Abstract
Background
Tenofovir diphosphate (TFV-DP) in dried blood spots (DBS) is associated with viral suppression in persons living with HIV (PLWH) taking tenofovir disoproxil fumarate (TDF). However, its value as a predictor of future viremia remained unknown.
Methods
Blood for plasma viral load (VL) and TFV-DP in DBS were collected (up to 3 visits within 48 weeks) in PLWH on TDF. TFV-DP cut points were selected using logistic prediction models maximizing the area under the receiver operation characteristic curve, and estimated adjusted odds ratio (aOR) of future viremia (≥20 copies/mL) were compared to the highest TFV-DP category.
Results
Among all 451 participants in the analysis, aOR of future viremia for participants with TFV-DP <800 and 800 to <1650 fmol/punch were 4.7 (95% CI, 2.6–8.7; P < .0001) and 2.1 (95% CI, 1.3–3.3; P = .002) versus ≥1650 fmol/punch, respectively. These remained significant for participants who were virologically suppressed at the time of the study visit (4.2; 95% CI, 1.5–12.0; P = .007 and 2.2; 95% CI, 1.2–4.0; P = .01).
Conclusions
TFV-DP in DBS predicts future viremia in PLWH on TDF, even in those who are virologically suppressed. This highlights the utility of this biomarker to inform about adherence beyond VL.
Clinical Trials Registration. NCT02012621.
Keywords: adherence, dried blood spots, tenofovir diphosphate, antiretroviral therapy, pharmacokinetics, predictive value
This study established that tenofovir diphosphate (TFV-DP) concentrations in dried blood spots predict future viremia in persons living with HIV taking TDF-based regimens, even in individuals who are virologically suppressed at the time of their visit.
Sustained antiretroviral therapy (ART) adherence is indispensable to achieve durable viral suppression in persons living with human immunodeficiency virus (PLWH) and to prevent disease progression and human immunodeficiency virus (HIV) transmission [1, 2]. However, despite its critical role, the accurate and consistent quantification of ART adherence remains challenging, thus limiting the ability to reliably monitor adherence in routine clinical practice [3]. Methods such as self-report usually overestimate adherence and are subject to recall errors and social desirability bias [4]. Similarly, pill counts and pharmacy refills, while more objective, are burdensome to implement and do not confirm drug ingestion [5, 6]. While viral load (VL) has been used as the main measure of ART adherence in HIV therapy, it is a delayed clinical outcome that cannot inform about the adherence gradients that preceded it, thus limiting the categorization of adherence into suppressive versus nonsuppressive. In addition, an undetectable VL does not predict change in viral status in the future, and providers may assume that discussions about adherence are not necessary [3, 7]. These limitations highlight the need for new and objective measures of ART adherence that can complement what is currently available in clinical practice.
Tenofovir diphosphate (TFV-DP), the phosphorylated anabolite of tenofovir (TFV), can be quantified in red blood cells and dried blood spots (DBS) in PLWH who are taking tenofovir disoproxil fumarate (TDF) and tenofovir alafenamide (TAF) [8, 9]. Within this matrix, TFV-DP has a unique pharmacology demonstrated by a long intracellular half-life of 17 days with large accumulation (25-fold) to steady state [8, 10]. The large dynamic range of accumulation renders it ideal to quantify levels of cumulative adherence and drug exposure over the preceding 4–8 weeks, analogous to the role that hemoglobin A1c has in patients with diabetes mellitus. This pharmacology has been leveraged such that TFV-DP in DBS is a strong predictor of efficacy to preexposure prophylaxis [11] and is also strongly associated with concomitant viral suppression in PLWH [12]. However, the value of this adherence and exposure biomarker as a tool to predict the development of viremia in the future, or as a complement to VL, has not been evaluated. To address this issue and further expand its potential clinical utility, we studied whether TFV-DP in DBS can predict the development of future viremia in PLWH who were receiving TDF-including ART.
