Summary
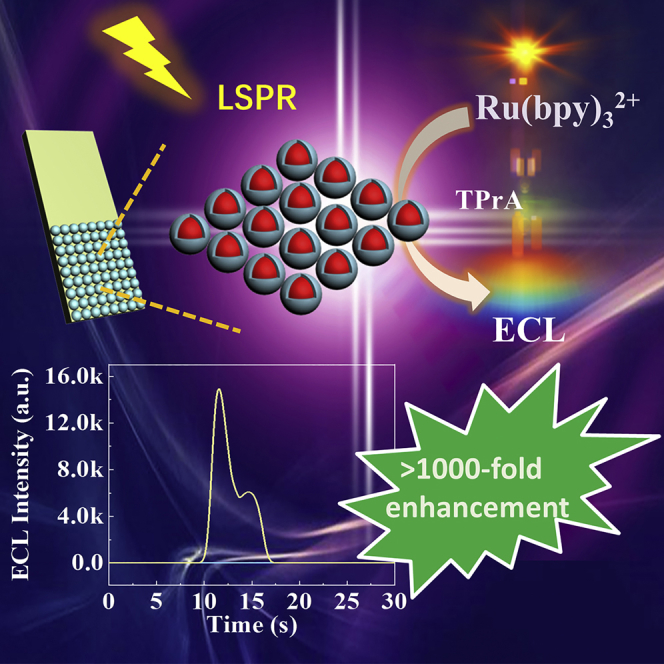
Enhancing electrochemiluminescence (ECL) with plasmonic materials is promising but still a long-standing barrier to improve its sensitivity for ultrasensitive bioassays, due to the lack of comprehensive understanding and effective strategies to fully utilize plasmonic effects for ECL enhancement. Herein, by insulating gold nanoparticles with silica shells (Au@SiO2 NPs), and finely tuning their core/shell sizes and controlling interparticle spacing via assembling them into a dense nanomembrane, we develop a novel 2D metasurface. Due to well-controlled high density “hot spots” and 2D ordered arrangement of the unit NPs in the nanomembrane, the metasurfaced ECL electrode shows over 1,000-fold plasmonic ECL enhancement for the classical Ru(bpy)32+-tripropylamine system, which is two orders of magnitude higher than ever reported (<30-fold). Such fabricated ECL biosensor demonstrates superior detection performance for prostate-specific antigen with a detection limit of 3 fg mL−1. Our results provide understanding of plasmonic effects for ECL enhancement and will benefit for biosensor construction for ultrasensitive bioassays.
Subject Areas: Electrochemical Engineering, Metamaterials, Nanostructure
Graphical Abstract
Highlights
-
•
A unique Au@SiO2 NP-based 2D metamaterial was constructed
-
•
The plasmon effects were fully utilized to enhance ECL excitation
-
•
The as-fabricated metasurfaced ECL electrode shows over 1,000-fold enhancement
Electrochemical Engineering; Metamaterials; Nanostructure
Introduction
Electrochemiluminescence (ECL) is the emission from an excited luminophore generated by an electrochemical redox reaction (Hu and Xu, 2010, Liu et al., 2015, Miao, 2008, Richter, 2004). In recent years, high-throughput, miniaturized biosensors based on ECL technology that are capable of multiplexing detection with high sensitivity, low detection limit, and good selectivity have become very powerful analytical methods and have been widely used in immunoassay and medical diagnostics. Although significant progresses have been achieved over the past decades, the mechanisms of ECL still need to be further studied and its detection sensitivity needs to be further improved for ultrasensitive bioassays (Wu et al., 2014). As an attractive solution, surface-enhanced electrochemiluminescence (SEECL) (Wang et al., 2015a), which merges photonics with photoelectronics at the nanoscale and provides an effective avenue to increase the sensitivity, has attracted enormous attention in the development of next-generation ECL biosensors.
Usually, localized surface plasmon resonance (LSPR) in plasmonic nanostructures can improve the sensitivity of SEECL biosensors via two main pathways: photonic enhancement and plasmon-induced energy transfer (PIRET) enhancement (Li et al., 2013, Wang et al., 2015a, Wu, 2018). For mostly reported regular or patterned plasmonic nanostructures, the light emitted by the luminescent molecule (e.g., Ru(bpy)32+) under the excitation of electricity is efficiently scattered multiple times, which increases the optical path length and photon flux in the luminescent molecules (Wu, 2018). This is referred to as photonic enhancement (or resonant light scattering), which contributes to the enhancement of ECL signals. In contrast, PIRET enhancement proceeds via a non-radiative process that is based on the near-field dipole-dipole interaction between the plasmonic nanostructures and the luminescent molecule. The PIRET does not require direct contact or band alignment; instead, the ECL efficiency is determined by the spectral overlap between the luminescent molecules' emission band edge and the LSPR absorbance (Li et al., 2013, Wu, 2018). Since the first report on surface plasmon-coupled ECL in 2004 (Zhang et al., 2004), Guo and Xu et al. have developed multiple strategies to fabricate SEECL systems based on the energy resonance transfer between Ru(bpy)32+ and plasmonic nanoparticles (NPs), or between fluorescent quantum dot and plasmonic NPs, which greatly promoted the development of SEECL systems for ultrasensitive biodetections (Li et al., 2016, Li et al., 2018, Lu et al., 2018, Wang et al., 2011, Wang et al., 2015b).
