Summary
The expression pattern of pathogenesis‐related genes PR‐1, PR‐2 and PR‐5, considered as markers for salicylic acid (SA)‐dependent systemic acquired resistance (SAR), was examined in the roots and shoots of tomato plants pre‐treated with SA and subsequently infected with root‐knot nematodes (RKNs) (Meloidogyne incognita). PR‐1 was up‐regulated in both roots and shoots of SA‐treated plants, whereas the expression of PR‐5 was enhanced only in roots. The over‐expression of PR‐1 in the whole plant occurred as soon as 1 day after SA treatment. Up‐regulation of the PR‐1 gene was considered to be the main marker of SAR elicitation. One day after treatment, plants were inoculated with active juveniles (J2s) of M. incognita. The number of J2s that entered the roots and started to develop was significantly lower in SA‐treated than in untreated plants at 5 and 15 days after inoculation. The expression pattern of PR‐1, PR‐2 and PR‐5 was also examined in the roots and shoots of susceptible and Mi‐1‐carrying resistant tomato plants infected by RKNs. Nematode infection produced a down‐regulation of PR genes in both roots and shoots of SA‐treated and untreated plants, and in roots of Mi‐carrying resistant plants. Moreover, in resistant infected plants, PR gene expression, in particular PR‐1 gene expression, was highly induced in shoots. Thus, nematode infection was demonstrated to elicit SAR in shoots of resistant plants. The data presented in this study show that the repression of host defence SA signalling is associated with the successful development of RKNs, and that SA exogenously added as a soil drench is able to trigger a SAR‐like response to RKNs in tomato.
Introduction
Root‐knot nematodes (RKNs), Meloidogyne spp., are among the most damaging and uncontrollable pests of cultivated crops, incurring estimated economic losses in excess of €80 billion/year in worldwide agriculture (Blok et al., 2008). RKNs are obligate sedentary endoparasites that spend most of their active lives within plant roots. RKNs enter the roots as motile second‐stage juveniles (J2s) that do not kill parasitized cells, but induce the formation of a few discrete giant or nurse cells. Such feeding sites serve principally to actively transfer solutes and nutrients to the developing nematode. J2s become sedentary and, through subsequent moults to J3s and J4s, develop into adult females which, at the end of their life cycle, lay eggs in an external gelatinous matrix, which is clearly visible outside the roots as an egg mass. Moreover, nematode action induces hypertrophy and hyperplasia of the surrounding tissues, thus causing the formation of the familiar galls on the roots (Williamson and Gleason, 2003). Current control strategies against RKNs rely mainly on the use of toxic chemical nematicides, although such chemicals are gradually being phased out because of governmental regulations and environmental concerns. Thus, there is an increasing demand to search for environmentally sustainable integrated strategies for the management of such pests. Resistant cultivars and induced resistance by natural plant activators will be widely used as sustainable control strategies to replace nematicides (Molinari, 2011). An extensive list of genes conferring resistance to RKNs (R genes) in annual and perennial crops has been reported in Williamson and Roberts (2009). The tomato gene Mi‐1 encodes a nucleotide‐binding, leucine‐rich repeat protein, and is currently the best characterized and most widely used resistance gene, as it confers effective resistance against the three most diffused RKN species: M. incognita, M. javanica and M. arenaria (Williamson, 1998). In the tomato cultivar Motelle, which carries Mi‐1, J2s enter the roots and move to the central cylinder, where a hypersensitive response (HR) with localized cell death occurs, instead of giant cell development (Paulson and Webster, 1972).
It has long been recognized that local and systemic salicylic acid (SA) accumulation following pathogen infection induces the expression of multiple pathogenesis‐related (PR) genes, which are markers of the onset of the so‐called systemic acquired resistance (SAR; Durrant and Dong, 2004). SA‐responsive PR genes include PR‐1 (unknown function), PR‐2 (β‐1,3‐glucanase) and PR‐5 (thaumatin‐like protein) (Cao et al., 1994). In tomato, it has been reported that SA induces, at least, PR‐1(P4) and PR‐1(P6) genes, and that both genes are used as molecular markers for the activation of the SA signalling pathway (Uehara et al., 2010). SA‐dependent signalling seems to be crucial for resistance against foliar biotrophic pathogens (Glazebook, 2005), although our knowledge on its role in root–biotroph interactions is still limited (Gutjahr and Paszkowski, 2009). Mi‐1‐mediated resistance seems to be regulated by an SA‐dependent defence pathway (Branch et al., 2004). However, transformation of resistant tomato with a construct expressing NahG, which encodes a bacterial salicylate hydroxylase that degrades SA into catechol, did not result in resistance failure and nematode development (Bhattarai et al., 2008). Indeed, few investigations have been reported to date on PR gene expression in incompatible responses to RKNs and cyst nematodes (CNs); moreover, these studies have focused only on root tissue (Mazarei et al., 2011; Tirumalaraju et al., 2011; Uehara et al., 2010). In this work, for the first time, we have analysed PR gene expression in both roots and shoots of resistant tomato, uninfected and infected with M. incognita.
Generally, successful RKN infection involves the local suppression of host defence signalling (Jammes et al., 2005), whereas broad and general activation of defence signalling is observed during CN infection (Hamamouch et al., 2011; Uehara et al., 2010; Wubben et al., 2008). In particular, in Arabidopsis, CN infection causes induction of the SAR marker gene PR‐1 in shoots, but not in roots (Wubben et al., 2008), whereas it has been suggested that RKNs suppress SA‐dependent SAR in leaves of infected plants (Hamamouch et al., 2011). We show that, in tomato, suppression of host defence SA signalling by RKNs involves both roots and shoots, as reported recently for rice (Kyndt et al., 2012).
Induced resistance is mainly based on the treatment of plants with natural or environmentally benign synthetic chemicals that are able to trigger SAR. The benefits, limitations and perspectives of induced resistance in conventional agriculture, mostly to fungi and bacteria, are summarized in Walters (2010). SA and its functional homologues, benzol‐(1,2,3)‐thiadiazole‐7‐carbothionic acid S‐methyl ester (BTH) and 2,6‐dichloroisonicotinic acid (INA), have already been tested as SAR elicitors against plant‐parasitic nematodes (Molinari and Baser, 2010; Owen et al., 2002; Sanz‐Alférez et al., 2008; Vieria dos Santos et al., 2013; Wubben et al., 2008). It has been demonstrated that SA applied as a soil drench to tomato plants is effective in defence activation against RKNs, although only at suitable concentrations (Molinari and Baser, 2010). Pretreatment with appropriate concentrations of SA was used in this study to test whether the inhibition of nematode infection was the result of SAR elicitation by monitoring (in the time period following SA application) the expression of SA‐responsive PR genes. The current investigation demonstrates that soil‐drenched SA induces a SAR‐like response in both roots and shoots of tomato, and this response results in a partial, yet significant, restriction of the number of J2s that establish feeding sites in the roots.
