Summary Statement:
The physiological concept, pathophysiological implications and clinical relevance and application of driving pressure and transpulmonary pressure to prevent ventilator-induced lung injury are discussed.
Concern over the potential for lung injury due to mechanical ventilation has fueled investigations on lung protection in the operating room 1-3. Based on the intensive care literature4, tidal volume (VT) and positive end-expiratory pressure (PEEP) settings have been the focus of intraoperative clinical trials1-3. Recent results in acute respiratory distress syndrome (ARDS)5 and surgical patients6 have suggested that the benefits associated with VT and PEEP settings are mediated by driving pressures. As our understanding of the physical and biological effects of mechanical ventilation evolves, the concepts of driving pressure and transpulmonary pressure have been increasingly used to quantify the mechanical forces acting over the lungs during mechanical ventilation and to guide clinical care. In this perspective, we discuss the definition of those concepts, their measurement in the clinical setting, their interpretation and their use in typical scenarios.
What are strain and stress and how do they apply to mechanical ventilation and ventilator-induced lung injury?
To prevent lung injury during mechanical ventilation, the factors causing most injury to the lungs must be identified. In the centuries-old engineering field of materials science, limits of maximal stress and strain are listed as key possible causes for materials to fail and rupture under the action of external loads. Recently, these concepts of stress and strain have been applied to increase understanding of mechanisms of injury during mechanical ventilation7-9 and better explain the positive clinical outcomes associated with lung protective ventilation5,7,9-11.
Stress is defined as a force divided by the area over which it is applied. Intuitively, if a fixed force is distributed throughout a large cross-sectional area of lung tissue, the force per unit area (i.e., stress) will be smaller than if that same force were distributed over a smaller area of lung tissue. More stress is expected to increase the risk of injury.
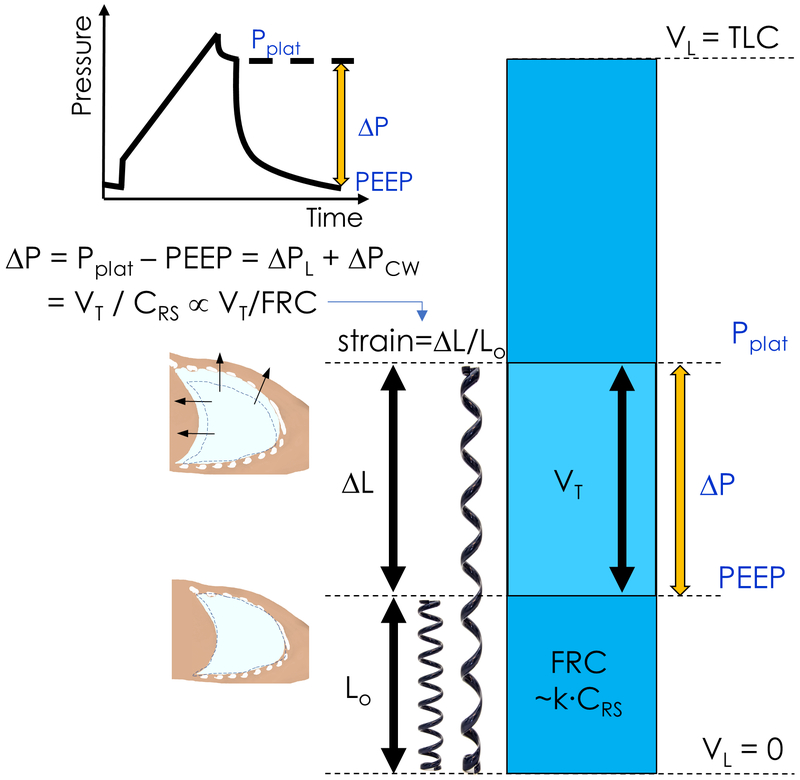
Strain is a measure of a change in the dimension of a structure from its original dimension. For instance, linear strain is defined as change in length divided by the original length (fig. 1). The most pertinent strain in ventilation is the volumetric strain created by inspiration and expiration. Volumetric strain is defined as change in volume divided by initial volume. In elastic materials, strain is directly proportional to stress. Volumetric strain during ventilation has both static and dynamic components and is heterogeneous throughout the lungs7.
Fig. 1.
Driving pressure (ΔP) is calculated as the difference between plateau pressure (Pplat) and positive end-expiratory pressure (PEEP). Driving pressure is composed of two pressures: that distributed to the lung itself, the transpulmonary pressure (ΔPL), and that applied to the chest wall (ΔPcw). Rearrangement of the standard respiratory system compliance (CRS) equation leads to driving pressure as equal to the tidal volume (VT) divided by CRS. Strain is a measure of material deformation relative to its original state. For example, the linear displacement of a spring (ΔL) relative to its rest length (Lo), or equivalently the ratio of VT to residual functional capacity (FRC). As CRS changes in proportion to FCR, i.e FRC=k × CRS, VT/CRS is an approximation of tidal volume normalized to FRC, and ΔP is proportional to lung strain. TLC=Total lung capacity. VL = lung volume.
What is the relevance of these concepts for prevention of lung injury?
