Abstract Abstract
Cirrhilabruswakandasp. nov. is described on the basis of the holotype and four paratypes collected between 50 and 80m depth over low-complexity reef and rubble bottoms at the east coast of Zanzibar, Tanzania, Africa. The new species belongs to a group of fairy wrasses from the western Indian Ocean, sharing a combination of characters that include: short pelvic fins (not or barely reaching anal-fin origin); relatively unmarked dorsal and anal fins; males with a strongly lanceolate caudal fin (except in C.rubrisquamis); both sexes with a pair of prominent facial stripes above and below the orbit; and both sexes with prominent purple scales and osseus elements that persist, and stain purple, respectively, even in preservation. This group of fairy wrasse is part of a larger complex that includes related species from the western Pacific Ocean. In addition to meristic and morphometric comparisons, we also compare mitochondrial DNA sequence data to the aforementioned, putatively related species.
Keywords: Coral reefs, deep reefs, Indian Ocean, rebreather diving, reef fish
Introduction
The labrid fish genus Cirrhilabrus Temminck & Schlegel, 1845 consists of small, colourful, planktivorous fishes found mostly on rubble slopes adjacent to coral reefs. Allen et al. (2015) listed 51 valid species in the genus. Eight other species have subsequently been described: Cirrhilabrusisosceles Tea et al., 2016, C.hygroxerus Allen & Hammer, 2016, C.rubeus Victor, 2016, C.efatensis Walsh et al., 2017, C.shutmani Tea & Gill, 2017, C.greeni Allen & Hammer, 2017, and C.cyanogularis Tea et al., 2018, bringing the valid species count to 59.
Members of this genus occur exclusively within the Indo-Pacific, attaining their highest diversity in the western Pacific Ocean and eastern Indian Ocean. In contrast, only seven nominal species have been reported from the western Indian Ocean, just slightly more than 10% of the genus. These are: Cirrhilabrusexquisitus Smith, 1957, C.blatteus Springer & Randall, 1974, C.rubriventralis Springer & Randall, 1974, C.rubrisquamis Randall & Emery, 1983, C.sanguineus Cornic, 1987, C.africanus Victor, 2016, and C.rubeus Victor, 2016. Of these, C.sanguineus, C.blatteus, and C.rubrisquamis are common only in mesophotic ecosystems, at depths greater than 40 m (Randall and Emery 1983; Springer and Randall 1974; Tea et al. 2018)
Mesophotic coral ecosystems (MCEs) characterise the deeper portions of coral reefs, found between 30 and 150 m (Rocha et al. 2018). While the number of studies conducted in MCEs of the Atlantic, Pacific, and northern Red Sea has increased in recent years (Loya et al. 2016), few researchers have investigated deep reefs of the western Indian Province. In a recent expedition organised by the California Academy of Sciences’ “Hope for Reefs” initiative, we had the opportunity to study the fish biodiversity in MCEs of Zanzibar, western Indian Ocean. While exploring deep reefs through technical rebreather diving, the authors discovered a new species of fairy wrasse belonging to the genus Cirrhilabrus. We herein describe Cirrhilabruswakanda sp. nov., the 60th recognised species of the genus and the eighth species recorded from the western Indian Ocean.
Materials and methods
Specimens of the new species were collected using hand nets while diving on mixed-gas, closed-circuit rebreathers (Hollis Prism 2). Methods of counting and measuring follow Randall and Masuda (1991). Gill raker counts follow Tea and Gill (2017) and are presented as upper (epibranchial) + lower (ceratobranchial); the angle raker is included in the second count. Data are presented as the range of all specimens examined, followed by data for the holotype in parentheses. Where counts were recorded bilaterally, both counts are given and separated from each other by a slash; the first count presented is the left count. Morphometric values are presented in Table 1, expressed as percentage of standard length. Institutional codes follow Sabaj (2016) and are as follows:
Table 1.
