Abstract
Background
The incidence of antimicrobial resistance has been increasing worldwide in the past decades, which includes resistance to bacteria that cause common childhood illnesses, such as acute respiratory infections and diarrhea. Numerous children with those common illnesses are treated with antibiotics. However, in such cases, antibiotic treatment is not required. Community-based studies focusing on antibiotic use among children are still limited. This study aimed to identify the prevalence of antibiotic use for common childhood illnesses and to investigate factors associated with antibiotic use in children under 5 years old as well as female caregivers in a rural community in Indonesia.
Methods
A cross-sectional study of 334 children in three villages of Banten Province, located in the western part of Java Island, was conducted in May 2018. Female caregivers who were responsible for providing medications to children were interviewed. We obtained information such as demographic data, any common clinical illness within the last 30 days, and antibiotic usage during an episode of illness. We excluded children with underlying disease that require a regular follow-up and children who were hospitalized in the last 30 days in the analysis. Antibiotic use answered by female caregivers was verified by checking its package or showing photos of various antibiotics to the female caregivers. Crushed antibiotics were confirmed with health professionals.
Results
A total of 203 children had clinical symptoms, and the most common symptom was fever and respiratory symptoms. In total, 49.3% received antibiotics, and 66% of them were prescribed by private health professionals. Only two children received antibiotics without a prescription. The most common antibiotic used among children was amoxicillin.
Conclusions
The high prevalence of antibiotic use was observed in children under 5 years of age, and the major source to obtain antibiotics was to consult health professionals. Training on appropriate antibiotic use must be conducted for health professionals in not only public but also private sectors.
Keywords: Antibiotics, Indonesia, Acute respiratory infection, Diarrhea, Child, Antimicrobial resistance
Background
The incidence of antimicrobial resistance (AMR) has been increasing worldwide in the past decades, which include resistance to bacteria that cause common infections in children, such as acute respiratory infections (ARI) and diarrhea [1–5]. Numerous children with ARI and diarrhea are treated with antibiotics. However, in these cases, antibiotic treatment may not be required [6–8]. Previous studies have shown that the use of antibiotic was associated with higher rates of resistance in children [9, 10].
A large variation was observed in the use of antibiotics among children between countries [11]. The global consumption of antibiotics had increased significantly, mainly due to the increase of antibiotic use in low- and middle-income countries [12]. Therefore, the appropriate use of antibiotics must be promoted in these countries to prevent the increasing trend of antibiotic resistance at the global level. However, in low- and middle-income countries, the inappropriate use of antibiotics is common [13–15]. Furthermore, the utilization of unprescribed antibiotics is a major issue particularly in low- and middle-income countries [16–18]. Studies about knowledge and practices of and attitudes toward the use of antibiotics have indicated that lower socioeconomic status and education level may be important factors for the misconceptions of antibiotic use among caregivers [19–21].
Despite numerous publications showing the prevalence of antibiotic use and its associated factors, community-based surveys focusing on children aged under 5 years are still limited in low- and middle-income countries, including Indonesia, where misuse and overuse of antibiotics are considered a more serious problem. The patterns of antibiotic use were studied in health care settings including primary care facilities [14, 22] and teaching hospitals [23]. A study conducted in Surabaya, Indonesia, has indicated that antibiotics without prescriptions are widely sold in pharmacies and kiosks [24]. Moreover, a community-based study in Yogyakarta has shown that self-medication with antibiotics was common in adult patients [25]. In 2015, the Ministry of Health of Indonesia had released the 2015–2019 strategic plans for implementing existing regulations and guidelines and rolling out of AMR-related activities. However, there is no national AMR surveillance currently implemented, and the data about AMR remains limited in the country [26–28]. Actions against AMR need to be further strengthened all over the country [26].
We conducted a study on antibiotic use in children under 5 years old in a rural community in Indonesia to identify the prevalence of antibiotic use for common childhood illnesses and to investigate factors associated with antibiotic use in children as well as female caregivers.
Results
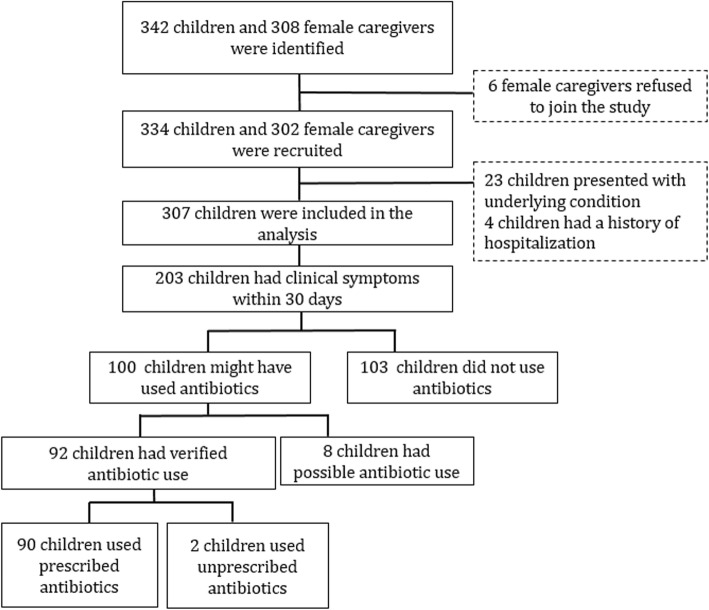
Out of 1645 children listed in the registration books at health-integrated posts, 342 children aged under 5 years in 308 households were selected for the study (Fig. 1). Out of 308 female caregivers, 302 (98.1%) agreed to participate in the study. There were 334 children in 302 households. Of these 334 children, 23 presented with underlying conditions, and four were hospitalized. These children were then excluded from the analysis. The remaining 307 children were included in the analysis. Of 307 children, 203 (66.1%) had at least one clinical symptom within the last 30 days.