During tidal breathing, the change in lung volume is represented by VT and the initial lung volume corresponds to the functional residual capacity (FRC). Global volumetric lung strain can, thus, be estimated as VT/FRC. This relationship shows that reduction of VT lowers lung strain, and also that FRC can have an effect on strain. The markedly low FRC of ARDS patients emphasizes the relevance of this concept. For instance, with a VT=500 ml, a healthy lung during anesthesia (FRC=2000 ml) would have a strain of 25% (=500/2000). That same VT in an ARDS patient (FRC=500 ml) would produce a strain of 100% (=500/500), a fourfold increase in strain and risk of injury.
These considerations also suggest that, while reducing VT is important in surgical and ARDS patients4,12, VT is not the final determinant of lung injury. This is because it does not take the size of lung parenchyma to which that VT applies (FRC) into account. Consequently, simply controlling VT is not enough to minimize injurious lung strain. These arguments are consistent with recent clinical outcome results in ARDS and surgical patients showing that the effect of VT on clinical outcomes are mediated by a variable associated with lung strain5,6,11.
The heterogeneity of lung expansion, e.g., as lung derecruitment develops, also increases the risk for lung injury. This is because this heterogeneity can produce regional strains larger than whole-lung strains in healthy and inflamed lungs of anesthetized ventilated large animals even if those whole-lung strains are acceptable7,13. Theoretical computations indicated that in heterogeneously inflated lungs, regional pressures could be substantially larger than whole-lung pressures, by as much as 3-4 times when an atelectatic area is surrounded by expanded lung14. Systemic inflammation, a common clinical finding, amplifies the injurious effect of strain9,15.
What is driving pressure and how is it measured?
Driving pressure is defined as plateau pressure (Pplat) minus PEEP (fig. 1)16. Plateau pressure is measured at the end of an inspiratory pause during volume-controlled constant flow ventilation and at the end of inspiration during pressure-controlled ventilation. Accordingly, in the absence of respiratory muscle effort by the patient, driving pressure is the pressure above PEEP applied to the entire respiratory system to achieve tidal ventilation. A caveat on the computation of plateau pressures is that they cannot be presumed to represent end-inspiratory alveolar pressures when end-inspiratory flows are not zero, indicating lack of equilibration between airway and alveolar pressures. During volume-controlled ventilation, an inspiratory pause ≥3 seconds provides best accuracy for Pplat measurements in normal and diseased lungs.17,18 Short inspiratory pauses of 0.5 second overestimate Pplat by 11% in ARDS patients and 17% in COPD patients.17 Examination of the airway pressure tracing available in current anesthesia machines for the presence of a plateau at the end of the inspiratory pause allows for better decision on reliability of Pplat measurement. Auto-PEEP is another potential source of error by leading to driving pressure overestimation as the end-expiratory pressure in alveolar units would be higher than the PEEP set in the ventilator and used to compute the driving pressure.
It is important to recognize that driving pressure and total airway pressure measured during mechanical ventilation have two components: one related to the expansion of the lungs, the other to the expansion of the chest wall. Each of these two components can change substantially during disease and surgical conditions and affect the interpretation of the driving pressure measurements.
What is transpulmonary pressure and how is it measured?
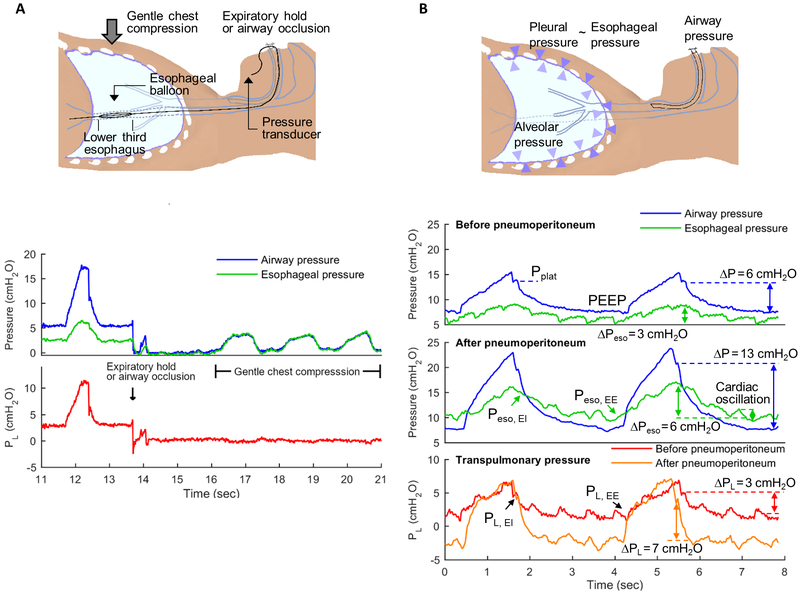
Transpulmonary pressure (PL) is defined as the pressure difference between the airway opening and the pleural surface (fig. 2)19,20. Accordingly, PL comprises the pressure to move air through the airways (airway opening – alveolar pressure) and the pressure to overcome the lung tissue elastic recoil (alveolar – pleural pressure), the latter most frequently associated with lung injury19. Although continuous estimation of PL is feasible, it is usually assessed at two critical points during the breathing cycle: the end of inspiration, relevant to prevent hyperinflation, and the end of expiration, relevant to avoid lung derecruitment. If respiratory flows are zero at these points, the airway pressures (Pplat at end-inspiration and PEEP at end-expiration) are presumed to represent alveolar pressures, a reasonable assumption in the absence of gas trapping21. This approach to measure PL may have led to the misconception that it exclusively expresses pressures at the alveolar level19,22,23. The essential concept is that in static, i.e., zero flow, conditions (end-inspiration and end-expiration) the PL approximates the lung tissue elastic recoil component, which is the relevant pressure to quantify stress applied to lung tissue beyond airways14, presumably responsible for injury during mechanical ventilation19.
