Abstract
The mosquito immune system has evolved in the presence of continuous encounters with fungi that range from food to foes. Herein, we review the field of mosquito-fungal interactions, providing an overview of current knowledge and topics of interest. Mosquitoes encounter fungi in their aquatic and terrestrial habitats. Mosquito larvae are exposed to fungi on plant detritus, within the water column, and at the water surface. Adult mosquitoes are exposed to fungi during indoor and outdoor resting, blood and sugar feeding, mating, and oviposition. Fungi enter the mosquito body through different routes, including ingestion and through active or passive breaches in the cuticle. Oral uptake of fungi can be beneficial to mosquitoes, as yeasts hold nutritional value and support larval development. However, ingestion of or surface contact with fungal entomopathogens leads to colonization of the mosquito with often lethal consequences to the host. The mosquito immune system recognizes fungi and mounts cellular and humoral immune responses in the hemocoel, and possibly epithelial immune responses in the gut. These responses are regulated transcriptionally through multiple signal transduction pathways. Proteolytic protease cascades provide additional regulation of antifungal immunity. Together, these immune responses provide an efficient barrier to fungal infections, which need to be overcome by entomopathogens. Therefore, fungi constitute an excellent tool to examine the molecular underpinnings of mosquito immunity and to identify novel antifungal peptides. In addition, recent advances in mycobiome analyses can now be used to examine the contribution of fungi to various mosquito traits, including vector competence.
Keywords: mycobiota, entomopathogen, infection, melanization, humoral immunity
Graphical Abstract
Introduction
Fungi hold nutritional value for larval and adult mosquitoes, and thus are mosquito prey (Asahina, 1964). Fungal commensals can form longer-term associations in the mosquito larval gut, with little to no impact on host survival (Tuzet and Manier, 1947). In contrast, some water molds (Chromista) and fungi utilize mosquitoes as their growth medium, most often with detrimental or lethal effects on their host (Braun, 1855; Galli-Valerio and Rochaz de Jongh, 1906; Eckstein, 1922; Roubaud and Toumanoff, 1930; Couch, 1935). The potential use of such fungal entomopathogens to control mosquito populations has largely driven the research on fungal-mosquito interactions over more than a century (reviewed in e.g. Christophers, 1952; Castillo and Roberts, 1980a,b; Jenkins 1964; Roberts, 1974; Lakon, 1919; Kanzok and Jacobs-Lorena, 2006; Scholte, 2005). While this holds true today (e.g. Lovett et al., 2019), mosquito microbiome studies have recently been extended to fungi (Kaufman et al., 2008; Muturi et al., 2016; Luis et al., 2019). These studies provide new insight into the range of mosquito-fungi encounters that may be fleeting, but may stimulate mosquito immunity through fungal surface molecules and/or secondary metabolites.
Entomopathogenic chromista and fungi infect mosquitoes through different routes, including active penetration of the body wall, through wounds, or through ingestion, dependent on the pathogen as well as the life stage of the mosquito (e.g. Clark et al., 1966, 1968; McCray et. al, 1973; Greenfield et al., 2014). Most commonly, entomopathogens isolated from field-caught mosquitoes belong to the genus Coelomomyces, which are ascomycetes fungi in the order of Blastocladiales (Castillo and Roberts, 1980; Couch and Umphlett, 1963). Coelomomyces fungi infect mosquito larvae through their aquatic zoospores. Zygospores attach to the larval intesegmental cuticle, and penetrate the host body wall by a penetration tube. Hyphal growth and sporangia formation occurs in the hemolymph, leading to larval death (reviewed in Araujo and Hughes, 2014; Scholte et al., 2004). However, their obligate two-host life cycle limits their use in mosquito control programs (Laird, 1967; Service, 1977). Currently, entomopathogens being proposed for mosquito control are ascomycetes fungi in the genera of Beauveria and Metarhizium in the order Hypocreales; Farenhorst et al., 2011; Lovett et al., 2019; Popko et al., 2018; Scholte et al., 2005). In general, fungi in these two genera infect mosquitoes via asexual spores called conidia, which germinate and produce penetration pegs that breach the host cuticle. Upon reaching the hemolymph, the fungus proliferates and forms yeast-like blastospores that rapidly colonize the host leading to its death (reviewed in Scholte et al., 2004).
Recognition of largely unknown fungal and water mold-derived molecules by mosquito pathogen recognition receptors (PRRs), activates mosquito epithelial, cellular and humoral immune responses. Mosquito cellular immune responses include phagocytosis, encapsulation, and nodulation (Bartholomay and Michel, 2018; Hillyer and Strand, 2014). Humoral immune responses include complement-like reactions, melanization, and the expression of antimicrobial peptides (Blandin et al., 2008; Nakhleh et al., 2017; Rhodes and Michel, 2017). These immune responses are regulated through proteolytic activation of key immune factors and/or on a transcriptional levels by several signal transduction pathways including Toll pathway, the immune deficiency (IMD) pathway, Janus Kinase (JAK)-signal transducer and activator of transcription (STAT), and Jun N-terminal kinase (JNK) and mitogen-activated protein kinase (MAPK) p38 pathway (Nakhleh et al., 2017; Rhodes and Michel, 2017). The most commonly observed immune response against fungi and water molds is melanization (Brey et al., 1988; Coluzzi, 1966; Mc Innis and Zattau, 1982; Clark et al. 1968). Molecular insights of mosquito antifungal immune responses are driven by comparative invertebrate immunology (Al Souhail et al., 2016; Chen et al., 2010; Valanne et al., 2011), and experiments performed using a small number of fungal laboratory infection models (Vizioli et al., 2001a; Ramirez et al., 2019; Rhodes et al., 2018; Yassine et al., 2012).
This review will summarize the potential environmental and molecular interactions of mosquito adults and larvae with fungi and water molds. The first two sections will provide an overview of their reported environmental encounters, and detail the potential routes of infection. The sum of these interactions across the symbiotic continuum shapes the mosquito anti-fungal immune responses, which we describe in the final section of this review.
1. Mosquito-fungus encounters in the environment
Fungi are ubiquitous and found in all mosquito habitats (Goh and Hyde, 1996). In addition, the aquatic environment of mosquito larvae provides habitat for water molds. This close proximity leads to a myriad of possible interactions between mosquitoes and fungi as well as water molds throughout the mosquito’s life history (Fig. 1). We mined the existing literature (see Supplementary Text for methods and references) and found records for 158 and 43 species of fungi and water molds, respectively having been observed in/isolated across 149 mosquito species (Tables S1 and S2, and references within). Only one third of these species have been isolated/observed in adult mosquitoes, reflecting the substantially lower number of fungus sampling efforts in adult mosquitoes compared to collections in immature life stages (Table S2). Two thirds of the isolated fungi belong to the orders Blastocladiales (39 species, 38 of which in the genus Coelomomyces), Eurotiales (28 species, including 16 Penicillium sp. and ten Aspergillus sp.), Hypocreales (24 species across 14 genera including Beauveria and Metarhizium), and Saccharomycetales (17 species with more than half belonging to the genus Candida). Only one sixth of the isolated water molds are known to be pathogenic, while nearly two thirds of the isolated fungi are either opportunistic, facultative or obligate pathogens (Table S1). However, these numbers are likely underestimates of the true range of mosquito-fungi interactions. Amplicon-based sequencing of adults from eleven mosquito species identified recently 347 and 1 species of fungi and water molds, respectively. Of these, 96 % were described for the first time in or on adult mosquitoes, and their impact on mosquito immunity is entirely unknown.
Figure 1: The multitude of potential fungus-mosquito encounters.
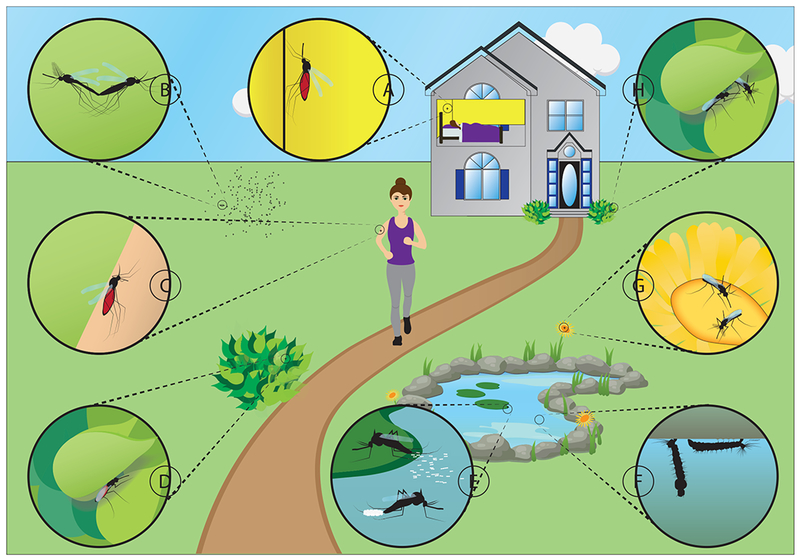
Adult mosquitoes are exposed to fungi on a multitude of surfaces during indoor and outdoor resting (A, D, H), blood and sugar feeding (C, G). Additional fungal encounters can occur during mating (B) and oviposition (E). Mosquito larvae in their aquatic environment are exposed to fungi on plant detritus, within the water column, and at the water surface (F).
This section describes the possible encounters with fungi and water molds across the mosquito life history, facilitated by mosquito behavior in their aquatic and terrestrial environments.
1.1. Larva-fungus interactions through ingestion
Fungi and water molds in the aquatic environment can enter the mosquito body passively through diverse larval feeding behaviors, including collecting-filtering, as well as grazing on and shredding of decaying and living organic matter (Fish and Carpenter, 1982; Merritt et al., 1992; Yee et al., 2004). Upon uptake, they either are digested, pass through the intestine unharmed, or manage to stay and grow within the mosquito host.
Fungi act as food and provide nutrients for the development of mosquito larvae. An example is the baker’s yeast, Saccharomyces cerevisiae that is commonly used for the rearing of mosquito larvae (Asahina, 1964). Seven individual yeast species that had been isolated previously from field-collected Culex theileri and Cx. pipiens larvae, supported the growth of Cx. pipiens larvae. This study thus confirmed the nutritional value of yeast ingestion by mosquito larvae (Steyn et al., 2016). Shift in fungal microbial populations on decaying oak leaf matter in the presence and absence of Aedes triseriatus larvae further suggests the ingestion of fungi through larval browsing and grazing (Fish and Carpenter, 1982; Kaufman et al., 2008).
Mosquito feeding behavior also leads to the establishment of commensals in the mosquito hindgut. At least four fungal species in the genus Smittium sp., belonging to the order of Harpellales, are able to attach and replicate in the hindgut of various mosquito species, with most often no effect on larval development or survival (Lopez-Lastra, 1997; Pereira et al., 2005; Sweeney, 1981; Tuzet and Manier, 1947; White et al., 2006).
During filter feeding and grazing, mosquito larvae do also ingest potential entomopathogenic fungi and water molds. The fungal entomopathogen Culicinomyces clavisporus causes infection in Culex fatigans larvae only upon ingestion of the fungal conidia (Sweeney, 1975). Likewise, Smittium morbosum infects mosquito larvae solely after oral uptake (Sato et al., 1989; Sweeney, 1981). M. anisopliae has deleterious effects on the larval hosts after oral uptake, which may be due to subsequent infection of the hemocoel, but at least in certain instances are due to toxicity in the gut (Cheng and Liu, 1990; Butt et al., 2013). The water molds Leptolegnia chapmanii and Lagenidium giganteum both infect the hemocoel of mosquito larvae after oral uptake of zoospores and by cuticular pentration of the body wall (Zattau and McInnis, 1987; McCray et al., 1973).
1. 2. Larva-fungus interactions through contact
Entomopathogenic fungi and water molds commonly infect mosquito larvae through active cuticular penetration. Fungi in the genus Coelomomyces, including C. opifexi are only known to infect mosquito larvae via cuticle penetration (Wong and Pillai, 1980). C. psorophorae spores infect only through attachment and cuticular penetration of the head, intersegmental regions, and the base of anal gills (Travland, 1979). In addition to aquatic fungal entomopathogens, soil-borne entomopathogens like M. anisopliae and B. bassiana, infect larvae of multiple mosquito species via direct penetration of the cuticle. Studies on M. anisopliae route of infection in mosquito larvae suggest that infection is mainly due to contact with spores at the water surface, mostly in and around the siphon (Crisan, 1971; Lacey et al., 1988). Likewise, dependent on formulation, B. bassiana conidia float on the water surface, and exposure preferentially happens aournd the perispiracular lobes (Clark et al., 1968).
1.3. Transstadial transmission of fungal communities
Transstadial transmission of bacterial microbiota from mosquito larvae to pupae and adults is limited, and has been reported in Culex tarsalis, Cx. pipiens, An. gambiae, and Ae. aegypti (Duguma et al., 2015; Moll et al., 2001). Studies conducted on yeast in Culex spp. have shown no transmission from larvae to pupae, or to adult stages (Díaz-Nieto et al., 2016; Steyn et al., 2016). These data suggest that transstadial transmission of fungi and water molds, especially if located solely in the midgut, does not occur and is not the source of fungal associations or infections in adult mosquitoes. One exception are the 36 species of Coelomomyces. While their persistence to adult mosquitoes occurs at a low frequency, transmission from infected larvae to pupae and adults has been observed repeatedly (Garland and Pillai, 1979; Lucarotti, 1987; Lucarotti and Andreadis, 1995).
