SUMMARY
A xyloglucan‐specific endo‐β‐1,4‐glucanase inhibitor cDNA, NbXEGIP1, was amplified from diseased leaves of Nicotiana benthamiana. The sequence was similar to the tomato xyloglucan‐specific endo‐β‐1,4‐glucanase inhibitor (XEGIP) and tobacco nectarin IV genes that have been described as binding and inactivating fungal Family 12 xyloglucan‐specific endo‐β‐1,4‐glucanases. Expression of NbXEGIP1 was not detected in healthy leaves, but the gene was induced during the later stages of infection by the fungi Colletotrichum destructivum and C. orbiculare. Induction of NbXEGIP1 also occurred during disease development by the bacterium Pseudomonas syringae pv. tabaci and during the hypersensitive response produced by P. syringae pv. tabaci expressing avrPto. A portion of NbXEGIP1 was cloned into a tobacco rattle virus vector for virus‐induced gene silencing in N. benthamiana. Silencing NbXEGIP1 did not affect the interactions with either Colletotrichum species but did significantly reduce population levels of P. syringae pv. tabaci in the compatible interaction and P. syringae pv. tabaci expressing avrPto in the incompatible interaction. In the susceptible response to P. syringae pv. tabaci, silencing of NbXEGIP1 also resulted in visibly wilted leaves several hours prior to necrosis, which was not observed in control plants. This was related to a significantly higher level of electrolyte leakage and higher expression of a defensin gene from infected NbXEGIP1‐silenced leaves compared with control leaves. Silencing appeared to be specific as it did not affect expression of a related gene, NbXEGIP2. NbXEGIP1 may act as an inhibitor of a bacterial enzyme that degrades the xyloglucan–cellulose plant cell‐wall network, and degradation of the cell wall results in host membrane disruption and signalling of defence responses.
INTRODUCTION
Xyloglucan‐specific endo‐β‐1,4‐glucanases are enzymes that can attack xyloglucan and cellulose but have 10 times more activity against xyloglucan than linear β‐glucan (Grishutin et al., 2004). They can greatly damage the plant cell wall as xyloglucan binds with cellulose microfibrils contributing to the structural integrity of the cell walls (Juge, 2006). The cross‐binding of xyloglucan with cellulose results in a network that prevents the turgor pressure inside a plant cell from rupturing the cell, while still allowing the cell to grow and develop (York et al., 2004). Xyloglucan‐specific endo‐β‐1,4‐glucanases are also important as the degradation of xyloglucan around cellulose microfibrils increases access for further cell‐wall degradation (Juge, 2006).
Plants produce xyloglucan‐specific endo‐β‐1,4‐glucanase inhibitor proteins (XEGIPs) that bind to the enzyme and inhibit its activity (Naqvi et al., 2005; Qin et al., 2003). XEGIPs are widespread among angiosperms and appear to be specific in inhibiting xyloglucan‐specific endo‐β‐1,4‐glucanase and not other cell‐wall‐degrading enzymes (Juge, 2006; Naqvi et al., 2005; Qin et al., 2003). Both purified tomato XEGIP and tobacco nectarin IV proteins were shown to inhibit a xyloglucan‐specific endo‐β‐1,4‐glucanase of Aspergillus aculeatus and are believed to have evolved as a defence mechanism against microbial degradation of the host cell wall by counteracting the endoglucanases of plant pathogens (Naqvi et al., 2005; Qin et al., 2003). The tomato XEGIP has 62% amino acid identity with an extracellular dermal glycoprotein (EDGP) from carrot (Satoh et al., 1992). Carrot EDGP is localized to dermal tissues and responds to wounding, and it was hypothesized that EDGP is involved in disease resistance. Direct evidence of an XEGIP in defence comes from silencing a potato homologue of the tomato XEGIP by agro‐infiltration that reduced transcript levels to 20–25% that of non‐silenced plants and resulted in faster lesion expansion by Phytophthora infestans (Jones et al., 2006). Water‐soaking was minimal in leaves of non‐silenced plants, but there was extensive water‐soaking in the silenced plants. It was believed that the potato XEGIP contributed to resistance by reducing the ability of P. infestans to loosen host cell walls, which limited pathogen penetration and reduced exposure of the host cell‐wall components to other P. infestans enzymes.
To assess the role of XEGIPs in bacterial and fungal diseases further, an examination was undertaken of an XEGIP homologue during the interactions of Nicotiana benthamiana with the fungi Colletotrichum destructivum and C. orbiculare, and the bacterium Pseudomonas syringae pv. tabaci. Colletotrichum destructivum and C. orbiculare are hemibiotrophic pathogens that have an initial symptomless biotrophic phase of infection with large primary hyphae and/or vesicles that invaginate the host plasma membrane followed by a necrotrophic phase several days later with secondary hyphae that results in water‐soaked lesions (Shen et al., 2001a,b). Pseudomonas syringae pv. tabaci is also a hemibiotrophic pathogen that initially grows biotrophically in the apoplast utilizing type III secretion system (TTSS) effectors to interfere with plant defence signal transduction in the living host cells but then becomes necrotrophic, breaking down parenchyma cells by enzymes and toxin, resulting in water‐soaked lesions with chlorotic halos (Agrios, 2005; Mudgett, 2005). A resistance response was also examined in N. benthamiana transformed with the resistance gene Pto, which involves a hypersensitive response (HR) due to the recognition of the TTSS effector AvrPto produced by P. syringae pv. tabaci transformed with avrPto (Rommens et al., 1995).
The role of a N. benthamiana XEGIP homologue, NbXEGIP1, in these plant–pathogen interactions was assessed by determining its expression pattern following pathogen inoculations and by silencing the gene through virus‐induced gene silencing (VIGS) with a tobacco rattle virus (TRV) vector (Liu et al., 2002). VIGS involves the induction of post‐transcriptional gene silencing in plants using a plant virus to generate double‐stranded (ds)RNA, and TRV is a good VIGS vector because it does not have a strong suppressor of silencing, spreads rapidly in the plant and infects meristematic tissue (Robertson, 2004). Nicotiana benthamiana has frequently been used for VIGS because VIGS is generally much more pronounced and persistent in N. benthamiana than in other plants (Lu et al., 2003). If the XEGIP homologue directly contributes to resistance, then one might expect that the gene will be induced by infection and silencing it should result in greater pathogen growth and greater symptom expression compared with non‐silenced plants.
RESULTS
NbXEGIP1 and NbXEGIP2 amplification and sequence analyses
Primers NbXEGIP1F1 and NbXEGIP1R1 were designed based on the nucleotide sequence of TC7425, which is annotated in the N. benthamiana Gene Index of the Computational Biology and Functional Genomics Laboratory database as being similar to a xyloglucan‐specific fungal endoglucanase inhibitor protein. A single band of the expected size of 708 bp was amplified with these primers using cDNA from N. benthamiana leaves infected with C. destructivum at 96 h post inoculation (hpi), and sequencing of the PCR product showed 100% nucleotide identity with the overlapping region of TC7425. BLASTN of the PCR product sequence against the GenBank non‐redundant database showed that the closest matches (86 and 84% nucleotide identity, respectively) were to sequences for a Solanum tuberosum putative xyloglucanase inhibitor (GenBank accession no. AY321357) and a Lycopersicon esculentum xyloglucan‐specific fungal endoglucanase inhibitor protein precursor (LeXEGIP) (GenBank accession no. AY155579). The gene was designated NbXEGIP1. Like LeXEGIP and other XEGIP genes, NbXEGIP1 encoded a protein with a signal peptide that is 23 amino acids long, indicating that the protein is secreted.
Based on the conserved regions of the nucleotide sequences of several XEGIP and EDGP‐like genes of Capsicum annuum, L. esculentum and S. tuberosum, primers NbXEGIP2F1 and NbXEGIP2R1 were designed. A single band with the expected size of 275 bp was amplified with these primers using cDNA from N. benthamiana leaves infected with C. destructivum at 96 hpi. The sequence of this PCR product did not show any close match with nucleotide sequences in the N. benthamiana Gene Index and only had 58% nucleotide identity with TC7425. However, BLASTN searches of the Tomato and Potato Gene Indices in the Computational Biology and Functional Genomics Laboratory database revealed 84% identity to a potato putative EDGP gene (TC132377) and 65% identity to the tomato XEGIP gene (TC154242). The gene was designated NbXEGIP2.