Fig.2.
Airway opening, esophageal (Peso) and transpulmonary pressures (PL) measurements. PL is defined as the difference between airway opening pressure (blue lines) and pleural pressure. Pleural pressure is frequently estimated from esophageal balloon pressure measurements (Peso). Using a specific protocol, the esophageal balloon is placed in the lower third of the esophagus (2A). Cardiac oscillations in Peso (2B, green lines) indicate accurate placement of the balloon, which can be confirmed by observation of similar airway pressure and Peso measurements as gentle chest compressions are performed during expiratory pause or with occluded airway opening (2A). PL can be estimated as the difference between airway and esophageal pressures (red and orange lines). Interventions such as pneumoperitoneum (2B, mid panel) produce a marked change in driving pressures (ΔP = plateau pressure, PPlat, minus positive end-expiratory pressure, PEEP). In this example, ΔP increased by 7 cmH2O. Yet, delta PL (end-inspiratory PL, PL EI, minus end-expiratory PL, PL EE) does not increase to the same degree as ΔP and PPlat. The change in delta PL in this example was 4 cm H2O. This demonstrates that part of the increase in ΔP and PPlat are due to the chest wall component and not to pressures applied to the lung parenchyma. This contribution of the chest wall is evidenced by the increased end-inspiratory (EI) to end-expiratory (EE) oscillation in Peso after as compared to before pneumoperitoneum. In addition, the esophageal pressure at end-expiration (Peso EE, at ~4 seconds on time scale) is positive before pneumoperitoneum while it is negative after pneumoperitoneum. This implies mechanical conditions consistent with lung collapse after pneumoperitoneum. Indeed, while PL did not increase by the same magnitude as ΔP, it also increased, indicating loss of lung compliance. Such conditions could prompt use of higher PEEP to prevent lung derecruitment.
While assessment of airway pressures to calculate PL is simple, estimates of pleural pressure are difficult to obtain. Esophageal manometry is currently the most widely accepted method to estimate pleural pressures in the clinical setting24-26. For this, a special balloon, either incorporated in a stand-alone catheter or as part of a naso- or orogastric tube, is positioned with a specific protocol24,27 in the lower third of the esophagus and connected to a pressure transducer (fig. 2A). Correct balloon position is confirmed by presence of cardiac oscillation in the esophageal pressure trace (fig. 2B) and measurement of airway opening and esophageal pressure swings with occluded airway opening (fig. 2A)28. Esophageal pressure measurements obtained in this manner more specifically assess periesophageal values, approximately at a third to half of the dorsal-to-ventral chest length26,29. In supine patients, they overestimate ventral pleural pressures and underestimate dorsal values given the ventral-dorsal increase of pleural pressure30.
Two approaches are used to apply esophageal pressure as a surrogate for pleural pressure and computation of PL. One assumes pleural pressure as equal to the absolute esophageal pressure directly read from the transducer measurements along the breathing cycle24. These measurements can be made at end-inspiration (transpulmonary pressure is equal to Pplat minus esophageal pressure at end-inspiration) and end-expiration (transpulmonary pressure is equal to PEEP minus esophageal pressure at end-expiration). Such esophageal pressure measurements can be affected by the weight of the mediastinum, abdominal pressure, and esophageal balloon positioning, and correction factors have been proposed to account for those31,32.
The second approach assumes that, while absolute esophageal and pleural pressures can differ, their changes are equivalent24,33. Using this approach, pleural pressures and PL can be measured in two ways, which present close agreement33: compliance-derived and release-derived. In the compliance-derived strategy, PL is calculated as the product of the plateau pressure and the ratio of compliances of the respiratory system and lung34,35. The compliance ratio is estimated during a tidal volume inflation (from PEEP to end-inspiratory pressures) from VT and changes in airway and esophageal pressures. This compliance-derived method assumes that in each patient the changes in esophageal and airway pressures are linear during tidal volume inflation and PEEP changes. In the release-derived strategy, PL is measured as the change in airway and esophageal pressure from atmospheric pressure due to tidal inflation and PEEP33. The release derived strategy involves opening of the ventilatory circuit to atmosphere, with risk of lung derecruitment and hypoxemia, while the compliance-based strategy does not. A key assumption of the second approach is that pleural pressures are zero at zero airway pressure. This would be questionable in resting conditions, and, more markedly, in conditions consistent with increased pleural pressures such as in obese and ARDS patients19, and presumably during laparoscopic and abdominal procedures. In such cases, that assumption could lead to inadequate use of PEEP. The approaches based on absolute or differential esophageal pressure to estimate pleural pressure do not provide equivalent measurements33,36 and direct comparison to a gold standard are needed.
Recently, an alternative method to assess PL without an esophageal balloon has been proposed and validated37. It is based on a PEEP-step maneuver and measurement of changes in end-expiratory lung volumes using the spirometer available in some ventilators37.