Although some progresses have been made in this field, the development of plasmonic SEECL biosensors is still severely hindered by the lack of comprehensive understanding of the underlying enhancement mechanisms and the corresponding strategies and methods to finely construct effective electrode interfaces and materials, and final sensors. Due to the lack of proper fabrication method and interfacial nanoengineering strategy, the resulting interfaces of electrodes were less effective and uncontrollable. Thus, in many cases, although plasmonic nanostructures had been optimized to achieve strong coupling with luminescent molecules, the plasmon effects cannot be fully utilized to enhance maximally the ECL because of the disorders of plasmonic nanostructures on the electrode surface, which dramatically weakened the photonic enhancement effect (Li et al., 2016, Wang et al., 2015a). Therefore, only a low enhancement in SEECL (less than ∼30-fold) has been achieved so far, and it remains a significant challenge to develop highly efficient SEECL systems for ultrasensitive bioassays and detections.
In this study, we finely fabricated the Au@SiO2 NP-based layered metamaterial and exploited it as a plasmonically active electrode material for SEECL and biosensing applications. By aqueous-phase core/shell nanoengineering of the unit Au@SiO2 NPs to finely tune the plasmonic coupling or effects and finally floating transfer the as-prepared ordered metamaterial onto the surface of an indium tin oxide (ITO) electrode, the resulting metasurfaced ECL electrode enhances significantly the ECL signals (over 1,000-fold) because of the synergy effect of photonic enhancement and PIRET. Locally, the abundance of finely controlled silica nanogaps in the dense nanomembrane creates rich “hot spots” and leads to significant enhancement of electromagnetic (EM) fields, which increases the plasmon coupling through PIRET (Jain et al., 2008, Lin et al., 2015, Shin et al., 2015). Moreover, the 2D order of the metamaterial and large size of AuNPs used (typically ∼75 nm in diameter) will benefit for the scattering of the incident light and “light trapping” of luminescent molecules, leading to an increase in photon flux and excitation efficiency in the luminescent molecules (Li et al., 2013, Wu, 2018). By varying the silica-shell thicknesses and AuNP sizes, the platform allows us to systematically probe the photonic enhancement and PIRET enhancement mechanisms and their effects on performances of SEECL sensors. Very impressively, by full exploitation and utilization of plasmonic effects via nanoengineering, the ECL signal was significantly improved with over 1,000-fold enhancement for the classical Ru(bpy)32+-tripropylamine (TPrA) ECL system, which is the highest ECL enhancement ever reported. For further practical use, a sandwich-type prostate-specific antigen (PSA) immunosensor was also constructed, in which Ru(bpy)32+-doped SiO2 (Ru@SiO2) NPs act as ECL luminophores, and the two kinds of antibodies specific to PSA are modified on the surface of the SEECL electrode and Ru@SiO2 NPs, respectively. In the presence of PSA, a sandwiched nanoarchitecture of Ru@SiO2-SEECL electrode is formed. The fabricated ECL immunosensor showed a very low detection limit of 3 fg mL−1 for PSA, demonstrating superior detection performance of the metasurfaced SEECL platform for ultrasensitive biodetections.
Results
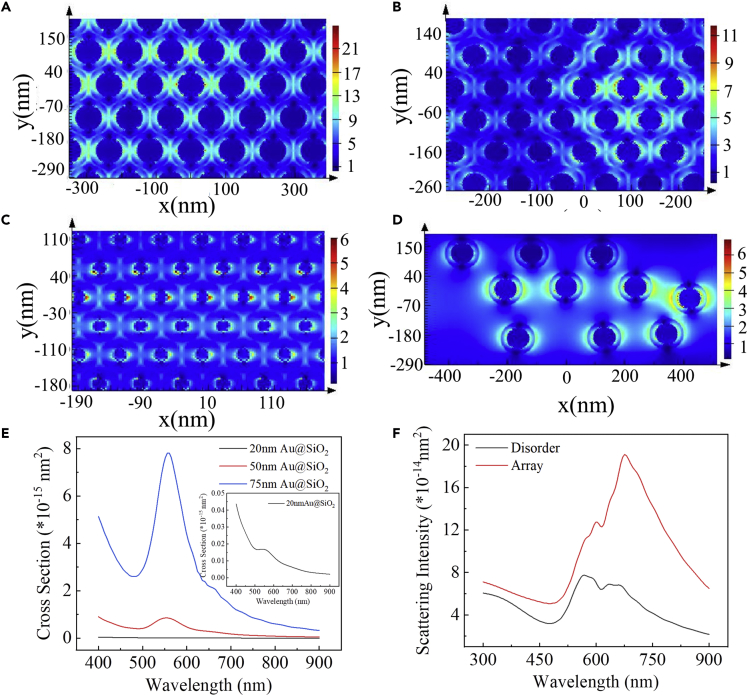
As reported previously (Li et al., 2013, Li et al., 2017, Wu, 2018), LSPR-induced electromagnetic (EM) field enhancement around the plasmonic nanostructures can efficiently improve the excitation efficiency of the adjacent luminescent molecule via a non-radiative process. Meanwhile, for regular or patterned plasmonic NPs, the light emitted by the luminescent molecule can efficiently scatter multiple times and increase the photon flux in the luminescent molecules. Therefore, we expected that the ordered close-packed Au@SiO2 NP-based monolayered metamaterial can efficiently improve the excitation of the luminescent molecule and enhance the ECL intensity tremendously over the plasmonic nanostructure. Bearing this in mind, we began our studies with a hypothesis-driven strategy by using finite difference time domain (FDTD) simulations to explore the EM enhancement and light-scattering properties of the ordered 2D plasmonic metamaterial of Au@SiO2. As clearly shown in Figure 1A, significant EM field enhancement of the monolayered 75-nm Au@21.5 nm SiO2 (up to 15-fold) can be locally generated under plasmonic