Abstract
The type II secretion system (T2SS) delivers toxins and a range of hydrolytic enzymes, including proteases, lipases, and carbohydrate-active enzymes, to the cell surface or extracellular space of Gram-negative bacteria. Its contribution to survival of both extracellular and intracellular pathogens as well as environmental species of proteobacteria is evident. This dynamic, multicomponent machinery spans the entire cell envelope and consists of a cytoplasmic ATPase, several inner membrane proteins, a periplasmic pseudopilus, and a secretin pore embedded in the outer membrane. Despite the trans-envelope configuration of the T2S nanomachine, proteins to be secreted engage with the system first once they enter the periplasmic compartment via the Sec or TAT export system. Thus, the T2SS is specifically dedicated to their outer membrane translocation. The many sequence and structural similarities between the T2SS and type IV pili suggest a common origin and argue for a pilus-mediated mechanism of secretion. This minireview describes the structures, functions, and interactions of the individual T2SS components and the general architecture of the assembled T2SS machinery and briefly summarizes the transport and function of a growing list of T2SS exoproteins. Recent advances in cryo-electron microscopy, which have led to an increased understanding of the structure-function relationship of the secretin channel and the pseudopilus, are emphasized.
INTRODUCTION
The type II secretion system (T2SS) is one of several extracellular secretion systems in Gram-negative bacteria. While highly prevalent in gamma- and betaproteobacteria, the T2SS is also recognized to a lesser extent in members of the delta and alpha classes (1, 2). It is known for its prolific protease secretion activity. In addition, the T2SS mediates extracellular delivery of a variety of toxins, lipases, and enzymes that break down complex carbohydrates, thus conferring a survival advantage to pathogenic as well as environmental species (2–4). The T2SS is not restricted to extracellular pathogens, such as Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Vibrio cholerae; it is also present and contributes to growth of intracellular pathogens, including Legionella pneumophila, which replicates in aquatic amoebae, alveolar macrophages, and epithelial cells (5–7). The obligate intracellular pathogen Chlamydia trachomatis also depends on T2SS components for extracellular secretion; however, its T2SS is atypical, as some components are missing or are too different from homologs in other species to be identified using BLAST algorithms (8, 9).
With 12 to 15 different components distributed in the cytoplasm, cytoplasmic membrane (CM), and outer membrane (OM), the large multiprotein T2SS spans the entire Gram-negative cell envelope (Fig. 1A). While many of the T2SS constituents are structurally and functionally related to those of type IV pilus systems (10), some of the components are unique to the T2SS and are therefore likely to have a specific role in the secretion process. Energy through the hydrolysis of ATP is provided by GspE, a cytoplasmic hexameric ATPase that interacts with the cytoplasmic domains of GspL and GspF, two CM components (Fig. 2) (11–21). GspL, in turn, forms a tight complex with GspM, a structural homolog (22–27). The CM complex also consists of GspC (Fig. 2), which reaches into the periplasmic space, making contact with the secretin that forms the OM conduit, consisting of 15 copies of GspD (Fig. 3A) (28–38). A gene for GspN, a fifth CM component, is present in the T2SS operons of a subset of Gram-negative species; however, its removal has often no discernible effect on secretion and its function remains unknown (39, 40). Interestingly, Xanthomonas campestris lacks GspC. Instead, it expresses GspN, which may substitute for GspC (41). In addition, the function of some T2SSs is supported by the CM proteins GspA and GspB, which contribute to GspD assembly and transport to the OM, possibly by increasing the pore size of the peptidoglycan or anchoring it to this structural meshwork (42–44). Finally, GspG forms a periplasmic pseudopilus that extends from the CM and is likely capped by the minor pseudopilins GspH, GspI, GspJ, and GspK, components that initiate the formation of the pseudopilus (Fig. 2) (45–50). Prior to assembly of the pseudopilus, which involves extracting the pseudopilins from the CM and polymerizing them into short helical pilus-like fibers, they are N-terminally cleaved and methylated by the prepilin peptidase GspO (PilD) (51–53).
Figure 1.
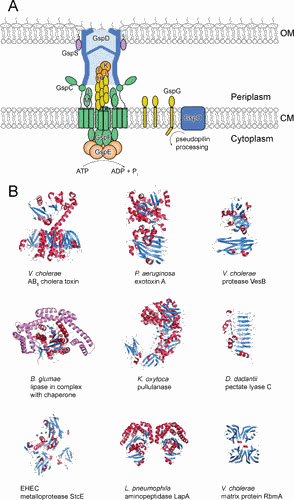
Overview of the general architecture of the T2SS and its substrates. (A) A schematic diagram of topology and location of the conserved core components of the T2SS. The accessory components GspN, GspA, and GspB are not shown. (B) A selection of the T2SS substrates of variable functions. Protein toxins include V. cholerae AB5 cholera toxin (139) and P. aeruginosa exotoxin A (140). Hydrolytic enzymes include V. cholerae VesB (68), B. glumae lipase in complex with chaperone (shown in purple) (71), K. oxytoca pullulanase (77), D. dadantii pectate lyase C (141), EHEC metalloprotease StcE (142), and L. pneumophila aminopeptidase LapA (91). V. cholerae biofilm matrix protein RbmA is a scaffolding protein (143, 144).
Figure 2.
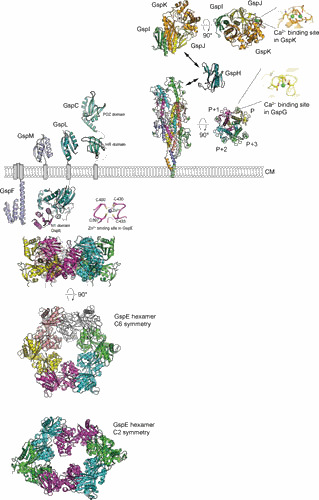
Structures of the T2SS assembly platform and pseudopilus components. The ATPase is hexameric V. cholerae GspE with C6 and C2 symmetries (20). A close-up view shows the Zn2+ binding site, which is required for the function of GspE (14, 145). Inner membrane components include the cytoplasmic domain of V. cholerae GspF (19), cytoplasmic domain of GspL in complex with N1 domain of V. cholerae GspE (16), periplasmic domain of V. parahaemolyticus GspL (26), periplasmic domain of V. cholerae GspM (25), the homology region (HR) domain of ETEC GspC (32), and the PDZ domain of V. cholerae GspC (29). The structure of periplasmic domain of P. aeruginosa GspL (XcpY) has been recently published (146). Regarding pseudopilus components, in the K. oxytoca GspG pseudopilus model based on the cryo-EM reconstruction (50), a close-up view shows the Ca2+ binding site of K. oxytoca GspG, V. cholerae minor pseudopilin GspH (47), and the trimeric complex of ETEC GspK-GspI-GspJ (48), and a close-up view shows a double-Ca2+ binding site of GspK. The structure of a homologous XcpX-XcpV-XcpW complex from P. aeruginosa has been recently reported (147).
Figure 3.
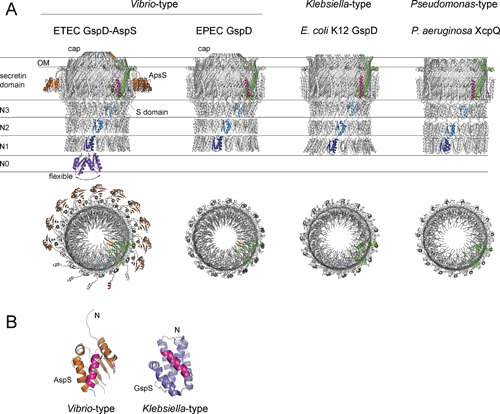
Structures of the T2SS secretins and pilotins. (A) The side and top views of ETEC GspD-AspS complex (37), EPEC GspD (36), E. coli K-12 GspD (34), and P. aeruginosa GspD (35). A single secretin protomer is highlighted, with N1, N2, and N3 domains in shades of blue, the secretin domain in green, and the S domain in magenta. Several AspS protomers (brown) were omitted to clearly show the location of the S domain. The cap subdomain in the Vibrio-type secretins is highlighted in orange. The N0 domains (purple) were not resolved in the available cryo-EM reconstructions due to flexibility. Instead, its approximate location is indicated (148). Note that the N1-N2 domains of EPEC GspD (36) and the N1 domain of P. aeruginosa GspD (35) have been placed as rigid fit models. (B) Structures of pilotins in complex with the secretin S domains (magenta). Structures of Vibrio-type ETEC AspS (37) and Klebsiella-type D. dadantii GspS (116) are shown.
Proteins to be secreted by the T2SS are initially produced as precursors with N-terminal signal peptides that are removed following translocation across the CM by the Sec or TAT pathways (54–57). While tethered to the CM or following release to the periplasmic compartment, they then undergo folding and, in some cases, oligomerization into larger complexes (58–65). Figure 1B shows examples of T2SS substrates with known structures. Many T2SS substrates, particularly proteases, are also produced with a removable propeptide, in addition to the signal peptide, that functions as an intramolecular chaperone and/or inhibitor (64, 66–68). Other T2SS substrates require dedicated, often CM-tethered, chaperones that assist in the folding and/or engagement with the T2SS prior to OM translocation (see the crystal structure of the Burkholderia glumae lipase in complex with a soluble form of its chaperone in Fig. 1B) (69–74).
