Abstract
In this work, the surfaces that repel liquid polydimethylsiloxane (PDMS) droplets in water were created by femtosecond laser treatment. We define this superwetting phenomenon as underwater “superpolymphobicity”. The resultant underwater superpolymphobic silicon surface shows a contact angle of 159 ± 1° and a sliding angle of 1.5 ± 0.5° to liquid PDMS droplets in water. This underwater superpolymphobicity can be achieved on a wide range of hydrophilic materials, including semiconductors, glass, and metals. The adhesion between the liquid polymer and a solid substrate is effectively prevented by the underwater superpolymphobic microstructures. The underwater superpolymphobicity will have a great significance in designing the adhesion between the polymer and a solid substrate, controlling the shape of the cured polymer materials, as well as nearly all the applications based on the polymer materials.
Introduction
In recent years, superhydrophobic and superoleophobic surfaces have attracted increasing interest because of their broad applications in liquid repellence,1 self-cleaning coating,2,3 droplet manipulation,4−6 oil/water separation,7−11 submarine drag reduction,12 antifogging/icing,13−16 anticorrosion,1 water harvesting,17,18 cell engineering,19−21 lab chip,22,23 and so on. A wide range of superhydrophobic and superoleophobic materials have been developed by the combination of proper surface microstructure and chemical composition.24−31 However, natural or artificial superhydrophobic and superoleophobic surfaces can only repel either water solutions or oils, but not polymers. Compared to water and oil solutions, liquid polymers usually have a more complex composition, lower fluidity, and higher viscosity. Moreover, many liquid polymers can be transformed into the solid state, different from water and oil liquids. There are few surfaces that can repel liquid polymers.24−27
Polymers have been widely used in various manufacturing industries, agriculture, national defence, and our daily lives. Some polymers have liquid states. For example, the uncured polydimethylsiloxane (PDMS) mixture of prepolymer and curing agent is in the liquid state.32,33 After curing at high temperature, it solidifies and its shape is formed permanently. Preventing the adhesion between liquid polymers and a solid substrate is important in polymer casting industry, polymer preparation, and three-dimensional printing technology. Following the definition of “super-hydro-phobicity” and “super-oleo-phobicity”, we coin a new term “super-polym-phobicity” (“polym” is usually short for “polymer”) to characterize that the contact angle (CA) of a liquid polymer droplet on a solid substrate is larger than 150°. However, the fabrication of superpolymphobic surfaces still remains a great challenge.
In this paper, hierarchical micro- and nanostructures were prepared on a wide range of materials by femtosecond laser processing, including silicon, glass, stainless steel, Al, and Cu. The wettabilities of underwater liquid PDMS droplets on the laser-structured surfaces were investigated. The resultant surface shows excellent underwater superpolymphobicity and has the ability to repel liquid PDMS in water. Such underwater superpolymphobicity is caused by the underwater Cassie wetting behavior between PDMS droplets and surface microstructures.
Experimental Section
Femtosecond Laser Treatment
Femtosecond laser processing is widely applied in the formation of micro/nanoscale structures on a solid substrate and controlling the wettability of material surfaces.34−43 A Ti:sapphire femtosecond laser system was utilized to induce micro/nanostructures on the surface of different substrates, including silicon, glass, stainless steel, Al, and Cu. The experimental setup for ablating a sample surface by femtosecond laser is shown in Figure 1a. The sample with initial smooth surface was mounted on a program-controlled translation stage. The laser beam (with the pulse width of 67 fs, the center wavelength of 800 nm, and the repetition rate of 1 kHz) was vertically focused onto the front surface of the samples by a plano-convex lens (focal length of 250 mm) in air. The size of the focused laser spot was about 100 μm. The typical line-by-line laser scanning manner was used (Figure 1b). The laser power, the scanning speed, and the space/interval of the laser-scanning lines were set constantly at 500 mW, 2.5 mm s–1, and 60 μm, respectively, in this experiment. The femtosecond laser-treated samples were finally cleaned with alcohol and distilled water, respectively.
Figure 1.
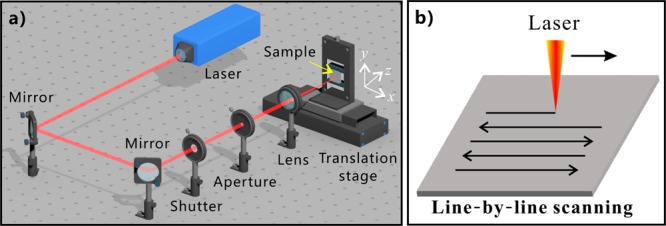
(a) Schematic of the experimental setup. (b) Line-by-line laser-scanning manner.