A dendrogram was constructed from 15 amino acid sequences with high identity to the predicted amino acid sequences of NbXEGIP1 and NbXEGIP2 from plants in the Solanaceae, as well as the carrot EDGP gene (Table 1, Fig. 1). The sequence of TAXI‐IV, which is not an XEGIP but is a Triticum aestivum xylanase inhibitor with some sequence similarity to NbXEGIP1 and NbXEGIP2, was used as an outgroup. Two clusters were observed in the dendrogram (Fig. 1). One cluster contained NbXEGIP1, tomato XEGIP, tobacco nectarin IV, carrot EDGP, and other similar proteins from tomato, tobacco and potato. The second cluster contained NbXEGIP2 and similar sequences from tomato, potato and pepper. The two clusters did not correlate with the gene annotations given that XEGIP, EDGP or nectarin IV‐like genes were found in both clusters. These results indicate that plants appear to contain multiple genes for xyloglucan‐specific fungal endoglucanase inhibitor proteins, which can be placed into at least two different groups for plants in the Solanaceae.
Table 1.
Xyloglucan‐specific endoglucanase inhibitor gene sequences used in this study.
| Accession number* | Source | Gene name/annotation |
|---|---|---|
| TC7425 | Nicotiana benthamiana | NbXEGIP1 |
| EF514216 | N. benthamiana | NbXEGIP2 |
| AAN87262 | Lycopersicon esculentum | LeXEGIP |
| TC156214 | L. esculentum | Nectarin IV like |
| TC177196 | L. esculentum | XEGIP like |
| AAP84703 | Solanum tuberosum | StXEGIP |
| TC128826 | S. tuberosum | XEGIP like |
| TC120914 | S. tuberosum | EDGP like |
| TC128827 | S. tuberosum | EDGP like |
| TC132377 | S. tuberosum | EDGP like |
| TC11291 | Nicotiana tabacum | Nectarin IV like |
| AAX81588 | Nicotiana langsdorffii×N. sanderae | Nectarin IV |
| TC3830 | Capsicum annuum | XEGIP like |
| BAA03413 | Daucus carota | EDGP precursor |
| BAD72882 | Triticum aestivum | TAXI‐IV |
The TC sequences were obtained from TGI databases at The Computational Biology and Functional Genomics Laboratory (http://compbio.dfci.harvard.edu/tgi/). The open reading frames by framefinder (+) in TGI databases were used to construct the dendrogram. The amino acid sequence of NbXEGIP2 was translated using the program GeneRunner (Hastings Software, Hastings. NY). All other sequences were obtained from GenBank (http://www.ncbi.nlm.nih.gov).
Figure 1.

Comparison of xyloglucanase inhibitor protein sequences from Solanaceae as well as the carrot EDGP protein. Wheat TAXI‐IV (BAD72882) was included as an outgroup. Amino acid sequences were aligned using CLUSTALX and a tree generated with the bootstrap neighbour‐joining‐tree procedure of CLUSTALX. Boostrap values represent the number of times out of 1000 that the branch was supported by bootstrap analysis. A description of each sequence is provided in Table 1.
Expression of NbXEGIP1 in N. benthamiana following infection by C. destructivum, C. orbiculare, and P. syringae pv. tabaci
No expression of NbXEGIP1 could be detected in water mock‐inoculated leaves of wild‐type N. benthamiana (Fig. 2). In addition, expression levels of NbXEGIP1 were not detectable prior to infection, during the penetration phase at 24 hpi or during the biotrophic phase at 48 hpi for the interactions with C. destructivum or C. orbiculare. However, an increase in expression was first detectable at 72 hpi with C. destructivum. This corresponded to the development of necrosis and the appearance of water‐soaked lesions. There was a further increase at 96 hpi, at which time the lesions had expanded and there was extensive leaf necrosis. A similar expression pattern occurred during the course of infection by C. orbiculare (Fig. 2). Because lesions expand more slowly with C. orbiculare than C. destructivum, samples could be collected at 120 hpi, showing that the increase in NbXEGIP1 expression continued. Thus, expression levels appeared to correlate directly with the amount of necrosis in the necrotrophic phase of infection.
Figure 2.

Relative RT‐PCR of NbXEGIP1 expression in wild‐type N. benthamiana leaves inoculated with C. destructivum ( ) or C. orbiculare (
) or C. orbiculare ( ). N. benthamiana leaves inoculated with water (
). N. benthamiana leaves inoculated with water ( ) were used as control. The quantity of NbXEGIP1 mRNA was determined relative to the amount of NbEF‐1αmRNA. Means are shown with standard error bars calculated from three or four replications.
) were used as control. The quantity of NbXEGIP1 mRNA was determined relative to the amount of NbEF‐1αmRNA. Means are shown with standard error bars calculated from three or four replications.
No expression of NbXEGIP1 could be detected in leaves mock‐infiltrated with MgCl2, but increased expression was first detectable by 48 hpi with P. syringae pv. tabaci (Fig. 3). Expression of NbXEGIP1 continued to increase as the disease progressed, reaching its highest expression at 96 hpi. Water‐soaked lesions and necrosis were first visible at 72 hpi with P. syringae pv. tabaci, and thus the increased expression of NbXEGIP1 at 48 hpi occurred prior to the appearance of symptoms.
Figure 3.

Relative RT‐PCR of NbXEGIP1 in wild‐type N. benthamiana plants inoculated with P. syringae pv. tabaci ( ). N. benthamiana leaves infiltrated with MgCl2 were used as control (
). N. benthamiana leaves infiltrated with MgCl2 were used as control ( ). The quantity of NbXEGIP1 mRNA was determined relative to the amount of NbEF‐1α mRNA. Means are shown with standard error bars calculated from three or four replications.
). The quantity of NbXEGIP1 mRNA was determined relative to the amount of NbEF‐1α mRNA. Means are shown with standard error bars calculated from three or four replications.
Expression of NbXEGIP1 during the hypersensitive response in N. benthamiana (Pto) following inoculation with P. syringae pv. tabaci (avrPto)
There was a transient increase in expression of NbXEGIP1 at 3 hpi in N. benthamiana (Pto) following mock‐infiltration with MgCl2 (Fig. 4). A larger transient increase at 3 hpi was observed following inoculation with P. syringae pv. tabaci (empty vector), which does not induce an HR because it contains a plasmid cloning vector without the avirulence gene, avrPto. This indicates that the infiltration process induces NbXEGIP1 expression, and this induction is greater when bacterial cells are present. However, expression dropped by 6 hpi with expression levels returning to pre‐treatment levels for MgCl2 and slightly above pre‐treatment levels with P. syringae pv. tabaci (empty vector). Therefore, increased expression of NbXEGIP1 at 3 hpi with P. syringae pv. tabaci (avrPto) is mostly due to this non‐specific effect. After 6 hpi, increased expression only occurred for leaves undergoing the HR induced by P. syringae pv. tabaci (avrPto). Expression of NbXEGIP1 peaked at 18 hpi, which was the time of the appearance of HR necrosis. It thus appears that NbXEGIP1 expression is induced during the HR with increases detectable before the appearance of necrosis.
Figure 4.
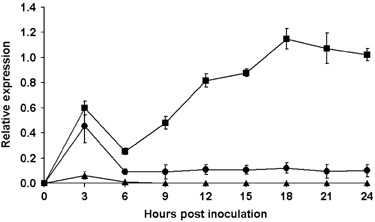
Relative RT‐PCR of NbXEGIP1 expression in leaves of N. benthamiana (Pto) inoculated with P. syringae pv. tabaci (avrPto) (









 ). Controls were leaves infiltrated with MgCl2 (
). Controls were leaves infiltrated with MgCl2 ( ) or P. syringae pv. tabaci containing the vector, pDSK519, without insert (
) or P. syringae pv. tabaci containing the vector, pDSK519, without insert ( ). The quantity of NbXEGIP1 mRNA was determined relative to the amount of NbEF‐1α mRNA. Means are shown with standard error bars calculated from three or four replications.