Proportional measurements of type specimens of Cirrhilabruswakanda sp. nov. expressed as a percentage of the standard length.
| Holotype | Paratypes | ||||
| CAS 246395 | CAS 246397 | CAS 246398 | CAS 246399 | CAS 246396 | |
| Sex | male | male | female | female | female |
| Standard length (mm) | 70.3 | 61.3 | 57.4 | 54.3 | 56.8 |
| Body depth | 30.9 | 31.7 | 29.8 | 31.9 | 31.8 |
| Body width | 11.8 | 12.9 | 12.6 | 13.9 | 14.5 |
| Head length | 31.0 | 30.6 | 31.2 | 30.1 | 27.7 |
| Snout length | 8.0 | 8.9 | 7.9 | 8.2 | 7.4 |
| Orbit diameter | 6.6 | 8.0 | 7.2 | 9.0 | 7.7 |
| Interorbital width | 8.5 | 9.6 | 7.7 | 9.3 | 9.1 |
| Upper jaw length | 6.9 | 8.2 | 6.5 | 7.4 | 8.2 |
| Caudal-peduncle depth | 15.1 | 16.3 | 14.8 | 16.3 | 16.5 |
| Caudal-peduncle length | 12.8 | 16.5 | 14.1 | 14.9 | 14.8 |
| Predorsal length | 32.6 | 33.8 | 31.9 | 31.7 | 33.7 |
| Preanal length | 60.4 | 59.5 | 59.6 | 58.5 | 61.4 |
| Prepelvic length | 34.4 | 33.1 | 31.5 | 35.7 | 36.4 |
| Dorsal-fin base | 58.2 | 56.6 | 55.3 | 63.2 | 57.0 |
| First dorsal spine | 5.4 | 6.5 | 6.2 | 5.2 | 6.4 |
| Longest dorsal spine | 11.9 | 14.3 | 12.4 | 13.6 | 12.7 |
| Longest dorsal ray | 19.0 | 18.3 | 16.8 | 16.7 | 17.2 |
| Anal-fin base | 26.1 | 25.3 | 25.4 | 27.6 | 24.6 |
| First anal spine | 6.0 | 6.4 | 5.2 | 5.7 | 6.4 |
| Second anal spine | 9.1 | 9.3 | 9.3 | 9.4 | 10.1 |
| Third anal spine | 10.5 | 11.1 | 10.8 | 10.9 | 11.4 |
| Longest anal ray | 16.8 | 17.8 | 14.5 | 15.1 | 17.9 |
| Caudal-fin length | 28.2 | 28.6 | 25.4 | 28.0 | 31.6 |
| Pectoral-fin length | 19.6 | 21.8 | 20.6 | 18.3 | 20.3 |
| Pelvic spine length | 11.2 | 12.1 | 11.7 | 11.0 | 11.3 |
| Pelvic fin length | 18.0 | 17.9 | 16.2 | 15.5 | 18.8 |
CASCalifornia Academy of Sciences.
DNA extraction and PCR amplification of the mitochondrial cytochrome c oxidase subunit I (COI) were performed following protocols detailed in Weigt et al. (2012). Forward and reverse contigs were aligned and trimmed separately using Geneious Prime 2019.1.1. (Biomatters, Auckland, New Zealand). Uncorrected pairwise distances for the COI marker were calculated in Geneious Prime. We compared the DNA sequences from four specimens of the new species to putatively related species of Cirrhilabrus with publicly available sequence data in GenBank (Cirrhilabrussanguineus: MH780162; Cirrhilabrusrubrisquamis: MH780161; Cirrhilabrusblatteus: MF123821).
Taxonomy
Cirrhilabrus wakanda sp. nov.
http://zoobank.org/2E9018A1-A98F-4F8C-AEA9-89D18BC69162
Vibranium fairy wrasse Figures 1 , 2 , 3 , 4 , 5A , 6A ; Table 1
Figure 1.
Cirrhilabruswakanda sp. nov., freshly euthanized male holotype (CAS 246395), 70.3 mm SL, male, collected at a depth of 75 m, east coast of Zanzibar, Africa (above). Note the pair of facial stripes above and below orbit. Photograph by H.T. Pinheiro and B. Shepherd.
Figure 2.
