Abstract
Due to the nature of the biological response to traumatic spinal cord injury, there are very limited therapeutic options available to patients. Recent advances in cell transplantation have demonstrated the therapeutic potential of transplanting supportive cell types following spinal cord injury. In particular, pluripotent stem cell derived neural cells are of interest for the future investigation. Use of pluripotent stem cells as the source allows many cell types to be produced from a population that can be expanded in vitro. In this review, we will discuss the signaling pathways that have been utilized to differentiate spinal neural phenotypes from pluripotent stem cells. Additionally, we will highlight methods that have been developed to direct the differentiation of pluripotent stem cells to specific neural fates. Further refinement and elaboration of these techniques might aid in elucidating the multitude of neuronal subtypes endogenous to the spinal cord, as well as produce further therapeutic options for spinal cord injury recovery.
Keywords: Stem Cell differentiation, Spinal Cord Injury, Interneurons, Motoneurons
Spinal Cord Injury:
Spinal cord injury (SCI) affects over 250,000 individuals in the US alone and has poor functional recovery due to glial scar formation and limited endogenous stem or progenitor cell pools(NSCISC, 2016). Current advances in medical care have prolonged the lives of individuals with SCI; however, SCI is typically unpreventable and frequently results from an accident. Poor rates of functional recovery result in a life-long cost of greater than $1 million for medical care and assistance with daily living tasks; therefore, it is important to continue to investigate therapeutic methods that will increase functional recovery after SCI.
SCI presents in two phases, the first of which is necrosis that occurs following trauma to the spinal cord(Lukovic et al., 2015; Ronaghi et al., 2010). Damage of the individual neurons in the spinal cord following SCI leads to excitotoxic chemical release in the surrounding tissue and, subsequently, the toxic environment leads to death in the surrounding tissue(Lukovic et al., 2015). Astrocytes react to the toxic environment by migrating to the injury site and producing an inhibitory extracellular matrix that forms the glial scar and serves to contain the spreading injury. In many cases, this scar tissue acts as a physical and biochemical barrier that prevents axon growth through the site of injury(Liddelow and Barres, 2017). There have been numerous strategies investigated to promote regeneration after SCI, but there has been little to no translation of these therapies to clinical application. There have been three main approaches for treatment of SCI: stimulation of growth or repair mechanisms, scaffolding to promote directed axon growth through the injury site, and/or cellular transplantation of neural cell types.
Use of both morphogens and scaffolds to promote directed recovery are desirable as they are simple and scalable; however, biomaterial scaffolds have not been integrated as a clinical standard of care. Recent studies evaluating rodent functional recovery, to SCI, has due to the reorganization of spinal interneurons (INs) into propriospinal relay circuits(Asboth et al., 2018; Courtine et al., 2009, 2008). Recovery in patients, therefore, might be mitigated more efficiently through transplantation of spinal INs or glia. Studies involving autologous or allogeneic transplantations of Schwann cells, oligodendrocyte progenitor cells, and olfactory ensheathing cells have shown modest increase in recovery in rodent models(Barnett and Riddell, 2004; Kanno et al., 2015; Lima et al., 2010; Tetzlaff et al., 2011). However, autologous transplantations of Schwann or olfactory ensheathing cells could hinder function in patients elsewhere, as it requires removal of tissue from a donor site(Barnett and Riddell, 2004; Lima et al., 2010; Tetzlaff et al., 2011). Autologous transplantation of these support cells would only offer limited trophic support, whereas allogenic use of stem cell sources would prove to be much more advantageous, as stem cells could be induced into specific cell fates for a variety of patients and injury types.
Since the discovery and isolation of mouse (m) and human (h) embryonic stem cells (ESCs), a vast number of different induction protocols to derive numerous cell types have been developed. Furthermore, development of induced pluripotent stem cells (iPSCs) has expanded the potential for both studying and developing therapeutic strategies for diseases or injuries that had no long-term effective treatment method. Pluripotent stem cells (PSCs, ESCs and IPSCs) have been cell sources of interest in the research field of SCI due to their capacity for self-renewal, as well as their potential to be directed to numerous cell fates, of which the spinal cord has many.
There are 12 cardinal classes of spinal INs, eight dorsal and four ventral, each of which serve specific roles and functions for proper afferent and efferent signaling(Briscoe and Ericson, 2001; Hernandez-Miranda et al., 2017; Jessell, 2000; Lu et al., 2015; Ziskind-Conhaim and Hochman, 2017). Additionally, motor neurons (MNs) are located in the ventral spinal cord, and although they are the most frequently derived and examined neural cell type, there has been increased research into investigating the potential therapeutic effects of different interneuron classes. Herein, we aim to describe the current methods being utilized to derive the various types of neurons that are found in the spinal cord from pluripotent stem cell sources.
Spinal Neurons
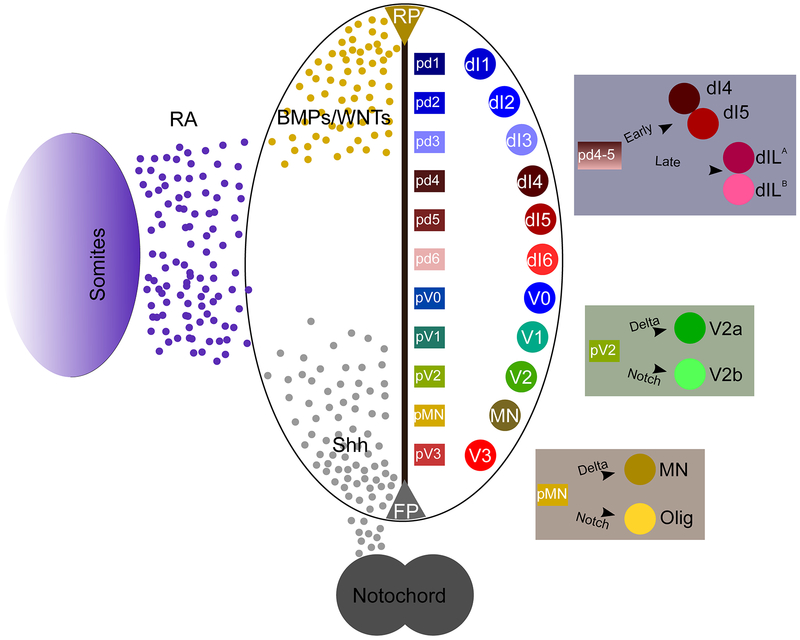
Within the ventral spinal cord there are the four cardinal classes of (INs) designated V0, V1, V2, and V3 based upon their dorsal to ventral locations, respectively. Each of these classes originate from progenitor pools (p0 – p3) that are located in similar dorsal to ventral orientation and can be identified by their expression of multiple transcription factors that are modulated along the dorsoventral axis by sonic hedgehog (Shh) signaling (Figure 1). Over time, these progenitor pools mature, and the subsequent ventral INs, V0, V1,V2, and V3, are then identifiable through their expression of Evx1, En1, Chx10/Gata2/3, and Sim1, respectively(Francius et al., 2013; Lu et al., 2015; Ziskind-Conhaim and Hochman, 2017). Among these four cardinal classes of INs is an ever-increasing number of subtypes being discovered and delineated through molecular codes, e.g. V2a, V2b, V2d, that each express some markers exclusive to the subtype. In addition to the ventral INs, MNs also develop in the ventral horn of the spinal cord arising from a progenitor MN (pMN) pool found between p2 and p3 domains. MNs, upon maturation will express Hb9 and eventually migrate toward specific motor columns depending on hox gene-mediated rostro-caudal patterning. MNs receive synaptic input from the many INs in the spinal cord, as well as descending corticospinal tracts from the brain, to provide proper excitation of muscle fibers(Crone et al., 2012; Francius et al., 2013; Lanuza et al., 2004).
