SUMMARY
Mexican lime plants transformed with the 3′‐terminal 549 nucleotides of the Citrus tristeza virus (CTV) genome in sense, antisense and intron‐hairpin formats were analysed for transgene‐derived transcript and short interfering RNA (siRNA) accumulation, and for CTV resistance. Propagations from all sense, antisense and empty‐vector transgenic lines were susceptible to CTV, except for a single sense‐line plant with a complex transgene integration pattern that showed transgene‐derived siRNAs in association with low levels of the transgene‐derived transcript. In contrast, nine of 30 intron‐hairpin lines showed CTV resistance, with 9%–56% of bud‐propagated plants, depending on the line, remaining uninfected on graft inoculation, and the others being susceptible. Although resistance was always associated with the presence of transgene‐derived siRNAs, their level in different sense and intron‐hairpin transformants was variable irrespective of the response to CTV infection. In intron‐hairpin lines with single transgene integration, CTV resistance was correlated with low accumulation of the transgene‐derived transcript rather than with high accumulation of transgene‐derived siRNAs.
INTRODUCTION
RNA silencing is a sequence‐specific mechanism of inhibition of gene expression evolutionarily conserved in most eukaryotes. In plants, post‐transcriptional gene silencing (PTGS) is an RNA pathway that relies on the perception of double‐stranded RNA (dsRNA) as the trigger of a series of reactions starting with dsRNA cleavage, mediated by RNase III‐like enzymes (DICER‐like, DCL), into RNA duplexes of 21–25 nucleotides called small interfering RNAs (siRNAs) (Bernstein et al., 2001; Hamilton and Baulcombe, 1999). On unwinding, one of the siRNA strands is incorporated into the RNA‐induced silencing complex (RISC) and programs it to inactivate complementary single‐stranded RNA (ssRNA) (Hammond et al., 2000). RNA interference (RNAi), a technology based on the use of dsRNA to trigger RNA silencing (Fire et al., 1998), can be achieved by transformation with sense and antisense sequences separated by an intron (intron‐hairpin design). On transcription, the resulting hairpin RNA acts as a strong inducer of PTGS (Smith et al., 2000). This strategy has been used to produce virus‐resistant transgenic plants (Hily et al., 2007; Kreuze et al., 2008; Prins et al., 2008; Waterhouse and Helliwell, 2003), with the accumulation of transgene‐derived siRNAs being regarded as a predictor of resistance to virus infection (Kalantidis et al., 2002). Virus‐infected plants also produce virus‐derived siRNAs presumably arising from dsRNA replicative intermediates and viral ssRNA with extensive secondary structure, or after being converted into dsRNA by a host RNA‐dependent RNA polymerase (Dalmay et al., 2000; Hamilton and Baulcombe, 1999; Molnar et al., 2005). These observations have led to the proposal that RNA silencing arose as an antiviral defence mechanism in primitive eukaryotes (Baulcombe, 2004). Viruses, on their side, have evolved counter‐defence strategies mainly based on the encoding of silencing suppressors (Díaz‐Pendón and Ding, 2008; Qu and Morris, 2005; Voinnet, 2005). The ectopic expression of some of these suppressors in transgenic plants, including several citrus species, induces morphological aberrations resembling viral symptoms (Fagoaga et al., 2005; Ghorbel et al., 2001; Siddiqui et al., 2008; Voinnet, 2005). These aberrations may result from interference of the suppressors with developmental functions orchestrated by another class of host‐encoded small RNAs, the microRNAs, suggesting that a similar interference could underlie symptom induction in natural viral infections (Kasschau et al., 2003; Voinnet, 2005). The success of RNAi against plant viruses depends on whether transgene‐induced PTGS can substantially attenuate or block virus gene expression and, more specifically, the accumulation of silencing suppressors.
Citrus tristeza virus (CTV), genus Closterovirus, family Closteroviridae, incites the decline and death of trees grafted on sour orange (Citrus aurantium L.), with the most severe strains also causing damage on scion varieties regardless of the rootstock (Bar‐Joseph et al., 1989; Moreno et al., 2008). The CTV genome consists of a positive‐sense ssRNA of approximately 19.3 kb organized in 12 open reading frames (ORFs) encoding at least 17 proteins, and 5′ and 3′ untranslated terminal regions (UTRs) (Karasev et al., 1995). The two 5′ proximal ORFs, which are translated from the genomic RNA, encode components of the replicase complex. The 10 3′ proximal ORFs, encoding p33, p6, p65, p61, p27, p25, p18, p13, p20 and p23 proteins, are expressed via 3′ co‐terminal subgenomic RNAs (Hilf et al., 1995) (Fig. 1A). The two p25 and p27 coat proteins, together with p65, p61 and p6, are required for virus assembly (Satyanarayana et al., 2000), while p23, an RNA‐binding protein with a putative zinc‐finger domain (López et al., 2000), is involved in regulating the balance of plus and minus RNA strands during replication (Satyanarayana et al., 2002). When ectopically expressed in transgenic citrus plants, p23 induces aberrations resembling CTV symptoms (Fagoaga et al., 2005; Ghorbel et al., 2001). Furthermore, p25, p20 and p23 act as RNA silencing suppressors in Nicotiana tabacum and Nicotiana benthamiana plants (Lu et al., 2004).
Figure 1.