What is the physiological interpretation of driving pressure and what are its clinical applications?
Driving pressures provide an easily measured correlate of global lung strain5,11. Driving pressure can be expressed as the ratio between VT and respiratory system compliance (fig. 1). Respiratory system compliance correlates with the aerated lung volume38. Accordingly, driving pressure can be interpreted as a measurement proportional to the VT normalized to aerated lung volume and, thus, to be related to global lung strain5. This concept also clarifies the contrast between the strictly volumetric information provided by VT and the additional information on lung strain (VT/initial lung volume) contained in the driving pressure (fig. 1).
In agreement with these physiological principles, recent studies confirmed that driving pressure explains clinical outcomes related to lung protective mechanical ventilation better than tidal volumes both in the intraoperative6,11 and the intensive care5 settings. Intraoperatively, a large registry study on patients undergoing non-cardiothoracic surgery with general anesthesia and mechanical ventilation indicated that the driving pressure presented a continuous and dose dependent relationship to the odds-ratio of major postoperative pulmonary complications (pneumonia, pulmonary edema, need for reintubation and adult respiratory distress syndrome, ARDS)11. A meta-analysis of randomized controlled trials of protective ventilation during general anesthesia indicated that the only ventilatory parameter associated with an increase in postoperative pulmonary complications was driving pressure with an odds ratio of 1.166.
In intensive care, an analysis of randomized trials of ventilation in ARDS patients found that an increase in driving pressure of 7 cmH2O was associated with increased mortality (relative risk=1.41), even if plateau pressures and VT were in ranges accepted as protective (plateau pressures≤30 cmH2O and VT ≤7 mL/kg) (relative risk=1.36)5. In that study, a driving pressure greater than 15 cmH2O was associated with increased mortality5. A subsequent investigation of ARDS patients with driving pressures above and below that threshold found that the higher driving pressure was associated with higher lung stress39.
While these are not prospective studies, the broad range of cases and patients included support the use of driving pressure as a marker of outcomes in mechanically ventilated patients. These studies also suggest that the traditional limits of airway pressure (e.g., ≤30cmH2O2,4) may not be enough to prevent lung injury. Instead, limiting or minimizing driving pressures could be a more relevant target. Current estimates for safe driving pressures range from 14 to 18 cmH2O5,6,11. Yet, there are caveats to such a concept to be discussed below.
Of note, spontaneously breathing patients during pressure-support ventilation can generate negative pleural pressures large enough to result in large VT and resulting end-inspiratory plateau pressures above set peak pressures. Such plateau pressures can be measured with an inspiratory hold and allow for assessment of driving pressures40. Importantly, such observation is indicative of large and potentially injurious transpulmonary pressures.
What is the physiological interpretation of transpulmonary pressure and what are its clinical applications?
Transpulmonary pressure is the physical quantity measuring the mechanical load applied to the lung during ventilation. Accordingly, transpulmonary pressure represents the stress applied to the lung parenchyma10,19 potentially conducive to ventilator-induced lung injury14,19,27 (note that pressure has units of force/area). Traditional teaching has focused on airway pressures as measures of risk for barotrauma and lung injury. Values such as 30-32 cmH2O have been cited as maximum safe limits during mechanical ventilation2,4. The concept of transpulmonary pressure, and the clinical and experimental evidence that followed24,41, emphasize that absolute airway pressures available in the anesthesia machine or mechanical ventilator are not the ultimate measure of lung stress. Instead, the transpulmonary pressure provides a more accurate measurement of lung stress and risk of injury42.
In healthy lungs, ventilator-induced lung injury occurs when stresses result in lung volumes nearing total lung capacity, corresponding to a PL~26 cmH2O20. In the clinical setting, upper limits for tidal changes in PL of 15-20 cmH2O in healthy patients and 10-12 cmH2O for ARDS patients have been recommended24.
Transpulmonary pressure has been used most frequently in the intensive care unit to guide PEEP setting in the most difficult patients, including patients with ARDS and obese patients25,43-45. The essential rationale is to adjust PEEP to values assuring a positive end-expiratory PL (e.g., end-expiratory PL=0-10 cmH2O). Based on the definition of PL, titration of mechanical ventilation to these values would avoid end-expiratory alveolar collapse.
Application of such transpulmonary pressure based approaches lead to improved oxygenation, respiratory system compliance and a trend to reduction in mortality in patients with ARDS25,43. Given the significant number of hypoxemic patients with unrecognized ARDS46, use of esophageal pressure monitoring might be considered in any patient with worsening hypoxemia. In obese patients with respiratory failure, low to negative PL predicted lung collapse and intratidal recruitment/derecruitment, providing guidance for PEEP selection and recruitment maneuvers45. In the intraoperative setting, PL has been used to determine optimal PEEP in patients undergoing laparoscopic bariatric surgery44.