). The quantity of NbXEGIP1 mRNA was determined relative to the amount of NbEF‐1α mRNA. Means are shown with standard error bars calculated from three or four replications.
Silencing NbXEGIP1 in N. benthamiana and the effect on the compatible interactions with C. destructivum, C. orbiculare and P. syringae pv. tabaci and incompatible interaction with P. syringae pv. tabaci (avrPto)
In leaves inoculated with C. destructivum, C. orbiculare or P. syringae pv. tabaci, the expression level of NbXEGIP1 was reduced in the NbXEGIP1‐silenced wild‐type N. benthamiana to 8–21% of that observed in the MgCl2 and TRV‐GFP vector controls (Table 2, Fig. 5). In N. benthamiana (Pto) inoculated with P. syringae pv. tabaci (avrPto), the expression level of NbXEGIP1 in NbXEGIP1‐silenced plants was 13–14% of that observed in the MgCl2 and TRV‐GFP vector controls (Table 2).
Table 2.
Relative RT‐PCR analysis of NbXEGIP1 and NbXEGIP2 expression in wild‐type N. benthamiana with MgCl2 control, TRV‐GFP control and TRV‐NbXEGIP1 treatments after subsequent inoculation with C. destructivum, C. orbiculare or P. syringae pv. tabaci and N. benthamiana (Pto) with the same treatments after inoculation with P. syringae pv. tabaci (avrPto).
| Treatment* | Relative gene expression† | |||||||
|---|---|---|---|---|---|---|---|---|
| C. destructivum (96 hpi) | C. orbiculare (96 hpi) | P. syringae pv. tabaci (72 hpi) | P. syringae pv. tabaci (avrPto) (24 hpi) | |||||
| NbXEGIP1 | NbXEGIP2 | NbXEGIP1 | NbXEGIP2 | NbXEGIP1 | NbXEGIP2 | NbXEGIP1 | NbXEGIP2 | |
| MgCl2 control | 2.09 a | 0.10 a | 1.65 a | 0.03 a | 0.81 a | 0 | 1.06 a | 0 |
| TRV‐GFP control | 2.13 a | 0.10 a | 1.60 a | 0.03 a | 0.86 a | 0 | 1.18 a | 0 |
| TRV‐NbXEGIP1 | 0.39 b | 0.12 a | 0.35 b | 0.04 a | 0.07 b | 0 | 0.15 b | 0 |
Wild‐type N. benthamiana and N. benthamiana (Pto) were infiltrated with A. tumefaciens containing TRV1 and TRV2 with a GFP insert (TRV‐GFP control), or containing TRV1 and TRV2 with a fragment of NbXEGIP1 (TRV‐NbXEGIP1) or with 10 mm MgCl2 without any A. tumefaciens (MgCl2 control). Plants were grown for 15–18 days after TRV treatment to allow silencing to spread in the plant and then inoculated with C. destructivum, C. orbiculare, P. syringae pv. tabaci or P. syringae pv. tabaci (avrPto).
Expression of NbXEGIP1 in MgCl2 control, TRV‐GFP control or TRV‐NbXEGIP1 N. benthamiana was determined by relative RT‐PCR. The expression of NbXEGIP2 was also examined in TRV‐NbXEGIP1 plants to examine the specificity of NbXEGIP1 silencing. Each measurement represents the mean relative expression from three experiments. Means in the same column followed by the same letter are not significantly different according to the anova Fisher multiple comparisons test at P < 0.05.
Figure 5.

Effect of virus‐induced gene silencing on expression of NbXEGIP1 in wild‐type N. benthamiana and N. benthamiana (Pto). Relative RT‐PCR of NbXEGIP1 co‐amplified with NbEF‐1α from cDNA of leaves at 96 h post‐inoculation (hpi) by C. destructivum (A) and C. orbiculare (B), at 72 hpi by P. syringae pv. tabaci (C) and 24 hpi by P. syringae pv. tabaci (avrPto) (D). Wild‐type N. benthamiana or N. benthamiana (Pto) were infiltrated with 10 mm MgCl2 (MgCl2), A. tumefaciens containing TRV1 and TRV2 with a GFP insert (TRV‐GFP), or A. tumefaciens containing TRV1 and TRV2 with a fragment of NbXEGIP1 (TRV‐NbXEGIP1). After 15–18 days from the TRV treatment to allow for silencing to spread, wild‐type N. benthamiana plants were inoculated with C. destructivum, C. orbiculare or P. syringae pv. tabaci, and N. benthamiana (Pto) plants were inoculated with P. syringae pv. tabaci (avrPto). Lane M is the 100‐bp ladder.
Silencing appeared to be specific as there was no significant difference in the expression of NbXEGIP2 in the NbXEGIP1‐silenced plants compared with the controls (Table 2). This was not unexpected as NbXEGIP1 and NbXEGIP2 have less than 60% nucleotide identity to each other. Detectable NbXEGIP2 expression levels were very low compared with that of NbXEGIP1 at 96 hpi with C. destructivum or C. orbiculare, and no NbXEGIP2 expression was detected with P. syringae pv. tabaci at 72 hpi or P. syringae pv. tabaci (avrPto) at 24 hpi (Table 2). Those values were typical for NbXEGIP2 expression during the interactions as its expression was only slightly induced by 72 hpi for C. destructivum, with a peak relative expression value of 0.14 at 96 hpi and was only slightly induced by 96 hpi for C. orbiculare, with a peak relative expression value of 0.04 at 120 hpi. No NbXEGIP2 expression was detected at any time point during infection by P. syringae pv. tabaci or the HR by P. syringae pv. tabaci (avrPto) (data not shown).
No significant difference in numbers of water‐soaked lesions was observed on leaves of NbXEGIP1‐silenced plants after inoculation with C. destructivum or C. orbiculare compared with MgCl2 and TRV‐GFP vector controls (Table 3). There was also no significant difference among the plants in the timing of the development of necrosis or in the appearance of the lesions among the different treatments.
Table 3.
Lesion numbers in N. benthamiana leaves from plants with MgCl2 control, TRV‐GFP or TRV‐NbXEGIP1 treatments following subsequent inoculation with C. destructivum or C. orbiculare.
| Treatment* | Lesions/cm2 † | |
|---|---|---|
| C. destructivum (72 hpi) | C. orbiculare (96 hpi) | |
| MgCl2 control | 3.23 a | 5.31 a |
| TRV‐GFP control | 3.01 a | 4.97 a |
| TRV‐NbXEGIP1 | 2.96 a | 5.14 a |
N. benthamiana plants were infiltrated with A. tumefaciens containing TRV1 and TRV2 with a GFP insert (TRV‐GFP control), or containing TRV1 and TRV2 with a fragment of NbXEGIP1 (TRV‐NbXEGIP1) or with 10 mm MgCl2 without any A. tumefaciens (MgCl2 control).
At 15–18 days after A. tumefaciens infiltration to induce silencing, the plants were sprayed with a spore suspension of C. destructivum or C. orbiculare. Lesion numbers were counted at 72 hpi for C. destructivum, and at 96 hpi for C. orbiculare. Lesion numbers per square centimetre were means pooled from two separate experiments with a total of 20 replications. Means in the same column followed by the same letter are not significantly different according to the anova Fisher multiple comparisons test at P < 0.05.