Figure 1:
Development of neuronal cell types is modulated by morphogen concentrations.
Within the dorsal spinal cord, eight canonical classes of INs are found: dI1 – dI6, dILA, and dILB. Of these eight, dI1–3 develop dependent on wingless related integration (WNT) and bone morphogenetic protein (BMP) signaling and are often grouped together as Class A neurons. Contrastingly, the Class B dorsal INs, dI4–6, arise independent of WNT and BMP signaling, and they are considered to be the default fate of INs(Hernandez-Miranda et al., 2017; Kim et al., 2009). Each of the dorsal INs develop in a very specific temporal pattern, with the Class A neurons developing first, followed by the Class B neurons, and lastly the dILA/B neurons arise from the dI4 and dI5 progenitor domains, respectively. Dorsal INs are known to integrate and interpret the incoming sensory information for either reflexive arc or ascending signal transmission(Ziskind-Conhaim and Hochman, 2017).
Spinal neurons can be delineated using various parameters outside of transcriptional codes, such as secreted neurotransmitters, direction of axonal projections, frequency of excitation, expression of various calcium binding proteins, and location along the rostro-caudal axis. Specification of excitatory and inhibitory INs can be determined through expression of vesicular transporters for glutamate or glycine and gamma-aminobutyric acid, respectfully. Further evaluation of IN subtypes can be conducted with fluorescent lineage tracing in combination with fluorescently labelled IN subtypes to illustrate the projections directions of neurons through various segments of the spinal cord(Haque et al., 2018). For comprehensive reviews regarding those nuances of characterization for INs and MNs please refer to Ziskind-Conhaim and Hochman, Francius et al. or Lu et al(Francius et al., 2013; Lu et al., 2015; Ziskind-Conhaim and Hochman, 2017).
Spinal Glia
Native to the spinal cord are numerous quantities of glial cells, and what was once conceived as an amalgamation of support cells that strictly hold together the neuronal networks has grown into wider understanding of a vital modulatory and support network. There are two primary structurally and metabolically supportive types of glia in the central nervous system (CNS), oligodendrocytes and astrocytes. Oligodendrocytes were once considered to only serve to produce myelin in the CNS white matter for rapid axon potential conduction; however, recently, oligodendrocytes have been shown to also play a role in the metabolic health of neurons by secreting lactate and pyruvate for neuronal consumption(Fünfschilling et al., 2012). Further misunderstandings have been made about the astrocyte, as their presence at the glial scar after injury was previously considered to be problematic, because they produce a variety of inhibitory factors found around the lesion site(Lukovic et al., 2015). However, in recent years there has been further work elucidating their quiescent functions, such as blood-brain barrier maintenance and neural metabolism, as well as reframing their reactive functions of inhibitory matrix formation as a protective measure following CNS injuries(Bayraktar et al., 2015; Ben Haim and Rowitch, 2016; Lukovic et al., 2015).
Similar to neurons, both astrocytes and oligodendrocytes develop from the neuroepithelium; however, they typically differentiate at a distinctly later time point(Billon, 2002; Tan et al., 2016). Following neurogenesis, glial restricted precursors differentiate into astrocytes and oligodendrocytes(Muroyama et al., 2005). Studies into astrocyte diversity have found differences amongst traditional classes of astrocytes, similar to the stratification of interneuron cardinal classes(Hochstim et al., 2008). In the spinal cord, there are both grey matter, or protoplasmic (A2), astrocytes as well as the white matter, or fibrous (A1), astrocytes. While the morphologies of these cells are quite distinct, discrete metrics defining astrocyte subpopulations remains elusive(Bayraktar et al., 2015). More frequently, they are identified based on their activity states: the quiescent, the inflammatory response, and the ischemic response astrocyte(Li et al., 2018; Liddelow et al., 2017; Liddelow and Barres, 2017). The A1 and A2 reactive astrocytes exhibit different responses to injury, and the field of astrocyte biology is growing to assess those nuances, for further information regarding this topic please consult Liddelow et al.(Liddelow and Barres, 2017).
Producing Distinct Neural Cell Types
The earliest publication, to our knowledge, regarding neuronal differentiation from an ESC source was performed in 1995 by Bain et al. in which, simple application of retinoic acid (RA) drove neural phenotype specification(Bain et al., 1995). Their approach was a novel adaptation from studying the mechanisms that could produce neurons from an embryonic carcinoma population, and since that initial publication there has been broader investigation into the underlying mechanisms and methodology to direct differentiation(Bain et al., 1994). Early on, there was a focus on the various components, such as morphogens and media components that could be utilized in neuronal differentiation to optimize protocols. One such example is the work of Okabe et al., which looked at the incorporation of insulin, transferrin, selenium, basic fibroblast growth factor (bFGF), and fibronectin to maintain neuroepithelium fates to increase the quantity of neural cells produced following induction(Okabe et al., 1996). Whereas temporal exposure of bFGF at the neuroepithelial stage had been reported as beneficial for proliferation, this was a crucial understanding in dissecting neuronal induction into discrete steps.
With advancements in the understanding of the molecular mechanisms driving neural developmental processes, as well as further development of small molecule inhibitors and activators, there has been wider use of different morphogens in induction. The most successful directed differentiation protocols in use for spinal neural populations in vitro exploit the specific developmental processes of neuroepithelialization, caudalization, and dorsoventral specification; however, the novelty amongst induction protocols is in modulating the strength of the signals involved in each of these steps.
During the neuroepithelialization phase of induction, in recent years, there has been expansion of the methods that aid driving various desired phenotypes. In hESC and hiPSC (hPSC) neural induction, dual SMAD inhibition is commonly utilized, as it has shown to increase the induction efficiency toward neural progenitor fates(Chambers et al., 2009; Su et al., 2018). SMAD inhibition is not widely used in mESC neural induction procedures, but there has been growth in the field regarding the production of neuromesodermal progenitors (NMPs) through modulation of FGF and TGFβ signaling pathways(Gouti et al., 2014). Additionally the usage of epidermal growth factor and FGF has been shown effective at propagation of the neuroepithelial states in hPSC derived neural progenitors(Borghese et al., 2010; Koch et al., 2009).
To drive caudalization, typically RA is utilized, as it endogenously originates from the somites around the lower hindbrain through the cervical vertebrae in mice(Del Corral et al., 2003; Ribes et al., 2009). In mammals RA and FGF signaling work through cross-repressive means in order to modulate the elongation of the body axis in vivo and as such derivation of more posterior spinal identities require FGF signaling(Berenguer et al., 2018; Del Corral et al., 2003; Gouti et al., 2014). In particular, NMP formation in vitro exploits these signaling modalities to produce caudal fates expressing hoxc8 with a combination of FGF signaling and WNT signaling(Gouti et al., 2014).