The use of transpulmonary pressure as a correlate of lung stress has limitations27. Compared to the simple measurement of driving pressure, esophageal manometry requires additional equipment and training in placement and interpretation, hindering its clinical use27. The esophageal pressure is affected by several factors such as posture, weight of the mediastinum, esophageal smooth muscle compliance and reactivity, and patient effort27. The esophageal balloon pressures reflect measurements at the location where the balloon is actually placed, i.e., at the height of the esophagus26. Regional variations in lung expansion are not necessarily accurately captured by esophageal manometry. Despite such limitations, recent data in supine large animals and cadavers support that end-expiratory esophageal balloon pressures are reliable estimates of end-expiratory pleural pressures at the level of the esophagus, and that end-inspiratory PL estimate end-inspiratory pressures in non-dependent lung26 providing a bedside measurement with value superior to other current clinical measurements to guide safe mechanical ventilation.
When do driving pressure and transpulmonary pressure diverge and how do we interpret these circumstances?
While driving pressures are easier to assess for guidance to avoid ventilator-induced lung injury, there are limitations. A major limitation is its dependence on the properties of the whole respiratory system and not exclusively the lungs. External to the lungs, the properties of the chest wall including the abdomen influence driving pressure measurements. This influence could be misleading as chest wall properties do not reflect increased risk of injury16. Thus, in conditions where the chest wall compliance is normal, changes in driving pressure will provide an appropriate surrogate for changes in transpulmonary pressures and lung strain. However, when chest wall compliance is abnormal, direct assessment of transpulmonary pressure could be required to appropriately quantify potentially damaging stress applied to the lungs. Common clinical situations in which chest wall compliance lead to a divergence between driving pressures and transpulmonary pressures are related to increased intra-abdominal pressure due to abdominal insufflation, intra-abdominal hypertension, obesity, ascites and body position47-50 and also to thoracic trauma, edema of intrathoracic and abdominal tissues, and pleural effusion27. In such cases, airway pressures by themselves may be misleading to set mechanical ventilation.
Laparoscopic surgery reduces the compliance of the chest wall, increasing airway pressures51,52. Yet, because airway pressures are distributed to the lung and chest wall according to their corresponding compliances, airway pressures are not fully transmitted to the lungs in terms of equivalent increases in transpulmonary pressures (fig. 2B). Robotic surgery, a specific type of laparoscopic surgery, presents analogous situations frequently exacerbated by the Trendelenburg position and use of special framework (fig. 2B). Direct human data in these conditions to provide quantification of the distribution of airway pressures to the lungs and chest wall are lacking.
Measurements of transpulmonary pressure have highlighted the possibility of distinct lung stresses during experimental intra-abdominal hypertension48,53. Increasing intra-abdominal pressure increased plateau pressure by about half of the applied intra-abdominal pressure, but produced minimal change in PL in healthy lungs, emphasizing that airway pressures do not reflect transpulmonary pressures53. Increased driving pressures with high intra-abdominal pressures without a corresponding PL increase have been also observed for unilateral atelectasis41,48. In contrast, both driving and transpulmonary pressures increased with high intra-abdominal pressures in the presence of lung injury48 indicating that lung mechanical properties and chest wall compliance affect changes in driving and transpulmonary pressures.
Obese patients frequently pose challenges for effective mechanical ventilation54. Increased abdominal weight exerts pressure on the diaphragm, increasing pleural pressure45. Measuring esophageal pressure in obese patients can help to determine optimal levels of PEEP and guide lung recruitment45. When directly guided by esophageal manometry44 or indirectly through electrical impedance tomography55, PEEP levels to achieve an end-expiratory PL≥0 cm H2O during laparoscopic bariatric surgeries were higher than routinely used PEEP values: 15-18 cmH2O prior to abdominal insufflation and 19-40 cm H2O after insufflation. These numbers are consistent with average supine esophageal pressures of 12.5±3.9 in the obese vs. 6.9±3.1 cm H2O in controls56.
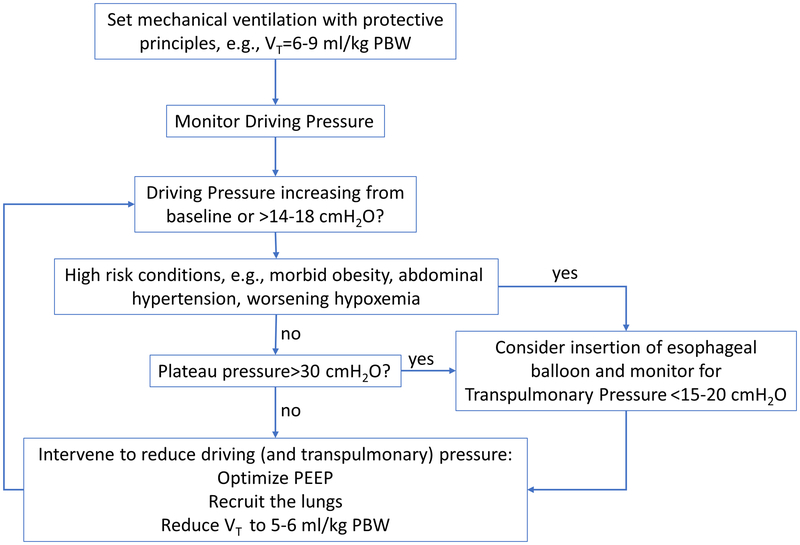
In summary, driving pressures are easily measured during routine clinical mechanical ventilation and should be monitored. Increases in driving pressures should prompt identification of potential causes and, if required, interventions to reduce them. In the several discussed clinical conditions in which driving and transpulmonary pressures diverge, if there is substantial risk for ventilator-induced lung injury, the use of methods to estimate transpulmonary pressure such as esophageal manometry is advisable to guide ventilatory management (fig. 3).
