Abstract
Purpose
There is heightened recognition that the environment is an important driver of human reproductive health. This article provides an overview of the nature and extent of the science in the field of reproductive environmental health and its implications for OB/GYN clinical practice.
Recent findings
Women of childbearing age incur ubiquitous contact to numerous toxic environmental contaminants. Even subtle perturbations caused by chemical exposures during critical and sensitive windows of development may lead to increased risks of disease and disability across the entire span of human life. The strength of the evidence is sufficiently high that leading scientists and clinicians have called for timely action to prevent harm.
Summary
OB/GYNs are uniquely poised to intervene in critical stages of human development (i.e., preconception and during pregnancy) to prevent harm. Efforts are underway to provide clinicians with the evidence-based foundation to develop recommendations for prevention. If adopted, current directions in toxicity testing, risk assessment and policy are likely to create important changes in how environmental chemicals are evaluated and regulated in the future. Together, these changes have the potential to assist in clinical assessment of patient risk and reductions in patient exposure to environmental contaminants linked to adverse reproductive health outcomes.
Keywords: reproductive environmental health, critical windows of development, environmental contaminants, developmental origins of disease and health
Introduction
The fact that exposure to chemicals can harm human reproduction has been known since Roman times when lead was first recognized to cause miscarriage and infertility in women and men. [1, 2] Over the past 60 years it has become clear that the placenta does not protect the fetus from damaging chemicals, that the fetus can be uniquely sensitive to chemical exposures, and that intergenerational harm can result from in utero chemical exposures (Table 1). These discoveries stemmed from exposure to drugs and higher levels of environmental chemical exposure than typically encountered by the general population. Hence it was generally assumed that environmental exposures experienced by an average person living in the U.S. would be below levels of reproductive harm.
Table 1.
Examples of human evidence that documents key principles in reproductive environmental health
| The placenta does not protect the fetus from damaging chemicals: Methylmercury |
| In the 1950s, methylmercury exposure in utero resulted in severe neonatal neurological impairment in children after pregnant mothers consumed high levels methylmercury in fish and shellfish contaminated from toxic industrial releases in Minimata, Japan. [3] More recent evidence documents that developmental and cognitive effects can occur in children exposed prenatally to mercury at low doses that do not result in effects in the mother, [4] [5] [6] [7] and that the adverse neurological effects of methylmercury exposure may be delayed. [8, 9] As of 1992 there were 2252 officially recognized cases of Minimata disease. [10] |
| The fetus can be uniquely sensitive to chemical exposures: Thalidomide |
| In the 1960s, thalidomide, a drug given to pregnant women for morning sickness, with no adverse maternal consequences, resulted in a high rate of congenital limb and gastrointestinal malformations when taken day 28-42 post conception. [11, 12] It is estimated that more than 10,000 children in 46 countries where the drug had been approved were born with deformities as a consequence their mothers using the drug during pregnancy. [13] |
| Intergenerational harm can result from in utero chemical exposures: Diethylstilbestrol (DES) |
| Diethylstilbestrol (DES), which was prescribed in up to 10 million pregnancies from 1938 to 1971 to prevent miscarriage, was subsequently found to be a “transplacental carcinogen” causally-linked to post-pubertal benign and malignant reproductive tract abnormalities in the daughters and sons of DES exposed mothers. These adverse health impacts manifested only decades after exposure. [14] Established health impacts of in utero DES exposure include: vaginal clear cell adenocarcinoma, vaginal epithelial changes, reproductive tract abnormalities (e.g., gross anatomical changes of the cervix, T-shaped and hypoplastic uteri), ectopic pregnancies, miscarriages, and premature births, and infertility in females exposed in utero; reproductive tract abnormalities (e.g., epididymal cysts, hypoplastic testis, cryptorchidism) in males exposed in utero; and increased risk for breast cancer in women who took the drug while pregnant. [15] Recent cohort studies indicate that women who were exposed to DES prenatally have an increased risk of breast cancer after age 40. [16]Animal data predict inter-generational impacts (i.e., among granddaughters of DES exposed women) to-date supported by limited human data. [17] |
A rapidly expanding body of scientific evidence has upended assumptions about the benign nature of “low-level” environmental exposures. [18–21] We now know that even subtle perturbations caused by chemical exposures may lead to important functional deficits and increased risks of disease and disability across the entire span of human life, particularly when exposure occurs during critical and sensitive windows of development (Figure 1). [22–26]
Figure 1. Windows of susceptibility.