Finally, our data suggest that RKN development is associated with a systemic repression of SA signalling in tomato, and that, conversely, PR gene up‐regulation in the immune response may be the effect and not the cause of the Mi‐1‐mediated failure of nematode infection.
Results
SA treatment reduces RKN parasitism
It has generally been recognized that SA plays a critical role in plant defence signalling. To examine whether SA affects RKN parasitism during a compatible interaction, the effect of SA treatment on tomato susceptibility to M. incognita was investigated. Individual plants were soil drenched with 20 mg SA on day 1 before inoculation with 300 active J2s. Five and 15 days after inoculation, the number of nematodes that had penetrated the roots was determined (Fig. 1). At day 5 after inoculation, very few invasive juveniles had become sedentary in the roots of untreated plants and even fewer sedentary juveniles (SJs) were present in SA‐treated plants. Moreover, fewer motile invasive forms were found in the roots of SA‐treated plants compared with controls (Fig. 1a). Ten days after the first detection, the number of juveniles developing in the roots increased dramatically (about 30‐fold), whereas the presence of motile invasive forms was reduced to a small fraction. However, the roots of SA‐treated plants exhibited a significant reduction in both motile forms and sedentary developing juveniles compared with untreated plants (Fig. 1b). Although the presence of adult females (AFs) was negligible at this stage, no evident differences could be determined between treated and untreated plants. Generally, in our system, the amount of juveniles that penetrated the roots 15 days after inoculation was about two‐thirds of the inoculated juveniles in the untreated plants and one‐half of the inoculated juveniles in the treated plants.
Figure 1.
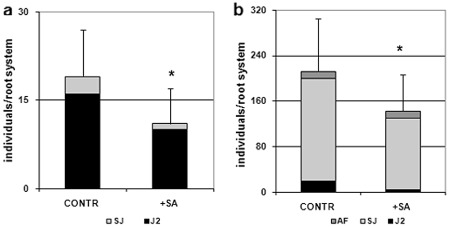
Numbers of nematodes that penetrated tomato roots of untreated (CONTR) and salicylic acid‐treated (+SA) plants, 5 days (a) and 15 days (b) after inoculation with 300 Meloidogyne incognita invasive juveniles (J2s). Motile juveniles (J2s), sedentary juveniles (SJs) and adult females (AFs) were determined. Values are expressed as averages (obtained from three biological replicates) of the numbers of individuals per root system ± SD (n = 18). Asterisks indicate that the means are significantly different, as determined by t‐test (P < 0.05).
The detection of infestation parameters was repeated 7 weeks after inoculation (Fig. 2) in order to allow nematodes to complete their life cycle and produce, in pot, a second generation of invasive juveniles (J2s), which, in turn, would be able to enter and develop in the roots, but not to reproduce. The number of inoculated J2s that were able to reproduce, i.e. to develop into gravid females and produce egg masses (EMs), was about 40% lower in the SA‐treated than untreated plants (EM/g root fresh weight, Fig. 2a). However, if we do not consider the number of inoculated J2s, but the J2s that were actually able to grow in the roots, the fractions of reproducing individuals were very similar between untreated and treated plants. Fewer nematodes per plant seemed to be able to develop in plants treated with SA, although—once nematodes started to develop—they seemed to have similar chances of reproducing in both treated and untreated plants. Fewer developing nematodes per plant produced fewer EMs, which results in an overall reduction in parasite reproduction, expressed as eggs/g root fresh weight (Fig. 2b). Moreover, the number of sedentary forms (SFs), expressed as SF/g root fresh weight, found in SA‐treated plants was much lower than that in untreated plants, even 7 weeks after inoculation (Fig. 2c). It should be mentioned that most of the SFs found in roots at this stage are from J2s hatched in pot, at least 30 days after SA treatment. Evidently, SA application is able to reduce nematode infection by about 50%. Conversely, the incompatible response shown by the Mi‐1‐carrying resistant cv. Motelle leads to negligible infestation (Fig. 2, RES data).
Figure 2.
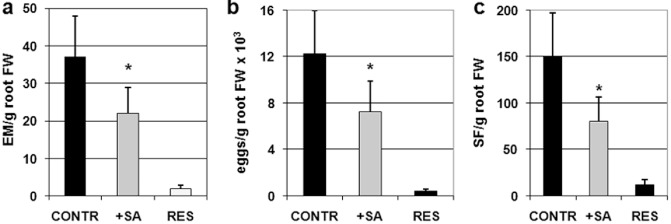
Infestation parameters of susceptible untreated (CONTR), susceptible salicylic acid‐treated (+SA) and resistant (RES) tomato plants, 7 weeks after inoculation with 300 Meloidogyne incognita invasive juveniles (J2s). (a) Egg masses per gram of root fresh weight (EM/g root FW). (b) Reproduction index (indicating the level of nematode reproduction) is expressed as eggs per gram of root fresh weight (eggs/g root FW). (c) Sedentary forms per gram of root fresh weight (SF/g root FW) represent the number of sedentary individuals, belonging to two parasite generations, and counted according to root biomass. Values are expressed as averages ± SD (n = 18). Asterisks indicate that the means of values belonging to SA‐treated and control plants are significantly different, as determined by t‐test (P < 0.05).
Expression of tomato SA‐responsive PR genes after SA application
Quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) was used to determine the transcript levels of three classes of PR genes (i.e. PR‐1, PR‐2 and PR‐5), which are commonly considered as responsive to SA‐dependent signalling (Cao et al., 1994). PR gene transcripts of roots and shoots were detected in potted plants soil drenched with 20 mg SA, 1, 3 and 5 days post‐treatment (dpt), and compared with untreated controls (Fig. 3). PR‐1 and PR‐5 were highly expressed in treated roots as early as 1 dpt (Fig. 3a); moreover, PR‐1 transcripts were approximately five‐fold higher in the shoots of treated relative to untreated plants (Fig. 3b). Conversely, at 3 dpt, a down‐regulation of the PR‐1 and PR‐5 genes occurred in both roots and shoots of treated plants. PR gene expression was generally comparable in treated and untreated plants at 5 dpt; only the PR‐5 gene seemed to be up‐regulated in treated roots and unaffected in treated shoots. PR‐2 expression was not induced in plants by SA application. It is likely that the early over‐expression of the PR‐1 gene in SA‐treated plants is a marker of SAR induction in both roots and shoots, which may result in a decreased susceptibility to RKNs.
