Summary
Many plant‐pathogenic bacteria suppress pathogen‐associated molecular pattern (PAMP)‐triggered immunity by injecting effector proteins into the host cytoplasm during infection through the type III secretion system (TTSS). This type III secretome plays an important role in bacterial pathogenicity in susceptible hosts. Xanthomonas axonopodis pv. manihotis (Xam), the causal agent of cassava bacterial blight, injects several effector proteins into the host cell, including TALE1Xam. This protein is a member of the Transcriptional Activator‐Like effector (TALE) protein family, formerly known as the AvrBs3/PthA family. TALE1Xam has 13.5 tandem repeats of 34 amino acids each, as well as two nuclear localization signals and an acidic activation domain at the C‐terminus. In this work, we demonstrate the importance of TALE1Xam in the pathogenicity of Xam. We use versions of the gene that lack different domains in the protein in structure–function studies to show that the eukaryotic domains at the 3′ end are critical for pathogenicity. In addition, we demonstrate that, similar to the characterized TALE proteins from other Xanthomonas species, TALE1Xam acts as a transcriptional activator in plant cells. This is the first report of the identification of a TALE in Xam, and contributes to our understanding of the pathogenicity mechanisms employed by this bacterium to colonize and cause disease in cassava.
Introduction
Plant‐pathogenic bacteria have developed diverse strategies to colonize and cause disease in a wide range of host plants. Their pathogenicity mainly depends on an arsenal of effector proteins that are secreted and translocated to the host cell through the type III secretion system (TTSS; Büttner and He, 2009; McCann and Guttman, 2008). Most effector proteins translocated by this system act as suppressors of a general defence response of plants against microbes, called pathogen‐associated molecular pattern (PAMP)‐triggered immunity (PTI; Jones and Dangl, 2006). However, many effectors are recognized in the host because of the evolution of the corresponding resistance (R) genes, constituting a branch of immunity in plants termed effector‐triggered immunity (ETI; Flor, 1971; Jones and Dangl, 2006). When these bacterial effector proteins are recognized, they are classically called avirulence (Avr) proteins, as they betray the presence of the bacterial pathogen to the host immune system. However, in the absence of the corresponding R gene, these effectors can increase bacterial fitness and aggressiveness, and are therefore required for maximal virulence (Block et al., 2008; Boch and Bonas, 2010).
Many effector proteins have been identified in the plant‐pathogenic species of the genus Xanthomonas. One of the best characterized is the family of effectors called the AvrBs3/PthA (avirulence and pathogenicity) protein family. These were recently designated as TAL effectors (TALEs), for Transcriptional Activator‐Like proteins, because they modulate gene expression in the host, acting like transcription factors (Boch and Bonas, 2010; Bogdanove et al., 2010; Kay et al., 2007). AvrBs3, the founder member of the family, has been characterized extensively in Xanthomonas euvesicatoria (Xeu), where it plays an important role in virulence of the pathogen and recognition by the host ETI system (Marois et al., 2002; Minsavage et al., 1990). At present, more than 40 proteins belonging to this conserved family have been identified from different species and pathovars of Xanthomonas and Ralstonia solanacearum (Heuer et al., 2007; Schornack et al., 2006; White et al., 2009). The intriguing characteristic of this family is the presence of nearly identical tandem repeats of 34 or 35 amino acids in the central region of the protein. The C‐terminal region of TALE proteins contains typical eukaryotic features, such as monopartite nuclear localization signals (NLSs) and a transcriptional acidic activation domain (AAD), the function of which is essential for both virulence and avirulence activities (Szurek et al., 2001; Yang et al., 2000). The NLSs are crucial for the interaction of AvrBs3 with the protein importin‐α from pepper, indicating that the AvrBs3‐like proteins recruit the host machinery to reach the nucleus (Szurek et al., 2001). In addition, mutations in the NLSs or AADs of AvrBs3 abolish the recognition by the R gene Bs3 in the host plant (Szurek et al., 2001). Once inside the nucleus, TALE proteins regulate the transcription of specific genes, as first demonstrated for AvrBs3 (Kay et al., 2007) and, more recently, for a few other members of the protein family (Boch et al., 2009; Römer et al., 2010). AvrBs3 activates the transcription of at least one gene involved in cell growth and proliferation, inducing developmental reprogramming in host cells by binding and activating its specific promoter target in pepper (Kay et al., 2007). In the case of the incompatible interaction, this same protein induces the expression of the corresponding R gene Bs3 (Römer et al., 2007). Most importantly, the amino acid nucleotide sequence code that defines the target DNA sequences of TALEs in their hosts has been deciphered recently (Boch et al., 2009). The variable number of repeats in the central region of this family of proteins, ranging between 1.5 and 28.5 (Boch and Bonas, 2010), as well as the specific sequence of the variable amino acids 12 and 13 in each repeat, are responsible for the binding to specific boxes in the promoters of host target genes (Boch et al., 2009; Moscou and Bogdanove, 2009).
Several TALE proteins have a strong effect in bacterial pathogenesis and physiological changes in susceptible hosts. For example, PthA from Xanthomonas axonopodis pv. citri (Xac) is necessary to cause citrus canker disease symptoms, including cell hypertrophy, hyperplasia and cell death (Brunings and Gabriel, 2003; Duan et al., 1999). Furthermore, Domingues et al. (2010) demonstrated that PthA and its variants PthA2 and PthA3 target citrus proteins involved in ubiquitination pathways and DNA repair. Likewise, a role in mRNA stability and translational regulation was proposed for PthA4, which targets citrus DNA‐ or RNA‐binding proteins involved in the remodelling of chromatin and mRNA modifications (de Souza et al., 2012).
Xanthomonas oryzae pv. oryzae (Xoo) also encodes several TALE family members whose inactivation results in severely reduced symptom development and bacterial growth. This is the case for pthXo1, which induces the rice susceptibility promoter Os8N3 (Yang et al., 2006), and avrXa7 and talC, which promote the expression of the Os11N3 promoter (Römer et al., 2010; Yu et al., 2011). In addition, the genes pthXo6 and pthXo7 induce the expression of OsTFX1 and OsTFIIAγ1, which encode for rice transcription factors (Sugio et al., 2007). Variations in these upregulated TALE (UPT) boxes account for the level of susceptibility and resistance of a particular rice cultivar to this bacterial pathogen, suggesting that the recognition of target sequences in the host is very specific, and mutations of these promoters facilitate the development of disease resistance in the plant. Indeed, impaired induction of the R gene xa13, the recessive allele of Os8N3, is the result of a substitution in the gene promoter (Chu et al., 2004; Yang et al., 2006).
The specific recognition of sequence targets by TALE proteins provides new means for the design of mechanisms of durable resistance. Indeed, Römer et al. (2009) demonstrated that a chimeric promoter region combining UPT boxes recognized by TALE proteins from different pathogens retains specificity for each of them, opening up the possibility of engineering R genes capable of recognizing multiple TALE proteins. Recent studies have demonstrated the ability of engineered TALE nucleases (TALENs) to generate double‐strand DNA breaks, which represents an accurate genetic tool to introduce site‐specific modifications in eukaryotic genes (Mahfouz et al., 2011, 2012). Moreover, the applications of TALEN modules for engineering modifications in plant genomes to generate resistance to plant diseases have been reported (Li et al., 2012).
Xanthomonas axonopodis pv. manihotis (Xam) is the causal agent of cassava bacterial blight, the most important bacterial disease affecting this crop (Lozano, 1986). The disease is distributed worldwide, causing losses that range between 12% and 100% in affected fields (Lozano, 1986). Xam is a foliar and vascular pathogen, capable of inducing a wide range of symptoms, including angular leaf spots, blight, shoot wilt, dieback, stem cankers, gum exudations and vascular necrosis (Lozano and Sequeira, 1974). Bacterial penetration of the leaf tissue takes place through natural openings (stomatal apertures) or wounds (Verdier et al., 2004). Xam multiplies in the intercellular spaces at the expense of surrounding cells and moves systemically through the xylem, causing the typical shoot wilt symptoms (Verdier et al., 2004).