excitation. Simultaneously, with the decrease of the Au cores (keep the silica shells as 21.5 nm), the EM field intensity reduced gradually (Figures 1B and 1C). In addition, due to the dense structure of the metamaterial, the simulated electric field distribution showed a high density of “hot spots” in the nanogaps between two adjacent Au@SiO2 NPs (Figures 1A–1C). These super-enhancing locations (hot spots), with ∼4-fold larger |E|2 values of the metamaterial than that of discrete NPs (Figure 1D), enhance significantly the EM field. All these simulated results presented above indicated that close-packed 75-nm Au@21.5 nm SiO2 NP-based monolayered metamaterial had superior EM field enhancement performance and LSPR coupling (collective wave-like charge density fluctuation and surfing of electrons) over dispersed nanostructures. As large nanocrystals or patterned plasmonic structures are also powerful scatterers, the re-radiation of the metasurface (the surface version of metamaterial, typically fabricated artificial materials via suitable periodic arrangement of micro/nanostructured metallic or dielectric inclusions) (Burokur et al., 2010) and light scattering (photonic enhancement) may be another advantage, compared with disordered morphologies (Li et al., 2013, Wu, 2018). As clearly seen in Figure 1E, the simulated scattering cross section dramatically augmented with the increase of the AuNPs' size; furthermore, the 2D well-organized metasurface has large aspect ratio and is favorable for light scattering. Thus, the scattering intensity of the ordered metasurface (Figure 1F) is much stronger than that of the disordered structures; this elastic scattering by plasmons is the source of the EM enhancement contribution, and the enhanced re-radiated dipolar fields in turn excite the luminescent molecules (Atwater and Polman, 2010, Li et al., 2013). According to the above simulation results, we reasoned that the 2D well-organized plasmonic metasurface might demonstrate promising characteristics as ECL electrode for ultrasensitive detection of biomolecular.
Figure 1.
Theoretical Simulation of the 2D Au@SiO2 NP-Assembled Metasurface
(A–D) (A) FDTD electromagnetic field simulation of 75-nm Au@21.5 nm SiO2 monolayered metasurface. FDTD electromagnetic field simulation of (B) 50-nm Au@21.5 nm SiO2, (C) 20-nm Au@21.5 nm SiO2, and (D) dispersive 75-nm Au@21.5 nm SiO2 nanoparticle-fabricated nanomembrane.
(E) Simulated scattering cross-sectional spectra of 20-nm Au@21.5 nm SiO2NPs, 50-nm Au@21.5 nm SiO2NPs, and 75-nm Au@21.5 nm SiO2NPs. Inset: enlarged scattering cross-sectional spectra of 20-nm Au@21.5 nm SiO2NPs.
(F) Simulated scattering intensity spectra of monolayered 75-nm Au@21.5 nm SiO2NP arrays and disorderly stacked 75-nm Au@21.5 nm SiO2NPs.
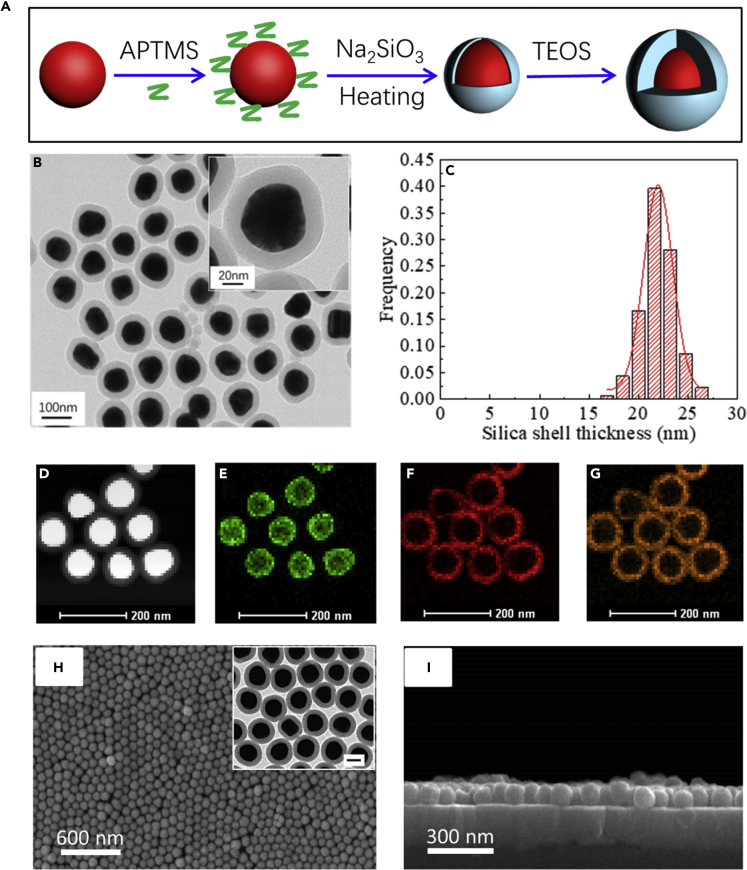
We then carried out proof of concept by preparing uniform Au@SiO2 NPs with controllable shell thicknesses (Figure 2A, please see “Transparent Methods” in the Supplemental Information). By controlling the pH and reaction time, the silica shell thicknesses can be adjusted from 4.0 ± 0.8 nm to 24.6± 2.7 nm (Figures 2B–2G and S1, mean ± transmission electron microscopy [TEM]). With the increase of silica shell thicknesses, the LSPR peak of the particles redshifted gradually (Figure S2). The free-standing monolayered Au@SiO2 nanomembrane (layered metamaterial) was then fabricated by the liquid-liquid interface self-assembly (LLISA) method. Briefly, colloidal Au@SiO2 NPs were added in a plastic container and then hexane was poured on top of the colloidal solution; the Au@SiO2 NPs were subsequently forced to assemble at hexane-water interface after methanol was rapidly added into the colloidal solution. Upon evaporation of hexane, a uniform monolayer of Au@SiO2 NPs was spontaneously formed over a large area (∼several square centimeters). Figure S3 shows photographs and optical images of the resulting nanomembrane after LLISA of the unit Au@SiO2 NPs, which presents impressively bright golden yellow color at the air-water interface. To fabricate an ECL electrode, the freshly prepared Au@SiO2 nanomembrane was transferred onto an ITO electrode by the method of floating transfer. As clearly seen from the scanning electron microscopic (SEM) top-/side-view images of the nanomembrane (Figures 2H and 2I), the unit Au@SiO2 NPs with uniform size were assembled into a dense monolayer nanomembrane with hexagonal close packing, which is beneficial to the photonic scattering and collective wave-like charge density fluctuation (Li et al., 2013, Wu, 2018).