Here we discuss the latest findings relating to the T2SS substrates, the structure and assembly of the secretin, and the mechanism of secretion, focusing on the role of the pseudopilus.
TRANSPORT AND FUNCTION OF T2SS SUBSTRATES
The secretion of exoproteins by the T2SS is considered a two-step process, where the two steps—transport across the CM and OM—can be genetically and physically separated (61, 62). All T2SS substrates have to be exposed to the periplasmic compartment to be recognized by the T2SS. Some enter the T2SS as soluble periplasmic intermediates, while others are extracted directly from the CM. Examples of the latter include the prolipoproteins pullulanase and SslE produced by Klebsiella pneumoniae and enteropathogenic Escherichia coli (EPEC), respectively, which are expressed with signal peptides that contain a lipobox with a conserved cysteine (75–77). The cysteine is acylated and the signal peptide is removed. The lipidated cysteine remains with the mature protein and is further modified by an N-acyltransferase prior to engagement with the T2SS (78). In contrast to many other, soluble T2SS substrates, which are released from the cells following OM transfer, these lipoproteins remain primarily associated with the bacterial cell surface (76, 79). Presumably they are retained with the OM through their lipidated N termini, because a pullulanase variant produced with a typical Sec signal peptide is solubly released following OM translocation (62). Another example of surface retention includes the cell association of heat-labile enterotoxin (LT) produced by enterotoxigenic Escherichia coli (ETEC), which binds via the B subunit oligomer to lipopolysaccharide in a Kdo core-dependent manner (80, 81). Although the B subunits of LT and cholera toxin are nearly identical, cholera toxin does not remain associated with the V. cholerae surface because its Kdo core is phosphorylated, thus preventing the binding. A third form of surface retention is typified by the pectin lyase PnlH, which is anchored in the Dickeya dadantii OM by a noncleavable TAT signal peptide (82). V. cholerae provides yet another means by which T2SS substrates associate with the cell surface. This involves the production of proteins with a C-terminally located tripartite motif, GlyGly-CTERM, which consists of residues rich in glycines and serines followed by a stretch of hydrophobic amino acids and positively charged residues (83, 84). A recent study has shown that the GlyGly-CTERM domain of one of these proteins, VesB, is cleaved off in the CM by an intramembrane protease, rhombosortase, and the newly generated C terminus is capped with a glycerol-phosphoethanolamine unit that may be acylated (84). This is followed by OM translocation and surface localization of VesB. Expression of VesB without its GlyGly-CTERM domain results in the release of VesB to the extracellular compartment, signifying the importance of the GlyGly-CTERM extension and C-terminal modification for VesB surface retention. While the above-listed methods exemplify ways to retain T2SS substrates on the bacterial surface, these proteins can also be found in various amounts in culture supernatants. This is due to release of OM vesicles, the formation of micelles, or removal of the proteins from the cell surface by extracellular proteases (79, 84).
The contribution of the T2SS to environmental growth and virulence of human, animal, and plant pathogens is apparent when one considers the secreted proteins and their activities (Table 1). Devastating diseases such as cholera and childhood diarrhea caused by ETEC are mediated by cholera toxin and LT and result in severe dehydration and even death when not treated (85, 86). By inducing watery diarrhea, the enterotoxins aid in the spread and transmission of these diseases. Another means by which T2SS substrates benefit bacteria is through nutrient acquisition in the eukaryotic host and environment. The release and generation of nutrients are the result of the action of pore-forming toxins such as aerolysin, which causes osmotic host cell lysis, and a range of enzymes, including proteases, lipases, DNases, and carbohydrate-degrading enzymes that digest host components and tissue (40, 63, 87–92). For example, plant cell wall-degrading enzymes, such as cellulases, pectinases, and pectate lyases, promote growth of phytopathogenic bacteria, resulting in crop losses (55, 87, 93). L. pneumophila aminopeptidase LapA is another recently identified example of a T2SS protein that contributes to nutrient acquisition by generating amino acids and thus supporting intracellular growth of L. pneumophila in amoebae (91). Degradative enzymes also aid pathogens in gaining access to new niches. Enzymes that target mucins, which cover and protect cells of the digestive tract, generate direct access to the epithelial cell surface for colonization of enteric pathogens and facilitate delivery of toxins (94–96). Recent work has also recognized the contribution of many proteases and other effectors in immune evasion. Burkholderia cenocepacia ZmpA and ZmpB are examples of proteases that cleave antimicrobial peptides, and the L. pneumophila T2SS reduces the output of cytokines and chemokines during infection, in part due to secretion and activity of the metalloprotease ProA (97–99). Another secreted metalloprotease, StcE, protects enterohemorrhagic Escherichia coli (EHEC) from host defense mechanisms, including complement-mediated killing, by cleaving C1 esterase inhibitor and neutrophil-associated proteins, while the A. baumannii metalloprotease CpaA interferes with blood coagulation by inactivating factor XII, which may result in the escape from clearance by intravascular clots and dissemination of A. baumannii (73, 100–103). In the environment, bacterial life frequently occurs as matrix-encased biofilm. The major component is often polysaccharides, but the matrix also contains specific proteins secreted by the T2SS that contribute to the formation, architecture, and stability of the biofilm (76, 104–109).
TABLE 1.
Examples of T2SS substrates
| Protein(s) | Type(s) | Activity(ies) | Reference(s) |
|---|---|---|---|
| Toxins | Enterotoxin (cholera toxin, E. coli heat-labile enterotoxin) | ADP-ribosylation of Gsα subunit leading to increased adenylate cyclase activity and raising cAMP levels, which activates protein kinase A, followed by phosphorylation of the CFTR channel. This leads to efflux of chloride ions and water release into the intestinal lumen and consequent secretory diarrhea. | 85, 86 |
| Exotoxin A | ADP-ribosylation of elongation factor 2, inhibition of protein synthesis in host cells | 149 | |
| Pore-forming toxin (aerolysin, cytolysin) | Host cell membrane depolarization and lysis | 63, 150–152 | |
| Proteases | Metalloprotease, serine protease, cysteine protease, aminopeptidase | Cleavage of proteins or peptides, breakdown of host extracellular matrix, tissue damage, detachment from host cells, nutrient acquisition, evasion of host defense system, translocation to new niche | 73, 90, 91, 96, 97, 99–101, 103, 149, 153–156 |
| Lipid-modifying enzymes | Lipase, phospholipase, glycerophospholipid cholesterol acyltransferase | Breakdown of lipids to fatty acids and glycerol, nutrient acquisition, translocation to new niche; breakdown of phospholipids and destabilization of host cell membranes | 40, 70, 73, 88, 91, 149, 157, 158 |
| Carbohydrate-active enzymes | Chitinase, amylase, cellulase, pullulanase, xylanase, pectinase, pectin methylesterase, pectate lyase, levansucrase | Breakdown of polysaccharides such as chitin, cellulose, pectin, amylose, pullulan, and xylan; targeting of O-GlcNAcylated proteins in the host; nutrient acquisition; depolymerization of plant cell wall; wilting; soft rot | 55, 87, 93, 157, 159, 160 |
| Phosphatases | Alkaline phosphatase, acid phosphatase | Dephosphorylation, phosphate acquisition, phosphate solubilization | 161–163 |
| Nucleic acid-targeting enzymes | DNase, RNase | Hydrolysis of DNA and RNA; generation of nutrients, including carbon, nitrogen, and phosphate, that support bacterial growth; evasion of neutrophil extracellular traps | 92, 164, 165 |
| Metal reductase | C-type cytochromes | Reduction of insoluble metal oxides; electron transport; anaerobic respiration | 166, 167 |
| Others | Chitin-binding protein, collagen-like protein, biofilm-associated proteins | Adherence, biofilm formation | 105, 106, 168, 169 |
SECRETIN STRUCTURE AND ASSEMBLY
The secretin of the T2SS forms the OM channel for the passage of secreted proteins. Topologically, it contains an N-terminal N0 domain, homologous repeat N1 to N3 domains, and a C-terminal secretin core domain (Fig. 3A). The majority of T2SS secretins have an additional C-terminal S domain required for interaction with pilotins, which delivers them to the OM (see below and Fig. 3B). Early electron microscopy (EM) studies revealed the general architecture of secretins as a multimeric channel with an open periplasmic chamber, a closed central gate, and a top chamber (31, 110–113). The crystal structures of the N0-N1-N2 domains of ETEC GspD (30) allowed modeling of the N0-N1-N2-N3 domains in the cryo-EM structure of V. cholerae GspD (31). However, only the recent progress in cryo-EM methodology enabled investigators to solve the full-length secretin structures of GspD from E. coli K-12 and V. cholerae (34), P. aeruginosa (35), and EPEC (36) and of the GspD-pilotin complex from ETEC (37) (Fig. 3A). The general structural features are well conserved between secretins of the Vibrio type, the Klebsiella type (114), and the more sequence-divergent Pseudomonas type (Fig. 3A) (35). The secretins form cylindrical structures ∼150 Å in diameter and ∼195 Å in length that display 15-fold symmetry, in contrast with previously reported 12-fold symmetry, a discrepancy likely caused by the limited resolution of the earlier studies. The major difference between the secretins is the presence of an extracellular gate (cap) of unknown function in the Vibrio-type secretins (Fig. 3A) (34, 36).
