SUMMARY
Xanthomonas campestris pv. vesicatoria secretes at least 20 effector proteins through the type III secretion system directly into plant cells. In this study, we uncovered virulence activities of the effector proteins AvrBs1, AvrBs3 and AvrBs4 using Agrobacterium‐mediated transient expression of the corresponding genes in Nicotiana benthamiana, followed by microscopic analyses. We showed that, in addition to the nuclear‐localized AvrBs3, the effector AvrBs1, which localizes to the plant cell cytoplasm, also induces a morphological change in mesophyll cells. Comparative analyses revealed that avrBs3‐expressing plant cells contain highly active nuclei. Furthermore, plant cells expressing avrBs3 or avrBs1 show a decrease in the starch content in chloroplasts and an increased number of vesicles, indicating an enlargement of the central vacuole and the cell wall. Both AvrBs1 and AvrBs3 cause an increased ion efflux when expressed in N. benthamiana. By contrast, expression of the avrBs3 homologue avrBs4 leads to large catalase crystals in peroxisomes, suggesting a possible virulence function of AvrBs4 in the suppression of the plant defence responses. Taken together, our data show that microscopic inspection can uncover subtle and novel virulence activities of type III effector proteins.
INTRODUCTION
The pathogenicity of most Gram‐negative plant pathogenic bacteria depends on the type III secretion (T3S) system that injects a battery of effector proteins into plant cells (Cornelis and Van Gijsegem, 2000; Tampakaki et al., 2004). Historically, many type III effectors have been identified by their avirulence (avr) activity, i.e. by betraying the presence of the bacterium to the plant's immune system. In this case, the Avr protein is recognized by a corresponding resistance (R) protein in the plant (Flor, 1971; Gabriel and Rolfe, 1990; Keen, 1990). Specific recognition often induces the hypersensitive response (HR), a rapid local plant cell death reaction that is concomitant with the halt of bacterial growth (Hammond‐Kosack and Jones, 1996; Klement, 1982; Staskawicz, 2001). However, functional studies of type III effector proteins have revealed that the suicidal HR elicitation activity arises through plant evolution, and that their primary role in susceptible plants is in virulence. There, effector proteins modify the cellular metabolism to benefit bacterial growth, for example by suppressing host defence (Abramovitch and Martin, 2004; Grant et al., 2006), or to cause bacterial dissemination (Wichmann and Bergelson, 2004). The list of effector proteins with a demonstrated virulence function has grown rapidly in recent years and includes, for example, PthA from Xanthomonas axonopodis pv. citri, which induces citrus canker and enhances the release of bacteria to the surface of infected leaves, thus promoting the spread of the pathogen to new infection sites (Swarup et al., 1991, 1992). Similar effects have been observed for Avrb6 from Xanthomonas campestris pv. malvacearum (Yang et al., 1994). Recent studies have suggested that the suppression or delay of plant defence reactions is one of the major virulence functions of type III effectors. The latter has been shown mainly for effectors from subspecies of Pseudomonas syringae, which, for example, suppress callose deposition at infection sites and cell death in non‐host plants, or inhibit the avr activity of a particular effector protein in resistant plants (Abbo et al., 1995; da Cunha et al., 2007; Kim et al., 2005; Mackey et al., 2003; Nomura et al., 2005; Oguiza and Asensio, 2005). In addition, several effector proteins from Xanthomonas species have also been found to suppress plant defence reactions (Brown et al., 1995; Fujikawa et al., 2006; Makino et al., 2006; Metz et al., 2005). Interestingly, there are indications that members of the AvrBs3 family can suppress cell death reactions (Fujikawa et al., 2006; Makino et al., 2006).
In our laboratory, we have studied X. campestris pv. vesicatoria, the causal agent of bacterial spot disease in pepper and tomato. The pathogenicity depends on the T3S system, which translocates at least 20 effectors into the plant cell (Thieme et al., 2005; F. Thieme and U. Bonas, unpublished data). A large number of avr genes have been cloned from X. campestris pv. vesicatoria (Gürlebeck et al., 2006; Minsavage et al., 1990; van't Slot and Knogge, 2002), three of which, avrBs1, avrBs3 and avrBs4, are the focus of this study. The avrBs3 gene encodes a 122‐kDa protein with a central domain composed of 17.5 nearly identical repeats of 34 amino acids (Bonas et al., 1989; Knoop et al., 1991). Analyses of AvrBs3 and homologues have shown that the number and order of the repeats determine R gene specificity (Bonas et al., 1993; Herbers et al., 1992). AvrBs3 is translocated from X. campestris pv. vesicatoria via the T3S system into the host cells, where it dimerizes and localizes to the nucleus (Gürlebeck et al., 2005, 2006; Szurek et al., 2002; Van den Ackerveken et al., 1996). The induction of HR in resistant pepper plants depends on the presence of at least one nuclear localization signal (NLS) and the acidic activation domain (AAD), both located in the C‐terminal region of the AvrBs3 protein (Van den Ackerveken et al., 1996), and is accompanied by a rapid macroscopic cell death. Yeast two‐hybrid, in vitro and in planta bimolecular fluorescence complementation studies have shown that importin α binds to the AvrBs3‐NLSs to mediate nuclear import inside the plant cell (Szurek et al., 2001, 2002; D. Gürlebeck and U. Bonas, unpublished data). In susceptible plants, AvrBs3‐delivering X. campestris pv. vesicatoria strains induce hypertrophy, which is an enlargement of mesophyll cells (Bonas et al., 1989; Marois et al., 2002). Macroscopically, this hypertrophy leads to pustules on the lower surface of infected leaves. As the NLSs and AAD of AvrBs3 are required for both HR and hypertrophy, we postulated that AvrBs3 acts as a transcriptional regulator in the nucleus (Gürlebeck et al., 2005, 2006; Marois et al., 2002; Szurek et al., 2001, 2002). Indeed, AvrBs3 induces the expression of plant genes necessary for the induction of cell enlargement (Kay et al., 2007; Marois et al., 2002).
Ongoing studies have revealed a diversity of molecular methods of action of type III effector proteins from phytopathogens, e.g. as E3‐ligases, cysteine proteases, phosphatases or transcriptional regulators (for a review, see Gürlebeck et al., 2006; Mudgett, 2005). However, in contrast with effectors from mammalian pathogens, effector gene knockout mutants of phytopathogenic bacteria rarely result in a visible decrease in virulence. Hence, the function of most X. campestris pv. vesicatoria effector proteins is still mysterious. For example, AvrBs1 lacks sequence homologies that suggest a biochemical function (Napoli and Staskawicz, 1987; Ronald and Staskawicz, 1988). Furthermore, despite its 97% amino acid identity to AvrBs3, the virulence function of AvrBs4 (Bonas et al., 1993; Schornack et al., 2004) has so far escaped observation.
In this article, we present our investigations to uncover the potential virulence functions of the X. campestris pv. vesicatoria type III effector proteins AvrBs1, AvrBs3 and AvrBs4. A combination of light and electron microscopy, immunohistochemistry and physiological analyses has revealed that the three type III effector proteins under study induce changes in plant cellular morphology and ion leakage, suggesting novel virulence activities for these Xanthomonas effectors.