METHODS
Participants and Study Design
A clinical cohort of PLWH was prospectively recruited from within the University of Colorado Hospital (UCH) Infectious Diseases Group Practice, in Aurora, Colorado [12]. Participants were required to be 18 years or older, to be taking a regimen including TDF (any type of regimen and any duration of time) and to have blood drawn for routine VL as recommended by their primary HIV provider. Enrollment occurred on a first-come, first-served basis at the time of a clinic visit, and up to 3 visits in a 48-week period were obtained. Once informed consent was obtained, 4–6 mL of whole blood for DBS were collected into 1 EDTA tube from the same peripheral venipuncture that was performed for the participant’s clinical blood draw including VL. Participants were also asked about their 3-month, 30-day, and 3-day self-reported adherence at every study visit using a validated visual analog scale [12, 13]. Timing of study visits was dependent on the follow-up visits scheduled by the participant’s clinical provider where VL was ordered, but were required to be at least 14 days apart to allow for 1 half-life of TFV-DP in DBS [8]. Enrollment for the study was initiated in June 2014 and the last follow-up visit for the last enrolled participant concluded in July 2017 [12]. Throughout this period, no major changes in ART prescription were observed, as TDF remained the most prescribed nucleoside analog in the study population and the proportion of nonnucleoside reverse transcriptase inhibitor (NNRTI), integrase strand-transfer inhibitor (INSTI), and protease inhibitor (PI)-based regimens remained stable (25%–30% of visits on NNRTI-based, 35%–45% of visits on INSTI-based, and 15%–25% of visits on PI-based regimens). Prior to the recruitment of the first participant, the study was approved by the Colorado Multiple Institutional Review Board (COMIRB No. 13–2104) and it was also registered with clinicaltrials.gov (NCT02012621) [12].
The primary outcome of our study was VL, and an undetectable VL was defined as HIV RNA <20 copies/mL. Our original study design called for all DBS samples to be assayed. However, as previously described, after 27 months in which study visits were consecutively run, logistical limitations required a reduced assay strategy [12]. From August 2016 through the end of the study, DBS samples were assayed for any participant with a VL >20 copies/mL at any of the 3 study visits, while DBS assays for participants with an undetectable VL at all 3 visits were discontinued [12]. This approach was chosen to accomplish outcome-dependent sampling, which would resemble a longitudinal extension of a case (detectable VL) control (undetectable VL) design by enriching the sample to maintain statistical power for a relatively rare study outcome (<21% of study visits) [12, 14].
Quantification of Tenofovir Diphosphate in Dried Blood Spots
The DBS for the drug concentrations of TFV-DP were prepared by pipetting 25 μL of whole blood 5 times onto a Whatman 903 Protein Saver card, as previously described [8, 12, 15]. DBS cards were stored frozen at −80°C until analysis. The concentration of TFV-DP was quantified from a 3-mm punch using a validated liquid chromatography/tandem mass spectrometry assay, for which long-term stability in frozen samples has been documented, as previously described [12, 15]. The lower limit of quantification of the assay was 25 fmol/sample [12, 15].
Viral Load Analysis
The quantitative analysis for VL was performed at the UCH clinical laboratory using the Roche Cobas 6800 HIV test, which has a linear range of 20 to 107 copies/mL. The UCH clinical laboratory is certified under the Clinical Laboratory Improvement Amendment of 1988.
Statistical Analysis
The maximum number of predictive paired TFV-DP and VL assessments per participant was 2, with visit 1 TFV-DP concentrations informing about visit 2 VL, and visit 2 TFV-DP concentrations informing about visit 3 VL. To enhance interpretation and ease of application in clinical practice, TFV-DP levels were categorized using the genetic algorithm in the CatPredi function of the R package CatPredi [16, 17]. The CatPredi function selects the optimal location and number of cut points in logistic prediction models by maximizing the area under the receiver operation characteristic curve [16, 17]. Accordingly, TFV-DP levels were assigned into 3 categories: (1) low, below the limit of quantification (BLQ) to <800; (2) medium, 800 to <1650; and (3) high, ≥1650 fmol/punch. Cut point optimization was performed using only the first record per participant in a univariable setting. To accommodate repeated measures over time, generalized estimating equations with a logit link were used to estimate the odds ratio (OR) of viremia (≥20 copies/mL) comparing each TFV-DP category to the highest reference category. An adjusted OR (aOR) was obtained by including covariates for age, sex, race, body mass index, estimated glomerular filtration rate (using MDRD equation), CD4+ T-cell count, and ART class. Adjustment variables were selected a priori based on previous findings on the pharmacology of TFV in plasma [18, 19], TFV-DP in peripheral blood mononuclear cells [20, 21] and DBS [10, 12]. To ensure adequate time for VL to reach suppression in participants who recently initiated ART [8, 10], visits in which the participant had not been on ART therapy for the previous 3 months were removed from the analysis. Data are presented as median (interquartile range) or number (percentage) unless otherwise specified. A P value <.05 was considered to be statistically significant. P values were not adjusted for multiple comparisons. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC) and R software version 3.4.4.
RESULTS
Study Population
A total of 807 participants were enrolled in the study, contributing a total of 1939 person-visits and 1936 DBS samples, with 444 participants (55%) completing all 3 visits, 244 (30%) completing 2 visits, and 119 (15%) completing only 1 study visit. Drug concentrations were analyzed in 532 participants, of whom 468 had more than 1 visit and could potentially be included in this analysis. After removing visits in which the participant had not been on ART for at least 3 months, 451 participants with both analyzed DBS and VL at the next visit were included in the analysis. Out of these, 201 (45%) were on a single-tablet regimen containing TDF for at least 1 of their visits (only 8 participants switched from a multitablet to a single-tablet regimen during the study). The demographic characteristics of this cohort are presented in Table 1.