1.4. Adult-fungus interactions through ingestion
Adult male and female mosquitoes commonly feed on plant nectars (Foster, 1995). These nectars contain many yeasts that likely are taken up by adult mosquitoes during feeding. The yeast nectar community is diverse and largely depends on inoculation through pollinator species including insects and birds (reviewed in Chappel and Fukami, 2018). Yeast community composition within the guts of pollinators overlaps with the yeast nectar community, strongly suggesting that such composition is largely driven by oral uptake and environmental filtering (Sandhu and Waraich, 1985). Several yeasts that have been identified in field-collected adult mosquitoes, including C. parapsilosis and Hansenula spp. in Ae. triseriatus, as well as Pichia spp. in Aedes japonicus (Bozic et al., 2017; Muturi et al., 2016; Ricci et al., 2011), overlap with nectar-associated yeasts. Most recently, amplicon-based sequening of adult Ae. albopictus identified a large number of yeast species (Luis et al., 2019). Together, these studies support the notion that nectar-associated yeasts are regularily ingested during nectar feeding by adult mosquitoes (Fig. 1G). Similarly, yeast may be taken up during blood feeding (Fig. 1C). Fungal cultures from human blood samples and blood-fed female mosquitoes share several yeast species, including C. parapsilosis, Rhodotorula spp., Saccharomyces spp., and Cryptococcus spp. (Bille et al., 1982; Chang et al., 2001; Muturi et al., 2016).
1.5. Adult-fungus interactions through substrate contact
Adult mosquitoes commonly rest in- and outdoors, and thus are exposed continuously to fungi through contact with contaminated surfaces. Mosquitoes, resting in tree cavities or on flower and leaf surfaces, come in touch with a diverse community of microorganisms including yeasts and filamentous fungi (Fig1. H, D, Levetin and Dorsey, 2006). Examples of such surface contact exposures is the occurance of infections of adult Culex pipiens with the fungal entomopathogen Entomophthora conglomerata and Entomophthora destruens in wine cellars and natural caves, repsectively (Novak, 1965; Weiser and Batko, 1966). This exposure route is also exploited for vector population control purposes. The provision of M. anisopliae and B. bassiana-impregnated netting, cloth, and mud panels as indoor resting sites increased the mortality of An. gambiae adults (Fig. 1A, Mnyone et al., 2010). Similarly, outdoor mosquito resting sites can be targeted using both entomopathogen species on the surface of clay pot traps, roof covers, and within resting stations like extra-domiciliary odor-baited stations (Luz et al., 2010; Lwetoijera et al., 2010; Mnyone et al., 2010). In addition, transfer of spores through contact with screens impregnated with B. bassiana in oviposition traps was used successfully to target female Ae. aegypti (Snetselaar et al., 2014).
Gravid female mosquitoes can locate suitable oviposition sites by the detection of secondary metabolites produced by certain fungi (Eneh et al., 2016; Geetha et al., 2003). During oviposition, these females are exposed to these and other soil-borne and/or aquatic fungi through direct contact (Fig. 1E). In how far these brief encounters contribute to fungal-mosquito interactions is currently unclear. The positive correlation of infection prevalence with number of ovipositions suggests that at least Entomophthora conglomerata may be able to infect Culex pipiens during oviposition (Kupriyanova, 1966). It is feasible that treatment of oviposition sites with fungal entomopathogens to control mosquito egg and early larval stages may also cause infection in adult females (Sousa et al., 2013).
1.6. Additional adult-fungus interactions
Mosquitoes can pass on spores that are attached to their own cuticle. Transfer of entomopathogenic fungal spores between individual mosquitoes through mating has been observed in several species. Infection of healthy male An. gambiae mosquitoes by M. anisopliae was observed post-mating with topically exposed female mosquitoes (Scholte et al., 2004a). Reduction in survival of healthy female Ae. aegypti mosquitoes post-mating was observed with both M. anisopliae and B. bassiana-exposed male mosquitoes, confirming conidial dissemination through mating in Ae. aegypti mosquitoes (Fig. 1B) (García-Munguía et al., 2011; Garza-Hernández et al., 2015; Reyes-Villanueva et al., 2011). As such, mosquitoes can be used to auto-disseminate fungal entomopathogens (Scholte et al., 2004a), and thus extend the reach of these biocontrol agents beyond the initial contact with the impregnated surface (Shah and Pell, 2003). Autodissemination may also extend to oviposition sites (Fig. 1E). A specialized case is the oviposition of resting sporangia of Coelomomyces sp., including C. psorophorae and C. stegomyiae by female mosquitoes. Infected females are sterile, but take blood meals, and actively oviposit sporangia, a behavior that contributes to the dissemination of the fungus to new aquatic environments (Lucarotti, 1987; Laird et al., 1992).
In addition to surfaces, indoor and outdoor air contains a highly diverse fungal spore community (Frohlich-Nowoisky et al., 2009), with some taxa also being found in mosquitoes (Guegan et al., 2018; Muturi et al., 2016). Conidia of B. bassiana can be air-borne (Feng et al., 1994), and thus may come in contact with mosquitoes through air rather than surfaces (Clark et al., 1968).
2. Getting in: Colonization of mosquitoes by fungal and water mold symbionts
Out of the 158 species of fungi for which we found published evidence of isolation from mosquitoes, roughly half can established longer-term interactions with their hosts. 67 species from six fungal orders are considered pathogens of mosquitoes, using the hemocoel and/or various tissues as growth medium (Section 1, Fig. 2, top and middle panel). Of these, only Entomophthora sp. and Coelomomyces sp. are obligate pathogens, while the rest are facultative pathogens that switch betweeen pathogenic and saprophytic or even endophytic life styles. In addition, 22 species of fungi in the orders Eurotiales, Hypocreales and Mucorales are opportunistic pathogens, who themselves cannot actively invade the mosquito hemocoel, but can establish an infection if entering through breaches in the cuticle (Fig. 2, top panel). Five Smittium species (order Harpellales) are commensals of mosquitoes, attaching to and growing within the hindgut of mosquito larvae (see Section 2.2, Fig. 2, bottom panel).
Figure 2: Entry routes of fungal entomopathogens into their mosquito hosts.
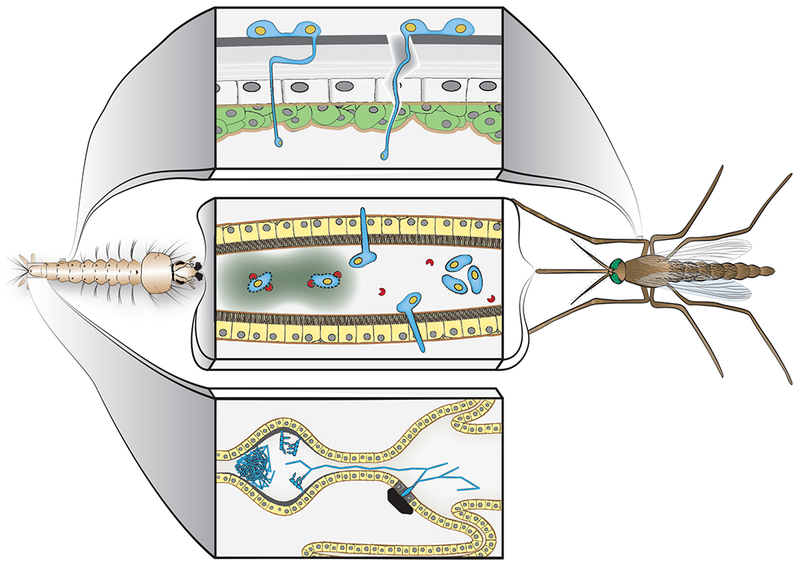
Entomopathogens enter mosquito larvae and adults through two distinct routes. Top panel: Fungal and water mold asexual spores attach to the mosquito cuticle and penetrate actively via penetration pegs. Opportunistic fungal pathogens can gain entry through wound sites. Center panel: Ingested spores may be degraded through digestive enzymes in the larval midgut, resulting in toxin release. Some spores may germinate and penetrate through the midgut epithelium, subseuqently disseminating throughout the larval body. It is unclear, whether this occurs in adult mosquitoes. Bottom panel: Trichospores of Smittium sp. attach to the cuticular lining of the hindgut in mosquito larvae, germinate and grow locally. Hyphae of Smittium morbosum can grow anteriorly, and penetrate the posterior midgut epithelium, where they are melanized.
Out of the 43 species of water molds that have been observed and/or isolated from mosquitoes, only five species (in the genera Lagenidium, Leptolegnia and Saprolegnia) are known facultative pathogens of mosquitoes. No commensal water molds have been described in mosquitoes. This section will briefly describe how some of these symbionts colonize their mosquito hosts, either through breaching the cuticle and/or through ingestion.
2.1. Active penetration of cuticle
As mentioned in Sections 1.2 and 1.5 above, insects often encounter entomopathogens through contact. Upon contact, colonization of the host is achieved through active penetration, a multistep process that somewhat varies dependent on fungal species (reviewed in Butt et al., 2016; Scholte et al., 2004; Lovett and St. Leger, 2017). In general, active penetration begins with the attachment of spores to the host epicuticle. Fungal spores germinate and form germ tubes that differentiate into specialized attachment organs, called appressoria, that then form a penetration tube, which penetrates the host cuticular layers through pressure exertion and secretion of cuticle-degrading enzymes. Upon reaching the hemolymph, the fungus proliferates as blastospores (yeast-like budded cells) or hyphal bodies (chains of budded cells). As the infection progresses, the insect host succumbs to the infection, either through fungal toxins or by starvation. In most fungal entomopathogens, hyphae break through the cuticle to produce either infective spores or resting structures on the insect cadaver (Fig. 2, top panel; recently reviewed in Lovett and St. Leger, 2017).
Infection by penetration is observed in mosquito larvae and adults, with histological examinations available for several species combinations of fungi/water molds and mosquitoes. Clark et al. (1968) describes the histological manifestation of B. bassiana infection in Cx. pipiens larvae, observing mycelial growth preferentially around the tracheal trunks, suggesting invasion in an around the trachea. Infection of Cx. pipiens larvae by B. bassiana led to the disintegration and deformation of the epicuticle, midgut epithelium, and muscles (Benzina et al., 2018). Cuticle penetration has also been commonly observed in mosquito larval infections by Coelomomyces sp. (Shoulkamy and Lucarotti, 1998; Wong and Pillai, 1980). C. psorophorae zygospore adhesion to Culiseta inornata larval cuticle is followed by germination and development of a narrow hyphal body that penetrates the host epidermal cells (Travland, 1979). An ultrastructural study of C. stegomyiae-infected Ae. aegypti larvae revealed hyphal growth in the muscles with deterioration of myofibrils, degeneration of midgut muscles, and fragmentation of the microvilli that line the malpighian tubules (Shoulkamy et al., 2001).
The infection process of water molds is similar. Motile zoospores of L. giganteum attach to the cuticle of mosquito larvae, a germ tube penetrates the body wall, hyphae grow in the hemocoel, and start to produce sporangia. The sporangia develop exit tubes that grow out through the cuticle, ultimately releasing zoospore vesicles into the aquatic environment. In addition, resting structures in the form of sexual oospores can also be produced (Fetter-Lasko and Washino, 1983; Brey et al., 1988). Attachment of L. giganteum zoospores followed by penetration of the larval cuticle has been observed in Aedes aegypti, An. gambiae, and Cx. pipiens larvae (Brey et al., 1988; Golkar et al., 1993). Similarily, zoospores of L. chapmanii have been shown to aggregate, and then enters via penetration tubes the head, body, anal papillae, and intersegmental folds of Ae. aegypti and Cx. quinquefasciatus larvae (Lord and Fukuda, 1988; Zattau and McInnis, 1987).
Exposure of adult An. stephensi mosquitoes to B. bassiana impregnated filter papers, led to the accumulation and germination of fungal spores on the proboscis, tarsi, legs and wings. During early stages of infection, hyphae invaded tissues and organs, including compound eyes, brain, salivary glands, mouthparts, muscles, midgut, ovaries, and malpighian tubules (Ishii et al., 2017). Conidiobolus coronatus infects Aedes taeniorhynchus and Cx. quinquefasciatus adult mosquitoes through cuticle penetration of the intersegmental interstices, head, and dorsal thoracic regions. Histological studies on infected mosquitoes revealed the dissemination of hyphal bodies in hemocoel, muscles, fat body, and gonads (Lowe et al., 1968; Lowe and Kennel, 1972). Infection of adult Aedes sierrensis mosquitoes with Tolypocladium cylindrosporum, showed fungal hyphae and hyphal bodies exclusively in the thorax, suggesting active penetration through the cuticle of the thoracic spiracles (Soarés, 1982).
2.2. Ingestion of fungal spores
Fungal spore uptake through ingestion has been commonly observed in mosquito larvae. Ingestion of C. clavisporus, B. bassiana, M. anisopliae, and Aspergillus clavatus spores was reported for Aedes, Culex, and Anopheles mosquito larvae (Miranpuri and Khachatourians, 1991; Scholte et al., 2004b; Seye et al., 2009). Upon ingestion, the spores may remain in the midgut and release toxins that cause pathology and larval killing (Fig. 2, center panel). Cross sections of Cx. pipiens larvae during early stages of infection by M. anisopliae revealed the accumulation of intact fungal spores in the gut, confirming spore ingestion. Digestion of fungal spores was observed in cross section of dead larvae where large number of spores were disrupted (Crisan, 1971). Ingestion of M. anisopliae spores by Cx. quinquefasciatus larvae induced larval mortality 24 hours post-exposure, suggesting the release of toxins during fungal spore digestion. Guts of infected larvae revealed the accumulation of non-germinated and partially digested fungal spores (Lacey et al., 1988). In neither study, oral uptake of M. anisopliae did result in disseminated fungal infection.
