Abstract
Background/Aims:
The prevalent comorbidity between neuropsychiatric and gastrointestinal (GI) disorders is believed to be significantly influenced by gut microbiota (GM). GM may also play a substantial role in comorbidity between substance abuse (e.g. Alcohol Use Disorder, AUD) and depression. The anti-parasitic drug Moxidectin (MOX) has been reported to reduce alcohol intake in male and female mice. This effect is purported to be centrally mediated with a significant contribution linked to purinergic, P2X4 purinergic receptors. However, MOX’s effects on GM in animal models of depression is not known.
Methods:
Adult male Wistar Kyoto (WKY) rats (5/group) were injected intraperitoneally (i.p.) once daily for 7 days with MOX (2.5mg/kg), or saline as control group. On day 8, approximately 20 h after the last MOX injection, animals were sacrificed, intestinal stools were collected and stored at −80°C DNA was extracted from the samples for 16S rRNA gene-based GM analysis using 16S Metagenomics application.
Results:
At taxa and species level, MOX affected a number of bacteria including a 30-fold increase in Bifidobacterium cholerium, a bacterium with a strong ability to degrade carbohydrates that resist digestion in the small intestine. There was a minimum of 2-fold increase in: five probiotic species of Lactobacillus, butyrate-forming Rosburia Facies and Butyrivibro proteovlasticus. In contrast, MOX depleted 11 species, including 2 species of Ruminoccus, which are positively associated with severity of irritable bowel syndrome, and 4 species of Provettela, which are closely associated with depressive-like behavior.
Conclusion:
Thus, MOX enhanced probiotic species, and suppressed the opportunistic pathogens. Since overall effect of MOX appears to be promoting GM associated with mood enhancement (e.g. Bifidobacterium and Lactobacillus) and suppressing GM associated with inflammation (e.g. Ruminoccus), potential antidepressant and anti-inflammatory effects of MOX in suitable animal models should be investigated.
Keywords: Microbiome-gut-brain axis, Dysbiosis, Antiparasitic, Depression, Animal Model, 16S rRNA gene, Metagenomics
Introduction
An imbalance in gut microbiota (GM) composition and diversity, commonly referred to as “dysbiosis”, can change the gut permeability and disrupt the gut-brain axis [1–3]. It is now well recognized that the bidirectional communication between the GM and brain is crucial for a healthy brain and phyisological functions [1–3]. High co-morbidity of gastrointestinal (GI) disorders, such as colitis or inflammatory bowel disease (IBD) and mood disorders, related to depression suggests that dysbiosis may be a common denominator (factor) in these conditions [4–8]. This hypothesis is further supported by studies that show pharmacotherapy of mood disorders may actually be beneficial to GI disorders through GM manipulation [9,10]. Conversely, it is likely that some of the drugs that are used in ulcerative colitis or IBD, would have mood elevating effects as depredepression is frequently associated with immune dysregulation and elevated inflammatory cytokines [11,12].
Moxidectin (MOX), derived from the actinomycete Streptomyces cyanogriseus subsp. Noncyanogenus [13,14] belonging to the milbemycin family, is a third-generation macrocyclic lactone with potent insecticide activity [15–18]. MOX has been used for the treatment of internal and external parasites in cattle, sheep, deer, and horses. Additionally, it was very recently approved in the United States for onchocerciasis (river-blindness) for people over the age of 11. MOX may also have antibacterial effects [19] and preclinical studies suggest its potential use for treatment of alcohol use disorders (AUD) [20,21]. However, practically no data is available on potential GM modifying effect of MOX, particularly in behavioral models.
Wistar Kyoto (WKY) rats, derived from Wistar rats, have been classically used as the control strain for spontaneously hypertensive rats [22]. Additional WKYs experiments showed exaggerated depressive-like behavior in the forced swim test (FST), a learned helplessness test [23–25]. These findings in addition to the ones that showed WKY rats are more susceptible to stress-induced ulcers [26,27], resulted in ascribing the WKY strain as a putative rat model of depression [23–25, 28–31]. Interestingly, the depression-ulcer association in these rats has been attributed to pro-inflammatory release of cytokines, secondary to an exaggerated systemic response to stressors [26,32,33]. Moreover, it was also shown that WKY rats are more likely to drink alcohol voluntarily than their control Wistar rats [34,35]. Thus, this strain may represent a suitable model for studying inter- relationships between stress, depression and alcohol intake. Since GM plays a role in all these conditions and MOX may affect at least some of these behaviors (e.g. alcohol intake), we decided to examine the effects of MOX on GM in WKY rats. Our hypothesis was that bacteria related to mood regulation and/or drinking behavior will be significantly affected by MOX.
Methods
Animals
Age matched, 3 month old adult male Wistar Kyoto (WKY) rats were purchased from Envigo (previously Harlan, Indianapolis, IN, USA) and were housed in a designated room, 2–3 per cage in standard polypropylene shoebox cages (42 × 20.5 × 20 cm) containing hardwood chip bedding (alpha-dry). Throughout the experiment, animals had access to food (Harlan Tek Lab) and water ad libitum. The room was maintained at 24–26 °C at 51–66% relative humidity, on a 12-h light/dark cycle (lights on at 7 am).
In order to acclimate the animals to the housing conditions, animals arrived at least one week prior to initiation of any experiment. During this period, subjects were gently handled in order to minimize any stress effects that might result from routine handling.
Drugs
MOX (10 mg/kg) solution (Boehringer Ingelheim, St. Joseph, MO) was diluted in a 0.9% sodium chloride solution (saline) to a concentration that would allow for an injection volume of 10 mL/kg of body weight at dose of 2.5 mg/kg as described previously [20]. Controls received saline.
