SUMMARY
Taxonomy: Superkingdom Eukaryota; Kingdom Metazoa; Phylum Nematoda; Class Chromadorea; Order Rhabditida; Suborder Tylenchina; Infraorder Tylenchomorpha; Superfamily Tylenchoidea; Family Pratylenchidae; Subfamily Radopholinae; Genus Radopholus.
Physical properties: Microscopic unsegmented worm; migratory endoparasite of plants. Strong sexual dimorphism; reproduction both by amphimixis and self‐fertilization.
Hosts: Over 250 different plant species, including citrus, black pepper and banana (main host plant).
Symptoms: Purple to black lesions and extensive cavities in plant roots, leading to reduced uptake of water and nutrients. In banana, this may result in poor vegetative growth, reduced bunch weight and toppling of plants.
Disease control: Nematicides, alternative cropping systems, nematode‐free planting material, some resistant cultivars.
Agronomic importance: Major problem in banana plantations in tropical regions worldwide.
INTRODUCTION
Radopholus similis (Cobb, 1893) Thorne (1949), or the burrowing nematode, was first discovered by the famous nematologist Nathan A. Cobb in 1891 when he examined banana roots from Fiji. In his publication of 1893, he described the males and females he found separately as two different species, Tylenchus similis and Tylenchus granulosus, respectively (Cobb, 1893). Later, he described a third species, Tylenchus biformis, when he found a population of males and females on sugarcane (Cobb, 1906). It was only in 1968 that the nomenclatural confusion concerning the three species described by Cobb was clarified, and the name Radopholus similis was given to all three (Sher, 1968). Later, two physiological races of R. similis were recognized: one parasitizing banana and many other hosts but not citrus, and another parasitizing both banana and citrus (R. citrophilus) (Huettel et al., 1984). However, as these races are not reproductively isolated, R. citrophilus was later correctly considered as a junior synonym of R. similis (Elbadri et al., 2002; Kaplan and Oppermann, 1997; 1997, 2000; Valette et al., 1998).
The genus Radopholus belongs to the Pratylenchidae within the Tylenchoidea superfamily of the Rhabditid order of the phylum Nematoda. The Pratylenchidae family comprises 12 genera belonging to the subfamilies Pratylenchinae, Apratylenchinae, Hirschmanniellinae, Radopholinae and Naccobinae (De Ley and Blaxter, 2002; Trinh et al., 2009). Only species belonging to three of these genera are of economic importance, i.e. Pratylenchus (lesion nematodes), Hirschmanniella (rice root nematodes) and Radopholus (burrowing nematodes). In the latter genus, R. similis is the only species of 30 described (De Waele and Elsen, 2007) that is recognized as an important plant pathogen world‐wide (Duncan and Moens, 2006).
Radopholus similis is a migratory endoparasitic nematode primarily found in plant roots and widely known as a destructive pest of banana, citrus and black pepper. It has been found on more than 250 different plant species throughout the tropics and subtropics, with banana as the main host plant (Sarah et al., 1996). These nematodes have caused large losses to the citrus industry and completely destroyed black pepper plantations in Indonesia (MacGowan, 1977). On banana, R. similis continues to be a major pest problem for the banana industry in many parts of the world, and it has also been introduced into heated glasshouses in Europe by way of trade with ornamental plants, in particular Anthurium[European and Mediterranean Plant Protection Organization (EPPO) Diagnostics Report, 2008].
LIFE CYCLE AND SURVIVAL STRATEGIES
The egg to egg life cycle of R. similis is usually completed within 3 weeks (Fig. 1). All developmental stages can be found inside the roots, almost exclusively in the root cortex, although the stele in banana can also be damaged. The nematode punctures the root epidermis cells with its stylet, digesting and sucking the cell contents as it burrows into the root. Entry is usually at the root tip or in the region of root hair production and takes less than 24 h. The burrowing nematode attacks only tender young feeder roots and not hardened, suberized, senescing or decayed roots. Once inside the root, the worms feed and reproduce. The male, with its rudimentary stylet, is not known to penetrate roots nor to injure the roots to any extent, whereas the female and second‐stage juveniles feed on roots. Females may lay one to six eggs a day predominantly inside the roots, although egg laying has also been observed outside the roots (Fig. 2). Lesions formed as a result of nematode activity enlarge and coalesce. Nematodes will only leave the root and migrate into the soil as a result of root decay or overcrowding. During this stage, whilst the worm is seeking a new food source of healthy roots, the area of infestation spreads to adjacent plants.
Figure 1.
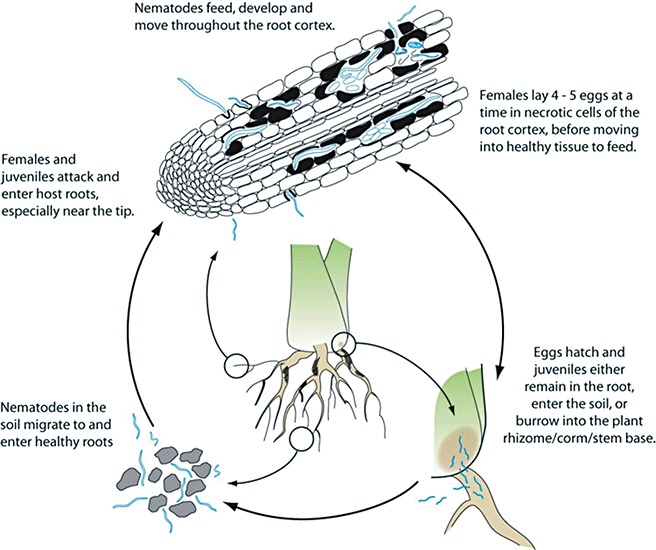
Life and infection cycle of Radopholus similis. Drawing by Vickie Brewster, reproduced by permission from Brooks (2008).
Figure 2.
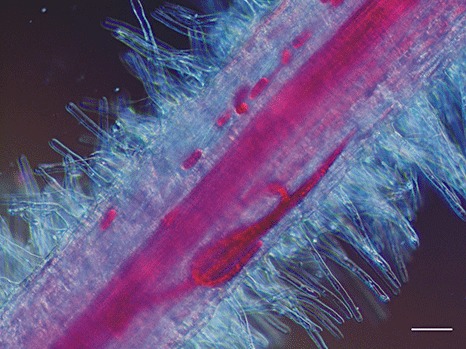
Fuchsin‐stained Radopholus similis female adults and eggs inside a root of Medicago truncatula L. Scale bar, 50 µm.
