Summary
The ability of the plant pathogen Xanthomonas campestris pv. campestris (Xcc) to cause disease is dependent on its ability to adapt quickly to the host environment during infection. Like most bacterial pathogens, Xcc has evolved complex regulatory networks that ensure expression and regulation of their virulence genes. Here, we describe the identification and characterization of a Fis‐like protein (named Flp), which plays an important role in virulence and type III secretion system (T3SS) gene expression in Xcc. Deletion of flp caused reduced virulence and hypersensitive response (HR) induction of Xcc and alterations in stress tolerance. Global transcriptome analyses revealed the Flp had a broad regulatory role and that most T3SS HR and pathogenicity (hrp) genes were down‐regulated in the flp mutant. β‐glucuronidase activity assays implied that Flp regulates the expression of hrp genes via controlling the expression of hrpX. More assays confirmed that Flp binds to the promoter of hrpX and affected the transcription of hrpX directly. Interestingly, the constitutive expression of hrpX in the flp mutant restored the HR phenotype but not full virulence. Taken together, the findings describe the unrecognized regulatory role of Flp protein that controls hrp gene expression and pathogenesis in Xcc.
Keywords: Fis‐like protein, hrpX promoter, type III secretion system, virulence, Xanthomonas
Introduction
Xanthomonads are Gram‐negative bacteria that are known to cause disease in a range of important crops worldwide. Xanthomonas campestris pv. campestris (Xcc) is one of the best studied of these as it is the causative agent of black rot disease in crucifers, which include many important vegetable brassica crops (Swings and Civerolo, 1993; Vicente and Holub, 2013). Xcc is also an important model for the study of microbe–plant interactions because of its genetic tractability and cultivability. For these reasons, the study of Xcc has provided a lot of insight into how plant pathogens can adapt to the host environment and cause disease. Studies have revealed many mechanisms that are important for disease and environmental adaptation, including extracellular enzymes (protease, mannanase, etc.), extracellular polysaccharides (EPS), diffusible signal factor (DSF)‐dependent cell–cell signalling, and proteins secreted by the type II secretion system (T2SS), type III secretion system (T3SS), type IV secretion system (T4SS) and more recently type VI secretion system (T6SS) (He and Zhang, 2008; Büttner and Bonas, 2010; Ryan et al., 2011, 2015; Notti and Stebbins, 2016; Zhou et al., 2017). In Xcc, one of the best‐studied mechanisms that contributes to virulence is the T3SS apparatus. This complex system is encoded by over 20 hypersensitive response and pathogenicity (hrp) genes that when silenced lead to the loss of Xcc's ability to cause full virulence and hypersensitive response (HR) induction (Alfano and Collmer, 1997; Lindgren, 1997). The activation of hrp genes, as well as some genes that encode secreted effector proteins, is controlled by two main regulators: HrpG (OmpR family regulator) and HrpX (AraC‐type transcriptional activator) (Huang et al., 2009; Koebnik et al., 2006; Wengelnik et al., 1996; Wengelnik and Bonas, 1996).
Several other proteins have been shown to control the expression of hrp genes. HpaS, a sensor kinase that putatively constitutes a two‐component signal transduction system with HrpG, positively regulates the expression of hrp genes (Li et al., 2014). The zinc uptake regulator (Zur), a key regulator of zinc homeostasis belonging to the Fur family of transcription factors, positively regulates the hrp gene expression via HrpX (Huang et al., 2009). HpaR1 (hrp‐associated regulator), a global regulator belonging to the YtrA subfamily of the GntR family, appears to indirectly regulate the expression of hrp genes via HrpG (An et al., 2011). In addition, HpaP, a novel regulatory protein with ATPase and phosphatase activity, regulates the expression of hrp genes by controlling the expression of hrpX. However, this is unlikely to be by a direct action (Cui et al., 2018).
Despite these advances, considerable work is still required to understand the regulatory mechanisms associated with control of T3SS and virulence in this important bacterial plant pathogen. In the present study, we report the identification and characterization of a Fis‐like protein (Flp), a previously unreported protein regulator of hrp and other virulence determinants in Xcc. We demonstrate that this protein contributes to virulence, HR induction and a series of other cellular functions in Xcc. We present evidences that Flp is likely to regulate the expression of hrp genes by controlling the expression of hrpX via directly binding to its promoter.
Results
Flp is important for pathogenicity in Xcc
In our earlier work aimed at establishing a general view of the factors that contribute to pathogenicity in Xcc, we screened a series of mutants which were constructed using a suicide vector (pK18mob) strategy (Windgassen et al., 2000). Virulence assays using the host plant cabbage (Brassica oleracea) showed that one of these mutants, 0520nk (Table S1), caused weaker symptoms of disease when compared with the wild‐type Xcc strain. The 0520nk mutant had a disrupted gene which corresponded to open reading frame (ORF) XC_0520 in the genome of Xcc strain 8004 (accession number CP000050). The XC_0520 gene encodes a putative DNA‐binding protein, which on further analysis with the SMART (Simple Modular Architecture Research Tool) program (http://smart.embl-heidelberg.de) has 40% identity with the Fis (factor for inversion stimulation) protein that is found in many other bacteria, including Pseudomonas aeruginosa, Escherichia coli and Yersinia pseudotuberculosis (Fig. S1).
In order to explore the detailed function of XC_0520 (herein Flp [Fis‐like protein]) in Xcc, we constructed a clean deletion removing the flp gene and designating the strain Δflp (see experimental procedures). Simultaneously, a complemented strain was constructed by introducing the recombinant plasmid, which carried the flp coding sequence, into the mutant Δflp. The resulting complemented strain was named CΔflp (Table S1). To confirm that deletion of flp influences the virulence in Xcc, the newly constructed strains were tested by inoculating into cabbage (B. oleracea) using leaf clippings (see Experimental procedures). Consistent with our previous findings, Δflp induced a significantly shorter lesion length compared with wild‐type (P < 0.05 by Student's t‐test) (Fig. 1). Importantly, the complementary strain CΔflp showed similar virulence symptoms (lesion length) to wild‐type (Fig. 1).
Figure 1.
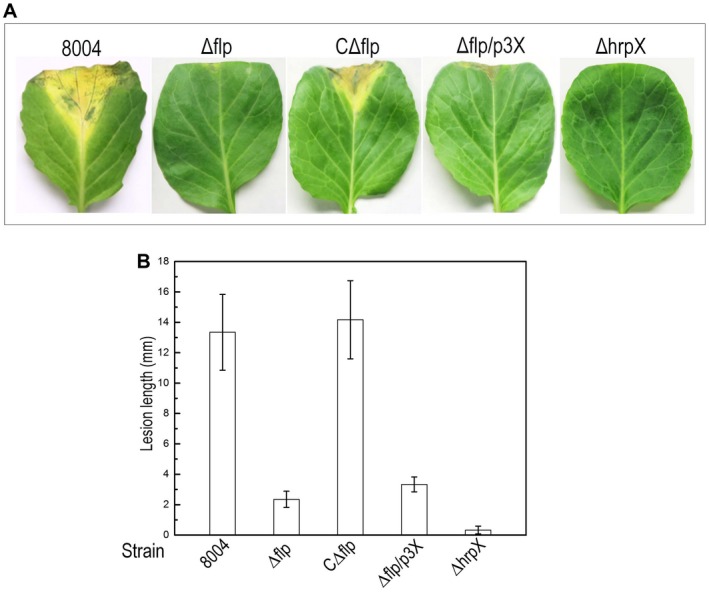
Flp plays a role in virulence in Xanthomonas campestris pv. campestris (Xcc). The Xcc wild‐type strain 8004, flp deletion mutant Δflp, complemented strains CΔflp and Δflp/p3X (Δflp constitutively expressing hrpX), and hrpX deletion mutant ΔhrpX (negative control) were cultured in NYG medium overnight and then adjusted to 1 × 106 CFU/mL in sterile distilled water. 30‐day‐old cabbage (Brassica oleracea 'Jingfeng No.1') was inoculated with bacterial suspensions of different Xcc strains by the leaf‐clipping method. (A) Infected cabbage leaves with Xcc strains showing symptoms as lesions 10 days post‐inoculation. (B) Lesion lengths were scored 10 days post‐inoculation. Values for means and standard deviation (SD) from 30 inoculated leaves in one experiment are indicated. The experiment was repeated three times.