Figure 3.
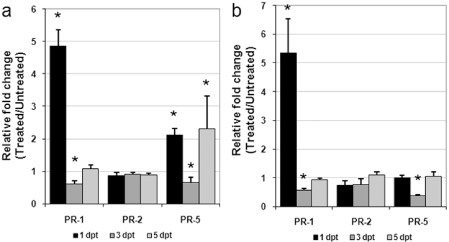
Time course analysis of pathogenesis‐related (PR) gene expression by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) in roots (a) and shoots (b) of susceptible tomato plants at 1, 3 and 5 days post‐treatment (dpt) with salicylic acid (SA). Data are the mean fold changes ± SD in PR transcript levels of tissues from SA‐treated plants relative to those from untreated control plants (the value of unity indicates no change). Means were determined from three biological replicates and three qRT‐PCRs were performed per sample, resulting in a total of nine replicates for statistical analysis. Asterisks indicate that the mean fold change is significantly different from unity as determined by Kolmogorov–Smirnov test (P < 0.05).
Expression of tomato SA‐responsive PR genes after RKN infestation
To examine the expression of SA‐responsive PR genes in compatible and incompatible Meloidogyne–tomato interactions, transcripts of such genes were determined in roots and shoots of both uninfested and infested plants, 5 days after inoculation with 300 M. incognita active J2s (Fig. 4). Nematode parasitism in both untreated and SA‐treated susceptible plants produced an inhibition of the expression of all the PR genes tested, whether in roots or shoots, although quantitatively higher in roots (Fig. 4a,b). The different level of infestation between untreated and SA‐treated plants, which has been shown to occur even at this early stage (Fig. 1a), was not associated with a difference in the expression of PR genes. A significant suppression of host defence SA signalling seems to be associated with the establishment of functional feeding sites. Conversely, the response of resistant plants to nematode inoculation, in terms of PR gene expression, was completely different in shoots. All the PR genes were over‐expressed in shoots as a result of parasite attack. In particular, the amount of PR‐1 transcripts was approximately 25‐fold higher in inoculated plants compared with uninoculated controls (Fig. 4b). In roots, PR‐2 and PR‐5 gene expression was apparently inhibited by nematode infection, as it occurred in susceptible plants (Fig. 4a). It is likely that nematode infection elicits a SAR response in resistant tomato, although this response seems to be expressed in shoots but not in roots.
Figure 4.
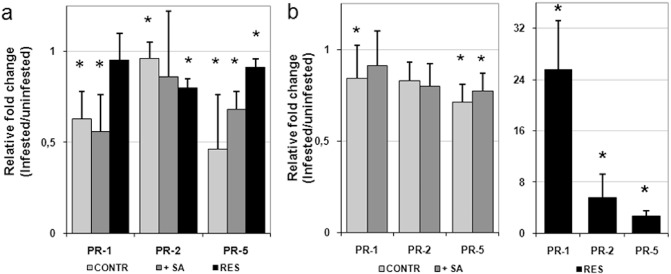
Pathogenesis‐related (PR) gene expression by quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) in roots (a) and shoots (b) of susceptible tomato plants, untreated (CONTR) and treated with salicylic acid (+SA), and of resistant tomato plants (RES), 5 days after inoculation with 300 invasive juveniles (J2s) of the root‐knot nematode Meloidogyne incognita. Data are the mean fold changes ± SD in PR transcript levels of tissues from infested plants relative to tissues from uninfested control plants (the value of unity indicates no change). Means were determined from three biological replicates and three qRT‐PCRs were performed per sample, resulting in a total of nine replicates for statistical analysis. Asterisks indicate that the mean fold change is significantly different from unity as determined by Kolmogorov–Smirnov test (P < 0.05).
Changes in key enzyme activities after RKN infestation
The PR‐2 gene encodes for the enzyme family known as β‐1,3‐endoglucanases, which may have a significant role in the defence response of plants to pathogens (Van Loon et al., 2006). We chose to assay the activity of this enzyme to determine whether the changes observed in the PR‐2 gene transcripts induced by nematode infection would somehow be reproduced in the enzyme activity of the encoded protein. Endoglucanase enzyme activity in roots did not reveal any apparent significant change as a result of nematode infection (Fig. 5a). Conversely, in shoots, changes in enzyme activity of the PR‐2 protein resembled those found in the gene transcripts, with about 50% inhibition in compatible interactions, whether or not plants had been treated with SA, and about 50% activation in the incompatible interaction (Fig. 5b). The effect of SA treatment on β‐1,3‐endoglucanase activity was different between roots and shoots; slight and insignificant inhibition occurred in roots of treated relative to untreated plants, whereas a marked inhibition occurred in shoots (results not shown). These results were in agreement with those obtained with the PR‐2 transcripts.
Figure 5.
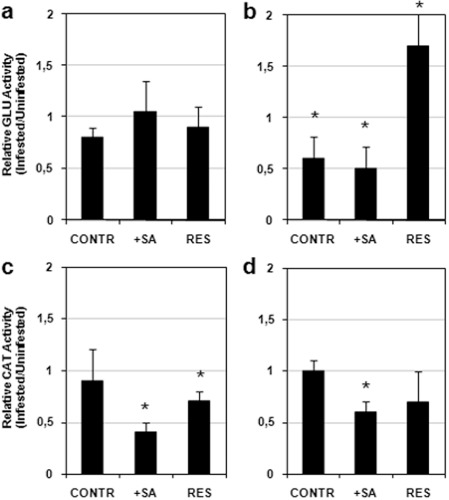
Glucanase (GLU) activity in roots (a) and shoots (b), and catalase (CAT) activity in roots (c) and shoots (d), of susceptible tomato plants, untreated (CONTR) and treated with salicylic acid (+SA), and of resistant tomato plants (RES), 5 days after inoculation with 300 invasive juveniles (J2s) of the root‐knot nematode Meloidogyne incognita. Data are the mean fold changes ± SD in enzyme activity of tissues from infested plants relative to tissues from uninfested control plants (the value of unity indicates no change). Means were determined from three biological replicates. Asterisks indicate that the mean fold change is significantly different from unity as determined by Kolmogorov–Smirnov test (P < 0.05).