Table 1.
Demographic Characteristics of Participants at First Evaluable Visit According to Tenofovir Diphosphate in Dried Blood Spots Categories
| TFV-DP (fmol/punch) | ||||
|---|---|---|---|---|
| Characteristic | All Participants (n = 451) | BLQ to <800 (n = 58) | 800 to <1650 (n = 165) | ≥1650 (n = 228) |
| Age, median (IQR) | 46 (37–53) | 42 (34–51) | 45 (39–51) | 49 (38–55) |
| Sex, n (%) | ||||
| Male | 380 (84) | 49 (84) | 141 (85) | 190 (83) |
| Female | 71 (16) | 9 (16) | 24 (15) | 38 (17) |
| Race/ethnicity, n (%) | ||||
| Black | 85 (19) | 18 (31) | 37 (22) | 30 (13) |
| White | 257 (57) | 23 (40) | 96 (58) | 138 (61) |
| Hispanic | 89 (20) | 14 (24) | 30 (18) | 45 (20) |
| Other | 20 (4) | 3 (5) | 2 (1) | 15 (7) |
| Body mass index, kg/m2, n (%) | ||||
| <18.5 | 18 (4) | 2 (3) | 3 (2) | 13 (6) |
| 18.5–25 | 191 (42) | 23 (40) | 60 (36) | 108 (47) |
| 25–30 | 149 (33) | 19 (33) | 58 (35) | 72 (32) |
| >30 | 93 (21) | 14 (24) | 44 (27) | 35 (15) |
| eGFR, mL/min/1.73 m2, median (IQR) | 87 (73–101) | 96 (80–112) | 89 (76–104) | 84 (70–97) |
| CD4+ T-cell count, cells/mm3, n (%) | ||||
| <200 | 48 (11) | 12 (21) | 18 (11) | 18 (8) |
| 200–350 | 66 (15) | 10 (17) | 24 (15) | 32 (14) |
| 350–500 | 66 (15) | 8 (14) | 22 (13) | 36 (16) |
| >500 | 271 (60) | 28 (48) | 101 (61) | 142 (62) |
| VL, copies/mL,a median (IQR) | 119 (37–710) | 1055 (140–34 575) | 71 (31–397) | 63 (32–187) |
| Type of ART, n (%) | ||||
| NNRTI-based | 119 (26) | 10 (17) | 63 (38) | 46 (20) |
| INSTI-based | 159 (35) | 22 (38) | 52 (32) | 85 (37) |
| b/PI-based | 111 (25) | 17 (29) | 28 (17) | 66 (29) |
| Multiclass | 62 (14) | 9 (16) | 22 (13) | 31 (14) |
| Time on current anchor drug, n (%) | ||||
| <6 months | 74 (16) | 6 (10) | 32 (19) | 36 (16) |
| ≥6 months | 377 (84) | 52 (90) | 133 (81) | 192 (84) |
| Pharmacologic booster, n (%) | ||||
| No | 223 (49) | 23 (40) | 101 (61) | 99 (43) |
| Yes | 228 (51) | 35 (60) | 64 (39) | 129 (57) |
| Self-reported adherence, %, median (IQR) | ||||
| 3-day | 100 (100–100) | 100 (72–100) | 100 (100–100) | 100 (100–100) |
| 30-day | 100 (93–100) | 90 (60–100) | 100 (92–100) | 100 (99–100) |
| 3-month | 98 (90–100) | 84 (62–90) | 98 (90–100) | 100 (95–100) |
Abbreviations: ART, antiretroviral therapy; BLQ, below limit of quantification; b/PI, boosted protease inhibitor; eGFR, estimated glomerular filtration rate; HIV, human immunodeficiency virus; INSTI, integrase strand-transfer inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; TFV-DP, tenofovir diphosphate; VL, viral load.
aLV for viremic participants (≥20 copies/mL).
Among the 451 participants contributing to the analysis, 205 (45%) contributed only 1 paired TFV-DP and VL assessment and 246 (55%) contributed 2 paired assessments, for a total of n = 697 paired assessments. The median time between visits was 17 (range 2–48) weeks. According to virologic suppression, 264 (59%) of the 451 participants were suppressed at all visits, 135 (30%) switched suppression status between visits (either suppressed to viremic or vice versa), and 52 (12%) had an VL >20 copies/mL at all visits. The median and range VL in viremic participants upon the first analyzed visit is shown in Table 1.