Fig. 3.
Approach for use of driving and transpulmonary pressures to guide mechanical ventilation during anesthesia. Limits presented are based on current experimental and clinical literature. Safety limits have not yet been defined in clinical trials.
Acknowledgments
Funding Statement: Supported by NIH R01 HL121228 (MFVM) and Harvard Anesthesia T32 grant 4T32GM007592-39 (ECW).
Footnotes
Clinical trial number and registry URL: Not applicable.
Prior presentations: Not applicable.
Conflicts of Interest: The authors declare no competing interests.
References
- 1.Ferrando C, Soro M, Unzueta C, Suarez-Sipmann F, Canet J, Librero J, Pozo N, Peiro S, Llombart A, Leon I, India I, Aldecoa C: Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet RespirMed 2018:Published Online January 19, 2018-Published Online January 19, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin JE, Pereira B, Jaber S, IMPROVE Study Group: A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013; 369:428–37 [DOI] [PubMed] [Google Scholar]
- 3.PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ: High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014; 384:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–8 [DOI] [PubMed] [Google Scholar]
- 5.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG: Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372:747–55 [DOI] [PubMed] [Google Scholar]
- 6.Neto AS, Hemmes SN, Barbas CS, Beiderlinden M, Fernandez-Bustamante A, Futier E, Gajic O, El-Tahan MR, Ghamdi AA, Gunay E, Jaber S, Kokulu S, Kozian A, Licker M, Lin WQ, Maslow AD, Memtsoudis SG, Reis Miranda D, Moine P, Ng T, Paparella D, Ranieri VM, Scavonetto F, Schilling T, Selmo G, Severgnini P, Sprung J, Sundar S, Talmor D, Treschan T, et al. : Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. LancetRespiratory Med 2016; 4:272–80 [DOI] [PubMed] [Google Scholar]
- 7.Motta-Ribeiro GC, Hashimoto S, Winkler T, Baron RM, Grogg K, Paula LFSC, Santos A, Zeng C, Hibbert K, Harris RS, Bajwa E, Melo MFV: Deterioration of Regional Lung Strain and Inflammation during Early Lung Injury. Am J Respir Crit Care Med 2018:May 22. doi: 10.1164/rccm.201710-2038OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Protti A, Cressoni M, Santini A, Langer T, Mietto C, Febres D, Chierichetti M, Coppola S, Conte G, Gatti S, Leopardi O, Masson S, Lombardi L, Lazzerini M, Rampoldi E, Cadringher P, Gattinoni L: Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med 2011; 183:1354–62 [DOI] [PubMed] [Google Scholar]
- 9.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Zheng H, Venegas JG, Vidal Melo MF: Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep*. Crit Care Med 2014; 42:e491–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Carlesso E, Caironi P: Stress and strain within the lung. Curr Opin Crit Care 2012; 18:42–7 [DOI] [PubMed] [Google Scholar]
- 11.Ladha K, Vidal Melo MF, McLean DJ, Wanderer JP, Grabitz SD, Kurth T, Eikermann M: Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015; 351:h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serpa Neto A, Hemmes SNT, Barbas CSV, Beiderlinden M, Biehl M, Binnekade JM, Canet J, Fernandez-Bustamante A, Futier E, Gajic O, Hedenstierna G, Hollmann MW, Jaber S, Kozian A, Licker M, Lin W-Q, Maslow AD, Memtsoudis SG, Reis Miranda D, Moine P, Ng T, Paparella D, Putensen C, Ranieri M, Scavonetto F, Schilling T, Schmid W, Selmo G, Severgnini P, Sprung J, et al. : Protective versus Conventional Ventilation for SurgeryA Systematic Review and Individual Patient Data Meta-analysis. Anesthesiology 2015; 123:66–78 [DOI] [PubMed] [Google Scholar]
- 13.Paula LF, Wellman TJ, Winkler T, Spieth PM, Guldner A, Venegas JG, Gama de Abreu M, Carvalho AR, Vidal Melo MF: Regional Tidal Lung Strain in Mechanically Ventilated Normal Lungs. J Appl Physiol Bethesda Md 1985 2016:jap.00861.2015 doi:10/f9n8g4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mead J, Takishima T, Leith D: Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970; 28:596–608 [DOI] [PubMed] [Google Scholar]
- 15.Costa EL, Musch G, Winkler T, Schroeder T, Harris RS, Jones HA, Venegas JG, Vidal Melo MF: Mild endotoxemia during mechanical ventilation produces spatially heterogeneous pulmonary neutrophilic inflammation in sheep. Anesthesiology 2010; 112:658–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tonetti T, Vasques F, Rapetti F, Maiolo G, Collino F, Romitti F, Camporota L, Cressoni M, Cadringher P, Quintel M, Gattinoni L: Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med 2017; 5:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barberis L, Manno E, Guérin C: Effect of end-inspiratory pause duration on plateau pressure in mechanically ventilated patients. Intensive Care Med 2003; 29:130–4 [DOI] [PubMed] [Google Scholar]