Other fungal entomopathogens spread from the midgut lumen throughout the mosquito body by penetration through the midgut epithelium (Fig. 2, center panel). For example, the conidia of C. clavisporus adhere to the gut walls of Cx. fatigans mosquito larvae, where they germinate and penetrate the gut epithelium (Sweeney, 1975). Ingestion of B. bassiana blastospores rather than conidia by Ae. aegypti larvae result in the spread of fungal spores throughout the larval body 24 hours post ingestion, with heavy colonization in the fore-, mid-, and hindgut (Miranpuri and Khachatourians, 1991). Exposure of Cx. quinquefasciatus larvae to A. clavatus led to the accumulation of fungal spores in the alimentary canal. Infection resulted in the destruction of gut cells and disruption of the gut epithelium in several locations, facilitating fungal dissemination in the mosquito body (Bawin et al., 2016). Ingestion Fusarium oxysporum spores by Aedes detritus and Cx. pipiens led to the colonization of the gut lumen and penetration of the epithelial lining (Hasan and Vago, 1972).
Several species in the genus Smittium establish symbiotic relationships due to attachment to the cuticular lining of the hindgut (Fig. 2, bottom panel; Lichtwardt, 1996). In most cases, the symbiotic relationship remains commensal, and the fungus is shed with every molt. Pathology can be induced by hyphal overgrowth causing complete blockage of the alimentary canal, as it is observed for Smittium culisetae (Williams and Lichtwardt, 1972). The only species within this genus that can be considered a facultative pathogen of mosquito larvae is Smittium morbosum (Coluzzi, 1966; Sweeney, 1981; Sato et al., 1989). Upon initial attachment to the hindgut, Smittium morbosum grows anteriorly, and hyphae penetrate the posterior midgut epithelium. Systemic infection is prevented by the mosquito immune system through strong melanization at the basal side of the midgut epithelium. However, this melanotic mass anchors the hyphae, and either allows transstadial transmission, or leads to incomplete molting, causing larval death. S. morbosum.
In addition to infection by cuticle penetration, as described in section 2.1, zoospores of the water mold L. chapmanii have been shown to be ingested by Ae. aegypti larvae (Pelizza et al., 2008; Zattau and McInnis, 1987). Upon ingestion, the zoospores germinated in the midgut, elongated and formed branched hyphae that penetrated the peritrophic matrix and midgut epithelium, reaching the hemocoel. Once in the hemocoel, infection proceeded similarily to infection initiated by culticle penetration (Zattau and McInnis, 1987).
To the best of our knowledge, there is currently no evidence that adult mosquitoes commonly ingest entomopathogenic fungi, or that oral uptake of fungal entomopathogens results in adult mosquito infection.
2.3. Fungal entry through injury
Fungal infection of mosquitoes can be facilitated through injuries that breach the cuticle, as these allow entry of opportunistic pathogens (Fig. 2, top panel). The entomopathogenic fungus Pythium sp. cannot infect mosquito larvae through penetration, but infects and kills mechanically injured Aedes, Anopheles, and Culex mosquito larvae (Clark et al., 1966). Experimental infections can also be achieved through intrathoracic conidial spore injection, as observed for B. bassiana in adult An. gambiae mosquitoes and M. anisopliae and A. clavatus in Cx. quinquefasciatus larvae (Bawin et al., 2014; Yassine et al., 2012). While this route of infection is perhaps occurs less frequently in the field, and is difficult to exploit for biological control, it can facilitate research of molecular mosquito-fungal interactions and antifungal immunity.
3. Antifungal immune mechanisms in mosquitoes
Once a water mold or a fungus has penetrated the mosquito cuticle and entered the hemocoel, it encounters both efficient cellular and humoral immune responses. Survival thus depends on its ability to largely avoid or eventually overcome these immune responses. Nevertheless, immune escape is incomplete. For example, B. bassiana is recognized, and its growth is limited by the mosquito immune system. The outcome of infection is thus strongly influenced by the immune competency of the host. Somewhat surprisingly, few data are available to date on the molecular interactions of mosquitoes with fungi. This section will provide a brief overview of the molecules and pathways currently known to be involved in fungus recognition and killing in various mosquito species.
3.1. Recognition
β-1,3-glucans are the known molecules on the surface of fungal cell walls that are recognized by insect immune systems (Lu and St. Leger, 2016). Studies in various insects have identified β-1,3-glucan recognition proteins (GRPs) and Gram-negative binding proteins (GNBPs) to bind to β-1,3-glucan and trigger downstream immune responses, including Toll pathway activation and/or melanization (Jiang et al., 2004; Matskevich et al., 2010; Ochiai and Ashida, 2000). The only molecule currently confirmed to bind β-1,3-glucan in mosquitoes is a GRP from Armigeres subalbatus (AsGRP). AsGRP binds to curdlan, an insoluble β-13-glucan polymer, and is required for melanization of some bacterial species (Wang et al., 2006, 2005). If AsGRP contributes to melanization of fungal species is unclear. GNBP2 was found to be transcriptionally upregulated in An. gamibae mosquitoes that were chanllenged by injection of dead B. bassiana conidia (Aguilar et al., 2005). Whether GNBP2 can directly bind to fungal surfaces however remains to be tested. The water mold L. chapmanii is also detected in mosquito larvae, as evidenced by localized immune responses to its surface (Zattau and McInnis, 1987). The cell wall of L. chapmanii, and that of all water molds, does not contain chitin, and it is unclear how and which molecules on its surface are responsible for immune recognition.
Thioester-containing proteins (TEPs) bind to many foreign surfaces and are critical for pathogen recognition and innate immunity in mosquitoes (Blandin and Levashina, 2004). The activation of the complement-like pathway in mosquitoes leads to the deposition of thioester-containing protein 1 (TEP1) on foreign surfaces (Fig. 3). TEP1 acts as an opsonin and promotes the phagocytosis of bacteria (Levashina et al., 2001; Moita et al., 2005) and lysis of the rodent malaria parasite, Plasmodium berghei (Blandin et al., 2004). Several studies point to the involvement of TEPs in antifungal immunity in mosquitoes. Infection of Ae. aegypti mosquitoes with B. bassiana and Cordyceps javanica resulted in upregulation of TEP22 in fat body and midgut of the infected mosquitoes (Ramirez et al., 2018a; 2019). Knockdown of TEP22 increased the susceptibility of Ae. aegypti to B. bassiana infection, confirming the role of TEP22 in antifungal immune response (Wang et al., 2015). In An. gambiae, TEP1 binds to B. bassiana hyphal bodies (Yassine et al., 2012). Knockdown of TEP1 decreased hyphal melanization and increased susceptibility of An. gambiae mosquitoes to infection by B. bassiana (Yassine et al., 2012). Together, these studies suggest that TEPs are important molecules for recognition of fungi in the mosquito hemocoel and critically contribute to the antifungal immune response in mosquitoes. However, the binding partner of TEPs on the surface of fungi, or any other surfaces awaits identification.
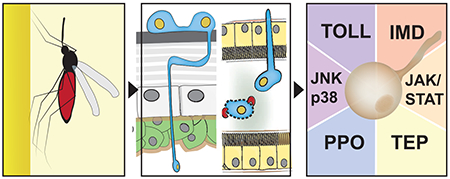
Figure 3: Immune modules that regulate mosquito antifungal immunity.
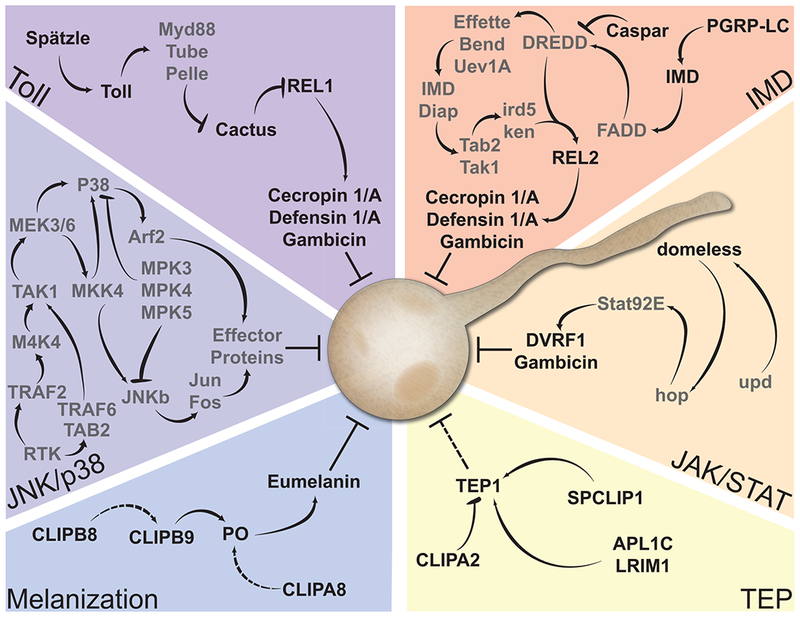
Fungi that penetrate epithelia and reach the hemolymph, as indicated by the germinating blastospore drawing, are attacked by multiple antifungal molecules. These molecules can be expression products of signal transduction pathways, including the Toll, IMD, JAK/STAT and MAPK pathways. Alternatively, these molecules are proteolytic activation products of humoral killing modules, circulating in the hemolymph, including PO and TEP1, which are products of the melanization and complement-like pathways, respectively. Proteins are bolded if their role in mosquito antifungal immunity is supported experimentally. Other key factors of the mosquito immune modules are listed in gray.
In addition to β-1,3-glucan, other sugar moieties on the fungal surface may be recognized. Neuraminic acid and/or sialic acid on the surface of B. bassiana blastospores are potential binding partners for lectins (Wanchoo et al., 2009). It remains to be seen whether mosquito-derived lectins can recognize these sugars and contribute to antifungal immunity or immunity against water molds.
3.2. Killing mechanisms
3.2.1. Cellular Immune Responses
Cellular immune responses, mediated by hemocytes, can kill fungal cells within the hemolymph in insects. Hemocytes phagocytize, encapsulate, and nodulate blastospores and hyphal bodies (Lavine and Strand, 2002). Phagocytosis is initiated by the recognition and binding of phagocytic cells to fungal particles, leading to particle engulfment through cytoskeleton modification and intracellular vesicular transport to the phagosome (Jiravanichpaisal et al., 2006). Phagocytosis of blastospores of the fungal species M. anisopliae, B. bassiana, Cordyceps farinosa, Metarhizium rileyi, Cordyceps fumosorosea, and C. albicans has been observed in several insect species (Hung et al., 1993; Hung and Boucias, 1992; Kawakami, 1965; Ouedraogo et al., 2003; Pendland and Boucias, 1996). In mosquitoes, granulocytes and less commonly prohemocytes exhibit phagocytic activity (Hillyer and Strand, 2014). Inoculation of An. albimanus with S. cerevisiae resulted in the melanization of yeast cells and encapsulation by plasmatocytes. However, phagocytosis of yeast cells was not observed (Hernández-Martínez et al., 2002).
Cellular immune responses have also been reported in response to water mold infections. L. giganteum increased transiently total hemocyte numbers in Cx. quinquefasciatus larvae, as well as causing a relative increase of granulocytes compared to over hemocyte cell types (Fei and Huai-en, 2001). This suggests that L. giganteum can stimulate cellular immunity in mosquitoes. Indeed, hemocytes in Ae. aegypti larvae form loose capsules on L. giganteum hyphae. In how far these cellular responses limit L. giganteum infection of mosquito larvae remains to be tested.
3.2.2. Antimicrobial Peptides (AMPs)
In addition to cellular immune responses, insects mount humoral immune responses against fungi, including the expression of antimicrobial peptides (AMPs, Fig. 3). Most insect AMPs are short amphipathic peptides that are active against gram-positive and/or gram-negative bacteria (Boman, 2003; Tonk and Vilcinskas, 2017). All mosquito genomes annotated to date, encode members of the defensin, cecropin, diptericin, and gambicin AMP families (Bartholomay et al., 2010; Christophides et al., 2002; García Gil de Muñoz et al., 2008; Neafsey et al., 2015; Waterhouse et al., 2007). In addition, a putative attacin gene has been identified in Ae. aegypti and An. gambiae (Waterhouse et al., 2007). Of these, defensin A and C (DEF1), cecropin A (CEC1), and gambicin (GAM1) are currently implicated in antifungal immunity. Infection of Cx. quinquefasciatus larvae with Metarhizium brunneum conidia and blastospores upregulates DEF1, CEC1, and GAM1 (Alkhaibari et al., 2018). Infection of adult Ae. aegypti with either B. bassiana or C. javanica upregulated defensin A and C , as well as cecropin D and G (Ramirez et al., 2019). Synthetic An. gambiae CEC1 exhibited antifungal activity against the yeasts S. cerevisiae, Cryptococcus neoformans, and C. albicans, and filamentous fungi belonging to Aspergillus and Fusarium genera (Delucca et al., 1997; Vizioli et al., 2000). Similarly, CEC1, purified from Ae. aegypti hemolymph exhibited antifungal activity against C. albicans, C. neoformans, S. cerevisiae, Fusarium culmorum, and Neurospora crassa (Lamberty et al., 2001; Lowenberger et al., 1999). DEF, isolated from female An. gambiae midgut tissues, inhibited hyphal growth of the filamentous fungi Botrytis cinerea, F. oxysporum, F. culmorum, and N. crassa (Vizioli et al., 2001b). GAM1, purified from An. gambiae 4a-3B cell conditioned media inhibited hyphal growth of N. crassa (Vizioli et al., 2001a). Beyond known AMPs, few studies have specifically searched for antifungal peptides (AFPs) in insects (Al Souhail et al., 2016; Faruck et al., 2016), and none have been conducted in mosquitoes.