Figure 2.
Preparation and Characterizations of the Au@SiO2 NPs and 2D Metamaterial
(A) The preparation process of Au@SiO2 NPs.
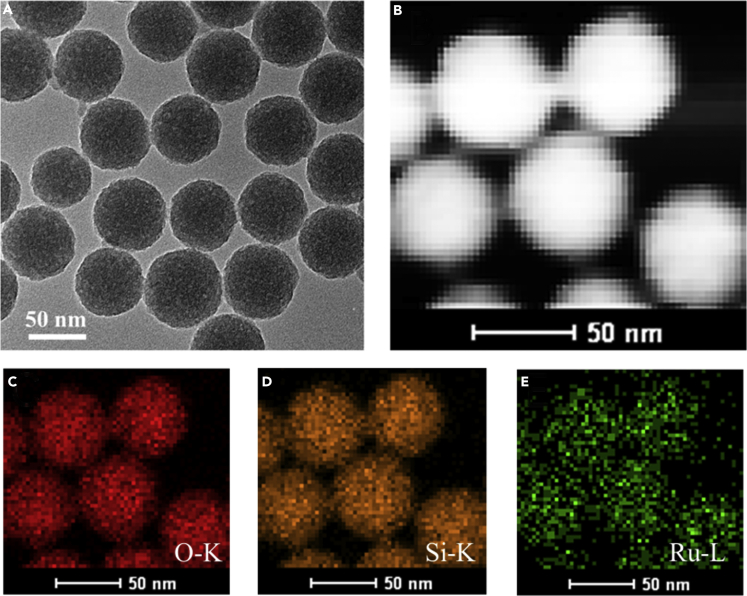
(B–G) (B) Transmission electron microscopic image, (C) silica shell thickness distribution, and (D) high-angle annular dark-field scanning transmission electron microscopic and (E–G) the corresponding energy-dispersive X-ray spectroscopic elemental mappings of 75-nm Au@21.5 nm SiO2 NPs. (E) Au, (F) Si, (G) O. Data are represented as mean ± transmission electron microscopy (TEM).
(H and I) Top- and side-view SEM images of the monolayered Au@SiO2 nanomembrane-assembled metasurface on ITO glasses. (H) Top view, (I) Side view. Inset in (H): transmission electron microscopic image of monolayered Au@SiO2 nanomembrane.
Scale bar in inset, 50 nm. See also Figures S1–S3.
Discussion
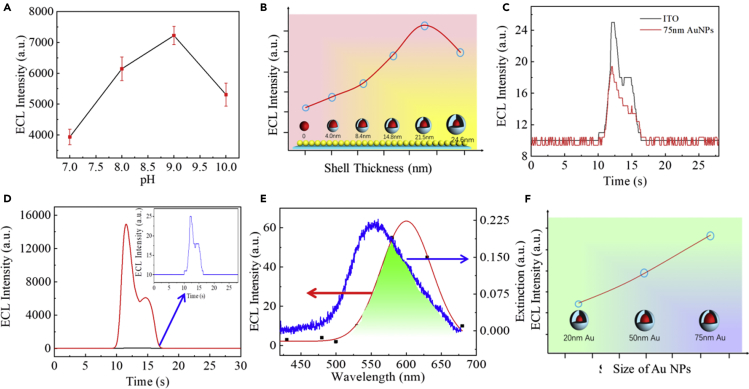
To investigate the ECL performance of the nanomembrane, a metasurfaced electrode for the Ru(bpy)32+-ECL system was constructed. As seen from Figures 3A and S4, the ECL of our system showed the highest intensity at an optimal pH of 9, and therefore the subsequent experiments were performed at the same conditions. Impressively, the as-fabricated monolayer Au@SiO2 nanomembrane electrode (MASNE) showed much higher ECL intensity compared with the bare ITO electrode (the effective surface areas of ITO electrodes are kept constant, 4 mm × 5 mm). Figures 3B and S5 shows the typical ECL responses of the metasurfaced MASNE system constructed from the 75-nm AuNPs with varied silica shell thickness ranging from 4.0 to 24.6 nm, recorded in the same ECL solution containing 20 μmol L−1 Ru(bpy)32+ and 0.1 mmol L−1 TPrA. As a comparison, a slight decrease in ECL response was observed after the decoration of the ITO electrode with monolayer (silica shell-free) AuNP nanomembrane (Figure 3C). The decrease in ECL is attributed to the energy transfer between Ru(bpy)32+ and bare AuNPs. In the presence of bare AuNPs, the excited Ru(bpy)32+* transfers the energy to AuNPs in a non-radiative way, and therefore partial ECL quenching was observed (Jebb et al., 2007, Wang et al., 2015a). However, this kind of non-radiative energy transfer can be effectively prevented after the coating of AuNPs with nanoscale insulating SiO2. As clearly seen in Figure 3D, the ECL intensity increases with an increase in silica shell thickness. However, with further increase of the silica shells the ECL intensity of the resulting MASNE decreases. Experimentally, when the shell thickness is ∼21.5±1.7 nm, the ECL intensity of the MASNE system reaches the highest value (∼1.6×104 a.u.), which is up to 1,000-fold enhancement than that of bare ITO (Figure 3D). Comparison ECL experiments by using Ru@SiO2 as luminophore instead of Ru(bpy)32+ solution to avoid the influence of quenching effect of bare AuNPs to Ru(bpy)32+ were also conducted. As shown in Figure S6, although the ECL enhancement is slightly smaller than that in Ru(bpy)32+ solution due to further increase of average distance (≥ 21.5 nm) between the embedded Ru(bpy)32+ in Ru@SiO2 NPs and the Au@21.5 nm SiO2 NPs, the ECL intensity of the 75-nm Au@21.5 nm SiO2 NP-modified ITO was still much higher than that of bare ITO and the 75-nm AuNP-modified ITO, which further demonstrated the superior performance of the Au@SiO2-modified ECL electrodes. However, due to the quenching effect induced between AuNPs and the embedded Ru(bpy)32+ in Ru@SiO2, the ECL intensity of AuNP-modified electrode is still lower than that of bare ITO. This is because