Characterization
The surface morphology of the samples after femtosecond laser treatment was observed by a scanning electron microscope (S-4100, Hitachi, Japan) and a laser confocal microscopy (VK-9700, Keyence, Japan). The wettabilities of in-air water droplets and underwater liquid PDMS droplets (∼10 μL) on the sample surfaces were investigated by a contact-angle measurement (SL2000KB, Kino, America). Regarding the underwater wettability, the samples were fixed in a man-made glass container which was filled with distilled water. The uncured liquid PDMS was prepared by mixing the PDMS prepolymer and curing agent (v/v = 10:1) (DC-184, Dow Corning Corporation).
Results and Discussion
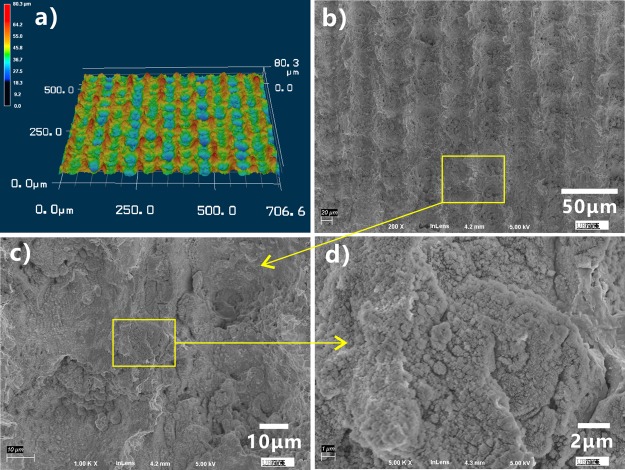
Figure 2 shows the morphology of a femtosecond laser-ablated silicon surface. The laser-treated silicon surface is characterized by hierarchical rough micro/nanostructures. A large number of micro-protuberances and micro-holes periodically distribute themselves on the resultant surface (Figure 2a,b). The sizes of the micro-protuberances and micro-holes are 40–60 μm. There are abundant nanoparticles with the diameter of a few tens of nanometer decorating on the surface of the micro-protuberances (Figure 2c,d).
Figure 2.
Microstructure of a laser-treated silicon surface. (a) Laser confocal microscopy image. (b–d) Scanning electron microscopy images of the laser-ablated silicon surface.
The formation of the micro/nanoscale hierarchical structures is ascribed to the material removal and particle resolidification during femtosecond laser ablation.33,44−46 When the femtosecond laser pulses are focused onto a sample surface, part of the laser energy is directly absorbed by electrons via the nonlinear effect, such as multiphoton absorption and avalanche ionization. Some energy is further transferred from the electrons to the lattice until the thermal equilibrium between electrons and ions occurs. A high-temperature/pressure plasma forms above the sample surface. As the plasma expands and bursts out of the laser-ablated spot, the sample surface will be strongly damaged. The material at the laser-focused point is removed with the plasma burst and is sputtered above the substrate in the form of ejected particles. This process usually leads to a microscale rough structure on the substrate. As the nanoscale-ejected particles that are at the molten state fall back to the sample surface and resolidify, the nanoparticles finally coat over the surface of the laser-ablation-induced microstructures, resulting in a kind of micro/nanoscale binary structures.