RESULTS
AvrBs1 and AvrBs3 cause chlorotic symptoms in the plant
To unravel the possible virulence functions of the individual effector proteins AvrBs1, AvrBs3 and AvrBs4 in the plant, we transiently expressed the corresponding genes or derivatives thereof for all performed studies in Nicotiana benthamiana under the control of the constitutive 35S promoter, mediated by Agrobacterium tumefaciens. As a negative control, we used an Agrobacterium strain containing an empty vector or expressed the Escherichia coli gene uidA for β‐glucuronidase (GUS) in N. benthamiana. N. benthamiana was used because it lacks the corresponding resistance genes and, in contrast with pepper and tomato, does not display basal defence reactions to Agrobacterium at early time points after infection. As shown in earlier studies, the three effector genes, as well as uidA, are well expressed in planta using Agrobacterium‐mediated transient expression (Ballvora et al., 2001; Escolar et al., 2001; Kay et al., 2005; Marois et al., 2002; Schornack et al., 2004; Szurek et al., 2002; Van den Ackerveken et al., 1996).
First, we observed the phenotypic reactions induced in N. benthamiana after Agrobacterium‐mediated expression of avrBs1, avrBs3, avrBs4 or uidA, which were monitored over a period of 5 days. Tissue reactions started to be visible at 4 days post‐infiltration (dpi) and increased within the next 24 h. Figure 1 shows macroscopically visible reactions induced by the effectors under study 5 dpi of the Agrobacterium strains. A swelling of the upper epidermis was observed in leaf areas that contained AvrBs1 or AvrBs3. In contrast, AvrBs4 and GUS did not induce swelling of the tissue. As expected, AvrBs3 caused hypertrophy on the lower surface of the leaf (Marois et al., 2002). In the case of AvrBs1, AvrBs4 and GUS, there was no visible pustule formation.
Figure 1.
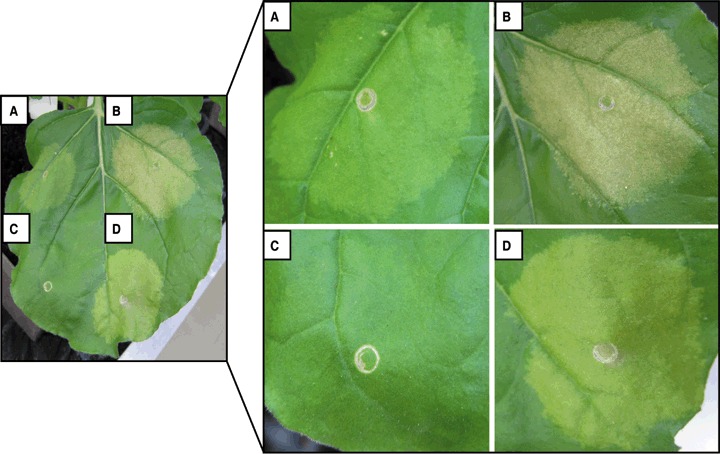
AvrBs1, AvrBs3 and AvrBs4 induce macroscopically visible reactions in Nicotiana benthamiana. Derivatives of Agrobacterium strain GV3101 delivering a T‐DNA with (A) avrBs4, (B) avrBs1, (C) uidA and (D) avrBs3 were inoculated into N. benthamiana. The upper surface of the leaf was photographed at 5 days post‐inoculation (dpi). Similar reactions were obtained in at least three independent experiments.
In addition, the expression of avrBs1 and avrBs3 led to chlorosis in N. benthamiana (Fig. 1B,D). Interestingly, AvrBs4, a close homologue of AvrBs3, caused very little chlorosis (Fig. 1A). Furthermore, the expression of avrBs1 resulted in weak necrotic reactions of the infiltrated tissue (Fig. 1B). The expression of uidA did not result in an altered plant phenotype (Fig. 1C), showing that the reactions caused by AvrBs1, AvrBs3 and AvrBs4 are specific and not caused by infection with Agrobacterium.
AvrBs1 and AvrBs3 induce increased ion leakage from N. benthamiana cells
To investigate whether there are additional physiological changes that may be induced by the type III effectors under study, we analysed the ion leakage of N. benthamiana cells expressing uidA, avrBs1, avrBs3 and avrBs4. Ion leakage is often used as a measure of cellular changes, e.g. cell death reactions after bacterial infection (Minsavage et al., 1990). Leaf material was collected 1 and 4 dpi in deionized water and the relative conductivity was determined. In comparison with uninfected tissue, uidA‐ and avrBs4‐expressing cells behaved similarly, whereas AvrBs3 and AvrBs1 induced a significant increase in ion leakage up to 4 dpi (Fig. 2).
Figure 2.

Determination of the ion leakage from plant cells following the expression of uidA, avrBs1, avrBs3 and avrBs4. Leaves of five Nicotiana benthamiana plants were infiltrated with infiltration medium and with derivatives of Agrobacterium strain GV3101 delivering a T‐DNA with uidA, avrBs4, avrBs3 and avrBs1. At 1 and 4 days post‐inoculation (dpi), leaf discs were harvested and the relative conductivity was determined. Columns represent the mean relative conductivity of five independent samples per treatment. Standard errors of the mean values are indicated as error bars. The experiment was repeated three times showing comparable results.
AvrBs1 induces morphological changes in mesophyll cells
Next, we investigated the effects of the different effectors on N. benthamiana cells in more detail using light microscopy. The leaf tissue was analysed 1, 2, 3, 4 and 5 dpi. The first morphological changes were only observed at 4 dpi, which further increased within 24 h (5 dpi). Representative example photographs are shown in Fig. 3. Mesophyll cells expressing uidA appeared morphologically comparable with uninfiltrated tissue of the same leaf. All inspected palisade cells were similar in size and shape and contained chloroplasts densely arranged to each other in the cytoplasm. The intercellular spaces were clearly visible. In contrast, tissue expressing avrBs3 contained strongly enlarged and bloated mesophyll cells and therefore reduced intercellular spaces. In particular, the palisade cells were elongated and wider than the cells in the negative controls.
Figure 3.

AvrBs1 and AvrBs3 cause morphological changes in mesophyll cells. Leaves of Nicotiana benthamiana with cells expressing uidA (B), avrBs3 (C), avrBs1 (D) or avrBs4 (E) were analysed at 1–5 days post‐inoculation (dpi) by light microscopy. (A) Untreated. Morphological changes were clearly visible at 5 dpi, shown here in representative example photographs of two magnifications as indicated. Size bars correspond to all photographs of the vertical panel. E, epidermis; P, palisade parenchyma; S, spongy parenchyma; V, vascular tissue. Areas containing visible chloroplasts are indicated by arrows, intercellular spaces by asterisks and dead cells by arrowheads.
Interestingly, cells expressing avrBs1 were also morphologically different from those of the negative controls. However, in contrast with avrBs3‐expressing cells, the palisade cells were not elongated but only bloated, thus changing their shape and leading to a decrease in the intercellular spaces. We also detected dead cells in avrBs1‐expressing tissue, which is in agreement with the macroscopically visible weak necrosis induced by AvrBs1 (1, 3). Cells expressing the avrBs3 homologue avrBs4 did not show substantial cellular changes (Fig. 3).
AvrBs1 localizes to the plant cell cytoplasm and AvrBs4 to the nucleus
To understand the mode of action of a certain effector, its localization within the host cell is of special interest. As shown in previous studies, AvrBs3 localizes to the nucleus of plant cells (Szurek et al., 2002). Because of its high similarity to AvrBs3, AvrBs4 was proposed to undergo the same subcellular localization. To prove this assumption, we performed a ‘nuclear targeting assay’ successfully used to show the nuclear localization of AvrBs3 (Szurek et al., 2002). For this, leaves of susceptible pepper (Early Cal Wonder) plants were infiltrated with the avrBs4‐expressing Xanthomonas strains Xcv 85* (pD200) and Xcv 85*ΔhrcV (pD200). The latter strain is a T3S mutant, which is unable to secrete and translocate effector proteins. Nuclei were isolated at 12 h post‐inoculation (hpi), fixed on microscopy glass slides and incubated with the AvrBs3‐specific antibody, which recognizes AvrBs3 and the 97% identical AvrBs4 protein. As expected, AvrBs4 protein was detected in the nuclei of plant cells infected with Xcv 85* (pD200), but not in plant cells infected with the T3S‐deficient strain Xcv 85*ΔhrcV (pD200). 4′,6‐Diamidino‐2‐phenylindole (DAPI) staining confirmed the localization of AvrBs4 to the nucleus (Fig. 4A, bottom panels).