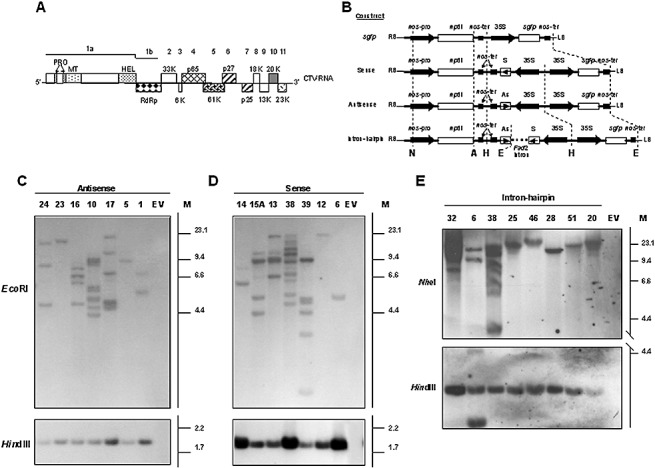
(A) Outline of the Citrus tristeza virus (CTV) genome organization (Karasev et al., 1995), with the numbers at the top indicating open reading frames (ORFs) and the boxes the corresponding proteins or catalytic domains within the polyprotein encoded by ORF 1a (see text for further details). (B) Diagram of the T‐DNA from the binary vector pBin19‐sgfp and constructs expressing a 3′‐moiety of p23 and the 3′UTR (3′p23+3′UTR) in sense, antisense or intron‐hairpin format. The 3′p23+3′UTR transgenes are controlled by the double‐enhanced 35S promoter of Cauliflower mosaic virus (CaMV) and the nopaline synthase terminator (nos‐ter), and flanked by the neomycin phosphotransferase II gene (nptII) between the nos promoter (nos‐pro) and nos‐ter, and by the green fluorescent protein gene (sgfp) between the 35S promoter and nos‐ter. A, E, H and N denote ApaI, EcoRI, HindIII and NheI restriction sites, respectively. (C) Southern blot hybridization of nucleic acid preparations from lime plants transformed with the 3′p23+3′UTR antisense construct (lines 24, 23, 16, 10, 17, 5 and 1), and with the empty vector pBin19‐sgfp (EV). (D) Southern blot hybridization of nucleic acid preparations from lime plants transformed with the 3′p23+3′UTR sense construct (lines 14, 15A, 13, 38, 39, 12 and 6), and with the empty vector pBin19‐sgfp (EV). In (C) and (D), DNA aliquots (20 µg) were digested with EcoRI, which cuts once in the T‐DNA near the left border (LB), or with HindIII, which excises the expression cassette. (E) Southern blot hybridization of nucleic acid preparations from lime plants transformed with the 3′p23+3′UTR intron‐hairpin construct (lines 32, 6, 38, 25, 46, 28, 51 and 20), and with the empty vector pBin19‐sgfp (EV). DNA was digested with NheI, which cuts once at the right of the intron‐hairpin cassette, or with HindIII, which excises the expression cassette. Digoxigenin‐labelled DNA probes were derived from the 3′p23+3′UTR fragment for the sense and antisense constructs, and from the Fad2 intron for the intron‐hairpin construct. M, DNA molecular weight marker II (Roche) (C, D and E).
Previously, we have reported that Mexican lime [Citrus aurantifolia (Christm.) Swing.] transformed with a silenced p23 transgene shows resistance to CTV challenge, with PTGS being associated with multiple p23 transgene integrations. Moreover, when propagations of these transgenic lines were graft or aphid inoculated with CTV, some plants were immune, others moderately resistant and the remainder susceptible to virus infection (Fagoaga et al., 2006). In this study, we have over‐expressed in Mexican lime the 3′‐terminal 549‐nucleotide region of the CTV genome comprising part of the p23 gene and the 3′UTR (3′p23+3′UTR) in sense, antisense and intron‐hairpin formats with the aim of: (i) comparing their ability to induce CTV resistance and (ii) examining the differential accumulation of transgene‐derived transcripts and siRNAs. We report that high levels of transgene‐derived siRNAs are not necessarily associated with resistance to CTV, which is better predicted by low levels of transgene‐derived transcripts.
RESULTS AND DISCUSSION
Genetic transformation of Mexican lime with 3′p23+3′UTR sense, antisense and intron‐hairpin cDNAs
More than 20 independent transformants of Mexican lime were generated for each sense, antisense, intron‐hairpin and empty‐vector (EV) construct class (Fig. 1B) by A. tumefaciens‐mediated transformation and shoot regeneration in kanamycin‐containing selective medium. Transgene loci number and integrity were assessed by restriction analysis and Southern blot hybridization. DNA restriction with HindIII, which excises the 3′p23+3′UTR‐derived expression cassettes (Fig. 1B), revealed that the regenerated transformants contained at least one intact copy of the sense, antisense or intron‐hairpin construct (Fig. 1C–E). After DNA digestion with EcoRI, which cuts once the antisense and sense T‐DNAs near the left border (Fig. 1B), a number of transgene loci (1–10 and 1–9, respectively) were estimated for these lines (Fig. 1C,D). Intron‐hairpin lines harboured one to seven transgene loci, as revealed by restriction with ApaI, which cuts once the intron‐hairpin T‐DNA between the nptII gene and nos‐ter, or with NheI, which cuts once within nos‐pro (Fig. 1B,E, and results not shown). Nine sense, 18 antisense and 30 intron‐hairpin lines showing distinct integration patterns, and nine control lines, were selected for CTV challenge.
PTGS‐mediated resistance against CTV requires the accumulation of transgene‐derived siRNAs
Uniform propagations (6–12 from each line) were graft inoculated with CTV‐T36, and virus accumulation in the leaves of at least three consecutive flushes was assessed by indirect DAS‐ELISA. In the second flush post‐inoculation, all propagations of the sense, antisense and control lines were infected with CTV, except for one propagation (out of seven) of the sense line 38, which remained uninfected 3 years after inoculation. In contrast, nine intron‐hairpin lines showed CTV resistance, with 9%–56% of the propagations remaining uninfected and the others being susceptible and symptomatic (Fig. 2; Table 1). This variable response to CTV challenge among clonal propagations is most probably a result of the complex interaction between PTGS elicited in a woody species (citrus) and the counter‐silencing response deployed by a phloem‐limited virus (Folimonova et al., 2008) with three silencing suppressors (Lu et al., 2004). Moreover, as indicated previously (Fagoaga et al., 2006), factors other than the genetic background of the transgenic plant, including differences in the physiological stage of individual propagations, may be critical for the efficiency of PTGS‐mediated resistance. Similarly, different ontological stages of individual propagations from the same line may explain the variable response reported for many transgenic host species against challenge with diverse pathogens and pests (Chu et al., 1999; Down et al., 2001; Gao et al., 2000; Lius et al., 1997; Smith et al., 1994; Xu et al., 2001). All propagations of the remaining 21 intron‐hairpin lines showed virus titres and symptoms comparable with those of the non‐transgenic controls (data not shown).