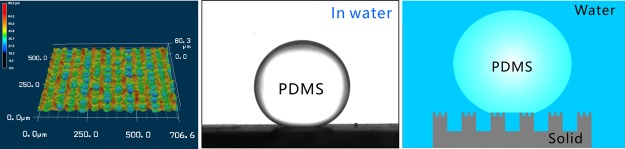
The wettabilities of water droplets and underwater liquid PDMS droplets on the sample surface were investigated by measuring CA and sliding angle (SA). The untreated flat silicon is inherently hydrophilic with the water CA (WCA) of 44 ± 3° to a small water droplet (Figure 3a). Once a water droplet was dispensed onto the laser-structured surface, the droplet would spread out quickly. The measured WCA is about 0° (Figure 3b), demonstrating the superhydrophilicity of the textured surface. The hydrophilicity of the silicon surface is enhanced by the laser-induced microstructure because the rough microstructure has the ability to amplify the natural wettability of a substrate.24−27 The flat silicon surface shows ordinary polymphobicity with a polymer CA (PCA) of 141.5 ± 2.5° and high adhesion to a liquid PDMS droplet in water (Figure 3c). The uncured liquid PDMS droplet can stick on the silicon surface as the sample is tilted at any angle. Regarding the laser-treated surface, an underwater PDMS droplet could keep a spherical shape on the surface (Figure 3d). The PCA is measured to be 159 ± 1° and the CA hysteresis is only 5 ± 1.8°. As long as the sample was tilted at 1.5 ± 0.5°, the PDMS droplet could slowly roll away (SA = 1.5 ± 0.5°) (Figure 3e). The results indicate that the laser-ablated silicon surface exhibits underwater superpolymphobicity and very low adhesion to liquid PDMS droplet; that is, the laser-ablated surface greatly repels liquid PDMS in water.
Figure 3.
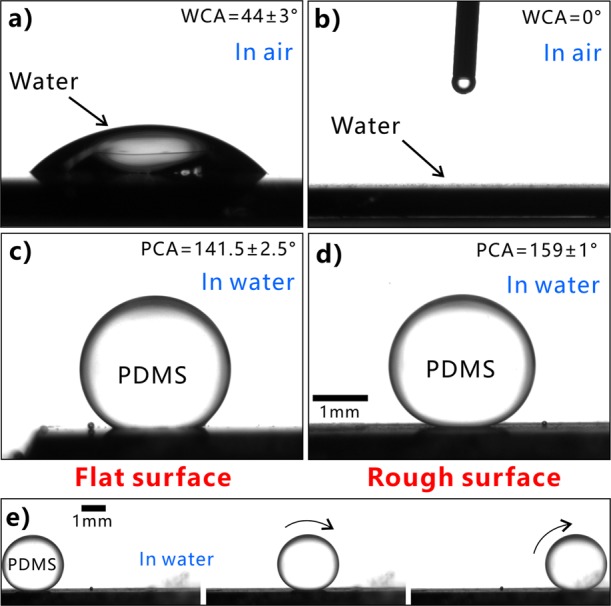
Wettabilies of the flat silicon and the laser-ablated silicon surfaces. (a,b) Water droplet on the silicon surfaces in air: (a) untreated surface and (b) laser-structured surface. (c,d) Liquid PDMS droplet on (c) untreated surface and (d) laser-ablated surface in water. (e) Process of a liquid PDMS droplet rolling on the rough surface in water.
The potential wetting model between the liquid PDMS droplet and the laser-structured surface is proposed to well understand the underwater superpolymphobicity of the laser-structured surface. The wettability of a liquid droplet on the flat solid substrate is generally explained by Young’s model.47Figure 4a shows the wetting state of a solid/PDMS/water three-phase system. The PCA (θPW) of an underwater polymer droplet on the flat surface can be expressed by
| 1 |
where γPA, γWA, and γPW are the free energies of polymer/air, water/air, and polymer/water interfaces, respectively. θP and θW are the CAs of polymer and water droplets in air. The liquid PDMS has a much smaller surface tension than water (γPA ≪ γWA), so the values of cos θP and cos θW are all positive, and γPA cos θP – γWA cos θW is negative.47 From eq 1, it can be predicted that flat silicon surface presents polymphobicity underwater.
Figure 4.
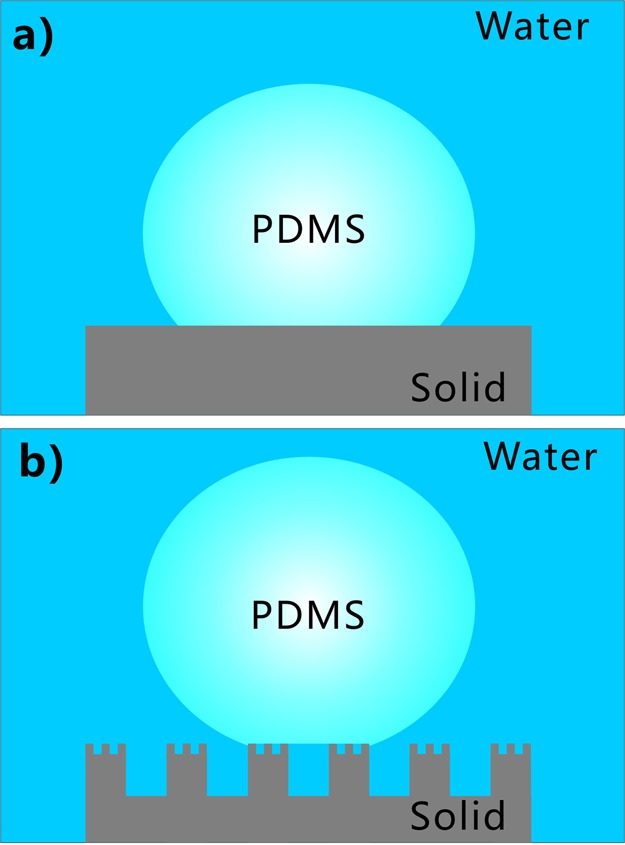
Wetting state between a liquid PDMS droplet and silicon surface in water. (a) Flat silicon surface. (b) Laser-induced rough silicon surface.