Figure 4.

Subcellular localization of AvrBs4 and AvrBs1 in the plant cell. (A) AvrBs4 localizes to the nucleus. Pepper Early Cal Wonder leaves were infected with the Xcv strains 85* and 85*ΔhrcV, both expressing avrBs4. Leaves were inoculated with bacterial suspensions of 109 colony‐forming units (cfu)/mL and harvested at 12 h post‐inoculation (hpi). Total cell extracts were fixed, immunostained for AvrBs4 and 4′,6‐diamidino‐2‐phenylindole (DAPI) stained for the nuclei. The arrowhead indicates an AvrBs4‐containing nucleus among visible chloroplasts. (B) AvrBs1 localizes to the cytoplasm. For localization studies, avrBs1‐gfp was transiently expressed in Nicotiana benthamiana using Agrobacterium tumefaciens strain GV3101 (avrBs1‐gfp). To visualize the nuclei, the leaves were infiltrated at 48 h post‐inoculation (hpi) with DAPI, harvested 1 h later, mounted in 1 × phosphate‐buffered saline (PBS) and viewed by a Zeiss confocal laser fluorescence microscope (Jena, Germany) using specific filter settings for enhanced green fluorescent protein (EGFP) and DAPI. In parallel, total protein extract from a leaf sample was separated by sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE), blotted onto nitrocellulose and incubated with anti‐GFP antibody (right panel).
Next, we investigated the subcellular localization of AvrBs1 using Agrobacterium‐mediated expression of avrBs1‐gfp in N. benthamiana. For this, we cloned avrBs1 into the binary vector pGWB5, which allows the expression of avrBs1 as a translational fusion to green fluorescent protein (GFP). Two dpi of strain GV3101 (avrBs1‐gfp) into N. benthamiana, leaf material was analysed by fluorescence microscopy. As shown in Fig. 4B, AvrBs1‐GFP localizes exclusively to the cytoplasm of the plant cells. Immunoblot analysis verified that the GFP signal in the cytoplasm was indeed caused by the fusion protein AvrBs1‐GFP and not GFP alone (Fig. 4B, right panel).
Cell enlargement is accompanied by enlargement of the vacuole
To further analyse the cellular changes induced by the effectors under study, we also performed electron microscopy to inspect membrane structures and the different organelles. Figure 5A gives an overview of the ultrastructures visualized within a plant cell using electron microscopy.
Figure 5.



Specific effects of AvrBs1, AvrBs3 and AvrBs4 in plant mesophyll cells. Nicotiana benthamiana plants were inoculated with Agrobacterium strains, leading to the expression of the genes as indicated. Leaf material was collected at 5 days post‐inoculation (dpi) (or as indicated) and inspected by electron microscopy. (A) Cytoplasm of an uninfected mesophyll cell. The visible organelles are indicated as follows: ch, chloroplast; cy, cytoplasm; g, Golgi apparatus; m, mitochondrion; p, peroxisome; tm, thylakoid membrane; white arrow, cristae mitochondriales; black arrows, double membranes of organelles. (B) Ultrastructure of cell wall and plasma membrane (B‐1), chloroplasts (B‐2), mitochondria (B‐3), dictyosomes (B‐4), peroxisomes (B‐5) and nuclei (B‐6). n, nucleolus; p, plasma; s, starch grain; v, nucleolar vacuole. Size bars correspond to 1 µm. (C) Analyses of peroxisomes of infected tissue. The two photographs on the left show the ultrastructure of peroxisomes of cells expressing avrBs4 or avrBs4Δ227. The diagram shows crystal formation in peroxisomes of tissue infected with avrBs4‐delivering Agrobacterium. Crystals were counted daily in 100 peroxisomes and are given as a percentage of the number of crystal‐containing peroxisomes. The right panel shows a crystal structure in a peroxisome immunolabelled by anti‐catalase antibody (black dots correspond to gold particles coated with secondary antibody). (D) Statistical results of the size measurements of 69 nuclei and nucleoli per infiltration site after analyses of ultrathin sections. Displayed are the empirical mean values of the sizes of the nuclei and nucleoli. The error bars reflect the standard errors of the mean (see Supporting Information for detailed results of statistical analysis).
As shown in Fig. 3, the enlargement of mesophyll cells expressing avrBs3 or avrBs1 does not appear to be the result of an excess production of cytoplasm, but rather of the enlargement of the central vacuole. This is supported by the detection of an increased amount of closed circular membrane structures within the vacuoles of cells expressing avrBs1 or avrBs3, in comparison with uidA‐ and avrBs4‐expressing cells (Fig. 5B‐1). In addition, avrBs1‐ and avrBs3‐expressing cells contained an increased number of dictyosomes and Golgi vesicles, which probably deliver components for cell wall synthesis (data not shown).
AvrBs1, AvrBs3 and AvrBs4 induce different changes within organelles
Our electron microscopy studies of plant cells expressing avrBs1, avrBs3 or avrBs4 did not hint at any degradation in chloroplasts (Fig. 5B‐2), mitochondria (Fig. 5B‐3), Golgi apparatus (Fig. 5B‐4), endoplasmic reticulum or peroxisomes (Fig. 5B‐5) when compared with the ultrastructure of these organelles in uidA‐expressing or uninfected cells. However, the chloroplasts of avrBs3‐ and avrBs1‐expressing cells contained little or no starch grains relative to chloroplasts of avrBs4‐ or uidA‐expressing tissue (Fig. 5B‐2).
Intriguingly, all peroxisomes of avrBs4‐expressing cells contained striking rhomboid structures (Fig. 5B‐5,C). For quantification of the crystals, we inspected 100 peroxisomes per day over a period of 5 dpi. At 1 and 2 dpi, none of the investigated peroxisomes contained crystals. At 3 dpi 5%, at 4 dpi 32% and at 5 dpi 100% of the observed peroxisomes contained a crystal (see diagram, Fig. 5C). Interestingly, cells expressing the deletion derivative avrBs4Δ227, lacking the NLSs and AAD, did not contain any rhomboid structures (Fig. 5C). In contrast with wild‐type AvrBs4, expression of the highly homologous avrBs3 gene also did not lead to crystals in peroxisomes (Fig. 5B‐5).
Earlier studies have shown that such crystals within peroxisomes consist of the protein catalase (Frederick and Newcomb, 1969; Huang and Beevers, 1973). To confirm this for the observed crystal structures in our studies, we immunolabelled ultrathin sections derived from avrBs4‐expressing leaf material using a polyclonal anti‐catalase antibody. As shown in Fig. 5C, the anti‐catalase antibody bound to the crystal in the peroxisome. Positive immunolabelling was observed in all inspected peroxisome crystals. Thus, AvrBs4 wild‐type protein leads to the accumulation of catalase in N. benthamiana cells.