- 18.D’Angelo E, Calderini E, Torri G, Robatto FM, Bono D, Milic-Emili J: Respiratory mechanics in anesthetized paralyzed humans: effects of flow, volume, and time. J Appl Physiol 1989; 67:2556–64 [DOI] [PubMed] [Google Scholar]
- 19.Loring SH, Topulos GP, Hubmayr RD: Transpulmonary Pressure: The Importance of Precise Definitions and Limiting Assumptions. Am J Respir Crit Care Med 2016; 194:1452–7 [DOI] [PubMed] [Google Scholar]
- 20.Protti A, Andreis DT, Monti M, Santini A, Sparacino CC, Langer T, Votta E, Gatti S, Lombardi L, Leopardi O, Masson S, Cressoni M, Gattinoni L: Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med 2013; 41:1046–55 [DOI] [PubMed] [Google Scholar]
- 21.Loring SH, O’Donnell CR, Behazin N, Malhotra A, Sarge T, Ritz R, Novack V, Talmor D: Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol Bethesda Md 1985 2010; 108:515–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gattinoni L, Carlesso E, Cadringher P, Valenza F, Vagginelli F, Chiumello D: Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir JournalSupplement 2003; 47:15s–25s [DOI] [PubMed] [Google Scholar]
- 23.Slutsky AS, Ranieri VM: Ventilator-induced lung injury. N Engl J Med 2013; 369:2126–36 [DOI] [PubMed] [Google Scholar]
- 24.Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, Mojoli F, Chiumello D, Piquilloud L, Grasso S, Jubran A, Laghi F, Magder S, Pesenti A, Loring S, Gattinoni L, Talmor D, Blanch L, Amato M, Chen L, Brochard L, Mancebo J, PLeUral pressure working Group (PLUG-Acute Respiratory Failure section of the European Society of Intensive Care Medicine): Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 2016; 42:1360–73 [DOI] [PubMed] [Google Scholar]
- 25.Talmor D, Sarge T, Malhotra A, O’Donnell CR, Ritz R, Lisbon A, Novack V, Loring SH: Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008; 359:2095–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida T, Amato MBP, Grieco DL, Chen L, Lima CAS, Roldan R, Morais CCA, Gomes S, Costa ELV, Cardoso PFG, Charbonney E, Richard J-CM, Brochard L, Kavanagh BP: Esophageal Manometry and Regional Transpulmonary Pressure in Lung Injury. Am J Respir Crit Care Med 2018; 197:1018–26 [DOI] [PubMed] [Google Scholar]
- 27.Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, Pelosi P, Talmor D, Grasso S, Chiumello D, Guerin C, Patroniti N, Ranieri VM, Gattinoni L, Nava S, Terragni PP, Pesenti A, Tobin M, Mancebo J, Brochard L: The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014; 189:520–31 [DOI] [PubMed] [Google Scholar]
- 28.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J: A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 1982; 126:788–91 [DOI] [PubMed] [Google Scholar]
- 29.Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ: Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 2001; 164:122–30 [DOI] [PubMed] [Google Scholar]
- 30.Agostoni E: Mechanics of the pleural space. Physiol Rev 1972; 52:57–128 [DOI] [PubMed] [Google Scholar]
- 31.Talmor D, Sarge T, OʼDonnell CR, Ritz R, Malhotra A, Lisbon A, Loring SH: Esophageal and transpulmonary pressures in acute respiratory failure*: Crit Care Med 2006; 34:1389–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guérin C, Richard J-C: Comparison of 2 Correction Methods for Absolute Values of Esophageal Pressure in Subjects With Acute Hypoxemic Respiratory Failure, Mechanically Ventilated in the ICU. Respir Care 2012; 57:2045. [DOI] [PubMed] [Google Scholar]
- 33.Chiumello D, Cressoni M, Colombo A, Babini G, Brioni M, Crimella F, Lundin S, Stenqvist O, Gattinoni L: The assessment of transpulmonary pressure in mechanically ventilated ARDS patients. Intensive Care Med 2014; 40:1670–8 [DOI] [PubMed] [Google Scholar]
- 34.Grasso S, Terragni P, Birocco A, Urbino R, Del Sorbo L, Filippini C, Mascia L, Pesenti A, Zangrillo A, Gattinoni L, Ranieri VM: ECMO criteria for influenza A (H1N1)-associated ARDS: role of transpulmonary pressure. Intensive Care Med 2012; 38:395–403 [DOI] [PubMed] [Google Scholar]
- 35.Staffieri F, Stripoli T, De Monte V, Crovace A, Sacchi M, De Michele M, Trerotoli P, Terragni P, Ranieri VM, Grasso S: Physiological effects of an open lung ventilatory strategy titrated on elastance-derived end-inspiratory transpulmonary pressure: study in a pig model*. Crit Care Med 2012; 40:2124–31 [DOI] [PubMed] [Google Scholar]
- 36.Gulati G, Novero A, Loring SH, Talmor D: Pleural pressure and optimal positive end-expiratory pressure based on esophageal pressure versus chest wall elastance: incompatible results*. Crit Care Med 2013; 41:1951–7 [DOI] [PubMed] [Google Scholar]
- 37.Persson P, Stenqvist O, Lundin S: Evaluation of lung and chest wall mechanics during anaesthesia using the PEEP-step method. Br J Anaesth 2018; 120:860–7 [DOI] [PubMed] [Google Scholar]