Ventralization is driven through both RA and the Shh pathway, which functionally modulates Gli transcriptional factors to act as repressors or activators through graded concentrations of Shh, producing the various ventral neural populations(Briscoe et al., 2000, 1999; Calder et al., 2015; Dessaud et al., 2010; Ericson et al., 1997; Ribes et al., 2009). Whereas RA is inexpensive at concentrations needed for induction, Shh levels required for induction of large quantities of cells in vitro could be costly, so Shh signaling typically is typically activated using small molecules, such as Smoothened agonist (SAG) or purmorphamine, the latter of which is weaker in potency(Sinha and Chen, 2006). Dorsalization of neuronal subtypes, as mentioned previously, is driven through WNT and BMP signaling, and the single publication (to our knowledge) on producing dorsal INs from stem cells relied on recombinant proteins(Gupta et al., 2018). Although it should be noted that plenty of small molecule BMP/TGFβ activators and inhibitors exist, and similar to the previously mentioned SMAD inhibitors used for hPSC induction(Chambers et al., 2009; Su et al., 2018).
Another common reagent used in induction protocols is DAPT ((N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester)), which acts as a gamma secretase inhibitor preventing the Notch intercellular domain from being cleaved and impacting gene expression. As such, investigators have thoroughly elucidated the effect of DAPT (Notch inhibition) on the regulation of neurogenesis or gliogenesis, as well as its effect on driving subtype specification(Ben-Shushan et al., 2014; Borghese et al., 2010; Butts et al., 2017; Tan et al., 2016).
Motor neurons
The widely adapted MN induction protocol, from Wichterle et al., was one of few initial examples of a specific spinal neuron type being induced from a mESC(Renoncourt et al., 1998; Wichterle et al., 2002). Embryoid body formation for the first two days allowed for the production of a three dimensional neuroepithelium. Subsequent caudalization with RA and ventralization with SAG demonstrated the simple stepwise process that could produce Lhx3+ MNs, similar in gene expression to those MNs of the hypaxial motor column(Peljto et al., 2010). This induction strategy produced predominately Lhx3+ MNs and little else, and more recently there has been further research conducted to produce specific motor column MNs from pluripotent stem cell sources(Peljto et al., 2010; Tan et al., 2016; Wichterle and Peljto, 2008). Recently, it has been demonstrated that temporal Notch inhibition can prevent the induction of oligodendrocyte fates during the pMN phase of induction(Ben-Shushan et al., 2014), as well as induce various MN phenotypes(Tan et al., 2016).
Although the initial methodology has undergone revision and review(Dasen et al., 2008; Okada et al., 2004; Peljto et al., 2010), it still paved the way for later extrapolation of these protocols to drive human embryonic and induced pluripotent stem cells to MN fates. Protocols outlining the induction of hESC/hiPSC derived-MNs frequently utilize SMAD inhibitors in the initial stages of differentiation to ensure efficient neurogenesis(Ben-Shushan et al., 2014; Butts et al., 2017; Chambers et al., 2009; Reinhardt et al., 2013). Following neuroepithelialization, RA treatment caudalizes the cells, and SAG is utilized for ventral specification. Distinct from the mESC-derived MNs, hPSCs are given two days of exclusive RA treatment to caudalize them. Further optimization of the induction protocol of hESC-MNs have explored the incorporation of DAPT during the progenitor phases of induction to drive progenitor MNs towards MN fates rather than glial lineages, such as oligodendrocytes(Ben-Shushan et al., 2014; Borghese et al., 2010). Often times residual glial progenitors in these cultures are capable of dividing and further differentiating, and this unregulated proliferation can overwhelm post-mitotic neurons. By producing transgenic mESC lines containing the antibiotic resistance gene, puromycin N-acetyltransferase (PAC), under the control of gene regulatory elements for either Olig2 or Hb9, McCreedy et al. produced two selectable cell lines capable of purification of mESC cultures to yield pMNs or MNs(McCreedy et al., 2014, 2012).
As a tool to investigate MN disorders, stem cell-derived MNs have been used to investigate diseases, such as spinal muscular atrophy and amyotrophic lateral sclerosis (ALS)(Dimos et al., 2008; Reinhardt et al., 2013; Sances et al., 2016). Recently, Sances et al. presented a review regarding the many attempts at using hiPSC techniques to produce pluripotent cells to derive MNs as a novel tool for evaluating ALS in vitro. As a therapeutic for spinal cord injury, these populations have been used to a limited capacity, as proper transplantation of ESC-derived MNs would require a fine level of integration into existing circuits. A better therapeutic option might be utilization of excitatory IN populations, such as V2a and V3 INs, that possess inherent reorganizational capacity, allowing them to bridge the signal from the cortical spinal tracts onto downstream targets(Courtine et al., 2009, 2008).
Ventral INs:
V3 INs:
A potentially crucial spinal neuron class that could be used for cell transplantation is the commissural projecting V3 IN(Borowska et al., 2013). Production of Sim1+ V3 INs has been demonstrated twice in the literature, both using mESC cells(Sternfeld et al., 2017; Xu and Sakiyama-Elbert, 2015). Initially, Xu and Sakiyama-Elbert demonstrated that the cell type could be produced by modulating the concentration of SAG and RA over varying induction periods. By evaluating the expression of Sim1 over 6 and 8 days, it was found that high levels of SAG with low levels of RA yielded higher expression of the definitive marker Sim1. In studies performed by Pfaff et al., a Sim1 driven fluorescent reporter (FR) cell line was used, and it indicated high levels of SAG, similar to that of Xu and Sakiyama-Elbert, generated the highest percentage of V3 INs.
Xu and Sakiyama-Elbert noted the induction efficiency of V3 INs was low, and as such, they produced a Sim1 selectable cell line. They inserted the antibiotic resistance gene PAC into the Sim1 locus of a mESC line(Xu et al., 2015). Similar to the MN selectable mESC lines, this allowed from selection of V3 INs using puromycin, allowing for scalability and the potential for future transplantation for therapeutic studies. Unfortunately, to our knowledge, the V3 IN induction procedure has not yet been adapted to hESCs or hiPSCs, nor has there been any publications regarding the transplantation of V3 INs.
V2a INs:
V2a INs were first differentiated using mESCs, through adapting the induction strategies from MN derivation(Brown et al., 2014). To produce a weaker Shh signal than the MN induction methods, Brown et al. investigated the use of both purmorphamine and SAG, noting the weaker signal from purmorphamine was ideal to drive the expression of the V2a definitive marker Chx10/Vsx2(Brown et al., 2014; Sinha and Chen, 2006). Whereas Brown et al. relied on lower levels of RA to drive Chx10 expression, they also demonstrated that the RA dosage did have a strong effect on rostro-caudal hox gene expression. To specify V2a phenotypes, DAPT was used to inhibit Notch signaling, as the V2 class separates into two known subtypes V2a and V2b INs through the Notch signaling pathway(Del Barrio et al., 2007; Ramos et al., 2010; Rocha et al., 2009). To produce highly enriched V2a IN populations, Iyer et al. used similar methods to Xu et al., inserting PAC into the locus of Chx10/Vsx2(Iyer et al., 2016). As a potential therapeutic, enriched populations of V2a INs could be transplanted in a SCI model to act as a propriospinal relay point for signal transmission. Recent transplantation studies involving this cell line have demonstrated integration with host tissue in a thoracic dorsal hemisection, and functional recovery in a C4 contusion hemisection SCI model(Thompson et al., 2018; Zholudeva et al., 2018).