Figure 2.

Response to graft inoculation with clonal Citrus tristeza virus (CTV)‐T36 strain of shoots from limes transformed with an empty vector control (right) and an intron‐hairpin 3′p23+3′UTR (ihp) construct (left). The control shoot shows leaf cupping, short internodes and yellowing, whereas the ihp transgenic shoot remains symptomless.
Table 1.
Properties of Mexican lime lines transformed with an intron‐hairpin construct derived from the 3′‐terminal 549 nucleotides of Citrus tristeza virus (CTV) RNA.
| Line | Number of transgene loci | Transgene integrity | Transcript accumulation* | siRNA accumulation* | Resistant/total propagations | |
|---|---|---|---|---|---|---|
| Resistant | 1 | 1 | Yes | +/− | + | 2/6 |
| 20 | 2 | No | ++ | ND | 1/6 | |
| 27 | 1–2† | Yes | +/− | ND | 3/10 | |
| 28 | 1 | Yes | + | ++ | 1/6 | |
| 29 | 1 | Yes | + | +++ | 5/9 | |
| 39 | 1 | Yes | + | ++ | 4/10 | |
| 43 | 2 | No | +/− | ND | 2/9 | |
| 46 | 1 | Yes | ++ | ++++ | 2/11 | |
| Susceptible | 5 | 3–4† | Yes | +/− | ND | 0/6 |
| 6 | 2 | Yes | + | +++ | 0/11 | |
| 12 | 1 | Yes | +/++ | +/++ | 0/6 | |
| 18 | 1–2† | Yes | + | ND | 0/6 | |
| 21 | 2 | Yes | − | ND | 0/6 | |
| 22 | 2 | No | ++ | ND | 0/6 | |
| 23 | 3 | Yes | − | ND | 0/6 | |
| 25 | 1 | Yes | ++ | +/++ | 0/6 | |
| 26 | 2 | Yes | +++++ | +++ | 0/11 | |
| 30 | 2 | No | − | ND | 0/6 | |
| 31 | 4 | Yes | +++ | ND | 0/8 | |
| 32 | 4 | Yes | − | ND | 0/6 | |
| 34 | 3 | Yes | ++ | +++ | 0/8 | |
| 35 | 2 | Yes | − | ND | 0/6 | |
| 38 | 5–7† | Yes | − | ND | 0/6 | |
| 41 | 2 | Yes | ++ | ND | 0/6 | |
| 42 | 1 | Yes | +++ | ++++ | 0/6 | |
| 45 | 2 | Yes | ++ | ND | 0/6 | |
| 48 | 2 | No | ++++ | ND | 0/6 | |
| 51 | 1 | Yes | +++ | +++ | 0/6 | |
| 52 | 1 | Yes | +++ | +++ | 0/6 | |
| Control | C1 | 0 | — | — | 0/11 | |
| C2 | 0 | — | — | 0/10 |
Relative accumulation from undetectable (−) to strong (+++++) signals. ND, not determined.
The number of hybridization bands varied depending on the restriction enzyme (ApaI or NheI) used for the Southern blot analysis of genomic DNA.
To search for a presumed relationship between resistance to CTV and transgene‐derived PTGS, the accumulation of transgene‐derived transcripts and siRNAs was examined in the non‐inoculated transformants that served as source for bud propagation and in non‐inoculated propagations from these source plants. Generally, in sense and antisense lines, there was an inverse correlation between the number of transgene integrations and transcript accumulation (1, 3), whereas no correlation between this number and siRNA accumulation was observed (1, 3 and data not shown). Only in the sense line 38, with a complex transgene integration pattern, were transgene‐derived siRNAs found to be associated with almost undetectable transcript accumulation (1, 3). This was the only line from the sense/antisense groups that showed CTV resistance, suggesting that siRNAs were required for efficient PTGS, in agreement with our previous finding that only p23 transgenic plants resistant to CTV accumulated transgene‐derived siRNAs (Fagoaga et al., 2006), and with data obtained with other plant–virus systems (Hily et al., 2007; Kalantidis et al., 2002; Kreuze et al., 2008). As a control, two sensitive intron‐hairpin lines (6 and 34, see below for other lines) showed high siRNA accumulation (Fig. 3B).
Figure 3.
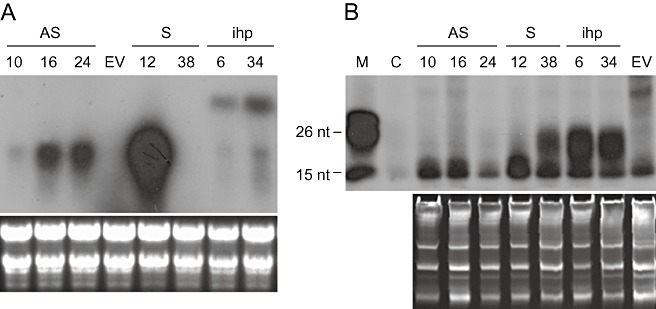
(A) Northern blot hybridization of the 3′p23+3′UTR transgene‐derived transcripts of non‐inoculated antisense (AS, lanes 10, 16 and 24), sense (S, lanes 12 and 38), intron‐hairpin (ihp, lanes 6 and 34) and empty vector (EV) lines. Total RNA was separated by electrophoresis in a formaldehyde‐agarose gel, transferred to a nylon membrane and probed with a digoxigenin‐labelled DNA from the 3′p23+3′UTR construct. (B) Analysis of siRNAs was performed by hybridization with a radioactively labelled 3′p23+3′UTR riboprobe for detecting the plus strand, digestion with RNase, separation of the resistant fragments by denaturing polyacrylamide gel electrophoresis (PAGE) in a 12% gel, dehydration and autoradiography. Lane M corresponds to radiolabelled DNA markers of 15–26 nucleotides. Lane C corresponds to the riboprobe digested with RNase. Equal RNA load was assessed by gel electrophoresis and ethidium bromide staining (bottom panels).
