To the Editor:
Bronchiolitis is the leading cause of hospitalizations in U.S. infants.1 In addition to the acute morbidity, cohort studies have also shown that 30%−40% of infants hospitalized for bronchiolitis (severe bronchiolitis) develop childhood asthma.1 Particularly, early life infection with rhinovirus (RV) – the second most common pathogen of bronchiolitis – is associated with an increased risk of childhood asthma.1 Yet, the mechanism through which RV raises asthma risk in infants is largely unknown. Experimental models and human (cross-sectional and retrospective) studies have reported that RV infection may induce type 2 cytokines (e.g., interleukin [IL]-4, IL-5, IL-13, thymic stromal lymphopoietin [TSLP]) and that the levels of these cytokines are elevated in the asthmatic airway.2–4 However, no prospective study has investigated the longitudinal relation of type 2 airway inflammation in children – let alone infants with bronchiolitis – to the development of childhood asthma. To address this knowledge gap, we prospectively examined the association of nasopharyngeal cytokines in infants with RV bronchiolitis with the risk of developing childhood asthma, by using data from a multicenter cohort of infants with severe bronchiolitis.
Details of the study design, samples, measurement, and analysis may be found in the Online Supplement (Supplemental Methods). Briefly, this multicenter prospective cohort study, the 35th Multicenter Airway Research Collaboration (MARC-35),5,6 enrolled 1,016 infants (aged <12 months) hospitalized for bronchiolitis at 17 sites across 14 US states (Table E1) during the 2011–2014 winter seasons. Bronchiolitis was defined by the American Academy of Pediatrics guidelines.7 In addition to the phenotypic data measurement via structured interview and medical record review, nasopharyngeal airway samples were collected within 24 hours of hospitalization using a standardized protocol.5,6 Levels of 10 nasopharyngeal cytokines (IL-4, IL-5, IL-10, IL-12, IL-13, IL-25, IL-33, interferon [IFN]-β, macrophage inflammatory protein[MIP]-1α, and TSLP) were quantified using electrochemiluminescence immunoassays. Respiratory viruses were tested using real-time PCR assays for RV and respiratory syncytial virus (RSV). To quantify the relative RV genomic load, cycle threshold (CT) values – the number of amplification cycles needed for a positive PCR test result – were used. The primary outcome was asthma at age 4 years based on a commonly-used epidemiologic definition – i.e., physician diagnosis of asthma plus either asthma medication use or asthma-related symptoms in the past year.8 For the current study, we analyzed 132 infants with RV bronchiolitis who underwent nasopharyngeal cytokine measurement. To examine the association of exposures (cytokine levels and RV genomic load) with asthma, we used generalized linear mixed-effects models, adjusting for potential confounders (age, sex, parental history of asthma, breathing problems prior to enrollment, IgE sensitization [aeroallergens or food], and virology [solo RV, RV/RSV coinfection]) and hospital-level clustering. As the models indicated statistically significant virology–exposure interactions, we then stratified the analysis by virology.
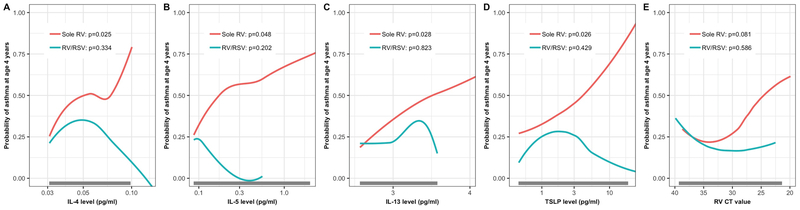
Of 132 infants with severe RV bronchiolitis, the median age was 4 (IQR 2–6) months, 64% were male, 40% were non-Hispanic white, and 55% had RSV coinfection. Asthma was observed in 30% of children at age 4 years (Table 1 and E2). There were statistically-significant interactions between virology and six cytokine levels (IL-4, IL-5, IL-13, IFN-β, MIP-1α, and TSLP) on the asthma risk (Pinteraction<0.05), indicating that exposure-asthma associations differ between solo RV infection and RV/RSV coinfection. Indeed, in infants with solo RV infection, the cytokine levels (IL-4, IL-5, and TSLP) and RV genomic load significantly differed between those with and without asthma (unadjusted P<0.05; Table E3) while there were no differences in those with RV/RSV (unadjusted P>0.05). Heatmap (Figure E1) also showed that distributions of these type 2 cytokines differed by virology and outcome. In the adjusted analysis (Figures 1 and E2), only infants with solo RV bronchiolitis had significant associations of higher type 2 cytokine levels (IL-4, IL-5, IL-13, and TSLP) with an increased risk of asthma (adjusted P<0.05). In the sensitivity analysis using normalized cytokine levels and the subgroup analysis excluding infants with a breathing problem prior to enrollment, the results were similar (Figures E3 and E4).
Table 1.