3.2.3. Melanization
In addition to AMP expression, the arthropod-specific melanization immune response is commonly observed against fungi (Fig. 3). Aromatic amino acids are converted to eumelanin, which is deposited on surfaces that are recognized by the immune system as foreign. This acellular encapsulation is thought to kill pathogens through site-directed cytotoxicity and/or by blocking nutrient uptake (Nappi and Christensen, 2005; Nappi and Vass, 1993). A key enzyme in melanization is prophenoloxidase (PPO), which is expressed from nine to ten paralogous genes in all mosquito genomes annotated to date (Bartholomay et al., 2010; Neafsey et al., 2015; Waterhouse et al., 2007). Infection of An. gambiae larvae with L. giganteum triggered a heavy melanization response of hyphae two hours post-infection, while Ae. aegypti larvae had a lower melanization response of less than 10% of hyphae (Golkar et al., 1993). Melanization in Ae. aegypti larvae was observed at the entry points of L. chapmanii zoospores and along the hyphal growth into the coelomic cavity (Zattau and McInnis, 1987). Yeast cells, deposited into the hemocoel of mosquitoes trigger an efficient melanization response. S. cerevisiae, injected into the hemocoel of An. albimanus is unable to grow and establish an infection in the hemolymph, due to the melanization of the yeast cells (Hernández-Martínez et al., 2002). Likewise, C. albicans is efficiently melanized and killed in the hemolymph of Cx. quinquefasciatus (Da Silva et al., 2000). Melanization in response to fungal entomopathogen infection has been observed in larvae of multiple mosquito species. Anopheles amicatus, Anopheles annulipes, Cx. fatigans, and Ae. tarsalis larvae form melanotic capsules around Culicinomyces at all sites of infection (Sweeney, 1975). The fungus is encapsulated on the cuticle of the larval foregut and hindgut, the fungal penetration sites between gut and hemolymph, and inside the hemolymph. Cuticular pigmentation and darkening of the head capsule was observed in early larval instars of An. stephensi when exposed to B. bassiana (Prasad and Veerwal, 2010). The melanization response in An. gambiae confers partial resistance to B. bassiana infection, as fungal load and mosquito death rate increased when the melanization response was experimentally decreased (Yassine et al., 2012). The resistance to fungal infection was in part mediated by TEP1, as its depletion from mosquito hemolymph resulted in less deposition of phenoloxidase on the surface of B. bassiana hyphal bodies. This study thus provided first molecular insight into mosquito antifungal immunity beyond AFPs, providing additional evidence that TEP1 functions as an opsonin for melanization (Yassine et al., 2012).
3.3. Regulation of antifungal immunity through signal transduction pathways
Several signal transduction pathways contribute to mosquito immunity, including Toll, IMD, JAK-STAT, JNK, and p38 signal transduction pathways (Fig. 3, Bian et al., 2005; Frolet et al., 2006; Meister et al., 2009, 2005; Shin et al., 2005). Below we briefly summarize current knowledge of these signal transduction pathways with regards to their (i) activation by fungal infections, (ii) control of antifungal molecule expression, and (iii) impact on fungal infection outcome.
3.3.1. Toll Pathway
The Toll pathway is a major immune signaling pathway in insects, whose role in antifungal immunity is well documented (Lemaitre et al., 1996). Named after its transmembrane receptor Toll, it consists of an extracellular protease cascade that activates the Toll ligand Spatzle, and an intracellular signal transduction cascade that translocates an NF-κB transcription factor into the nucleus (Hoffmann and Reichhart, 2002; Lemaitre, 2004; Rhodes and Michel, 2017; Valanne et al., 2011). In mosquitoes, the NF-κB transcription factor downstream of the Toll pathway is REL1 (Barillas-Mury et al., 1996; Meister et al., 2005; Shin et al., 2005). B. bassiana infection activates the Toll pathway in Ae. aegypti and An. gambiae adult mosquitoes (Dong et al., 2012; Rhodes et al., 2018; Shin et al., 2006). Cordyceps javanica and Beauveria brongniartii also activate the Toll pathway in Ae. aegypti adult mosquitoes (Ramirez et al., 2018a). Manipulation of the pathway in mosquitoes strongly influences fungal infection outcome. In Ae. aegypti, knockdown of Toll5A and Spz1C decreased survival in B. bassiana-infected mosquitoes (Shin et al., 2006). Similarly, Rel1 knockdown in Ae. aegypti and An. gambiae mosquitoes decreased mosquito survival to infection by B. bassiana (Bian et al., 2005; Rhodes et al., 2018; Shin et al., 2005). Knockdown of Cactus, a negative regulator of the Toll pathway increased survival of An. gambiae mosquito to infections with B. bassiana (Rhodes et al., 2018). The Toll pathway regulates the expression of several known AMPs with antifungal activity, including DEF1, CEC1, and GAM1 (Barillas-Mury et al., 1996; Garver et al., 2009; Luna et al., 2006; Shin et al., 2005; Zhang et al., 2017). In addition, the Toll pathway regulates the basal expression levels of TEP1 (Frolet et al., 2006). As the Toll pathway controls about 10% of the mosquito transcriptome, its influence on fungal infection likely goes beyond the regulation of these few antifungal factors.
3.3.2. IMD Pathway
The IMD pathway is a major immune signal transduction pathway in the gut and regulates malaria parasite killing in mosquitoes (Antonova et al., 2009; Chen et al., 2012; Garver et al., 2009, 2012; Meister et al., 2005, 2009). Its downstream NF-κB transcription factor in mosquitoes is called REL2 (Fig. 3, Antonova et al., 2009; Meister et al., 2005). Infection of Ae. Aegypti mosquitoes with B. bassiana, B. brongniartii, and C. javanica upregulated Rel2 transcript levels in the midgut and fat body six days post-infection (Ramirez et al., 2018a; Ramirez et al., 2019), suggesting the activation of the IMD pathway upon fungal challenge.
The IMD pathway confers resistance to fungal infections in mosquitoes. Knockdown of Rel2 in B. bassiana and C. javanica-infected Ae. aegypti adult mosquitoes decreased mosquito survival to infection and increased fungal load in infected mosquitoes (Ramirez et al., 2018b). Similar to the Toll pathway, the IMD pathway regulates the expression of the DEF1, CEC1, and GAM1 (Antonova et al., 2009; Garver et al., 2009; Luna et al., 2006; Meister et al., 2005, 2009; Ramirez et al., 2018b; Zhang et al., 2017). Altered expression of these antifungal molecules may contribute to the antifungal resistance that is exerted through the IMD pathway.
3.3.3. JAK-STAT Pathway
In mosquitoes, the JAK-STAT pathway is involved in antiviral and anti-Plasmodium immunity (Carissimo et al., 2015; Gupta et al., 2009; Souza-Neto et al., 2009). The pathway is activated by ligand-binding to the domeless (Dome) receptor, and ultimately translocates the transcription factor Stat92E to the nucleus (Fig. 3, Zeidler and Bausek, 2013). Infection of adult Ae. aegypti with B. bassiana upregulated the expression of Dome and the STAT-regulated anti-dengue restriction factor 1 (DVRF1). Similarly, the expression of STAT was upregulated in Ae. aegypti mosquitoes infected by B. bassiana, Be. brongniartii, and Isaria amoenerosea (Ramirez et al., 2018b, 2018c). Knockdown of Dome or DVRF1 decreased mosquito survival to B. bassiana infection (Dong et al., 2012). In Ae. aegypti cells, the JAK-STAT pathway upregulates GAM1 expression in response to C. albicans exposure (Zhang et al., 2017), which may contribute to the JAK-STAT pathway-mediated antifungal immunity in mosquitoes.
3.3.3. MAPK Pathways
Less is known about the role of the JNK and p38 MAP-kinase signaling pathways in mosquito immunity including responses to fungal infection. In Ae aegypti mosquitoes, infection with C. albicans upregulates MAP kinase kinase 4 (MAPK4), which is upstream to JNK and p38 kinases (Wu and Cho, 2014). Likewise, infection with Be. brongniartii upregulates the expression of JNK in the midgut and fat body (Ramirez et al., 2018a). It is unclear whether either of these pathways contribute to antifungal immunity in mosquitoes. However, in Drosophila, p38 mutant flies exhibited increased susceptibility to infection by B. bassiana and Aspergillus fumigatus (Chen et al., 2010).
3.4. Regulation of antifungal immunity through protease cascades
In addition to transcriptional regulation, several mosquito humoral immune responses are regulated through proteolytic activation of key immune factors and enzymes, including TEP1 and PPO (Nakhleh et al., 2017; Rhodes and Michel, 2017). Proteolytic activity is regulated through cascades of Clip-domain containing serine proteinases (CLIPs) and their proteolytically inactive homologs (clip-SPHs, most often CLIPAs). TEP1 function is augmented by two clip-SPHs, SPCLIP1 and CLIPA2. SPCLIP1 is essential for TEP1 deposition on the surface of microbes and acts as a positive regulator of the complement-like pathway (Povelones et al., 2013). CLIPA2 acts as a negative regulator through inhibiting the production of active TEP1 during infection (Yassine et al., 2014). Knockdown of CLIPA2 decreased susceptibility of An. gambiae to B. bassiana in a TEP1-dependent manner, confirming the role of TEP1 in antifungal immunity (Kamareddine et al., 2016).
An. gambiae CLIPA8 is a positive regulator of phenoloxidase activation and required for melanization in response to P. berghei parasites, bacteria, and B. bassiana (Schnitger et al., 2007; Yassine et al., 2012). Knockdown of CLIPA8 abolished the melanization of B. bassiana hyphae, and decreased resistance to B. bassiana infection (Yassine et al., 2012). While several CLIPBs are required for PO activation in mosquitoes (An et al., 2011; Zhang et al., 2016), their role in antifungal immunity in mosquitoes is untested.
4. Summary and outlook
The mosquito immune system has evolved in the context of continuous encounters between mosquitoes and fungi ranging from food to foes. Many of these encounters indeed are beneficial to the mosquito. Yeast hold nutritional value for mosquito larvae (Asahina, 1964; Steyn et al., 2016), and may generate a hypoxic environment in the larval midgut required for mosquito development (Valzania et al., 2018). Encounters with potential opportunistic fungal pathogens may remain benign for the mosquito due to physical and physiological barriers, including the cuticle and digestive enzymes (Gillespie et al., 2000). However, an significant number of fungal entomopathogens can overcome barriers to infection and colonize the mosquito hemocoel with often lethal consequences to the host. It is thus not surprising to find all branches of the mosquito immune system to be engaged in antifungal immunity. The current body of literature describes some of these interactions on a histological, and to a much lesser extent molecular level.
Important knowledge gaps remain. (i) Out of the many potential mosquito-fungal encounters, little is known of the mosquito immune system’s role in fungal interactions beyond defense against a small number of well-studied entomopathogens in adult infections. Comparative studies using multiple mosquito and fungal species combinations would be valuable to address whether a core of anti-fungal immunity mechanisms are engaged across mosquito species, and what biotic and abiotic factors influence the efficacy of these immune responses. Mosquito larval immunity is largely unexplored, and its contribution to antifungal defense is unclear. It is likely that exposure of the larval immune system impacts adult immunity. This is important to explore, as it could impact vector-borne pathogen susceptibility and transmission rates.
(ii) The molecular identity of antifungal molecules in both mosquito larvae and adults is largely unexplored. Naturally occurring AFPs constitute a numerous and structurally highly diverse group of peptides (van der Weerden et al., 2013), and it is likely that mosquitoes produce antifungals beyond canonical AMPs. Beyond knowledge gain, mosquito AFPs could have practical applications, including their use as biomarkers of exposure, and natural product fungicides (Rautenbach et al., 2016; van der Weerden et al., 2013).
(iii) During colonization, entomopathogenic fungi clearly interact with mosquito epithelia, including the epidermis, tracheal linings, and the gut epithelium. However, the role of epithelial immunity in limiting fungal colonization is unknown. Mosquito epithelial immune responses contribute to maintenance of a healthy microbiota and defense against vector-borne disease pathogens, and likely also limit fungal infections through similar mechanisms.
(iv) In laboratory studies, boosting mosquito basal immunity reduces fungal entomopathogen infections (Rhodes et al., 2018; Yassine et al., 2012). Different strains and species of fungal entomopathogens differ in their virulence against the same mosquito species (Blanford et al., 2012). Entomopathogen virulence is also influenced by abiotic factors, such as temperature (Heinig et al., 2015). However, in contrast to field and laboratory studies on variation in host susceptibility to vector-borne pathogens (Collins et al., 1986; Gubler, 1979; Gubler and Rosen, 1976; Huff, 1929, 1927; Niaré et al., 2002; Wallis et al., 1985), mosquito host genotype influence on fungal entomopathogen infection is, to our knowledge, unexplored. Such studies may help to identify non-canonical antifungal immune responses in mosquitoes. Such knowledge could further aid biocontrol programs to select for strains that even more readily overcome mosquito immunity.