AvrBs3 causes an increased transcriptional activity in plant cell nuclei
Next, we inspected the nuclei of plant cells after expression of the three effector genes under study. For each effector and negative controls, we measured the sizes of 69 nuclei and their nucleoli followed by statistical analyses (Fig. 5D and Supporting Information). The nuclei of uninfected cells and of cells expressing uidA, avrBs1 or avrBs4 were significantly smaller in size (mean size, 60–110 µm2) than the nuclei of avrBs3‐expressing cells (mean size, 160 µm2; Fig. 5D). Moreover, the nucleoli in these nuclei were significantly larger (mean size, 17 µm2) than the nucleoli in control tissue, uidA‐, avrBs4‐ and avrBs1‐expressing tissue (mean size, 4–9 µm2) (Fig. 5D). Strikingly, in 52% of the inspected nucleoli in avrBs3‐expressing cells, a central, bright area was detectable (Fig. 5B‐6). However, in cells expressing avrBs1 or avrBs4, only 6% and 9%, respectively, of the nucleoli showed a bright area. Similar results were obtained for uninfected plant tissue (4%).
DISCUSSION
In this study, microscopic techniques allowed us to identify X. campestris pv. vesicatoria type III effector‐induced plant phenotypes. Cellular changes induced by the type III effectors AvrBs1, AvrBs3 and AvrBs4 were examined in N. benthamiana after transient expression of single effector genes via Agrobacterium. This allows the study of single effectors, whereas Xanthomonas campestris pv. vesicatoria injects a large cocktail of different effectors, some of which may act similarly. Furthermore, N. benthamiana lacks the corresponding resistance genes (Bs1, Bs3, Bs4) and, in contrast with pepper and tomato, does not display basal defence reactions to Agrobacterium. Thus, the observed changes presumably represent specific virulence activities of the individual effectors.
AvrBs1 and AvrBs3 induce hypertrophy of mesophyll cells and increased ion leakage
Microscopy showed that AvrBs1 causes plant cell enlargement reminiscent of the effect induced by AvrBs3 (Marois et al., 2002). This is interesting as the two proteins show no similarity on the amino acid level. Furthermore, the two proteins show different localization within plant cells. AvrBs3 localizes to the nucleus, whereas AvrBs1 localizes to the cytoplasm. Strikingly, the AvrBs3 homologue AvrBs4 induces almost no enlargement.
As described for plant cell growth in general (Cosgrove, 1993), expression of avrBs1 and avrBs3 also caused an expansion of the central vacuole and an enlargement of the cell wall. The number of chloroplasts did not appear to be affected, as degrading or dividing plastids were not observed. In addition to an increased number of dictyosomes and Golgi vesicles, which probably contain material for cell wall synthesis, membrane structures were observed within the central vacuole, which may be involved in the enlargement of the vacuole. Furthermore, chloroplasts of enlarged plant cells expressing avrBs1 or avrBs3 did not contain any starch grains, in contrast with cells expressing uidA or avrBs4 and cells of untreated tissue. It is probable that little starch storage takes place because carbohydrates are used to generate energy for cell growth, and pre‐existing starch grains are catabolized for energy generation.
Interestingly, slowly growing avrBs1‐expressing X. campestris pv. vesicatoria strains (which do not carry AvrBs3) induced some pustules in pepper (data not shown), which could be caused by AvrBs1. Thus, the observed change in N. benthamiana is likely to be a virulence effect of the effector rather than an avr effect. However, as X. campestris pv. vesicatoria secretes a large number of effectors (Thieme et al., 2005), we cannot exclude the possibility that another, yet unidentified, effector is responsible for cell enlargement.
An open question is how X. campestris pv. vesicatoria benefits from the induction of hypertrophy. One possibility is that plant cell growth generates squeezing forces that release the bacteria onto the leaf surface from where they can infect other parts of the plant. Indeed, two members of the AvrBs3 family (Avrb6 from X. campestris pv. malvacearum and PthA from X. citri) have been shown to enhance bacterial release to the plant surface (Yang et al., 1994). However, Avrb6 and PthA induce cell proliferation (hyperplasia) resulting in rupture of the tissue (Brunings and Gabriel, 2003; Gürlebeck et al., 2006; Swarup et al., 1991), which was not observed in our microscopic studies. Another possibility is that cellular growth accompanied by a decrease in the intercellular spaces expels the bacteria through stomata. This could explain the enhanced fitness and distribution of X. campestris pv. vesicatoria strains expressing avrBs1 and avrBs3 in field studies (Wichmann and Bergelson, 2004).
Interestingly, the nematode Meloidogyne javanica also induces enlarged plant cells, termed giant cells, in its host plant tomato (Gal et al., 2006). The growth of giant cells depends on expansins, which cause a loosening of the cell wall (Gal et al., 2006; McQueen‐Mason, 1997). This is reminiscent of the finding that AvrBs3 induces the expression of expansins in susceptible pepper plants (Marois et al., 2002). The giant cells represent a feeding site, providing the nematode with nutrients (Williamson and Hussey, 1996). Thus, it is possible that AvrBs3‐ and AvrBs1‐induced cell enlargement improves the nutrient supply of X. campestris pv. vesicatoria. In contrast with the nematode‐induced polynucleated giant cells, the X. campestris pv. vesicatoria effectors studied here did not induce nuclear divisions in mesophyll cells.
The expression of avrBs1 and avrBs3 in N. benthamiana caused an increased ion leakage, which is normally measured during HR (Minsavage et al., 1990). Indeed, expression of avrBs1 led to macroscopically and microscopically visible cell death reactions. However, in avrBs3‐expressing tissue, dead cells were observed only rarely, although the ion leakage was comparable with that induced by AvrBs1. These data suggest that the ion efflux caused by AvrBs1 and AvrBs3 is induced independent of cell death. Hence, the induction of ion leakage may represent another virulence activity of AvrBs1 and AvrBs3. It is also possible that ion leakage occurs as a result of hypertrophy and reflects an enhanced release of nutrients, which would be of advantage for the pathogen.
AvrBs3 induces a stronger nuclear activity than the similar AvrBs4 protein
AvrBs3 and AvrBs4 localize to the plant cell nucleus, where both may act as transcriptional regulators (Kay et al., 2007; Marois et al., 2002; Szurek et al., 2002; this study). Strikingly, the nuclei and nucleoli of cells expressing avrBs3 were significantly enlarged compared with the nuclei of avrBs4‐expressing cells or untreated cells. Furthermore, the nucleoli of avrBs3‐expressing cells displayed electron‐transparent regions, termed ‘nucleolar vacuoles’. The expansion of the nucleoli and the formation of nucleolar vacuoles point to an increased need for ribosomes, and thus to an enhanced transcriptional activity (Gonzalez‐Camacho and Medina, 2006; Jordan, 1984; Shaw and Doonan, 2005). Previously, it has been shown that AvrBs3 and other family members induce the transcription of plant genes (Gu et al., 2005; Kay et al., 2007; Marois et al., 2002; Sugio et al., 2007; Szurek et al., 2002; Yang et al., 2006). Interestingly, our results suggest that AvrBs4 induces less transcription than AvrBs3. As AvrBs1 localizes to the plant cell cytoplasm and only a few nucleoli were enlarged or contained a nuclear vacuole in avrBs1‐expressing cells, AvrBs1 probably uses a different, indirect molecular mechanism compared with AvrBs3 to induce hypertrophy.
In addition to our effector studies, it is interesting to note that uidA, which is often used as a negative control or reporter gene, has effects in the plant cell. First, it seems to have a positive effect on starch grain storage. Secondly, it leads to larger nuclei. Thus, in future studies, it must be kept in mind that a reporter gene may have effects, which we simply do not see.
AvrBs4—a microbial defence against plant defence?