Fig. 1.
Flowchart of the recruitment of study participants
Sociodemographic characteristics of the female caregivers and children
Table 1 shows a summary of the sociodemographic characteristics of the female caregivers and children. The mothers of the children comprised over 94% of the female caregivers. The median age of the female caregivers was 30.3 years. About 60% of female caregivers had education at the primary school level or lower. Approximately 64% of households had an income lower than the minimum salary in Rangkasbitung district. About one third of the female caregivers claimed that their children had health insurance. The ages of the children were not equally distributed. That is, there is a higher proportion of older children. The proportion of boys was slightly higher than that of girls (56% vs. 44%).
Table 1.
Characteristics of the female caregivers and children
| Characteristics | n (%) |
|---|---|
| Female caregivers (n = 302) | |
| Age (years), median (IQR) | 30.3 (25.3–35.7) |
| Relationship with the child | |
| Mother | 285 (94.4) |
| Grandmother | 10 (3.3) |
| Others | 7 (2.3) |
| Two or more children aged < 5 years | 31 (10.3) |
| Level of education | |
| None/primary | 175 (57.9) |
| Secondary | 86 (28.5) |
| Higher than secondary | 41 (13.6) |
| Income of the family in the previous month† | |
| Lower than the minimum salary in Rangkasbitung district | 193 (63.9) |
| Higher than the minimum salary in Rangkasbitung district | 95 (31.5) |
| No answer | 14 (4.6) |
| Living with extended family | 139 (46.0) |
| With health insurance for child/children | 104 (34.4) |
| Children (n = 334) | |
| Age in year | |
| < 1 | 44 (13.2) |
| 1 | 32 (9.6) |
| 2 | 96 (28.7) |
| 3 | 53 (15.9) |
| 4 | 109 (32.6) |
| Gender | |
| Male | 187 (56.0) |
| Female | 147 (44.0) |
IQR Interquartile range
†The minimum salary in Rangkasbitung district in 2017–2018 was IDR 2,312,384 or $158.1
Characteristics of children with any clinical signs and those who used antibiotics
The median age of 203 children who had clinical symptoms within the last 30 days was 2 years and 10 months, and 54.7% were boys. More than 90% of children who had respiratory symptoms, diarrhea, or fever visited a health facility. A total of 100 children took either verified or possible antibiotics (Table 2). This meant 49.3% of children with clinical symptoms or 32.6% of children included in the analysis took the drug. Of those who received antibiotics, 63 were verified to have taken antibiotics based on the remaining package (n = 44) or via the identification of the photos of antibiotics (n = 19) by the female caregivers. Of the 63 children whose antibiotic use was verified, 62 took syrup and one took tablets. A total of 29 children used crushed drugs, which the health care staff verified as antibiotics. However, the name of these crushed antibiotics was not identified. Among 100 children, 8 were considered to have received possible antibiotics as recalled by the female caregivers.
Table 2.
Proportion of antibiotic use among children who developed clinical symptoms within the last 30 days (n = 203)
| Characteristics | Antibiotic use | ||
|---|---|---|---|
| Total | Verified | Overall† | |
| n | n (%) | n (%) | |
| Total | 203 | 92 (45.3) | 100 (49.3) |
| Health facility visit | |||
| Visited | 186 | 90 (48.4) | 98 (52.7) |
| Did not visit | 17 | 2 (11.8) | 2 (11.8) |
| Symptoms†† | |||
| R + D + F | 37 | 22 (59.5) | 23 (62.2) |
| R + D | 2 | 1 (50.0) | 1 (50.0) |
| R + F | 100 | 46 (46.0) | 48 (48.0) |
| R | 20 | 5 (25.0) | 6 (30.0) |
| D + F | 4 | 2 (50.0) | 2 (50.0) |
| D | 4 | 1 (25.0) | 1 (25.0) |
| F | 36 | 15 (41.7) | 19 (52.8) |
| Age | |||
| < 1 year | 35 | 19 (54.3) | 21 (60.0) |
| 1 year | 23 | 11 (47.8) | 13 (56.5) |
| 2 years | 54 | 22 (40.7) | 24 (44.4) |
| 3 years | 32 | 17 (53.1) | 18 (56.3) |
| 4 years | 59 | 23 (39.0) | 24 (40.7) |
†Overall antibiotic use is a combination of verified and possible antibiotic use
††R Respiratory symptoms (cough or difficulty of breathing), D Diarrhea (frequent loose or liquid stool). F Fever (fever or feverish)
The name of the antibiotics used in 63 children was identified, of whom 53 (84.1%) used amoxicillin. Other antibiotics included trimethoprim and sulfamethoxazole (n = 4), cefadroxil (n = 4), chloramphenicol (n = 1), and metronidazole (n = 1).