Paratypes of Cirrhilabruswakanda sp. nov., not to scale A1 CAS 246397, 61.3 mm SL, male, freshly euthanized A2 CAS 246397, male in preservation B1 CAS 246398, 57.38 mm SL, female, freshly euthanized B2 CAS 246398, female in preservation C1 CAS 246399, 54.32 mm SL, female, freshly euthanized C2 CAS 246399, female in preservation. Photographs by H.T. Pinheiro and B. Shepherd (A1, B1, C1), and J. Fong (A2, B2, C2).
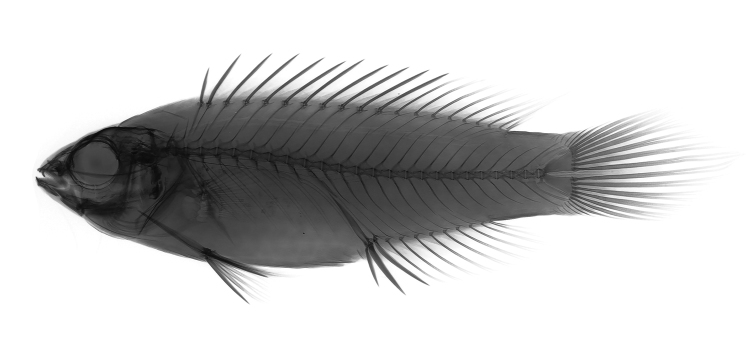
Figure 3.
Cirrhilabruswakanda sp. nov., CAS 246395, 70.3 mm SL, male holotype, x-ray. Radiograph by J. Fong.
Figure 4.
Cirrhilabruswakanda sp. nov., in situ photographs at 75 m depth, in the east coast of Zanzibar, Tanzania, Africa. Specimens not retained. Note intensity of yellow on the heads of males (A), transitioning males (B), and females (C). Photographs by L.A. Rocha.
Figure 5.
A selection of Cirrhilabrus species from the western Indian Ocean group of the Cirrhilabrusjordani complex ACirrhilabruswakanda sp. nov., in situ photograph from the east coast of Zanzibar, Africa BCirrhilabrusblatteus, in situ photograph from the Red Sea, off the coast of Eilat; C: Cirrhilabrussanguineus, aquarium photograph of a specimen from Mauritius DCirrhilabrusrubrisquamis, aquarium photograph of a specimen from the Maldives. Photographs by L.A. Rocha (A); E. Brokovich (B), and Y.K. Tea (C, D).
Figure 6.
A selection of Cirrhilabrus species in preservation showing the purple staining qualities. Not to scale ACirrhilabruswakanda sp. nov., 70.3 mm SL, male holotype, CAS 246395 BCirrhilabrusblatteus, 65.1 mm SL, male, CAS 235080 CCirrhilabrusearlei, 56.5 mm SL, male paratype, CAS 213114. Photographs by L.A. Rocha (A) and B.W. Frable (B, C).
Holotype.
CAS 246395 (field code: HTP 900), 70.3 mm SL male, GenBank MN010585, east coast of Zanzibar, Tanzania, Africa (GPS coordinates: 6°10'30"S; 39°32'28"E), 75 m, collected by H.T. Pinheiro, B. Shepherd, and L.A. Rocha, 14 December 2018; Figure 1.
Paratypes.
CAS 246396 (HTP 883), 56.8 mm SL female, GenBank MN010586, east coast of Zanzibar, Tanzania, Africa, 70 m, 07 December 2018; CAS 246397 (HTP 901), 61.3 mm SL male, GenBank MN010587, east coast of Zanzibar, Tanzania, Africa, 75 m, 14 December 2018; Figure 2 (A1, A2); CAS 246398 (HTP 902), 57.4 mm SL female, GenBank MN010588, east coast of Zanzibar, Tanzania, Africa, 75 m, 14 December 2018; Figure 2 (B1, B2); CAS 246399 (HTP 903), 54.3 mm SL female, east coast of Zanzibar, Tanzania, Africa, 75 m, 14 December 2018; Figure 2 (C1, C2). All type specimens collected by H.T. Pinheiro, B. Shepherd, and L.A. Rocha.