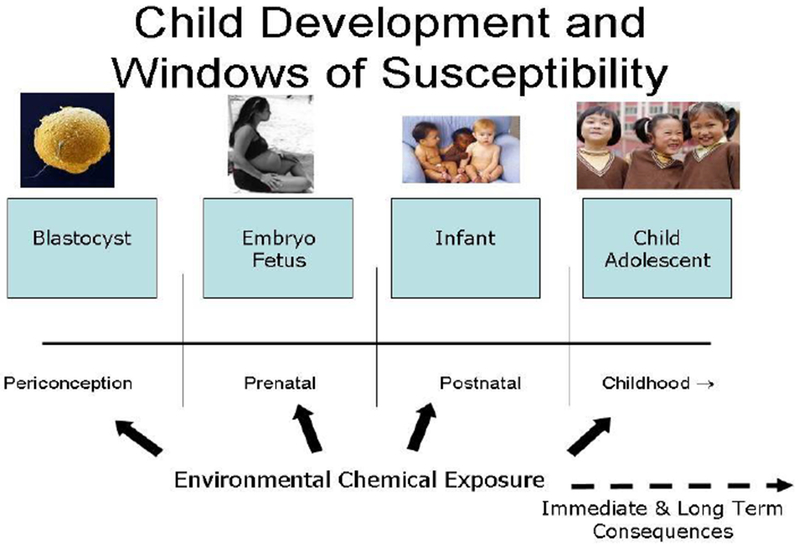
Permission needed: (Modified from Louis et al.[(39)])
“Reproductive environmental health” addresses exposures to environmental contaminants (synthetic chemicals and metals), particularly during critical and sensitive periods of development and their potential effects on all aspects of future reproductive health throughout the life course, including conception, fertility, pregnancy, child and adolescent development and adult health. [26] This article provides an overview of the nature and extent of the science in the field of reproductive environmental health and its implications for OB/GYN clinical practice.
Trends in Reproductive Health
Scientific indicators of declining reproductive function and increasing rates of reproductive illnesses since the mid-20th century suggest our reproductive health and, ultimately, our reproductive capacity are under strain. [24, 26–28] A spectrum of female and male reproductive disorders as well as poor birth outcomes and childhood disorders are increasing (Table 2). [29, 30] The burgeoning evidence from human populations is further amplified by signals from wildlife showing birth defects and other altered reproductive performance in wild populations of annelids, mollusks, crustaceans, insects, fish, amphibians, and other species. [31–33]
Table 2.
Trends in reproductive health indicators [29] Permission needed
| Reproductive Diseases/Disorders | Increase | Period | Location | Ref. | |
|---|---|---|---|---|---|
| Testicular cancer | 1 – 6% | 1953 - 1999 | Europe | [34] | |
| Testicular cancer | 60% | 1973 - 2003 | USA | [35] | |
| Certain childhood cancers | 20 – 24% | 1976 - 2005 | USA | [36] | |
| Autism | 57% | 2002 - 2006 | USA | [37] | |
| Attention Deficit Hyperactivity Disorder | 3% per year | 1997 - 2006 | USA | [38] | |
| Birth defects: | |||||
| Cryptorchidism | 200% | 1970 - 1993 | USA | [39] | |
| Gastroschisis | 300% | 1978 - 2005 | California | [40] | |
| Congenital hypothyroidism | 138% | 1987 - 2003 | New York | [41] | |
| Reproductive Function | Time | Location | Ref. | ||
| Reported difficulty conceiving and maintaining pregnancy | |||||
| All ages | 60% more women | 1982; 2002 | USA | [42, 43] | |
| <25 years old | 200% more women | 1982; 2002 | USA | [42, 43] | |
| Prematurity | 2.9% shorter gestation | 1992 - 2002 | USA | [44] | |
| Pre-eclampsia | 19-36% | 1968-2002 | Norway | [45] | |
| Gestational Diabetes | 122% | 1989-2004 | USA | [46] | |
| Premature puberty: | |||||
| Age at onset of breast development | 1 – 2 years younger | 1940 - 1994 | USA, Denmark | [47, 48] | |
| Age at onset of menstruation | 2.5 – 4 months younger | 1940 - 1994 | USA | [47] | |
| Sperm count | ~1% decline per year | 1931 - 1994 | Western countries | [49, 50] | |
| Serum testosterone | 1% decline per year | 1987 - 2004 | Boston, USA | [51, 52] |
These trends in reproductive health have occurred in roughly the same time frame in which human exposure to both natural and synthetic chemicals has dramatically increased. Approximately 87,000 chemical substances are registered for use in U.S. commerce as of 2006, with about 3,000 chemicals manufactured or imported in excess of 1 million pounds each, [53] and 700 new industrial chemicals introduced into commerce each year. [54]
Today, women of childbearing age have ubiquitous contact to toxic environmental contaminants and their exposure can reach the fetus through placental transfer and continue to expose the newborn through breast-feeding. [55] Population-based studies conducted by the U.S. Centers for Disease Control and Prevention (CDC) demonstrate widespread exposure to many chemicals found in homes, communities and workplaces that can disrupt the normal functioning of hormones and/or have neurological or other toxicities. [56, 57] A recent analysis of CDC population prevalence data found virtually all pregnant women in the U.S. have body burdens of all of the following reproductive/developmental toxicants: lead, mercury, toluene, perchlorate, bis-phenol A (BPA), and some phthalates, pesticides, perfluorochemicals (PFCs), polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs). [58] Similar findings from studies in Europe, [59] and populations in the Arctic far from pollution sources, [60] indicate that all human populations are exposed to some level of synthetic chemicals.