Inhibition of root catalase activity has already been reported to be associated with an early defence reaction occurring in Mi‐1‐bearing tomato plants challenged with RKNs (Molinari and Loffredo, 2006). On the contrary, no changes in catalase activity have been reported during tomato–RKN compatible interactions (Molinari, 2008; Molinari and Abd‐Elgawad, 2007; Molinari and Loffredo, 2006). Our results confirm that the inhibition of catalase activity specifically occurs in both roots and shoots of Mi‐1‐bearing tomato. Interestingly, a consistent inhibition of this activity was also found in roots and shoots of SA‐treated susceptible plants, whereas untreated plants showed no changes (Fig. 5c,d).
Discussion
Although it has long been demonstrated that pretreatment with SA can reduce nematode infection in tomato (Molinari and Baser, 2010), knowledge of the molecular events supporting such a protective role by SA is poor. We therefore decided to carry out a time course analysis of the expression of the SA‐responsive PR genes (PR‐1, PR‐2 and PR‐5) in roots and shoots, spanning a few days after SA application. Basically, over‐expression of PR‐1, observed at 1 dpt, was the earliest and most remarkable response of the whole plant. Given that PR‐1 has been commonly used as a molecular marker for SAR induction (Bowling et al., 1994), in our experimental system, it can be assumed that exogenously provided SA actually induces SAR in tomato plants. PR‐5 expression is also induced early, although at lower levels and only in roots. Comparably, in Arabidopsis thaliana, pretreatment of plants with SA significantly decreased their susceptibility to CNs, whilst simultaneously inducing PR‐1 gene expression in both roots and shoots (Wubben et al., 2008). Moreover, SA‐derived elicitors, such as INA and BTH, were able to activate the expression of PR‐1 and PR‐2 as SAR markers in tomato, although these treatments reduced the production of galls by only 25% following M. javanica infection (Sanz‐Alférez et al., 2008).
SAR activation could be detected, as PR‐1 over‐expression, in the leaves of test plants as soon as 1 day after SA treatment. Conversely, 3 days after SA treatment, a marked repression of PR‐1 transcription occurred after the initial peak, as has been reported to occur in tomato leaves absorbing SA via the transpiration stream (Van Kan et al., 1995). Therefore, this study has shown that, once SAR signalling is generated, defence against nematodes is activated, regardless of the possible temporary feedback inhibition of defence gene expression. Changes in PR‐1 gene expression in the time period following SA treatment were no different between roots and shoots. Conversely, the different response of PR‐5 gene expression may indicate that different control mechanisms of this gene may exist in leaves relative to roots, as already suggested by Hamamouch et al. (2011). PR‐2 gene expression does not seem to be regulated by SA treatment.
The dosages and application methods by which SA is supplied to plants are very important for the successful inhibition of nematode infection, as reported previously (Molinari and Baser, 2010). In our experimental system, SA, supplied as a soil drench to plants, markedly decreased the number of invasive J2s that succeeded in developing and reproducing, when compared with untreated plants. SA‐induced inhibition of nematode infestation is an effect of SAR activation by plants and not of SA nematicidal activity in soil. Indeed, SA dissolved in water to a concentration of 10 mm did not exhibit direct nematicidal effects on RKN J2s (Nandi et al., 2003). In our case, the SA concentration in the pots was <1.5 mm; however, the bioavailability of SA in soil is always lower than in water solution, as SA is actively absorbed by soil humic acids (Traversa et al., 2012). Moreover, SA has been demonstrated to reduce RKN infection also by root dip, with no direct contact between the chemical and the invasive RKN J2s (Molinari and Baser, 2010). Conversely, spraying of tomato leaves with SA did not result in any accumulation of PR proteins and, consequently, did not inhibit nematode infection (Van Kan et al., 1995; Vieria dos Santos et al., 2013).
The results of the present study clearly show that successful M. incognita attack suppresses SA signalling, as revealed by the down‐regulation, in either roots or shoots of infested tomato plants, of all the SA‐responsive PR genes tested. It should be noted that gene transcripts were determined 5 days after inoculation (5 dpi) in order to gather information on the early stages of nematode infection. The early stages (0–6 days) of giant cell formation are characterized by cell elongation, expansion and nuclear division; only at the 9‐day time point do giant cells start to fully support nematode feeding by dramatically increasing their osmoticum and cell wall thickening (Jones, 1981). Comparably, only at day 9 of M. incognita infection in Arabidopsis has a significant up‐regulation of PR‐1, PR‐2 and PR‐5 been reported to occur in roots; contrarily, and in accordance with our results, the down‐regulation of all PR genes occurred in shoots on any tested day (5, 9, 14) after inoculation (Hamamouch et al., 2011). Over‐expression of the PR‐2 and PR‐5 genes in roots at 9 dpi may be explained by the potential functions of these genes, which include cell wall modification and osmotic regulation, respectively (Van Loon et al., 2006). Our results are also in full agreement with the general finding that several genes are down‐regulated after nematode infection and many of these are involved in the pathogen defence response (Gheysen and Fenoll, 2002). The restriction of PR gene expression found in M. incognita‐infected plants confirms that successful RKN parasitism, at its earliest stages, may involve both local and systemic suppression of the host defence response (Jammes et al., 2005). On the contrary, potato CN infection of tomato and Arabidopsis has been found to be associated with an increase in, at least, some SA‐responsive PR genes, even at the earliest stages of parasitism (Uehara et al., 2010; Wubben et al., 2008). CNs do not form discrete giant cells for their feeding; rather, they induce the breakdown of the cell walls between the initial feeding site and its neighbouring cells, resulting in the development of a multinucleate syncytium (Williamson and Gleason, 2003). Moreover, a major difference between RKN and CN parasitism occurs at the earliest stages of infection. After penetrating the roots, CNs move to the vascular cylinder and pierce cell walls with their stylets, thus disrupting cells, whereas RKNs move intercellularly and do not cause any remarkable tissue damage. The early necroses caused by CNs may trigger a rapid but transient increase in SA and an early activation of SA signalling (Van Kan et al., 1995), which should not occur in Meloidogyne–plant interactions.
