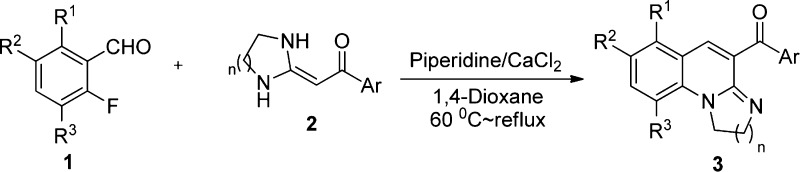
Abstract
A one-step protocol without transition-metal catalysts with simple post-treatment for the synthesis of 1,3-diazaheterocycle-fused [1,2-a]quinoline derivatives via the cascade reaction of 2-fluorobenzaldehyde (1) and heterocyclic ketene aminals (2) was developed. In the one-step cascade reaction, C=C and C–N bonds were constructed, and the targeted compound can be efficiently obtained by filtering without column chromatography. This protocol describes a valuable route to concisely and feasibly obtain 1,3-diazaheterocycle-fused [1,2-a]quinoline derivatives. The synthetic methodology is particularly attractive because of the following features: low-cost solvent, mild temperature, atom economy, high yield, and potential biological activity of the product.
Introduction
One of the most important aspects of modern chemical synthesis is that it is necessary to develop methods with a low environmental impact.1−4 The United States Environmental Protection Agency (EPA) defined the “green chemistry” concept as “the design of chemical products and processes that reduce or eliminate the use or generation of hazardous substances”.5 In the green chemistry field, the ideal synthesis6,7 should be a combination of a number of environmental, health, safety, and economic factors. Among them, group-assisted purification8−10 chemistry allows the synthesis of organic compounds without using traditional purification technologies, including column chromatography and recrystallization. This technology makes more efforts to find environmentally benign reagents and reactions so as to reduce the waste generated from silica and solvents, particularly toxic solvents.
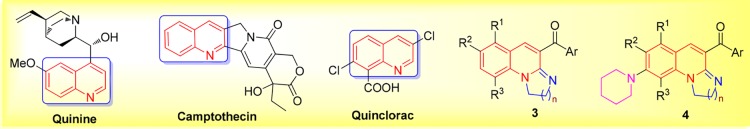
Quinolines is an important class of well-known heterocyclic scaffolds; they are embedded in many biological systems and have interesting applications in agriculture, materials, chemical industry, and medicine, for instance, as antimalarial (Figure 1, quinine),11,12 anticancer (Figure 1, camptothecin),13 antibacterial,14,15 insecticide (Figure 1, quinclorac), and so forth.16−18 Consequently, various methods of synthesis of quinolines have been reported.19−27 Among them, the transition-metal-catalyzed C–H bond or N–H bond activation approaches have provided numerous strategies for the synthesis of quinoline.28−32
Figure 1.
Biological activity of quinolines and the targeted compounds.
Heterocyclic ketene aminals (HKAs) have been widely used as a type of versatile building blocks to construct various fused heterocyclic compounds including quinolones,33,34 isoquinolin-1-imine,35 indoles,36 indolin-2-ones,37,38 isocoumarins,39 pyridines,40−42 pyrroles, and so forth43−47 Many of these compounds have a wide variety of biological activities, such as antitumor,48−51 herbicidal, pesticidal,52,53 antileishmanial,54 and antibacterial.55,56 Therefore, it is vital and urgent to develop a green synthetic methodology with benign conditions and straight-forward post-treatment for the synthesis of quinoline derivatives by construction of C=C and C–N bonds through the one-step cascade reaction.
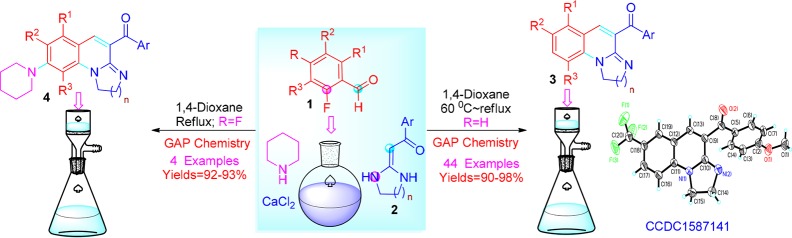
Here, we describe a cascade strategy for the regioselective convergent synthesis of a series of 1,3-diazaheterocycle-fused [1,2-a]quinoline derivatives (3–4). To the best of our knowledge, the synthesis of the polycyclic[1,2-a]quinoline derivatives by the cascade reaction of 2-fluorobenzaldehyde 1 and HKAs 2 (Scheme 1) has not been reported to date.
Scheme 1. Strategy for the Cascade Reaction Synthesis of [1,2-a]Quinolines 3 and 4.
Results and Discussion
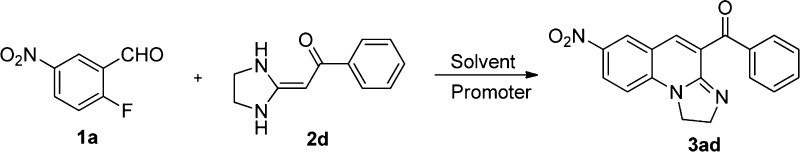
In this work, we developed a one-pot protocol to synthesize a series of polycyclic quinoline derivatives. To obtain the optimal reaction conditions for the synthesis of 1,3-diazaheterocycle-fused [1,2-a]quinoline 3ad, the reaction of 2-fluoro-5-nitrobenzaldehyde (1a) with HKA (2d) was chosen as the model reaction. First, the reaction mixture of 1a with 2d was ground under catalyst-free and solvent-free conditions at room temperature (Table 1, entry 1). Then, the reaction temperature reached the melting point of 1a (mp 60 °C), (Table 1, entry 2), and the results showed that the reaction can proceed nonselectively to yield many byproducts. The solvent-free condition is a disadvantage to the reaction, and we cannot obtain a pure product. Subsequently, by performing the reaction in the stoichiometric amount of base, such as K2CO3, t-BuOK, Cs2CO3, Et3N, and piperidine in the aprotic solvent 1,4-dioxane at reflux (Table 1, entries 3–7), the results revealed that piperidine was the optimal catalyst for this selective synthesis of [1,2-a]quinoline 3ad with an excellent yield (91%) (Table 1, entry 7). Afterward, we screened several aprotic solvents, including acetonitrile (CH3CN), tetrahydrofuran (THF), N,N-dimethylformamide (DMF), or the protonic solvents ethanol and water (Table 1, entries 8–12). The results indicated that the most suitable solvent for this defluorinaton and cyclization reaction was 1,4-dioxane. Next, we screened the temperature and found that the optimal temperature was 60 °C. In the postprocessing stage, after the reaction solution was cooled and filtered, we observed the hydrofluoric acid salt of piperidine mixed in the products. Considering the environmental hazards, we added CaCl2 to absorb the hydrofluoric acid produced by the defluorination reaction with piperidine as a catalyst. Unexpectedly, the product 3ad was obtained with a 97% yield (Table 1, entries 14) when the time of reaction was shortened to 30 min. Ultimately, the optimal reaction conditions for preparation of 3ad were 1,4-dioxane as the solvent, with piperidine and CaCl2 as promoters, at 60 °C for 30 min.
Table 1. Optimized Conditions for the Synthesis of [1,2-a]Quinoline 3ada.
| entry | solvent | promoter | T (°C) | time (h) | yieldb (%) |
|---|---|---|---|---|---|
| 1 | rt | 0.5 | n.r. | ||
| 2 | 60 | 0.5 | trace | ||
| 3 | 1,4-dioxane | K2CO3 | reflux | 2 | 23 |
| 4 | 1,4-dioxane | Cs2CO3 | reflux | 2 | 21 |
| 5 | 1,4-dioxane | t-BuOK | reflux | 2 | 19 |
| 6 | 1,4-dioxane | Et3N | reflux | 2 | 75 |
| 7 | 1,4-dioxane | piperidine | reflux | 2 | 91 |
| 8 | acetonitrile | piperidine | reflux | 2 | 54 |
| 9 | THF | piperidine | reflux | 3 | 68 |
| 10 | DMF | piperidine | 110 | 2 | 40 |
| 11 | EtOH | piperidine | reflux | 2 | 90 |
| 12 | H2O | piperidine | reflux | 5 | 14 |
| 13 | 1,4-dioxane | piperidine | 60 | 2 | 92 |
| 14 | 1,4-dioxane | piperidine/CaCl2 | 60 | 0.5 | 97 |
Reaction conditions: 1a (1.1 mmol), 2d (1.0 mmol), promoter (1.5 mmol), solvent (15 mL).
Isolated yield based on HKA 2d. n.r. = no reaction.
With the optimized conditions at hand, we explored the scope and limitations of the reactions involving different 2-fluorobenzaldehydes (1a–1c) with various HKAs (2a–2p) (Table 2, entries 1–44). For HKAs 2, the electron-withdrawing groups (Cl or Br) on the aromatic ring could accelerate the reaction rate, otherwise the electron-donating groups (CH3 or CH3O) were the opposite because of the influence of the substituting groups on the electrophilicity of the α-C of HKAs 2 (Scheme 1). Unexpectedly, the reaction rate showed that Cl > Br > H > F > CH3 > OCH3, which maybe attributed to the intermolecular hydrogen bonding of fluorineon HKAs (2a & 2h) with the diamino group of piperidine. Moreover, the size of the diazaheterocycle of HKAs 2 showed that the five-membered ring provided the highest yields, and then the six-membered and seven-membered rings (e.g., Table 2, entries 2 vs 9 vs 15). Additionally, when the different groups (R1 = NO2, CF3, and F) were introduced into the C5 position of 2-fluorobenzaldehyde 1, the yields of 3 decreased (e.g., Table 2, entries 4, 20 & 34) as the electron-withdrawing ability of the substituent groups decreased (NO2 > CF3 > F). The starting material 2-fluorobenzaldehyde 1 bearing a moderately electron-withdrawing group (such as F, 1c) had difficulty to react with HKAs 2, unless the reaction was carried out under reflux conditions and CaCl2 was indispensable. We conjectured that, for 2-fluorobenzaldehyde 1, the strong electron-withdrawing substituent group at the C5 position could facilitate the removal of the fluorine atom at the C2 position. At the same time, this leads to the enhancement of the electrophilicity of the formyl group at the C1 position, which benefits the attack with keto-carbonyl at the α-C position of HKAs 2.
Table 2. Cascade Reaction Synthesis of [1,2-a]Quinoline Derivatives 3a.