Intron‐hairpin lines accumulating high levels of transgene‐derived siRNAs are susceptible to CTV challenge
The association between siRNA accumulation and resistance to CTV was further examined in non‐inoculated intron‐hairpin transgenic lines, the propagations of which showed different resistance/susceptibility when challenged with CTV (Table 1). Unexpectedly, siRNA levels were highly variable in different transformants irrespective of their response to CTV: resistant lines 1, 39 and 29 showed low, medium and high siRNA levels, respectively, whereas the susceptible line 26 displayed a level of siRNAs comparable with that of line 29 (Fig. 4; Table 1). Similar results were obtained with riboprobes of both polarities and with different non‐inoculated source and propagated plants from the same line (not shown). The lack of correlation between the accumulation of transgene‐derived siRNAs and resistance against DNA viruses has been observed previously; however, in these cases, siRNA levels were low in all susceptible transformants, suggesting that there is an siRNA threshold below which the virus can overcome resistance (Noris et al., 2004; Ribeiro et al., 2007).
Figure 4.
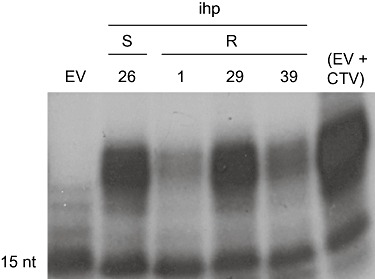
Accumulation of siRNAs in non‐inoculated Mexican lime transformed with the intron‐hairpin (ihp) 3′p23+3′UTR construct, the propagations of which resulted in susceptibility (S) or resistance (R) to Citrus tristeza virus (CTV) challenge. EV and (EV + CTV) correspond to non‐inoculated and CTV‐inoculated controls transformed with the empty vector, respectively. siRNAs were detected by a RNase protection assay, as in Fig. 3B. A RNase‐resistant fragment of approximately 15 nucleotides present in all preparations indicates similar RNA loading.
The accumulation of transgene‐derived siRNAs could not be followed after CTV infection because of the high level of virus‐derived siRNAs present in CTV‐infected control plants, indicative of a strong natural PTGS‐mediated antiviral response (Fig. 4 and data not shown). This high titre of siRNAs resulting from DCL‐mediated digestion suggests that only a fraction of them are effective for viral RNA inactivation.
Intron‐hairpin transgene‐derived transcripts rather than siRNAs predict resistance to CTV
Considering that many intron‐hairpin transgenic lines (including line 26) showed more than one transgene integration and that a high transgene copy number in Arabidopsis could trigger alternative pathways for RNAi involving extreme siRNA dosage (Dunoyer et al., 2007), we focused our analysis on the levels of transgene‐derived transcripts and siRNAs in all lines with a single integration showing different response to CTV challenge (Fig. 5A; Table 1). Transgene‐derived siRNAs accumulated at generally high but variable levels in independent transformants, possibly as a result of position/RNA sensing effects (Schubert et al., 2004), but no correlation with resistance to CTV was observed in inoculated propagations. Indeed, the resistant line 1 showed the lowest transgene‐derived siRNA accumulation and the susceptible lines 42, 51 and 52 the highest (Fig. 5C; Table 1). The level of intron‐hairpin transcripts was also variable among individual lines, but CTV resistance was associated with low transcript accumulation (Fig. 5B; Table 1).
Figure 5.
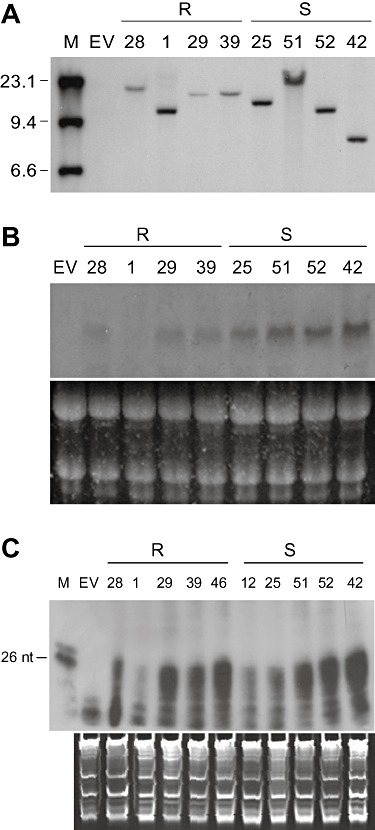
Analysis of nucleic acid preparations from non‐inoculated transgenic lime plants carrying a single integration of the intron‐hairpin 3′p23+3′UTR construct, the propagations of which were resistant (R) or susceptible (S) to Citrus tristeza virus (CTV) challenge. (A) Southern blot hybridization of DNA digested with ApaI, which cuts once at the left of the intron‐hairpin cassette, followed by electrophoresis in a 1% agarose gel and probing with a digoxigenin‐labelled DNA fragment derived from the Fad2 intron. M, digoxigenin‐labelled DNA molecular weight marker II (Roche). (B) Northern blot hybridization of the transgene‐derived transcript separated by electrophoresis in a formaldehyde‐containing 1% agarose gel and probed with a digoxigenin‐labelled DNA from the 3′p23+3′UTR construct. (C) RNase protection assay of the transgene‐derived siRNAs (as in Fig. 3B). M, radiolabelled DNA markers of 24 and 26 nucleotides. EV, nucleic acid preparation from control plants transformed with the empty vector. Equal RNA load was assessed by gel electrophoresis and ethidium bromide staining (B and C, bottom panels).