Diagnosis.
Cirrhilabruswakanda shares similar meristic characters to other members of this genus. However, it is readily distinguished from all other Cirrhilabrus in having the following combination of colouration and morphological characters: caudal fin strongly lanceolate in males; both sexes with a series of purple scales (in life and in preservation) arranged in a chain-link pattern across dorsal two-thirds of body.
Description.
Dorsal-fin rays XI,9; anal-fin rays III,9; dorsal and anal-fin soft rays branched except first ray unbranched in two individuals; last dorsal and anal-fin ray branched to base; pectoral-fin rays 14–15 (15/15), upper two unbranched; pelvic-fin rays I,5; principal caudal-fin rays 7+6, uppermost and lowermost unbranched; upper procurrent caudal-fin rays 6, lower procurrent caudal-fin rays 6; lateral line interrupted, with dorsoanterior series of pored scales 16–19 (17/17) and midlateral posterior peduncular series 8–9 (9/9); scales above lateral line to origin of dorsal fin 2; scales below lateral line to origin of anal fin 6; median predorsal scales 4–5 (4); median prepelvic scales 5; rows of scales on cheek 2; circumpeduncular scales 15–16 (15); gill rakers 8–9 (8) + 8–9 (8) = 16–18 (16); pseudobranchial filaments 8–10; vertebrae 9+16; epineurals 13 (Figure 3).
Body moderately elongate and compressed, depth 3.1–3.4 (3.2) in SL, width 2.1–2.6 (2.6) in depth; head length 3.2–3.6 (3.2) in SL; snout pointed, its length 3.4–3.9 (3.9) in HL; orbit diameter 3.6–4.7 (4.7) in HL; depth of caudal peduncle 1.7–2.1 (2.1) in HL. Mouth small, terminal, and oblique, with maxilla almost reaching vertical at front edge of orbit; dentition typical of genus with three pairs of canine teeth present anteriorly at side of upper jaw, first forward-projecting, next two strongly recurved and outcurved, third longest; an irregular row of very small conical teeth medial to upper canines; lower jaw with a single stout pair of canines anteriorly which protrude obliquely outward and are slightly lateral to medial pair of upper jaw; no teeth on roof of mouth.
Posterior margin of preoperculum with 30–32 (32) very fine serrated; margins of posterior and ventral edges of preoperculum free to about level of middle pupil. Anterior nostril in short membranous tube, located nearer to orbit than snout tip; posterior nostril larger, roughly ovoid to rectangular, located just medial and anterior to upper edge of eye. Scales cycloid; head scaled except snout and interorbital space; four large scales on opercle; a broad naked zone on membranous edge of preopercle; a row of large, elongate, pointed scales along base of dorsal fin, one per element, scales progressively shorter posteriorly on soft portion of fin; anal fin with a similar basal row of scales; last pored scale of lateral line (posterior to hypural plate) enlarged and pointed; one scale above and below last pored scale also enlarged; a horizontal series of greatly enlarged scales extend two-thirds distance to central posterior margin of caudal fin; pectoral fins naked except for a few small scales at extreme base; a single large scale at base of each pelvic fin, about three-fourths length of pelvic spine.
Origin of dorsal fin above third lateral-line scale, predorsal length 3.0–3.2 (3.1) in SL; first 1–4 dorsal-fin spines progressively longer, fifth to sixth subequal, eighth to tenth longest, 2.1–2.6 (2.6) in HL; interspinous membranes of dorsal fin in males extend beyond dorsal-fin spines, with each membrane extending in a pointed filament beyond spine; fifth dorsal-fin soft ray longest, 1.6–1.9 (1.6) in HL, remaining rays progressively shorter; origin of anal fin below base of ninth dorsal-fin spine; third anal-fin spine longest, 2.4–3.0 (3.0) in HL; interspinous membranes of anal fin extended as on dorsal fin; anal-fin soft rays relatively uniform in length, sixth longest, 1.5–2.1 (1.8) in HL; dorsal and anal-fin rays barely reaching caudal-fin base; caudal fin of males lanceolate; pectoral fins short, reaching vertical between bases of fifth or sixth dorsal-fin spines, longest ray 1.4–1.6 (1.6) in HL; origin of pelvic fins below lower base of pectoral fins; pelvic fins short, not reaching past anal fin origin, longest ray 1.5–1.9 (1.7) in HL.