| entry | 1 (R1/R2/R3) | 2 (n/Ar) | T (°C) | time (h) | 3 | yieldb (%) |
|---|---|---|---|---|---|---|
| 1 | 1a (H/NO2/H) | 2a (1/4-FC6H4) | 60 | 0.5 | 3aa | 94 |
| 2 | 1a (H/NO2/H) | 2b (1/4-ClC6H4) | 60 | 0.5 | 3ab | 98 |
| 3 | 1a (H/NO2/H) | 2c (1/4-BrC6H4) | 60 | 0.5 | 3ac | 97 |
| 4 | 1a (H/NO2/H) | 2d (1/C6H5) | 60 | 0.5 | 3ad | 97 |
| 5 | 1a (H/NO2/H) | 2e (1/4-MeC6H4) | 60 | 0.5 | 3ae | 95 |
| 6 | 1a (H/NO2/H) | 2f (1/4-MeOC6H4) | 60 | 0.5 | 3af | 93 |
| 7 | 1a (H/NO2/H) | 2g (1/thiophene-2-yl) | 60 | 0.5 | 3ag | 92 |
| 8 | 1a (H/NO2/H) | 2h (2/4-FC6H4) | 60 | 0.5 | 3ah | 94 |
| 9 | 1a (H/NO2/H) | 2i (2/4-ClC6H4) | 60 | 0.5 | 3ai | 96 |
| 10 | 1a (H/NO2/H) | 2j (2/4-BrC6H4) | 60 | 0.5 | 3aj | 95 |
| 11 | 1a (H/NO2/H) | 2k (2/C6H5) | 60 | 0.5 | 3ak | 96 |
| 12 | 1a (H/NO2/H) | 2l (2/4-MeC6H4) | 60 | 0.5 | 3al | 92 |
| 13 | 1a (H/NO2/H) | 2m (2/4-MeOC6H4) | 60 | 0.5 | 3am | 91 |
| 14 | 1a (H/NO2/H) | 2n (2/thiophene-2-yl) | 60 | 0.5 | 3an | 91 |
| 15 | 1a (H/NO2/H) | 2o (3/4-ClC6H4) | 60 | 0.5 | 3ao | 93 |
| 16 | 1a (H/NO2/H) | 2p (3/4-MeC6H4) | 60 | 0.5 | 3ap | 92 |
| 17 | 1b (H/CF3/H) | 2a (1/4-FC6H4) | 75 | 1 | 3ba | 95 |
| 18 | 1b (H/CF3/H) | 2b (1/4-ClC6H4) | 75 | 1 | 3bb | 97 |
| 19 | 1b (H/CF3/H) | 2c (1/4-BrC6H4) | 75 | 1 | 3bc | 97 |
| 20 | 1b (H/CF3/H) | 2d (1/C6H5) | 75 | 1 | 3bd | 95 |
| 21 | 1b (H/CF3/H) | 2e (1/4-MeC6H4) | 75 | 1 | 3be | 92 |
| 22 | 1b (H/CF3/H) | 2f (1/4-MeOC6H4) | 75 | 1 | 3bf | 92 |
| 23 | 1b (H/CF3/H) | 2g (1/thiophene-2-yl) | 75 | 1 | 3bg | 93 |
| 24 | 1b (H/CF3/H) | 2h (2/4-FC6H4) | 75 | 1 | 3bh | 94 |
| 25 | 1b (H/CF3/H) | 2i (2/4-ClC6H4) | 75 | 1 | 3bi | 96 |
| 26 | 1b (H/CF3/H) | 2j (2/4-BrC6H4) | 75 | 1 | 3bj | 95 |
| 27 | 1b (H/CF3/H) | 2k (2/C6H5) | 75 | 1 | 3bk | 95 |
| 28 | 1b (H/CF3/H) | 2l (2/4-MeC6H4) | 75 | 1 | 3bl | 94 |
| 29 | 1b (H/CF3/H) | 2m (2/4-MeOC6H4) | 75 | 1 | 3bm | 93 |
| 30 | 1b (H/CF3/H) | 2n (2/thiophene-2-yl) | 75 | 1 | 3bn | 92 |
| 31 | 1c (F/F/F) | 2a (1/4-FC6H4) | reflux | 2 | 3ca | 93 |
| 32 | 1c (F/F/F) | 2b (1/4-ClC6H4) | reflux | 2 | 3cb | 95 |
| 33 | 1c (F/F/F) | 2c (1/4-BrC6H4) | reflux | 2 | 3cc | 94 |
| 34 | 1c (F/F/F) | 2d (1/C6H5) | reflux | 2 | 3cd | 94 |
| 35 | 1c (F/F/F) | 2e (1/4-MeC6H4) | reflux | 2 | 3ce | 92 |
| 36 | 1c (F/F/F) | 2f (1/4-MeOC6H4) | reflux | 2 | 3cf | 93 |
| 37 | 1c (F/F/F) | 2g (1/thiophene-2-yl) | reflux | 2 | 3cg | 92 |
| 38 | 1c (F/F/F) | 2h (2/4-FC6H4) | reflux | 2 | 3ch | 92 |
| 39 | 1c (F/F/F) | 2i (2/4-ClC6H4) | reflux | 2 | 3ci | 95 |
| 40 | 1c (F/F/F) | 2j (2/4-BrC6H4) | reflux | 2 | 3cj | 95 |
| 41 | 1c (F/F/F) | 2k (2/C6H5) | reflux | 2 | 3ck | 94 |
| 42 | 1c (F/F/F) | 2l (2/4-MeC6H4) | reflux | 2 | 3cl | 92 |
| 43 | 1c (F/F/F) | 2m (2/4-MeOC6H4) | reflux | 2 | 3cm | 90 |
| 44 | 1c (F/F/F) | 2n (2/thiophene-2-yl) | reflux | 2 | 3cn | 91 |
Conditions: 1 (1.1 mmol) and 2 (1.0 mmol) were heated in the solvent 1,4-dioxane (15 mL) with piperidine (1.5 mmol) and CaCl2 (0.5 mmol) as catalysts.
Isolated yield based on HKAs 2.
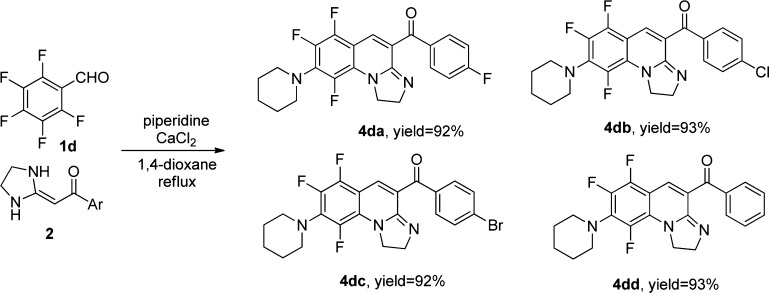
Furthermore, when 2,3,4,5,6-pentafluorobenzaldehyde 1d reacted with the five-membered ring HKAs 2, we synthesized a new product 4, which had the piperidine as the substituent (Table 3, entries 1–4). The possible reason is that the electron-withdrawing formyl group at the C1 position of 1d makes the fluorine atom at the C4 position to be easily replaced by piperidine. The reaction was performed under reflux for 6 h, and that was more difficult than the synthesis of compounds 4. Unfortunately, the six-membered and seven-membered rings HKAs 2 could not react with 1d.
Table 3. Cascade Reaction Synthesis of [1,2-a]Quinoline Derivatives 4a,b.
Conditions: 1 (1.1 mmol) and 2 (1.0 mmol) were heated in the solvent 1,4-dioxane (15 mL) with piperidine (1.5 mmol) and CaCl2 (0.5 mmol) as catalysts.
Isolated yield based on HKAs 2.
The chemical structures of polycyclic quinoline derivatives 3 and 4 were fully characterized by Fourier transform infrared (FTIR), proton nuclear magnetic resonance (1H NMR), carbon-13 nuclear magnetic resonance (13C NMR), and high-resolution mass spectroscopy (HRMS) spectral analysis. To further verify the structure of the target products, 3bf was selected as a representative compound and unequivocally confirmed by X-ray diffraction analysis, as shown in Figure 2 (CCDC 1587141).
Figure 2.
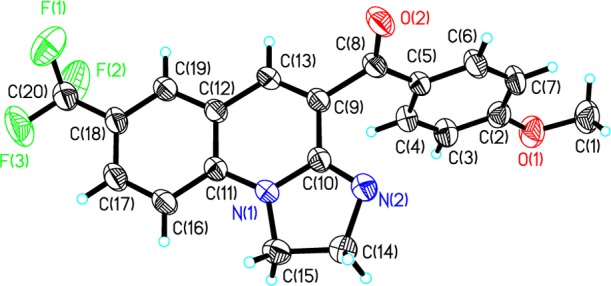
ORTEP diagram of 3bf; ellipsoids are drawn at the 30% probability level.
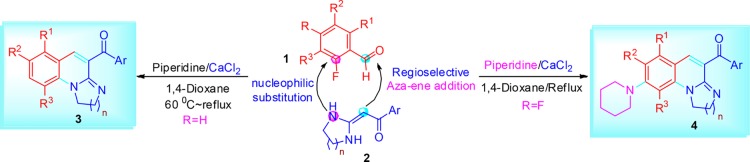
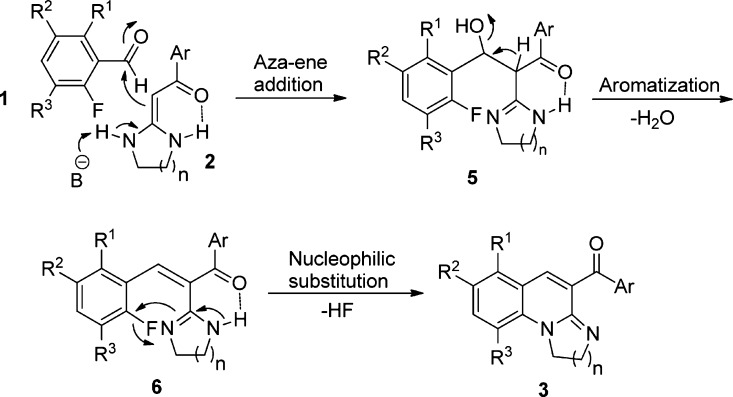
A proposed mechanism for synthesis of 1,3-diazaheterocycle-fused [1,2-a]quinoline derivatives 3 by the cascade reaction of 2-fluorobenzaldehyde 1 and HKAs 2 is presented in Scheme 2. First, HKAs 2, with a strong electron-withdrawing keto-carbonyl at the α-position and two electron-donating diamino groups on the diazaheterocycle, can serve as a nucleophilic component to react with the electrophilic formyl group of 2-fluorobenzaldehyde 1 to form the intermediate 5 via an aza–ene addition. Then, the intermediate 5 is converted into intermediate 6 via aromatization and results in the formation of one C=C bond. Thereafter, intramolecular nucleophilic substitution of fluorine intermediate 6 produces the target products 3. The outcome of the cascade reaction is one C=C bond, one C–N bond, and one diazaheterocycle-fused ring.
Scheme 2. Mechanism Hypotheses for the Synthesis of Target Compounds 3.
To testify this mechanism, we perform the reaction in 1,4-dioxane at 60 °C promoted by piperidine and CaCl2 for about 10 min, and the mixture was cooled to room temperature. Then, the reaction mixture was injected in high-performance liquid chromatography–HRMS system. The molecular ion peak appeared in high-resolution mass spectrometry (HRMS (TOF ES+) m/z: calcd for C18H15FN3O3 [M + H]+, 340.1092; found, 340.1089) (see the Supporting Information, which is the HRMS spectra of compound 6). On the basis of the results, we believe that the proposed mechanism is reasonable.
Conclusions
To summarize, we developed a method for the efficient synthesis of 1,3-diazaheterocycle-fused [1,2-a]quinoline derivatives via one-step cyclization of 2-fluorobenzaldehyde 1 and HKAs 2. This is a concise, rapid, and environmentally friendly method to prepare [1,2-a]quinoline derivatives without extra post-treatment. The reaction has some attractive features, including simple and mild conditions, atom economy, and operational simplicity. Moreover, these series of bicyclic[1,2-a]quinolines may possess potential biological activities for use in medical treatment of diseases. Our future investigations will be aimed at discovering in vitro biological activities of compounds 3 and 4.
Experimental Section
General Methods
All received reagents and solvents were used without further purification unless otherwise stated. Melting points were determined on an XT-4A melting point apparatus and were uncorrected. NMR spectra were recorded on Bruker DRX300 (1H: 300 MHz, 13C: 75 MHz), Bruker DRX400 (1H: 400 MHz, 13C: 100 MHz), Bruker DRX500 (1H: 500 MHz, 13C: 125 MHz), and Bruker DRX600 (1H: 600 MHz, 13C: 150 MHz) instruments with DMSO-d6 and CDCl3 as the solvents. The chemical shifts (δ) are expressed in parts per million relative to the residual deuterated solvent signal, and coupling constants (J) are given in hertz. IR spectra were recorded on an FT-IR Thermo Nicolet Avatar 360 instrument using KBr pellets. HRMS (electrospray ionization) was performed on an Agilent LC/MSD TOF instrument.
All received reagents and solvents were used without further purification unless otherwise stated. The materials (1a–d) were purchased from Aldrich Corporation Limited. HKAs 2 were prepared according to a procedure described in the literature.39,40 The structure of HKAs 2 was confirmed by 1H NMR, 13C NMR, and HRMS spectra.
General Procedure for the Synthesis of Compounds 3–4
A mixture of 2-fluorobenzaldehyde 1 (1.1 mmol), HKAs 2 (1.0 mmol), and piperidine (1.5 mmol) is mixed by stirring at different temperatures (1a as starting material, the temperature of the reaction was 60 °C; 1b was 75 °C; 1c and 1d at reflux) in 1,4-dioxane (15 mL). When the solution of the reaction was clear, CaCl2 (0.5 mmol) was added. After completion of the reaction, as indicated by thin-layer chromatography (CH2Cl2–EtOAc, 1:10 v/v), the mixture was cooled to room temperature and filtered. The solid was then washed with a small amount of ethanol (ca. 5 mL) and dissolved in CHCl3 (20 mL). Then, the organic phase was washed with saturated salt water (25 mL) and NaHCO3 (25 mL), dried over anhydrous Na2SO4, concentrated, and petroleum ether was added for recrystallization to obtain the pure product 3 or 4.
4-(4′-Fluorophenyl)methanoneyl-7-nitro-1,2-dihydroimi-dazo[1,2-a]quinoline (3aa)
Yellow solid, mp 229–230 °C; IR (KBr): 3438, 1636, 1613, 1517, 1330, 1263, 1154, 853 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 3.88–3.95 (m, 2H, CH2N), 4.01–4.08 (m, 2H, NCH2), 6.98 (d, J = 9.3 Hz, 1H, ArH), 7.35 (t, J = 8.9 Hz, 2H, ArH), 7.80 (s, 1H, CH), 7.97–8.02 (m, 2H, ArH), 8.24–8.28 (m, 1H, ArH), 8.45 (d, J = 2.4 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 45.5, 53.5, 112.4, 115.8 (d, J = 21.8 Hz), 119.1, 124.9, 127.2, 129.4, 132.4, 132.6 (d, J = 9.8 Hz), 136.2, 139.5, 143.9, 152.8, 165.4 (d, J = 251.3 Hz), 190.9; HRMS (TOF ES+) m/z: calcd for C18H13FN3O3 [M + H], 338.0935; found, 338.0935.