We have shown previously that the pathogenicity of Xam is associated with a plasmid (p44) that is widely distributed among Xam strains (Verdier, 1988). A region of 8 kb was missing from the plasmid in a nonpathogenic Xam strain CFBP1851Δp44 (previously named ORST4), suggesting its role in pathogenicity (Verdier, 1988). This 8‐kb region was contained within a 12‐kb HindIII plasmid fragment (psF2; Verdier et al., 1996). A restriction map for the plasmid fragment psF2 was established and partial sequence analysis allowed the generation of a polymerase chain reaction (PCR)‐based assay for the specific detection of Xam pathogenic strains (Verdier et al., 1998). In addition, DNA probes originated from p44 were developed for Xam and were used to assess the population diversity in different regions worldwide, mainly South America (Gonzalez et al., 2002; Restrepo et al., 1999, 2000a, b; Restrepo and Verdier, 1997).
PthBXam, which is contained within the psF2 plasmid fragment, encodes a protein belonging to the TALE family in Xam; this effector has been renamed as TALE1Xam. A conclusive demonstration of the role of TALE1Xam in the pathogenicity of Xam on cassava is still lacking, as is an exhaustive study of the structural and functional characteristics of this gene. Here, we demonstrate the importance of TALE1Xam as a pathogenicity factor for this bacterium, assess the importance of the eukaryotic domains in bacterial fitness and disease development, and determine its role as a transcriptional activator in plant cells.
Results
The tale1Xam locus on the p44 plasmid encodes a TALE protein
A 10 736‐bp fragment encompassed in the psF2 fragment of p44 was sequenced. Within this fragment, we detected TALE1Xam of 3099 bp in length. Several bioinformatic analyses were carried out to analyse and characterize the tale1Xam region. TALE1Xam contains a central repeat region composed of 13.5 repeats; it also contains two NLSs, with a motif K–[KR]–X–[KR], and a transcriptional AAD, with a motif T–V–M–x–E–Q–D–[EA]–[DA]–P–F–A–G–A–A–D–D–F–P–A–F–N–E(3), at the C‐terminus (Fig. 1a). The flanking sequences were analysed to detect possible rearrangements, and revealed the presence of several repetitive sequences in the region: (i) two direct imperfect repeats of 1300 bp flanking tale1Xam; (ii) two 200‐bp‐long tandem repeats; and (iii) eight inverted repeats/palindromic sequences upstream and downstream of the tale1Xam gene. An insertion element belonging to the Tn3 family and encoding a putative resolvase element is present downstream of tale1Xam (Fig. 1b). The presence of various repetitive sequences and the resolvase flanking TALE1Xam, which have also been detected in members of this family in other Xanthomonas species (Kim et al., 2006; Shiotani et al., 2007), suggest that this region is highly prone to horizontal transfer and recombinational events which might lead to the generation of gene homologues.
Figure 1.
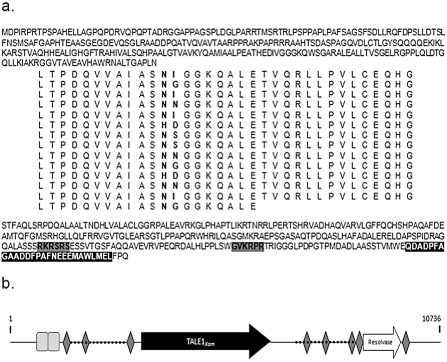
Analysis of the TALE1Xam locus in the p44 plasmid of Xanthomonas axonopodis pv. manihotis (Xam) strain CFBP1851. (a) Amino acid sequence of TALE1Xam. The hypervariable amino acids at positions 12 and 13 in each repeat are shown in bold. Nuclear localization signals (NLSs) and the acidic activation domain (AAD) are highlighted in grey and black, respectively. (b) Schematic analysis of the HindIII region containing the tale1Xam gene in p44. Dotted lines indicate the 1.3‐kb nearly perfect direct repeats and the upstream squares indicate the 240‐bp‐long tandem repeats. Diamonds indicate the inverted repeat (IR) regions. tale1Xam and putative resolvase genes are indicated by the black and white arrows, respectively.
Phylogenetic relationships among TALEs
Phylogenetic relationships of TALE1Xam and other members of the TALE family were established. Maximum parsimony, maximum likelihood and Bayesian analysis were used to increase the robustness of the approach, and the central repeat region was excluded because of ambiguities in the alignment. TALE1Xam groups with PthC and PthB, both from Xanthomonas citri pv. aurantifollii (Xcau; Fig. 2). The diversity of the TALE was divided into two large clades: the X. oryzae clade, which includes TALEs from X. oryzae pv. oryzae (Xoo) and X. oryzae pv. oryzicola (Xoc), and the X. axonopodis/campestris clade, which contains Xeu and Xac TALEs. In general, TALEs from the same pathovar belong to the same clade, except for Avrb6 and PthN from Xanthomonas campestris pv. malvacearum (Xcm), which cluster in the X. axonopodis and X. oryzae clades, respectively. TALE1Xam shares ancestry with TALEs in the Xoc–Xoo clade, which suggests that TALE1Xam could have recently diverged from this clade of effectors. The fact that TALE1Xam does not group with the campestris/axonopodis clade again suggests horizontal gene transfer (HGT) events. Notably, when compared with the C‐terminus, the N‐terminus shows less variation among TALEs. This may be a result of selection intrinsic to the function fulfilled by these two regions, where the N‐terminal region of TALEs is necessary for secretion and translocation, and the C‐terminus plays an important role for import into the host nucleus and for transcriptional activation. Alternatively, the different phylogenetic signals between the N‐ and C‐termini may suggest that this difference in variation could be a result of HGT and/or recombination.
Figure 2.

Evolutionary relationships of 30 Transcriptional Activator‐Like effector (TALE) family members. The relationships among the different TALEs were inferred using three approaches: maximum parsimony, maximum likelihood and Bayesian analysis. Maximum parsimony analysis resulted in eight trees (tree length, 1514) supported by bootstrap analysis (1000 replicates); only 725 characters were informative. Maximum likelihood was inferred using the model K81uf+G selected in ModelTest 3.7 (log L = −9489.03). Bayesian analysis was run with default parameters, resulting in a mean ln L = −9517.65. All the phylogenies were congruent under Kishino–Hasegawa and Shimodaira–Hasegawa tests (P < 0.05). The values on the branches represent bootstrap values for maximum parsimony and maximum likelihood, and posterior probabilities for Bayesian analysis. TALE genes used in this analysis are listed in Table S1.
We evaluated both termini independently using a partition homogeneity test. When the phylogenetic signals obtained from C‐ and N‐termini were compared, a significant difference (P < 0.05) was observed, suggesting an HGT event. In addition, we performed an evaluation of the maximum likelihood tree topologies for both terminal regions independently using the Kishino–Hasegawa and Shimodaira–Hasegawa tests. Both tests showed significant differences (P < 0.05; data not shown), suggesting that the tree topologies were incompatible for C‐ and N‐terminal regions. From these analyses, we make the assumption that a recombination event may have taken place between the two terminal regions of these TALEs, either by HGT or by other mechanisms.