(v) Our knowledge of mosquito-fungus interactions is mostly confined to entomopathogens, due to their potential for biological control of mosquitoes. In contrast to bacterial microbiomes, investigations of naturally occurring fungal associations with mosquito larvae and adults are just beginning. So far, a handful of studies have identified yeasts and filamentous fungi associated with mosquitoes. Future studies should explore whether the transient nature of these associations impact mosquito physiology, and shape the immune status of mosquitoes.
Supplementary Material
Highlights.
Mosquito-fungus interactions occur in all life stages.
Fungal entomopathogens are taken up passively or enter the mosquito actively.
Fungal infections activate all branches of the mosquito immune system.
New technology enables research of mosquito-fungus interactions beyond biocontrol.
Acknowledgements
We thank Carol Sevin, Research librarian at Kansas State University, for helping us to retrieve literature on mosquito interactions with B. bassiana and M. anisopliae. Dr. K.M. is partially supported by the National Institutes of Health grant number R01AI140760, and P.T. is supported through the USDA-ARS Specific Cooperative Agreement 58-5430-4-022. In addition, this work was supported by the USDA National Institute of Food and Agriculture, Hatch project 1003627. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies. This is contribution 19-190-J from the Kansas Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar R, Jedlicka AE, Mintz M, Mahairaki V, Scott AL, Dimopoulos G, 2005. Global gene expression analysis of Anopheles gambiae responses to microbial challenge. Insect Biochemistry and Molecular Biology, Genetic manipulation of insects 35, 709–719. [DOI] [PubMed] [Google Scholar]
- Al Souhail Q, Hiromasa Y, Rahnamaeian M, Giraldo MC, Takahashi D, Valent B, Vilcinskas A, Kanost MR, 2016. Characterization and regulation of expression of an antifungal peptide from hemolymph of an insect, Manduca sexta. Dev. Comp. Immunol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhaibari AM, Maffeis T, Bull JC, Butt TM, 2018. Combined use of the entomopathogenic fungus, Metarhizium brunneum, and the mosquito predator, Toxorhynchites brevipalpis, for control of mosquito larvae: Is this a risky biocontrol strategy? Journal of Invertebrate Pathology 153, 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Budd A, Kanost MR, Michel K, 2011. Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cellular and Molecular Life Sciences 68, 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova Y, Alvarez KS, Kim YJ, Kokoza V, Raikhel AS, 2009. The role of NF-κB factor REL2 in the Aedes aegypti immune response. Insect Biochemistry and Molecular Biology 39, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahina S, 1964. Food material and feeding procedures for mosquito larvae. Bull World Health Organ 31, 465–466. [PMC free article] [PubMed] [Google Scholar]
- Araújo JPM, Hughes DP, 2014. Diversity of entomopathogens Fungi: Which groups conquered the insect body? [DOI] [PubMed]
- Barillas-Mury C, Charlesworth A, Gross I, Richman A, Hoffmann JA, Kafatos FC, 1996. Immune factor Gambif1, a new Rel family member from the human malaria vector, Anopheles gambiae. Embo J 15, 4691–4701. [PMC free article] [PubMed] [Google Scholar]
- Bartholomay LC, Michel K, 2018. Mosquito Immunobiology: The intersection of vector health and vector competence. Annual Review of Entomology 63, 145–167. [DOI] [PubMed] [Google Scholar]
- Bartholomay LC, Waterhouse RM, Mayhew GF, Campbell CL, Michel K, Zou Z, Ramirez JL, Das S, Alvarez K, Arensburger P, Bryant B, Chapman SB, Dong Y, Erickson SM, Karunaratne SHPP, Kokoza V, Kodira CD, Pignatelli P, Shin SW, Vanlandingham DL, Atkinson PW, Birren B, Christophides GK, Clem RJ, Hemingway J, Higgs S, Megy K, Ranson H, Zdobnov EM, Raikhel AS, Christensen BM, Dimopoulos G, Muskavitch MAT, 2010. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science 330, 88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawin T, Seye F, Boukraa S, Zimmer J-Y, Raharimalala FN, Ndiaye M, Compere P, Delvigne F, Francis F, 2016. Histopathological effects of Aspergillus clavatus (Ascomycota: Trichocomaceae) on larvae of the southern house mosquito, Culex quinquefasciatus (Diptera: Culicidae). Fungal Biology 120, 489–499. [DOI] [PubMed] [Google Scholar]
- Benzina F, Hamid S, Mohand-Kaci H, Bissaad F, Halouane F, 2018. Histological changes in the larvae of the domestic mosquito Culex pipiens treated with the entomopathogenic fungus Beauveria bassiana. Scientific Research and Essays 13, 1–10. [Google Scholar]
- Bian G, Shin SW, Cheon H-M, Kokoza V, Raikhel AS, 2005. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proceedings of the National Academy of Sciences 102, 13568–13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bille J, Stockman L, Roberts GD, 1982. Detection of yeasts and filamentous fungi in blood cultures during a 10-year period (1972 to 1981). Journal of clinical microbiology 16, 968–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S, Levashina EA, 2004. Thioester-containing proteins and insect immunity. Molecular Immunology, Innate Immunity 40, 903–908. [DOI] [PubMed] [Google Scholar]
- Blandin S, Shiao S-H, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA, 2004. Complement-like protein TEP1 is a seterminant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116, 661–670. [DOI] [PubMed] [Google Scholar]
- Blandin SA, Marois E, Levashina EA, 2008. Antimalarial responses in Anopheles gambiae: from a complement-like protein to a complement-like pathway. Cell Host & Microbe 3, 364–374. [DOI] [PubMed] [Google Scholar]
- Blanford S, Jenkins NE, Read AF, Thomas MB, 2012. Evaluating the lethal and pre-lethal effects of a range of fungi against adult Anopheles stephensi mosquitoes. Malaria Journal 11, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman HG, 2003. Antibacterial peptides: basic facts and emerging concepts. J. Intern. Med 254, 197–215. [DOI] [PubMed] [Google Scholar]
- Bozic J, Capone A, Pediconi D, Mensah P, Cappelli A, Valzano M, Mancini MV, Scuppa P, Martin E, Epis S, Rossi P, Favia G, Ricci I, 2017. Mosquitoes can harbour yeasts of clinical significance and contribute to their environmental dissemination. Environ Microbiol Rep 9, 642–648. [DOI] [PubMed] [Google Scholar]
- Braun A, 1855. Algarum unicellularium genera nova et minus cognita: praemissis observationibus de algis unicellularibus in genere. Engelmann. [Google Scholar]
- Brey PT, Lebrun RA, Papierok B, Ohayon H, Vennavalli S, Hafez J, 1988. Defense reactions by larvae of Aedes aegypti during infection by the aquatic fungus Lagenidium giganteum (Oomycete). Cell Tissue Res. 253, 245–250. [DOI] [PubMed] [Google Scholar]
- Butt TM, Greenfield BPJ, Greig C, Maffeis TGG, Taylor JWD, Piasecka J, Dudley E, Abdulla A, Dubovskiy IM, Garrido-Jurado I, Quesada-Moraga E, Penny MW, Eastwood DC, 2013. Metarhizium anisopliae pathogenesis of mosquito larvae: a verdict of accidental death. PLoS ONE 8, e81686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt TM, Coates CJ, Dubovskiy IM, Ratcliffe NA, 2016. Entomopathogenic fungi: new insights into host-pathogen interactions. Adv. Genet 94, 307–364. [DOI] [PubMed] [Google Scholar]
- Carissimo G, Pondeville E, McFarlane M, Dietrich I, Mitri C, Bischoff E, Antoniewski C, Bourgouin C, Failloux A-B, Kohl A, Vernick KD, 2015. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proceedings of the National Academy of Sciences 112, E176–E185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo JM, Roberts DW, 1980. Coelomomyces pathogens of Culicidae (mosquitos). Bull World Health Organ 58, 53–67. [PMC free article] [PubMed] [Google Scholar]
- Castillo JM, Roberts DW, 1980. Fungal pathogens, except Coelomomyces, of Culicidae (mosquitos). Bull. World Health Organ. 58 Suppl, 69–83. [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Leaw SN, Huang AH, Wu TL, Chang TC, 2001. Rapid identification of yeasts in positive blood cultures by a multiplex PCR method. Journal of Clinical Microbiology 39, 3466–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell CR, Fukami T, 2018. Nectar yeasts: a natural microcosm for ecology. Yeast 35, 417–423. [DOI] [PubMed] [Google Scholar]
- Chen J, Xie C, Tian L, Hong L, Wu X, Han J, 2010. Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc Natl Acad Sci U S A 107, 20774–20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dong Y, Sandiford S, Dimopoulos G, 2012. Transcriptional mediators Kto and Skd are involved in the regulation of the IMD pathway and anti-Plasmodium defense in Anopheles gambiae. PLOS ONE 7, e45580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang N-T, Liu S-D, 1990. Virulence of entomogenous fungus Metarhizium anisopliae var. anisopliae to Aedes aegypti and Aedes albopictus larvae. Chinese Journal of Microbiology and Immunology 23, 253–257. [PubMed] [Google Scholar]
- Christophers SR, 1952. The recorded parasites of mosquitoes. Rivista di parassitologia 13, 21–28. [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, Blass C, Brey PT, Collins FH, Danielli A, Dimopoulos G, Hetru C, Hoa NT, Hoffmann JA, Kanzok SM, Letunic I, Levashina EA, Loukeris TG, Lycett G, Meister S, Michel K, Moita LF, Muller H-M, Osta MA, Paskewitz SM, Reichhart J-M, Rzhetsky A, Troxler L, Vernick KD, Vlachou D, Volz J, von Mering C, Xu J, Zheng L, Bork P, Kafatos FC, 2002. Immunity-related genes and gene families in Anopheles gambiae. Science 298, 159–165. [DOI] [PubMed] [Google Scholar]
- Clark TB, Kellen WR, Fukuda T, Lindegren JE, 1968. Field and laboratory studies on the pathogenicity of the fungus Beauveria bassiana to three genera of mosquitoes. J. Invertebr. Pathol 11, 1–7. [DOI] [PubMed] [Google Scholar]
- Clark TB, Kellen WR, Lindegren JE, Sanders RD, 1966. Pythium sp. (Phycomycetes: Pythiales) pathogenic to mosquito larvae. Journal of Invertebrate Pathology 8, 351–354. [Google Scholar]
- Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, Collins WE, Campbell CC, Gwadz RW, 1986. Genetic selection of a Plasmodium-refractory strain of the malaria vector An. gambiae. Science 234, 607–610. [DOI] [PubMed] [Google Scholar]
- Coluzzi M, 1966. Experimental infections with Rubetella fungi in Anopheles gambiae and other moquitoes. in: Corradetti A (Ed.), Proceedings of the First International Congress of Parasitology Pergamon, pp. 592–593. [Google Scholar]
- Couch JN, 1935. A New Saprophytic species of Lagenidium, with notes on other forms. Mycologia 27, 376–387. [Google Scholar]
- Couch JN, Umphlett CJ, 1963. Coelomomyces Infections, in: Insect Pathology. Elsevier, pp. 149–188. [Google Scholar]
- Crisan EV, 1971. Mechanism responsible for release of toxin by Metarhizium spores in mosquito larvae. Journal of Invertebrate Pathology 17, 260–264. [DOI] [PubMed] [Google Scholar]
- Da Silva JB, De Albuquerque CMR, De Araujo EC, Peixoto CA, Hurd H, 2000. Immune defense mechanisms of Culex quinquefasciatus (Diptera: Culicidae) against Candida albicans infection. Journal of Invertebrate Pathology 76, 257–262. [DOI] [PubMed] [Google Scholar]
- DeLucca AJ, Bland JM, Jacks TJ, Grimm C, Cleveland TE, & Walsh TJ, 1997. Fungicidal activity of cecropin A. Antimicrobial agents and chemotherap. 41, 481–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Nieto LM, D’Alessio C, Perotti MA, Beron CM, 2016. Culexpipiens development is greatly influenced by native bacteria and exogenous yeast. PLOS ONE 11, e0153133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikgolz VE, Toledo AV, Topa PE, Lopez Lastra CC, 2005. Evaluation of histological techniques for the detection of fungal infections caused by Leptolegnia chapmanii (Oomycetes: Saprolegniales) in Aedes aegypti (Diptera: Culicidae) larvae. Folia Microbiologica 50, 125–127. [DOI] [PubMed] [Google Scholar]
- Dong Y, Morton JC, Ramirez JL, Souza-Neto JA, Dimopoulos G, 2012. The entomopathogenic fungus Beauveria bassiana activate toll and JAK-STAT pathway-controlled effector genes and anti-dengue activity in Aedes aegypti. Insect Biochem. Mol. Biol. 42, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguma D, Hall MW, Rugman-Jones P, Stouthamer R, Terenius O, Neufeld JD, Walton WE, 2015. Developmental succession of the microbiome of Culex mosquitoes. BMC Microbiology 15, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F, 1922. Beitraege zur Erkenntnis der Stechmueckenparasiten. Centralblatt fUr Bakteriologie, Parasitenkunde und lnfektionskrankheiten. 1. Abt., Originale. 88, 128–135. [Google Scholar]