It is notable that AvrBs4, despite its high degree of identity to AvrBs3, induces different plant reactions. What could be the virulence activity of AvrBs4? The work presented here revealed that the expression of avrBs4 in N. benthamiana leads to large rhomboid structures in peroxisomes. Such structures have been shown in earlier studies to be a result of the accumulation of catalase (Frederick and Newcomb, 1969; Huang and Beevers, 1973), which was confirmed in our experiments. Catalase detoxifies hydrogen peroxide (H2O2), which is generated in peroxisomes during oxidative processes and the disproportionation of reactive oxygen species (ROS; del Rio et al., 2006). Pathogen attack induces an enhanced production of H2O2 (del Rio et al., 2006), which is toxic for pathogens and acts as a signal molecule triggering plant defence reactions (Alvarez et al., 1998; Apostol et al., 1989; Gechev and Hille, 2005; Montillet et al., 2005; Willekens et al., 1995). Interestingly, the application of catalase prevents necrotic reactions during pathogen infection and inhibits ROS formation (Devlin and Gustine, 1992; Masuta et al., 1991; Willekens et al., 1995). In addition, a reduced catalase content leads to an increased resistance of the plant against pathogens (Chamnongpol et al., 1998; Mittler et al., 1999). Furthermore, signal molecules, which induce plant defence reactions, inhibit the catalase activity in tobacco (Conrath et al., 1995). Taken together, the present knowledge about plant catalases suggests that the AvrBs4‐induced increase in the catalase content in plant peroxisomes may reflect a virulence function of AvrBs4, i.e. in the inhibition of plant defences. Consistent with this, there are a growing number of type III effectors from Pseudomonas and Xanthomonas which suppress basal and specific plant defence reactions (Abramovitch and Martin, 2004; Abramovitch et al., 2006; Bretz et al., 2003; DebRoy et al., 2004; Espinosa et al., 2003; Fujikawa et al., 2006; Keshavarzi et al., 2004; Kim et al., 2005; Makino et al., 2006; Metz et al., 2005). Future studies must show whether the AvrBs4‐induced increase in the catalase content in the plant is a control point of plant defence reactions.
In conclusion, our studies demonstrate that, in addition to screening for cell death induction and suppression by effectors, microscopic analysis is an excellent technique to uncover potential virulence activities of type III effector proteins.
EXPERIMENTAL PROCEDURES
Bacterial strains and growth conditions
The A. tumefaciens strains used in this study were GV3101 (uidA), GV3101 (avrBs3), GV3101 (avrBs1), GV3101 (avrBs4) and GV3101 (avrBs4Δ227) [designated GV3101 (p35SGUSint), GV3101 (pVSF300), GV3101 (pVS100), GV3101 (pVS200F) and GV3101 (pVS227) in earlier studies; Ballvora et al., 2001; Escolar et al., 2001; Gürlebeck et al., 2005; Marois et al., 2002; Schornack et al., 2004; Szurek et al., 2001; Van den Ackerveken et al., 1996]. For localization studies of AvrBs1, the avrBs1 gene was amplified from strain Xcv 85‐10 using the oligonucleotides aBs1‐fe (CACCATGTCCGACATGAAAGTTAATTTC) and aBs1‐rsl (CGCTTCTCCTGCATTTGTAACATG), and cloned into pENTR/D TOPO (Invitrogen GmbH, Karlsruhe, Germany), resulting in plasmid pENTR(avrBs1‐sl). Subsequently, the gene was recombined via GATEWAY technology (Invitrogen GmbH) into pGWB5 (C‐terminal GFP tag; Nakagawa et al., 2007). A. tumefaciens strain GV3101 was transformed with pGWB5::avrBs1 by electroporation, resulting in strain GV3101 (avrBs1‐gfp).
For nuclear targeting assays, the X. campestris pv. vesicatoria (also designated as Xanthomonas euvesicatoria; Jones et al., 2004) strains Xcv 85* (pDSK200) and Xcv 85*ΔhrcV (pDSK200), which both express avrBs4, were used (Ballvora et al., 2001). A. tumefaciens cells were cultivated at 30 °C in yeast extract broth (YEB) medium (Van den Ackerveken et al., 1996) and Xcv in nutrient yeast glycerol (NYG) medium (Daniels et al., 1984). Antibiotics were added to the media at the following final concentrations: kanamycin, 25 µg/mL; rifampicin, 100 µg/mL; spectinomycin, 50 µg/mL.
Plant material and inoculations
Pepper (Capsicum annuum) plants of cultivar Early Cal Wonder and N. benthamiana plants were grown as described previously (Bonas et al., 1989; Marois et al., 2002; Szurek et al., 2002). Briefly, 6‐week‐old plants were used for bacterial inoculations. Inoculated N. benthamiana plants were kept in a Percival growth chamber (Percival Scientific, Perry, IA, USA) at 22 °C, 16 h of light and 70% humidity. For transient expression assays, N. benthamiana leaves were co‐infiltrated with Agrobacterium strain C58CI (pCH32/pBin6Ip19; Voinnet, 2001), leading to the expression of the silencing inhibitor p19, and strain GV3101, delivering the T‐DNA with the appropriate gene. Bacteria were resuspended at an optical density at 600 nm (OD600) of 1.0 (1.25 × 109) in infiltration medium [5 mm 2‐(N‐morpholino)ethanesulphonic acid (MES), pH 5.3, 10 mm MgCl2, 150 mm acetosyringone], and inoculated with a needle‐less syringe into the intercellular spaces of abaxial leaf surfaces. Plant reactions were scored over 7 days. For AvrBs1 localization studies, N. benthamiana leaves were infiltrated with Agrobacterium strain GV3101 (avrBs1‐gfp), leading to the expression of AvrBs1‐GFP. The strain was resuspended to an OD600 of 0.5 prior to infiltration. Xanthomonas strains were inoculated as described previously (Szurek et al., 2002).
Microscopy
For localization studies by fluorescence microscopy, Agrobacterium‐infected N. benthamiana leaves were infiltrated 2 dpi with DAPI, harvested 1 h later, mounted in 1 × phosphate‐buffered saline (PBS), and viewed by a Zeiss confocal laser fluorescence microscope LSM510 (Jena, Germany) using specific filter settings for enhanced green fluorescent protein (EGFP) and DAPI.
The nuclear targeting assay was performed as described by Szurek et al. (2002).
For ultrastructural studies, leaf segments were collected at 1, 2, 3, 4 and 5 dpi, fixed with 3% glutaraldehyde in sodium cacodylate buffer (SCB), pH 7.2, for 3 h at room temperature, washed with SCB, post‐fixed with 1% osmium tetroxide in SCB, dehydrated in a graded ethanol series, and embedded in epoxy resin (Spurr, 1969). The material was sectioned with an ultramicrotome S (Leica, Bensheim, Germany). Semi‐thin sections (1 µm) were stained with 1% toluidine blue and observed with an Axioskop 20 light microscope (Zeiss). Ultra‐thin sections (80 nm) were transferred to coated copper grids, stained with uranyl acetate and lead citrate, and inspected with an EM 900 transmission electron microscope (Zeiss SMT, Oberkochen, Germany) at an acceleration voltage of 80 kV. Electron micrographs were taken with a slow scan camera (Variospeed SSCCD camera SM‐1k‐120, TRS, Moorenweis, Germany). For morphometric measurements, images of mesophyll nuclei containing a nucleolus were used to avoid the analysis of peripheral sections. Measurements were carried out using analySIS Software (SIS, Münster, Germany).
For immunocytochemistry studies of the ultrastructural localization of catalase, 2‐mm leaf discs were high‐pressure frozen, freeze‐substituted and embedded in HM20 (Polysciences Europe, Eppelheim, Germany), as described previously (Thieme et al., 2007). Ultrathin sections were immunolabelled using a polyclonal α‐catalase antibody (Biotrend, Cologne, Germany) and donkey α‐sheep immunoglobulin G (IgG) conjugated to 6‐nm gold particles (Biotrend, Cologne, Germany). After staining with uranyl acetate and lead citrate, sections were analysed as described above.