Data about the proportion of verified, and possible antibiotic use by health facility visit, symptoms, and age are shown in Table 2. Most children (98.0%) received antibiotics from health facilities. Seventeen children did not visit any of the health facilities. Among them, only two (11.8%) took leftover antibiotics at home without a prescription. Proportions of antibiotic use varied among children with different clinical symptoms (Table 2). The highest proportion (62.2%) of antibiotic use was observed in children who had a combination of respiratory symptoms, diarrhea, and fever, whereas the lower proportion of antibiotic use was observed in those with respiratory symptoms only and diarrhea only (30.0% and 25.0%, respectively). Children aged under 1 year had the highest proportion (60.0%) of antibiotic use. However, the proportions were also high in older children ranging from 40.7 to 56.5% (Table 2).
Children who visited the clinic of a private doctor had a higher proportion of antibiotic use (60.0%) than those who visited primary health centers (51.6%). However, the result was not statistically significant (Table 3). Similarly, the odds ratio (OR) for taking antibiotics at the private clinics of midwives/nurses was lower than that at private doctor’s clinics though there was no statistical significance. Children who did not visit the health facility had the lowest proportion of antibiotic use (11.8%), and the differences were statistically significant when compared with children who visited one of the health facilities (p < 0.001).
Table 3.
Association between antibiotic use and the type of health facility visited, clinical symptoms and age of children (n = 203)
| Characteristics | Description | Total n |
Antibiotic use | aOR†† (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Yes† n (%) |
No n (%) |
|||||
| Type of health facility visited | Private doctor clinic | 55 | 33 (60.0) | 22 (40.0) | Ref. | – |
| Private midwife/nurse clinic | 69 | 33 (47.8) | 36 (52.2) | 0.42 (0.20–1.04) | 0.18 | |
| Primary health centers | 62 | 32 (51.6) | 30 (48.4) | 0.64 (0.29–1.59) | 0.36 | |
| Did not go to any health facility | 17 | 2 (11.8) | 15 (88.2) | 0.08 (0.01–0.54) | < 0.001* | |
| Clinical symptoms | ||||||
| Fever | No | 26 | 8 (30.8) | 18 (69.2) | Ref. | |
| Yes | 177 | 92 (52.0) | 85 (48.0) | 2.48 (0.97–7.03) | 0.06 | |
| Respiratory symptoms | No | 44 | 22 (50.0) | 22 (50.0) | Ref. | |
| Yes | 159 | 78 (49.1) | 81 (50.9) | 0.88 (0.44–1.98) | 1.00 | |
| Diarrhea | No | 156 | 73 (46.8) | 83 (53.2) | Ref. | |
| Yes | 47 | 27 (57.4) | 20 (42.6) | 1.83 (0.89–4.32) | 0.24 | |
| Age of the child | < 2 years | 58 | 34 (58.6) | 24 (41.4) | Ref. | |
| 2–4 years | 145 | 66 (45.5) | 79 (54.5) | 0.57 (0.30–1.25) | 0.12 | |
aOR adjusted odds ratio, 95% CI confidence interval, Ref. Reference
P values for the type of health facility visited were assessed between a private doctor’s clinic and each of the other category
†The category “Yes” includes both verified and possible antibiotic use
††Adjusted for age of the child and living with extended family, or living with extended family only (for age of the child)
*p value < 0.05
When comparing children with and without a certain symptom (i.e., fever, respiratory symptoms, and diarrhea) (Table 3), no difference was observed between children with and without respiratory symptoms. Children with fever had a higher proportion of antibiotic use (52.0%) than those without fever (30.8%). However, the result was not statistically different (p = 0.06). Moreover, no difference was noted in the proportion of antibiotic use between children aged less than 2 years, and those aged 2–4 years (Table 3).
In Table 4, we compared the characteristics of female caregivers as well as children who used and did not use antibiotics. The female caregivers who did not live with extended family had a significantly higher proportion of antibiotics use (57.7% vs. 40.4%, p = 0.014) and odds ratio to have antibiotics was also significantly low among those who live with extended family (OR = 0.47, 95% CI = 0.25–0.97). No difference was observed in the proportion of antibiotic use in terms of the other characteristics of the female caregivers (relationship with the child, age group, educational level, family income, and with health insurance for children) (Table 4).
Table 4.