PTGS‐mediated resistance using p25+p20+3′UTR, p25+p23+3′UTR or p23+3′UTR with an intron‐hairpin format has been assayed previously in N. benthamiana. Because N. benthamiana is a CTV non‐host, transgenic plants were challenged with recombinant Potato virus X or Grapevine virus A vectors carrying CTV‐derived sequences (Batuman et al., 2006; Roy et al., 2006). Resistance or susceptibility responses were dependent on the CTV‐specific fragment inserted in the virus vector, which, in all cases, represented less than 5% of the CTV genome. When Citrus macrophylla plants transformed with the p23+3′UTR intron‐hairpin construct were challenged with CTV by graft inoculation, all lines were susceptible (Batuman et al., 2006), contrasting with our findings with a similar intron‐hairpin construct. However, although we propagated transgenic Mexican limes on a CTV‐resistant rootstock (Troyer citrange), Batuman et al. (2006) propagated a different transgenic citrus species on a CTV‐susceptible rootstock (C. volkameriana), which, on inoculation, might provide a continuous virus supply. Interestingly, C. macrophylla transformants showed high accumulation of transgene‐derived transcripts (Batuman et al., 2006), as did our transformed Mexican limes susceptible to CTV. Transgene‐specific siRNAs were not examined in C. macrophylla transformants, rendering it impossible to make a direct comparison with the Mexican lime transformants.
Taken together, our results show that CTV resistance of intron‐hairpin transgenic plants cannot be predicted by high transgene‐derived siRNA levels. Our results also suggest that only a fraction of the transgene‐derived siRNAs, perhaps those resulting from HEN1‐mediated methylation (Yang et al., 2006; Yu et al., 2005) and/or those programming RISC (Omarov et al., 2007; Pantaleo et al., 2007), are indeed competent for RNA silencing, with the other fraction being quickly degraded, as proposed to occur with most virus‐derived siRNAs in infected plants (Qu and Morris, 2005).
EXPERIMENTAL PROCEDURES
Recombinant vectors and plant transformation
The 3′p23+3′UTR fragment (549 nucleotides, positions 18 748–19 296 of GENBANK accession no. U16304) was obtained from an infectious cDNA clone of the CTV‐T36 strain (Satyanarayana et al., 2001) by polymerase chain reaction (PCR) amplification with Pfu DNA polymerase (Stratagene (La Jolla, CA, USA)) and the sense and antisense primers RF‐402/BamHI (5′‐CTTGGATCCGCGGAGATATTTGCGAT‐3′) and RF‐403/BamHI (5′‐CTTGGATCCTGGACCTATGTTGGCCCC‐3′), respectively, containing BamHI restriction sites (italic). After BamHI digestion, the cDNA was cloned into pMOG180 between the 35S promoter of Cauliflower mosaic virus (CaMV) and the nopaline synthase terminator (nos‐ter) in either sense or antisense orientation. For the intron‐hairpin construct, the sense cDNA fragment, the first intron from the Arabidopsis Fad2 gene (GENBANK accession no. AJ271841) and the antisense cDNA fragment were PCR amplified with primers RF‐402/BamHI and RF‐403/EcoRI (5′‐CTTGAATTCTGGACCTATGTTGGCCCC‐3′), RF‐404/EcoRI (5′‐CTTGAATTCGTCCGTCGCTTCTCTTCCATTTCTTCTC‐3′) and RF‐405/ClaI (5′‐CTTATCGATCTGCAGAAAACCAAAAGCAAAAG‐3′), and RF‐403/ClaI (5′‐CTTATCGATTGGACCTATGTTGGCCCC‐3′) and RF‐402/XhoI (5′‐CTTCTCGAGGCGGAGATATTTGCGAT‐3′), respectively, with restriction sites in italic. The three amplification products were separated by electrophoresis in 1% agarose gels, excised and digested with the corresponding restriction enzymes, generating the 549‐nucleotide BamHI/EcoRI sense, 1130‐nucleotide EcoRI/ClaI intron and 549‐nucleotide ClaI/XhoI antisense fragments, which were ligated stepwise into the pBluescript II KS+ plasmid (Stratagene) digested accordingly. The resulting 2228‐nucleotide fragment was PCR amplified with primer RF‐402/BamHI, electrophoresed and excised from the gel. After BamHI digestion, the fragment was inserted into BamHI‐digested pMOG180 between the CaMV 35S promoter and nos‐ter.
The three expression cassettes were excised with HindIII from pMOG180 and subcloned into the corresponding site of the binary plasmid pBin19‐sgfp between the marker cassettes nos‐pro/nptII/nos‐ter and 35S‐pro/sgfp/nos‐ter (Fig. 1B), and the resulting plasmids and pBin19‐sgfp control were electroporated into the disarmed Agrobacterium tumefaciens strain EHA105, which was used to transform Mexican lime, as described previously (Ghorbel et al., 2001).
Southern blot, Northern blot and siRNA analyses
Transgene copy number and integrity were assessed by Southern blot analysis using leaf DNA digested with HindIII, EcoRI, ApaI or NheI, electrophoresed in 1% agarose gels and blotted onto nylon membranes. After UV irradiation, the membranes were probed with digoxigenin‐labelled DNA of the 3′p23+3′UTR fragment for the sense and antisense constructs, or the Fad2 intron for the intron‐hairpin construct. Probes were prepared by PCR, and hybridization signals were detected by chemiluminescence with the CPD‐Star substrate (Roche Diagnostics (Mannheim, Germany)).