ECL emission is a competing result of two effects: one is the quenching effect induced by energy transfer between the luminophores and AuNPs and the other is PIRET enhancement. When AuNPs approach to Ru(bpy)32+ closely, non-radiative energy transfer plays an important role and then the ECL intensity enhancement is very limited. With the increase of SiO2 shell thickness, energy transfer between the Ru(bpy)32+ and AuNPs decrease rapidly, thus non-radiative energy transfer can be effectively prevented and the radiative mode dominates, resulting in significant ECL enhancement (Li et al., 2018). However, further increase in the thickness of SiO2 shell will cause an exponential decay of the EM field with distance, which in turn decreases the ECL intensity. The plasmonic effects on the ECL enhancement were implied because the ECL response of the control (AuNP-free) monolayer SiO2 nanomembrane electrode (detailed characterization see Figure S7) showed only ∼9-fold enhancement than that of the bare ITO electrode due to “nanoelectrode” surface effect (which increases the effective surface area of the electrode) and the enrichment of Ru(bpy)32+ (Wang et al., 2015a) in this case (Figure S8).
Figure 3.
Performance of MASNE in Ru(bpy)32+-Based Plasmonic ECL System
(A) The effect of pH value of the solution on ECL of the 75-nm Au@21.5 nm SiO2-modified MASNE with 0.1 M PBS containing 20 μM Ru(bpy)32+ and 0.1 mM tripropylamine (TPrA).
(B) The effect of silica shell thicknesses on the ECL intensity. Comparison of ECL intensity between ITO and MASNE electrodes at the same concentration of Ru(bpy)32+-based ECL system (0.1 M PBS solution [pH = 9] containing 20 μmol L−1 Ru(bpy)32+ and 0.1 mmol L−1 TPrA).
(C) ECL intensity of the bare AuNP monolayer-based electrode and bare ITO electrode.
(D) Comparison of ECL intensity of MASNE and bare ITO electrode in Ru(bpy)32+-based plasmonic ECL system. Inset: enlarged image of ECL intensity of bare ITO electrode.
(E) Spectra overlap between the emission spectrum of Ru(bpy)32+ and extinction spectrum of 75-nm Au@21.5 nm SiO2-based nanomembrane.
(F) The effect of size of AuNPs (keep the silica shell thicknesses constant, ∼21.5 nm) on the ECL intensity.
See also Figures S4–S16.
The plasmonic nature of the fabricated MASNE was further manifested by in situ dark-field scattering imaging. As seen from Figure S9, the metasurface of the MASNE showed intense plasmonic scattering of yellow-green color. Upon the generation of excited Ru(bpy)32+∗ via electrochemical reaction, ECL emission excites surface plasmons optically on the metasurface, due to the spectral overlap between the LSPR band of Au@SiO2 and the spectrum of Ru(bpy)32+ (Figure 3E). Upon resonant excitation, the LSPR on the electrode surface breaks the diffraction limit and can concentrate light down to a nanoscale region (Jiang et al., 2014); the strong light localization makes the optical electric field near the metasurface largely enhanced, which is consistent with the FDTD simulation in Figure 1A (up to 15-fold). Figure S10 shows the simulated excitation wavelength-dependent EM field enhancement of the metasurface. The results indicated that the 550-nm excitation light results in a higher EM field enhancement when compared with 450- and 700-nm incident light, which is consistent with the LSPR spectra of the Au@SiO2 nanomembrane. This kind of strong EM field enhancement and near-field dipole-dipole LSPR coupling could increase both the excitation rate and emission factor of Ru(bpy)32+, resulting in significant enhancement in the ECL intensity.