Regarding the laser-ablated surface with micro/nanoscale structures, water can fully wet the microstructures because of the superhydrophilicity and occupy the whole space between surface microstructures as the sample is dipped into water. The water layer trapped in microstructures can provide a repulsive force to the PDMS droplet because of the insolubility between water and liquid PDMS. The trapped water cushion allows the liquid PDMS droplet to only touch the peaks of the surface microstructures. In fact, the liquid PDMS is on a composite solid–water interface. The contact model between the underwater PDMS droplet and rough surface microstructure agrees well with the underwater Cassie state (Figure 4b).47 Therefore, the laser-structured silicon surface exhibits underwater superpolymphobicity. The high PCA (θPW*) of an underwater polymer droplet on the textured silicon surface can be expressed by
| 2 |
where f is the projected area fraction of the polymer touching the surface microstructures, θPW is the PCA on a flat surface underwater. From eq 2, the f can be calculated as 0.306 based on the measured values of CAs (θPW* = 159°, θPW = 141.5°), demonstrating that the underwater liquid PDMS droplet is in contact with a small area of the laser-induced surface microstructures.
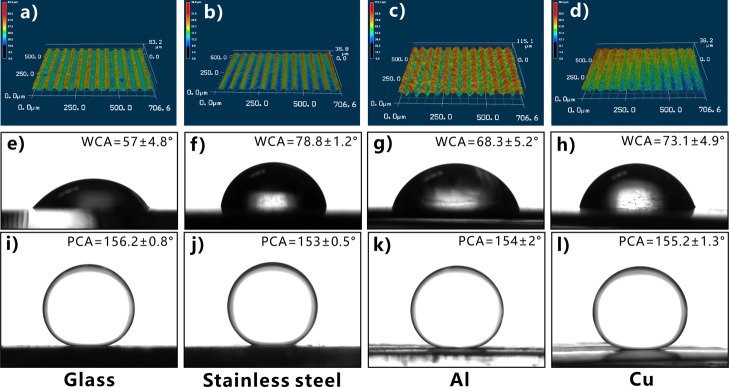
Femtosecond laser pulse has two unique characteristics: ultrashort pulse width and ultrahigh peak intensity. Such features endow the femtosecond laser with the ability to ablate almost all of the known materials, so various hierarchical microstructures can be easily created on the surfaces of different material substrates through one-step femtosecond laser ablation.34−37 In addition to the silicon surface, underwater superpolymphobicity can also be achieved on a wide range of other hydrophilic materials by femtosecond laser treatment. For example, Figure 5a–d shows the surface microstructures of laser-ablated glass, stainless steel, Al, and Cu substrates. Those materials are intrinsically hydrophilic (Figure 5e–h) and become superhydrophilic after laser treatment. When the laser-structured samples are submerged in water and liquid PDMS droplets are dispensed onto the sample surfaces, all the PDMS droplets are spherical with the PCA higher than 150° (Figure 5i–l). Therefore, the hydrophilic substrates exhibit excellent underwater superpolymphobicity after femtosecond laser ablation.
Figure 5.
Underwater superpolymphobicity of different substrates after femtosecond laser treatment. (a–d) Laser confocal microscopy images of the laser-structured sample surfaces. (e–h) Water droplets on the untreated flat sample surfaces in air. (i–l) Liquid PDMS droplets on the laser-structured sample surfaces in water. Substrates: (a,e,i) glass, (b,f,j) stainless steel, (c,g,k) Al, and (d,h,l) Cu.