Total leaf RNA was extracted with buffer‐saturated phenol and aliquots were fractionated with 2 m LiCl (Carpenter and Simon, 1998); the insoluble fraction was electrophoresed in 1% agarose gels containing formaldehyde, blotted onto nylon membranes, fixed by UV irradiation and probed with a digoxigenin‐labelled DNA of the 3′p23+3′UTR construct to detect transgene‐derived transcripts. To obtain siRNA‐enriched preparations, total RNA aliquots were fractionated by chromatography on non‐ionic cellulose (CF11, Whatman Inc. (Maidstone, Kent, UK)) (Martínez de Alba et al., 2002), and transgene‐derived siRNAs (30 µg) were detected by a ribonuclease protection assay (RPA) (Ambion Inc. (Applied Biosystems, Austin, TX, USA)) using 32P‐labelled riboprobes specific for both 3′p23+3′UTR polarity strands (hybridized at 42 °C), with the probe fragments protected from RNase digestion being identified by denaturing polyacrylamide gel electrophoresis (PAGE) and autoradiography (Fagoaga et al., 2006).
Challenge of the transgenic plants and analysis of resistance
Buds from sense, antisense, intron‐hairpin and control transformants were propagated by grafting onto Troyer citrange [C. sinensis (L.) Osb. ×P. trifoliata Raf.] seedlings and kept in a contained glasshouse at 24–26 °C/18–20 °C (day/night), 60–80% relative humidity and natural light. When new shoots from propagated buds were 20–30 cm long, 6–12 uniform propagations from each line were graft inoculated 1–2 cm below the bud union with two bark chips from a Mexican lime plant infected with a clonal CTV‐T36 isolate (Satyanarayana et al., 2001). Graft inoculation was repeated twice at monthly intervals to ensure 100% infection in control plants. Three months after the last inoculation, at least one bark chip per plant was removed and the presence of the virus was confirmed by reverse‐transcription polymerase chain reaction (RT‐PCR) with specific primers (Domínguez et al., 2002). Virus accumulation in the leaves of at least three consecutive flushes (in 1 year) was assessed by indirect double‐antibody sandwich‐enzyme‐linked immunosorbent assay (DAS‐ELISA) with the monoclonal antibodies 3DF1 + 3CA5 (Cambra et al., 1990). A plant was considered to be infected when the absorbance at 405 nm was at least two‐fold that of non‐inoculated controls.
ACKNOWLEDGEMENTS
We thank J. A. Pina, M. T. Gorris and J. E. Peris for their excellent technical assistance and Dr W. O. Dawson (University of Florida) for providing the clonal CTV‐T36 isolate. This research was partially supported by grants AGL2006‐03673 from the Ministerio de Ciencia y Tecnología, and Prometeo/2008/121 from the Generalitat Valenciana.
REFERENCES
- Bar‐Joseph, M. , Marcus, R. and Lee, R.F. (1989) The continuous challenge of citrus tristeza virus control. Annu. Rev. Phytopathol. 27, 291–316. [Google Scholar]
- Batuman, O. , Mawassi, M. and Bar‐Joseph, M. (2006) Transgenes consisting of a dsRNA of an RNAi suppressor plus the 3′UTR provide resistance to Citrus tristeza virus sequences in Nicotiana benthamiana but not in citrus. Virus Genes, 33, 319–327. [DOI] [PubMed] [Google Scholar]
- Baulcombe, D. (2004) RNA silencing in plants. Nature, 431, 356–363. [DOI] [PubMed] [Google Scholar]
- Bernstein, E. , Caudy, A.A. , Hammond, S.M. and Hannon, G.J. (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature, 409, 363–366. [DOI] [PubMed] [Google Scholar]
- Cambra, M. , Garnsey, S.M. , Permar, T.A. , Henderson, C. , Gumpf, D. and Vela, C. (1990) Detection of Citrus tristeza virus (CTV) with a mixture of monoclonal antibodies. Phytopathology, 80, 1034. [Google Scholar]
- Carpenter, C.D. and Simon, A.E. (1998) Preparation of RNA. Methods Mol. Biol. 82, 85–89. [DOI] [PubMed] [Google Scholar]
- Chu, P.W.G. , Anderson, B.J. , Khan, M.R.I. , Shukla, D. and Higgins, T.J.V. (1999) Production of Bean yellow mosaic virus resistant subterranean clover (Trifolium subterraneum) plants by transformation with the virus coat protein gene. Ann. Appl. Biol. 135, 469–480. [Google Scholar]
- Dalmay, T. , Hamilton, A. , Rudd, S. , Angell, S. and Baulcombe, D.C. (2000) An RNA‐dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell, 101, 543–553. [DOI] [PubMed] [Google Scholar]
- Díaz‐Pendón, J.A. and Ding, S.W. (2008) Direct and indirect roles of viral suppressors of RNA silencing in pathogenesis. Annu. Rev. Phytopathol. 46, 303–326. [DOI] [PubMed] [Google Scholar]
- Domínguez, A. , Hermoso de Mendoza, A. , Guerri, J. , Cambra, M. , Navarro, L. , Moreno, P. and Peña, L. (2002) Pathogen‐derived resistance to Citrus tristeza virus (CTV) in transgenic Mexican lime (Citrus aurantifolia (Christ.) Swing.) plants expressing its p25 coat protein gene. Mol. Breed. 10, 1–10. [Google Scholar]
- Down, R.E. , Ford, L. , Bedford, S.J. , Gatehouse, L.N. , Newell, C. , Gatehouse, J.A. and Gatehouse, A.M.R. (2001) Influence of plant development and environment on transgene expression in potato and consequences for insect resistance. Transgenic Res. 10, 223–236. [DOI] [PubMed] [Google Scholar]