Population levels of P. syringae pv. tabaci in wild‐type N. benthamiana were significantly lower in NbXEGIP1‐silenced plants than in MgCl2 and TRV‐GFP vector controls (Table 4). Although the bacterial populations were lower with silencing of NbXEGIP1, leaves infected with P. syringae pv. tabaci showed more severe wilting symptoms approximately 4–6 h prior to water‐soaking and necrosis in NbXEGIP1‐silenced N. benthamiana than in the MgCl2 and TRV‐GFP vector controls (Fig. 6). An examination of electrolyte leakage from leaves infected with P. syringae pv. tabaci at that time after inoculation revealed that the relative conductivity was significantly higher in the NbXEGIP1‐silenced plants than in the MgCl2 or TRV‐GFP vector controls (Table 5). The higher levels of ion leakage indicated that P. syringae pv. tabaci infection resulted in greater host membrane disruption when NbXEGIP1 was silenced.
Table 4.
Bacterial populations in wild‐type N. benthamiana with MgCl2 control, TRV‐GFP control and TRV‐NbXEGIP1 treatments after subsequent inoculation with P. syringae pv. tabaci and N. benthamiana (Pto) with the same treatments after inoculation with P. syringae pv. tabaci (avrPto).
| Treatment* | CFU/cm2 † | |
|---|---|---|
| P. syringae pv. tabaci (72 hpi) | P. syringae pv. tabaci (avrPto) (20 hpi) | |
| MgCl2 control | 1.01 × 108 a | 7.75 × 105 a |
| TRV‐GFP control | 1.10 × 108 a | 5.99 × 105 a |
| TRV‐NbXEGIP1 | 7.32 × 107 b | 1.67 × 105 b |
Wild‐type N. benthamiana and N. benthamiana (Pto) were infiltrated with A. tumefaciens containing TRV1 and TRV2 with a GFP insert (TRV‐GFP control), or containing TRV1 and TRV2 with a fragment of NbXEGIP1 (TRV‐NbXEGIP1) or with 10 mm MgCl2 without any A. tumefaciens (MgCl2 control).
At 15–18 days after A. tumefaciens infiltration to induce silencing, the two youngest fully developed leaves of wild‐type N. benthamiana were infiltrated with P. syringae pv. tabaci and N. benthamiana (Pto) were infiltrated with P. syringae pv. tabaci (avrPto). Samples were collected at 72 hpi for P. syringae pv. tabaci and at 20 hpi for P. syringae pv. tabaci (avrPto). CFU/cm2 of leaf were means pooled from four separate experiments with a total of 38 replications. Means in the same column followed by the same letter are not significantly different according to the anova Fisher multiple comparisons test at P < 0.05.
Figure 6.

Appearance of youngest fully matured leaf of N. benthamiana at 66 hpi with P. syringae pv. tabaci. Plants had been infiltrated 15–18 days earlier with 10 mm MgCl2 (MgCl2 control), A. tumefaciens containing TRV1 and TRV2 with a GFP insert (TRV‐GFP control), or A. tumefaciens containing TRV1 and TRV2 with a fragment of NbXEGIP1 (TRV‐NbXEGIP1).
Table 5.
Electrolyte leakage from wild‐type N. benthamiana leaf discs from plants with MgCl2 control, TRV‐GFP or TRV‐NbXEGIP1 treatments at 66 h post‐inoculation with P. syringae pv. tabaci.
N. benthamiana plants were infiltrated with A. tumefaciens containing TRV1 and TRV2 with a GFP insert (TRV‐GFP control), or containing TRV1 and TRV2 with a fragment of NbXEGIP1 (TRV‐NbXEGIP1) or with 10 mm MgCl2 without any A. tumefaciens (MgCl2 control).
At 15–18 days after A. tumefaciens infiltration to induce silencing, the two youngest fully developed leaves of each plant were infiltrated with P. syringae pv. tabaci. Relative conductivity values were means pooled from three separate experiments with a total of 15 replications. Means in the same column followed by the same letter are not significantly different according to the anova Fisher multiple comparisons test at P < 0.05.
The numbers of P. syringae pv. tabaci (avrPto) in N. benthamiana (Pto) were significantly lower in the NbXEGIP1‐silenced plants than in the controls (Table 4). However, there was no significant difference in the timing or appearance of HR development for leaves of NbXEGIP1‐silenced plants compared with controls.
The lower bacterial numbers in the interactions of the NbXEGIP1‐silenced plants with P. syringae pv. tabaci and P. syringae pv. tabaci (avrPto) may be related to the activation of other defences. To examine this, the expression of a defensin gene, NbDef2.2, which is strongly induced in N. benthamiana leaves during bacterial infection (Bahramnejad, 2007) was determined. Expression of NbDef2.2 in NbXEGIP1‐silenced wild‐type N. benthamiana was four times higher than in the MgCl2 and TRV‐GFP control plants at 72 hpi with P. syringae pv. tabaci (Table 6). However, there was no significant difference in NbDef2.2 expression between the NbXEGIP1‐silenced N. benthamiana (Pto) and the MgCl2 and TRV‐GFP controls at 24 hpi with P. syringae pv. tabaci (avrPto) (Table 6). No expression of NbDef2.2 was detected in the MgCl2 control, TRV‐GFP control or NbXEGIP1‐silenced plants inoculated with C. destructivum or C. orbiculare at 96 hpi (data not shown).
Table 6.
Relative RT‐PCR analysis of NbDef2.2 expression in wild‐type N. benthamiana with MgCl2 control, TRV‐GFP control and TRV‐NbXEGIP1 treatments after subsequent inoculation with P. syringae pv. tabaci and N. benthamiana (Pto) with the same treatments after inoculation with P. syringae pv. tabaci (avrPto).
| Treatment* | Relative gene expression† | |
|---|---|---|
| P. syringae pv.tabaci (72 hpi) | P. syringae pv. tabaci (avrPto) (20 hpi) | |
| MgCl2 control | 0.16 a | 0.40 a |
| TRV‐GFP control | 0.15 a | 0.53 a |
| TRV‐NbXEGIP1 | 0.70 b | 0.50 a |
Wild‐type N. benthamiana and N. benthamiana (Pto) were infiltrated with A. tumefaciens containing TRV1 and TRV2 with a GFP insert (TRV‐GFP control), or containing TRV1 and TRV2 with a fragment of NbXEGIP1 (TRV‐NbXEGIP1) or with 10 mm MgCl2 without any A. tumefaciens (MgCl2 control). Plants were grown for 15–18 days after TRV treatment to allow silencing to spread in the plant and then inoculated with P. syringae pv. tabaci or P. syringae pv. tabaci (avrPto).
Expression of NbDef2.2 in MgCl2 control, TRV‐GFP control or TRV‐NbXEGIP1 N. benthamiana was determined by relative RT‐PCR. Each measurement represents the mean relative expression from three experiments. Means in the same column followed by the same letter are not significantly different according to the anova Fisher multiple comparisons test at P < 0.05.
DISCUSSION
NbXEGIP1 showed increased expression late in the infection by C. destructivum and C. orbiculare, which corresponded to the development of the necrotrophic stage, whereas expression of NbXEGIP1 increased prior to the appearance of necrosis for the compatible and incompatible interactions with P. syringae pv. tabaci. The lack of detectable expression of NbXEGIP1 by RT‐PCR in healthy leaves and leaves during the early stages of infection would be consistent with a role for NbXEGIP1 in the plant's response during the later stages of infection when the host cells would be considerably disrupted by the activities of the pathogens. The transient increase in expression at 3 h after infiltration with MgCl2 also indicates that the gene can be induced by other stresses, such as wounding.
It has been proposed that XEGIP expression is stimulated by biotic and abiotic stresses, such as wounding, which induced expression of the carrot EDGP gene (Satoh et al., 1992; York et al., 2004). Even though NbXEGIP1 is similar to the carrot EDGP and nectarin IV genes, carrot EDGP gene expression was not induced due to infection by Erwinia carotovora, and an arabidopsis homologue of nectarin IV was not induced during infection by Alternaria brassicola (Naqvi et al., 2005; Satoh et al., 1992). However, a homologue of carrot EDGP in Ageratum conyzoides showed increased expression following inoculation with Agrobacterium tumefaciens (Ditt et al., 2001). It appears that there is considerable variation in the regulation of XEGIP genes by biotic stresses. This is also illustrated during compatible and incompatible interactions of N. benthamiana with P. syringae pv. tabaci, in which NbXEGIP1 expression was induced but NbXEGIP2 expression was not.