Association between antibiotic use and characteristics of female caregivers (n = 203)
| Characteristics | Description | Total n |
Antibiotic use | aOR†† (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Yes† n (%) |
No n (%) |
|||||
| Relationship with the child | ||||||
| Mother | 192 | 94 (49.0) | 98 (51.0) | Ref. | 0.91 | |
| Grandmother | 7 | 4 (57.1) | 3 (42.9) | 1.42 (0.24–11.68) | ||
| Others | 4 | 2 (50.0) | 2 (50.0) | 0.77 (0.14–5.77) | ||
| Age of female caregivers | < 30 years | 114 | 58 (50.9) | 56 (49.1) | Ref. | |
| ≥ 30 years | 89 | 42 (47.2) | 47 (52.8) | 0.83 (0.45–1.71) | 0.60 | |
| Educational level of female caregiver | None or primary | 91 | 41 (45.1) | 50 (54.9) | Ref. | |
| Secondary or above | 112 | 59 (52.7) | 53 (47.3) | 1.04 (0.56–2.11) | 0.28 | |
| Lower than the minimum salary in Rangkasbitung district | No | 64 | 32 (50.0) | 32 (50.0) | Ref. | |
| Yes | 130 | 65 (50.0) | 65 (50.0) | 0.96 (0.51–2.03) | 1.00 | |
| Living with extended family | No | 104 | 60 (57.7) | 44 (42.3) | Ref. | |
| Yes | 99 | 40 (40.4) | 59 (59.6) | 0.47 (0.25–0.97) | 0.01* | |
| With health insurance for child(ren) | No | 133 | 70 (52.6) | 63 (47.4) | Ref. | |
| Yes | 69 | 29 (42.0) | 40 (58.0) | 0.90 (0.48–1.89) | 0.15 | |
aOR adjusted odds ratio, 95% CI confidence interval, Ref. Reference
The minimum salary in Rangkasbitung was compared with the household income in last month
†The category “Yes” includes both verified and possible antibiotic use
††Adjusted for age of the child and living with extended family, or age of the child only (for living with extended family)
*p value < 0.05
Discussion
In the present study, about 33% of children received antibiotics within the last 30 days (about four courses per child per year), which is similar to the rate of antibiotic courses provided to children less than 2 years (4.9 courses per child per year) in a prospective cohort study conducted in eight low- and middle-income countries [11]. About half of the children presented with common clinical symptoms, including fever, respiratory symptoms, and diarrhea, received antibiotics. The Demographic and Health Survey of Indonesia conducted in 2012 has also shown that the prevalence of antibiotic use ranged from 12.5% for diarrhea to 39% for ARI that occurred within 2 weeks before the survey [29].
The Integrated Management Childhood Illness (IMCI) was developed by the World Health Organization (WHO) and the United Nations International Children’s Emergency Fund (UNICEF) to guide the management of common childhood illnesses in resource-limited settings [30]. Indonesia has adopted IMCI and has been implementing it nationwide [31]. Amoxicillin must be taken when the diagnosis of pneumonia, as characterized by rapid breathing, is made. We did not assess the severity of the condition of the children with ARI in this study; however, most ARI cases reported in this study might be mild, which do not require antibiotic treatment partly because we excluded hospitalized cases in the analysis. A previous study in low-middle-income countries also reported 44% of acute respiratory illness were treated with antibiotics [11].
Similarly, only acute diarrhea with bloody stool or suspected cholera cases requires oral antibiotic treatment, such as cotrimoxazole and tetracycline. Whether diarrhea was bloody or suggestive of cholera was not assessed in this study. However, Indonesia reported low cholera incidence in the last decade [32] and dysentery was frequently observed in the rainy season (December to January) [33]. The proportion of the symptoms mentioned above is considered to be low among all diarrhea cases during the non-epidemic period. Therefore, most antibiotics provided to children with ARI and acute diarrhea might not be necessary. Although no statistical significance was observed, odds for taking antibiotics was the highest in comparing children with fever and those without. When children who had ARI with and without fever were compared, a higher proportion of antibiotic use was observed in those with ARI and fever than in those with ARI without fever. A previous study in low- and middle-income countries reported that fever was independently associated with antibiotics treatment [11]. Health facilities in the study sites also might be using fever as criteria to give antibiotics.
Only two children received unprescribed antibiotics. On the contrary, another community-based study in Indonesia showed that more than half of the antibiotics used were not prescribed [25]. However, this study was conducted for the adult population in 2010. There might be different patterns of non-prescribed antibiotic use between children and adults. The recent strict enforcement of regulations to limit the over the counter availability of antibiotics might have reduced the prevalence of non-prescribed use of antibiotics [26, 34]. Continuous studies must be conducted to monitor the trend of using non-prescribed antibiotics in different age groups.
Although most children received antibiotics from health care facilities with the prescription, the overuse of antibiotics in health care facilities is a major concern, as reported in previous studies in Indonesia [14, 22]. About two thirds of antibiotics were prescribed by private doctors or private midwives/nurses. IMCI has not been fully implemented even in primary government health centers due to various challenges, including the shortage of trained staff [35]. It would be more difficult to train health care professionals, such as midwives, in the private clinic.
The most common antibiotic used among children in the present study was amoxicillin, which is the first-line treatment recommended for pneumonia in the IMCI in Indonesia in 2015. Amoxicillin is not recommended for children with acute diarrhea. However, it was commonly provided to children with diarrhea in the present study. Recently, the WHO classifies antibiotics in the WHO essential medicines list into three groups: Access, Watch, and Reserve (AWaRe) [36]. Access antibiotics are first-line or second-line antibiotics for common infectious diseases. Most of the antibiotics used for children in the present study were Access antibiotics. Antibiotics in the Watch list, such as third-generation cephalosporins and quinolones were not prescribed even by private doctors. However, further training on the proper use of antibiotics must be conducted for health professionals in both the public and private sectors.