Characteristics and clinical presentation of 132 infants hospitalized for rhinovirus bronchiolitis, by asthma status at age 4 years
| No asthma | Asthma | P-value | |
|---|---|---|---|
| Variables | n=92 (70%) | n=40 (30%) | |
| Characteristics at hospitalization for bronchiolitis | |||
| Age (mo), median, (IQR) | 4 (2–6) | 5 (3–9) | 0.02 |
| Male sex | 55 (60) | 29 (72) | 0.23 |
| Race/ethnicity | 0.21 | ||
| Non-Hispanic white | 42 (46) | 11 (28) | |
| Non-Hispanic black | 20 (22) | 14 (35) | |
| Hispanic | 27 (29) | 14 (35) | |
| Other | 3 (3) | 1 (3) | |
| Parental history of asthma | 29 (32) | 22 (55) | 0.02 |
| Parental history of eczema | 14 (15) | 13 (33) | 0.04 |
| Maternal smoking during pregnancy | 11 (12) | 4 (10) | 0.60 |
| Mode of birth (c-section) | 24 (26) | 14 (35) | 0.40 |
| Prematurity (32–37 weeks) | 19 (21) | 10 (25) | 0.75 |
| Previous breathing problems before the index hospitalization* | 20 (22) | 17 (43) | 0.03 |
| History of eczema | 10 (11) | 11 (28) | 0.03 |
| Ever attended daycare | 28 (30) | 13 (33) | 0.98 |
| Household sibling | 74 (80) | 35 (88) | 0.46 |
| Breastfed | 42 (46) | 21 (53) | 0.64 |
| Smoke exposure at home | 13 (14) | 2 (5) | 0.22 |
| Corticosteroid use in lifetime | 11 (12) | 16 (40) | 0.001 |
| Presentation and course at hospitalization for bronchiolitis | |||
| Weight at presentation (kg), median (IQR) | 6.2 (4.9–7.7) | 7.4 (5.7–8.8) | 0.02 |
| Laboratory testing | |||
| Coinfection with RSV | 58 (63) | 15 (38) | 0.01 |
| Blood eosinophil count/ul, median (IQR) | 170 (70–30) | 100 (20–200) | 0.16 |
| Serum total 25OHD (ng/ml), median (IQR) | 24.8 (16.9–31.2) | 26.7 (18.5–34.0) | 0.51 |
| Serum LL-37 (ng/ml), median (IQR) | 54.0 (40–70) | 62.0 (42.0–75.0) | 0.29 |
| sIgE sensitization† | 9 (10) | 10 (25) | 0.04 |
| Food sensitization | 9 (10) | 10 (25) | 0.04 |
| Aeroallergen sensitization | 1 (1) | 0 (0) | 0.99 |
| Clinical course | |||
| Intensive care use‡ | 12 (13) | 5 (13) | 0.99 |
| Use of mechanical ventilation | 2 (2) | 2 (5) | 0.75 |
| Chronic outcome | |||
| Recurrent wheeze by age 3 years§ | 27 (29) | 31 (78) | <0.001 |
Data are no. (%) of infants unless otherwise indicated. Percentages may not equal 100, because of rounding and missingness.
Abbreviations: IQR, interquartile range; RSV, respiratory syncytial virus; sIgE, specific immunoglobulin E; 25OHD, 25-hydroxyvitamin D.
Defined as an infant having cough that wakes him/her at night and/or causes emesis, or when the infant has wheezing or shortness of breath without cough before the index hospitalization
Defined by having one or more positive values for serum allergen-specific IgE at the index hospitalization
Defined as admission to intensive care unit and/or use of mechanical ventilation (continuous positive airway pressure and/or intubation during inpatient stay, regardless of location) at any time during the index hospitalization
Defined as having at least two corticosteroid-requiring exacerbations in six months or having at least four wheezing episodes in one year that last at least one day and affect sleep
Figure 1. Association of nasopharyngeal type 2 cytokine levels and rhinovirus genomic load in infants hospitalized for bronchiolitis with risks of asthma at age 4 years, according to virology.
The fitted lines represent locally estimated scatterplot smoothed (loess) curves for infants with solo RV infection and those with RV/RSV coinfection. Only in infants with solo bronchiolitis, there were significant associations of nasopharyngeal A) IL-4, B) IL-5, C) IL-13, D) TSLP levels and E) RV genomic load (lower cycle threshold [CT] value indicates higher genomic loads) with a significantly higher risk of asthma at age 4 years with the use of generalized linear mixed-effects model. The grey bar represents the range in which 95% of data are present. The results of all cytokines are presented in Figures E2 and E3 (Online Supplement).
Abbreviations: IL, interleukin; RSV, respiratory syncytial virus; RV, rhinovirus; TSLP, thymic stromal lymphopoietin.