The initial step forward to producing V2a INs from mESCs, as a tool for potential therapy, was recently extrapolated to hESC/hIPSCs by Butts et al.(Butts et al., 2017). Briefly, hESCs and hiPSCs were differentiated using methods analogous to hESC derived MNs, with SMAD inhibitors and basal medium for the first segment of neuro-epithelialization(Ben-Shushan et al., 2014; Peljto et al., 2010). Subsequently, the hESCs were caudalized with RA, ventralized with purmorphamine, and directed to V2a phenotype with DAPT. Upon transplantation of the hESC derived V2a INs into an uninjured mouse spinal cord, the authors demonstrated that the transplant was capable of extending axons up to 5 mm through the length of the spinal cord. This recent adaptation to the differentiation repertoire of hESC/hIPSCs has further opened the door for potential cell transplantation techniques for SCI.
V1 INs:
To date, there is only one peer reviewed publication, to our knowledge, that has focused on producing V1 INs. Sternfeld et al. attempted to derive the many distinct ventral neuronal populations, such as MN, V3 IN, and V1 IN to integrate them into neural circuitoids(Sternfeld et al., 2017). To produce V1 INs, they relied upon a FR system to evaluate concentrations of SAG for induction. Whereas their En1+ neurons were most likely V1 INs, as this is the definitive marker for this IN class in the spinal cord, it is unclear if this induction procedure has been optimized or vetted.
V0 INs:
To date, there is no publication outlining a robust, optimized differentiation strategy for V0 INs. However, in order to evaluate the cell types produced under retinoic acid only induction methods, Kim et al. revealed that there was high expression of Evx1+ V0 INs(Kim et al., 2009). This process also yielded high expression of dI4/6, another cell type that does not require strong dorsal or ventral signaling cues.
Dorsal INs:
Class A INs:
In a recent publication by Gupta et al., production of the Class A dorsal INs from hESCs relies on similar methods as the hESC induction protocol for MNs, without dual SMAD inhibition(Gupta et al., 2018). Maturation occurred through caudalization with RA and dorsalization with BMP4. This is the only publication inducing dorsal spinal INs, to our knowledge, and no follow-up work has been conducted with either mESCs or transplantation. We suspect that, as these Class A INs (dI1–3) arise in development before the Class B INs (dI4–6), similar strategies with modulations to the duration of morphogens present or the duration of induction might hold the key in producing the Class B dorsal INs.
Oligodendrocytes
The production of oligodendrocytes from mESCs and hESCs has been well-established through various protocols. The initial induction protocols from Bain et al. demonstrated the potential to produce oligodendrocytes and Liu et al. further demonstrated the potential for these oligodendrocytes to mature and incorporate into demyelinated spinal cords(Bain et al., 1995; Su Liu, Yun Qu, Todd J. Stewart, Michael J. Howard, Shushovan Chakrabortty, Terrence F. Holekamp, 2000). As the induction methodology at this point had not been refined to specifically induce oligodendrocytes, Billon et al. employed a lineage selectable mESC method to yield higher numbers of oligodendrocytic progenitors (OPCs)(Billon, 2002). Billion et al. used a Sox2 selectable line to ensure a robust, proliferative progenitor pool was maintained while differentiated cells were removed from culture. While this work largely demonstrated the shift in the proliferative population over time from a pluripotent to a more multipotent cell fate, it helped establish a larger foundation of OPC generation for future studies.
Adaptation of the OPC differentiation protocol using hESCs has since been performed by several groups, the earliest of which, to our knowledge, was by Nistor et al.(Nistor et al., 2005). Keirstead et al. further showed that these hESC derived OPCs, when transplanted, produced a functionally mature oligodendrocyte phenotype(Keirstead, 2005). When transplanted into injured spinal cord models, OPCs matured and helped drive functional recovery by myelinating axons. This technique was adapted in a brief phase I clinical trial that consisted of five patients before it was quickly cancelled 1 year after inception. The decision to cancel the trial was primarily motivated by financial concerns of the sponsoring company, Geron. The ESC technology was eventually licensed to Asterias, who later reported that there were no adverse events for the 5 patients after 3 years of clinical follow up. Asterias Biotherapeutics recently reported the 24 month data for the 25 patients enrolled in their Phase I/IIa SciSTAR study. There were no serious adverse events and evidence of cell engraftment by MRI. In the ASI-A cohort with the high dose (10 million cells), 83% (5/6) of these subjects have recovered improved motor function of two or more motor levels on at least one side. They are currently in discussions with the FDA about an expanded clinical trial.
Additional work has considered the potential of using pMNs, as this progenitor domain produces both MNs and OPCs. Transplantation of pMNs allows for the maturation of the progenitor domain into integrated and functional MNs and oligodendrocytes; however, this method relies strictly on endogenous signals. Modulation of the ratio of MN:OPCs produced, has been demonstrated to be driven through the Notch-Delta signaling pathway. Oligodendrocytes, similarly to Schwann cells, have proven to be a potential therapeutic option specifically for their capacity to remyelinate axons following SCI, yet this limited capacity is far from the only functional recovery deficit in SCI.
Astrocytes:
Directed induction of astrocytes is a recently growing area of interest, though far less robust as astrocytes were initially viewed as an unwanted byproduct of neural induction. However, it has since been shown that either primary or derived astrocytes might be extremely beneficial to the longevity of the neuronal cultures(Johnson et al., 2007; Thompson et al., 2017). Derivation of astrocytes has been studied in both mESC and hESC cultures, although usage of mESCs has led to breakthroughs in enhancing the derivation process(Benveniste et al., 2005; Kleiderman et al., 2016; Krencik et al., 2011; Roybon et al., 2013).
In 2011, Krencik et al. published an extended differentiation protocol for hESCs to help standardize the differentiation process(Krencik et al., 2011; Krencik and Zhang, 2011). Initial differentiation protocols merely demonstrated the expression of glia fibrillary acidic protein (GFAP) as a marker for astrocytes following a 12 week induction. GFAP is not a strong indicator of the astrocyte population, as it is also expressed in oligodendroglia, and varies in its expression levels depending on the reactive state of the astrocyte(Krencik and Zhang, 2011; Lukovic et al., 2015). Krencik et al. went on to demonstrate that through an extensive 6 month differentiation process various markers of astrocytes are expressed as the astroglia mature into a functionally capable cell type, as determined by calcium waves and synaptogenesis. By using mESC derived astrocytes, Roybon et al. demonstrated that inclusion of FGF1 could drive further maturation of the derived astrocytes(Roybon et al., 2013). When Roybon et al. extrapolated this finding to the induction of hPSCs, they demonstrated that hPSC derived astrocytes had higher expression of excitatory amino acid transporter, suggesting they were more like primary tissue derived astrocytes. Induction protocols for specific subtypes of neurons have been demonstrated, as previously mentioned; yet, many publications on hPSC derived astrocytes have primarily been concerned with generating a monolithic cell type that is simply “an astrocyte”(Kleiderman et al., 2016). Since the early 1980s, there was a compelling quantity of research being established suggesting that there were various phenotypes amongst astrocytes, such as protoplasmic and fibrous astrocytes. Roybon et al. did not examine the formation of protoplasmic and fibrous astrocyte subtypes, but did highlight the potential reactive state could be modulated using FGF and TNFα(Roybon et al., 2013).