Tenofovir Diphosphate in Dried Blood Spots
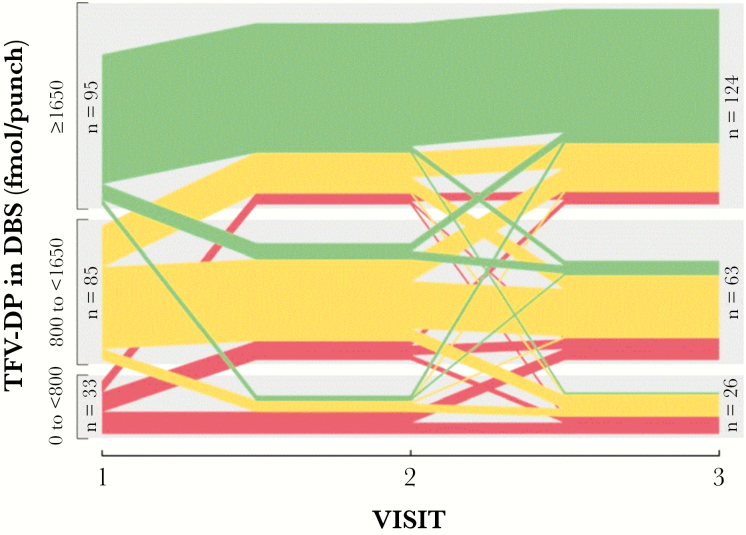
Included in the analysis were 697 samples of TFV-DP quantified in DBS, with median 1704 (range BLQ–7432) fmol/punch. TFV-DP concentrations BLQ were observed in 4 samples (0.6% of total) from 4 different participants. Based on the categorization of TFV-DP concentrations at visit 1 for the 451 participants included in the analysis, 228 (50%) of them had TFV-DP in the highest category, 165 (37%) had TFV-DP in the medium range, and 58 (13%) of participants had TFV-DP <800 fmol/punch. Regarding all 697 visits included in the analysis, 55/368 (15%) of the participants with TFV-DP in the high concentration category (≥1650 fmol/punch) became viremic at a future visit. This is in comparison to 63/247 (26%) in the medium (800 to <1650 fmol/punch) and 44/82 (54%) in the low (BLQ to <800 fmol/punch) TFV-DP categories. For the smaller subset of 213 participants who had DBS analyzed from all 3 visits, Figure 1 shows the trajectory of TFV-DP concentrations by study visit. The proportion of participants in the highest TFV-DP category (≥1650 fmol/punch) increased from 47% at visit 1 to 58% by visit 3, with the majority of the increase arising from participants in the midlevel category (800 to <1650 fmol/punch). The proportion (≤13%) of participants with TFV-DP concentrations <800 fmol/punch remained relatively stable across visits, with participants moving to higher TFV-DP categories over time being replaced by individuals with declining TFV-DP levels (Figure 1).
Figure 1.
Trajectory of TFV-DP in DBS in participants in whom drug concentrations were available for all 3 study visits (N = 213) according to drug concentration category. Colors represent TFV-DP categories with green indicating higher concentrations (≥1650 fmol/punch), yellow medium concentrations (800 to <1650 fmol/punch), and red low concentrations (BLQ to <800 fmol/punch) of TFV-DP. Each line within each category represents 1 participant. Individuals with the same progression of TFV-DP over time are grouped together forming thicker color bands. At visit 2, n = 115, n = 74, and n = 24 participants were included in the high, medium, and low concentrations, respectively. Abbreviations: BLQ, below limit of quantification; DBS, dried blood spots; TFV-DP, tenofovir diphosphate.
Predictive Value of Tenofovir Diphosphate for Future Viremia
In a univariable model, TFV-DP levels in DBS categorized into low, medium, and high were predictive of viremia at the next study visit (P < .0001). This association remained highly significant after adjusting for covariates (P < .0001), with the aOR for the highest TFV-DP category compared to the lower 2, increasing in both magnitude and significance. With TFV-DP concentrations BLQ to <800 versus ≥1650 fmol/punch, the odds of future viremia were 4.2 (95% confidence interval [CI], 2.3–7.5) and 4.7 (95% CI, 2.6–8.7) in the unadjusted and adjusted models, respectively (P < .0001 in both models). Full pairwise comparison results for both models are shown in Table 2. Comparisons for the adjusted model only are illustrated in Supplementary Figure 1. Along with TFV-DP categories, significant predictors of future viremia included ART class (P = .0002) and CD4+ T-cell count (P < .0001) (Supplementary Table 1).
Table 2.