4-(4′-Chlorophenyl)methanoneyl-7-nitro-1,2-dihydroimi-dazo[1,2-a]quinoline (3ab)
Yellow solid, mp 273–274 °C; IR (KBr): 2938, 1635, 1610, 1330, 1265, 1093, 871 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 3.92–3.95 (m, 2H, CH2N), 4.02–4.05 (m, 2H, NCH2), 7.00 (d, J = 9.0 Hz, 1H, ArH), 7.59 (d, J = 8.4 Hz, 2H, ArH), 7.84 (s, 1H, CH), 7.91 (d, J = 8.7 Hz, 2H, ArH), 8.26–8.29 (m, 1H, ArH), 8.48 (d, J = 2.7 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 45.5, 53.4, 112.5, 119.1, 125.1, 127.3, 128.8, 129.1, 131.4, 134.4, 136.8, 138.8, 139.6, 143.9, 152.8, 191.3; HRMS (TOF ES+) m/z: calcd for C18H13N3O3Cl [M + H], 354.0640; found, 354.0638.
4-(4′-Bromophenyl)methanoneyl-7-nitro-1,2-dihydroimi-dazo[1,2-a]quinoline (3ac)
Yellow solid, mp 281–282 °C; IR (KBr): 3439, 2938, 1636, 1613, 1325, 1264, 1091, 868 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 3.89–3.95 (m, 2H, CH2N), 4.02–4.09 (m, 2H, NCH2), 7.01 (d, J = 9.0 Hz, 1H, ArH), 7.74 (d, J = 8.7 Hz, 2H, ArH), 7.83 (d, J = 6.9 Hz, 2H, ArH), 7.84 (s, 1H, CH), 8.27–8.31 (m, 1H, ArH), 8.49 (d, J = 2.4 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 45.5, 53.5, 112.5, 119.1, 125.1, 127.3, 128.1, 129.1, 131.4, 131.8, 134.8, 136.7, 139.6, 144.0, 152.8, 191.6; HRMS (TOF ES+) m/z: calcd for C18H13N3O3Br [M + H], 398.0134; found, 398.0137.
4-(Phenyl)methanoneyl-7-nitro-1,2-dihydroimidazo[1,2-a]quinoline (3ad)
Yellow solid, mp 284–285 °C; IR (KBr): 3439, 2927, 1635, 1592, 1325, 1262, 1094, 729 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 3.90–3.96 (m, 2H, CH2N), 4.03–4.09 (m, 2H, NCH2), 7.01 (d, J = 9.3 Hz, 1H, ArH), 7.54 (t, J = 7.5 Hz, 2H, ArH), 7.69 (t, J = 7.4 Hz, 1H, ArH), 7.80 (s, 1H, CH), 7.91 (d, J = 7.5 Hz, ArH), 8.27–8.30 (m, 1H, ArH), 8.48 (d, J = 2.4 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 50.8, 58.8, 117.7, 124.4, 130.2, 132.4, 134.0, 134.8, 135.0, 139.2, 140.9, 141.1, 144.8, 149.2, 158.1, 197.7; HRMS (TOF ES+) m/z: calcd for C18H14N3O3 [M + H], 320.1029; found, 320.1029.
4-(p-Tolyl)methanoneyl-7-nitro-1,2-dihydroimidazo[1,2-a]quinoline (3ae)
Yellow solid, mp 283–284 °C; IR (KBr): 3439, 2935, 1657, 1635, 1610, 1517, 1325, 1288, 1264, 1090, 870 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 2.39 (s, 3H, CH3), 3.92–3.95 (m, 2H, CH2N), 4.02–4.05 (m, 2H, NCH2), 7.00 (d, J = 9.0 Hz, 1H, ArH), 7.34 (d, J = 7.8 Hz, 2H, ArH), 7.75 (s, 1H, CH), 7.80 (d, J = 7.8 Hz, 2H, ArH), 8.27 (d, J = 8.7 Hz, 1H, ArH), 8.47 (s, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 21.2, 45.5, 53.5, 112.3, 119.1, 124.8, 127.0, 129.3, 129.7, 129.9, 133.1, 135.5, 139.5, 143.8, 144.6, 152.8, 191.9; HRMS (TOF ES+) m/z: calcd for C19H16N3O3 [M + H], 334.1186; found, 334.1186.
4-(4′-Methoxyphenyl)methanoneyl-7-nitro-1,2-dihydroi-midazo[1,2-a]quinoline (3af)
Yellow solid, mp 260–261 °C; IR (KBr): 1651, 1613, 1592, 1330, 1260, 1172, 585 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 3.85 (s, 3H, OCH3), 3.89–3.96 (m, 2H, CH2N), 4.03–4.09 (m, 2H, NCH2), 7.00 (d, J = 9.3 Hz, 1H, ArH), 7.05 (d, J = 8.7 Hz, 2H, ArH), 7.72 (s, 1H, CH), 7.88 (d, J = 8.7 Hz, 2H, ArH), 8.25–8.29 (m, 1H, ArH), 8.46 (d, J = 2.4 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 45.5, 53.5, 55.6, 112.3, 114.0, 119.2, 124.7, 126.9, 128.4, 130.1, 132.0, 135.0, 139.5, 143.7, 152.9, 163.8, 190.7; HRMS (TOF ES+) m/z: calcd for C19H16N3O4 [M + H], 350.1135; found, 350.1132.
4-(Thiophen-2′-yl)methanoneyl-7-nitro-1,2-dihydroimid-azo[1,2-a]quinoline (3ag)
Orange solid, mp 274–275 °C; IR (KBr): 3076, 2975, 1641, 1589, 1410, 1324, 1262, 1054, 742 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 3.97–4.00 (m, 2H, CH2N), 4.04–4.06 (m, 2H, NCH2), 7.00 (d, J = 9.0 Hz, 1H, ArH), 7.25–7.28 (m, 1H, CH), 7.86 (s, 1H, CH), 7.89–7.90 (m, 1H, CH), 8.14–8.16 (m, 1H, CH), 8.26–8.30 (m, 1H, ArH), 8.48 (d, J = 2.4 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 45.6, 53.5, 112.4, 119.0, 125.0, 127.3, 129.0, 135.7, 136.6, 136.9, 139.5, 142.5, 143.9, 152.5, 184.1; HRMS (TOF ES+) m/z: calcd for C16H12N3O3S [M + H], 326.0593; found, 326.0595.
5-(4′-Fluorophenyl)methanoneyl-8-nitro-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3ah)
Yellow solid, mp 224–225 °C; IR (KBr): 2934, 1634, 1596, 1508, 1327, 1277, 907, 585 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.90–1.92 (m, 2H, CH2), 3.27–3.31 (m, 2H, CH2N), 3.97–3.99 (m, 2H, NCH2), 7.30–7.42 (m, 3H, ArH), 7.63 (s, 1H, CH), 7.95–8.00 (m, 2H, ArH), 8.25–8.29 (m, 1H, ArH), 8.44 (d, J = 2.4 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.3, 42.9, 44.4, 112.4, 115.8 (d, J = 22.5 Hz), 119.5, 124.2, 125.8, 130.8, 132.2 (d, J = 9.8 Hz), 132.7, 136.2, 140.4, 145.1, 146.7, 165.2 (d, J = 250.5 Hz), 192.5; HRMS (TOF ES+) m/z: calcd for C19H15N3O3F [M + H], 352.1091; found, 352.1088.
5-(4′-Chlorophenyl)methanoneyl-8-nitro-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3ai)
Yellow solid, mp 272–273 °C; IR (KBr): 2928, 1662, 1640, 1324, 1277, 1094, 899, 834 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.86–1.90 (m, 2H, CH2), 3.25–3.28 (m, 2H, CH2N), 3.92–3.96 (m, 2H, NCH2), 7.36 (d, J = 9.3 Hz, 1H, ArH), 7.59 (s, 1H, CH), 7.70 (d, J = 8.7 Hz, 2H, ArH), 7.89 (d, J = 8.4 Hz, 2H, ArH), 8.24–8.28 (m, 1H, ArH), 8.42 (d, J = 2.7 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.4, 43.1, 44.3, 112.1, 119.4, 124.2, 125.8, 128.8, 130.3, 130.9, 134.7, 136.7, 138.4, 140.2, 145.3, 146.5, 193.0; HRMS (TOF ES+) m/z: calcd for C19H15N3O3Cl [M + H], 368.0796; found, 368.0795.
5-(4′-Bromophenyl)methanoneyl-8-nitro-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3aj)
Yellow solid, mp 264–265 °C; IR (KBr): 2966, 1653, 1613, 1326, 1273, 743 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.86–1.90 (m, 2H, CH2), 3.25–3.28 (m, 2H, CH2N), 3.92–3.96 (m, 2H, NCH2), 7.36 (d, J = 9.3 Hz, 1H, ArH), 7.59 (s, 1H, CH), 7.70 (d, J = 8.7 Hz, 2H, ArH), 7.81 (d, J = 8.4 Hz, 2H, ArH), 8.24–8.28 (m, 1H, ArH), 8.42 (d, J = 2.7 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.4, 43.1, 44.3, 112.1, 119.5, 124.2, 125.8, 127.6, 130.3, 131.0, 131.8, 135.1, 136.7, 140.3, 145.3, 146.5, 193.2; HRMS (TOF ES+) m/z: calcd for C19H15N3O3Br [M + H], 412.0291; found, 412.0291.
5-(Phenyl)methanoneyl-8-nitro-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3ak)
Yellow solid, mp 285–286 °C; IR (KBr): 2954, 2849, 1640, 1595, 1324, 1277, 1095, 904, 718 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.88 (m, 2H, CH2), 3.27 (m, 2H, CH2N), 3.95 (m, 2H, NCH2), 7.37 (d, J = 9.3 Hz, 1H, ArH), 7.51 (t, J = 7.5 Hz, 2H, ArH), 7.56 (s, 1H, CH), 7.64 (t, J = 7.1 Hz, 1H, ArH), 7.90 (d, J = 7.5 Hz, ArH), 8.27–8.25 (m, 1H, ArH), 8.42 (s, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.4, 43.1, 44.3, 112.1, 119.5, 124.1, 125.6, 128.7, 129.1, 129.8, 133.6, 135.9, 137.2, 140.3, 145.2, 146.5, 194.0; HRMS (TOF ES+) m/z: calcd for C19H16N3O3 [M + H], 334.1186; found, 334.1186.
5-(p-Tolyl)methanoneyl-8-nitro-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3al)
Yellow solid, mp 282–283 °C; IR (KBr): 2959, 1642, 1595, 1323, 1277, 1093, 904 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.86–1.89 (m, 2H, CH2), 2.37 (s, 3H, CH3), 3.26–3.29 (m, 2H, CH2N), 3.92–3.96 (m, 2H, NCH2), 7.30 (d, J = 8.1 Hz, 2H, ArH), 7.35 (d, J = 9.3 Hz, 1H, ArH), 7.51 (s, 1H, CH), 7.78 (d, J = 8.1 Hz, 2H, ArH), 8.22–8.26 (m, 1H, ArH), 8.40 (d, J = 2.7 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.4, 21.2, 43.1, 44.3, 112.0, 119.5, 124.0, 125.6, 129.2, 129.2, 129.5, 133.5, 137.4, 140.2, 144.1, 145.2, 146.4, 193.5; HRMS (TOF ES+) m/z: calcd for C20H18N3O3 [M + H], 348.1342; found, 348.1341.
5-(4′-Methoxyphenyl)methanoneyl-8-nitro-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3am)
Yellow solid, mp 221–222 °C; IR (KBr): 2934, 1641, 1595, 1328, 1277, 1162, 986 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.87–1.90 (m, 2H, CH2), 3.27–3.30 (m, 2H, CH2N), 2.83 (s, 3H, OCH3), 3.92–3.96 (m, 2H, NCH2), 7.02 (d, J = 8.7 Hz, 2H, ArH), 7.35 (d, J = 9.3 Hz, 1H, ArH), 7.49 (s, 1H, CH), 7.85 (d, J = 8.7 Hz, 2H, ArH), 8.22–8.26 (m, 1H, ArH), 8.40 (d, J = 2.7 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.4, 43.1, 44.3, 55.6, 112.0, 113.9, 119.6, 123.9, 125.5, 128.9, 129.2, 131.6, 137.5, 140.2, 145.2, 146.4, 163.4, 192.4; HRMS (TOF ES+) m/z: calcd for C20H18N3O4 [M + H], 364.1291; found, 364.1293.