Pretreatment with SA and consequent SAR elicitation did not impair the ability of the nematodes, which had established successful feeding sites, to suppress PR gene expression. Therefore, the decrease in susceptibility to nematodes in treated plants might be a result of defence priming occurring early after SA treatment, as attested by PR‐1 gene over‐expression. How SAR activation may reduce the number of J2s developing in roots is a matter for future investigation. It is noteworthy that root catalase activity was as markedly inhibited in infected SA‐treated plants as in the incompatible reaction of the Mi‐1‐bearing cv. Motelle. It is generally known that SA application to plants induces H2O2 accumulation and that catalase may be inhibited by SA and H2O2, that is, its own substrate (Molinari, 2007). However, H2O2 and other reactive oxygen species (ROS) have been reported to accumulate in nematode‐penetrated cells, although at an enhanced rate in hyper‐responsive cells (Melillo et al., 2006). The presence of elevated levels of SA in treated plants may enhance the production of ROS by nematode action and generate limited hypersensitive‐like reactions in roots, with catalase inhibition being a specific symptom. The establishment of a more hostile environment may thus reduce nematode infection.
Mi‐1‐bearing resistant tomato plants (cv. Motelle) infected with M. incognita were subsequently used to determine the expression of SA‐responsive PR genes in a true incompatible reaction. The most evident difference between compatible and incompatible reactions was found in shoots. PR genes were all significantly up‐regulated after nematode infection and, in particular, PR‐1 gene transcripts of shoots from infested plants were about 25‐fold higher than those from uninfested plants. No information on the expression of PR genes in resistant tomato infested by RKNs was previously available, with the exception of a report in which a generic up‐regulation of defence‐related genes was mentioned (Bhattarai et al., 2008). Investigations carried out on interactions between endoparasitic sedentary nematodes and different resistant crops are few and deal only with gene expression in infected roots. In a study on M. arenaria–peanut compatible and incompatible interactions, the largest subset of differentially expressed sequences in roots of the infected resistant cultivar NemaTAM represented defence signal transduction, including the PR‐1 gene (Tirumalaraju et al., 2011). Recent studies have focused on gene expression in roots of soybean and tomato resistant and susceptible to CNs (Mazarei et al., 2011; Uehara et al., 2010). PR‐1(P6) was markedly up‐regulated in infected resistant tomato, whereas several genes belonging to the disease and defence category were up‐regulated in infected resistant soybean; susceptible cultivars did not show the same level of gene up‐regulation. In a study on Mi‐1‐bearing and susceptible tomato attacked by avirulent potato aphids, transcripts of PR‐1 were detected earlier and accumulated at a higher level in the incompatible than compatible response (Martinez de Ilarduya et al., 2003). Therefore, for the first time, our results clearly show that SAR is induced in shoots of resistant plants infected by RKNs. It is likely that root tissue necroses induced by HR occurring in incompatible interactions generate SAR upward‐moving signals. Interestingly, SA‐responsive PR genes were not up‐regulated in roots at this stage of pathogenesis, although it cannot be ruled out that they were at the earliest stages. Over‐production of SA was found in roots and shoots of resistant tomato attacked by M. incognita (Vasyukova et al., 2003); herein, it is indirectly indicated by catalase inhibition. Alternatively, Mi‐1‐mediated resistance has also been reported to take place when the SA level in roots is highly reduced, as in Mi‐1 NahG plants, expressing a bacterial salicylate hydroxylase that degrades SA to catechol (Bhattarai et al., 2008). Therefore, SA generation might be a side‐effect rather than the triggering event of the Mi‐1‐mediated resistance response. However, any increase in SA level in roots is likely to be transient, as it has been found that most SA absorbed by roots is quickly distributed into the leaves (Molinari and Loffredo, 2006). Transfer of root‐synthesized SA, in the free or conjugated form, from infected resistant roots may explain the SAR induction observed in shoots. Conversely, in infected susceptible plants, SA‐dependent SAR in leaves may be suppressed by developing nematodes, as suggested previously by Hamamouch et al. (2011).
Further investigation is needed to gain an insight into SA metabolism influenced by nematode action in plants. The suppression of SA signalling is likely to be of particular relevance for biotrophic pathogens (Glazebook, 2005). Secretion of the enzyme chorismate mutase, which prevents the accumulation of SA, has been indicated as a possible virulence factor of sedentary nematodes (Bekal et al., 2003). Continued investigation will address this topic as the suppression of PR genes is likely to be associated with the active suppression of SA metabolism and signalling on the part of developing nematodes.
Experimental Procedures
Plant material and SA treatment
Seeds of the tomato (Solanum lycopersicon L.) cv. Roma VF, accessions ‘Regina Torre Canne’ and ‘Fiaschetto Torre Guaceto’, collected from growers in Apulia, Italy, all fully susceptible to RKNs, and cv. Motelle, carrying the Mi‐1 gene conferring resistance to RKNs, were used in the experiments. Tomato seeds were surface sterilized and sown in a sterilized mixture of peat and soil at 23–25 °C in a glasshouse. Single tomato seedlings were transplanted into 100‐cm3 clay pots filled with steam‐sterilized river sand and allowed to grow to the fifth to sixth compound leaf stage. Pots were randomly placed on temperature‐controlled benches (soil temperature, 23–25 °C) located in a glasshouse, provided with a regular regime of 12 h of light/day and regularly watered with Hoagland's solution. SA was applied as a soil drench only to the susceptible plants. An aqueous solution of SA was added to each pot in order to set the dosage at 20 mg SA/plant (Molinari and Baser, 2010). Only freshly prepared stock solutions of potassium salicylate (approximately 3 mg/mL, pH 6.0) were employed. Groups of untreated and SA‐treated plants were collected 1, 3 and 5 days after treatment for RNA isolation from shoots and roots.
Nematode inoculation and determination of infestation levels
One avirulent field population (MifieldV) was used in this study to inoculate susceptible and resistant tomato plants; this population was species identified as M. incognita by isozyme electrophoretic patterns of esterase and malate dehydrogenase (Molinari et al., 2005). The field population had been reared previously in a glasshouse on susceptible tomato plants. Invasive J2s were obtained by incubation of EMs in tap water at 27 °C; 3‐day‐old J2s were collected and used for inoculation. Resistant, SA‐treated and untreated susceptible plants were inoculated with 300 J2s per plant. Inoculation of susceptible plants was carried out on day 1 after SA treatment (Molinari and Baser, 2010). Juveniles that penetrated and established into the roots were determined 5 and 15 days after inoculation by the sodium hypochloride–acid fuchsin method under a stereoscope (Byrd et al., 1983). Juveniles were distinguished as still, motile vermiform individuals (second stage, J2s), swollen individuals that had become sedentary (third and fourth stages, SJs) and AFs.