TALE1Xam restores pathogenicity to the nonpathogenic strain CFBP1851Δp44
Southern blot analyses indicated that the region of 8 kb missing from the p44 plasmid in the nonpathogenic Xam strain CFBP1851Δp44 encompasses TALE1Xam, suggesting its role in pathogenicity (Fig. S1, see Supporting Information). To determine whether the loss of pathogenicity observed in CFBP1851Δp44 with respect to the wild‐type (WT) strain CFBP1851 was indeed caused by the lack of tale1Xam, a genetic complementation assay was carried out. Transformation of strain CFBP1851Δp44 with the full‐length tale1Xam gene cloned into pBAV226 (Vinatzer et al., 2006) resulted in the restoration of pathogenicity on the susceptible cassava line HMC‐1 (Fig. 3a), indicating that the nonpathogenic phenotype of CFBP1851Δp44 is caused by the loss of tale1Xam in the deleted 8‐kb region of the plasmid. The development of characteristic disease symptoms on cassava plants was also observed after stem inoculation with the complemented strain (Fig. S2, see Supporting Information).
Figure 3.

TALE1Xam restores the pathogenicity of the nonpathogenic strain CFBP1851Δp44, as well as its ability to growth and move systemically. (a) Disease symptoms observed on the cassava susceptible cultivar HMC‐1 at 8 days post‐inoculation with Xanthomonas axonopodis pv. manihotis (Xam) pathogenic strain CFBP1851, nonpathogenic strain CFBP1851Δp44 and its derivative strain complemented with the tale1 Xam gene cloned into the expression vector pBAV226. (b) The growth and movement of CFBP1851, CFBP1851Δp44 and complemented strains through the mid‐vein of the main leaflet of leaves from the cassava cultivar MCol1522 were monitored at 0, 4 and 8 days post‐inoculation at four different sites from the inoculation point. CFU, colony‐forming unit.
To determine whether the complementation with tale1Xam had an impact on bacterial multiplication and systemic movement in planta, bacterial growth was determined at different distances from the inoculation point and at 0, 4 and 8 days post‐inoculation (dpi; Fig. 3b). The WT strain CFBP1851 moved through the central vein and was detected in sites 3 and 4 at 8 dpi. In contrast, the nonpathogenic derivative strain CFBP1851Δp44 was reduced in its ability to grow and move systemically through the vascular system. CFBP1851Δp44 was not detected in sites 3 and 4 at any of the time points tested. However, the complemented strain was able to grow and move at levels comparable with those of the WT CFBP1851 strain (Fig. 3b). These results suggest that the absence of TALE1Xam affects both bacterial growth and bacterial movement in the host plant. Hence, TALE1Xam may play an important role in plant defence suppression at the vascular level, which is an important line of defence in cassava against Xam (Kpemoua, 1995).
The eukaryotic domains of TALE1Xam are essential for pathogenicity in Xam
To test whether the eukaryotic domains at the C‐terminus of TALE1Xam are critical for pathogenicity in Xam, four different versions of the protein (TALE1XamΔNLS1, TALE1XamΔNLS2, TALE1XamΔNLS1/2 and TALE1XamΔAAD expressed from pBAV226) were used to complement CFBP1851Δp44. The resulting strains were inoculated on susceptible cassava plants. The strain complemented with the complete protein induced angular leaf lesions typical of the disease. However, bacteria transformed with any of the four deletion constructs did not induce the disease phenotype of the WT bacterium on host plants (Fig. 4). These results indicate that both AADs and NLSs of TALE1Xam are necessary for Xam CFBP1851 pathogenicity.
Figure 4.

The eukaryotic domains of TALE1Xam are necessary for the pathogenicity of Xanthomonas axonopodis pv. manihotis (Xam). Disease symptoms observed on the cassava susceptible cultivar HMC‐1 at 8 days post‐inoculation (dpi) with Xam pathogenic strain CFBP1851 (a), nonpathogenic strain CFBP1851Δp44 (b) and their derivative strains complemented with TALE1Xam (c), and the deletion derivatives as follows: TALE1Xam with deletions in NLS1 (d), NLS2 (e), both NLSs (f) and the AAD (g). (h) MgSO4 was inoculated as negative control. AAD, acidic activation domain; NLS, nuclear localization signal.
Multiple effector genes have been shown to suppress host defence responses. We tested the ability of TALE1Xam to suppress the hypersensitive response (HR) elicited by a constitutive gain‐of‐function version of the Pto protein (L205D; Rathjen et al., 1999) and a constitutively active mitogen‐activated protein kinase involved in plant defence (MEK2; Jin et al., 2003). TALE1Xam was unable to suppress HR under the tested conditions (data not shown).
TALE1Xam acts as a transcriptional activator in plant cells
To test whether TALE1Xam performs a transcriptional activator function similar to other TALEs, we first predicted the optimal putative target promoter sequence for this protein using the code recently deciphered for TALE repeats (TATAAACAAATCAAT; Boch et al., 2009; Moscou and Bogdanove, 2009). The putative target promoter was cloned upstream of the minimal Bs3 promoter and the β‐glucuronidase (GUS) reporter gene. This construct was transiently co‐transformed into Nicotiana benthamiana leaves with a construct expressing TALE1Xam under the control of the 35S promoter using Agrobacterium‐mediated transient expression. GUS activity was detected only when TALE1Xam was co‐expressed with the GUS construct under the control of the hybrid TALE1Xam target promoter and minimal Bs3 promoter (Fig. 5). No GUS activity was observed when the TALE1Xam target promoter was absent or when TALE1Xam was not co‐expressed. This result suggests that TALE1Xam recognizes and binds to a specific target promoter sequence in the host, activating its transcription.
Figure 5.

TALE1Xam is a transcriptional activator in plant cells. Agrobacterium‐mediated transient expression of β‐glucuronidase (GUS). Nicotiana benthaminana leaves were inoculated with A. tumefaciens strain GV3101 carrying pBAV139 (tale1Xam) (a), pGWB3 (minimal Bs3 promoter + uidA gene) (b), pGWB3 (TALE1Xam putative target + minimal Bs3 promoter + uidA gene) (c) and co‐inoculated with pBAV139 (tale1Xam) and pGWB3 (minimal Bs3 promoter + uidA gene) (d) and pBAV139 (tale1Xam) and pGWB3 (TALE1Xam putative target + minimal Bs3 promoter + uidA gene) (e). Transient expression was evaluated at 48 h post‐inoculation (hpi).
Discussion
TALE1Xam, a new member of the AvrBs3 family, is a determinant of pathogenicity in Xam
Several TALE family members play a role in virulence. Examples of these include pthA in Xac, which is necessary for this pathogen to cause citrus canker disease and is sufficient to cause division, enlargement and death of host cells (Duan et al., 1999). Similarly, the hax genes (hax2–hax4) in Xanthomonas campestris pv. armoraciae (Xca) contribute additively to the aggressiveness of the pathogen in radish Raphanus sativus (Kay et al., 2005). tale1Xam encodes for a new member of the Xanthomonas TALE family and, in this study, it has been found to be a key pathogenicity determinant of Xam in cassava. So far, tale1Xam has not been demonstrated to be an avirulence factor (Restrepo et al., 2000a). It is important to note that most effector proteins from different phytopathogenic bacterial species only partially contribute to pathogenicity because of functional redundancy (Badel et al., 2006; Kvitko et al., 2009). However, we have demonstrated that TALE1Xam is an effector protein crucial for pathogenicity in Xam, as it complements the complete lack of pathogenicity of strain CFBP1851Δp44. This correlates well with the presence of at least one TALE in most pathogenic Xam strains evaluated in previous studies (Gonzalez et al., 2002). Xam strain CFBP1851Δp44 harbours other rearrangements in various genomic locations (R. Bart and B. Staskawicz, University of California, Berkeley, personal communication) which may affect virulence; however, TALE1Xam was clearly demonstrated to be a critical pathogenicity determinant.