- Eneh LK, Saijo H, Borg-Karlson A-K, Lindh JM, Rajarao GK, 2016. Cedrol, a malaria mosquito oviposition attractant is produced by fungi isolated from rhizomes of the grass Cyperus rotundus. Malaria Journal 15, 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farenhorst M, Hilhorst A, Thomas MB, Knols BGJ, 2011. Development of fungal applications on netting substrates for malaria vector C=control. J Med Entomol 48, 305–313. [DOI] [PubMed] [Google Scholar]
- Faruck MO, Yusof F, Chowdhury S, 2016. An overview of antifungal peptides derived from insect. Peptides, Invertebrate Neuropeptides XVI 80, 80–88. [DOI] [PubMed] [Google Scholar]
- Fei M, Huai-en B, 2001. Observation on hemocytes of Culex pipiens quinquefascaitus larvae infected by Lagenidium giganteum. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi= Chinese journal of parasitology & parasitic diseases 19, 330–332. [PubMed] [Google Scholar]
- Feng MG, Poprawski TJ, Khachatourians GG, 1994. Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Science and Technology 4, 3–34. [Google Scholar]
- Fetter-Lasko JL, Washino RK, 1983. In Situ Studies on Seasonality and Recycling Pattern in California of Lagenidium giganteum Couch, an Aquatic Fungal Pathogen of Mosquitoes. Environ Entomol 12, 635–640. [Google Scholar]
- Fish D, Carpenter SR, 1982. Leaf Litter and Larval Mosquito Dynamics in Tree-Hole Ecosystems. Ecology 63, 283–288. [Google Scholar]
- Foster WA, 1995. Mosquito sugar feeding and reproductive energetics. Annual Review of Entomology 40, 443–474. [DOI] [PubMed] [Google Scholar]
- Frohlich-Nowoisky J, Pickersgill DA, Despres VR, Poschl U, 2009. High diversity of fungi in air particulate matter. Proceedings of the National Academy of Sciences 106, 12814–12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolet C, Thoma M, Blandin S, Hoffmann JA, Levashina EA, 2006. Boosting NF-κB-Dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 25, 677–685. [DOI] [PubMed] [Google Scholar]
- Galli-Valerio B, Rochaz de Jongh J, 1906. Uber die Wirkung von Aspergillus niger und A. glaucus auf die Larven von Culex und Anopheles. 2, Mitteilung. Centralblatt fur Bakteriologie, Parasitenkunde und Infektionskrankheiten. 1. Abt, Originale. 40, 630–633. [Google Scholar]
- García Gil de Muñoz FL, Martínez-Barnetche J, Lanz-Mendoza H, Rodríguez MH, Hernández-Hernández FC, 2008. Prostaglandin E2 modulates the expression of antimicrobial peptides in the fat body and midgut of Anopheles albimanus. Archives of Insect Biochemistry and Physiology 68, 14–25. [DOI] [PubMed] [Google Scholar]
- García-Munguía AM, Garza-Hernández JA, Rebollar-Tellez EA, Rodríguez-Pérez MA, Reyes-Villanueva F, 2011. Transmission of Beauveria bassiana from male to female Aedes aegypti mosquitoes. Parasites & Vectors 4, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland CD, Pillai JS, 1979. Coelomomyces opifexi Pillai & smith (Coelomomycetaceae: Blastocladiales) V. Development of the fungus in pupae and adults of Aedes australis. New Zealand Journal of Zoology 6, 279–283. [Google Scholar]
- Garver LS, Bahia AC, Das S, Souza-Neto JA, Shiao J, Dong Y, Dimopoulos G, 2012. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti-Plasmodium action. PLoS Pathogens 8, e1002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver LS, Dong Y, Dimopoulos G, 2009. Caspar Controls Resistance to Plasmodium falciparum in Diverse Anopheline Species. PLoS Pathogens 5, e1000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Hernández JA, Reyes-Villanueva F, Russell TL, Braks MAH, Garcia-Munguia AM, Rodríguez-Pérez MA, 2015. Copulation Activity, Sperm Production and Conidia Transfer in Aedes aegypti Males Contaminated by Metarhizium anisopliae: A Biological Control Prospect. PLOS Neglected Tropical Diseases 9, e0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha I, Paily KP, Padmanaban V, Balaraman K, 2003. Oviposition response of the mosquito, Culex quinquefasciatus to the secondary metabolite(s) of the fungus, Trichoderma viride. Mem. Inst. Oswaldo Cruz 98, 223–226. [DOI] [PubMed] [Google Scholar]
- Gillespie JP, Bailey AM, Cobb B, Vilcinskas A, 2000. Fungi as elicitors of insect immune responses. Archives of Insect Biochemistry and Physiology 44, 49–68. [DOI] [PubMed] [Google Scholar]
- Goh TK, Hyde KD, 1996. Biodiversity of freshwater fungi. Journal of Industrial Microbiology & Biotechnology 17, 328–345. [Google Scholar]
- Golkar L, Lebrun RA, Ohayon H, Gounon P, Papierok B, Brey PT, 1993. Variation of larval susceptibility to Lagenidium giganteum in three mosquito species. Journal of Invertebrate Pathology 62, 1–8. [DOI] [PubMed] [Google Scholar]
- Greenfield Bethany P. J., Lord Alex M., Dudley Ed, Butt Tariq M., 2014. Conidia of the insect pathogenic fungus, Metarhizium anisopliae, fail to adhere to mosquito larval cuticle. Royal Society Open Science 1, 140193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ, 1979. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am. J. Trop. Med. Hyg 28, 1045–1052. [DOI] [PubMed] [Google Scholar]
- Gubler DJ, Rosen L, 1976. Variation among geographic strains of Aedes Albopictus in suceptibility to infection with Dengue viruses. The American Journal of Tropical Medicine and Hygiene 25, 318–325. [DOI] [PubMed] [Google Scholar]
- Guegan M, Zouache K, Demichel C, Minard G, Tran Van V, Potier P, Mavingui P, Valiente Moro C, 2018. The mosquito holobiont: fresh insight into mosquito-microbiota interactions. Microbiome 6, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta L, Molina-Cruz A, Kumar S, Rodrigues J, Dixit R, Zamora RE, Barillas-Mury C, 2009. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host & Microbe 5, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S, Vago C, 1972. The pathogenicity of Fusarium oxysporum to mosquito larvae. Journal of Invertebrate Pathology 20, 268–271. [DOI] [PubMed] [Google Scholar]
- Heinig RL, Paaijmans KP, Hancock PA, Thomas MB, 2015. The potential for fungal biopesticides to reduce malaria transmission under diverse environmental conditions. J Appl Ecol 52, 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Martínez S, Lanz H, Rodrguez MH, González-Ceron L, Tsutsumi V, 2002. Cellular-mediated reactions to foreign organisms inoculated into the hemocoel of Anopheles albimanus (Diptera: Culicidae). J Med Entomol 39, 61–69. [DOI] [PubMed] [Google Scholar]
- Hillyer JF, Strand MR, 2014. Mosquito hemocyte-mediated immune responses. Current Opinion in Insect Science, Vectors and medical and veterinary entomology 3, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JA, Reichhart J-M, 2002. Drosophila innate immunity: an evolutionary perspective. Nature Immunology 3, 121–126. [DOI] [PubMed] [Google Scholar]
- Huff CG, 1929. The effects of selection upon susceptibility to bird malaria in Culex pipiens Linn. Annals of Tropical Medicin and Parasitology 23, 427–442. [Google Scholar]
- Huff CGG, 1927. Studies on the infectivity of plasmodia of birds for mosquitoes, with special reference to the problem of immunity in the mosquito. The American Journal of Hygiene 7, 706–734. [Google Scholar]
- Hung S-Y, Boucias DG, 1992. Influence of Beauveria bassiana on the cellular defense response of the beet armyworm, Spodoptera exigua. Journal of Invertebrate Pathology 60, 152–158. [DOI] [PubMed] [Google Scholar]
- Hung S-Y, Boucias DG, Vey AJ, 1993. Effect of Beauveria bassiana and Candida albicans on the Cellular Defense Response of Spodoptera exigua. Journal of Invertebrate Pathology 61, 179–187. [DOI] [PubMed] [Google Scholar]
- Impoinvil DE, Kongere JO, Foster WA, Njiru BN, Killeen GF, Githure JI, Beier JC, Hassanali A, Knols BGJ, 2004. Feeding and survival of the malaria vector Anopheles gambiae on plants growing in Kenya. Medical and Veterinary Entomology 18, 108–115. [DOI] [PubMed] [Google Scholar]
- Ishii M, Kanuka H, Badolo A, Sagnon N, Guelbeogo WM, Koike M, Aiuchi D, 2017. Proboscis infection route of Beauveria bassiana triggers early death of Anopheles mosquito. Scientific Reports 7, 3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins DW, 1964. Pathogens, parasites and predators of medically important arthropods. annotated list and bibliography. Bulletin of the World Health Organization 30, 5–150. [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ma C, Lu Z-Q, Kanost MR, 2004. β-1,3-Glucan recognition protein-2 (βGRP-2) from Manduca sexta: an acute-phase protein that binds β-1,3-glucan and lipoteichoic acid to aggregate fungi and bacteria and stimulate prophenoloxidase activation. Insect Biochemistry and Molecular Biology 34, 89–100. [DOI] [PubMed] [Google Scholar]
- Jiravanichpaisal P, Lee BL, Soderhall K, 2006. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 211, 213–236. [DOI] [PubMed] [Google Scholar]
- Kamareddine L, Nakhleh J, Osta MA, 2016. Functional interaction between apolipophorins and complement regulate the mosquito immune response to systemic infections. Journal of Innate Immunity 8, 314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzok SM, Jacobs-Lorena M, 2006. Entomopathogenic fungi as biological insecticides to control malaria. Trends in Parasitology 22, 49–51. [DOI] [PubMed] [Google Scholar]
- Kaufman MG, Chen S, Walker ED, 2008. Leaf-Associated Bacterial and Fungal Taxa Shifts in Response to Larvae of the Tree Hole Mosquito, Ochlerotatus triseriatus. Microb Ecol 55, 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, 1965. Phagocytosis in muscardine-diseased larvae of the silkworm, Bombyx mori (Linnaeus). Journal of Invertebrate Pathology 7, 203–208. [DOI] [PubMed] [Google Scholar]
- Kupriyanova ES, 1966. On an Entomophthora fungus parasitising mosquitos of the complex of Culexpipiens L. Zoologicheskii Zhurnal 45. [Google Scholar]
- Lacey CM, Lacey LA, Roberts DR, 1988. Route of invasion and histopathology of Metarhizium anisopliae in Culex quinquefasciatus. Journal of Invertebrate Pathology 52, 108–118. [DOI] [PubMed] [Google Scholar]
- Laird M, Mogi M, Sota T, 1992. Northernmost occurrences of the protistan pathogen, Coelomomyces stegomyiae var. stegomyiae. J Am Mosq Control Assoc 8, 430–432. [PubMed] [Google Scholar]
- Lakon G, 1919. Die Insektenfeinde aus der Familie der Entomophthoreen. Zeitschrift fur Angewandte Entomologie 5, 161–216. [Google Scholar]
- Lamberty M, Zachary D, Lanot R, Bordereau C, Robert A, Hoffmann JA, Bulet P, 2001. Constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. J. Biol. Chem 276, 4085–4092. [DOI] [PubMed] [Google Scholar]
- Lavine MD, Strand MR, 2002. Insect hemocytes and their role in immunity. Insect Biochemistry and Molecular Biology, Recent Progress in Insect Molecular Biology 32, 1295–1309. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, 2004b. The road to Toll. Nature Reviews Immunology 4, 521–527. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart J-M, Hoffmann JA, 1996. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC, 2001. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 104, 709–718. [DOI] [PubMed] [Google Scholar]
- Levetin E, Dorsey K, 2006. Contribution of leaf surface fungi to the air spora. Aerobiologia 22, 3–12. [Google Scholar]
- Lopez Lastra CC, 1997. Primera cita de Smittium culisitae Y. S. culicis (Trichomycetes: Harpellales) en larvas de mosquitos (Diptera: Culicidae) de la republica Agentina. Bol. Soc. Argent. Bot 33, 43–46. [Google Scholar]
- López Lastra CC, Scorsetti AC, Marti GA, Garcia JJ, 2004. Host range and specificity of an Argentinean isolate of the aquatic fungus Leptolegnia chapmanii (Oomycetes: Saprolegniales), a pathogen of mosquito larvae (Diptera: Culicidae). Mycopathologia 158, 311–315. [DOI] [PubMed] [Google Scholar]
- Lord JC, Fukuda T, 1988. An ultrastructural study of the invasion of Culex quinquefasciatus larvae by Leptolegnia chapmanii (Oomycetes: Saprolegniales). Mycopathologia 104, 67–73. [DOI] [PubMed] [Google Scholar]