The sample size of the study was not enough to obtain data about the association between the characteristics of female caregivers and antibiotic use. Age, educational level, family income, and health insurance for children were not significantly associated with antibiotic use. However, a higher proportion of antibiotics use was observed in female caregivers who did not live with extended family than in those who lived with extended family. The reason for such difference was not clear. A caregiver living with extended family might obtain more appropriate advice from other family members.
The present study had some limitations. First, we collected information about symptoms and antibiotic use from female caregivers. Although we only assessed the episodes within the last 30 days before the interview, responses of the caregivers were still subject to recall bias. Second, we could not validate antibiotic use in some cases or specify types of some crushed antibiotics. Third, it was not possible to classify ARI into different severity categories, including pneumonia, and bloody diarrhea and suspected cholera were not identified among diarrhea cases. Thus, we could not know how many children with ARI and diarrhea required antibiotic treatment. Despite these limitations, results from the present study provided important insights for future interventions and data about antibiotic use in children in the rural Indonesian community.
Conclusion
The high prevalence of antibiotic use was observed in children under 5 years of age with common clinical symptoms such as fever, respiratory symptoms, and diarrhea. The major source to obtain antibiotics was to consult health professionals, and 66% of antibiotics were from private doctors, midwives, or nurses. Training on appropriate antibiotic use must be conducted for health professionals in not only public but also private sectors.
Methods
Study setting and design
A cross-sectional survey was conducted in May 2018 in Rangkasbitung district, Banten Province, located in the western part of Java Island, Indonesia (Fig. 2). The total population in Rangkasbitung district was 122,646 in 2016 [37]. The main industry of the district is agriculture. Rangkasbitung district is reported as a non-endemic malaria area; thus, antimalaria treatment is not recommended [31]. In Indonesia, the primary health center is called puskesmas, which covers a catchment of approximately 30,000 people [38]. A health center comprised several health professionals, including physicians, nurses, and midwives. There are lower level facilities under each primary health center, including the village health post (poskesdes) and the health-integrated posts (posyandu) [39]. The former is a facility with at least one midwife, and basic health services such as family planning, maternal care, and immunization are provided [40]. The latter is mainly operated by volunteer health workers (cadres) with occasional support from midwives, and anthropometry for children under five years old, family planning, immunization, nutrition, and sanitation programs are provided [41]. We selected all three villages (desa) in the catchment area of Mekarsari primary health center. There is one village health post and 24 health-integrated posts under the center.
Fig. 2.
Map of the study area
Participants of the study
Our sample size was simply calculated by assuming an expected prevalence of antibiotic use of 25% with 5% precision and 5% level of significance. We also considered 5% as the non-response rate in the survey to have a target sample size of 300 children. We stratified listed children by age group (< 1 year, 1–2 years, and 3–4 years). The number of children in each stratum was proportionally allocated to the number of children registered in each post. We conducted a face-to-face household interview with a female caregiver, which was defined as a female household member who was responsible for providing medications to children. When the female caregiver was not around, we visited the household again until a maximum of three visits. If we could not meet the female caregiver within three visits, the alternative child in the list was included in the study. We identified and interviewed one female caregiver in each household when two or more female caregivers lived in the same household.
Data collection
A standardized questionnaire was developed before the survey, which consisted of two parts. The first part was for the female caregivers that included demographic characteristics, level of education, and household income, and the other part was for children that included illness history and antibiotic usage during an episode. The questionnaires and informed consent form were initially developed in English and then translated into local languages (Bahasa and Sundanese). The questionnaire was applied to the ESRI Survey 123 (ESRI Inc., Redlands, CA) for inputting data during the interview. Field staffs obtained informed consents from female caregivers before the interview. The regional minimum salary (Upah Minimum Regional/UMR) was used as a cut-off to assess the economic status of the household [42, 43]. We interviewed female caregivers about the presence of clinical signs among children within 30 days before the interview. The data included fever or feverish (fever), cough or difficulty of breathing (respiratory symptoms), and frequent loose or liquid stools (diarrhea). If there were more than one illness episodes within 30 days before the interview, we collected the information related to illness episode including antibiotics use only about the most recent episode.
We used three steps to verify antibiotic use and to identify the type of antibiotics provided to children. First, female caregivers were asked to show a drug package or bottle to the interviewers if available. Second, if package or bottle was not available, the photos of various antibiotics commonly used in the study area were shown to the female caregivers to identify the antibiotics that were provided. If the types of antibiotics were not verified using these steps, we asked them if they recalled the name of the drug. If the name of the drug mentioned by the female caregivers was that for antibiotics, we categorized it as might have used antibiotics. In the study area, children sometimes receive antibiotics in a crushed powder (puyer). If the crushed powder was provided, we checked the medical records at the health center or contacted to health professionals who prescribed them. If antibiotic use was confirmed based on the information obtained from the medical records or health professionals, we categorized them as verified use of antibiotics.