Development of the protoplasmic and fibrous astrocyte phenotypes had been evaluated first by Davies et al. and then Thompson et al.(Davies et al., 2008, 2006; Thompson et al., 2017). Initial derivation of astrocyte phenotypes relied on driving glial restricted progenitors towards either protoplasmic or fibrous astrocytic fate with signaling from either BMP or CNTF, respectively. By doing this, Davies et al. demonstrated that protoplasmic astrocytes have a higher impact on growth responses when transplanted into injury sites(Davies et al., 2008). Thompson et al. adapted both the Roybon et al. and Davies et al. protocols to induce mESCs toward either protoplasmic or fibrous fates(Thompson et al., 2017). They found that over time, protoplasmic and fibrous astrocytes produced different extracellular matrix compositions, which greatly affected the growth of neurons on them. Follow-up studies by Thompson et al. used ECM produced from mESC-derived astrocytes as a transplantable therapeutic for SCI model in rats(Thompson et al., 2018). By transplanting mESC derived protoplasmic astrocyte ECM, there was a change to the scar environment and an overall increase in neuronal processes into the injury site. By using the astrocyte ECM as a vehicle for transplantation of purified V2a INs, transplanted cells demonstrated integration into the spinal cord and further increase in neuronal extension into and out of the injury site. Investigation into the functional outcomes of the animals was not conducted.
Challenges to overcome:
To date, there has been an expansive evaluation of the transplantation efficacy of various cell populations that might influence recovery following SCI. Frequently, the potentially therapeutic cell populations being investigated for cell transplantation for support following SCI are astrocytes or oligodendrocytes, with the overall goal of promoting recovery through neurotrophic support and re-myelination of injured tissues(Davies et al., 2008; Nicaise, 2015; Su Liu, Yun Qu, Todd J. Stewart, Michael J. Howard, Shushovan Chakrabortty, Terrence F. Holekamp, 2000). Both astrocytes and oligodendrocytes are well known for their supportive potential following SCI; however, there is interest in the potential usage of excitatory INs for propriospinal relay networks in functional recovery(Asboth et al., 2018; Courtine et al., 2009, 2008). As mentioned previously, recent work has investigated the potential to transplant specific INs involved in injured tissues such as high cervical SCI models and V2a INs, which are required for proper inspiration, with the intention of the reorganizational capacity of those INs providing functional recovery(Crone et al., 2012; Thompson et al., 2018; Zholudeva et al., 2018). However, these procedures have only been conducted on a limited number of excitatory cell types. Transplantation of inhibitory neurons, counterintuitively, can also be beneficial to recovery, as mESC and hESC derived GABAergic neuron transplantation has shown to reduce neuropathic pain, tactile hypersensitivity, and increase bladder functionality following SCI(Fandel et al., 2016; Hwang et al., 2016; Kim et al., 2010). As both excitatory and inhibitory neuron transplantations potentially could confer functional benefits, it is unclear if there is a superior cell type for transplantation in clinical settings based on current research. Glial and neuronal transplantations demonstrate integration and functional benefits, but methods should be refined in a number of areas before clinical translation occurs, such as population heterogeneity.
Heterogeneity in cultures of induced pluripotent sources is a well-documented obstacle, typically mediated through cell enrichment procedures. As mentioned previously, two popular cell purification and enrichment procedures, in the literature reviewed here, are antibiotic resistance and fluorescent reporter methods(Iyer et al., 2016; McCreedy et al., 2014; Sternfeld et al., 2017; Xu et al., 2015). Both of these methods are limited by the expression of definitive markers that are in some instances temporally regulated and might have off-target expression. Using an Hb9-eGFP reporter mouse, Caldeira et al. showed that Hb9 might be temporally regulated in the development of an unidentified IN subtype(Caldeira et al., 2017). It is unclear if the Hb9 INs were present in derived MN cultures, yet it demonstrates the potential area for refinement in this technique. Through further vetting of induction strategies and the cell types produced these effects likely could be mitigated. Enrichment procedures typically involve the enrichment of entire cell classes, e.g. V3 IN and MNs. Although Wichterle has highlighted the production of specific MN subtypes is possible, subtype induction specificity often times is not heavily scrutinized amongst induction procedures(Tan et al., 2016). Induction to a specific IN subtype has been demonstrated amongst only one of the classes of INs, V2a INs, which has recently been demonstrated to be made up of two subtypes(Hayashi et al., 2018). Furthermore, recent studies by Bikoff et al. and Sweeney et al., have suggested that there are possibly >50 V1 IN subtypes present in the spinal cord with identities associated with their rostro-caudal hox gene expression, similar to MNs(Bikoff et al., 2016; Sweeney et al., 2018). As the diversity in subtypes was patterned based on hox codes, similar diversity could be inherent in other IN subtypes. Furthermore, the functional roles of many IN subtypes of spinal IN remain unknown, or poorly elucidated.
Conclusion:
Many studies have looked to stem cells as a potential therapeutic cell source for spinal cord injury. To produce various neural phenotypes, most methods incorporate a development inspired induction strategy of driving neuroepithelization, caudalization and dorsoventral patterning. Doing so with PSCs often relies on use of morphogens and small molecules to drive specific neurogenic pathways. Exploiting inherent graded signal pathways with PSCs yield proper phenotypic specification in vitro. Of the various spinal INs and glia, there are few canonical phenotypes that have not been produced using PSCs; however, due to the growing delineations that are being made based on molecular codes and gene expression profiles there is an abundance in diversity being uncovered. Because of this diversity in subtypes, there are many that could be used in a therapeutic capacity for transplantation following SCI, as the benefit of any particular phenotype has yet to be elucidated by experimentation.
Acknowledgements:
This work was supported by the National Institutes of Health R01NS051706 (SSE).