- Lovett B, Bilgo E, Millogo SA, Ouattarra AK, Sare I, Gnambani EJ, Dabire RK, Diabate A, St. Leger RJ, 2019. TransgenicMetarhizium rapidly kills mosquitoes in a malaria-endemic region of Burkina Faso. Science 364, 894–897. [DOI] [PubMed] [Google Scholar]
- Lovett B, Leger RJS, 2017. The Insect Pathogens. Microbiology Spectrum 5, 1–19. [DOI] [PubMed] [Google Scholar]
- Lowe RE, Rumbaugh RG, Patterson RS, 1968. Entomophthora coronata as a pathogen of mosquitoes. Journal of Invertebrate Pathology 11. [DOI] [PubMed] [Google Scholar]
- Lowe RE, Kennel EW, 1972. Pathogenicity of the fungus Entomophthora coronata Culex pipiens quinquefasciatus and Aedes taeniorhynchus. Mosquito news. 32, 614–620. [Google Scholar]
- Lowenberger C, Charlet M, Vizioli J, Kamal S, Richman A, Christensen BM, Bulet P, 1999. Antimicrobial activity spectrum, cDNA cloning, and mRNA expression of a newly isolated member of the Cecropin family from the mosquito vector Aedes aegypti. Journal of Biological Chemistry 274, 20092–20097. [DOI] [PubMed] [Google Scholar]
- Lu H-L, St. Leger RJ, 2016. Chapter Seven - Insect immunity to entomopathogenic fungi, in: Lovett B, St. Leger Raymond J. (Eds.), Advances in Genetics, Genetics and Molecular Biology of Entomopathogenic Fungi. Academic Press; 94, 251–285. [DOI] [PubMed] [Google Scholar]
- Lucarotti CJ, & Andreadis TG, 1995. Reproductive strategies and adaptations for survival among obligatory microsporidian and fungal parasites of mosquitoes: a comparative analysis of Amblyospora and Coelomomyces. J Am Mosq Control. Assoc 11, 111–121. [PubMed] [Google Scholar]
- Lucarotti CJ, 1987. Coelomomyces stegomyiae Infection in Adult Aedes aegypti. Mycologia 79, 362–369. [DOI] [PubMed] [Google Scholar]
- Luis P, Vallon L, Tran F-H, Hugoni M, Tran-Van V, Mavingui P, Minard G, Moro CV, 2019. Aedes albopictus mosquitoes host a locally structured mycobiota with evidence of reduced fungal diversity in invasive populations. Fungal Ecology 39, 257–266. [Google Scholar]
- Luna C, Hoa NT, Lin H, Zhang L, Nguyen HLA, Kanzok SM, Zheng L, 2006. Expression of immune responsive genes in cell lines from two different Anopheline species. Insect Molecular Biology 15, 721–729. [DOI] [PubMed] [Google Scholar]
- Luz C, Mnyone LL, Sangusangu R, Lyimo IN, Rocha LFN, Humber RA, Russell TL, 2010. A new resting trap to sample fungus-infected mosquitoes, and the pathogenicity of Lecanicillium muscarium to culicid adults. Acta Tropica 116, 105–107. [DOI] [PubMed] [Google Scholar]
- Lwetoijera DW, Sumaye RD, Madumla EP, Kavishe DR, Mnyone LL, Russell TL, Okumu FO, 2010. An extra-domiciliary method of delivering entomopathogenic fungus, Metarhizium anisopliae IP 46 for controlling adult populations of the malaria vector, Anopheles arabiensis. Parasites & Vectors 3, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matskevich AA, Quintin J, Ferrandon D, 2010. The Drosophila PRR GNBP3 assembles effector complexes involved in antifungal defenses independently of its Toll-pathway activation function. European Journal of Immunology 40, 1244–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccray EM, Umphlett CJ, Fay RW, 1973. Laboratory studies on a new fungal pathogen of mosquitoes. Mosquito News 33, 54–60. [Google Scholar]
- Mc Innis T, Zattau WC, 1982. Experimental infection of mosquito larvae by a species of the aquatic fungus Leptolegnia. Journal of Invertebrate Pathology 39, 98–104. [DOI] [PubMed] [Google Scholar]
- Meister S, Agianian B, Turlure F, Relogio A, Morlais I, Kafatos FC, Christophides GK, 2009. Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathogens. 5, e1000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister S, Kanzok SM, Zheng X. -l., Luna C, Li T-R, Hoa NT, Clayton JR, White KP, Kafatos FC, Christophides GK, Zheng L, 2005. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proceedings of the National Academy of Sciences 102, 11420–11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED, 1992. Feeding Behavior, Natural Food, and Nutritional Relationships of Larval Mosquitoes. Annual Review of Entomology 37, 349–374. 10.1146/annurev.en.37.010192.002025 [DOI] [PubMed] [Google Scholar]
- Meyling NV, Eilenberg J, 2007. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biological Control 43, 145–155. [Google Scholar]
- Miranpuri GS, Khachatourians GG, 1991. Infection sites of the entomopathogenic fungus Beauveria bassiana in the larvae of the mosquito Aedes aegypti. Entomologia Experimentalis et Applicata 59, 19–27. [Google Scholar]
- Mnyone LL, Kirby MJ, Lwetoijera DW, Mpingwa MW, Simfukwe ET, Knols BG, Takken W, Russell TL, 2010. Tools for delivering entomopathogenic fungi to malaria mosquitoes: effects of delivery surfaces on fungal efficacy and persistence. Malaria Journal 9, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita LF, Wang-Sattler R, Michel K, Zimmermann T, Blandin S, Levashina EA, Kafatos FC, 2005. In vivo identification of novel regulators and conserved pathways of phagocytosis in An. gambiae. Immunity 23, 65–73. [DOI] [PubMed] [Google Scholar]
- Moll RM, Romoser WS, Modrakowski MC, Moncayo AC, Lerdthusnee K, 2001. Meconial peritrophic membranes and the fate of midgut bacteria during mosquito (Diptera: Culicidae) metamorphosis. J Med Entomol 38, 29–32. [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Bara JJ, Rooney AP, Hansen AK, 2016. Midgut fungal and bacterial microbiota of Aedes triseriatus and Aedes japonicus shift in response to La Crosse virus infection. Molecular Ecology 25, 4075–4090. [DOI] [PubMed] [Google Scholar]
- Nakhleh J, El Moussawi L, Osta MA, 2017. Chapter Three - The melanization response in insect immunity, in: Ligoxygakis P (Ed.), Advances in Insect Physiology, Insect Immunity. Academic Press; 52, 83–109. [Google Scholar]
- Nappi AJ, Christensen BM, 2005. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochemistry and Molecular Biology 35, 443–459. [DOI] [PubMed] [Google Scholar]
- Nappi AJ, Vass E, 1993. Melanogenesis and the Generation of Cytotoxic Molecules During Insect Cellular Immune Reactions. Pigment Cell Research 6, 117–126. [DOI] [PubMed] [Google Scholar]
- Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, Amon J, Arca B, Arensburger P, Artemov G, Assour LA, Basseri H, Berlin A, Birren BW, Blandin SA, Brockman AI, Burkot TR, Burt A, Chan CS, Chauve C, Chiu JC, Christensen M, Costantini C, Davidson VLM, Deligianni E, Dottorini T, Dritsou V, Gabriel SB, Guelbeogo WM, Hall AB, Han MV, Hlaing T, Hughes DST, Jenkins AM, Jiang X, Jungreis I, Kakani EG, Kamali M, Kemppainen P, Kennedy RC, Kirmitzoglou IK, Koekemoer LL, Laban N, Langridge N, Lawniczak MKN, Lirakis M, Lobo NF, Lowy E, MacCallum RM, Mao C, Maslen G, Mbogo C, McCarthy J, Michel K, Mitchell SN, Moore W, Murphy KA, Naumenko AN, Nolan T, Novoa EM, O’Loughlin S, Oringanje C, Oshaghi MA, Pakpour N, Papathanos PA, Peery AN, Povelones M, Prakash A, Price DP, Rajaraman A, Reimer LJ, Rinker DC, Rokas A, Russell TL, Sagnon N, Sharakhova MV, Shea T, Simao FA, Simard F, Slotman MA, Somboon P, Stegniy V, Struchiner CJ, Thomas GWC, Tojo M, Topalis P, Tubio JMC, Unger MF, Vontas J, Walton C, Wilding CS, Willis JH, Wu Y-C, Yan G, Zdobnov EM, Zhou X, Catteruccia F, Christophides GK, Collins FH, Cornman RS, Crisanti A, Donnelly MJ, Emrich SJ, Fontaine MC, Gelbart W, Hahn MW, Hansen IA, Howell PI, Kafatos FC, Kellis M, Lawson D, Louis C, Luckhart S, Muskavitch MAT, Ribeiro JM, Riehle MA, Sharakhov IV, Tu Z, Zwiebel LJ, Besansky NJ, 2015. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science 347, 1258522–1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaré O, Markianos K, Volz J, Oduol F, Touré A, Bagayoko M, Sangaré D, Traoré SF, Wang R, Blass C, Dolo G, Bouaré M, Kafatos FCC, Kruglyak L, Touré YT, Vernick KDD, Niare O, Markianos K, Volz J, Oduol F, Toure A, Bagayoko M, Sangare D, Traore SF, Wang R, Blass C, Dolo G, Bouare M, Kafatos FCC, Kruglyak L, Toure YT, Vernick KDD, 2002. Genetic loci affecting resistance to human malaria parasites in a West African mosquito vector population. Science 298, 213–216. [DOI] [PubMed] [Google Scholar]
- Novák D, 1965. Zum Auftreten der Mykosen bei Stechmüken in Mähren (Diptera: Culicidae). 1 15, 135–137. [Google Scholar]
- Ochiai M, Ashida M, 2000. The binding domain and the cDNA cloning of β-1,3-glucan recognition protein from the silkworm, Bombyx mori. J. Biol. Chem 275, 4995–5002. [DOI] [PubMed] [Google Scholar]
- Ouedraogo RM, Cusson M, Goettel MS, Brodeur J, 2003. Inhibition of fungal growth in thermoregulating locusts, Locusta migratoria, infected by the fungus Metarhizium anisopliae var acridum. Journal of Invertebrate Pathology 82, 103–109. [DOI] [PubMed] [Google Scholar]
- Pelizza SA, López Lastra CC, Becnel JJ, Humber RA, García JJ, 2008. Further research on the production, longevity and infectivity of the zoospores of Leptolegnia chapmanii Seymour (Oomycota: Peronosporomycetes). Journal of Invertebrate Pathology 98, 314–319. [DOI] [PubMed] [Google Scholar]
- Pendland JC, Boucias DG, 1996. Phagocytosis of lectin-opsonized fungal cells and endocytosis of the ligand by insect Spodoptera exigua granular hemocytes: an ultrastructural and immunocytochemical study. Cell and Tissue Research 285, 57–67. [Google Scholar]
- Pereira E. da S., Ferreira RLM, Hamada N, Lichtwardt RW, 2005. Trichomycete fungi (Zygomycota) associated with mosquito larvae (Diptera: Culicidae) in natural and artificial habitats in Manaus, AM Brazil. Neotropical Entomology 34, 325–329. [Google Scholar]
- Popko DA, Henke JA, Mullens BA, Walton WE, 2018. Evaluation of Two Entomopathogenic Fungi for Control of Culex quinquefasciatus (Diptera: Culicidae) in Underground Storm Drains in the Coachella Valley, California, United States. J Med Entomol 55, 654–665. [DOI] [PubMed] [Google Scholar]
- Povelones M, Bhagavatula L, Yassine H, Tan LA, Upton LM, Osta MA, Christophides GK, 2013. The CLIP-Domain Serine Protease Homolog SPCLIP1 Regulates Complement Recruitment to Microbial Surfaces in the Malaria Mosquito Anopheles gambiae. PLOS Pathogens 9, e1003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad A, Veerwal B, 2010. Biotoxicity of entomopathogenic fungus Beauveria bassiana (Balsamo) vuillemin, against early instars of anopheline mosquitoes. Journal of Herbal Medicine. 4, 181–188. [Google Scholar]
- Ramirez JL, Dunlap CA, Muturi EJ, Barletta ABF, Rooney AP, 2018a. Entomopathogenic fungal infection leads to temporospatial modulation of the mosquito immune system. PLoS Negl Trop Dis. 12, e0006433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JL, Muturi EJ, Barletta ABF, Rooney AP, 2018b. The Aedes aegypti IMD pathway is a critical component of the mosquito antifungal immune response. Developmental & Comparative Immunology. In press. [DOI] [PubMed] [Google Scholar]
- Ramirez JL, Muturi EJ, Dunlap C, Rooney AP, 2018c. Strain-specific pathogenicity and subversion of phenoloxidase activity in the mosquito Aedes aegypti by members of the fungal entomopathogenic genus Isaria. Sci Rep. 8, 9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JL, Muturi EJ, Barletta ABF, Rooney AP, 2019. The Aedes aegypti IMD pathway is a critical component of the mosquito antifungal immune response. Developmental & Comparative Immunology 95, 1–9. [DOI] [PubMed] [Google Scholar]
- Rautenbach M, Troskie AM, Vosloo JA, 2016. Antifungal peptides: To be or not to be membrane active. Biochimie 130, 132–145. [DOI] [PubMed] [Google Scholar]
- Reyes-Villanueva F, Garza-Hernandez JA, Garcia-Munguia AM, Tamez-Guerra P, Howard AF, Rodriguez-Perez MA, 2011. Dissemination ofMetarhizium anisopliae of low and high virulence by mating behavior in Aedes aegypti. Parasites & Vectors 4, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes VL, Thomas MB, Michel K, 2018. The interplay between dose and immune system activation determines fungal infection outcome in the African malaria mosquito, Anopheles gambiae. Developmental & Comparative Immunology 85, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes VLM, Michel K, 2017. Modulation of Mosquito Immune Defenses as a Control Strategy, in: Arthropod Vector: Controller of Disease Transmission, Elsevier. 1, 59–89. [Google Scholar]