Most populations of R. similis reproduce best at intermediate (25 °C) or high (30 °C) rather than low (15–20 °C) temperatures (Fallas and Sarah, 1995). Populations introduced into Europe appear to have adapted, reproducing at temperatures too low for tropical populations. In Florida citrus plantations, root infection is greatest in the deeper soil layers where the optimum temperature remains more stable throughout the year. The burrowing nematode's ability to survive adverse conditions is enhanced by three factors: (i) a wide host range; (ii) a short life cycle, allowing rapid reproduction during favourable periods; and (iii) the ability of females to reproduce without males (MacGowan, 1977). The latter reproduction strategy was thought to be parthenogenesis, but it has been shown that R. similis rather is a hermaphrodite. In females that have not mated with a male 50–60 days after the fourth moult, self‐fertilization takes place with rod‐shaped spermatids produced in the ovatestis and matured in the spermatheca (Kaplan and Opperman, 2000). The study of this nematode is relatively easy, as it multiplies quite quickly on carrot discs (Speijer and De Waele, 1997), and nematodes can even be conserved by cryopreservation (Elsen et al., 2007).
OCCURRENCE, SYMPTOMS AND DAMAGE
Radopholus similis is considered to be among the 10 most damaging plant‐parasitic nematodes world‐wide (EPPO Diagnostics Report, 2008). It is of great economic importance in Central and South America, the Caribbean, Africa, Asia and the Pacific, causing what is variously called pepper yellows on pepper, spreading decline on citrus and root rot, blackhead and toppling disease on banana. In West Africa, for example, the yield of plantains can be reduced by more than 50% over the first two crop cycles (Fogain, 2000), whereas, in South‐east Asia, the yield of export bananas cultivated in the Philippines can be reduced by up to 60% (Davide and Marasigan, 1985) as a result of R. similis infection. As a result of the devastating effects R. similis can have on agricultural crops, EPPO has classified the pathotype that can attack banana as an A2 quarantine organism, and the pathotype that can attack citrus as A1, as the latter pathotype is absent from Europe.
Root damage by R. similis has the typical symptoms caused by lesion nematodes: reddish, brownish to black lesions caused by cell wall collapse as nematodes move inter‐ and intracellularly (Duncan and Moens, 2006) (Fig. 3). The roots have extensive cavities and the phloem and cambium may be completely destroyed, leaving nematode‐filled spaces separating the remaining stele from the cortex. External cracks may appear over the lesion (EPPO Diagnostics Report, 2008). Tissue rot occurs following secondary infections with fungi and bacteria (Duncan and Moens, 2006). Infected plants also show poor growth, reduced leaf size and colour alterations. Because of the infection with R. similis, the root system of the plants is weakened, which leads to the toppling over of infected plants, especially those bearing fruits, after a strong gust of wind (Fig. 3). This is the most obvious symptom in the banana field of nematode infection (EPPO Diagnostics Report, 2008).
Figure 3.

Damage caused by Radopholus similis. (a) Toppling over or uprooting of banana plants. (b) Lesions in banana roots as a consequence of R. similis feeding.
PROGRESS IN CONTROL MEASURES
One of the most effective means of controlling R. similis is to prevent the spread of this pest to new areas. A successful quarantine programme was therefore started several decades ago in both Europe and the USA. In other regions, however, the pathogen was often introduced with the Cavendish banana cultivar, which is more susceptible to R. similis than other cultivars (Marin et al., 1998a; Price, 2006). The reduction of nematode populations in the soil before planting and the use of nematode‐free planting or grafting material are therefore of primary importance in the control of R. similis. Burrowing nematode populations can be reduced to an undetectable level by fallow or crop rotation with non‐host plants (e.g. sweet potato, pineapple). Six or seven weeks of flooding can be equally effective. However, the latter method is often difficult to apply as flooding requires a permanent water supply and the land must be levelled (Sarah et al., 1996).
Nematicides (generally organophosphates or carbamates) are traditionally the primary way to control nematodes. Nematicides can increase banana yields by 50% compared with untreated controls (Fogain, 2000). In large commercial plantations of banana, nematode control is based on two to four nematicide treatments each year, having toxic effects on the environment (Chabrier and Quénéhervé, 2003). During the 1970s and 1980s, an estimated 10 000 Costa Rican banana workers were sterilized as a result of the use of a nematicide. The chemical was banned and the Costa Rican government has attempted to control, regulate and monitor pesticide use on banana plantations, but has not always been effective because of inadequate funding and lack of control over transnationals. Companies have placed more effort into the training of personnel and the installation of shower and laundry facilities for workers (Mackey, 1997). In 2007, Wageningen University, the Brazilian Agricultural Research Corporation (EMBRAPA), the International Network for Improvement of Banana and Plantain (INIBAP), the Agricultural Research Centre for International Development (CIRAD) of France and the Catholic University of Leuven, Belgium built an international consortium of stakeholders in the banana production chain to formulate the Pesticide Reduction Plan for Banana. The aim is to secure banana production for small holders and to reduce pesticide input in banana production by at least 50% in the next decade. More attention is being paid to an integrated pest management approach. In Martinique, for example, an alternative cropping system has been developed based on the cleanup of contaminated fields prior to planting. This cleanup is carried out through either a fallow period or an appropriate crop rotation, and then nematode‐free in vitro banana plants are planted. As a consequence, growers are able to cultivate bananas for at least 2–3 years without nematicide application (Chabrier and Quénéhervé, 2008). As R. similis can also infect other underground plant organs, such as corms, the recolonization of bananas by R. similis can be slowed down by treating the corm‐based planting material with hot water (Elsen et al., 2004). Moreover, the introduction of ditches could efficiently isolate field sectors and partially protect other banana plants from infection (Chabrier and Quénéhervé, 2008). The latter method is based on barrier treatments historically used in Florida (Poucher et al., 1967).
Host resistance, defined as the ability of a plant to limit pathogen reproduction, is an effective means of nematode control in many crops. In bananas and plantains, there has been little progress in breeding for resistance to nematodes because of the genetic complexity within the genus Musa and the easy choice of chemical nematicides in the past. Increasing awareness about the negative effects of pesticides has led to a restricted number of permitted nematicides in large parts of the world. Therefore, different banana cultivars and hybrids are being screened for nematode resistance. Promising cultivars have been identified, even partially resistant to other nematodes as well, which will hopefully lead to an improved sustainability of banana production (2006, 2009; Elsen et al., 2002; Fogain and Gowen, 1998; Marin et al., 1998b; Moens et al., 2005; 2009a, 2009b; Stanton, 1999). Next to the identification of resistant cultivars, more and more knowledge is emerging about the chemical and genetic background of resistance. For example, the analysis of resistant plants of a hybrid banana population has indicated that the resistance is, in this case, controlled by two dominant genes (Dochez et al., 2009). Resistant cultivars have been observed to show significant increases in condensed tannins and flavan‐3,4‐diols in roots relative to susceptible cultivars (Collingborn et al., 2000). Another study has shown that resistant cultivars have constitutively significantly higher levels of lignin in the vascular bundle and cell wall‐bound ferulic acid esters in the cortex (Wuyts et al., 2007).