To prove this conjecture, we studied the ECL intensity variation with the decrease of AuNP core sizes (while keeping the silica shell as a constant, ∼21 nm; see Figures S11 and S12 for detailed characterization of Au@SiO2). It is worth noting that because simultaneous cyclic voltammetry (CV) characterizations of the different ECL electrodes were nearly identical (See Figure S13), the observed ECL signal difference was not a main reflection of the change of surface area caused by different Au@SiO2 preparations. As seen from Figures 3F and S14, smaller AuNPs result in lower ECL intensity (∼13,600 a.u. and 9,920 a.u. for 50- and 20-nm AuNPs, respectively); this is because smaller AuNP cores generate weaker near-EM field, which is consistent with our prediction (FDTD simulation, see Figures 1B and 1C). Furthermore, the 2D well-organized metasurface we fabricated has large aspect ratio and is favorable for light scattering. As seen from Figure S15, the ECL intensity of the ordered metasurface is much stronger than that of the disordered structures (for detailed SEM images of the disordered structures see Figure S16); this elastic scattering by plasmons is the source of the EM enhancement contribution, and the enhanced re-radiated dipolar fields in turn excites the ECL emission of molecules (Atwater and Polman, 2010, Li et al., 2013). Thus the ECL emission can be effectively enhanced. All the results presented above indicated that the strong localized EM field and LSPR coupling (collective wave-like charge density fluctuation and surfing of electrons) play a crucial role in the metasurface-mediated huge ECL enhancement. In addition, the high-density of “hot spots” in the nanogaps between two adjacent Au@SiO2 NPs also played a key role in the improvement of ECL enhancement.
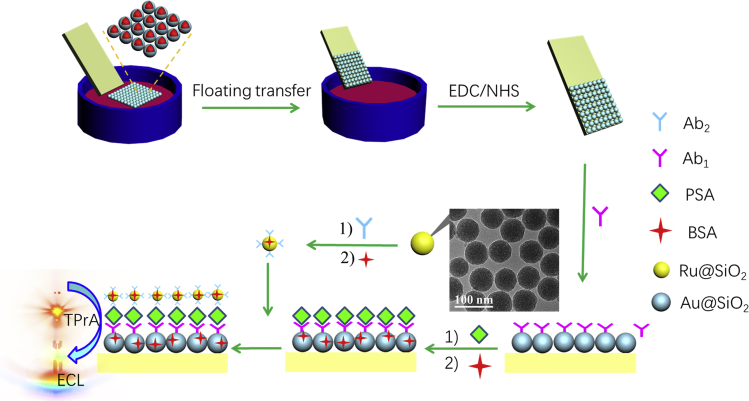
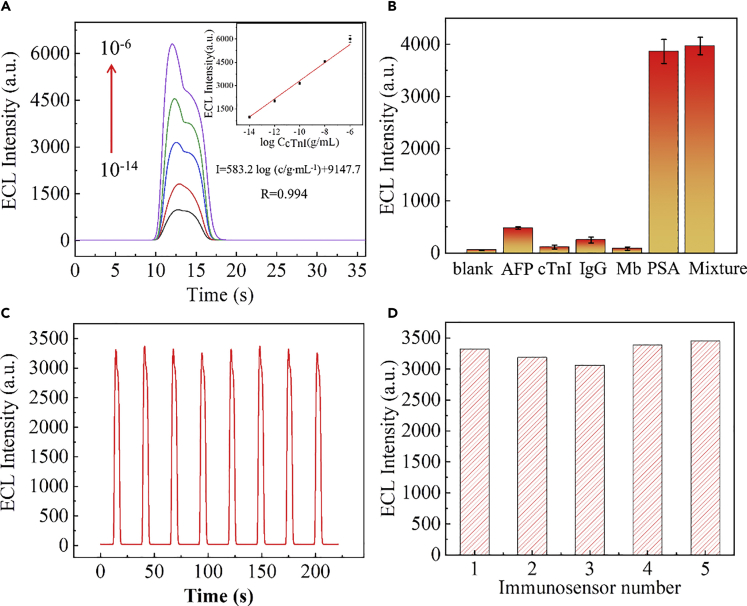
The applicability and detection performance of the as-prepared (MASNE) metasurfaced ECL biosensors were further examined by applying for PSA detection. A schematic diagram of the MASNE ECL biosensor for PSA detection is shown in Scheme 1 (for detailed CV characterizations after every modification step see Figure S17). First, the primary antibody to PSA (Ab1) was immobilized on the surface of an MASNE electrode with 1-ethyl 3-(3-(dimethylamino)propyl) carbodiimide hydrochloride/n-hydroxysuccinimide (EDC/NHS), which provided specific binding sites for PSA. The Ab1-modified MASNE electrode was then immersed into the secondary antibody (Ab2)-decorated Ru@SiO2 solution. In the presence of PSA, a sandwich-type PSA ECL immunosensor was formed. As the number of Ru@SiO2 NPs (for detailed characterization see Figure 4) immobilized on the surface of MASNE electrode is proportional to the PSA concentration (signal-on), the proposed detection method could be used for quantitative determination of PSA. As shown in Figure 5A, the intensity of ECL increased with the increase of PSA concentration until the binding saturation is reached, which indicated that the prepared ECL immunosensor was appropriate and reliable for PSA detection. In addition, the ECL intensity increased linearly with the logarithm of PSA concentration ranging from 10 fg mL−1 to 1 μg mL−1 with a regression equation of I = 583.2log (c/g mL−1) + 9147.7 and a correlation coefficient of 0.994 (n = 3). Under the optimized condition, a limit of detection of 3 fg mL−1 (S/N = 3) was obtained (Gao et al., 2017), which is much lower (or comparable) than those previously reported by other methods, especially for the reported sandwich-type PSA ECL biosensors (Table S1). To evaluate the practical application of the prepared PSA SEECL immunosensor, the selectivity was performed by introducing alpha-fetoprotein (AFP), cardiac troponin I (cTnI), immunoglobulin G (IgG), and myoglobin (Mb) as interfering proteins into the detection system.