Flp influences the regulation of extracellular polysaccharide, motility, stress tolerance and extracellular enzymes production
To explore if Flp manipulated specific functions that are known to be required for pathogenesis in Xcc, we conducted a series of basic phenotypic tests to assess extracellular polysaccharide (EPS) production, extracellular enzymes (cellulase and amylase), cell motility and the adaption to stress and antimicrobials.
The results showed that the flp‐mutant strain Δflp displayed decreased EPS production (Fig. 2A) and motility (swimming and swarming, tested on 0.28% w/v agar plates and 0.6% w/v agar plates, respectively) (Fig. 2B). However, EPS production and motility of the CΔflp complemented strain were similar to the wild‐type under the conditions tested (Fig. 2A,B).
Figure 2.
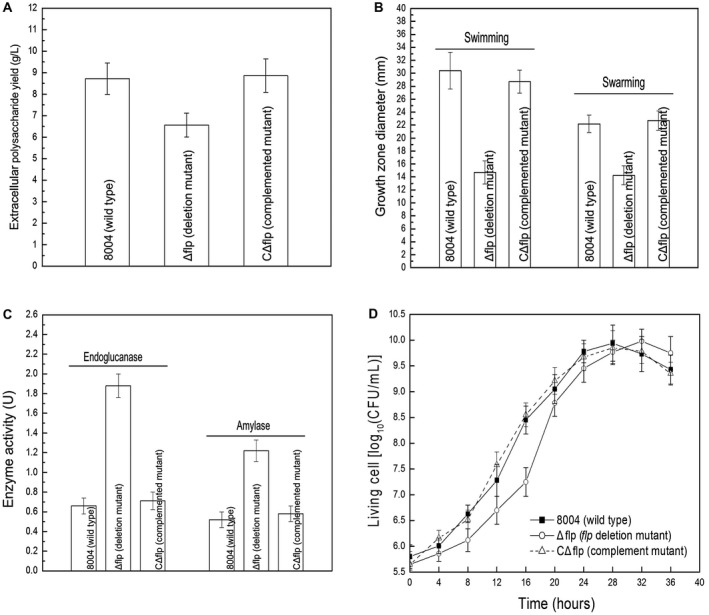
Flp positively regulates EPS production and motility, and negatively regulates the activities of extracellular enzymes in Xanthomonas campestris pv. campestris (Xcc). (A) The flp‐mutant Δflp produced significantly less EPS (P < 0.05 by Student's t‐test) compared to the wild‐type strain. Mean weight of EPS extracted from the Δflp mutant, the wild‐type strain and the CΔflp complemented strain. (B) Mean measurements of colony diameters of Xcc strains on ‘swim’ (0.28% agar) medium and ‘swarm’ (0.6% agar) medium after 3 and 4 days' incubation at 28 °C, respectively. Data are shown as mean ± SD (standard deviation). (C) Mean relative quantity of extracellular endoglucanase (cellulase) and amylase in Xcc strains inoculated into 100 mL NY medium. (D) Growth of Xcc strains in complex media. The strains were inoculated into NYG medium with the same final density of 0.01 (OD600). Growth of the strains was recorded at intervals of 4 h.
When extracellular enzymes in the Xcc wild‐type strain, Δflp mutant strain and CΔflp complemented strain were compared, positive differences were seen. The results show that the Δflp mutant exhibits a significant enhancement in cellulase and amylase secretion (P < 0.05 by Student's t‐test) (Fig. 2C). Moreover, enhancement in the activity of extracellular enzymes could be restored to wild‐type levels in the CΔflp complemented strain (Fig. 2C).
The growth characteristics of the Xcc strains in liquid medium nutrient‐yeast‐glycerol (NYG) were also investigated. Results revealed that the flp‐mutant Δflp displayed small changes in growth properties. The mutant had a reduced growth rate compared to that of the wild‐type strain in the early exponential phase (Fig. 2D). However, the growth rate was recovered in the mid‐exponential phase and the Δflp mutant grew with a rate exceeded that of the wild‐type in the late exponential phase.
Additionally, differences were seen when the wild‐type strain, the Δflp mutant and the CΔflp complemented strain were assessed for their ability to adapt to environmental stresses (Fig. 3). For these experiments, kill curve assays were used in which the quantity of living cells on agar plates were supplied with various different concentrations of environmental stresses, including sodium dodecyl sulphate (SDS), H2O2, NaCl, phenol and heavy metal salt CdCl2 and CuSO4 (see Experimental procedures). These tests demonstrated that compared to the wild‐type, the Δflp mutant showed reduced survival in the presence of phenol, CdCl2, CuSO4 and SDS but not H2O2 and NaCl (Fig. 3). Importantly, in all cases the complementation strain responded similarly to the wild‐type (Fig. 3).
Figure 3.
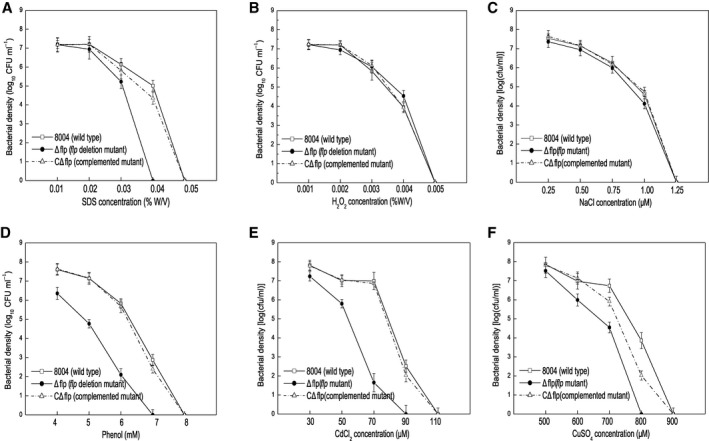
Flp is required for stress tolerance in Xanthomonas campestris pv. campestris (Xcc). Survival experiments performed by subculturing strains overnight on fresh NYG agar plates supplemented with different concentrations of SDS (A), oxidant H2O2 (B), hyperosmosis NaCl (C), phenol (D) and heavy metal salts CdCl2 (E) and CuSO4 (F). The surviving bacterial colonies on the plates were counted after incubation for 3 days.
Taken together, these findings suggest that Flp regulates positively the EPS production, motility and some stress tolerance but appears to negatively regulate extracellular enzymes in Xcc under the conditions tested. Despite these observations, the mechanism of regulation by Flp in these cases remains enigmatic.
Flp has an influence over the expression of genes involved in virulence and various adaptation processes in Xcc
To gain a greater understanding of the regulatory role of Flp in Xcc a set of global gene expression profiles was generated using RNA‐Seq. For this, selected Xcc strains were grown to the mid‐exponential phase (OD600 = 0.6) in XVM2, a medium that mimics more closely the nutrition environment of the plant (Astua‐Monge et al., 2005). Following bacterial RNA extraction, library construction and sequencing, differential gene expression analysis was conducted on the generated data (see Experimental procedures). Of the 4273 annotated genes from the genome of Xcc strain 8004, 279 genes presented differentially expressed (fold changes ≥ 2.0), with 121 genes up‐regulated and 158 genes down‐regulated according to the transcriptome data (Fig. 4A, Table S2). In order to verify the transcriptome data, 16 genes that showed changes were selected randomly and reverse transcription‐polymerase chain reaction (RT‐PCR) was performed to examine the relative expression levels of these genes. All selected genes showed expression changes that were comparable with the transcriptome data (Table 1).
Figure 4.
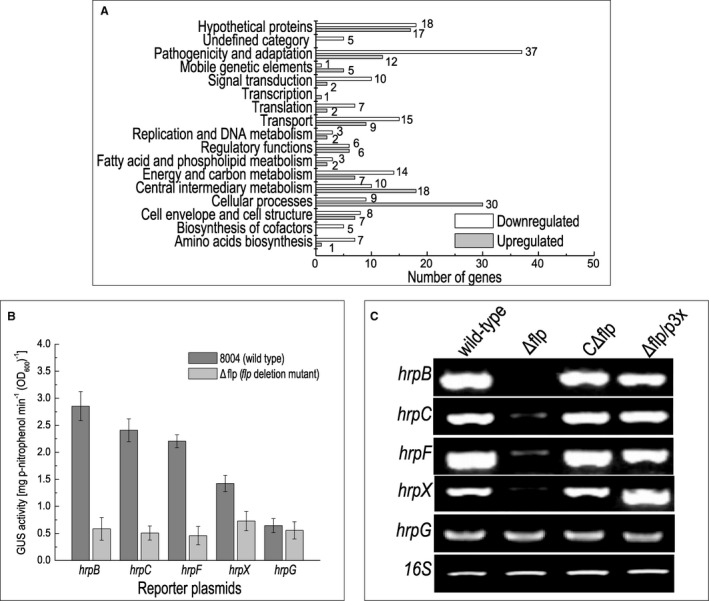
Flp is a global regulatory protein that affects the expression of a number of genes, including T3SS genes. (A) Functional categories of differential expressed genes in Δflp mutant. Genome‐scale transcriptome profiling of Xanthomonas campestris pv. campestris (Xcc) strains cultured in XVM2 were investigated by RNA sequencing, and 279 genes were found differentially expressed by two‐fold or more in Δflp mutant (Table S2). These genes were broadly categorized according to their biological function (He et al., 2007). Notably, due to cross‐talk between different metabolism pathways, some genes might be counted into different categories simultaneously. (B) ß‐glucuronidase (GUS) activity of hrp gene promoter‐gusA reporters in Δflp mutation and wild‐type backgrounds. Data shown are mean and standard deviation of triplicate measurements. The experiment was repeated twice, and similar results were obtained. (C) The expression levels of hrp genes in Xcc wild‐type strain 8004, Δflp, CΔflp and Δflp/p3X as measured by semi‐quantitative RT‐PCR.
Table 1.
Confirmation of RNA‐Seq gene expression data by semi‐quantitative RT‐PCR.
| ID | Gene | Description | Expression level |
Semi RT‐PCR WT/Δflp |
|---|---|---|---|---|
| XC_3001 | hpa2 | Lysozyme‐related protein Hpa2 | 2.97↓ |