- Ricci I, Mosca M, Valzano M, Damiani C, Scuppa P, Rossi P, Crotti E, Cappelli A, Ulissi U, Capone A, Esposito F, Alma A, Mandrioli M, Sacchi L, Bandi C, Daffonchio D, Favia G, 2011. Different mosquito species host Wickerhamomyces anomalus (Pichia anomala): perspectives on vector-borne diseases symbiotic control. Antonie van Leeuwenhoek. 99, 43–50. [DOI] [PubMed] [Google Scholar]
- Roberts D, 1974. Fungal Infections of Mosquitoes. Le Controle des Moustiques/Mosquito Control 143–193. [Google Scholar]
- Roubaud E, Toumanoff, 1930. Essais d’infection experimentale de larves de culicides par quelques champignons entomophytes. 23, 1025–1027. [Google Scholar]
- Sandhu DK, Waraich MK, 1985. Yeasts associated with pollinating bees and flower nectar. Microbial Ecology. 11, 51–58. [DOI] [PubMed] [Google Scholar]
- Sato H (Tokyo U. of A. and T., Shimada N, Aoki J, 1989. Light and electron microscopy of Smittium morbosum (Trichomycetes), newly recorded from Japan. Transactions of the Mycological Society of Japan (Japan). [Google Scholar]
- Saunders GA, Washburn JO, Egerter DE, Anderson JR, 1988. Pathogenicity of fungi isolated from field-collected larvae of the western treehole mosquito, Aedes sierrensis (Diptera: Culicidae). Journal of Invertebrate Pathology 52, 360–363. [DOI] [PubMed] [Google Scholar]
- Schnitger AKD, Kafatos FC, Osta MA, 2007. The melanization reaction is not required for survival of Anopheles gambiae mosquitoes after bacterial infections. Journal of Biological Chemistry. 282, 21884–21888. [DOI] [PubMed] [Google Scholar]
- Scholte E-J, 2005. An Entomopathogenic Fungus for Control of Adult African Malaria Mosquitoes. Science. 308, 1641–1642. [DOI] [PubMed] [Google Scholar]
- Scholte E-J, Knols BG, Takken W, 2004a. Autodissemination of the entomopathogenic fungus Metarhizium anisopliae amongst adults of the malaria vector Anopheles gambiae s.s. Malaria Journal. 3, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte E-J, Knols BGJ, Samson RA, Takken W, 2004b. Entomopathogenic fungi for mosquito control: a review. J. Insect Sci. 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank A, Vainstein MH, 2010. Metarhizium anisopliae enzymes and toxins. Toxicon 56, 1267–1274. [DOI] [PubMed] [Google Scholar]
- Seye F, Faye O, Ndiaye M, Njie E, Afoutou JM, 2009. Pathogenicity of the fungus, Aspergillus clavatus, isolated from the locust, Oedaleus senegalensis, against larvae of the mosquitoes Aedes aegypti, Anopheles gambiae and Culex quinquefasciatus. J Insect Sci. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PA, Pell JK, 2003. Entomopathogenic fungi as biological control agents. Applied Microbiology and Biotechnology 61, 413–423. [DOI] [PubMed] [Google Scholar]
- Shin SW, Bian G, Raikhel AS, 2006. A Toll Receptor and a Cytokine, Toll5A and Spz1C, are involved in Toll antifungal immune signaling in the mosquito Aedes aegypti. Journal of Biological Chemistry 281, 39388–39395. [DOI] [PubMed] [Google Scholar]
- Shin SW, Kokoza V, Bian G, Cheon H-M, Kim YJ, Raikhel AS, 2005. REL1, a Homologue of Drosophila Dorsal, regulates Toll antifungal immune pathway in the female mosquito Aedes aegypti. Journal of Biological Chemistry 280, 16499–16507. [DOI] [PubMed] [Google Scholar]
- Shoulkamy MA, Abdelzaher HMA, Shahin AAB, 2001. Ultrastructural changes in the muscles, midgut, hemopoietic organ, imaginal discs and malpighian tubules of the mosquito Aedes aegypti larvae infected by the fungus Coelomomyces stegomyiae. Mycopathologia 149, 99–106. [DOI] [PubMed] [Google Scholar]
- Shoulkamy MA, Lucarotti CJ, 1998. Pathology of Coelomomyces stegomyiae in Larval Aedes aegypti. Mycologia 90, 559–564. [DOI] [PubMed] [Google Scholar]
- Snetselaar J, Andriessen R, Suer RA, Osinga AJ, Knols BG, Farenhorst M, 2014. Development and evaluation of a novel contamination device that targets multiple life-stages of Aedes aegypti. Parasit Vectors. 7, 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soarés GG, 1982. Pathogenesis of infection by the hyphomycetous fungus, Tolypocladium cylindrosporum in Aedes sierrensis and Culex tarsalis [Dip.: Culicidae]. Entomophaga. 27, 283–299. [Google Scholar]
- Sousa NA, Lobo LS, Rodrigues J, Luz C, 2013. New insights on the effectiveness of Metarhizium anisopliae formulation and application against Aedes aegypti eggs. Letters in Applied Microbiology. 57, 193–199. [DOI] [PubMed] [Google Scholar]
- Souza-Neto JA, Sim S, Dimopoulos G, 2009. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proceedings of the National Academy of Sciences. 106, 17841–17846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn A, Roets F, Botha A, 2016. Yeasts associated with Culexpipiens and Culex theileri mosquito larvae and the effect of selected yeast strains on the ontogeny of Culex pipiens. Microbial Ecology. 71, 747–760. [DOI] [PubMed] [Google Scholar]
- Sur B, Bihari V, Sharma A, Basu SK, 1999. Survey of termite-inhabited soil and mosquito breeding sites in Lucknow, India for potential mycopathogens of Anopheles stephensi. Mycopathologia 144, 77–80. [DOI] [PubMed] [Google Scholar]
- Sweeney A, 1975. The Mode of Infection of the Insect Pathogenic Fungus Culicinomyces in Larvae of the Mosquito Culex Fatigans. Australian Journal of Zoology. 23, 49. [Google Scholar]
- Sweeney AW, 1981. An undescribed species of Smittium (Trichomycetes) pathogenic to mosquito larvae in Australia. Transactions of the British Mycological Society 77, 55–60. [Google Scholar]
- Tajedin L, Hashemi J, Abaei M, Hosseinpour L, Rafei F, Basseri H, 2009. Study on fungal flora in the midgut of the larva and adult of the different populations of the malaria vector Anopheles stephensi. Iran J Arthropod Borne Dis. 3, 36–40. [PMC free article] [PubMed] [Google Scholar]
- Tonk M, Vilcinskas A, 2017. The Medical Potential of Antimicrobial Peptides from Insects. Curr Top Med Chem. 17, 554–575. [DOI] [PubMed] [Google Scholar]
- Travland LB, 1979. Initiation of infection of mosquito larvae (Culiseta inornata) by Coelomomycespsorophorae. Journal of Invertebrate Pathology. 33, 95–105. [Google Scholar]
- Tuzet O, Manier JF, 1947. Orphella culici n. sp., entophyte parasite du rectum des larves de Culex hortensis Fclb. Comptes rendus des seances de la Societe de biologie et des ses filiales 225, 264–265 [PubMed] [Google Scholar]
- Valanne S, Wang J-H, Ramet M, 2011. The Drosophila Toll Signaling Pathway. The Journal of Immunology. 186, 649–656. [DOI] [PubMed] [Google Scholar]
- Valzania L, Martinson VG, Harrison RE, Boyd BM, Coon KL, Brown MR, Strand MR, 2018. Both living bacteria and eukaryotes in the mosquito gut promote growth of larvae. PLOS Neglected Tropical Diseases. 12, e0006638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Weerden NL, Bleackley MR, Anderson MA, 2013. Properties and mechanisms of action of naturally occurring antifungal peptides. Cellular and Molecular Life Sciences. 70, 3545–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizioli J, Bulet P, Charlet M, Lowenberger C, Blass C, Muller H-M, Dimopoulos G, Hoffmann J, Kafatos FC, Richman A, 2000. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect Molecular Biology. 9, 75–84. [DOI] [PubMed] [Google Scholar]
- Vizioli J, Bulet P, Hoffmann JA, Kafatos FC, Muller H-M, Dimopoulos G, 2001a. Gambicin: A novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae. Proc Natl Acad Sci U S A. 98, 12630–12635. 10.1073/pnas.221466798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizioli J, Richman AM, Uttenweiler-Joseph S, Blass C, Bulet P, 2001b. The defensing peptide of the malaria vector mosquito Anopheles gambiae: antimicrobial activities and expression in adult mosquitoes. Insect Biochemistry and Molecular Biology. 31, 241–248. [DOI] [PubMed] [Google Scholar]
- Wallis GP, Aitken THG, Amato GD, Tabachnick AJ, 1985. Selection for susceptibility and refractoriness of Aedes aegypti to oral infection with yellow fever virus. Am J Trop Med Hyg. 34, 1225–1231. [DOI] [PubMed] [Google Scholar]
- Wanchoo A, Lewis MW, Keyhani NO, 2009. Lectin mapping reveals stage-specific display of surface carbohydrates in in vitro and haemolymph-derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiology. 155, 3121–3133. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang S, 2017. Insect Pathogenic Fungi: Genomics, Molecular Interactions, and Genetic Improvements. Annual Review of Entomology. 62, 73–90. [DOI] [PubMed] [Google Scholar]
- Wang X, Fuchs JF, Infanger L-C, Rocheleau TA, Hillyer JF, Chen C-C, Christensen BM, 2005. Mosquito innate immunity: involvement of β 1,3-glucan recognition protein in melanotic encapsulation immune responses in Armigeres subalbatus. Molecular and Biochemical Parasitology. 139, 65–73. [DOI] [PubMed] [Google Scholar]
- Wang X, Rocheleau TA, Fuchs JF, Christensen BM, 2006. Beta 1, 3-glucan recognition protein from the mosquito, Armigeres subalbatus, is involved in the recognition of distinct types of bacteria in innate immune responses. Cellular Microbiology. 8, 1581–1590. [DOI] [PubMed] [Google Scholar]
- Wang Y-H, Hu Y, Xing L-S, Jiang H, Hu S-N, Raikhel AS, Zou Z, 2015. A critical role for CLSP2 in the modulation of antifungal immune response in mosquitoes. PLoS Pathog. 11, e1004931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse RM, Kriventseva EV, Meister S, Xi Z, Alvarez KS, Bartholomay LC, Barillas-Mury C, Bian G, Blandin S, Christensen BM, Dong Y, Jiang H, Kanost MR, Koutsos AC, Levashina EA, Li J, Ligoxygakis P, MacCallum RM, Mayhew GF, Mendes A, Michel K, Osta MA, Paskewitz S, Shin SW, Vlachou D, Wang L, Wei W, Zheng L, Zou Z, Severson DW, Raikhel AS, Kafatos FC, Dimopoulos G, Zdobnov EM, Christophides GK, 2007. Evolutionary Dynamics of Immune-Related Genes and Pathways in Disease-Vector Mosquitoes. Science. 316, 1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser J, Batko A, 1966. A new parasite of Culexpipiens L., Entomophthora destruens sp. nov. (Phycomycetes, Entomophthoraceae). Folia Parasitologica 13. [Google Scholar]
- Williams MC, Lichtwardt RW, 1972. Infection of Aedes aegypti Larvae by Axenic Cultures of the Fungal Genus Smittium (Trichomycetes). American Journal of Botany 59, 189–193. [Google Scholar]
- White MM, Siri A, Lichtwardt RW, 2006. Trichomycete insect symbionts in Great Smoky Mountains National Park and vicinity. Mycologia 98, 333–352. [DOI] [PubMed] [Google Scholar]
- Wong TL, Pillai JS, 1978. Coelomomyces opifexi Pillai & Smith (Coelomomycetaceae: Blastocladiales) IV. Host range and relative susceptibility of Aedes australis and Opifex fuscus larvae. New Zealand Journal of Zoology 5, 807–810. [Google Scholar]
- Wong TL, Pillai JS, 1980. Coelomomyces opifexi Pillai & Smith (Coelomomycetaceae: Blastocladiales) VI. Observations on the mode of entry into Aedes australis larvae. New Zealand Journal of Zoology 7, 135–139. [Google Scholar]
- Wu RC-C, Cho W-L, 2014. Cloning and characterization of microbial activated Aedes aegypti MEK4 (AaMEK4): influences of noncatalytic domains on enzymatic activity. Insect Molecular Biology. 23, 644–655. [DOI] [PubMed] [Google Scholar]
- Yassine H, Kamareddine L, Chamat S, Christophides GK, Osta MA, 2014. A serine protease homolog negatively regulates TEP1 consumption in systemic infections of the malaria vector Anopheles gambiae. JIN. 6, 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine H, Kamareddine L, Osta MA, 2012. The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLOS Pathogens. 8, e1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA, 2004. Larval feeding behavior of three co-occurring species of container mosquitoes. J Vector Ecol. 29, 315–322. [PMC free article] [PubMed] [Google Scholar]
- Zattau WC, McInnis T, 1987. Life cycle and mode of infection of Leptolegnia chapmanii (Oomycetes) parasitizing Aedes aegypti. Journal of Invertebrate Pathology 50, 134–145. [DOI] [PubMed] [Google Scholar]
- Zeidler MP, Bausek N, 2013. The Drosophila JAK-STAT pathway. JAK-STAT. 2, e25353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Rudian, Zhu Y, Pang X, Xiao X, Zhang Renli, Cheng G, 2017. Regulation of Antimicrobial Peptides in Aedes aegypti Aag2 Cells. Front. Cell. Infect. Microbiol. 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, An C, Sprigg K, Michel K, 2016. CLIPB8 is part of the prophenoloxidase activation system in Anopheles gambiae mosquitoes. Insect Biochemistry and Molecular Biology. 71, 106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