Different from water and oil liquids, many liquid polymers such as PDMS can be cured and become a solid state. We can selectively design the adhesion between liquid polymer and solid substrate or change the shape of the liquid polymer by using superpolymphobic microstructures. The shape of the liquid polymer will be fixed permanently when it solidifies, e.g., liquid PDMS can be cured at high temperature. Therefore, superpolymphobicity of the laser-induced microstructures can be applied to control the shape of cured polymer materials and enable designing the polymer–substrate adhesion.
Conclusions
In conclusion, underwater superpolymphobicity was achieved on various hydrophilic substrates by simple femtosecond laser processing, including semiconductors, glass, and metals. Femtosecond laser ablation endows silicon surface with hierarchical micro/nanostructures. The liquid PDMS droplet on the resultant surface has a PCA of 159 ± 1° and SA of 1.5 ± 0.5° in water, demonstrating that the laser-structured surfaces show excellent underwater superpolymphobicity and extremely low adhesion to the underwater PDMS droplet. The adhesion between the liquid polymer and a solid substrate can be effectively prevented by the underwater superpolymphobic microstructures. The underwater superpolymphobicity results from the underwater Cassie wetting state between the liquid PDMS droplet and the laser-induced surface microstructure. Following the formation mechanism of underwater superpolymphobicity and the building principle of underwater superpolymphobic microstructures reported in this paper, we believe the underwater superpolymphobicity can also be achieved on material surfaces by using various microfabrication methods besides laser processing. Current research will have wide potential applications in reducing the polymer/solid adhesion and controlling the shape of the polymer materials.
Acknowledgments
This work is supported by the Bill & Melinda Gates Foundation under the grant no. OPP1119542, National Key Research and Development Program of China under the grant no. 2017YFB1104700, the National Science Foundation of China under the grant nos. 51335008, 61875158, and 61805192, the Fundamental Research Funds for the Central Universities under the grant no. xzy012019042, and the China Postdoctoral Science Foundation under the grant no. 2016M600786.
The authors declare no competing financial interest.
References
- Pan S.; Kota A. K.; Mabry J. M.; Tuteja A. Superomniphobic Surfaces for Effective Chemical Shielding. J. Am. Chem. Soc. 2013, 135, 578–581. 10.1021/ja310517s. [DOI] [PubMed] [Google Scholar]
- Nishimoto S.; Bhushan B. Bioinspired Self-Cleaning Surfaces with Superhydrophobicity, Superoleophobicity, and Superhydrophilicity. RSC Adv. 2013, 3, 671–690. 10.1039/c2ra21260a. [DOI] [Google Scholar]
- Wu D.; Wu S.-z.; Chen Q.-D.; Zhao S.; Zhang H.; Jiao J.; Piersol J. A.; Wang J.-N.; Sun H.-B.; Jiang L. Facile Creation of Hierarchical PDMS Microstructures with Extreme Underwater Superoleophobicity for Anti-Oil Application in Microfluidic Channels. Lab Chip 2011, 11, 3873–3879. 10.1039/c1lc20226j. [DOI] [PubMed] [Google Scholar]
- Wang M.; Chen C.; Ma J.; Xu J. Preparation of Superhydrophobic Cauliflower-Like Silica Nanospheres with Tunable Water Adhesion. J. Mater. Chem. 2011, 21, 6962–6967. 10.1039/c1jm10283d. [DOI] [Google Scholar]