References
- Asboth L, Friedli L, Beauparlant J, Martinez-Gonzalez C, Anil S, Rey E, Baud L, Pidpruzhnykova G, Anderson MA, Shkorbatova P, Batti L, Pagès S, Kreider J, Schneider BL, Barraud Q, Courtine G, 2018. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci 21:576–588. 10.1038/s41593-018-0093-5 [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI, 1995. Embryonic stem cells express neuronal properties in vitro. Dev. Biol 10.1006/dbio.1995.1085 [DOI] [PubMed] [Google Scholar]
- Bain G, Ray WJ, Yao M, Gottlieb DI, 1994. From Embryonal Carcinoma Cells to Neurons: The P19 Pathway. BioEssays 16:343–348. [DOI] [PubMed] [Google Scholar]
- Barnett SCS, Riddell JJS, 2004. Olfactory ensheathing cells and the treatment of CNS injury: advantages nd possible caveats. J. Anat 204:57–67. 10.1111/j.1469-7580.2004.00257.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar OA, Fuentealba LC, Alvarez-Buylla A, Rowitch DH, 2015. Astrocyte development and heterogeneity. Cold Spring Harb. Perspect. Biol 7 10.1101/cshperspect.a020362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shushan E, Feldman E, Reubinoff BE, 2014. Notch Signaling Regulates Motor Neuron Differentiation of Human Embryonic Stem Cells. Stem Cells 2:403–415. 10.1002/stem.295 [DOI] [PubMed] [Google Scholar]
- Ben Haim L, Rowitch DH, 2016. Functional diversity of astrocytes in neural circuit regulation. Nat. Rev. Neurosci 18:31–41. 10.1038/nrn.2016.159 [DOI] [PubMed] [Google Scholar]
- Benveniste RJ, Keller G, Germano I, 2005. Embryonic stem cell-derived astrocytes expressing drug-inducible transgenes: differentiation and transplantion into the mouse brain. J. Neurosurg 103:115–123. 10.3171/jns.2005.103.1.0115 [DOI] [PubMed] [Google Scholar]
- Berenguer M, Lancman JJ, Cunningham TJ, Dong PDS, Duester G, 2018. Mouse but not zebrafish requires retinoic acid for control of neuromesodermal progenitors and body axis extension. Dev. Biol 441:127–131. 10.1016/j.ydbio.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikoff JB, Gabitto MI, Rivard AF, Drobac E, MacHado TA, Miri A, Brenner-Morton S, Famojure E, Diaz C, Alvarez FJ, Mentis GZ, Jessell TM, 2016. Spinal Inhibitory Interneuron Diversity Delineates Variant Motor Microcircuits. Cell 165:207–219. 10.1016/j.cell.2016.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, 2002. Normal timing of oligodendrocyte development from genetically engineered, lineage-selectable mouse ES cells. J. Cell Sci. 115:3657–3665. 10.1242/jcs.00049 [DOI] [PubMed] [Google Scholar]
- Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brüstle O, 2010. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells 28:955–964. 10.1002/stem.408 [DOI] [PubMed] [Google Scholar]
- Borowska J, Jones CT, Zhang H, Blacklaws J, Goulding M, Zhang Y, 2013. Functional Subpopulations of V3 Interneurons in the Mature Mouse Spinal Cord. J. Neurosci 33:18553–18565. 10.1523/JNEUROSCI.2005-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J, Ericson J, 2001. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol 11:43–49. 10.1016/S0959-4388(00)00172-0 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J, 2000. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101:435–445. 10.1016/S0092-8674(00)80853-3 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, 1999. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature 398 10.1038/19315 [DOI] [PubMed] [Google Scholar]
- Brown CR, Butts JC, McCreedy DA, Sakiyama-Elbert SE, 2014. Generation of v2a interneurons from mouse embryonic stem cells. Stem Cells Dev. 23:1765–76. 10.1089/scd.2013.0628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts JC, McCreedy DA, Martinez-Vargas JA, Mendoza-Camacho FN, Hookway TA, Gifford CA, Taneja P, Noble-Haeusslein L, McDevitt TC, 2017. Differentiation of V2a interneurons from human pluripotent stem cells. Proc. Natl. Acad. Sci 114:4969–4974. 10.1073/pnas.1608254114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira V, Dougherty KJ, Borgius L, Kiehn O, 2017. Spinal Hb9::Cre-derived excitatory interneurons contribute to rhythm generation in the mouse. Sci. Rep 7:1–12. 10.1038/srep41369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder EL, Tchieu J, Steinbeck JA, Tu E, Keros S, Ying S-W, Jaiswal MK, Cornacchia D, Goldstein PA, Tabar V, Studer L, 2015. Retinoic Acid-Mediated Regulation of GLI3 Enables Efficient Motoneuron Derivation from Human ESCs in the Absence of Extrinsic SHH Activation. J. Neurosci 35:11462–11481. 10.1523/JNEUROSCI.3046-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SMSM, Fasano CACA, Papapetrou EP, Tomishima M, Sadelain M, Studer L, 2009. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol 27:275–280. 10.1038/nbt.1529.Highly [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, Van Den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR, 2009. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci 12:1333–1342. 10.1038/nn.2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV, 2008. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med 14:69–74. 10.1038/nm1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone SA, Viemari J-CJ-C, Droho S, Mrejeru A, Ramirez J-MJ-M, Sharma K, 2012. Irregular Breathing in Mice following Genetic Ablation of V2a Neurons. J. Neurosci 32:7895–7906. 10.1523/JNEUROSCI.0445-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM, 2008. Hox Repertoires for Motor Neuron Diversity and Connectivity Gated by a Single Accessory Factor, FoxP1. Cell 134:304–316. 10.1016/j.cell.2008.06.019 [DOI] [PubMed] [Google Scholar]
- Davies JE, Huang C, Proschel C, Noble M, Mayer-Proschel M, Davies SJA, 2006. Astrocytes derived from glial-restricted precursors promote spinal cord repair. J. Biol 5 10.1186/jbiol35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JE, Pröschel C, Zhang N, Noble M, Mayer-Pröschel M, Davies SJA, 2008. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. J. Biol 7 10.1186/jbiol85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Barrio MG, Taveira-Marques R, Muroyama Y, Yuk D-I, Li S, Wines-Samuelson M, Shen J, Smith HK, Xiang M, Rowitch D, Richardson WD, 2007. A regulatory network involving Foxn4, Mash1 and delta-like 4/Notch1 generates V2a and V2b spinal interneurons from a common progenitor pool. Development 134:3427–3436. 10.1242/dev.005868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corral RD, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K, 2003. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40:65–79. 10.1016/S0896-6273(03)00565-8 [DOI] [PubMed] [Google Scholar]
- Dessaud E, Ribes V, Balaskas N, Yang LL, Pierani A, Kicheva A, Novitch BG, Briscoe J, Sasai N, 2010. Dynamic assignment and maintenance of positional identity in the ventral neural tube by the morphogen sonic hedgehog. PLoS Biol. 8 10.1371/journal.pbio.1000382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K, 2008. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. TL - 321. Science (80-.). 321 VN-:1218–1221. 10.1126/science.1158799 [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, Van Heyningen V, Jessell TM, Briscoe J, 1997. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90:169–180. 10.1016/S0092-8674(00)80323-2 [DOI] [PubMed] [Google Scholar]