|
| XC_2324 | c‐di‐GMP phosphodiesterase A | 4.33↓ |

|
|
| XC_3657 | copB | Copper resistance protein B | 2.58↓ |

|
| XC_3129 | pmrC | Inner membrane protein | 3.59↓ |

|
| XC_3597 | hns | DNA‐binding protein | 3.03↓ |

|
| XC_3437 | lptD | LPS‐assembly protein | 2.29↓ |

|
| XC_2004 | avrXccC | Avirulence protein | 8.31↓ |

|
| XC_3694 | ompW | Outer membrane protein | 2.59↓ |

|
| XC_2827 | hpaR | MarR family transcriptional regulator | 2.25↓ |

|
| XC_0158 | Xylosidase/arabinosidase | 2.51↓ |

|
|
| XC_2659 | gcd | Quinoprotein glucose dehydrogenase | 7.31↓ |

|
| XC_1273 | trkA | Voltage‐gated potassium channel | 2.36↓ |

|
| XC_1314 | lptC | Lipopolysaccharide export system protein LptC | 2.51↓ |

|
| XC_3652 | fabB | β‐ketoacyl‐[ACP] synthase | 2.05↑ |

|
| XC_0783 | celS | Cellulase S | 2.51↑ |

|
| 16S rRNA | Internal reference |

|
16 genes of the transcriptome data were chosen to validate the integral accuracy via semi‐quantitative RT‐PCR. RNA, extracted from the cultures of Xanthomonas campestris pv. campestris wild‐type (8004) and Δflp, respectively, was reverted into cDNA, and then cDNA was used as the template in semi‐quantitative PCR. In this study, false discovery rate (FDR) ≤ 0.05 and absolute value of log2 fold change ≥ 1 were used as the cut‐off values. The acquired results were accordant to the transcriptome data.↑, up‐regulated; ↓, down‐regulated.
Functional clustering analysis, according to the annotation of Xcc strain 8004 genome (Qian et al., 2005), was carried out where the majority of the 279 genes regulated by Flp were assigned to the functional categories ‘pathogenicity and adaption’, ‘cellular process’, ‘central intermediary metabolism’, ‘transport’, ‘energy and carbon metabolism’, ‘cell envelope and cell structure’, ‘regulatory functions’ and ‘signal transduction’. The remaining genes were predicted to encode hypothetical proteins or have not been given a functional category to date (Fig. 4A; Table S2). The most dominant functional categories which genes were assigned to were ‘pathogenicity and adaption’ and ‘cellular processes’ (Fig. 4A). Notably, the deletion of Flp had an impact on genes belonging to the type III secretion system (T3SS). The expression of 16 hrp genes, XC_3003 (hrcC), XC_3004 (hrcT), XC_3006 (hrcN), XC_3007 (hrpB5), XC_3009 (hrcJ), XC_3010 (hrpB2), XC_3012 (hrcU), XC_3013 (hrcV), XC_3015 (hrcQ), XC_3016 (hrcR), XC_3018 (hpaA), XC_3019 (hrpD5), XC_3022 (hpaB), XC_3023 (hrpW), XC_3025 (hrpF) and XC_3076 (hrpX) was decreased in the Flp mutant compared to the wild‐type (Fig. 4A, Table S2).
Given that mutation of Flp leads to a significant reduction in virulence, it is feasible that the impact of Flp on the expression of pathogenicity related genes at the transcriptional level accounts for the phenotypes seen in the flp mutant.
Flp regulates the expression of T3SS genes by altering the expression of key regulator HrpX
The gene transcription profile data presented suggest that Flp regulated the expression of the T3SS via hrp gene expression. To confirm this idea, we quantified the expression of several hrp operons (hrpB, hrpC, hrpF), and hrpX and hrpG, the key regulators of T3SS. This was achieved by using promoterless‐gusA transcriptional fusion reporters that we have deployed in previous studies (An et al., 2011; Cui et al., 2018; Huang et al., 2009). Here we constructed a group of reporter plasmids for hrpB, hrpC, hrpF, hrpX and hrpG where the promoter sequence fused in front of the gusA gene so that the activity of gusA (Huang et al., 2009). The reporter plasmids were introduced into the wild‐type and Δflp strains, respectively (Table S1). The reporter strains were cultured in XVM2 medium for 8 h, and the activities of β‐glucuronidase GUS were determined (see Experimental procedures). The results demonstrate that GUS activities for hrpB, hrpC, hrpF and hrpX reporters, but not the hrpG reporter, were significantly reduced in the Δflp deletion strain compared to the wild‐type (P < 0.05 by Student's t‐test) (Fig. 4B). A similar result was seen using an RT‐PCR assay, in which we compared RNA amounts of hrpX between wild‐type and Δflp cultured in media XVM2 (Fig. 4C). These observations indicate that Flp regulates the expression of hrp genes and T3SS, apparently by controlling the expression of hrpX.
Flp enhances hrpX expression via binding to its promoter
The findings outlined above raise the question of how the Flp influences hrpX expression. One possible explanation is that Flp binds directly to the promoter of hrpX. To explore the potential interaction between Flp and hrpX promoter we conducted a set of electrophoretic mobility shift assays (EMSA) (see Experimental procedures). The 6 × His‐tagged expression construct of Flp was generated and the protein subsequently purified by nickel affinity column chromatography (see Experimental procedures). The purified 6 × His‐tagged Flp fusion protein caused a mobility shift of DNA probes spanning the promoter of hrpX [from +70 bp downstream to –131 bp upstream against the transcription initiation site (TIS) with a pair of 5ʹ FAM‐labelled primers]. The binding of the hrpX promoter appeared to increase with increasing concentrations of Flp protein (scale from 0 to 1500 nM) to the EMSA assays (Fig. 5A). Furthermore, the shifted bands also could be competed by excess of the unlabelled probes (Fig. 5A). Taken together, the data suggests that under the conditions used Flp binds to the upstream region of hrpX.
Figure 5.
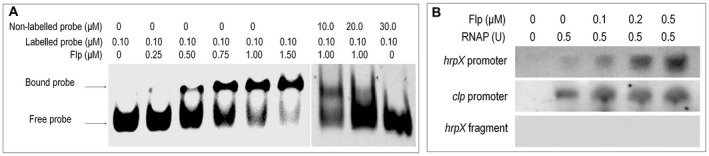
Flp regulates HrpX by interacting with the promoter sequence of hrpX. (A) Electrophoretic mobility shift assays (EMSA) to examine the interaction between the hrpX promoter sequence and Flp protein. In this experiment, DNA is acquired from purified PCR products of the hrpX promoter sequence with 5ʹ‐FAM labelled primers. The DNA is then incubated with purified Flp protein (protein final density 0, 0.25, 0.5, 0.75, 1 and 1.5 µM) and the hrpX promoter DNA is 0.10 µmol). A competition assay was also conducted with unlabelled DNA. When the concentration of unlabelled DNA increased from 10 to 30 µM, the Flp‐DNA complexity decreased, indicating the unlabelled DNA of hrpX brings competition to the 5'‐FAM‐labelled hrpX in forming DNA–protein complex with Flp. F, free DNA strips; R, retarded DNA strips. (B) In vitro transcription experiment using Flp protein (final density arranged from 0, 0.1, 0.20 and 0.5 µM) in the transcription system. A template DNA fragment containing the clp promoter (Liu et al., 2019) and a 121‐bp hrpX fragment extending from +1 to +121 relative to the TIS were used as controls.
The fact that Flp binds to the promoter of hrpX suggests that Flp may directly regulate the expression of the hrpX gene. To validate this, we performed an in vitro transcription assay. Template DNA fragments of 311 bp, extending from –131 to +179 relative to the TIS of the hrpX promoter, were incubated with RNA polymerase holoenzyme from Escherichia coli with increasing amounts of purified 6 × His‐tagged Flp protein. The results show that, although a certain amount of hrpX transcripts could be generated without Flp protein, the hrpX transcription level was obviously increased when Flp protein was added to the reaction (Fig. 5B), suggesting that Flp could enhance hrpX transcription in vitro.
Whether or not Flp binds to the hrpX promoter in vivo was further estimated by chromatin immunoprecipitation (ChIP) assay. To do this, a wild‐type background strain expressing the Flp protein fused with 3 × Flag‐tag (3 × Flag::Flp) at the N‐terminus of Flp was first constructed (see Experimental procedures). Xcc strains were grown in XVM2 medium for 12 h and used for the ChIP assay. A western blot assay revealed that the 3 × Flag::Flp fusion protein could be eluted from the 3 × Flag::Flp expression strain Δflp/pFlp‐Flag, but not the control strain 8004/pLAFR3 (Fig. 6A). Using the eluted DNA from 3 × Flag::Flp protein as a template, a PCR product was obtained by the primer pair (Table S3) designed for amplification of the DNA fragment containing the hrpX promoter, but no product could be obtained by the primers (Table S3) for the promoter of the XC_0784 gene (Fig. 6B), indicating that binding of Flp to hrpX promoter exists in Xcc cells.
Figure 6.
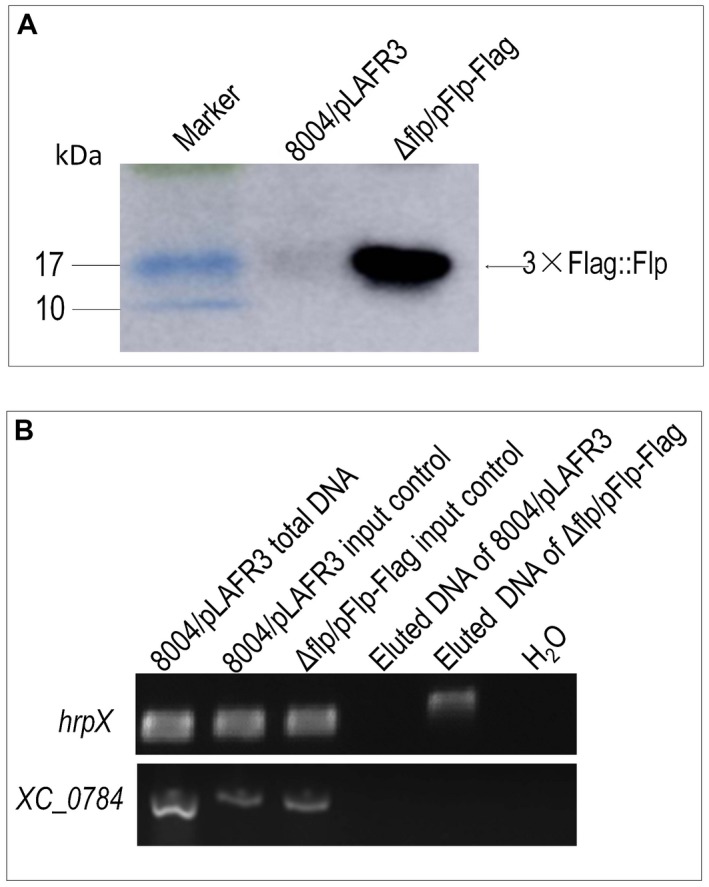