Developmental Origins of Health and Disease
The body of evidence linking environmental exposure or other stimulus or insult during a critical period of growth and development to the propensity to develop disease or dysfunction later in life evolved independently in the fields of nutrition and environmental health. [17, 61] In the field of nutrition, the hypothesis of “developmental programming” stemmed from epidemiologic studies beginning in the mid-1980s in the UK by Barker and colleagues that identified strong relationships between maternal under nutrition, low birth weight and long-term risk of metabolic disease. [62–65] A large body of experimental and epidemiologic data have substantiated and further refined scientific understanding of these linkages. [17, 61]
In 1971, the transplacental carcinogenicity of in utero exposure to a synthetic non-steroidal pharmaceutical with estrogenic activity was first identified, and DES remains one of the most scientifically robust illustrations of the linkage between developmental exposure to a hormonally active exogenous chemical and latent onset of adult disease. [17] Since the latter part of the 20th century “endocrine disrupting chemicals” (EDCs) beyond DES have received increased scrutiny because: they are ubiquitous in the environment; hormonal regulation is critical to human reproduction and development; and chemicals that interfere with this process can cause permanent disruption. [27, 66]
The U.S. Environmental Protection Agency (USEPA) defines endocrine disruptors as compounds which “interfere with the synthesis, secretion, transport, binding, action, or elimination of natural hormones in the body that are responsible for the maintenance of homeostasis (normal cell metabolism), reproduction, development, and/or behavior.” (70) Examples of EDCs commonly found in food, water, air, house dust, and/or personal care products include phthalates, BPA, PBDEs, perchlorate and some pesticides.
Between 2007 and 2010, four major scientific reviews/proceedings examined the state of the science regarding the relationship between exposure to environment contaminants and human reproductive health and all concluded that while many scientific questions remain, the current evidence warrants timely action to prevent harm. [25, 26, 55, 67] A key point of intersection of these four efforts is the critical mass of scientific evidence that the embryo, fetus and developing human are highly vulnerable to exposure to even small amounts of environmental toxicants. [25, 26, 55, 67]
For example, in 2009, the Endocrine Society, the premier professional organization devoted to research on hormones and the clinical practice of endocrinology, conducted a comprehensive review of the human and experimental evidence linking environmental exposure to EDCs to human health. The Endocrine Society review concluded, “The evidence for adverse reproductive outcomes (infertility, cancers, malformations) from exposure to endocrine disrupting chemicals is strong, and there is mounting evidence for effects on other endocrine systems, including thyroid, neuroendocrine, obesity and metabolism, and insulin and glucose homeostasis.” [55] Subsequently, evidence that strengthens these conclusions has continued to mount, exemplified by: (1) the first animal evidence to report a link between neonatal exposure to high doses of BPA with development of polycystic ovarian syndrome -like abnormalities in adulthood; [68] and epidemiologic evidence confirming the role of prenatal exposure to pesticides and childhood neurobehavioral deficits, [69] and childhood leukemia (30% to 200% increased risk). [70] [71]
Epigenetics
While it has long been known that genetic mutations can alter gene expression and lead to disease (i.e., ionizing radiation), the field of “epigenetics” has greatly extended our understanding of the mechanisms through which EDCs and other environmental contaminants can perturb the fetal environment and influence health throughout the lifecourse.[72] “Epigenetics” includes any process that alters gene activity without mutating the DNA sequence, and leads to modifications that can be transmitted to daughter cells. (80) Environmental modifications of gene expression can affect embryonic imprinting, cellular differentiation, and phenotypic expression. [73] Nascent human research has begun to expand mechanistic data from animal studies on the effect of chemical contaminants on the epigenome and human health. [74, 75]
Implications for OB/GYN Clinical Practice
Current research in reproductive environmental health is inextricably linked to healthy pregnancies, children and future generations but the science has yet to be harnessed to improvements in patient outcomes. Among physicians, OB/GYNs are uniquely poised to intervene in critical stages of human development (i.e., preconception and during pregnancy) to prevent harm. Health care professionals are increasingly called on to serve as a science-based source of guidance on how to avoid potentially adverse exposures. [76, 77] Many OB/GYN patients are already asking questions about their exposure to environmental contaminants and many more lack awareness of potential environmental risks to their fertility and their future children’s health. The role of clinicians extends beyond the clinic walls.[78] In 2009, the Endocrine Society recommended that “Until such time as conclusive scientific evidence exists to either prove or disprove harmful effects of substances, a precautionary approach should be taken in the formulation of EDC [endocrine-disrupting chemicals] policy.” [55] Subsequently, the American Medical Association (AMA) adopted a resolution that reflected the findings and recommendations of the Endocrine Society’s review and called on the AMA to work with the federal government to enact new policies to decrease the public exposure to EDCs.
Bridging the Gap Between Environmental and Clinical Health Sciences
One key challenge to translating the state of the evidence linking exposure to environmental toxicants to human health into clinical practice is that the evidence stream and decision context in environmental health sciences differs from clinical sciences and practice. In the clinical setting, in vivo, in vitro and human experimental evidence and an analysis of risks and benefits have informed human exposure decisions prior to the substances entry into the marketplace (Figure 2). In stark contrast, population exposure to exogenous substances in the environment typically occurs prior to regulatory scrutiny of a compound and in the absence of risk-benefit analysis because of our current regulatory structure for governing manufactured chemicals (Figure 2). Ethical considerations virtually preclude experimental human data from the environmental health evidence stream.
Figure 2.