5-(Thiophen-2′-yl)methanoneyl-8-nitro-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3an)
Yellow solid, mp 224–225 °C; IR (KBr): 2960, 1645, 1594, 1511, 1329, 1279, 1054, 737 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.89–1.92 (m, 2H, CH2), 3.31–3.35 (m, 2H, CH2N), 3.92–3.96 (m, 2H, NCH2), 7.20–7.23 (m, 1H, CH), 7.34 (d, J = 9.3 Hz, 1H, ArH), 7.58 (s, 1H, CH), 7.77–7.79 (m, 1H, CH), 8.05–8.07 (m, 1H, CH), 8.22–8.40 (m, 1H, ArH), 8.40 (d, J = 2.7 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.4, 43.1, 44.4, 112.0, 119.4, 124.2, 125.8, 128.9, 129.7, 135.5, 135.8, 136.6, 140.2, 143.1, 145.3, 146.1, 186.1; HRMS (TOF ES+) m/z: calcd for C17H14N3O3S [M + H], 340.0750; found, 340.0752.
6-(4′-Chlorophenyl)methanoneyl-9-nitro-1,2,3,4-tetrahy-dro-[1,3]diazepino[1,2-a]quinoline (3ao)
Yellow solid, mp 232–233 °C; IR (KBr): 2930, 1657, 1626, 1597, 1340, 1285, 1087, 840 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.78 (m, 2H, CH2), 2.04–2.07 (m, 2H, CH2), 3.62–3.66 (m, 2H, CH2N), 4.11–4.15 (m, 2H, NCH2), 7.38 (d, J = 9.3 Hz, 1H, ArH), 7.56 (d, J = 8.7 Hz, 2H, ArH), 7.62 (s, 1H, CH), 7.90 (d, J = 8.4 Hz, 2H, ArH), 8.22–8.26 (m, 1H, ArH), 8.45 (d, J = 2.7 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 23.6, 25.0, 47.2, 49.2, 112.8, 120.0, 124.1, 125.6, 128.8, 130.6, 130.8, 134.8, 137.0, 138.2, 140.1, 146.9, 148.5, 192.9; HRMS (TOF ES+) m/z: calcd for C20H17ClN3O3 [M + H], 382.0953; found, 382.0952.
6-(p-Tolyl)methanoneyl-9-nitro-1,2,3,4-tetrahydro-[1,3]diazepino[1,2-a]quinoline (3ap)
Yellow solid, mp 236–237 °C; IR (KBr): 1663, 1636, 1594, 1500, 1486, 1327, 1265, 1206, 1090, 861, 819 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.79 (m, 2H, CH2), 2.04–2.08 (m, 2H, CH2), 3.63–3.66 (m, 2H, CH2N), 4.11–4.15 (m, 2H, NCH2), 7.31 (d, J = 8.1 Hz, 1H, ArH), 7.37 (d, J = 9.3 Hz, 2H, ArH), 7.54 (s, 1H, CH), 7.79 (d, J = 8.1 Hz, 2H, ArH), 8.21–8.25 (m, 1H, ArH), 8.43 (d, J = 2.7 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 21.2, 23.6, 25.1, 47.2, 49.2, 112.8, 120.2, 123.9, 125.3, 129.1, 129.2, 129.7, 133.5, 137.8, 140.0, 143.9, 146.8, 148.5, 193.5; HRMS (TOF ES+) m/z: calcd for C21H20N3O3 [M + H], 362.1499; found, 362.1500.
4-(4′-Fluorophenyl)methanoneyl-7-(trifluoromethyl)-1,2-dihydroimidazo[1,2-a]quinoline (3ba)
Yellow solid, mp 198–199 °C; IR (KBr): 3438, 1663, 1636, 1599, 1386, 1336, 1207, 1155, 1115, 1077, 998, 859, 610 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 3.85–3.93 (m, 2H, CH2N), 3.98–4.04 (m, 2H, NCH2), 7.01 (d, J = 8.7 Hz, 1H, ArH), 7.30–7.38 (m, 2H, ArH), 7.72 (s, 1H, CH), 7.72–7.76 (dd, J1 = 9.0 Hz, J2 = 1.8 Hz, 1H, ArH), 7.92 (d, J = 1.5 Hz, 1H, ArH), 7.94–8.01 (m, 2H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 45.3, 53.3, 112.5, 115.8 (d, J2 = 21.8 Hz), 119.3, 119.8–120.7 (m), 124.4 (d, J1 = 269.3 Hz), 126.4, 128.2, 129.0, 132.5 (d, J3 = 9.8 Hz), 136.3, 142.1, 153.2, 165.3 (d, J1 = 251.3 Hz), 191.2; HRMS (TOF ES+) m/z: calcd for C19H13N2OF4 [M + H], 361.0958; found, 361.0958.
4-(4′-Chlorophenyl)methanoneyl-7-(trifluoromethyl)-1,2-dihydroimidazo[1,2-a]quinoline (3bb)
Yellow solid, mp 232–233 °C; IR (KBr): 3442, 1661, 1635, 1580, 1334, 1206, 1159, 1112, 1076, 997, 840, 519 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 3.87–3.91 (m, 2H, CH2N), 3.98–4.02 (m, 2H, NCH2), 7.02 (d, J = 8.6 Hz, 1H, ArH), 7.59 (d, J = 8.3 Hz, 2H, ArH), 7.75 (s, 1H, ArH), 7.76 (s, 1H, ArH), 7.90 (d, J = 8.5 Hz, 2H, ArH), 7.94 (s, 1H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 45.7, 53.7, 112.9, 119.6, 120.5 (d, J2 = 32.5 Hz), 124.8 (d, J1 = 270.0 Hz), 126.9, 128.8, 129.1, 129.2, 131.7, 135.1, 137.2, 139.1, 142.6, 153.6, 192.0; HRMS (TOF ES+) m/z: calcd for C19H13N2OF3Cl [M + H], 377.0663; found, 377.0664.
4-(4′-Bromophenyl)methanoneyl-7-(trifluoromethyl)-1,2-dihydroimidazo[1,2-a]quinoline (3bc)
Yellow solid, mp 237–238 °C; IR (KBr): 1662, 1635, 1582, 1399, 1334, 1206, 1159, 1111, 1075, 996, 837, 765 cm–1; 1H NMR (400 MHz, DCCl3) (δ, ppm): 4.22–4.27 (t, J = 12.2 Hz, 2H, CH2N), 4.35–4.42 (t, J = 12.4 Hz, 2H, NCH2), 7.04 (d, J = 11.2 Hz, 1H, ArH), 7.66 (s, 1H, CH), 7.82 (m, 2H, ArH), 7.85–7.88 (m, 2H, ArH), 8.02 (d, J = 10.0 Hz, 2H, ArH); 13C NMR (125 MHz, DCCl3) (δ, ppm): 45.8, 53.9, 111.9, 119.2, 122.5 (m), 125.3, 126.7, 128.6, 129.0, 129.2, 131.4, 131.9, 134.9, 138.1, 142.0, 154.0, 191.5; HRMS (TOF ES+) m/z: calcd for C19H13N2OF3Br [M + H], 421.0157; found, 421.0158.
4-(Phenyl)methanoneyl-7-(trifluoromethyl)-1,2-dihydro-imidazo[1,2-a]quinoline (3bd)
Yellow solid, mp 212–213 °C; IR (KBr): 1666, 1635, 1578, 1387, 1333, 1204, 1160, 1117, 1073, 996, 817, 519 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 3.85–3.92 (m, 2H, CH2N), 3.98–4.05 (m, 2H, NCH2), 7.02 (d, J = 8.7 Hz, 1H, ArH), 7.51–7.56 (m, 2H, ArH), 7.67 (d, J = 7.2 Hz, 1H, ArH), 7.71 (s, 1H, CH), 7.73–7.76 (dd, J1 = 8.7 Hz, J2 = 1.5 Hz, 1H, ArH), 7.89 (s, 1H, ArH), 7.92 (d, J = 5.4 Hz, 2H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 45.3, 53.3, 112.5, 119.3, 120.0 (d, J = 32.3 Hz), 124.4 (d, J = 277.5 Hz), 126.3, 128.2, 128.7, 129.3, 129.5, 133.8, 135.8, 135.9, 142.1, 153.2, 192.6; HRMS (TOF ES+) m/z: calcd for C19H14N2OF3 [M + H], 343.1052; found, 343.1050.
4-(p-Tolyl)methanoneyl-7-(trifluoromethyl)-1,2-dihydro-imidazo[1,2-a]quinoline (3be)
Yellow solid, mp 249–250 °C; IR (KBr): 1660, 1637, 1333, 1206, 1157, 1110, 1075, 997, 828, 762 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 2.38 (s, 3H, CH3), 3.85–3.91 (m, 2H, CH2N), 3.97–4.04 (m, 2H, NCH2), 7.00 (d, J = 8.7 Hz, 1H, ArH), 7.32 (d, J = 8.7 Hz, 2H, ArH), 7.66 (s, 1H, CH), 7.73 (d, J = 8.4 Hz, 1H, ArH), 7.78 (d, J1 = 8.1 Hz, 2H, ArH), 7.92 (s, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 21.2, 45.3, 53.3, 112.4, 119.3, 119.7, 124.4 (d, J = 269.3 Hz), 126.2, 128.1, 129.3, 129.5, 129.6, 133.3, 135.5, 142.0, 144.4, 153.2, 192.1; HRMS (TOF ES+) m/z: calcd for C20H16N2OF3 [M + H], 357.1209; found, 357.1210.
4-(4′-Methoxyphenyl)methanoneyl-7-(trifluoromethyl)-1,2-dihydroimidazo[1,2-a]quinoline (3bf)
Yellow solid, mp 228–229 °C; IR (KBr): 2945, 1658, 1635, 1596, 1387, 1334, 1265, 1205, 1155, 1109, 856 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 3.85 (s, 3H, CH3), 3.88–3.92 (m, 2H, CH2N), 4.00–4.04 (m, 2H, NCH2), 7.03 (d, J = 9.0 Hz, 1H, ArH), 7.05 (d, J = 8.6 Hz, 2H, ArH), 7.65 (s, 1H, CH), 7.75 (d, J = 8.5 Hz, 1H, ArH), 7.88 (d, J1 = 8.5 Hz, 2H, ArH), 7.92 (s, 1H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 45.7, 53.6, 56.0, 112.8, 114.4, 119.8, 120.2, 125.9, 126.5, 128.4, 129.0, 130.1, 132.4, 135.5, 142.3, 153.7, 164.2, 192.4; HRMS (TOF ES+) m/z: calcd for C20H16N2O2F3 [M + H], 373.1158; found, 373.1160.
4-(Thiophen-2′-yl)methanoneyl-7-(trifluoromethyl)-1,2-dihydroimidazo[1,2-a]quinoline (3bg)
Orange solid, mp 208–209 °C; IR (KBr): 3069, 1650, 1633, 1413, 1334, 1204, 1159, 1118, 1073, 821, 743 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 3.92–3.96 (m, 2H, CH2N), 4.00–4.04 (m, 2H, NCH2), 7.00 (d, J = 8.7 Hz, 1H, CH), 7.26 (t, J = 4.3 Hz, 1H, CH), 7.73–7.75 (m, 1H, CH), 7.78 (s, 1H, CH), 7.88 (d, J = 3.7 Hz, 1H, ArH), 7.93 (s, 1H, ArH), 8.13 (d, J = 4.8 Hz, 1H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 45.8, 53.6, 56.0, 112.8, 119.6, 120.4 (d, J = 32.5 Hz), 124.8 (d, J = 270.0 Hz), 126.8, 128.7, 129.0, 129.3, 136.2, 136.6, 136.9, 142.4, 143.0, 153.4, 184.7; HRMS (TOF ES+) m/z: calcd for C17H12N2OF3S [M + H], 349.0616; found, 349.0614.
5-(4′-Fluorophenyl)methanoneyl-8-(trifluoromethyl)-2,3-dihydro-1H-pyrimido[1,2-a]-quinoline (3bh)
Yellowy solid, mp 172–173 °C; IR (KBr): 1668, 1642, 1596, 1319, 1210, 1154, 1116, 846, 814 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 1.87–1.89 (m, 2H, CH2), 3.25–3.27 (m, 2H, CH2N), 3.90–3.92 (m, 2H, NCH2), 7.32 (t, J = 8.6 Hz, 2H, ArH), 7.37 (d, J = 8.9 Hz, 1H, ArH), 7.49 (s, 1H, CH), 7.75 (d, J1 = 8.7 Hz, 1H, ArH), 7.89 (s, 1H, ArH), 7.95–7.98 (m, 2H, ArH); 13C NMR (123 MHz, DMSO-d6) (δ, ppm): 19.9, 43.5, 44.3, 112.4, 116.1 (d, J = 22.5 Hz), 119.9, 121.3 (d, J = 33.8 Hz), 124.7 (d, J = 268.8 Hz), 126.1 (d, J = 3.8 Hz), 127.4 (d, J = 2.5 Hz), 130.4, 132.4 (d, J = 10.0 Hz), 133.3, 137.0, 143.8, 147.2, 165.5 (d, J = 252.5 Hz), 193.2; HRMS (TOF ES+) m/z: calcd for C20H15N2OF4 [M + H], 375.1115; found, 375.1113.