The genetic engineering of bananas may be an important strategy for the reduction of nematicides. It has been shown that a transformed Cavendish banana expressing a rice cystatin, a nematode cysteine proteinase inhibitor, has 70% less nematodes per gram of root relative to the control 8 weeks after infection (Atkinson et al., 2004). The commercial use of genetically modified (GM) crops in Africa is currently limited to maize, cotton and soybean in Egypt, Burkina Faso and South Africa (Karembu et al., 2009). It can be expected that GM crops will increasingly find their way into the field as several developing countries have recently put in place policies and regulatory frameworks to support the responsible and safe use of biotechnology. Field trials with genetically modified banana cultivars with viral resistance and resistance against black sigatoka are currently ongoing in Egypt and Uganda, respectively (Karembu et al., 2009). Other promising reports come from recent studies on biological control measures. The reduction of R. similis infection has been observed from the application of Fusarium oxysporum, Paecilomyces lilacinus, Bacillus firmus and arbuscular mycorrhizal fungi to banana (Athman et al., 2007; Elsen et al., 2008; Kilama et al., 2007; Mendoza and Sikora, 2009; Mendoza et al., 2008; Sikora et al., 2008; Vu et al., 2006). These antagonists could be useful in controlling R. similis infection in banana seedlings.
THE FIRST STEPS TOWARDS A MOLECULAR UNDERSTANDING OF THE PATHOGEN
Questioning the taxonomic classification of R. similis within the Pratylenchidae
Plant parasitism among nematodes has arisen (at least) three times independently during evolution, resulting in the following three different taxonomic orders: Rhabditida, Triplonchida and Dorylaimida (Blaxter et al., 1998). The genus Radopholus belongs to the family of the Pratylenchidae within the Tylenchoidea superfamily of the Rhabditid order (De Ley and Blaxter, 2002). The inclusion of the burrowing endoparasitic nematodes in a single family Pratylenchidae is classically defined by morphological characteristics that are probably the result of convergent evolution related to similar feeding modes. Several molecular analyses have proven that the Pratylenchidae family is polyphyletic and that R. similis is more closely related to ectoparasitic and cyst‐forming endoparasitic nematodes of the Hoplolaimidae family (Bert et al., 2008; Holterman et al., 2009; Subbotin et al., 2006) (Fig. 4). Alternative phylogenetic positions of R. similis were tested in the molecular analysis of Holterman et al. (2009), but these were statistically rejected. On closer inspection, there do exist a number of morphological characters that would support the placement of R. similis within the Hoplolaimidae. The strong sexual dimorphism in the cephalic region is a common feature of R. similis and Hoplolaimidae, but is absent in other Pratylenchidae (Siddiqi, 2000). Moreover, Radopholus and Radopholoides are the only members of the Pratylenchidae with a protrusible gubernaculum, a trait that is commonly found in Hoplolaimidae (Siddiqi, 2000). With developing understanding of plant–nematode interactions in the economically very important genera Radopholus and Pratylenchus, incorrect assumptions of the monophyly of burrowing endoparasitic nematodes versus convergence have critical implications when extrapolating the insights of one group (i.e. Pratylenchus spp.) to another (i.e. Radopholus spp.) (Bert et al., 2008).
Figure 4.
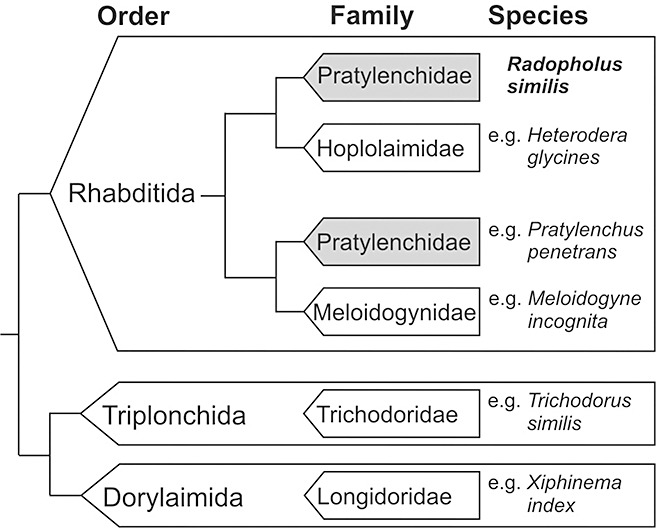
Schematic representation of the phylogenetic relationships among some selected plant‐parasitic nematode taxa following the nomenclature of De Ley and Blaxter (2002). Radopholus similis has an aberrant phylogenetic position within the Pratylenchidae (indicated in grey) (Bert et al., 2008; Holterman et al., 2009; Subbotin et al., 2006). Examples of the most well‐known species are given for each family.
From expressed sequence tag (EST) generation to detailed gene characterization
Expressed sequence tag (EST) analysis is an effective way to obtain a first overview of the transcriptome of an organism, and has become increasingly popular in nematology (Wylie et al., 2004). For R. similis, 7007 ESTs have been generated and analysed recently (Jacob et al., 2008). About 31% of the unigenes lack homology to any sequence in the database and are so‐called orphan sequences. Among those are some of the most abundantly expressed genes, such as cluster 1 (Jacob et al., 2008). This unknown cluster represents a disproportionately large amount (8%) of the total number of ESTs and was later demonstrated to be of mitochondrial origin (Jacob et al., 2009). The majority of the unigenes showed homology to sequences from the database, and several genes possibly important in parasitism were identified, such as endo‐1,4‐β‐glucanase, endoxylanase, fatty acid and retinol binding proteins, and some genes with unknown function that are exclusive to parasitic nematodes (Jacob et al., 2008).