Scheme 1.
Fabrication Procedures for the MASNE-Based PSA Immunosensors
Figure 4.
Morphological Characterizations of the Ru@SiO2 NPs
(A–E) (A) Transmission electron microscopic image, (B) high-angle annular dark-field scanning transmission electron microscopic image, and (C–E) the corresponding energy-dispersive X-ray spectroscopic elemental mappings of Ru@SiO2 NPs.
Figure 5.
Detection Performance of the Constructed PSA ECL Immunosensor
(A) Linear relationship between the ECL intensity and PSA concentrations.
(B) Selectivity of the ECL immunosensor: blank, AFP (10 ng mL−1), cTnI (10 ng mL−1), IgG (10 ng mL−1), Mb (10 ng mL−1), PSA (1 ng mL−1), and mixture (containing all the above analytes). AFP, alpha-fetoprotein; cTn1, cardiac troponin I; IgG, immunoglobulin G; Mb, myoglobin.
(C) Stabilization of the ECL emission (0.1 nM).
(D) Reproducibility of five independent immunosensors (0.1 nM).
See also Figures S17, Table S1.
The concentration of the interfering proteins was chosen as 10 ng mL−1 (much higher than those in healthy people's serum to ensure that it met with the testing requirements of actual samples), whereas the concentration of PSA was set as 1 ng mL−1. As shown in Figure 5B, only the presence of PSA led to an obvious ECL enhancement, whereas the ECL responses of the interfering proteins showed almost no difference compared with the blank solution. These results demonstrated that the developed metasurfaced SEECL immunosensor had a good selectivity for PSA detection. Moreover, as seen from Figures 5C and 5D, the PSA SEECL biosensor showed very stable ECL intensity and reproducibility (the relative standard deviation of the tested five ECL electrodes were ∼5%), which indicated the reliability of the immunosensor for ECL PSA detection. To further assess the practicability of the as-proposed immunosensor, standard addition method was applied to analyze the PSA concentrations in human serum. The detecting solution was prepared by adding PSA of different concentrations (10, 0.1, 0.001 ng mL−1) into diluted serum samples. As shown in Table 1, the as-fabricated PSA SEECL biosensors showed great performance with a recovery of 95.1%–107.6%. The results identified the feasibility of the SEECL immunosensor for promising clinical ultrasensitive detection of PSA.
Table 1.
Quantitative Determination of PSA in Healthy Human Serum Sample
| Sample Number | Added (ng⋅mL−1) | Found (ng⋅mL−1) | Recovery (%) |
|---|---|---|---|
| 1 | 10 | 9.63 | 96.3% |
| 2 | 0.1 | 0.0951 | 95.1% |
| 3 | 0.001 | 0.001076 | 107.6% |
Conclusion
In summary, we developed a superior ECL-sensing platform by surface decoration of ECL electrodes with a dense monolayered metamaterial nanomembrane, made by self-assembly of plasmonic and size-tunable Au@SiO2 NPs. The finely prepared metasurfaced ECL electrode with well-controlled core/shell sizes and interparticle spacing supports simultaneously photonic enhancement (resonant light scattering), PIRET, and silica shell-mediated “hot spot” effect, enhancing synergistically the ECL efficiency. The optimized metasurface electrode showed over 1,000-fold ECL enhancement for the classical Ru(bpy)32+-TPrA system, which is the highest ECL enhancement ever reported. The as-fabricated metasurfaced ECL biosensor demonstrated superior detection performance in detecting cancer biomarker PSA with a detection limit of 3 fg mL−1. This work provides a new insight into the understanding of full utilization of plasmonic effects on ECL and opens a way to design a high-performance ECL-sensing platform for ultrasensitive bioassays.
Limitations of the Study
As the arrangement of NPs plays an important role in the performance of biosensors, 2D nanomembrane with poor quality may not result in superior (up to 1,000-fold) enhancement.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
C.L. thanks Prof. Guobao Xu for helpful discussion of the manuscript. Y.J. acknowledges support by the Natural Science Foundation of China (Grant Nos. 21675146 and 21475125), the National Key Research and Development Program of China (Grant No. 2016YFA0201300), and the Instrument Developing Project of the Chinese Academy of Sciences (Grant No. YZ201666).
Author Contributions
Y.J. and C.L. conceived and designed the project. C.L. prepared the metamaterials, conducted the structural and optical characterizations, and fabricated the immunosensor. S.W. performed the ECL experiments. C.L., S.W., M.S., H.L., C.X., and Y.J. analyzed and discussed the data. Y.J., H.L., and C.L. wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Published: July 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.06.042.