Other cell‐wall‐degrading enzyme inhibitor genes of plants can also have a wide range of responses to pathogen attack. For wheat, the endoxylanase inhibitor gene, Taxi‐I, is not pathogen‐inducible, whereas the xylanase inhibitor gene, Xip‐I, is greatly induced in Erysiphe graminis‐infected leaves but not in Fusarium graminearum‐inoculated spikelets (Igawa et al., 2005). Two other endoxylanase inhibitor genes of wheat, Taxi‐III and Taxi‐IV, showed increased expression following infection by both E. graminis and F. graminearum (Igawa et al., 2004). Thus, not only are some cell‐wall‐degrading enzyme inhibitors induced by disease, but some can show differential induction depending upon the pathogen attacking the plant.
Although NbXEGIP1 was induced during infection by both C. destructivum and C. orbiculare, silencing of this gene did not alter the host reaction to either Colletotrichum species. Colletotrichum species, such as C. gloeosporioides and C. lindemuthianum, are known to produce many cell‐wall‐degrading enzymes, like endoglucanases and xylanases (Acosta‐Rodriguez et al., 2005; Ortega, 1994). For other plant pathogenic fungi, such as Alternaria alternata and Macrophomina phaseolina, it has been established that they express endo‐β‐1,4‐glucanase during invasion of the plant, and production of the enzyme is related to symptom expression, indicating a role in virulence (Eshel et al., 2002; Jones and Wang, 1997). It is possible that if there are xyloglucan‐specific endo‐β‐1,4‐glucanases produced by C. destructivum or C. orbiculare, then the enzymes are not important during infection or are not affected by NbXEGIP1.
Silencing NbXEGIP1 during the susceptible response to P. syringae pv. tabaci increased the amount of leaf wilting compared with the controls, and this was related to increased electrolyte leakage, indicating greater membrane damage when NbXEGIP1 was silenced. A possible explanation for the effect of silencing NbXEGIP1 is that bacterial xyloglucan‐specific endo‐β‐1,4‐glucanases are being secreted during infection that sufficiently affect host cell‐wall integrity so that host membrane disruption would occur. With less NbXEGIP1 potentially to bind and inactivate it, xyloglucan‐specific endo‐β‐1,4‐glucanases produced by P. syringae pv. tabaci in the apoplast would be more active, either directly weakening the host cell wall by reducing the cross‐binding of xyloglucan with cellulose or indirectly weakening the host cell wall by opening up the wall and permitting greater access to other microbial cell‐wall‐degrading enzymes. The weakened host wall would be unable to retain the turgor pressure of the cell, resulting in host membrane damage, electrolyte leakage and wilting of the leaves. NbXEGIP1 thus appears to limit the amount of host membrane damage being produced during bacterial infection.
Although NbXEGIP1 contributes to resistance by reducing host cell damage due to P. syringae pv. tabaci infection, silencing NbXEGIP1 expression reduced rather than increased bacterial cell numbers in the leaf. The lower bacterial numbers in the NbXEGIP1‐silenced N. benthamiana may be related to the greater induction of expression of defence‐related genes, such as NbDef2.2, compared with the controls after inoculation with P. syringae pv. tabaci. NbDef2.2 encodes a cationic defensin that showed the greatest up‐regulation among eight N. benthamiana defensin genes following treatment with ethylene, wounding or inoculation with P. syringae pv. tabaci or P. syringae pv. tabaci (avrPto) (Bahramnejad, 2007). Although higher levels of this defensin may be partially responsible for the lower population of P. syringae pv. tabaci in NbXEGIP1‐silenced N. benthamiana leaves, there may be a number of defence‐related compounds that are induced more in the NbXEGIP1‐silenced plants. NbDef2.2 expression was chosen as an indicator of the activation of potential defence responses.
One explanation for the effect of NbXEGIP1 silencing on P. syringae pv. tabaci is that xyloglucan‐specific endo‐β‐1,4‐glucanases produced by P. syringae pv. tabaci are affecting signal molecules, such as hydroxyproline‐rich glycopeptides (HypSys) and AtPep1 (Huffaker et al., 2006; Narvaez‐Vasquez et al., 2005). Cell‐wall damage may cause proteases to process HypSys precursors sequestered in the cell walls into active peptides that interact with receptors to signal defence responses to herbivores and/or pathogens (Pearce et al., 2007). In petunia, a processed HypSys induced expression of an antimicrobial defensin gene, PhD1 (Lay et al., 2003; Pearce et al., 2007). Wounding can also induce defensin gene expression by other plant peptides involved in defence signalling, such as AtPep1 in Arabidopsis, that are widespread in monocots and dicots (Huffaker et al., 2006). With less NbXEGIP1 in the silenced plants, greater cell‐wall damage could occur by xyloglucan‐specific endo‐β‐1,4‐glucanases, releasing defence signalling molecules and resulting in more antimicrobial products, such as defensins, that could result in lower populations of P. syringae pv. tabaci in NbXEGIP1‐silenced leaves.
Silencing NbXEGIP1 also resulted in lower population levels of P. syringae pv. tabaci (avrPto) during the resistance response associated with an HR. Perhaps greater activity of a bacterial xyloglucan‐specific endo‐β‐1,4‐glucanase results in a stronger induction of the HR or more amplification of defence signalling during the HR. However, there was no significant difference in NbDef2.2 expression between the NbXEGIP1‐silenced plants and the MgCl2 and TRV‐GFP controls at 24 hpi with P. syringae pv. tabaci (avrPto). This could be due to different defence signalling and activation of defence‐related genes other than NbDef2.2 during the HR as compared with the compatible interaction.
Tomato XEGIP and tobacco nectarin IV proteins have only been demonstrated to inhibit fungal xyloglucan‐specific endo‐β‐1,4‐glucanase in Family 12 glycoside hydrolases, and it appears that XEGIPs are highly specific in their inhibition (Naqvi et al., 2005; Qin et al., 2003; York et al., 2004). However, silencing a potato XEGIP resulted in greater susceptibility to the stramenopile P. infestans (Jones et al., 2006). The Stramenopila are phylogenetically distinct from fungi, and these results indicate that an XEGIP gene can be effective against xyloglucan‐specific endo‐β‐1,4‐glucanases of non‐fungal plant pathogens, such as P. infestans. No Family 12 endoglucanase genes have yet been found in P. infestans (Jones et al., 2006). However, a Family 12 endoglucanase gene was obtained from P. sojae, suggesting that there may be a similar enzyme in P. infestans.
Endoglucanases are also important in the virulence of at least some plant pathogenic bacteria. Endoglucanase mutants of Ralstonia solancearum have a reduced ability to cause wilting of tomato, and tissue maceration is reduced in endoglucanase mutants of Erwinia carotovora (Denny et al., 1990; Walker et al., 1994). The genome of P. syringae pv. tabaci has not been sequenced, but the genome of a related bacterial plant pathogen, Pseudomonas syringae pv. tomato DC3000, contains a Family 8 endoglucanase, wssD (locus ID: PSPTO 1029), but no Family 12 endoglucanse (http://www.pseudomonas.com). However, there are Family 12 endoglucanases described for a variety of plant pathogenic bacteria, such as cellulase S of Xanthomonas axonopodis pv. citri (GenBank accession no. NP_643814) and Xanthomonas oryzae pv. oryzae (GenBank accession no. AAW74331), and celB of Erwinia carotovora subsp. carotovora (Park et al., 1998). It is therefore possible that NbXEGIP1 is inhibiting the action of an as yet undescribed Family 12 endoglucanase of P. syringae pv. tabaci, or there may be a novel interaction of NbXEGIP1 with an endoglucanase from another glucoside hydrolase family given that silencing NbXEGIP1 affects the growth of this pathogen in the plant.