Different factors suitable for the characterization of the level of infestation at the end of the nematode life cycle were detected in plants collected 7 weeks after inoculation. The roots were washed free of soil debris, weighed and chopped into pieces (∼1 cm). Samples (2 g) were immersed in a solution (0.1 g/L) of eosin yellow dye (Roberts et al., 1990) and stored for at least 1 h in a refrigerator. Red‐coloured EMs were then counted under a stereoscope (×6 magnification) and referred to as EMs per gram of root fresh weight. SFs and eggs were extracted from additional samples according to the methods described in Molinari (2009) and counted. SFs present in the roots at this stage included developing SJs, adult and gravid females originating from both the inoculated J2s and a second generation of J2s hatched in pot from the eggs produced by the inoculated J2s. Normally, however, this second generation is not able to reproduce in our system within the experimental time frame used. The numbers of SFs and eggs were expressed per gram of root fresh weight. Values of infestation factors are averages (±SD) obtained from three different biological replicates in which six pots per treatment were employed. Statistical differences in the mean (n = 18) were determined by t‐test with an α level of 0.05.
RNA isolation and qRT‐PCR
Total RNA for qRT‐PCR was isolated from the bulked shoots and root systems of six tomato plants of each biological replicate. The tissue samples were ground in liquid nitrogen using a porcelain mortar. Three biological replicates were completed for SA‐treated plants, 1, 3 and 5 days after treatment, and for nematode‐infected plants, 5 days after inoculation. Untreated and uninfected plants were used as controls. Total RNA was extracted using an RNeasy Plant Mini Kit (Qiagen GmbH, Hilden, Germany) following the manufacturer's instructions, and further treated with RNase‐free DNase I (Qiagen) to eliminate any contaminating genomic DNA. RNA was verified by 1.0% agarose gel electrophoresis and quantified using a NanoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA). First‐strand cDNA was synthesized from 1 μg of total RNA using the QuantiTect Reverse Transcription Kit (Qiagen) with random hexamers, following the manufacturer's instructions. A single 25‐μL PCR included 1 × FastStart SYBR Green Master Mix (Roche Diagnostics, Milan, Italy), 2 μL of cDNA template and 10 μm of each forward and reverse primer. The PCR cycling parameters were as follows: pre‐incubation at 95 °C for 10 min, 40 cycles at 95 °C for 30 s, 58 °C for 30 s and 72 °C for 30 s, and a final extension step at 72 °C for 7 min. The qRT‐PCRs were performed in triplicate using an Mx3000P instrument (Stratagene Corp., La Jolla, CA, USA); the negative controls included water and qPCR Master mix. The specificity of the PCR products was verified by dissociation melting curve analysis after 40 cycles, and agarose gel electrophoresis. The actin gene (BT013524) was used as the reference gene (Correa‐Aragunde et al., 2006). Actin gene expression of tomato tissues has been demonstrated not to vary after SA treatment and nematode infestation. The GenBank accession used for PR‐1 has been described in Uehara et al. (2010) as PR‐1(P6). PR‐1 is actually a small multigenic family; however, the induction of PR‐1(P6) in plants infected by nematodes has been reported to be more consistent than the induction of the homologous gene PR‐1(P4) (Uehara et al., 2010). The primers used were as follows: for the PR‐1 gene (GenBank accession no. Y08804): forward primer, 5′‐GGATCGGACAACGTCCTTAC‐3′; reverse primer, 5′‐GCAACATCAAAAGGGAAATAAT‐3′; for the PR‐2 gene (accession no. NM001247229): forward primer, 5′‐AAGTATATAGCTGTTGGTAATGAA‐3′; reverse primer, 5′‐ATTCTCATCAAACATGGCGAA‐3′; for the PR‐5 gene (accession no. NM001247422): forward primer, 5′‐GCAACAACTGTCCATACACC‐3′; reverse primer, 5′‐AGACTCCACCACAATCACC‐3′. The relative fold change was calculated according to the 2–ΔΔCT method (Livak and Schmittgen, 2001). The means (n = 9) of the control and treated groups were compared by the nonparametric Kolmogorov–Smirnov test (P < 0.05).
Protein extraction and enzyme activity assays
Plants were harvested 5 days after nematode inoculation. Plants from each pot were thoroughly rinsed with tap water. The roots and shoots of plants taken from each treatment (six pots), i.e. SA‐treated and untreated, uninoculated and inoculated susceptible plants and uninoculated and inoculated resistant plants, were separated, dried, weighed and collected on ice. Tissues were then placed in porcelain mortars and reduced to powder through immersion in liquid nitrogen. Three different samples of both powdered roots and shoots were produced from each treatment. Samples were suspended (1:5, w/v) in a grinding buffer consisting of 0.1 m potassium phosphate buffer (pH 6.0) with the protease inhibitor phenylmethanesulphonyl fluoride (PMSF, 1 mm). Suspensions were ground further using a Polytron® PT‐10‐35 (Kinematica GmbH, Lucern, Switzerland). Coarse homogenates were filtered through four layers of gauze and centrifuged at 9000 g for 15 min. Aliquots of supernatants from each sample were filtered through 0.45‐μm nitrocellulose filters applied to 10‐mL syringes. These filtrates were ultrafiltered at 4 °C through 2‐mL Vivaspin microconcentrators (ultrafiltration membranes, 10 000 molecular weight cut‐off; Sartorius Stedim Biotech GmbH, Goettingen, Germany). Retained protein suspensions were used for enzyme assays. Detection of protein content was carried out by the enhanced alkaline copper protein assay with bovine serum albumin as the standard (Lowry et al., 1951). The catalase activity of tissue extracts was measured as the initial rate of disappearance of hydrogen peroxide (Chance and Mahley, 1955), using 20 mm H2O2 and 20 μL tissue extracts (about 0.05 mg protein for roots and 0.1 mg protein for shoots) in 0.1 m sodium phosphate, pH 7.0 (final volume, 0.5 mL); the rate of H2O2 disappearance was observed as a decrease in the absorbance at 240 nm, and the oxidation of 1 mmol H2O2/min (ε = 0.038 mm −1 cm−1) represented one unit of enzyme. β‐1,3‐Endoglucanase (glucanase) was measured by determining the amount of glucose released from laminarin (Sigma Chemical Company, Milano, Italia) used as substrate. Laminarin (0.4 mg) and 20 μL of tissue extracts (about 0.05 mg protein for roots and 0.1 mg protein for shoots) were added to 400 μL of 0.1 m sodium acetate (pH 5.2), and the mixture was incubated at 37 °C for 30 min. The mixtures were then added to 0.3 mL of Nelson alkaline copper reagent and kept at 100 °C for 10 min. All samples were then assayed for reducing sugars by the Nelson method (Ashwell, 1957); the results were expressed as μmoles of glucose equivalents released per minute based on a standard curve created with established amounts (10–200 μg/mL) of commercial glucose (Sigma Chemical Company). Assays were provided with negative (grinding buffer) and positive (laminarinase, 2 U/mL) controls.