Chromatin immunoprecipitation (ChIP) assay showing that Flp binds to the hrpX promoter region in vivo. A Xanthomonas campestris pv. campestris strain encoding Flp fused with the 3 × Flag peptide was created. The resulting Δflp/pFlp‐Flag strain was cultured in XVM2 medium for 12 h and ChIP samples were prepared. Anti‐Flag was added to the ChIP samples and incubated overnight. The bound DNA fragments and proteins were eluted. 8004/pLAFR3 was used as the control strain. (A) Western blotting of the eluted 3 × Flag::Flp fusion protein. Protein samples were separated by sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane. The presence of the fusion proteins was detected by anti‐Flag monoclonal antibody. (B) PCR detection of eluted DNA. DNA fragments containing the hrpX promoter were PCR amplified using the eluted DNA from the 3 × Flag::Flp protein as the template. Template DNA from a non‐conjugated ChIP sample was used as input control. Simultaneously, DNA fragments containing a cellulase S encoding gene XC_0784 were amplified as positive and negative controls, respectively.
Flp regulates T3SS in planta by altering the expression of hrpX, which has an impact on the induction of HR and plant defence
Given that mutation of Flp leads to a reduction in the expression level of hrp genes, the influence of flp on the induction of HR was evaluated using an infiltration assay. Bacterial suspensions of the wild‐type, Δflp, CΔflp and a hrpX mutant (negative control) were inoculated into the leaves of non‐host pepper (Capsicum annuum cv. ECW‐10R) through the use of a pressurized syringe (see Experimental procedures). Following 8 h inoculation, the Δflp strain resembled the negative control by showing no visible HR symptoms (Fig. 7A). Conversely, the wild‐type and CΔflp strains induced comparable HR symptoms (Fig. 7A). It was not until after 16 h post‐inoculation that the Δflp strain appeared to induce any visual HR symptoms. These results suggest that the Δflp mutant had a delayed and weakened HR compared to the wild‐type.
Figure 7.
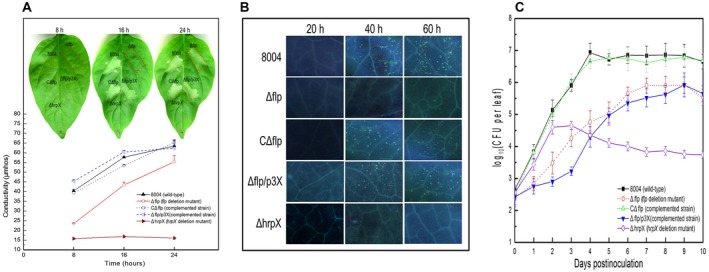
Flp is important for response to plant immunity and plant colonization. (A) Flp is required for HR induction in Xanthomonas campestris pv. campestris (Xcc). HR symptoms observed after infiltration (upper part). The infiltration was performed on the fully expanded pepper (Capsicum annuum cv. ECW‐10R). The HR symptoms of strains Xcc wild‐type (8004), Δflp, CΔflp, Δflp/p3X and ΔhrpX were recorded at 8, 16 and 24 h post‐inoculation. There was electrolyte leakage from pepper leaves inoculated with Xcc strains (Down element). The conductivity of the infiltrating spots was measured by a DDS‐307A conductometer at 8, 16 and 24 h post‐inoculation, with four 0.4 cm2 leaf disks collected from the infiltrated area for each sample. Three samples were taken for each measurement in each experiment. Data are shown as mean and standard deviation. (B) Callose deposition within cabbage leaves inoculated with Xcc strains 8004, Δflp, CΔflp, Δflp/p3X and ΔhrpX using the infiltration method. Leaves were collected at intervals of 20 h and stained with aniline blue. The stained samples were observed under an Olympus BH‐2 epifluorescent microscope. Represented images are presented. (C) In planta growth curves of Xcc strains. 8004, Δflp, CΔflp, Δflp/p3X and ΔhrpX were inoculated onto cabbage leaves using leaf clipping methods. Four leaves were collected from every group of clipped leaves daily and homogenized in sterile water. The homogenates were diluted and plated on NYG plates. Bacterial CFU were counted after incubation for 3 days. Data are the means and standard deviations from three replicates.
To provide a quantitative assessment of HR induction an electrolyte leakage assay was used (see Experimental procedures). Here, leaf tissues within the infiltration areas were collected at three time points (8, 16 and 24 h) post‐inoculation of the Xcc strains. The results show that wild‐type and CΔflp induced similar levels of electrolyte leakage, whereas Δflp and the hrpX mutant induced much lower levels at 8 and 16 h, compared to the wild‐type (Fig. 7A). However, at 24 h post‐inoculation, it appeared that Δflp generated a very similar to the wild‐type and CΔflp strains but the hrpX mutant still retained lower levels (Fig. 7A).
In order to confirm Flp influence on T3SS in planta was due to modulation of hrpX expression, we tested an flp mutant that constitutively expressed hrpX in several HR induction assays. To achieve this, the entire ORF and Shine–Dalgarno (SD) sequence of the hrpX gene was introduced into the pLARF3 plasmid (under the control of the lacZ promoter) to generate the p3X construct (Huang et al., 2009). This construct was introduced into the Δflp mutant to obtain the Δflp/p3X strain. Importantly, the Δflp/p3X strain constitutively expressing hrpX retained the ability to induce HR (Fig. 7A).
Callose deposition is required for disease resistance against many pathogens, including Xcc. It has been shown previously that Xcc induces defence responses in host plant Arabidopsis in a T3SS‐dependent manner (Rong et al., 2010). Therefore, to examine potential differences in defence response we monitored callose deposition in the wild‐type, Δflp, CΔflp, Δflp/p3X and hrpX mutant strains. As before, we inoculated wild‐type, Δflp, CΔflp, Δflp/p3X and hrpX strains into cabbage leaves using the infiltration method (see Experimental procedures). Subsequently, leaves were collected at 20, 40 and 60 h post‐infiltration and callose disposition was examined (see Experimental procedures). Like HR induction the wild‐type, CΔflp and Δflp/p3X showed similar callose disposition across the leaves tested (Fig. 7B). The Δflp showed reduced levels of callose disposition compared to the wild‐type but the level, which was greater than what the hrpX produced, was restored to the wild‐type by the constitutive expression of hrpX (Fig. 7B).
To determine if growth in planta contributes to the HR induction and plant defence phenotypes observed, we determined the growth of strains. We examined the growth of wild‐type, Δflp, CΔflp, Δflp/p3X and hrpX strains following inoculation into cabbage by leaf‐cutting methods and recorded the growth variation within 10 days post‐inoculation (see Experimental procedures). The results show that the Δflp and Δflp/p3X have slower growth in planta compared to wild‐type and CΔflp strains (Fig. 7C). Notably, a comparison between Δflp and Δflp/p3X growth profiles showed that the latter was slower initially in colonization, which might be due to the deregulation of HrpX in this strain. Conversely, the hrpX mutant grew at a similar growth rate to the wild‐type and complementary strain CΔflp strains, indicating that HrpX is not initially required for host colonization.
These results together indicate that a constitutive expression of hrpX could restore T3SS, HR and host plant defence induction ability of the Δflp strain, which is consistent with the idea that Flp regulates T3SS by modulating the expression of hrpX in planta. Furthermore, Flp appears to be important for plant colonization.
Flp influences previously uncharacterized virulence factors independently of HrpX regulation in Xcc
The data presented above demonstrate that Flp regulates hrpX by directly interacting with the promoter and therefore has an impact on T3SS. However, results from the transcriptome profile and hrpX complementation of the Δflp strain suggest that Flp plays a greater role in virulence than just through regulation by HrpX/T3SS.
To confirm this idea we compared the transcriptome profile of the Δflp strain (generated in this study) and the hrpX mutant strain (generated in Jiang et al., 2018), which identified a set of target genes that appeared to be regulated by Flp and not HrpX. To confirm this we examined the expression of XC_1273, XC_2659, XC_2827, XC_3129, XC_3694 and XC_4002 by RT‐PCR by extracting RNA from wild‐type, Δflp, CΔflp and Δflp/p3X cultured in XVM2, respectively. The RT‐PCR results confirmed that expression of these selected genes was consistent with the data from the transcriptome analyses and appeared to be under the regulation of Flp (Fig. 8A). Furthermore, the expression of these genes was restored to wild‐type levels by in trans expression of flp in the Δflp mutant background (CΔflp) (Fig. 8A). Interestingly, the expression levels of genes (XC_1273, XC_2659, XC_3694, XC_4002) were not restored to wild‐type levels in the Δflp mutant background expressing hrpX (Fig. 8A). This suggests that genes are under the control of Flp and not HrpX.
Figure 8.
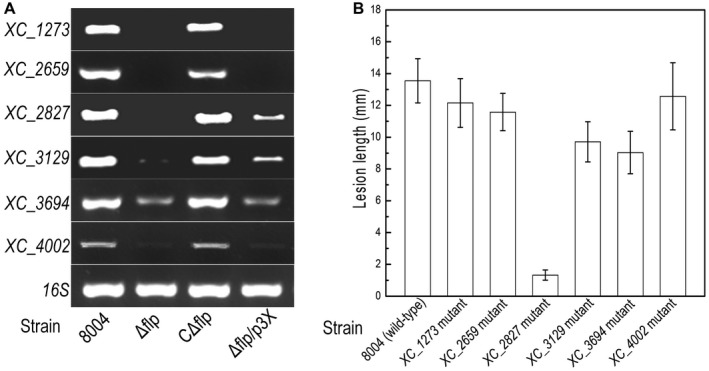