Interesting targets for nematode control are genes that occur exclusively in nematodes, as effects on other organisms are expected to be minimal. As many as 9% of all unigenes of the EST analysis were nematode specific, being promising targets (Jacob et al., 2008). Examples of such genes are transthyretin‐like genes, characterized by Jacob et al. (2007). The expression of four ttl genes was analysed spatially and temporally. Expression was located either in the ventral nerve chord or in the region around the vulva, suggesting a function in the central nerve system. Moreover, ttl genes are expressed in all life stages except embryos, and a search through all nematode ESTs revealed a higher abundance in parasitic nematodes and, especially, in parasitic stages. However, the majority of the Caenorhabditis elegans ttl genes do not show an RNA interference (RNAi) phenotype (Rogers et al., 2008), making ttl genes less attractive as a possible target for nematode control.
Characterization of plant cell wall‐degrading enzymes
To invade plant tissue, plant‐parasitic nematodes face the physical barrier formed by the plant cell wall. To overcome this barrier, these nematodes produce cell wall‐degrading and cell wall‐modifying proteins in the pharyngeal glands and secrete these enzymes through the stylet. The combinatorial effect of this enzymatic activity and the physical damage caused by the protrusions of the stylet enable the nematode to break down the cell walls. Over the last decade, different genes encoding cell wall‐degrading enzymes have been isolated from various plant‐parasitic nematodes, mainly sedentary. These genes generally occur in extensive gene families, e.g. recently the genome of Meloidogyne incognita has been shown to possess more than 60 cell wall‐degrading enzymes (Abad et al., 2008).
Cellulases or endo‐1,4‐β‐glucanases of the glycosyl hydrolase family 5 (GHF5), which can degrade cellulose, have been found in the nematode genera Heterodera, Globodera and Meloidogyne (Abad et al., 2008; Bera‐Maillet et al., 2000; 2002, 2004; Goellner et al., 2000; Ledger et al., 2006; Rosso et al., 1999; Smant et al., 1998; Yan et al., 2001). In addition to these sedentary nematodes, GHF5 endoglucanases have also been found in the migratory nematodes Pratylenchus penetrans (Uehara et al., 2001), Pratylenchus coffeae and Ditylenchus africanus (Kyndt et al., 2008). In R. similis, four putative endoglucanases have been characterized, two containing a carbohydrate‐binding module (CBM) (Haegeman et al., 2008). Interestingly, these endoglucanases are most similar to endoglucanases from cyst nematodes (Kyndt et al., 2008), confirming the close relationship of Radopholus and Hoplolaimidae. All genes are expressed in the gland cell region of the nematode. One gene shows strong expression in eggs and juveniles, whereas the others are only expressed in adults: a very strong expression in females in contrast with males that have either no or a very low expression. This low expression of endoglucanase genes in males can be linked to the lifestyle of the nematodes, as males have a degenerate stylet and are thought to be non‐feeding (Haegeman et al., 2008). Males only migrate in search for females and therefore most probably follow the path burrowed by the females (Steiner and Buhrer, 1933).
In contrast with the endoglucanases, a characterized putative endoxylanase did not show any differential expression between females and males (Haegeman et al., 2009a). Endoxylanases are able to degrade xylan, the main component of hemicellulose. In nematodes, endoxylanases have only been found in Meloidogyne species (Abad et al., 2008; Mitreva‐Dautova et al., 2006), and so the endoxylanase identified in R. similis is the first of its kind to be identified in migratory nematodes. Moreover, it is the first animal endoxylanase containing a putative CBM. Interestingly, when the gene was downregulated by RNAi, the treated nematodes were less infective, with an average decrease in infection of 60%. This suggests that the endoxylanase gene is important for R. similis to effectively infect its host plant. Moreover, it proves that the RNAi technique is also effective in migratory nematodes and could be promising in the future to combat different types of nematode. It should, however, be noted that the efficacy of the technique depends on the targeted gene region, and that, in one of the replicas of the experiment, dsRNA failed to reduce the expression level of the gene (Haegeman et al., 2009a). This indicates that the selection of the target region and optimization are necessary to obtain consistent positive results.
Radopholus similis harbours an endosymbiotic Wolbachia bacterium
Although other endosymbiotic bacteria have been identified in plant‐parasitic nematodes (Noel and Atibalentja, 2006; Vandekerckhove et al., 2000), R. similis is the only nematode to date that has been found to harbour a Wolbachia‐like bacterium (Haegeman et al., 2009b; Jacob et al., 2008). Wolbachia is an endosymbiotic bacterium that belongs to the Rickettsiaceae and occurs widely in insects and filarial nematodes. In insects, the bacterium acts as a true parasite that can cause reproductive manipulations, whereas, in filarial nematodes, Wolbachia is a mutualist as the nematodes cannot survive without this endosymbiont which acts as a source of essential metabolites (Fenn and Blaxter, 2006). Wolbachia from R. similis is quite diverged from all other known Wolbachia, and suggests a more widespread occurrence of this bacterium. Its role remains unknown, although all individual nematodes seem to be infected (Haegeman et al., 2009b). The possible function of the symbiont in R. similis remains an intriguing question. As Wolbachia in filarial nematodes is thought to supply its nematode host with essential metabolites and is necessary for survival, it could be expected that this is also the case for R. similis.
CONCLUSIONS
Over 80 million tonnes of bananas are produced annually world‐wide, and this number is increasing (FAO, 2009), although the yield can be seriously reduced as a result of infection with R. similis. As nematicide treatments are being banned, an active search for alternative equally effective control measures is ongoing. Promising results have recently been obtained using in vitro nematode‐free plantlets (Chabrier and Quénéhervé, 2008), the screening for new banana cultivars and hybrids for natural resistance (2006, 2009; Elsen et al., 2002; Fogain and Gowen, 1998; Moens et al., 2005; 2009a, 2009b; Stanton, 1999) and biological control measures (Athman et al., 2007; Elsen et al., 2008; Kilama et al., 2007; Mendoza and Sikora, 2009; Mendoza et al., 2008; Sikora et al., 2008; Vu et al., 2006). Nevertheless, more research is needed to better contain this pest, and molecular knowledge will certainly attribute to our understanding of the pathogen.