Catalase and glucanase activities were expressed as enzyme units per milligram protein and per gram of tissue fresh weight. Data show the relative enzyme activities of inoculated relative to uninoculated plants. Values less or more than unity indicate inhibition or activation, respectively, of the relative enzyme activity as a result of nematode infestation 5 days after inoculation. Statistical differences in the mean (n = 9) were determined by the Kolmogorov–Smirnov test (P < 0.05).
Acknowledgements
This research was carried out within the project CISIA, sub‐project: OR3 SOS‐POM, funded by the National Research Council (CNR) of Italy. The authors wish to thank Yole M. DeBellis, native tongue English copyeditor and language consultant of the University of Bari, for the revision of the manuscript.
References
- Ashwell, G. (1957) Colorimetric analysis of sugars. Methods Enzymol. 3, 73–105. [Google Scholar]
- Bekal, S. , Niblack, T.L. and Lambert, K.N.A. (2003) A chorismate mutase from the soybean cyst nematode Heterodera glycines shows polymorphisms that correlate with virulence. Mol. Plant–Microbe Interact. 16, 439–446. [DOI] [PubMed] [Google Scholar]
- Bhattarai, K.K. , Xie, Q.G. , Mantelin, S. , Bishnoi, U. , Girke, T. , Navarre, D.A. and Kaloshian, I. (2008) Tomato susceptibility to root‐knot nematodes requires an intact jasmonic acid signaling pathway. Mol. Plant–Microbe Interact. 21, 1205–1214. [DOI] [PubMed] [Google Scholar]
- Blok, V.C. , Jones, J.T. , Phillips, M.S. and Trudgill, D.L. (2008) Parasitism genes and host range disparities in biotrophic nematodes: the conundrum of polyphagy versus specialisation. BioEssays, 30, 249–259. [DOI] [PubMed] [Google Scholar]
- Bowling, S.A. , Guo, A. , Cao, H. , Gordon, A.S. , Klessig, D.F. and Dong, X. (1994) A mutation in Arabidopsis thaliana that leads to constitutive expression of systemic acquired resistance. Plant Cell, 6, 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch, C. , Hwang, C.F. , Navarre, D.A. and Williamson, V.M. (2004) Salicylic acid is part of the Mi‐1‐mediated defense response to root‐knot nematode in tomato. Mol. Plant–Microbe Interact. 17, 351–356. [DOI] [PubMed] [Google Scholar]
- Byrd, D.W., Jr , Kirkpatrick, T. and Barker, K.R. (1983) An improved technique for clearing and staining plant tissue for detection of nematodes. J. Nematol. 15, 142–143. [PMC free article] [PubMed] [Google Scholar]
- Cao, H. , Bowling, S.A. , Gordon, A.S. and Dong, X. (1994) Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell, 6, 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance, B. and Mahley, A.C. (1955) Assay of catalases and peroxidases In: Methods in Enzymology, Vol. 2 (Colowick S.P. and Kaplan N.O., eds), pp. 764–775. New York: Academic Press. [Google Scholar]
- Correa‐Aragunde, N. , Graziano, M. , Chevalier, C. and Lamattina, L. (2006) Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J. Exp. Bot. 57, 581–588. [DOI] [PubMed] [Google Scholar]
- Durrant, W.E. and Dong, X. (2004) Systemic acquired resistance. Annu. Rev. Phytopathol. 42, 185–209. [DOI] [PubMed] [Google Scholar]
- Gheysen, G. and Fenoll, C. (2002) Gene expression in nematode feeding sites. Annu. Rev. Phytopathol. 40, 191–219. [DOI] [PubMed] [Google Scholar]
- Glazebook, J. (2005) Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43, 205–227. [DOI] [PubMed] [Google Scholar]
- Gutjahr, C. and Paszkowski, U. (2009) Weights in the balance: jasmonic acid and salicylic acid signaling in root–biotroph interactions. Mol. Plant–Microbe Interact. 22, 763–772. [DOI] [PubMed] [Google Scholar]
- Hamamouch, N. , Li, C. , Seo, P.J. , Park, C. and Davis, E.L. (2011) Expression of Arabidopsis pathogenesis‐related genes during nematode infection. Mol. Plant Pathol. 12, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jammes, F. , Lecomte, P. , de Almeida‐Engler, J. , Bitton, F. , Martin‐Magniette, M.L. , Renou, J.P. , Abad, P. and Favery, B. (2005) Genome‐wide expression profiling of the host response to root‐knot nematode infection in Arabidopsis . Plant J. 44, 447–458. [DOI] [PubMed] [Google Scholar]
- Jones, M.G.K. (1981) The development and function of plant cells modified by endoparasitic nematodes In: Plant Parasitic Nematodes, Vol. III (Zuckerman B.M. and Rhode R.A., eds), pp. 255–280. New York: Academic Press. [Google Scholar]
- Kyndt, T. , Nahar, K. , Haegeman, A. , De Vleesschauwer, D. , Höfte, M. and Gheysen, G. (2012) Comparing systemic defence‐related gene expression changes upon migratory and sedentary nematode attack in rice. Plant Biol. 14, 73–82. [DOI] [PubMed] [Google Scholar]
- Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real time quantitative PCR and the 2–ΔΔCT method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lowry, O.H. , Rosebrough, N.J. , Farr, A.L. and Randall, R.J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- Martinez de Ilarduya, O. , Xie, Q. and Kaloshian, I. (2003) Aphid‐induced defense responses in Mi‐1‐mediated compatible and incompatible tomato interactions. Mol. Plant–Microbe Interact. 16, 699–706. [DOI] [PubMed] [Google Scholar]
- Mazarei, M. , Liu, W. , Al‐Ahmad, H. , Arelli, P.R. , Pantalone, V.R. and Stewart Jr, C.N. (2011) Gene expression profiling of resistant and susceptible soybean lines infected with soybean cyst nematode. Theor. Appl. Genet. 123, 1193–1206. [DOI] [PubMed] [Google Scholar]