Flp influences previously uncharacterized virulence factors independently of HrpX regulation in Xanthomonas campestris pv. campestris (Xcc). (A) Semi‐quantitative RT‐PCR analysis of expression of the selected genes in different Xcc strains: wild‐type 8004, Δflp, CΔflp and Δflp/p3X. (B) Mean lesion lengths caused by different Xcc mutant strains (1273nk, 2659nk, 2827nk, 3129nk, 3694nk, 4002nk) on cabbage leaves. Lesion lengths were measured at 10 days post‐inoculation. Values for means and standard deviation (SD) from 30 inoculated leaves in one experiment are indicated. The experiment was repeated three times.
The role of the Flp‐regulated genes in virulence to Chinese cabbage was tested by the use of a panel of insertional mutants (Table S1). The virulence of each mutant was tested by measurement of the lesion length after bacteria that were introduced into the vascular system of Chinese cabbage by leaf clipping. Mutation of three genes (XC_2827, XC_3129, XC_3694) gave a significant reduction in virulence (P < 0.05 by Student's t‐test) (Fig. 8B). Nevertheless, the remaining genes (XC_1273, XC_2659, XC_4002) showed no difference to the wild‐type strain (Fig. 8B). Despite a significant impact on virulence, none of the genes led to a complete loss of virulence. Many of these genes had not been previously associated with Xcc virulence.
This subset of new virulence factors for Xcc includes outer membrane protein (XC_3694) and inner membrane protein (XC_3129), both under the control of Flp. Interestingly, many of these proteins have homologues in other plant‐associated bacteria, including other Xanthomonas species and Stenotrophomonas.
Discussion
Fis protein was initially identified as a factor for inversion stimulation of the homologous Hin and Gin site‐specific DNA recombinases of E. coli (Kahmann et al., 1985). Subsequently, its diverse roles have been described, including roles in regulating bacterial virulence factors and optimizing bacterial adaptation to various environments. Fis is an abundant bacterial nucleoid‐associated protein that influences DNA topology by directly binding and bending DNA (Dillon and Dorman, 2001). It has been suggested that Fis alteration of DNA can occur in multiple tandem sites in a non‐random fashion (Schneider et al., 2001; Kahramanoglou et al., 2011). Fis has been shown to display a preference for binding to regions upstream of ORFs which can influence gene expression (Kahramanoglou et al., 2011). In addition, Fis can also directly activate and repress transcription at promoters by interacting with RNA polymerase (Browning et al., 2010). Despite extensive studies showing that Fis serves as a global transcription factor that activates a diverse range of virulence functions, including quorum sensing, capsule production, adhesion and type III secretion in many mammalian pathogens, e.g. pathogenic E. coli (Falconi et al., 2001; Goldberg et al., 2001), Shigella flexneri (Falconi et al., 2001), Salmonella enterica serovar Typhimurium (Kelly et al., 2004) and Yersinia pseudotuberculosis (Green et al., 2016), few works have been carried out in plant bacterial pathogens. Additionally, no such protein has been identified or characterized in Xcc or other bacteria from the Xanthomonas genus. In this study, we identified the ORF XC_0520 from Xcc that encodes a small protein with 40% amino acid sequence identity to the Fis protein characterized in other bacterial species. This Fis‐like protein (or Flp) is identical in all three sequenced Xcc strains (8004, ATCC33913 and B100). Here, the deletion of Flp in Xcc caused a series of changes in virulence and HR‐associated phenotypes. Although virulence factor regulation by Fis has been seen in other bacterial plant pathogens such as Dickeya dadantii (Ouafa et al., 2012) and Dickeya zeae (Lv et al., 2018), no role has been attributed to its regulation of HR, suggesting a differentiate role in Xcc.
Our transcriptome analysis revealed that expression of T3SS genes, including master regulator HrpX, was down‐regulated in a flp mutant. We therefore investigated the regulatory effect of Flp on hrp genes. Using GUS‐based reporter plasmids, electrophoretic mobility shift and in vitro transcription assays, we demonstrated that Flp regulates the expression of hrp genes and T3SS by controlling the expression of hrpX directly. In Xanthomonas spp., the hrp genes are highly conserved and comprise more than 20 genes. The expression of hrp genes is mainly controlled by two key regulators, HrpG and HrpX. HrpG and HrpX form a regulatory cascade: HrpG regulates the expression of hrpX and HrpX then activates the expression of other hrp genes as well as some effector genes (Huang et al., 2009; Koebnik et al., 2006; Wengelnik et al., 1996; Wengelnik and Bonas, 1996). Besides HrpG and HrpX, several regulators have been identified as being involved in the regulation of the expression of hrp genes. However, these regulators and their regulatory mechanisms are distinct in different Xanthomonas species, e.g. a histone‐like nucleoid‐structuring (H‐NS) protein XrvC and a LysR‐type transcriptional regulator GamR directly control the transcription of both hrpG and hrpX in Xanthomonas oryzae pv. oryzae (Liu et al., 2016; Rashid et al., 2016), a T3SS component HrcT positively regulates the expression of hrpX via binding to its promoter in X. oryzae pv. oryzicola (Liu et al., 2014), and the zinc uptake regulator Zur and a GntR family transcriptional regulator HpaR1 indirectly control the expression of hrp genes via HrpX and HrpG, respectively, in Xcc (An et al., 2011; Huang et al., 2009). Here our experimental evidences suggest a novel regulatory pathway control T3SS in Xcc. However, our analysis cannot eliminate the possibility that Flp may interact with multiple ORFs to control the transcription of hrp genes. Furthermore, given that Flp functions as a global regulator, illustrated by our global transcriptional analysis, it is possible that Flp directly/indirectly regulates hrp expression via other avenues.
Our transcriptome analysis also demonstrated that Flp affects the expression of a series of other genes, such as flagellar and pilus biosynthesis, nutrition transport, stress response, amino acid and cofactor biosynthesis, galactose and starch metabolism. These genes are unlikely to be under the control of HrpX that contribute to virulence and other phenotypes such as motility and cellular stress response. This is supported by the fact that expression of HrpX in the Flp mutant cannot rescue virulence in planta (Fig. 1). Importantly, the transcriptome data, as well as RT‐PCR, mutation and pathogenicity experiments, unveiled a range of previously uncharacterized proteins, e.g. XC_3694 (putative outer membrane protein) and XC_3129 (putative inner membrane protein), which are required for full virulence in Xcc. These virulence factors appear to be regulated by a Flp‐dependent and no HrpX‐dependent mechanism. However, whether Flp have directly regulatory effects on these proteins remains unknown. Given many of them have homologues in other plant pathogens, the roles of the novel virulence factors in the pathogenicity of Xcc merit further investigation.
Previous studies in various bacteria have shown that Fis plays a pleiotropic role in bacterial virulence (Duprey et al., 2014). Our data further illustrate this in Xcc as Flp has a role in regulating HrpX and T3SS but also in a range of previously uncharacterized virulence factors that appear to be regulated independently of HrpX. Additionally, we demonstrated that Flp is required for swimming and swarming motility, stress tolerance, and extracellular polysaccharide and extracellular enzymes (cellulase and amylase) production. Further studies are needed to examine the role of Flp in the regulation of these phenotypes but this suggests several other questions need to be addressed: What are the environmental cues that activate the expression and activity of Flp? What genes are directly regulated by Flp? Does Flp have a conserved binding site? Does Flp regulate a different set of genes during plant colonization? Does Flp modulate the activity of HrpG directly? How does Flp affect gene expression of the new genes identified as Xcc virulence?
Our work demonstrates the complexity of the signalling pathways involved in the regulation of virulence in Xcc and describes Flp, a Fis‐like protein, an extensive regulator that controls hrp gene expression, HR induction and pathogenesis. The characterization of virulence protein regulators such as Flp is required to develop strategies for disease control in plant pathogens such as Xcc.
Experimental Procedures
Bacterial strains, plasmids and growth conditions