5-(4′-Chlorophenyl)methanoneyl-8-(trifluoromethyl)-2,3-dihydro-1H-pyrimido[1,2-a]-quinoline (3bi)
Yellowy solid, mp 181–182 °C; IR (KBr): 2931, 1670, 1640, 1592, 1319, 1208, 1161, 1115, 815 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.85–1.88 (m, 2H, CH2), 3.22–3.26 (m, 2H, CH2N), 3.89–3.92 (m, 2H, NCH2), 7.37 (d, J = 8.7 Hz, 1H, ArH), 7.51 (s, 1H, CH), 7.55 (d, J = 8.4 Hz, 2H, ArH), 7.75 (d, J = 9.0 Hz, 1H, ArH), 7.87–7.90 (m, 3H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.5, 43.0, 43.9, 112.1, 119.5, 121.0 (d, J = 33.0 Hz), 124.3 (d, J = 270.0 Hz), 125.8, 127.0, 128.8, 130.4, 130.8, 134.9, 136.2, 138.2, 143.4, 146.8, 193.2; HRMS (TOF ES+) m/z: calcd for C20H15N2OF3Cl [M + H], 391.0819; found, 391.0816.
5-(4′-Bromophenyl)methanoneyl-8-(trifluoromethyl)-2,3-dihydro-1H-pyrimido[1,2-a]-quinoline (3bj)
Yellowy solid, mp 195–196 °C; IR (KBr): 2951, 1671, 1641, 1590, 1318, 1277, 1112, 814 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.86 (m, 2H, CH2), 3.23 (m, 2H, CH2N), 3.88–3.92 (m, 2H, NCH2), 7.37 (d, J = 9.0 Hz, 1H, ArH), 7.51 (s, 1H, CH), 7.70 (d, J = 8.4 Hz, 2H, ArH), 7.76 (d, J = 9.3 Hz, 1H, ArH), 7.80 (d, J = 8.4 Hz, 2H, ArH), 7.90 (s, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.5, 43.0, 43.8, 112.1, 119.5, 120.5 (d, J2 = 32.3 Hz), 124.3 (d, J1 = 269.3 Hz), 125.8, 127.1, 127.5, 130.4, 130.9, 131.8, 135.2, 136.2, 143.4, 146.8, 193.5; HRMS (TOF ES+) m/z: calcd for C20H15N2OF3Br [M + H], 435.0314; found, 435.0317.
5-(Phenyl)methanoneyl-8-(trifluoromethyl)-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3bk)
Yellowy solid, mp 187–188 °C; IR (KBr): 1667, 1640, 1589, 1343, 1320, 1213, 1160, 1099, 814 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 1.87–1.89 (m, 2H, CH2), 3.24–3.26 (m, 2H, CH2N), 3.90–3.93 (m, 2H, NCH2), 7.37 (d, J = 8.9 Hz, 1H, ArH), 7.48–7.52 (m, 3H, CH), 7.63 (t, J = 7.4 Hz, 1H, ArH), 7.75 (d, J = 8.8 Hz, 1H, ArH), 7.88–7.90 (m, 3H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 19.9, 43.4, 44.3, 112.4, 120.0, 121.4 (d, J = 32.5 Hz), 124.8 (d, J = 267.5 Hz), 126.0, 127.3, 129.0, 129.4, 130.3, 133.8, 136.5, 137.2, 143.7, 147.3, 194.6; HRMS (TOF ES+) m/z: calcd for C20H16N2OF3 [M + H], 357.1209; found, 357.1205.
5-(p-Tolyl)methanoneyl-8-(trifluoromethyl)-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3bl)
Yellowy solid, mp 226–227 °C; IR (KBr): 1663, 1642, 1319, 1209, 1160, 1112, 1083, 828 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 1.86–1.88 (m, 2H, CH2), 2.37 (s, 3H, CH3), 3.24–3.26 (m, 2H, CH2N), 3.89–3.92 (m, 2H, NCH2), 7.30 (d, J = 8.0 Hz, 2H, ArH), 7.36 (d, J = 8.9 Hz, 1H, ArH), 7.43 (s, 1H, CH), 7.74 (d, J = 8.7 Hz, 1H, ArH), 7.78 (d, J = 8.1 Hz, 2H, ArH), 7.88 (s, 1H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 19.9, 21.6, 43.4, 44.3, 112.4, 120.0, 121.3 (d, J = 32.5 Hz), 124.8 (d, J = 270.0 Hz), 125.9, 127.1, 127.2, 129.6, 129.9, 134.1, 137.4, 143.7, 144.4, 147.2, 194.2; HRMS (TOF ES+) m/z: calcd for C21H18N2OF3 [M + H], 371.1365; found, 371.1363.
5-(4′-Methoxyphenyl)methanoneyl-8-(trifluoromethyl)-2,3-dihydro-1H-pyrimido[1,2-a]-quinoline (3bm)
White solid, mp 192–193 °C; IR (KBr): 1662, 1641, 1593, 1319, 1264, 1157, 1029, 839 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 1.87–1.88 (m, 2H, CH2), 3.26 (m, 2H, CH2N), 3.84 (s, 3H, CH3), 3.90–3.91 (m, 2H, NCH2), 7.01–7.03 (m, 2H, ArH), 7.36 (d, J = 8.8 Hz, 1H, ArH), 7.41 (s, 1H, CH), 7.74 (d, J = 8.8 Hz, 1H, ArH), 7.84–7.88 (m, 3H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 19.9, 43.4, 44.3, 55.9, 112.3, 114.3, 120.0, 121.3 (d, J = 32.5), 124.8 (d, J = 270.0 Hz), 125.9, 127.2, 127.2, 129.5, 129.7, 137.5, 143.7, 147.2, 163.8, 193.1; HRMS (TOF ES+) m/z: calcd for C21H18N2O2F3 [M + H], 387.1314; found, 387.1317.
5-(Thiophen-2′-yl)methanoneyl-8-(trifluoromethyl)-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3bn)
Light red solid, mp 209–210 °C; IR (KBr): 1645, 1586, 1343, 1319, 1209, 1156, 1102, 821, 732 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.87–1.91 (m, 2H, CH2), 3.29–3.32 (m, 2H, CH2N), 3.88–3.92 (m, 2H, NCH2), 7.19–7.22 (m, 1H, CH), 7.35 (d, J = 8.7 Hz, 1H, CH), 7.50 (s, 1H, CH), 7.72–7.76 (m, 2H, CH), 7.88 (d, J = 1.8 Hz, 1H, ArH), 8.04 (dd, J1 = 4.8 Hz, J2 = 1.2 Hz, 1H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.5, 43.0, 43.9, 112.0, 119.4, 120.9 (q, J2 = 32.3 Hz), 124.3 (d, J1 = 269.3 Hz), 125.7, 126.1, 127.0, 128.7, 129.8, 135.2, 135.5, 136.2, 143.3 (d, J3 = 9.8 Hz), 146.5, 186.3; HRMS (TOF ES+) m/z: calcd for C18H14N2OF3S [M + H], 363.0773; found, 363.0777.
6,7,9-Trifluoro-4-(4′-fluorophenyl)methanoneyl-1,2-dihy-droimidazo[1,2-a]quinoline (3ca)
Red solid, mp 177–178 °C; IR (KBr): 1668, 1636, 1598, 1496, 1393, 1267, 1157, 858, 603 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 3.84 (t, J = 10.3 Hz, 2H, CH2N), 4.20–4.25 (m, 2H, NCH2), 7.35 (t, J = 8.7 Hz, 2H, ArH), 7.61 (s, 1H, CH), 7.70–7.76 (m, 1H, ArH), 7.97–8.00 (m, 2H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 48.6, 54.1, 108.8 (t, J = 25.0 Hz), 111.5 (d, J = 15.0 Hz), 116.2 (d, J = 22.5 Hz), 126.4 (d, J = 13.8 Hz), 127.3, 131.2, 132.7, 132.9 (d, J = 10.0 Hz), 140.9 (t, J = 11.9 Hz), 141.9 (m), 142.7 (d, J = 12.5 Hz), 143.9 (t, J = 18.8 Hz), 153.7, 165.8 (d, J = 251.3 Hz), 191.1; 19F NMR (565 MHz, DMSO-d6) (δ, ppm): −148.4 (t, J = 16.9 Hz), −147.0 (d, J = 22.6 Hz), −132.8 (d, J = 11.3 Hz), −104.5; HRMS (TOF ES+) m/z: calcd for C18H11N2OF4 [M + H], 347.0802; found, 347.0801.
6,7,9-Trifluoro-4-(4′-chlorophenyl)methanoneyl-1,2-dihy-droimidazo[1,2-a]quinoline (3cb)
Red solid, mp 186–187 °C; IR (KBr): 1664, 1638, 1595, 1499, 1393, 1269, 1090, 776 cm–1; 1H NMR (600 MHz, DMSO-d6) (δ, ppm): 3.81–3.85 (m, 2H, CH2N), 4.21–4.26 (m, 2H, NCH2), 7.60 (d, J = 8.5 Hz, 2H, ArH), 7.65 (s, 1H, CH), 7.73–7.78 (m, 1H, ArH), 7.91 (d, J = 8.5 Hz, 2H, ArH); 13C NMR (150 MHz, DMSO-d6) (δ, ppm): 48.7 (d, J = 9.0 Hz), 54.2, 109.0 (t, J = 25.5 Hz), 111.6 (d, J = 10.5 Hz), 126.6 (d, J = 10.0 Hz), 127.8, 129.3, 131.0, 131.8, 134.8, 139.4, 141.1, 142.5 (d, J = 60.0 Hz), 144.0 (d, J = 8.8 Hz), 153.8, 191.7; HRMS (TOF ES+) m/z: calcd for C18H11N2OF3Cl [M + H], 363.0506; found, 363.0503.
6,7,9-Trifluoro-4-(4′-bromophenyl)methanoneyl-1,2-dihy-droimidazo[1,2-a]quinoline (3cc)
Orange solid, mp 203–204 °C; IR (KBr): 1663, 1635, 1586, 1496, 1269, 1124, 774, 609 cm–1; 1H NMR (600 MHz, DMSO-d6 + DCCl3) (δ, ppm): 3.83 (t, J = 10.3 Hz, 2H, CH2N), 4.21–4.26 (m, 2H, NCH2), 7.64 (s, 1H, CH), 7.73–7.74 (m, 3H, ArH), 7.82 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, DMSO-d6 + DCCl3) (δ, ppm): 48.7 (d, J = 9.0 Hz), 54.2, 109.0 (t, J = 25.5 Hz), 111.6 (d, J = 10.5 Hz), 126.6, 127.9, 128.6, 131.0, 131.8, 132.3, 135.1, 141.1, 142.7, 143.9 (d, J = 51.0 Hz), 153.8, 191.8; HRMS (TOF ES+) m/z: calcd for C18H11N2OF3Br [M + H], 407.0001; found, 407.0000.
6,7,9-Trifluoro-4-(phenyl)methanoneyl-1,2-dihydroimid-azo[1,2-a]quinoline (3cd)
Orange solid, mp 195–196 °C; IR (KBr): 1667, 1636, 1498, 1392, 1270, 803, 609 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 3.84 (t, J = 10.3 Hz, 2H, CH2N), 4.21–4.26 (m, 2H, NCH2), 7.54 (d, J = 7.7 Hz, 2H, ArH), 7.59 (s, 1H, CH), 7.67–7.76 (m, 2H, ArH), 7.90 (d, J = 7.5 Hz, 2H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 48.6 (d, J = 8.8 Hz), 54.1, 108.7 (t, J = 24.4 Hz), 111.5 (d, J = 11.3 Hz), 126.4 (d, J = 12.5 Hz), 127.1, 129.1, 129.8, 131.4, 134.3, 135.9, 140.9, 141.9, 142.8, 143.9 (d, J = 33.8 Hz), 153.7, 192.6; HRMS (TOF ES+) m/z: calcd for C18H12N2OF3 [M + H], 329.0896; found, 329.0894.