During the past decade, a tremendous amount of molecular information has become available for several nematodes, mostly under the form of EST projects. For plant‐parasitic nematodes, the focus has been mainly on sedentary nematodes, with the completion of the sequence of two root‐knot nematode genomes as the greatest achievement so far (Abad et al., 2008; Opperman et al., 2008). Recently, the focus for EST projects has shifted towards migratory nematodes, expanding the molecular information available for plant‐parasitic nematodes. As the migratory nematode R. similis is a major pest in the tropical world, ESTs have also been generated for this species (Jacob et al., 2008). In addition, several studies have shown that R. similis is closely related to cyst nematodes (Bert et al., 2008; Holterman et al., 2009; Subbotin et al., 2006). Given the results of the current and previous molecular phylogenetic analyses, and the morphological heterogeneity of the Pratylenchidae, a revision of the family seems necessary (Holterman et al., 2009). This apparent relationship to cyst nematodes makes it very interesting to study the molecular background of R. similis, as comparisons between sedentary nematodes and their close migratory relative could shed more light on the processes important in cyst development and the evolutionary transition from a migratory to a sedentary lifestyle. Moreover, molecular comparisons between migratory and sedentary nematodes can reveal conserved nematode‐specific sequences. These can be considered as useful targets for combating nematode infection, for example through in planta RNAi (Gheysen and Vanholme, 2007; Rosso et al., 2009). This technique could be promising, as it has been demonstrated that in vitro RNAi is effective in reducing the infectivity of R. similis (Haegeman et al., 2009a).
Present address: Soil Service of Belgium npo, W. de Croylaan 48, 3001 Heverlee, Belgium.
REFERENCES
- Abad, P. , Gouzy, J. , Aury, J.M. , Castagnone‐Sereno, P. , Danchin, E.G.J. , Deleury, E. , Perfus‐Barbeoch, L. , Anthouard, V. , Artiguenave, F. , Blok, V.C. , Caillaud, M.C. , Coutinho, P.M. , Dasilva, C. , De Luca, F. , Deau, F. , Esquibet, M. , Flutre, T. , Goldstone, J.V. , Hamamouch, N. , Hewezi, T. , Jaillon, O. , Jubin, C. , Leonetti, P. , Magliano, M. , Maier, T.R. , Markov, G.V. , McVeigh, P. , Pesole, G. , Poulain, J. , Robinson‐Rechavi, M. , Sallet, E. , Segurens, B. , Steinbach, D. , Tytgat, T. , Ugarte, E. , Van Ghelder, C. , Veronico, P. , Baum, T.J. , Blaxter, M. , Bleve‐Zacheo, T. , Davis, E.L. , Ewbank, J.J. , Favery, B. , Grenier, E. , Henrissat, B. , Jones, J.T. , Laudet, V. , Maule, A.G. , Quesneville, H. , Rosso, M.N. , Schiex, T. , Smant, G. , Weissenbach, J. and Wincker, P. (2008) Genome sequence of the metazoan plant‐parasitic nematode Meloidogyne incognita . Nat. Biotechnol. 26, 909–915. [DOI] [PubMed] [Google Scholar]
- Athman, S.Y. , Dubois, T. , Coyne, D. , Gold, C.S. , Labuschagne, N. and Viljoen, A. (2007) Effect of endophytic Fusarium oxysporum on root penetration and reproduction of Radopholus similis in tissue culture‐derived banana (Musa spp.) plants. Nematology, 9, 599–607. [Google Scholar]
- Atkinson, H.J. , Grimwood, S. , Johnston, K. and Green, J. (2004) Prototype demonstration of transgenic resistance to the nematode Radopholus similis conferred on banana by a cystatin. Transgenic Res. 13, 135–142. [DOI] [PubMed] [Google Scholar]
- Bera‐Maillet, C. , Arthaud, L. , Abad, P. and Rosso, M.N. (2000) Biochemical characterization of MI‐ENG1, a family 5 endoglucanase secreted by the root‐knot nematode Meloidogyne incognita . Eur. J. Biochem. 267, 3255–3263. [DOI] [PubMed] [Google Scholar]
- Bert, W. , Leliaert, F. , Vierstraete, A.R. , Vanfleteren, J.R. and Borgonie, G. (2008) Molecular phylogeny of the Tylenchina and evolution of the female gonoduct (Nematoda : Rhabditida). Mol. Phylogenet. Evol. 48, 728–744. [DOI] [PubMed] [Google Scholar]
- Blaxter, M.L. , De Ley, P. , Garey, J.R. , Liu, L.X. , Scheldeman, P. , Vierstraete, A. , Vanfleteren, J.R. , Mackey, L.Y. , Dorris, M. , Frisse, L.M. , Vida, J.T. and Thomas, W.K. (1998) A molecular evolutionary framework for the phylum Nematoda. Nature, 392, 71–75. [DOI] [PubMed] [Google Scholar]
- Brooks, F.E. (2008) Burrowing nematode. Plant Health Instr. doi: 10.1094/PHI‐I‐2008‐1020‐01 [Google Scholar]
- Chabrier, C. and Quénéhervé, P. (2003) Control of the burrowing nematode (Radopholus similis Cobb) on banana: impact of the banana field destruction method on the efficiency of the following fallow. Crop Prot. 22, 121–127. [Google Scholar]
- Chabrier, C. and Quénéhervé, P. (2008) Preventing nematodes from spreading: a case study with Radopholus similis (Cobb) Thorne in a banana field. Crop Prot. 27, 1237–1243. [Google Scholar]
- Cobb, N.A. (1893) Nematodes, Mostly Australian and Fijian. Sydney: F. Cunninghame & Co. [Google Scholar]
- Cobb, N.A. (1906) Fungus Maladies of the Sugar Cane with Notes on Associated Insects and Nematodes. Honolulu, HI: Hawaiian Sugar Planters Association, Division of Pathology and Physiology Bulletin. [Google Scholar]
- Collingborn, F.M.B. , Gowen, S.R. and Mueller‐Harvey, I. (2000) Investigations into the biochemical basis for nematode resistance in roots of three Musa cultivars in response to Radopholus similis infection. J. Agric. Food Chem. 48, 5297–5301. [DOI] [PubMed] [Google Scholar]
- Davide, R.G. and Marasigan, L.Q. (1985) Yield loss assessment and evaluation of resistance of banana cultivars to the nematodes Radopholus similis Thorne and Meloidogyne incognita Chitwood. Philipp. Agric. 68, 335–349. [Google Scholar]