In the secretin monomer, the domains are arranged in line that is tilted at ∼30° (Fig. 3A). The N0 domain is not modeled in the available reconstructions, as it was disordered, although weak smeared density was observed in some two-dimensional (2D) class averages (34). Together, the secretin core domains form a double-barrel structure that includes inner and outer barrels. Each secretin monomer contributes 4 shorter beta-sheets to the inner barrel and 4 longer beta-sheets to the outer barrel. The β-hairpin extensions from the inner barrel form the periplasmic gate in the secretin channel. A mechanism of periplasmic gate opening during secretion has been suggested based on a partially open structure of a G453A mutant of V. cholerae GspD, which showed an upward rotation of the β-hairpins (34). This is consistent with a recent structure of a homologous type III secretion system secretin in the open form, which revealed that the β-hairpins of the periplasmic gate are in upward position (115). The aromatic and aliphatic residues on the top of the outer barrel contribute to the hydrophobic belt that creates the transmembrane region. The S domain provides overall stability to the secretin oligomer by interacting with an adjacent protomer. The N3 domains form a ring below the secretin core domains with which they make extensive contacts. The interactions between N1-N2 and N2-N3 domain rings, on the other hand, are limited to the linkers and several specific contacts, which led to lower resolution of 3D reconstructions in this region.
The majority of T2SSs contain a Klebsiella-type pilotin, a fatty-acylated protein which has an α-helical structure that provides a hydrophobic groove for interactions with the second α-helix from the S domain of the secretin (Fig. 3B) (116). In contrast, Vibrio-type secretins utilize an alternative, structurally unrelated pilotin, although this pilotin fulfills the same function (114, 117). The structure of the Vibrio-type pilotin in complex with the secretin adopts an open conformation compared to the apo-pilotin to accommodate the α-helix from the S domain (Fig. 3B) (37). While pilotins direct secretins to the OM via the lipoprotein sorting system (118), the mechanism by which the secretin protomers acquire the assembled state and insert into the OM independently of the BAM complex is less clear (119, 120). However, a recent study that subjected the N3 domain of the Klebsiella oxytoca GspD to mutational analysis underscores the importance of the N3 domain in the early steps of secretin assembly (121). The results suggest that the N3 domain provides stability to the prepore, a prerequisite for OM insertion and pore formation of the secretin.
PSEUDOPILUS AND ASSEMBLY PLATFORM
Topologically, all pseudopilins consist of an N-terminal hydrophobic helix that extends into variable C-terminal globular domains. The major pseudopilin GspG contains a conserved Ca2+ binding site (Fig. 2) (50, 122). Disruption of Ca2+ binding by either amino acid substitutions or Ca2+ ion removal with chelating agents affects the stability of the pseudopilin monomer, pseudopilus assembly, and substrate secretion (50, 122). Interestingly, the minor pseudopilin GspK also contains a Ca2+ binding site (Fig. 2); however, the functional significance of this feature has yet to be determined (48). The minor pseudopilins GspK, GspI, and GspJ form a quasihelical trimeric complex that is thought to be located at the pseudopilus tip (Fig. 2) (48). It has been demonstrated that this complex serves as a priming site for pseudopilus assembly (123). The minor pseudopilin GspH possibly acts as an adapter between the GspK-GspI-GspJ tip and the poly-GspG fiber (47). The most recent model of the K. oxytoca GspG pseudopilus based on a cryo-EM reconstruction revealed a right-handed helical fiber with a 10-Å rise (50). The N-terminal hydrophobic helices of the GspG subunits arrange within the core of the pseudopilus, with the C-terminal domains and the Ca2+ binding sites located at the surface (Fig. 2). Interestingly, the N-terminal hydrophobic helix is connected to the C-terminal domain by an extended linker, a feature distinct from the type IV pilus.
While a number of GspE homologs from the type IV pili systems have been structurally characterized in hexameric form (124–126), the structure of hexameric GspE has been elusive. Employing a “scaffold” Hcp1 fusion strategy allowed visualization of GspE in two hexameric conformations: a symmetrical C6 form and an extended C2 form (Fig. 2) (20). These conformations may reflect structural transitions in GspE during ATP hydrolysis and transfer of mechanical energy to support pseudopilus assembly. The details of this process are not completely understood, although it is believed to involve GspF, GspL, and GpsM, which have all been shown to interact with the pseudopilus (13, 18, 127, 128). While the structures of the extramembrane domains of GspC, GspF, GspL, and GspM have been solved (Fig. 2), the structure of the assembly platform formed by these components has not yet been reported.
IMPLICATIONS FOR MECHANISM
Despite the progress in understanding the structure-function relationship of the various components of the T2SS, the mechanism by which this important secretion system transports both soluble and lipidated proteins across the OM remains poorly understood. As folding of the T2SS substrates is a prerequisite for engagement with the T2SS, a secretion signal is thought to be formed in the folded structures; however, the structures of T2SS substrates greatly vary and a common, general secretion signal has yet to be identified. The protein-protein interaction domain PDZ of GspC has been suggested to recognize and recruit the T2SS substrates once they arrive in the periplasmic compartment, yet interactions between the T2SS substrates and GspD, GspH-GspI-GspJ-GspK (which forms the tip of the pseudopilus), and the CM proteins GspL and GspM have also been demonstrated (129–134). These interactions are, for the most part, consistent with two prevailing models for driving proteins through the secretin pore: the piston machinery and the Archimedes screw (135). In the piston model the pseudopilus tip supposedly pushes the substrates through the secretin in a linear fashion, although this mechanism cannot fully account for the required retraction of the pseudopilus and recharging of substrate, as the T2SS lacks a retraction ATPase (48, 129, 132, 135, 136). In the Archimedes screw model, the rotary motion via interactions with the poly-GspG shaft of the pseudopilus threads the T2SS substrates out through the secretin pore; however, this model requires a continuous degradation and replenishment of GspG (137, 138). Dedicated removal of GspG-bound calcium and subsequent destabilization of GspG may result in degradation (50), but given the rigid structure of the double-barrel secretin domain, the dimensions of the pseudopilus, and the mounting evidence for the rotation of the pseudopilus itself, perhaps a composite model should be considered, in which the rotary motion of the pseudopilus drives the secretion of the substrates that are pushed out by the pseudopilus tip.
CONCLUSION
In conclusion, while structural information is now available for most of the T2SS components and many T2SS substrates and the general architecture of the T2SS is understood, there are still multiple unanswered questions about the precise stoichiometry of this secretion complex, the detailed mechanism of pseudopilus assembly and possibly disassembly, and the molecular basis for substrate recognition. Before long, however, answers to these quandaries are expected to be revealed, as the field is progressing rapidly due to technology advances.
ACKNOWLEDGMENTS
We thank Iain Hay and Trevor Lithgow for the models of EPEC GspD and P. aeruginosa XcpQ secretins.
This work was supported in part by grants R01AI127085 from National Institute of Allergy and Infectious Diseases (to M.S.) and W81XWH-18-1-0587 from the Department of Defense (to M.S.).
Contributor Information
Konstantin V. Korotkov, Department of Molecular and Cellular Biochemistry, University of Kentucky, Lexington, KY 40506
Maria Sandkvist, Department of Microbiology and Immunology, University of Michigan Medical School, Ann Arbor, MI 48109.
Eric Cascales, CNRS Aix-Marseille Université, Mediterranean Institute of Microbiology, Marseille, France.