Statistical analyses
Statistical analyses were performed using SPSS12 software (Brosius, 2004). For all tests, the significance level was 0.05. Normal distribution was ensured using the Kolmogorov–Smirnov test. The mean values were compared using an analysis of variance (anova) test including a post hoc test (Tamhane).
Electrolyte leakage measurement
At 1 and 4 days after Agrobacterium infiltration, two leaf discs (d = 9 mm) per infiltrated area were harvested and incubated for 15 h in 6 mL of deionized water in a 15‐mL plastic tube (Greiner BIO‐one GmbH, Frickenhausen, Germany) by head‐over‐head inversion. The conductivity was measured using a conductometer 703 (Knick, Berlin, Germany). Samples were boiled for 5 min, cooled to 22 °C, and the total conductivity was determined. The relative conductivity reflecting the ion leakage from plant tissue into water was calculated as follows: sample conductivity/total conductivity × 100%. Experiments were repeated three times with five independent samples for each strain.
Immunoblot analyses
Protein samples were separated by 10% sodium dodecylsulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) (Laemmli, 1970) and blotted on to a nitrocellulose membrane. Western blots were reacted with polyclonal anti‐GFP antibody (Invitrogen GmbH) and anti‐rabbit secondary antibody (Sigma‐Aldrich, Hamburg, Germany), followed by enhanced chemiluminescence.
Supporting information
Table S1 Descriptive statistics for size of nuclei for several treatments.
Table S2 anova test.
Table S3 Tamhane test as post hoc test.
Table S4 Descriptive statistics for size of nucleoli for several treatments.
Table S5 anova test.
Table S6 Tamhane test as post hoc test.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
ACKNOWLEDGEMENTS
We gratefully acknowledge B. Rosinsky, our gardener, and T. Nakagawa, who provided the pGWB5 vector. We thank D. Büttner and, especially, E. Marois for suggestions on the manuscript. We also wish to thank S. Schornack for fruitful discussions. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 648) to U.B. and G.H.
REFERENCES
- Abbo, S. , Dunford, R.P. , Foote, T.N. , Reader, S.M. , Flavell, R.B. and Moore, G. (1995) Organization of retro‐element and stem‐loop repeat families in the genomes and nuclei of cereals. Chromosome Res. 3, 5–15. [DOI] [PubMed] [Google Scholar]
- Abramovitch, R.B. and Martin, G.B. (2004) Strategies used by bacterial pathogens to suppress plant defenses. Curr. Opin. Plant Biol. 7, 356–364. [DOI] [PubMed] [Google Scholar]
- Abramovitch, R.B. , Janjusevic, R. , Stebbins, C.E. and Martin, G.B. (2006) Type III effector AvrPtoB requires intrinsic E3 ubiquitin ligase activity to suppress plant cell death and immunity. Proc. Natl. Acad. Sci. USA, 103, 2851–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, M.E. , Pennell, R.I. , Meijer, P.J. , Ishikawa, A. , Dixon, R.A. and Lamb, C. (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell, 92, 773–784. [DOI] [PubMed] [Google Scholar]
- Apostol, I. , Heinstein, P.F. and Low, P.S. (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: role in defense and signal transduction. Plant Physiol. 90, 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballvora, A. , Pierre, M. , Van den Ackerveken, G. , Schornack, S. , Rossier, O. , Ganal, M. , Lahaye, T. and Bonas, U. (2001) Genetic mapping and functional analysis of the tomato Bs4 locus, governing recognition of the Xanthomonas campestris pv. vesicatoria AvrBs4 protein. Mol. Plant–Microbe Interact. 14, 629–638. [DOI] [PubMed] [Google Scholar]
- Bonas, U. , Stall, R.E. and Staskawicz, B. (1989) Genetic and structural characterization of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria . Mol. Gen. Genet. 218, 127–136. [DOI] [PubMed] [Google Scholar]
- Bonas, U. , Conrads‐Strauch, J. and Balbo, I. (1993) Resistance in tomato to Xanthomonas campestris pv. vesicatoria is determined by alleles of the pepper‐specific avirulence gene avrBs3 . Mol. Gen. Genet. 238, 261–269. [DOI] [PubMed] [Google Scholar]
- Bretz, J.R. , Mock, N.M. , Charity, J.C. , Zeyad, S. , Baker, C.J. and Hutcheson, S.W. (2003) A translocated protein tyrosine phosphatase of Pseudomonas syringae pv. tomato DC3000 modulates plant defence response to infection. Mol. Microbiol. 49, 389–400. [DOI] [PubMed] [Google Scholar]
- Brosius, F. (2004) SPSS 12—Das mitp‐Standardwerk. Bonn: mitp‐Verlag. [Google Scholar]
- Brown, I. , Mansfield, J. and Bonas, U. (1995) hrp genes in Xanthomonas campestris pv. vesicatoria determine ability to suppress papillae deposition in pepper mesophyll cells. Mol. Plant–Microbe Interact. 8, 825–836. [Google Scholar]
- Brunings, A.M. and Gabriel, D.W. (2003) Xanthomonas citri: breaking the surface. Mol. Plant Pathol. 4, 141–157. [DOI] [PubMed] [Google Scholar]
- Chamnongpol, S. , Willekens, H. , Moeder, W. , Langebartels, C. , Sandermann, H. Jr. , Van Montagu, M. , Inzé, D. and Van Camp, W. (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc. Natl. Acad. Sci. USA, 95, 5818–5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrath, U. , Chen, Z. , Ricigliano, J.R. and Klessig, D.F. (1995) Two inducers of plant defense responses, 2,6‐dichloroisonicotinec acid and salicylic acid, inhibit catalase activity in tobacco. Proc. Natl. Acad. Sci. USA, 92, 7143–7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis, G.R. and Van Gijsegem, F. (2000) Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54, 735–774. [DOI] [PubMed] [Google Scholar]
- Cosgrove, D.J. (1993) Water uptake by growing cells: an assessment of the controlling roles of wall relaxation, solute uptake, and hydraulic conductance. Int. J. Plant Sci. 154, 10–21. [DOI] [PubMed] [Google Scholar]
- Da Cunha, L. , Sreerekha, M.V. and Mackey, D. (2007) Defense suppression by virulence effectors of bacterial phytopathogens. Curr. Opin. Plant Biol. 10, 349–357. [DOI] [PubMed] [Google Scholar]
- Daniels, M.J. , Barber, C.E. , Turner, P.C. , Sawczyc, M.K. , Byrde, R.J.W. and Fielding, A.H. (1984) Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 3, 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DebRoy, S. , Thilmony, R. , Kwack, Y.‐B. , Nomura, K. and He, S.Y. (2004) A family of conserved bacterial effectors inhibits salicylic acid‐mediated basal immunity and promotes disease necrosis in plants. Proc. Natl. Acad. Sci. USA, 101, 9927–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin, W.S. and Gustine, D.L. (1992) Involvement of the oxidative burst in phytoalexin accumulation and the hypersensitive reaction. Plant Physiol. 100, 1189–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escolar, L. , Van Den Ackerveken, G. , Rossier, O. , Pieplow, S. and Bonas, U. (2001) Type III secretion and in planta recognition of the Xanthomonas avirulence proteins AvrBs1 and AvrBsT. Mol. Plant Pathol. 2, 287–296. [DOI] [PubMed] [Google Scholar]