Odds Ratio of Risk of Future HIV Load >20 copies/mL by Concentration of Tenofovir Diphosphate in Dried Blood Spots at Current Visit (N = 697 Paired Assessments)
| TFV-DP (fmol/punch) | Paired Assessments, n (%) | OR (95% CI) | P Value | aOR (95% CI)a | P Value |
|---|---|---|---|---|---|
| BLQ to <800 | 82 (12) | 4.2 (2.3–7.5) | <.0001 | 4.7 (2.6–8.7) | <.0001 |
| 800 to <1650 | 247 (35) | 1.8 (1.2–2.7) | .007 | 2.1 (1.3–3.3) | .002 |
| ≥1650 | 368 (53) | 1 | REF | 1 | REF |
Abbreviations: aOR, adjusted odds ratio; BLQ, below limit of quantification; CI, confidence interval; OR, odds ratio; REF, reference; TFV-DP, tenofovir diphosphate.
aAdjusted for age, sex, race, body mass index, estimated glomerular filtration rate, CD4+ T-cell count, and antiretroviral therapy class. The OR and aOR between categories BLQ to <800 vs 800 to <1650 fmol/punch were 2.3 (1.3–4.1), P = .003 and 2.3 (1.2–4.2), P = .008, respectively.
A subanalysis was performed on participants who were virologically suppressed at the time of their study visit to investigate the ability of TFV-DP to predict viremia at future visits, even among currently suppressed individuals. This suppressed cohort consisted of 354 participants (from the 451 available) contributing 501 paired assessments. In an adjusted model, TFV-DP in DBS in this subgroup was predictive of viremia at the next clinical visit (P = .005). The aOR for TFV-DP concentrations BLQ to <800 versus ≥1650 fmol/punch levels was 4.2 (95% CI, 1.5–12.0), P = .007, and for TFV-DP 800 to <1650 versus ≥1650 fmol/punch was 2.2 (95% CI, 1.2–4.0), P = .01, Supplementary Figure 2.
Sensitivity Analyses
An initial sensitivity analysis focused on the subset of participants in whom DBS were consecutively assayed prior to the modification of the assay strategy, which consisted of 284 participants providing 452 paired assessments. Viremia for this subgroup was 21% versus 23% in the outcome-enriched population. Model results for this subgroup were similar to the full cohort, although the pairwise comparison between the medium and low TFV-DP concentrations lost significance in the adjusted model (Table 3). In an additional sensitivity analysis, we utilized TFV-DP categories derived from the mean TFV-DP concentrations at steady state from volunteers without HIV randomized to 33%, 67%, or 100% TDF adherence [10]. In this analysis, not all pairwise comparisons remained significant; however, all of the aOR were consistent with lower TFV-DP levels predicting higher aOR of future viremia (Supplementary Table 2).
Table 3.
Odds Ratio of Risk of Future HIV Load >20 Copies/mL by Concentration of Tenofovir Diphosphate in DBS at Current Visit in a Subset of 284 Participants with Consecutively Analyzed DBS (N = 452 Paired Assessments)
| TFV-DP (fmol/punch) | Paired Assessments, n (%) | OR (95% CI) | P Value | aOR (95% CI)a | P Value |
|---|---|---|---|---|---|
| BLQ to <800 | 57 (13) | 4.4 (2.1–9.4) | <.0001 | 4.5 (2.0–10.1) | .0003 |
| 800 to <1650 | 167 (37) | 1.9 (1.1–3.4) | .02 | 2.2 (1.2–4.3) | .01 |
| ≥1650 | 228 (50) | 1 | REF | 1 | REF |
Abbreviations: aOR, adjusted odds ratio; ART, antiretroviral therapy; BLQ, below limit of quantification; CI, confidence interval; DBS, dried blood spots; HIV, human immunodeficiency virus; OR, odds ratio; REF, reference; TFV-DP, tenofovir diphosphate.
aAdjusted for age, sex, race, body mass index, estimated glomerular filtration rate, CD4+ T-cell count, and antiretroviral therapy class. The OR and aOR between categories BLQ to <800 vs 800 to <1650 fmol/punch were 2.3 (1.2–4.5), P = .02 and 2.0 (0.9–4.3), P = .08, respectively.
Discussion
In this study, we established the value of TFV-DP in DBS, obtained at the time of a clinic visit, as a predictor of future viremia >20 copies/mL in PLWH taking TDF. Our data demonstrated that the odds of future viremia increased with lower drug concentration categories, which remained significant after adjusting for individual characteristics that could influence TFV-DP in DBS [12]. In addition, TFV-DP remained a significant predictor of future viremia when we restricted our analysis to PLWH who were virologically suppressed at the time of their visit. Extending on previous findings of the association of TFV-DP in DBS with viral suppression at the time of concomitant sampling [12], this study shows new clinical utility for this biomarker in predicting future viremia in PLWH. In particular, our findings highlight the value that an objective measure of adherence could add to the information that is currently provided by VL in clinical care. While VL has historically been regarded as the ultimate clinical outcome in patients who reach a sufficient degree of ART adherence, modern ART has become more potent and pharmacologically forgiving to missed doses, allowing for virologic suppression with adherence levels around 80%–85% [22–25], or even as low as 50% [26]. Thus, an undetectable VL no longer reflects perfect adherence [7], and relying on VL alone could lead to inadequate conclusions about a patient’s adherence and lead to negative consequences.