Experimental Design
Following one week of acclimation, the animals were randomly divided into two groups, control and experimental (n=5 each), and were housed in separate cages. Animals belonging to the same group were also randomly selected and housed together (2–3 animals/cage). This housing method assured that the animals in both groups were exposed to identical environment and would not be exposed to cross- contamination between the treated vs the control group. The dose of the drug and the number of animals used in each group were based on previous studies [20,36].
Animals were injected intraperitoneally (i.p.) daily (around noon) for 7 consecutive days. On day 8, approximately 20 h after the last MOX or saline injection, the animals were sacrificed by decapitation, alternating between the groups. Colons containing stools were collected, quick-frozen on dry ice and stored at −80°C. This method of rapid freezing is considered best practice for preserving stool DNA samples for further microbial analyses [37].
Stool DNA Extraction
Total DNA was isolated from stool samples using Norgen’s Stool DNA Isolation Kit and the Precellys Dual-24 Homogenizer (Bertin Technologies) as described previously [36]. Purification was based on spin column chromatography using resin as the separation matrix. Briefly, 200 mg stool samples were bead-homogenized after adding 1 mL of Lysis Buffer L. One hundred μL of lysis additive was added and vortexed, followed by centrifugation at 20,000 x g for 5 min. The clear supernatant was transferred (600 μL) to a DNAase- free microcentrifuge tube. Next, the samples were centrifuged and 100 μL of Binding Buffer I was added to the clean supernatant and incubated on ice for 10 minutes. Equal amounts of 70% ethanol were then added to the clean supernatant from Binding Buffer I lysate after centrifugation. The protocol was then followed for complete DNA isolation. The purified DNA was quantified and analyzed for purity using the NanoDrop™ 2000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Twenty μL of purified DNA was then quick-frozen on dry ice and shipped to Norgen Biotek (Thorold, ON, Canada) for 16S rRNA gene analysis.
16S rRNA Gene Sequencing and Analysis
Briefly, the V3-V4 hypervariable region of the bacterial 16S rRNA gene was amplified from 12.5 ng of stool DNA. The amplicons were then cleaned, sequenced according to the Illumina MiSeq 16S Metagenomic Sequencing Library Preparation protocol (http://support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation) [38]. The final library was paired-end sequenced at 2 × 300 bp using a MiSeq Reagent Kit v3 on the Illumina MiSeq platform. For bioinformatic analysis, the sequencing data was analyzed using the Illumina 16S metagenomics app (Illumina 16S Metagenimics Pippeline (v1.0.1) (https://basespace.illumina.com/apps/593593/16S-Metagenomics/perferredversion) [39] which performs taxonomic classification of 16S rRNA targeted amplicons by using an Illumina-curated version of the GeenGenes taxonomic database. The app provides interactive visualization and raw classification output for per-sample and aggregate analyses. Classification was performed using the Illumina 16 S Metagenomics workflow, which is also available in the MiSeq Reporter software. The algorithm uses a high- performance implementation of the Ribosomal Database Project (RDP) Classifier as described by Wang et al. [40].
Statistical Analysis
Comparisons were performed between two groups with equal variance (MOX and saline-treated animals), student t-tests were applied for detecting significant differences in specific measured parameters. The cut-off for statistical significance was p<0.05, two-tailed t-tests.
Results
Diversity and Richness
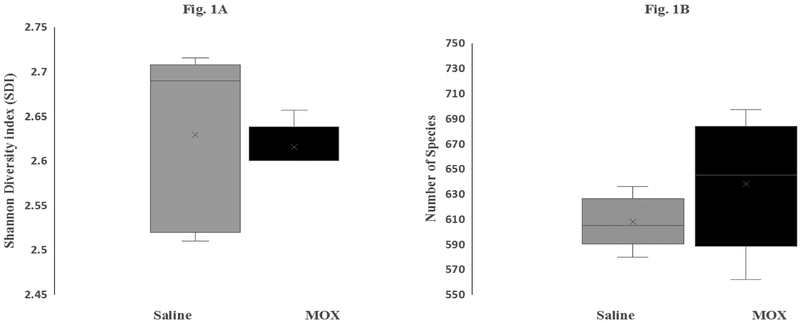
Figure 1 depicts the effects of chronic low dose MOX on GM diversity (A) and richness (B). A total of 1,121 different GM species were identified in both saline and MOX groups. Overall, there was no significant difference in either diversity [Figure 1A] as estimated by the Shannon Diversity Index (SDI) (saline control vs. MOX; 2.62 vs. 2.60, P=0.99) or species richness [Figure 1B] as measured by mean species number. Although a total of 1,121species were identified, only less than 700 were considered qualified (i.e. made the cut off at 0.01% abundance).
Figure 1:
Effects of 7day MOX (2.5mg/kg i.p.) on GM species diversity (1A) and species richness (1B). Box-and-Whisker represent values of Shannon Diversity Index (n=5) for (1A) and species number (1B). The ‘x’ designation in the middle of the bar represents mean value for each group, horizontal line across the bars indicates median values. The minimum and maximum quartile values are represented by a horizontal line in the bottom and top of the bars.