- Fandel TM, Trivedi A, Nicholas CR, Zhang H, Chen J, Martinez AF, Noble-Haeusslein LJ, Kriegstein AR, 2016. Transplanted Human Stem Cell-Derived Interneuron Precursors Mitigate Mouse Bladder Dysfunction and Central Neuropathic Pain after Spinal Cord Injury. Cell Stem Cell 19:544–557. 10.1016/j.stem.2016.08.020 [DOI] [PubMed] [Google Scholar]
- Francius C, Harris A, Rucchin V, Hendricks TJ, Stam FJ, Barber M, Kurek D, Grosveld FG, Pierani A, Goulding M, Clotman F, 2013. Identification of Multiple Subsets of Ventral Interneurons and Differential Distribution along the Rostrocaudal Axis of the Developing Spinal Cord. PLoS One 8 10.1371/journal.pone.0070325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfschilling U, Supplie LM, Mahad D, Boretius S, Saab AS, Edgar J, Brinkmann BG, Kassmann CM, Tzvetanova ID, Möbius W, Diaz F, Meijer D, Suter U, Hamprecht B, Sereda MW, Moraes CT, Frahm J, Goebbels S, Nave KA, 2012. Glycolytic oligodendrocytes maintain myelin and long-term axonal integrity. Nature 485:517–521. 10.1038/nature11007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J, 2014. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 12 10.1371/journal.pbio.1001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Sivalingam D, Hain S, Makkar C, Sosa E, Clark A, Butler SJ, 2018. Deriving Dorsal Spinal Sensory Interneurons from Human Pluripotent Stem Cells. Stem Cell Reports 10:390–405. 10.1016/j.stemcr.2017.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F, Rancic V, Zhang W, Clugston R, Ballanyi K, Gosgnach S, 2018. WT1-Expressing Interneurons Regulate Left–Right Alternation during Mammalian Locomotor Activity. J. Neurosci 38:5666–5676. 10.1523/JNEUROSCI.0328-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Hinckley CA, Driscoll SP, Moore NJ, Levine AJ, Hilde KL, Sharma K, Pfaff SL, 2018. Graded Arrays of Spinal and Supraspinal V2a Interneuron Subtypes Underlie Forelimb and Hindlimb Motor Control. Neuron 97:869–884.e5. 10.1016/j.neuron.2018.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Miranda LR, Müller T, Birchmeier C, 2017. The dorsal spinal cord and hindbrain: From developmental mechanisms to functional circuits. Dev. Biol 432:34–42. 10.1016/j.ydbio.2016.10.008 [DOI] [PubMed] [Google Scholar]
- Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ, 2008. Identification of Positionally Distinct Astrocyte Subtypes whose Identities Are Specified by a Homeodomain Code. Cell 133:510–522. 10.1016/j.cell.2008.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Hahm S-C, Choi K-A, Park S-H, Jeong H, Yea J-H, Kim J, Hong S, 2016. Intrathecal Transplantation of Embryonic Stem Cell-Derived Spinal GABAergic Neural Precursor Cells Attenuates Neuropathic Pain in a Spinal Cord Injury Rat Model. Cell Transplant. 25:593–607. 10.3727/096368915X689460 [DOI] [PubMed] [Google Scholar]
- Iyer NR, Huettner JE, Butts JC, Brown CR, Sakiyama-Elbert SE, 2016. Generation of highly enriched V2a interneurons from mouse embryonic stem cells. Exp. Neurol 277:305–316. 10.1016/j.expneurol.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM, 2000. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat. Rev. Genet 1:20–29. 10.1038/35049541 [DOI] [PubMed] [Google Scholar]
- Johnson MA, Weick JP, Pearce RA, Zhang S-C, 2007. Functional Neural Development from Human Embryonic Stem Cells: Accelerated Synaptic Activity via Astrocyte Coculture. J. Neurosci 27:3069–3077. 10.1523/JNEUROSCI.4562-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno H, Pearse DD, Ozawa H, Itoi E, Bunge MB, 2015. Schwann cell transplantation for spinal cord injury repair: Its significant therapeutic potential and prospectus. Rev. Neurosci 26:121–128. 10.1515/revneuro-2014-0068 [DOI] [PubMed] [Google Scholar]
- Keirstead HS, 2005. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cell Transplants Remyelinate and Restore Locomotion after Spinal Cord Injury. J. Neurosci 25:4694–4705. 10.1523/JNEUROSCI.0311-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Jung Jung SE, Nam TS, Jeon YH, Lee DR, Lee JS, Leem JW, Kim DW, 2010. Transplantation of GABAergic neurons from ESCs attenuates tactile hypersensitivity following spinal cord injury. Stem Cells 28:2099–2108. 10.1002/stem.526 [DOI] [PubMed] [Google Scholar]
- Kim M, Habiba A, Doherty JM, Mills JC, Mercer RW, Huettner JE, 2009. Regulation of mouse embryonic stem cell neural differentiation by retinoic acid. Dev. Biol 328:456–471. 10.1016/j.ydbio.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiderman S, Sá JV, Teixeira AP, Brito C, Gutbier S, Evje LG, Hadera MG, Glaab E, Henry M, Sachinidis A, Alves PM, Sonnewald U, Leist M, 2016. Functional and phenotypic differences of pure populations of stem cell-derived astrocytes and neuronal precursor cells. Glia 64:695–715. 10.1002/glia.22954 [DOI] [PubMed] [Google Scholar]
- Koch P, Opitz T, Steinbeck JA, Ladewig J, Brüstle O, 2009. A rosette-type, self-renewing human ES cell-derived neural stem cell with potential for in vitro instruction and synaptic integration. Proc. Natl. Acad. Sci. U. S. A 106:3225–30. 10.1073/pnas.0808387106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Weick JP, Liu Y, Zhang Z-J, Zhang S-C, 2011. Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat. Biotechnol 29:528–534. 10.1038/nbt.1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Zhang S-C, 2011. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat. Protoc 6:1710–1717. 10.1038/nprot.2011.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M, 2004. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron 42:375–386. 10.1016/S0896-6273(04)00249-1 [DOI] [PubMed] [Google Scholar]
- Li S, Uno Y, Rudolph U, Cobb J, Liu J, Anderson T, Levy D, Balu DT, Coyle JT, 2018. Astrocytes in primary cultures express serine racemase, synthesize D-serine and acquire A1 reactive astrocyte features. Biochem. Pharmacol 151:245–251. 10.1016/j.bcp.2017.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Barres BA, 2017. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46:957–967. 10.1016/j.immuni.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA, 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G, Santana Maia CA, Capucho C, Hasse-Ferreira A, Peduzzi JD, 2010. Olfactory Mucosal Autografts and Rehabilitation for Chronic Traumatic Spinal Cord Injury. Neurorehabil. Neural Repair 24:10–22. 10.1177/1545968309347685 [DOI] [PubMed] [Google Scholar]
- Lu DC, Niu T, Alaynick WA, 2015. Molecular and cellular development of spinal cord locomotor circuitry. Front. Mol. Neurosci 8:1–18. 10.3389/fnmol.2015.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukovic D, Stojkovic M, Moreno-Manzano V, Jendelova P, Sykova E, Bhattacharya SS, Erceg S, 2015. Concise review: Reactive astrocytes and stem cells in spinal cord injury: Good guys or bad guys? Stem Cells 33:1036–1041. 10.1002/stem.1959 [DOI] [PubMed] [Google Scholar]
- McCreedy DA, Brown CR, Butts JC, Xu H, Huettner JE, Sakiyama-Elbert SE, 2014. A new method for generating high purity motoneurons from mouse embryonic stem cells. Biotechnol. Bioeng 111:2041–2055. 10.1002/bit.25260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreedy DA, Rieger CR, Gottlieb DI, Sakiyama-Elbert SE, 2012. Transgenic enrichment of mouse embryonic stem cell-derived progenitor motor neurons. Stem Cell Res 8 10.1016/j.scr.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH, 2005. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature 438:360–363. 10.1038/nature04139 [DOI] [PubMed] [Google Scholar]