Data analysis
We excluded children from the analysis of antibiotic use if female caregivers stated that the child had any disease or condition that requires a regular medical follow-up, or was hospitalized because of the illness within the last 30 days. We defined health center as any health facility under the government system, including primary health center, village health post, and health-integrated post. Prevalence of antibiotic use was calculated as the proportion of the number of children who received antibiotics (both verified and possible antibiotics) to the number of children with clinical symptoms. The characteristics of children who used and did not use antibiotics and the female caregivers were compared using the chi-square test. The statistically significant level was set at less than 5% (p < 0.05). The association between antibiotic use and the type of health facility visited, clinical symptoms, age, and characteristics of the female caregivers were evaluated using logistic regression with generalized estimating equations to account for the effect of having the same female caregivers. Odds ratios were then adjusted with two variables that showed p value < 0.1 in the univariate analysis: age of children and living with extended families or not. Extended family was defined as a family that extends beyond the nuclear family to include other relatives. To test statistical significance, 95% confidence intervals (CI) were calculated. The analysis was performed using either IBM SPSS Statistics for Windows, Version 21.0 (IBM, Armonk, NY) or Microsoft R Open 3.5.1 (Microsoft and R core team, Redmond, WA) with geepack package.
Acknowledgements
We would like to thank the field team for their valuable work; and the head of Mekarsari primary health center and staffs for their technical support; and all midwives and cadres in Mekarsari for their kindness to support the study; and Minsarnawati Tanaca and Tri Bayu Purnama for helping us to create the map of the study area. First author expresses gratitude to Lembaga Pengelola Dana Pendidikan (LPDP), Ministry of Finance, The Republic of Indonesia for supporting PhD scholarship and this research.
Abbreviations
- AMR
Antimicrobial Resistance
- ARI
Acute Respiratory Infections
- AWaRe
Access, Watch, and Reserve
- IMCI
Integrated Management of Childhood Illness
- Poskesdes
Pos Kesehatan Desa
- Posyandu
Pos Pelayanan Terpadu
- Puskesmas
Pusat Kesehatan Masyarakat
- UMR
Upah Minimum Regional
- UNICEF
the United Nations International Children’s Emergency Fund
- WHO
World Health Organization
Authors’ contributions
RNA, HO, TK, FA, and DUI conceived and designed the study. RNA and TK developed data collection instruments and trained facilitators and staff involved in the study. RNA, TK, and HO analyzed the data, and RNA wrote the first draft of the manuscript. HO, TK, MS, FA, and DUI critically revised draft. All authors read and approved the final manuscript.
Funding
This study was supported by Lembaga Pengelola Dana Pendidikan (LPDP), Ministry of Finance, the Republic of Indonesia, and the Japan Society for the Promotion of Science (JSPS), Core-to-Core Program B, Asia-Africa Science Platforms. The funders did not play a role in the study design, data collection and analysis, or the preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by two ethics committees, including the Health Research and Development Bureau of the Ministry of Health of the Republic of Indonesia (No. LB.02.01/2/KE.174/2018) and Tohoku University Graduate School of Medicine (No. 2017-1-868). An explanation of the purpose of the study, voluntary involvement, and confidentiality was provided prior to the start of the study. Written informed consents were obtained from the participants during data collection.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Raihana Nadra Alkaff, Email: hanaalkaff@gmail.com.
Taro Kamigaki, Phone: +81-(0)22-717-8211, Email: kamigakit@med.tohoku.ac.jp.
Mayuko Saito, Email: msaitop@med.tohoku.ac.jp.
Fajar Ariyanti, Email: jaryanti06@yahoo.com.
Dewi Utami Iriani, Email: dhe_ut@yahoo.com.
Hitoshi Oshitani, Email: oshitanih@med.tohoku.ac.jp.