Colouration of males in life.
Based on colour photographs and specimens when freshly dead, and field photos of live individuals (Figures 1; 2A1; 4A; 4B; 5A): head ochreous yellow; lower part of head whitish to pale pink (yellowish when freshly dead), purple stripe present from mid-upper lip to mid-upper edge of orbit; second stripe of similar colour present from lower edge of maxilla to mid-lower edge of orbit; interorbital and upper part of snout yellowish, with a series of very fine white stripes; preoperculum prominently purple on outer edge; iris bright yellow, greenish on the upper edge, with orange ring around pupil; body pale mauve to purplish-pink, with a faint region of paler yellowish-pink below middle part of dorsal fin; body with a network of dark purple scales arranged in a chain-link pattern from just after dorsal fin origin to edge of caudal peduncle, absent from lower third of body; dorsal-fin bluish-purple, bright fuchsia on distal half; posterior dorsal fin yellowish hyaline with a faint blue medial band, sometimes broken into spots; distal edge of dorsal fin narrowly bright blue; caudal fin bluish hyaline with a pair of concentric bright blue chevrons converging at lanceolatus terminus; coloured portion of chevron marking bright fuchsia to magenta; anal-fin similar to dorsal fin, distal edge narrowly bright blue; pelvic fins hyaline to translucent magenta; pectoral fins pinkish hyaline.
Colouration of females and juveniles in life.
Similar to males described above. Head and body more subdued in colouration, pinkish-purple to lilac (Figure 4C), deepening to yellow post mortem (Figures 2B1; 2C1).
Colouration in preservative.
(Figures 2A2; 2B2; 2C2; 6A): head and body pale tan; fine white stripes on interorbital and nape remain; infraorbitals, frontals, and pre-maxilla weakly purple; preoperculum, dentary, angular, and articular bones strongly purple; scales in chain-link formation deep purple; median fins translucent, except rays weakly purple; pelvic and pectoral fins translucent hyaline.
Etymology.
The specific epithet refers to the fictional East African nation of Wakanda, home of the superhero Black Panther, as is the case for the new species, which has remained hidden from the world for a long time. To be treated as a noun in apposition. The common name refers to the fictional metal vibranium, a rare substance found on Wakanda that is woven into Black Panther’s suit. The purple chain-link scale pattern of the new species is reminiscent of this detail.
Distribution and habitat.
Cirrhilabruswakanda is presently known only from the east coast of Zanzibar, Tanzania. The species inhabits deep shelves consisting of small patch reefs dominated by rhodolith and sponge beds, at depths between 50 and 80 m.
Comparisons.
Pairwise comparison of mitochondrial sequence data suggests that Cirrhilabruswakanda is most closely related to C.rubrisquamis Randall & Emery (1983), differing by 0.6% in mitochondrial COI (uncorrected pairwise distance). Such marginal differences in sequence data between closely related sister species is not uncommon in Cirrhilabrus, even when stark morphological differences are present (Tea et al., 2016; Victor, 2016; Allen & Hammer, 2017). It also appears to be closely related to C.blatteus Springer & Randall (1974) (1.9% difference in COI) and C.sanguineus Cornic (1987) (1.5% difference in COI). These four species share the following character combination: short pelvic fins (not or barely reaching anal-fin origin); relatively unmarked dorsal and anal fins; males with a strongly lanceolate caudal fin (except in C.rubrisquamis); both sexes with a pair of prominent facial stripes above and below the orbit; and both sexes with prominent purple scales and osseus elements that persist, and stain purple, respectively, even in preservation.