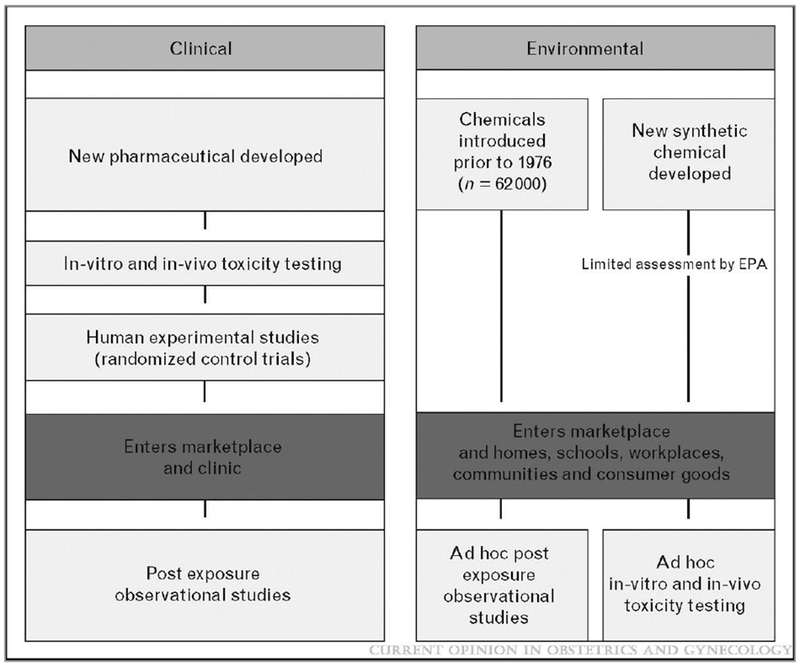
Comparison of streams of evidence in clinical and environmental health sciences
To help bridge this gap between environmental and clinical health sciences, in 2009, the University of California, San Francisco Program on Reproductive Health and the Environment undertook a collaborative project to craft the Navigation Guide, a methodology to provide the evidence-based foundation for the development of recommendations for prevention. [79] The Navigation Guide is the product of a year-long collaboration of 22 clinical and environmental health scientists and/or practitioners, from governmental and non-governmental organizations in the U.S. and Europe. The Navigation Guide proceeds from an evidence-based medicine framework but reflects the differences in evidence and decision contexts described above. Uptake of the Navigation Guide in clinical and policy arenas will provide a missing tool to support evidence-based decision making to ensure healthy pregnancies, children, and future generations.
Current Directions in Toxicity Testing, Risk Assessment and Regulatory Policy
Just as the thalidomide tragedy led to strengthened regulatory oversight of the safety and efficacy of all prescription drugs, [13] recent advances in toxicity testing, [80, 81] risk assessment, [18–20, 82] and policy [83] are likely to create important change in the how environmental chemicals are evaluated and regulated in the future.
Toxicity Testing
Toxicity testing in the 21st century is anticipated to move away from identifying apical endpoints in animal models and towards identifying biologic perturbations in key toxicity pathways without the use of whole body animal testing. [54] The proof of principle that it is feasible to move toward using upstream mechanistic indicators as the basis for risk assessment and decision-making was recently demonstrated for several classes of early perturbations, i.e., thyroid hormone disruption, antiandrogen effects and immune system disruption. [84] This fundamental shift will align toxicity testing with clinical care which already recognizes the importance of perturbations in individual hormone levels to disease, for example, in the relationship between hypothyroidism during pregnancy and increase risk for neurocognitive deficits in children. [85]
Assessing harm from chemical exposures
Assessing harm from exposure to environmental contaminants is rapidly moving beyond traditional methods that generally look at how exposure to one chemical, acting through a dominant mechanism of action, can impact a healthy adult. [86] In 2008, the National Academy of Sciences (NAS) issued two groundbreaking reports concerning new science that extends our understanding of the relationship between exposure to environmental contaminants and health. [18] [19] The NAS reports address the need to: incorporate increased recognition of how concurrent chemical exposures, age, underlying health status and other biological factors can modify risk; [18] and move from a “common mechanisms of action” approach to a “common adverse outcome” focus, because there may be not just one but many pathways to the same adverse health outcome. [19] The “common adverse outcome” principle is exemplified by the health risk of exposure to phthalate esters, a class of ubiquitous chemicals used in a wide range of personal care and other consumer products. Phthalate esters disturb androgen action through multiple mechanisms which all lead to a common adverse health impact, i.e., altered male reproductive outcomes. [19] Studies also show that exposure to other chemicals that act on the same common adverse health outcome, male reproductive development, but through different mechanisms, can have added effects. [19] If adopted, these changes would accelerate our understanding of the health risks of current levels of exposure to environmental contaminants.
Policy
Just because a product is readily available on the shelf at the store is no assurance that it is non-toxic. The vast majority of chemicals in commerce have entered the marketplace without comprehensive and standardized information on their reproductive or other chronic toxicities (Figure 2). [83] The inadequacies of the regulatory framework are receiving increased attention. In 2007, “REACH”, a new European Community Regulation on chemicals and their safe use (EC 1907/2006) entered into force. In 2009, the USEPA established “Essential Principles for Reform of Chemicals Management Legislation” to help inform legislative efforts now underway to reauthorize and significantly strengthen the effectiveness of chemical regulation. [87] Because many exposures are not controllable at the individual level (e.g. air pollution), these society wide actions are critical to reducing exposure to harmful chemicals.