Taxa-Level Distribution
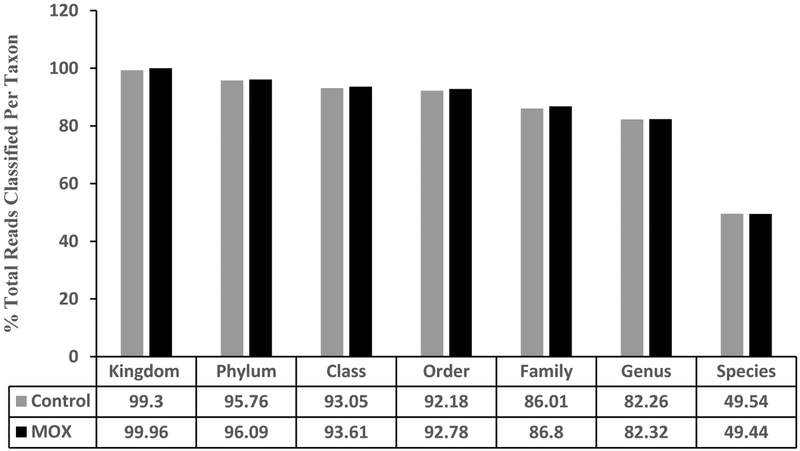
There were a total number of 29 Phyla, 56 classes, 106 orders, 234 families, 600 genera and 1,121 species identified in the two groups. There were no differences between the saline and MOX group in percent reads, i.e., percentage of identified sequences belonging to each taxon [Figure 2].
Figure 2:
Effects of 7day MOX (2.5mg/kg i.p.) on GM distribution of taxa. Values are percent total reads/taxa/group (n=5). Note: the samples from each group were pooled and hence overall there were two groups to be compared (control vs treated).
Phylum-Level Effects
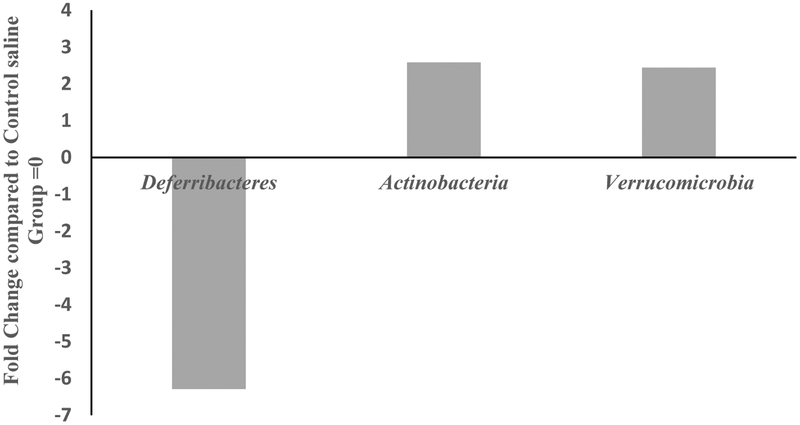
MOX significantly reduced abundances of one phylum, Deferibacteres, and enriched two phyla: Actinobacteria and Verrucomicrobia (Fig 3). Overall there were 29 different phyla identified in the two groups. Deferibacteres, Actinobacteria and Verrucomicrobia are all low- abundance phyla accounting for less than 2% of the total phyla reads. MOX selectively reduced Deferibacteres by 6.5-fold and enriched both Actinobacteria and Verrucomicrobia by 2-fold compared to control group.
Figure 3:
Effects of 7day MOX (2.5mg/kg i.p.) on fold change in abundance of GM phylum. Values are the fold change of mean compared to control group (n=5). Note: the samples from each group were pooled and hence overall there were two groups to be compared (control vs treated).
Class-Level Effects
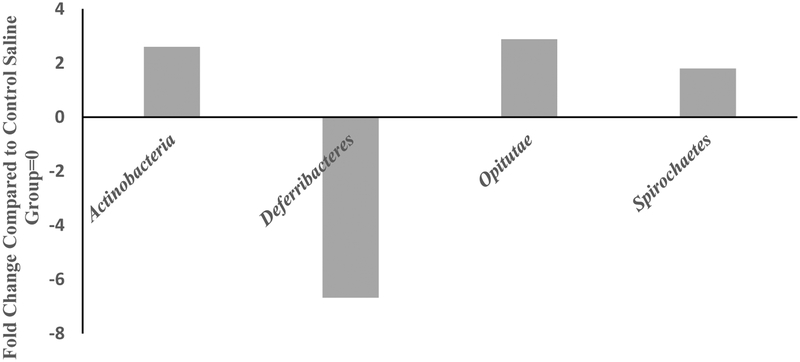
MOX significantly reduced the levels of two classes, Deferrribacteres by 6.5-fold compared to the saline group (Fig 4) and increased Actinobacteria, Opitutae and Spirochaetes by 2.5, 3 and 2-fold respectively. There was a total of 56 different classes identified between the two groups.
Figure 4:
Effects of 7day MOX (2.5mg/kg i.p.) on fold change in abundance of GM class. Values are the fold change over saline control group mean (n=5). Note: the samples from each group were pooled and hence overall there were two groups to be compared (control vs treated).
Order-Level Effects
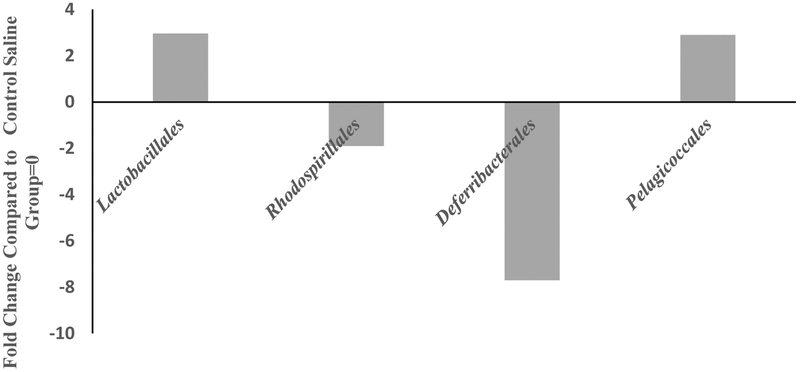
MOX significantly increased the abundance of Lactobacillales and Pelagicoccales by 3-fold each and reduced abundance of Rhodospirillales and Deferribacterales by 2 and 8-fold, respectively, compared to saline control group (Fig 5). There was a total of 106 different classes identified in both groups.