In Cirrhilabruswakanda the purple scale pattern presents as a scattered, chain-link motif (Figure 1; 2; 4; 5A). In the other related species, the purple scales are manifested as: two rows dorsally and laterally in C.blatteus (Figure 5B); an oblique mid-dorsal saddle in C.sanguineus (Figure 5C); a crosshatch network anteriorly in C.rubrisquamis(Figure 5D). Aside from details in live colouration, Cirrhilabruswakanda differs from: C.blatteus in having a higher number of pored lateral line scales (24–28 vs. 21–24); C.sanguineus in having one fewer median prepelvic scale (5 vs. 6) and fewer pseudobranchial filaments (8–10 vs. 11), and further from C.rubrisquamis in having a lanceolate caudal fin.
The four species are part of a larger complex of fairy wrasses that includes five other species from the western Pacific Ocean: Cirrhilabrusjordani, C.earlei, C.roseafascia, C.lanceolatus, and C.shutmani. Together, these nine species form the Cirrhilabrusjordani complex. Previous morphological and molecular studies have also shown support for this grouping (Tea and Gill 2017; Tea et al. 2018).
Remarks.
Cirrhilabruswakanda possess several osseus elements and fin rays that stain naturally purple in ethanol (Figure 6A). Only a handful of other Cirrhilabrus share this character. Springer and Randall (1974) first noted this occurrence in Cirrhilabrusblatteus (Figure 6B). Subsequently, Randall (1995) made note of its reoccurrence in Cirrhilabrusrubrisquamis and Cirrhilabrussanguineus. Tea et al. (2018) expanded this list to include Cirrhilabrusearlei (Figure 6C). Incidentally, these species are all closely related members of the jordani complex, with C.wakanda, C.rubrisquamis, C.sanguineus and C.blatteus occurring in the western Indian Ocean, and C.earlei occurring in the western Pacific Ocean. However, since the purple post-preservation staining is not found in the other Pacific Ocean species (C.jordani, C.shutmani, C.roseafascia, and C.lanceolatus), the distribution of this character within the jordani complex sensu lato is paraphyletic and is therefore not synapomorphic for this group of fairy wrasses.
Material examined.
Cirrhilabrusblatteus – Red Sea, off Saudi Arabia: CAS 235080, 56.2 mm SL; 63.4 mm SL; 65.1 mm SL; Cirrhilabrusearlei – Palau: CAS 213114, 56.5 mm SL.
Supplementary Material
Acknowledgements
This work was funded by the generous support of donors who endorsed the California Academy of Sciences’ Hope for Reefs Initiative. We thank M. V. Bell, A. Fusillo, J. Armstrong, and the staff of the Rising Sun Diving Centre and Breezes Beach Club for providing diving and logistic operations in the field, C. Castillo for logistical and programmatic support, and C. Rocha and G. Arango for support from the CAS genomics lab. We also thank Zanzibar’s Institute of Marine Science of the University of Dar es Salam (through Dr. Saleh Yahya) and the Secretary of the Zanzibar Research Committee for issuing scientific collecting and export permits. D. Catania provided CAS collection numbers, J. Fong provided x-radiographs and type series photos, and B.W. Frable and E. Brokovich provided photographs of additional material examined.