- Dunoyer, P. , Himber, C. , Ruiz‐Ferrer, V. , Alioua, A. and Voinnet, O. (2007) Intra‐ and intercellular RNA interference in Arabidopsis thaliana requires components of the microRNA and heterochromatic silencing pathways. Nat. Genet. 39, 848–856. [DOI] [PubMed] [Google Scholar]
- Fagoaga, C. , López, C. , Hermoso de Mendoza, A. , Moreno, P. , Navarro, L. , Flores, R. and Peña, L. (2006) Post‐transcriptional gene silencing of the p23 silencing suppressor of Citrus tristeza virus confers resistance to the virus in transgenic Mexican lime. Plant Mol. Biol. 60, 155–167. [DOI] [PubMed] [Google Scholar]
- Fagoaga, C. , López, C. , Moreno, P. , Navarro, L. , Flores, R. and Peña, L. (2005) Viral‐like symptoms induced by the ectopic expression of the p23 gene of Citrus tristeza virus are citrus‐specific and do not correlate with the pathogenicity of the virus strain. Mol. Plant–Microbe Interact. 18, 435–445. [DOI] [PubMed] [Google Scholar]
- Fire, A. , Xu, S.Q. , Montgomery, M.K. , Kostas, S.A. , Driver, S.E. and Mello, C.C. (1998) Potent and specific genetic interference by double‐stranded RNA in Caenorhabditis elegans . Nature, 391, 806–811. [DOI] [PubMed] [Google Scholar]
- Folimonova, S.Y. , Folimonov, A.S. , Tatineni, S. and Dawson, W.O. (2008) Citrus tristeza virus: survival at the edge of the movement continuum. J. Virol. 82, 6546–6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, A.‐G. , Hakimi, S.M. , Mittanck, C.A. , Wu, Y. , Woerner, B.M. , Stark, D.M. , Shah, D.M. , Liang, J. and Rommens, C.M.T. (2000) Fungal pathogen protection in potato by expression of a plant defensin peptide. Nat. Biotechnol. 18, 1307–1310. [DOI] [PubMed] [Google Scholar]
- Ghorbel, R. , López, C. , Fagoaga, C. , Moreno, P. , Navarro, L. , Flores, R. and Peña, L. (2001) Transgenic citrus plants expressing the citrus tristeza virus p23 protein exhibit viral‐like symptoms. Mol. Plant Pathol. 2, 27–36. [DOI] [PubMed] [Google Scholar]
- Hamilton, A.J. and Baulcombe, D.C. (1999) A species of small antisense RNA in post‐transcriptional gene silencing in plants. Science, 286, 950–952. [DOI] [PubMed] [Google Scholar]
- Hammond, S.M. , Bernstein, E. , Beach, D. and Hannon, G.J. (2000) An RNA‐directed nuclease mediates post‐transcriptional gene silencing in Drosophila cells. Nature, 404, 293–296. [DOI] [PubMed] [Google Scholar]
- Hilf, M.E. , Karasev, A.V. , Pappu, H.R. , Gumpf, D.J. , Niblett, C.L. and Garnsey, S.M. (1995) Characterization of citrus tristeza virus subgenomic RNAs in infected tissue. Virology, 208, 576–582. [DOI] [PubMed] [Google Scholar]
- Hily, J.M. , Ravelonandro, M. , Damsteegt, V. , Bassett, C. , Petri, C. , Liu, Z. and Scorza, R. (2007) Plum pox virus coat protein gene intron‐hairpin‐RNA (ihpRNA) constructs provide resistance to plum pox virus in Nicotiana benthamiana and Prunus domestica . J. Am. Soc. Hortic. Sci. 132, 850–858. [Google Scholar]
- Kalantidis, K. , Psaradakis, S. , Tabler, M. and Tsagris, M. (2002) The occurrence of CMV‐specific short RNAs in transgenic tobacco expressing virus‐derived double‐stranded RNA is indicative of resistance to the virus. Mol. Plant–Microbe Interact. 15, 826–833. [DOI] [PubMed] [Google Scholar]
- Karasev, A.V. , Boyko, V.P. , Gowda, S. , Nikolaeva, O.V. , Hilf, M.E. , Koonin, E.V. , Niblett, C.L. , Cline, K. , Gumpf, D.J. , Lee, R.F. , Garnsey, S.M. , Lewandowsky, D.J. and Dawson, W.O. (1995) Complete sequence of the citrus tristeza virus RNA genome. Virology, 208, 511–520. [DOI] [PubMed] [Google Scholar]
- Kasschau, K.D. , Xie, Z. , Allen, E. , Llave, C. , Chapman, E.J. , Krizan, K.A. and Carrington, J.C. (2003) P1/HC‐Pro, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA function. Dev. Cell, 4, 205–217. [DOI] [PubMed] [Google Scholar]
- Kreuze, J.F. , Klein, I.S. , Lázaro, M.U. , Chuquiyuri, W.J.C. , Morgan, G.L. , Mejía, P.G.C. , Ghislain, M. and Valkonen, J.P.T. (2008) RNA silencing‐mediated resistance to a crinivirus (Closteroviridae) in cultivated sweetpotato (Ipomoea batatas L.) and development of sweetpotato virus disease following co‐infection with a potyvirus. Mol. Plant Pathol. 9, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lius, S. , Manshardt, R.M. , Fitch, M.M.M. , Slightom, J.L. , Sanford, J.C. and Gonsalves, D. (1997) Pathogen‐derived resistance provides papaya with effective protection against papaya ringspot virus. Mol. Breed. 3, 161–168. [Google Scholar]
- López, C. , Navas‐Castillo, J. , Gowda, S. , Moreno, P. and Flores, R. (2000) The 23 kDa protein coded by the 3′‐terminal gene of citrus tristeza virus is an RNA‐binding protein. Virology, 269, 462–470. [DOI] [PubMed] [Google Scholar]