6,7,9-Trifluoro-4-(p-tolyl)methanoneyl-1,2-dihydroimid-azo[1,2-a]quinoline (3ce)
Red-orange solid, mp 185–186 °C; IR (KBr): 1662, 1635, 1496, 1392, 1272, 1193, 603 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 2.39 (s, 3H, CH3), 3.83 (t, J = 10.3 Hz, 2H, CH2N), 4.19–4.25 (m, 2H, NCH2), 7.33 (d, J = 8.0 Hz, 2H, ArH), 7.53 (s, 1H, CH), 7.69–7.75 (m, 1H, ArH), 7.79 (d, J = 8.1 Hz, 2H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 21.6, 48.6 (d, J = 8.8 Hz), 54.1, 108.6 (t, J = 25.0 Hz), 111.6, 126.4, 126.7, 129.7, 130.0, 131.7, 133.5, 140.9, 141.9 (d, J = 30.0 Hz), 142.8, 143.9, 145.0, 153.7, 192.0; HRMS (TOF ES+) m/z: calcd for C19H14N2OF3 [M + H], 343.1052; found, 343.1050.
6,7,9-Trifluoro-4-(4′-methoxyphenyl)methanoneyl-1,2-di-hydroimidazo[1,2-a]quinoline (3cf)
Orange solid, mp 191–192 °C; IR (KBr): 1633, 1598, 1497, 1260, 1162, 1026, 603 cm–1; 1H NMR (300 MHz, DMSO-d6 + DCCl3) (δ, ppm): 3.82–3.89 (m, 2H, CH2N), 3.85 (s, 3H, CH3), 4.19–4.27 (m, 2H, NCH2), 7.02 (d, J = 9.0 Hz, 2H, ArH), 7.46 (d, J = 1.5 Hz, 1H, CH), 7.57–7.67 (m, 1H, ArH), 7.83–7.88 (m, 2H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 48.2 (d, J = 8.3 Hz), 5.71, 55.5, 108.0 (t, J = 24.8 Hz), 111.1 (d, J = 10.5 Hz), 113.9, 126.0, 128.3, 131.3, 131.9, 139.8, 140.8, 143.1 (d, J = 18.8 Hz), 144.0, 153.4, 163.8, 190.3; HRMS (TOF ES+) m/z: calcd for C19H14N2O2F3 [M + H], 359.1001; found, 359.0999.
6,7,9-Trifluoro-4-(thiophen-2-yl)methanoneyl-1,2-dihyd-roimidazo[1,2-a]quinoline (3cg)
Orange solid, mp 170–171 °C; IR (KBr): 1640, 1496, 1413, 1280, 1127, 733 cm–1; 1H NMR (600 MHz, DMSO-d6) (δ, ppm): 3.86–3.89 (m, 2H, CH2N), 4.22–4.25 (m, 2H, NCH2), 7.25–7.26 (m, 1H, CH), 7.63 (s, 1H, CH), 7.66–7.72 (m, 1H, ArH), 7.86–7.87 (m, 1H, CH), 8.13–8.14 (m, 1H, CH); 13C NMR (150 MHz, DMSO-d6) (δ, ppm): 48.8, 54.2, 108.9 (m), 111.5 (m), 126.5 (d, J = 12.0 Hz), 127.1, 129.4, 130.8, 136.9, 137.2, 141.1–142.7 (m), 142.0–142.4 (m), 142.9, 143.6–144.0 (m), 153.5, 184.4; HRMS (TOF ES+) m/z: calcd for C16H10N2OF3S [M + H], 335.0460; found, 335.0464.
7,8,10-Trifluoro-5-(4′-fluorophenyl)methanoneyl-2,3-di-hydro-1H-pyrimido[1,2-a]quinoline (3ch)
Yellow solid, mp 179–180 °C; IR (KBr): 2845, 1665, 1639, 1599, 1492, 1265, 1151, 992, 844 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 1.75 (m, 2H, CH2), 3.22 (m, 2H, CH2N), 4.14 (m, 2H, NCH2), 7.32 (t, J = 8.7 Hz, 2H, ArH), 7.43 (s, 1H, CH), 7.66–7.72 (m, 1H, ArH), 7.94–7.97 (m, 2H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 20.1, 43.5, 48.5 (d, J = 17.5 Hz), 108.2 (m), 112.4 (d, J = 18.8 Hz), 116.0 (t, J = 25.6 Hz), 121.3, 127.4, 132.4 (d, J = 10.0 Hz), 133.2 (d, J = 10.0 Hz), 138.5, 141.5 (d, J = 21.3 Hz), 143.5 (d, J = 6.3 Hz), 145.6, 146.8, 165.5 (d, J = 251.3 Hz), 192.5; 19F NMR (470 MHz, DMSO-d6) (δ, ppm): −149.6, −145.5 (t, J = 9.4 Hz), −123.2, −105.3; HRMS (TOF ES+) m/z: calcd for C19H13N2OF4 [M + H], 361.0958; found, 361.0959.
7,8,10-Trifluoro-5-(4′-chlorophenyl)methanoneyl-2,3-di-hydro-1H-pyrimido[1,2-a]quinoline (3ci)
Yellow solid, mp 199–200 °C; IR (KBr): 2924, 1663, 1638, 1600, 1492, 1264, 1088, 991, 842 cm–1; 1H NMR (600 MHz, DMSO-d6 + HClO4) (δ, ppm): 2.16 (m, 2H, CH2), 3.54 (m, 2H, CH2N), 4.67 (m, 2H, NCH2), 7.71 (d, J = 8.2 Hz, 2H, ArH), 7.99 (d, J = 8.3 Hz, 2H, ArH), 8.26–8.31 (m, 1H, ArH), 8.48 (s, 1H, CH); 13C NMR (150 MHz, DMSO-d6 + HClO4) (δ, ppm): 18.3, 39.1, 50.8 (d, J = 19.5 Hz), 112.7 (m), 113.6 (d, J = 16.5 Hz), 124.8, 125.1, 129.6, 132.7, 134.9, 135.1, 140.3, 143.5 (d, J = 6.3 Hz), 145.6, 146.8, 150.7, 190.9; 19F NMR (565 MHz, DMSO-d6 + HClO4) (δ, ppm): −145.3 (m), −138.3 (m), −115.8; HRMS (TOF ES+) m/z: calcd for C19H13N2OF3Cl [M + H], 377.0663; found, 377.0665.
7,8,10-Trifluoro-5-(4′-bromophenyl)methanoneyl-2,3-di-hydro-1H-pyrimido[1,2-a]quinoline (3cj)
Yellow solid, mp 193–194 °C; IR (KBr): 2948, 1669, 1638, 1587, 1493, 1263, 1163, 1070, 906, 844 cm–1; 1H NMR (600 MHz, DMSO-d6) (δ, ppm): 1.74 (m, 2H, CH2), 3.21 (m, 2H, CH2N), 4.14 (m, 2H, NCH2), 7.47 (s, 1H, ArH), 7.71–7.72 (m, 3H, ArH), 7.79–7.81 (m, 2H, ArH); 13C NMR (150 MHz, DMSO-d6 + HClO4) (δ, ppm): 20.2, 43.6, 48.6 (d, J = 16.5 Hz), 108.4 (m), 112.5 (d, J = 18.0 Hz), 121.8, 127.5, 128.0, 131.5, 132.2, 135.6, 138.3, 141.9 (d, J = 12.0 Hz), 143.5, 144.0, 147.0, 193.3; HRMS (TOF ES+) m/z: calcd for C19H13N2OF3Br [M + H], 421.0158; found, 421.0159.
7,8,10-Trifluoro-5-(phenyl)methanoneyl-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3ck)
Yellow solid, mp 191–192 °C; IR (KBr): 2958, 1669, 1638, 1597, 1492, 1267, 1198, 1165, 990, 665 cm–1; 1H NMR (600 MHz, DMSO-d6) (δ, ppm): 1.72–1.76 (m, 2H, CH2), 3.21 (m, 2H, CH2N), 4.13–4.16 (m, 2H, NCH2), 7.42 (s, 1H, CH), 7.48–7.52 (m, 2H, ArH), 7.64 (t, J = 7.4 Hz, 1H, ArH), 7.67–7.72 (m, 1H, ArH), 7.85–7.88 (m, 2H, ArH); 13C NMR (150 MHz, DMSO-d6) (δ, ppm): 20.2, 43.6, 48.6, 108.2 (m), 112.5 (t, J = 10.5 Hz), 121.2, 127.4, 129.1, 129.5, 134.0, 136.4, 138.9, 141.8 (m), 143.4 (m), 145.6 (m), 147.0, 194.0; HRMS (TOF ES+) m/z: calcd for C19H14N2OF3 [M + H], 343.1052; found, 343.1054.
7,8,10-Trifluoro-5-(p-tolyl)methanoneyl-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3cl)
Yellow solid, mp 196–197 °C; IR (KBr): 1663, 1602, 1492, 1268, 1163, 990, 836 cm–1; 1H NMR (300 MHz, DMSO-d6) (δ, ppm): 1.69–1.76 (m, 2H, CH2), 2.37 (s, 3H, CH3), 3.19–3.22 (m, 2H, CH2N), 4.10–4.16 (m, 2H, NCH2), 7.30 (d, J = 7.8 Hz, 2H, ArH), 7.35 (s, 1H, CH), 7.62–7.73 (m, 1H, ArH), 7.75 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (75 MHz, DMSO-d6) (δ, ppm): 19.7, 21.2, 43.0, 48.2, 107.6 (m), 112.0 (d, J = 20.3 Hz), 120.4, 127.0, 129.2, 129.2, 133.5, 138.6, 140.6 (m), 143.7 (m), 142.6–145.8 (m), 144.0, 146.4, 193.1; HRMS (TOF ES+) m/z: calcd for C20H16N2OF3 [M + H], 357.1209; found, 357.1208.
7,8,10-Trifluoro-5-(4′-methoxyphenyl)methanoneyl-2,3-dihydro-1H-pyrimido[1,2-a]quinoline (3cm)
Yellow solid, mp 174–175 °C; IR (KBr): 1659, 1597, 1493, 1257, 1162, 1019, 849 cm–1; 1H NMR (500 MHz, DMSO-d6) (δ, ppm): 1.74–1.76 (m, 2H, CH2), 3.23–3.24 (m, 2H, CH2N), 3.85 (s, 3H, CH3), 4.14–4.15 (m, 2H, NCH2), 7.03 (d, J = 8.8 Hz, 2H, ArH), 7.34 (s, 1H, CH), 7.63–7.73 (m, 1H, ArH), 7.84 (d, J = 8.7 Hz, 2H, ArH); 13C NMR (125 MHz, DMSO-d6) (δ, ppm): 20.1, 43.4, 48.5, 56.0, 108.0 (m), 112.4, 114.3, 120.7, 127.3, 129.3, 131.9, 139.0, 141.6 (m), 143.3 (m), 143.7–145.5 (m), 146.8, 163.9, 192.3; HRMS (TOF ES+) m/z: calcd for C20H16N2O2F3 [M + H], 373.1158; found, 373.1158.
7,8,10-Trifluoro-5-(thiophen-2′-yl)methanoneyl-2,3-dihy-dro-1H-pyrimido[1,2-a]quinoline (3cn)
Yellow solid, mp 178–179 °C; IR (KBr): 2959, 1641, 1599, 1492, 1409, 1256, 1197, 983, 857 cm–1; 1H NMR (600 MHz, DMSO-d6) (δ, ppm): 1.75–1.78 (m, 2H, CH2), 3.26–3.28 (m, 2H, CH2N), 4.12–4.15 (m, 2H, NCH2), 7.20–7.22 (m, 1H, CH), 7.42 (s, 1H, CH), 7.66–7.72 (m, 1H, ArH), 7.75–7.76 (m, 1H, CH), 8.04–8.05 (m, 1H, CH); 13C NMR (150 MHz, DMSO-d6) (δ, ppm): 20.2, 43.5, 48.6, 108.4 (m), 112.3 (t, J = 7.5 Hz), 121.2, 127.5 (d, J = 7.5 Hz), 129.2, 135.8, 136.0, 138.3, 141.7–142.0 (m), 143.4–143.5 (m), 143.5, 143.8–145.5 (m), 146.6, 186.2; HRMS (TOF ES+) m/z: calcd for C17H12N2OF3S [M + H], 349.0616; found, 349.0618.