Citation
Tea Y-K, Pinheiro HT, Shepherd B, Rocha LA (2019) Cirrhilabrus wakanda, a new species of fairy wrasse from mesophotic ecosystems of Zanzibar, Tanzania, Africa (Teleostei, Labridae). ZooKeys 863: 85–96. https://doi.org/10.3897/zookeys.863.35580
References
- Allen GR, Erdmann M, Dailami M. (2015) Cirrhilabrusmarinda, a new species of wrasse (Pisces: Labridae) from eastern Indonesia, Papua New Guinea, and Vanuatu. Journal of the Ocean Science Foundation 15: 1–13. 10.5281/zenodo.896902 [DOI] [Google Scholar]
- Allen GR, Hammer MP. (2016) Cirrhilabrushygroxerus, a new species of fairy wrasse (Pisces: Labridae) from the Timor Sea, northern Australia. Journal of the Ocean Science Foundation 22: 41–52. 10.5281/zenodo.60551 [DOI] [Google Scholar]
- Allen GR, Hanmer MP. (2017) Cirrhilabrusgreeni, a new species of wrasse (Pisces: Labridae) from the Timor Sea, northern Australia. Journal of the Ocean Science Foundation 29: 55–65. 10.5281/zenodo.1115674 [DOI] [Google Scholar]
- Loya Y, Eyal G, Treibitz T, Lesser MP, Appeldoorn R. (2016) Theme section on mesophotic coral ecosystems: advances in knowledge and future perspectives. Coral Reefs 35: 1–9. 10.1007/s00338-016-1410-7 [DOI] [Google Scholar]
- Randall JE. (1995) A review of the wrasses of the genus Cirrhilabrus (Perciformes: Labridae) from the western Indian Ocean. Revue Française d’Aquariologie Herpêtologie 22: 19–26. [Google Scholar]
- Randall JE, Emery AR. (1983) A new labrid fish of the genus Cirrhilabrus from the Chagos Archipelago, Indian Ocean. Journal of Aquariculture & Aquatic Sciences 3: 21–24. [Google Scholar]
- Randall JE, Masuda H. (1991) Two new species of labrid fishes of the genus Cirrhilabrus from islands of the tropical Pacific. aqua, Journal of Ichthyology and Aquatic Biology 4: 89–98. [Google Scholar]
- Rocha LA, Pinheiro HT, Shepherd B, Papastamatiou YP, Luiz OJ, Pyle RL, Bongaerts P. (2018) Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361: 281–284. 10.1126/science.aaq1614 [DOI] [PubMed] [Google Scholar]
- Sabaj MH. (2016) Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an Online Reference. Version 6.5 (16 August 2016). American Society of Ichthyologists and Herpetologists, Washington, D.C. Electronically accessible. http://www.asih.org/ [accessed 16 February 2019]
- Springer VG, Randall JE. (1974) Two new species of the labrid fish genus Cirrhilabrus from the Red Sea. Israel Journal of Zoology 23: 45–54. [Google Scholar]
- Tea YK, Frable BW, Gill AC. (2018) Cirrhilabruscyanogularis, a new species of fairy wrasse from the Philippines and Indonesia (Teleostei: Labridae). Zootaxa 4418: 577–587. 10.11646/zootaxa.4418.6.5 [DOI] [PubMed] [Google Scholar]
- Tea YK, Frable BW, Van Der Wal C. (2018) Redescription and phylogenetic placement of Cirrhilabrussanguineus (Teleostei: Labridae), with first documentation of the female form. Zootaxa 4526: 358–372. 10.11646/zootaxa.4526.3.5 [DOI] [PubMed] [Google Scholar]
- Tea YK, Gill AC. (2017) Cirrhilabrusshutmani, a new species of fairy wrasse from the Babuyan Islands, northern Philippines (Teleostei: Labridae). Zootaxa 4341: 77–88. 10.11646/zootaxa.4418.6.5 [DOI] [PubMed] [Google Scholar]
- Tea YK, Senou H, Greene BD. (2016) Cirrhilabrusisosceles, a new species of wrasse (Teleostei: Labridae) from the Ryukyu Archipelago and the Philippines, with notes on the C.lunatus complex. Journal of the Ocean Science Foundation 21: 45–54. 10.5281/zenodo.53228 [DOI] [Google Scholar]
- Victor BC. (2016) Two new species in the spike-fin fairy-wrasse complex (Teleostei: Labridae: Cirrhilabrus) from the Indian Ocean. Journal of the Ocean Science Foundation 23: 21–50. 10.5281/zenodo.163217 [DOI] [Google Scholar]
- Walsh F, Tea YK, Tanaka H. (2017) Cirrhilabrusefatensis, a new species of wrasse (Teleostei: Labridae) from Vanuatu, South Pacific Ocean. Journal of the Ocean Science Foundation 26: 68–79. 10.5281/zenodo.570930 [DOI] [Google Scholar]
- Weigt LA, Baldwin CC, Driskell A, Smith DG, Ormos A, Reyier EA. (2012) Using DNA barcoding to assess Caribbean reef fish biodiversity: Expanding taxonomic and geographic coverage. PLoS ONE 7: e41059. 10.1371/journal.pone.0041059 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.