- Lu, R. , Folimonov, A. , Shintaku, M. , Li, W.‐X. , Falk, B.W. , Dawson, W.O. and Ding, S.‐W. (2004) Three distinct suppressors of RNA silencing encoded by a 20‐kb viral RNA genome. Proc. Natl. Acad. Sci. USA, 101, 15742–15747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de Alba, A.E. , Flores, R. and Hernández, C. (2002) Two chloroplastic viroids induce the accumulation of small RNAs associated with post‐transcriptional gene silencing. J. Virol. 76, 13094–13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar, A. , Csorba, T. , Lakatos, L. , Varallyay, E. , Lacomme, C. and Burgyan, J. (2005) Plant virus‐derived small interfering RNAs originate predominantly from highly structured single‐stranded viral RNAs. J. Virol. 79, 7812–7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, P. , Ambrós, S. , Albiach‐Martí, M.R. , Guerri, J. and Peña, L. (2008) Plant diseases that changed the world—Citrus tristeza virus: a pathogen that changed the course of the citrus industry. Mol. Plant Pathol. 9, 251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noris, E. , Lucioli, A. , Tavazza, R. , Caciagly, P. , Accotto, G.P. and Tavazza, M. (2004) Tomato yellow leaf curl Sardinia virus can overcome transgene‐mediated RNA silencing of two essential viral genes. J. Gen. Virol. 85, 1745–1749. [DOI] [PubMed] [Google Scholar]
- Omarov, R.T. , Ciomperlik, J.J. and Scholthof, H.B. (2007) RNAi‐associated ssRNA‐specific ribonucleases in tombusvirus P19 mutant‐infected plants and evidence for a discrete siRNA‐containing effector complex. Proc. Natl. Acad. Sci. USA, 104, 1714–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo, V. , Szittya, G. and Burgyán, J. (2007) Molecular bases of viral RNA targeting by viral small interfering RNA‐programmed RISC. J. Virol. 81, 3797–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins, M. , Laimer, M. , Noris, E. , Schubert, J. , Wassenegger, M. and Tepfer, M. (2008) Strategies for antiviral resistance in transgenic plants. Mol. Plant Pathol. 9, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, F. and Morris, T.J. (2005) Suppressors of RNA silencing encoded by plant viruses and their role in viral infections. FEBS Lett. 579, 5958–5964. [DOI] [PubMed] [Google Scholar]
- Ribeiro, S.G. , Lohuis, H. , Goldbach, R. and Prins, M. (2007) Tomato chlorotic mottle virus is a target of RNA silencing but the presence of specific short interfering RNAs does not guarantee resistance in transgenic plants. J. Virol. 81, 1563–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, G. , Sudarshana, M.R. , Ullman, D.E. , Ding, S.‐W. , Dandekar, A.M. and Falk, B.W. (2006) Chimeric cDNA sequences from Citrus tristeza virus confer RNA silencing‐mediated resistance in transgenic Nicotiana benthamiana plants. Phytopathology, 96, 819–827. [DOI] [PubMed] [Google Scholar]
- Satyanarayana, T. , Bar‐Joseph, M. , Mawassi, M. , Albiach‐Martí, M.R. , Ayllón, M.A. , Gowda, S. , Hilf, M.E. , Moreno, P. , Garnsey, S.M. and Dawson, W.O. (2001) Amplification of Citrus tristeza virus from a cDNA clone and infection of citrus trees. Virology, 280, 87–96. [DOI] [PubMed] [Google Scholar]
- Satyanarayana, T. , Gowda, S. , Ayllón, M.A. , Albiach‐Martí, M.R. , Rabindran, S. and Dawson, W.O. (2002) The p23 protein of citrus tristeza virus controls asymmetrical RNA accumulation. J. Virol. 76, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana, T. , Gowda, S. , Mawassi, M. , Albiach‐Martí, M.R. , Ayllón, M.A. , Robertson, C. , Garnsey, S.M. and Dawson, W.O. (2000) Closterovirus encoded HSP70 homolog and p61 in addition to both coat proteins function in efficient virion assembly. Virology, 278, 253–265. [DOI] [PubMed] [Google Scholar]
- Schubert, D. , Lechtenberg, B. , Forsbach, A. , Gils, M. , Bahadur, S. and Schmidt, R. (2004) Silencing in Arabidopsis T‐DNA transformants: the predominant role of a gene‐specific RNA sensing mechanism versus position effects. Plant Cell, 16, 2561–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui, S.A. , Sarmiento, C. , Truve, E. , Lehto, H. and Lehto, K. (2008) Phenotypes and functional effects caused by various viral RNA silencing suppressors in transgenic Nicotiana benthamiana and N. tabacum . Mol. Plant–Microbe Interact. 21, 178–187. [DOI] [PubMed] [Google Scholar]
- Smith, H.A. , Swaney, S.L. , Parks, T.D. , Wernsman, E.A. and Dougherty, W.G. (1994) Transgenic plant virus resistance mediated by untranslatable sense RNAs: expression, regulation, and fate of nonessential RNAs. Plant Cell, 6, 1441–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, N.A. , Singh, S.P. , Wang, M.B. , Stoutjesdijk, P.A. , Green, A.G. and Waterhouse, P.M. (2000) Total silencing by intron‐spliced hairpin RNAs. Nature, 407, 319–320. [DOI] [PubMed] [Google Scholar]
- Voinnet, O. (2005) Induction and suppression of RNA silencing: insights from viral infections. Nat. Rev. Genet. 6, 206–220. [DOI] [PubMed] [Google Scholar]
- Waterhouse, P.M. and Helliwell, C.A. (2003) Exploring plant genomes by RNA‐induced gene silencing. Nat. Rev. Genet. 4, 29–38. [DOI] [PubMed] [Google Scholar]
- Xu, J.P. , Schubert, J. and Altpeter, F. (2001) Dissection of RNA‐mediated ryegrass mosaic virus resistance in fertile transgenic perennial ryegrass (Lolium perenne L.). Plant J. 26, 265–274. [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Ebright, Y.W. , Yu, B. and Chen, X. (2006) HEN1 recognizes 21–24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide. Nucleic Acids Res. 34, 667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, B. , Yang, Z. , Li, J. , Minakhina, S. , Yang, M. , Padgett, R.W. , Steward, R. and Chen, X. (2005) Methylation as a crucial step in plant microRNA biogenesis. Science, 307, 932–935. [DOI] [PMC free article] [PubMed] [Google Scholar]