Another possible explanation for the reduced bacterial populations, wilting and electrolyte leakage due to silencing NbXEGIP1 is that it encodes an inhibitor that regulates the activity of endogenous endoglucanases of N. benthamiana induced by P. syringae pv. tabaci infection or during the HR. However, it is considered unlikely that plants have endogenous targets for XEGIPs as plant endoglucanases are not closely related to xyloglucan‐specific endo‐β‐1,4‐glucanases (York et al., 2004). For example, the tomato XEGIP did not inhibit a tomato xlyoglucan glucosylase that uses xlyoglucan as a substrate (Qin et al., 2003). Therefore, it appears more likely that NbXEGIP1 acts to inhibit a bacterial enzyme that can damage host cells rather than a host enzyme.
EXPERIMENTAL PROCEDURES
Biological materials and pathogen inoculations
Nicotiana benthamiana plants were grown at 22 °C until the eight‐leaf stage with a photoperiod of 8/16 h dark/light at 150 µmol/m2/s. Colletotrichum destructivum isolate N150P3 and C. orbiculare isolate A20767P1 (Chen et al., 2003) were cultured on sodium chloride–yeast extract–sucrose agar medium (SYAS) (Mandanhar et al., 1986) at 22 °C under continuous fluorescent light, and conidia were washed from 7–12‐day‐old cultures. For C. destructivum, entire plants were sprayed with a 1 × 105 conidia/mL suspension in sterile distilled water, and for C. orbiculare, they were inoculated with 2 × 106 conidia/mL. The plants were then incubated at room temperature in containers to maintain high humidity. Pseudomonas syringae pv. tabaci strain 11528R was grown overnight in Kings medium B (KB) (King et al., 1954) containing 50 mg/L rifampicin, and suspended in 10 mm MgCl2 to 1 × 106 CFU/mL. Pseudomonas syringae pv. tabaci strain 11528R transformed with vector pDSK519 or vector pDSK519 containing avrPto (Thilmony et al., 1995) was grown overnight in KB containing 50 mg/L rifampicin and 20 mg/L kanamycin, and suspended in 10 mm MgCl2 to 1 × 108 CFU/mL. For the compatible bacterial interaction, the two youngest fully matured leaves of wild‐type N. benthamiana were infiltrated with a suspension of P. syringae pv. tabaci strain 11528R using a needle‐less syringe (Klement, 1963). For the incompatible bacterial interaction, the two youngest fully matured leaves of N. benthamiana transformed with Pto (Rommens et al., 1995) were inoculated with P. syringae pv. tabaci (avrPto) in the same manner. The interaction between N. benthamiana (Pto) and P. syringae pv. tabaci strain 11528R transformed with the vector pDSK519 without an insert served as the empty vector control. Inoculated plants were kept at 22 °C with a photoperiod of 8/16 h dark/light at 150 µmol/m2/s.
The area of the inoculated leaves was measured with a leaf area meter (Model 3100, LI‐COR, Lincoln, NE). For quantifying Colletotrichum infection, the number of lesions was counted at 72 and 96 hpi for C. destructivum and C. orbiculare, respectively, and expressed on a per cm2 leaf basis. For quantifying Pseudomonas populations, leaf discs of 0.8 cm diameter collected at 72 hpi for P. syringae pv. tabaci with wild‐type N. benthamiana and 20 hpi for P. syringae pv. tabaci (avrPto) with N. benthamiana (Pto), respectively, were ground in 1 mL 10 mm MgCl2 containing 0.01% Tween 80 and then dilution‐plated onto KB containing the antibiotics described above to determine the number of colony forming units.
RNA extraction
For extraction of RNA for relative RT‐PCR, leaf samples were frozen in liquid nitrogen and stored at –80 °C. Total RNA was prepared according to the method of Chen et al. (2000) except that phenol/chloroform was added prior to the homogenization buffer, which consisted of 200 mm Tris base, 400 mm KCl, 200 mm sucrose, 35 mm MgCl2·6H2O and 25 mm EDTA, pH 9.0. The RNA was resuspended in 25–50 µL DEPC‐treated dH2O and stored at –80 °C.
Sequence alignments
A dendrogram was created using amino acid sequences homologous to LeXEGIP (Qin et al., 2003) from plants in the family Solanaceae. Sequences were obtained from the databases at GenBank at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov) and The Computational Biology and Functional Genomics Laboratory (http://compbio.dfci.harvard.edu/index.html) (Table 1). In addition, the predicted protein sequences of NbXEGIP1 and NbXEGIP2 were included along with the carrot extracellular dermal glycoprotein (EDGP) (Satoh et al., 1992) and the Triticum aestivum xylanase inhibitor IV (TAXI‐IV) (Igawa et al., 2004). The sequences were aligned, and a dendrogram was created with CLUSTAL X (Thompson et al., 1997) using default parameters. The CLUSTAL X alignment was subjected to distance analysis using the PHYLIP program with the neighbour‐joining algorithm and 1000 bootstrap replications. Identification of a putative signal peptide in the predicted protein sequence of NbXEGIP1 was done using SignalP (http://www.cbs.dtu.dk/services/SignalP/).
RT‐PCR
Single‐stranded cDNA was synthesized using moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen, Burlington, ON, Canada) and oligo (dT) primer with total RNA following the manufacturer's instructions. All PCR reactions were performed using a Mastercycler Personal (Eppendorf, Mississauga, ON, Canada). RT‐PCRs were performed in 15‐µL reaction volumes with 1 µL cDNA, 1.5 units Tsg polymerase (Biobasic, Toronto, ON, Canada), 1.5 µL 10×Tsg polymerase buffer, 2 mm dNTP, 2.5 mm MgSO4 and 1.0 µm of each primer. All RNA samples used for reverse transcription were tested for the presence of genomic DNA by using them directly as the PCR template, prior to cDNA synthesis, under the same PCR conditions.
For the amplification of NbXEGIP1, primers NbXEGIP1F2 (5′‐GGTATGGCTGGCCTTGGTAG) and NbXEGIP1R1 (5′‐AGATCAATGGATGGCACAGC) were designed from the nucleotide sequence of the annotated xyloglucan‐specific fungal endoglucanase inhibitor gene of N. benthamiana, TC7425 (Table 1), to amplify a PCR product of 510 bp. Amplification conditions were 3 min at 94 °C followed by 30 cycles of 30 s at 94 °C, 1 min at 55 °C and 1 min at 72 °C, and one cycle for 10 min at 72 °C. The PCR product was purified using GenElute™ Gel Purification Kit (Sigma, St Louis, MO), cloned into pBluescript (Stratagene, La Jolla, CA), and then transformed into Escherichia coli DH5α. The insert was then sequenced (Laboratory Services Division, University of Guelph).
For the amplification of NbXEGIP2, primers NbXEGIP2F1 (5′‐AACCTGTCCCTTGTGGT) and NbXEGIP2R1(5′‐CCTTTGCTAGTTTCTCAAG) were designed based on the conserved regions of the nucleotide sequences of TC3830, TC156214, TC120914, TC132377, TC128826 and TC128827 (Table 1) to amplify a PCR product of 275 bp. The PCR conditions were 94 °C for 3 min followed by 30 cycles of 30 s at 94 °C, 1 min at 45 °C, 1 min at 72 °C, and a final extension for 10 min at 72 °C. The PCR product was directly sequenced (Laboratory Services Division).
Relative RT‐PCR
Semi‐quantitative relative RT‐PCR was done with the translation elongation factor 1α (NbEF‐1α) as an internal constitutive control according to Dean et al. (2002). EF‐1α forward primer TobefS (5′‐CTCCAAGGCTAGGTATGATG) and reverse primer TobefA (5′‐CTTCGTGGTGCATCTCAAC) were designed by Dean et al. (2002) to yield a 375‐bp PCR product that could be co‐amplified with the gene of interest.