These findings are concordant with previous cross-sectional and retrospective studies that suggested potential interrelations between RV infection, type 2 cytokines (e.g., IL-4, IL-5, IL-13, TSLP), and asthmatic airway inflammation.2–4 The current prospective study builds on these earlier reports, and extends them by demonstrating the prospective association between type 2 cytokine levels in the airway of infants with solo RV bronchiolitis and the risk of developing asthma. The mechanisms underlying these findings warrant further investigation. It is possible that severe RV infection is an early marker of TH2 bias in predisposed infants, which leads to augmented RV infection and replication.3,4 Yet, our data also showed no significant correlations between serum total IgE levels and nasopharyngeal cytokine levels (Table E4). Alternatively, the association may be causal—i.e., severe RV bronchiolitis modulates host airway response towards type 2 inflammation and damages the airways during early infancy – a crucial period of lung development.1 Furthermore, these possibilities are not mutually exclusive and may jointly contribute to disrupted epithelial barrier, exaggerated inflammation, and airway injury, thereby leading to the development of asthma. Notwithstanding the complexity, our data, in conjunction with earlier studies, provide an evidence base for early identification of children at high risk for asthma, as well as the development of targeted prevention strategies (e.g., immunomodulators).
Our study has potential limitations. First, bronchiolitis involves inflammation of the lower airways in addition to the upper airways. Although our study was based on the nasopharyngeal samples, studies have shown that upper airway sampling provides reliable representation of the lung inflammatory profiles.9 Second, the study did not have information from “healthy controls”. However, the objective was to examine the role of RV infection and host response on the development of asthma among infants with severe bronchiolitis. Third, we used an epidemiologic definition of asthma at age 4 years while diagnosis of asthma at this age can be challenging. To address this important point, the study population is currently being followed longitudinally up to age 6 years. Finally, although MARC-35 samples consist of racially/ethnically- and geographically-diverse US sample, our findings may not be generalizable beyond infants with severe RV bronchiolitis (e.g., infants with solo RSV infection). Regardless, bronchiolitis accounts for 130,000 hospitalizations annually, with RV being the second most common causative virus.1 Additionally, further understanding of infants at highest risk could better delineate the mechanisms of asthma development in larger populations.
In summary, higher type 2 cytokine (IL-4, IL-5, IL-13, and TSLP) levels in the airway of infants with solo RV infection were associated with a greater risk of developing childhood asthma. Our data should advance research not only into understanding the mechanisms linking early RV infection to incident asthma but also into developing potential strategies for asthma prevention.
Supplementary Material
ACKNOWLEDGMENTS
We thank the MARC-35 study hospitals and research personnel for their ongoing dedication to bronchiolitis and asthma research (Table E1 in the Online Supplement). We also thank Janice A. Espinola, MPH, and Ashley F. Sullivan, MS, MPH (Massachusetts General Hospital, Boston, MA), as well as Alkis Togias, MD (National Institute of Allergy and Infectious Diseases) for their contributions to the study.
Financial Support: This work was supported by grants UG3/UH3 OD-023253, U01 AI-087881, R01AI-114552, R01 AI-134940, R01 AI-137091, and R21 HL-129909 from the National Institutes of Health (Bethesda, MD). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: Dr. Hasegawa received asthma-related research grants from Novartis and Teva, unrelated to the current work. Dr. Celedón received research materials from GSK and Merck (inhaled steroids) and Pharmavite (vitamin D and placebo capsules) to provide medications free of cost to participants in NIH-funded studies, unrelated to the current work. The other authors have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Hasegawa K, Mansbach JM, Camargo CA Jr. Infectious pathogens and bronchiolitis outcomes. Exp Rev Anti Infect Ther. 2014;12(7):817–828. [DOI] [PubMed] [Google Scholar]
- 2.Custovic A, Belgrave D, Lin L, et al. Cytokine responses to rhinovirus and development of asthma, allergic sensitization, and respiratory infections during childhood. Am J Respir Crit Care Med. 2018;197(10):1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi GA, Colin AA. Infantile respiratory syncytial virus and human rhinovirus infections: respective role in inception and persistence of wheezing. Eur Respir J. 2015;45(3):774–789. [DOI] [PubMed] [Google Scholar]
- 4.Vandini S, Calamelli E, Faldella G, Lanari M. Immune and inflammatory response in bronchiolitis due to respiratory Syncytial Virus and Rhinovirus infections in infants. Paediatr Respir Rev. 2017;24:60–64. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa K, Stewart CJ, Celedon JC, Mansbach JM, Tierney C, Camargo CA Jr. Circulating 25-hydroxyvitamin D, nasopharyngeal airway metabolome, and bronchiolitis severity. Allergy. 2018;73(5):1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart CJ, Mansbach JM, Wong MC, et al. Associations of nasopharyngeal metabolome and microbiome with severity among infants with bronchiolitis: A multi-omic analysis. Am J Respir Crit Care Med. 2017;196(7):882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474–1502. [DOI] [PubMed] [Google Scholar]
- 8.Camargo CA Jr., Ingham T, Wickens K, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127(1):e180–187. [DOI] [PubMed] [Google Scholar]
- 9.Poole A, Urbanek C, Eng C, et al. Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. J Allergy Clin Immunol. 2014;133(3):670–678 e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.