Figure 5:
Effects of 7day MOX (2.5mg/kg i.p.) on fold change in abundance of GM order. Values are the fold change over saline control group mean (n=5). There was a total of 106 different orders. Numbers of the columns refer to the fold-change. Note: the samples from each group were pooled and hence overall there were two groups to be compared (control vs treated).
Family-Level Effects
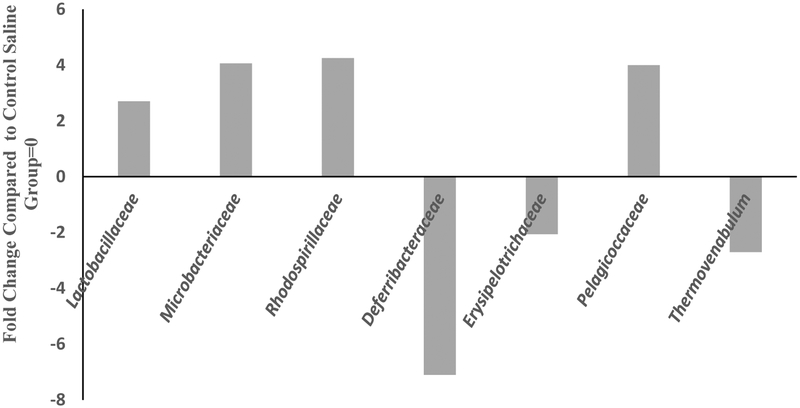
MOX significantly enriched Lactobacillaceae, Microbacteriaceae, Rhodospirillaceae and Pelagicoccaceae by 3, 4, 4, and 4-fold, respectively. Deferrribacteraceae, Erysipelotrichaceae and Thermovenabulum were reduced by 8, 2.5, and 3.5- fold respectively, compared to the control group at the family level [Figure 6]. There was a total of 234 different families. It should be noted that for analysis at the family level, the samples were pooled and hence overall there were two groups to be compared (control vs treated). Since a statistical analysis could not be performed in such cases, we used a conservative cutoff point of a minimum of 2-fold difference between the groups, which could imply important changes.
Figure 6:
Effects of 7day MOX (2.5mg/kg i.p.) on fold change in abundance of GM family. Values are the fold change over control group mean (n=5). There was a total of 234 families.
Genus-Level Effects
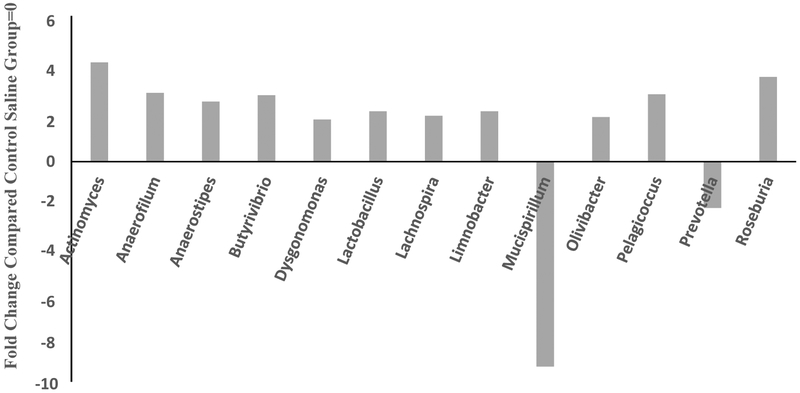
MOX significantly enriched abundance of 13 genera, particularly, Actinomyces, Anaerofilum, Anaerostripes, Lachnobacterium, Butyrivibria, Pelagicoccus and Rosburia by 4.3, 3, 2.6, 2.9, 2.9 and 3.7-fold, respectively. Conversely, MOX decreased levels of Mucispirillum and Prevottella by 9 and 2-fold, respectively [Figure 7]. All comparisons were with respect to saline control group. There was a total of 611 different genera. It should be noted that for analysis at the genus level, the samples were pooled and hence overall there were two groups to be compared (control vs treated). Since a statistical analysis could not be performed in such cases, we used a conservative cutoff point of a minimum of 2-fold difference between the groups, which could imply important changes.
Figure 7:
Effects of 7day MOX (2.5mg/kg i.p.) on fold change in abundance of gut bacteria genus. Values are the fold change over control group mean (n=5). All comparisons are with respect to saline group. There was a total of 611 different genera.
