, B.A. , McCue, K. , Schaeffer, L. and Wold, B. (2008) Mapping and quantifying mammalian transcriptomes by RNA‐Seq. Nat. Methods, 5, 621–628. [DOI] [PubMed] [Google Scholar]
- Mott, G.A. , Middleton, M.A. , Desveaux, D. and Guttman, D.S. (2014) Peptides and small molecules of the plant–pathogen apoplastic arena. Front. Plant Sci. 5, 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möbius, N. and Hertweck, C. (2009) Fungal phytotoxins as mediators of virulence. Curr. Opin. Plant Biol. 12, 390–398. [DOI] [PubMed] [Google Scholar]
- Nicastro, G. , Orsomando, G. , Ferrari, E. , Manconi, L. , Desario, F. , Amici, A. , Naso, A. , Carpaneto, A. , Pertinhez, T.A. , Ruggieri, S. and Spisni, A. (2009) Solution structure of the phytotoxic protein PcF: the first characterized member of the Phytophthora PcF toxin family. Protein Sci. 18, 1786–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsomando, G. , Lorenzi, M. , Raffaelli, N. , Dalla Rizza, M. , Mezzetti, B. and Ruggieri, S. (2001) Phytotoxic protein PcF, purification, characterization, and cDNA sequencing of a novel hydroxyproline‐containing factor secreted by the strawberry pathogen Phytophthora cactorum . J. Biol. Chem. 276, 21 578–21 584. [DOI] [PubMed] [Google Scholar]
- Orsomando, G. , Lorenzi, M. , Ferrari, E. , de Chiara, C. , Spisni, A. and Ruggieri, S. (2003) PcF protein from Phytophthora cactorum and its recombinant homologue elicit phenylalanine ammonia lyase activation in tomato. Cell. Mol. Life Sci. 60, 1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsomando, G. , Brunetti, L. , Pucci, K. , Ruggeri, B. and Ruggieri, S. (2011) Comparative structural and functional characterization of putative protein effectors belonging to the PcF toxin family from Phytophthora spp. Protein Sci. 20, 2047–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottmann, C. , Luberacki, B. , Küfner, I. , Koch, W. , Brunner, F. , Weyand, M. , Mattinen, L. , Pirhonen, M. , Anderluh, G. , Seitz, H.U. , Nürnberger, T. and Oecking, C. (2009) A common toxin fold mediates microbial attack and plant defense. Proc. Natl. Acad. Sci. USA, 106, 10 359–10 364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, M. and Kuc, J. (1992) Peroxidase‐generated hydrogen peroxide as a source of antifungal activity in vitro and on tobacco leaf disks. Phytopathology, 82, 696–699. [Google Scholar]
- Rooney, H.C. , Van't Klooster, J.W. , van der Hoorn, R.A. , Joosten, M.H. , Jones, J.D. and de Wit, P.J. (2005) Cladosporium Avr2 inhibits tomato Rcr3 protease required for Cf‐2‐dependent disease resistance. Science, 308, 1783–1786. [DOI] [PubMed] [Google Scholar]
- Stassen, J.H. and Van den Ackerveken, G. (2011) How do oomycete effectors interfere with plant life? Curr. Opin. Plant Biol. 14, 407–414. [DOI] [PubMed] [Google Scholar]
- Stergiopoulos, I. and de Wit, P.J. (2009) Fungal effector proteins. Annu. Rev. Phytopathol. 47, 233–263. [DOI] [PubMed] [Google Scholar]
- Thomma, B.P.H.J. , Nürnberger, T. and Joosten, M.H.A.J. (2011) Of PAMPs and effectors: the blurred PTI–ETI dichotomy. Plant Cell, 23, 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler, B.M. , Tripathy, S. , Zhang, X. , Dehal, P. , Jiang, R.H. , Aerts, A. , Arredondo, F.D. , Baxter, L. , Bensasson, D. , Beynon, J.L. , Chapman, J. , Damasceno, C.M. , Dorrance, A.E. , Dou, D. , Dickerman, A.W. , Dubchak, I.L. , Garbelotto, M. , Gijzen, M. , Gordon, S.G. , Govers, F. , Grunwald, N.J. , Huang, W. , Ivors, K.L. , Jones, R.W. , Kamoun, S. , Krampis, K. , Lamour, K.H. , Lee, M.K. , McDonald, W.H. , Medina, M. , Meijer, H.J. , Nordberg, E.K. , Maclean, D.J. , Ospina‐Giraldo, M.D. , Morris, P.F. , Phuntumart, V. , Putnam, N.H. , Rash, S. , Rose, J.K. , Sakihama, Y. , Salamov, A.A. , Savidor, A. , Scheuring, C.F. , Smith, B.M. , Sobral, B.W. , Terry, A. , Torto‐Alalibo, T.A. , Win, J. , Xu, Z. , Zhang, H. , Grigoriev, I.V. , Rokhsar, D.S. and Boore, J.L. (2006) Phytophthora genome sequences uncover evolutionary origins and mechanisms of pathogenesis. Science, 313, 1261–1266. [DOI] [PubMed] [Google Scholar]
- Wawra, S. , Belmonte, R. , Löbach, L. , Saraiva, M. , Willems, A. and van West, P. (2012) Secretion, delivery and function of oomycete effector proteins. Curr. Opin. Microbiol. 15, 685–691. [DOI] [PubMed] [Google Scholar]
- van West, P. , Kamoun, S. , van't Klooster, J.W. and Govers, F. (1999) Internuclear gene silencing in Phytophthora infestans . Mol. Cell, 3, 339–348. [DOI] [PubMed] [Google Scholar]
- Whisson, S.C. , Boevink, P.C. , Moleleki, L. , Avrova, A.O. , Morales, J.G. , Gilroy, E.M. , Armstrong, M.R. , Grouffaud, S. , van West, P. , Chapman, S. , Hein, I. , Toth, I.K. , Pritchard, L. and Birch, P.R. (2007) A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature, 450, 115–118. [DOI] [PubMed] [Google Scholar]
- Win, J. , Morgan, W. , Bos, J. , Krasileva, K.V. , Cano, L.M. , Chaparro‐Garcia, A. , Ammar, R. , Staskawicz, B.J. and Kamoun, S. (2007) Adaptive evolution has targeted the C‐terminal domain of the RXLR effectors of plant pathogenic oomycetes. Plant Cell, 19, 2349–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaya‐Molina, L.X. , Ortega, M.A. and Dorrance, A.E. (2011) Easy and efficient protocol for oomycete DNA extraction suitable for population genetic analysis. Biotechnol. Lett. 33, 715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Protein sequence alignment of SCR96, SCR99, SCR121 and PcF identified in this study. The gene sequences of scr96, scr99, scr121 and PcF were cloned from Phytophthora cactorum isolate 10 300. The deduced protein sequences were aligned and shaded for consensus (50% threshold for shading) using BioEdit.
Fig. S2 The growth of Phytophthora cactorum wild‐type isolate 10 300 on nutrient pea broth (NPB) medium (Mcleod et al., 2008) supplemented with geneticin (G418) at different concentrations. Photographs were taken at 4 days post‐inoculation (dpi).
Fig. S3 Phytophthora cactorum transformant GFP1 expressing green fluorescent protein. (a) Mycelia. (b) Sporangia. (c) A germinating cyst. (d) Oospores.
Table S1 Summary of Illumina reads mapping to Phytophthora cactorum reference transcriptome by blast‐Like Alignment Tool (BLAT) analysis.
Table S2 Phytophthora cactorum isolates used in this study.
Table S3 List of primers used in this study.