The bacterial strains and plasmids used in this work are listed in Table S1. Escherichia coli strains were cultured in Luria Bertani medium (Miller, 1972) at 37 °C. Xcc strains were cultured in NYG medium (Daniels et al., 1984), NY medium (NYG medium but without glycerol), and the mimic medium XVM2 (Wengelnik and Bonas, 1996) at 28 °C and 200 rpm. Antibiotics were used according to the concentrations as required: kanamycin at 25 µg/mL, rifampicin at 50 µg/mL, ampicillin at 100 µg/mL, spectinomycin at 50 µg/mL and Tet at 5 µg/mL for Xcc strains and 15 µg/mL for E. coli strains.
Nucleus acid manipulations
The nucleic acid manipulations followed the procedures described by Sambrook et al. (1989). Conjugation between the Xcc and E. coli strains was performed as described by Turner et al. (1985). The restriction endonucleases, T4 DNA ligase and Pfu polymerase were provided by Promega (Shanghai, China). The total RNAs were extracted from the cultures of the Xcc strains with a total‐RNA extraction kit (Invitrogen, Waltham, MA, USA) and cDNA generated using a cDNA synthesis kit (Invitrogen). These kits were used with reference to the manufacturer's instructions. For semi‐quantitative RT‐PCR, the obtained cDNA was diluted and used as a template with selected primers for target genes (Table S3).
Construction of mutant strains
In order to construct the in‐frame deleted mutant of flp (XC_0520), 333 bp (EcoRI and BamHI) of the upstream and 339 bp (BamH1 and HindIII) of the downstream sequences of the flp gene were amplified by PCR using the relevant primers (Table S3). After being digested by restriction enzymes, these DNA fragments were fused with the suicide plasmid pK18mobsacB (Schäfer et al., 1994) and transformed into E. coli DH5α. The acquired recombined plasmid was introduced into Xcc with the help of plasmid pRK2073 (Table S1). The original ORF of XC_0520 will be deleted from genomes through allelic exchange and homologous recombination.
To complement the flp deletion mutant, an 297‐bp DNA fragment of the flp gene coding sequence was PCR‐amplified from Xcc strain 8004 and inserted into the pLAFR3 vector at the BamHI/HindIII restriction site, creating the plasmid pLCflp (Table S1). This plasmid was introduced into the mutant by triparental mating.
Pathogenicity tests, HR assays, leakage assays and in‐plant growth curve
The virulence of the Xcc strain to the host cabbage plant (Brassica oleracea 'Jingfeng No. 1') was tested by the leaf‐clipping method (Wang et al., 2007). Cabbage seedlings were planted and grown in the greenhouse for 30 days and the leaves were used for inoculation. Xcc strains, collected from overnight culture, were washed and adjusted to the same final density (OD600 = 0.6, approximately 1 × 109 CFU/mL). The bacterial resuspension was then diluted to 1 × 106 CFU/mL. The lesion and symptoms were measured 10 days post‐inoculation (Dow et al., 2003).
HR was tested on pepper leaves (Capsicum annuum cv. ECW‐10R) as previously described (Li et al., 2014). Briefly, bacteria suspensions (1 × 108 CFU/mL) were infiltrated into the abaxial side of the pepper leaves. These inoculated plants were kept in the greenhouse at 28 °C to observe the HR symptoms and gauge conductivity. For conductivity measurements, samples (leaf discs of 0.4 cm2) were collected using a hole‐puncher. These acquired samples were soaked in 10 mL ultrapure water with shaking at 200 rpm. The leaf discs were then removed and the conductivity of water was measured with a DDS‐307A conductivity meter.
Bacterial in planta growth was estimated as previously described (Li et al., 2014). Briefly, Xcc strains were inoculated onto cabbage leaves using leaf clippings. At intervals of 24 h, four clipped leaves from every group of inoculated plants were collected and homogenized. Homogenate was serially diluted using NYG medium and 100 μL dilution was used for spread plate counting (NYG medium). The amounts of bacteria were calculated after 2 days of incubation.
Callose deposition assay
Callose deposition assay was performed on cabbage leaves as recently described by Hamdoun et al. (2018). Thirty‐day‐old cabbage leaves were inoculated with Xcc strains by infiltration (detailed above). At intervals of 20 h, the leaves were collected and individually soaked in 70% ethanol for 2 h, then 50% ethanol for 2 h and finally sterile distilled water for 2 h. When the chlorophyll was completely leached, the leaves were stained in 0.01% aniline blue. Callose deposition was observed using an Olympus BH‐2 epifluorescent microscope.
GUS activity assays
GUS activity in bacterial strains was measured as described by Henderson et al. (1985). Wild‐type and mutant strains harbouring reporter plasmids were cultured in XVM2 media at 28 °C for 8 h. Bacteria cells were collected by centrifugation and resuspended in 375 μL of 1 mM p‐nitrophenyl‐β‐d‐glucuronide extraction buffer (50 mM sodium dihydrogen phosphate, 0.1% Triton X‐100 and 10 mM β‐mercaptoethanol, pH 7.2) and incubated at 37 °C for 10 min, and then terminated by 200 mL of 2.5 M 2‐amino‐2‐methyl‐1,3‐propanediol. Enzyme assays were carried out in triplicate from at least three independent cultures.
Protein manipulation
In order to obtain purified Flp protein, the flp gene was cloned and introduced into the expression vector pQE30 [harbouring a lac promoter and a ribosome binding site [RBS] in front of the multiple cloning site (MCS)]. The Flp protein was expressed and purified with the methods modified from An et al. (2011). To remove imidazole, 6 × His‐tagged Flp was dialysed against 200 volumes of Tris‐HCl buffer [10 mM Tris‐HCl (pH 8.0) and 1 mM dithiothreitol (DTT)] at 4 °C.
Electrophoretic mobility shift assays
The method that was deployed for EMSA assays was modified from that previously described by Su et al. (2016). Here fragments of the gene encoding HrpX were PCR amplified with the primers described in Table S3. These DNA fragments were labelled with FAM at the 5ʹ terminal. The Flp protein and selected DNA fragments were mixed with the binding buffer and incubated for 20 min at 28 °C. The reaction samples were loaded onto a 6% polyacrylamide‐Tris‐glycine‐EDTA gel. Electrophoresis was performed in TGE buffer (pH 7.6) and visualized using an autoradiograph.
In vitro transcription assays
In vitro transcription assays were performed as previously described (Su et al., 2016). Promoter sequence fragments (311‐bp) of hrpX were acquired using PCR (see Table S3). 6 × His‐tagged Flp protein and DNA fragments were incubated for 30 min at room temperature in transcription buffer. Then, a NTP mixture (250 μM each of ATP, CTP and GTP; 250 μM biotin‐16‐UTP) and 0.5 U of E. coli RNA polymerase holoenzyme (New England BioLabs, Ipswich, MA, USA) were added to initiate transcription. After incubation at 28 °C for 30 min, the reactions were terminated and the transcription products were analysed by electrophoresis. The transcripts obtained were visualized using a phosphor imager screen (GE AI600).
Stress tolerance assay
The minimal inhibitory concentration (MIC) method (Su et al., 2016) was employed to test the sensitivity of the Xcc strains to several environmental stresses, including sodium dodecyl sulphate (SDS), hydrogen peroxide (H2O2), hyperosmosis (NaCl), phenol and heavy metal salts (CdCl2, CuSO4) stress. Briefly, Xcc strains were cultured overnight and diluted to an OD600 of 0.1, then 100 μL of the diluted culture was plated on NYG plates supplemented with different concentrations of each reagent, respectively. The surviving colonies on the plates were counted after 3 days of incubation at 28 °C.
Exopolysaccharide and extracellular enzymes assays
Exopolysaccharide (EPS) and extracellular enzymes assays were performed as previously described (Su et al., 2016; Tang et al., 1991). To estimate EPS production, Xcc strains were inoculated into 100 mL NY liquid medium containing glucose (2% w/v) at 28 °C, 200 rpm for 5 days. EPS was precipitated from the culture supernatant with ethanol and dried at 55 °C using an oven and weighed. For quantitative estimation of the activity of the extracellular enzymes endoglucanase (cellulase) and amylase, Xcc strains were cultured in NYG medium for 12 h. For endoglucanase, 10 μL of enzyme‐containing extracts was added to 200 μL of indicator buffer containing 1% (wt/vol) carboxymethylcellulose (CMC, Sangon, Shangshai, China) as the substrate. The reactions were carried out for 30 min at 28 °C. The released reducing sugars were measured as d‐glucose equivalents, as described by Miller (1959). One unit (U) of the endoglucanase activity was defined as the amount of enzyme releasing 1 μmol of reducing sugar per minute. Amylase activity quantification was conducted in the same way as for the endoglucanase measurement, except that the substrate 1% (wt/vol) CMC was replaced by 1% (wt/vol) starch solution.