Conclusion
Women of childbearing age have ubiquitous exposure to toxic environmental contaminants. Exposure to environmental contaminants incurred during critical and sensitive windows of development can adversely impact the health of infants, children, women, men and potentially future generations. While many scientific questions remain, the strength of the evidence is sufficiently high that leading scientists and clinicians have called for timely action to prevent harm.
OB/GYNs are uniquely poised to intervene in critical stages of human development (i.e., preconception and during pregnancy) to prevent harm. Efforts are underway to provide clinicians with the evidence-based foundation to develop recommendations for prevention. If adopted, current directions in toxicity testing, risk assessment and policy are likely to create important changes in how environmental chemicals are evaluated and regulated in the future. Together, these changes have the potential to assist in clinical assessment of patient risk and reductions in patient exposure to environmental contaminants linked to adverse reproductive health outcomes.
Acknowledgments
The Passport Foundation and the Clarence E. Heller Foundation provided support for this work.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Cunningham M, Chronic occupational lead exposure: the potential effect on sexual function and reproductive ability in male workers. Aaohn J, 1986. 34(6): p. 277–9. [PubMed] [Google Scholar]
- 2.Hauser R and Sokol R, Science Linking Environmental Contaminant Exposures with Fertility and Reproductive Health Impacts in the Adult Male. Fertil Steril, 2008. 89(2 Suppl): p. e59–65. [DOI] [PubMed] [Google Scholar]
- 3.Rusyniak DE, Furbee RB, and Pascuzzi R, Historical neurotoxins: what we have learned from toxins of the past about diseases of the present. Neurol Clin, 2005. 23(2): p. 337–52. [DOI] [PubMed] [Google Scholar]
- 4.Grandjean P, et al. , Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol, 1997. 19(6): p. 417–28. [DOI] [PubMed] [Google Scholar]
- 5.Grandjean P, et al. , Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ Res, 1998. 77(2): p. 165–72. [DOI] [PubMed] [Google Scholar]
- 6.Grandjean P, et al. , Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environ Health Perspect, 1999. 107(7): p. 587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lederman SA, et al. , Relation between Cord Blood Mercury Levels and Early Child Development in a World Trade Center Cohort. Environ Health Perspect, 2008. 116(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Research Council, Toxicological Effects of Methylmercury. 2000, Washington, DC: National Academy Press. [Google Scholar]
- 9.USEPA, Methylmercury (MeHg) (CASRN 22967-92-6), I.R.I. System, Editor. Last Revised — 07/27/2001: Washington, DC. [Google Scholar]
- 10.Harada M, Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol, 1995. 25(1): p. 1–24. [DOI] [PubMed] [Google Scholar]
- 11.McBride WG, Thalidomide and Congenital Abnormalities. The Lancet, 1961. 278(7216): p. 1358–1358. [Google Scholar]
- 12.William GM, Thalidomide embryopathy. Teratology, 1977. 16(1): p. 79–82. [DOI] [PubMed] [Google Scholar]
- 13.FDA. This Week In FDA History - July 15, 1962. May/20/2009. Available from: http://www.fda.gov/AboutFDA/WhatWeDo/History/ThisWeek/ucm117836.htm
- 14.Newbold RR, Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol, 2004. 199(2): p. 142–50. [DOI] [PubMed] [Google Scholar]
- 15.NIH, N.C.I. DES Research Update 1999: Current Knowledge, Future Directions, Meeting Summary. 1999; Available from: http://women.cancer.gov/planning/previous/DES/chapter1.html.
- 16.Palmer JR, et al. , Prenatal diethylstilbestrol exposure and risk of breast cancer. Cancer Epidemiol Biomarkers Prev, 2006. 15(8): p. 1509–14. [DOI] [PubMed] [Google Scholar]
- 17.Newbold R and Heindel J, Developmental exposures and implications for disease, in Environmental Impacts on Reproductive Health and Fertility, Woodruff TJ, Janssen SJ, Giullette LJ, Giudice LC, Editor. 2010, Cambridge University Press: Cambridge, UK: p. 92–102. [Google Scholar]; * Authorative and current synthesis of the relationship between developmental exposure to environmental contaminants and human health.
- 18.National Academy of Science, Science and Decisions: Advancing Risk Assessment. 2008, National Research Council Committee on Improving Risk Analysis Approaches Used by the U.S. EPA: Washington, DC. [Google Scholar]; * One of two groundbreaking authoritative reports by the National Academy of Sciences on advances advances in assessing harm from chemical exposures.
- 19.National Research Council, Phthalates and Cumulative Risk Assessment: The Task Ahead, ed. Committee on the Health Risks of Phthalates. 2008, Washington, D.C.: National Academies Press. [PubMed] [Google Scholar]; * Second of two groundbreaking authoritative reports by the National Academy of Sciences on advances advances in assessing harm from chemical exposures.