- Espinosa, A. , Guo, M. , Tam, V.C. , Fu, Z.Q. and Alfano, J.R. (2003) The Pseudomonas syringae type III‐secreted protein HopPtoD2 possesses protein tyrosine phosphatase activity and suppresses programmed cell death in plants. Mol. Microbiol. 49, 377–387. [DOI] [PubMed] [Google Scholar]
- Flor, H.H. (1971) Current status of the gene‐for‐gene concept. Annu. Rev. Phytopathol. 9, 275–296. [Google Scholar]
- Frederick, S.E. and Newcomb, E.H. (1969) Cytochemical localization of catalase in leaf microbodies (peroxisomes). J. Cell. Biol. 43, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa, T. , Ishihara, H. , Leach, J.E. and Tsuyumu, S. (2006) Suppression of defense response in plants by the avrBs3/pthA gene family of Xanthomonas spp. Mol. Plant–Microbe Interact. 19, 342–349. [DOI] [PubMed] [Google Scholar]
- Gabriel, D.W. and Rolfe, B.G. (1990) Working models of specific recognition in plant–microbe interactions. Annu. Rev. Phytopathol. 28, 365–391. [Google Scholar]
- Gal, T.Z. , Aussenberg, E.R. , Burdman, S. , Kapulnik, Y. and Koltai, H. (2006) Expression of a plant expansin is involved in the establishment of root knot nematode parasitism in tomato. Planta, 224, 155–162. [DOI] [PubMed] [Google Scholar]
- Gechev, T.S. and Hille, J. (2005) Hydrogen peroxide as a signal controlling plant programmed cell death. J. Cell. Biol. 168, 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez‐Camacho, F. and Medina, F.J. (2006) The nucleolar structure and the activity of NopA100, a nucleolin‐like protein, during the cell cycle in proliferating plant cells. Histochem. Cell. Biol. 125, 139–153. [DOI] [PubMed] [Google Scholar]
- Grant, S.R. , Fisher, E.J. , Chang, J.H. , Mole, B.M. and Dangl, J.L. (2006) Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol. 60, 425–449. [DOI] [PubMed] [Google Scholar]
- Gu, K. , Yang, B. , Tian, D. , Wu, L. , Wang, D. , Sreekala, C. , Yang, F. , Chu, Z. , Wang, G.L. , White, F.F. and Yin, Z. (2005) R gene expression induced by a type‐III effector triggers disease resistance in rice. Nature, 435, 1122–1125. [DOI] [PubMed] [Google Scholar]
- Gürlebeck, D. , Szurek, B. and Bonas, U. (2005) Dimerization of the bacterial effector protein AvrBs3 in the plant cell cytoplasm prior to nuclear import. Plant J. 42, 175–187. [DOI] [PubMed] [Google Scholar]
- Gürlebeck, D. , Thieme, F. and Bonas, U. (2006) Type III effector proteins from the plant pathogen Xanthomonas and their role in the interaction with the host plant. J. Plant Physiol. 163, 233–255. [DOI] [PubMed] [Google Scholar]
- Hammond‐Kosack, K.E. and Jones, J.D.G. (1996) Resistance gene‐dependent plant defense responses. Plant Cell, 8, 1773–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbers, K. , Conrads‐Strauch, J. and Bonas, U. (1992) Race‐specificity of plant resistance to bacterial spot disease determined by repetitive motifs in a bacterial avirulence protein. Nature, 356, 172–174. [Google Scholar]
- Huang, A.H. and Beevers, H. (1973) Localization of enzymes within microbodies. J. Cell. Biol. 58, 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J.B. , Lacy, G.H. , Bouzar, H. , Stall, R.E. and Schaad, N.W. (2004) Reclassification of the xanthomonads associated with bacterial spot disease of tomato and pepper. Syst. Appl. Microbiol. 27, 755–762. [DOI] [PubMed] [Google Scholar]
- Jordan, E.G. (1984) Nucleolar nomenclature. J. Cell. Sci. 67, 217–220. [DOI] [PubMed] [Google Scholar]
- Kay, S. , Boch, J. and Bonas, U. (2005) Characterization of AvrBs3‐like effectors from a Brassicaceae pathogen reveals virulence and avirulence activities and a protein with a novel repeat architecture. Mol. Plant–Microbe Interact. 18, 838–848. [DOI] [PubMed] [Google Scholar]
- Kay, S. , Hahn, S. , Marois, E. , Hause, G. and Bonas, U. (2007) A bacterial effector acts as a plant transcription factor and induces a cell size regulator. Science, 318, 648–651. [DOI] [PubMed] [Google Scholar]
- Keen, N.T. (1990) Gene‐for‐gene complementarity in plant–pathogen interactions. Annu. Rev. Genet. 24, 447–463. [DOI] [PubMed] [Google Scholar]
- Keshavarzi, M. , Soylu, S. , Brown, I. , Bonas, U. , Nicole, M. , Rossiter, J. and Mansfield, J. (2004) Basal defenses induced in pepper by lipopolysaccharides are suppressed by Xanthomonas campestris pv. vesicatoria . Mol. Plant–Microbe Interact. 17, 805–815. [DOI] [PubMed] [Google Scholar]
- Kim, H.S. , Desveaux, D. , Singer, A.U. , Patel, P. , Sondek, J. and Dangl, J.L. (2005) The Pseudomonas syringae effector AvrRpt2 cleaves its C‐terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proc. Natl. Acad. Sci. USA, 102, 6496–6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement, Z. (1982) Hypersensitivity In: Phytopathogenic Prokaryotes, Vol. 2. (Mount M.S. and Lacy G.H., eds), pp. 149–177. New York: Academic Press. [Google Scholar]
- Knoop, V. , Staskawicz, B. and Bonas, U. (1991) Expression of the avirulence gene avrBs3 from Xanthomonas campestris pv. vesicatoria is not under the control of hrp genes and is independent of plant factors. J. Bacteriol. 173, 7142–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Mackey, D. , Belkhadir, Y. , Alonso, J.M. , Ecker, J.R. and Dangl, J.L. (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2‐mediated resistance. Cell, 112, 379–489. [DOI] [PubMed] [Google Scholar]
- Makino, S. , Sugio, A. , White, F. and Bogdanove, A.J. (2006) Inhibition of resistance gene‐mediated defense in rice by Xanthomonas oryzae pv. oryzicola . Mol. Plant–Microbe Interact. 19, 240–249. [DOI] [PubMed] [Google Scholar]
- Marois, E. , Van den Ackerveken, G. and Bonas, U. (2002) The Xanthomonas type III effector protein AvrBs3 modulates plant gene expression and induces cell hypertrophy in the susceptible host. Mol. Plant–Microbe Interact. 15, 637–646. [DOI] [PubMed] [Google Scholar]
- Masuta, C. , Van den Bulcke, M. , Bauw, G. , Van Montagu, M. and Caplan, A.B. (1991) Differential effects of elicitors on the viability of rice suspension cells. Plant Physiol. 97, 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen‐Mason, S. (1997) Plant cell walls and the control of growth. Biochem. Soc. Trans. 25, 204–214. [DOI] [PubMed] [Google Scholar]
- Metz, M. , Dahlbeck, D. , Morales, C.Q. , Al Sady, B. , Clark, E.T. and Staskawicz, B.J. (2005) The conserved Xanthomonas campestris pv. vesicatoria effector protein XopX is a virulence factor and suppresses host defense in Nicotiana benthamiana . Plant J. 41, 801–814. [DOI] [PubMed] [Google Scholar]
- Minsavage, G.V. , Dahlbeck, D. , Whalen, M.C. , Kearney, B. , Bonas, U. , Staskawicz, B.J. and Stall, R.E. (1990) Gene‐for‐gene relationships specifying disease resistance in Xanthomonas campestris pv. vesicatoria–pepper interactions. Mol. Plant–Microbe Interact. 3, 41–47. [Google Scholar]