Our analysis shows similar phylogenetic relationships between TALEs to those reported in recent studies by Moreira et al. (2010) and Yu et al. (2011). In our study, we used a larger number of genes to include all the functional members of the TALE family known so far. Phylogenetically, the group/cluster containing TALE1Xam grouped with the X. oryzae‐related clade that includes TALEs from Xanthomonas pathovars causing bacterial blight diseases and exhibiting a vascular lifestyle inside their hosts. Notably, TALE1Xam forms a well‐supported clade with TALEs from Xanthomonas species that affect dicot hosts, suggesting that its divergence within the X. oryzae clade could be a result of host adaptation. In general, we observed the maintenance of two major clades of TALEs, consistent with our data which suggest that HGT acts as a diversifying force for this family of effectors. Our hypothesis is supported by the presence of proteins, such as resolvases, which are also detected in the downstream region of other plasmid‐borne TALEs, such as PthB from Xcau, and PthA1, PthA2, PthA3 and PthA4 from Xac (Brunings and Gabriel, 2003; El Yacoubi et al., 2007).
The pathogenicity function of TALE1Xam relies on its eukaryotic domains
Previous studies on AvrBs3 and other members of the TALE family have shown that eukaryotic domains are crucial for both virulence and avirulence activities in the host. In the case of AvrBs3, NLS motifs are critical for the interaction with the host protein importin and for subsequent translocation to the nucleus (Szurek et al., 2001). Mutations in these domains disrupt the nuclear import function, and hence the modulation of the host transcriptome (Szurek et al., 2001; Van den Ackerveken et al., 1996). Likewise, mutations in the same regions in the Xoo proteins AvrXa7 and AvrXa10 impair the recognition by the cognate R genes Xa7 and Xa10, respectively (Yang et al., 2000; Zhu et al., 1999), and reduce the pathogenicity function of AvrXa7 (Ponciano et al., 2004). Deletions of the TALE1Xam eukaryotic domains cause a loss in the pathogenic activity of this protein. This result demonstrates that, as for other TALEs, TALE1Xam must be localized inside the nucleus to bind a specific DNA target sequence and to activate the transcription of certain genes using the host transcriptional machinery. Interestingly, each single monopartite NLS alone is not sufficient for TALE1Xam pathogenicity function in cassava, which suggests that the presence of both NLSs is required for TALE1Xam function. One possible explanation for these observations is that the deletion mutant proteins are unstable. However, we have recently observed that TALE1Xam is an autoactive protein in the yeast two‐hybrid system and all the deletion mutants retain this ability (Gil et al., 2011). This suggests that the proteins are stable, and therefore the most plausible explanation for these observations is that the AADs and NLSs are all required for the pathogenicity function of TALE1Xam.
TALE1Xam functions as a transcriptional activator
TAL‐dependent transcriptional activation of host genes has been demonstrated for several bacterial TALEs to promote disease development. This is the case for Xoo TALEs PthXo1 and AvrXa7, which act as transcription factors modulating the expression of the N3 nodulin family glucose transporters Os8N3 and Os11N3, respectively (also called OsSWEET11 and 14; White and Yang, 2009; Yang et al., 2006; Yu et al., 2011). In addition, Sugio et al. (2007) have demonstrated that PthXo6 induces the expression of the bZIP transcription factor OsTFX1 in rice, contributing to disease susceptibility and full bacterial virulence. As TALE1Xam specifically induces the expression of a reporter gene under the control of the predicted target promoter, we can infer that this bacterial effector has a transcriptional activation function in its host when it recognizes a specific target promoter sequence through the repeat variable di‐residues (RVDs) in the central repeat region of the protein.
The characterization of the tale1Xam gene provides new insights and perspectives in the understanding of the molecular interactions between Xam and cassava. In addition to TALE1Xam, other TALEs are present in the genome of Xam strains, with some strains having at least four to five TALE family members, and the most pathogenic strains carrying at least one copy (Gonzalez et al., 2002). Future studies should aim to determine the importance of other TALE members and to test whether they play major or minor roles in Xam pathogenicity. For example, strain CFBP1851 has an additional member of the TALE family (Fig. S1; Gonzalez et al., 2002) and it would be interesting to determine its role in the virulence of this strain (Gonzalez et al., 2002).
The recent discovery of the code ciphered in the repeat domain from TALE proteins has allowed us to predict the optimal putative target sequences in the host (Fig. 6a). Possible binding sites for TALE1Xam were predicted in the promoter regions (500 bp upstream from the translation start site) using the prediction program talvez (A. L. Pérez‐Quintero et al., unpublished data; http://bioinfo.mpl.ird.fr/cgi‐bin/talvez/talvez.cgi) on the available draft genome of cassava (version Cassava 4, Cassava Genome Project; http://www.phytozome.net/cassava). This program uses positional weight matrices for a given RVD sequence based on known RVD‐DNA specificities (Boch et al., 2009; Deng et al., 2012; Mak et al., 2012; Moscou and Bogdanove, 2009), scores possible binding sites using a log‐likelihood function and performs better than other TALE target prediction programs (Doyle et al., 2012) in control tests using known TAL–DNA interactions (A. L. Pérez‐Quintero et al., unpublished data). Although no gene in cassava contains the optimal target site validated in this work, several genes were found to contain other possible binding sites with prediction scores as high as those obtained for known targets in other systems (Moscou and Bogdanove, 2009; Römer et al., 2010). The top 100 predicted targets comprised a heterogeneous group of genes, with no over‐represented gene ontology terms as determined by singular enrichment analysis using the AgriGO platform (Du et al., 2010). However, some common annotations were found among the predicted targets, including transcription factors (10%) and proteins with hydrolase (7%) or kinase (5%) function (Fig. 6b). Notably, 23% of the predicted targets have no known annotation, suggesting that TALE1Xam interferes with yet unknown host functions. It should be noted that no SWEET genes were present in the top 100 predicted targets, indicating that Xam major virulence TALEs rely on different mechanisms to cause disease than those identified in Xoo. It is possible that phenotypic effects caused in cassava by TALE1Xam are caused by the induction of more than one of the predicted targets, as has been shown in other systems (Boch and Bonas, 2010). In addition, the induction of transcription factors involved in various signalling pathways could produce large‐scale effects on infection. Bioinformatic target predictions may become more accurate as a greater number of cassava varieties are sequenced. In addition, efforts to identify the target(s) of this protein in cassava using transcriptomics are underway.
Figure 6.

Putative target of TALE1Xam in cassava. (a) The putative target sequence in the host was predicted on the basis of the hypervariable amino acids at positions 12 and 13 in each repeat. (b) Pie chart showing the most common annotations for the top 100 predicted targets for TALE1Xam in the cassava genome using the program Talvez. Annotations are based on the closest Arabidopsis homologue and were obtained from phytozome (http://www.phytozome.net).
The origin of TALEs, their diversity in the Xam population and their effect on the evolution of Xam genomes or the virulence of Xam strains are far from being understood. Answers to these and other questions might come from a deeper analysis of Xam genomes, as well as a deeper knowledge of the ecology of cassava bacterial blight disease. Sequencing projects focused on Xam strains with specific geographical origins will also improve our understanding of the interplay between the ecology and adaptation of Xam, and may reveal new targets for disease control.
Experimental Procedures
Bacterial strains, plasmids, media and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. Xam strains CFBP1851 and CFBP1851Δp44 were grown on Luria peptone glucose agar (LPGA) plates and liquid Phi ϕ medium (Link et al., 1997). Bacteria were incubated at 30 °C for 24 h in liquid medium and for 48 h in solid medium. Escherichia coli DH5α was used for all cloning procedures and transformed by electroporation. Media were supplemented with kanamycin (50 μg/mL), gentamycin (10 μg/mL) or tetracycline (10 μg/mL) when appropriate. Bacteria were transformed by electroporation (E. coli, A. tumefaciens and Xam).