Species-Level Effects
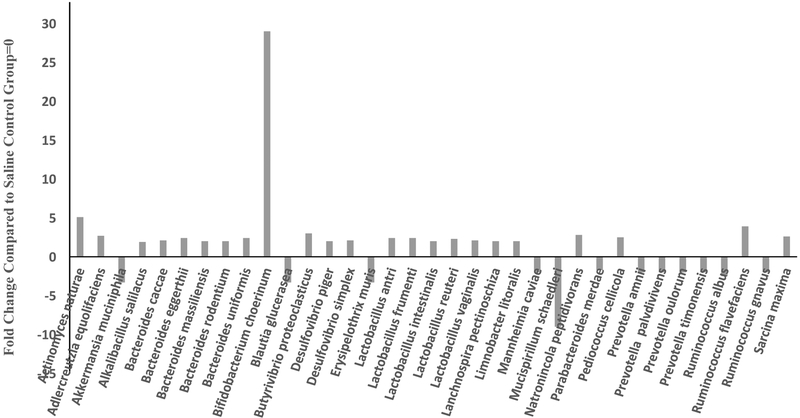
MOX significantly enriched abundances of 22 species particularly, Bifidobacterium cholerinum Actinomyces naturae, butyrate forming and biohydrogenating bacteria Butyrivibrio proteoclasticus, and Sarcina maxima, and an important cellulose-decomposing bacterium Ruminococcus flavvefaciens by 30, 5, 2, 2 and 4-fold, respectively. Other species, including five species of Bacteroides, five species of Lactobacillus, two species of Desulfovibrio were enriched by over 2-fold. However, levels of four species of Prevotella, and two species each of Ruminococcus, Akkermansia muciniphila, Lachnospira pectinoschiza, Blautia glucerasea and Erysipelothrix muris were decreased by over 2-fold, compared to control group [Figure 8]. Of particular interest was Mucispirillum schaedleri, which was reduced by 9-fold compared to control group [Figure 8].
Fig 8.
Effects of 7day MOX (2.5mg/kg i.p.) on fold change in abundance of GM species. Values are the fold change over control group mean (n=5). There was a total of 1138 different species
There were a total of 1138 different species. It should be noted that for analysis at the species level, the samples were pooled and hence overall there were two groups to be compared (control vs treated). Since a statistical analysis could not be performed in such cases, we used a conservative cutoff point of a minimum of 2-fold difference between the groups, which could imply important changes.
Discussion
The results of this study indicate that daily treatment of WKY rats with MOX for 7 days promoted certain gut bacteria associated with mood enhancement (e.g. Bifidobacterium and Lactobacillus) and suppressed those associated with inflammation (e.g. Ruminoccus). Hence, it might be suggested that MOX has the potential of exerting antidepressant and anti-inflammatory effects through the GM. Moreover, the behavioral disparity seen in WKY rats might at least be partially related to the dysbiosis in GM of this rat strain. Although potential antidepressant and anti-inflammatory properties of MOX have yet to be investigated, it is of interest that MOX has been shown to reduce alcohol intake in a mouse model and may be effective in addressing alcohol craving and AUD [20,21]. In support of this hypothesis, AUD is often times associated with depression, which has a strong underlying inflammatory component [11,41]. Thus, a common underlying factor for all three disorders, depression, inflammation and AUD, may be manipulated through interference with the GM, i.e. dysbiosis. As alluded to earlier, and supported by substantial literature, brain-gut axis is now thought to play an important role in mood and immune (inflammatory) disorders [42–45]. A recent review has also extended the role of microbiomes to eating disorders as well as AUD [46]. Although MOX is not typically considered antimicrobial, its effectiveness against some bacteria has been documented [47,48]. Thus, taken together, it may be suggested that MOX through its interactions with GM, could be of potential therapeutic value, especially in AUD-induced depression.
Interestingly, it has been reported that ketamine, a glutamate NMDA antagonist with fast and sustained antidepressant effects, can also reduce alcohol intake in two rat models of alcohol drinking [49,50]. However, use of ketamine for AUD might be problematic as ketamine might have abuse potential of its own [51, 52]. Of relevance is that recently it was shown that ketamine strongly interacts with GM, and some of the same species (e.g. Bifidobacterium, Lactobacillus and Ruminoccus) that are affected by MOX were also similarly affected by ketamine [36]. Since ketamine has also been advocated as potential therapy in inflammatory diseases (e.g. colitis and IBD) [53, 54], the usefulness of MOX in such diseases warrants further investigation. If effective, MOX may have an added advantage over other novel drugs, especially for AUD, as its safety has been established [55].
Another brain-gut connection may be through short chain fatty acids (SCFAs) produced by gut bacteria, such as low-abundant Lactobaccilus, Sarcina, Bifidobacterium and Butyrivibrio in the gut [56–59]. SCFAs (e.g. propionate and butyrate) can influence intestinal gluconeogenesis with beneficial effects on glucose and energy homeostasis [60]. Curiously, the Westernized diet, implicated in metabolic syndrome, is linked to lower levels of Lactobacillus and Sarcina, and higher levels of Ruminococcus [61, 62], all of which were affected by MOX. Thus, MOX increased the levels of beneficial “probiotics” Lactobacillus and Sarcina, and decreased levels of the “opportunistic” Ruminococcus, and hence may be of benefit in metabolic syndrome as well. Some of the effects of MOX on GM are similar to recently reported GM effects of Tianasi Liquid, a Chinese herbal medicine. Tianasi Liquid, advocated for its metabolomic effects, also increases the relative abundance of Lactobacillus, and Lachnospira. It is important, however, to note that Lactobacillus genus members are unable to synthesize amino acids and/or purines and thus rely on nutrient rich environments and other bacteria for supply of essential building blocks [62–65]. Therefore, the presence of other probiotic bacteria such as Lachnospira may be required for Lactobaccillus to produce therapeutic benefits [62,64].
In addition, changes in “butyrogenic” bacteria may also influence inflammatory as well as neurological and neuropsychiatric diseases [65–67]. Key downstream effects of these beneficial microbes include their ability to decrease concentration of nuclear factor (NF)-κB and pro-inflammatory cytokines but increase the concentrations of anti- inflammatory cytokines [68–72]. Moreover, these bacteria can bring about changes in tryptophan and kynurenines levels, thus affecting other factors involved in mood regulation [68–72]. Since MOX significantly increases the levels of butyrogenic bacteria (e.g. Butyrivibrio Proteoclasticus, Provettela, Lactobaccillu, Bifidobacterium), it may be suggested that its interaction with this system is another contributory mechanism to its beneficial effects.