6,7,9-Trifluoro-4-(4′-fluorophenyl)methanoneyl-8-(piperi-din-1-yl)-1,2-dihydroimidazo-[1,2-a]quinoline (4da)
Red solid, mp 170–171 °C; IR (KBr): 2935, 2851, 1653, 1628, 1482, 1271, 1232, 1156, 1119, 1001, 848, 768, 602 cm–1; 1H NMR (500 MHz, DMSO-d6 + CDCl3) (δ, ppm): 1.62–1.68 (m, 6H, CH2), 3.23 (m, 4H, CH2), 3.84 (t, J = 10.2 Hz, 2H, CH2N), 4.21–4.26 (m, 2H, NCH2), 7.28 (t, J = 8.7 Hz, 2H, ArH), 7.48 (s, 1H, CH), 7.90–7.93 (m, 2H, ArH); 13C NMR (125 MHz, DMSO-d6 + CDCl3) (δ, ppm): 24.0, 26.5, 48.8, 52.2, 53.9, 115.9 (d, J2 = 22.5 Hz), 127.3, 128.7, 132.7 (d, J3 = 10.0 Hz), 133.1, 133.6, 154.0, 165.7 (d, J1 = 252.5 Hz), 191.1; 19F NMR (471 MHz, DMSO-d6 + DCCl3) (δ, ppm): −105.0, −145.9, −148.5, −156.9 (d, J = 18.8 Hz); HRMS (TOF ES+) m/z: calcd for C23H20N3OF4 [M + H], 430.1537; found, 430.1533.
6,7,9-Trifluoro-4-(4′-chlorophenyl)methanoneyl-8-(piperidin-1-yl)-1,2-dihydroimidazo-[1,2-a]quinoline (4db)
Red solid, mp 160–161 °C; IR (KBr): 2932, 2854, 1628, 1483, 1269, 1120, 1090, 1000, 844, 766 cm–1; 1H NMR (300 MHz, DMSO-d6 + CDCl3) (δ, ppm): 1.61 (m, 6H, CH2), 3.22 (m, 4H, CH2), 3.84 (t, J = 10.2 Hz, 2H, CH2N), 4.17–4.26 (m, 2H, NCH2), 7.47 (s, 1H, CH), 7.49 (d, J = 8.7 Hz, 2H, ArH), 7.82 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (75 MHz, DMSO-d6 + CDCl3) (δ, ppm): 23.6, 26.1, 48.3, 51.8, 53.6, 103.2, 126.0, 126.5, 128.5, 128.8, 131.0, 134.7, 138.6, 153.6, 191.0; HRMS (TOF ES+) m/z: calcd for C23H20ClN3OF3 [M + H], 446.1242; found, 446.1239.
6,7,9-Trifluoro-4-(4′-bromophenyl)methanoneyl-8-(piperidin-1-yl)-1,2-dihydroimidazo-[1,2-a]quinoline (4dc)
Orange solid, mp 181–181 °C; IR (KBr): 2933, 2855, 1654, 1633, 1478, 1386, 1270, 1156, 1121, 997, 832, 761 cm–1; 1H NMR (500 MHz, DMSO-d6 + CDCl3) (δ, ppm): 1.62 (m, 6H, CH2), 3.24 (m, 4H, CH2), 3.83 (t, J = 10.2 Hz, 2H, CH2N), 4.21–4.26 (m, 2H, NCH2), 7.52 (s, 1H, CH), 7.69 (d, J = 8.4 Hz, 2H, ArH), 7.76 (d, J = 8.4 Hz, 2H, ArH); 13C NMR (150 MHz, DMSO-d6 + CDCl3) (δ, ppm): 23.80, 26.6, 44.7, 50.8, 52.4, 104.9, 114.7, 124.0, 126.6, 127.0, 128.1, 131.9, 132.4, 135.6, 137.4, 138.1, 139.7, 140.2, 140.5, 141.6, 146.7, 155.2, 190.9; 19F NMR (565 MHz, DMSO-d6 + DCCl3) (δ, ppm): −143.9, −144.0, −148.7 (d, J = 16.9 Hz); HRMS (TOF ES+) m/z: calcd for C23H20N3OF3Br [M + H], 490.0735; found, 490.0737.
6,7,9-Trifluoro-4-(phenyl)methanoneyl-8-(piperidin-1-yl)-1,2-dihydroimidazo[1,2-a]quinoline (4dd)
Orange-red solid, mp 186–187 °C; IR (KBr): 2938, 2853, 1633, 1480, 1456, 1268, 1119, 1000, 656 cm–1; 1H NMR (600 MHz, DMSO-d6) (δ, ppm): 1.62 (m, 6H, CH2), 3.23 (m, 4H, CH2), 3.86 (t, J = 10.3 Hz, 2H, CH2N), 4.21–4.26 (m, 2H, NCH2), 7.43 (s, 1H, CH), 7.46–7.49 (m, 2H, ArH), 7.60–7.62 (m, 1H, ArH), 7.83 (d, J = 7.5 Hz, 2H, ArH); 13C NMR (150 MHz, DMSO-d6) (δ, ppm): 23.7, 26.5, 44.7, 50.7, 52.3, 104.8 (d, J3 = 19.5 Hz), 114.8, 124.0, 129.3, 129.9, 133.9, 136.5, 137.2, 137.9, 139.8, 140.4, 155.2, 191.7; 19F NMR (471 MHz, DMSO-d6 + DCCl3) (δ, ppm): −143.9, −144.6, −149.0 (d, J = 22.6 Hz); HRMS (TOF ES+) m/z: calcd for C23H21N3OF3 [M + H], 412.1631; found, 412.1633.
Acknowledgments
This work was supported by the Program for Changjiang Scholars and Innovative Research Team in University (no. IRT17R94), the National Natural Science Foundation of China (nos. 21662042, 81760621, 21362042, U1202221), the Natural Science Foundation of Yunnan Province (2017FA003), the Reserve Talent Foundation of Yunnan Province for Middle-aged and Young Academic and Technical Leaders (no. 2012HB001), Donglu Schloars of Yunnan University, Excellent Young Talents, Yunnan University, and High-Level Talents Introduction Plan of Yunnan Province.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01856.
Spectroscopic and analytical data as well as the original copy of 1H and 13C NMR spectra of all new compounds and X-ray crystallographic data (CIF file) of compound 3bf (CCDC 1587141) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Agana B. A.; Reeve D.; Orbell J. D. An approach to industrial water conservation–a case study involving two large manufacturing companies based in Australia. J. Environ. Manage. 2013, 114, 445–460. 10.1016/j.jenvman.2012.10.047. [DOI] [PubMed] [Google Scholar]
- Song Q.-W.; Zhou Z.-H.; He L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. 10.1039/c7gc00199a. [DOI] [Google Scholar]
- Marteel-Parrish A. E. Teaching Green and Sustainable Chemistry: A Revised One-Semester Course Based on Inspirations and Challenges. J. Chem. Educ. 2014, 91, 1084–1086. 10.1021/ed400393b. [DOI] [Google Scholar]
- Singh J.; Laurenti R.; Sinha R.; Frostell B. Progress and challenges to the global waste management system. Waste Manage. Res. 2014, 32, 800–812. 10.1177/0734242x14537868. [DOI] [PubMed] [Google Scholar]
- Chemat F.; Vian M. A.; Cravotto G. Green extraction of natural products: concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. 10.3390/ijms13078615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B. M.; Osipov M.; Dong G. Palladium-Catalyzed Dynamic Kinetic Asymmetric Transformations of Vinyl Aziridines with Nitrogen Heterocycles: Rapid Access to Biologically Active Pyrroles and Indoles. J. Am. Chem. Soc. 2010, 132, 15800–15807. 10.1021/ja1071509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.; Cue B. W. Jr.. Green Techniques for Organic Synthesis and Medicinal Chemistry, John Wiley & Sons, Ltd., 2012. [Google Scholar]
- An G.; Seifert C.; Li G. N-Phosphonyl/phosphinyl imines and group-assisted purification (GAP) chemistry/technology. Org. Biomol. Chem. 2015, 13, 1600–1617. 10.1039/c4ob02254h. [DOI] [PubMed] [Google Scholar]
- Qiao S.; Mo J.; Wilcox C. B.; Jiang B.; Li G. Chiral GAP catalysts of phosphonylated imidazolidinones and their applications in asymmetric Diels–Alder and Friedel–Crafts reactions. Org. Biomol. Chem. 2017, 15, 1718–1724. 10.1039/c6ob02801b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.-C.; Chen Z.-Q.; Hao X.-P.; Yan S.-J.; Huang R.; Lin J. Regioselective synthesis of 9,10-dihydro-6H-chromeno[4,3-d]imidazo-[1,2-a]pyridin-6-one derivatives. RSC Adv. 2014, 4, 6110–6115. 10.1039/c3ra46428h. [DOI] [Google Scholar]
- Song C. E.An Overview of Cinchona Alkaloids in Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, 2009; pp 1–10. [Google Scholar]
- Wootton J. C.; Feng X.; Ferdig M. T.; Cooper R. A.; Mu J.; Baruch D. I.; Magill A. J.; Su X.-Z. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature 2002, 418, 320–323. 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- Murphy A. G.; Casey R.; Maguire A.; Tosetto M.; Butler C. T.; Conroy E.; Reynolds A. L.; Sheahan K.; O’Donoghue D.; Gallagher W. M.; Fennelly D.; Kennedy B. N.; O’Sullivan J. Preclinical validation of the small molecule drug quininib as a novel therapeutic for colorectal cancer. Sci. Rep. 2016, 6, 34523. 10.1038/srep34523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinakaran M.; Senthilkumar P.; Yogeeswari P.; China A.; Nagaraja V.; Sriram D. Novel ofloxacin derivatives: synthesis, antimycobacterial and toxicological evaluation. Bioorg. Med. Chem. Lett. 2008, 18, 1229–1236. 10.1016/j.bmcl.2007.11.110. [DOI] [PubMed] [Google Scholar]
- Zhuang Z.-P.; Kung M.-P.; Wilson A.; Lee C.-W.; Plössl K.; Hou C.; Holtzman D. M.; Kung H. F. Structure-Activity Relationship of Imidazo[1,2-a]pyridines as Ligands for Detecting β-Amyloid Plaques in the Brain. J. Med. Chem. 2003, 46, 237–243. 10.1021/jm020351j. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Ang W.; Long H.; Chang Y.; Li Z.; Zhou L.; Yang T.; Deng Y.; Luo Y. Scaffold Hopping Toward Agomelatine: Novel 3,4-Dihydroisoquinoline Compounds as Potential Antidepressant Agents. Sci. Rep. 2016, 6, 34711. 10.1038/srep34711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meanwell N. A. 2015 Philip S. Portoghese Medicinal Chemistry Lectureship. Curing Hepatitis C Virus Infection with Direct-Acting Antiviral Agents: The Arc of a Medicinal Chemistry Triumph. J. Med. Chem. 2016, 59, 7311–7351. 10.1021/acs.jmedchem.6b00915. [DOI] [PubMed] [Google Scholar]
- Hu Y.; Green N.; Gavrin L. K.; Janz K.; Kaila N.; Li H.-Q.; Thomason J. R.; Cuozzo J. W.; Hall J. P.; Hsu S.; Nickerson-Nutter C.; Telliez J.-B.; Lin L.-L.; Tam S. Inhibition of Tpl2 kinase and TNFalpha production with quinoline-3-carbonitriles for the treatment of rheumatoid arthritis. Bioorg. Med. Chem. Lett. 2006, 16, 6067–6072. 10.1016/j.bmcl.2006.08.102. [DOI] [PubMed] [Google Scholar]
- Zheng J.; Li Z.; Huang L.; Wu W.; Li J.; Jiang H. Palladium-Catalyzed Intermolecular Aerobic Annulation of o-Alkenylanilines and Alkynes for Quinoline Synthesis. Org. Lett. 2016, 18, 3514–3517. 10.1021/acs.orglett.6b01008. [DOI] [PubMed] [Google Scholar]
- Yu X.; Wang J.; Xu Z.; Yamamoto Y.; Bao M. Copper-Catalyzed Aza-Diels–Alder Reaction and Halogenation: An Approach To Synthesize 7-Halogenated Chromeno-quinolines. Org. Lett. 2016, 18, 2491–2494. 10.1021/acs.orglett.6b01065. [DOI] [PubMed] [Google Scholar]
- Neuhaus J. D.; Morrow S. M.; Brunavs M.; Willis M. C. Diversely Substituted Quinolines via Rhodium-Catalyzed Alkyne Hydroacylation. Org. Lett. 2016, 18, 1562–1565. 10.1021/acs.orglett.6b00390. [DOI] [PubMed] [Google Scholar]
- Min L.; Pan B.; Gu Y. Synthesis of Quinoline-Fused 1-Benzazepines through a Mannich-Type Reaction of a C,N-Bisnucleophile Generated from 2-Aminobenzaldehyde and 2-Methylindole. Org. Lett. 2016, 18, 364–367. 10.1021/acs.orglett.5b03287. [DOI] [PubMed] [Google Scholar]