Peter J. Christie, Department of Microbiology and Molecular Genetics, McGovern Medical School, Houston, Texas
REFERENCES
- 1.Abby SS, Cury J, Guglielmini J, Néron B, Touchon M, Rocha EP. 2016. Identification of protein secretion systems in bacterial genomes. Sci Rep 6:23080. 10.1038/srep23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cianciotto NP, White RC. 2017. Expanding role of type II secretion in bacterial pathogenesis and beyond. Infect Immun 85:e00014-17. 10.1128/IAI.00014-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korotkov KV, Sandkvist M, Hol WGJ. 2012. The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol 10:336–351. 10.1038/nrmicro2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikora AE. 2013. Proteins secreted via the type II secretion system: smart strategies of Vibrio cholerae to maintain fitness in different ecological niches. PLoS Pathog 9:e1003126. 10.1371/journal.ppat.1003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liles MR, Edelstein PH, Cianciotto NP. 1999. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol Microbiol 31:959–970. 10.1046/j.1365-2958.1999.01239.x. [DOI] [PubMed] [Google Scholar]
- 6.Hales LM, Shuman HA. 1999. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect Immun 67:3662–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossier O, Cianciotto NP. 2001. Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect Immun 69:2092–2098. 10.1128/IAI.69.4.2092-2098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen BD, Valdivia RH. 2012. Virulence determinants in the obligate intracellular pathogen Chlamydia trachomatis revealed by forward genetic approaches. Proc Natl Acad Sci U S A 109:1263–1268. 10.1073/pnas.1117884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snavely EA, Kokes M, Dunn JD, Saka HA, Nguyen BD, Bastidas RJ, McCafferty DG, Valdivia RH. 2014. Reassessing the role of the secreted protease CPAF in Chlamydia trachomatis infection through genetic approaches. Pathog Dis 71:336–351. 10.1111/2049-632X.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCallum M, Burrows LL, Howell PL. 2018. The dynamic structures of the type IV pilus. Microbiol Spectr 7:PSIB-0006-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandkvist M, Bagdasarian M, Howard SP, DiRita VJ. 1995. Interaction between the autokinase EpsE and EpsL in the cytoplasmic membrane is required for extracellular secretion in Vibrio cholerae. EMBO J 14:1664–1673. 10.1002/j.1460-2075.1995.tb07155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandkvist M, Keith JM, Bagdasarian M, Howard SP. 2000. Two regions of EpsL involved in species-specific protein-protein interactions with EpsE and EpsM of the general secretion pathway in Vibrio cholerae. J Bacteriol 182:742–748. 10.1128/JB.182.3.742-748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Py B, Loiseau L, Barras F. 2001. An inner membrane platform in the type II secretion machinery of Gram-negative bacteria. EMBO Rep 2:244–248. 10.1093/embo-reports/kve042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robien MA, Krumm BE, Sandkvist M, Hol WGJ. 2003. Crystal structure of the extracellular protein secretion NTPase EpsE of Vibrio cholerae. J Mol Biol 333:657–674. 10.1016/j.jmb.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Camberg JL, Sandkvist M. 2005. Molecular analysis of the Vibrio cholerae type II secretion ATPase EpsE. J Bacteriol 187:249–256. 10.1128/JB.187.1.249-256.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abendroth J, Murphy P, Sandkvist M, Bagdasarian M, Hol WGJ. 2005. The X-ray structure of the type II secretion system complex formed by the N-terminal domain of EpsE and the cytoplasmic domain of EpsL of Vibrio cholerae. J Mol Biol 348:845–855. 10.1016/j.jmb.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 17.Camberg JL, Johnson TL, Patrick M, Abendroth J, Hol WGJ, Sandkvist M. 2007. Synergistic stimulation of EpsE ATP hydrolysis by EpsL and acidic phospholipids. EMBO J 26:19–27. 10.1038/sj.emboj.7601481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arts J, de Groot A, Ball G, Durand E, El Khattabi M, Filloux A, Tommassen J, Koster M. 2007. Interaction domains in the Pseudomonas aeruginosa type II secretory apparatus component XcpS (GspF). Microbiology 153:1582–1592. 10.1099/mic.0.2006/002840-0. [DOI] [PubMed] [Google Scholar]
- 19.Abendroth J, Mitchell DD, Korotkov KV, Johnson TL, Kreger A, Sandkvist M, Hol WGJ. 2009. The three-dimensional structure of the cytoplasmic domains of EpsF from the type 2 secretion system of Vibrio cholerae. J Struct Biol 166:303–315. 10.1016/j.jsb.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu C, Turley S, Marionni ST, Park YJ, Lee KK, Patrick M, Shah R, Sandkvist M, Bush MF, Hol WGJ. 2013. Hexamers of the type II secretion ATPase GspE from Vibrio cholerae with increased ATPase activity. Structure 21:1707–1717. 10.1016/j.str.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C, Korotkov KV, Hol WGJ. 2014. Crystal structure of the full-length ATPase GspE from the Vibrio vulnificus type II secretion system in complex with the cytoplasmic domain of GspL. J Struct Biol 187:223–235. 10.1016/j.jsb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michel G, Bleves S, Ball G, Lazdunski A, Filloux A. 1998. Mutual stabilization of the XcpZ and XcpY components of the secretory apparatus in Pseudomonas aeruginosa. Microbiology 144:3379–3386. 10.1099/00221287-144-12-3379. [DOI] [PubMed] [Google Scholar]
- 23.Sandkvist M, Hough LP, Bagdasarian MM, Bagdasarian M. 1999. Direct interaction of the EpsL and EpsM proteins of the general secretion apparatus in Vibrio cholerae. J Bacteriol 181:3129–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert V, Hayes F, Lazdunski A, Michel GP. 2002. Identification of XcpZ domains required for assembly of the secreton of Pseudomonas aeruginosa. J Bacteriol 184:1779–1782. 10.1128/JB.184.6.1779-1782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abendroth J, Rice AE, McLuskey K, Bagdasarian M, Hol WGJ. 2004. The crystal structure of the periplasmic domain of the type II secretion system protein EpsM from Vibrio cholerae: the simplest version of the ferredoxin fold. J Mol Biol 338:585–596. 10.1016/j.jmb.2004.01.064. [DOI] [PubMed] [Google Scholar]
- 26.Abendroth J, Kreger AC, Hol WGJ. 2009. The dimer formed by the periplasmic domain of EpsL from the type 2 secretion system of Vibrio parahaemolyticus. J Struct Biol 168:313–322. 10.1016/j.jsb.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lallemand M, Login FH, Guschinskaya N, Pineau C, Effantin G, Robert X, Shevchik VE. 2013. Dynamic interplay between the periplasmic and transmembrane domains of GspL and GspM in the type II secretion system. PLoS One 8:e79562. 10.1371/journal.pone.0079562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lybarger SR, Johnson TL, Gray MD, Sikora AE, Sandkvist M. 2009. Docking and assembly of the type II secretion complex of Vibrio cholerae. J Bacteriol 191:3149–3161. 10.1128/JB.01701-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korotkov KV, Krumm B, Bagdasarian M, Hol WGJ. 2006. Structural and functional studies of EpsC, a crucial component of the type 2 secretion system from Vibrio cholerae. J Mol Biol 363:311–321. 10.1016/j.jmb.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 30.Korotkov KV, Pardon E, Steyaert J, Hol WGJ. 2009. Crystal structure of the N-terminal domain of the secretin GspD from ETEC determined with the assistance of a nanobody. Structure 17:255–265. 10.1016/j.str.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reichow SL, Korotkov KV, Hol WGJ, Gonen T. 2010. Structure of the cholera toxin secretion channel in its closed state. Nat Struct Mol Biol 17:1226–1232. 10.1038/nsmb.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korotkov KV, Johnson TL, Jobling MG, Pruneda J, Pardon E, Héroux A, Turley S, Steyaert J, Holmes RK, Sandkvist M, Hol WGJ. 2011. Structural and functional studies on the interaction of GspC and GspD in the type II secretion system. PLoS Pathog 7:e1002228. 10.1371/journal.ppat.1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Pineau C, Gu S, Guschinskaya N, Pickersgill RW, Shevchik VE. 2012. Cysteine scanning mutagenesis and disulfide mapping analysis of arrangement of GspC and GspD protomers within the type 2 secretion system. J Biol Chem 287:19082–19093. 10.1074/jbc.M112.346338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan Z, Yin M, Xu D, Zhu Y, Li X. 2017. Structural insights into the secretin translocation channel in the type II secretion system. Nat Struct Mol Biol 24:177–183. 10.1038/nsmb.3350. [DOI] [PubMed] [Google Scholar]
- 35.Hay ID, Belousoff MJ, Lithgow T. 2017. Structural basis of type 2 secretion system engagement between the inner and outer bacterial membranes. mBio 8:e01344-17. 10.1128/mBio.01344-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hay ID, Belousoff MJ, Dunstan RA, Bamert RS, Lithgow T. 2018. Structure and membrane topography of the Vibrio-type secretin complex from the type 2 secretion system of enteropathogenic Escherichia coli. J Bacteriol 200:e00521-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin M, Yan Z, Li X. 2018. Structural insight into the assembly of the type II secretion system pilotin-secretin complex from enterotoxigenic Escherichia coli. Nat Microbiol 3:581–587. 10.1038/s41564-018-0148-0. [DOI] [PubMed] [Google Scholar]