Some species of Mucispirillum may cause intestinal inflammation by causing a “leaky” gut, where there is an increase in gut permeability due to degradation of mucin (colonic mucus layer), which is crucial in maintaining the physical barrier that separates trillions of gut bacteria from the host [73–75]. Leaky gut is also a key contributor to the co-morbidity of depression and intestinal disorders [76–78]. In addition, Mucispirillum is positively associated with increases in plasma levels of LPS and is considered colitogenic, because of which, it is used as a microbial marker of active colitis [73–75,78,79]. On the other hand, low levels of Sarcina have been implicated in inflammatory processes of relevance to depression [62]. Thus, the dramatic reduction (over 9-fold) of Mucispirillum, and over a 2-fold increase in Sarcina by MOX, strengthens its potential usefulness in leaky gut conditions, in addition to its usefulness in depression associated with gut dysbiosis. Moreover, a recent study suggests the use of MOX for the treatment of glioma, a rather severe and aggressive form of central inflammatory disease [80].
Finally, since MOX is often used in quarantine practices [19], our results indicate potential confounding effects of such treatment on behavioral or neurochemical measurements associated with those experiments. Hence, careful consideration of inclusion of appropriate control groups in such cases is warranted.
In summary, our findings indicate that chronic administration of MOX results in significant increases in the levels of probiotic bacteria (e.g. Lactobacillus, Bifidobacterium and Butyrivibrio), and significant decreases in opportunistic pathogens (e.g. Provettela and Mucispirallum) in WKY rats, a putative animal model of depression. Since these microorganisms have been implicated in mood regulation and inflammatory diseases, the utility of MOX in these conditions, individually or as a co-morbid condition warrants further investigation.
Acknowledgment
Supported by R03AA022479 from NIAAA (YT); AA022448 (D.L.D.), USC School of Pharmacy.
Availability of supporting data
The datasets used and/or analyzed during the current study are
Footnotes
Conflict of Interest
The authors state that they have no conflict of interest.
References
- 1.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012; 10:735–742. [DOI] [PubMed] [Google Scholar]
- 2.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012; 13:701–712. [DOI] [PubMed] [Google Scholar]
- 3.Bienenstock J, Kunze W, Forsythe P. Microbiota and the gut-brain axis. Nutr Rev. 2015; 73(Suppl 1):28–31. [DOI] [PubMed] [Google Scholar]
- 4.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006; 27:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, Khalili H, Pan A, Higuchi LM, De Sliva P, Richter JM, et al. Association between depressive symptoms and incidence of Crohn’s disease and ulcerative colitis: results from the Nurses’ Health Study. Clinical Gastroenterol Hepatol. 2013; 11:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015; 9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2018; 23:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudzki L, Ostrowska L, Pawlak D, Małus A, Pawlak K, Waszkiewicz N, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019; 100:213–222. [DOI] [PubMed] [Google Scholar]
- 9.Sobin WH, Heinrich TW, Drossman DA. Central Neuromodulators for Treating Functional GI Disorders: A Primer. Am J Gastroenterol. 2017; 112:693–702. [DOI] [PubMed] [Google Scholar]
- 10.Bonilla S, Nurko S. Focus on the use of antidepressants to treat pediatric functional abdominal pain: current perspectives. Clin Exp Gastroenterol. 2018; 11:365–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley LL, Tizabi Y. Neuroinflammation, neurodegeneration, and depression. Neurotox Res. 2013; 23:131–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nazimek K, Strobel S, Bryniarski P, Kozlowski M, Filipczak-Bryniarska I, Bryniarski K. The role of macrophages in anti-inflammatory activity of antidepressant drugs. Immunobiology. 2017; 222 :823–830. [DOI] [PubMed] [Google Scholar]
- 13.McKellar QA, Benchaoui HA. Avermectins and milbemycins. J Vet Pharmacol Ther. 1996; 19:331–351. [DOI] [PubMed] [Google Scholar]
- 14.Cotreau MM, Warren S, Ryan JL, Fleckenstein L, Vanapalli SR, Brown KR, et al. The antiparasitic moxidectin: Safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol. 2003;43:1108–1115. [DOI] [PubMed] [Google Scholar]
- 15.Perez M, Blazquez AG, Real R, Mendoza G, Prieto JG, Merino G, et al. In vitro and in vivo interaction of moxidectin with BCRP/ABCG2. Chem Biol Interact. 2009; 180:106–112. [DOI] [PubMed] [Google Scholar]
- 16.Prichard R, Ménez C, Lespine A. Moxidectin and the avermectins: Consanguinity but not identity. Int J Parasitol Drugs and Drug Resist. 2012;14:134–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barda B, Ame SM, Ali SM, Albonico M, Puchkov M, Huwyler J, et al. Efficacy and tolerability of moxidectin alone and in co-administration with albendazole and tribendimidine versus albendazole plus oxantel pamoate against Trichuris trichiura infections: a randomised, non-inferiority, single-blind trial. Lancet Infect Dis. 2018; 18:864–873. [DOI] [PubMed] [Google Scholar]
- 18.Maheu-Giroux M, Joseph SA. Moxidectin for deworming: from trials to implementation. Lancet Infect Dis. 2018; 18:817–819. [DOI] [PubMed] [Google Scholar]
- 19.Korte SW, Franklin CL, Dorfmeyer RA, Ericsson AC. Effects of Fenbendazole- impregnated Feed and Topical Moxidectin during Quarantine on the Gut Microbiota of C57BL/6 Mice. J Am Assoc Lab Anim Sci. 2018; 57: 229–235. [PMC free article] [PubMed] [Google Scholar]