- 20.Weiss B, et al. , The new tapestry of risk assessment. Neurotoxicology, 2008. 29(5): p. 883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cory-Slechta DA, Weiss B, and Cranmer J, The environmental etiologies of neurobehavioral deficits and disorders: weaving complex outcomes and risk modifiers into the equation. Neurotoxicology, 2008. 29(5): p. 759–60. [DOI] [PubMed] [Google Scholar]
- 22.Palanza P, et al. , Prenatal exposure to endocrine disrupting chemicals: effects on behavioral development. Neurosci Biobehav Rev, 1999. 23(7): p. 1011–27. [DOI] [PubMed] [Google Scholar]
- 23.Welshons WV, et al. , Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect, 2003. 111(8): p. 994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crain DA, et al. , Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril, 2008. 90(4): p. 911–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grandjean P, et al. , The faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol, 2008. 102(2): p. 73–5. [DOI] [PubMed] [Google Scholar]; ** Interdisciplinary consensus statement of 200 experts which reviews the state of the science and concludes: “The accumulated research evidence suggests that prevention efforts against toxic exposures to environmental chemicals should focus on protecting the embryo, foetus and small child as highly vulnerable populations. Given the ubiquitous exposure to many environmental chemicals, there needs to be renewed efforts to prevent
- 26.Woodruff TJ, et al. , Proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility: Executive summary. Fertil Steril, 2008. 89(2 Suppl): p. e1–e20. [DOI] [PubMed] [Google Scholar]; ** Summary of the proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility which convened over 400 scientists, health care professionals, community groups, political representatives and the media to hear presentations on the impact of environmental contaminants on reproductive health and fertility and to discuss opportunities to improve health through research, education, communication and policy.
- 27.Colborn T, Dumanoski D, and Myers JP, Our Stolen Future. 1996: Penguin Books USA, Inc; 1–306. [Google Scholar]
- 28.Schettler T, et al. , Generations at Risk. Reproductive Health and the Environment. 1999, Cambridge, MA: MIT Press. [Google Scholar]
- 29.Woodruff TJ, Schwartz J, and Giudice LC, Research agenda for environmental reproductive health in the 21st century. J Epidemiol Community Health, 2010. 64(4): p. 307–10. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Current review of trends and future research directions in the field of reproductive environmental health.
- 30.Donahue SM, et al. , Trends in birth weight and gestational length among singleton term births in the United States: 1990–2005. Obstet Gynecol, 2010. 115(2 Pt 1): p. 357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Important analysis of the most recent data. From 1990 to 2005, birth weight decreased among term births in the U.S., and these declines were not explained by trends in maternal and neonatal characteristics, changes in obstetrical practices, or concurrent decreases in gestational length.
- 31.Guillette LJ Jr. and Edwards TM, Environmental Influences on Fertility: Can We Learn Lessons from Studies of Wildlife? Fertil Steril, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Oehlmann J, et al. , A critical analysis of the biological impacts of plasticizers on wildlife. Philos Trans R Soc Lond B Biol Sci, 2009. 364(1526): p. 2047–62. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Recent review of the biological effects of the most widely used plasticizers, including dibutyl phthalate, diethylhexyl phthalate, dimethyl phthalate, butyl benzyl phthalate and bisphenol A (BPA), on wildlife.
- 33.Hamlin HJ and Guillette LJ Jr., Birth defects in wildlife: the role of environmental contaminants as inducers of reproductive and developmental dysfunction. Syst Biol Reprod Med, 2010. 56(2): p. 113–21. [DOI] [PubMed] [Google Scholar]; * Recent review of wildlife evidence of adverse reproductive outcomes linked to exposure to environmental contaminants and implications for human health.
- 34.Bray F, et al. , Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int J Cancer, 2006. 118(12): p. 3099–111. [DOI] [PubMed] [Google Scholar]
- 35.Shah MN, et al. , Trends in testicular germ cell tumours by ethnic group in the United States. Int J Androl, 2007. 30(4): p. 206–13; discussion 213–4. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Environmental Protection Agency. American’s Children and the Environment. 2008. [cited 2008 December 29]; Available from: http://www.epa.gov/envirohealth/children/child_illness/d6-graph.htm.