The predictive value of TFV-DP in DBS for the development of future viremia offers a wide range of potential clinical applications. Specifically, this biomarker could identify PLWH who have low drug concentrations (ie, <800 fmol/punch) while remaining virologically suppressed. Since viremia is a delayed clinical outcome that requires long periods of suboptimal adherence before it becomes evident [27–30], early identification of patients with insufficient adherence could prevent virologic breakthrough and its adverse clinical consequences (ie, virologic failure, drug resistance, HIV transmission, burst in inflammation), which could be irreversible by the time viremia has ensued [2, 29, 31, 32]. Thus, low TFV-DP concentrations could trigger an in-depth reevaluation of a patient’s drug adherence, such as a focused discussion between the patient and the provider, a careful review of pharmacy refills, or an earlier follow-up visit for reevaluation of drug concentrations, all of which would have otherwise not happened by relying on VL alone. Comparatively, high TFV-DP concentrations (ie, ≥1650 fmol/punch) with high or persistent viremia could prompt an earlier consideration of drug resistance testing. Frequently, the decision to perform HIV drug resistance testing in the clinic can be delayed if there is a concern for (or suspicion of) nonadherence, leading to a discussion with the patient and repeated VL testing several weeks (or months) later. This attempt to regain virologic suppression can perpetuate viremia and impact the success of genotyping [33] or lead to worse clinical outcomes [34–36]. Collectively, these frequently encountered and challenging clinical scenarios illustrate the additional value that this adherence biomarker could provide beyond VL in HIV care.
In addition to identifying PLWH who might develop viral breakthrough or drug resistance, additional clinical applications of this biomarker could include feedback to both patients and providers regarding drug intake prior to a clinic visit. For patients, given the current emphasis on the undetectable equals untransmittable premise for the prevention of HIV transmission [37], the concentrations of TFV-DP would provide reassurance regarding optimal adherence and exposure in the periods between VL analyses. For providers, TFV-DP could offer a confirmation of a provider’s perception about the patient’s adherence (ie, low TFV-DP in the setting of viremia despite high adherence by self-report), or be used to remotely monitor drug adherence and exposure, in particular because self-collection of DBS has been successfully achieved for other purposes in HIV research [38, 39]. As the field moves towards the development of clinically useful measures for adherence monitoring, future research will be indispensable to evaluate the clinical impact of this adherence biomarker in clinical practice.
Our study offers several strengths, which include the longitudinal prospective sample and data collection in a diverse clinical cohort, making our results applicable to the demographic groups most affected by the HIV/AIDS epidemic. In addition, quantification of adherence using TFV-DP could offer the opportunity to infer adherence to the full ART regimen in individuals taking single-tablet formulations. It could also help identify if a specific ART regimen might be the ideal choice for a treatment-naive individual, or determine if any modifications to TDF —and eventually TAF—dosing may be considered (especially given the upcoming availability of generic drugs). Furthermore, DBS samples are easy to collect and practical for routine clinical care, and efforts are underway to create a point-of-care assay using a miniature mass spectrometer at an anticipated cost that would be lower than VL. Among the limitations of our study are the observational nature of the cohort and its possible impact on the number of follow-up visits for some participants who missed their second or third visit after enrollment. Similarly, the timing between DBS and future VL sampling was variable (and sometimes long), and may not have reflected adherence near the VL measurement. However, we believe that any potential influence from this would have biased our results towards the null, in which case the magnitude of our association would have been even stronger. In addition, not all participants were included in the analysis; however, our sampling strategy of assaying all DBS samples from participants with documented viremia focused on the most informative participants, which has been shown to produce accurate estimates when compared to an analysis of the full cohort [14], and our sensitivity analysis limited to the period prior to when our assay strategy was modified confirmed our results. Additionally, categorization of a continuous variable can lead to loss of information, which could result in different cut points across various subpopulations. However, our sensitivity analysis using cut points previously established in an independent population (volunteers without HIV) [10] demonstrated similar predictive qualities across categories. Lastly, our study was focused only on participants who were taking a TDF-based regimen. While some of our DBS samples were collected while on TAF, the sample size and number of events in this cohort would not allow for a similar analysis of participants on ART including TAF. Future research to assess the predictive value of TFV-DP in DBS derived from TAF will be indispensable to move this biomarker forward in clinical practice given its widespread use in clinical practice.