- Yao X.; Gao J.; Song Y.; Jiang L. Superoleophobic Surfaces with Controllable Oil Adhesion and Their Application in Oil Transportation. Adv. Funct. Mater. 2011, 21, 4270–4276. 10.1002/adfm.201100775. [DOI] [Google Scholar]
- Liu X.; Liang Y.; Zhou F.; Liu W. Extreme Wettability and Tunable Adhesion: Biomimicking Beyond Nature?. Soft Matter 2012, 8, 2070–2086. 10.1039/c1sm07003g. [DOI] [Google Scholar]
- Xue Z.; Cao Y.; Liu N.; Feng L.; Jiang L. Special Wettable Materials for Oil/Water Separation. J. Mater. Chem. A 2014, 2, 2445–2460. 10.1039/c3ta13397d. [DOI] [Google Scholar]
- Feng L.; Zhang Z.; Mai Z.; Ma Y.; Liu B.; Jiang L.; Zhu D. A Super-Hydrophobic and Super-Oleophilic Coating Mesh Film for the Separation of Oil and Water. Angew. Chem., Int. Ed. 2004, 43, 2012–2014. 10.1002/anie.200353381. [DOI] [PubMed] [Google Scholar]
- Yong J.; Huo J.; Chen F.; Yang Q.; Hou X. Oil/Water Separation Based on Natural Materials with Super-Wettability: Recent Advances. Phys. Chem. Chem. Phys. 2018, 20, 25140–25163. 10.1039/c8cp04009e. [DOI] [PubMed] [Google Scholar]
- Yong J.; Chen F.; Yang Q.; Bian H.; Du G.; Shan C.; Huo J.; Fang Y.; Hou X. Oil-Water Separation: A Gift from the Desert. Adv. Mater. Interfaces 2016, 3, 1500650. 10.1002/admi.201500650. [DOI] [Google Scholar]
- Yong J.; Fang Y.; Chen F.; Huo J.; Yang Q.; Bian H.; Du G.; Hou X. Femtosecond Laser Ablated Durable Superhydrophobic PTFE Films with Micro-Through-Holes for Oil/Water Separation: Separating Oil from Water and Corrosive Solutions. Appl. Surf. Sci. 2016, 389, 1148–1155. 10.1016/j.apsusc.2016.07.075. [DOI] [Google Scholar]
- Shi F.; Niu J.; Liu J.; Liu F.; Wang Z.; Feng X.-Q.; Zhang X. Towards Understanding Why a Superhydrophobic Coating Is Needed by Water Striders. Adv. Mater. 2007, 19, 2257–2261. 10.1002/adma.200700752. [DOI] [Google Scholar]
- Gao X.; Yan X.; Yao X.; Xu L.; Zhang K.; Zhang J.; Yang B.; Jiang L. The Dry-Style Antifogging Properties of Mosquito Compound Eyes and Artificial Analogues Prepared by Soft Lithography. Adv. Mater. 2007, 19, 2213–2217. 10.1002/adma.200601946. [DOI] [Google Scholar]
- Nosonovsky M.; Hejazi V. Why Superhydrophobic Surfaces Are Not Always Icephobic. ACS Nano 2012, 6, 8488–8491. 10.1021/nn302138r. [DOI] [PubMed] [Google Scholar]
- Kreder M. J.; Alvarenga J.; Kim P.; Aizenberg J. Design of Anti-Icing Surfaces: Smooth, Textured or Slippery?. Nat. Rev. Mater. 2016, 1, 15003. 10.1038/natrevmats.2015.3. [DOI] [Google Scholar]
- Lv J.; Song Y.; Jiang L.; Wang J. Bio-Inspired Strategies for Anti-Icing. ACS Nano 2014, 8, 3152–3169. 10.1021/nn406522n. [DOI] [PubMed] [Google Scholar]
- Parker A. R.; Lawrence C. R. Water Capture by a Desert Beetle. Nature 2001, 414, 33–34. 10.1038/35102108. [DOI] [PubMed] [Google Scholar]
- Garrod R. P.; Harris L. G.; Schofield W. C. E.; McGettrick J.; Ward L. J.; Teare D. O. H.; Badyal J. P. S. Mimicking a Stenocara Beetle’s Back for Microcondensation Using Plasmachemical Patterned Superhydrophobic-Superhydrophilic Surfaces. Langmuir 2007, 23, 689–693. 10.1021/la0610856. [DOI] [PubMed] [Google Scholar]
- Shen L.; Wang B.; Wang J.; Fu J.; Picart C.; Ji J. Asymmetric Free-Standing Film with Multifunctional Anti-Bacterial and Self-Cleaning Properties. ACS Appl. Mater. Interfaces 2012, 4, 4476–4483. 10.1021/am301118f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Liu M.; Bai H.; Chen P.; Xia F.; Han D.; Jiang L. Antiplatelet and Thermally Responsive Poly(N-isopropylacrylamide) Surface with Nanoscale Topography. J. Am. Chem. Soc. 2009, 131, 10467–10472. 10.1021/ja9019935. [DOI] [PubMed] [Google Scholar]