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source |
|---|---|---|
| Xanthomonas axonopodis pv. manihotis (Xam) | ||
| CFBP1851 | Wild‐type carrying native plasmid p44 | CFBP |
| CFBP1851Δ p44 | CFPBP1851 derivative nonpathogenic strain carrying an 8‐kb deletion in plasmid p44 | Verdier (1988) |
| Plasmids | ||
| pENTR/D‐TOPO | Gateway™ cloning vector | Invitrogen |
| PDONR207 | Gateway™ cloning vector | Invitrogen |
| pBAV226 | Gateway™ Pseudomomas syringae expression vector (nptII promoter and C‐terminal HA‐tag) | Vinatzer et al. (2006) |
| pBAV139 | Gateway™ binary plant expression vector (35S promoter and C‐terminal HA‐tag) | Vinatzer et al. (2006) |
| pME6010 | Cloning vector | Heeb et al., 2000 |
| pF3 | 5.4‐kb EcoRI fragment from the p44‐kb native plasmid in Xam ligated into pBluescript | Restrepo and Verdier (1997) |
Generation of deletion mutants of TALE1Xam
Deletions in the eukaryotic domains (NLSs 1 and 2, and AAD) from the C‐terminal region of tale1Xam were produced using site‐directed mutagenesis, based on overlap extension polymerase chain reaction (OE‐PCR; Higuchi et al., 1988). For this, all PCRs were carried out using high‐fidelity Platinum Pfx DNA polymerase (Invitrogen, Carlsbad, CA, USA). The flanking 3′ primer contained an AscI restriction site. The N‐terminal domain was amplified from pF3, a pBluescript derivative vector that carries a 5.4‐kb fragment issued from the native plasmid p44 in Xam strain CFBP1851 containing the tale1Xam gene (Restrepo and Verdier, 1997), using oligonucleotides specific for this domain, and adding the CACC sequence at the 5′ end for cloning into pENTR/D‐TOPO (Invitrogen) and an XhoI restriction site at the 3′ end. The different C‐terminal deletion fragments were cloned into pGEM T‐easy. The central repeat region was excised from pF3 with SphI and XhoI, and then ligated with the N‐terminal domain in pENTR/D‐TOPO, previously digested with the same enzymes. Different versions of the C‐terminal fragment were excised from pGEM T‐easy with XhoI and AscI, and then ligated with the repeats and N‐terminal domain in pENTR/D‐TOPO, assembling the full‐length gene versions. The resulting pENTR/D‐TOPO clones were used in a Gateway™ LR cloning reaction in order to introduce the tale1Xam versions into pBAV226, a Gateway™ bacterial expression vector with the nptII promoter (Vinatzer et al., 2006). The tale1Xam deletion mutants were then used for complementation of CFBP1851Δp44. The WT tale1Xam gene was amplified by PCR from pF3 and cloned into pDONR207 vector (Invitrogen). All clones were confirmed by sequencing at Macrogen, Inc. (Seoul, South Korea).
Plant material and inoculation assays
Susceptible cassava plants, from cultivars HMC‐1 (a.k.a. ICA Armenia) and MCol‐1522, were grown in a glasshouse at approximately 25 °C/16 °C (day/night temperatures) with a 12‐h photoperiod. Nicotiana benthamiana plants were grown in a controlled‐environment chamber at 25 °C, 85% relative humidity and with a 16‐h photoperiod. Bacterial suspensions of Xam [optical density (OD) = 1, ∼3 × 109 colony‐forming units (CFU)] were prepared in 10 mm MgSO4 with a 12‐h culture and were subsequently diluted to the appropriate cell concentrations for individual experiments. Young leaves from 1‐month‐old cassava plants were inoculated by placing 20 μL of the bacterial suspension into 2‐mm‐diameter holes previously made on the leaf using a cork borer, as described previously (Restrepo et al., 2000a). Symptom appearance was monitored every day up to 15 dpi.
Quantification of in planta bacterial growth
To study the growth and movement of Xam through the leaf tissue of susceptible cassava plants, the main vein of the main leaflet was pricked using a syringe needle. A 10‐μL drop of an OD = 0.2 (1 × 108 CFU/mL) bacterial suspension was deposited on the wound. The leaflets were sampled at 0, 4 and 8 dpi. The number of bacterial cells in the leaves was determined at the top 4 cm of each leaf, which was cut into four 1‐cm sections, and labelled as sites 1, 2, 3 and 4 (site 1 being the section adjacent to the inoculation point). The leaf pieces were then processed individually by grinding in 100 μL of 10 mm MgSO4. Serial dilutions were made and spread onto LPGA plates. The plates were incubated at 28 °C until single colonies could be counted. The number of CFUs per section (equivalent to about 1 cm2) was counted and standard errors were calculated. The experiment was repeated independently three times.
Agrobacterium‐mediated transcriptional activation assay
To test the transcriptional activation activity of TALE1Xam, Agrobacterium transient expression assay was carried out. The TALE1Xam predicted target was cloned upstream of the minimal Bs3 promoter (positions −344 to −1 from the Bs3 open reading frame in Capsicum annuum cv. ECW‐30R) into the T‐DNA vector pGWB3 (Nakagawa et al., 2007) in order to fuse these elements in frame with the uidA gene. A construct containing only the minimal Bs3 promoter was used as negative control. In addition, the tale1Xam gene was cloned into the binary vector pBAV139 (Vinatzer et al., 2006). After transformation into A. tumefaciens strain GV3101, these constructs were co‐inoculated or inoculated separately into N. benthamiana leaves. After 48 h, the inoculated leaves were collected and subjected to a qualitative GUS assay by vacuum infiltration with the GUS staining solution [50 mm sodium citrate pH 7.0, 0.1 M HCl, 0.2% Tween 20 and 1 mm 5‐bromo‐4‐chloro‐3‐indolyl‐β‐d‐glucuronide (X‐Gluc)]. Leaves were incubated overnight in this solution and subsequently cleared with 100% ethanol.
Bioinformatic analyses
The entire 10 736‐bp sequence encompassing the region containing tale1Xam has been deposited in GenBank (Accession number HQ113297). This replaces the former sequence AF012325 that had been deposited previously. Inverted repeats, tandem repeats and palindromic sequences in tale1Xam were searched using the EMBOSS suite (Sarachu and Colet, 2005). The threshold values were reduced to increase the probability of repeat finding, and the output was analysed based on the size, number and score. Using the IS Finder database (http://www‐is.biotoul.fr/is.html), Blast searches were performed to reveal the presence of elements related to insertion sequences (ISs). The candidate ISs were confirmed with BlastX (parameters by default) against the nonredundant protein database. As a member of the TALE family, TALE1Xam nucleotide and amino acid sequences were compared against nucleotide and protein databases at GenBank (http://www.ncbi.nlm.nih.gov/) using BlastX to identify the members of this family from Xanthomonas species. All the sequences employed for this study are summarized in Table S1 (see Supporting Information).
Phylogenetic analyses
N‐terminal and C‐terminal regions for all the sequences were extracted in two separate multifasta files using the web EMBOSS suite. Nucleotide sequence alignments were constructed without the central repeat region, using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/). A BLOSUM30 matrix was used for sequence comparisons and the alignment was manually revised using MacClade 4.08 (Maddison and Maddison, 2005).