- 38.Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M: Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis 1987; 136:730–6 [DOI] [PubMed] [Google Scholar]
- 39.Chiumello D, Carlesso E, Brioni M, Cressoni M: Airway driving pressure and lung stress in ARDS patients. Crit Care Lond Engl 2016; 20:276-016-1446–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellani G, Grassi A, Sosio S, Foti G: Plateau and driving pressure in the presence of spontaneous breathing. Intensive Care Med 2018. doi:10/gfs4b5 [DOI] [PubMed] [Google Scholar]
- 41.Morais CCA, Koyama Y, Yoshida T, Plens GM, Gomes S, Lima CAS, Ramos OPS, Pereira S, Kawaguchi N, Yamamoto H, Uchiyama A, Borges JB, Vidal Melo MF, Tucci MR, Amato MBP, Kavanagh BP, Costa ELV, Fujino Y: High Positive End-Expiratory Pressure Renders Spontaneous Effort Noninjurious. Am J Respir Crit Care Med 2018; 197:1285–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, Tallarini F, Cozzi P, Cressoni M, Colombo A, Marini JJ, Gattinoni L: Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 2008; 178:346–55 [DOI] [PubMed] [Google Scholar]
- 43.Baedorf Kassis E, Loring SH, Talmor D: Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med 2016; 42:1206–13 [DOI] [PubMed] [Google Scholar]
- 44.Eichler L, Truskowska K, Dupree A, Busch P, Goetz AE, Zollner C: Intraoperative Ventilation of Morbidly Obese Patients Guided by Transpulmonary Pressure. Obes Surg 2018; 28:122–9 [DOI] [PubMed] [Google Scholar]
- 45.Fumagalli J, Berra L, Zhang C, Pirrone M, Santiago RRDS, Gomes S, Magni F, Dos Santos GAB, Bennett D, Torsani V, Fisher D, Morais C, Amato MBP, Kacmarek RM: Transpulmonary Pressure Describes Lung Morphology During Decremental Positive End-Expiratory Pressure Trials in Obesity. Crit Care Med 2017; 45:1374–81 [DOI] [PubMed] [Google Scholar]
- 46.Bellani G, Laffey J, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley D, Ranieri M, Rubenfeld G, Thompson B, Wrigge H, Slutsky A, Pesenti A, for the LUNG SAFE Investigators and the ESICM Trials Group: Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315:788–800 [DOI] [PubMed] [Google Scholar]
- 47.Aldenkortt M, Lysakowski C, Elia N, Brochard L, Tramer MR: Ventilation strategies in obese patients undergoing surgery: a quantitative systematic review and meta-analysis. Br J Anaesth 2012; 109:493–502 [DOI] [PubMed] [Google Scholar]
- 48.Cortes-Puentes GA, Keenan JC, Adams AB, Parker ED, Dries DJ, Marini JJ: Impact of Chest Wall Modifications and Lung Injury on the Correspondence Between Airway and Transpulmonary Driving Pressures. Crit Care Med 2015; 43:e287–95 [DOI] [PubMed] [Google Scholar]
- 49.Keenan JC, Cortes-Puentes GA, Zhang L, Adams AB, Dries DJ, Marini JJ: PEEP titration: the effect of prone position and abdominal pressure in an ARDS model. Intensive Care Med Exp 2018; 6:3-018-0170–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pelosi P, Vargas M: Mechanical ventilation and intra-abdominal hypertension: “Beyond Good and Evil.” Crit Care Lond Engl 2012; 16:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cinnella G, Grasso S, Spadaro S, Rauseo M, Mirabella L, Salatto P, De Capraris A, Nappi L, Greco P, Dambrosio M: Effects of recruitment maneuver and positive end-expiratory pressure on respiratory mechanics and transpulmonary pressure during laparoscopic surgery. Anesthesiology 2013; 118:114–22 [DOI] [PubMed] [Google Scholar]
- 52.Loring SH, Behazin N, Novero A, Novack V, Jones SB, O’Donnell CR, Talmor DS: Respiratory mechanical effects of surgical pneumoperitoneum in Humans. J Appl Physiol Bethesda Md 1985 2014; 117:1074–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortes-Puentes GA, Gard KE, Adams AB, Faltesek KA, Anderson CP, Dries DJ, Marini JJ: Value and limitations of transpulmonary pressure calculations during intra-abdominal hypertension. Crit Care Med 2013; 41:1870–7 [DOI] [PubMed] [Google Scholar]
- 54.Fernandez-Bustamante A, Hashimoto S, Serpa Neto A, Moine P, Vidal Melo MF, Repine JE: Perioperative lung protective ventilation in obese patients. BMC Anesthesiol 2015; 15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nestler C, Simon P, Petroff D, Hammermuller S, Kamrath D, Wolf S, Dietrich A, Camilo LM, Beda A, Carvalho AR, Giannella-Neto A, Reske AW, Wrigge H: Individualized positive end-expiratory pressure in obese patients during general anaesthesia: a randomized controlled clinical trial using electrical impedance tomography. Br J Anaesth 2017; 119:1194–205 [DOI] [PubMed] [Google Scholar]
- 56.Behazin N, Jones SB, Cohen RI, Loring SH: Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol Bethesda Md 1985 2010; 108:212–8 [DOI] [PMC free article] [PubMed] [Google Scholar]