- Ivanova E. P.; Hasan J.; Webb H. K.; Gervinskas G.; Juodkazis S.; Truong V. K.; Wu A. H. F.; Lamb R. N.; Baulin V. A.; Watson G. S.; Watson J. A.; Mainwaring D. E.; Crawford R. J. Bactericidal Activity of Black Silicon. Nat. Commun. 2013, 4, 2838. 10.1038/ncomms3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.-H.; Lee B.-H.; Im H.; Jeon S.-B.; Kim D.; Seol M.-L.; Hwang H.; Choi Y.-K. Controlled Anisotropic Wetting of Scalloped Silicon Nanogroove. RSC Adv. 2016, 6, 41914–41918. 10.1039/c6ra06379a. [DOI] [Google Scholar]
- Vitale A.; Quaglio M.; Marasso S. L.; Chiodoni A.; Cocuzza M.; Bongiovanni R. Direct Photolithography of Perfluoropolyethers for Solvent-Resistant Microfluidics. Langmuir 2013, 29, 15711–15718. 10.1021/la402755q. [DOI] [PubMed] [Google Scholar]
- Wen L.; Tian Y.; Jiang L. Bioinspired Super-Wettability from Fundamental Research to Practical Applications. Angew. Chem., Int. Ed. 2015, 54, 3387–3399. 10.1002/anie.201409911. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Su B.; Jiang L. Interfacial Material System Exhibiting Superwettability. Adv. Mater. 2014, 26, 6872–6897. 10.1002/adma.201400883. [DOI] [PubMed] [Google Scholar]
- Su B.; Tian Y.; Jiang L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. 10.1021/jacs.5b12728. [DOI] [PubMed] [Google Scholar]
- Liu M.; Wang S.; Jiang L. Nature-Inspired Superwettability Systems. Nat. Rev. Mater. 2017, 2, 17036. 10.1038/natrevmats.2017.36. [DOI] [Google Scholar]
- Bellanger H.; Darmanin T.; Taffin de Givenchy E.; Guittard F. Chemical and Physical Pathways for the Preparation of Superoleophobic Surfaces and Related Wetting Theories. Chem. Rev. 2014, 114, 2694–2716. 10.1021/cr400169m. [DOI] [PubMed] [Google Scholar]
- Wang J.-N.; Zhang Y.-L.; Liu Y.; Zheng W.; Lee L. P.; Sun H.-B. Recent Developments in Superhydrophobic Graphene and Graphene-Related Materials: From Preparation to Potential Applications. Nanoscale 2015, 7, 7101–7114. 10.1039/c5nr00719d. [DOI] [PubMed] [Google Scholar]
- Jiang T.; Guo Z.; Liu W. Biomimetic Superoleophobic Surfaces: Focusing on their Fabrication and Applications. J. Mater. Chem. A 2015, 3, 1811–1827. 10.1039/c4ta05582a. [DOI] [Google Scholar]
- Teisala H.; Tuominen M.; Kuusipalo J. Superhydrophobic Coatings on Cellulose-Based Materials: Fabrication, Properties, and Applications. Adv. Mater. Interfaces 2014, 1, 1300026. 10.1002/admi.201300026. [DOI] [Google Scholar]
- Yong J.; Chen F.; Li M.; Yang Q.; Fang Y.; Huo J.; Hou X. Remarkably Simple Achievement of Superhydrophobicity, Superhydrophilicity, Underwater Superoleophobicity, Underwater Superoleophilicity, Underwater Superaerophobicity, and Underwater Superaerophilicity on Femtosecond Laser Ablated PDMS Surfaces. J. Mater. Chem. A 2017, 5, 25249–25257. 10.1039/c7ta07528f. [DOI] [Google Scholar]
- Yong J.; Chen F.; Huo J.; Fang Y.; Yang Q.; Zhang J.; Hou X. Femtosecond Laser Induced Underwater Superaerophilic and Superaerophobic PDMS sheet with Through-Microholes for Air Bubbles Selectively Passing Through and Further Collecting Underwater Gas. Nanoscale 2018, 10, 3688–3696. 10.1039/c7nr06920k. [DOI] [PubMed] [Google Scholar]
- Sugioka K.; Cheng Y. Ultrafast Lasers-Reliable Tools for Advanced Materials Processing. Light: Sci. Appl. 2014, 3, e149 10.1038/lsa.2014.30. [DOI] [Google Scholar]