- 37.Centers for Disease Control and Prevention, Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ, 2009. 58(10): p. 1–20. [PubMed] [Google Scholar]
- 38.Pastor PN and Reuben CA, Diagnosed attention deficit hyperactivity disorder and learning disability: United States, 2004–2006. Vital Health Stat 10, 2008(237): p. 1–14. [PubMed] [Google Scholar]
- 39.Paulozzi LJ, International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect, 1999. 107(4): p. 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vu LT, et al. , Increasing prevalence of gastroschisis: population-based study in California. J Pediatr, 2008. 152(6): p. 807–11. [DOI] [PubMed] [Google Scholar]
- 41.Harris KB and Pass KA, Increase in congenital hypothyroidism in New York State and in the United States. Mol Genet Metab, 2007. 91(3): p. 268–77. [DOI] [PubMed] [Google Scholar]
- 42.Swan SH and Hertz-Picciotto I, Reasons for infecundity. Fam Plann Perspect, 1999. 31(3): p. 156–7. [PubMed] [Google Scholar]
- 43.Brett K, Fecundity in 2002 NSFG women 15–24 years of age (personal communication) 2008, National Center for Health Statistics: Hyattsville, MD. [Google Scholar]
- 44.Davidoff MJ, et al. , Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol, 2006. 30(1): p. 8–15. [DOI] [PubMed] [Google Scholar]
- 45.Dahlstrom BL, et al. , Changes in the prevalence of pre-eclampsia in Akershus County and the rest of Norway during the past 35 years. Acta Obstet Gynecol Scand, 2006. 85(8): p. 916–21. [DOI] [PubMed] [Google Scholar]
- 46.Getahun D, et al. , Gestational diabetes in the United States: temporal trends 1989 through 2004. Am J Obstet Gynecol, 2008. 198(5): p. 525 e1–5. [DOI] [PubMed] [Google Scholar]
- 47.Euling SY, et al. , Role of environmental factors in the timing of puberty. Pediatrics, 2008. 121 Suppl 3: p. S167–71. [DOI] [PubMed] [Google Scholar]
- 48.Aksglaede L, et al. , Recent decline in age at breast development: the Copenhagen Puberty Study. Pediatrics, 2009. 123(5): p. e932–9. [DOI] [PubMed] [Google Scholar]
- 49.Jorgensen N, et al. , Coordinated European investigations of semen quality: results from studies of Scandinavian young men is a matter of concern. Int J Androl, 2006. 29(1): p. 54–61; discussion 105–8. [DOI] [PubMed] [Google Scholar]
- 50.Carlsen E, et al. , Evidence for decreasing quality of semen during past 50 years. Bmj, 1992. 305(6854): p. 609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andersson A, et al. , Trends in Leydig cell function in Danish men. Human Reproduction, 2005. 20(suppl. 1): p. i26. [Google Scholar]
- 52.Perheentupa A, et al. Clear Birth Cohort Effect in Serum Testosterone and SHBG Levels in Finnish Men. in Endocrine Society Meeting 2006. 2006. [Google Scholar]
- 53.U.S. EPA. What is the TSCA Chemical Substance Inventory. 2006. [cited 2007 Apr 4]; Available from: http://www.epa.gov/opptintr/newchems/pubs/invntory.htm.
- 54.National Research Council, Toxicity Testing in the 21st Century: A Vision and Strategy. 2007, Washington, DC: The National Academies Press. [Google Scholar]; * The most recent and most authorative compilation of the current directions in toxicity testing.
- 55.Diamanti-Kandarakis E, et al. , Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev, 2009. 30(4): p. 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The most recent definitive review of the human and experimental evidence linking environmental exposure to endocrine disrupting chemicals to human health by the Endocrine Society, the premier professional organization devoted to research on hormones and the clinical practice of endocrinology, comprised of over 14,000 research scientists and physicians from over 100 countries.
- 56.CDC, Third national report on human exposure to environmental chemicals. 2005, Centers for Disease Control and Prevention, National Center for Environmental Health: Atlanta, GA. [Google Scholar]
- 57.CDC, Fourth National Report on Human Exposure to Environmental Chemicals. . 2009, Centers for Disease Control and Prevention: Atlanta, GA. [Google Scholar]; * Most recent report by CDC of the NHANES data.
- 58.Woodruff T, Zota A, and Schwartz J, Environmental chemicals in pregnant women in the US: NHANES 2003-2004. Environ Health Perspect, (in review). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Most recent analysis of a sub-set of NHANES data that reflects population-wide prevalence of exposure to selected enviromental contaminants among pregnant women in the U.S.
- 59.Porta M, et al. , Monitoring concentrations of persistent organic pollutants in the general population: The international experience. Environment International, 2008. 34(4): p. 546–561. [DOI] [PubMed] [Google Scholar]
- 60.Artic Monitoring and Assessment Programme (AMAP), AMAP Assessment 2009: Human Health in the Arctic. 2009: Oslo. [Google Scholar]; * Evidence of environmental contaminants geographically far from their point of origin.
- 61.Warner MJ and Ozanne SE, Mechanisms involved in the developmental programming of adulthood disease. Biochem J, 2010. 427(3): p. 333–47. [DOI] [PubMed] [Google Scholar]; * Current review of human and experimental evidence for developmental programming. in the field of nutrition.
- 62.Barker DJ and Osmond C, Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet, 1986. 1(8489): p. 1077–81. [DOI] [PubMed] [Google Scholar]
- 63.Barker DJ, Maternal and fetal origins of coronary heart disease. J R Coll Physicians Lond, 1994. 28(6): p. 544–51. [PMC free article] [PubMed] [Google Scholar]
- 64.Barker DJ, Fetal origins of coronary heart disease. Bmj, 1995. 311(6998): p. 171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barker DJ, Fetal programming of coronary heart disease. Trends Endocrinol Metab, 2002. 13(9): p. 364–8. [DOI] [PubMed] [Google Scholar]
- 66.Colborn T, vom Saal FS, and Soto AM, Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect, 1993. 101(5): p. 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.President’s Cancer Panel, Reduction Environmental Cancer Risk: What We Can Do Now, National Cancer Institute, Editor. 2010, U.S. Department of Health and Human Services: Bethesda, MD. [Google Scholar]; ** Between September 2008 and January 2009, the (NCI’s) President’s Cancer Panel convened four meetings to assess the state of environmental cancer research, policy, and programs addressing known and potential effects of environmental exposures on cancer. This report summarizes the Panel’s findings and conclusions based on the testimony received and additional information gathering, and concluded that “the true burden of environmentally induced cancer has been grossly underestimated.”