Relative RT‐PCR was performed in 15‐µL reaction volumes with 1 µL cDNA, 1.5 units Tsg polymerase (Biobasic, Toronto, ON, Canada), 1.5 µL 10×Tsg polymerase buffer, 2 mm dNTP and 2.5 mm MgCl, with 0.5 µm of EF‐1α primer pair and 1 µm of each NbXEGIP1, NbXEGIP2 or NbDef2.2 primer. For relative RT‐PCR of NbXEGIP1, forward primer NbXEGIP1F1 (5′‐GCCTGCTTCCTGATAACACA), which was 198 bp upstream from NbXEGIP1F2, was paired with primer NbXEGIP1R1 to amplify an RT‐PCR product of 708 bp. Relative RT‐PCR of NbXEGIP1 was done at 94 °C for 3 min followed by 26 cycles of 30 s at 94 °C, 1 min at 62 °C, 1 min at 72 °C, and a final extension for 10 min at 72 °C. For relative RT‐PCR of NbXEGIP2, primers NbXEGIP2F1 and NbXEGIP2R1 were used at 94 °C for 3 min followed by 30 cycles of 30 s at 94 °C, 1 min at 45 °C, 1 min at 72 °C, and a final extension for 10 min at 72 °C. For relative RT‐PCR of NbDef2.2, primers NbDef2.2F1 (5′‐AAAAGCTTGTATCAGTGAG) and NbDef2.2R1 (5′‐GGATTGAAGTGCCACAC) were used at 94 °C for 3 min followed by 30 cycles of 30 s at 94 °C, 1 min at 56 °C, 1 min at 72 °C, and a final extension for 10 min at 72 °C.
Electrophoresis and quantification of the PCR products were done as per Dean et al. (2002). To determine that the number of amplification cycles was not too great for quantification, the cDNA samples were also amplified with three fewer cycles, and the bands again quantified. The results with the different number of cycles were compared to demonstrate that the expression patterns were not significantly different. To confirm the identity of the RT‐PCR products, the bands from the gel for both NbXEGIP1 and NbXEGIP2 were purified with GenElute™ Gel Purification Kit (Sigma) and directly sequenced (Laboratory Services Division, University of Guelph).
Gene silencing and analysis of silenced plants
The cloned 510‐bp RT‐PCR fragment of NbXEGIP1 obtained with primers NbXEGIP1F2 and NbXEGIP1R1 was digested from pBluescript using EcoRI and subcloned into EcoRI‐digested TRV‐RNA2 (PYL156 or pTRV2) vector (Liu et al., 2002). The pTRV2 construct was transformed into E. coli DH5α and selected on LB agar containing 50 mg/L kanamycin. The plasmid was extracted from E. coli and then transformed into Agrobacterium tumefaciens strain GV3101 by electroporation and selected on LB agar containing 50 mg/L kanamycin and 5 mg/L tetracycline. The antibiotic‐resistant GV3101 colonies were checked for the presence of the insert by PCR. Using the same approach, the green fluorescent protein (GFP) gene from pEZS‐CL (Yoshimoto et al., 2004) was inserted into pTRV2 and transformed into A. tumefaciens as a control.
Agrobacterium tumefaciens inoculations were done following the procedure of Liu et al. (2002) with some modifications. Transformed A. tumefaciens were grown for 48 h at 28 °C on LB containing 50 mg/L kanamycin and 5 mg/L tetracycline. Agrobacterium tumefaciens cultures containing pTRV1 and A. tumefaciens containing pTRV2 with NbXEGIP1 were suspended in 10 mm MgCl2 to an OD600 of 0.8 and then mixed in a 1 : 1 ratio. The bacterial mixture was infiltrated into the lower leaf of four‐leaf stage plants using a 1‐mL needle‐less syringe. As controls, 10 mm MgCl2 or A. tumefaciens containing pTRV1 mixed with A. tumefaciens containing pTRV2 with GFP were infiltrated onto N. benthamiana. At 15–18 days after A. tumefaciens infiltration, the two youngest fully matured leaves of each plant were sprayed either with spore suspensions of Colletotrichum spp. or infiltrated with P. syringae pv. tabaci or P. syringae pv. tabaci (avrPto) suspensions as described above. Lesion numbers were determined at 72 hpi for C. destructivum and 96 hpi for C. orbiculare. Bacterial populations were checked at 72 hpi for P. syringae pv. tabaci and 20 hpi for P. syringae pv. tabaci (avrPto). Relative RT‐PCR analysis of NbXEGIP1, NbXEGIP2 and NbDef2.2 expression was performed as described above at 96 hpi for Colletotrichum spp., 72 hpi for P. syringae pv. tabaci and 24 hpi for P. syringae pv. tabaci (avrPto) for plants treated with MgCl2, A. tumefaciens (pTRV1/pTRV2‐GFP) or A. tumefaciens (pTRV1/pTRV2‐NbXEGIP1).
For NbXEGIP1‐silenced and control leaves inoculated with P. syringae pv. tabaci, five 8‐mm‐diameter discs were excised per leaf at 66 hpi. The leaf discs were first washed in 15 mL sterile distilled water for 5 min, and then incubated for 2 h in 15 mL of fresh sterile distilled water with shaking at 60 r.p.m. The amount of electrolytes in the water was measured using a conductivity meter (PC 300 series, Oakton, Vernon Hills, IL). After the measurements, the material was autoclaved for 15 min at 121 °C, and then the amount of electrolytes was measured again after the water had returned to room temperature. Relative conductivity was calculated from the electrical conductivity at 2 h incubation divided by the electrical conductivity after autoclaving. Three separate experiments with a total of 15 leaves were examined.
ACKNOWLEDGMENTS
Pseudomonas syringae pv. tabaci 11528R and P. syringae pv. tabaci 11528R containing avrPto or vector pDSK519 were kindly provided by Dr G. Martin, Boyce Thompson Institute, Cornell University, Ithaca, NY. Transgenic N. benthamiana containing Pto was kindly provided by Dr R. Michelmore, Genome Center, University of California, Davis, CA. The plasmid pEZS‐CL was kindly provided by Dr M. Raizada, Department of Plant Agriculture, University of Guelph, Guelph, ON, Canada. Funding for this study was provided by the Natural Science and Engineering Research Council of Canada.