- 38.Majewski DD, Worrall LJ, Strynadka NC. 2018. Secretins revealed: structural insights into the giant gated outer membrane portals of bacteria. Curr Opin Struct Biol 51:61–72. 10.1016/j.sbi.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Possot OM, Vignon G, Bomchil N, Ebel F, Pugsley AP. 2000. Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE. J Bacteriol 182:2142–2152. 10.1128/JB.182.8.2142-2152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson TL, Waack U, Smith S, Mobley H, Sandkvist M. 2016. Acinetobacter baumannii is dependent on the type II secretion system and its substrate LipA for lipid utilization and in vivo fitness. J Bacteriol 198:711–719. 10.1128/JB.00622-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee HM, Wang KC, Liu YL, Yew HY, Chen LY, Leu WM, Chen DC, Hu NT. 2000. Association of the cytoplasmic membrane protein XpsN with the outer membrane protein XpsD in the type II protein secretion apparatus of Xanthomonas campestris pv. campestris. J Bacteriol 182:1549–1557. 10.1128/JB.182.6.1549-1557.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li G, Miller A, Bull H, Howard SP. 2011. Assembly of the type II secretion system: identification of ExeA residues critical for peptidoglycan binding and secretin multimerization. J Bacteriol 193:197–204. 10.1128/JB.00882-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strozen TG, Stanley H, Gu Y, Boyd J, Bagdasarian M, Sandkvist M, Howard SP. 2011. Involvement of the GspAB complex in assembly of the type II secretion system secretin of Aeromonas and Vibrio species. J Bacteriol 193:2322–2331. 10.1128/JB.01413-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanderlinde EM, Strozen TG, Hernández SB, Cava F, Howard SP. 2017. Alterations in peptidoglycan cross-linking suppress the secretin assembly defect caused by mutation of GspA in the type II secretion system. J Bacteriol 199:e00617-17. 10.1128/JB.00617-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sauvonnet N, Vignon G, Pugsley AP, Gounon P. 2000. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J 19:2221–2228. 10.1093/emboj/19.10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durand E, Bernadac A, Ball G, Lazdunski A, Sturgis JN, Filloux A. 2003. Type II protein secretion in Pseudomonas aeruginosa: the pseudopilus is a multifibrillar and adhesive structure. J Bacteriol 185:2749–2758. 10.1128/JB.185.9.2749-2758.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanez ME, Korotkov KV, Abendroth J, Hol WGJ. 2008. Structure of the minor pseudopilin EpsH from the type 2 secretion system of Vibrio cholerae. J Mol Biol 377:91–103. 10.1016/j.jmb.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korotkov KV, Hol WGJ. 2008. Structure of the GspK-GspI-GspJ complex from the enterotoxigenic Escherichia coli type 2 secretion system. Nat Struct Mol Biol 15:462–468. 10.1038/nsmb.1426. [DOI] [PubMed] [Google Scholar]
- 49.Douzi B, Durand E, Bernard C, Alphonse S, Cambillau C, Filloux A, Tegoni M, Voulhoux R. 2009. The XcpV/GspI pseudopilin has a central role in the assembly of a quaternary complex within the T2SS pseudopilus. J Biol Chem 284:34580–34589. 10.1074/jbc.M109.042366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.López-Castilla A, Thomassin JL, Bardiaux B, Zheng W, Nivaskumar M, Yu X, Nilges M, Egelman EH, Izadi-Pruneyre N, Francetic O. 2017. Structure of the calcium-dependent type 2 secretion pseudopilus. Nat Microbiol 2:1686–1695. 10.1038/s41564-017-0041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pugsley AP, Dupuy B. 1992. An enzyme with type IV prepilin peptidase activity is required to process components of the general extracellular protein secretion pathway of Klebsiella oxytoca. Mol Microbiol 6:751–760. 10.1111/j.1365-2958.1992.tb01525.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 52.Pugsley AP. 1993. Processing and methylation of PuIG, a pilin-like component of the general secretory pathway of Klebsiella oxytoca. Mol Microbiol 9:295–308. 10.1111/j.1365-2958.1993.tb01691.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 53.Nunn DN, Lory S. 1993. Cleavage, methylation, and localization of the Pseudomonas aeruginosa export proteins XcpT, -U, -V, and -W. J Bacteriol 175:4375–4382. 10.1128/jb.175.14.4375-4382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pugsley AP, Kornacker MG, Poquet I. 1991. The general protein-export pathway is directly required for extracellular pullulanase secretion in Escherichia coli K12. Mol Microbiol 5:343–352. 10.1111/j.1365-2958.1991.tb02115.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 55.He SY, Schoedel C, Chatterjee AK, Collmer A. 1991. Extracellular secretion of pectate lyase by the Erwinia chrysanthemi out pathway is dependent upon Sec-mediated export across the inner membrane. J Bacteriol 173:4310–4317. 10.1128/jb.173.14.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voulhoux R, Ball G, Ize B, Vasil ML, Lazdunski A, Wu LF, Filloux A. 2001. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J 20:6735–6741. 10.1093/emboj/20.23.6735. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ball G, Antelmann H, Imbert PR, Gimenez MR, Voulhoux R, Ize B. 2016. Contribution of the twin arginine translocation system to the exoproteome of Pseudomonas aeruginosa. Sci Rep 6:27675. 10.1038/srep27675. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirst TR, Holmgren J. 1987. Transient entry of enterotoxin subunits into the periplasm occurs during their secretion from Vibrio cholerae. J Bacteriol 169:1037–1045. 10.1128/jb.169.3.1037-1045.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirst TR, Holmgren J. 1987. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc Natl Acad Sci U S A 84:7418–7422. 10.1073/pnas.84.21.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardy SJ, Holmgren J, Johansson S, Sanchez J, Hirst TR. 1988. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc Natl Acad Sci U S A 85:7109–7113. 10.1073/pnas.85.19.7109. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pugsley AP. 1992. Translocation of a folded protein across the outer membrane in Escherichia coli. Proc Natl Acad Sci U S A 89:12058–12062. 10.1073/pnas.89.24.12058. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poquet I, Faucher D, Pugsley AP. 1993. Stable periplasmic secretion intermediate in the general secretory pathway of Escherichia coli. EMBO J 12:271–278. 10.1002/j.1460-2075.1993.tb05653.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardie KR, Schulze A, Parker MW, Buckley JT. 1995. Vibrio spp. secrete proaerolysin as a folded dimer without the need for disulphide bond formation. Mol Microbiol 17:1035–1044. 10.1111/j.1365-2958.1995.mmi_17061035.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 64.Braun P, Tommassen J, Filloux A. 1996. Role of the propeptide in folding and secretion of elastase of Pseudomonas aeruginosa. Mol Microbiol 19:297–306. 10.1046/j.1365-2958.1996.381908.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 65.Voulhoux R, Taupiac MP, Czjzek M, Beaumelle B, Filloux A. 2000. Influence of deletions within domain II of exotoxin A on its extracellular secretion from Pseudomonas aeruginosa. J Bacteriol 182:4051–4058. 10.1128/JB.182.14.4051-4058.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Häse CC, Finkelstein RA. 1991. Cloning and nucleotide sequence of the Vibrio cholerae hemagglutinin/protease (HA/protease) gene and construction of an HA/protease-negative strain. J Bacteriol 173:3311–3317. 10.1128/jb.173.11.3311-3317.1991. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McIver KS, Kessler E, Olson JC, Ohman DE. 1995. The elastase propeptide functions as an intramolecular chaperone required for elastase activity and secretion in Pseudomonas aeruginosa. Mol Microbiol 18:877–889. 10.1111/j.1365-2958.1995.18050877.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Gadwal S, Korotkov KV, Delarosa JR, Hol WGJ, Sandkvist M. 2014. Functional and structural characterization of Vibrio cholerae extracellular serine protease B, VesB. J Biol Chem 289:8288–8298. 10.1074/jbc.M113.525261. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hobson AH, Buckley CM, Aamand JL, Jørgensen ST, Diderichsen B, McConnell DJ. 1993. Activation of a bacterial lipase by its chaperone. Proc Natl Acad Sci U S A 90:5682–5686. 10.1073/pnas.90.12.5682. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martínez A, Ostrovsky P, Nunn DN. 1999. LipC, a second lipase of Pseudomonas aeruginosa, is LipB and Xcp dependent and is transcriptionally regulated by pilus biogenesis components. Mol Microbiol 34:317–326. 10.1046/j.1365-2958.1999.01601.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 71.Pauwels K, Lustig A, Wyns L, Tommassen J, Savvides SN, Van Gelder P. 2006. Structure of a membrane-based steric chaperone in complex with its lipase substrate. Nat Struct Mol Biol 13:374–375. 10.1038/nsmb1065. [PubMed] [DOI] [PubMed] [Google Scholar]