Pairwise distance analysis was carried out using PAUP*4.0b10 (Swofford and Sullivan, 2003). The following approaches were carried out simultaneously for tree construction: neighbour‐joining method (Saitou and Nei, 1987) and maximum parsimony analyses using heuristic searches, both methods employing bootstrap analysis with 1000 replicates in order to determine the reliability of the tree. PhyML was employed for maximum likelihood analysis (Guindon and Gascuel, 2003). Appropriate evolutionary models for use in maximum likelihood analysis were determined for the dataset using ModelTest version 3.7 and selected on the basis of the hierarchical likelihood ratio test (hLRT). Bayesian analysis was performed using the program MrBayes version 3.1.2 (Huelsenbeck and Ronquist, 2001). Trees were visualized and annotated using FigTree version 1.3.1 (Rambaut and Drummond, 2010).
Supporting information
Fig. S1 Southern blot analysis of genomic DNA from Xanthomonas axonopodis pv. manihotis (Xam) strains CFBP1851, CFBP1851Δp44 and CFBP1851Δp44 + TALE1Xam. Total DNA was digested with BamHI and separated on a 1% agarose gel. The blot was hybridized with a 595‐bp probe corresponding to the 3′ end of tale1 Xam.
Fig. S2 TALE1Xam restores the pathogenicity of the nonpathogenic strain CFBP1851Δp44 after inoculation on stems of cassava plants. Disease symptoms observed on cassava plants after inoculations on the stem of the cassava cultivar MCol1522, monitored at 7, 15, 20 and 28 days post‐inoculation, with the Xanthomonas axonopodis pv. manihotis (Xam) pathogenic strain CFBP1851, nonpathogenic strain CFBP1851Δp44 and its derivative strain complemented with the tale1 Xam gene.
Table S1 Members of the Transcriptional Activator‐Like effector (TALE) family used in the phylogenetic analyses.
Acknowledgements
The authors wish to thank C. Boucher [Institut National de la Recherche Agronomique (INRA), Toulouse, France], K. Assigbétsé and G. Cuny [Institut de Recherche pour le Développement (IRD), Montpellier, France] and Joe Tohme [International Center for Tropical Agriculture (CIAT), Cali, Colombia] for support to V. Verdier and for fruitful discussions at the beginning of this work. They would also like to thank R. Michelmore for supporting the work of J. Gil; Doug Dahlbeck and Brian Staskawicz (University of California Berkeley, CA, USA) for the transcriptional activation constructs and Jan Leach for critical reading of the manuscript. This work was funded by grants from the Colombian Administrative Department of Science and Technology Colciencias (Contract no. 264‐2008) and the International Center for Genetic Engineering and Biotechnology (ICGEB, Italy, Contract No. CRP/07/011). The ECOS Nord (Evaluation‐Orientation of the Scientific Cooperation) program supported the scientific exchange through actions C07A09 and C11A02. The Biological Sciences Department and the Faculty of Sciences of Universidad de Los Andes provided support to L. F. Castiblanco. The IRD supported the Jeune Equipe Associee between IRD, Universidad de los Andes and Universidad Nacional.
References
- Badel, J. , Shimizu, R. , Oh, H. and Collmer, A. (2006) A Pseudomonas syringae pv. tomato avrE1/hopM1 mutant is severely reduced in growth and lesion formation in tomato. Mol. Plant–Microbe Interact. 19, 99–111. [DOI] [PubMed] [Google Scholar]
- Block, A. , Li, G. , Fu, Z.Q. and Alfano, J.R. (2008) Phytopathogen type III effector weaponry and their plant targets. Curr. Opin. Plant Biol. 11, 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boch, J. and Bonas, U. (2010) Xanthomonas AvrBs3 family‐type III effectors: discovery and function. Annu. Rev. Phytopathol. 48, 419–436. [DOI] [PubMed] [Google Scholar]
- Boch, J. , Scholze, H. , Schornack, S. , Landgraf, A. , Hahn, S. , Kay, S. , Lahaye, T. , Nickstadt, A. and Bonas, U. (2009) Breaking the code of DNA binding specificity of TAL‐type III effectors. Science, 326, 1509–1512. [DOI] [PubMed] [Google Scholar]
- Bogdanove, A.J. , Schornack, S. and Lahaye, T. (2010) TAL effectors: finding plant genes for disease and defense. Curr. Opin. Plant Biol. 13, 394–401. [DOI] [PubMed] [Google Scholar]
- Brunings, A.M. and Gabriel, D.W. (2003) Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 4, 141–157. [DOI] [PubMed] [Google Scholar]
- Büttner, D. and He, S.Y. (2009) Type III protein secretion in plant pathogenic bacteria. Plant Physiol. 150, 1656–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, Z. , Ouyang, Y. , Zhang, J. , Yang, H. and Wang, S. (2004) Genome‐wide analysis of defense‐responsive genes in bacterial blight resistance of rice mediated by the recessive R gene xa13 . Mol. Genet. Genomics, 271, 111–120. [DOI] [PubMed] [Google Scholar]
- Deng, D. , Yan, C. , Pan, X. , Mahfouz, M. , Wang, J. , Zhu, J.‐K. , Shi, Y. and Yan, N. (2012) Structural basis for sequence‐specific recognition of DNA by TAL effectors. Science, 335, 720–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues, M.N. , De Souza, T.A. , Cernadas, R.A. , De Oliveira, M.L.P. , Docena, C. , Farah, C.S. and Benedetti, C.E. (2010) The Xanthomonas citri effector protein PthA interacts with citrus proteins involved in nuclear transport, protein folding and ubiquitination associated with DNA repair. Mol. Plant Pathol. 11, 663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, E.L. , Booher, N.J. , Standage, D.S. , Voytas, D.F. , Brendel, V.P. , VanDyk, J.K. and Bogdanove, A.J. (2012) TAL Effector‐Nucleotide Targeter (TALE‐NT) 2.0: tools for TAL effector design and target prediction. Nucleic Acids Res. 40, W117–W122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Z. , Zhou, X. , Ling, Y. , Zhang, Z. and Su, Z. (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 38, W64–W70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan, Y.P. , Castaneda, A. , Zhao, G. , Erdos, G. and Gabriel, D.W. (1999) Expression of a single, host‐specific, bacterial pathogenicity gene in plant cells elicits division, enlargement, and cell death. Mol. Plant–Microbe Interact. 12, 556–560. [Google Scholar]
- El Yacoubi, B. , Brunings, A.M. , Yuan, Q. , Shankar, S. and Gabriel, D.W. (2007) In planta horizontal transfer of a major pathogenicity effector gene. Appl. Environ. Microbiol. 73, 1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor, H.H. (1971) Current status of the gene‐for‐gene concept. Annu. Rev. Phytopathol. 9, 275–296. [Google Scholar]
- Gil, J. , Bohórquez, L. , Castiblanco, L. , Bernal, A. and López, C. (2011) La proteína PthB de Xanthomonas axonopodis pv. manihotis es autoactiva en ensayos de doble híbrido. Acta Biol. Colomb. 16, 109–120. [Google Scholar]
- Gonzalez, C. , Restrepo, S. , Tohme, J. and Verdier, V. (2002) Characterization of pathogenic and nonpathogenic strains of Xanthomonas axonopodis pv. manihotis by PCR‐based DNA fingerprinting techniques. FEMS Microbiol. Lett. 215, 23–31. [DOI] [PubMed] [Google Scholar]
- Guindon, S. and Gascuel, O. (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 52, 696–704. [DOI] [PubMed] [Google Scholar]
- Heeb, S. , Itoh, Y. , Nishijyo, T. , Schnider, U. , Keel, C. , Wade, J. , Walsh, U. , O′Gara, F. and Haas, D. (2000) Small, stable shuttle vectors based on the minimal pVS1 replicon for use in Gram‐negative, plant‐associated bacteria. Mol. Plant‐Microbe Interact. 13, 232–237. [DOI] [PubMed] [Google Scholar]
- Heuer, H. , Yin, Y.‐N. , Xue, Q.‐Y. , Smalla, K. and Guo, J.‐H. (2007) Repeat domain diversity of avrBs3‐like genes in Ralstonia solanacearum strains and association with host preferences in the field. Appl. Environ. Microbiol. 73, 4379–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi, R. , Krummel, B. and Saiki, R. (1988) A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 16, 7351–7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck, J. and Ronquist, F. (2001) MrBayes—Bayesian inference of phylogenetic trees. Bioinformatics, 17, 754–755. [DOI] [PubMed] [Google Scholar]
- Jin, H. , Liu, Y. , Yang, K.Y. , Kim, C.Y. , Baker, B. and Zhang, S. (2003) Function of a mitogen‐activated protein kinase pathway in N gene‐mediated resistance in tobacco. Plant J. 33, 719–731. [DOI] [PubMed] [Google Scholar]
- Jones, J.D.G. and Dangl, J.L. (2006) The plant immune system. Nature, 444, 323–329. [DOI] [PubMed] [Google Scholar]
- Kay, S. , Boch, J. and Bonas, U. (2005) Characterization of AvrBs3‐like effectors from a Brassicaceae pathogen reveals virulence and avirulence activities and a protein with a novel repeat architecture. Mol. Plant–Microbe Interact. 18, 838–848. [DOI] [PubMed] [Google Scholar]
- Kay, S. , Hahn, S. , Marois, E. , Hause, G. and Bonas, U. (2007) A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science, 318, 648–651. [DOI] [PubMed] [Google Scholar]
- Kim, J.‐G. , Choi, S. , Oh, J. , Moon, J.S. and Hwang, I. (2006) Comparative analysis of three indigenous plasmids from Xanthomonas axonopodis pv. glycines. Plasmid, 56, 79–87. [DOI] [PubMed] [Google Scholar]
- Kpemoua, K.E. (1995) Etude comparative du développement de Xanthomonas campestris pv. manihotis chez les variétés de manioc sensibles et résistances. Approche histologique, ultrastructurale et cytochimique des mécanismes de la pathogenèse. Nantes: Faculté des Sciences et Techniques, Université de Nantes 1.