In summary, our study established the value of TFV-DP in DBS to predict future viremia in PLWH, even in those who are virologically suppressed at the time of their visit. These findings provide new insight on its possible use as a complement to VL to monitor efficacy of ART, and set the framework for future implementation of this adherence biomarker in clinical practice. Further research to determine whether TFV-DP in DBS can improve clinical outcomes in PLWH on ART is needed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author Contributions. M. M. performed data and statistical analysis and interpretation, generated figures and tables, and cowrote the first version of the manuscript and performed all edits in subsequent versions. R. P. C. and S. S. C. performed participant consent, data and sample collection, data management, data analysis and interpretation and made substantial edits and critical revisions of the manuscript. E. M. G. participated in study design, assisted with the adherence data interpretation and performed manuscript editing and critical revision. J. H. Z., L. E., and L. R. B. led the sample processing, pharmacologic analysis and data validation for the drug concentrations, and made substantial edits and critical revisions of the manuscript. J. J. K. participated in the study design, adherence and pharmacologic data interpretation and performed manuscript editing and critical revisions. P. L. A. coled the study conception and design, assisted with obtaining the funding, supported the study monitoring and logistics, directed and supported all aspects of the pharmacologic and drug concentration analysis, collaborated with data interpretation, and made substantial edits and critical revisions of the original manuscript and all its subsequent versions. S. M. substantially contributed to the study design and conceptualization, performed the sample size calculation, data management, led the statistical analysis and interpretation, generated figures and tables, and made substantial edits and critical revisions of the manuscript. J. C. M. led the conception and study design, obtained the funding and regulatory approvals, led all aspects regarding study monitoring, logistics, data and sample collection, result interpretation, cowrote the first manuscript draft and contributed to all the edits for the subsequent drafts.
Acknowledgments. We thank the study participants and the personnel at the Colorado Antiviral Pharmacology Laboratory for their invaluable assistance and support of this study. We also thank Dr Steven Johnson, director of the University of Colorado Hospital HIV program and the medical assistants (Nancy Olague, Brittany Limon, Ariel Cates, Maureen Sullivan, and Missy Sorrell) at the University of Colorado Hospital-Infectious Disease Group Practice for their invaluable contributions and support of this study.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (grant numbers K23 AI104315 to J. C. M. and R01 AI122298 to P. L. A.).
Potential conflicts of interest. J. J. K and P. L. A. have received research support from Gilead Sciences paid to their institution. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV.ttps://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed 10 June 2014.
- 2. Cohen MS, Chen YQ, McCauley M, et al. ; HPTN 052 Study Team Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016; 375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castillo-Mancilla JR, Haberer JE. Adherence measurements in HIV: new advancements in pharmacologic methods and real-time monitoring. Current HIV/AIDS Reports 2018; 15:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simoni JM, Kurth AE, Pearson CR, Pantalone DW, Merrill JO, Frick PA. Self-report measures of antiretroviral therapy adherence: a review with recommendations for HIV research and clinical management. AIDS Behav 2006; 10:227–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bisson GP, Gross R, Bellamy S, et al. Pharmacy refill adherence compared with CD4 count changes for monitoring HIV-infected adults on antiretroviral therapy. PLoS Med 2008; 5:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okatch H, Beiter K, Eby J, et al. Brief report: apparent antiretroviral overadherence by pill count is associated with HIV treatment failure in adolescents. J Acquir Immune Defic Syndr 2016; 72:542–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ammassari A, Trotta MP, Shalev N, Marconi P, Antinori A. Beyond virological suppression: the role of adherence in the late HAART era. Antivir Ther 2012; 17:785–92. [DOI] [PubMed] [Google Scholar]
- 8. Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses 2013; 29:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castillo-Mancilla J, Coyle RP, Zheng JH, et al. Tenofovir diphosphate arising from TAF is quantifiable in dried blood spots. Conference on Retroviruses and Opportunistic Infections,Seattle, WA, February 13-16, 2017: Abstract 405. http://www.croiconference.org/sites/default/files/uploads/croi2017-abstract-eBook.pdf. Accessed 6 April 2019. [Google Scholar]
- 10. Anderson PL, Liu AY, Castillo-Mancilla JR, et al. Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2018; 62:e01710–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grant RM, Anderson PL, McMahan V, et al. ; iPrEx study team Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis 2014; 14:820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castillo-Mancilla J, Morrow M, Coyle RP, et al. Tenofovir diphosphate in dried blood spots is strongly associated with viral suppression in individuals with HIV infection [published online ahead of print 23 August, 2018]. Clin Infect Dis doi: 10.1093/cid/ciy708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clinical Trials 2004; 5:74–9. [DOI] [PubMed] [Google Scholar]