Transcriptome analysis of the Flp mutant
Transcriptome analysis were performed as previously described (Cui et al., 2018). Briefly, RNA was prepared from cell culture of OD600 = 0.6. Contaminating genomic DNA was removed using RNase‐free DNase I. After the quantity determination and quality assessment, total RNA was sent to Novogene (Beijing, China) for library construction and strand‐specific RNA sequencing. Sequencing libraries were generated using a NEBNext Ultra™ Directional RNA Library Prep Kit for Illumina (New England BioLabs), and sequenced on an Illumina (CA, USA) HiSeq 2000 platform. Clean reads were mapped to the reference genome and the RPKM (reads per kilobase per million mapped reads) method was used to calculate the gene expression levels. False discovery rate FDR ≤ 0.05 and |log2FC| (log2 of the fold changes) ≥1 were considered for differentially expressed genes.
ChIP assay
ChIP assay was performed as previously described with minor modifications (Liu et al., 2019). In brief, a strain producing an Flp protein fused with 3 × Flag‐tag (3 × Flag::Flp) at the N‐terminus of Flp was first constructed. To do this, a DNA fragment encoding Flp fused with 3 × Flag peptide was obtained using PCR with the primer set Fflag‐F/R (Table S3) and cloned into the BamHI/HindIII sites of the vector pLAFR3. The acquired recombinant plasmid pFlp‐Flag was introduced into Xcc flp deletion strain Δflp, resulting in strain Δflp/pFlp‐Flag. Xcc wild‐type strain 8004 containing the empty vector pLAFR3 (8004/pLAFR3) was used as a negative control. Xcc strains were grown in XVM2 medium for 12 h and cross‐linked using formaldehyde. Bacterial cells were collected and then lysed by sonication. For each ChIP sample, 50 μL of anti‐Flag (agarose conjugated) was added to the bacterial lysates and incubated overnight. Unbound DNA fragments were washed and the bound DNA fragments and proteins were eluted by 0.25 M glycine (pH 2.5).
Supporting information
Fig. S1 The homology of Flp and its position in evolution. (A) Sequence alignments between Flp and Fis proteins in Yersinia pseudotuberculosis, Dickeya zeae and E. coli. The sequences of these proteins were acquired from NCBI website and the alignment was proceeded with the software NTI Vector. (B) The position of Flp in evolutionary tree. A series of Fis family proteins were acquired from NCBI, and the evolutionary tree of these proteins was compiled with MEGA6.0. The position on the branches of the tree indicated the distance in evolution.
Table S1 Bacterial strains and plasmids used in this work. Note: aRifr, Kanr, Tetr and Spcr indicate resistance to rifampicin, kanamycin, tetracycline and spectinomycin, respectively.
Table S2 Genes expressed by the Δflp mutant strain when grown in XVM2.
Table S3 Primers used in this study.
Acknowledgements
This work was supported by the 973 Program of the Ministry of Science and Technology of China (2015CB150601), the National Natural Science Foundation of China (31860021) and the Ba Gui Scholar Program of Guangxi Zhuang Autonomous Region of China (2014A002).
Contributor Information
Guang‐Tao Lu, Email: lugt@gxu.edu.cn.
Ji‐Liang Tang, Email: jltang@gxu.edu.cn.
References
- Alfano, J.R. and Collmer, A. (1997) The type III (Hrp) secretion pathway of plant pathogenic bacteria: trafficking harpins, Avr proteins, and death. J. Bacteriol. 179, 5655–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, S.Q. , Lu, G.T. , Su, H.Z. , Li, R.F. , He, Y.Q. , Jiang, B.L. , Tang, D.J. and Tang, J.L. (2011) Systematic mutagenesis of all predicted gntR genes in Xanthomonas campestris pv. campestris reveals a GntR family transcriptional regulator controlling hypersensitive response and virulence. Mol. Plant Microbe Interact. 24, 1027–1039. [DOI] [PubMed] [Google Scholar]
- Astua‐Monge, G. , Freitas‐Astua, J. , Bacocina, G. , Roncoletta, J. , Carvalho, S.A. and Machado, M.A. (2005) Expression profiling of virulence and pathogenicity genes of Xanthomonas axonopodis pv. citri . J. Bacteriol. 187, 1201–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning, D.F. , Grainger, D.C. and Busby, S.J. (2010) Effects of nucleoid‐associated proteins on bacterial chromosome structure and gene expression. Curr. Opin. Microbiol. 13, 773–780. [DOI] [PubMed] [Google Scholar]
- Büttner, D. and Bonas, U. (2010) Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 34, 107–133. [DOI] [PubMed] [Google Scholar]
- Cui, P. , Li, R.F. , Zhang, D.P. , Tang, J.L. and Lu, G.T. (2018) HpaP, a novel regulatory protein with ATPase and phosphatase activity, contributes to full virulence in Xanthomonas campestris pv. campestris . Environ. Microbiol. 20, 1389–1404. [DOI] [PubMed] [Google Scholar]
- Daniels, M.J. , Barber, C.E. , Turner, P.C. , Sawczyc, M.K. , Byrde, R.J. and Fielding, A.H. (1984) Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 3, 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon, S.C. and Dorman, C.J. (2010) Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol., 8, 185–195 [DOI] [PubMed] [Google Scholar]
- Dow, J.M. , Crossman, L. , Findlay, K. , He, Y.Q. , Feng, J.X. and Tang, J.L. (2003) Biofilm dispersal in Xanthomonas campestris is controlled by cell–cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA. 100, 10995–11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprey, A. , Reverchon, S. and Nasser, W. (2014) Bacterial virulence and Fis: adapting regulatory networks to the host environment. Trends Microbiol., 22, 92–99 [DOI] [PubMed] [Google Scholar]
- Falconi, M. , Prosseda, G. , Giangrossi, M. , Beghetto, E. and Colonna, B. ( 2001) Involvement of FIS in the H‐NS‐mediated regulation of virF gene of Shigella and enteroinvasive Escherichia coli . Mol. Microbiol. 42, 439–452. [DOI] [PubMed] [Google Scholar]
- Goldberg, M.D. , Johnson, M. , Hinton, J.C. and Williams, P.H. (2001) Role of the nucleoid‐associated protein Fis in the regulation of virulence properties of enteropathogenic Escherichia coli . Mol. Microbiol. 41, 549–559. [DOI] [PubMed] [Google Scholar]
- Green, E.R. , Clark, S. , Crimmins, G.T. , Mack, M. , Kumamoto, C.A. and Mecsas, J. (2016) Fis is essential for Yersinia pseudotuberculosis virulence and protects against reactive oxygen species produced by phagocytic cells during infection. PLoS Pathog. 12, e1005898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdoun, S. , Gao, M. , Gill, M. , Kwon, A. , Norelli, J.L. and Lu, H. (2018) Signalling requirements for Erwinia amylovora‐induced disease resistance, callose deposition and cell growth in the non‐host Arabidopsis thaliana . Mol. Plant Pathol. 19, 1090–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y.W. and Zhang, L.H. (2008) Quorum sensing and virulence regulation in Xanthomonas campestris . FEMS Microbiol. Rev. 32, 842–857. [DOI] [PubMed] [Google Scholar]
- He, Y.Q. , Zhang, L. , Jiang, B.L. , Zhang, Z.C. , Xu, R.Q. , Tang, D.J. , Qin, J. , Jiang, W. , Zhang, X. , Liao, J. , Cao, J.R. , Zhang, S.S. , Wei, M.L. , Liang, X.X. , Lu, G.T. , Feng, J.X. , Chen, B. , Cheng, J. and Tang, J.L. (2007) Comparative and functional genomics reveals genetic diversity and determinants of host specificity among reference strains and a large collection of Chinese isolates of the phytopathogen Xanthomonas campestris pv. campestris . Genome Biol. 8, R218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, R.F. , Benson, J.M. , Hahn, F.F. , Hobbs, C.H. , Jones, R.K. , Mauderly, J.L. , McClellan, R.O. and Pickrell, J.A. (1985) New approaches for the evaluation of pulmonary toxicity: bronchoalveolar lavage fluid analysis. Fundam. Appl. Toxicol. 5, 451–458. [DOI] [PubMed] [Google Scholar]
- Huang, D.L. , Tang, D.J. , Liao, Q. , Li, X.Q. , He, Y.Q. , Feng, J.X. , Jiang, B.L. , Lu, G.T. and Tang, J.L. (2009) The Zur of Xanthomonas campestris is involved in hypersensitive response and positively regulates the expression of the hrp cluster via hrpX but not hrpG . Mol. Plant‐Microbe Interact. 22, 321–329. [DOI] [PubMed] [Google Scholar]
- Jiang, B.L. , Jiang, G.F. , Liu, W. , Yang, L.C. , Yang, L.Y. , Wang, L. , Hang, X.H. and Tang, J.L. (2018) RpfC regulates the expression of the key regulator hrpX of the hrp/T3SS system in Xanthomonas campestris pv. campestris . BMC Microbiol. 18, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahmann, R. , Rudt, F. , Koch, C. and Mertens, G. (1985) G inversion in bacteriophage Mu DNA is stimulated by a site within the invertase gene and a host factor. Cell, 41, 771–780. [DOI] [PubMed] [Google Scholar]