- 20.Huynh N, Arabian N, Naito A, Louie S, Jakowec MW, Asatryan L, et al. Preclinical development of moxidectin as a novel therapeutic for alcohol use disorder. Neuropharmacol. 2016; 113(Pt A): 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoja S, Huynh N, Asatryan L, Jakowec MW, Davies DL. Preclinical evaluation of avermectins as novel therapeutic agents for alcohol use disorders. Psychopharmacol (Berl). 2018; 235:1697–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J. 1963; 27:282–93. [DOI] [PubMed] [Google Scholar]
- 23.Paré WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav. 1992; 51:1051–1056. [DOI] [PubMed] [Google Scholar]
- 24.Paré WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994; 55:433–439. [DOI] [PubMed] [Google Scholar]
- 25.López-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacol. 2000; 22:191–199. [DOI] [PubMed] [Google Scholar]
- 26.Paré WP. Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol Behav. 1989; 46:993–938. [DOI] [PubMed] [Google Scholar]
- 27.Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, et al. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002; 115:229–242. [DOI] [PubMed] [Google Scholar]
- 28.Getachew B, Hauser SR, Taylor RE, Tizabi Y. Desipramine blocks alcohol-induced anxiety- and depressive-like behaviors in two rat strains. Pharmacol Biochem Behav. 2008; 91:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Getachew B, Hauser SR, Taylor RE, Tizabi Y. Alcohol-induced depressive-like behavior is associated with cortical norepinephrine reduction. Pharmacol Biochem Behav. 2010; 96:395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tizabi Y, Hauser SR, Tyler KY, Getachew B, Madani R, Sharma Y, et al. Effects of nicotine on depressive-like behavior and hippocampal volume of female WKY rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010; 34:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hauser SR, Getachew B, Taylor RE, Tizabi Y. Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol Biochem Behav. 2011; 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gądek-Michalska A, Tadeusz J, Rachwalska P, Bugajski J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol Rep. 2013; 65:1655–1662. [DOI] [PubMed] [Google Scholar]
- 33.Muscatello MR, Bruno A, Scimeca G, Pandolfo G, Zoccali RA. Role of negative affects in pathophysiology and clinical expression of irritable bowel syndrome. World J Gastroenterol. 2014; 20:7570–7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morganstern I, Tejani-Butt S. Differential patterns of alcohol consumption and dopamine-2 receptor binding in Wistar-Kyoto and Wistar rats. Neurochem Res. 2010; 35:1708–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaroslavsky I, Tejani-Butt SM. Voluntary alcohol consumption alters stress-induced changes in dopamine-2 receptor binding in Wistar-Kyoto rat brain. Pharmacol Biochem Behav. 2010; 94:471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Getachew B, Aubee JI, , Schottenfeld RS, Csoka AB, Thompson KL, Tizabi Y. Ketamine Interactions with Gut-Microbiota in Rats: Relevance to its Antidepressant and Anti-inflammatory properties. BMC Microbiol. 2018; 18:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathay C, Hamot G, Henry E, Georges L, Bellora C, Lebrun L, et al. Method optimization for fecal sample collection and cecal DNA extraction. Biopreserv Biobank. 2015; 13:79–93. [DOI] [PubMed] [Google Scholar]
- 38.Illumina MiSeq 16S metagenomic sequencing library preparation protocol.2017.
- 39.Illumina 16S metagenomics pipeline (v1.0.1). Accessed 20 Aug 2017.
- 40.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007; 73:5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillemacher T, Bachmann O, Kahl KG, Frieling H. Alcohol, microbiome, and their effect on psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2018; 85:105–115. [DOI] [PubMed] [Google Scholar]
- 42.Gorky J, Schwaber. The role of the gut-brain axis in alcohol use disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2016; 65:234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cenit MC, Sanz Y, Codoñer-Franch P. Influence of gut microbiota on neuropsychiatric disorders. World J Gastroenterol. 2017; 23:5486–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bastiaanssen TFS, Cowan CSM, Claesson MJ, Dinan TG, Cryan JF. Making Sense of the Microbiome in Psychiatry. Int J Neuropsychopharmacol. 2019; 22:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Ting-Ting, Lai Jian-Bo, Du Yan-Li, Xu Yi, Ruan Lie-Min, Hu Shao-Hua Current Understanding of Gut Microbiota in Mood Disorders: An Update of Human Studies. Front Genet. 2019:10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Temko JE, Bouhlal S, Farokhnia M, Lee MR, Cryan JF, Leggio L. The Microbiota, the Gut and the Brain in Eating and Alcohol Use Disorders: A ‘Ménage à Trois’?. Alcohol Alcohol. 2017; 52:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim LE, Vilcheze C, Ng C, Jacobs WR, Jr, Ramon-Garcia S, Thompson CJ. Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug- resistant clinical strains. Antimicrob Agents Chemother. 2013; 57:1040–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woerde DJ, Martin PA, Govendir M. Susceptibility of rapidly growing mycobacteria isolated from Australian cats to ivermectin, moxidectin, ceftiofur, and florfenicol. J Feline Med Surg. 2015;17:1065–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rezvani AH, Levin ED, Cauley M, Getachew B, Tizabi Y. Ketamine Differentially Attenuates Alcohol Intake in Male Versus Female Alcohol Preferring (P) Rats. J Drug Alcohol Res. 2017; 6: 236030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruda-Kucerova J, Babinska Z, Luptak M, Getachew B, Tizabi Y. Both ketamine and NBQX attenuate alcohol drinking in male Wistar rats. Neurosci Lett. 2018; 666:175–180. Babinska Z, Ruda-Kucerova J. Differential characteristics of ketamine self- administration in the olfactory bulbectomy model of depression in male rats. Exp Clin Psychopharmacol. 2017; 25:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Babinska Z, Ruda-Kucerova J. Differential characteristics of ketamine self- administration in the olfactory bulbectomy model of depression in male rats. Exp Clin Psychopharmacol. 2017; 25:84–93. [DOI] [PubMed] [Google Scholar]