- Kvitko, B. , Park, D. , Velásquez, A. , Wei, C. , Russell, A. , Martin, G. , Schneider, D.J. and Collmer, A . (2009) Deletions in the repertoire of Pseudomonas syringae pv. tomato DC3000 type III secretion effector genes reveal functional overlap among effectors. PLoS Pathog. 5, e1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T. , Liu, B. , Spalding, M.H. , Weeks, D.P. and Yang, B. (2012) High‐efficiency TALEN‐based gene editing produces disease‐resistant rice. Nat. Biotech. 30, 390–392. [DOI] [PubMed] [Google Scholar]
- Link, A. , Phillips, D. and Church, G. (1997) Methods for generating precise deletions and insertions in the genome of wild‐type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179, 6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano, J.C. (1986) Cassava bacterial blight: a manageable disease. Plant Dis. 70, 1089–1093. [Google Scholar]
- Lozano, J.C. and Sequeira, L. (1974) Bacterial blight of cassava in Colombia: etiology. Phytopathology, 64, 74–82. [Google Scholar]
- Maddison, D. and Maddison, W. (2005) MacClade 4: Analysis of Phylogeny and Character Evolution, Version 4.08. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Mahfouz, M. , Li, L. , Piatek, M. , Fang, X. , Mansour, H. , Bangarusamy, D. and Zhu, J.‐K. (2012) Targeted transcriptional repression using a chimeric TALE‐SRDX repressor protein. Plant Mol. Biol. 78, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfouz, M.M. , Li, L. , Shamimuzzaman, M. , Wibowo, A. , Fang, X. and Zhu, J.‐K. (2011) De novo‐engineered transcription activator‐like effector (TALE) hybrid nuclease with novel DNA binding specificity creates double‐strand breaks. Proc. Natl. Acad. Sci. USA, 108, 2623–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, A.N.‐S. , Bradley, P. , Cernadas, R.A. , Bogdanove, A.J. and Stoddard, B.L. (2012) The crystal structure of TAL effector PthXo1 bound to its DNA target. Science, 335, 716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois, E. , Van den Ackerveken, G. and Bonas, U. (2002) The Xanthomonas type III effector protein AvrBs3 modulates plant gene expression and induces cell hypertrophy in the susceptible host. Mol. Plant–Microbe Interact. 15, 637–646. [DOI] [PubMed] [Google Scholar]
- McCann, H.C. and Guttman, D.S. (2008) Evolution of the type III secretion system and its effectors in plant–microbe interactions. New Phytol. 177, 33–47. [DOI] [PubMed] [Google Scholar]
- Minsavage, G. , Dahlbeck, D. , Whalen, M. , Kearney, B. , Bonas, U. , Staskawicz, B. and Stall, R.E. (1990) Gene‐for‐gene relationships specifying disease resistance in Xanthomonas campestris pv. Vesicatoria–pepper interactions. Mol. Plant–Microbe Interact. 3, 41–47. [Google Scholar]
- Moreira, L. , Almeida, N. , Potnis, N. , Digiampietri, L. , Adi, S. , da Bortolossi, J., da Silva, A., de Silva, A., de Moraes, F., de Oliveira, J., Souza, R. , Facincani, A. , Ferraz, A. , Ferro, M. , Furlan, L. , Gimenez, D. , Jones, J. , Kitajima, E. , Laia, M. , Leite, R. , Nishiyama, M. , Rodrigues Neto, J. , Nociti, L. , Norman, D. , Ostroski, E. , Pereira, H. , Staskawicz, B. , Tezza, R. , Ferro, J. , Vinatzer, B. and Setubal, J. (2010) Novel insights into the genomic basis of citrus canker based on the genome sequences of two strains of Xanthomonas fuscans subsp. aurantifolii . BMC Genomics, 11, 238–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou, M. and Bogdanove, A. (2009) A simple cipher governs DNA recognition by TAL effectors. Science, 326, 1501. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T. , Kurose, T. , Hino, T. , Tanaka, K. , Kawamukai, M. , Niwa, Y. , Toyooka, K. , Matsuoka, K. , Jinbo, T. and Kimura, T. (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104, 34–41. [DOI] [PubMed] [Google Scholar]
- Ponciano, G. , Linholm, K. , Bai, J. , Cruz, C.V. and Leach, J.E. (2004) Molecular characterization of the avrXa7 locus from Xanthomonas oryzae pv. oryzae field isolates. Physiol. Mol. Plant Pathol. 64, 145–153. [Google Scholar]
- Rambaut, A. and Drummond, A. (2010) FigTree: Tree Figure Drawing Tool. Edinburgh: University of Edinburgh, Institute of Evolutionary Biology. [Google Scholar]
- Rathjen, J.P. , Chang, J.H. , Staskawicz, B.J. and Michelmore, R.W. (1999) Constitutively active Pto induces a Prf‐dependent hypersensitive response in the absence of avrPto . EMBO J. 18, 3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo, S. and Verdier, V. (1997) Geographical differentiation of the population of Xanthomonas axonopodis pv. manihotis in Colombia. Appl. Environ. Microbiol. 63, 4427–4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo, S. , Duque, M. , Tohme, J. and Verdier, V. (1999) AFLP fingerprinting: an efficient technique for detecting genetic variation of Xanthomonas axonopodis pv. manihotis . Microbiology, 145, 107–114. [DOI] [PubMed] [Google Scholar]
- Restrepo, S. , Duque, M.C. and Verdier, V. (2000a) Characterization of pathotypes among isolates of Xanthomonas axonopodis pv. manihotis in Colombia. Plant Pathol. 49, 680–687. [Google Scholar]
- Restrepo, S. , Velez, C.M. and Verdier, V. (2000b) Measuring the genetic diversity of Xanthomonas axonopodis pv. manihotis within different fields in Colombia. Phytopathology, 90, 683–690. [DOI] [PubMed] [Google Scholar]
- Römer, P. , Hahn, S. , Jordan, T. , Strauss, T. , Bonas, U. and Lahaye, T. (2007) Plant pathogen recognition mediated by promoter activation of the pepper Bs3 resistance gene. Science, 318, 645–648. [DOI] [PubMed] [Google Scholar]
- Römer, P. , Recht, S. and Lahaye, T. (2009) A single plant resistance gene promoter engineered to recognize multiple TAL effectors from disparate pathogens. Proc. Natl. Acad. Sci. USA, 106, 20 526–20 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer, P. , Recht, S. , Strauß, T. , Elsaesser, J. , Schornack, S. , Boch, J. , Wang, S. and Lahaye, T. (2010) Promoter elements of rice susceptibility genes are bound and activated by specific TAL effectors from the bacterial blight pathogen, Xanthomonas oryzae pv. oryzae . New Phytol. 187, 1048–1057. [DOI] [PubMed] [Google Scholar]