- Mittler, R. , Herr, E.H. , Orvar, B.L. , Van Camp, W. , Willekens, H. , Inzé, D. and Ellis, B.E. (1999) Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc. Natl. Acad. Sci. USA, 96, 14 165–14 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montillet, J.L. , Chamnongpol, S. , Rusterucci, C. , Dat, J. , Van De Cotte, B. , Agnel, J.P. , Battesti, C. , Inze, D. , Van Breusegem, F. and Triantaphylides, C. (2005) Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 138, 1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett, M.B. (2005) New insights to the function of phytopathogenic bacterial type III effectors in plants. Annu. Rev. Plant Biol. 56, 209–531. [DOI] [PubMed] [Google Scholar]
- Nakagawa, T. , Kurose, T. , Hino, T. , Tanaka, K. , Kawamukai, M. , Niwa, Y. , Toyooka, K. , Matsuoka, K. , Jinbo, T. and Kimura, T. (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104, 34–41. [DOI] [PubMed] [Google Scholar]
- Napoli, C. and Staskawicz, B. (1987) Molecular characterization and nucleic acid sequence of an avirulence gene from race 6 of Pseudomonas syringae pv. glycinea . J. Bacteriol. 169, 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura, K. , Melotto, M. and He, S.Y. (2005) Suppression of host defense in compatible plant–Pseudomonas syringae interactions. Curr. Opin. Plant Biol. 8, 361–368. [DOI] [PubMed] [Google Scholar]
- Oguiza, J.A. and Asensio, A.C. (2005) The VirPphA/AvrPtoB family of type III effectors in Pseudomonas syringae . Res. Microbiol. 156, 298–303. [DOI] [PubMed] [Google Scholar]
- Del Rio, L.A. , Sandalio, L.M. , Corpas, F.J. , Palma, J.M. and Barroso, J.B. (2006) Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol. 141, 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald, P.C. and Staskawicz, B.J. (1988) The avirulence gene avrBs1 from Xanthomonas campestris pv. vesicatoria encodes a 50‐kDa protein. Mol. Plant–Microbe Interact. 1, 191–198. [PubMed] [Google Scholar]
- Schornack, S. , Ballvora, A. , Gürlebeck, D. , Peart, J. , Baulcombe, D. , Ganal, M. , Baker, B. , Bonas, U. and Lahaye, T. (2004) The tomato resistance protein Bs4 is a predicted non‐nuclear TIR‐NB‐LRR protein that mediates defense responses to severely truncated derivatives of AvrBs4 and overexpressed AvrBs3. Plant J. 37, 46–60. [DOI] [PubMed] [Google Scholar]
- Shaw, P. and Doonan, J. (2005) The nucleolus. Playing by different rules? Cell Cycle, 4, 102–105. [DOI] [PubMed] [Google Scholar]
- Van't Slot, K.A.E. and Knogge, W. (2002) A dual role of microbial pathogen‐derived effector proteins in plant disease and resistance. Crit. Rev. Plant Sci. 21, 229–271. [Google Scholar]
- Spurr, A.R. (1969) A low‐viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43. [DOI] [PubMed] [Google Scholar]
- Staskawicz, B.J. (2001) Genetics of plant–pathogen interactions specifying plant disease resistance. Plant Physiol. 125, 73–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio, A. , Yang, B. , Zhu, T. and White, F.F. (2007) Two type III effector genes of Xanthomonas oryzae pv. oryzae control the induction of the host genes OsTFIIAgamma1 and OsTFX1 during bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 25, 10 720–10 725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup, S. , De Feyter, R. , Brlansky, R.H. and Gabriel, D.W. (1991) A pathogenicity locus from Xanthomonas citri enables strains from several pathovars of Xanthomonas campestris to elicit cankerlike lesions on citrus. Phytopathology, 81, 802–809. [Google Scholar]
- Swarup, S. , Yang, Y.N. , Kingsley, M.T. and Gabriel, D.W. (1992) A Xanthomonas citri pathogenicity gene, pthA, pleiotropically encodes gratuitous avirulence on nonhosts. Mol. Plant–Microbe Interact. 5, 204–213. [DOI] [PubMed] [Google Scholar]
- Szurek, B. , Marois, E. , Bonas, U. and Van den Ackerveken, G. (2001) Eukaryotic features of the Xanthomonas type III effector AvrBs3: protein domains involved in transcriptional activation and the interaction with nuclear import receptors from pepper. Plant J. 26, 523–534. [DOI] [PubMed] [Google Scholar]
- Szurek, B. , Rossier, O. , Hause, G. and Bonas, U. (2002) Type III‐dependent translocation of the Xanthomonas AvrBs3 protein into the plant cell. Mol. Microbiol. 46, 13–23. [DOI] [PubMed] [Google Scholar]
- Tampakaki, A.P. , Fadouloglou, V.E. , Gazi, A.D. , Panopoulos, N.J. and Kokkinidis, M. (2004) Conserved features of type III secretion. Cell. Microbiol. 6, 805–816. [DOI] [PubMed] [Google Scholar]
- Thieme, F. , Koebnik, R. , Bekel, T. , Berger, C. , Boch, J. , Büttner, D. , Caldana, C. , Gaigalat, L. , Goesmann, A. , Kay, S. , Kirchner, O. , Lanz, C. , Linke, B. , McHardy, A.C. , Meyer, F. , Mittenhuber, G. , Nies, D.H. , Niesbach‐Klösgen, U. , Patschkowski, T. , Rückert, C. , Rupp, O. , Schneiker, S. , Schuster, S.C. , Vorhölter, F.J. , Weber, E. , Pühler, A. , Bonas, U. , Bartels, D. and Kaiser, O. (2005) Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J. Bacteriol. 187, 7254–7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme, J. , McNulty, I. , Vogt, S. and Paterson, D. (2007) X‐ray spectromicroscopy—a tool for environmental sciences. Environ. Sci. Technol. 41, 6885–6889. [DOI] [PubMed] [Google Scholar]
- Van den Ackerveken, G. , Marois, E. and Bonas, U. (1996) Recognition of the bacterial avirulence protein AvrBs3 occurs inside the host plant cell. Cell, 87, 1307–1316. [DOI] [PubMed] [Google Scholar]
- Voinnet, O. (2001) RNA silencing as a plant immune system against viruses. Trends Genet. 17, 449–459. [DOI] [PubMed] [Google Scholar]
- Wichmann, G. and Bergelson, J. (2004) Effector genes of Xanthamonas axonopodis pv. vesicatoria promote transmission and enhance other fitness traits in the field. Genetics, 166, 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens, H. , Inzé, D. , Van Montagu, M. and Van Camp, W. (1995) Catalases in plants. Mol. Breed. 1, 207–228. [Google Scholar]
- Williamson, V.M. and Hussey, R.S. (1996) Nematode pathogenesis and resistance in plants. Plant Cell, 8, 1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B. , Sugio, A. and White, F.F. (2006) Os8N3 is a host disease‐susceptibility gene for bacterial blight of rice. Proc. Natl. Acad. Sci. USA, 103, 10 503–10 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y.N. , De Feyter, R. and Gabriel, D.W. (1994) Host‐specific symptoms and increased release of Xanthomonas citri and X. campestris pv. malvacearum from leaves are determined by the 102‐bp tandem repeats of pthA and avrb6, respectively. Mol. Plant–Microbe Interact. 7, 345–355. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Descriptive statistics for size of nuclei for several treatments.
Table S2 anova test.
Table S3 Tamhane test as post hoc test.
Table S4 Descriptive statistics for size of nucleoli for several treatments.
Table S5 anova test.
Table S6 Tamhane test as post hoc test.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