- De Ley, P. and Blaxter, M. (2002) Systematic position and phylogeny In: The Biology of Nematodes (Lee D.L., ed.), pp. 1–30. London: Taylor & Francis. [Google Scholar]
- De Waele, D. and Elsen, A. (2007) Challenges in tropical plant nematology. Annu. Rev. Phytopathol. 45, 457–485. [DOI] [PubMed] [Google Scholar]
- Dochez, C. , Dusabe, J. , Whyte, J. , Tenkouano, A. , Ortiz, R. and De Waele, D. (2006) New sources of resistance to Radopholus similis in Musa germplasm from Asia. Australas. Plant Pathol. 35, 481–485. [Google Scholar]
- Dochez, C. , Tenkouano, A. , Ortiz, R. , Whyte, J. and De Waele, D. (2009) Host plant resistance to Radopholus similis in a diploid banana hybrid population. Nematology, 11, 329–335. [Google Scholar]
- Duncan, L.W. and Moens, M. (2006) Migratory endoparasitic nematodes In: Plant Nematology (Perry R.N. and Moens M., eds), pp. 123–152. Oxfordshire: CABI Publishing. [Google Scholar]
- Elbadri, G.A.A. , De Ley, P. , Waeyenberge, L. , Vierstraete, A. , Moens, M. and Vanfleteren, J. (2002) Intraspecific variation in Radopholus similis isolates assessed with restriction fragment length polymorphism and DNA sequencing of the internal transcribed spacer region of the ribosomal RNA cistron. Int. J. Parasitol. 32, 199–205. [DOI] [PubMed] [Google Scholar]
- Elsen, A. , Stoffelen, R. , Tuyet, N.T. , Baimey, H. , Dupre de Boulois, H. and De Waele, D. (2002) In vitro screening for resistance to Radopholus similis in Musa spp. Plant Sci. 163, 407–416. [Google Scholar]
- Elsen, A. , Goossens, B. , Belpaire, B. , Neyens, A. , Speijer, P. and De Waele, D. (2004) Recolonisation by nematodes of hot water treated cooking banana planting material in Uganda. Nematology, 6, 215–221. [Google Scholar]
- Elsen, A. , Vallterra, S.F. , Van Vauwe, T. , Thuy, T.T.T. , Swennen, R. , De Waele, D. and Panis, B. (2007) Cryopreservation of Radopholus similis, a tropical plant‐parasitic nematode. Cryobiology, 55, 148–157. [DOI] [PubMed] [Google Scholar]
- Elsen, A. , Gervacio, D. , Swennen, R. and De Waele, D. (2008) AMF‐induced biocontrol against plant parasitic nematodes in Musa sp.: a systemic effect. Mycorrhiza, 18, 251–256. [DOI] [PubMed] [Google Scholar]
- European and Mediterranean Plant Protection Organization (EPPO) Diagnostics Report (2008) Radopholus similis. EPPO Bull. 38, 374–378. [Google Scholar]
- Fallas, G. and Sarah, J.L. (1995) Effect of temperature on the in vitro multiplication of seven Radopholus similis isolates from different banana producing zones of the world. Fundam. Appl. Nematol. 18, 445–449. [Google Scholar]
- FAO (2009) Food and Agriculture Organization of the United Nations. Available at: http://faostat.fao.org[accessed during Nov 2009.
- Fenn, K. and Blaxter, M. (2006) Wolbachia genomes: revealing the biology of parasitism and mutualism. Trends Parasitol. 22, 60–65. [DOI] [PubMed] [Google Scholar]
- Fogain, R. (2000) Effect of Radopholus similis on plant growth and yield of plantains (Musa, AAB). Nematology, 2, 129–133. [Google Scholar]
- Fogain, R. and Gowen, S.R. (1998) ‘Yangambi km5’ (Musa AAA, Ibota subgroup): a possible source of resistance to Radopholus similis and Pratylenchus goodeyi . Fundam. Appl. Nematol. 21, 75–80. [Google Scholar]
- Gao, B. , Allen, R. , Maier, T. , Davis, E.L. , Baum, T.J. and Hussey, R.S. (2002) Identification of a new beta‐1,4‐endoglucanase gene expressed in the esophageal subventral gland cells of Heterodera glycines . J. Nematol. 34, 12–15. [PMC free article] [PubMed] [Google Scholar]
- Gao, B.L. , Allen, R. , Davis, E.L. , Baum, T.J. and Hussey, R.S. (2004) Developmental expression and biochemical properties of a beta‐1,4‐endoglucanase family in the soybean cyst nematode, Heterodera glycines . Mol. Plant Pathol. 5, 93–104. [DOI] [PubMed] [Google Scholar]
- Gheysen, G. and Vanholme, B. (2007) RNAi from plants to nematodes. Trends Biotechnol. 25, 89–92. [DOI] [PubMed] [Google Scholar]
- Goellner, M. , Smant, G. , De Boer, J.M. , Baum, T.J. and Davis, E.L. (2000) Isolation of beta‐1,4‐endoglucanase genes from Globodera tabacum and their expression during parasitism. J. Nematol. 32, 154–165. [PMC free article] [PubMed] [Google Scholar]
- Haegeman, A. , Jacob, J. , Vanholme, B. , Kyndt, T. and Gheysen, G. (2008) A family of GHF5 endo‐1,4‐beta‐glucanases in the migratory plant‐parasitic nematode Radopholus similis . Plant Pathol. 57, 581–590. [Google Scholar]
- Haegeman, A. , Vanholme, B. and Gheysen, G. (2009a) Characterization of a putative endoxylanase in the migratory plant‐parasitic nematode Radopholus similis . Mol. Plant Pathol. 10, 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegeman, A. , Vanholme, B. , Jacob, J. , Vandekerckhove, T.T.M. , Claeys, M. , Borgonie, G. and Gheysen, G. (2009b) An endosymbiotic bacterium in a plant‐parasitic nematode: member of a new Wolbachia supergroup. Int. J. Parasitol. 39, 1045–1054. [DOI] [PubMed] [Google Scholar]
- Holterman, M. , Karssen, G. , Van Den Elsen, S. , Van Megen, H. , Bakker, J. and Helder, J. (2009) Small subunit rDNA‐based phylogeny of the Tylenchida sheds light on relationships among some high‐impact plant‐parasitic nematodes and the evolution of plant feeding. Phytopathology, 99, 227–235. [DOI] [PubMed] [Google Scholar]
- Huettel, R.N. , Dickson, D.W. and Kaplan, D.T. (1984) Radopholus citrophilus sp. n. (Nematoda), a sibling species of Radopholus similis . Proc. Helm. Soc. Wash. 51, 35. [Google Scholar]