- 72.Coulthurst SJ, Lilley KS, Hedley PE, Liu H, Toth IK, Salmond GP. 2008. DsbA plays a critical and multifaceted role in the production of secreted virulence factors by the phytopathogen Erwinia carotovora subsp. atroseptica. J Biol Chem 283:23739–23753. 10.1074/jbc.M801829200. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harding CM, Kinsella RL, Palmer LD, Skaar EP, Feldman MF. 2016. Medically relevant Acinetobacter species require a type II secretion system and specific membrane-associated chaperones for the export of multiple substrates and full virulence. PLoS Pathog 12:e1005391. 10.1371/journal.ppat.1005391. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kinsella RL, Lopez J, Palmer LD, Salinas ND, Skaar EP, Tolia NH, Feldman MF. 2017. Defining the interaction of the protease CpaA with its type II secretion chaperone CpaB and its contribution to virulence in Acinetobacter species. J Biol Chem 292:19628–19638. 10.1074/jbc.M117.808394. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pugsley AP, Chapon C, Schwartz M. 1986. Extracellular pullulanase of Klebsiella pneumoniae is a lipoprotein. J Bacteriol 166:1083–1088. 10.1128/jb.166.3.1083-1088.1986. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Baldi DL, Higginson EE, Hocking DM, Praszkier J, Cavaliere R, James CE, Bennett-Wood V, Azzopardi KI, Turnbull L, Lithgow T, Robins-Browne RM, Whitchurch CB, Tauschek M. 2012. The type II secretion system and its ubiquitous lipoprotein substrate, SslE, are required for biofilm formation and virulence of enteropathogenic Escherichia coli. Infect Immun 80:2042–2052. 10.1128/IAI.06160-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.East A, Mechaly AE, Huysmans GHM, Bernarde C, Tello-Manigne D, Nadeau N, Pugsley AP, Buschiazzo A, Alzari PM, Bond PJ, Francetic O. 2016. Structural basis of pullulanase membrane binding and secretion revealed by X-ray crystallography, molecular dynamics and biochemical analysis. Structure 24:92–104. 10.1016/j.str.2015.10.023. [PubMed] [DOI] [PubMed] [Google Scholar]
- 78.Zückert WR. 2014. Secretion of bacterial lipoproteins: through the cytoplasmic membrane, the periplasm and beyond. Biochim Biophys Acta 1843:1509–1516. 10.1016/j.bbamcr.2014.04.022. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.d’Enfert C, Chapon C, Pugsley AP. 1987. Export and secretion of the lipoprotein pullulanase by Klebsiella pneumoniae. Mol Microbiol 1:107–116. 10.1111/j.1365-2958.1987.tb00534.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 80.Horstman AL, Kuehn MJ. 2002. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. J Biol Chem 277:32538–32545. 10.1074/jbc.M203740200. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horstman AL, Bauman SJ, Kuehn MJ. 2004. Lipopolysaccharide 3-deoxy-d-manno-octulosonic acid (Kdo) core determines bacterial association of secreted toxins. J Biol Chem 279:8070–8075. 10.1074/jbc.M308633200. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferrandez Y, Condemine G. 2008. Novel mechanism of outer membrane targeting of proteins in Gram-negative bacteria. Mol Microbiol 69:1349–1357. 10.1111/j.1365-2958.2008.06366.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 83.Haft DH, Varghese N. 2011. GlyGly-CTERM and rhombosortase: a C-terminal protein processing signal in a many-to-one pairing with a rhomboid family intramembrane serine protease. PLoS One 6:e28886. 10.1371/journal.pone.0028886. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gadwal S, Johnson TL, Remmer H, Sandkvist M. 2018. C-terminal processing of GlyGly-CTERM containing proteins by rhombosortase in Vibrio cholerae. PLoS Pathog 14:e1007341. 10.1371/journal.ppat.1007341. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sandkvist M, Michel LO, Hough LP, Morales VM, Bagdasarian M, Koomey M, DiRita VJ, Bagdasarian M. 1997. General secretion pathway (eps ) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J Bacteriol 179:6994–7003. 10.1128/jb.179.22.6994-7003.1997. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tauschek M, Gorrell RJ, Strugnell RA, Robins-Browne RM. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc Natl Acad Sci U S A 99:7066–7071. 10.1073/pnas.092152899. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lindeberg M, Collmer A. 1992. Analysis of eight out genes in a cluster required for pectic enzyme secretion by Erwinia chrysanthemi: sequence comparison with secretion genes from other gram-negative bacteria. J Bacteriol 174:7385–7397. 10.1128/jb.174.22.7385-7397.1992. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kagami Y, Ratliff M, Surber M, Martinez A, Nunn DN. 1998. Type II protein secretion by Pseudomonas aeruginosa: genetic suppression of a conditional mutation in the pilin-like component XcpT by the cytoplasmic component XcpR. Mol Microbiol 27:221–233. 10.1046/j.1365-2958.1998.00679.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 89.Sikora AE, Lybarger SR, Sandkvist M. 2007. Compromised outer membrane integrity in Vibrio cholerae type II secretion mutants. J Bacteriol 189:8484–8495. 10.1128/JB.00583-07. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park BR, Zielke RA, Wierzbicki IH, Mitchell KC, Withey JH, Sikora AE. 2015. A metalloprotease secreted by the type II secretion system links Vibrio cholerae with collagen. J Bacteriol 197:1051–1064. 10.1128/JB.02329-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.White RC, Gunderson FF, Tyson JY, Richardson KH, Portlock TJ, Garnett JA, Cianciotto NP. 2018. Type II secretion-dependent aminopeptidase LapA and acyltransferase PlaC are redundant for nutrient acquisition during Legionella pneumophila intracellular infection of amoebas. mBio 9:e00528-18. 10.1128/mBio.00528-18. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilton M, Halverson TWR, Charron-Mazenod L, Parkins MD, Lewenza S. 2018. Secreted phosphatase and deoxyribonuclease are required by Pseudomonas aeruginosa to defend against neutrophil extracellular traps. Infect Immun 86:e00403-18. 10.1128/IAI.00403-18. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chapon V, Czjzek M, El Hassouni M, Py B, Juy M, Barras F. 2001. Type II protein secretion in gram-negative pathogenic bacteria: the study of the structure/secretion relationships of the cellulase Cel5 (formerly EGZ) from Erwinia chrysanthemi. J Mol Biol 310:1055–1066. 10.1006/jmbi.2001.4787. [PubMed] [DOI] [PubMed] [Google Scholar]
- 94.Silva AJ, Pham K, Benitez JA. 2003. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149:1883–1891. 10.1099/mic.0.26086-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Luo Q, Kumar P, Vickers TJ, Sheikh A, Lewis WG, Rasko DA, Sistrunk J, Fleckenstein JM. 2014. Enterotoxigenic Escherichia coli secretes a highly conserved mucin-degrading metalloprotease to effectively engage intestinal epithelial cells. Infect Immun 82:509–521. 10.1128/IAI.01106-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hews CL, Tran SL, Wegmann U, Brett B, Walsham ADS, Kavanaugh D, Ward NJ, Juge N, Schüller S. 2017. The StcE metalloprotease of enterohaemorrhagic Escherichia coli reduces the inner mucus layer and promotes adherence to human colonic epithelium ex vivo. Cell Microbiol 19:e12717. 10.1111/cmi.12717. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kooi C, Sokol PA. 2009. Burkholderia cenocepacia zinc metalloproteases influence resistance to antimicrobial peptides. Microbiology 155:2818–2825. 10.1099/mic.0.028969-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 98.McCoy-Simandle K, Stewart CR, Dao J, DebRoy S, Rossier O, Bryce PJ, Cianciotto NP. 2011. Legionella pneumophila type II secretion dampens the cytokine response of infected macrophages and epithelia. Infect Immun 79:1984–1997. 10.1128/IAI.01077-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mallama CA, McCoy-Simandle K, Cianciotto NP. 2017. The type II secretion system of Legionella pneumophila dampens the MyD88 and Toll-like receptor 2 signaling pathway in infected human macrophages. Infect Immun 85:e00897-16. 10.1128/IAI.00897-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lathem WW, Grys TE, Witowski SE, Torres AG, Kaper JB, Tarr PI, Welch RA. 2002. StcE, a metalloprotease secreted by Escherichia coli O157:H7, specifically cleaves C1 esterase inhibitor. Mol Microbiol 45:277–288. 10.1046/j.1365-2958.2002.02997.x. [DOI] [PubMed] [Google Scholar]
- 101.Szabady RL, Lokuta MA, Walters KB, Huttenlocher A, Welch RA. 2009. Modulation of neutrophil function by a secreted mucinase of Escherichia coli O157:H7. PLoS Pathog 5:e1000320. 10.1371/journal.ppat.1000320. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tilley D, Law R, Warren S, Samis JA, Kumar A. 2014. CpaA a novel protease from Acinetobacter baumannii clinical isolates deregulates blood coagulation. FEMS Microbiol Lett 356:53–61. 10.1111/1574-6968.12496. [PubMed] [DOI] [PubMed] [Google Scholar]