- Wakade S. B.; Tiwari D. K.; Prabhakar Ganesh P. S. K.; Phanindrudu M.; Likhar P. R.; Tiwari D. K. Transition-Metal-Free Quinoline Synthesis from Acetophenones and Anthranils via Sequential One-Carbon Homologation/Conjugate Addition/Annulation Cascade. Org. Lett. 2017, 19, 4948–4951. 10.1021/acs.orglett.7b02429. [DOI] [PubMed] [Google Scholar]
- Chi Y.; Yan H.; Zhang W.-X.; Xi Z. Synthesis of Quinoline Derivatives via Cu-Catalyzed Cascade Annulation of Heterocumulenes, Alkynes, and Diaryliodonium Salts. Org. Lett. 2017, 19, 2694–2697. 10.1021/acs.orglett.7b01025. [DOI] [PubMed] [Google Scholar]
- Mao X.-F.; Zhu X.-P.; Li D.-Y.; Liu P.-N. Cu-Catalyzed Cascade Annulation of Alkynols with 2-Azidobenzaldehydes: Access to 6H-Isochromeno[4,3-c]quinoline. J. Org. Chem. 2017, 82, 7032–7039. 10.1021/acs.joc.7b00937. [DOI] [PubMed] [Google Scholar]
- Xu H.; Zhou P.; Zhou B.; Zhou J.; Shen Y.; Lu L.-L.; Yu F.-C. Convenient one-step synthesis of pyrrolo[3,4-c]quinolin-1-ones via TMSCl-catalyzed cascade reactions of isatins and β-enamino ketones. RSC Adv. 2016, 6, 73760–73768. 10.1039/c6ra15492a. [DOI] [Google Scholar]
- Xu H.; Zhou B.; Zhou P.; Zhou J.; Shen Y.; Yu F.-C.; Lu L.-L. Insights into the unexpected chemoselectivity in Bronsted acid catalyzed cyclization of isatins with enaminones: convenient synthesis of pyrrolo[3,4-c]quinolin-1-ones and spirooxindoles. Chem. Commun. 2016, 52, 8002–8005. 10.1039/c6cc02659a. [DOI] [PubMed] [Google Scholar]
- Midya S. P.; Sahoo M. K.; Landge V. G.; Rajamohanan P. R.; Balaraman E. Reversed reactivity of anilines with alkynes in the rhodium-catalysed C-H activation/carbonylation tandem. Nat. Commun. 2015, 6, 8591. 10.1038/ncomms9591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manan R. S.; Zhao P. Merging rhodium-catalysed C-H activation and hydroamination in a highly selective [4+2] imine/alkyne annulation. Nat. Commun. 2016, 7, 11506. 10.1038/ncomms11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller S. T.; Kiho T.; Narayan A. R. H.; Sarpong R. Protic-solvent-mediated cycloisomerization of quinoline and isoquinoline propargylic alcohols: syntheses of (+/-)-3-demethoxyerythratidinone and (+/-)-cocculidine. Angew. Chem., Int. Ed. 2013, 52, 11129–11133. 10.1002/anie.201304687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastalir M.; Glatz M.; Pittenauer E.; Allmaier G.; Kirchner K. Sustainable Synthesis of Quinolines and Pyrimidines Catalyzed by Manganese PNP Pincer Complexes. J. Am. Chem. Soc. 2016, 138, 15543–15546. 10.1021/jacs.6b10433. [DOI] [PubMed] [Google Scholar]
- Jiang H.; Cheng Y.; Wang R.; Zheng M.; Zhang Y.; Yu S. Synthesis of 6-alkylated phenanthridine derivatives using photoredox neutral somophilic isocyanide insertion. Angew. Chem., Int. Ed. 2013, 52, 13289–13292. 10.1002/anie.201308376. [DOI] [PubMed] [Google Scholar]
- Yu F.; Yan S.; Hu L.; Wang Y.; Lin J. Cascade reaction of isatins with heterocyclic ketene aminals: synthesis of imidazopyrroloquinoline derivatives. Org. Lett. 2011, 13, 4782–4785. 10.1021/ol201783d. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-C.; Liu Z.-C.; Yang R.; Zhang J.-H.; Yan S.-J.; Lin J. Regioselective construction of 1,3-diazaheterocycle fused [1,2-a][1,8]naphthyridine derivatives via cascade reaction of quinolines with heterocyclic ketene aminals: a joint experimental-computational approach. Org. Biomol. Chem. 2013, 11, 7276–7288. 10.1039/c3ob41200h. [DOI] [PubMed] [Google Scholar]
- Yan S.; Huang C.; Su C.; Ni Y.; Lin J. Facile Route to 1,3-Diazaheterocycle-Fused [1,2-b]Isoquinolin-1(2H)-one Derivatives via Substitution-Cyclization Reactions. J. Comb. Chem. 2010, 12, 91–94. 10.1021/cc900121c. [DOI] [PubMed] [Google Scholar]
- Zhou B.; Liu Z.-C.; Qu W.-W.; Yang R.; Lin X.-R.; Yan S.-J.; Lin J. An environmentally benign, mild, and catalyst-free reaction of quinones with heterocyclic ketene aminals in ethanol: site-selective synthesis of rarely fused [1,2-a]indolone derivatives via an unexpected anti-Nenitzescu strategy. Green Chem. 2014, 16, 4359–4370. 10.1039/c4gc00676c. [DOI] [Google Scholar]
- Yu F.; Huang R.; Ni H.; Fan J.; Yan S.; Lin J. Three-component stereoselective synthesis of spirooxindole derivatives. Green Chem. 2013, 15, 453–462. 10.1039/c2gc36552a. [DOI] [Google Scholar]
- Chen X.-B.; Liu X.-M.; Huang R.; Yan S.-J.; Lin J. Three-Component Synthesis of Indanone-Fused Spirooxindole Derivatives. Eur. J. Org. Chem. 2013, 21, 4607–4613. 10.1002/ejoc.201300376. [DOI] [Google Scholar]
- Yan S.-j.; Chen Y.-j.; Liu L.; Tang Y.-j.; Lin J. Synthesis of novel tetracyclo-isocoumarins via AcOH-catalyzed cascade reaction of heterocyclic ketene aminals with 2,2-dihydroxy-2H-indene-1,3-dione. Tetrahedron Lett. 2011, 52, 465–467. 10.1016/j.tetlet.2010.11.100. [DOI] [Google Scholar]
- Li M.; Zhou Z.-M.; Wen L.-R.; Qiu Z.-X. Chemistry of Heterocyclic Ketene Aminals: Construction of Imidazo(pyrido)[1,2-a]pyridines and Imidazo(pyrido)[3,2,1-ij][1,8]naph-thyridines via DABCO-Catalyzed Tandem Annulations. J. Org. Chem. 2011, 76, 3054–3063. 10.1021/jo102167g. [DOI] [PubMed] [Google Scholar]
- Li M.; Shao P.; Wang S.-W.; Kong W.; Wen L.-R. Four-Component Cascade Heteroannulation of Heterocyclic Ketene Aminals: Synthesis of Functionalized Tetrahydroimidazo[1,2-a ]pyridine Derivatives. J. Org. Chem. 2012, 77, 8956–8967. 10.1021/jo3013836. [DOI] [PubMed] [Google Scholar]
- Chen L.; Huang R.; Du X.-X.; Yan S.-J.; Lin J. One-Pot Synthesis of Highly Functionalized Bicyclic Imidazopyridinium Derivatives in Ethanol. ACS Sustainable Chem. Eng. 2017, 5, 1899–1905. 10.1021/acssuschemeng.6b02622. [DOI] [Google Scholar]
- Zeng C.-C.; Liu F.-J.; Ping D.-W.; Hu L.-M.; Cai Y.-L.; Zhong R.-G. One-Pot Electrochemical Synthesis of Fused Indole Derivatives Containing Active Hydroxyl Groups in Aqueous Medium. J. Org. Chem. 2009, 74, 6386–6389. 10.1021/jo901091s. [DOI] [PubMed] [Google Scholar]
- Yaqub M.; Arif N.; Perveen R.; Batool J.; Riaz M. T.; Yaseen M. One-pot three component cascade synthesis of fused ring quinazoline-2,4-dione derivatives employing heterocyclic ketene aminals as a versatile synthone. Asian J. Chem. 2015, 27, 1013–1018. 10.14233/ajchem.2015.18004. [DOI] [Google Scholar]
- Chen X.-B.; Liu Z.-C.; Lin X.-R.; Huang R.; Yan S.-J.; Lin J. Highly Diastereoselective Convergent Synthesis of Polycyclic Pyrroles with Consecutive Quaternary Stereocenters: Cascade Construction of Multiple C-C and C-Hetero Bonds. ACS Sustainable Chem. Eng. 2014, 2, 2391–2398. 10.1021/sc5004105. [DOI] [Google Scholar]
- Ma Y.-L.; Wang K.-M.; Huang R.; Lin J.; Yan S.-J. An environmentally benign double Michael addition reaction of heterocyclic ketene aminals with quinone monoketals for diastereoselective synthesis of highly functionalized morphan derivatives in water. Green Chem. 2017, 19, 3574–3584. 10.1039/c7gc01435j. [DOI] [Google Scholar]
- Chen X.-B.; Liu Z.-C.; Yang L.-F.; Yan S.-J.; Lin J. A Three-Component Catalyst-Free Approach to Regioselective Synthesis of Dual Highly Functionalized Fused Pyrrole Derivatives in Water–Ethanol Media: Thermodynamics versus Kinetics. ACS Sustainable Chem. Eng. 2014, 2, 1155–1163. 10.1021/sc500170d. [DOI] [Google Scholar]
- Yan S.-J.; Liu Y.-J.; Chen Y.-L.; Liu L.; Lin J. An efficient one-pot synthesis of heterocycle-fused 1,2,3-triazole derivatives as anti-cancer agents. Bioorg. Med. Chem. Lett. 2010, 20, 5225–5228. 10.1016/j.bmcl.2010.06.141. [DOI] [PubMed] [Google Scholar]
- Huang C.; Yan S.-J.; Zeng X.-H.; Dai X.-Y.; Zhang Y.; Qing C.; Lin J. Biological evaluation of polyhalo 1,3-diazaheterocycle fused isoquinolin-1(2H)-imine derivatives Eur. J. Med. Chem. 2011, 46, 1172–1180. 10.1016/j.ejmech.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Yu F.-C.; Lin X.-R.; Liu Z.-C.; Zhang J.-H.; Liu F.-F.; Wu W.; Ma Y.-L.; Qu W.-W.; Yan S.-J.; Lin J. Beyond the Antagonism: Self-Labeled Xanthone Inhibitors as Modeled “Two-in-One” Drugs in Cancer Therapy. ACS Omega 2017, 2, 873–889. 10.1021/acsomega.6b00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X.-X.; Huang R.; Yang C.-L.; Lin J.; Yan S.-J. Synthesis and evaluation of the antitumor activity of highly functionalised pyridin-2-ones and pyrimidin-4-ones. RSC Adv. 2017, 7, 40067–40073. 10.1039/c7ra06466g. [DOI] [Google Scholar]
- Chen N.; Meng X.; Zhu F.; Cheng J.; Shao X.; Li Z. Tetrahydroindeno [1′,2′:4,5]pyrrolo[1,2-a]imidazole-5(1H)-ones as Novel Neonicotinoid Insecticides: Reaction Selectivity and Substituent Effects on the Activity Level. J. Agric. Food Chem. 2015, 63, 1360–1369. 10.1021/jf505281p. [DOI] [PubMed] [Google Scholar]
- Bao H.; Shao X.; Zhang Y.; Deng Y.; Xu X.; Liu Z.; Li Z. Specific Synergist for Neonicotinoid Insecticides: IPPA08, a cis-Neonicotinoid Compound with a Unique Oxabridged Substructure. J. Agric. Food Chem. 2016, 64, 5148–5155. 10.1021/acs.jafc.6b01512. [DOI] [PubMed] [Google Scholar]
- Suryawanshi S. N.; Pandey S.; Rashmirathi; Bhatt B. A.; Gupta S. Chemotherapy of leishmaniasis Part VI: Synthesis and bioevaluation of some novel terpenyl S,N- and N,N-acetals. Eur. J. Med. Chem. 2007, 42, 511–516. 10.1016/j.ejmech.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Kondo H.; Taguchi M.; Inoue Y.; Sakamoto F.; Tsukamoto G. Synthesis and antibacterial activity of thiazolo-, oxazolo-, and imidazolo[3,2-a][1,8]naphthyri-dinecarboxylic acids. J. Med. Chem. 1990, 33, 2012–2015. 10.1021/jm00169a033. [DOI] [PubMed] [Google Scholar]
- Abdelhalim M. M.; El-Saidi M. M. T.; Rabie S. T.; Elmegeed G. A. Synthesis of novel steroidal heterocyclic derivatives as antibacterial agents. Steroids 2007, 72, 459–465. 10.1016/j.steroids.2007.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.