- Nicaise C, 2015. Transplantation of stem cell-derived astrocytes for the treatment of amyotrophic lateral sclerosis and spinal cord injury. World J. Stem Cells 7:380 10.4252/wjsc.v7.i2.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS, 2005. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 49:385–396. 10.1002/glia.20127 [DOI] [PubMed] [Google Scholar]
- NSCISC, 2016. Spinal Cord Injury (SCI) 2016 Facts and Figures at a Glance., The Journal of Spinal Cord Medicine. 10.1080/10790268.2016.1210925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RDG, 1996. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech. Dev 59:89–102. 10.1016/0925-4773(96)00572-2 [DOI] [PubMed] [Google Scholar]
- Okada Y, Shimazaki T, Sobue G, Okano H, 2004. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev. Biol 275:124–142. 10.1016/j.ydbio.2004.07.038 [DOI] [PubMed] [Google Scholar]
- Peljto M, Dasen JS, Mazzoni EO, Jessell TM, Wichterle H, 2010. Functional diversity of ESC-derived motor neuron subtypes revealed through intraspinal transplantation. Cell Stem Cell 7:355–366. 10.1016/j.stem.2010.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos C, Rocha S, Gaspar C, Henrique D, 2010. Two notch ligands, Dll1 and Jag1, are differently restricted in their range of action to control neurogenesis in the mammalian spinal cord. PLoS One 5 10.1371/journal.pone.0015515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt P, Glatza M, Hemmer K, Tsytsyura Y, Thiel CS, Höing S, Moritz S, Parga JA, Wagner L, Bruder JM, Wu G, Schmid B, Röpke A, Klingauf J, Schwamborn JC, Gasser T, Schöler HR, Sterneckert J, 2013. Derivation and Expansion Using Only Small Molecules of Human Neural Progenitors for Neurodegenerative Disease Modeling. PLoS One 8 10.1371/journal.pone.0059252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoncourt Y, Carroll P, Filippi P, Arce V, Alonso S, 1998. Neurons derived in vitro from ES cells express homeoproteins characteristic of motoneurons and interneurons. Mech. Dev 79:185–197. 10.1016/S0925-4773(98)00189-0 [DOI] [PubMed] [Google Scholar]
- Ribes V, Le Roux I, Rhinn M, Schuhbaur B, Dolle P, 2009. Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development 136:665–676. 10.1242/dev.016204 [DOI] [PubMed] [Google Scholar]
- Rocha SF, Lopes SS, Gossler A, Henrique D, 2009. Dll1 and Dll4 function sequentially in the retina and pV2 domain of the spinal cord to regulate neurogenesis and create cell diversity. Dev. Biol 328:54–65. 10.1016/j.ydbio.2009.01.011 [DOI] [PubMed] [Google Scholar]
- Ronaghi M, Erceg S, Moreno-Manzano V, Stojkovic M, 2010. Challenges of stem cell therapy for spinal cord injury: Human embryonic stem cells, endogenous neural stem cells, or induced pluripotent stem cells? Stem Cells 28:93–99. 10.1002/stem.253 [DOI] [PubMed] [Google Scholar]
- Roybon L, Lamas NJ, Garcia AD, Yang EJ, Sattler R, Lewis VJ, Kim YA, Kachel CA, Rothstein JD, Wichterle H, Henderson CE, 2013. Human stem cell-derived spinal cord astrocytes with defined mature or reactive phenotypes Laurent. Cell Rep. 4:1035–1048. 10.1016/j.celrep.2013.06.021.Human [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sances S, Bruijn LI, Chandran S, Eggan K, Ho R, Klim JR, Livesey MR, Lowry E, Macklis JD, Rushton D, Sadegh C, Sareen D, Wichterle H, Zhang SC, Svendsen CN, 2016. Modeling ALS with motor neurons derived from human induced pluripotent stem cells. Nat. Neurosci 19:542–553. 10.1038/nn.4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Chen JK, 2006. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat. Chem. Biol 2:29–30. 10.1038/nchembio753 [DOI] [PubMed] [Google Scholar]
- Sternfeld MJ, Hinckley CA, Moore NJ, Pankratz MT, Hilde KL, Driscoll SP, Hayashi M, Amin ND, Bonanomi D, Gifford WD, Sharma K, Goulding M, Pfaff SL, 2017. Speed and segmentation control mechanisms characterized in rhythmically-active circuits created from spinal neurons produced from genetically-tagged embryonic stem cells. Elife 6:1–29. 10.7554/eLife.21540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Su, Qu Yun, Stewart Todd J., Howard Michael J., Chakrabortty Shushovan, Holekamp Terrence F., and M. JW, 2000. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc. Natl. Acad. Sci 49:385–396. 10.1073/pnas.97.11.6126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Zhang Y, Liao B, Zhong X, Chen X, Wang H, Guo Y, Shan Y, Wang L, Pan G, 2018. Antagonism between the transcription factors NANOG and OTX2 specifies rostral or caudal cell fate during neural patterning transition. J. Biol. Chem 293:4445–4455. 10.1074/jbc.M117.815449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney LB, Bikoff JB, Gabitto MI, Brenner-Morton S, Baek M, Yang JH, Tabak EG, Dasen JS, Kintner CR, Jessell TM, 2018. Origin and Segmental Diversity of Spinal Inhibitory Interneurons. Neuron 97:341–355. 10.1016/j.neuron.2017.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan GC, Mazzoni EO, Wichterle H, 2016. Iterative Role of Notch Signaling in Spinal Motor Neuron Diversification. Cell Rep. 16:907–916. 10.1016/j.celrep.2016.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, Kawaja MD, Fehlings MG, Kwon BK, 2011. A Systematic Review of Cellular Transplantation Therapies for Spinal Cord Injury. J. Neurotrauma 28:1611–1682. 10.1089/neu.2009.1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RE, Lake A, Kenny P, Saunders M, Sakers K, Iyer N, Dougherty JD, Sakiyama-Elbert SE, 2017. Different Mixed Astrocyte Populations Derived from Embryonic Stem Cells have Variable Neuronal Growth Support Capacities. Stem Cells Dev. 26:scd.2017.0121. 10.1089/scd.2017.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RE, Pardieck J, Smith L, Kenny P, Crawford L, Shoichet M, Sakiyama-Elbert S, 2018. Effect of hyaluronic acid hydrogels containing astrocyte-derived extracellular matrix and/or V2a interneurons on histologic outcomes following spinal cord injury. Biomaterials 162:208–223. 10.1016/j.biomaterials.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Peljto M, 2008. Differentiation of mouse embryonic stem cells to spinal motor neurons. Curr. Protoc. Stem Cell Biol. 10.1002/9780470151808.sc01h01s5 [DOI] [PubMed] [Google Scholar]
- Wichterle H, Wichterle H, Lieberam I, Lieberam I, Porter JA, Porter JA, Jessell TM, Jessell TM, 2002. Directed differentiation of embryonic stem cells into motor neurons. Cell 110:385–97. 10.1016/S0092-8674(02)00835-8 [DOI] [PubMed] [Google Scholar]
- Xu H, Iyer N, Huettner JE, Sakiyama-Elbert SE, 2015. A puromycin selectable cell line for the enrichment of mouse embryonic stem cell-derived V3 interneurons. Stem Cell Res. Ther 6:1–17. 10.1186/s13287-015-0213-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Sakiyama-Elbert SE, 2015. Directed Differentiation of V3 Interneurons from Mouse Embryonic Stem Cells. Stem Cells Dev. 24:2723–2732. 10.1089/scd.2015.0122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholudeva LV, Iyer NR, Qiang L, Spruance VM, Randelman ML, White NW, Bezdudnaya T, Fischer I, Sakiyama-Elbert SE, Lane MA, 2018. Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury. J. Neurotrauma neu.2017.5439. 10.1089/neu.2017.5439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Hochman S, 2017. Diversity of molecularly-defined spinal interneurons engaged in mammalian locomotor pattern generation. J. Neurophysiol. jn.00322.2017. 10.1152/jn.00322.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]