References
- 1.Mastro TD, Nomani NK, Ishaq Z, Ghafoor A, Shaukat NF, Esko E, et al. Use of nasopharyngeal isolates of Streptococcus pneumoniae and Haemophilus influenzae from children in Pakistan for surveillance for antimicrobial resistance. Pediatr Infect Dis J. 1993;12(10):824–830. doi: 10.1097/00006454-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Felmingham D, Farrell DJ, Reinert RR, Morrissey I. Antibacterial resistance among children with community-acquired respiratory tract infections (PROTEKT 1999-2000) J Infect. 2004;48(1):39–55. doi: 10.1016/S0163-4453(03)00140-3. [DOI] [PubMed] [Google Scholar]
- 3.Vila J, Vargas M, Casals C, Urassa H, Mshinda H, Schellemberg D, et al. Antimicrobial resistance of diarrheagenic Escherichia coli isolated from children under the age of 5 years from Ifakara, Tanzania. Antimicrob Agents Chemother. 1999;43(12):3022–3024. doi: 10.1128/AAC.43.12.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandomando I, Jaintilal D, Pons MJ, Valles X, Espasa M, Mensa L, et al. Antimicrobial susceptibility and mechanisms of resistance in Shigella and Salmonella isolates from children under five years of age with diarrhea in rural Mozambique. Antimicrob Agents Chemother. 2009;53(6):2450–2454. doi: 10.1128/AAC.01282-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah M, Kathiiko C, Wada A, Odoyo E, Bundi M, Miringu G, et al. Prevalence, seasonal variation, and antibiotic resistance pattern of enteric bacterial pathogens among hospitalized diarrheic children in suburban regions of central Kenya. Trop Med Health. 2016;44:39. doi: 10.1186/s41182-016-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757–762. doi: 10.1086/322627. [DOI] [PubMed] [Google Scholar]
- 7.Bojalil R, Calva JJ. Antibiotic misuse in diarrhea. A household survey in a Mexican community. J Clin Epidemiol. 1994;47(2):147–156. doi: 10.1016/0895-4356(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 8.Bounxou Keohavong MV, Phoummalaysith B, Louangpradith V, Inthaphatha S, Kariya T, Saw YM, Yamamoto E, Hamajima N. Antibiotic prescription for under-fives with common cold or upper respiratory tract infection in Savannakhet Province, Lao PDR. Trop Med Health. 2019;47:16. doi: 10.1186/s41182-019-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasrin D, Collignon PJ, Roberts L, Wilson EJ, Pilotto LS, Douglas RM. Effect of beta lactam antibiotic use in children on pneumococcal resistance to penicillin: prospective cohort study. BMJ. 2002;324(7328):28–30. doi: 10.1136/bmj.324.7328.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melander E, Ekdahl K, Jonsson G, Molstad S. Frequency of penicillin-resistant pneumococci in children is correlated to community utilization of antibiotics. Pediatr Infect Dis J. 2000;19(12):1172–1177. doi: 10.1097/00006454-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Rogawski ET, Platts-Mills JA, Seidman JC, John S, Mahfuz M, Ulak M, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ. 2017;95(1):49–61. doi: 10.2471/BLT.16.176123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desalegn AA. Assessment of drug use pattern using WHO prescribing indicators at Hawassa University Teaching and Referral Hospital, south Ethiopia: a cross-sectional study. BMC Health Serv Res. 2013;13:170. doi: 10.1186/1472-6963-13-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrajati R, Tilaqza A, Supardi S. Factors related to rational antibiotic prescriptions in community health centers in Depok City, Indonesia. J Infect Public Health. 2017;10(1):41–48. doi: 10.1016/j.jiph.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Xu J, Wang F, Wang B, Liu L, Hou W, et al. Overprescribing in China, driven by financial incentives, results in very high use of antibiotics, injections, and corticosteroids. Health Aff (Millwood) 2012;31(5):1075–1082. doi: 10.1377/hlthaff.2010.0965. [DOI] [PubMed] [Google Scholar]
- 16.Togoobaatar G, Ikeda N, Ali M, Sonomjamts M, Dashdemberel S, Mori R, et al. Survey of non-prescribed use of antibiotics for children in an urban community in Mongolia. Bull World Health Organ. 2010;88(12):930–936. doi: 10.2471/BLT.10.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekwochi U, Chinawa JM, Osuorah CD, Odetunde OI, Obu HA, Agwu S. The use of unprescribed antibiotics in management of upper respiratory tract infection in children in Enugu, South East Nigeria. J Trop Pediatr. 2014;60(3):249–252. doi: 10.1093/tropej/fmt111. [DOI] [PubMed] [Google Scholar]
- 18.Ocan M, Aono M, Bukirwa C, Luyinda E, Ochwo C, Nsambu E, et al. Medicine use practices in management of symptoms of acute upper respiratory tract infections in children (≤12 years) in Kampala city, Uganda. BMC Public Health. 2017;17(1):732. doi: 10.1186/s12889-017-4770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teck KC, Ghazi HF, Bin Ahmad MI, Binti Abdul Samad N, Ee Yu KL, Binti Ismail NF, et al. Knowledge, Attitude, and Practice of Parents Regarding Antibiotic Usage in Treating Children’s Upper Respiratory Tract Infection at Primary Health Clinic in Kuala Lumpur, Malaysia: Pilot Study. Health Serv Res Manag Epidemiol. 2016;3:2333392816643720. doi: 10.1177/2333392816643720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal S, Yewale VN, Dharmapalan D. Antibiotics Use and Misuse in Children: A Knowledge, Attitude and Practice Survey of Parents in India. J Clin Diagn Res. 2015;9(11):SC21–SC24. doi: 10.7860/JCDR/2015/14933.