Supplemental Information
References
- Atwater H.A., Polman A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010;9:205. doi: 10.1038/nmat2629. [DOI] [PubMed] [Google Scholar]
- Burokur S.N., Daniel J.-P., Ratajczak P., de Lustrac A. Tunable bilayered metasurface for frequency reconfigurable directive emissions. Appl. Phys. Lett. 2010;97:064101. [Google Scholar]
- Gao W., Qi L., Liu Z., Majeed S., Kitte S.A., Xu G. Efficient lucigenin/thiourea dioxide chemiluminescence system and its application for selective and sensitive dopamine detection. Sens. Actuators B. 2017;238:468–472. [Google Scholar]
- Hu L., Xu G. Applications and trends in electrochemiluminescence. Chem. Soc. Rev. 2010;39:3275–3304. doi: 10.1039/b923679c. [DOI] [PubMed] [Google Scholar]
- Jain P.K., Huang X., El-Sayed I.H., El-Sayed M.A. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008;41:1578–1586. doi: 10.1021/ar7002804. [DOI] [PubMed] [Google Scholar]
- Jebb M., Sudeep P.K., Pramod P., Thomas K.G., Kamat P.V. Ruthenium(II) trisbipyridine functionalized gold nanorods. morphological changes and excited-state interactions. J. Phys. Chem. B. 2007;111:6839–6844. doi: 10.1021/jp070701j. [DOI] [PubMed] [Google Scholar]
- Jiang R., Li B., Fang C., Wang J. Metal/semiconductor hybrid nanostructures for plasmon-enhanced applications. Adv. Mater. 2014;26:5274–5309. doi: 10.1002/adma.201400203. [DOI] [PubMed] [Google Scholar]
- Li J., Cushing S.K., Zheng P., Meng F., Chu D., Wu N. Plasmon-induced photonic and energy-transfer enhancement of solar water splitting by a hematite nanorod array. Nat. Commun. 2013;4:2651. doi: 10.1038/ncomms3651. [DOI] [PubMed] [Google Scholar]
- Li L., Chen Y., Zhu J.-J. Recent advances in electrochemiluminescence analysis. Anal. Chem. 2017;89:358–371. doi: 10.1021/acs.analchem.6b04675. [DOI] [PubMed] [Google Scholar]
- Li M.-X., Feng Q.-M., Zhou Z., Zhao W., Xu J.-J., Chen H.-Y. Plasmon-enhanced electrochemiluminescence for nucleic acid detection based on gold nanodendrites. Anal. Chem. 2018;90:1340–1347. doi: 10.1021/acs.analchem.7b04307. [DOI] [PubMed] [Google Scholar]
- Li M.-X., Zhao W., Qian G.-S., Feng Q.-M., Xu J.-J., Chen H.-Y. Distance mediated electrochemiluminescence enhancement of CdS thin films induced by the plasmon coupling of gold nanoparticle dimers. Chem. Commun. (Camb.) 2016;52:14230–14233. doi: 10.1039/c6cc08441a. [DOI] [PubMed] [Google Scholar]
- Lin L., Zapata M., Xiong M., Liu Z., Wang S., Xu H., Borisov A.G., Gu H., Nordlander P., Aizpurua J. Nanooptics of plasmonic nanomatryoshkas: shrinking the size of a core–shell junction to subnanometer. Nano Lett. 2015;15:6419–6428. doi: 10.1021/acs.nanolett.5b02931. [DOI] [PubMed] [Google Scholar]
- Liu Z., Qi W., Xu G. Recent advances in electrochemiluminescence. Chem. Soc. Rev. 2015;44:3117–3142. doi: 10.1039/c5cs00086f. [DOI] [PubMed] [Google Scholar]
- Lu H.-J., Pan J.-B., Wang Y.-Z., Ji S.-Y., Zhao W., Luo X.-L., Xu J.-J., Chen H.-Y. Electrochemiluminescence energy resonance transfer system between RuSi nanoparticles and hollow Au nanocages for nucleic acid detection. Anal. Chem. 2018;90:10434–10441. doi: 10.1021/acs.analchem.8b02347. [DOI] [PubMed] [Google Scholar]
- Miao W. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008;108:2506–2553. doi: 10.1021/cr068083a. [DOI] [PubMed] [Google Scholar]
- Richter M.M. Electrochemiluminescence (ECL) Chem. Rev. 2004;104:3003–3036. doi: 10.1021/cr020373d. [DOI] [PubMed] [Google Scholar]
- Shin Y., Song J., Kim D., Kang T. Facile preparation of ultrasmall void metallic nanogap from self-assembled gold–silica core–shell nanoparticles monolayer via kinetic control. Adv. Mater. 2015;27:4344–4350. doi: 10.1002/adma.201501163. [DOI] [PubMed] [Google Scholar]
- Wang D., Guo L., Huang R., Qiu B., Lin Z., Chen G. Surface enhanced electrochemiluminescence of Ru(bpy)32+ Sci. Rep. 2015;5:7954. doi: 10.1038/srep07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Li Y., Lin Z., Qiu B., Guo L. Surface-enhanced electrochemiluminescence of Ru@SiO2 for ultrasensitive detection of carcinoembryonic antigen. Anal. Chem. 2015;87:5966–5972. doi: 10.1021/acs.analchem.5b01038. [DOI] [PubMed] [Google Scholar]
- Wang J., Shan Y., Zhao W.-W., Xu J.-J., Chen H.-Y. Gold nanoparticle enhanced electrochemiluminescence of CdS thin films for ultrasensitive thrombin detection. Anal. Chem. 2011;83:4004–4011. doi: 10.1021/ac200616g. [DOI] [PubMed] [Google Scholar]
- Wu N. Plasmonic metal–semiconductor photocatalysts and photoelectrochemical cells: a review. Nanoscale. 2018;10:2679–2696. doi: 10.1039/c7nr08487k. [DOI] [PubMed] [Google Scholar]
- Wu P., Hou X., Xu J.-J., Chen H.-Y. Electrochemically generated versus photoexcited luminescence from semiconductor nanomaterials: bridging the valley between two worlds. Chem. Rev. 2014;114:11027–11059. doi: 10.1021/cr400710z. [DOI] [PubMed] [Google Scholar]
- Zhang J., Gryczynski Z., Lakowicz J.R. First observation of surface plasmon-coupled electrochemiluminescence. Chem. Phys. Lett. 2004;393:483–487. doi: 10.1016/j.cplett.2004.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.