- Melillo, M.T. , Leonetti, P. , Bongiovanni, M. , Castagnone‐Sereno, P. and Bleve‐Zacheo, T. (2006) Modulation of ROS activities and H2O2 accumulation during compatible and incompatible tomato/root‐knot nematode interactions. New Phytol. 170, 501–512. [DOI] [PubMed] [Google Scholar]
- Molinari, S. (2007) New developments in understanding the role of salicylic acid in plant defence. CAB Rev. 2, 1–10. [Google Scholar]
- Molinari, S. (2008) Salicylic acid as an elicitor of resistance to root‐knot nematodes in tomato. Acta Hort. 789, 119–126. [Google Scholar]
- Molinari, S. (2009) Bioassays on plant–nematode interactions In: Plant Bioassays (Narwal S.S., Sampietro D.A., Catalàn C.A.N., Vattuone M.A. and Politycka B., eds), pp. 293–326. Enfield, CT: Science Publisher. [Google Scholar]
- Molinari, S. (2011) Natural genetic and induced plant resistance, as a control strategy to plant‐parasitic nematodes alternative to pesticides. Plant Cell Rep. 30, 311–323. [DOI] [PubMed] [Google Scholar]
- Molinari, S. and Abd‐Elgawad, M.M. (2007) Catalase inhibition as a biochemical marker of resistance to root‐knot nematodes in tomato. Nematol. Medit. 35, 237–242. [Google Scholar]
- Molinari, S. and Baser, N. (2010) Induction of resistance to root‐knot nematodes by SAR elicitors in tomato. Crop Prot. 29, 1354–1362. [Google Scholar]
- Molinari, S. and Loffredo, E. (2006) The role of salicylic acid in defense response of tomato to root‐knot nematodes. Physiol. Mol. Plant Pathol. 68, 69–78. [Google Scholar]
- Molinari, S. , Lamberti, F. , Crozzoli, R. , Sharma, S.B. and Sanchez Portales, L. (2005) Isozyme patterns of exotic Meloidogyne spp. populations. Nematol. Medit. 33, 61–65. [Google Scholar]
- Nandi, B. , Kundu, K. , Banerjee, N. and Sinha Babu, S.P. (2003) Salicylic acid‐induced suppression of Meloidogyne incognita infestation of okra and cowpea. Nematology, 5, 747–752. [Google Scholar]
- Owen, K.J. , Green, C.D. and Deverall, B.J. (2002) A benzothiadiazole applied to foliage reduces development and egg deposition by Meloidogyne spp. in glasshouse‐grown grapevine roots. Australas. Plant Pathol. 31, 47–53. [Google Scholar]
- Paulson, R.E. and Webster, J.M. (1972) Ultrastructure of the hypersensitive reaction in roots of tomato Lycopersicon esculentum L., to infection by the root‐knot nematode, Meloidogyne incognita . Physiol. Plant Pathol. 2, 227–232. [Google Scholar]
- Roberts, P.A. , Dalmasso, G.B. , Cap, G.B. and Castagnone‐Sereno, P. (1990) Resistance in Lycopersicon peruvianum to isolates of Mi gene‐compatible Meloidogyne populations. J. Nematol. 22, 585–589. [PMC free article] [PubMed] [Google Scholar]
- Sanz‐Alférez, S. , Mateos, B. , Alvarado, R. and Sanchez, M. (2008) SAR induction in tomato plants is not effective against root‐knot nematode infection. Eur. J. Plant Pathol. 120, 417–425. [Google Scholar]
- Tirumalaraju, S.V. , Jain, M. and Gallo, M. (2011) Differential gene expression in roots of nematode‐resistant and ‐susceptible peanut (Arachis hypogea) cultivars in response to early stages of peanut root‐knot nematode (Meloidogyne arenaria) parasitization. J. Plant Physiol. 168, 481–492. [DOI] [PubMed] [Google Scholar]
- Traversa, A. , Molinari, S. and Loffredo, E. (2012) Quantitative aspects of the interaction between chemical activators of systemic acquired resistance in plants and humic acids. Agrochimica, 56, 112–119. [Google Scholar]
- Uehara, T. , Sugiyama, S. , Matsura, H. , Arie, T. and Masuta, C. (2010) Resistant and susceptible responses in tomato to cyst nematode are differentially regulated by salicylic acid. Plant Cell Physiol. 51, 1524–1536. [DOI] [PubMed] [Google Scholar]
- Van Kan, J.A.L. , Cozijnsen, T. , Danhash, N. and De Wit, P.J.G.M. (1995) Induction of tomato stress protein mRNAs by ethephon, 2,6‐dichloroisonicotinic acid and salicylate. Plant Mol. Biol. 27, 1205–1213. [DOI] [PubMed] [Google Scholar]
- Van Loon, L.C. , Rep, M. and Pieterse, C.M.J. (2006) Significance of inducible defense‐related proteins in infected plants. Annu. Rev. Phytopathol. 44, 135–162. [DOI] [PubMed] [Google Scholar]
- Vasyukova, N.I. , Zinov'eva, S.V. , Udalova, Z.V. , Panina, Y.S. and Ozeretskovskaya, O.L. (2003) The role of salicylic acid in systemic resistance of tomato to nematodes. Dokl. Biol. Sci. 391, 343–345. [DOI] [PubMed] [Google Scholar]
- Vieria dos Santos, M.C. , Curtis, R.H.C. and Abrantes, I. (2013) Effect of plant elicitors on the reproduction of the root‐knot nematode Meloidogyne chitwoodi on susceptible hosts. Eur. J. Plant Pathol. 136, 193–202. [Google Scholar]
- Walters, D.R. (2010) ) Induced resistance: destined to remain on the sidelines of crop protection? Phytoparasitica, 38, 1–4. [Google Scholar]
- Williamson, V.M. (1998) Root‐knot nematodes resistance genes in tomato and their potential for future use. Annu. Rev. Phytopathol. 36, 277–293. [DOI] [PubMed] [Google Scholar]
- Williamson, V.M. and Gleason, C.A. (2003) Plant–nematode interactions. Curr. Opin. Plant Biol. 6, 327–333. [DOI] [PubMed] [Google Scholar]
- Williamson, V.M. and Roberts, P.A. (2009) Mechanisms and genetics of resistance In: Root‐Knot Nematodes (Perry R.N., Moens M. and Starr J.L., eds), pp. 301–325. Wallingford, Oxfordshire: CAB International. [Google Scholar]
- Wubben, M.J.E. , Jin, J. and Baum, T.J. (2008) Cyst nematode parasitism of Arabidopsis thaliana is inhibited by salicylic acid (SA) and elicits uncoupled SA‐independent pathogenesis‐related gene expression in roots. Mol. Plant–Microbe Interact. 21, 424–432. [DOI] [PubMed] [Google Scholar]