- 52.Wright KN, Kabbaj M. Sex differences in sub-anesthetic ketamine’s antidepressant effects and abuse liability. Curr Opin Behav Sci. 2018; 23:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White M, Shan N, Lindley K, Lloyd-Thomas A, Thomas M. Pain management in fulminating ulcerative colitis. Pediatric Anesthesia. 2006; 16:1148–1152. [DOI] [PubMed] [Google Scholar]
- 54.Loix S, Kock M, Henin P. The anti-inflammatory effects of ketamine: State of the art. Acta anaesthesiol Belg. 2011; 62:47–58. [PubMed] [Google Scholar]
- 55.Janko C, Geyer J. Moxidectin has a lower neurotoxic potential but comparable brain penetration in P-glycoprotein-deficient CF-1 mice compared to ivermectin. J Vet Pharmacol Ther. 2013; 36:275–284. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Martínez I, Walter J, Keshavarzian A, Rose DJ. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe. 2013; 23:74–81. [DOI] [PubMed] [Google Scholar]
- 57.Ijssennagger N, Belzer C, Hooiveld GJ, Dekker J, van Mil SW, Müller M, et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci U S A. 2015; 112:10038–10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourassa MW, Alim I, Bultman SJ, Ratan RR. Butyrate, neuroepigenetics and the gut icrobiome: can a high fiber diet improve brain health? Neurosci Lett. 2016; 625; 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell.2016; 165 1332–1345. [DOI] [PubMed] [Google Scholar]
- 60.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits.Cell. 2014; 156:84–96. [DOI] [PubMed] [Google Scholar]
- 61.Martínez I, Stegen JC, Maldonado-Gómez MX, Eren AM, Siba PM, Greenhill AR, et al. The gut microbiota of rural Papua New Guineans: composition, diversity patterns, and ecological processes. Cell Rep. 2015;11:527–538. [DOI] [PubMed] [Google Scholar]
- 62.Hausmann B, Knorr KH, Schreck K, Tringe SG, Glavina Del Rio T, Loy AM, et al. Consortia of low-abundance bacteria drive sulfate reduction-dependent degradation of fermentation products in peat soil microcosms. ISME J. 2016; 10:2365–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Clarke SF, Murphy EF, O’Sullivan O, Ross RP, O’Toole PW, Shanahan F, et al. Targeting the microbiota to address diet-induced obesity: a time dependent challenge. PLoS One. 2013; 8:e65790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, et al. Consumption of fermented milk product with probiotic modulates activity. Brain Gastroenterology. 2013;144:1394–401,1401.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016; 99:110–132. [DOI] [PubMed] [Google Scholar]
- 66.Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X, et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disord. 2017; 207:300–304. [DOI] [PubMed] [Google Scholar]
- 67.Abildgaard A, Kern T, Pedersen O, Hansen T, Wegener G, Lund S. The antidepressant-like effect of probiotics and their faecal abundance may be modulated by the cohabiting gut microbiota in rats. Eur Neuropsychopharmacol. 2019; 29:98–110. [DOI] [PubMed] [Google Scholar]
- 68.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res. 2008; 43:164–174. [DOI] [PubMed] [Google Scholar]
- 69.Danato KA, Gareau MG, Wang YJ, Sherman PM. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signalling. Microbiol. 2010; 156: 3288–3297. [DOI] [PubMed] [Google Scholar]
- 70.Desbonnet L, Garrett L, Clarke G, Kiely B, Cryan JF, Dinan TG. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010; 170:1179–1188. [DOI] [PubMed] [Google Scholar]
- 71.Hegazy SK, El-Bedewy MM. Effect of probiotics on pro-inflammatory cytokines and NF-kappaB activation in ulcerative colitis. World J Gastroenterol. 2010;16: 4145–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yan F, Polk DB. Disruption of NF-kappaB signalling by ancient microbial molecules: novel therapies of the future? Gut. 2010; 59:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robertson BR, O’Rourke JL, Neilan BA, Vandamme P, On SL, Fox JG, et al. Mucispirillum schaedleri gen. nov., sp. nov., a spiral-shaped bacterium colonizing the mucus layer of the gastrointestinal tract of laboratory rodents. Int J Syst. Evol Microbiol. 2005; 55:1199–2204. [DOI] [PubMed] [Google Scholar]
- 74.Berry D, Schwab C, Milinovich G, Reichert J. Ben Mahfoudh K, Decker T, Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J. 2012; 6:2091–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moreno-Indias I, Yorres M, Sanchez-Alcohlado L, Cardona F, Almendros I, Gozal D, et al. Normoxic recovery mimicking treatment of sleep apnea does not reverse intermittent hypoxia-induced bacterial dysbiosis and low-grade endotoxemia in mice. Sleep. 2016; 39:1891–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008; 57:1470–1481. [DOI] [PubMed] [Google Scholar]
- 77.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008; 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009; 587:4153–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rooks MG, Veiga P, Wardwell-Scott LH, Tickle T, Segata N, Michaud M, et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014; 8:1403–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song D, Liang H, Qu B, Li Y, Liu J, Chen C, et al. Moxidectin inhibits glioma cell viability by inducing G0/G1cell cycle arrest and apoptosis. Oncol Rep. 2018; 40: 1348–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]