- 103.Waack U, Warnock M, Yee A, Huttinger Z, Smith S, Kumar A, Deroux A, Ginsburg D, Mobley HLT, Lawrence DA, Sandkvist M. 2018. CpaA is a glycan-specific adamalysin-like protease secreted by Acinetobacter baumannii that inactivates coagulation factor XII. mBio 9:e01606-18. 10.1128/mBio.01606-18. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Overhage J, Lewenza S, Marr AK, Hancock RE. 2007. Identification of genes involved in swarming motility using a Pseudomonas aeruginosa PAO1 mini-Tn5-lux mutant library. J Bacteriol 189:2164–2169. 10.1128/JB.01623-06. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duncan C, Prashar A, So J, Tang P, Low DE, Terebiznik M, Guyard C. 2011. Lcl of Legionella pneumophila is an immunogenic GAG binding adhesin that promotes interactions with lung epithelial cells and plays a crucial role in biofilm formation. Infect Immun 79:2168–2181. 10.1128/IAI.01304-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johnson TL, Fong JC, Rule C, Rogers A, Yildiz FH, Sandkvist M. 2014. The type II secretion system delivers matrix proteins for biofilm formation by Vibrio cholerae. J Bacteriol 196:4245–4252. 10.1128/JB.01944-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fong JNC, Yildiz FH. 2015. Biofilm matrix proteins. Microbiol Spectr 3:MB-0004-2014. 10.1128/microbiolspec.MB-0004-2014. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fong JC, Rogers A, Michael AK, Parsley NC, Cornell WC, Lin YC, Singh PK, Hartmann R, Drescher K, Vinogradov E, Dietrich LE, Partch CL, Yildiz FH. 2017. Structural dynamics of RbmA governs plasticity of Vibrio cholerae biofilms. eLife 6:e26163. 10.7554/eLife.26163. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ennouri H, d’Abzac P, Hakil F, Branchu P, Naïtali M, Lomenech AM, Oueslati R, Desbrières J, Sivadon P, Grimaud R. 2017. The extracellular matrix of the oleolytic biofilms of Marinobacter hydrocarbonoclasticus comprises cytoplasmic proteins and T2SS effectors that promote growth on hydrocarbons and lipids. Environ Microbiol 19:159–173. 10.1111/1462-2920.13547. [PubMed] [DOI] [PubMed] [Google Scholar]
- 110.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, Ghazi A, Pugsley AP. 1999. Secretin PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci U S A 96:8173–8177. 10.1073/pnas.96.14.8173. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nouwen N, Stahlberg H, Pugsley AP, Engel A. 2000. Domain structure of secretin PulD revealed by limited proteolysis and electron microscopy. EMBO J 19:2229–2236. 10.1093/emboj/19.10.2229. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chami M, Guilvout I, Gregorini M, Rémigy HW, Müller SA, Valerio M, Engel A, Pugsley AP, Bayan N. 2005. Structural insights into the secretin PulD and its trypsin-resistant core. J Biol Chem 280:37732–37741. 10.1074/jbc.M504463200. [PubMed] [DOI] [PubMed] [Google Scholar]
- 113.Tosi T, Estrozi LF, Job V, Guilvout I, Pugsley AP, Schoehn G, Dessen A. 2014. Structural similarity of secretins from type II and type III secretion systems. Structure 22:1348–1355. 10.1016/j.str.2014.07.005. [PubMed] [DOI] [PubMed] [Google Scholar]
- 114.Dunstan RA, Heinz E, Wijeyewickrema LC, Pike RN, Purcell AW, Evans TJ, Praszkier J, Robins-Browne RM, Strugnell RA, Korotkov KV, Lithgow T. 2013. Assembly of the type II secretion system such as found in Vibrio cholerae depends on the novel pilotin AspS. PLoS Pathog 9:e1003117. 10.1371/journal.ppat.1003117. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hu J, Worrall LJ, Hong C, Vuckovic M, Atkinson CE, Caveney N, Yu Z, Strynadka NCJ. 2018. Cryo-EM analysis of the T3S injectisome reveals the structure of the needle and open secretin. Nat Commun 9:3840. 10.1038/s41467-018-06298-8. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gu S, Rehman S, Wang X, Shevchik VE, Pickersgill RW. 2012. Structural and functional insights into the pilotin-secretin complex of the type II secretion system. PLoS Pathog 8:e1002531. 10.1371/journal.ppat.1002531. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Strozen TG, Li G, Howard SP. 2012. YghG (GspSβ) is a novel pilot protein required for localization of the GspSβ type II secretion system secretin of enterotoxigenic Escherichia coli. Infect Immun 80:2608–2622. 10.1128/IAI.06394-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Collin S, Guilvout I, Nickerson NN, Pugsley AP. 2011. Sorting of an integral outer membrane protein via the lipoprotein-specific Lol pathway and a dedicated lipoprotein pilotin. Mol Microbiol 80:655–665. 10.1111/j.1365-2958.2011.07596.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 119.Collin S, Guilvout I, Chami M, Pugsley AP. 2007. YaeT-independent multimerization and outer membrane association of secretin PulD. Mol Microbiol 64:1350–1357. 10.1111/j.1365-2958.2007.05743.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 120.Dunstan RA, Hay ID, Wilksch JJ, Schittenhelm RB, Purcell AW, Clark J, Costin A, Ramm G, Strugnell RA, Lithgow T. 2015. Assembly of the secretion pores GspD, Wza and CsgG into bacterial outer membranes does not require the Omp85 proteins BamA or TamA. Mol Microbiol 97:616–629. 10.1111/mmi.13055. [PubMed] [DOI] [PubMed] [Google Scholar]
- 121.Guilvout I, Brier S, Chami M, Hourdel V, Francetic O, Pugsley AP, Chamot-Rooke J, Huysmans GH. 2017. Prepore stability controls productive folding of the BAM-independent multimeric outer membrane secretin PulD. J Biol Chem 292:328–338. 10.1074/jbc.M116.759498. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Korotkov KV, Gray MD, Kreger A, Turley S, Sandkvist M, Hol WGJ. 2009. Calcium is essential for the major pseudopilin in the type 2 secretion system. J Biol Chem 284:25466–25470. 10.1074/jbc.C109.037655. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cisneros DA, Bond PJ, Pugsley AP, Campos M, Francetic O. 2012. Minor pseudopilin self-assembly primes type II secretion pseudopilus elongation. EMBO J 31:1041–1053. 10.1038/emboj.2011.454. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reindl S, Ghosh A, Williams GJ, Lassak K, Neiner T, Henche AL, Albers SV, Tainer JA. 2013. Insights into FlaI functions in archaeal motor assembly and motility from structures, conformations, and genetics. Mol Cell 49:1069–1082. 10.1016/j.molcel.2013.01.014. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mancl JM, Black WP, Robinson H, Yang Z, Schubot FD. 2016. Crystal structure of a type IV pilus assembly ATPase: insights into the molecular mechanism of PilB from Thermus thermophilus. Structure 24:1886–1897. 10.1016/j.str.2016.08.010. [PubMed] [DOI] [PubMed] [Google Scholar]
- 126.McCallum M, Tammam S, Khan A, Burrows LL, Howell PL. 2017. The molecular mechanism of the type IVa pilus motors. Nat Commun 8:15091. 10.1038/ncomms15091. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gray MD, Bagdasarian M, Hol WGJ, Sandkvist M. 2011. In vivo cross-linking of EpsG to EpsL suggests a role for EpsL as an ATPase-pseudopilin coupling protein in the type II secretion system of Vibrio cholerae. Mol Microbiol 79:786–798. 10.1111/j.1365-2958.2010.07487.x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nivaskumar M, Santos-Moreno J, Malosse C, Nadeau N, Chamot-Rooke J, Tran Van Nhieu G, Francetic O. 2016. Pseudopilin residue E5 is essential for recruitment by the type 2 secretion system assembly platform. Mol Microbiol 101:924–941. 10.1111/mmi.13432. [PubMed] [DOI] [PubMed] [Google Scholar]
- 129.Shevchik VE, Robert-Baudouy J, Condemine G. 1997. Specific interaction between OutD, an Erwinia chrysanthemi outer membrane protein of the general secretory pathway, and secreted proteins. EMBO J 16:3007–3016. 10.1093/emboj/16.11.3007. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bouley J, Condemine G, Shevchik VE. 2001. The PDZ domain of OutC and the N-terminal region of OutD determine the secretion specificity of the type II out pathway of Erwinia chrysanthemi. J Mol Biol 308:205–219. 10.1006/jmbi.2001.4594. [PubMed] [DOI] [PubMed] [Google Scholar]
- 131.Douzi B, Ball G, Cambillau C, Tegoni M, Voulhoux R. 2011. Deciphering the Xcp Pseudomonas aeruginosa type II secretion machinery through multiple interactions with substrates. J Biol Chem 286:40792–40801. 10.1074/jbc.M111.294843. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reichow SL, Korotkov KV, Gonen M, Sun J, Delarosa JR, Hol WGJ, Gonen T. 2011. The binding of cholera toxin to the periplasmic vestibule of the type II secretion channel. Channels (Austin) 5:215–218. 10.4161/chan.5.3.15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pineau C, Guschinskaya N, Robert X, Gouet P, Ballut L, Shevchik VE. 2014. Substrate recognition by the bacterial type II secretion system: more than a simple interaction. Mol Microbiol 94:126–140. 10.1111/mmi.12744. [PubMed] [DOI] [PubMed] [Google Scholar]
- 134.Michel-Souzy S, Douzi B, Cadoret F, Raynaud C, Quinton L, Ball G, Voulhoux R. 2018. Direct interactions between the secreted effector and the T2SS components GspL and GspM reveal a new effector-sensing step during type 2 secretion. J Biol Chem 293:19441–19450. 10.1074/jbc.RA117.001127. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nunn D. 1999. Bacterial type II protein export and pilus biogenesis: more than just homologies? Trends Cell Biol 9:402–408. 10.1016/S0962-8924(99)01634-7. [DOI] [PubMed] [Google Scholar]