- Sugioka K.; Cheng Y. Femtosecond Laser Three-Dimensional Micro- and Nanofabrication. Appl. Phys. Rev. 2014, 1, 041303. 10.1063/1.4904320. [DOI] [Google Scholar]
- Yong J.; Chen F.; Yang Q.; Hou X. Femtosecond Laser Controlled Wettability of Solid Surfaces. Soft Matter 2015, 11, 8897–8906. 10.1039/c5sm02153g. [DOI] [PubMed] [Google Scholar]
- Yong J.; Chen F.; Yang Q.; Jiang Z.; Hou X. A Review of Femtosecond-Laser-Induced Underwater Superoleophobic Surfaces. Adv. Mater. Interfaces 2018, 5, 1701370. 10.1002/admi.201701370. [DOI] [Google Scholar]
- Chen F.; Zhang D.; Yang Q.; Yong J.; Du G.; Si J.; Yun F.; Hou X. Bioinspired Wetting Surface via Laser Microfabrication. ACS Appl. Mater. Interfaces 2013, 5, 6777–6792. 10.1021/am401677z. [DOI] [PubMed] [Google Scholar]
- Yong J.; Singh S. C.; Zhan Z.; Chen F.; Guo C. Substrate-Independent, Fast, and Reversible Switching between Underwater Superaerophobicity and Aerophilicity on the Femtosecond Laser-Induced Superhydrophobic Surfaces for Selectively Repelling or Capturing Bubbles in Water. ACS Appl. Mater. Interfaces 2019, 11, 8667–8675. 10.1021/acsami.8b21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong J.; Singh S. C.; Zhan Z.; Chen F.; Guo C. How To Obtain Six Different Superwettabilities on a Same Microstructured Pattern: Relationship between Various Superwettabilities in Different Solid/Liquid/Gas Systems. Langmuir 2019, 35, 921–927. 10.1021/acs.langmuir.8b03726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong J.; Singh S. C.; Zhan Z.; Huo J.; Chen F.; Guo C. Reducing Adhesion for Dispensing Tiny Water/Oil Droplets and Gas Bubbles by Femtosecond Laser-Treated Needle Nozzles: Superhydrophobicity, Superoleophobicity, and Superaerophobicity. ChemNanoMat 2019, 5, 241–249. 10.1002/cnma.201800495. [DOI] [Google Scholar]
- Jiao Y.; Lv X.; Zhang Y.; Li C.; Li J.; Wu H.; Xiao Y.; Wu S.; Hu Y.; Wu D.; Chu J. Pitcher Plant-Bioinspired Bubble Slippery Surface Fabricated by Femtosecond Laser for Buoyancy-Driven Bubble Self-Transport and Efficient Gas Capture. Nanoscale 2019, 11, 1370–1378. 10.1039/c8nr09348b. [DOI] [PubMed] [Google Scholar]
- Li G.; Lu Y.; Wu P.; Zhang Z.; Li J.; Zhu W.; Hu Y.; Wu D.; Chu J. Fish Scale Inspired Design of Underwater Superoleophobic Microcone Arrays by Sucrose Solution Assisted Femtosecond Laser Irradiation for Multifunctional Liquid Manipulation. J. Mater. Chem. A 2015, 3, 18675–18683. 10.1039/c5ta05265c. [DOI] [Google Scholar]
- Vorobyev A. Y.; Guo C. Multifunctional Surfaces Produced by Femtosecond Laser Pulses. J. Appl. Phys. 2015, 117, 033103. 10.1063/1.4905616. [DOI] [Google Scholar]
- Wu B.; Zhou M.; Li J.; Ye X.; Li G.; Cai L. Superhydrophobic Surfaces Fabricated by Microstructuring of Stainless Steel using a Femtosecond Laser. Appl. Surf. Sci. 2009, 256, 61–66. 10.1016/j.apsusc.2009.07.061. [DOI] [Google Scholar]
- Yong J.; Huo J.; Yang Q.; Chen F.; Fang Y.; Wu X.; Liu L.; Lu X.; Zhang J.; Hou X. Femtosecond Laser Direct Writing of Porous Network Microstructures for Fabricating Super-Slippery Surfaces with Excellent Liquid Repellence and Anti-Cell Proliferation. Adv. Mater. Interfaces 2018, 5, 1701479. 10.1002/admi.201701479. [DOI] [Google Scholar]
- Yong J.; Chen F.; Yang Q.; Huo J.; Hou X. Superoleophobic Surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. 10.1039/c6cs00751a. [DOI] [PubMed] [Google Scholar]