- Kahramanoglou, C. , Seshasayee, A.S. , Prieto, A.I. , Ibberson, D. , Schmidt, S. , Zimmermann, J. , Benes, V. , Fraser, G.M. and Luscombe, N.M. (2011) Direct and indirect effects of H-NS and Fis on global gene expression control in Escherichia coli. Nucleic Acids Res., 39, 2073–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, A. , Goldberg, M.D. , Carroll, R.K. , Danino, V. , Hinton, J.C. and Dorman, C.J. ( 2004) A global role for Fis in the transcriptional control of metabolism and type III secretion in Salmonella enterica serovar Typhimurium. Microbiology, 150, 2037–2053. [DOI] [PubMed] [Google Scholar]
- Koebnik, R. , Kruger, A. , Thieme, F. , Urban, A. and Bonas, U. (2006) Specific binding of the Xanthomonas campestris pv. vesicatoria AraC‐type transcriptional activator HrpX to plant‐inducible promoter boxes. J. Bacteriol. 188, 7652–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R.F. , Lu, G.T. , Li, L. , Su, H.Z. , Feng, G.F. , Chen, Y. , He, Y.Q. , Jiang, B.L. , Tang, D.J. and Tang, J.L. (2014) Identification of a putative cognate sensor kinase for the two‐component response regulator HrpG, a key regulator controlling the expression of the hrp genes in Xanthomonas campestris pv. campestris . Environ. Microbiol. 16, 2053–2071. [DOI] [PubMed] [Google Scholar]
- Lindgren, P.B. (1997) The role of hrp genes during plant‐bacterial interactions. Annu. Rev. Phytopathol. 35, 129–152. [DOI] [PubMed] [Google Scholar]
- Liu, Z.Y. , Zou, L.F. , Xue, X.B. , Cai, L.L. , Ma, W.X. , Xiong, L. , Ji, Z.Y. and Chen, G.Y. (2014) HrcT is a key component of the type III secretion system in Xanthomonas spp. and also regulates the expression of the key hrp transcriptional activator HrpX. Appl. Environ. Microbiol. 80, 3908–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Long, J. , Shen, D. and Song, C. (2016) Xanthomonas oryzae pv. oryzae requires H‐NS‐family protein XrvC to regulate virulence during rice infection. FEMS Microbiol. Lett. 363, 10. [DOI] [PubMed] [Google Scholar]
- Liu, G.F. , Su, H.Z. , Sun, H.Y. , Lu, G.T. and Tang, J.L. (2019) Competitive control of endoglucanase gene engXCA expression in the plant pathogen Xanthomonas campestris by the global transcriptional regulators HpaR1 and Clp. Mol. Plant Pathol. 20, 51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, M. , Chen, Y. , Liao, L. , Liang, Z. , Shi, Z. , Tang, Y. , Ye, S. , Zhou, J. and Zhang, L. (2018) Fis is a global regulator critical for modulation of virulence factor production and pathogenicity of Dickeya zeae. Sci. Rep., 8, 341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G.L. (1959) Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428. [Google Scholar]
- Miller, J.H. (1972) Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Notti, R.Q. and Stebbins, C.E. (2016) The structure and function of type III secretion systems. Microbiol. Spectr. 4, VMBF‐0004‐2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouafa, Z.A. , Reverchon, S. , Lautier, T. , Muskhelishvili, G. and Nasser, W. (2012) The nucleoid-associated proteins H-NS and FIS modulate the DNA supercoiling response of the pel genes, the major virulence factors in the plant pathogen bacterium Dickeya dadantii. Nucleic Acids Res., 40, 4306–4319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, W. , Jia, Y. , Ren, S.X. , He, Y.Q. , Feng, J.X. , Lu, L.F. , Sun, Q. , Ying, G. , Tang, D.J. , Tang, H. , Wu, W. , Hao, P. , Wang, L. , Jiang, B.L. , Zeng, S. , Gu, W.Y. , Lu, G. , Rong, L. , Tian, Y. , Yao, Z. , Fu, G. , Chen, B. , Fang, R. , Qiang, B. , Chen, Z. , Zhao, G.P. , Tang, J.L. and He, C. ( 2005) Comparative and functional genomic analyses of the pathogenicity of phytopathogen Xanthomonas campestris pv. campestris . Genome Res. 15, 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid, M.M. , Ikawa, Y. and Tsuge, S. (2016) GamR, the LysR‐Type galactose metabolism regulator, regulates hrp gene expression via transcriptional activation of two key hrp regulators, HrpG and HrpX, in Xanthomonas oryzae pv. oryzae . Appl. Environ. Microbiol. 82, 3947–3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, W. , Feng, F. , Zhou, J. and He, C. (2010) Effector‐triggered innate immunity contributes Arabidopsis resistance to Xanthomonas campestris . Mol. Plant Pathol. 11, 783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, R.P. , Vorhölter, F.J. , Potnis, N. , Jones, J.B. , Van Sluys, M.A. , Bogdanove, A.J. and Dow, J.M. (2011) Pathogenomics of Xanthomonas: understanding bacterium‐plant interactions. Nat. Rev. Microbiol. 9, 344–355. [DOI] [PubMed] [Google Scholar]
- Ryan, R.P. , An, S.Q. , Allan, J.H. , McCarthy, Y. and Dow, J.M. (2015) The DSF family of cell–cell signals: an expanding class of bacterial virulence regulators. PLoS Pathog. 11, e1004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J. , Fritsch, E.F. and Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edition Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Schneider, R. , Lurz, R. , Lüder, G. , Tolksdorf, C. , Travers, A. and Muskhelishvili, G. (2001) An architectural role of the Escherichia coli chromatin protein FIS in organising DNA. Nucleic Acids Res., 29, 5107–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer, A. , Tauch, A. , Jäger, W. , Kalinowski, J. , Thierbach, G. and Pühler, A. (1994) Small mobilizable multi‐purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum . Gene, 145, 69–73. [DOI] [PubMed] [Google Scholar]
- Su, H.Z. , Wu, L. , Qi, Y.H. , Liu, G.F. , Lu, G.T. and Tang, J.L. (2016) Characterization of the GntR family regulator HpaR1 of the crucifer black rot pathogen Xanthomonas campestris pathovar campestris . Sci. Rep. 6, 19862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swings, J.G. and Civerolo, E.L. (1993) Xanthomonas. London: Chapman & Hall. [Google Scholar]
- Tang, J.L. , Liu, Y.N. , Barber, C.E. , Dow, J.M. , Wootton, J.C. and Daniels, M.J. (1991) Genetic and molecular analysis of a cluster of rpf genes involved in positive regulation of synthesis of extracellular enzymes and polysaccharide in Xanthomonas campestris pathovar campestris . Mol. Gen. Genet. 226, 409–417. [DOI] [PubMed] [Google Scholar]
- Turner, P. , Barber, C.E. and Daniels, M.J. (1985) Evidence for clustered pathogenicity genes in Xanthomonas campestris pv. campestris . Mol. Gen. Genet. 199, 338–343. [Google Scholar]
- Vicente, J.G. and Holub, E.B. (2013) Xanthomonas campestris pv. campestris (cause of black rot of crucifers) in the genomic era is still a worldwide threat to brassica crops. Mol. Plant Pathol. 14, 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Tang, X. and He, C. (2007) The bifunctional effector AvrXccC of Xanthomonas campestris pv. campestris requires plasma membrane‐anchoring for host recognition. Mol. Plant. Pathol. 8, 491–501. [DOI] [PubMed] [Google Scholar]
- Wengelnik, K. and Bonas, U. (1996) HrpXv, an AraC‐type regulator, activates expression of five of the six loci in the hrp cluster of Xanthomonas campestris pv. vesicatoria . J. Bacteriol. 178, 3462–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengelnik, K. , Van den Ackerveken, G. and Bonas, U. (1996) HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two‐component response regulators. Mol. Plant Microbe Interact. 9, 704–712. [DOI] [PubMed] [Google Scholar]
- Windgassen, M. , Urban, A. and Jaeger, K.E. (2000) Rapid gene inactivation in Pseudomonas aeruginosa . FEMS Microbiol. Lett. 193, 201–205. [DOI] [PubMed] [Google Scholar]
- Zhou, L. , Zhang, L.H. , Camara, M. and He, Y.W. (2017) The DSF family of quorum sensing signals: diversity, biosynthesis, and turnover. Trends Microbiol. 25, 293–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 The homology of Flp and its position in evolution. (A) Sequence alignments between Flp and Fis proteins in Yersinia pseudotuberculosis, Dickeya zeae and E. coli. The sequences of these proteins were acquired from NCBI website and the alignment was proceeded with the software NTI Vector. (B) The position of Flp in evolutionary tree. A series of Fis family proteins were acquired from NCBI, and the evolutionary tree of these proteins was compiled with MEGA6.0. The position on the branches of the tree indicated the distance in evolution.
Table S1 Bacterial strains and plasmids used in this work. Note: aRifr, Kanr, Tetr and Spcr indicate resistance to rifampicin, kanamycin, tetracycline and spectinomycin, respectively.
Table S2 Genes expressed by the Δflp mutant strain when grown in XVM2.
Table S3 Primers used in this study.