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding L, Sun Q, Sun W, Du Y, Li Y, Bian X, et al. Antibiotic use in rural China: a cross-sectional survey of knowledge, attitudes and self-reported practices among caregivers in Shandong province. BMC Infect Dis. 2015;15:576. doi: 10.1186/s12879-015-1323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadi U, Duerink DO, Lestari ES, Nagelkerke NJ, Werter S, Keuter M, et al. Survey of antibiotic use of individuals visiting public healthcare facilities in Indonesia. Int J Infect Dis. 2008;12(6):622–629. doi: 10.1016/j.ijid.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Hadi U, Duerink DO, Lestari ES, Nagelkerke NJ, Keuter M, Huis In’t Veld D, et al. Audit of antibiotic prescribing in two governmental teaching hospitals in Indonesia. Clin Microbiol Infect. 2008;14(7):698–707. doi: 10.1111/j.1469-0691.2008.02014.x. [DOI] [PubMed] [Google Scholar]
- 24.Hadi U, van den Broek P, Kolopaking EP, Zairina N, Gardjito W, Gyssens IC, et al. Cross-sectional study of availability and pharmaceutical quality of antibiotics requested with or without prescription (Over The Counter) in Surabaya, Indonesia. BMC Infect Dis. 2010;10:203. doi: 10.1186/1471-2334-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widayati A, Suryawati S, de Crespigny C, Hiller JE. Self medication with antibiotics in Yogyakarta City Indonesia: a cross sectional population-based survey. BMC Res Notes. 2011;4:491. doi: 10.1186/1756-0500-4-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parathon H, Kuntaman K, Widiastoety TH, Muliawan BT, Karuniawati A, Qibtiyah M, et al. Progress towards antimicrobial resistance containment and control in Indonesia. BMJ. 2017;358:j3808. doi: 10.1136/bmj.j3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farida H, Severin JA, Gasem MH, Keuter M, Wahyono H, van den Broek P, et al. Nasopharyngeal carriage of Streptococcus pneumonia in pneumonia-prone age groups in Semarang, Java Island, Indonesia. PLoS One. 2014;9(1):e87431. doi: 10.1371/journal.pone.0087431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadinegoro SR, Prayitno A, Khoeri MM, Djelantik IG, Dewi NE, Indriyani SA, et al. Nasopharyngeal carriage of streptococcus pneumoniae in healthy children under five years old in central Lombok Regency, Indonesia. Southeast Asian J Trop Med Public Health. 2016;47(3):485–493. [PubMed] [Google Scholar]
- 29.Statistics Indonesia-Badan Pusat Statistik-BPS, National Population and Family Planning Board-BKKBN/Indonesia, Kementerian Kesehatan-Kemenkes-Ministry of Health/Indonesia, and ICF International . Indonesia Demographic and Health Survey 2012. 2013. [Google Scholar]
- 30.World Heath Organization. Revised WHO classification and treatment of childhood pneumonia at health facilities. [https://www.who.int/maternal_child_adolescent/documents/child-pneumonia-treatment/en/] Accessed on 8 Mar 2018.
- 31.Ministry of Health . Chart Book: Integrated Management of Childhood Illnesses (Indonesian) 2015. [Google Scholar]
- 32.Ahmed MU, Baquilod M, Deola C, Tu ND, Anh DD, Grasso C, et al. Cholera prevention and control in Asian countries. BMC Proc. 2018;12(Suppl 13):62. doi: 10.1186/s12919-018-0158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agtini MD, Soeharno R, Lesmana M, Punjabi NH, Simanjuntak C, Wangsasaputra F, et al. The burden of diarrhoea, shigellosis, and cholera in North Jakarta, Indonesia: findings from 24 months surveillance. BMC Infect Dis. 2005;5:89. doi: 10.1186/1471-2334-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ministry of Health . Decree of MoH Republic of Indonesia no. 2406/ MENKES/PER/XII/2011 on General Guidelines on the use of Antibiotic (Indonesian) 2011. [Google Scholar]
- 35.Titaley CR, Jusril H, Ariawan I, Soeharno N, Setiawan T, Weber MW. Challenges to the implementation of the integrated management of childhood illness (IMCI) at community health centres in West Java province, Indonesia. WHO South East Asia J Public Health. 2014;3(2):161–170. doi: 10.4103/2224-3151.206732. [DOI] [PubMed] [Google Scholar]
- 36.Sharland M, Pulcini C, Harbarth S, Zeng M, Gandra S, Mathur S, et al. Classifying antibiotics in the WHO Essential Medicines List for optimal use-be AWaRe. Lancet Infect Dis. 2018;18(1):18–20. doi: 10.1016/S1473-3099(17)30724-7. [DOI] [PubMed] [Google Scholar]
- 37.Statistics Lebak, Badan Pusat Statistik Lebak . Lebak Regency Regional Statistics (Indonesian) 2017. [Google Scholar]
- 38.Abdullah A, Hort K, Abidin AZ, Amin FM. How much does it cost to achieve coverage targets for primary healthcare services? A costing model from Aceh, Indonesia. Int J Health Plann Manage. 2012;27(3):226–245. doi: 10.1002/hpm.2099. [DOI] [PubMed] [Google Scholar]
- 39.National Research Council . Reducing Maternal and Neonatal Mortality in Indonesia: Saving Lives, Saving the Future. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 40.Ministry of Health . Technical guidelines for the development and implementation of village health posts (Indonesian) 2012. [Google Scholar]
- 41.Ministry of Health . Let’s go to the Posyandu Every Month: Posyandu Cadre Handbook (Indonesian) 2012. [Google Scholar]
- 42.Ministry of Finance . Government Regulation No.78 of 2015 concerning Wages (Indonesian) 2015. [Google Scholar]
- 43.Government of Banten Province . Banten Governor Decree Number: 561/Kep-442-Huk/2017 concerning Establishment of district/city minimum wages in Banten province (Indonesian) 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.