- 14. Schildcrout JS, Schisterman EF, Mercaldo ND, Rathouz PJ, Heagerty PJ. Extending the case-control design to longitudinal data: stratified sampling based on repeated binary outcomes. Epidemiology 2018; 29:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng JH, Rower C, McAllister K, et al. Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrio I, Rodriguez-Alvarez MX, Arostegui I. Optimal categorisation of continuous variables in prediction models. Package “CatPredi’. 2017. Available at https://cran.r-project.org/web/packages/CatPredi/CatPredi.pdf [DOI] [PubMed] [Google Scholar]
- 17. Barrio I, Arostegui I, Rodríguez-Álvarez MX, Quintana JM. A new approach to categorising continuous variables in prediction models: proposal and validation. Stat Methods Med Res 2017; 26:2586–602. [DOI] [PubMed] [Google Scholar]
- 18. Gervasoni C, Meraviglia P, Landonio S, et al. Low body weight in females is a risk factor for increased tenofovir exposure and drug-related adverse events. PLoS One 2013; 8:e80242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cattaneo D, Minisci D, Baldelli S, et al. Effect of cobicistat on tenofovir disoproxil fumarate (TDF): what is true for TAF may also be true for TDF. J Acquir Immune Defic Syndr 2018; 77:86–92. [DOI] [PubMed] [Google Scholar]
- 20. Pruvost A, Negredo E, Théodoro F, et al. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother 2009; 53:1937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lahiri CD, Tao S, Jiang Y, et al. Impact of protease inhibitors on intracellular concentration of tenofovir-diphosphate among HIV-1 infected patients. AIDS 2015; 29:1113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Viswanathan S, Detels R, Mehta SH, Macatangay BJ, Kirk GD, Jacobson LP. Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). AIDS Behav 2015; 19:601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kobin AB, Sheth NU. Levels of adherence required for virologic suppression among newer antiretroviral medications. Ann Pharmacother 2011; 45:372–9. [DOI] [PubMed] [Google Scholar]
- 24. Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis 2006; 43:939–41. [DOI] [PubMed] [Google Scholar]
- 25. Braithwaite RS, Kozal MJ, Chang CC, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS 2007; 21:1579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenblum M, Deeks SG, van der Laan M, Bangsberg DR. The risk of virologic failure decreases with duration of HIV suppression, at greater than 50% adherence to antiretroviral therapy. PLoS One 2009; 4:e7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ford N, Darder M, Spelman T, Maclean E, Mills E, Boulle A. Early adherence to antiretroviral medication as a predictor of long-term HIV virological suppression: five-year follow up of an observational cohort. PLoS One 2010; 5:e10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis 2002; 34:1115–21. [DOI] [PubMed] [Google Scholar]
- 29. Gardner EM, Burman WJ, Steiner JF, Anderson PL, Bangsberg DR. Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS 2009; 23:1035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One 2008; 3:e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Edén A, Fuchs D, Hagberg L, et al. HIV-1 viral escape in cerebrospinal fluid of subjects on suppressive antiretroviral treatment. J Infect Dis 2010; 202:1819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Graham SM, Masese L, Gitau R, et al. Antiretroviral adherence and development of drug resistance are the strongest predictors of genital HIV-1 shedding among women initiating treatment. J Infect Dis 2010; 202:1538–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gonzalez-Serna A, Min JE, Woods C, et al. Performance of HIV-1 drug resistance testing at low-level viremia and its ability to predict future virologic outcomes and viral evolution in treatment-naive individuals. Clin Infect Dis 2014; 58: 1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seyler C, Adjé-Touré C, Messou E, et al. Impact of genotypic drug resistance mutations on clinical and immunological outcomes in HIV-infected adults on HAART in West Africa. AIDS 2007; 21:1157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palella FJ Jr, Armon C, Buchacz K, et al. ; HOPS (HIV Outpatient Study) Investigators The association of HIV susceptibility testing with survival among HIV-infected patients receiving antiretroviral therapy: a cohort study. Ann Intern Med 2009; 151:73–84. [DOI] [PubMed] [Google Scholar]
- 36. Petersen ML, Tran L, Geng EH, et al. Delayed switch of antiretroviral therapy after virologic failure associated with elevated mortality among HIV-infected adults in Africa. AIDS 2014; 28:2097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. The Lancet HIV. U = U taking off in 2017. Lancet HIV 2017; 4:e475. [DOI] [PubMed] [Google Scholar]
- 38. Spielberg F, Critchlow C, Vittinghoff E, et al. Home collection for frequent HIV testing: acceptability of oral fluids, dried blood spots and telephone results. HIV early detection study group. AIDS 2000; 14:1819–28. [DOI] [PubMed] [Google Scholar]
- 39. Frank AP, Wandell MG, Headings MD, Conant MA, Woody GE, Michel C. Anonymous HIV testing using home collection and telemedicine counseling. A multicenter evaluation. Arch Intern Med 1997; 157:309–14. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.