- Jacob, J. , Vanholme, B. , Haegeman, A. and Gheysen, G. (2007) Four transthyretin‐like genes of the migratory plant‐parasitic nematode Radopholus similis: members of an extensive nematode‐specific family. Gene, 402, 9–19. [DOI] [PubMed] [Google Scholar]
- Jacob, J. , Mitreva, M. , Vanholme, B. and Gheysen, G. (2008) Exploring the transcriptome of the burrowing nematode Radopholus similis . Mol. Genet. Genomics 280, 1–17. [DOI] [PubMed] [Google Scholar]
- Jacob, J. , Vanholme, B. , Van Leeuwen, T. and Gheysen, G. (2009) A unique genetic code change in the mitochondrial genome of the parasitic nematode Radopholus similis . BMC Res. Notes 2, 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, D.T. and Oppermann, C.H. (1997) Genome similarity implies that citrus‐parasitic burrowing nematodes do not represent a unique species. J. Nematol. 29, 430–440. [PMC free article] [PubMed] [Google Scholar]
- Kaplan, D.T. and Opperman, C.H. (2000) Reproductive strategies and karyotype of the burrowing nematode, Radopholus similis . J. Nematol. 32, 126–133. [PMC free article] [PubMed] [Google Scholar]
- Kaplan, D.T. , Vanderspool, M.C. and Opperman, C.H. (1997) Sequence tag site and host range assays demonstrate that Radopholus similis and R. citrophilus are not reproductively isolated. J. Nematol. 29, 421–429. [PMC free article] [PubMed] [Google Scholar]
- Kaplan, D.T. , Thomas, W.K. , Frisse, L.M. , Sarah, J.L. , Stanton, J.M. , Speijer, P.R. , Marin, D.H. and Opperman, C.H. (2000) Phylogenetic analysis of geographically diverse Radopholus similis via rDNA sequence reveals a monomorphic motif. J. Nematol. 32, 134–142. [PMC free article] [PubMed] [Google Scholar]
- Karembu, M. , Nguthi, F. and Ismail, H. (2009) Biotech Crops in Africa: The Final Frontier. Nairobi: ISAAA AfriCenter. [Google Scholar]
- Kilama, P. , Dubois, T. , Coyne, D. , Nicre, B. , Gold, C.S. and Adipala, E. (2007) Antagonism of Paecilomyces spp. isolated from banana (Musa spp.) roots and rhizosphere against Radopholus similis . Nematropica, 37, 215–225. [Google Scholar]
- Kyndt, T. , Haegeman, A. and Gheysen, G. (2008) Evolution of GHF5 endoglucanase gene structure in plant‐parasitic nematodes: no evidence for an early domain shuffling event. BMC Evol. Biol. 8, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledger, T.N. , Jaubert, S. , Bosselut, N. , Abad, P. and Rosso, M.N. (2006) Characterization of a new beta‐1,4‐endoglucanase gene from the root‐knot nematode Meloidogyne incognita and evolutionary scheme for phytonematode family 5 glycosyl hydrolases. Gene, 382, 121–128. [DOI] [PubMed] [Google Scholar]
- MacGowan, J.B. (1977) The burrowing nematode Radopholus similis (C, 1893) T 1949. Nematology Circ . Fla. Dpt. Agric. 27. [Google Scholar]
- Mackey, C. (1997) Going bananas for eco safety. Americas, 49, 3. [Google Scholar]
- Marin, D.H. , Sutton, T.B. and Barker, K.R. (1998a) Dissemination of bananas in Latin America and the Caribbean and its relationship to the occurrence of Radopholus similis . Plant Dis. 82, 964–974. [DOI] [PubMed] [Google Scholar]
- Marin, D.H. , Sutton, T.B. , Barker, K.R. , Kaplan, D.T. and Opperman, C.H. (1998b) Burrowing nematode resistance of black sigatoka resistant hybrids. Nematropica, 28, 241–247. [Google Scholar]
- Mendoza, A.R. and Sikora, R.A. (2009) Biological control of Radopholus similis in banana by combined application of the mutualistic endophyte Fusarium oxysporum strain 162, the egg pathogen Paecilomyces lilacinus strain 251 and the antagonistic bacteria Bacillus firmus . Biocontrol, 54, 263–272. [Google Scholar]
- Mendoza, A.R. , Kiewnick, S. and Sikora, R.A. (2008) In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis, the root‐knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci . Biocontrol Sci. Tech. 18, 377–389. [Google Scholar]
- Mitreva‐Dautova, M. , Roze, E. , Overmars, H. , De Graaff, L. , Schots, A. , Helder, J. , Goverse, A. , Bakker, J. and Smant, G. (2006) A symbiont‐independent endo‐1,4‐beta‐xylanase from the plant‐parasitic nematode Meloidogyne incognita . Mol. Plant–Microbe Interact. 19, 521–529. [DOI] [PubMed] [Google Scholar]
- Moens, T. , Araya, M. , Swennen, R. and De Waele, D. (2005) Screening of Musa cultivars for resistance to Helicotylenchus multicinctus, Meloidogyne incognita, Pratylenchus coffeae and Radopholus similis . Australas. Plant Pathol. 34, 299–309. [Google Scholar]
- Noel, G.R. and Atibalentja, N. (2006) ‘Candidatus Paenicardinium endonii’, an endosymbiont of the plant‐parasitic nematode Heterodera glycines (Nemata: Tylenchida), affiliated to the phylum Bacteroidetes. Int. J. Syst. Evol. Microbiol. 56, 1697–1702. [DOI] [PubMed] [Google Scholar]
- Opperman, C.H. , Bird, D.M. , Williamson, V.M. , Rokhsar, D.S. , Burke, M. , Cohn, J. , Cromer, J. , Diener, S. , Gajan, J. , Graham, S. , Houfek, T.D. , Liu, Q. , Mitros, T. , Schaff, J. , Schaffer, R. , Scholl, E. , Sosinski, B.R. , Thomas, V.P. and Windham, E. (2008) Sequence and genetic map of Meloidogyne hapla: a compact nematode genome for plant parasitism. Proc. Natl Acad. Sci. USA, 105, 14 802–14 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poucher, C. , Ford, H.W. , Suit, R.F. and DuCharme, E.P. (1967) Burrowing nematode in citrus. Fl. Dept. Agric. Bull. 7. [Google Scholar]
- Price, N.S. (2006) The banana burrowing nematode, Radopholus similis (Cobb) Thorne, in the Lake Victoria region of East Africa: its introduction, spread and impact. Nematology, 8, 801–817. [Google Scholar]
- Quénéhervé, P. , Salmon, F. , Topart, P. and Horry, J.P. (2009a) Nematode resistance in bananas: screening results on some new Mycosphaerella resistant banana hybrids. Euphytica, 165, 137–143. [Google Scholar]