- Saitou, N. and Nei, M. (1987) The neighbor‐joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425. [DOI] [PubMed] [Google Scholar]
- Sarachu, M. and Colet, M. (2005) wEMBOSS: a web interface for EMBOSS. Bioinformatics, 21, 540–541. [DOI] [PubMed] [Google Scholar]
- Schornack, S. , Meyer, A. , Romer, P. , Jordan, T. and Lahaye, T. (2006) Gene‐for‐gene‐mediated recognition of nuclear‐targeted AvrBs3‐like bacterial effector proteins. J. Plant Physiol. 163, 256–272. [DOI] [PubMed] [Google Scholar]
- Shiotani, H. , Fujikawa, T. , Ishihara, H. , Tsuyumu, S. and Ozaki, K. (2007) A pthA homolog from Xanthomonas axonopodis pv. citri responsible for host‐specific suppression of virulence. J. Bacteriol. 189, 3271–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza, T.A. , Soprano, A.S. , Lira, N.P.V. , Quaresma, A.J.C. , Pauletti, B.A. , Leme, A.F.P. and Benedetti, C.E. (2012) The TAL effector PthA4 interacts with nuclear factors involved in RNA‐dependent processes including a HMG protein that selectively binds poly(U) RNA. PLoS ONE 7, e32305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio, A. , Yang, B. , Zhu, T. and White, F.F. (2007) Two type III effector genes of Xanthomonas oryzae pv. oryzae control the induction of the host genes OsTFIIAγ1 and OsTFX1 during bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 104, 10 720–10 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford, D. and Sullivan, J. (2003) Phylogeny inference based on parsimony and other methods using PAUP* In: The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny (Salemi M. and Vandamme A.‐M., eds), pp. 160–181. Cambridge: Cambridge University Press. [Google Scholar]
- Szurek, B. , Marois, E. , Bonas, U. and Van den Ackerveken, G. (2001) Eukaryotic features of the Xanthomonas type III effector AvrBs3: protein domains involved in transcriptional activation and the interaction with nuclear import receptors from pepper. Plant J. 26, 523–534. [DOI] [PubMed] [Google Scholar]
- Van den Ackerveken, G. , Marois, E. and Bonas, U. (1996) Recognition of the bacterial avirulence protein AvrBs3 occurs inside the host plant cell. Cell, 87, 1307–1316. [DOI] [PubMed] [Google Scholar]
- Verdier, V. (1988) Contribution à l'étude de la variabilité de Xanthomonas campestris pv. manihotis (Arthaud Berthet et Bondar) Starr. agent causal de la bactériose vasculaire du manioc (Manihot esculenta Crantz). PhD thesis, University Paris Sud‐Orsay (ed), Orsay, France, 242p.
- Verdier, V. , Cuny, G. , Assigbete, K. , Geiger, J.P. and Boucher, C. (1996) Characterization of pathogenicity gene pthB in Xanthomonas campestris pv. manihotis In: Biology of plant‐microbe interactions: Proceedings of the 8th International Symposium on Molecular Plant‐Microbe Interactions. (Stacey G., Mullin B. and Gresshoff P.M., eds). Knoxville, TN: International Society for Molecular Plant‐Microbe Interactions. Abstract 671. [Google Scholar]
- Verdier, V. , Mosquera, G. and Assigbetse, K. (1998) Detection of the cassava bacterial blight pathogen, Xanthomonas axonopodis pv. manihotis, by polymerase chain reaction. Plant Dis. 82, 79–83. [DOI] [PubMed] [Google Scholar]
- Verdier, V. , Restrepo, S. , Mosquera, G. , Jorge, V. and Lopez, C. (2004) Recent progress in the characterization of molecular determinants in the Xanthomonas axonopodis pv. Manihotis–cassava interaction. Plant Mol. Biol. 56, 573–584. [DOI] [PubMed] [Google Scholar]
- Vinatzer, B.A. , Teitzel, G.M. , Lee, M.W. , Jelenska, J. , Hotton, S. , Fairfax, K. , Jenrette, J. and Greenberg, J.T. (2006) The type III effector repertoire of Pseudomonas syringae pv. syringae B728a and its role in survival and disease on host and non‐host plants. Mol. Microbiol. 62, 26–44. [DOI] [PubMed] [Google Scholar]
- White, F.F. and Yang, B. (2009) Host and pathogen factors controlling the rice‐Xanthomonas oryzae interaction. Plant Physiol. 150, 1677–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, F.F. , Potnis, N. , Jones, J.B. and Koebnik, R. (2009) The type III effectors of Xanthomonas . Mol. Plant Pathol. 10, 749–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Zhu, W. , Johnson, L.B. and White, F.F. (2000) The virulence factor AvrXa7 of Xanthomonas oryzae pv. oryzae is a type III secretion pathway‐dependent nuclear‐localized double‐stranded DNA‐binding protein. Proc. Natl. Acad. Sci. USA, 97, 9807–9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Sugio, A. and White, F.F. (2006) Os8N3 is a host disease‐susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 103, 10 503–10 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. , Streubel, J. , Balzergue, S. , Champion, A. , Boch, J. , Koebnik, R. , Feng, J. , Verdier, V. and Szurek, B. (2011) Colonization of rice leaf blades by an African strain of Xanthomonas oryzae pv. oryzae depends on a new TAL effector that induces the rice nodulin‐3 Os11N3 gene. Mol. Plant–Microbe Interact. 24, 1102–1113. [DOI] [PubMed] [Google Scholar]
- Zhu, W. , Yang, B. , Wills, N. , Johnson, L.B. and White, F.F. (1999) The C terminus of AvrXa10 can be replaced by the transcriptional activation domain of VP16 from the herpes simplex virus. Plant Cell, 11, 1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Southern blot analysis of genomic DNA from Xanthomonas axonopodis pv. manihotis (Xam) strains CFBP1851, CFBP1851Δp44 and CFBP1851Δp44 + TALE1Xam. Total DNA was digested with BamHI and separated on a 1% agarose gel. The blot was hybridized with a 595‐bp probe corresponding to the 3′ end of tale1 Xam.
Fig. S2 TALE1Xam restores the pathogenicity of the nonpathogenic strain CFBP1851Δp44 after inoculation on stems of cassava plants. Disease symptoms observed on cassava plants after inoculations on the stem of the cassava cultivar MCol1522, monitored at 7, 15, 20 and 28 days post‐inoculation, with the Xanthomonas axonopodis pv. manihotis (Xam) pathogenic strain CFBP1851, nonpathogenic strain CFBP1851Δp44 and its derivative strain complemented with the tale1 Xam gene.
Table S1 Members of the Transcriptional Activator‐Like effector (TALE) family used in the phylogenetic analyses.