REFERENCES
- Acosta‐Rodriguez, I. , Pino‐Escobedo, C. , Zavala‐Paramo, M.G. , Lopez‐Romero, E. and Cano‐Camacho, H. (2005) Degradation of cellulose by the bean‐pathogenic fungus Colletotrichum lindemuthianum. Production of extracellular cellulolytic enzymes by cellulose induction. Int. J. Gen. Mol. Microbiol. 87, 301–310. [DOI] [PubMed] [Google Scholar]
- Agrios, G.N. (2005) Plant Pathology, 5th edn. San Diego: Elsevier Academic Press. [Google Scholar]
- Bahramnejad, B. (2007) The relationship of genes for defensin, 4,5‐DOPA dioxygenase and a ribonuclease‐like protein to diseases and abiotic stresses. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada. [Google Scholar]
- Chen, G.Y.J. , Jin, S. and Goodwin, P.H. (2000) An improved method for the isolation of total RNA from Malva pusilla tissues infected with Colletotrichum gloeosporioides . J. Phytopathol. 148, 57–60. [Google Scholar]
- Chen, N. , Goodwin, P.H. and Hsiang, T. (2003) Use of green fluorescent protein to quantify the growth of Colletotrichum during infection of tobacco. J. Microbiol. Methods, 53, 13–122. [DOI] [PubMed] [Google Scholar]
- Dean, J.D. , Goodwin, P.H. and Hsiang, T. (2002) Comparison of relative RT‐PCR and Northern blot analyses to measure expression of β‐1,3‐glucanase in Nicotiana benthamiana infected with Colletotrichum destructivum . Plant Mol. Biol. Rep. 20, 347–356. [Google Scholar]
- Denny, T.P. , Carney, B.F. and Schell, M.A. (1990) Inactivation of multiple virulence genes reduces the ability of Pseudomonas solanacearum to cause wilt symptoms. Mol. Plant–Microbe Interact. 3, 293–300. [Google Scholar]
- Ditt, R.F. , Nester, E.W. and Comai, L. (2001) Plant gene expression response to Agrobacterium tumefaciens . Proc. Natl Acad. Sci. USA, 98, 10954–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel, D. , Miyara, I. , Ailing, T. , Dinoor, A. and Prusky, D. (2002) pH regulates endoglucanase expression and virulence of Alternaria alternata in persimmon fruit. Mol. Plant–Microbe Interact. 15, 774–779. [DOI] [PubMed] [Google Scholar]
- Grishutin, S.G. , Gusakov, A.V. , Markov, A.V. , Ustinov, B.B. , Semenova, M.V. and Sinitsyn, A.P. (2004) Specific xyloglucanases as a new class of polysaccharide‐degrading enzymes. Biochem. Biophys Acta-Gen. Subjects, 1674, 268–281. [DOI] [PubMed] [Google Scholar]
- Huffaker, A. , Pearce, G. and Ryan, C.A. (2006) An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl Acad. Sci. USA, 103, 10098–10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa, T. , Ochiai‐Fukuda, T. , Takahashi‐Ando, N. , Ohsato, S. , Shibata, T. , Yamaguchi, I. and Kimura, M. (2004) New TAXI‐type xylanase inhibitor genes are inducible by pathogens and wounding in hexaploid wheat. Plant Cell Physiol. 45, 1347–1360. [DOI] [PubMed] [Google Scholar]
- Igawa, T. , Tokai, T. , Kudo, T. , Yamaguchi, I. and Kimura, M. (2005) A wheat xylanase inhibitor gene, Xip‐I, but not Taxi‐I, is significantly induced by biotic and abiotic signals that trigger plant defense. Biosci. Biotech. Biochem. 69, 1058–1063. [DOI] [PubMed] [Google Scholar]
- Jones, R.W. and Wang, H.Y. (1997) Immunolocalization of a beta‐1,4‐endoglucanase from Macrophomina phaseolina expressed in planta. Can. J. Microbiol. 43, 491–495. [Google Scholar]
- Jones, R.W. , Ospina‐Giraldo, M. and Deahl, K. (2006) Gene silencing indicates a role for potato endoglucanase inhibitor protein in germplasm resistance to late blight. Am. J. Potato Res. 83, 41–46. [Google Scholar]
- Juge, N. (2006) Plant protein inhibitors of cell wall degrading enzymes. Trends Plant Sci. 11, 359–367. [DOI] [PubMed] [Google Scholar]
- King, E.O. , Ward, M.K. and Raney, D.E. (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44, 301–307. [PubMed] [Google Scholar]
- Klement, Z. (1963) Rapid detection of the pathogenicity of phytopathogenic Pseudomonads . Nature, 199, 299–300. [DOI] [PubMed] [Google Scholar]
- Lay, F.T. , Brugliera, F. and Anderson, M.A. (2003) Isolation and properties of floral defensins from ornamental tobacco and petunia. Plant Physiol. 131, 1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. , Schiff, M. , Marathe, R. and Dinesh‐Kumar, S.P. (2002) Tobacco Rar1, EDS1 and NPR1/NIM1 like genes are required for N‐mediated resistance to tobacco mosaic virus. Plant J. 30, 415–429. [DOI] [PubMed] [Google Scholar]
- Lu, R. , Martin‐Hernandez, A.M. , Peart, J.R. , Malcuit, I. and Baulcombe, D.C. (2003) Virus‐induced gene silencing in plants. Methods, 30, 296–303. [DOI] [PubMed] [Google Scholar]
- Mandanhar, J. , Hartman, G. and Sinclair, J. (1986) Colletotrichum destructivum, the anamorph of Glomerella glycines . Phytopathology, 76, 282–285. [Google Scholar]
- Mudgett, M.B. (2005) New insights to the function of phytopathogenic bacterial type III effectors in plants. Annu. Rev. Plant Biol. 56, 509–531. [DOI] [PubMed] [Google Scholar]
- Naqvi, S.M.S. , Harper, A. , Carter, C. , Ren, G. , Guirgis, A. , York, W. and Thornburg, R.W. (2005) Nectarin IV, a potent endoglucanase inhibitor secreted into the nectar of ornamental tobacco plants. Isolation, cloning and characterization. Plant Physiol. 139, 1389–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez‐Vasquez, J. , Pearce, G. and Ryan, C.A. (2005) The plant cell wall matrix harbors a precursor of defense signaling peptides. Proc. Natl Acad. Sci. USA, 102, 12974–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega, J. (1994) Cell‐wall degrading enzymes produced by the phytopathogenic fungus Colletotrichum gloeoporioides . Tex. J. Sci. 46, 329–335. [Google Scholar]
- Park, Y.W. , Lim, S.T. and Yun, H.D. (1998) Cloning and characterization of a CMCase gene, celB, of Erwinia carotovora subsp. carotovora LY34 and its comparison to celA. Mol. Cells, 8, 280–285. [PubMed] [Google Scholar]
- Pearce, G. , Siems, W.F. , Bhattacharya, R. , Chen, Y.C. and Ryan, C.A. (2007) Three hydroxyproline‐rich glycopeptides derived from a single petunia polyprotein precursor activate defensin I, a pathogen defense response gene. J. Biol. Chem. 282, 17777–17784. [DOI] [PubMed] [Google Scholar]
- Qin, Q. , Bergmann, C.W. , Rose, J.K.C. , Saladie, M. , Kolli, V.S.K. , Albersheim, P. , Darvill, A.G. and York, W.S. (2003) Characterization of a tomato protein that inhibits a xyloglucan‐specific endoglucanase. Plant J. 34, 327–338. [DOI] [PubMed] [Google Scholar]
- Robertson, D. (2004) VIGS vectors for gene silencing: many targets, many tools. Annu. Rev. Plant Biol. 55, 495–519. [DOI] [PubMed] [Google Scholar]
- Rommens, C.M.T. , Salmeron, J.M. , Oldroyd, G.E.D. and Staskawicz, B.J. (1995) Intergeneric transfer and functional expression of the tomato disease resistance gene Pto. Plant Cell, 7, 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, S. , Sturm, A. , Fujii, T. and Chrispeels, M.J. (1992) cDNA cloning of an extracellular dermal glycoprotein of carrot and its expression in response to wounding. Planta, 188, 432–438. [DOI] [PubMed] [Google Scholar]
- Shen, S. , Goodwin, P.H. and Hsiang, T. (2001a) Hemibiotrophic infection and identity of the fungus, Colletotrichum destructivum, causing anthracnose of tobacco. Mycol. Res. 105, 1340–1347. [Google Scholar]
- Shen, S. , Goodwin, P.H. and Hsiang, T. (2001b) Infection of Nicotiana spp. by the anthracnose fungus, Colletotrichum orbiculare . Eur. J. Plant Pathol. 107, 767–773. [Google Scholar]
- Thilmony, R.L. , Chen, Z.T. , Bressan, R.A. and Martin, G.B. (1995) Expression of the tomato Pto gene in tobacco enhances resistance to Pseudomonas syringae pv tabaci expressing AvrPto . Plant Cell, 7, 1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D. , Gibson, T.J. , Plewniak, F. , Jeanmougin, F. and Higgins, D.G. (1997) The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24, 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, D.S. , Reeves, P.J. and Salmond, G.P.C. (1994) The major secreted cellulase, CelV, of Erwinia carotovora subsp. carotovora is an important soft rot virulence factor. Mol. Plant–Microbe Interact. 7, 425–431. [Google Scholar]
- York, W.S. , Qin, Q. and Rose, J.K.C. (2004) Proteinaceous inhibitors of endo‐β‐glucanases. Biochim. Biophys. Acta, 1696, 223–233. [DOI] [PubMed] [Google Scholar]
- Yoshimoto, K. , Hanaoka, H. , Sato, S. , Kato, T. , Tabata, S. , Noda, T. and Ohsumi, Y. (2004) Processing of ATG8s, ubiquitin‐like proteins, and their deconjugation by ATG4s are essential for plant autophagy. Plant Cell, 16, 2967–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]