- Quénéhervé, P. , Valette, C. , Topart, P. , Montcel, H.T. and Salmon, F. (2009b) Nematode resistance in bananas: screening results on some wild and cultivated accessions of Musa spp. Euphytica, 165, 123–136. [Google Scholar]
- Rogers, A. , Antoshechkin, I. , Bieri, T. , Blasiar, D. , Bastiani, C. , Canaran, P. , Chan, J. , Chen, W.J. , Davis, P. , Fernandes, J. , Fiedler, T.J. , Han, M. , Harris, T.W. , Kishore, R. , Lee, R. , Mckay, S. , Mulller, H.M. , Nakamura, C. , Ozersky, P. , Petcherski, A. , Schindelman, G. , Schwarz, E.M. , Spooner, W. , Tuli, M.A. , Van Auken, K. , Wang, D. , Wang, X. , Williams, G. , Yook, K. , Durbin, R. , Stein, L.D. , Spieth, J. and Sternberg, P. (2008) WormBase 2007. Nucleic Acids Res. 36, D612–D617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso, M.N. , Favery, B. , Piotte, C. , Arthaud, L. , De Boer, J.M. , Hussey, R.S. , Bakker, J. , Baum, T.J. and Abad, P. (1999) Isolation of a cDNA encoding a beta‐1,4‐endoglucanase in the root‐knot nematode Meloidogyne incognita and expression analysis during plant parasitism. Mol. Plant–Microbe Interact. 12, 585–591. [DOI] [PubMed] [Google Scholar]
- Rosso, M.N. , Jones, J.T. and Abad, P. (2009) RNAi and functional genomics in plant parasitic nematodes. Annu. Rev. Phytopathol. 47, 207–232. [DOI] [PubMed] [Google Scholar]
- Sarah, J.L. , Pinochet, J. and Stanton, J. (1996) The Burrowing Nematode of Bananas, Radopholus similis Cobb, 1913. Montpellier: INIBAP. [Google Scholar]
- Sher, S.A. (1968) Revision of the genus Radopholus Thorne, 1949 (Nematoda: Tylenchoidea). Proc. Helm. Soc. Wash. 35, 219–237. [Google Scholar]
- Siddiqi, M.R. (2000) Tylenchida: Parasites of Plants and Insects. Oxfordshire: CABI Publishing. [Google Scholar]
- Sikora, R.A. , Pocasangre, L. , Felde, A. , Niere, B. , Vu, T.T. and Dababat, A.A. (2008) Mutualistic endophytic fungi and in‐planta suppressiveness to plant parasitic nematodes. Biol. Control, 46, 15–23. [Google Scholar]
- Smant, G. , Stokkermans, J.P.W. , Yan, Y.T. , De Boer, J.M. , Baum, T.J. , Wang, X.H. , Hussey, R.S. , Gommers, F.J. , Henrissat, B. , Davis, E.L. , Helder, J. , Schots, A. and Bakker, J. (1998) Endogenous cellulases in animals: isolation of beta‐1,4‐endoglucanase genes from two species of plant‐parasitic cyst nematodes. Proc. Natl Acad. Sci. USA, 95, 4906–4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speijer, P.R. and De Waele, D. (1997) Screening of Musa Germplasm for Resistance and Tolerance to Nematodes. Montpellier: INIBAP. [Google Scholar]
- Stanton, J.M. (1999) Assessment of resistance and tolerance of in vitro‐propagated banana plants to burrowing nematode, Radopholus similis . Aust. J. Exp. Agric. 39, 891–895. [Google Scholar]
- Steiner, G. and Buhrer, E.M. (1933) The nematode Tylenchus similis, Cobb. as a parasite of the tea plant (Thea sinensis, Linn.), its sexual dimorphism, and its nemic associates in the same host. Parasitol. Res. 5, 412–420. [Google Scholar]
- Subbotin, S.A. , Sturhan, D. , Chizhov, V.N. , Vovlas, N. and Baldwin, J.G. (2006) Phylogenetic analysis of Tylenchida Thorne, 1949 as inferred from D2 and D3 expansion fragments of the 28S rRNA gene sequences. Nematology, 8, 455–474. [Google Scholar]
- Thorne, G. (1949) On the classification of the Tylenchida new order (Nematoda: Phasmidia). Proc. Helm. Soc. Wash. 16, 37–73. [Google Scholar]
- Trinh, P.Q. , Waeyenberge, L. , Nguyen, C.N. , Baldwin, J.G. , Karssen, G. and Moens, M. (2009) Apratylenchus vietnamensis gen. n., sp n. and A. binhi gen. n., sp n., sedentary Pratylenchidae (Nematoda: Tylenchida) from coffee in Vietnam, with proposal of Apratylenchinae subfam. n. Nematology, 11, 565–581. [Google Scholar]
- Uehara, T. , Kushida, A. and Momota, Y. (2001) PCR‐based cloning of two beta‐1,4‐endoglucanases from the root‐lesion nematode Pratylenchus penetrans . Nematology, 3, 335–341. [Google Scholar]
- Valette, C. , Mounport, D. , Nicole, M. , Sarah, J.L. and Baujard, P. (1998) Scanning electron microscope study of two African populations of Radopholus similis (Nematoda: Pratylenchidae) and proposal of R. citrophilus as a junior synonym of R. similis . Fundam. Appl. Nematol. 21, 139–146. [Google Scholar]
- Vandekerckhove, T.T.M. , Willems, A. , Gillis, M. and Coomans, A. (2000) Occurrence of novel verrucomicrobial species, endosymbiotic and associated with parthenogenesis in Xiphinema americanum‐group species (Nematoda, Longidoridae). Int. J. Syst. Evol. Microbiol. 50, 2197–2205. [DOI] [PubMed] [Google Scholar]
- Vu, T. , Hauschild, R. and Sikora, R.A. (2006) Fusarium oxysporum endophytes induced systemic resistance against Radopholus similis on banana. Nematology, 8, 847–852. [Google Scholar]
- Wuyts, N. , Lognay, G. , Verscheure, M. , Marlier, M. , De Waele, D. and Swennen, R. (2007) Potential physical and chemical barriers to infection by the burrowing nematode Radopholus similis in roots of susceptible and resistant banana (Musa spp.). Plant Pathol. 56, 878–890. [Google Scholar]
- Wylie, T. , Martin, J.C. , Dante, M. , Mitreva, M.D. , Clifton, S.W. , Chinwalla, A. , Waterston, R.H. , Wilson, R.K. and McCarter, J.P. (2004) Nematode.net: a tool for navigating sequences from parasitic and free‐living nematodes. Nucleic Acids Res. 32, D423–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Y.T. , Smant, G. and Davis, E. (2001) Functional screening yields a new beta‐1,4‐endoglucanase gene from Heterodera glycines that may be the product of recent gene duplication. Mol. Plant–Microbe Interact. 14, 63–71. [DOI] [PubMed] [Google Scholar]