- 136.Forest KT. 2008. The type II secretion arrowhead: the structure of GspI-GspJ-GspK. Nat Struct Mol Biol 15:428–430. 10.1038/nsmb0508-428. [PubMed] [DOI] [PubMed] [Google Scholar]
- 137.Nivaskumar M, Bouvier G, Campos M, Nadeau N, Yu X, Egelman EH, Nilges M, Francetic O. 2014. Distinct docking and stabilization steps of the pseudopilus conformational transition path suggest rotational assembly of type IV pilus-like fibers. Structure 22:685–696. 10.1016/j.str.2014.03.001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nivaskumar M, Francetic O. 2014. Type II secretion system: a magic beanstalk or a protein escalator. Biochim Biophys Acta 1843:1568–1577. 10.1016/j.bbamcr.2013.12.020. [PubMed] [DOI] [PubMed] [Google Scholar]
- 139.O’Neal CJ, Amaya EI, Jobling MG, Holmes RK, Hol WGJ. 2004. Crystal structures of an intrinsically active cholera toxin mutant yield insight into the toxin activation mechanism. Biochemistry 43:3772–3782. 10.1021/bi0360152. [PubMed] [DOI] [PubMed] [Google Scholar]
- 140.Wedekind JE, Trame CB, Dorywalska M, Koehl P, Raschke TM, McKee M, FitzGerald D, Collier RJ, McKay DB. 2001. Refined crystallographic structure of Pseudomonas aeruginosa exotoxin A and its implications for the molecular mechanism of toxicity. J Mol Biol 314:823–837. 10.1006/jmbi.2001.5195. [PubMed] [DOI] [PubMed] [Google Scholar]
- 141.Yoder MD, Jurnak F. 1995. Protein motifs. 3. The parallel beta helix and other coiled folds. FASEB J 9:335–342. 10.1096/fasebj.9.5.7896002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 142.Yu AC, Worrall LJ, Strynadka NC. 2012. Structural insight into the bacterial mucinase StcE essential to adhesion and immune evasion during enterohemorrhagic E. coli infection. Structure 20:707–717. 10.1016/j.str.2012.02.015. [PubMed] [DOI] [PubMed] [Google Scholar]
- 143.Giglio KM, Fong JC, Yildiz FH, Sondermann H. 2013. Structural basis for biofilm formation via the Vibrio cholerae matrix protein RbmA. J Bacteriol 195:3277–3286. 10.1128/JB.00374-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Maestre-Reyna M, Wu WJ, Wang AH. 2013. Structural insights into RbmA, a biofilm scaffolding protein of V. cholerae. PLoS One 8:e82458. 10.1371/journal.pone.0082458. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rule CS, Patrick M, Camberg JL, Maricic N, Hol WGJ, Sandkvist M. 2016. Zinc coordination is essential for the function and activity of the type II secretion ATPase EpsE. Microbiologyopen 5:870–882. 10.1002/mbo3.376. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fulara A, Vandenberghe I, Read RJ, Devreese B, Savvides SN. 2018. Structure and oligomerization of the periplasmic domain of GspL from the type II secretion system of Pseudomonas aeruginosa. Sci Rep 8:16760. 10.1038/s41598-018-34956-w. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Y, Faucher F, Zhang W, Wang S, Neville N, Poole K, Zheng J, Jia Z. 2018. Structure-guided disruption of the pseudopilus tip complex inhibits the type II secretion in Pseudomonas aeruginosa. PLoS Pathog 14:e1007343. 10.1371/journal.ppat.1007343. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Korotkov KV, Delarosa JR, Hol WGJ. 2013. A dodecameric ring-like structure of the N0 domain of the type II secretin from enterotoxigenic Escherichia coli. J Struct Biol 183:354–362. 10.1016/j.jsb.2013.06.013. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wretlind B, Pavlovskis OR. 1984. Genetic mapping and characterization of Pseudomonas aeruginosa mutants defective in the formation of extracellular proteins. J Bacteriol 158:801–808. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bo JN, Howard SP. 1991. Mutagenesis and isolation of Aeromonas hydrophila genes which are required for extracellular secretion. J Bacteriol 173:1241–1249. 10.1128/jb.173.3.1241-1249.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paranjpye RN, Lara JC, Pepe JC, Pepe CM, Strom MS. 1998. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 cells and virulence in iron-overloaded mice. Infect Immun 66:5659–5668. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sikora AE, Zielke RA, Lawrence DA, Andrews PC, Sandkvist M. 2011. Proteomic analysis of the Vibrio cholerae type II secretome reveals new proteins, including three related serine proteases. J Biol Chem 286:16555–16566. 10.1074/jbc.M110.211078. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Szabady RL, Yanta JH, Halladin DK, Schofield MJ, Welch RA. 2011. TagA is a secreted protease of Vibrio cholerae that specifically cleaves mucin glycoproteins. Microbiology 157:516–525. 10.1099/mic.0.044529-0. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Golovkine G, Faudry E, Bouillot S, Voulhoux R, Attrée I, Huber P. 2014. VE-cadherin cleavage by LasB protease from Pseudomonas aeruginosa facilitates type III secretion system toxicity in endothelial cells. PLoS Pathog 10:e1003939. 10.1371/journal.ppat.1003939. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.DuMont AL, Cianciotto NP. 2017. Stenotrophomonas maltophilia serine protease StmPr1 induces matrilysis, anoikis, and protease-activated receptor 2 activation in human lung epithelial cells. Infect Immun 85:e00544-17. 10.1128/IAI.00544-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Truchan HK, Christman HD, White RC, Rutledge NS, Cianciotto NP. 2017. Type II secretion substrates of Legionella pneumophila translocate out of the pathogen-occupied vacuole via a semipermeable membrane. mBio 8:e00870-17. 10.1128/mBio.00870-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jha G, Rajeshwari R, Sonti RV. 2005. Bacterial type two secretion system secreted proteins: double-edged swords for plant pathogens. Mol Plant Microbe Interact 18:891–898. 10.1094/MPMI-18-0891. [PubMed] [DOI] [PubMed] [Google Scholar]
- 158.Nascimento R, Gouran H, Chakraborty S, Gillespie HW, Almeida-Souza HO, Tu A, Rao BJ, Feldstein PA, Bruening G, Goulart LR, Dandekar AM. 2016. The type II secreted lipase/esterase LesA is a key virulence factor required for Xylella fastidiosa pathogenesis in grapevines. Sci Rep 6:18598. 10.1038/srep18598. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Overbye LJ, Sandkvist M, Bagdasarian M. 1993. Genes required for extracellular secretion of enterotoxin are clustered in Vibrio cholerae. Gene 132:101–106. 10.1016/0378-1119(93)90520-D. [DOI] [PubMed] [Google Scholar]
- 160.Francetic O, Belin D, Badaut C, Pugsley AP. 2000. Expression of the endogenous type II secretion pathway in Escherichia coli leads to chitinase secretion. EMBO J 19:6697–6703. 10.1093/emboj/19.24.6697. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aragon V, Kurtz S, Cianciotto NP. 2001. Legionella pneumophila major acid phosphatase and its role in intracellular infection. Infect Immun 69:177–185. 10.1128/IAI.69.1.177-185.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ball G, Durand E, Lazdunski A, Filloux A. 2002. A novel type II secretion system in Pseudomonas aeruginosa. Mol Microbiol 43:475–485. 10.1046/j.1365-2958.2002.02759.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 163.Putker F, Tommassen-van Boxtel R, Stork M, Rodríguez-Herva JJ, Koster M, Tommassen J. 2013. The type II secretion system (Xcp) of Pseudomonas putida is active and involved in the secretion of phosphatases. Environ Microbiol 15:2658–2671. [PubMed] [DOI] [PubMed] [Google Scholar]
- 164.Rossier O, Dao J, Cianciotto NP. 2009. A type II secreted RNase of Legionella pneumophila facilitates optimal intracellular infection of Hartmannella vermiformis. Microbiology 155:882–890. 10.1099/mic.0.023218-0. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mulcahy H, Charron-Mazenod L, Lewenza S. 2010. Pseudomonas aeruginosa produces an extracellular deoxyribonuclease that is required for utilization of DNA as a nutrient source. Environ Microbiol 12:1621–1629. [PubMed] [DOI] [PubMed] [Google Scholar]
- 166.DiChristina TJ, Moore CM, Haller CA. 2002. Dissimilatory Fe(III) and Mn(IV) reduction by Shewanella putrefaciens requires ferE, a homolog of the pulE (gspE ) type II protein secretion gene. J Bacteriol 184:142–151. 10.1128/JB.184.1.142-151.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shi L, Deng S, Marshall MJ, Wang Z, Kennedy DW, Dohnalkova AC, Mottaz HM, Hill EA, Gorby YA, Beliaev AS, Richardson DJ, Zachara JM, Fredrickson JK. 2008. Direct involvement of type II secretion system in extracellular translocation of Shewanella oneidensis outer membrane cytochromes MtrC and OmcA. J Bacteriol 190:5512–5516. 10.1128/JB.00514-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kirn TJ, Jude BA, Taylor RK. 2005. A colonization factor links Vibrio cholerae environmental survival and human infection. Nature 438:863–866. 10.1038/nature04249. [PubMed] [DOI] [PubMed] [Google Scholar]
- 169.Cadoret F, Ball G, Douzi B, Voulhoux R. 2014. Txc, a new type II secretion system of Pseudomonas aeruginosa strain PA7, is regulated by the TtsS/TtsR two-component system and directs specific secretion of the CbpE chitin-binding protein. J Bacteriol 196:2376–2386. 10.1128/JB.01563-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]