- 68.Fernandez MO, et al. , Neonatal Exposure to Bisphenol A and Reproductive and Endocrine Alterations Resembling the Polycystic Ovarian Syndrome in Adult Rats. Environ Health Perspect, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Most recent experimental evidence linking BPA exposure to adverse reproductive health outcomes.
- 69.Harari R, et al. , Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ Health Perspect, 2010. 118(6): p. 890–6. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The results of this study confirm previous results of other environmental studies of neurodevelopmental toxicity and the theory of window of vulnerability of central nervous system during uterine life.
- 70.Turner MC, Wigle DT, and Krewski D, Residential pesticides and childhood leukemia: a systematic review and meta-analysis. Environ Health Perspect, 2010. 118(1): p. 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Current systematic review and meta-analysis of 15 human studies.
- 71.Wigle DT, Turner MC, and Krewski D, A Systematic Review and Meta-analysis of Childhood Leukemia and Parental Occupational Pesticide Exposure. Environmenal Health Perspectives, 2009. (epub). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Current systematic review and meta-analysis of 31 human studies.
- 72.Baccarelli A and Bollati V, Epigenetics and environmental chemicals. Curr Opin Pediatr, 2009. 21(2): p. 243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Current review of the evidence linking environmental contaminant expoure to epigenetic mechanisms
- 73.Weinhold B, Epigenetics: the science of change. Environ Health Perspect, 2006. 114(3): p. A160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pilsner JR, et al. , Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ Health Perspect, 2009. 117(9): p. 1466–71. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Early human data on epigenetic mechanisms.
- 75.Wright RO, et al. , Biomarkers of lead exposure and DNA methylation within retrotransposons. Environ Health Perspect, 2010. 118(6): p. 790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Early human data on epigenetic mechanisms.
- 76.Solomon G and Janssen S, Talking with Patients and the Public about Endocrine-Disrupting Chemicals, in Endocrine-Disrupting Chemicals: From Basic Research to Clinical Practice, Gore A, Editor. 2007, Humana Press; Totowa. [Google Scholar]
- 77.Solomon G and Janssen S, Communicating with patients and the public about environmental exposures and reproductive risk, in Environmental Impacts on Reproductive Health and Fertility, Woodruff TJ, Janssen SJ, Giullette LJ, Giudice LC, Editor. 2010, Cambridge University Press: Cambridge, UK: p. 214–26. [Google Scholar]; * Authoritative discussion of the role of clinicians by two physician leaders in the fields of environmenal and occupational health, including case studies and other practical information for the practicing clinician.
- 78.Gould R and Russell C, Taking Action to Prevent Harm. County Medical Associations and Environmental Health, in Medicine and the Environment. Practice, Prevention and Policy, Lee P, Editor. 2010. Journal of the San Francisco Medical Society: San Francisco. [Google Scholar]; * Describes active role of physicans policy arenas
- 79.Sutton P, et al. , The Navigation Guide: An evidence-based methodology to support clinical recommendations about the potential risks to reproductive health from exposure to chemicals in the environment (in review), 2010. [Google Scholar]; * Describes the first methodology to link an evidence-based medicine framework to the evidence stream in environmental health sciences.
- 80.Lein P, Locke P, and Goldberg A, Meeting report: alternatives for developmental neurotoxicity testing. Environ Health Perspect, 2007. 115(5): p. 764–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cory-Slechta DA, Studying toxicants as single chemicals: does this strategy adequately identify neurotoxic risk? Neurotoxicology, 2005. 26(4): p. 491–510. [DOI] [PubMed] [Google Scholar]
- 82.Callahan MA and Sexton K, If cumulative risk assessment is the answer, what is the question? Environ Health Perspect, 2007. 115(5): p. 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilson MP, Chia DA, and Ehlers BC, Green chemistry in California: a framework for leadership in chemicals policy and innovation. New Solut, 2006. 16(4): p. 365–72. [DOI] [PubMed] [Google Scholar]
- 84.Woodruff TJ, et al. , Meeting report: moving upstream-evaluating adverse upstream end points for improved risk assessment and decision-making. Environ Health Perspect, 2008. 116(11): p. 1568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Escobar GM, Obregon MJ, and del Rey FE, Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab, 2004. 18(2): p. 225–48. [DOI] [PubMed] [Google Scholar]
- 86.Woodruff T, Bridging epidemiology and model organisms to increase understaning of endocrine disrupting chemicals and human health effects. 2010. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]; * Comprehensive and current summary of key scientific concepts relevant to integrating human and experimental evidence in scientific decision-making about the relationship between endocrine disrupting chemicals and adverse human health effects, including two case studies, i.e., thyroid disrupting chemicals and anti-androgen chemicals.
- 87.USEPA. Essential Principles for Reform of Chemicals Management Legislation. 2010. 6/14/2010]; Available from: http://www.epa.gov/oppt/existingchemicals/pubs/principles.html.; * Definitive statement by USEPA on the need for reforming the Toxic Substances Control Act (TSCA) which governs the regulation of most manufactured chemicals.


